Abstract
Ovarian cancer is a highly lethal form of cancer in females, largely due to extensive metastases that often accompany the initial diagnosis. Exosomes are microvesicles size from 30 to 100nm, which can be secreted by most cells. These special extracellular vesicles play a vital role in the metastasis of ovarian cancer. In this study, we conducted a comprehensive review of current research pertaining to the role of exosomes in ovarian cancer, utilizing the PubMed® and Web of Science databases. Our review highlights the progress in elucidating the mechanisms by which exosomes facilitate ovarian cancer progression. Additionally, we discuss the potential of exosomes as a novel therapeutic target for ovarian cancer treatment. Overall, our review provides valuable insights into the current state of research on exosomes in ovarian cancer therapy.
Keywords: Ovarian cancer, exosome, cancer therapy, metastasis, drug carrier
Introduction
Worldwide, ovarian cancer accounts for the top five of cancer-related mortality in women. About 80% of cases are diagnosed at advanced international federation of gynecology and obstetrics (FIGO) stages (Ⅲ, Ⅳ) with poor prognosis. In 2021, The American Cancer Society estimated 21,410 new cases of ovarian cancer diagnosed and 13,770 death1. Current diagnostic methods for monitoring ovarian cancer include pelvic examination, transvaginal ultrasound, and the serological marker CA125 (carbohydrate antigen 125). However, these methods are not sufficient for early diagnosis of ovarian cancer due to the limitation in both sensitivity and specificity2. It has generally been accepted that ovarian cancer cells migrate directly into the peritoneal cavity and omentum through the peritoneal fluid. In addition, epithelial ovarian cancer (EOC) can also spread through blood-borne metastatic routes3.
Many studies have shown that cell-to-cell communication in the tumor microenvironment passes through extracellular vesicles (EVs), mainly by a particular type of EVs called exosomes4-6. These special EVs carry various biomolecules, including proteins, glycans, lipids, metabolites, RNA and DNA4. Multiple evidences point out that exosomes play an important role in the metastasis of ovarian cancer by promoting ovarian cancer cell adhesion, invasion, angiogenesis, facilitating immune evasion and chemoresistance and are the main mediators of tumor-stroma crosstalk7-10. Tumor-derived exosomes can support tumor cell survival and promote metastasis by reprogramming or educating other cells8, 11. It has been shown that ovarian cancer metastasis is frequently facilitated by the interaction of exosomes with the tumor microenvironment12-14. Release of exosomal contents can modulate the fate of recipient cells. MicroRNAs (miRNAs) and non-coding RNAs are the main RNA species transported by exosomes, and in addition, mRNA, rRNA and tRNA have also been reported in exosomes15. In this review, we summarize recent advances on the role of exosomes in ovarian cancer metastasis and the potential applications of exosomes in diagnosis and treatment of ovarian cancer.
Role of exosomes in the invasion and metastasis of ovarian cancer
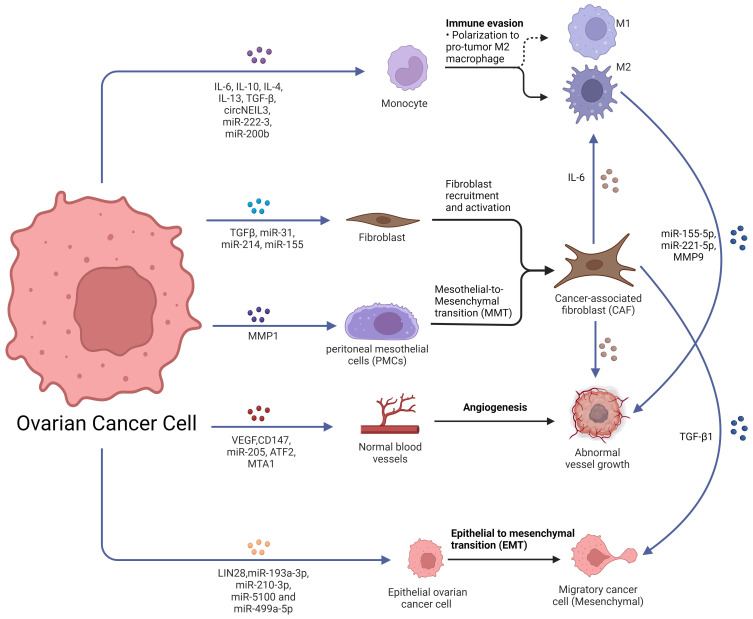
Unlike other tumors, which typically metastasize through hematogenous or lymphatic pathways, ovarian cancer cells usually metastasize in the peritoneal cavity and greater omentum in the initial stages by some passive mechanism. Much evidence suggests that ovarian cancer metastasis is closely linked to intercellular communication in the tumor microenvironment, and these links can be achieved by soluble factors and EVs (Fig. 1).
Figure 1.
Role of exosomes in ovarian cancer metastasis. Ovarian cancer-derived exosomes with their cargoes promote TAMs polarization, fibroblast recruitment and activation and transfer normal fibroblasts and PMCs into CAFs. Ovarian cancer-derived exosomes promote angiogenesis and EMT. Furthermore, CAF-derived exosomes also promote TAMs polarization, angiogenesis and EMT. TAMs-derived exosomes promoted the malignant phenotype of EOC. Created with BioRender.com.
Pre-metastatic niche
The crosstalk between tumor cells and stroma is a crucial feature of tumor progression and metastasis. Pre-metastatic niches (PMNs) are formed by changes occurring in distant organs that prepare for colonization and growth of circulating tumor cells (CTCs), leading to the formation of distant metastases 16. Notably, in addition to intercellular communication in the microenvironment of the primary tumor, tumor can transmit signals to distant organs to promote the formation of PMNs. In PMN models, organs colonized at the time of tumor metastasis are predetermined by the initiation and coordination of primary tumor-secreted factors 17.
The primary tumor secretes cytokines and exosomes which target specific organs, inducing their changes to create a hospitable environment for CTCs (Fig. 2A) 18. Evidence suggests that ovarian cancer-derived exosomes can change PMNs by promoting angiogenesis, immunosuppression, stromal remodeling and transition of cancer-associated fibroblasts (CAFs), helping ovarian cancer to thrive in new environments 19-22.
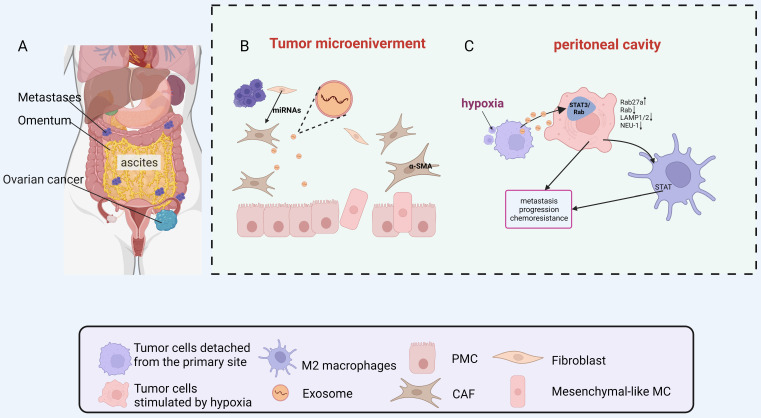
Figure 2.
Metastasis of ovarian cancer to the peritoneal cavity through malignant ascites. A. Metastases distributed in the peritoneal cavity. B. MiRNAs secreted by ovarian cancer cells were taken in the PMCs, and PMCs were converted into CAFs. C. The hypoxic ovarian cancer cells deliver exosomes to induce the polarization of M2 macrophages and promote a more aggressive phenotype of cancer cell. Abbreviations: PMC: peritoneal mesothelial cells; CAF: cancer-associated fibroblast; STAT: Signal transducer and activator of transcription; MC: mesothelial cell; miRNA: microRNA. Created with BioRender.com.
Exosomes mediate epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT) is a process characterized by loss of polarity of the epithelium and transformation into mesenchymal cells, a phenomenon observed early in tumor invasion and metastasis 23. MiRNAs such as miR-193a-3p, miR-210-3p, miR-5100 and miR-499a-5p can be transmitted via exosomes to help promote tumor metastasis and EMT 24, 25. Exosomes secreted by ovarian cancer cells are also involved in EMT. LIN28, a proto-oncogene secreted in exosomes derived from ovarian cancer cell line IGROV1, has been shown to promote ovarian cancer cells migration and invasion, increasing the expression of EMT-related genes such as ZEB1, NOTCH1, WNT5A, NODAL and SNAI226, 27. Additionally, transforming growth factor beta Ⅰ (TGF-β Ⅰ) secreted by CAF-derived exosomes has been demonstrated to induce EMT in ovarian cancer cells in vitro28. However, further validation is needed through in vivo experiments.
The relationship between cancer-associated fibroblasts and exosomes
CAFs are activated fibroblast subsets that play an essential role in the progression and metastasis of various tumors22, 29. Tumor cells induce CAFs formation through the TGF-β/SMAD pathway, whereby free TGF-β secreted by tumor cells binds to TGF-β Ⅰ and Ⅱ receptors in fibroblasts, prompting downstream SMAD2 and SMAD3 phosphorylation and further forming a complex with SMAD4, resulting in up-regulation of α-SMA transcription30. Furthermore, tumor-derived exosomes carry many miRNAs that influence gene transcription by being taken up by fibroblasts and prompt the differentiation of fibroblast into CAFs5, 31. Several studies have already reported the role of exosomes in tumor stromal cell activation32-34, yet the mechanism by which ovarian cancer cell-derived exosomes convert normal fibroblasts into CAFs has been less investigated. For example, exosomes derived from ovarian cancer cell line SKOV3 were found to significantly promote proliferation and motility of normal fibroblasts, as well as increase expression of typical markers for CAFs such as α-SMA and tissue inhibitor of metalloproteinase 2 (TIMP2) 35. Additionally, peritoneal mesothelial cells (PMCs) can be converted into CAFs through mesothelial-to-mesenchymal transition (MMT) processes during ovarian cancer peritoneal metastasis (Fig. 2B). Omentum mesothelial cells (MCs) incubated with SKOV3 cell conditioned medium acquired a spindle morphology with increased expression of MMT markers such as α-SMA, fibronectin, collagen Ⅰ, vascular endothelial growth factor (VEGF) and TGF-β Ⅰ 36, indicating accumulation of large numbers of CAFs from PMCs via MMT processes is essential for tumor peritoneal metastasis 36-38. During the development of MMT, exosomes participate in adhesion between tumor cells and MCs. They also facilitate the co-invasion of PMC derived CAFs and tumor cells into the peritoneal matrix 39.
CAF-derived exosomes have been found to be involved in the metastasis of tumor cells and immune escape40-42. Yan et al. discovered that exosomes derived from CAFs in metastatic ovarian cancer tissues had significantly higher levels of TGF-β Ⅰ compared to exosomes from fibroblasts in normal omental tissue. This increased TGF-β Ⅰ was found to promote the process of EMT in ovarian cancer cells 42. Moreover, these exosomes can induce the transformation of normal fibroblasts into CAFs by modulating the expression of miRNA-31, miRNA-214, and miRNA-155, which are differentially expressed between normal fibroblasts and ovarian cancer-associated fibroblasts43.
Exosomes promote angiogenesis
Tumor-derived exosomes have been found to be involved in the process of angiogenesis, which is essential for tumor progression and metastasis10, 44. These exosomes are known to contain proangiogenic proteins such as angiogenin, VEGF, interleukin-6 (IL-6), and interleukin-8 (IL-8)45, 46, as well as encapsulate angiogenic factors which can be translocated to endothelial cells47, 48. As an example, exosomes highly expressing miR-205 secreted by ovarian cancer cells can promote tumor metastasis by inducing angiogenesis via the PTEN-AKT pathway11. Similarly, miR-130a has been found to be involved in promoting angiogenesis by chemoresistant ovarian cancer cell secreted exosomes 47. The activation of transcription factor 2 (ATF2) and metastasis-associated protein 1 (MTA1) upregulated angiogenesis in ovarian cancer-derived exosomes49, indicating the vital role of these vesicles in tumor angiogenesis.
In addition to tumor-derived exosomes, those secreted by tumor-associated macrophages (TAMs) and CAFs have also been found to be involved in the promotion of angiogenesis and metastasis50-52. For instance, M2 macrophage-derived exosomal miR-155-5p and miR-221-5p have been reported to induce angiogenesis of pancreatic ductal adenocarcinoma in an E2F2 dependent manner53. Although there are few relevant reports on ovarian cancer, it is likely that these vesicles may also play a role in ovarian cancer progression.
Relationship between exosomes and TAMs
In contrast to other solid tumors, ovarian cancers in advanced FIGO stage are often characterized by a unique tumor microenvironment termed "malignant ascites", which contains a variety of components including single floating cells or multicellular spheroids, immune cells, fibroblasts, adipocytes, MCs, EVs, cytokines, growth factors and lipid mediators54, 55. The predominant immune cell population in this environment consists of macrophages; mainly tissue-resident macrophages and infiltrating recruited macrophages from bone marrow-derived monocytes56. These cells can be converted into TAMs through soluble factors present in the tumor microenvironment such as IL-6, interleukin-10 (IL-10), interleukin-4 (IL-4), interleukin-13 (IL-13) and TGF-β57-60.
TAMs are generally considered to be immunosuppressive cells that can promote tumor phenotypes and thus facilitate tumor growth and progression 61. They constitute the primary immune cell population within the ovarian cancer microenvironment, showing two main phenotypes depending on stimulus: anti‑tumorigenic macrophages M1-like or pro‑tumorigenic macrophages M2-like62. However, within this environment, TAMs are often polarized towards immunosuppressive M2-like phenotypes with high expression levels for scavenger receptor class B (CD163), mannose receptor (CD204), IL-10, chemokines CCL18 or CCL2263.
Ovarian cancer-derived exosomes are rich in various miRNAs and have been found to play an essential role in converting an M2 like phenotype. For example, EOC derived exosome miR-222-3p has been shown to promote TAMs polarization to inhibit anti-tumor immunity via activation of the signal transducer and activator of transcription 3 (STAT3) pathway64 , while elevated miR-200b levels in the plasma of ovarian cancer patients have been reported to promote macrophage M2 polarization but inhibit M1 polarization65, with higher expression levels enhancing proliferation and invasion of tumor cells.
TAMs have been found to affect both tumor cells and other immune cells in order to achieve tumor escape. Zhu et al. discovered that exosomes derived from macrophages under hypoxia and enriched with miR-223 could promote the malignant phenotype of EOC through the PTEN-PI3K/AKT pathway66. Additionally, TAMs-derived exosomes containing miR-29a-3p and miR-21-5p could inhibit STAT3, regulate Treg/Th17 cells, create an imbalance of these cell populations and ultimately generate an immune suppressive microenvironment that facilitates EOC progression and metastasis67.
Relationship between Hypoxia and Exosomes
During peritoneal metastasis of ovarian cancer, tumor cells detach from the primary site to form single cells or multicellular tumorspheres into the peritoneal cavity. In this process, tumor cells are exposed to hypoxic conditions due to the inability to obtain a vascular supply 68. Hypoxic conditions can lead to the acquisition of a more aggressive malignant phenotype by tumor cells, allowing them to survive and colonize the peritoneal cavity to form metastases69, 70. Research on ovarian cancer has provided additional evidence to support the idea that hypoxia can trigger an increase in exosome production. Studies have shown that hypoxia can prompt ovarian cancer cells to release more exosomes, which in turn promote a more aggressive cancer phenotype and contribute to chemoresistance through the activation of STAT3/Rab proteins 71. These findings suggest that exosomes may play a crucial role in driving changes in tumor cells within a hypoxic microenvironment (Fig. 2C).
Notably, exosomes secreted by hypoxic ovarian cancer cells have been found to act on both ovarian cancer cells and other cells in the tumor microenvironment, such as CAFs and TAMs. For instance, high expression of miR‑940 in exosomes derived from EOC cells could induce macrophages to differentiate into the M2 phenotype, thereby promoting the proliferation and metastasis of EOC 72. Furthermore, Chen et al. demonstrated that exosomes secreted by hypoxic EOC cells can induce M2 macrophage polarization, thus promoting the proliferation and migration of EOC. Moreover, hypoxia-inducible factors were found to be crucial in this process. Microarray analysis of normoxic and hypoxic exosomes revealed that miR-21-3p, miR-125b-5p, and miR-181d-5p were enriched in hypoxic exosomes, which may be the key mediators of the tumor-promoting phenotype 73.
Relationship between exosomes and blood-borne metastatic ovarian cancer
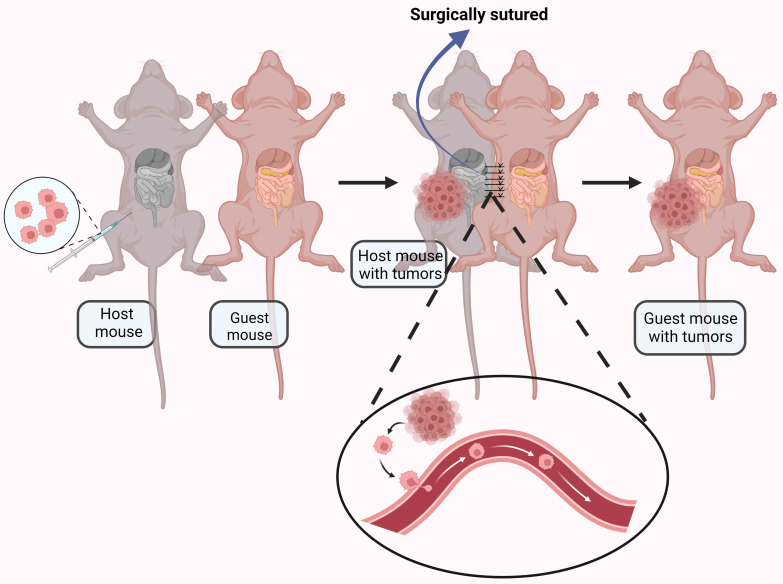
It is generally accepted that ovarian cancer metastasis results from direct surface spread and dissemination of detached tumor cells into the peritoneal cavity74. However, CTCs have observed in the blood of ovarian cancer patients 75, 76, and a significant number of ovarian cancer patients have distant metastasis at initial diagnosis 77, suggesting hematogenous metastasis may be possible. To explore this phenomenon, a parabiosis model was used to analyze underlying mechanisms of ovarian cancer spread78. A mouse with ovarian cancer was surgically anastomosed with a guest mouse without cancer. Metastasize to the omentum was found in the guest mouse, indicating hematogenous metastasis (Fig. 3). The paired mice shared blood but not lymphatic vessels, providing evidence for alternative strategies for prevention and treatment of ovarian cancer metastasis.
Figure 3.
An ovarian cancer-bearing mouse and a healthy mouse were anastomosed together by surgical suturing. The ovarian cancer cells were transferred to the healthy mouse through the blood vessels connected between the mouse. Created with BioRender.com.
Exosome-derived cargoes are a critical factor in tumor metastasis. Exosome-derived cargoes are known to play an important role in tumor metastasis. Several studies have investigated crucial biomolecules carried by circulating exosomes in ovarian cancer plasma79, 80. A study showed that circulating exosomal circular forkhead box protein P1 (circFoxp1) was positively associated with distant metastasis, residual tumor diameter, and clinical response81. These findings suggest that hematogenous metastasis to the greater omentum may be as likely as direct dissemination in some cases—a potential mode of spread often overlooked.
Clinical application of exosomes in ovarian cancer
Exosomes in early diagnosis of ovarian cancer
At the initial diagnosis, most ovarian cancer patients have already developed peritoneal metastases3. Cancer metastasis is a lengthy process long before the second tumor engraftment, and exosomes play a critical role in this process. Therefore, exosome detection may offer the possibility of early diagnosis. Compared with non-exosomal detection methods, exosomes possess unique advantages such as their stable biological structure that prevents cargoes from RNase degradation. Numerous studies have demonstrated the potential of exosomes as biomarkers for ovarian metastasis (Table 1). For instance, CD24 and epithelial cell adhesion molecule (EpCAM) were found to be highly expressed in tumor-derived exosomes and were thus associated with ovarian cancer development55. Moreover, all samples tested positive for CD24 in ascites-derived exosomes even when the corresponding tumors were negative, which suggests that the ascites exosome test could be a sensitive method for diagnosing ovarian cancer. A National Cancer Institute (NCI-60) study further revealed that only 213 proteins were shared among 60 tumor cell lines from 9 different cancers 82, implying that exosome-specific proteins could potentially serve as tumor markers. In an effort to identify potential biomarkers of EOC, a cohort study used liquid chromatography tandem mass spectrometry (LC-MS/MS) with tandem mass tagging (TMT) to perform a comprehensive proteomic analysis of patient plasma derived from ovarian cancer-associated exosomes. They identified 50 genes which exhibited differential expression between EOC patients and those with benign diseases; bioinformatics analysis eventually selected GSN, FGG, FGA and LBP as potential diagnostic and prognostic biomarkers for EOC83. Additionally, miRNAs extracted from circulating exosomes have been explored as diagnostic markers for ovarian cancer since 200884, with increasing number of studies being conducted on this topic over recent years85-87. For example, miR-99a-5p was significantly increased in microarray analysis of ovarian cancer derived exosomes; serum miR-99a levels in patients suffering from this disease were 1.7 and 2.8 times higher than those observed in benign tumor patients or healthy individuals respectively with high specificity (0.75) and sensitivity (0.85), thereby indicating its utility as a potential marker for early diagnosis of ovarian cancer88. Taken together, these findings point to the potential clinical value of utilizing exosomes for early diagnosis of ovarian cancer despite current limitations regarding extraction methods or detection techniques thereof. Nonetheless its attractive promise remains intact making it an option moving forward into clinical application.
Table 1.
Potential exosomal biomarkers of diagnosis of ovarian cancer.
| Type of factors | Factors | Source | Subtypes | Reference |
|---|---|---|---|---|
| protein | GSN, FGG, FGA and LBP | Plasma | EOCs | 83 |
| protein | CD24 EpCAM | Ascites | NA | 55 |
| miRNA | miR-99a-5p | Serum | EOCs | 88 |
| miRNA | miR-145, miR-200c, miR-21 and miR-93 | Serum | EOCs | 85 |
| miRNA | miR-21, miR-100, miR-200b, miR-320, miR-16, miR-93, miR-126 and miR-223 | Plasma | EOCs | 86 |
| miRNA | miR-21, miR-141, miR-200a/b/c, miR-203, miR-205 and miR-214 | Serum | HGSC | 84 |
| miRNA | miR-200a, miR-200b and miR-200c | Serum | EOCs | 87 |
Abbreviations: NA: not available; miRNA: microRNA; HGSC: high-grade serous carcinoma; EOC: epithelial ovarian cancer.
Exosomes in the treatment of ovarian cancer
Exosomes as therapeutic targets
Tumor-derived exosomes have been implicated in promoting tumor progression and drug resistance 10, 89. Recently, research on their potential therapeutic applications has become a hot topic. For instance, GW4869, an inhibitor of exosome release, was found to attenuate CD44 expression and reverse EMT in PMCs by inhibiting exosome release in ovarian cancer cells, thereby inhibiting ovarian cancer invasion 13. In another study, researchers utilized GW4869 treatment to inhibit miR-205 uptake by human umbilical vein endothelial cells (HUVECs), which effectively suppressed the invasion and angiogenesis of ovarian cancer. The same effect could also be achieved by filipin (an inhibitor of lipid raft-dependent and caveolar endocytosis) 11. Furthermore, stromal-derived exosomal miR-21 has been shown to promote paclitaxel resistance in ovarian cancer cells through APF1 90. These findings suggest that targeting and inhibiting exosome-derived miRNAs may serve as an adjuvant therapy for treating metastasis and drug resistance in ovarian cancer.
Exosomes as Drug Carriers
Exosomes possess several advantages, such as high tolerance, long circulation half-life, low toxicity, low immunogenicity, and inherent homing ability 91. Moreover, they can be genetically engineered to cross biological barriers such as the blood-brain barrier and carry a variety of drug molecules; thus preventing drug degradation 92. Exosomal formulations of antineoplastic agents have already been tested. For instance, exosomes containing triptolide were found to inhibit ovarian cancer cell proliferation as well as tumor growth and have good therapeutic potential 93. Additionally, berry anthocyanins encapsulated with exosomes as carriers exhibited promising therapeutic effects on both drug-sensitive and drug-resistant ovarian cancer cells; when combined with cisplatin it synergistically improved therapeutic activity 94. Furthermore, exosomal formulations of paclitaxel (PAC) for oral delivery can significantly reduce the side effects associated with PAC. Nie et al. used a chemical engineering approach to construct a responsive exosome nano-bioconjugate that cooperated with tumor immunotherapy 95. They conjugated azide-modified M1 macrophage-derived exosomes with dibenzocyclooctynes-modified antibodies of CD47 and SIRPα through pH-sensitive linker. Notably, in their experiment, BALB/c mice injected with 4T1 tumor cells were randomly divided into four groups: 1) PBS control, 2) free antibody, 3) M1 exosome, and 4) exosome nano-bioconjugates. All mice in forth group survived throughout the observation period without metastasis, whereas all those in the PBS group died within 24 days. Numerous studies have highlighted the potential value of exosomes as drug carriers for treating ovarian cancer (summarized in Table 2). However, their massive acquisition and engineering remain major challenges that need to be addressed.
Table 2.
Role of exosomes as drug carriers in ovarian cancer.
| Source of exosomes | Loading approach | Cargo | Role | Reference |
|---|---|---|---|---|
| SKOV3 | Sonication | Triptolide | Reduce the cytotoxic and apototic effects on SKOV3 cells. Enhance the inhibition of proliferation and growth. | 93 |
| Milk | Incubation | Berry anthocyanidins | Enhance the therapeutic activity of paclitaxel in vivo | 94 |
| Milk | Incubation | Cisplatin | Enhance the anti-cancer effect through avoiding endosome trapping | 96 |
| Macrophages from umbilical cord blood | Sonication | Cisplatin | Enhance the cytotoxicity in drug-resistant A2780/DDP cells and drug-sensitive A2780 cells. | 97 |
| Fibroblasts | Electroporation | miR-199a-3p | Suppressed c-Met expression, inhibited cell proliferation and invasion | 98 |
Summary
Metastasis of ovarian cancer is a complex process driven by various factors, including matrix remodeling, immunosuppression, angiogenesis, and changes in the tumor microenvironment. Recent studies have shed light on the molecular mechanisms underlying ovarian cancer metastasis and have made significant progress in exploring the role of exosomes in regulating this process. Exosomes offer new possibilities for early diagnosis and treatment of ovarian cancer through targeted medication, which can improve drug efficacy while reducing toxicity. Significant strides have been made in the application of engineered exosomes for the treatment of ovarian cancer. These remarkable achievements are a testament to the potential of exosomes as a therapeutic tool in the fight against this deadly disease. However, challenges still remain in applying exosomes to clinical practice due to limitations in detection and extraction methods. Further exploration into these issues is necessary to enable better clinical use of exosomes for diagnosis and treatment of ovarian cancer.
Acknowledgments
The authors would like to thank all the funding and journal staff who contributed to this review.
Funding
This work is supported by The Natural Science Foundation of Zhejiang Province (LY21H160047), China Postdoctoral Science Foundation (2022M723207) and the Medical Scientific Research Foundation of Zhejiang Province of China (2023KY666).
Author Contributions
YFZ drafted the manuscript, finished the three figures. CW, ZYM, FFL and CWX reviewed and made significant revisions to the manuscript. AJL and WWP as the corresponding authors, provided direction and guidance throughout the preparation of this manuscript. All authors read and approved the final manuscript.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: a cancer journal for clinicians. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Yang K, Zhou Q, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochimica et biophysica acta Reviews on cancer. 2021;1875:188503. doi: 10.1016/j.bbcan.2021.188503. [DOI] [PubMed] [Google Scholar]
- 3.Yousefi M, Dehghani S, Nosrati R, Ghanei M, Salmaninejad A, Rajaie S. et al. Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles. Cellular oncology (Dordrecht) 2020;43:515–38. doi: 10.1007/s13402-020-00513-9. [DOI] [PubMed] [Google Scholar]
- 4.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature cell biology. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Yi X, Du S, Gong L, Wu Q, Cai J. et al. Tumour-derived exosomal piR-25783 promotes omental metastasis of ovarian carcinoma by inducing the fibroblast to myofibroblast transition. Oncogene. 2023;42:421–33. doi: 10.1038/s41388-022-02560-y. [DOI] [PubMed] [Google Scholar]
- 6.Mo Y, Leung LL, Mak CSL, Wang X, Chan WS, Hui LMN. et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Molecular cancer. 2023;22:4. doi: 10.1186/s12943-022-01703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Z, Mao J, Barrero RA, Wang P, Zhang F, Wang T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules (Basel, Switzerland) 2020. 25. [DOI] [PMC free article] [PubMed]
- 8.Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. Journal of hematology & oncology. 2020;13:156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Ren X, E J, Zhou Y, Bian R. Exosome-transmitted circIFNGR2 Modulates Ovarian Cancer Metastasis via miR-378/ST5 Axis. Molecular and cellular biology. 2023;43:22–42. doi: 10.1080/10985549.2022.2160605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J. et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–20. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Z, Zhao R, Li B, Qi Y, Qiu W, Guo Q. et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Molecular cancer. 2022;21:16. doi: 10.1186/s12943-021-01485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E. et al. Exosomes Promote Ovarian Cancer Cell Invasion through Transfer of CD44 to Peritoneal Mesothelial Cells. Molecular cancer research: MCR. 2017;15:78–92. doi: 10.1158/1541-7786.MCR-16-0191. [DOI] [PubMed] [Google Scholar]
- 14.Guan X, Zong ZH, Liu Y, Chen S, Wang LL, Zhao Y. circPUM1 Promotes Tumorigenesis and Progression of Ovarian Cancer by Sponging miR-615-5p and miR-6753-5p. Molecular therapy Nucleic acids. 2019;18:882–92. doi: 10.1016/j.omtn.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ. et al. Reassessment of Exosome Composition. Cell. 2019;177:428–45.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Molecular cancer. 2019;18:124. doi: 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frawley T, Piskareva O. Extracellular Vesicle Dissemination of Epidermal Growth Factor Receptor and Ligands and Its Role in Cancer Progression. Cancers. 2020. 12. [DOI] [PMC free article] [PubMed]
- 20.Han Q, Tan S, Gong L, Li G, Wu Q, Chen L, Omental cancer-associated fibroblast-derived exosomes with low microRNA-29c-3p promote ovarian cancer peritoneal metastasis. Cancer science. 2023. [DOI] [PMC free article] [PubMed]
- 21.Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668–81. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Advanced drug delivery reviews. 2016;99:186–96. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends in cell biology. 2020;30:764–76. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L. et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Molecular cancer. 2019;18:40. doi: 10.1186/s12943-019-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He S, Li Z, Yu Y, Zeng Q, Cheng Y, Ji W. et al. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Experimental cell research. 2019;379:203–13. doi: 10.1016/j.yexcr.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 Pathway in Cancer. Frontiers in genetics. 2017;8:31. doi: 10.3389/fgene.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enriquez VA, Cleys ER, Da Silveira JC, Spillman MA, Winger QA, Bouma GJ. High LIN28A Expressing Ovarian Cancer Cells Secrete Exosomes That Induce Invasion and Migration in HEK293 Cells. BioMed research international. 2015;2015:701390. doi: 10.1155/2015/701390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Zhang X, Wang J, Li M, Cao C, Tan J. et al. TGFβ1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget. 2017;8:96035–47. doi: 10.18632/oncotarget.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue H, Li W, Chen R, Wang J, Lu X, Li J. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol. 2021;160:530–8. doi: 10.1016/j.ygyno.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nature reviews Cancer. 2020;20:174–86. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J. et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. Journal of experimental & clinical cancer research: CR. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringuette Goulet C, Bernard G, Tremblay S, Chabaud S, Bolduc S, Pouliot F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Molecular cancer research: MCR. 2018;16:1196–204. doi: 10.1158/1541-7786.MCR-17-0784. [DOI] [PubMed] [Google Scholar]
- 33.Hu T, Hu J. Melanoma-derived exosomes induce reprogramming fibroblasts into cancer-associated fibroblasts via Gm26809 delivery. Cell Cycle. 2019;18:3085–94. doi: 10.1080/15384101.2019.1669380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning X, Zhang H, Wang C, Song X. Exosomes Released by Gastric Cancer Cells Induce Transition of Pericytes Into Cancer-Associated Fibroblasts. Medical science monitor: international medical journal of experimental and clinical research. 2018;24:2350–9. doi: 10.12659/MSM.906641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giusti I, Di Francesco M, D'Ascenzo S, Palmerini MG, Macchiarelli G, Carta G. et al. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer biology & therapy. 2018;19:722–34. doi: 10.1080/15384047.2018.1451286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandoval P, Jiménez-Heffernan JA, Rynne-Vidal Á, Pérez-Lozano ML, Gilsanz Á, Ruiz-Carpio V. et al. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol. 2013;231:517–31. doi: 10.1002/path.4281. [DOI] [PubMed] [Google Scholar]
- 37.Rynne-Vidal A, Jiménez-Heffernan JA, Fernández-Chacón C, López-Cabrera M, Sandoval P. The Mesothelial Origin of Carcinoma Associated-Fibroblasts in Peritoneal Metastasis. Cancers. 2015;7:1994–2011. doi: 10.3390/cancers7040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Rio D, Masi I, Caprara V, Spadaro F, Ottavi F, Strippoli R. et al. Ovarian Cancer-Driven Mesothelial-to-Mesenchymal Transition is Triggered by the Endothelin-1/β-arr1 Axis. Frontiers in cell and developmental biology. 2021;9:764375. doi: 10.3389/fcell.2021.764375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pascual-Antón L, Cardeñes B, Sainz de la Cuesta R, González-Cortijo L, López-Cabrera M, Cabañas C, Mesothelial-to-Mesenchymal Transition and Exosomes in Peritoneal Metastasis of Ovarian Cancer. International journal of molecular sciences. 2021. 22. [DOI] [PMC free article] [PubMed]
- 40.Dou D, Ren X, Han M, Xu X, Ge X, Gu Y. et al. Cancer-Associated Fibroblasts-Derived Exosomes Suppress Immune Cell Function in Breast Cancer via the miR-92/PD-L1 Pathway. Frontiers in immunology. 2020;11:2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Jiang Y, Wang K, Lu X, Wang Y, Chen J. Cancer-associated fibroblasts-derived exosomes promote lung cancer progression by OIP5-AS1/ miR-142-5p/ PD-L1 axis. Molecular immunology. 2021;140:47–58. doi: 10.1016/j.molimm.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Yan L, Wang P, Fang W, Liang C. Cancer-associated fibroblasts-derived exosomes-mediated transfer of LINC00355 regulates bladder cancer cell proliferation and invasion. Cell biochemistry and function. 2020;38:257–65. doi: 10.1002/cbf.3462. [DOI] [PubMed] [Google Scholar]
- 43.Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME. et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer discovery. 2012;2:1100–8. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 45.Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. The international journal of biochemistry & cell biology. 2019;109:59–68. doi: 10.1016/j.biocel.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Yang K, Zhou Q, Qiao B, Shao B, Hu S, Wang G. et al. Exosome-derived noncoding RNAs: Function, mechanism, and application in tumor angiogenesis. Molecular therapy Nucleic acids. 2022;27:983–97. doi: 10.1016/j.omtn.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Yan-Qing W, Xiao Y, Shi-Yi L, Meng-Qin Y, Shu X. et al. Exosomes secreted by chemoresistant ovarian cancer cells promote angiogenesis. Journal of ovarian research. 2021;14:7. doi: 10.1186/s13048-020-00758-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Sheng Y, Li B, Wang Q, Liu X, Han J. Ovarian cancer derived PKR1 positive exosomes promote angiogenesis by promoting migration and tube formation in vitro. Cell biochemistry and function. 2021;39:308–16. doi: 10.1002/cbf.3583. [DOI] [PubMed] [Google Scholar]
- 49.Yi H, Ye J, Yang XM, Zhang LW, Zhang ZG, Chen YP. High-grade ovarian cancer secreting effective exosomes in tumor angiogenesis. International journal of clinical and experimental pathology. 2015;8:5062–70. [PMC free article] [PubMed] [Google Scholar]
- 50.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cellular immunology. 2020;353:104119. doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 51.Mao X, Xu J, Wang W, Liang C, Hua J, Liu J. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Molecular cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Xu M, Li X, Su X, Xiao X, Keating A. et al. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. Journal of hematology & oncology. 2018;11:82. doi: 10.1186/s13045-018-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X. et al. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Molecular therapy: the journal of the American Society of Gene Therapy. 2021;29:1226–38. doi: 10.1016/j.ymthe.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ford CE, Werner B, Hacker NF, Warton K. The untapped potential of ascites in ovarian cancer research and treatment. British journal of cancer. 2020;123:9–16. doi: 10.1038/s41416-020-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D. et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107:563–71. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 56.Worzfeld T, Pogge von Strandmann E, Huber M, Adhikary T, Wagner U, Reinartz S. et al. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Frontiers in oncology. 2017;7:24. doi: 10.3389/fonc.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batchu RB, Gruzdyn OV, Kolli BK, Dachepalli R, Umar PS, Rai SK. et al. IL-10 Signaling in the Tumor Microenvironment of Ovarian Cancer. Advances in experimental medicine and biology. 2021;1290:51–65. doi: 10.1007/978-3-030-55617-4_3. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Liu G, Li Y, Pan Y. Metabolic Reprogramming Induces Macrophage Polarization in the Tumor Microenvironment. Frontiers in immunology. 2022;13:840029. doi: 10.3389/fimmu.2022.840029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Gao S, Song L, Liu M, Sun Z, Liu J. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell malignant progression by suppressing the HMGB1-TLR4 axis. Molecular immunology. 2021;130:113–21. doi: 10.1016/j.molimm.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Lecker LSM, Berlato C, Maniati E, Delaine-Smith R, Pearce OMT, Heath O. et al. TGFBI Production by Macrophages Contributes to an Immunosuppressive Microenvironment in Ovarian Cancer. Cancer Res. 2021;81:5706–19. doi: 10.1158/0008-5472.CAN-21-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cortés M, Sanchez-Moral L, de Barrios O, Fernández-Aceñero MJ, Martínez-Campanario MC, Esteve-Codina A. et al. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. The EMBO journal. 2017;36:3336–55. doi: 10.15252/embj.201797345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Frontiers in immunology. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowak M, Klink M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells. 2020. 9. [DOI] [PMC free article] [PubMed]
- 64.Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L. et al. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget. 2016;7:43076–87. doi: 10.18632/oncotarget.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiong J, He X, Xu Y, Zhang W, Fu F. MiR-200b is upregulated in plasma-derived exosomes and functions as an oncogene by promoting macrophage M2 polarization in ovarian cancer. Journal of ovarian research. 2021. 14. [DOI] [PMC free article] [PubMed]
- 66.Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q. et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. Journal of experimental & clinical cancer research: CR. 2019;38:81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X. et al. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer immunology research. 2018;6:1578–92. doi: 10.1158/2326-6066.CIR-17-0479. [DOI] [PubMed] [Google Scholar]
- 68.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nature reviews Cancer. 2014;14:430–9. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun H, Meng Q, Shi C, Yang H, Li X, Wu S. et al. Hypoxia-Inducible Exosomes Facilitate Liver-Tropic Premetastatic Niche in Colorectal Cancer. Hepatology (Baltimore, Md) 2021;74:2633–51. doi: 10.1002/hep.32009. [DOI] [PubMed] [Google Scholar]
- 70.Lin Z, Song J, Gao Y, Huang S, Dou R, Zhong P. et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox biology. 2022;52:102312. doi: 10.1016/j.redox.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA. et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806–21. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Ying X, Wang X, Wu X, Zhu Q, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncology reports. 2017;38:522–8. doi: 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer letters. 2018;435:80–91. doi: 10.1016/j.canlet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL. et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. The Journal of clinical investigation. 2014;124:4614–28. doi: 10.1172/JCI74778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asante DB, Calapre L, Ziman M, Meniawy TM, Gray ES. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer letters. 2020;468:59–71. doi: 10.1016/j.canlet.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Cheng X, Zhang L, Chen Y, Qing C. Circulating cell-free DNA and circulating tumor cells, the "liquid biopsies" in ovarian cancer. Journal of ovarian research. 2017;10:75. doi: 10.1186/s13048-017-0369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim MC, Kang S, Lee KS, Han SS, Park SJ, Seo SS. et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol. 2009;112:28–34. doi: 10.1016/j.ygyno.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 78.Pradeep S, Kim SW, Wu SY, Nishimura M, Chaluvally-Raghavan P, Miyake T. et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26:77–91. doi: 10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang C, Wu S, Mou Z, Zhou Q, Dai X, Ou Y. et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Molecular therapy: the journal of the American Society of Gene Therapy. 2022;30:1054–70. doi: 10.1016/j.ymthe.2022.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tong Y, Liu X, Xia D, Peng E, Yang X, Liu H. et al. Biological Roles and Clinical Significance of Exosome-Derived Noncoding RNAs in Bladder Cancer. Frontiers in oncology. 2021;11:704703. doi: 10.3389/fonc.2021.704703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo Y, Gui R. Circulating exosomal circFoxp1 confers cisplatin resistance in epithelial ovarian cancer cells. Journal of gynecologic oncology. 2020;31:e75. doi: 10.3802/jgo.2020.31.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK, Meckes DG Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 2016;7:86999–7015. doi: 10.18632/oncotarget.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang W, Ou X, Wu X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: A potential role in the coagulation cascade, diagnosis and prognosis. International journal of oncology. 2019;54:1719–33. doi: 10.3892/ijo.2019.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 85.Kim S, Choi MC, Jeong JY, Hwang S, Jung SG, Joo WD. et al. Serum exosomal miRNA-145 and miRNA-200c as promising biomarkers for preoperative diagnosis of ovarian carcinomas. Journal of Cancer. 2019;10:1958–67. doi: 10.7150/jca.30231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan C, Stevic I, Müller V, Ni Q, Oliveira-Ferrer L, Pantel K. et al. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Molecular oncology. 2018;12:1935–48. doi: 10.1002/1878-0261.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuberi M, Mir R, Das J, Ahmad I, Javid J, Yadav P. et al. Expression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2015;17:779–87. doi: 10.1007/s12094-015-1303-1. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura A, Sawada K, Nakamura K, Kinose Y, Nakatsuka E, Kobayashi M. et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC cancer. 2018;18:1065. doi: 10.1186/s12885-018-4974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weiner-Gorzel K, Dempsey E, Milewska M, McGoldrick A, Toh V, Walsh A. et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer medicine. 2015;4:745–58. doi: 10.1002/cam4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS. et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta pharmacologica Sinica. 2017;38:754–63. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding C. et al. The Effect of Triptolide-Loaded Exosomes on the Proliferation and Apoptosis of Human Ovarian Cancer SKOV3 Cells. BioMed research international. 2019;2019:2595801. doi: 10.1155/2019/2595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L. et al. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food & function. 2017;8:4100–7. doi: 10.1039/c7fo00882a. [DOI] [PubMed] [Google Scholar]
- 95.Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A. et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angewandte Chemie (International ed in English) 2020;59:2018–22. doi: 10.1002/anie.201912524. [DOI] [PubMed] [Google Scholar]
- 96.Zhou G, Gu Y, Zhu Z, Zhang H, Liu W, Xu B. et al. Exosome Mediated Cytosolic Cisplatin Delivery Through Clathrin-Independent Endocytosis and Enhanced Anti-cancer Effect via Avoiding Endosome Trapping in Cisplatin-Resistant Ovarian Cancer. Frontiers in medicine. 2022;9:810761. doi: 10.3389/fmed.2022.810761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang X, Liu L, Tang M, Li H, Guo X, Yang X. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug development and industrial pharmacy. 2020;46:1150–62. doi: 10.1080/03639045.2020.1776320. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi M, Sawada K, Miyamoto M, Shimizu A, Yamamoto M, Kinose Y. et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochemical and biophysical research communications. 2020;527:153–61. doi: 10.1016/j.bbrc.2020.04.076. [DOI] [PubMed] [Google Scholar]