Abstract
Multiple molecular targets have been identified to mediate membrane-delimited and nongenomic effects of natural and synthetic steroids, but the influence of steroid metabolism on neuroactive steroid signaling is not well understood. To begin to address this question, we set out to identify major metabolites of a neuroprotective synthetic steroid 20-oxo-5β-pregnan-3α-yl l-glutamyl 1-ester (pregnanolone glutamate, PAG) and characterize their effects on GABAA and NMDA receptors (GABARs, NMDARs) and their influence on zebrafish behavior. Gas chromatography–mass spectrometry was used to assess concentrations of PAG and its metabolites in the hippocampal tissue of juvenile rats following intraperitoneal PAG injection. PAG is metabolized in the peripheral organs and nervous tissue to 20-oxo-17α-hydroxy-5β-pregnan-3α-yl l-glutamyl 1-ester (17-hydroxypregnanolone glutamate, 17-OH-PAG), 3α-hydroxy-5β-pregnan-20-one (pregnanolone, PA), and 3α,17α-dihydroxy-5β-pregnan-20-one (17-hydroxypregnanolone, 17-OH-PA). Patch-clamp electrophysiology experiments in cultured hippocampal neurons demonstrate that PA and 17-OH-PA are potent positive modulators of GABARs, while PAG and 17-OH-PA have a moderate inhibitory effect at NMDARs. PAG, 17-OH-PA, and PA diminished the locomotor activity of zebrafish larvae in a dose-dependent manner. Our results show that PAG and its metabolites are potent modulators of neurotransmitter receptors with behavioral consequences and indicate that neurosteroid-based ligands may have therapeutic potential.
Keywords: negative allosteric modulator, thigmotaxis, steroid, glutamate, zebrafish
Introduction
Brain function relies on synaptic transmission, a complex and tightly regulated process. The majority of fast synaptic transmission in the central nervous system is mediated by presynaptically released neurotransmitters glutamate and γ-aminobutyric acid (GABA) that activate postsynaptically localized ionotropic receptors. Excitatory postsynaptic potentials are associated with the activation of AMPA/kainate and NMDA receptors (NMDARs) and membrane depolarization. In contrast, inhibitory postsynaptic potentials are mediated by the activation of GABAA receptors (GABARs) and membrane hyperpolarization. Fast synaptic transmission is subject to modulation by intrinsic and extrinsic factors that may facilitate or depress signal transduction across any given synapse. Among these modulating factors are neurosteroids – a specific group of steroids synthesized by neurons and glia in the mammalian central nervous system (CNS) independently of peripheral glands.1−3 Neurosteroids are synthesized from cholesterol by its conversion to pregnenolone and subsequently by a series of specific steroidogenic enzymes that direct the synthesis of one or several distinct steroids in a particular cell.4,5 These steroids occur in the nervous system either unconjugated or conjugated (e.g., as sulfate esters or as fatty acid esters).6 Since steroidogenic enzymes are expressed with distinct anatomical and cell-type preference that is developmentally regulated,4 neurosteroid concentrations in the CNS may locally differ.
Multiple molecular mechanisms have been identified to mediate steroid membrane-delimited, nongenomic effects in the nervous tissue: steroids may act via proper membrane receptors coupled to G proteins7 through specific membrane-associated steroid receptors,8 muscarinic receptors,9 and, in addition, they may allosterically modulate ionotropic receptors for neurotransmitters such as GABARs,10 nicotinic receptors,11 and NMDARs.12 For some neurosteroids, multiple molecular targets have been identified. For example, pregnenolone sulfate is an inhibitor of responses mediated by GABAR,13 but it potentiates NMDAR responses.12
In the past, we have prepared synthetic analogues of the endogenous NMDAR inhibitor pregnanolone sulfate and found that some of them were potent NMDAR inhibitors.14−19 In addition, selected C3 substituted analogues (hemiesters of dicarboxylic acids, including pregnanolone glutamate, PAG) showed neuroprotective activity without psychomimetic symptoms.20−22 Neurosteroid neuroprotective effects may involve a number of different mechanisms, including direct modulation of NMDARs and GABARs.23,24 Since steroids are endogenously metabolized, it is possible that part of the biological effect is due to the action of metabolites. Therefore, we set out to elucidate the effect of PAG and its metabolites on the excitatory and inhibitory receptors and spontaneous locomotion of zebrafish larvae. We show that PAG is converted to conjugated and unconjugated metabolites with modulatory action at GABARs and NMDARs that influence locomotor activity of zebrafish larvae.
Results
Metabolomics
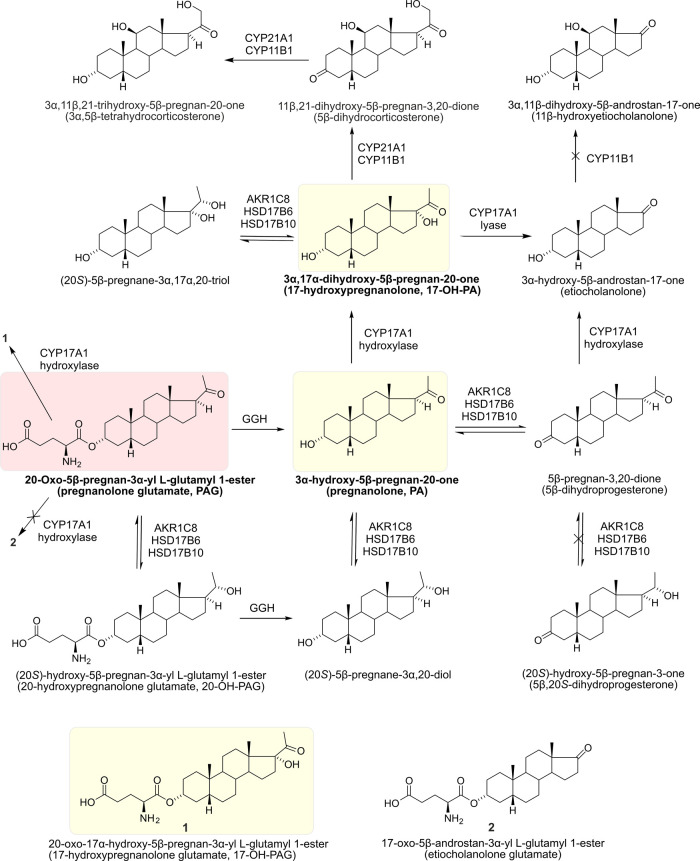
To characterize the pathways of PAG metabolism relevant to the PAG neuroprotective effect in the hippocampus (HC), we determined the steroid content in HC of 12-day-old male albino Wistar rats that were either naive, controls (15 min following intraperitoneal (i.p.) application of (2-hydroxypropyl)-β-cyclodextrin (CDX)), or the experimental group (15 min following i.p. application of PAG (1 mg/kg + CDX)) (see Methods for details). The results of our metabolomic analysis are summarized in Figure 1 and Table 1. Our main findings include the observation that the original compound PAG is able to cross the blood–brain barrier (BBB) to some extent, being present in HC at concentrations in the nM range and representing 6% of the 5β steroids introduced to HC by the PAG treatment. However, the majority of the injected PAG is quickly metabolized, in part to the unconjugated pregnanolone (PA) that, as a result of the PAG treatment, is found in HC at high nM concentrations, and to 17-hydroxylated PA (17-OH-PA) that reaches remarkably high concentrations (in the μM range) in HC after the PAG treatment.
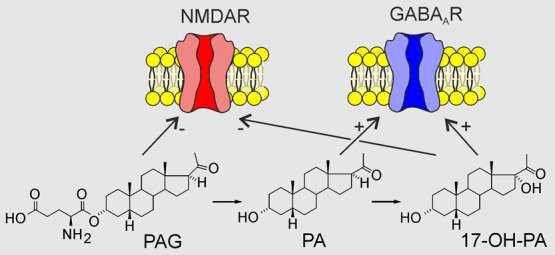
Figure 1.
Simplified scheme of PAG conversion after its i.p. administration in male rat pups. Arrows indicate the conversion of PAG to its metabolites in individual metabolic steps. Crossed arrows indicate enzymatic steps whose products were not elevated after i.p. PAG administration compared to controls. The structure of PAG is highlighted in red and the structures of its major metabolites (PA, 17-OH-PA, and 17-OH-PAG) studied for their effect on GABARs and NMDARs are highlighted in yellow.
Table 1. Steroid Concentrations in HC Following i.p. Injection of PAGa.
| steroid | naive (N) (nM) | control (C) (nM) | PAG (P) (nM) | Δ5β (%) |
|---|---|---|---|---|
| conjugated PA | 1.2 (0.741, 1.93) | 0.642 (0.402, 0.991) | 128 (46.2, 534) | 5.98 |
| F = 91.7, p < 0.001, ηp2 = 0.943; P > C, P > N | ||||
| PA | 0.794 (0.552, 1.15) | 0.735 (0.489, 1.11) | 200 (72, 786) | 9.39 |
| F = 120.9, p < 0.001, ηp2 = 0.957; P > C, P > N | ||||
| 5β-dihydroprogesterone | 0.324 (0.138, 0.816) | 0.081 (0.0295, 0.178) | 1.75 (0.67, 6.3) | 0.08 |
| F = 13.4, p = 0.001, ηp2 = 0.71; P > C | ||||
| conjugated (20S)-5β-pregnane-3α,20-diol | 0.579 (0.223, 1.49) | 0.117 (0.0472, 0.253) | 11.3 (5.09, 26) | 0.53 |
| F = 38.1, p < 0.001, ηp2 = 0.884; P > C, P > N | ||||
| (20S)-5β-pregnane-3α,20-diol | 1.07 (0.751, 1.54) | 0.346 (0.232, 0.501) | 26.3 (13.5, 59.8) | 1.22 |
| F = 98, p < 0.001, ηp2 = 0.942; C < N, P > C, P > N | ||||
| 5β,20S-tetrahydroprogesterone | 0.705 (0.489, 0.955) | 0.425 (0.255, 0.629) | 0.288 (0.144, 0.465) | |
| F = 4.4, p = 0.036, ηp2 = 0.426; P < N | ||||
| conjugated 17-OH-PA | 0.025 (0.013, 0.052) | 0.0097 (0.0048, 0.019) | 0.81 (0.250, 4.13) | 0.04 |
| F = 30.3, p < 0.001, η2 = 0.465; P > C, P > N | ||||
| 17-OH-PA | 8.34 (4.74, 14.3) | 2.88 (1.09, 5.76) | 1570 (515, 7060) | 73.98 |
| F = 86.2, p < 0.001, ηp2 = 0.94; P > C, P > N | ||||
| (20S)-5β-pregnane-3α,17α,20-triol | 8.07 (3.83, 16.4) | 2.01 (0.445, 5.07) | 17.7 (8.74, 36.5) | 0.74 |
| F = 7.3, p = 0.01, ηp2 = 0.57; P > C | ||||
| conjugated etiocholanolone | 0.429 (0.267, 0.7) | 0.272 (0.174, 0.415) | 0.465 (0.305, 0.723) | |
| F = 1.8, p = 0.208, ηp2 = 0.248 | ||||
| etiocholanolone | 0.257 (0.204, 0.323) | 0.183 (0.151, 0.221) | 2.29 (1.76, 3.08) | 0.10 |
| F = 189.5, p < 0.001, ηp2 = 0.974; P > C, P > N | ||||
| 11β-hydroxyetiocholanolone | 5.84 (3.28, 12.4) | 1.88 (1.03, 3.38) | 3.16 (1.88, 5.61) | |
| F = 3.8, p = 0.056, ηp2 = 0.407 | ||||
| 3α,5β-tetrahydrocorticosterone | 114 (69.2, 177) | 50.3 (22.8, 91.6) | 219 (143, 327) | 7.95 |
| F = 8.3, p = 0.006, ηp2 = 0.603; P > C | ||||
F, p, and ηp2 represent F-statistic, p-value, and effect size, respectively. Steroid concentrations in HC of 12-day-old rats in naive animals (naive), in animals 15 min following i.p. injection of CDX (control), and in animals 15 min following i.p. injection of PAG (1 mg/kg of body weight) in CDX (PAG). The values in parentheses represent 95% confidence intervals (see Methods for details). % Δ5β refers to the percentage of total significantly increased 5β steroids in rat HC above the basal level (control group) after PAG application. Values are shown as means with retransformed 95% confidence intervals. Differences between groups were evaluated using one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons.
Deconjugation of 5β-Steroid Glutamates before Their Penetration across the BBB and Extensive Extra-Adrenal 17-Hydroxylation of PA in the Periphery
Our data show that PAG is metabolized mainly to 17-OH-PA. After deconjugation of the glutamate moiety of PAG by γ-glutamyl hydrolase (GGH)25 and 17-hydroxylation by steroid C17-hydroxylase-C17,20-lyase (CYP17A1; these enzymes are most active in the small intestine), the 17-OH-PA penetrates across the BBB into the CNS. The results in Table 1 demonstrate that this metabolite represents 74% of the overall 5β-steroid increase in HC following PAG application. In contrast to extra-adrenal peripheral tissues, the adrenal glands of rats do not exhibit CYP17A1 activity.26 Also, the activity of GGH and CYP17A1 is negligible in the CNS.27−32 Therefore, it is unlikely that brain CYP17A1 activity participates in the formation of hippocampal 17-OH-PA after PAG application. We also did not find detectable concentrations of Δ5-steroid 17-hydroxypregnenolone and Δ4-steroid 17-hydroxyprogesterone in HC independently of the PAG application. Data in Table 1 further suggest the conversion of (20S)-5β-pregnane-3α,20-diol to (20S)-5β-pregnane-3α,17α,20-triol, judging from similar concentrations of these steroids in HC.
Low C17,20-Lyase Activity
The CYP17A1 enzyme catalyzes the conversion of C21-deoxysteroids to their 17-hydroxy counterparts in the first step, while in the second step (C17,20-lyase), it catalyzes the cleavage between the carbons C17 and C20, producing C19 steroids (androstanes). The low concentration of etiocholanolone in HC of 12-day-old rats after PAG administration indicates relatively low CYP17A1 activity in the C17,20-lyase step despite a roughly tenfold increase in the concentration of this 5β-reduced C19 steroid (Table 1). This result indicates the functioning of the so-called “backdoor” pathway for 5β-steroids in the rat since PA is converted to 17-OH-PA and then to etiocholanolone. The finding of “backdoor” pathway functioning for the 5β-steroids is surprising, as references to the ″backdoor″ pathway have only been published for 5α-steroids.33,34 The classical “frontdoor” pathway assumes CYP17A1-catalyzed conversion of the C21 Δ5 and Δ4 17-deoxy-steroids to their 17-hydroxy counterparts and then to the corresponding C19 metabolites. Finally, the 17-hydroxy-steroids and their corresponding C19 metabolites are reduced in the C5 and C3 positions by 5β-reductase (AKR1D1) and type 8, subfamily 1C aldoketoreductase (AKR1C8), respectively.35 The participation of the “frontdoor” pathway in the metabolism of the i.p. administered PAG is not possible as this pathway involves the conversion of pregnenolone to progesterone, and the identified compounds cannot be metabolites of endogeneous progesterone.
Conversion of PA to 3α,5β-Tetrahydrocorticosterone
Another unexpected result was the peripheral conversion of PAG to 3α,5β-tetrahydrocorticosterone with gradual participation of corticosterone synthesizing enzymes such as steroid 21-hydroxylase (CYP21A1) and steroid type 1 11β-hydroxylase (CYP11B1). Even this relatively polar steroid had to penetrate to the brain via the BBB and could not be synthesized locally due to the lack of enzyme activity necessary for its synthesis. The passive transport of 3α,5β-tetrahydrocorticosterone is in all probability facilitated by the high BBB permeability of only 12-day-old rats. Moreover, the percentage of this steroid in HC after the PAG application is relatively high (about 8% of the total 5β-steroid increase, see Table 1).36
20α-Hydroxysteroid Dehydrogenase Activity and Interconversion between 3α- and 3-oxo-steroids
The data in Table 1 show a remarkable conversion of PAG and PA into their 3α,20S-dihydroxymetabolites by AKR1C837 even if this conversion is less marked than 17-hydroxylation. Our data are consistent with the results of other authors who reported 20α-HSD (hydroxysteroid dehydrogenase) activity transforming 20-oxo-pregnanes to their 20S-hydroxy-counterparts in the rat hypothalamus.38 Concerning the interconversion between 3α- and 3-oxo- steroids, a small part of PA was also converted to 5β-dihydroprogesterone. This conversion may be catalyzed by type 16 retinol dehydrogenase (RDH16), steroid type 6 17β-hydroxysteroid dehydrogenase (HSD17B6), and/or steroid type 10 17β-hydroxysteroid dehydrogenase (HSD17B10).35
Concentration of the Major Metabolites in Serum
To increase our understanding of the transport of the main metabolites of PAG across the BBB, we determined their levels in serum 15 min following i.p. application of PAG (1 mg/kg + CDX) (see Methods for details). Table 2 shows serum concentrations of PA and 17-OH-PA in their conjugated and unconjugated forms.
Table 2. Steroid Concentrations in the Serum Following i.p. Injection of PAGa.
| steroid | PAG (P) (nM) |
|---|---|
| conjugated PA | 127 (102, 168) |
| PA | 212 (189, 250) |
| conjugated 17-OH-PA | 246 (96.1, 504) |
| 17-OH-PA | 10.9 (9.12, 12.7) |
Steroid concentrations in the serum of 12-day-old rats 15 min following i.p. injection of PAG (1 mg/kg of body weight) in CDX (PAG). The values in parentheses represent 95% confidence intervals (see Methods for details). Values are shown as means with retransformed 95% confidence intervals.
Electrophysiology
Effect of Charged Steroids on GABARs and NMDARs
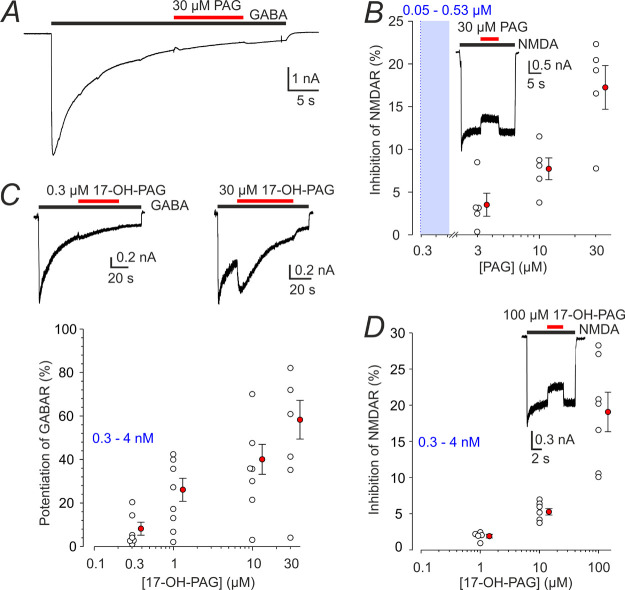
In subsequent experiments, we characterized the pharmacodynamics of PAG and its main metabolites, namely, 17-OH-PA, PA, and 17-hydroxypregnanolone glutamate (17-OH-PAG) at GABARs and NMDARs in cultured rat hippocampal neurons. Figure 2A,B shows that PAG (30 μM) had virtually no effect on responses induced by 1 μM GABA while inhibiting responses to 100 μM NMDA. Compound 17-OH-PAG at concentrations much higher than those found in HC after i.p. PAG administration had only a mild potentiating effect on responses induced by 1 μM GABA and a minor inhibitory effect on responses to 100 μM NMDA (Figure 2C,D). We also tested the effect of PAG and 17-OH-PAG on responses to brief GABA applications (as used in subsequent experiments) and we found no effects (data not shown).
Figure 2.
Effect of PAG and 17-OH-PAG on GABAR and NMDAR responses. (A) Representative recording from a cultured hippocampal neuron shows a response to 1 μM GABA coapplied with PAG (30 μM) (the duration of GABA and steroid application is indicated by black and red bars, respectively). (B) Graph shows the degree of inhibition by PAG of responses to 100 μM NMDA observed in individual neurons (open symbols). Inset shows the effect of 30 μM PAG on a response to 100 μM NMDA. (C) Response to 1 μM GABA was recorded in the absence and presence of 0.3 or 30 μM of 17-OH-PAG. The plot of the relative 17-OH-PAG (0.3–30 μM)-induced potentiation of current responses to 1 μM GABA, as assessed for the first GABA and steroid co-application in each neuron. The data assessed in individual cells (open symbols) are plotted normalized with respect to the response induced by 1 μM GABA just preceding GABA and steroid co-application. (D) The graph shows the degree of inhibition by 17-OH-PAG (1–100 μM) of responses to 100 μM NMDA observed in individual neurons (open symbols). Inset shows the effect of 100 μM 17-OH-PAG on a response to 100 μM NMDA. The red-filled symbols in B–D give mean ± SEM. The blue box and numbers indicate a 5–95% confidence interval of the steroid concentrations found in HC following i.p. PAG application (data from Table 1). DMSO concentration in the control and extracellular solution (ECS) with steroids was 1%.
Effect of Uncharged Steroids at GABARs and NMDARs
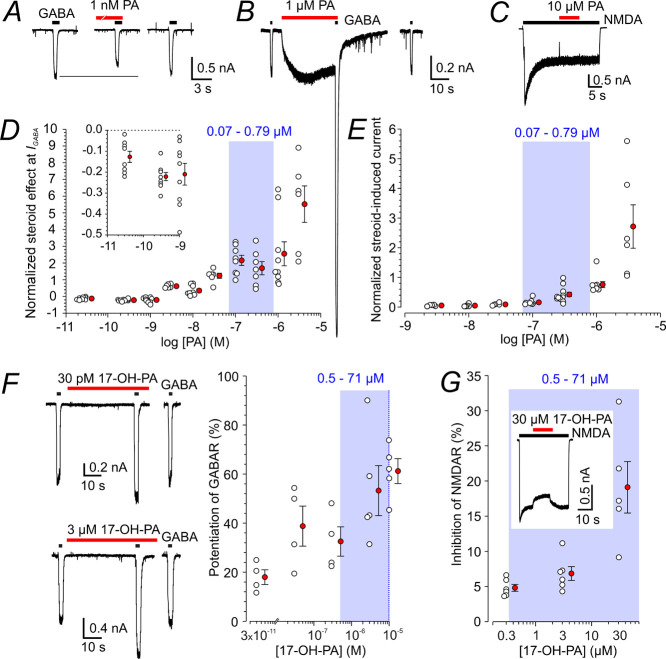
Next, we explored the effect of PA, an uncharged metabolite of PAG, on GABARs and NMDARs. Dose–response analysis of the PA effect on short responses to 1 μM GABA indicates that at the steroid concentrations of 0.03 to 1 nM, the peak responses to GABA and PA co-application made following PA pre-application for 20 s were significantly diminished, by 13 to 22%. At the PA concentrations of 3 nM to 3 μΜ, GABAR responses were significantly potentiated, by 35 to 552% (Figure 3A,B,D). We have noticed that the degree of steroid potentiation of GABAR responses fades with repeated applications (Figure S1). Therefore, we analyzed the potentiation using only the first control (GABA) application and a single test (GABA and steroid) co-application usually made within 1–2 min after the start of the whole-cell recording. This criterion was applied to the analysis of the effect of all the steroids used in this study. Despite analyzing only the first response, the degree of PA potentiation of responses to GABA was quite variable, e.g., at 1 μM PA, it ranged from 1.7-fold to 7.4-fold. We have not analyzed the background of the variability; one possible explanation could involve different levels of GABAR δ-subunit expression that is associated with high sensitivity to neurosteroid modulation.39,40 The positive allosteric effect of PA on responses to a low concentration of GABA agrees well with the data described previously.41−47
Figure 3.
Modulation of GABAR and NMDAR responses by PA and 17-OH-PA. (A) Representative responses are shown for the first 1 μM GABA application and when co-applied with 1 nM PA following 1 nM PA pre-application for 40 s. (B) Responses are shown for the first 1 μM GABA application and to the first co-application of 1 μM GABA and 1 μM PA made immediately following 1 μM PA pre-application for 30 s. Note that PA (1 μM) alone induced a membrane current that was of a similar amplitude as the current induced by 1 μM GABA. (C) Response to 100 μM NMDA before and during 10 μM PA co-application. (D) The plot of the relative PA (30 pM to 3 μM) effect on responses to 1 μM GABA. Responses to PA and GABA co-application were assessed following 40 s PA pre-application only for the first steroid and GABA co-application in each neuron. Data from individual cells (open symbols) are plotted normalized with respect to the control response induced by 1 μM GABA. Inset shows the data for 30 pM to 1 nM PA on the expanded y-axis. (E) Plot of the relative PA (3 nM to 3 μM)-induced current assessed for the first steroid application in each neuron. Data from individual cells (open symbols) are plotted normalized with respect to the control response induced by 1 μM GABA. (F) First response to 1 μM GABA recorded after attaining whole-cell configuration in the absence and in the presence of 30 pM (above) or 3 μM (below; different cell) 17-OH-PA. The plot of the relative 17-OH-PA (30 pM to 10 μM) potentiation of responses to 1 μM GABA assessed for the first steroid and GABA co-application in each neuron. Data from individual cells (open symbols) are plotted normalized with respect to the response induced by 1 μM GABA. (G) Plot of the relative 17-OH-PA (0.3–30 μM) effect on steady-state responses to 100 μM NMDA (open symbols). Inset shows a response to 100 μM NMDA induced before and during 30 μM 17-OH-PA co-application. The red-filled symbols next to the open symbols in D-G give mean ± SEM. Blue boxes and numbers in blue in D–G indicate a 5–95% confidence interval of PA and 17-OH-PA concentration measured in HC following i.p. PAG application (Table 1). DMSO concentration in the control and ECS with steroids was 1%.
In agreement with previous results showing that PA may induce currents by direct GABAR activation,48 we show that 0.1 μM PA (in the absence of GABA) induces currents with a mean amplitude of ∼20% of those induced by 1 μM GABA, while at a concentration of 3 μM, PA induced responses that were on average 2.7-fold larger than control GABA responses (Figure 3B,E). Similar to the steroid-induced potentiation of responses to GABA, the amplitude of currents induced by direct steroid activation of GABARs decreased with repeated steroid applications, and therefore, the amplitudes were analyzed for only the first response to PA. In contrast to a strong modulatory effect of PA at GABAR, this steroid had virtually no effect on responses to 100 μM NMDA (Figure 3C).
Unlike PA, 17-OH-PA shows no GABAR inhibition at low concentrations and instead potentiates responses to 1 μM GABA at a wide range of concentrations, but the degree of potentiation is relatively low (61% at 10 μM 17-OH-PA) (Figure 3F). In addition, no direct activation of membrane currents was observed when 17-OH-PA (30 pM to 10 μM) was applied in the absence of GABA. 17-OH-PA had only a mild inhibitory effect on responses induced by 100 μM NMDA (Figure 3G).
We assume that in our experiments, we activated a mixed population of GABARs with different subunit compositions. Additional experiments (Figure S2) revealed that, on average, responses of hippocampal neurons to 1 μM GABA represented only 8 ± 2% (n = 6) of the amplitude of the maximal response induced by the co-application of 1 mM GABA and 50 μM GABAR allosteric modulator propofol.49,50 In addition, we detected no constitutive GABAR activity as the application of 200 μM picrotoxin had no effect on the membrane current (Figure S2). Together, these results suggest that the dominant contribution to GABA-induced currents is from synaptic receptors composed of α1β2/3γ2 subunits.51 Specific effects of neurosteroid metabolites on GABARs of different subunit compositions should be addressed in more detail in the future.
The similar structure of PA and 17-OH-PA, both of which are high-affinity allosteric modulators of GABARs, led us to hypothesize that these steroids may compete for the same site of action at the receptor, although they act with different efficacies. In this case, occlusion of the potentiating effects of the two steroids (PA and 17-OH-PA) would be expected to result in an intermediate degree of potentiation compared to the effect of either steroid alone. If distinct sites mediate the potentiating effects, the summation of effects is expected. Figure S3 shows the results of experiments in which we tested how the co-application of 17-OH-PA together with PA will compare with the potentiation induced by PA only.
Our results indicate that 17-OH-PA at a concentration similar to that found in the hippocampal tissue of juvenile rats following i.p. PAG application (see Table 1) does not significantly alter the degree of potentiation induced by PA. Furthermore, no correlation was found for the potentiation induced by 0.1 μM PA and 1 μM 17-OH-PA (Figure S3). The molecular basis of PA and 17-OH-PA action at the GABAR may be more complicated since our results do not clearly support either the occlusion or the summation of the effects of PA and 17-OH-PA.
In Vivo Analysis of Locomotion in Zebrafish Larvae Exposed to Steroids
Results of our acute electrophysiological experiments show that PA and 17-OH-PA, at concentrations found in the nervous tissue following i.p. PAG application, have a strong potentiating effect on GABARs. Furthermore, 17-OH-PA, PAG, and 17-OH-PAG have a mild inhibitory effect on NMDARs. While the inhibitory effect of PAG and 17-OH-PAG on NMDARs is stable in time, the potentiating effect of PA and 17-OH-PA on GABAR responses is markedly reduced after repeated or prolonged steroid application. To examine long-lasting modulatory effects of PAG and its metabolites, we analyzed spontaneous locomotor activity in zebrafish larvae. For this purpose, we adopted a version of the open-field test that provides temporal and spatial resolution regarding the position and movement over time following the introduction of the larvae to a novel environment (square chamber).52 The experimental protocol consisted of an initial period (1 h), during which the larvae were exposed to the test medium and a subsequent period (1 h), during which the locomotor activity of the larvae (in the same medium) was monitored.
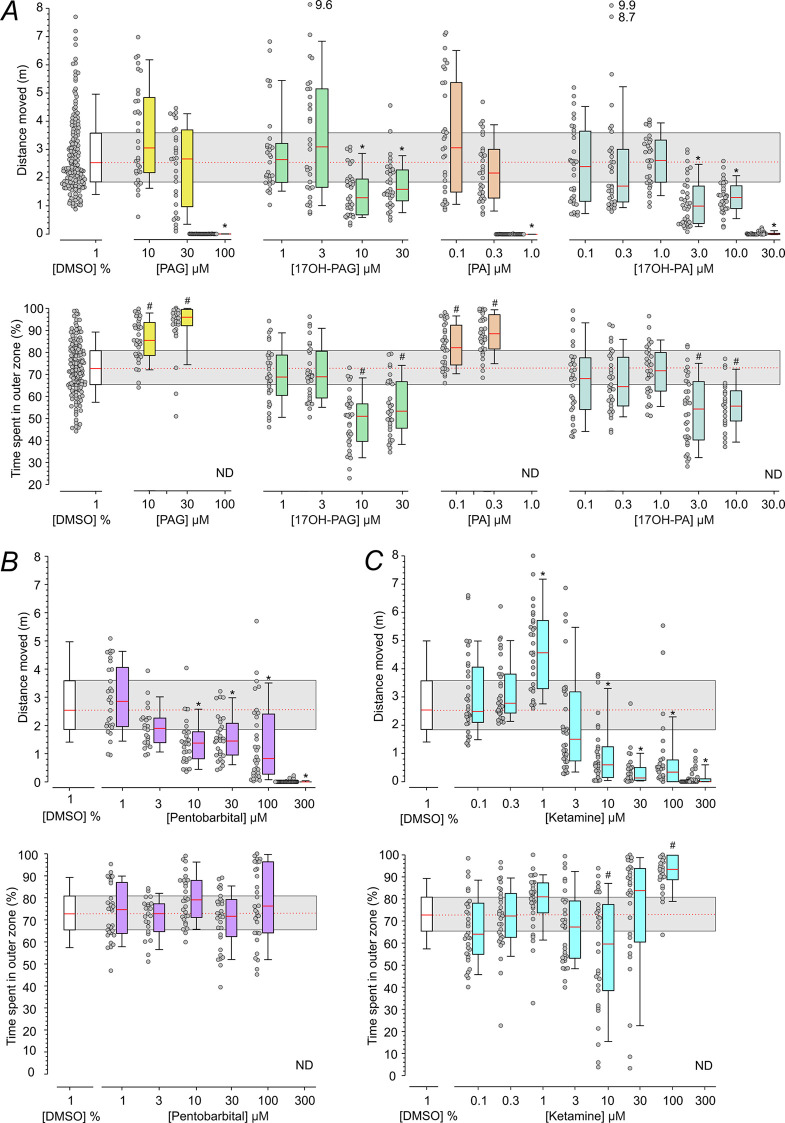
Figure 4A summarizes the effects of steroids on the locomotor activity of zebrafish larvae. We found that 100 μM PAG nearly completely eliminated movements when compared to the vehicle (DMSO)-treated larvae. The effects of PAG at concentrations of 10 and 30 μM were not significantly different from vehicle (DMSO)-treated larvae (Figure 4A). A charged PAG metabolite 17-OH-PAG had a significant effect at concentrations of 10 and 30 μM; however, when compared to PAG (100 μM), it induced only a mild decrease in the distance the larvae moved. PA, an uncharged metabolite of PAG, nearly completely eliminated movement when applied at 1 μM; however, this steroid did not affect locomotor activity when used at a concentration of 0.1 or 0.3 μM. 17-OH-PA had no effect at 0.1, 0.3, or 1 μM, reduced the activity at a concentration of 3 or 10 μM, and nearly completely eliminated movement when applied at 30 μM. Control experiments were performed to determine whether the inhibitory effects of 100 μM PAG and 1 μM PA on the locomotor activity were reversible. Spontaneous locomotor activity recovered within 1 h following the replacement of the medium containing 100 μM PAG or 1 μM PA (leading to the cessation of movements) for the control medium (with no DMSO).
Figure 4.
Effects of PAG and its metabolites on locomotor activity of zebrafish larvae. (A) Distance the larvae moved and thigmotaxis (measured as the % of time spent in the outer zone during the 1 h monitoring period). Larvae were treated with the vehicle (1% DMSO), PAG (10–100 μM), 17-OH-PAG (1–30 μM), PA (0.1–1 μM), or 17-OH-PA (0.1–30 μM). DMSO concentration in the media with steroids ranged from 0.001 to 1%. (B) The distance moved and thigmotaxis of larvae treated with the vehicle (1% DMSO; same data as in A) and pentobarbital (1–300 μM). (C) Distance moved and thigmotaxis of larvae treated with the vehicle (1% DMSO; same data as in A) and ketamine (0.1–300 μM). In the box plots (A–C), the boundary of the box closest to zero indicates the 25th percentile, the red line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers above and below the box indicate the 10th and 90th percentiles. Dot plots show the distance moved by each individual. *indicates statistically significant differences in the median values of the distance moved (Kruskal–Wallis One-Way ANOVA on ranks followed by pairwise comparison of the distance larvae moved in the presence of steroid versus in the medium containing vehicle (1% DMSO); Dunn’s Method). #indicates statistically significant differences in the mean values of the time spent in the outer zone (One Way ANOVA followed by pairwise comparison of the time larvae spent in the outer zone in the presence of steroids versus in the medium containing vehicle (1% DMSO); Holm–Sidak test) (same experiment as the measurements of distance moved). (A–C) n = number of fish per condition/drug concentration: DMSO, n = 159; PAG, n = 30–36; 17-OH-PAG, n = 30–33; PA, n = 31–35; 17-OH-PA, n = 31–34; pentobarbital, n = 23–38; and ketamine n = 29–36; assessed in two independent measurements for each condition.
Measurements of the locomotor activity were used to determine the time larvae spent in the center of the chamber to analyze thigmotaxis. Thigmotaxis provides information on anxiety and is characterized by the propensity of animals to avoid the center of an arena and stay or move in proximity to the boundaries of a novel environment. This evolutionarily conserved behavior is displayed by a wide range of species including humans,53 rodents,54 and fish.55 Our analysis indicates that vehicle (DMSO)-treated larvae spent significantly more time (72.8 ± 1.0%) in the outer zone relative to the inner zone (27.2 ± 1.0%; Paired t-test; n = 159). The time larvae spent in the outer zone is significantly increased above the chance level set by the relative surface area of the outer zone (64.0%; One-sample t-test; p < 0.001). We observed that for larvae treated with PAG or PA, the time spent in the outer zone relative to vehicle (DMSO)-treated larvae was significantly increased (anxiogenic effect) at concentrations that did not affect the distance larvae moved (10–30 and 0.1–0.3 μM, respectively; Figure 4A). Both 17-OH-PAG and 17-OH-PA exerted an anxiolytic effect by significantly decreasing the time spent in the outer zone relative to vehicle (DMSO)-treated larvae at concentrations of 10–30 and 3 μM, respectively (Figure 4A).
Control experiments were performed to exclude the effect of the time of day and DMSO on the locomotor activity of the larvae. Like many other animals, zebrafish larvae have a circadian rhythm of locomotor activity.56,57Figure S4 shows the results of experiments in which the locomotor activity was assessed at different times of day relevant to pharmacological experiments. We found no differences in the locomotor activity recorded in the presence of the control medium during the monitoring period for epochs of 14:00–15:00 to 18:00–19:00. Since steroids were dissolved in DMSO, the effect of this vehicle was also tested. No significant differences in locomotor activity were found for larvae maintained in E3 medium containing 1% DMSO (the maximal concentration used in pharmacological experiments) before and during the monitoring period for epochs of 14:00–15:00 to 18:00–19:00. Furthermore, no differences were found in the locomotor activity recorded in the control and DMSO containing medium (see Figure S4). Locomotor activity assessed in vehicle (DMSO)-treated larvae in epochs 14:00–15:00 to 18:00–19:00 was pooled and served as the control (vehicle (DMSO)-treated larvae) in the pharmacological experiments, as shown in Figure 4.
The effect of steroids on the locomotor activity observed here may be attributed to their positive allosteric effect at GABARs and/or negative allosteric effect at NMDARs. Therefore, we assessed the behavioral effects of drugs that selectively potentiate GABARs (pentobarbital) or inhibit NMDARs (ketamine).58,59 At low concentrations, pentobarbital (1–3 μM) had no effect on the locomotor activity when compared to the vehicle (DMSO)-treated larvae; at 10–100 μM, it diminished the distance larvae moved, and at 300 μM, it nearly completely eliminated larvae movement (Figure 4B). The effects were reversible within a 1 h washout period. Thigmotaxis assay showed no effect of pentobarbital (1–100 μM) on the time larvae spent in the outer zone relative to the inner zone when compared to the vehicle (DMSO)-treated larvae (Figure 4B). Ketamine at 0.1–0.3 μM had no effect on the locomotor activity when compared to the vehicle (DMSO)-treated larvae. At 1 μM, it increased the distance the larvae moved, and at 10–300 μM, it diminished the larval movement (Figure 4C). The effect of ketamine (300 μM) on the spontaneous locomotor activity recovered within a 1 h washout period. In larvae treated with 100 μM ketamine, the time spent in the outer zone relative to vehicle (DMSO)-treated larvae was significantly increased (anxiogenic effect) (Figure 4C). The observed effects of PAG and its metabolites on locomotor activity of zebrafish larvae resemble the inhibitory behavioral effects of pentobarbital and ketamine, underscoring the ability of neurosteroids to powerfully modulate CNS function.
Discussion
The goal of the present study was to analyze the pathways of metabolism of the synthetic neuroactive steroid PAG, assess the concentration of PAG and its metabolites in HC of rat pups, determine the effect of these metabolites on GABAR and NMDAR function, and characterize the influence of steroid treatment on the locomotor activity and thigmotaxis in larval zebrafish.
Our results show that PAG is rapidly (within 15 min) metabolized to steroids that reach high concentrations in the hippocampal tissue in rat pups. PAG itself accounts for only ∼6% of the overall 5β-steroid increase induced in HC by i.p. PAG application, and its unconjugated form (PA) accounts for ∼10%. The most abundant PAG metabolite in HC was 17-OH-PA (∼74%), while its conjugated form 17-OH-PAG was present at relatively low concentrations (∼0.04%). Of the direct metabolites of PAG, 0.5 and 1.2% were present in the conjugated and unconjugated form of (20S)-5β-pregnane-3α,20-diol, respectively. The relative increase in the concentration of steroids in the hippocampal tissue following i.p. injection of PAG compared to their control values was most prominent for PA, 17-OH-PA, and (20S)-5β-pregnane-3α,20diol in their conjugated and unconjugated form (Table 1).
Previous data, including ours, demonstrate close association between the circulating steroids and steroids in the brain60 and the cerebrospinal fluid.61 In agreement with these reports, our present data suggest that a considerable part of PAG penetrates through the BBB and accounts for 6% of the overall 5β-steroid increase in HC after the PAG application. The serum concentrations of PA and 17-OH-PAG were higher than the concentration of PAG, indicating that PAG was rapidly metabolized. Our data suggest that parallel conversions of PAG to PA and PAG to 17-OH-PAG occur, and the resulting 17-OH-PAG is then deconjugated to 17-OH-PA (Table 2). Surprisingly, the levels of 17-OH-PA are substantially higher in the brain than in the blood. Steroid levels can be significantly higher in the brain compared to the blood in various vertebrate species, including humans, for various steroids.62−64
The proportion of the overall 5β-steroid increase in HC after the PAG application found for 20-hydroxypregnanolone glutamate with polarity comparable to PAG is less than 1%. As expected, the proportion is even lower for the more polar 17-OH-PAG as steroid penetrability through the BBB negatively correlates with the polarity of the substance.61 Concerning the penetrability of steroid conjugates from the periphery into the CNS, pregnenolone sulfate and, to a lesser extent, the more polar dehydroepiandrosterone sulfate readily penetrate through the BBB despite the known preference (∼10-fold) of organic anion transporter for the efflux of steroid sulfates from the CNS over their influx.65 In all probability, the penetration of less polar steroid glutamates should be easier compared to the more polar steroid sulfates.
Endogenous neurosteroids and their synthetic analogues are known to affect several classes of metabotropic receptors as well as ligand- and voltage-gated ion channels and other synaptic targets that may directly or indirectly affect brain development and function and have clinical potential as anesthetics, anti-depressants, anti-epileptics, neuroprotective agents, and cognitive enhancers.2,3,66−71 Our electrophysiological analysis was confined to PAG and its close metabolites PA and 17-OH-PA that are commercially available. 17-OH-PAG was synthesized from 17-OH-PA (steroid with the hydroxyl group at C17 that is sterically hindered). It was not possible to synthesize 3α-glutamyl ester of (20S)-5β-pregnane-3α,17α,20-triol, as both C3 and C20 hydroxyl groups are secondary and as such, both hydroxyl groups would exhibit identical reactivity under the condition of coupling with l-glutamate. While PAG is inhibitory via negative modulation of NMDARs,21,22 PA is a positive allosteric modulator acting at GABARs.72,73 To our knowledge, the effect of 17-OH-PA on GABARs has not been studied yet. The results of our experiments indicate that this steroid is a positive allosteric modulator of GABARs with an apparent affinity similar to PA.
Typically, endogenous charged (sulfated) steroids with the “bent” structure at the A/B ring (such as pregnanolone sulfate) show inhibitory action at NMDARs (but see ref (74)) and GABARs, while their uncharged forms potentiate GABARs and have little to no effect at NMDARs.3,46 In agreement with this overall neurosteroid structure–function pattern, the results of our electrophysiological experiments showed that PA and 17-OH-PA potentiated GABARs responses while PAG and 17-OH-PAG inhibited NMDAR responses. Surprisingly, however, the charged PAG did not affect GABARs even at high concentrations, and 17-OH-PAG potentiated GABARs. The potentiating effect of 17-OH-PAG at GABARs must be interpreted cautiously since 17-OH-PA, a potent positive allosteric modulator of GABARs (see Figure 3F), may be present as a contaminant in the 17-OH-PAG solution. Unexpectedly, uncharged 17-OH-PA had an inhibitory effect on NMDARs. If we consider concentrations at which PAG and its metabolites are found in the hippocampal tissue, the action of PA and 17-OH-PA as positive allosteric modulators of GABARs and the inhibitory effect 17-OH-PA at NMDARs are most relevant for the in vivo effects.
In vivo analysis of the effect of PAG and its metabolites indicates that all of the steroids studied affected the distance the larvae moved and thigmotaxis. The effect of PAG and PA on the swim length occurred in a narrow concentration range (less than a three-fold concentration difference between no effect and complete elimination of movement) in contrast to pentobarbital and ketamine, selective modulators of GABARs and NMDARs, respectively, that affected the distance the larvae moved in a broad concentration range (Figure 4). Both PAG and PA exhibited an anxiogenic effect at concentrations that did not affect the swim length, similar to that observed for high concentrations of ketamine; no obvious effect on thigmotaxis was observed for pentobarbital. Metabolites 17-OH-PAG and 17-OH-PA had similar effects characterized by anxiolytic action with only a mild reduction of the distance the larvae moved.
The behavioral effects of the steroids studied are indicative of sedation, sleep, or anesthesia and cannot be unequivocally attributed to the effect on either GABARs or NMDARs. It has been shown earlier that certain pregnane steroids induce rapid sedation and anesthesia in rodents75 by a mechanism that was later associated with the enhancement of responses mediated by GABARs.2 Ketamine, a selective NMDAR inhibitor, has similar effects, that include dissociative anesthesia, pain relief, sedation, and amnesia.76−79 Incomplete inhibition of spontaneous zebrafish locomotion was reported earlier for MK-801, a compound with a similar mode of action at the NMDAR as ketamine.76,80−82 Inhibition of locomotion is also observed in zebrafish with knockouts of both paralogues the obligatory GluN1 subunit of the NMDAR.82 Thus, while it is clear that neurosteroids with modulatory action at GABARs and NMDARs can profoundly influence CNS function, further research is needed to better understand how individual steroids and their metabolites act in concert to alter the balance of excitation and inhibition in neural circuits, with clinically relevant consequences for behavior.
Materials and Methods
Analysis of PAG and Its Metabolites in the Nervous Tissue
Samples
PAG was dissolved in a solution of 3 g of CDX (Sigma-Aldrich, St. Louis, MO, USA) and 157 mg of citric acid (Sigma-Aldrich) in 30 mL of distilled water. The pH was adjusted to 7.36 with NaOH. The solution of PAG was administered intraperitoneally (i.p.) at a concentration of 1 mg/mL at a dose of 1 mg/kg. To characterize PAG metabolism, we determined steroid content in the hippocampal tissue of male 12-day-old albino Wistar rats that were either naive, controls (15 min following i.p. application of CDX in a volume corresponding to the 1 mg/kg PAG dose), or the experimental group (15 min following i.p. application of 1 mg/kg PAG in CDX).
Steroid Analysis: Sample Preparation
Hippocampal tissue (left + right HC, 17–100 mg) was minced and then transferred into screw-cap tubes. Then, 2 mL of methanol with a mixture of internal standards was added and the samples were incubated for one week at 4 °C with agitation for 2 min once a day. Afterward, the extract was transferred into fresh screw-cap tubes and dried in a vacuum centrifuge at 45 °C. Then, 1 mL of water of chromatographic purity was added and the samples were further treated as described before.83
Steroid Analysis: GC–MS/MS Quantification
The GC–MS/MS-based steroid quantification method was as described earlier.83 The processing of rat hippocampal tissue samples was as described in detail.36
Cell Cultures: Primary Hippocampal Neurons
Hippocampi were dissected from newborn (P0-P1) male Wistar rat pups. Cells were dissociated in papain, washed, and plated in Neurobasal A medium supplemented with B27 (Gibco) at a density of 50,000 cells/cm2 on top of a confluent layer of cortical astrocytes grown on collagen/poly-d-lysine-covered glass coverslips.71
Electrophysiology
Electrophysiological experiments were performed on cultured hippocampal neurons, as described previously.20 Whole-cell voltage-clamp recordings were made with a patch-clamp amplifier (Axopatch 200B; Axon Instruments. Inc., Foster City, CA) after series resistance (<10 MΩ) and capacitance compensation of 80–90%. Agonist-induced responses were recorded at a holding potential of −60 mV, low-pass filtered at 2 kHz, digitally sampled at 5 kHz, and analyzed with pClamp software version 10 (Molecular Devices). Patch pipettes (3–5 MΩ) pulled from borosilicate glass were filled with a Cs-based intracellular solution containing the following (in mM): 140 CsCl, 5 NaCl, 10 HEPES, 1 MgCl2, 5 EGTA, and 0.2 ATP-Mg salt (pH-adjusted to 7.35). Extracellular solution (ECS) used to record GABA receptor responses contained the following (in mM): 160 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, (pH adjusted to 7.3 with NaOH), and ECS used to record NMDA receptor responses contained the following (in mM): 160 NaCl, 2.5 KCl, 10 HEPES, 10 glucose, 0.7 CaCl2, and 0.2 EDTA (pH-adjusted to 7.3 with NaOH). GABAR responses were induced by GABA in the presence of 10 μM CNQX, 50 μM D-AP5, and 0.5 μM tetrodotoxin (TTX). NMDAR responses were induced by NMDA in the continuous presence of 10 μM glycine, 10 μM CNQX, 10 μM bicuculline, and 0.5 μM TTX.
Steroids were dissolved in dimethyl sulfoxide (DMSO) and added to the ECS at the indicated concentrations, with the final DMSO concentration of 1%. The same concentration of DMSO (1%) was present in control solutions. The final dilution of steroids in ECS was made at 50 °C, and the solution was sonicated for 1 min (Sonorex Digitec DT 100/H, Badelin electronic, Berlin, Germany). Application of solutions was made using a microprocessor-controlled multi-barrel fast perfusion system. The solution exchange rate was estimated to be τ ∼ 12.0 ms for dish-attached cells.84 Experiments were performed at room temperature (21–25 °C).
Experiments on Zebrafish
Zebrafish Maintenance
Handling and maintenance of zebrafish comply with the Directive 2010/63/EU on the protection of animals used for scientific purposes and with national and institutional guidelines. Adult wild-type (WT) zebrafish (Danio rerio, AB strain) were obtained from the Zebrafish International Resource Center (www.zirc.org) (ZIRC, Eugene, OR, USA). Fish were mated, raised, and staged according to published protocols85 as well as recommendations for zebrafish husbandry86 and housed in a ZebTEC aquatic system (Tecniplast) at 28 °C in the Institute of Molecular Genetics CAS, Prague, zebrafish facility. The fish were fed live brine shrimp and dried flake food daily (Skretting, Tooele, USA). The fish were kept on a 14 h light–10 h dark cycle (lights on at 8:00; lights off at 22:00). For in-system breeding, crosses of males and females were set up the previous evening in tanks with partitions taken off the following morning at the time of light onset to stimulate spawning and fertilization. Fertilized eggs were collected, placed in Petri dishes, and washed thoroughly with buffered egg water (in mM): 5 NaCl, 0.17 KCl, 0.33 CaCl2, 0.33 MgSO4, and 0.0001% methylene blue, hereafter referred to as E3 medium. After experiments, larvae were euthanized with an overdose of tricaine (Sigma-Aldrich) and disposed of according to local regulations.
Locomotor Activity
All experiments were done in groups of 20 zebrafish larvae at 6 days postfertilization. The experimental protocol consisted of two phases. In the first phase, 20 larvae were transferred from the dish containing their siblings to a new one containing control or test E3 and kept in an incubator at 28 °C for 1 h. In the second phase, larvae were transferred individually to wells prepared by attaching a grid of 20 square apertures (each 10 mm × 10 mm × 3 mm (w × d × h) with a septum of 2 mm made from poly(d,l-lactic acid) made by a 3D printer (Original Prusa i3 MK3S, Prusa Research, Prague, Czech Republic) and attached to the bottom of a polystyrene Petri dish (Thermo Scientific 100 × 20 mm; Cat # 130182). Larvae were allowed to acclimate for 5 min in the Petri dish that was temperature-controlled (28 ± 0.5 °C), illuminated (100 lux), and placed in a custom-made holder attached to an optically and vibration-isolated table. Locomotor activity was tracked continuously for a duration of 1 h between 14:00 and 19:00. Swimming behavior was monitored using a high-resolution (6.41 MPix) USB3 Vision industrial camera U3-3880SE-M-GL (CMOS sensor Sony IMX178; Imaging Development Systems GmbH (IDS), Obersulm, Germany) employing an f = 50 mm lens (Lensagon, CK10M5020S43; Lensation GmbH, Karlsruhe, Germany). In the optical path, a lens (f = 250 mm; 200 mm in diameter) was placed close to the dish with larvae. The uEye camera control software (IDS) was modified to allow for real time encoding of the high-resolution input video and sequential capture of repeated experiments. To enable real time recording of high-resolution and high-framerate video input, the control software was extended to leverage hardware-accelerated video encoding by the graphics chip. The computer used for video capture (Intel i9-9900K, nVidia GeForce 2060 Super) was able to encode input video at 50 frames per second in real time into the HEVC compressed video format. Video recordings were stored on the computer for later analysis. For the processing, each video frame was converted to images separately for each well. A ResNet deep learning model was trained on a set of hand-annotated images representing the larvae position and shape as defined by 8 points, one for the head placed between the eyes and seven equidistant points along the tail, starting in the center of the swimming bladder. A classification Python script employing TensorFlow and Keras libraries was then used for inference of the larva position in the plane. Inferred coordinates were further processed on a 128 CPU core computer using an in-house R script with an optional shiny interface allowing locomotion tracking as well as additional (non)interactive analyses. For each group, we calculated the distance traveled and time spent in the outer zone representing 64% and the inner zone (of a square shape) representing 36% of the well surface area (1 cm2). For drug treatment trials, drugs in DMSO were diluted in E3 to a final concentration as described in the Results section (≤1% DMSO). Tracks were analyzed for the total distance moved; to remove system noise, an input filter of 0.134 mm (minimum distance moved) was used (i.e., filtered data). Locomotion analysis was only conducted on larvae that responded in 10–90% of the mean distance moved.
Chemicals
Most steroids and deuterated standards used for the metabolomic analysis were purchased from Steraloids (Newport, RI, USA). The deuterated standard D7 cortisone [2,2,4,6,6,12,12-D7] and trimethylchlorosilane (TMCS) for the hydrolysis of steroid conjugates were from Sigma-Aldrich. Sylon BTZ, methoxyamine hydrochloride, and all other solvents and chemicals were from Merck (Darmstadt, Germany). All solvents were of high-performance liquid chromatography (HPLC) grade.
Steroids used in the electrophysiological experiments and to test the locomotor activity in the zebrafish: PA was prepared as described,19 17-OH-PA was purchased from Alfa chemistry (New York, USA, Catalogue No. A18D02141), and PAG was prepared according to a published protocol.18 Synthesis of 20-oxo-17α-hydroxy-5β-pregnan-3α-yl l-glutamyl 1-ester and 17-OH-PAG is described below. Ketamine hydrochloride and pentobarbital were purchased from Bioveta a.s. (Ivanovice na Hane, Czech Republic).
Synthesis of 17α-Hydroxy-20-oxo-5β-pregnan-3α-yl l-glutamyl 1-ester
3α,17α-Dihydroxy-5β-pregnan-20-one (17-OH-PA) (Alfa chemistry New York, USA, 840 mg, 2.5 mM) and Boc-Glu(OBzl)-OH (1036 mg, 0.8 mM) were dissolved in dry benzene (60 mL) and dry dichloromethane (4 mL). Then, 4-dimethylaminopyridine (30 mg, 0.3 mM) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (740 mg, 4.8 mM) were added under an inert atmosphere, and the reaction mixture was stirred at room temperature. After 12 h, the reaction mixture was poured into a saturated solution of sodium bicarbonate (50 mL) and the product was extracted with ethyl acetate (3 × 15 mL). Combined organic extracts were washed with water (2 × 50 mL), 5% water solution of hydrochloric acid, and saturated water solution of hydrogen sodium sulfate and dried over anhydrous sodium sulfate. The solvent was removed in vacuo. Chromatography on silica gel (50% ether in petroleum ether) gave white foam of 20-oxo-17-hydroxy-5β-pregnan-3α-yl (2S)-5-(benzyloxy)-2-[(tert-butoxycarbonyl)amino]-5-oxo-pentanoate (780 mg, 48%): 1H NMR (401 MHz, CDCl3): δ 7.36 (s, 5H-Ar), 5.13 (s, 2H-N), 4.77 (td, J = 11.1, 5.6 Hz, 1H-3β), 4.29 (dd, J = 13.0, 7.3 Hz, 1H-2′), 2.71–2.60 (m, 1H), 2.46 (m, 2H-4′), 2.27 (s, 3H-21), 1.44 (s, 9H, t-But), 0.93 (s, 3H-19), 0.70 (s, 3H-18). 13C NMR (101 MHz, CDCl3): δ 211.9, 172.7, 171.8, 155.7, 134.0, 128.72 (2 × C), 128.42, 128.37 (2 × C), 105.1, 90.2, 75.8, 66.6, 50.7, 48.7, 42.1, 40.4, 35.8, 35.1, 34.8, 33.8, 32.2, 30.6, 30.5, 28.5 (3 × C), 28.1, 28.1, 27.0, 26.6, 26.6, 24.2, 23.3, 20.4, 15.7.
Palladium on charcoal (80 mg, 10%) was added to the solution of the above-described compound (780 mg, 1.2 mM) in absolute MeOH (45 mL). The reaction mixture was hydrogenated under slight overpressure of hydrogen. After 8 h, the reaction mixture was filtered through diatomaceous earth to remove the catalyst. The filtration column was washed with acetone. Combined filtrates were evaporated in vacuo. The residue was dissolved in ether. Evaporation in vacuum gave white foam of 20-oxo-17-hydroxy-5β-pregnan-3α-yl N-(tert-butoxycarbonyl)-l-glutamyl 1-hemiester (379 mg, 58%): 1H NMR (401 MHz, CDCl3): δ 5.21 (d, J = 8.1 Hz, 1H-N), 4.78 (tt, J = 11.3, 4.7 Hz, 1H-3β), 4.39–4.20 (m, 1H-2′), 2.66 (ddd, J = 14.5, 11.7, 2.6 Hz, 1H), 2.51–2.35 (m, 2H-4′), 2.26 (s, 3H-21), 1.45 (s, 9H, t-But), 0.94 (s, 3H-19), 0.69 (s, 3H-18). 13C NMR (101 MHz, CDCl3): δ 212.0, 176.8, 171.7, 155.7, 90.3, 80.4, 75.7, 53.1, 50.7, 48.7, 42.0, 40.3, 35.8, 35.1, 34.7, 33.7, 32.2, 30.6, 30.2, 28.5, 28.3 (3 × C), 28.1, 27.0, 26.6, 26.6, 24.1, 23.4, 20.5, 15.7.
Then, trifluoroacetic acid (4 mL, 53.6 mM) was added dropwise to a stirred solution of the above-described compound (465 mg, 0.85 mM) in dichloromethane (4 mL). The reaction mixture was stirred for 2 h at room temperature and allowed to stand overnight at 5 °C. Then, it was evaporated to dryness, and excess trifluoroacetic acid was removed by evaporation with benzene. Residue was dissolved in a mixture of pyridine (4 mL) and MeOH (4 mL). One hour later, the mixture was poured into water (40 mL). The precipitated product (159 mg, 40%) was separated by filtration: m.p. 212–214 °C, [α]D + 36.5 (c 0.14, CHCl3/MeOH, 4:1). 1H NMR (401 MHz, CDCl3): δ 7.82 (s, 2H-N), 4.78–4.86 (m, 1H-3β), 3.97 (dd, J = 8.1, 3.7 Hz, 1H-2′), 2.72 (ddd, J = 14.7, 11.5, 3.0 Hz, 1H-4′), 2.52 (t, J = 6.5 Hz, 2H), 2.24 (s, 3H-21), 1.03 (s, 2H-19), 0.65 (s, 3H-18). 13C NMR (101 MHz, MeOD): δ 211.9, 177.7, 168.8, 104.4, 89.6, 76.1, 52.5, 50.2, 46.6, 41.4, 39.5, 35.3, 34.1, 33.8, 32.1, 31.2, 30.3, 26.2, 26.0, 25.8, 25.7, 25.6, 22.8, 22.0, 19.8, 13.8. IR spectrum (CHCl3): 3517 (OH, COOH); 1746, 1739 (C=O, COOH); 1703 (C=O, acetate), 1355 (C=O, acetate). MS: ESI m/z 486.3 (100%, M + 23). HR-MS (ESI) m/z: for C26H41O6NNa [M + Na] calcd, 486.28261; found 386.28255. For C26H41NO6: calcd, C, 67.36%; H, 8.91%; N, 3.02%. Found: C, 66.13%; H, 8.82%; N, 2.77%.
Statistical Analysis
Data are presented as mean ± SEM or median with 10th, 25th, 75th, and 90th percentile (box-and-whisker plots). Throughout the study, n refers to the number of cells or larvae tested. The statistical analysis was performed with SigmaPlot 14.0. Data distribution was assessed using the Shapiro–Wilk Normality test and equal variance was tested by the Brown–Forsythe test. We used parametric statistics (paired t-test, unpaired t-test, or ANOVA) if data distribution was normal, and nonparametric statistics (Mann–Whitney rank-sum test, Wilcoxon signed-rank test, or Kruskal–Wallis one-way ANOVA on ranks) if data did not have a normal distribution; p ≤ 0.05 was considered statistically significant throughout the study.
In the metabolomic data, the determined steroid concentrations in the hippocampal tissue were transformed by power transformations to attain symmetric data distribution and homoscedasticity (constant variance). Then, the values of transformed means and their confidence intervals were re-transformed to the original scale.
Institutional Review Board Statement
The study was conducted according to the guidelines of the European Union directive 2010/63/EU and Act No 246/1992 Coll. on the protection of animals against cruelty and was approved by the Animal Care and Use Committee of the Institute of Physiology of the Czech Academy of Sciences and by the Central Committee of the Czech Academy of Sciences (approval number 16/2020, approved 20 June 2020).
Acknowledgments
This work was supported by the Czech Science Foundation (GACR): 23-04922S; Technology Agency of the Czech Republic: TN02000109; ERDF/ESF project: PharmaBrain (No. CZ.02.1.01/0.0/0.0/16_025/0007444); Research Project of the CAS RVO: 67985823 and RVO 61388963; and BIOCEV – Biotechnology and Biomedicine Centre of Academy of Sciences and Charles University in Vestec, project supported from European Regional Development Fund; Ministry of Education, Youth and Sport grant LM2023052. We thank R. Markova and N. Pavlu for their excellent technical assistance.
Glossary
Abbreviations
- 17-OH-PA
3α,17α-dihydroxy-5β-pregnan-20-one
- 17-OH-PAG
20-oxo-17α-hydroxy-5β-pregnan-3α-yl l-glutamyl 1-ester
- AKR1C8
type 8, subfamily 1C aldoketoreductase
- AKR1D1
aldoketoreductase family 1 member D1
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BBB
blood–brain barrier
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CYP11B1
steroid 11β-hydroxylase
- CYP17A1
steroid 17-hydroxylase-C17,20-lyase
- CYP21A1
steroid 21-hydroxylase
- DMSO
dimethyl sulfoxide
- ECS
extracellular solution
- EDTA
ethylenediaminetetraacetic acid
- GABA
γ-aminobutyric acid
- GABAR
GABAA receptor
- GGH
γ-glutamyl hydrolase
- HC
hippocampus
- HPLC
high-performance liquid chromatography
- HSD17B6
steroid type 6 17β-hydroxysteroid dehydrogenase
- HSD17B10
steroid type 10 17β-hydroxysteroid dehydrogenase
- ICS
intracellular solution
- i.p.
intraperitoneal
- MK801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
- NMDA
N-methyl-d-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- PA
3α-hydroxy-5β-pregnan-20-one
- PAG
20-oxo-5β-pregnan-3α-yl l-glutamyl 1-ester
- RDH16
type 16 retinol dehydrogenase
- THDOC
3α,21-dihydroxy-5α-pregnan-20-one
- TTX
tetrodotoxin
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.3c00131.
Time course of the potentiation of GABAR responses by pregnanolone with repeated GABA and steroid co-applications; effects of GABA, GABA + propofol, and picrotoxin on neuronal GABARs; combined effect of PA and 17-OH-PA at GABARs; and locomotor activity of zebrafish larvae in relationship to the time of day (PDF)
Author Contributions
V.A., P.Ba., and A.B.: Performed experiments on zebrafish. V.L.A., K.F., M.K., V.V., B.K., and B.H.K.: Performed electrophysiological experiments and their analysis. M.H., K.V.: Performed metabolomic analysis. E.K., H.C.: Synthesized the steroids. M.M., D.C., P.Bo., J.M., and J.C.: Wrote video recording and tracking analysis programs. I.D.: Constructed zebrafish set-up. L.V.: Designed and supervised this project. L.V., T.S.: Wrote the manuscript. All authors have read and agreed to the submitted version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Baulieu E. E. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997, 52, 1–32. [PubMed] [Google Scholar]
- Belelli D.; Lambert J. J. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Korinek M.; Kapras V.; Vyklicky V.; Adamusova E.; Borovska J.; Vales K.; Stuchlik A.; Horak M.; Chodounska H.; Vyklicky L. Neurosteroid modulation of N-methyl-D-aspartate receptors: molecular mechanism and behavioral effects. Steroids 2011, 76, 1409–1418. 10.1016/j.steroids.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Mellon S. H.; Griffin L. D.; Compagnone N. A. Biosynthesis and action of neurosteroids. Brain Res. Brain Res. Rev. 2001, 37, 3–12. 10.1016/S0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan A. G.; Do-Rego J. L.; Beaujean D.; Luu-The V.; Pelletier G.; Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999, 51, 63–81. [PubMed] [Google Scholar]
- Jo D. H.; Abdallah M. A.; Young J.; Baulieu E. E.; Robel P. Pregnenolone, dehydroepiandrosterone, and their sulfate and fatty acid esters in the rat brain. Steroids 1989, 54, 287–297. 10.1016/0039-128X(89)90003-2. [DOI] [PubMed] [Google Scholar]
- Orchinik M.; Murray T. F.; Franklin P. H.; Moore F. L. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 3830–3834. 10.1073/pnas.89.9.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez V. D.; Zheng J. Membrane sex-steroid receptors in the brain. Front. Neuroendocrinol. 1996, 17, 402–439. 10.1006/frne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Klangkalya B.; Chan A. Structure-activity relationships of steroid hormones on muscarinic receptor binding. J. Steroid Biochem. 1988, 29, 111–118. 10.1016/0022-4731(88)90384-6. [DOI] [PubMed] [Google Scholar]
- Majewska M. D. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–394. 10.1016/0301-0082(92)90025-A. [DOI] [PubMed] [Google Scholar]
- Valera S.; Ballivet M.; Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 9949–9953. 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S.; Gibbs T. T.; Farb D. H. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D- aspartate receptor. Mol. Pharmacol. 1991, 40, 333–336. [PubMed] [Google Scholar]
- Majewska M. D.; Schwartz R. D. Pregnenolone-sulfate: an endogenous antagonist of the gamma- aminobutyric acid receptor complex in brain?. Brain Res. 1987, 404, 355–360. 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Adla S. K.; Slavikova B.; Smidkova M.; Tloustova E.; Svoboda M.; Vyklicky V.; Krausova B.; Hubalkova P.; Nekardova M.; Holubova K.; Vales K.; Budesinsky M.; Vyklicky L.; Chodounska H.; Kudova E. Physicochemical and biological properties of novel amide-based steroidal inhibitors of NMDA receptors. Steroids 2017, 117, 52–61. 10.1016/j.steroids.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Slavikova B.; Chodounska H.; Nekardova M.; Vyklicky V.; Ladislav M.; Hubalkova P.; Krausova B.; Vyklicky L.; Kudova E. Neurosteroid-like Inhibitors of N-Methyl-d-aspartate Receptor: Substituted 2-Sulfates and 2-Hemisuccinates of Perhydrophenanthrene. J. Med. Chem. 2016, 59, 4724. 10.1021/acs.jmedchem.6b00079. [DOI] [PubMed] [Google Scholar]
- Kudova E.; Chodounska H.; Slavikova B.; Budesinsky M.; Nekardova M.; Vyklicky V.; Krausova B.; Svehla P.; Vyklicky L. A New Class of Potent N-Methyl-D-Aspartate Receptor Inhibitors: Sulfated Neuroactive Steroids with Lipophilic D-Ring Modifications. J. Med. Chem. 2015, 58, 5950–5966. 10.1021/acs.jmedchem.5b00570. [DOI] [PubMed] [Google Scholar]
- Cerny J.; Bozikova P.; Balik A.; Marques S. M.; Vyklicky L. NMDA Receptor Opening and Closing-Transitions of a Molecular Machine Revealed by Molecular Dynamics. Biomolecules 2019, 9, 546. 10.3390/biom9100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovska J.; Vyklicky V.; Stastna E.; Kapras V.; Slavikova B.; Horak M.; Chodounska H.; Vyklicky L. Access of inhibitory neurosteroids to the NMDA receptor. Br. J. Pharmacol. 2012, 166, 1069–1083. 10.1111/j.1476-5381.2011.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastna E.; Chodounska H.; Pouzar V.; Kapras V.; Borovska J.; Cais O.; Vyklicky L. Synthesis of C3, C5, and C7 pregnane derivatives and their effect on NMDA receptor responses in cultured rat hippocampal neurons. Steroids 2009, 74, 256–263. 10.1016/j.steroids.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Vyklicky V.; Smejkalova T.; Krausova B.; Balik A.; Korinek M.; Borovska J.; Horak M.; Chvojkova M.; Kleteckova L.; Vales K.; Cerny J.; Nekardova M.; Chodounska H.; Kudova E.; Vyklicky L. Preferential Inhibition of Tonically over Phasically Activated NMDA Receptors by Pregnane Derivatives. J. Neurosci. 2016, 36, 2161–2175. 10.1523/JNEUROSCI.3181-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambousek L.; Bubenikova-Valesova V.; Kacer P.; Syslova K.; Kenney J.; Holubova K.; Najmanova V.; Zach P.; Svoboda J.; Stuchlik A.; Chodounska H.; Kapras V.; Adamusova E.; Borovska J.; Vyklicky L.; Vales K. Cellular and behavioural effects of a new steroidal inhibitor of the N-methyl-d-aspartate receptor 3alpha5beta-pregnanolone glutamate. Neuropharmacology 2011, 61, 61–68. 10.1016/j.neuropharm.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Vales K.; Rambousek L.; Holubova K.; Svoboda J.; Bubenikova-Valesova V.; Chodounska H.; Vyklicky L.; Stuchlik A. 3alpha5beta-Pregnanolone glutamate, a use-dependent NMDA antagonist, reversed spatial learning deficit in an animal model of schizophrenia. Behav. Brain Res. 2012, 235, 82–88. 10.1016/j.bbr.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Ishikawa M.; Yoshitomi T.; Covey D. F.; Zorumski C. F.; Izumi Y. Neurosteroids and oxysterols as potential therapeutic agents for glaucoma and Alzheimer’s disease. Neuropsychiatry 2018, 8, 344–359. 10.4172/Neuropsychiatry.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guennoun R.; Labombarda F.; Gonzalez Deniselle M. C.; Liere P.; De Nicola A. F.; Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. 10.1016/j.jsbmb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Shafizadeh T. B.; Halsted C. H. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. J. Nutr. 2007, 137, 1149–1153. 10.1093/jn/137.5.1149. [DOI] [PubMed] [Google Scholar]
- Payne A. H.; Hales D. B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004, 25, 947–970. 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Le Goascogne C.; Sananes N.; Gouezou M.; Takemori S.; Kominami S.; Baulieu E. E.; Robel P. Immunoreactive cytochrome P-450(17 alpha) in rat and guinea-pig gonads, adrenal glands and brain. J. Reprod. Fertil. 1991, 93, 609–622. 10.1530/jrf.0.0930609. [DOI] [PubMed] [Google Scholar]
- Vianello S.; Waterman M. R.; Dalla Valle L.; Colombo L. Developmentally regulated expression and activity of 17alpha-hydroxylase/C-17,20-lyase cytochrome P450 in rat liver. Endocrinology 1997, 138, 3166–3174. 10.1210/endo.138.8.5297. [DOI] [PubMed] [Google Scholar]
- Dalla Valle L.; Toffolo V.; Vianello S.; Belvedere P.; Colombo L. Expression of cytochrome P450c17 and other steroid-converting enzymes in the rat kidney throughout the life-span. J. Steroid Biochem. Mol. Biol. 2004, 91, 49–58. 10.1016/j.jsbmb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Mukai H.; Tsurugizawa T.; Ogiue-Ikeda M.; Murakami G.; Hojo Y.; Ishii H.; Kimoto T.; Kawato S. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology 2006, 84, 255–263. 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Missaghian E.; Kempna P.; Dick B.; Hirsch A.; Alikhani-Koupaei R.; Jegou B.; Mullis P. E.; Frey B. M.; Fluck C. E. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009, 202, 99–109. 10.1677/JOE-08-0353. [DOI] [PubMed] [Google Scholar]
- Emanuelsson I.; Almokhtar M.; Wikvall K.; Gronbladh A.; Nylander E.; Svensson A. L.; Fex Svenningsen A.; Norlin M. Expression and regulation of CYP17A1 and 3beta-hydroxysteroid dehydrogenase in cells of the nervous system: Potential effects of vitamin D on brain steroidogenesis. Neurochem. Int. 2018, 113, 46–55. 10.1016/j.neuint.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Luu-The V. Assessment of steroidogenesis and steroidogenic enzyme functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 176–182. 10.1016/j.jsbmb.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Miller W. L.; Auchus R. J. The ″backdoor pathway″ of androgen synthesis in human male sexual development. PLoS Biol. 2019, 17, e3000198 10.1371/journal.pbio.3000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudova E.; Mares P.; Hill M.; Vondrakova K.; Tsenov G.; Chodounska H.; Kubova H.; Vales K. The neuroactive steroid pregnanolone glutamate: Anticonvulsant effect, metabolites and its effect on neurosteroid levels in developing rat brains. Pharmaceuticals 2022, 15, 49. 10.3390/ph15010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning T. M.; Wangtrakuldee P.; Auchus R. J. Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes. Endocr. Rev. 2019, 40, 447–475. 10.1210/er.2018-00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eechaute W. P.; Dhooge W. S.; Gao C. Q.; Calders P.; Rubens R.; Weyne J.; Kaufman J. M. Progesterone-transforming enzyme activity in the hypothalamus of the male rat. J. Steroid Biochem. Mol. Biol. 1999, 70, 159–167. 10.1016/S0960-0760(99)00106-5. [DOI] [PubMed] [Google Scholar]
- Stell B. M.; Brickley S. G.; Tang C. Y.; Farrant M.; Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 14439–14444. 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S.; Losi G.; Homanics G. E. GABA(A) receptor delta subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology 2002, 43, 646–650. 10.1016/S0028-3908(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Covey D. F.; Nathan D.; Kalkbrenner M.; Nilsson K. R.; Hu Y.; Zorumski C. F.; Evers A. S. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid(A) receptor modulation and anesthesia. J. Pharmacol. Exp. Ther. 2000, 293, 1009–1016. [PubMed] [Google Scholar]
- Le Foll F.; Castel H.; Louiset E.; Vaudry H.; Cazin L. Multiple modulatory effects of the neuroactive steroid pregnanolone on GABAA receptor in frog pituitary melanotrophs. J. Physiol. 1997, 504, 387–400. 10.1111/j.1469-7793.1997.387be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. J.; Belelli D.; Hill-Venning C.; Peters J. A. Neurosteroids and GABAA receptor function. Trends Pharmacol. Sci. 1995, 16, 295–303. 10.1016/S0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Sooksawate T.; Simmonds M. A. Increased membrane cholesterol reduces the potentiation of GABA(A) currents by neurosteroids in dissociated hippocampal neurones. Neuropharmacology 1998, 37, 1103–1110. 10.1016/S0028-3908(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Li P.; Bracamontes J. R.; Manion B. D.; Mennerick S.; Steinbach J. H.; Evers A. S.; Akk G. The neurosteroid 5beta-pregnan-3alpha-ol-20-one enhances actions of etomidate as a positive allosteric modulator of alpha1beta2gamma2L GABAA receptors. Br. J. Pharmacol. 2014, 171, 5446–5457. 10.1111/bph.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Chung M.; Malayev A.; Purdy R. H.; Gibbs T. T.; Farb D. H. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999, 830, 72–87. 10.1016/S0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Wang M.; He Y.; Eisenman L. N.; Fields C.; Zeng C. M.; Mathews J.; Benz A.; Fu T.; Zorumski E.; Steinbach J. H.; Covey D. F.; Zorumski C. F.; Mennerick S. 3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J. Neurosci. 2002, 22, 3366–3375. 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H.; Cottrell G. A.; Hather N. Y.; Lambert J. J.; Nooney J. M.; Peters J. A. Modulation of the GABAA receptor by progesterone metabolites. Proc. R. Soc. London, Ser. B 1987, 231, 359–369. 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Collins G. G. Effects of the anaesthetic 2,6-diisopropylphenol on synaptic transmission in the rat olfactory cortex slice. Br. J. Pharmacol. 1988, 95, 939–949. 10.1111/j.1476-5381.1988.tb11724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S.; Mienville J. M.; Costa E. Actions of benzodiazepine and beta-carboline derivatives on gamma-aminobutyric acid-activated Cl- channels recorded from membrane patches of neonatal rat cortical neurons in culture. J. Pharmacol. Exp. Ther. 1987, 243, 1195–1201. [PubMed] [Google Scholar]
- Germann A. L.; Pierce S. R.; Burbridge A. B.; Steinbach J. H.; Akk G. Steady-State Activation and Modulation of the Concatemeric alpha1beta2gamma2L GABA(A) Receptor. Mol. Pharmacol. 2019, 96, 320–329. 10.1124/mol.119.116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh R. N.; Cummins R. A. The Open-Field Test: a critical review. Psychol. Bull. 1976, 83, 482–504. 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- Kallai J.; Makany T.; Csatho A.; Karadi K.; Horvath D.; Kovacs-Labadi B.; Jarai R.; Nadel L.; Jacobs J. W. Cognitive and affective aspects of thigmotaxis strategy in humans. Behav. Neurosci. 2007, 121, 21–30. 10.1037/0735-7044.121.1.21. [DOI] [PubMed] [Google Scholar]
- Treit D.; Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol. Biochem. Behav. 1988, 31, 959–962. 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Schnorr S. J.; Steenbergen P. J.; Richardson M. K.; Champagne D. L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. 10.1016/j.bbr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- MacPhail R. C.; Brooks J.; Hunter D. L.; Padnos B.; Irons T. D.; Padilla S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. 10.1016/j.neuro.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Cahill G. M.; Hurd M. W.; Batchelor M. M. Circadian rhythmicity in the locomotor activity of larval zebrafish. Neuroreport 1998, 9, 3445–3449. 10.1097/00001756-199810260-00020. [DOI] [PubMed] [Google Scholar]
- Akk G.; Steinbach J. H. Activation and block of recombinant GABA(A) receptors by pentobarbitone: a single-channel study. Br. J. Pharmacol. 2000, 130, 249–258. 10.1038/sj.bjp.0703335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F.; Miljkovic Z.; Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J. Neurophysiol. 1987, 58, 251–266. 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Sze Y.; Gill A. C.; Brunton P. J. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J. Neuroendocrinol. 2018, 30, e12644 10.1111/jne.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kancheva R.; Hill M.; Novak Z.; Chrastina J.; Kancheva L.; Starka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience 2011, 191, 22–27. 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Irwin R. W.; Solinsky C. M.; Loya C. M.; Salituro F. G.; Rodgers K. E.; Bauer G.; Rogawski M. A.; Brinton R. D. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer’s disease. PLoS One 2015, 10, e0128313 10.1371/journal.pone.0128313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixo M.; Andersson A.; Winblad B.; Purdy R. H.; Backstrom T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997, 764, 173–178. 10.1016/S0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Liere P.; Cornil C. A.; de Bournonville M. P.; Pianos A.; Keller M.; Schumacher M.; Balthazart J. Steroid profiles in quail brain and serum: Sex and regional differences and effects of castration with steroid replacement. J. Neuroendocrinol. 2019, 31, e12681 10.1111/jne.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaiser M. Z.; Dolman D. E. M.; Begley D. J.; Abbott N. J.; Cazacu-Davidescu M.; Corol D. I.; Fry J. P. Uptake and metabolism of sulphated steroids by the blood-brain barrier in the adult male rat. J. Neurochem. 2017, 142, 672–685. 10.1111/jnc.14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A.; Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005, 28, 278–283. 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Grobin A. C.; Gizerian S.; Lieberman J. A.; Morrow A. L. Perinatal allopregnanolone influences prefrontal cortex structure, connectivity and behavior in adult rats. Neuroscience 2006, 138, 809–819. 10.1016/j.neuroscience.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Zorumski C. F.; Paul S. M.; Covey D. F.; Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol. Stress 2019, 11, 100196 10.1016/j.ynstr.2019.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D. S.; Estes W. A. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol. Sci. 2016, 37, 543–561. 10.1016/j.tips.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch E. D.; Hollmann M. W. Steroid Anesthesia Revisited: Again. Anesth. Analg. 2015, 120, 983–984. 10.1213/ANE.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T.; Korinek M.; Krusek J.; Hrcka Krausova B.; Candelas Serra M.; Hajdukovic D.; Kudova E.; Chodounska H.; Vyklicky L. Endogenous neurosteroids pregnanolone and pregnanolone sulfate potentiate presynaptic glutamate release through distinct mechanisms. Br. J. Pharmacol. 2021, 178, 3888–3904. 10.1111/bph.15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty D.; Thomas P.; Field M.; Andersen O. J.; Gold M. G.; Biggin P. C.; Gielen M.; Smart T. G. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol. 2017, 24, 977–985. 10.1038/nsmb.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir C. J.; Ling A. T.; Belelli D.; Wildsmith J. A.; Peters J. A.; Lambert J. J. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br. J. Anaesth. 2004, 92, 704–711. 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Kysilov B.; Hrcka Krausova B.; Vyklicky V.; Smejkalova T.; Korinek M.; Horak M.; Chodounska H.; Kudova E.; Cerny J.; Vyklicky L. Pregnane-based steroids are novel positive NMDA receptor modulators that may compensate for the effect of loss-of-function disease-associated GRIN mutations. Br. J. Pharmacol. 2022, 179, 3970–3990. 10.1111/bph.15841. [DOI] [PubMed] [Google Scholar]
- Selye H. Anesthetic Effect of Steroid Hormones. Proc. Soc. Exp. Biol. Med. 1941, 46, 116–121. 10.3181/00379727-46-11907. [DOI] [Google Scholar]
- MacDonald J. F.; Bartlett M. C.; Mody I.; Pahapill P.; Reynolds J. N.; Salter M. W.; Schneiderman J. H.; Pennefather P. S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J. Physiol. 1991, 432, 483–508. 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. M.; Roback M. G.; Kennedy R. M.; Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann. Emerg. Med. 2011, 57, 449–461. 10.1016/j.annemergmed.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Zanos P.; Moaddel R.; Morris P. J.; Riggs L. M.; Highland J. N.; Georgiou P.; Pereira E. F. R.; Albuquerque E. X.; Thomas C. J.; Zarate C. A. Jr.; Gould T. D. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris P. J.; Moaddel R.; Zanos P.; Moore C. E.; Gould T. D.; Zarate C. A.; Thomas C. J. Jr.; Thomas C. J. Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Org. Lett. 2017, 19, 4572–4575. 10.1021/acs.orglett.7b02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Patel R.; Friedman T. C.; Jones K. S. The Behavioral and Pharmacological Actions of NMDA Receptor Antagonism are Conserved in Zebrafish Larvae. Int. J. Comp. Psychol. 2010, 23, 82–90. [PMC free article] [PubMed] [Google Scholar]
- Li F.; Lin J.; Liu X.; Li W.; Ding Y.; Zhang Y.; Zhou S.; Guo N.; Li Q. Characterization of the locomotor activities of zebrafish larvae under the influence of various neuroactive drugs. Ann. Transl. Med. 2018, 6, 173. 10.21037/atm.2018.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoodsma J. D.; Chan K.; Bhandiwad A. A.; Golann D. R.; Liu G.; Syed S. A.; Napoli A. J.; Burgess H. A.; Sirotkin H. I.; Wollmuth L. P. A Model to Study NMDA Receptors in Early Nervous System Development. J. Neurosci. 2020, 40, 3631–3645. 10.1523/JNEUROSCI.3025-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.; Hana V. Jr; Velikova M.; Parizek A.; Kolatorova L.; Vitku J.; Skodova T.; Simkova M.; Simjak P.; Kancheva R.; Koucky M.; Kokrdova Z.; Adamcova K.; Cerny A.; Hajek Z.; Duskova M.; Bulant J.; Starka L. A method for determination of one hundred endogenous steroids in human serum by gas chromatography-tandem mass spectrometry. Physiol. Res. 2019, 68, 179–207. 10.33549/physiolres.934124. [DOI] [PubMed] [Google Scholar]
- Vyklicky V.; Korinek M.; Balik A.; Smejkalova T.; Krausova B.; Vyklicky L.. Analysis of Whole-Cell NMDA Receptor Currents. In Ionotropic Glutamate Receptor Technologies; Springer, 2016; pp 205–219. [Google Scholar]
- Westerfield M.The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, 2000. [Google Scholar]
- Alestrom P.; D’Angelo L.; Midtlyng P. J.; Schorderet D. F.; Schulte-Merker S.; Sohm F.; Warner S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. 10.1177/0023677219869037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.