Abstract
Background
Disorders of the sense of smell have received greater attention because of the frequency with which they occur as a symptom of SARS-CoV-2 infection. Olfactory dysfunction can lead to profound reduction in quality of life and may arise from many different causes.
Methods
A selective literature review was conducted with consideration of the current version of the guideline issued by the Association of the Scientific Medical Societies in Germany.
Results
The cornerstones of diagnosis are the relevant medical history and psychophysical testing of olfactory function using standardized validated tests. Modern treatment strategies are oriented on the cause of the dysfunction. While treatment of the underlying inflammation takes precedence in patients with sinunasal dysosmia, olfactory training is the primary treatment option for other forms of the disorder. The prognosis is determined not only by the cause of the olfactory dysfunction and the patient’s age, but also by the olfactory performance as measured at the time of diagnosis.
Conclusion
Options for the treatment of olfactory dysfunction are available but limited, depending on the cause. It is therefore important to carry out a detailed diagnostic work-up and keep the patient informed of the expected course and prognosis.
Despite the widely held assumption that the human sense of smell is relatively poor, humans are actually more sensitive than other mammals to a range of odors (1).
The general functions of the sense of smell.
The human sense of smell is important for the recognition of danger, perception of the flavors of food and drink, and social interaction.
Olfaction is unique among the senses in that the olfactory cells regenerate continuously (e1, 2). Another special feature of the human sense of smell is its duality: odor molecules reach the olfactory mucosa not only orthonasally, on breathing in through the nose, but also retronasally by way of the throat, both on breathing out and when eating and drinking, mainly while swallowing (3, e2, e3). The orthonasal route is important for the perception of ambient odor molecules, the retronasal pathway for the perception of flavor (e4). Besides its function as a warning system for fire or potentially poisonous chemicals, the sense of smell also helps to detect when food has gone off. This explains why patients with olfactory dysfunction report difficulties with eating, when cooking, and in recognition of danger (e5), together with a general sense of insecurity in their daily lives, including the area of personal hygiene (4). Olfaction is also important in social interactions, e.g., in partnership and sexuality, and loss of the sense of smell can lead to social insecurity and, in approximately one third of those affected, to signs of depression (e6, e7). Olfactory dysfunction is thus frequently associated with a distinct deterioration in quality of life (e6).
The impact of limited olfactory capacity.
Loss of the sense of smell may be associated with distinct deterioration in the quality of life and depressive symptoms.
An important role in perception of odors is played by the chemosensory system of the trigeminal nerve, which is activated by almost all odors in high concentration, triggering sensations such as stinging, pricking, tingling, coolness, warmth, or burning. Persons who lose their sense of smell or were born without it still possess this trigeminal perception.
Learning goals
After completing this article, the reader should be able to answer the following questions:
What are the principal causes of olfactory dysfunction?
How can the sense of smell be measured in clinical routine?
What are the main principles in the treatment of olfactory dysfunction?
Classification of olfactory dysfunction
Olfactory dysfunction is divided into quantitative disorders (readily measurable) and qualitative disorders (much less amenable to measurement) (5). The quantitative disorders can be subdivided by olfactory performance, e.g., according to sensitivity to odors (olfactory threshold), discrimination between odors, or identification of odors. Normal function of the sense of smell is termed normosmia (with the olfactory capacity of young adults often serving as reference value); reduction, hyposmia; and complete loss, anosmia.
Qualitative olfactory dysfunction is separated into two subgroups. The term parosmia describes disorders featuring altered perception of odors from an extant source, while phantosmia is the detection of odors in the absence of a source. As a rule, parosmia involves perception of odors as unpleasant and disgusting, e.g., coffee smells “spoilt” or “fecal.” Phantosmias are often experienced as “smoky” or “burnt.” These erroneous impressions are extremely disconcerting in day-to-day life. The confusing perceptions mean that parosmia and phantosmia both often seriously impair the patients’ quality of life (5, 6).
Quantitative and qualitative olfactory dysfunction may occur in isolation, but are often present in combination. For example, an odor may initially trigger parosmia, followed by persisting phantosmia (e8, 8). Qualitative olfactory dysfunction is found in all causes of loss of the sense of smell and also occurs in persons with demonstrably intact olfactory capacity (normosmia) (e9). Nevertheless, an accumulation of parosmias is found in postinfectious olfactory dysfunction. Phantosmia occurs more frequently with post-traumatic olfactory dysfunction (9).
The classification of olfactory dysfunction.
Reduced perception of odors is described as hyposmia, complete loss of the sense of smell as anosmia. In parosmia odors are perceived incorrectly, while in phantosmia odors are perceived in the absence of a source.
The causes of olfactory dysfunction
Olfactory disorders are classified according to the underlying cause. In addition to age-related dysfunction, they are divided into conditions of acquired and congenital origin (10). Exclusive categorization of olfactory disorders as conductive or sensorineural should no longer be practiced, because, for example, olfactory dysfunction in chronic sinusitis or following an infection often features both components (11, e10).
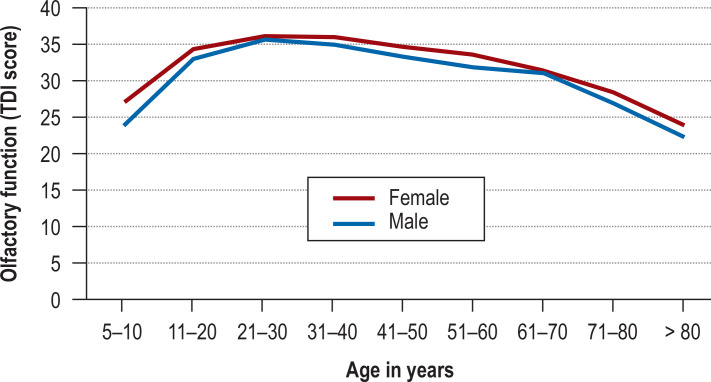
In common with hearing and sight, human olfactory function often deteriorates with advancing age (figure). Reduced olfactory performance is found in up to 75% of persons over the age of 80 years. The underlying causes include decreased regenerative capacity of the olfactory epithelium, increased apoptosis of olfactory cells, and altered central nervous processing (12). In addition to age-related impairment there are many other causes of acquired olfactory dysfunction (table 1): it can occur after an infection of the upper respiratory tract, for instance COVID-19 (postinfectious); following craniocerebral trauma (post-traumatic); with an underlying sinunasal condition (e.g., chronic rhinosinusitis with or without nasal polyposis); in the presence of an underlying neurological or neurodegenerative disease; in association with medications or other toxic substances; after radiotherapy or surgery; and with a tumor in the frontobasal region. Olfactory dysfunction may also be classified as congenital or—after exclusion of all known causative factors—idiopathic (11).
Figure.
Age-dependent change in olfactory function in subjectively normosmic persons (n = 3355), stratified by gender (modified from [23])
TDI, Summed results, olfactory threshold + discrimination test + identification test
Table 1. The most commonly occurring causes of acquired olfactory dysfunction in otorhinolaryngology*.
| Cause | Relative frequency |
| Sinunasal conditions (inflammations of the nose and nasal sinuses, non-inflammatory respiratory disorders) | 67% |
| Viral infection of the upper respiratory tract | 14% |
| Idiopathic | 8% |
| Trauma | 6% |
| Iatrogenic | 3% |
*Modified from (10)
The causes of olfactory dysfunction.
Age-related decrease in olfactory performance; chronic rhinosinusitis; following upper respiratory tract infection; craniocerebral trauma; medications; underlying neurodegenerative disease; frontobasal tumors; or idiopathic
The congenital causes of olfactory dysfunction are divided into isolated and syndromal hyposmia and anosmia (e11). The most widely known examples of syndromal congenital anosmia are Kallmann syndrome (olfactory dysfunction together with hypogonadotropic hypogonadism) (e11) and congenital insensitivity to pain (e12, e13). Genetic variants of both isolated and syndromal congenital anosmia have been described (e14, e15). Congenital olfactory dysfunction is typically first diagnosed at 12 to 14 years of age (e16). A common radiological finding in congenital olfactory dysfunction is hypoplasia or aplasia of the olfactory bulb (e17). Suspicion of congenital olfactory dysfunction on clinical examination or imaging should prompt investigation by an interdisciplinary panel including pediatricians, endocrinologists, and, if possible, geneticists.
With regard to the neurological or neurodegenerative causes of olfactory disorders, over 90% of men and women with idiopathic Parkinson’s disease (IPD) have olfactory dysfunction, which is viewed as a supportive diagnostic criterion in the clinical diagnosis of IPD. Olfactory dysfunction may occur more than 10 years before the onset of the motor symptoms (e19), so early IPD should be borne in mind as a possibility in patients with olfactory impairment of unclear origin, particularly if other non-motor symptoms are present, such as REM sleep disorders, depression, or a family history of IPD (e20, 13).
Epidemiology.
Reduced olfactory function is a common occurrence. The prevalence of quantitative olfactory dysfunction in the general population is around 20%, that of anosmia around 5%.
Olfactory dysfunction is found to a lesser degree in other movement disorders, e.g., multiple system atrophy, supranuclear ophthalmoplegia, and corticobasal degeneration. Only a small number of studies have so far been conducted on olfactory function in familial Parkinson’s disease. Moderate hyposmia has been described in Huntington’s disease (e21) and mild olfactory dysfunction in patients with hereditary ataxia (e22). Mild dysfunction has also been observed in motor neurone disease (e23).
Severe olfactory dysfunction is found in many different forms of dementia (e24, e25). Olfactory dysfunction is an early symptom of Alzheimer’s disease, occurring in patients whose cognitive dysfunction is as yet only mild. Difficulty in identifying odors is a predictor of conversion to dementia (conversion rate 47%, odds ratio [OR] 5.1) (e26). Idiopathic olfactory dysfunction is often diagnosed in the prodromal phase of neurodegenerative diseases.
Olfactory dysfunction is also encountered in inflammatory disorders of the central nervous system: the incidence in multiple sclerosis is reported as 20–45% (e27). Patients with temporal lobe epilepsy tend to be affected by restriction of centrally mediated abilities such as odor identification and discrimination. Those with an acute depressive episode show a distinct reduction in olfactory sensitivity (e28), but after successful drug treatment there is no longer a significant difference from healthy persons. Limitations of the sense of smell are also known to occur in patients with schizophrenia and their first-degree relatives (e29).
Epidemiology
The prevalence of quantitative olfactory dysfunction in the general public is around 20% (7, 14, 15). The reports range widely, however, because of the different methods used to measure olfactory performance (e30). Epidemiological studies estimate a prevalence of about 15% for olfactory dysfunction in the USA (15, e31). European studies in which olfactory performance was assessed state the prevalence of anosmia as around 5%, that of hyposmia as 15% (14, e32, e33).
Measurement of olfactory function.
Psychophysical assessment of olfactory function with simple screening tests for identification of odors plays a central part in the basic diagnostic work-up for olfactory dysfunction.
The prevalence of isolated qualitative olfactory disorders is lower than that of quantitative dysfunction. While the prevalence of isolated phantosmia is assumed to be between 1% and 9%, the rate of parosmia is reported as 2–4% (e9). In contrast, parosmia occurs with much higher frequency in the context of quantitative olfactory dysfunction, depending on the cause of the dysfunction. The rate of parosmia is highest in postinfectious olfactory dysfunction (49–68%), but it is also observed in post-traumatic (14–53%), idiopathic (14–55%), and sinunasal (28–30%) dysfunction (16– 19, e34). A problem with the documentation of qualitative olfactory dysfunction is that so far it has been assessed only by questioning the persons affected.
COVID-19-associated olfactory dysfunction
Around 50% of individuals with SARS-CoV-2-related olfactory dysfunction have loss of the sense of smell (29, e41), a rate higher than found in other viral infections (5). The loss is thought to be caused by damage to the supporting cells in the olfactory mucosa (e37), which leads indirectly to loss of function or death of the olfactory receptor neurons.
In contrast to other virus-related olfactory disorders, in COVID-19, particularly the Delta variant, nasal breathing is rarely impeded. In the Omicron variant olfactory dysfunction occurs less frequently, affecting around 15% of those infected (e40). In about 40–60% of those affected, parosmia arises several weeks or months later, especially in young patients and those with better olfactory performance. Phantosmia occurs less frequently (8).
Detailed investigation.
For more detailed analysis of the progress of olfactory disorders, an odor identification or odor discrimination test can be accompanied by determination of the olfactory threshold. Objective depiction of olfactory function is achieved by documentation of olfactory event-related potentials.
The outcome of olfactory dysfunction in COVID-19 is thought to be generally favorable: a majority of patients report improvement within 2–3 weeks (29). Systematic investigations with psychophysical testing have shown that the initially impaired olfactory performance was much improved or restored to normal in 80–85% of patients at 6 months and in 95% at 12 months (e40). These patients are frequently regarded as fully recovered on the basis of their subjective assessments, but objective measurement often shows residual deficits (e41). Although overall the prognosis is therefore good, because of the large number of persons infected the SARS-CoV-2 pandemic has led to a significant increase in the prevalence of olfactory dysfunction. Details of the treatment of COVID-19-related olfactory dysfunction can be found in the Box.
Measurement of olfaction
The quantitative determination of olfactory performance can be achieved by means of subjective assessment, psychophysical tests, or electrophysiological methods. Structural and functional imaging techniques are also used for evaluation of olfaction.
COVID-19-associated olfactory dysfunction.
The course of olfactory dysfunction in COVID-19 is viewed as generally favorable: most report improvement within 2–3 weeks.
Subjective assessment is the swiftest and simplest way of estimating olfactory function and is, like the medical history, of great importance. However—probably owing to the variation in both burden of suffering and self-esteem—subjective ratings are imprecise and often do not correspond to the objective olfactory capacity (19, e35).
Psychophysical tests
Psychophysical tests of olfactory performance often evaluate three different olfactory functions (11). Threshold testing enables determination of the lowest concentration at which an odorous substance, e.g., n-butanol or phenethyl alcohol, is detected. The staircase procedure is often used for this purpose: the samples are presented repeatedly in different concentrations until the odor can confidently be distinguished from solvent (20, 21, e36, e38). The discrimination test assesses the ability to tell odors apart: the participants are given various odor triplets to sniff, with two of the samples identical and the third different. In the identification test, various odors are presented and have to be characterized using one of a list of (typically four) terms (e38). These tests are best administered in a forced-choice process, where the study participants have to give a response even if they detect no odor.
In this testing scheme the olfactory threshold tends to describe the function of the periphery of the olfactory system, while odor identification and odor discrimination rather reflect the central nervous processing of odors (5). The identification test can also be administered by the study participants themselves (e38). Numerous versions of the identification test have been developed, varying mainly in the number of different odors used, and the test has to be adapted to avoid odors unfamiliar to the region or cultural group involved (5).
Measurement of the negative impact of olfactory dysfunction.
Validated questionnaires on the impact on the patient’s quality of life are available for documentation of the subjective severity of olfactory dysfunction and of its course.
It is important that the diagnostic acuity and the reliability of the tests increase with the number of odors used (22). Screening tests (table 2) are limited in their ability to assess the course of olfactory function, so additional documentation of the olfactory threshold is advisable (11, 5).
Table 2. Swift tests for assessment of olfaction.
| Test, Author | Test type | Number ofitems | Reliability coefficient | Commercially available |
|
Brief Smell Identification Test (B-SIT) Doty et al., 1996 (e75) |
Identification test | 12 | 0.73 | Yes |
|
Alcohol Sniff Test Davidson et al., 1997 (e76) |
Threshold test | 1 | 0.80 | No |
|
Four-Minute Odor Identification Test Hummel et al., 2001 (e77) |
Identification test | 12 | 0.78 | Yes |
|
Quick Smell Test (Q-SIT) Jackman and Doty, 2005 (e78) |
Identification test | 3 | 0.87 | Yes |
|
Short Olfactory Screening Test Mueller and Renner, 2006 (e79) |
Identification test | 5 | 0.77 | Yes |
|
Odorized Marker Screening Test Vodicka et al., 2007 (e80) |
Identification test | 5 | Not published | No |
|
Short Connecticut Smell Test (CST) Toledano et al., 2009 (e81) |
Threshold test | 1 | Not published | No |
|
Q-Sticks Test Hummel et al., 2010 (e82) |
Identification test | 3 | Not published | Yes |
|
OLFACAT Smell Test Mullol et al., 2015 (e83) |
Identification test, questions on perception and identification | 4 | Not published | Yes |
The following tests, some of which are commercially available, are used worldwide: the CCCRC test, a combined threshold and identification test; the UPSIT, a single-use disposable odor identification test in different variations with three to 40 odors that can be self-administered and is therefore extremely useful in, for example, patients with acute SARS-CoV-2 infection; and the reusable Sniffin’ Sticks test, which captures the olfactory threshold, odor discrimination, and odor identification (etable 1). All of these tests are of verified reliability and validity (5); for the Sniffin’ Sticks, for example, there are normative data from over 9000 healthy men and women, enabling age- and gender-dependent classification of olfactory performance into normosmia, hyposmia, and anosmia (23).
eTable 1. Comprehensive tests for assessment of olfaction.
| Test, author | Test type | Number of items | Reliability coefficient | Commercially available |
|
Connecticut Chemosensory Clinical Research Center (CCCRC) Test Cain et al., 1983 (21) |
Threshold, identification | 1 10 |
Threshold: 0.68 Identification: 0.60 |
No |
|
University of Pennsylvania Smell Identification Test (UPSIT)
Doty et al., 1984 (e84) |
Identification | 40 | 0.94 | Yes |
|
T&T Olfactometer Toyota et al.,1978 (e85) and Takagi, 1989 (e86) |
Threshold, identification | 5 | Threshold: 0.56–0.71 Identification: 0.33–0.45 |
Yes |
|
Sniffin’ Sticks Test Hummel et al., 1997 (e87) |
Threshold, discrimination, identification | 16 per subtest | 0.72 | Yes |
|
Tasting powders Heilmann et al., 2002 (e43) |
Retronasal identification | 20 | 0.76 | No |
|
European Test of Olfactory Capabilities (ETOC)
Thomas-Danguin et al., 2003 (e88) |
Threshold, identification | 16 | 0.90 | No |
|
Barcelona Smell Test Cardesin et al., 2006 (e89) |
Odor perception, identification, odor memory | 24 | Not published | No |
|
Candy Smell Test Renner et al., 2009 (24) |
Retronasal identification | 23 | 0.75 | No |
|
Extended Sniffin’ Sticks Test Haehner et al., 2009 (e90) |
Threshold, discrimination, identification | 32 | 0.93 | Yes |
|
Sniffin’ Test of Odor Memory (TOM) Croy et al., 2015 (e91) |
Odor memory | 8 | 0.70 | Yes |
It is important to use different olfactory tests in the course of COVID-19 (e40), for example, bearing in mind that odor identification may be largely normal but the olfactory threshold impaired (e41).
The determination of retronasal olfactory function (identification of aromas), however, is not an established element of routine clinical examination, although validated, reliable odor identification tests and tests for determination of the retronasal olfactory threshold are available, e.g., the “tasting powders” (e43) and the Candy Smell Test (24) (etable 1). In these tests, odorous substances are given by mouth in the form of powders or sorbitol candies and identified, analogous to orthonasal tests, from a list of options in a forced-choice model (e43, e44).
The description of qualitative olfactory dysfunction rests essentially on questioning of those affected (e45). Measurement by the SSParoT method, for example, has been proposed as a means of standardizing the severity of parosmia (e46).
Electrophysiological procedures and functional imaging
While psychophysical testing of olfactory performance plays a major role in daily clinical practice, objective methods are needed whenever the person’s cooperation in psychophysical tests is problematic. This may be the case, for example, in children, in persons with cognitive disorders, or in the context of medicolegal investigations.
Smelling tests.
Tests widely available across the world are the CCCRC Test, a combined threshold and identification test; the UPSIT, a single-use odor identification test in different variations of three to 40 odors; and the reusable Sniffin’ Sticks test.
Among the electrophysiological techniques, recording of olfactory event-related potentials (OERP) from the EEG has been studied closely (e47). Owing to its technical complexity, however, this method is available at only a small number of centers. Nevertheless, it is currently the only means of assessing olfactory function objectively.
In contrast, magnetic resonance imaging (MRI) is widely available and enables the structural examination of areas of the brain that are intimately involved with the processing of odors, such as the olfactory bulb and the orbitofrontal cortex (25). In these structures, for example, small volumes point to the presence of a reduction in olfactory capacity. With the aid of imaging, a possible prognosis can then be outlined (e48). Cranial MRI naturally also clarifies whether, for instance, an intracranial tumor such as olfactory nerve meningioma is present that could cause olfactory dysfunction (e49). Not only structural MRI but also functional olfactory MRI can be performed (e50); however, the results at individual level are difficult to interpret (e51).
Measurement of the detrimental effect of olfactory dysfunction
Olfactory dysfunction can have a negative impact on the quality of life. This can hardly be assessed by psychophysical tests but is instead ascertained with the aid of questionnaires. One instrument often used to evaluate the olfaction-specific quality of life is the Questionnaire of Olfactory Dysfunction (QOD) with 52 items (26, e52). A short version with seven questions is also available (27).
Retronasal perception of odors has a greater influence on the quality of life than orthonasal detection (e53, 28). Other questionnaires, such as the Importance of Olfaction Questionnaire, measure the individual significance of the sense of smell (e54), which decreases with increasing age and with the increasing duration of olfactory dysfunction (28, e54).
The prognosis of olfactory dysfunction
Olfactory disorders may become less marked (e55) and may, as seen for example in COVID-19-associated dysfunction, disappear entirely (e56, 29).
Treatment of sinunasal olfactory dysfunction.
Treatment of the underlying inflammatory disease is recommended.
The prognosis of and spontaneous recovery from olfactory dysfunction depends on, among other factors, the duration of the dysfunction, its cause, the presence/absence of parosmia at initial examination, the patient’s smoking status, and, most important, their age (e57, e58). The prognosis is therefore most favorable in younger non-smokers with a postviral olfactory disorder, relatively good olfactory function, only brief loss of olfactory function, and parosmic changes (17).
Among patients whose loss of the sense of smell persists for a longer period, e,g., 18 months, only around 30% will experience a spontaneous clinically relevant improvement in olfactory performance within 12 months (e59).
Treatment
While for patients with olfactory dysfunction in connection with sinunasal conditions it is recommended that the underlying disease be treated (e60), there are few therapeutic options and recommendations for olfactory disorders of other causes (7, e61).
Although many different kinds of treatment have been tested in clinical studies, apart from management of the inflammatory disease only olfactory training, i.e., the deliberate sniffing of various odors several times each day, possesses proven therapeutic value (5).
Drug treatment of sinunasal olfactory dysfunction
Topical corticosteroids form the basis of treatment (11, e60, e62) (evidence: eTable 2). They not only ameliorate the underlying chronic inflammation, e.g., rhinosinusitis with nasal polyposis, but also have a significant effect on olfactory function (10). Systemic steroids are given only for a short time to confirm the diagnosis of inflammation-related olfactory dysfunction and reduce the inflammation before continuing with topical treatment (e63) (evidence: eTable 3). The review and meta-analysis by Banglawala et al. (e64) included 28 randomized controlled trials (RCT) of topical and systemic corticoid therapy. Meta-analysis of the latter (five studies) showed significant improvement of both subjective (SMD -2.22, 95% confidence interval [-3.94; -0.49]) and objective (SMD 0.65 [0.28; 1.01]) olfactory function compared with placebo. As for topical treatment, 70% of the studies reviewed found improvement. When giving topical therapy, it is advisable to administer the nasal spray using a long applicator (e63, e65). With a normal applicator, the filtering function of the nose practically prevents the spray from reaching the olfactory cleft (e66, e67). The same effect can be achieved by administering the nasal drops in the so-called Kaiteki position (https://goo.gl/ZqxhDN) (e68). Corticosteroids are currently recommended only for sinunasal causes (5, e60).
eTable 2. Evidence for treatment with intranasal topical corticoids in sinunasal olfactory dysfunction caused by chronic rhinosinusitis with polyposis (RCT).
| Author | Study participants | Clinical endpoint | Results |
|
Xu et al., 2020 (e92) |
n = 127 1) Methylprednisolone 24 mg + budesonide NS for 1 week 2) Budesonide nasal drops + budesonide NS for 1 week 3) Budesonide NS for 1 week |
VAS (0–10) | Significant VAS improvement in all groups compared with baseline No difference between the groups |
|
Zeng et al., 2019 (e93) |
n = 187* 1) Fluticasone propionate NS for 3 months 2) Clarithromycin 250 mg for 3 months |
VAS (0–10) | Significant VAS improvement in both groups compared with baseline No difference between the groups |
|
Khan et al., 2019 (e94) |
n = 310 1) Mometasone furoate NS 1/d 2) Mometasone furoate NS 2/d 3) Placebo |
Subjective assessment (0–3) | Significant improvement compared with baseline only for 2 × daily nasal spray |
|
Zhou et al., 2016 (e95) |
n = 748 1) Mometasone furoate NS 2/d for 16 weeks 2) Placebo mometasone furoate |
Subjective assessment (0–3) | Significant improvement compared with placebo |
|
Bangwala et al., 2014 (e64) |
n = 419 Review: 28 RCT Meta-analysis: 5 RCT |
Subjective assessment (0–3) Objective testing |
Significant improvement of olfaction by oral and topical steroids (improvement in 70% of the topical studies) |
|
Jankowski et al., 2009 (e96) |
n = 246 1) Fluticasone propionate NS 2/d for 8 months 2) Fluticasone propionate NS 2/d for 1 month, Fluticasone propionate NS 1/d + placebo NS for 7 months 3) Placebo NS for 2 months, then fluticasone propionate NS 2/d for 6 months |
VAS (0–100) Mean sense of smell disorder score |
Significant improvement of both scores in corticoid groups compared with placebo |
|
Ehnhage et al., 2009 (e97) |
n = 68 1) Fluticasone propionate nasal drops for 10 weeks 2) Placebo for 10 weeks |
Subjective assessment (0–3) Butanol threshold test |
No significant improvement compared with placebo |
|
Small et al., 2008 (e98) |
n = 447 1) Mometasone furoate NS for 4 months 2) Placebo |
Subjective assessment (0–3) | Significant improvement compared with placebo |
*Also included patients who had chronic rhinosinusitis without polyposis
NS, Nasal spray; RCT, randomized controlled trial; VAS, visual analog scale
Treatment of postviral, post-traumatic, and idiopathic olfactory dysfunction.
To date, the only treatment option is olfactory training: sniffing various odors several times each day.
Various monoclonal antibodies (“biologics”) have recently been approved for the treatment of rhinosinusitis with nasal polyposis. Because of their specific action on the inflammation they also exert a positive effect on the associated olfactory dysfunction (30), but they are not licensed for the treatment of olfactory dysfunction alone.
Olfactory training
Olfactory training has become established as the treatment of choice for non-sinunasal olfactory dysfunction (7, e69) (evidence: eTable 4). A meta-analysis (e70) of 13 RCT featuring very heterogeneous groups revealed a strong association for the improvement of odor identification (g = 0.83), odor discrimination (g = 0.89), and overall olfaction (g = 1.10), together with a mild to moderate effect for the olfactory threshold (g = 0.34). Olfactory training should be carried out carefully and consistently, smelling four different odors for 30 seconds each twice daily over a period of 4–6 months or longer. The effect is even better if the odors are replaced by different ones after 3 months (e71). Studies have shown that the initial olfactory performance and the cause of the olfactory dysfunction are associated with achievement of a relevant improvement in olfactory function after the training (e72, e73, 31). A less pronounced improvement is found for olfactory dysfunction of post-traumatic or idiopathic origin.
Further treatment options
Other topical treatments that have been evaluated are
sodium citrate, vitamin A drops, theophylline, palmitoyl ethanolamide/luteolin, and platelet-rich plasma. The systemic treatments that have been investigated include zinc, pentoxifylline, theophylline, cavoverin, α-lipoic acid, and vitamin B (e61, e74). Acupuncture has also been used to treat olfactory dysfunction (e74). Although many of these treatment options showed positive effects in the initial case series, as a rule there is a lack of robust clinical trials, particularly RCT and meta-analyses—although isolated RCT have been carried out for, among others, theophylline, vitamin A, and α-lipoic acid (5, 11).
Drug treatment of sinunasal olfactory dysfunction.
Topical corticosteroids are the basic treatment. They not only ameliorate the underlying chronic inflammatory condition but also have a significant effect on olfactory function.
Supplementary Material
Case report
A 46-year-old man who worked as a self-employed retailer presented with olfactory dysfunction of approximately 3 years’ standing. He reported that the dysfunction had developed gradually and that he had hardly noticed it at first. He denied possible causes such as an infection, trauma, or medications, and no comorbidities, difficulty in nose breathing, or any other nasal problems were present. ENT examination including nasal endoscopy found no abnormalities. Investigation of orthonasal olfactory function using the Sniffin’ Sticks procedure, comprising threshold, discrimination, and identification tests, revealed functional anosmia. Suprathreshold concentrations of tasting powders in the primary flavors were correctly identified, showing normogeusia. As the findings of magnetic resonance imaging were normal, the diagnosis was idiopathic functional anosmia. Regular olfactory training was recommended. Due to the absence of symptoms, no neurological investigations took place.
The anosmia remained unchanged over the course of several follow-up visits. At 3 years after diagnosis the patient reported the recent onset of tremors in the left extremities at rest, together with minor clumsiness of the left hand and occasional pain in the right thigh. Clinical examination by a neurologist found slight, moderately frequent trembling of the left extremities at rest, slight left-sided bradydiadochokinesis, discrete rigor of the left arm, and reduced left arm swing. The diagnosis was suspected idiopathic Parkinson’s disease and the patient was transferred to the department of neurology for further investigation.
Box. Treatment of COVID-19-associated olfactory dysfunction.
If COVID-19-associated olfactory dysfunction persists, the treatment of choice is a consistent, structured program of olfactory training (10). One goal is to stimulate the regeneration of olfactory receptor neurons in the olfactory mucosa. The patient should sniff four odors, e.g., rose, lemon, eucalyptus, and cloves, twice daily for 20–30 seconds each time over a period of 4–12 months. The odors should be changed every 3–4 months (e71). There are conflicting reports on treatment with intranasal corticosteroids (32).
Further information on CME. Participation in the CME certification program is possible only via the Internet: cme.aerzteblatt.de.This unit can be accessed until 2 March 2024. Submissions by letter, e-mail, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 2 March 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the rate of olfactory dysfunction among patients with idiopathic Parkinson’s disease?
10%
20%
30%
50%
90%
Question 2
Which of the following nerves has a major influence on olfaction?
The ophthalmic nerve
The mandibular nerve
The abducens nerve
The facial nerve
The trigeminal nerve
Question 3
What is parosmia?
The perception of smells in the absence of an odor source
Reduced olfactory capacity
Qualitatively altered perception of smells
Hyperactive olfaction
Faulty osmosis
Question 4
Which of the following is the principal determinant of perceived aroma?
The surface of the tongue
The composition of the salivary fluid
The dental status
Retronasal olfaction
The alignment of the nasal septum
Question 5
What is the prevalence of anosmia in the general population?
0.2%
5%
10%
20%
40%
Question 6
In what type of olfactory dysfunction is the rate of parosmia highest?
In post-traumatic olfactory dysfunction
In postinfectious olfactory dysfunction
In sinunasal olfactory dysfunction
In idiopathic olfactory dysfunction
In congenital olfactory dysfunction
Question 7
Which of the following is a promising treatment option for post-traumatic olfactory dysfunction?
Antibiotics
Nasal sprays containing corticoids
Regular olfactory training for at least 6 months
Saline nasal rinses
Realignment of the septum
Question 8
What is tested using Sniffin’ Sticks?
The subjective assessment of olfactory function
The smell-related quality of life
Olfactory event-related potentials
The threshold, discrimination, and identification of odors
The assessment of nasal breathing
Question 9
At what age is olfactory function best?
5–10 years
21–30 years
41–50 years
61–70 years
Over 80 years
Question 10
Which of the following olfactory dysfunctions is generally treated with topical or systemic corticoids?
Post-traumatic olfactory dysfunction 2 years after the causative event
Chronic rhinosinusitis with nasal polyposis
Congenital anosmia
Olfactory dysfunction in connection with COVID-19
Olfactory dysfunction following chemotherapy
►Participation is possible only via the Internet: cme.aerzteblatt.de
eTable 3. Evidence for treatment with oral corticoids in sinunasal olfactory dysfunction caused by chronic rhinosinusitis with polyposis (RCT).
| Author | Study participants | Clinical endpoint | Results |
|
Papadakis et al., 2021 (e99) |
n = 140 1) Dexamethasone for 7 days + 12 weeks budesonide NS 2) Budesonide NS for 12 weeks |
VAS (0–10) Sniffin’ Sticks identification test |
Significant improvement in VAS and identification test compared with solely topical administration of steroids |
|
Ecevit et al., 2015 (e100) |
n = 22 1) Prednisolone 60 mg/d for 7 days, then dose reduction up to day 16 2) Placebo |
VAS (0–10) Butanol threshold |
Significant improvement in VAS and threshold test compared with placebo |
|
Banglawala et al., 2014 (e64) |
n = 419 Review: 28 RCT Meta-analysis: 5 RCT |
Subjective assessment Objective testing |
Significant improvement of olfactory capacity by oral (subjective: SMD −2.22, 95% CI [−3.94; −0.49]; objective: SMD 0.65, 95% CI [0.28; 1.01] and topical steroids |
|
Alobid et al., 2014 (e101) |
n = 92 1) Prednisone 30 mg for 12 weeks + budesonide NS for 12 weeks 2) No steroids |
Barcelona Smell Test | Significant improvement compared with baseline only in oral prednisone group |
|
Kirtsreesakul et al., 2012 (e102) |
n = 114 1) Prednisolone 50 mg for 2 weeks + mometasone furoate NS for 10 weeks 2) Placebo for 2 weeks + mometasone furoate NS for 10 weeks |
Subjective assessment (0–3) | Significant improvement compared with baseline only in oral prednisone group |
|
Vaidyanathan et al., 2011 (e103) |
n = 60 1) Prednisolone 25 mg for 2 weeks + topical steroids for 26 weeks 2) Placebo for 2 weeks + topical steroids for 26 weeks |
VAS (0–100) Pocket Smell Test (PST) |
Significant improvement in VAS and PST compared with placebo |
|
Van Zele et al., 2010 (e104) |
n = 47 1) Methylprednisolone 32 mg for 20 days 2) Placebo for 20 days |
VAS (0–10) | Significant improvement in VAS compared with placebo |
|
Benitez et al., 2006 (e105) |
n = 84 1) Prednisone 30 mg for 2 weeks + budesonide NS for 10 weeks 2) No steroids |
Subjective assessment (0–3) | Significant improvement compared with baseline only in oral prednisone group |
|
Wright et al., 2007 (e106) |
n = 26 1) Prednisone 30 mg for 2 weeks + ESS 2) Placebo + ESS |
VAS (0–10) | Significant improvement compared with baseline only in oral prednisone group |
| Hissaria et al., 2006 (e107) | n = 40 1) Prednisolone 50 mg for 2 weeks 2) Placebo for 2 weeks |
Modified 31-item Rhinosinusitis Outcome Measure Questionnaire |
Significant improvement compared with baseline only in oral prednisone group |
ESS, Endoscopic sinus surgery; NS, nasal spray; RCT, randomized controlled trial; VAS, visual analog scale
eTable 4. Evidence for the efficacy of olfactory training in the treatment of olfactory dysfunction.
| Author | Design | Study participants | Clinical endpoint | Results |
|
Pieniak et al., 2022 (e108) |
Review | n = 3134 (PIOD, PTOD, IOD, sinunasal, IPD, medication, elderly) Review: 48 studies |
Sniffin’ Sticks or other psychophysical tests | Improvement of olfactory function in all patient groups except for sinunasal etiology, ‧greatest efficacy for discrimination and identification |
|
Yaulaci et al., 2022 (e109) |
Prospective, non-randomized study | n = 51 (PIOD) 1) Olfactory training 2) No treatment for 12 weeks |
Sniffin’ Sticks | Clinically significant improvement in 40% of training group versus 6% of control group |
|
Lechner et al., 2022 (e110) |
RCT | n = 63 (PIOD) 1) Olfactory training 2) No treatment for 12 weeks |
Brief Smell Identification Test (BSIT) | Non-significant improvement of BSIT score in training group versus control group, ‧OR 2.38 |
|
Choi et al., 2021 (e111) |
Prospective, non-randomized study | n = 104 (PIOD) 1) Olfactory training 2) No treatment for 3 months |
Sniffin’ Sticks | Significant improvement of TDI, threshold, and identification in training group |
|
Qiao et al., 2020 (e112) |
RCT | n = 125 (PIOD) 1) Olfactory training 2) Training with household odors (e.g., perfume, vinegar) for 24 weeks |
Sniffin’ Sticks | Significant improvement of TDI, discrimination, and identification in both groups |
|
Saatci et al., 2020 (e113) |
RCT | n = 60 (PIOD) 1) Olfactory training (4 odors) 2) Training ball with 4 odors for 12 weeks |
Sniffin’ Sticks | Significant improvement of TDI, discrimination, and identification in both groups |
|
Kattar et al., 2020 (e114) |
Systematic review and meta-analysis | n = 990 (PIOD) Review: 16 studies; meta-analysis: 4 studies |
Sniffin’ Sticks | Significant improvement in all studies, OR 2.77 |
|
Sorokowska et al., 2017 (e70) |
Systematic review and meta-analysis | n = 1005 (PIOD, PTOD, IOD, sinunasal, IPD, elderly) Meta-analysis: 13 studies | Sniffin’ Sticks | Significant association between training and improvement of TDI (g = 1.10), discrimination (g = 0.89) and identification (g = 0.83) |
|
Pekala et al., 2016 (e115) |
Systematic review and meta-analysis | n = 639 (PIOD, PTOD, IOD, sinunasal, IPD, elderly) Review: 10 studies; meta-analysis: 3 studies | Olfactory improvement using psychophysical tests | Significant association between training and improvement of TDI, discrimination, and identification, OR 2.75 |
|
Jiang et al., 2019 (e116) |
RCT | n = 111 (PTOD) 1) Olfactory training (4 odors) 2) Olfactory training (1 odor – PEA) for 6 months |
UPSIT PEA threshold |
Significant improvement of PEA threshold in both groups, UPSIT improvement only in PEA group |
|
Langdon et al., 2018 (e117) |
RCT | n = 42 (PTOD) 1) Olfactory training (6 odors) 2) No treatment for 12 weeks |
Barcelona Smell Test Butanol threshold test VAS |
Significant improvement in training group compared with control group for threshold test; no change in VAS, Barcelona Smell Test |
|
Oleszkiewicz et al., 2018 (e118) |
RCT | n = 108 (PIOD, IOD) Olfactory training with 1) 4 odors 2) 4 odor combinations 3) 3 × 4 odors, changed every 2 months |
Sniffin’ Sticks | Significant improvement of TDI, threshold, and identification in all groups, no effect of training procedure |
|
Patel et al., 2017 (e119) |
RCT | n = 43 (PIOD, IOD) 1) Olfactory training (4 odors) 2) No treatment for 6 months |
UPSIT | Significant in 32% of the training group (versus 13% in control group) |
|
Damm et al., 2014 (e72) |
RCT | n = 171 (PIOD) 1) Olfactory training with high-concentration odors 2) Olfactory training with low-concentration odors Cross-over after 18 weeks |
Sniffin’ Sticks | Significant improvement of TDI in 26% of the training group with high-concentration odors (versus 15% in group with low-concentration odors) |
|
Poletti et al, 2017 (e120) |
Prospective, pseudo-randomized study | n = 96 (PIOD, PTOD) Olfactory training with 1) 4 high-molecular odors 2) 4 low-molecular odors for 5 months |
Sniffin’ Sticks | Significant improvement of TDI in both groups, high-molecular odors associated with significantly better threshold in PIOD |
|
Konstantinidis et al., 2016 (e121) |
Prospective, partially randomized study | n = 111 (PIOD) 1) Olfactory training for 16 weeks 2) Olfactory training for 56 weeks 3) No treatment |
Sniffin’ Sticks | Significant improvement of TDI in training groups compared with controls; highest increase in long-term group (9.1 for 16 weeks, 11.4 for 56 weeks) |
|
Gellrich et al., 2018 (e122) |
Prospective cohort study | n = 30 (PIOD) 1) Olfactory training for 12 weeks |
Sniffin’ Sticks Gray matter (GM) volume on MRI |
Significant improvement of TDI and significant GM volume increase in hippocampus, thalamus, and cerebellum |
|
Hummel et al., 2018 (e123) |
Prospective cohort study | n = 23 (PIOD, IOD) 1) Olfactory training for 4–6 months |
Sniffin’ SticksEOG | Significant improvement in identification, but not in TDIEOG response significantly improved by training |
|
Altundag et al., 2015 (e71) |
Prospective cohort study | n = 85 (PIOD) 1) Olfactory training with 3 sets of 4 odors 2) Olfactory training with 4 odors 3) No treatment for 36 weeks |
1) Sniffin’ Sticks 2) VAS |
Significant improvement of TDI in training groups compared with controls, with greatest improvement rate in group with changing odors (TDI 8.2 versus 6.1 versus 1.7) |
|
Konstantinidis et al., 2013 (e73) |
Prospective cohort study | n=119 (PTOD, PIOD) 1) Olfactory training (4 odors) 2) No olfactory training for 4 months |
Sniffin’ Sticks | Significant improvement of TDI in training groups compared with controls, particularly in PIOD (TDI 6.25 versus 1.5 PIOD, 5.1 versus 1.2 PTOD) |
|
Haehner et al., 2013 (e124) |
Prospective cohort study | n = 70 (PD) 1) Olfactory training 2) No training |
Sniffin’ Sticks | Significant improvement of TDI and discrimination in training group compared with controls (TDI by 2.4 versus −0.6) |
|
Hummel et al. 2009 (e125) |
Prospective cohort study | n = 56 (PIOD, PTOD, IOD) 1) Olfactory training 2) No treatment for 12 weeks |
Sniffin’ Sticks | Significant improvement of TDI and olfactory threshold in training group compared with controls |
EOG, Electro-olfactogram; IOD, idiopathic olfactory dysfunction; IPD, idiopathic Parkinson’s disease; PIOD, postinfectious olfactory dysfunction; PTOD, post-traumatic olfactory dysfunction;
RCT, randomized controlled trial; TDI, summed score olfactory threshold + discrimination test + identification test; UPSIT, University of Pennsylvania Smell Identification Test
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement
B.S. has received consultancy fees from Pohl-Boskamp, Bristol-Myers-Squibb, and Glaxo-Smith-Kline; lecture fees from Merck und Sanofi; and support for training courses from Bristol-Myers-Squibb, Merck, ALK, Sanofi, Pohl-Boskamp, MSD, and Novartis.
T.H. has received financial support from the Smell and Taste Lab, Geneva, Switzerland and from Takasago. He has received consultancy fees from Primavera, Oy-Mittelberg. T.H. is a committee member of the Olfactology/Gustology Working Group of the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery. He has received support in the form of donations in kind from Burghart, Holm, and aspuraclip, Schönefeld.
The remaining authors declare that no conflict of interest exists.
References
- 1.McGann JP. Poor human olfaction is a 19th-century myth. Science. 2017;12 doi: 10.1126/science.aam7263. 356 (6338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durante MA, Kurtenbach S, Sargi ZB, et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat Neurosci. 2020;23:323–326. doi: 10.1038/s41593-020-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozin P. „Taste-smell confusions“ and the duality of the olfactory sense. Percept Psychophys. 1982;31:397–401. doi: 10.3758/bf03202667. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer L, Schriever VA, Croy I. Human olfactory dysfunction: causes and consequences. Cell Tissue Res. 2021;383:569–579. doi: 10.1007/s00441-020-03381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinology. 2017;(Suppl. 25):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 6.Fikentscher R, Rasinski C. (Parosmias—definition and clinical picture) Parosmien—Begriffsbestimmung und klinisches Bild. Laryngol Rhinol Otol (Stuttg) 1986;65 [PubMed] [Google Scholar]
- 7.Patel ZM, Holbrook EH, Turner JH, et al. International consensus statement on allergy and rhinology. Olfaction. Int Forum Allergy Rhinol. 20221;2:327–680. doi: 10.1002/alr.22929. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrino R, Mainland JD, Kelly CE, Parker JK, Hummel T. Prevalence and correlates of parosmia and phantosmia among smell disorders. Chem Senses. 2021 doi: 10.1093/chemse/bjab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mainland JD, Barlow LA, Munger SD, et al. Identifying treatments for taste and smell disorders: gaps and opportunities. Chem Senses. 2020;45:493–502. doi: 10.1093/chemse/bjaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damm M, Schmitl L, Müller CA, Welge-Lüssen A, Hummel T. Diagnostik und Therapie von Riechstörungen. HNO. 2019;67:274–281. doi: 10.1007/s00106-019-0614-x. [DOI] [PubMed] [Google Scholar]
- 11.Damm M, Hüttenbrink KB, Hummel T, et al. AWMF Leitlinien Riech- und Schmeckstörungen. www.awmforg/uploads/tx_szleitlinien/017-050l_S2k_Riech-und-Schmeckst%C3%B6rungen_2017-03pdf (last accessed on 3 February 2023) [Google Scholar]
- 12.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg D, Godau J, Seppi K, et al. The PRIPS study: screening battery for subjects at risk for Parkinson‘s disease. Eur J Neurol. 2013;20:102–108. doi: 10.1111/j.1468-1331.2012.03798.x. [DOI] [PubMed] [Google Scholar]
- 14.Landis BN, Hummel T. New evidence for high occurrence of olfactory dysfunctions within the population. Am J Med. 2006;119:91–92. doi: 10.1016/j.amjmed.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL. Epidemiology of smell and taste dysfunction. Handb Clin Neurol. 2019;164:3–13. doi: 10.1016/B978-0-444-63855-7.00001-0. [DOI] [PubMed] [Google Scholar]
- 16.Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss—a randomized controlled multicenter study. Laryngoscope. 2014;124:826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 17.Liu DT, Sabha M, Damm M, et al. Parosmia is associated with relevant olfactory recovery after olfactory training. Laryngoscope. 2021;131:618–623. doi: 10.1002/lary.29277. [DOI] [PubMed] [Google Scholar]
- 18.Ohla K, Veldhuizen MG, Green T, et al. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology. 2022 doi: 10.4193/Rhin21.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS. Ratings of overall olfactory function. Chem Senses. 2003;28:691–694. doi: 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Wylie C, Potter M, Beston R, Cope B, Majam K. Clinical validation of the olfactory detection threshold module of the Snap & Sniff® olfactory test system. Int Forum Allergy Rhinol. 2019;9:986–992. doi: 10.1002/alr.22377. [DOI] [PubMed] [Google Scholar]
- 21.Cain WS, Gent J, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. Am J Otolaryngol. 1983;4:252–256. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- 22.Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995;20:645–656. doi: 10.1093/chemse/20.6.645. [DOI] [PubMed] [Google Scholar]
- 23.Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated sniffin‘ sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276:719–728. doi: 10.1007/s00405-018-5248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renner B, Mueller CA, Dreier J, Faulhaber S, Rascher W, Kobal G. The candy smell test: a new test for retronasal olfactory performance. Laryngoscope. 2009;119:487–495. doi: 10.1002/lary.20123. [DOI] [PubMed] [Google Scholar]
- 25.Seubert J, Freiherr J, Frasnelli J, Hummel T, Lundstrom JN. Orbitofrontal cortex and olfactory bulb volume predict distinct aspects of olfactory performance in healthy subjects. Cereb Cortex. 2013;23:2448–2456. doi: 10.1093/cercor/bhs230. [DOI] [PubMed] [Google Scholar]
- 26.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 2005;262:231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 27.Zou L, Haehner A, Menzel S, Gunder N, Hummel T. Reliability and validity of a brief version of the Questionnaire of Olfactory Disorders (brief QOD) in patients with olfactory dysfunction. Rhinology. 2022;60:56–62. doi: 10.4193/Rhin21.059. [DOI] [PubMed] [Google Scholar]
- 28.Liu DT, Besser G, Prem B, et al. Self-perceived taste and flavor perception: associations with quality of life in patients with olfactory loss. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820965242. 194599820965242. [DOI] [PubMed] [Google Scholar]
- 29.Prajapati DP, Shahrvini B, Said M, Srinivas S, DeConde AS, Yan CH. Assessment of patient recognition of coronavirus disease 2019 (COVID-19)-associated olfactory loss and recovery: a longitudinal study. Int Forum Allergy Rhinol. 2021;11:1529–1537. doi: 10.1002/alr.22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullol J, Bachert C, Amin N, et al. Olfactory outcomes with dupilumab in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2022;10:1086–1095e5. doi: 10.1016/j.jaip.2021.09.037. [DOI] [PubMed] [Google Scholar]
- 31.Konstantinidis I, Tsakiropoulou E, Constantinidis J. Long term effects of olfactory training in patients with post-infectious olfactory loss. Rhinology. 2016;54:170–175. doi: 10.4193/Rhino15.264. [DOI] [PubMed] [Google Scholar]
- 32.Hintschich CA, Dietz M, Haehner A, Hummel T. Topical administration of mometasone i not helpful in Post-COVID-19 olfactory dysfunction. Life. 2022;12 doi: 10.3390/life12101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Graziadei PP, Karlan MS, Graziadei GA, Bernstein JJ. Neurogenesis of sensory neurons in the primate olfactory system after section of the fila olfactoria. Brain Res. 1980;186:289–300. doi: 10.1016/0006-8993(80)90976-2. [DOI] [PubMed] [Google Scholar]
- E2.Brillat-Savarin JA. La Physiologie du Goût: „Die Physiologie des Geschmacks oder Transcendentalgastronomische Betrachtungen“, German 1865. Leipzig: Philipp Reclam jun 1826 [Google Scholar]
- E3.Frasnelli J, van Ruth S, Kriukova I, Hummel T. Intranasal concentrations of orally administered flavors. Chem Senses. 2005;30:575–582. doi: 10.1093/chemse/bji051. [DOI] [PubMed] [Google Scholar]
- E4.Small DM. Flavor is in the brain. Physiol Behav. 2012;107:540–552. doi: 10.1016/j.physbeh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- E5.Nordin S, Blomqvist EH, Olsson P, Stjarne P, Ehnhage A. Effects of smell loss on daily life and adopted coping strategies in patients with nasal polyposis with asthma. Acta Otolaryngol. 2011;131:826–832. doi: 10.3109/00016489.2010.539625. [DOI] [PubMed] [Google Scholar]
- E6.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses. 2014;39:185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- E7.Schäfer L, Mehler L, Hähner A, Walliczek U, Hummel T, Croy I. Sexual desire after olfactory loss: quantitative and qualitative reports of patients with smell disorders. Physiol Behav. 2019;201:64–69. doi: 10.1016/j.physbeh.2018.12.020. [DOI] [PubMed] [Google Scholar]
- E8.Frasnelli J, Landis BN, Heilmann S, et al. Clinical presentation of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 2004;261:411–415. doi: 10.1007/s00405-003-0703-y. [DOI] [PubMed] [Google Scholar]
- E9.Nordin S, Brämerson A, Millqvist E, Bende M. Prevalence of parosmia: the Skövde population-based studies. Rhinology. 2007;45:50–53. [PubMed] [Google Scholar]
- E10.Fikentscher R, Fikentscher R, Roseburg B. Systematik und Terminologie der Riech- und Schmeckstörungen Medizinische Olfaktologie und Gustologie. Halle: KTB, MLU Halle-Wittenberg, Wiss. Beiträge. 1988 [Google Scholar]
- E11.Karstensen HG, Tommerup N. Isolated and syndromic forms of congenital anosmia. Clin Genet. 2012;81:210–215. doi: 10.1111/j.1399-0004.2011.01776.x. [DOI] [PubMed] [Google Scholar]
- E12.Marchi M, Provitera V, Nolano M, et al. A novel SCN9A splicing mutation in a compound heterozygous girl with congenital insensitivity to pain, hyposmia and hypogeusia. J Peripher Nerv Syst. 2018;23:202–206. doi: 10.1111/jns.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Mansouri M, Chafai Elalaoui S, Ouled Amar Bencheikh B, et al. A novel nonsense mutation in SCN9A in a Moroccan child with congenital insensitivity to pain. Pediatr Neurol. 2014;51:741–744. doi: 10.1016/j.pediatrneurol.2014.06.009. [DOI] [PubMed] [Google Scholar]
- E14.Keydar I, Ben-Asher E, Feldmesser E, et al. General olfactory sensitivity database (GOSdb): candidate genes and their genomic variations. Hum Mutat. 2013;34:32–41. doi: 10.1002/humu.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Alkelai A, Olender T, Dode C, et al. Next-generation sequencing of patients with congenital anosmia. Eur J Hum Genet. 2017;25:1377–1387. doi: 10.1038/s41431-017-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Schriever VA, Hummel T. Etiologies of olfactory dysfunction in a pediatric population: based on a retrospective analysis of data from an outpatient clinic. Eur Arch Otorhinolaryngol. 2020;277:3213–3216. doi: 10.1007/s00405-020-06087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E17.Abolmaali ND, Hietschold V, Vogl TJ, Huttenbrink KB, Hummel T. MR evaluation in patients with isolated anosmia since birth or early childhood. AJNR Am J Neuroradiol. 2002;23:157–164. [PMC free article] [PubMed] [Google Scholar]
- E18.Haehner A, Boesveldt S, Berendse HW, et al. Prevalence of smell loss in Parkinson‘s disease—a multicenter study. Parkinsonism Relat Disord. 2009;15:490–494. doi: 10.1016/j.parkreldis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- E19.Haehner A, Masala C, Walter S, Reichmann H, Hummel T. Incidence of Parkinson‘s disease in a large patient cohort with idiopathic smell and taste loss. J Neurol. 2019;266:339–345. doi: 10.1007/s00415-018-9135-x. [DOI] [PubMed] [Google Scholar]
- E20.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‚idiopathic‘ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128(Pt 1):126–137. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- E21.Nordin S, Paulsen JS, Murphy C. Sensory- and memory-mediated olfactory dysfunction in Huntington‘s disease. J Int Neuropsychol Soc. 1995;1:281–290. doi: 10.1017/s1355617700000278. [DOI] [PubMed] [Google Scholar]
- E22.Moscovich M, Munhoz RP, Teive HA, et al. Olfactory impairment in familial ataxias. J Neurol Neurosurg Psychiatry. 2012;83:970–974. doi: 10.1136/jnnp-2012-302770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Viguera C, Wang J, Mosmiller E, Cerezo A, Maragakis NJ. Olfactory dysfunction in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2018;5:976–981. doi: 10.1002/acn3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Driver-Dunckley E, Adler CH, Hentz JG, et al. Olfactory dysfunction in incidental Lewy body disease and Parkinson‘s disease. Parkinsonism Relat Disord. 2014;20:1260–1262. doi: 10.1016/j.parkreldis.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Pardini M, Huey ED, Cavanagh AL, Grafman J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch Neurol. 2009;66:92–96. doi: 10.1001/archneurol.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Conti MZ, Vicini-Chilovi B, Riva M, et al. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer‘s disease. Arch Clin Neuropsychol. 2013;28:391–399. doi: 10.1093/arclin/act032. [DOI] [PubMed] [Google Scholar]
- E27.Lucassen EB, Turel A, Knehans A, Huang X, Eslinger P. Olfactory dysfunction in Multiple Sclerosis: a scoping review of the literature. Mult Scler Relat Disord. 2016;6:1–9. doi: 10.1016/j.msard.2015.12.002. [DOI] [PubMed] [Google Scholar]
- E28.Negoias S, Croy I, Gerber J, et al. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–421. doi: 10.1016/j.neuroscience.2010.05.012. [DOI] [PubMed] [Google Scholar]
- E29.Moberg PJ, Kamath V, Marchetto DM, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2014;40:50–59. doi: 10.1093/schbul/sbt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Gudziol H, Forster G. Zur Durchführung präoperativer Riechtests aus medicolegaler Sicht. Laryngorhinootologie. 2002;81:586–590. doi: 10.1055/s-2002-33360. [DOI] [PubMed] [Google Scholar]
- E31.„Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015;125:1102–1106“. doi: 10.1002/lary.24999. [DOI] [PubMed] [Google Scholar]
- E32.Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255:1121–1126. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- E33.Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the Skövde population-based study. Laryngoscope. 2004;114:733–737. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- E34.Reden J, Maroldt H, Fritz A, Zahnert T, Hummel T. A study on the prognostic significance of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 2007;264:139–144. doi: 10.1007/s00405-006-0157-0. [DOI] [PubMed] [Google Scholar]
- E35.Lötsch J, Hummel T. Clinical usefulness of self-rated olfactory performance—a data science-based assessment of 6000 patients. Chem Senses. 2019;44:357–364. doi: 10.1093/chemse/bjz029. [DOI] [PubMed] [Google Scholar]
- E36.Croy I, Lange K, Krone F, Negoias S, Seo HS, Hummel T. Comparison between odor thresholds for phenyl ethyl alcohol and butanol. Chem Senses. 2009;34:523–527. doi: 10.1093/chemse/bjp029. [DOI] [PubMed] [Google Scholar]
- E37.Khan M, Yoo SJ, Clijsters M, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184 doi: 10.1016/j.cell.2021.10.027. 5932-49.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Doty RL. Measurement of chemosensory function. World J Otorhinolaryngol Head Neck Surg. 2018;4:11–28. doi: 10.1016/j.wjorl.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E39.Mueller CA, Grassinger E, Naka A, Temmel AF, Hummel T, Kobal G. A self-administered odor identification test procedure using the „Sniffin‘ Sticks“. Chem Senses. 2006;31:595–598. doi: 10.1093/chemse/bjj064. [DOI] [PubMed] [Google Scholar]
- E40.Tan BKJ, Han R, Zhao JJ, Tan NKW, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ. 2022;378 doi: 10.1136/bmj-2021-069503. e069503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Mangal V, Murari T, Vashisht R, Iqbal SM, Meghana K, Gujrathi S, et al. Olfactory Dysfunction Among Asymptomatic Patients with SARS CoV2 Infection: A Case-Control Study. Indian J Otolaryngol Head Neck Surg. 2021;73:1–6. doi: 10.1007/s12070-021-02366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E42.Whitcroft KL, Cuevas M, Andrews P, Hummel T. Monitoring olfactory function in chronic rhinosinusitis and the effect of disease duration on outcome. Int Forum Allergy Rhinol. 2018;218:769–776. doi: 10.1002/alr.22104. [DOI] [PubMed] [Google Scholar]
- E43.Heilmann S, Strehle G, Rosenheim K, Damm M, Hummel T. Clinical assessment of retronasal olfactory function. Arch Otolaryngol Head Neck Surg. 2002;128:414–418. doi: 10.1001/archotol.128.4.414. [DOI] [PubMed] [Google Scholar]
- E44.Özay H, Çakir A, Ecevit MC. Retronasal olfaction test methods: a systematic review. Balkan Med J. 2019;36:49–59. doi: 10.4274/balkanmedj.2018.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E45.Han P, Su T, Qin M, Chen H, Hummel T. A systematic review of olfactory related questionnaires and scales. Rhinology. 2020;59:133–143. doi: 10.4193/Rhin20.291. [DOI] [PubMed] [Google Scholar]
- E46.Liu DT, Welge-Lüssen A, Besser G, Mueller CA, Renner B. Assessment of odor hedonic perception: the sniffin‘ sticks parosmia test (SSParoT) Sci Rep. 2020;10 doi: 10.1038/s41598-020-74967-0. 18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E47.Stuck BA, Beule A, Damm M, et al. [Position paper „Chemosensory testing for expert opinion in smell disorders“] Laryngorhinootologie. 2014;93:327–329. doi: 10.1055/s-0033-1364034. [DOI] [PubMed] [Google Scholar]
- E48.Macel D, Huart C, Duprez T, Hummel T, Rombaux P. Prognostic value of olfactory bulb volume measurement for recovery in post-infectious and post-traumic olfactory loss. B-ENT. 2012;8(Suppl. 18) [Google Scholar]
- E49.Rudmik L, Smith KA, Soler ZM, Schlosser RJ, Smith TL. Routine magnetic resonance imaging for idiopathic olfactory loss: a modeling-based economic evaluation. JAMA Otolaryngol Head Neck Surg. 2014;140:911–917. doi: 10.1001/jamaoto.2014.1883. [DOI] [PubMed] [Google Scholar]
- E50.Hura N, Yi JS, Lin SY, Roxbury CR. Magnetic resonance imaging as a diagnostic and research tool in patients with olfactory dysfunction: a systematic review. Am J Rhinol Allergy. 2022:;36:668–683. doi: 10.1177/19458924221096913. [DOI] [PubMed] [Google Scholar]
- E51.Han P, Zang Y, Akshita J, Hummel T. Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr. 2019;32:987–997. doi: 10.1007/s10548-019-00729-5. [DOI] [PubMed] [Google Scholar]
- E52.Zou LQ, Hummel T, Otte MS, et al. Association between olfactory function and quality of life in patients with olfactory disorders: a multicenter study in over 760 participants. Rhinology. 2021;59:164–172. doi: 10.4193/Rhin20.403. [DOI] [PubMed] [Google Scholar]
- E53.Oleszkiewicz A, Park D, Resler K, et al. Quality of life in patients with olfactory loss is better predicted by flavor identification than by orthonasal olfactory function. Chem Senses. 2019;44:371–377. doi: 10.1093/chemse/bjz027. [DOI] [PubMed] [Google Scholar]
- E54.Croy I, Buschhuter D, Seo HS, Negoias S, Hummel T. Individual significance of olfaction: development of a questionnaire. Eur Arch Otorhinolaryngol. 2010;267:67–71. doi: 10.1007/s00405-009-1054-0. [DOI] [PubMed] [Google Scholar]
- E55.Davidson TM, Murphy C, Jalowayski AA. Smell impairment. Can it be reversed? Postgrad Med. 1995;98:107-9–12-8. [PubMed] [Google Scholar]
- E56.Loftus PA, Roland LT, Gurrola JG 2nd, Cheung SW, Chang JL. Temporal profile of olfactory dysfunction in COVID-19. OTO Open. 2020;4 doi: 10.1177/2473974X20978133. 2473974x20978133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E57.London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63:159–166. doi: 10.1002/ana.21293. [DOI] [PubMed] [Google Scholar]
- E58.Hummel T, Lötsch J. Prognostic factors of olfactory dysfunction. Arch Otolaryngol Head Neck Surg. 2010;136:347–351. doi: 10.1001/archoto.2010.27. [DOI] [PubMed] [Google Scholar]
- E59.Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132:265–269. doi: 10.1001/archotol.132.3.265. [DOI] [PubMed] [Google Scholar]
- E60.Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- E61.Doty RL. Treatments for smell and taste disorders: a critical review. Handb Clin Neurol. 2019;164:455–479. doi: 10.1016/B978-0-444-63855-7.00025-3. [DOI] [PubMed] [Google Scholar]
- E62.Stuck BA, Popert U, Beule A, et al. Rhinosinusitis AWMF Leitlinien. www.awmf.org/leitlinien/detail/ll/017-49.html (last accessed on.6 February 2023) 2017 [Google Scholar]
- E63.Damm M. Sinunasale Dysosmien Riech- und Schmeckstörungen. In: Hummel T, Welge-Luessen A, editors. Thieme. Stuttgart: 2009. pp. 61–76. [Google Scholar]
- E64.Banglawala SM, Oyer SL, Lohia S, Psaltis AJ, Soler ZM, Schlosser RJ. Olfactory outcomes in chronic rhinosinusitis with nasal polyposis after medical treatments: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2014;4:986–994. doi: 10.1002/alr.21373. [DOI] [PubMed] [Google Scholar]
- E65.Shu CH, Lee PL, Shiao AS, Chen KT, Lan MY. Topical corticosteroid applied with a squirt system being more effective than with nasal spray for steroid-dependent olfactory impairment. Laryngoscope. 2012;122:747–750. doi: 10.1002/lary.23212. [DOI] [PubMed] [Google Scholar]
- E66.Scheibe M, Bethge C, Witt M, Hummel T. Intranasal administration of drugs. Arch Otolaryngol Head Neck Surg. 2008;134:643–646. doi: 10.1001/archotol.134.6.643. [DOI] [PubMed] [Google Scholar]
- E67.Kubba H, Spinou E, Robertson A. The effect of head position on the distribution of drops within the nose. Am J Rhinol. 2000;14:83–86. doi: 10.2500/105065800781692949. [DOI] [PubMed] [Google Scholar]
- E68.Mori E, Merkonidis C, Cuevas M, Gudziol V, Matsuwaki Y, Hummel T. The administration of nasal drops in the „Kaiteki“ position allows for delivery of the drug to the olfactory cleft: a pilot study in healthy subjects. Eur Arch Otorhinolaryngol. 2016;273:939–943. doi: 10.1007/s00405-015-3701-y. [DOI] [PubMed] [Google Scholar]
- E69.Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 2019;145:846–853. doi: 10.1001/jamaoto.2019.1728. [DOI] [PubMed] [Google Scholar]
- E70.Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017;55:17–26. doi: 10.4193/Rhino16.195. [DOI] [PubMed] [Google Scholar]
- E71.Altundag A, Cayonu M, Kayabasoglu G, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. 2015;125:1763–1766. doi: 10.1002/lary.25245. [DOI] [PubMed] [Google Scholar]
- E72.Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124:826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- E73.Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123:85–90. doi: 10.1002/lary.24390. [DOI] [PubMed] [Google Scholar]
- E74.Drews T, Hummel T. Treatment strategies for smell loss. Curr Otorhinolaryngol Rep. 2016;4:122–129. [Google Scholar]
- E75.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT) Laryngoscope. 1996;106(3 Pt 1):353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- E76.Davidson TM, Freed C, Healy MP, Murphy C. Rapid clinical evaluation of anosmia in children: the Alcohol Sniff Test. Ann NY Acad Sci. 1998;855:787–792. doi: 10.1111/j.1749-6632.1998.tb10659.x. [DOI] [PubMed] [Google Scholar]
- E77.Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110:976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- E78.Jackman AH, Doty RL. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope. 2005;115:2209–2212. doi: 10.1097/01.mlg.0000183194.17484.bb. [DOI] [PubMed] [Google Scholar]
- E79.Mueller C, Renner B. A new procedure for the short screening of olfactory function using five items from the „Sniffin‘ Sticks“ identification test kit. Am J Rhinol. 2006;20:113–116. [PubMed] [Google Scholar]
- E80.Vodicka J, Pellant A, Chrobok V. Screening of olfactory function using odourized markers. Rhinology. 2007;45:164–168. [PubMed] [Google Scholar]
- E81.Toledano A, Ruiz C, Navas C, et al. Development of a short olfactory test based on the Connecticut Test (CCCRC) Rhinology. 2009;47:465–469. doi: 10.4193/Rhin08.133. [DOI] [PubMed] [Google Scholar]
- E82.Hummel T, Pfetzing U, Lötsch J. A short olfactory test based on the identification of three odors. J Neurol. 2010;257:1316–1321. doi: 10.1007/s00415-010-5516-5. [DOI] [PubMed] [Google Scholar]
- E83.Mullol J, Alobid I, Marino-Sanchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study) BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001256. e001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E84.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- E85.Toyota B, Kitamura T, Takagi SF. Igaku-Shoin. Tokyo: 1978. Olfactory disorders–olfactometry and therapy. [Google Scholar]
- E86.Takagi SF. Standardized olfactometries in Japan–a review over ten years. Chem Senses. 1989;14:5–46. [Google Scholar]
- E87.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‚Sniffin‘ sticks‘: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- E88.Thomas-Danguin T, Rouby C, Sicard G, et al. Development of the ETOC: a European test of olfactory capabilities. Rhinology. 2003;41:142–151. [PubMed] [Google Scholar]
- E89.Cardesin A, Alobid I, Benitez P, et al. Barcelona Smell Test—24 (BAST-24): validation and smell characteristics in the healthy Spanish population. Rhinology. 2006;44:83–89. [PubMed] [Google Scholar]
- E90.Haehner A, Mayer AM, Landis BN, et al. High test-retest reliability of the extended version of the „Sniffin‘ Sticks“ test. Chem Senses. 2009;34:705–711. doi: 10.1093/chemse/bjp057. [DOI] [PubMed] [Google Scholar]
- E91.Croy I, Zehner C, Larsson M, Zucco GM, Hummel T. Test-retest reliability and validity of the Sniffin‘ TOM odor memory test. Chem Senses. 2015;40:173–179. doi: 10.1093/chemse/bju069. [DOI] [PubMed] [Google Scholar]
- E92.Xu Z, Luo X, Xu L, et al. Effect of short-course glucocorticoid application on patients with chronic rhinosinusitis with nasal polyps. World Allergy Organ J. 2020;13 doi: 10.1016/j.waojou.2020.100131. 100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E93.Zeng M, Wang H, Wang H, et al. Comparison of efficacy of fluticasone propionate versus clarithromycin for postoperative treatment of different phenotypic chronic rhinosinusitis: a randomized controlled trial. Rhinology. 2019;57:101–109. doi: 10.4193/Rhin17.226. [DOI] [PubMed] [Google Scholar]
- E94.Khan AR, Arif MA. Mometasone furoate intra nasal spray for the treatment of bilateral nasal polyposis. J Med Sci. 2019;27:203–209. [Google Scholar]
- E95.Zhou B, He G, Liang J, et al. Mometasone furoate nasal spray in the treatment of nasal polyposis in Chinese patients: a double-blind, randomized, placebo-controlled trial. Int Forum Allergy Rhinol. 2016;6:88–94. doi: 10.1002/alr.21650. [DOI] [PubMed] [Google Scholar]
- E96.Jankowski R, Klossek JM, Attali V, Coste A, Serrano E. Long-term study of fluticasone propionate aqueous nasal spray in acute and maintenance therapy of nasal polyposis. Allergy. 2009;64:944–950 . doi: 10.1111/j.1398-9995.2009.01938.x. [DOI] [PubMed] [Google Scholar]
- E97.Ehnhage A, Olsson P, Kölbeck KG, et al. Functional endoscopic sinus surgery improved asthma symptoms as well as PEFR and olfaction in patients with nasal polyposis. Allergy. 2009;64:762–769. doi: 10.1111/j.1398-9995.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- E98.Small CB, Stryszak P, Danzig M, Damiano A. Onset of symptomatic effect of mometasone furoate nasal spray in the treatment of nasal polyposis. J Allergy Clin Immunol. 2008;121:928–932. doi: 10.1016/j.jaci.2007.11.018. [DOI] [PubMed] [Google Scholar]
- E99.Papadakis CE, Chimona TS, Chaidas K, Ladias A, Zisoglou M, Proimos EK. Effect of oral steroids on olfactory function in chronic rhinosinusitis with nasal polyps. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138:343–348. doi: 10.1016/j.anorl.2020.06.028. [DOI] [PubMed] [Google Scholar]
- E100.Ecevit MC, Erdag TK, Dogan E, Sutay S. Effect of steroids for nasal polyposis surgery: a placebo-controlled, randomized, double-blind study. Laryngoscope. 2015;125:2041–2045. doi: 10.1002/lary.25352. [DOI] [PubMed] [Google Scholar]
- E101.Alobid I, Benítez P, Cardelús S, et al. Oral plus nasal corticosteroids improve smell, nasal congestion, and inflammation in sino-nasal polyposis. Laryngoscope. 2014;124:50–56. doi: 10.1002/lary.24330. [DOI] [PubMed] [Google Scholar]
- E102.Kirtsreesakul V, Wongsritrang K, Ruttanaphol S. Does oral prednisolone increase the efficacy of subsequent nasal steroids in treating nasal polyposis? Am J Rhinol Allergy. 2012;26:455–462. doi: 10.2500/ajra.2012.26.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E103.Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Ann Intern Med. 2011;154:293–302. doi: 10.7326/0003-4819-154-5-201103010-00003. [DOI] [PubMed] [Google Scholar]
- E104.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: Two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125 doi: 10.1016/j.jaci.2010.02.020. 1069-76. e4. [DOI] [PubMed] [Google Scholar]
- E105.Benítez P, Alobid I, De Haro J, et al. A short course of oral prednisone followed by intranasal budesonide is an effective treatment of severe nasal polyps. Laryngoscope. 2006;116:770–775. doi: 10.1097/01.mlg.0000205218.37514.0f. [DOI] [PubMed] [Google Scholar]
- E106.Wright ED, Agrawal S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope. 2007;117(11 pt 2 suppl 115):1–28. doi: 10.1097/MLG.0b013e31814842f8. [DOI] [PubMed] [Google Scholar]
- E107.Hissaria P, Smith W, Wormald PJ, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol. 2006;118:128–123. doi: 10.1016/j.jaci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- E108.Pieniak M, Oleszkiewicz A, Avaro V, Calegari F, Hummel T. Olfactory training—thirteen years of research reviewed. Neurosci Biobehav Rev. 2022;141 doi: 10.1016/j.neubiorev.2022.104853. 104853. [DOI] [PubMed] [Google Scholar]
- E109.Yaylaci A, Azak E, Önal A, Aktürk DR, Karadenizli A. Effects of classical olfactory training in patients with COVID-19-related persistent loss of smell. Eur Arch Otorhinolaryngol. 2022;29:1–7. doi: 10.1007/s00405-022-07570-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E110.Lechner M, Liu J, Counsell N, et al. The COVANOS trial—insight into post-COVID olfactory dysfunction and the role of smell training. Rhinology. 2022 doi: 10.4193/Rhin21.470. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- E111.Choi BY, Jeong H, Noh H, Park JY, Cho JH, Kim JK. Effects of olfactory training in patients with postinfectious olfactory dysfunction. Clin Exp Otorhinolaryngol. 2021;14:88–92. doi: 10.21053/ceo.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E112.Qiao XF, Bai YH, Wang GP, Li X, Zheng W. Clinical effects of two combinations of olfactory agents on olfactory dysfunction after upper respiratory tract infection during olfactory training. Rev Assoc Med Bras (1992) 2020;66:18–24. doi: 10.1590/1806-9282.66.1.18. [DOI] [PubMed] [Google Scholar]
- E113.Saatci O, Altundag A, Duz OA, Hummel T. Olfactory training ball improves adherence and olfactory outcomes in post-infectious olfactory dysfunction. Eur Arch Otorhinolaryngol. 2020 277:2125–2132. doi: 10.1007/s00405-020-05939-3. [DOI] [PubMed] [Google Scholar]
- E114.Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2021;164:244–254. doi: 10.1177/0194599820943550. [DOI] [PubMed] [Google Scholar]
- E115.Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2016;6:299–307. doi: 10.1002/alr.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E116.Jiang RS, Twu CW, Liang KL. The effect of olfactory training on odor identification in patients with traumatic anosmia. Int Forum Allergy Rhinol. 2019;9:1244–1251. doi: 10.1002/alr.22409. [DOI] [PubMed] [Google Scholar]
- E117.Langdon C, Lehrer E, Berenguer J, et al. Olfactory training in post-traumatic smell impairment: mild improvement in threshold performances: results from a randomized controlled trial. J Neurotrauma. 2018;35:2641–2652. doi: 10.1089/neu.2017.5230. [DOI] [PubMed] [Google Scholar]
- E118.Oleszkiewicz A, Hanf S, Whitcroft KL, Haehner A, Hummel T. Examination of olfactory training effectiveness in relation to its complexity and the cause of olfactory loss. Laryngoscope. 2018;128:1518–1522 . doi: 10.1002/lary.26985. [DOI] [PubMed] [Google Scholar]
- E119.Patel ZM, Wise SK, DelGaudio JM. Randomized controlled trial demonstrating cost-effective method of olfactory training in clinical practice: essential oils at uncontrolled concentration. Laryngoscope Investig Otolaryngol. 2017;2:53–56. doi: 10.1002/lio2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E120.Poletti SC, Michel E, Hummel T. Olfactory training using heavy and light weight molecule odors. Perception. 2017;46:343–351. doi: 10.1177/0301006616672881. [DOI] [PubMed] [Google Scholar]
- E121.Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123:E85–E90. doi: 10.1002/lary.24390. [DOI] [PubMed] [Google Scholar]
- E122.Gellrich J, Han P, Manesse C, Betz A, et al. Brain volume changes in hyposmic patients before and after olfactory training. Laryngoscope. 2018;128:1531–1536. doi: 10.1002/lary.27045. [DOI] [PubMed] [Google Scholar]
- E123.Hummel T, Stupka G, Haehner A, Poletti SC. Olfactory training changes electrophysiological responses at the level of the olfactory epithelium. Rhinology. 2018;56:330–335. doi: 10.4193/Rhin17.163. [DOI] [PubMed] [Google Scholar]
- E124.Haehner A, Tosch C, Wolz M. Olfactory training in patients with Parkinson’s disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061680. e61680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E125.Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119:496–499. doi: 10.1002/lary.20101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case report
A 46-year-old man who worked as a self-employed retailer presented with olfactory dysfunction of approximately 3 years’ standing. He reported that the dysfunction had developed gradually and that he had hardly noticed it at first. He denied possible causes such as an infection, trauma, or medications, and no comorbidities, difficulty in nose breathing, or any other nasal problems were present. ENT examination including nasal endoscopy found no abnormalities. Investigation of orthonasal olfactory function using the Sniffin’ Sticks procedure, comprising threshold, discrimination, and identification tests, revealed functional anosmia. Suprathreshold concentrations of tasting powders in the primary flavors were correctly identified, showing normogeusia. As the findings of magnetic resonance imaging were normal, the diagnosis was idiopathic functional anosmia. Regular olfactory training was recommended. Due to the absence of symptoms, no neurological investigations took place.
The anosmia remained unchanged over the course of several follow-up visits. At 3 years after diagnosis the patient reported the recent onset of tremors in the left extremities at rest, together with minor clumsiness of the left hand and occasional pain in the right thigh. Clinical examination by a neurologist found slight, moderately frequent trembling of the left extremities at rest, slight left-sided bradydiadochokinesis, discrete rigor of the left arm, and reduced left arm swing. The diagnosis was suspected idiopathic Parkinson’s disease and the patient was transferred to the department of neurology for further investigation.