Abstract
Purpose
Tru-cut biopsy is a minimally invasive technique used to obtain tissue samples for the diagnosis of tumors, especially in patients where primary surgery is not indicated. The aim of this study was to assess the adequacy, accuracy and safety of the tru-cut biopsy for diagnosis in gynecological cancer.
Methods
A retrospective population-based review of 328 biopsies was conducted. The indications for tru-cut biopsies were diagnosis of primary tumors, metastases of gynecological and non-gynecological tumors, and suspected recurrences. A tissue sample was considered adequate when the quality/quality was sufficient to identify the subtype/origin of the tumor. Potential factors affecting adequacy were analyzed using logistic regressions analyses. Accuracy was defined as agreement between the diagnosis of the tru-cut biopsy and the postoperative histology. The therapy plan was registered, and the clinical applicability of the tru-cut biopsy was investigated. Complications within 30 days after the biopsy procedure were registered.
Results
In total, 300 biopsies were identified as tru-cut biopsies. The overall adequacy was 86.3%, varying between 80.8% and 93.5%, respectively, when performed by a gynecological oncologist or a gynecologist with a subspecialty in ultrasound diagnosis. Sampling of a pelvic mass had a lower adequacy (81.6%) compared with sampling of the omentum (93.9%) or carcinomatosis (91.5%). The accuracy was 97.5%, and the complication rate was 1.3%.
Conclusion
The tru-cut biopsy is a safe and reliable diagnostic method with a high accuracy and a good adequacy, depending on the site of the tissue sample, indications for the biopsy and the experience of the operator.
Keywords: tru-cut biopsy, gynecological malignancy, ultrasound, adequacy, accuracy
Plain Language Summary
In ovarian cancer and other cancer diagnoses, the outcomes depend on timely diagnosis, as well as access to appropriate surgery and systemic therapy. These parameters can be considered indicators of the effectiveness of a country’s health-care system. A global assessment by world region and Human Development Index showed that inadequate public-health systems with fewer facilities for diagnosis could have an impact on ascertaining cases and could also negatively contribute to lower survival. In contrast, many high-income countries either had centralized diagnostics and treatment for ovarian cancer, or they were in the process of centralization, which had previously been linked to improved survival.
Correct characterization of the tumor and its spread before initial intervention can help to select effective therapeutic approaches for each patient. Histotype-specific and/or stage-dependent treatment options are needed, in combination with a patient’s characteristics such as age, comorbidity, and personal or family wishes.
Introduction
In the era of molecular and genetic needs for cancer treatment, diagnostic analyses must also be made when primary surgery is not indicated or when preoperative diagnosis is crucial for further treatment. Ideally, minimally invasive techniques should be chosen to obtain cytological or tissue specimens.1,2 Tru-cut biopsy is a highly sensitive and established method to obtain material to diagnose primary, locally recurrent or metastatic lesions in various anatomical regions, with an accuracy varying between 76% and 99%.3,4 In many centers, laparoscopy is used to identify tumor extension and for tissue sampling.5,6 On the other hand, ultrasound-guided biopsy methods, with flexibility to use transabdominal, transvaginal, and transrectal approaches offer benefits and have minimal risk of complications, high accessibility and low costs, compared to computer tomography (CT) or magnetic resonance (MR) guidance.7–9 The role of tru-cut biopsy in gynecological oncology has been addressed by several authors.2,10–12 In patients with poor performance status, with suspicion of recurrent disease, when surgery is not the first option of treatment, or when general anesthesia has to be avoided, the tru-cut biopsy is a less invasive option than laparoscopy.13–17
This review aims to look at the adequacy, accuracy and safety of the tru-cut biopsy in a large group of patients with gynecological malignancy, in relation to clinical specialty of visualization method, and to identify factors that might affect the performance, with a focus on ultrasound guidance. In daily practice, an ultrasound-guided tru-cut-biopsy is performed by different health-care providers such as gynecological oncologists, gynecologists or radiologists with ultrasound competence and Computed Tomography-guidance (CT-guided), depending on the local or regional resources.
Differences in adequacy were investigated in relation to type of operator, anatomic location chosen for the biopsy and guidance method. The goal setting was to identify the optimal instance for reference of the patients and to facilitate a safe and rapid track to diagnose gynecological malignancies and initiate treatment.
Materials and Methods
Study Population
The study was conducted as a retrospective population-based review. The study was approved by the Swedish Ethical Review Authority (2020/2818). The primary data source was charts from patients who underwent a tru-cut biopsy between 1st of January 2015 and 31st of December 2020 at the tertiary center for oncologic gynecological surgery in the Department of Obstetrics and Gynecology Skåne University Hospital, Lund. Sweden. Patients were identified from the Laboratory Information Management System (LIMS) used by pathologists, by searching for tru-cut biopsies required by the gynecological department. The clinical characteristics of the patients were collected from the patient data system (Melior.220–9.3.0.400–20210909.3, Cerner Corporation, Kansas City, USA): age, body mass index (BMI), CA–125, and Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) stage (when applicable), and primary diagnosis (when recurrence was suspected). The pathologists performed histopathological diagnoses on formalin-fixed and paraffin-embedded material.
The indications for the tru-cut biopsies were divided into three main groups, suspicion of A) new tumors of gynecological origin, B) tumors of non-gynecological origin for further referral, and C) recurrences from gynecological tumors.
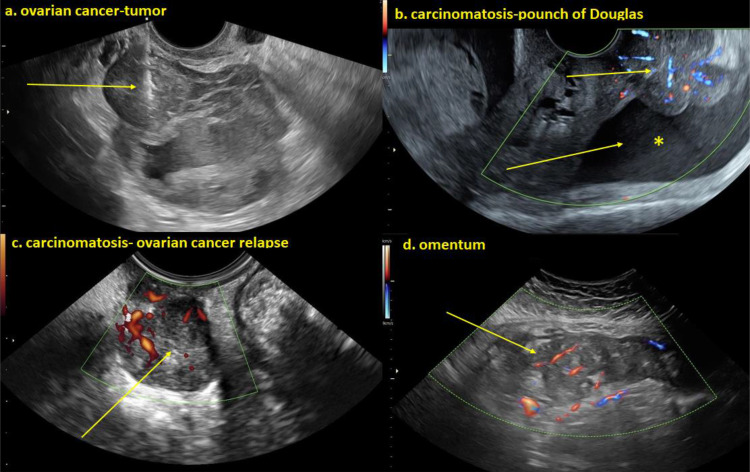
Five different groups of operators performed biopsies: gynecological oncologists, gynecologists or radiologists subspecialized in ultrasound diagnosis, radiologists specialized in performing CT-guided biopsies and general gynecologists. The biopsy sites consisted of pelvic tumors, peritoneal carcinomatosis, omental cake, lymph nodes, and others (including the liver and thorax). Some of the biopsy sites are exemplified in Figure 1.
Figure 1.
(a) Heterogeneous irregular cystic-solid ovarian tumor and the tru-cut biopsy needle visualized at sampling of the solid component of the lesion. (b) Irregular solid richly vascularized carcinomatosis deposits on peritoneal surface and free fluid *In the pouch of Douglas (c) Heterogeneous solid richly vascularized metastatic tumor relapse in the pouch of Douglas. (d) Irregular solid heterogeneous moderately vascularized omentum metastasis of ovarian cancer.
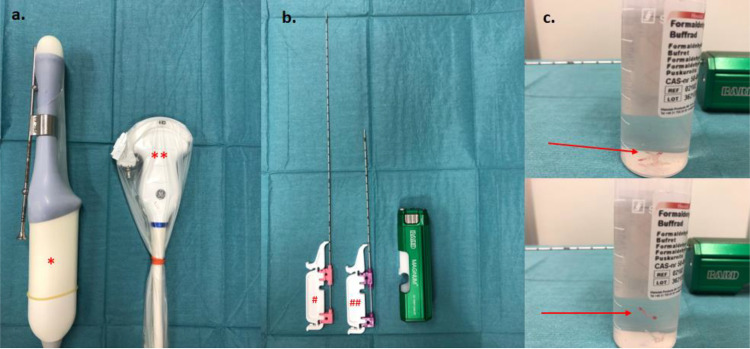
Patients who underwent tru-cut biopsy were initially assessed by vaginal and abdominal ultrasound scan or corresponding imaging technique, for tumor extent, whereby the optimal site for biopsy was selected. Consecutively, tissue samples were collected, for example using a 30 cm 18–gauge needle (for the transvaginal approach) or a 20 cm 16–gauge needle (for the transabdominal approach) mounted on a Bard® Magnum® Ref MG1522 core biopsy instrument, under machine-integrated ultrasound needle guidance. Examples of used equipment are presented in Figure 2. When the transabdominal procedure was used, 8–12 mL of local anesthesia was infiltrated prior to sampling. No anesthetic was applied prior to vaginal sampling, and no analgesics or antibiotics were administered prior to the procedures. Patients were informed and recommended contacting the gynecology emergency unit in the event of possible adverse outcomes such as bleeding, severe pain, fever or infection in the days following the procedure.
Figure 2.
(a) Transvaginal RIC5–9–D *and transabdominal C2-9–D curvilinear **Probes prepared with protective cover and probe guide. (b) Bard® Magnum® Ref MG1522 tru-cut core biopsy instrument and 18G 30cm needle for transvaginal #Respectively 16G 20 cm needle for transabdominal ##Sampling. (c) tru-cut biopsy in formaldehyde solution.
In patients where no tumor site was considered safely accessible by either the transvaginal or transabdominal ultrasound approach, or in the case of distant metastasis (liver, lungs), diagnostic biopsies were made either by CT-guided sampling or by diagnostic laparoscopy.
The Adequacy and Accuracy Analyses
Only tissue samples where quality and quantity led to diagnoses, including immunohistochemistry analyses, in order to identify the subtype/origin of the tumor, were considered adequate.
Potential factors that could have affected adequacy were registered, as follows: age, BMI, ascites, CA–125, indication, site of biopsy, biopsy approach and operator qualifications.
The accuracy of the biopsy method was analyzed for patients where a tru-cut sample was considered adequate and therefore could be compared with the postoperative histopathological diagnosis.
The results of somatic BRCA mutation-analysis were registered when this where performed on the biopsy material, even if the BRCA analyses were not considered as a part of the study aims.
All therapy strategies were registered and therapeutical decisions based on tru-cut biopsy results were analyzed, in order to investigate the clinical applicability of the procedure.
The therapy strategies were documented and divided into seven categories: primary surgery, neoadjuvant chemotherapy and interval surgery, palliative chemotherapy, best supportive care, recurrence surgery, curative radiotherapy and follow-up.
All complications occurred within 30 days after the biopsies were registered and were analyzed in relation to the operator, the site of the biopsy and the biopsy approach.
Statistical Analyses
Descriptive and analytical statistics were performed using the statistical software IBM Corp. Released in 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp Nominal and ordinal data were coded to enable subsequent statistics to be obtained. Qualitative data were described in counts (n) and percentages (%) and quantitative nonparametric data in medians and range intervals.
Age and BMI were analyzed as continuous data, while ascites was dichotomized into two groups, below and above 1000 mL. CA–125 was logarithmized due to squid data distribution.
The different parameters affecting adequacy were analyzed separately, comparing one parameter at time with the whole group, using logistic regression analysis.
For accuracy analyses, the postoperative final histopathological diagnosis constituted the diagnostic reference standard (gold standard), while the tru-cut biopsy diagnosis was the diagnostic test.
Results
Study Population
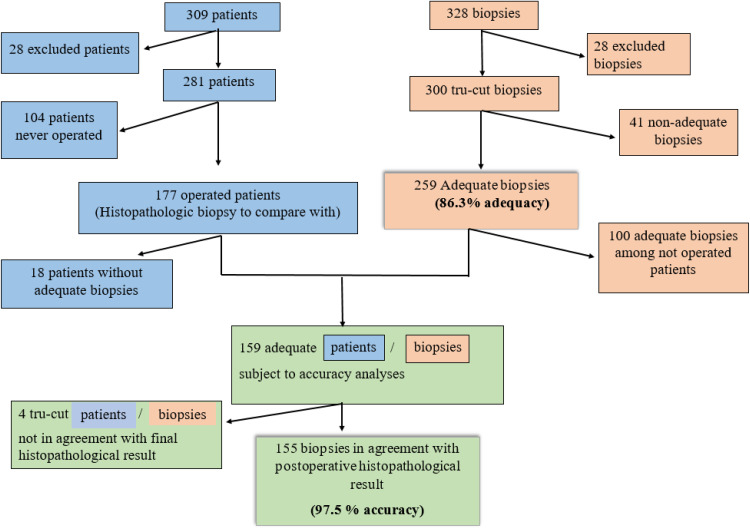
A total of 328 biopsies were identified in the LIMS database. Twenty-eight biopsies were excluded due to either insufficient data, non-gynecological (as breast tissue), or other techniques than tru-cut biopsy (Figure 3).
Figure 3.
The flowchart of patients (blue) and biopsies (brown). Correspondent biopsy for each patient- flow chart to analyze accuracy (green).
In total, 300 tru-cut biopsy procedures were performed on 281 patients (Figure 3). In 15 patients additional biopsies were needed, as follows: 13 patients needed a second biopsy, one patient underwent a third biopsy and one patient underwent five biopsies due to low quantity and/or quality of the samples. Clinical data, type of diagnosis, biopsy site, approach and the type of operator are shown in Table 1. In 286 out of 300 cases, two or more cores/tissue cylinders were sampled.
Table 1.
Characteristics of the Study Population (Patients and Biopsies) with Respect to Indication, Biopsy Site, Approach and Operator, with Corresponding Adequacy for Each Variable
| Parameter Patients | Median (Range) | N (%) 281 | |
|---|---|---|---|
| Age (years) | 71 years (24–96) | ||
| BMI (kg/m²) | 25 (15–47) | ||
| CA-125 (U/mL) | 99 (6–32,832) | ||
| ECOG | |||
| ≤1 | 194 (69.0) | ||
| ≥2 | 82 (29.2) | ||
| Missing data | 5 (1.8) | ||
| Ascites | |||
| None | 185 (65.8) | ||
| <1000 mL | 25 (8.9) | ||
| ≥1000 mL | 65 (23.1) | ||
| Laparocentesis | 6 (2.1) | ||
| Parameter | N (%) | Corresponding adequacy N (%) | p |
| Biopsies | 300 | 259 (86.3) | |
| Diagnosis | |||
| Ovarian cancer | 149 (49.7) | 132 (88.6) | p=0.283 |
| Uterine malignancy | 54 (18) | 44 (81.5) | |
| Cervical cancer | 23 (7.7) | 19 (82.6) | |
| Vulvar cancer | 12 (4.0) | 12 (100) | |
| Breast cancer (with peritoneal carcinomatosis) | 2 (0.7) | 2 (100%) | |
| Gastrointestinal cancer | 22 (7.3) | 20 (90.9) | |
| Benign biopsy | 26 (8.7) | 22 (84.6) | |
| Other | 12 (4.0) | 8 (66.3) | |
| Indication | |||
| Primary diagnosis | 196 (65.3) | 175 (89.3) | p=0.039 |
| Metastatic tumor | 12 (4) | 8 (66.7) | |
| Recurrence | 92 (30.7) | 76 (82.6) | |
| Operator | |||
| Gynecological oncologist | 120 (40) | 97 (80.8) | p=0.026 |
| Ultrasound gynecologist | 123 (41) | 115 (93.5) | |
| Ultrasound radiologist | 48 (16) | 40 (83.3) | |
| CT-guided | 7 (2.3) | 6 (85.7) | |
| General gynecologist | 2 (0.7) | 1 (50) | |
| Site of biopsy | |||
| Pelvic tumor | 174 (58.0) | 142 (81.6) | p=0.093 |
| Carcinomatosis | 47 (15.7) | 43 (91.5) | |
| Omental cake | 33 (11.0) | 31 (93.9) | |
| Lymph node | 15 (5.0) | 14 (93.3) | |
| Others | 31 (10.3) | 29 (93.5) | |
| Approach | |||
| Transvaginal | 180 (60) | 157 (87.2) | p=0.722 |
| Transabdominal | 90 (30) | 75 (83.3) | |
| Transvaginal + transabdominal | 2 (0.7) | 2 (100) | |
| Others* | 28 (9.3) | 25 (89.3) |
Notes: *Extra-abdominal lymph nodes. Bold values denote statistical significance at the p < 0.05 level.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Adequacy
An adequate tumor sample was obtained in 259 out of 300 biopsies, with an overall adequacy of 86.3%. In 41 of the tru-cut biopsies (34 patients), the result was inadequate or not representative for the tumor type or origin (Figure 3). For 15 of these patients, one or several biopsies were repeated. Of the remaining 19 patients, one (1) patient underwent open biopsy (superficial, subcutaneous metastasis), seven (7) patients underwent laparoscopy or laparotomy, in two (2) patients a fine needle biopsy was performed, for one (1) patient with actinomycosis the diagnosis was settled by microbiology culture, and in two (2) patients the diagnosis was settled by cytology. In six (6) patients no final diagnosis was registered: two with poor performance status and advanced disease died before the completion of diagnosis, in three a follow-up was planned due to clinical benign nature of the tumor, and one moved abroad and was lost to follow-up.
The adequacy of the tru-cut biopsy was negatively influenced by the biopsy site, with a 66% higher risk of a failed biopsy from pelvic tumors but not by age, BMI, CA–125 or quantity of ascites. The procedure approach did not significantly affect the adequacy. Ultrasound-guided biopsies performed by gynecologist subspecialized in ultrasound diagnostics provided 3.3 times higher odds of giving an adequate sample (Table 2). When the data were adjusted for biopsy site, approach and indication, the chance for a successful tru-cut biopsy was still higher when performed by gynecologist subspecialized in ultrasound diagnosis (OR 2.9; 95CI: 1.249–6.722, p=0.13).
Table 2.
Dependency of Adequacy on Selected Parameters as Evaluated Using Logistic Regression
| Variable | Constant | Odds Ratio | 95% Confidence Interval | P value | Impact on Adequacy |
|---|---|---|---|---|---|
| Age | −0.008 | 0.992 | 0.969–1.016 | 0.525 | None |
| BMI | 0.52 | 1.053 | 0.984–1.127 | 0.135 | None |
| Log2(CA-125) | 1.073 | 0.929–1.239 | 0.338 | None | |
| Ascites | |||||
| <1000mL | −0.617 | 0.540 | 0.217–1.344 | 0.185 | None |
| ≥1000mL | 0.617 | 1.857 | 0.744–4.617 | 0.185 | None |
| Indication | |||||
| Diagnose | 0.685 | 1.984 | 1.020–3.859 | 0.044 | Positive |
| Metastasis | −1.22 | 0.295 | 0.085–1.028 | 0.55 | None |
| Relapse | −0.432 | 0.649 | 0.328–1.284 | 0.214 | None |
| Site of biopsy | |||||
| Pelvic tumor | −1.075 | 0.341 | 0.157–0.744 | 0.007 | Negative |
| Carcinomatosis | 0.611 | 1.841 | 0.624–5.435 | 0.268 | None |
| Omentum | 0.975 | 2.65 | 0.610–11.527 | 0.193 | None |
| Lymph nodes | 0.827 | 2.286 | 0.292–17.864 | 0.43 | None |
| Approach | |||||
| Transvaginal | 0.186 | 1.205 | 0.619–2.343 | 0.583 | None |
| Transabdominal | −0.347 | 0.707 | 0.354–1.408 | 0.324 | None |
| Operator | |||||
| Gynecological oncologist | −0.758 | 0.469 | 0.241–0.912 | 0.026 | Negative |
| Ultrasound gynecologist | 1.192 | 3.294 | 1.465–7.408 | 0.004 | Positive |
| Ultrasound radiologist | −0.243 | 0.753 | 0.324–1.750 | 0.51 | None |
| CT-guide | −0.053 | 0.949 | 0.111–8.080 | 0.962 | None |
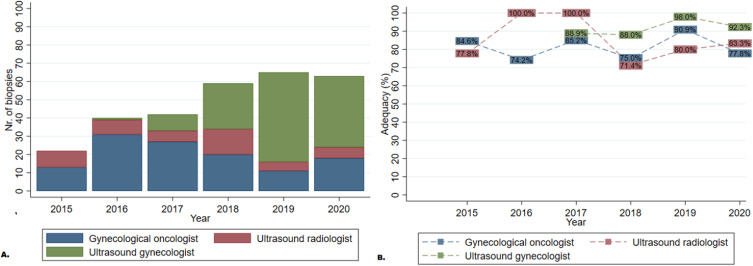
Gynecological oncologists and radiologists performed tru-cut biopsy during the entire six-year review period, while the gynecologist subspecialized in ultrasound diagnosis started to routinely perform the procedure in 2017, covering just 4 years of the study period, as shown in Figure 4. The gynecological oncologist more often targeted pelvic tumors than peritoneal carcinomatosis or omental cake (74.2% vs 9.2% and 5%, respectively, p=0.000) and compared with other operators, more often used transvaginal guided ultrasonography (73.3% vs 51.1%, p=0.001).
Figure 4.
Clinical development showing the number of biopsies (A) and adequacy (B) by operator during the study period.
The biopsies performed by the radiology department were mainly transabdominal (81.3%), and the biopsy site was other than pelvic tumors in 85.7% of CT-guided and in 79.2% of ultrasound-guided biopsies.
Logistic regression analyses comparing ultrasound-guided tru-cut biopsies between gynecological oncologists vs gynecologist and radiologists, both with ultrasound competence showed a higher odds ratio for the last two, OR: 3.6 (1.544–8.364), p=0.003 for the gynecologist and OR: 1.25 (0.518–3.017), for the radiologist (p=0.62), using the gynecological oncologist as reference.
Accuracy
Out of 281 patients, 177 underwent surgery at some point and had a postoperative histopathological biopsy to compare it with, whereas 104 patients never had surgery (palliative, best supportive care, benign). In 18 out of 177 patients, the tru-cut biopsies were not adequate and were excluded from the accuracy analyses. The remaining 159 patients with an adequate tru-cut biopsy and a postoperative histopathological diagnosis to compare it with were included in the accuracy analyses (Figure 3).
In four out of 159 patients, the tru-cut biopsy was not in agreement with the postoperative histopathological result, resulting in an accuracy of 97.5%. It is notable that three of these tumors were of non-gynecological origin (Table 3).
Table 3.
The Tru-Cut Biopsy Diagnosis and Final Diagnosis in Patients with Unclear Diagnosis Despite an Adequate Tru-Cut Biopsy Sample
| Tru-Cut Biopsy Diagnosis | Final Diagnosis |
|---|---|
| Unclear mucinous cancer | Pancreatic cancer metastasis |
| Malign epithelial tumor | Breast cancer with peritoneal metastases |
| Dedifferentiated tumor | Neuroendocrine tumor of the lungs |
| Benign ovarian tissue | Low-grade serous carcinoma |
Safety and Clinical Applicability of the Tru-Cut Biopsy
In four (4/300) of cases, complications occurred following the procedure (1.3%). All complications were related to infection. The patients were clinically stable and did not require surgery (Table 4).
Table 4.
Tru-Cut Biopsy Characteristics, Therapy and Hospital Stay for Patients with Complications
| Nr. (Age) |
Indication | Place of Biopsy | Approach | Operator | Complication | Readmission | Therapeutic Approach |
|---|---|---|---|---|---|---|---|
| 1. (69) | AOC suspicion (diagnostic) | Omentum | Transabdominal | Gynecological oncologist | Intestinal perforation | 26 hours after biopsy | Intravenous antibiotics |
| 2. 86 | AOC suspicion (diagnostic) | Pelvic tumor | Transvaginal | Gynecological oncologist | Superficial, puncture infection | 5 days after biopsy | Intravenous antibiotics |
| 3. (80) | AOC suspicion (diagnostic) | Pelvic tumor | Transvaginal | Gynecological oncologist | Pelvic abscess | 3 days after biopsy | Intravenous antibiotics |
| 4. (25) | Krukenberg tumor (diagnostic) |
Pelvic tumor | Transvaginal | Ultrasound gynecologist | Pain Pelvic abscess | 4 hours after biopsy | Intravenous antibiotics |
Abbreviation: AOC, Advanced ovarian cancer.
Out of 259 adequate biopsies, 254 (98.1%) led to a therapy plan, but not in five cases, despite an adequate tissue sample being obtained. In four cases, the accuracy of tru-cut biopsy was not proper as shown in accuracy analyses and in the fifth case, a suspicion of vulvar cancer relapse, the tru-cut biopsy of the groin lymph nodes was benign and lead to no therapeutic approach. Later on, a therapy plan was established after a cytological relapse confirmation.
For cases with an adequate sample, the therapy plan consisted of upfront surgery in 51/254 of cases, neoadjuvant chemotherapy and interval surgery in 20/254, palliative chemotherapy in 105/254, best supportive care in 40/254, relapse surgery in 11/254, curative radiotherapy in 5/254, and follow-up in 22/254.
BRCA Analyses
In 21 cases breast cancer gene mutation (BRCA) analyses were required. In all cases the material was considered adequate for the BRCA analyses. Three tumors were found to be BRCA 2 mutated and one BRCA 1 mutated.
Discussion
This study is a retrospective review of the experience of performing a tru-cut biopsy during 6 years of clinical practice in a single tertiary institution. Many similar studies have shown a high rate of successful retrieval of tumor material with this method, when performed under ideal circumstances in clinical practice (as performed in highly specialized health care). Zikan et al describe a high adequacy of 91.3% when the biopsy is performed by ultrasound specialists, which is comparable with 93.5% in our study when comparing with the same specialist category.3 However, in daily practice, a highly specialized ultrasound-guided biopsy is not always available. The overall adequacy in our study was 86.3%, with differences depending on indication, site of biopsy and operator that is comparable with another Swedish study describing an adequacy of 88%.12 The gynecological oncologist has the advantage of performing a tru-cut biopsy without delay, when the patient is presenting at the office, with a relative good adequacy (80.8%), enabling a swifter planning of treatment. This is an advantage if accessibility to an ultrasound or radiology department is limited. However, since the risk of an inadequate sample is 53% higher, this might also lead to a delay due to the necessity for planning and performing a new biopsy for some patients, with possible implications for overall survival.18 Therefore, these results indicate that preparing for a specialist ultrasound with a tru-cut biopsy might be worthwhile, since this procedure gives the most adequate material.
Differences in the tru-cut procedure were registered between the gynecology and radiology departments, with a higher incidence of use of the transabdominal approach for the radiology department. Tumor sites other than the pelvis were more often targeted and specific localizations, such as liver biopsy, were exclusively performed in the radiology department. This was partly a result of the diagnostic arrangement, and emphasizes the importance of multidisciplinary collaboration in cancer patient care.
The adequacy was negatively affected when the biopsy was sampled from pelvic tumors. This might be related to tumor heterogeneity and to the presence of partly necrotic and cystic components.19,20 The indication of primary diagnostics of gynecological tumors was a positive predictive factor, compared with suspicion of metastasis from non-gynecological cancer or recurrent disease. Most of the patients with primary diagnosis had FIGO stages III and IV, which implies a high tumor burden, whereas recurrences might be smaller in size, impairing the ability to collect representative material.
Verschuere et al describe an increase in successful biopsies/accuracy where at least two cylinders were sampled, compared with just one cylinder. In our study, two or more cylinders were sampled in the majority of cases (95.6%), and no differences in the number of collected cylinders were noted.4
The accuracy in the present study was high (97.5%), with just four cases where the tru-cut biopsy was not in total agreement with the postoperative/final histopathology diagnosis. In two cases the primary tumor was breast and lung cancer, with unusual dissemination to the peritoneal cavity. In both cases a malignant diagnosis was identified but needed further investigation through a stereotactic breast biopsy and a thoracoscopic biopsy, respectively.21,22 The other two cases were a mucinous and low-grade type tumor. The difficulty in diagnosing this entity is even described for other histopathological procedures as frozen section diagnosis, where difficulty diagnosing mucinous histopathology and distinguishing between borderline tumors and low-grade histology was observed.23,24 The difficulty in correctly diagnosing the origin of a mucinous tumor may be explained by the fact that mucinous tumors in different organs can be very similar.25 In the fourth case, a benign tru-cut biopsy was followed by a low-grade serous cancer in the postoperative final histopathological result. We considered the biopsy to be adequate due to the quality and quantity of the ovarian tissue in the sample, but this might be questioned since the postoperative final histopathological result of the same ovary showed a mixed benign tissue with noninvasive and invasive low-grade serous tumors, which might be seen due to the heterogenic nature of the low-grade serous tumors.26
The tru-cut biopsy is demonstrated to be a safe method with a complication rate between 1.1% and 4.8%, mainly minor complications.2,3 In our study an incidence of minor complications of 1.3% was registered, with no major complications. In our study, three out of four patients with complications were disclosed more than 24 h after their biopsy and one case presented, while being at the hospital, 4 hours after the biopsy. In the last case the reported symptoms were not related to infection, but later the actual symptoms of infection with fever and elevated leukocytosis started, after 36 h. Since complications are rare and can become symptomatic after more than 24 h, our opinion is that the tru-cut biopsy is safe to perform in an outpatient setting, thus avoiding additional costs due to unnecessary post-biopsy observation admissions.
Tru-cut biopsy is particularly advantageous in patients with advanced disease and poor performance status when surgery is not an option. These patients need palliative treatment or best supportive care planning, which nevertheless require confirmation of the malignancy type. Out of 259 adequate biopsies, 254 (98.1%) biopsies led to a therapy plan. Among these, for 100 patients, who never had surgery the histopathological diagnosis and therapy plan were decided based on the tru-cut biopsy only.
About 20% of ovarian/fallopian tube cancer patients carry germline mutations in the BRCA genes and about 0.4% have mutations in DNA mismatch repair genes.27,28 Poly(ADP-ribose) polymerase (PARP) treatment demonstrated its efficiency in treatment of both primary and recurrent ovarian cancer.29–31 In recurrent ovarian and inoperable cancer, histopathological confirmation is highly necessary, and might be lifesaving. In our review, the BRCA status was required from tru-cut biopsies sample in 21 cases, and all cases were adequate samples for analysis. BRCA screening was not a clinical routine at that time and only performed for a subset of these patients. Since our study was not designed to consider this particular aspect of the tru-cut procedure, further studies are needed to investigate this ability.
In this review we wanted to investigate the reliability of the tru-cut biopsy from a daily clinical practice perspective. The accessibility to highly specialized diagnostic tools is not uniform in centers treating gynecological cancers. Ideal cancer care involves specialized medical oncologists, surgeons, radiology, ultrasonography and histopathology departments with special interests and qualifications in gynecological malignancies, specialist nurses, and rehabilitation and psychotherapy specialists. This is only possible in highly specialized hospitals, which can offer a good preoperative evaluation of the tumor extent, and which have a histopathology department with access to molecular analysis, and additional fast track tools as mentioned above.
To our knowledge, this is the first study comparing the performance of a tru-cut biopsy from a time perspective and including several medical specialists who carry out these procedures.
This study benefits from a large study population, and to our knowledge is the largest study ever performed in a single cancer center. All histopathology analyses were carried out by a subspecialized gynecological pathology team to ensure that expert knowledge was available. Also, the fact that the patients’ clinical records, including their ultrasound, radiological and histopathological records, are all connected to one entity might be considered as an advantage as well.
A limitation of the study is its retrospective nature and the uncertainty of inter-individual variations between the specialists within each group. Several gynecological oncologists and radiologists performed this procedure, whereas only one individual gynecologist subspecialized in ultrasound diagnosis (KL) carried out all the ultrasound-guided biopsies.
Conclusion
The tru-cut biopsy is a safe and reliable diagnostic method with high accuracy and good adequacy, although these factors depend on the site of the tissue sample, indications for the biopsy and the experience of the operator.
Acknowledgment
Sara Mikkelsen for statistical support.
This paper is based on first author’s PhD thesis (MA).32
Funding Statement
Skåne County Council’s Research and Development Foundation funded this study. The funding sources solely contributed the means for carrying out the study but were not involved in the execution, analyses, interpretation of the data, or decision to submit the results.
Data Sharing Statement
Raw data are available from the lead author (Mihaela Asp) upon request.
Ethical Approval and Informed Consent
All patient information was handled according to the World Medical Association’s Declaration from the 2008 Declaration of Helsinki and in compliance with national law. The Swedish Ethical Review Authority approved this study with apl.no 2020–02818. Consent for publication: not applicable.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Mihaela Asp: Conceptualization, Investigation, Methodology, Data collection, Data-curation, Writing original draft preparation, Writing review and editing, Validation, Visualization, Editing the manuscript, Formal analysis.
Ingrida Mockute: Data collection, Writing original draft, Literature review.
Anna Måsbäck: Resources, Validation, Reviewing, Supervision, Histopathological expertise.
Susanne Malander: Validation, Reviewing, Supervision, Funding acquisition, Oncological expertise.
Karina Liuba: Data collection, Writing original draft, Ultrasound expertise, Conceptualization, Literature review.
Päivi Kannisto: Validation, Methodology, Reviewing, Supervision, Funding Acquisition.
Disclosure
The authors report no conflicts of interest.
References
- 1.Timmerman D, Planchamp F, Bourne T, et al. ESGO/ISUOG/IOTA/ESGE consensus statement on pre-operative diagnosis of ovarian tumors. Int J Gynecol Cancer. 2021;31(7):961–982. doi: 10.1136/ijgc-2021-002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascilini F, Quagliozzi L, Moro F, et al. Role of transvaginal ultrasound-guided biopsy in gynecology. Int J Gynecol Cancer. 2020;30(1):128–132. doi: 10.1136/ijgc-2019-000734 [DOI] [PubMed] [Google Scholar]
- 3.Zikan M, Fischerova D, Pinkavova I, Dundr P, Cibula D. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet Gynecol. 2010;36(6):767–772. doi: 10.1002/uog.8803 [DOI] [PubMed] [Google Scholar]
- 4.Verschuere H, Froyman W, Van den Bosch T, et al. Safety and efficiency of performing transvaginal ultrasound-guided tru-cut biopsy for pelvic masses. Gynecol Oncol. 2021;161(3):845–851. doi: 10.1016/j.ygyno.2021.03.026 [DOI] [PubMed] [Google Scholar]
- 5.Fagotti A, Ferrandina G, Fanfani F, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol. 2006;13(8):1156–1161. doi: 10.1245/ASO.2006.08.021 [DOI] [PubMed] [Google Scholar]
- 6.Vizzielli G, Costantini B, Tortorella L, et al. Influence of intraperitoneal dissemination assessed by laparoscopy on prognosis of advanced ovarian cancer: an exploratory analysis of a single-institution experience. Ann Surg Oncol. 2014;21(12):3970–3977. doi: 10.1245/s10434-014-3783-6 [DOI] [PubMed] [Google Scholar]
- 7.Francque SM, De Pauw FF, Van den Steen GH, Van Marck EA, Pelckmans PA, Michielsen PP. Biopsy of focal liver lesions: guidelines, comparison of techniques and cost-analysis. Acta Gastroenterol Belg. 2003;66(2):160–165. [PubMed] [Google Scholar]
- 8.Arezzo F, Loizzi V, La Forgia D, et al. The role of ultrasound guided sampling procedures in the diagnosis of pelvic masses: a narrative review of the literature. Diagnostics. 2021;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonomo F, Bussolaro S, de Almeida Fiorillo C, et al. Ultrasound-guided tru-cut biopsy in gynecological and non-gynecological pelvic masses: a single-center experience. J Clin Med. 2022;11:9. doi: 10.3390/jcm11092534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischerova D, Cibula D, Dundr P, et al. Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. Int J Gynecol Cancer. 2008;18(4):833–837. doi: 10.1111/j.1525-1438.2007.01015.x [DOI] [PubMed] [Google Scholar]
- 11.Vlasak P, Bouda J, Kostun J, et al. Diagnostic reliability, accuracy and safety of ultrasound-guided biopsy and ascites puncture in primarily inoperable ovarian tumours. Anticancer Res. 2020;40(6):3527–3534. doi: 10.21873/anticanres.14341 [DOI] [PubMed] [Google Scholar]
- 12.Epstein E, Van Calster B, Timmerman D, Nikman S. Subjective ultrasound assessment, the ADNEX model and ultrasound-guided tru-cut biopsy to differentiate disseminated primary ovarian cancer from metastatic non-ovarian cancer. Ultrasound Obstet Gynecol. 2016;47(1):110–116. doi: 10.1002/uog.14892 [DOI] [PubMed] [Google Scholar]
- 13.Cibula D, Pötter R, Planchamp F, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. 2018;127(3):404–416. doi: 10.1016/j.radonc.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. doi: 10.1093/annonc/mdv484 [DOI] [PubMed] [Google Scholar]
- 15.Razumova Z, Bizzarri N, Kacperczyk-Bartnik J, Pletnev A, Gonzalez Martin A, Persson J. Report from the European Society of Gynaecological Oncology (ESGO) 2020 State-of-The-art virtual meeting. Int J Gynecol Cancer. 2021;31(5):658–669. doi: 10.1136/ijgc-2021-002577 [DOI] [PubMed] [Google Scholar]
- 16.Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. 2019;30(5):672–705. doi: 10.1093/annonc/mdz062 [DOI] [PubMed] [Google Scholar]
- 17.Lin SY, Xiong YH, Yun M, et al. Transvaginal ultrasound-guided core needle biopsy of pelvic masses. J Ultrasound Med. 2018;37(2):453–461. doi: 10.1002/jum.14356 [DOI] [PubMed] [Google Scholar]
- 18.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts ME, Aynardi JT, Chu CS. Uterine leiomyosarcoma: a review of the literature and update on management options. Gynecol Oncol. 2018;151(3):562–572. doi: 10.1016/j.ygyno.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 20.Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58(2):133–147. doi: 10.1016/j.cyto.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 21.Hanane K, Salma B, Khadija B, et al. Peritoneal carcinomatosis, an unusual and only site of metastasis from lung adenocarcinoma. Pan Afr Med J. 2016;23:60. doi: 10.11604/pamj.2016.23.60.8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beniey M. Peritoneal metastases from breast cancer: a scoping review. Cureus. 2019;11(8):e5367. doi: 10.7759/cureus.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asp M, Peber E, Kannisto P, Måsbäck A, Malander S, Wiedmann MKH. Ovarian tumor frozen section, a multidisciplinary affair. Acta Oncol. 2022;61:1–8. doi: 10.1080/0284186X.2021.2009562 [DOI] [PubMed] [Google Scholar]
- 24.Song T, Choi CH, Kim HJ, et al. Accuracy of frozen section diagnosis of borderline ovarian tumours. Gynecol Oncol. 2011;122(1):127–131. doi: 10.1016/j.ygyno.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 25.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seidman JD, Savage J, Krishnan J, Vang R, Kurman RJ. Intratumoral heterogeneity accounts for apparent progression of noninvasive serous tumors to invasive low-grade serous carcinoma: a study of 30 low-grade serous tumors of the ovary in 18 patients with peritoneal carcinomatosis. Int J Gynecol Pathol. 2020;39(1):43–54. doi: 10.1097/PGP.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 27.Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2(4):482–490. doi: 10.1001/jamaoncol.2015.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian ovarian cancer study group. J Clin Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2021;22(12):1721–1731. doi: 10.1016/S1470-2045(21)00531-3 [DOI] [PubMed] [Google Scholar]
- 30.González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962 [DOI] [PubMed] [Google Scholar]
- 31.Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403–2415. doi: 10.1056/NEJMoa1909707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asp M. Advanced ovarian cancer. A multimodal diagnostic approach to predict outcome [Doctoral Thesis (compilation), Department of Clinical Sciences, Lund]. Lund University, Faculty of Medicine; 2022. Available from: https://lucris.lub.lu.se/ws/portalfiles/portal/127566180/Advanced_Ovarian_Cancer.pdf. Accessed May 10, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available from the lead author (Mihaela Asp) upon request.