Abstract
Background:
Papillary thyroid carcinoma (PTC) is more frequently reported in patients with Hashimoto’s thyroiditis (HT), which may be associated with the presence of solid cell nests (SCNs) and focal PTC-like nuclear alterations in the thyroid gland. The point of this consideration was to assess the morphological and immunohistochemical features of SCNs and follicular epithelial changes in Vietnamese patients with HT.
Materials and Methods:
Hematoxylin–eosin and immunohistochemistry were performed on 20 samples of HT patients who underwent thyroidectomy and were diagnosed with Hashimoto’s thyroiditis at Military Medical Hospital 103 from 6/2018 to 6/2019. The expression of five markers (P63, Calcitonin, TTF1, CK19 and HBME-1) in SCNs and follicular epithelial changes were evaluated.
Results:
Ninety per cent of samples had SCNs with an average of 10 SCNs per section. Only type 1 and type 4 SCNs were presented (85% and 55%, respectively) and all SCNs were composed of main cells (p63-positive). Fifteen of the 18 cases having SCNs possessed nuclear features of PTC. C-cell hyperplasia was found in one case with 20 clusters. All SCNs showed strong staining with CK19 and weak staining with HBME-1. Follicular epithelial changes were Hürthle cell metaplasia, PTC-like nuclear alterations, atypical solid nodules, papillary and glomerular-like forms (40%, 100%, 25% and 50%, respectively). Follicular cells of glomerular-like forms (new alteration) especially were positive with CK19 (2+ ~ 3+), HBME-1 (1+) and TTF1, while the components in these follicles were negative with CK19, HBME-1 and TTF1. Among PTC-like nuclear alterations, all the atypical solid nodules related to HT showed markers related to PTC and without SCNs.
Conclusions:
Increasing the number of SCNs, as well as PTC-like nuclear alterations of main cells in SCNs and follicular epithelial changes were co-expressed CK19 and HBME-1. Therefore, the need for HT management should be considered.
Keywords: Hashimoto’s thyroiditis, papillary thyroid carcinoma, solid cell nests
INTRODUCTION
Hashimoto’s thyroiditis (HT), as first portrayed by Dr. Hakaru Hashimoto in 1912, is an autoimmune disease of the thyroid gland. The main histological feature of HT is the infiltration of primary lymphocytes, organised in lymphoid follicles with prominent germinal centres. Other highlights incorporate Hürthle cell change, destruction and atrophy of follicular cells, and interstitial fibrosis.[1-4]
It is reported that there was a relationship between HT and thyroid cancer. In particular, the relative percentage range in the literature was 0%–30%.[5-9] The increased frequency of cancer (especially PTC) related to HT according to the writing recommends whether HT is a risk factor for thyroid carcinoma or not.[9,10] In terms of aetiology, PTC is related to HT because both diseases may be derived from the same populace of p63-positive pluripotent stem cells within the title of solid cell nests (SCNs).[11] In 1907, SCNs were first described by Getzowa, who defined SCNs as small thyroid structures and remnants during embryonic development of the human body. SCNs were also considered small, morphologically inconspicuous aggregates and positive with p63, which were populous in HT.[12]
The follicular epithelial changes that occur in HT have been emphasised since its original description in 1912.[13] Except for classical histological features as the most important criteria to make the diagnosis, another unusual feature of HT which has been mentioned as a potential source of confusion for diagnosis is focal clear nuclear change with some degree of overlapping comparable to that perceived in PTC.[14] The presence of atypical follicular cell changes in HT has been already said within the writing without an in-depth consideration of their highlights and/or conceivable noteworthiness.
We examined 20 specimens of HT. Their blocks were stained by haematoxylin and eosin (H&E) and IHC to assess the characteristics of morphology and immunohistochemistry of the SCNs and the modified follicular cells on the infiltration of the lymphocytic background.
MATERIALS AND METHODS
Materials
All cases accessioned under the diagnosis of HT within the surgical pathology files at the Department of Histopathology, Military Medical Hospital 103 from June 2018 to June 2019 were reviewed. Of a total of 860 cases in which thyroid surgery was done, HT was found incidentally in 20 patients who underwent lobectomy or subtotal thyroidectomy for other reasons, including thyroid neoplasms, and thyroid follicular nodular disease. In comparison to H and E staining slides, we selected carefully one sample from more than two samples of each patient, according to the same criteria such as a typical HT on H and E staining slides and a two-dimensional sample size of 1 × 1 cm. H and E and immunohistochemistry stainings for P63, calcitonin, TTF1, cytokeratin 19 (CK19), and mesothelial cell antibody (HBME1) were performed on 20 samples.
Methods
We checked all the accessible slides of each case to affirm the conclusion of HT, assess its expansion and characterise follicular cell changes. The age and sex of all the patients were recorded. As it appeared the classic pathologic highlights of HT, comprising of atrophic follicles with inexhaustible Hürthle cells without or diminished colloid and extensive lymphocytic infiltrate with germinal center formation. The degree of thyroid inclusion by HT was assessed and classified as central or diffuse, as already portrayed within the writing.[8] Cases where lymphocytic penetrated without the other components of HT were avoided from the consideration.
Immunohistochemical staining of the Leica detection framework was performed on successive segments (3 μm thick) from each documented tissue block. Essential antibodies, sources and weakening are recorded in Table 1.
Table 1.
Five antibodies used in the study
| Antibody | Species | Catalogue N0 | Clone | Antibody Concentration | Source | Company | Country |
|---|---|---|---|---|---|---|---|
| CK 19 | Mouse Anti-human | PA0799 | B170 | 0.07 mg/L | Mono | Leica | UK |
| HBME-1 | Mouse Anti-human | PA0458 | HBME1 | Not applicable | Mono | Leica | UK |
| TTF1 | Mouse Anti-human | PA0364 | SPT24 | 2.6 mg/L | Mono | Leica | UK |
| Calcitonin | Mouse | PA0406 | POLYCLONAL | Not applicable | mono | Leica | UK |
| p63 | Mouse Anti-human | PA0103 | 7JUL | 20 mg/L | Mono | Leica | UK |
According to the manufacture’s instruction, all sections were deparaffinised with xylene and rehydrated through a series of descending graded alcohols. Antigen retrieval was performed in 1 mmol/L EDTA PH 8.0. Sections did not get the antigen retrieval programme with HBME-1. Endogenous peroxidase activity was blocked by using 3% H2O2 for 20 min. They were then incubated with primary antibody for 1 hour at room temperature, followed by a peroxidase-conjugated polymer (Leica, United Kingdom) for 30 min. To reveal the immunostaining, we used the sections which were incubated with the Leica REAL DAB + chromogen for 5 min (Leica, United Kingdom), then counterstained with haematoxylin, followed by dehydration and mounting. Slides were examined using a BX50 optical microscope (Olympus, Japan) by taking photos using iPhone X (Apple Inc, USA).
Appropriate positive controls were used for cytokeratin 19, p63, TTF1, HBME-1, calcitonin. We evaluated the expression CK19, HBME-1 on IHC staining:
1 (+): the whole membrane colour is weak or incomplete.
2 (+): the whole-cell membrane colour is average.
3 (+): the whole-cell membrane colour is strong.
We examined p63 staining in all slides to mark SCNs and compared IHC with H and E specimens to count and identify characterised expression patterns of SCNs. SCNs were classified into four types according to Asioli in 2009.[15] We also assessed the expression of calcitonin, TTF1, CK19, HBME-1 on SCNs, and PTC-like changes in HT.
Ethical considerations
The Ethical Review Committee of Military Medical University, Vietnam approved the protocol of the study. The study was in line with the Declaration of Helsinki. A descriptive study was performed after all participants had been informed fully and agreed to sign written consent.
Statistical analysis
The collected data were processed by the method of medical statistics using SPSS 22.0 software based on biomedical statistics method.
RESULTS
All patients were female, aged from 28 to 69 (53 ± 12) years old. The age group of 46–60 years was the most prevalent (40%) [Figure 1].
Figure 1.
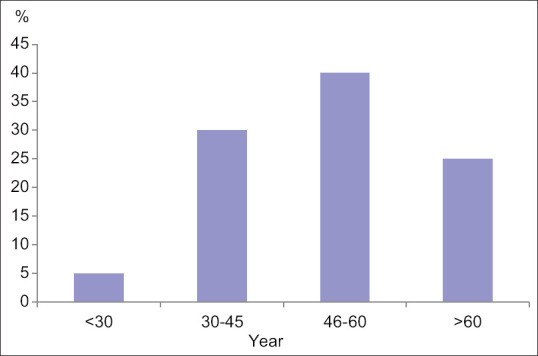
Age distribution of patients
Morphological characteristics of SCNs
A summary of the morphological features of SCNs is provided in Table 2. There were a total of 18 slides (90%) having SCNs. The number of SCNs per section ranging from 0 to 35 (median 10 nests) was divided into three groups: The first group with zero SCNs per section, the second group 1–3 SCNs per section and the third group with over three SCNs per section (10%, 30% and 60%, respectively). Only type 1 and type 4 SCNs were seen in our patients (85% and 55%, respectively).
Table 2.
Morphological characteristics of SCNs
| Characteristics | Number | Ratio |
|---|---|---|
| Number of SCNs | ||
| 0 | 2 | 10% |
| 1–3 | 6 | 30% |
| >3 | 12 | 60% |
| Mean±SD | 10±10.3 | |
| Type | ||
| 1 | 17 | 85% |
| 4 | 11 | 55% |
| PTC-like nuclear changes in SCNs | 15/18 | 83.3% |
Most SCNs were found near or in the lymphoid follicles and there was one case in which SCNs were located between thyroid follicles. The majority of SCNs had changes in the nucleus, including nuclear enlargement, chromatin clearing and nuclear groove (83.3%). Some examples of HT with the morphological feature of SCNs are shown in Figure 2.
Figure 2.
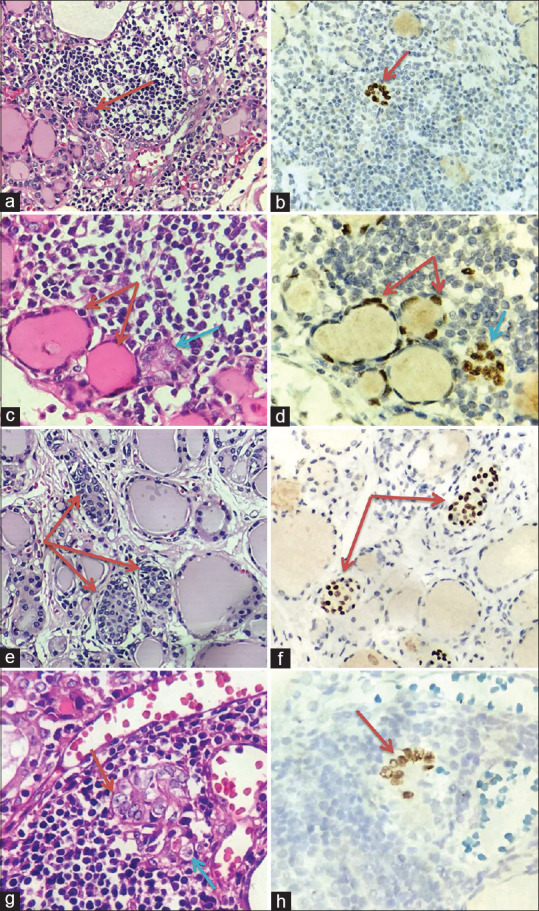
Morphological characteristics of solid cell nests (SCNs). (a) Type 1 of SCNs HT: floret-like shape (Red arrow, Hematoxylin and Eosin (H&E), Original magnification x 200). (b) P63 positivity in type 1 of SCNs (Red arrow, Original magnification x 400). (c) Type 4 of SCNs contain both solid of main cells (Blue arrow) and cystic of follicular epithelium (Red arrow), (H&E, Original magnification x 400). (d) P63 positivity in type 4 of SCNs (Red and blue arrow, Original magnification x 400). (e) Type 1 of SCNs located between thyroid follicles (Red arrow, H&E, Original magnification x 200). (f) P63 positivity in type 1 of SCNs located between thyroid follicles (Red arrow, Original magnification x 400). (g) PTC-like nuclear changes of SCNs: prominent nucleoli and nuclear groove (Red and blue arrow, H&E, Original magnification x 400). (h) P63 positivity in PTC-like nuclear changes of SCNs (Red arrow, Original magnification x 400)
Histopathological characteristics of follicular cell changes
As shown in Table 3, of all cases with HT, there were 8 (40%) cases that showed diffuse involvement and 12 (60%) cases that showed focal involvement of follicular cell changes surrounding lymphoid follicles. When we observed slides on the optical microscope of 20 cases, four changes were reported such as Hürthle cell metaplasia, PTC-like nuclear alterations, papillary structure and glomerular-like structure [Figure 3]. In all cases, PTC-like nuclear alterations accounted for the majority (60%). Compared with the focal lesion, papillary and glomerular-like forms are encountered more frequently in diffuse lesions.
Table 3.
Histopathological characteristics of follicular epithelial changes in Hashimoto’s Thyroiditis
| Characteristics | Location of follicular cell changes | Total | |
|---|---|---|---|
|
| |||
| Diffuse (n=8) | Surrounded lymphoid follicles (n=12) | ||
| Hürthle cell metaplasia | 6 (30%) | 2 (10%) | 8 (40%) |
| PTC-like nuclear alteration | 8 (40%) | 12 (60%) | 20 (100%) |
| Papillary structure | 3 (15%) | 2 (10%) | 5 (25%) |
| Glomerular-like structure | 7 (35%) | 3 (15%) | 10 (50%) |
Figure 3.
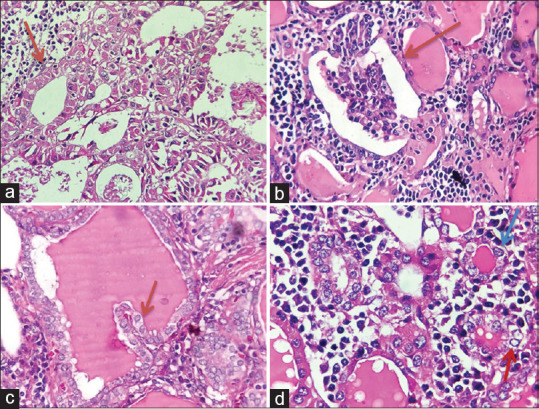
Histopathological characteristics of follicular cell changes. (a) Hürthle cell metaplasia in HT: Epithelial cells have eosinophilic, large cytoplasm (Red arrow), (H&E, Original magnification x 200). (b) Glomerular-like structure: grown from thyroid follicles which were damaged by lymphocytic infiltrations and inflammatory cells of interstitial tissues invade into these follicles forming the component in them similar to glomerulus (Red arrow, H&E, Original magnification x 200). (c) Papillary structure: Papillary with fibrovascular cores (Red arrow, H&E, Original magnification x 400). (d) PTC-like nuclear alterations: Nuclear groove (Blue arrow) and overlapping nuclear (Red arrow), (H&E, Original magnification x 400)
PTC-like nuclear alterations detected in 20 (100%) were composed of two types of changes, including follicular formed by PTC-like nuclear cells and atypical solid nodules. The papillary structure defined as short papillary with fibrovascular cores was detected in 5 cases (25%). Fifty per cent of cases had glomerular-like structures that were grown from thyroid follicles which were damaged by lymphocytic infiltrations and inflammatory cells of interstitial tissues invade into these follicles forming components in them similar to the glomerulus. Characteristics of glomerular-like structures are enlarged follicles, atrophy of follicular cells which can also be PTC-like nuclear features. The component of glomeruli involves lymphocytes, macrophages and fibrocytes of interstitial tissue.
The immunohistochemical characteristics in SCNs and follicular cell changes
The immunohistochemical results for HBME-1, CK19, TTF1, p63, and Calcitonin in the SCNs are summarised in Table 4.
Table 4.
Characteristics of the expression of immune markers in SCNs
| Marker | Positive (Number/Ratio) | Negative (Number/Ratio) | Total | ||
|---|---|---|---|---|---|
| P63 | 18 (100%) | 0 | 18 | ||
| Calcitonin | 0 | 18 (100%) | 18 | ||
| TTF1 | 18 (100%) | 0 | 18 | ||
|
| |||||
| (1+) | (2+) | (3+) | |||
|
| |||||
| CK19 | 0 | 0 | 18 (100%) | 0 | 18 |
| HBME-1 | 16 (88.9%) | 1 (5.6%) | 0 | 1 (5.6%) | 18 |
When analysing the results of immunohistochemistry with five markers, p63-positive expression in SCNs was detected in 18 cases, while SCNs were negative for calcitonin in all cases [Figure 4]. In 18 cases which had SCNs, all SCNs were positive with TTF1, local and strongly positive with CK19 and local and weakly positive with HBME-1 expression in 16 cases (88.9%). Co-expression of two markers (CK19 and HBME-1) was observed in 16 (94.4%) of 18 cases having SCNs.
Figure 4.
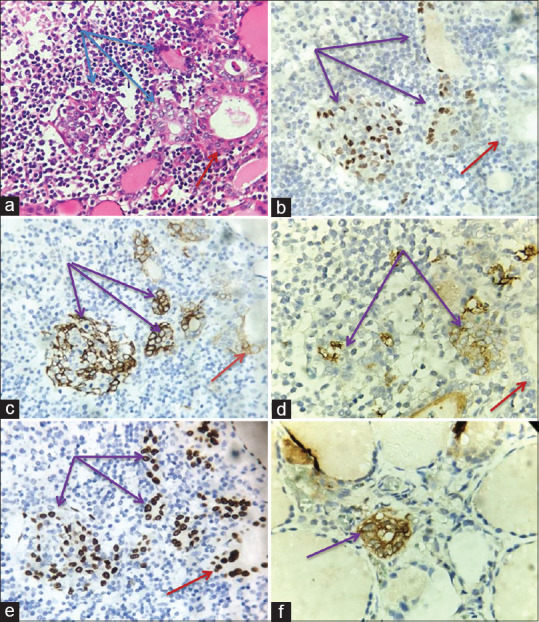
The immunohistochemical characteristics in SCNs. (a) SCNs (Blue arrow) and PTC-like nuclear alterations (Red arrow) are located next to the lymph follicles in HT (H&E, Original magnification x 200). (b)P63 positivity in SCNs (Purple arrow) and negativity in PTC-like nuclear alterations (Red arrow) (Original magnification x 200). (c) CK19 positivity (3+) in SCNs (Purple arrow) and positivity (1+) in PTC-like nuclear alterations (Red arrow), (Immunoperoxidase, Original magnification x 200). (d) HBME-1 positivity (2+) in SCNs (Purple arrow) and negativity in PTC-like nuclear alterations (Red arrow), (Immunoperoxidase, Original magnification x 200). (e) TTF1 positivity in SCNs (Purple arrow) and PTC-like nuclear alterations (Red arrow), (Immunoperoxidase, Original magnification x 200). (f) Calcitonin positivity in SCNs (Purple arrow, Immunoperoxidase, Original magnification x 400)
The staining intensity of the above five markers in follicular cell changes was summarised in Table 5. All follicular cell changes were negative for p63, calcitonin, but positive for TTF1. CK19 was frequently stained weakly or moderately in Hürthle cell metaplasia and papillary structures (50% and 60%). By contrast, frequencies of strongly CK19-positive staining were higher for PTC-like nuclear alterations and glomerular-like structures (95% and 30%). Among these PTC-like nuclear alterations, all atypical solid nodules expressed intensively with CK19. The recolouring intensity of HBME-1 was frequently negative or weakly positive in 50% (4/8) Hürthle cell metaplasia, 95% (19/20) PTC-like nuclear alterations, 80% (4/5) papillary structures and 100% (10/10) glomerular-like structures, in comparison 50% (4/8) Hürthle cell metaplasia, 5% (1/20) PTC-like nuclear alterations, 20% (1/5) papillary structures were moderately positive with HBME-1. Co-expression of two markers (CK19 and HBME-1) was seen in all lesions.
Table 5.
Characteristics of the expression of five markers in follicular epithelial changes
| Marker | Positive (Number/Ratio) | Negative (Number/Ratio) | ||
|---|---|---|---|---|
| P63 | 0 | 20 (100%) | ||
| Calcitonin | 0 | 20 (100%) | ||
| TTF1 | 20 (100%) | 0 | ||
|
| ||||
| CK19 | (1+) | (2+) | (3+) | 0 |
|
| ||||
| Hürthle cell metaplasia (8) | 4 (50%) | 4 (50%) | 0 | 0 |
| PTC-like nuclear alteration (20) | 0 | 1 (5%) | 19 (95%) | 0 |
| Papillary structure (5) | 3 (60%) | 2 (40%) | 0 | 0 |
| Glomerular-like structure (10) | 0 | 5 (50%) | 3 (30%) | 2 (20%) |
|
| ||||
| HBME-1 | (1+) | (2+) | (3+) | |
|
| ||||
| Hürthle cell metaplasia (8) | 4 (50%) | 4 (50%) | 0 | 0 |
| PTC-like nuclear alteration (20) | 19 (95%) | 1 (5%) | 0 | 0 |
| Papillary structure (5) | 3 (60%) | 1 (20%) | 0 | 1 (20%) |
| Glomerular-like structure (10) | 8 (80%) | 0 | 0 | 2 (20%) |
In the glomerular-like structures, only their follicular cells were positive with CK19 and HBME-1, TTF1 and the component of glomeruli were negative for CK19, HBME-1 and TTF1. Heterogeneous expression of CK19 was reported frequently in each lesion [Figure 5].
Figure 5.
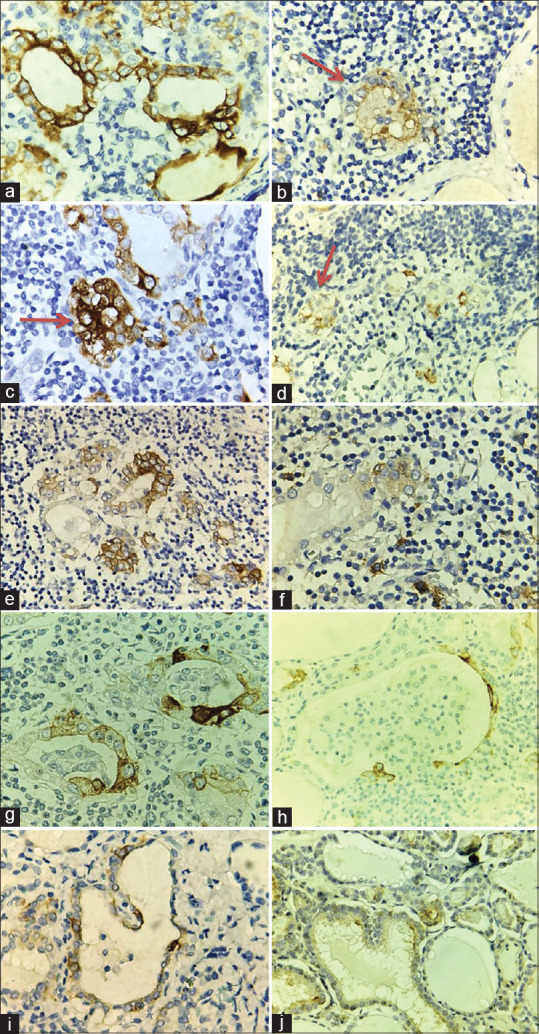
The immunohistochemical characteristics follicular cell changes. (a) CK19 positivity (3+) in Follicles formed PTC-like nuclear cells (Immunoperoxidase, Original magnification x 400). (b) HBME-1 positivity (2+) in Follicles formed PTC-like nuclear cells (Red arrow, Immunoperoxidase, Original magnification x 400). (c) CK19 positivity (3+) in an atypical solid nodule (Red arrow, Immunoperoxidase, Original magnification x 400). (d) HBME-1 positivity (2+) in an atypical solid nodule (Red arrow, Immunoperoxidase, Original magnification x 400). (e) CK19 positivity (3+) in Hürthle cell metaplasia (Immunoperoxidase, Original magnification x 400. (f) HBME-1 positivity (2+) in Hürthle cell metaplasia (Immunoperoxidase, Original magnification x 400). (g) CK19 positivity (3+) in the Glomerular-like structure (Immunoperoxidase, Original magnification x 400. (h) HBME-1 positivity (2+) in the Glomerular-like structure (Immunoperoxidase, Original magnification x 400). (i) CK19 positivity (2+) in papillary strucrue, (Immunoperoxidase, Original magnification x 400). (j) HBME-1 positivity (2+) in papillary strucrue (Immunoperoxidase, Original magnification x 400)
DISCUSSION
Morphological and immunohistochemical characteristics of SCNs
SCNs, identified as small nests of cells, are similar to squamous cells or transitional epithelium. Such nests surrounded by stroma usually differ clearly from the thyroid follicles. Multiple foci (2–8 per section) of SCNs are common.[16] SCNs are found incidentally within the ordinary thyroid gland and may be related to the neoplastic or non-neoplastic injuries of the thyroid gland, the incidence of SCNs in adults is from 3% of routinely examined thyroids to 60% of the glands subjected to serial blocking.[17] SCNs have been reported in 9.5% to 41% of patients with multinodular thyroid diseases.[18] The association between focal lymphocytic thyroiditis (FLT) and SCNs was reported in 1985 by Prod’hom when the author examined the thyroid tissue of 500 routine autopsies. SCNs were found in 56 (11.2%) of all cases and 18 (30.5%) of 59 FLT cases.[19] In our study, SCNs were found in 90% of patients with HT. This incidence was much higher than that of SCNs in the normal thyroid gland. Our result with an average of 10 (0–35) SCNs per section was similar to the figure of other authors when finding more than 10 SCNs per slide on microscopic examinations.[15]
According to Asioli (2009), SCNs are classified into four types. In our study, 20 cases of HT had only type 1 and type 4, accounting for 85% and 55%. Type 1 (floret-like) is composed of main cells, characterised by round to oval or elongated cells with scant cytoplasm, centrally located oval to fusiform nuclei and occasional nuclear grooves [Figure 2a]. Type 4 SCNs (mixed follicles) have a follicular appearance and contain both follicular cells and small cells [Figure 2c]. While type 2 was extremely recognised in H and E staining slides and type 3 did not present in any sections.
SCNs are composed of two cell populations – predominant main cells (positive for p63) and a minority of C-cells (positive for calcitonin). Main cells are defined as polygonal/elongated to round to spindle cells with squamoid features (but no intercellular bridges). Their nuclei are centrally located, oval to fusiform, with finely granular chromatin, uneven nuclear membrane and occasional nuclear grooves. In contrast, C-cells are a minor population of cells with clear cytoplasm and centrally located, small compact nuclei. In our study on 20 patients with HT, all the cells in SCNs were main cells because of p63 expression [Figure 2b, d] Besides, we found that there was only a patient who had 20 nests positive with calcitonin [Figure 4f]. This patient has been considered to be C-cell hyperplasia because C-cells in the normal thyroid gland are rather rare with about 0.1% of the total thyroid follicular cells population. C-cells belong to thyroid cysts and are located between the basal membrane and the follicular epithelium. Thus, the appearance of C-cells in positions different from their normal site could be considered C-cell hyperplasia.[18]
SCNs associated with HT may show some atypical nuclear features including prominent nuclear grooves, enlarged overlapping nuclei and nuclear clearing. These features are sometimes mistaken for papillary thyroid microcarcinomas especially when the SCNs are numerous.[9] The nuclei of SCNs featured some worrisome aspects like chromatin clearing and nuclear membrane irregularity with occasional grooves and cup-shaped nuclei. These features may explain the misleading diagnosis of a papillary microcarcinoma performed by the first pathologist. The morphological details described previously were consistent with SCNs in a background of HT.[20] In the study by Bellevivine (2012), SCNs in a background of HT were described as typical nuclear features of PTC, which became a trap for pathologists to distinguish them from micropapillary carcinoma on H and E staining slides. The author found that these foci were positive for p63 and confirmed these were SCNs and not micropapillary carcinoma.[20] In our study, 88.9% of SCNs had atypical nuclei mimicking PTC nuclear features. To further distinguish these SCNs from papillary microcarcinoma, p63 staining was performed and malignant characteristics were excluded like invasion and reactive stroma.
The immunohistochemical characteristics of SCNs were portrayed in detail by Reis-Filho in 2003. Most cells of SCNs showing a basal or stem cell phenotype are unequivocally brightened by p63, whereas C-cells and all other thyroid structures were consistently negative. Additionally, most cells communicated carcinoembryonic antigen and all cytokeratins but for cytokeratin 20 and needed TTF-1, thyroglobulin and calcitonin. To differentiate, C-cells of SCNs show features of parafollicular differentiation (positive for calcitonin, AE1/AE3, and cytokeratin 7 and focal immunoreactivity for TTF-1).[21] All cells of SCNs were positive for p63 and strongly positive for CK19 and TTF1 [Figure 4c and e]. Moreover, there was co-expression of CK19 and HBME-1 in the main cells of SCNs (94.4%). According to Scognamiglio (2006), PTC can be distinguished from encapsulated follicular adenoma by papillary nuclear features, co-expression of CK19 and HBME-1, which has the specificity of 100% for the diagnosis of PTC.[22] As such immunophenotype of PTC expression, SCNs in HT could be a potential malignant.
Morphological and immunohistochemical characteristics of follicular cell changes
HT was moreover known as constant lymphocytic or autoimmune thyroiditis. The coexisting of HT with PTC has been taken note for decades. In any case, there is still no clear proof of whether HT was a chance that can advance the improvement of PTC. Histologically, in expansion to an eosinophilic variation of follicular cells which is ordinarily related with thyroid follicular atrophy or damage, we seem moreover watch follicular cells dysplasia (FED) within the shape of scattered microfollicles missing follicular colloid or irregularly shaped follicles in HT tissues, particularly within the zones of thick encompassing lymphocytic infiltration. These follicles were as often as possible lined by Hürthle cells or cells with softly recoloured cytoplasm. A few follicular cells were histologically comparative to PTC with extended, clear nuclear and grooves. Such injuries were ordinarily little without joining into a film. In a few cases, the expansion was more prominent, but still on the foundation of HT illnesses. In the study of 20 cases of HT, we found that follicular changes that were seen in all cases surrounding FLT or spread throughout the thyroid gland with the interstitial lymphoid infiltrates. The follicular cells having the PTC-like nuclear were seen in all 20 cases; Hürthle cell metaplasia, papillary and glomerular-like structures accounted for 40%, 25% and 50% respectively [Table 3]. Although Hürthle cell metaplasia and PTC-like nuclear alterations with variant names like follicular epithelial dysplasia, clear nuclear changes in HT have been mentioned in many types of research, papillary and glomerular-like structures were not noted in any studies [Figure 3].
A study was conducted on 12 patients with HT, who had clear nuclear changes like PTC of thyroid follicular cells. The monitoring of 12 patients in the period from 1.5 to 9 years showed that there was no evidence of recurrence and metastases. In this study, Arkadi (1995) declared that changes in thyroid follicular epithelium in HT, including PTC-like nuclear alterations, were the response of the thyroid follicular cells to the autoimmune process with potential malignancy. Thus, these changes should be examined in context with the rest of the thyroid gland to avoid overdiagnosis of cancer.[14]
In the research of Heng Ma (2014) performed on 18 patients with HT, there wasscattered CK19 expression in follicular epithelial dysplasia, in particular, 5 cases (27.8%) were moderate to strongly positive and 13 cases (72,7%) were weakly positive. In Hürthle cells, the expression of CK19 protein was focal and weak. Moreover, most CK19-positive ones were often located near lymphoma follicles and showed an extremely eosinophilic change. While there was only 11.1% of follicular epithelial dysplasia weakly positive to HBME-1 and negative to HBME-1 in Hürth cell metaplasia.[4]
Another research performed on 23 HT patients with focal or diffuse Hürthle cell change and PTC-like nuclear alterations of thyroid follicular cells showed that focal expression of cytokeratin 19 (CK19) and HBME-1 was 43% and 26%, respectively and limited only in PTC-like nuclear alterations. There were four cases with PTC foci identified by strong and diffuse expression of markers specific to PTC, which promoted retrospective studies and examination of the H&E-stained slides to fully evaluate atypical nuclear. Thus, such omitting lesions would be avoided. This study also showed that immunophenotypic changes of focal PTC-like nuclear alterations suggest the possibility of premalignant transformation in many cases of HT.[23]
HT suggests the possibility of early, focal premalignant transformation in some cases.[22] In our study, the thyroid follicular epithelial changes were negative for p63, calcitonin, and positive for TTF1. These follicles with PTC-type nuclear changes were moderate to strong positive for CK19 (100%), weak to moderate positive for HBME-1 (100%) [Figure 5a and b]. Five cases (25%), that had atypical solid nodules were strongly positive with CK19, moderately positive with HBME-1 [Figure 5c and d], positive with TTF1 but negative with p63 and calcitonin. It is true that the follicular epithelium or cell nests with PTC-like nuclear features exhibited the molecular markers of PTC but did not show that the molecular markers of SCNs are potential malignant transformations into PTC.
CONCLUSIONS
An increasing number of SCNs were detected in HT, mainly type 1 and type 4. SCNs located next to and in lymphoid follicles were mostly composed of main cells having PTC-like nuclear change, p63 and TTF1 positive expression, CK19 strongly and HBME-1 weakly positive staining.
In HT, there are many changes in follicular cells such as PTC-like nuclear alterations, Hürthle cell metaplasia, micropapillary and glomerular-like structure. All changes were negative with p63, calcitonin, and the majority of these changes were co-expressive with CK19 and HBME-1. Among these transformations, the micropapillary and glomerular-like structure had not been described in the previous literature. Moreover, another alteration being seen in HT was cell nests which are similar to SCNs in morphology. They did not show p63 and calcitonin but were strongly positive with CK19 and moderately positive with HBME-1.
The co-expressed CK19 and HBME-1 of the main cells with PTC-like nuclear changes in SCNs and follicular epithelial changes. The increased number of SCNs and histopathological and immunophenotypic changes of thyroid follicular epithelial cells on HT are not certain enough to conclude the cancerous progression in the future. However, it should be considered a high risk factor for the clinician to manage Hashimoto patients.
Authors contribution
Tran Ngoc Dung; Nguyen Khac Tuyen: Conceptualization, Project Administration, Supervision, Validation.
Nguyen Thuy Linh: Conceptualization, Methodology, Writing – Review & Editing; Corresponding; Last endorsement of the adaptation to be distributed
Nguyen Manh Hung, Lewis A. Hassell: Data Curation, Formal Analysis, Investigation.
Truong Dinh Tien, Pham Van Thinh, Nguyen Khanh Van, Lewis A. Hassell: interpretation of data, Methodology, writing – Original Draft Preparation.
Ethical considerations
The study was approved by the Ethical Review Committee of Vietnam Military Medical University. The study was in line with the Declaration of Helsinki. Written informed consent has been signed by all participants after full explanation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank all the staff in the Department of Histopathology, Military Medical Hospital 103 for collecting the samples.
REFERENCES
- 1.Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, Iwama S. Hashimoto's thyroiditis:Celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid. 2013;23:142–50. doi: 10.1089/thy.2012.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livolsi VA. The pathology of autoimmune thyroid disease:A review. Thyroid. 1994;4:333–9. doi: 10.1089/thy.1994.4.333. [DOI] [PubMed] [Google Scholar]
- 3.Paunovic I, Isic T, Havelka M, Tatic S, Cvejic D, Savin S. Combined immunohistochemistry for thyroid peroxidase, galectin-3, CK19 and HBME-1 in differential diagnosis of thyroid tumors. APMIS. 2012;120:368–79. doi: 10.1111/j.1600-0463.2011.02842.x. [DOI] [PubMed] [Google Scholar]
- 4.Ma H, Yan J, Zhang C, Qin S, Qin L, Liu L, et al. Expression of papillary thyroid carcinoma-associated molecular markers and their significance in follicular epithelial dysplasia with papillary thyroid carcinoma-like nuclear alterations in Hashimoto's thyroiditis. Int J Clin Exp Pathol. 2014;7:7999–8007. [PMC free article] [PubMed] [Google Scholar]
- 5.Segal K, Ben-Bassat M, Avraham A, Har-El G, Sidi J. Hashimoto's thyroiditis and carcinoma of the thyroid gland. Int Surg. 1985;70:205–9. [PubMed] [Google Scholar]
- 6.Eisenberg BL, Hensley SD. Thyroid cancer with coexistent Hashimoto's thyroiditis:Clinical assessment and management. Arch Surg. 1989;124:1045–7. doi: 10.1001/archsurg.1989.01410090055012. [DOI] [PubMed] [Google Scholar]
- 7.Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, Lawrence AM, et al. The incidence of thyroid carcinoma in Hashimoto's thyroiditis. Am Surg. 1987;53:442–5. [PubMed] [Google Scholar]
- 8.Ott RA, Calandra DB, McCall A, Shah KH, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in patients with Hashimoto's thyroiditis and solitary cold nodules. Surgery. 1985;98:1202–6. [PubMed] [Google Scholar]
- 9.Sclafani AP, Valdes M, Cho H. Hashimoto's thyroiditis and carcinoma of the thyroid:Optimal management. Laryngoscope. 1993;103:845–9. doi: 10.1288/00005537-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Okayasu I, Fujiwara M, Hara Y, Tanaka Y, Rose NR. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–8. doi: 10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Preto A, Reis-Filho JS, Ricardo S, Soares P. P63 expression in papillary and anaplastic carcinomas of the thyroid gland:Lack of an oncogenetic role in tumorigenesis and progression. Pathol Res Pract. 2002;198:449–54. doi: 10.1078/0344-0338-00281. [DOI] [PubMed] [Google Scholar]
- 12.Burstein DE, Chandandeep N, Beverly YW, Pamela U. Immunohistochemical detection of p53 homolog p63 in solid cell nests, papillary thyroid carcinoma, and hashimoto's thyroiditis:A stem cell hypothesis of papillary carcinoma oncogenesis. Hum Pathol. 2004;36:590–1. doi: 10.1016/j.humpath.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto H. Zur kenntniss der lymphomatosen veranderung der Schilddruse (Struma lymphomatosa) Arch Klin Chir. 1912;97:219–48. [Google Scholar]
- 14.Berho M, Suster S. Clear nuclear changes in Hashimoto's thyroiditis. A clinicopathologic study of 12 cases. Ann Clin Lab Sci. 1995;25:513–21. [PubMed] [Google Scholar]
- 15.Asioli S, Erickson LA, Lloyd RV. Solid cell nests in Hashimoto's thyroiditis sharing features with papillary thyroid microcarcinoma. Endocr Pathol. 2009;20:197–203. doi: 10.1007/s12022-009-9095-x. [DOI] [PubMed] [Google Scholar]
- 16.Manzoni M, Roversi G, Di Bella C, Pincelli AI, Cimino V, Perotti M, et al. Solid cell nests of the thyroid gland:Morphological, immunohistochemical and genetic features. Histopathology. 2016;68:866–74. doi: 10.1111/his.12858. [DOI] [PubMed] [Google Scholar]
- 17.Harach HR. Solid cell nests of the human thyroid in early stages of postnatal life. Cells Tissues Organs. 1986;127:262–4. doi: 10.1159/000146296. [DOI] [PubMed] [Google Scholar]
- 18.Autelitano F, Santeusanio G, Tondo UD, Costantino AM, Renda F, Autelitano M. Immunohistochemical study of solid cell nests of the thyroid gland found from an autopsy study. Cancer. 1987;59:477–83. doi: 10.1002/1097-0142(19870201)59:3<477::aid-cncr2820590321>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Prod’hom G, Hedinger C. Relationship between solid cell nests and focal lymphocytic thyroiditis. Ann Pathol. 1985;5:265–70. [PubMed] [Google Scholar]
- 20.Bellevicine C, Ippolito S, Arpaia D, Ciancia G, Pettinato G, Troncone G, et al. Ultimobranchial body remnants (solid cell nests) as a pitfall in thyroid pathology. J Clin Endocrinol Metab. 2012;97:2209–10. doi: 10.1210/jc.2012-1034. [DOI] [PubMed] [Google Scholar]
- 21.Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simoes M. p63 expression in solid cell nests of the thyroid:Further evidence for a stem cell origin. Mod Pathol. 2003;16:43–8. doi: 10.1097/01.MP.0000047306.72278.39. [DOI] [PubMed] [Google Scholar]
- 22.Scognamiglio T, Hyjek E, Kao J, Chen YT. Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. Am J Clin Pathol. 2006;126:700–8. doi: 10.1309/044V-86JN-2W3C-N5YB. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami Y, Michigishi T, Nonomura A, Nakamura S, Ishizaki T. Pathology of chronic thyroiditis:A new clinically relevant classification. Pathol Annu. 1994;29:135–58. [PubMed] [Google Scholar]


