ABSTRACT
BACKGROUND:
The objective of the study was to investigate risk factors affecting mortality rates in patients with Fournier’s gangrene (FG) and develop methods to increase the survival rate.
METHODS:
We collected data of 73 patients treated for FG between February 2012 and June 2021 at Istanbul Professor Doctor Cemil Taşçıoğlu City Hospital General Surgery Clinic. The data of living patients (Group 1, n=56) and deceased patients (Group 2, n=17) were analyzed separately. Demographic data of patients were sex, age, infection rate, Uludag FG severity index (UFGSI) scores and FG severity index (FGSI) scores, urea serum levels, the source of infection, the presence of diabetes, obesity, the presence of diversion stoma, duration of vacuum-assisted closure treatment in days, hospitalization time in days, intensive care period in days, and isolated bacterial species.
RESULTS:
The mortality rate was 23%. A significant difference in age and dissemination score of the infection was found between the two groups. According to UFGSI and FGSI scores, the scores of the two groups of patients were significantly higher. The UFGSI had 100% sensitivity and 68% sensitivity. FGSI had 82% sensitivity and 58% specificity. The cutoff values for UFGSI and FGSI were 8 and 6, respectively.
CONCLUSION:
Age and dissemination scores of diseases were important factors that cause mortality in patients with FG. However, an accurate scoring system is important in predicting patients to be treated in the intensive care unit (ICU). Patients with a UFGSI score above 8 face a higher risk of death and should be treated in the ICU.
Keywords: Fournier’s gangrene, Fournier’s gangrene severity index, Uludag Fournier’s gangrene severity index
INTRODUCTION
Fournier’s gangrene (FG) is the fast-developing necrotizing fasciitis of the perineum in the form of polymicrobial infection, which occurs due to gastrointestinal or urethral mucosal prolapse infections. The symptom of FG starts abruptly with severe pain and spreads rapidly from the fascia of the anterior abdominal wall to muscles of the gluteal and femoral regions. FG was first described in 1883 by the French dermatologist and venereologist Jean Alfred Fournier. Due to the complications of FG, it is vital to detect its symptoms early. Furthermore, the symptoms of patients should not be overlooked. If the diagnosis and treatment of FG delay, the patient’s prognosis may be worse. The visible symptoms of patients with FG may vary depending on the time they come to the hospital. In the early period, the symptom begins with localized skin hardening, redness, and swelling in the pelvic region. More prominent dermal manifestations occur at later stages, which include sepsis and a systemic inflammatory response syndrome. Other common lesions include deep local erythema, hyperemia, itching, fever, scrotal swelling, and non-specific abdominal pain. The disease is often overlooked since the definitive diagnosis of minimal skin lesions in the early stage of FG is difficult. However, some symptoms such as cyanosis, malodorous flow, repulsive fecaloid odor, and necrosis may appear because of the rapid and aggressive nature of the disease.[1,2] Black necrosis of the skin is a clear sign of gangrene development. The decrease or even disappearance of pain with the development of gangrene is a specific symptom.[3]
The incidence of FG increases after 50 years of age.[3] However, FG is closely related to low socioeconomic status, and it is more prevalent in low-income societies.[1] In terms of etiology, the infection is mostly associated with colorectal origin (30–50%) followed by the urological (20–40%) and dermal (20%) origins.[4] Moreover, it is frequently associated with systemic diseases, chronic alcoholism, and malignancies. Diabetes mellitus (DM) has been detected in 20–70% of FG patients. In the second place, chronic alcoholism has been observed in 25–50% of FG patients.[4] A commonly associated risk facing FG patients is the deterioration of immune resistance due to a decrease in cellular immunity.[1] Successful therapy depends on the extent of rapid diagnosis. The treatment approach comprises hemodynamic stabilization, appropriate parenteral antibiotics, and urgently aggressive surgical debridement. Early triple combined with therapy and aggressive surgical debridement are recommended.
Despite the application of this triple combined treatment, mortality rates are still high among patients suffering from FG, 3–45%,[5] despite the advancements in understanding the etiology and the pathophysiology of FG. Mortality rates are increase due to diabetes, alcoholism, acquired immunodeficiency, trauma, genitourinary infections, and immunosuppression.[6] Most disease-related causes of death include sepsis, renal failure, coagulopathy, coma caused by diabetic ketoacidosis, and multiple organ failure. Advanced age and the dissemination score of infection are basic prognostic factors that negatively affect the survival of the patient. Delayed treatment, septic shock on hospital admission, positive blood culture, increased urea level, an anorectal infection source, diabetes, and immunosuppressive disorders are other negative factors mentioned in many studies.
For this reason, the objective of the study was to investigate the factors affecting mortality in FG patients and develop methods to increase FG patient survival rates. We hypothesized that mortality expectancy increased in patients with high FG severity index (FGSI) and Uludağ FGSI (UFGSI) scores. In addition, we hypothesized that mortality expectancy increased in patients with prolonged intensive care period (ICP) and comorbidities apart from DM and obesity.
MATERIALS AND METHODS
Study Population
The study includes a total of 73 patients who have been treated for FG for over 30 years. Included in the study were one patient who did not receive VAC treatment and 72 patients who received VAC treatment after aggressive surgical debridement. The debridement area of one patient not receiving VAC treatment was not large and could be primarily closed 5 days after the debridement. Excluded from the study are the patients hospitalised with a preliminary diagnosis of FG but did not have fascia, subcutaneous and skin necrosis during debridement.
Treatment Methodology and Study Parameters
Ethical approval was obtained from Professor Dr. Cemil Taşçıoğlu City Hospital Ethics Board, İstanbul, Türkiye. Information of 73 patients treated between February 2012 and June 2021 for FG was recorded in Taşçıoğlu City Hospital General Surgery Clinic, Istanbul. The survivors (Group 1, n=56) were analyzed separately from the deceased (Group 2, n=17). Demographic data analyzed are sex, age, the extent of infection, UFGSI and FGSI scores, serum urea levels, the source of the infection, the presence of DM, obesity, and other comorbidities, the presence of diversion stoma, duration of VAC, treatment duration, hospital (inpatient) bed-days, ICP bed–days, and isolated bacterial species.
Some scoring systems have been used with a moderate level of success. Although no reliable tool is available for estimating FG severity. A reliable scoring system should contain clear and effective patient data, which should identify high morbidity and mortality rates.[7] Hahn et al.[8] discussed that the FGSI offered the accurate physiological and chronic health assessment score relating to the FG prognosis. They showed that the FGSI score can be predicted with a 75% accuracy for mortality and 78% for survival. The FGSI score draws attention in the literature. It is a valid and effective score to determine FG results. For this reason, ıt is generally used in many studies. Yilmazlar et al.[9] developed a new scoring system by combining FGSI with age and dissemination score. The most important feature of this scoring system, called the UFGSI score, is the grading of the disease spread.
Surgical debridement was performed extensively until viable tissues were thoroughly perfused. A series of re-exploration were performed every 24–48 h for required debridement. Aggressive debridement aims to remove all necrotic areas, stop the spread of infection, and reduce systemic toxicity.[1] Fecal diversion is necessary to avoid contamination of the debridement site with feces. The urinary contamination of the debridement area is prevented by applying a catheter to the bladder. Application of this procedure is recommended if there are sphincter involvement or large wounds in the perineum. Although no consensus is available for colostomy. Thus, we opened a colostomy in the second debridement or in the next session to evaluate the sphincters more thoroughly. Meanwhile, the inflammation decreased considerably in patients who received colostomy operations.
The common result is extensive tissue defects due to aggressive surgical debridement. Thus, wound care in FG is the most important part of the treatment. In the past two decades, the VAC procedure has contributed significantly to the treatment of this disease, providing minimal skin defects and accelerating wound healing.[10]
VAC treatment was conducted for all our patients except one after completing surgical debridement procedures. VAC exchange transactions were repeated in 3 to 4 day intervals. The final stage of management is to close the large wound after the development of the healthy granulation tissue following the VAC treatment. The closure of the large wound is possible using delayed primary sutures, rotation flap, and V-Y local advancement flaps. The closure with split-thickness skin grafts is commonly performed and the preferred method in case of large wounds. We cured all the patients in Group 1 using four different methods, according to the largeness of the wound (Table 1).
Table 1.
Characteristics of the groups and related factors according to mortality rate
| Group 1(No mortality) | Group 2 (Exitus) | p | |
|---|---|---|---|
|
| |||
| n (%) | n (%) | ||
| Sex | |||
| Female | 18 (32.1) | 13 (76.5) | 0.001 |
| Male | 38 (67.9) | 4 (23.5) | |
| Infection source | |||
| Urogenital | 25 (44.6) | 11 (64.7) | 0.120 |
| Anorectal | 31 (55.4) | 6 (35.3) | |
| Diabetes Mellitus | |||
| No | 14 (25.0) | 3 (17.6) | 0.394 |
| Yes | 42 (75.0) | 14 (82.4) | |
| Comorbidity | |||
| No | 27 (48.2) | 0 (0.0) | 0.000 |
| Yes | 29 (51.8) | 17 (100) | |
| Obesity | |||
| No | 40 (71.4) | 9 (52.9) | 0.131 |
| Yes | 16 (28.6) | 8 (47.1) | |
| Isolated bacteria type | |||
| None | 28 (50.0) | 5 (29.4) | 0.291 |
| E.Coli | 19 (33.9) | 9 (52.9) | |
| Others | 9 (16.1) | 3 (17.6) | |
| Definitive closure: None | 1 (1.8) | 14 (82.3) | |
| Primary closure | 15 (26.8) | 1 (5.8) | 0.00 |
| Split thickness skin graft19 (33.9) | 2 (11.9) | ||
| V-Y flap | 16 (28.6) | 0 (0) | |
| Rotation flap | 5 (8.9) | 0 (0) | |
| Stoma | |||
| No | 48 (85.7) | 15 (88.2) | 0.576 |
| Yes 8 (14.3) | 2 (11.8) | ||
Statistical Analysis
The results were evaluated using a t-test for parametric values. In addition, the results were evaluated using the Mann–Whitney U-test, Chi-square, receiver operating characteristic (ROC) tests, and regression analysis. The value considered statistically significant is level of p<0.05. The statistical analyses were carried out using SPSS software (version 23.0, IBM Corporation, Armonk, NY, USA). The Shapiro–Wilk, detrended plots, skewness/kurtosis, and coefficient of variance tests were used for normal distribution. Non-parametric tests were used because the selected groups were not in a normal distribution. Tables 1–3 showed the detailed descriptive statistics.
Table 3.
The distribution and statistical significance of the scores between groups according to FGSI and UFGSI scoring systems
| Group 1 (No mortality) | Group 2 (Exitus) | p | ||
|---|---|---|---|---|
| Temparature Score | 0 | 51 | 11 | |
| 1 | 4 | 6 | 0.012 | |
| 3 | 1 | 0 | ||
| Heart Rate Score | 0 | 51 | 11 | 0.012 |
| 2 | 5 | 6 | ||
| Respiratory Rate Score | 0 | 52 | 14 | 0.200 |
| 1 | 4 | 3 | ||
| Serum Potassium Score | 0 | 21 | 4 | |
| 1 | 33 | 10 | 0.131 | |
| 2 | 2 | 2 | ||
| 4 | 0 | 1 | ||
| Serum Sodium Score | 0 | 47 | 15 | 0.501 |
| 2 | 9 | 2 | ||
| Serum Creatinine Score | 0 | 35 | 7 | 0.365 |
| 2 | 17 | 8 | ||
| 3 | 3 | 2 | ||
| 4 | 1 | 0 | ||
| Haematocrit Score | 0 | 45 | 11 | |
| 1 | 4 | 0 | 0.066 | |
| 2 | 7 | 6 | ||
| White Blood Score | 0 | 16 | 3 | |
| 1 | 13 | 5 | 0.659 | |
| 2 | 26 | 8 | ||
| 4 | 1 | 1 | ||
| Serum Bicarbonate Score | 0 | 39 | 3 | 0.003 |
| 1 | 2 | 1 | ||
| 2 | 8 | 9 | ||
| 3 | 5 | 2 | ||
| 4 | 2 | 2 | ||
| Dissemination Score | ||||
| Limited to urogenital or anorectal region | 1 | 30 | 4 | 0.011 |
| Limited in the pelvis | 2 | 18 | 5 | |
| Passing out pelvis | 6 | 8 | 8 | |
| Age Score | ||||
| <60 years | 0 | 37 | 4 | 0.002 |
| ≥60 years | 1 | 19 | 13 | |
FGSI: Fournier’s Gangrene Severity Index; UFGSI: Uludag Fournier’s Gangrene Severity Index.
RESULTS
A total of 73 patients, 42 (57.5%) males and 31 (42.5%) females, were included in this study. The observed mortality rate was 23% (n=17). Statistically, a significant sex-based difference was observed between Group 1 and Group 2 (Table 1). The mean age of the total patient population was 57.29±13.36 years. The mean age in Group 1 (53.66±11.185 years) was significantly lower than in Group 2 (69.24±13.264 years, p=0.000, Table 2). A significant difference between the two groups in terms of age score of UFGSI parameters was observed (p=0.002, Table 3). The infection spread score, one of the UFGSI parameters decreased significantly in Group 1 (p=0.011, Table 3). According to the UFGSI score, the scores of Groups 1 and 2 patients significantly decreased (p=0.00). According to the FGSI score, the scores of Groups 1 and 2 patients significantly decreased (p=0.009). Hematocrit and bicarbonate values (UFGSI and FGSI parameters) were significantly decreased in Group 2 (p=0.000, p=0.015, Table 2). The temperature, heart rate, and serum bicarbonate scores (UFGSI and FGSI scores) significantly increased in Group 2 (p=0.012, p=0.012, and p=0.003, Table 3).
Table 2.
The means and statistical significance of the groups’ characteristics
| Group 1 (No mortality) | Group 2 (Exitus) | p | |
|---|---|---|---|
| Age (years) | 53.66±11.185 | 69.24±13.264 | 0.000 |
| Temperature (°C) | 36.980±.6560 | 37.135±1.0920 | 0.762 |
| Heart rate (/min) | 90.89±11.615 | 97.88±17.273 | 0.069 |
| Respiratory rate (/min) | 23.04±1.981 | 24.59±5.087 | 0.303 |
| Serum potassium (milimol/L) | 4.1888±.81888 | 3.8841±1.11534 | 0.060 |
| Serum sodium (mmol/L) | 135.43±4.902 | 135.76±6.572 | 0.896 |
| Serum creatinine (mg/100 ml) | 1.1630±.63819 | 1.0653±.69921 | 0.411 |
| Haematocrit (%) | 37.771±6.3465 | 31.494±4.2163 | 0.000 |
| White blood count (X1000/mm3) | 20.2553±7.99483 | 21.4424±7.7787 | 0.569 |
| Serum bicarbonate, venous (mmol/L) | 22.693±4.1384 | 20.741±6.3382 | 0.015 |
| Urea (mg/100 ml) | 49.82±29.969 | 66.29±39.920 | 0.090 |
| vacuum-assisted closure therapy duration (day) | 26.95±16.843 | 34.47±19.644 | 0.185 |
| Length of the hospital stay (day) | 39.68±20.065 | 44.65±23.447 | 0.794 |
| Intensive care period (day) | 4.61±9.244 | 25.12±22.561 | 0.000 |
Groups 1 and 2 comprise 42 and 14 patients with DM, respectively. No significant difference was observed between the incidence of DM in Group 1 and Group 2 (Table 1). In Group 1, 14 patients were obese and suffered from DM. In Group 2, seven patients are obese and suffered from DM. A significant difference between the groups regarding obesity was not found (Table 1). In Group 1, a total of 29 patients had comorbidities aside from DM or obesity and 17 in Group 2. A significant difference between the groups was found (p=0.000, Table 1). Due to respiratory failure, the entire 46 patients required mechanical ventilation support during the intensive care unit (ICU), and 17 of these patients died. Moreover, ICP significantly increased in Group 2 based on Group 1 results (p=0.000, Table 2).
Bacteria were isolated from FG wound infections in 40 patients. Bacteria were identified with deep tissue biopsy culture in 28 patients of Group 1 and 12 patients of Group 2. Escherichia coli, isolated from 28 patients, was the most common infection causing bacteria. Other bacteria, such as Acinetobacter, Streptococcus, Staphylococcus aureus, Pseudomonas, and Klebsiella, were identified in 12 patients. A significant difference between the groups regarding bacterial isolation was not observed (Table 1).
To predict mortality, the UFGSI had 100% sensitivity and 68% specificity. However, FGSI had 82% sensitivity and 58% specificity. ROC graph was drawn using the cutoff valuations to predict mortality (sensitivity, specificity, likelihood ratio, positive predictive value, and negative predictive value) in the UFGSI and FGSI scoring systems, as revealed in Figure 1 and Table 4.
Figure 1.
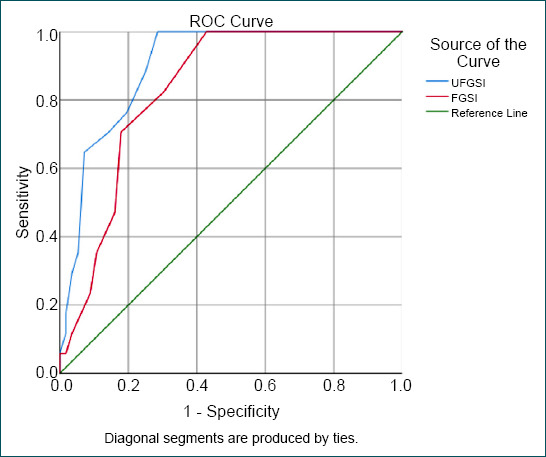
The predictive values of Uludag Fournier’s gangrene severity index and Fournier’s gangrene severity index scoring systems were evaluated using receiver operating characteristic curve analysis.
Table 4.
FGSI and UFGSI scoring systems for predicting mortality in patients with Fournier’s gangrene
| Cut off 95% CI | Sensitivity | Specifity | Likelihood ratio | (+) Predictive value | (-) Predictive value | |
|---|---|---|---|---|---|---|
| UFGSI | ≥8 | 100% | 68% | 3.11 | 48% | 100% |
| FGSI | ≥6 | 82% | 58% | 2.71 | 45% | 93% |
FGSI: Fournier’s Gangrene Severity Index; UFGSI: Uludag Fournier’s Gangrene Severity Index; CI: Confidence interval.
DISCUSSION
Scholars have continued to debate about the accurate treatment procedure for FG as well as the predictable mortality rate associated with FG. The previous studies have reported factors affecting mortality. However, the female gender faces higher mortality risks from the disease than their male counterparts.[11] Similar to the previous studies, this study reported that the female gender faced significant mortality risks (Table 1). In contrast, Sarkut et al.[12] reported that female gender did not a factor affecting prognosis of patients with FG. Apart from gender, age is also an important issue that influences mortality.[5,7] We indicated a correlation between age and mortality. This result is consistent with the results stated by Yilmazlar et al.[9] This correlation is the most conspicuous finding in the study. In contrast, Marín et al.[13] did not indicate a correlation between age and mortality in the study reported by themselves.
The effect of some comorbid on mortality was investigated in other studies.[7,13] In this study, DM was identified in 56 patients. In exitus patients, 14 had DM in Group 2 and 42 had DM in Group 1 (Table 1). The frequency of DM increased in Group 1. Even so, a significant difference was not seen between DM and mortality. Similarly, a significant difference was not found between DM and mortality in other studies as well.[7,9,13] The results of this study indicated that multiple comorbid diseases such as malignancy, heart failure, and respiratory failure significantly affected the mortality of FG (p=0.000) (Table 1). This result is another of our striking findings.
Some clinical studies comparing FGSI and UFGSI indicated mortality rates in patients with FG.[7,13] In this study, both scores were observed to correlate with mortality. Moreover, 100% sensitivity was obtained for UFGSI. In addition, 82% sensitivity was obtained for FGSI. We concluded that UFGSI is more sensitive than FGSI in predicting mortality. The specificity was 68% for UFGSI and 58% for FGSI. The UFGSI scoring system was first described by Yilmazlar et al.[9] They declared sensitivity of 94% and specificity of 81%. The results reported here in terms of sensitivity are compatible with those described by Yilmazlar et al. However, our results are not compatible with the specificity results described by Roghmann et al.[7] as their figures were 85% for sensitivity and 67% for the specificity of UFGSI. However, specificity results are compatible with those described by Roghmann et al.[7] In the two studies mentioned above, the FGSI sensitivity and specificity were 88–67% and 65–100%, respectively.[9] A sensitivity of 87% and a specificity of 77% for FGSI were described by Czymek et al.[11] In a series of 120 cases, Yilmazlar et al.[5] reported that no surviving patient was identified in those with a UFGSI score higher than 13. The total mortality rate was reported as 20.8% by Yilmazlar et al.[5] They indicated that the cutoff values pertain to UFGSI and FGSI were 9 and 7, respectively. The cutoff values pertain to UFGSI and FGSI according to the results of our study were 8 and 6, respectively (Table 4). The cutoff value of 8 for UFGSI is another one of the most striking results of this study, revealing that 17 of 33 cases with a UFGSI score ≥9 died, and 16 cases with a UFGSI score ≥8 were to be discharged (Fig. 2). In addition, 14 of 31 cases with an FGSI score ≥6 died, and 17 cases with an FGSI score ≥6 could be discharged (Fig. 3). Since many cases with higher scores than cutoff values were to be discharged, we considered that a prompt debridement and effective treatment were performed in cases with higher scores than cutoff values of UFGSI and FGSI.
Figure 2.
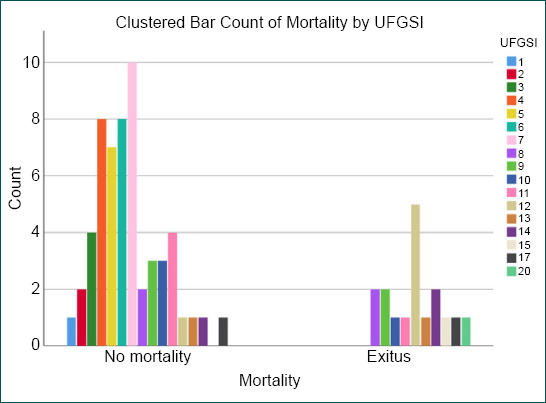
The distribution of patients according to the Uludag Fournier’s gangrene severity index scoring system.
Figure 3.
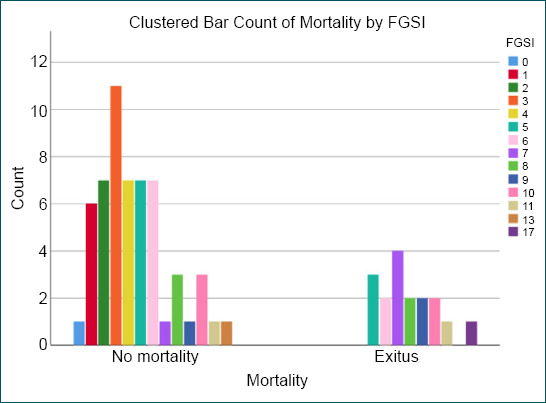
The distribution of patients according to the Fournier’s gangrene severity index scoring system.
Hematocrit and serum bicarbonate levels were significantly different between the groups (p=0.000 and p=0.015, respectively, Table 2). Temperature, heart, and serum bicarbonate scores also varied significantly between the two groups (p=0.012, p=0.012, and p=0.003, respectively, Table 3). Some studies associate an increase in mortality rate with serum creatinine, hematocrit, and potassium levels.[13,14] Roghmann et al.[7] reported a relationship between mortality, creatinine, and hematocrit levels.
In this study, disease dissemination significantly increased in Group 2 relative to Group 1 (p=0.011, Table 3). Moreover, an association is recorded between the dissemination of disease and mortality, consistent with the studies by Yilmazlar et al.[5,9] Roghmann et al.[7] suggested using disease dissemination as a scoring system to predict prognosis and patient outcome. In the study, HT was not significantly different between the two groups, whereas ICP significantly increased in Group 2 relative to Group 1 (p=0.000, Table 2). Moreover, ICP was associated with mortality; this demonstrated another one of the most striking results of the present study.
In this study, bacteria were isolated from 70.5% of the patients in Group 2. The most found bacteria, E. coli, was isolated from FG wounds of 38.3% of patients. This bacterial reproduction was similar to those reported in the literature.[7,15] Bacterial reproduction was not found to be significantly associated with mortality (Table 1). The most frequent origin of FG has been anorectal disease (50.68%) followed by urogenital disease (49.32%, Table 1). The effect of infection source of mortality was not statistically significant.
A fecal diversion procedure containing sigmoid loop colostomy was performed in eight patients in Group 1 and two patients in Group 2 (Table 1). In the practice, we routinely perform enemas before changing VAC dressings every 3–4 days. We never recommend fecal diversion unless there are extensive sphincter damages or large perineal wounds as suggested by Ozturk et al.[16] It is more appropriate to make the decision for stoma during the second debridement, when the sphincters are better evaluated and the patient is more hemodynamically stable. Colostomy, which is frequently preferred by some surgeons and used when necessary by some surgeons, has been accepted as an effective factor in prognosis for many years.[17,18] Contrary to this belief, evidence-based information has shown that stoma opening is not effective on prognosis.[16] In our study, the fecal diversion was not found to be a statistically significant risk factor for mortality. Debridement regions are kept clean and quickly heal thanks to VAC therapy.
This study was designed retrospectively. Moreover, we enrolled a limited number of patients. A wide series of FG has been very little, which includes low case numbers. The factors affecting mortality were analyzed in patients receiving the same treatment strategies for FG. The uniformity of the patients and the similarity in treatment approach represent the greatest strength of the study.
Conclusion
All issues related to the hypothesis were investigated, and all predictions (high mortality expectancy in patients with prolonged ICP, comorbidities apart from DM and obesity, and high FGSI and UFGSI scores) were confirmed. Age and dissemination scores of diseases were important prognostic factors causing mortality in FG. Recently, various scoring systems have been used to predict mortality in a series of patients with FG. However, the age and dissemination scores of UFGSI should be considered. Being over 60 years of age and having a disease spreading outside the pelvis are important parameters for predicting mortality. Patients with a UFGSI score below 8 are more likely to survive and rarely require ICU. Patients with a UFGSI score above 8 have a higher risk of death and should be treated in the ICU. The FG scoring system is important. However, the importance of an accurate scoring system should not be overlooked in predicting patients to be treated in the ICU. Patients with a UFGSI score above 8 should be under the direct care of an experienced clinical team, including general surgeons, plastic surgeons, and intensive care specialists in an ICU.
Footnotes
Ethics Committee Approval: This study was approved by the İstanbul Prof. Dr. Cemil Taşçıoğlu City Hospital Clinical Research Ethics Committee (Date: 02.08.2021, Decision No: 287).
Peer-review: Externally peer-reviewed.
Authorship Contributions: Concept: S.E., S.K.; Design: S.E., S.K.; Supervision: resource Resource: S.E., S.H., B.G., M.Y.; Materials: S.E., S.H., B.G., M.Y.; Data: S.E., S.H., A.A.; Analysis: S.E., S.H., M.G.D.; Literature search: S.E., B.G., M.G.D.; Writing: S.E., A.A., M.Y.; Critical revision: S.E., A.A., M.G.D.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Sroczynski M, Sebastian M, Rudnicki J, Sebastian A, Agrawal AK. A complex approach to the treatment of Fournier's gangrene. Adv Clin Exp Med. 2013;22:131–5. [PubMed] [Google Scholar]
- 2.Djafari AA, Rahavian A, Javanmard B, Montazeri S, Shahabi V, Hojjati SA, et al. Factors related to mortality in patients with Fournier's gangrene or necrotising fasciitis;a 10-year cross-sectional study. Arch Acad Emerg Med. 2021;9:e33. doi: 10.22037/aaem.v9i1.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corcoran AT, Smaldone MC, Gibbons EP, Walsh TJ, Davies BJ. Validation of the Fournier's gangrene severity index in a large contemporary series. J Urol. 2008;180:944–8. doi: 10.1016/j.juro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Thwaini A, Khan A, Malik A, Cherian J, Barua J, Shergill I, et al. Fournier's gangrene and its emergency management. Postgrad Med J. 2006;82:516–9. doi: 10.1136/pgmj.2005.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yilmazlar T, Isik Ö, Öztürk E, Özer A, Gülcü B, Ercan I. Fournier's gangrene:review of 120 patients and predictors of mortality. Ulus Travma Acil Cerrahi Derg. 2014;20:333–7. doi: 10.5505/tjtes.2014.06870. [DOI] [PubMed] [Google Scholar]
- 6.El-Qushayri AE, Khalaf KM, Dahy A, Mahmoud AR, Benmelouka AY, Ghozy S, et al. Fournier's gangrene mortality:a 17-year systematic review and meta-analysis. Int J Infect Dis. 2020;92:218–25. doi: 10.1016/j.ijid.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Roghmann F, von Bodman C, Löppenberg B, Hinkel A, Palisaar J, Noldus J. Is there a need for Fournier's s gangrene severity index?comparison of scoring systems for outcome prediction in patients with Fournier's gangrene. BJU Int. 2012;110:1359–65. doi: 10.1111/j.1464-410X.2012.11082.x. [DOI] [PubMed] [Google Scholar]
- 8.Hahn HM, Jeong KS, Park DH, Park MC, Lee IJ. Analysis of prognostic factors affecting poor outcomes in 41 cases of Fournier gangrene. Ann Surg Treat Res. 2018;95:324–32. doi: 10.4174/astr.2018.95.6.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmazlar T, Ozturk E, Ozguc H, Ercan I, Vuruskan H, Oktay B. Fournier's gangrene:an analysis of 80 patients and a novel scoring system. Tech Coloproctol. 2010;14:217–23. doi: 10.1007/s10151-010-0592-1. [DOI] [PubMed] [Google Scholar]
- 10.Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum-assisted closure therapy in the management of Fournier's gangrene. Am J Surg. 2009;197:660–5. doi: 10.1016/j.amjsurg.2008.04.018. discussion 665. [DOI] [PubMed] [Google Scholar]
- 11.Czymek R, Frank P, Limmer S, Schmidt A, Jungbluth T, Roblick U, et al. Fournier's gangrene:Is the female gender a risk factor?Langenbecks Arch Surg. 2010;395:173–80. doi: 10.1007/s00423-008-0461-9. [DOI] [PubMed] [Google Scholar]
- 12.Sarkut P, Işık Ö, Öztürk E, Gülcü B, Ercan İ, Yılmazlar T. Gender does not affect the prognosis of Fournier's gangrene:a case-matched study. Ulus Travma Acil Cerrahi Derg. 2016;22:541–4. doi: 10.5505/tjtes.2016.27095. [DOI] [PubMed] [Google Scholar]
- 13.Marín AG, Fuentes FT, Ayuso MC, Lillo JA, Ballesteros JC, López MP. Predictive factors for mortality in Fournier's gangrene:a series of 59 cases. Cir Esp. 2015;93:12–7. doi: 10.1016/j.ciresp.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Lin TY, Ou CH, Tzai TS, Tong YC, Chang CC, Cheng HL, et al. Validation and simplification of Fournier's gangrene severity index. Int J Urol. 2014;21:696–701. doi: 10.1111/iju.12426. [DOI] [PubMed] [Google Scholar]
- 15.Wróblewska M, Kuzaka B, Borkowski T, Kuzaka P, Kawecki D, Radziszewski P. Fournier's gangrene-current concepts. Pol J Microbiol. 2014;63:267–73. [PubMed] [Google Scholar]
- 16.Ozturk E, Sonmez Y, Yilmazlar T. What are the indications for a stoma in Fournier's gangrene? Colorectal Dis. 2011;13:1044–7. doi: 10.1111/j.1463-1318.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 17.Yavagal S, de Farias TF, Medina CA, Takacs P. Normal vulvovaginal, perineal, and pelvic anatomy with reconstructive considerations. Semin Plast Surg. 2011;25:121–9. doi: 10.1055/s-0031-1281481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronder C, Cowey A, Hill J. Delayed stoma formation in Fournier's gangrene. Colorectal Dis. 2004;6:518–20. doi: 10.1111/j.1463-1318.2004.00663.x. [DOI] [PubMed] [Google Scholar]


