Abstract
Background:
Population-based cancer survival provides insight into the effectiveness of health systems to care for all residents with cancer, including those in marginalized groups.
Methods:
Using CONCORD-2 data, we estimated 5-year net survival among patients diagnosed 2004–2009 with one of 10 common cancers, and children diagnosed with acute lymphoblastic leukemia (ALL), by socioeconomic status (SES) quintile, age (0–14, 15–64, ≥65 years), and country (Canada or United States).
Results:
In the lowest SES quintile, survival was higher among younger Canadian adults diagnosed with liver (23% vs 15%) and cervical (78% vs 68%) cancers and with leukemia (62% vs 56%), including children diagnosed with ALL (92% vs 86%); and higher among older Americans diagnosed with colon (62% vs 56%), female breast (87% vs 80%), and prostate (97% vs 85%) cancers. In the highest SES quintile, survival was higher among younger Americans diagnosed with stomach cancer (33% vs 27%) and younger Canadians diagnosed with liver cancer (31% vs 23%); and higher among older Americans diagnosed with stomach (27% vs 22%) and prostate (99% vs 92%) cancers.
Conclusions:
Among younger Canadian cancer patients in the lowest SES group, greater access to health care may have resulted in higher cancer survival, while higher screening prevalence and access to health insurance (Medicare) among older Americans during the period of this study may have resulted in higher survival for some screen-detected cancers. Higher survival in the highest SES group for stomach and liver may relate to treatment differences. Survival differences by age and SES between Canada and the United States may help inform cancer control strategies.
Keywords: breast, Canada, cancer, cervix, colon, leukemia, liver, net, population-based, prostate, survival, United States
Introduction
Cancer is the leading cause of death in Canada, and it may become the leading cause of death in the United States.1,2 The numbers of new diagnoses and deaths from cancer are likely to continue to rise because of growing and aging populations in both countries.2,3 An increase in the number of new cancer patients and survivors poses a challenge to the health care systems in Canada and the United States with a need to detect, diagnose, and treat cancers and provide appropriate follow-up care for survivors. Implementation of effective cancer-related health care services and cancer control initiatives is critical to responding to these challenges.
Population-based cancer survival estimates include all patients diagnosed with cancer in a defined geographic area, such as a state or province, regardless of their age, race, immigrant status, income, or access to health insurance and health care. As such, population-based cancer survival is a measure of the overall effectiveness of the health care system to deliver services to all patients and survivors, including marginalized groups. Along with incidence and mortality data, survival is a key metric for evaluating cancer care and cancer control initiatives in the population.4-6
Population-based cancer survival for many leading cancers is among the highest in the world in Canada and the United States.6 However, survival has been shown to be associated with social and economic status in high-income countries,7-10 including those with universal health insurance, such as Canada,11-13 with survival tending to be lower among those with lower incomes. Such disparities represent large numbers of potentially avoidable premature deaths, and they place a large economic burden on communities that are economically or socially marginalized.14
When comparing survival with the United States, Gorey15-19 and Boyd20 have reported a Canadian survival advantage for several common cancers among the very poor. The authors posited that this advantage may have resulted from better access to health care because of universal health insurance coverage in Canada. These comparative studies were limited in their geographic coverage and did not account for the availability of health insurance (Medicare) to older adults in the United States.
CONCORD is a program for the global surveillance of population-based cancer survival.6 In 2015, CONCORD-2 published 5-year survival trends for patients diagnosed from 1995–2009 with 1 of 10 common cancers in 67 countries, including Canada and the United States. The study provided a unique opportunity to compare cancer survival between Canada and the United States. We speculated that the previously reported survival advantage among Canadian patients in the lowest economic group may have been limited to patients younger than 65 years, for whom health insurance coverage was higher in Canada than in the United States. Because older adults in the United States are eligible for Medicare, we further speculated that survival should be comparable in Canada and the United States among this age group because both Canadians and Americans had access to health insurance. To aid in the interpretation of these results, we have also reported corresponding cancer incidence rates.
Methods and Materials
We used CONCORD-2 data for patients diagnosed with cancer during 2004–2009 and followed up to December 31, 2009, from 33 statewide registries, covering approximately 73% of the US population, and 10 provincial registries, covering more than 99% of the Canadian population, and which agreed for their data to be included in this study. Site and histology information, coded to the International Classification of Diseases for Oncology, third edition (ICD-O-3),21 was used to examine cancers of the stomach, colon, rectum, liver, lung, female breast, cervix, ovary, and prostate in adults, and adult leukemia and childhood acute lymphoblastic leukemia (ALL) using CONCORD-2 cancer site definitions (Table 1).
Table 1.
CONCORD-2 Study Cancer Site Definitions6
| Cancer site | Incidence (ICD-O-3) 21 |
|---|---|
| Stomach | C16·0–C16·6, C16·8–C16·9 |
| Colon | C18·0–C18·9, C19·9 |
| Rectum | C20.9, C21·0–C21·2, C21·8 |
| Liver (and intrahepatic bile duct) | C22·0–C22·1 |
| Lung (and bronchus) | C34·0–C34·3, C34·8–C34·9 |
| Breast | C50·0–C50·6, C50·8–C50·9 |
| Cervix | C53·0–C53·1, C53·8–C53·9 |
| Ovary | C48·0–C48·2, C56·9, C57·0–C57·4, C57·7–C57·9 Prostate C61·9 |
| Leukemia | 9670, 9687, 9727, 9728, 9729, 9800, 9801, 9805, 9820, 9823, 9826, 9832, 9833, 9835, 9836, 9837, 9840, 9860, 9861, 9866, 9867, 9870, 9871, 9872, 9873, 9874, 9891, 9895, 9896, 9897, 9910, 9920, 9930, 9931, 9940, 9984, 9987 |
| Childhood ALL | 9727, 9728, 9729, 9835, 9836, 9837 |
ALL, acute lymphoblastic leukemia; ICD-O-3, International Classification of Diseases for Oncology, third edition.
Statistical Analysis
Detailed descriptions of the CONCORD-2 study, including quality control procedures, data evaluation and statistical methods, have been published.4,6 Briefly, we estimated 5-year net survival (%) for each cancer, using the complete approach22 and the Pohar Perme estimator.23,24 To produce survival estimates that were robustly comparable between countries, we adjusted for background mortality in each country using life tables by age (single year), sex, calendar year, and socioeconomic status (SES), and by race in the United States.25,26 SES was categorized into quintiles and ordered from lowest to highest at the national level in the United States and within individual provinces in Canada. For the United States, the SES quintiles were created from county-level SES index scores, which included factors such as income, poverty, unemployment, education, and house value.27 For Canada, SES was defined by neighborhood income assigned at the postal code level.28 Survival estimates for all ages combined were age-standardized using the International Cancer Survival Standard (ICSS) weights.29 Between-country differences in the survival estimates in the lowest and highest SES groups were commented on when the 95% CIs did not overlap, and if the survival estimates differed by at least 5%.
Results
Table 2 shows the number of adults and children diagnosed with one of the 10 cancers of interest during 2004–2009 in Canada and the United States by SES quintile (all cases, lowest, highest) and age group. Our analyses included 4,163,672 patients diagnosed in the United States and 587,785 diagnosed in Canada, including 12,047 and 1,355 children, respectively.
Table 2.
Number of Adults (15–99 Years) Diagnosed with 1 of 10 Common Cancers and Children (0–14 Years) Diagnosed with Acute Lymphoblastic Leukemia (ALL) During 2004–2009 in Canada and the United States, by SES Quintile and Age Group
| SES quintile | ||||||
|---|---|---|---|---|---|---|
| Cancer site | Country | Total patients | Lowest | Highest | ||
| 15–64 y | 65–99 y | 15–64 y | 65–99 y | |||
| Stomach | Canada | 18,187 | 1,276 | 2,710 | 1,010 | 1,870 |
| US | 101,475 | 7,599 | 12,936 | 6,590 | 11,309 | |
| Colon | Canada | 89,037 | 5,038 | 12,860 | 5,528 | 10,565 |
| US | 534,721 | 42,031 | 74,391 | 30,990 | 54,866 | |
| Rectum | Canada | 30,741 | 2,513 | 3,708 | 2,601 | 2,954 |
| US | 164,021 | 17,309 | 17,864 | 14,027 | 12,489 | |
| Liver | Canada | 10,665 | 1,174 | 1,451 | 658 | 1,010 |
| US | 92,571 | 9,209 | 8,490 | 8,436 | 7,778 | |
| Lung | Canada | 133,060 | 10,517 | 21,685 | 6,436 | 12,914 |
| US | 955,184 | 78,536 | 143,529 | 44,126 | 94,163 | |
| Breast | Canada | 123,360 | 12,200 | 10,449 | 16,057 | 9,141 |
| US | 926,271 | 95,309 | 76,455 | 105,882 | 63,236 | |
| Cervix | Canada | 8,086 | 1,493 | 399 | 1,067 | 222 |
| US | 60,263 | 10,791 | 2,852 | 7,305 | 1,721 | |
| Ovary | Canada | 17,079 | 1,572 | 1,734 | 1,727 | 1,487 |
| US | 116,459 | 10,500 | 11,135 | 11,555 | 9,372 | |
| Prostate | Canada | 132,175 | 7,077 | 14,474 | 12,381 | 17,001 |
| US | 1,033,091 | 76,963 | 127,391 | 79,985 | 99,332 | |
| Leukemia | Canada | 24,040 | 1,713 | 2,983 | 1,894 | 2,718 |
| US | 167,569 | 13,033 | 19,972 | 12,456 | 15,579 | |
| ALL (children) | Canada | 1,355 | 262 | NA | 273 | NA |
| US | 12,047 | 2,050 | NA | 2,228 | NA | |
NA, not applicable; SES, socioeconomic status.
Note: Cancers are ordered by International Classification of Diseases for Oncology, third edition (ICD-O-3) codes.
Participating provincial registries: Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland*, Nova Scotia, Ontario, Prince Edward Island, Quebec, Saskatchewan.
Newfoundland did not report SES data and is only represented in the totals for all deprivation quintiles combined.
Participating state registries: Alabama, Alaska, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Iowa, Kentucky, Louisiana, Mississippi, Montana, New Hampshire, New Jersey, New Mexico, New York, Ohio, Oklahoma, Oregon, Pennsylvania, Tennessee, Texas, Maryland, Nebraska, North Carolina, South Carolina, Utah, West Virginia, Washington, and Wyoming.
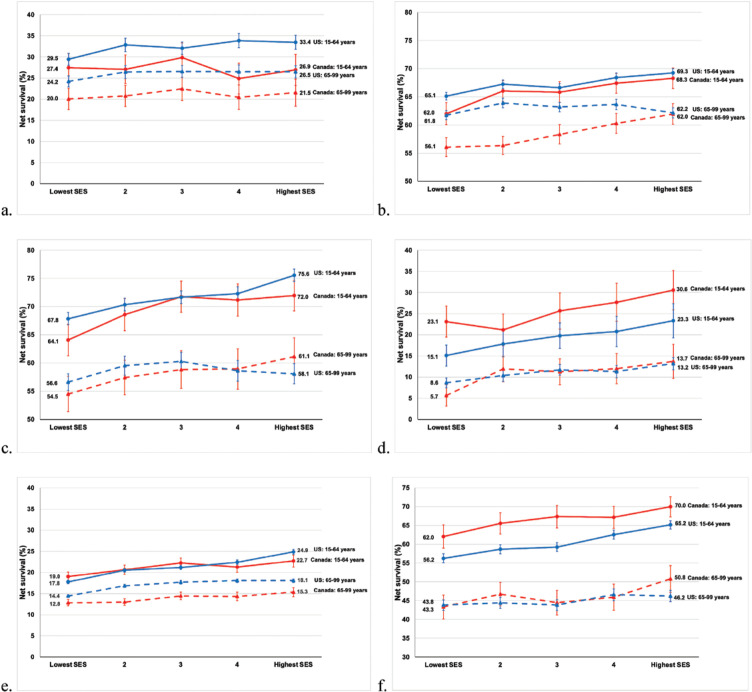
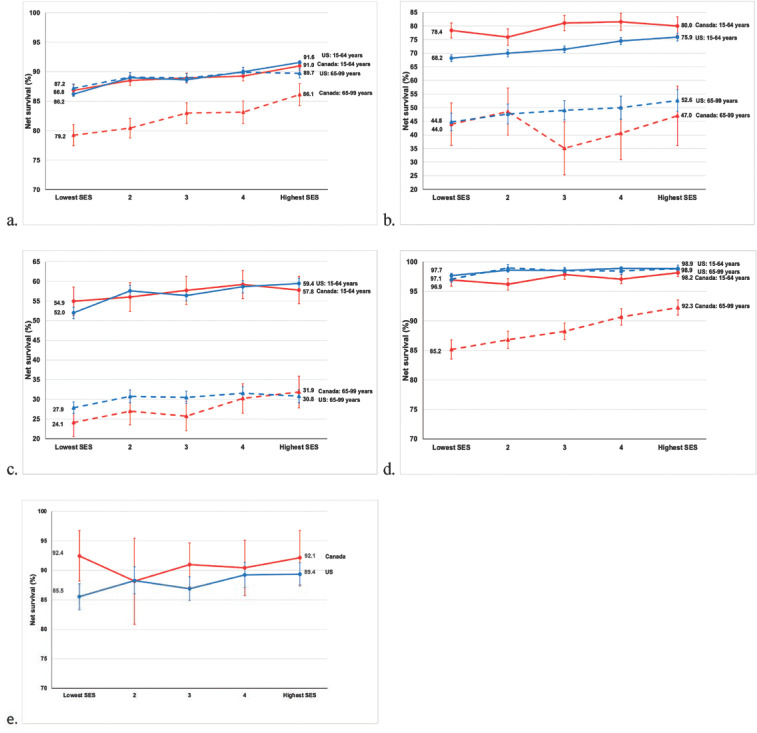
Table 3 shows 5-year survival (%) for adults diagnosed with one of the 10 cancers of interest, and children diagnosed with ALL during 2004–2009 in Canada and the United States by age and SES for all patients combined, and for patients in the lowest SES and highest SES quintiles. In the lowest SES group, 5-year survival was higher among younger adults in Canada than in the United States for cancers of the liver (23.1% vs 15.1%) and cervix (78.4% vs 68.2%) and for leukemia (62.0% vs 56.2%). Between-country differences in survival estimates narrowed between the lowest and highest SES quintiles for cervical cancer in younger women (Figure 1). Among children diagnosed with ALL in the lowest SES group, 5-year survival was higher in Canada (92.4%) than in the United States (85.5%). Among older adults in the lowest SES group, 5-year survival was higher in the United States than in Canada among patients diagnosed with cancers of the colon (61.8% vs 56.1%), female breast (87.2% vs 79.2%), and prostate (97.1% vs 85.2%). Between-country differences in survival estimates narrowed between the lowest and highest SES quintiles for colon and prostate cancers in older adults (Figures 1 and 2). In the highest SES quintile, survival was higher among younger Americans diagnosed with stomach cancer (33.4% vs 26.9%) and younger Canadians diagnosed with liver cancer (30.6% vs 23.3%). Survival was higher among older Americans diagnosed with stomach (26.5% vs 21.5%) and prostate (98.9% vs 92.3%) cancers.
Table 3.
Five-Year Survival for Adults (10 Cancers of Interest) and Children (ALL), 2004–2009
| Age–standardized | |||||||
|---|---|---|---|---|---|---|---|
| All | Lowest SES | Highest SES | |||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | ||
| Stomach | |||||||
| Canada | 24.5 | 23.6 – 25.5 | 23.7 | 21.7 – 25.8 | 24.4 | 22.0 – 26.8 | |
| United States | 29.2 | 28.7 – 29.6 | 26.7 | 25.8 – 27.7 | 30.1 | 29.0 – 31.2 | |
| Colon | |||||||
| Canada | 62.1 | 61.6 – 62.7 | 59.5 | 58.2 – 60.7 | 64.9 | 63.6 – 66.2 | |
| United States | 65.2 | 65.0 – 65.4 | 63.4 | 62.9 – 63.9 | 65.8 | 65.2 – 66.4 | |
| Rectum | |||||||
| Canada | 63.0 | 62.0 – 64.0 | 58.8 | 56.7 – 61.0 | 65.8 | 63.5 – 68.0 | |
| United States | 64.5 | 64.0 – 64.9 | 61.3 | 60.3 – 62.3 | 66.0 | 64.9 – 67.1 | |
| Liver | |||||||
| Canada | 17.1 | 15.9 – 18.3 | 13.6 | 11.5 – 5.7 | 21.1 | 18.1 – 24.1 | |
| United States | 14.9 | 14.4 – 15.3 | 11.7 | 10.8 – 12.5 | 17.8 | 16.8 – 18.8 | |
| Lung | |||||||
| Canada | 17.4 | 17.1 – 17.7 | 16.1 | 15.4 – 16.8 | 18.9 | 18.0 – 19.8 | |
| United States | 19.1 | 18.9 – 19.2 | 16.0 | 15.7 – 16.3 | 21.6 | 21.2 – 21.9 | |
| Breast | |||||||
| Canada | 85.1 | 84.6 – 85.6 | 82.8 | 81.7 – 83.8 | 87.9 | 86.8 – 89.1 | |
| United States | 89.1 | 88.9 – 89.3 | 86.8 | 86.3 – 87.3 | 90.5 | 90.1 – 91.0 | |
| Cervix | |||||||
| Canada | 66.8 | 65.2 – 68.5 | 66.6 | 63.4 – 69.7 | 66.3 | 61.8 – 70.8 | |
| United States | 63.0 | 62.3 – 63.6 | 59.3 | 57.9 – 60.6 | 67.1 | 65.5 – 68.8 | |
| Ovary | |||||||
| Canada | 39.8 | 38.6 – 41.0 | 37.0 | 34.4 – 39.6 | 42.4 | 39.6 – 45.1 | |
| United States | 41.1 | 40.7 – 41.6 | 37.5 | 36.5 – 38.6 | 42.6 | 41.5 – 43.7 | |
| Prostate | |||||||
| Canada | 91.2 | 90.8 – 91.6 | 89.3 | 88.3 – 90.4 | 93.6 | 92.7 – 94.5 | |
| United States | 97.8 | 97.6 – 98.0 | 96.7 | 96.3 – 97.1 | 98.1 | 97.6 – 98.5 | |
| Leukemia (adults) | |||||||
| Canada | 55.6 | 54.6 – 56.6 | 52.4 | 50.2 – 54.7 | 59.5 | 57.2 – 61.7 | |
| United States | 52.5 | 52.1 – 52.9 | 49.8 | 48.9 – 50.7 | 55.2 | 54.3 – 56.2 | |
| ALL (children) | |||||||
| Canada | 91.1 | 88.9 – 93.2 | 92.4 | 88.1 – 96.7 | 92.1 | 87.5 – 96.7 | |
| United States | 88.0 | 87.1 – 88.9 | 85.5 | 83.3 – 87.7 | 89.4 | 87.4 – 91.3 | |
| Younger adults (15–64 years) | |||||||
|---|---|---|---|---|---|---|---|
| All | Lowest SES | Highest SES | |||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | ||
| Stomach | |||||||
| Canada | 27.6 | 26.0 – 29.1 | 27.4 | 24.1 – 30.8 | 26.9 | 23.3 – 30.6 | |
| United States | 32.2 | 31.7 – 32.6 | 29.5 | 28.1 – 30.9 | 33.4 | 31.8 – 35.1 | |
| Colon | |||||||
| Canada | 65.9 | 65.1 – 66.7 | 62.0 | 60.0 – 64.0 | 68.3 | 66.5 – 70.1 | |
| United States | 67.4 | 67.2 – 67.6 | 65.1 | 64.4 – 65.8 | 69.3 | 68.5 – 70.0 | |
| Rectum | |||||||
| Canada | 69.5 | 68.2 – 70.7 | 64.1 | 61.3 – 66.9 | 72.0 | 69.2 – 74.7 | |
| United States | 71.8 | 71.4 – 72.1 | 67.8 | 66.8 – 68.9 | 75.6 | 74.4 – 76.7 | |
| Liver | |||||||
| Canada | 25.0 | 23.1 – 26.8 | 23.1 | 19.5 – 26.8 | 30.6 | 25.9 – 35.2 | |
| United States | 19.4 | 19.0 – 19.7 | 15.1 | 13.9 – 16.2 | 23.3 | 22.0 – 24.7 | |
| Lung | |||||||
| Canada | 21.0 | 20.4 – 21.5 | 19.0 | 18.0 – 20.1 | 22.7 | 21.3 – 24.1 | |
| United States | 21.2 | 21.0 – 21.3 | 17.8 | 17.4 – 18.1 | 24.9 | 24.3 – 25.5 | |
| Breast | |||||||
| Canada | 88.9 | 88.6 – 89.3 | 86.8 | 85.9 – 87.8 | 91.0 | 90.3 – 91.7 | |
| United States | 89.3 | 89.2 – 89.4 | 86.2 | 85.8 – 86.6 | 91.6 | 91.3 – 91.8 | |
| Cervix | |||||||
| Canada | 79.5 | 78.1 – 80.8 | 78.4 | 75.6 – 81.2 | 80.0 | 76.6 – 83.4 | |
| United States | 71.8 | 71.5 – 72.2 | 68.2 | 67.0 – 69.4 | 75.9 | 74.6 – 77.3 | |
| Ovary | |||||||
| Canada | 57.3 | 55.7 – 58.8 | 54.9 | 51.3 – 58.5 | 57.8 | 54.3 – 61.3 | |
| United States | 57.1 | 56.6 – 57.5 | 52.0 | 50.5 – 53.4 | 59.4 | 58.0 – 60.8 | |
| Prostate | |||||||
| Canada | 97.3 | 96.9 – 97.7 | 96.9 | 95.9 – 98.0 | 98.2 | 97.5 – 98.8 | |
| United States | 98.6 | 98.5 – 98.7 | 97.7 | 97.3 – 98.1 | 98.9 | 98.6 – 99.1 | |
| Leukemia (adults) | |||||||
| Canada | 66.5 | 65.2 – 67.8 | 62.0 | 58.9 – 65.1 | 70.0 | 67.3 – 72.6 | |
| United States | 60.7 | 60.3 – 61.0 | 56.2 | 55.0 – 57.4 | 65.2 | 64.0 – 66.3 | |
| Older adults (65–99 years) | |||||||
|---|---|---|---|---|---|---|---|
| All | Lowest SES | Highest SES | |||||
| NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | ||
| Stomach | |||||||
| Canada | 21.3 | 20.1 – 22.5 | 20.0 | 17.5 – 22.6 | 21.5 | 18.4 – 24.7 | |
| United States | 26.2 | 25.8 – 26.7 | 24.2 | 22.9 – 25.5 | 26.5 | 25.0 – 27.9 | |
| Colon | |||||||
| Canada | 58.5 | 57.7 – 59.2 | 56.1 | 54.4 – 57.8 | 62.0 | 60.1 – 63.8 | |
| United States | 63.0 | 62.7 – 63.2 | 61.8 | 61.0 – 62.6 | 62.2 | 61.3 – 63.1 | |
| Rectum | |||||||
| Canada | 58.0 | 56.6 – 59.5 | 54.5 | 51.4 – 57.6 | 61.1 | 57.8 – 64.5 | |
| United States | 58.8 | 58.3 – 59.3 | 56.6 | 55.1 – 58.1 | 58.1 | 56.3 – 59.9 | |
| Liver | |||||||
| Canada | 10.7 | 9.2 – 12.2 | 5.7 | 3.1 – 8.2 | 13.7 | 9.7 – 17.8 | |
| United States | 10.9 | 10.5 – 11.3 | 8.6 | 7.5 – 9.8 | 13.2 | 11.8 – 14.6 | |
| Lung | |||||||
| Canada | 13.8 | 13.4 – 14.2 | 12.8 | 12.1 – 13.6 | 15.3 | 14.3 – 16.4 | |
| United States | 16.9 | 16.7 – 17.0 | 14.4 | 14.1 – 14.8 | 18.1 | 17.6 – 18.5 | |
| Breast | |||||||
| Canada | 82.1 | 81.3 – 82.9 | 79.2 | 77.5 – 81.0 | 86.1 | 84.3 – 88.0 | |
| United States | 89.0 | 88.7 – 89.2 | 87.2 | 86.4 – 87.9 | 89.7 | 88.9 – 90.5 | |
| Cervix | |||||||
| Canada | 43.2 | 39.0 – 47.4 | 44.0 | 36.2 – 51.8 | 47.0 | 36.1 – 57.9 | |
| United States | 48.2 | 47.1 – 49.3 | 44.8 | 41.6 – 48.0 | 52.6 | 48.6 – 56.6 | |
| Ovary | |||||||
| Canada | 27.7 | 26.0 – 29.3 | 24.1 | 20.6 – 27.6 | 31.9 | 27.9 – 35.9 | |
| United States | 30.1 | 29.6 – 30.5 | 27.9 | 26.4 – 29.4 | 30.8 | 29.1 – 32.5 | |
| Prostate | |||||||
| Canada | 88.7 | 88.1 – 89.4 | 85.2 | 83.5 – 86.8 | 92.3 | 91.0 – 93.6 | |
| United States | 98.4 | 98.2 – 98.6 | 97.1 | 96.5 – 97.7 | 98.9 | 98.3 – 99.5 | |
| Leukemia (adults) | |||||||
| Canada | 46.2 | 44.7 – 47.6 | 43.3 | 40.1 – 46.5 | 50.8 | 47.2 – 54.3 | |
| United States | 44.9 | 44.4 – 45.3 | 43.8 | 42.4 – 45.2 | 46.2 | 44.7 – 47.7 | |
ALL, acute lymphoblastic leukemia; NS, net survival; SES, socioeconomic status (quintile).
Figure 1.
Five-Year Net Survival (%) for Adults (Aged 15–99 Years) Diagnosed with 1 of 6 Common Cancers During 2004–2009 in Canada and the United States; Separately for Younger Adults (Aged 15–64 Years) and Older Adults (Aged 65–99 years) by Socioeconomic Status (SES) Quintiles (a, stomach; b, colon; c, rectum; d, liver; e, lung; f, leukemia)
Figure 2.
Five-year Net Survival (%) for Adults (Aged 15–99 years) Diagnosed with 1 of 4 Common Cancers and Children (Aged 0–14 Years) Diagnosed with Acute Lymphoblastic Leukemia (ALL) During 2004–2009 in Canada and the United States; Separately for Younger Adults (Aged 15–64 Years) and Older Adults (Aged 65–99 Years) by Socioeconomic Status (SES) Quintiles (a, breast [women]; b, cervix; c, ovary; d, prostate; e, ALL)
Discussion
Canada and the United States are countries with similar cultural and socioeconomic backgrounds, and, in many ways, similar medical care. However, they differ in their approach to health insurance and cancer screening. Between-country survival differences within the lowest SES group suggest a role for greater access to health care in younger adults in Canada and more frequent screening in older adults in the United States.
Access to Health Insurance
In Canada, health insurance is available to all residents through provincial and territorial health insurance plans jointly funded by the provinces, territories, and the federal government. In the United States, health insurance is available through a combination of private insurers and public programs. The US federal and state governments directly fund, or help to fund, insurance programs such as Medicare and Medicaid, which cover older adults and the very poor and disabled, respectively. Most adults under the age of 65 years obtain health insurance through their employer or purchase private insurance directly. However, during the time period of our study, approximately 17% of adults under the age of 65 years in the lowest income group were uninsured.30
Previous studies have shown that, in the United States, the uninsured and those on Medicaid were more likely to be diagnosed with advanced-stage cancers, to receive less optimal treatment, and to have lower survival than those with insurance.31-35 These uninsured and underinsured men and women were less likely to be screened or to receive antiviral treatment for their hepatitis infections (a risk factor for liver cancer) and were less likely to be referred for and receive evidenced-based treatment, including liver transplant, following a diagnosis of liver cancer.36,37 Among women, cervical cancer screening was lowest among US women without insurance and women who reported no usual source of health care.38 Between-country cervical cancer survival differences narrowed in younger women with increasing SES, likely reflecting the increased prevalence of cervical cancer screening with incressing SES among women in both countries.38,39 In the United States, survival among adults, adolescents, and children diagnosed with leukemia was reported to be lower among the uninsured and those on Medicaid than among patients who were insured or who were in higher SES families.40-42
Between-country survival differences did not narrow, or narrowed only somewhat, with increasing SES for younger adults diagnosed with liver cancer or leukemia, including children diagnosed with ALL. For these cancers, Canadian survival estimates in adults were consistently slightly higher than US estimates across all SES groups.
Cancer Screening
The higher survival for colon, female breast, and prostate cancers among older adults in the United States compared to Canada may reflect the different approaches to screening in the 2 countries. The United States uses opportunistic screening (ie, requests for screening come from individuals or health care providers) whereas Canada uses a combination of opportunistic and population-based, programmatic screening, with a significant emphasis on the latter in most provincial/territorial jurisdictions for colorectal, female breast, and cervical cancers. With programmatic screening, the provincial public health sector invites all eligible residents to participate in screening. For these cancers, screening use was lowest in the lowest SES groups and increased with increasing income in both countries.38,39 Cancer screening use was generally consistent with each country's specific guidelines, particularly regarding age at initiation, with higher screening prevalence reported in the United States than in Canada for these cancers in all age groups.43
Colorectal cancer screening has been shown to reduce colorectal cancer incidence and death rates.44 In the 2000s, both the Canadian Task Force on Preventive Health Care (CTFPHC) and the US Preventive Services Task Force (USPSTF) recommended colorectal cancer screening beginning at age 50 years.45,46 However, while Medicare began covering colorectal cancer screening for eligible adults in the United States beginning in the late 1990s, programmatic colorectal cancer screening in Canada did not begin until 2007, when Alberta, Manitoba, and Ontario became the first provinces to announce programmatic screening. The lower incidence rates (Table 4) and higher colon cancer survival among older adults in the United States may reflect high screening prevalence in the United States compared to Canada and the fact that widespread programmatic screening for colorectal cancer in Canada occurred largely after the period covered by this study.
Table 4.
Incidence Rates Among Participating Registries (2004–2009)
| Cancer Sites | United States | Canada | United States | Canada | United States | Canada |
|---|---|---|---|---|---|---|
| All ages | All ages | 15–64 y | 15–64 y | ≥65 y | ≥65 y | |
| Stomach | 10.8 | 12.5 | 4.8 | 5.2 | 39.3 | 47.7 |
| Colon | 54 | 59.7 | 22.6 | 22.2 | 204.9 | 239.8 |
| Liver | 9.7 | 7.3 | 6.1 | 3.8 | 26.9 | 24 |
| Female breast | 173.6 | 157.9 | 124.6 | 111.8 | 408.7 | 379.1 |
| Cervix | 10.6 | 10 | 10.3 | 10 | 11.9 | 10.1 |
| Prostate | 224.6 | 198.7 | 104.1 | 81.1 | 803.4 | 763.5 |
| Leukemia | 16.9 | 17.2 | 8.1 | 8.2 | 59.1 | 60.4 |
| Childhood ALL (0–14 years) | 4.2 | 4.2 | NA | NA | NA | NA |
ALL, acute lymphoblastic leukemia; NA, not applicable.
Both the USPSTF and the CTFPHC recommended breast cancer screening during the 2000s, although the age at initiation (40 years vs 50 years, respectively) and frequency of screening (annual vs biannual, respectively) differed between the United States and Canada.47-49 The earlier initiation and higher frequency of screening in the United States than in Canada may have contributed to both higher breast cancer incidence (Table 4) and higher survival among older women in the United States.
The introduction of the prostate-specific antigen (PSA) test during the late 1980s was associated with an increase in the incidence of prostate cancer in both Canada and the United States.50 During the period of this study, neither the USPSTF nor the CTFPHC recommended prostate cancer screening for men at average risk.51,52 However, in 2003, the American Cancer Society recommended that annual PSA testing and digital rectal examination should be offered to asymptomatic men who have a life expectancy of at least 10 years starting at age 50.53 Higher prevalence of PSA testing may account for higher prostate cancer incidence (Table 4) and higher survival in older men in the United States compared to Canada.
Between-country survival differences narrowed with increasing SES in older adults for colon, breast, and prostate cancers, as survival increased with increasing SES, most noteably for Canadians. This narrowing of differences likely reflects the increased prevalence of screening/testing for these cancers with increasing income in both countries.38,39 Mortality provides critical evidence of the effectiveness of cancer screening. The lower death rates for colorectal, female breast, and prostate cancers in the United States compared to Canada are consistent with data from the United States showing that more intensive screening, and perhaps more aggressive treatment among older patients, can lead to higher survival and lower death rates.54,55 However, the decision to screen older adults requires balancing the potential harms of screening and follow-up diagnostic tests with the possibility of benefits.55
Treatment
Survival was higher in the highest SES group in the United States than in Canada following a diagnosis of stomach cancer in both younger and older adults. Survival was higher in Canada following a diagnosis of liver cancer among younger adults in the highest SES groups. Higher survival for these cancers, and the fact that survival was modestly but consistently higher across all SES quintiles for cervical cancer, liver cancer, and leukemia in Canada and stomach cancer in the United States, may relate to treatment differences.
Conclusion
Our study found that 5-year survival differed according to SES between Canada and the United States for several common cancers. Among younger adults in the lowest SES group, greater access to health insurance and health care may have resulted in somewhat higher cancer survival in Canada for liver and cervical cancers and leukemia, including children with ALL; while higher cancer screening prevalence, coupled with access to health insurance (including Medicare), may have resulted in higher survival among older adults in the United States for colon, female breast, and prostate cancers. An examination of stage distribution and stage-specific survival for these cancers may provide insight as to whether these survival differences reflect true benefits to patients through better treatment and more intensive screening or merely reflect lead time biases resulting from more frequent and earlier interactions with the health care community. However, survival for lung cancer and ovarian cancer, which are often detected at an advanced stage of disease, and where opportunities for earlier diagnosis and treatment options are limited, showed similar survival patterns by SES in both Canada and the United States.
The relevance of this study from the US perspective lies in the fact that it included patients diagnosed with cancer just prior to the implementation of the Patient Protection and Affordable Care Act of 2010, which expanded health care and health insurance to more Americans.56 We would expect survival in the United States to improve in the lowest SES group—particularly in those states that expanded Medicaid coverage in 2010—and nationwide following the expansion of insurance coverage in 2014. In Canada, we would expect colon cancer survival to improve as program-matic colorectal cancer screening was rolled out throughout Canada. Comparative analyses of population-based cancer survival can help to measure progress in the achievement of these objectives and help identify opportunities to improve health systems and guide public health actions to improve cancer outcomes.
Strengths and Limitations
Our study had several notable strengths. First, CONCORD-2 provided very high population coverage of cancer survival for Canada (99%) and the United States (73%). Second, CONCORD-2 produced robustly comparable survival estimates.4 All registries collected a uniform set of high-quality cancer survival data and survival estimates were comparable because death ascertainment was virtually complete.57 During this period, participating registries ascertained almost all deaths among their cancer patients through linkages with their respective state or provincial vital records offices and with their national death indices. In addition, participating registries followed a common protocol in which their data were centrally evaluated and analyzed, including the use of life tables, which adjusted for differences in background mortality.
However, our study had several limitations. Neither US nor Canadian cancer registries collect patient-level SES. Therefore, SES was ecologically defined using county of diagnosis in the United States and postal code in Canada. In addition, SES was defined somewhat differently in Canada (income) and the United States (SES index). SES quintiles reflect the socioeconomic gradient in each country. We recommend caution when comparing between-country SES-specific survival estimates. This study did not collect stage data from participating registries because stage data were not available from all cancer registries during the period of this study.
CONCORD Canada–US Working Group Members
America (North)—Canada: A. Eckstrand, C. Nikiforuk (Alberta Cancer Registry); R. R. Woods (British Columbia Cancer Registry); G. Noonan, D. Turner (Manitoba Cancer Registry); E. Kumar, B. Zhang (New Brunswick Provincial Cancer Registry); F. R. McCrate, S. Ryan (Newfoundland & Labrador Cancer Registry); M. MacIntyre, N. Saint-Jacques (Nova Scotia Cancer Registry); A. Anam, P. De (Ontario Cancer Registry); C. A. McClure, K. A. Vriends (Prince Edward Island Cancer Registry); C. Bertrand, A. V. Ramanakumar (Registre Québécois du Cancer); S. Kozie, H. Stuart-Panko (Saskatchewan Cancer Agency); United States: T. Freeman, J. T. George (Alabama Statewide Cancer Registry); R. M. Avila, D. K. O'Brien (Alaska Cancer Registry); A. Holt (Arkansas Central Cancer Registry); L. Almon (Metropolitan Atlanta Registry); S. Kwong, C. Morris (California State Cancer Registry); R. Rycroft (Colorado Central Cancer Registry); L. Mueller, C. E. Phillips (Connecticut Tumor Registry); H. Brown, B. Cromartie (Delaware Cancer Registry); A. G. Schwartz, F. Vigneau (Metropolitan Detroit Cancer Surveillance System); G. M. Levin, B. Wohler (Florida Cancer Data System); R. Bayakly (Georgia Cancer Registry); K. C. Ward (Georgia Cancer Registry; Metropolitan Atlanta Registry); S. L. Gomez, M. McKinley (Greater Bay Area Cancer Registry); R. Cress (Cancer Registry of Greater California); J. Davis, B. Hernandez (Hawaii Tumor Registry); C. J. Johnson (Cancer Data Registry of Idaho); L. P. Ruppert (Indiana State Cancer Registry); S. Bentler, M. E. Charlton (State Health Registry of Iowa); B. Huang, T. C. Tucker (Kentucky Cancer Registry); D. Deapen, L. Liu (Los Angeles Cancer Surveillance Program); M. C. Hsieh, X. C. Wu (Louisiana Tumor Registry); M. Schwenn (Maine Cancer Registry); K. Stern (Maryland Cancer Registry); S. T. Gershman, R. C. Knowlton (Massachusetts Cancer Registry); G. Alverson, T. Weaver (Michigan State Cancer Surveillance Program); J. Desai (Minnesota Cancer Reporting System); D. B. Rogers (Mississippi Cancer Registry); J. Jackson-Thompson (Missouri Cancer Registry and Research Center); D. Lemons, H. J. Zimmerman (Montana Central Tumor Registry); M. Hood, J. Roberts-Johnson (Nebraska Cancer Registry); C. A. Geiger, J. R. Rees (New Hampshire State Cancer Registry); K. S. Pawlish, A. Stroup (New Jersey State Cancer Registry); C. Key, C. Wiggins (New Mexico Tumor Registry); A. R. Kahn, M. J. Schymura (New York State Cancer Registry); S. Radhakrishnan, C. Rao (North Carolina Central Cancer Registry); L. K. Giljahn, R. M. Slocumb (Ohio Cancer Incidence Surveillance System); C. Dabbs, R. E. Espinoza (Oklahoma Central Cancer Registry); K. G. Aird, T. Beran (Oregon State Cancer Registry); J. J. Rubertone, S. J. Slack (Pennsylvania Cancer Registry); J. Oh (Rhode Island Cancer Registry); T. A. Janes, S. M. Schwartz (Seattle Cancer Surveillance System); S. C. Chiodini, D. M. Hurley (South Carolina Central Cancer Registry); M. A. Whiteside (Tennessee Cancer Registry); S. Rai, M. A. Williams (Texas Cancer Registry); K. Herget, C. Sweeney (Utah Cancer Registry); A. T. Johnson (Vermont Cancer Registry); M. B. Keitheri Cheteri, P. Migliore Santiago (Washington State Cancer Registry); S. E. Blankenship, S. Farley (West Virginia Cancer Registry); R. Borchers, R. Malicki (Wisconsin Department of Health Services); J. Espinoza, J. Grandpre (Wyoming Cancer Surveillance Program); H. K. Weir, R. Wilson (Centers for Disease Control and Prevention); B. K. Edwards, A. Mariotto (National Cancer Institute).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention, the US National Cancer Institute, or the Canadian Partnership Against Cancer.
Funding to the CONCORD Central Analytic Team at the London School of Hygiene and Tropical Medicine for the survival analyses was provided by the US Centers for Disease Control and Prevention (12FED03123 and ACO12036) and the Canadian Partnership Against Cancer. The sponsors did not participate in the design or conduct of the study, collection, management, analysis, or interpretation of the data. This manuscript has been cleared by the US Centers for Disease Control and Prevention and the National Cancer Institute.
References
- 1.Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths—trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157. doi: 10.5888/pcd13.160211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019. Canadian Cancer Society; 2019. https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2019-statistics/canadian-cancer-statistics-2019-en.pdf [Google Scholar]
- 3.Weir HK, Thompson TD, Stewart SL, White MC. Cancer incidence projections in the United States between 2015 and 2050. Prev Chronic Dis. 2021;18:E59. doi: 10.5888/pcd18.210006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allemani C, Harewood R, Johnson CJ, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123(Suppl 24):4982–4993. doi: 10.1002/cncr.31025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr. 2014;2014(49):187–197. doi: 10.1093/jncimonographs/lgu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allemani C, Weir HK, Carreira H, et al. ; CONCORD Working Group . Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ingh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950-2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372. doi: 10.1155/2017/2819372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petridou ET, Sergentanis TN, Perlepe C, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol. 2015;26(3):589–597. doi: 10.1093/annonc/mdu572 [DOI] [PubMed] [Google Scholar]
- 9.Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr. 2014;2014(49):236–243. doi: 10.1093/jncimonographs/lgu020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113(3):582–591. doi: 10.1002/cncr.23567 [DOI] [PubMed] [Google Scholar]
- 11.embere N, Campitelli MA, Sherman M, et al. Influence of socioeconomic status on survival of hepatocellular carcinoma in the Ontario population; a population-based study, 1990-2009. PLoS One. 2012;7(7):e40917. doi: 10.1371/journal.pone.0040917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth CM, Li G, Zhang-Salomons J, Mackillop WJ. The impact of socioeconomic status on stage of cancer at diagnosis and survival: a population-based study in Ontario, Canada. Cancer. 2010;116(17):4160–4167. doi: 10.1002/cncr.25427 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Schwartz N, Young S, Klein-Geltink J, Truscott R. Comprehensive cancer survival by neighborhood-level income in Ontario, Canada, 2006–2011. J Registry Manag. 2020;47(3):150–160. [PubMed] [Google Scholar]
- 14.Weir HK, Li C, Henley SJ, Joseph D. Years of life and productivity loss from potentially avoidable colorectal cancer deaths in U.S. counties with lower educational attainment (2008-2012). Cancer Epidemiol Biomarkers Prev. 2017;26(5):736–742. doi: 10.1158/1055-9965.EPI-16-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorey KM, Kliewer E, Holowaty EJ, Laukkanen E, Ng EY. An international comparison of breast cancer survival: Winnipeg, Manitoba and Des Moines, Iowa, metropolitan areas. Ann Epidemiol. 2003;13(1):32–41. doi: 10.1016/s1047-2797(02)00259-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM. An international comparison of cancer survival: relatively poor areas of Toronto, Ontario and three US metropolitan areas. J Public Health Med. 2000;22(3):343–348. doi: 10.1093/pubmed/22.3.343 [DOI] [PubMed] [Google Scholar]
- 17.Gorey KM, Holowaty EJ, Fehringer G, Laukkanen E, Richter NL, Meyer CM. An international comparison of cancer survival: metropolitan Toronto, Ontario, and Honolulu, Hawaii. Am J Public Health. 2000;90(12):1866–1872. doi: 10.2105/ajph.90.12.1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorey KM, Holowaty EJ, Laukkanen E, Fehringer G, Richter NL. An international comparison of cancer survival: advantage of Toronto's poor over the near poor of Detroit. Can J Public Health. 1998;89(2):102–104. doi: 10.1007/BF03404398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorey KM, Holowaty EJ, Fehringer G, et al. An international comparison of cancer survival: Toronto, Ontario, and Detroit, Michigan, metropolitan areas. Am J Public Health. 1997;87(7):1156–1163. doi: 10.2105/ajph.87.7.1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol. 1999;17(7):2244–2255. doi: 10.1200/JCO.1999.17.7.2244 [DOI] [PubMed] [Google Scholar]
- 21.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin M, Whelan S.. International Classification of Diseases for Oncology. 3rd ed. World Health Organization; 2000. [Google Scholar]
- 22.Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40(3):326–335. doi: 10.1016/j.ejca.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 23.Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics. 2012;68(1):113–120. doi: 10.1111/j.1541-0420.2011.01640.x [DOI] [PubMed] [Google Scholar]
- 24.Clerc-Urmès I GM, Hédelin G. Net survival estimation with stns. Stata J. 2014;14:87–102. [Google Scholar]
- 25.Mariotto AB, Zou Z, Johnson CJ, Scoppa S, Weir HK, Huang B. Geographical, racial and socio-economic variation in life expectancy in the US and their impact on cancer relative survival. PLoS One. 2018;13(7):e0201034. doi: 10.1371/journal.pone.0201034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C. Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations. BMC Public Health. 2015;15:1240. doi: 10.1186/s12889-015-2534-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. doi: 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 28.Examining Disparities in Cancer Control: A System Performance Special Focus Report. Canadian Partnership Against Cancer; 2014. https://s22457.pcdn.co/wp-content/uploads/2019/01/Examining-disparities-in-cancer-control-EN.pdf [Google Scholar]
- 29.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardizing survival ratios. Eur J Cancer. 2004;40(15):2307–2316. doi: 10.1016/j.ejca.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Moonesinghe R, Zhu J, Truman BI; Centers for Disease Control and Prevention (CDC) . Health insurance coverage—United States, 2004 and 2008. MMWR Suppl. 2011;60(1):35–37. [PubMed] [Google Scholar]
- 31.Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32(28):3118–3125. doi: 10.1200/JCO.2014.55.6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010;16(6):614–621. doi: 10.1097/PPO.0b013e3181ff2aec [DOI] [PubMed] [Google Scholar]
- 33.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenlee RT, Howe HL. County-level poverty and distant stage cancer in the United States. Cancer Causes Control. 2009;20(6):989–1000. doi: 10.1007/s10552-009-9299-x [DOI] [PubMed] [Google Scholar]
- 35.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. doi: 10.1016/S1470-2045(08)70032-9 [DOI] [PubMed] [Google Scholar]
- 36.Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18(6):377–383. doi: 10.1111/j.1365-2893.2010.01401.x [DOI] [PubMed] [Google Scholar]
- 37.Yu JC, Neugut AI, Wang S, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116(7):1801–1809. doi: 10.1002/cncr.24936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep.2012;61(3):41–45. [PubMed] [Google Scholar]
- 39.Kerner J, Liu J, Wang K, et al. Canadian cancer screening disparities: a recent historical perspective. Curr Oncol. 2015;22(2):156–163. doi: 10.3747/co.22.2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry AM, Brunner AM, Zou T, et al. Association between insurance status at diagnosis and overall survival in chronic myeloid leukemia: a population-based study. Cancer. 2017;123(13):2561–2569. doi: 10.1002/cncr.30639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent EE, Sender LS, Largent JA, Anton-Culver H. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control. 2009;20(8):1409–1420. doi: 10.1007/s10552-009-9367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petridou ET, Sergentanis TN, Perlepe C, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol. 2015;26(3):589–597. doi: 10.1093/annonc/mdu572 [DOI] [PubMed] [Google Scholar]
- 43.Kadiyala S, Strumpf EC. Are United States and Canadian cancer screening rates consistent with guideline information regarding the age of screening initiation?. Int J Qual Health Care. 2011;23(6):611–620. doi: 10.1093/intqhc/mzr050 [DOI] [PubMed] [Google Scholar]
- 44.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canadian Task Force on Preventive Health Care. Colorectal cancer screening. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2001;165(2):206–208. [PMC free article] [PubMed] [Google Scholar]
- 46.US Preventive Services Task Force. Screening for colorectal cancer: Recommendation and rationale. Ann Intern Med. 2002;137(2):129–131. doi: 10.7326/0003-4819-137-2-200207160-00014 [DOI] [PubMed] [Google Scholar]
- 47.Lipskie TL. A summary of cancer screening guidelines. Chronic Dis Can. 1998;19(3):112–130. [PubMed] [Google Scholar]
- 48.Ringash J; Canadian Task Force on Preventive Health Care . Preventive health care, 2001 update: screening mammography among women aged 40-49 years at average risk of breast cancer. CMAJ. 2001;164(4):469–476. [PMC free article] [PubMed] [Google Scholar]
- 49.US Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137(5 part 1):344–346. [DOI] [PubMed] [Google Scholar]
- 50.McDavid K, Lee J, Fulton JP, Tonita J, Thompson TD. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Rep. 2004;119(2):174–186. doi: 10.1177/003335490411900211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US Preventive Services Task Force. Screening for prostate cancer: recommendations and rationale. Ann Intern Med. 2002;137:915–916. [DOI] [PubMed] [Google Scholar]
- 52.Prostate cancer (2014). Canadian Task Force on Preventive Health Care website. Accessed October 13, 2021. https://canadiantaskforce.ca/guidelines/published-guidelines/prostate-cancer [Google Scholar]
- 53.Smith RA, Cokkinides V, Eyre HJ; American Cancer Society . American Cancer Society guidelines for the early detection of cancer, 2004. CA Cancer J Clin. 2004;54(1):41–52. doi: 10.3322/canjclin.54.1.41 [DOI] [PubMed] [Google Scholar]
- 54.Sherman R, Firth R, Charlton M, eds. Cancer in North America, 2014-2018, Volume Three: Registry-specific Cancer Mortality in the United States and Canada. North American Association of Central Cancer Registries; 2021. [Google Scholar]
- 55.Kotwal AA, Schonberg MA. Cancer screening in the elderly: a review of breast, colorectal, lung, and prostate cancer screening. Cancer J. 2017;23(4):246–253. doi: 10.1097/PPO.0000000000000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The Patient Protection and Affordable Care Act. 2010. Public Law 111–148. March 23, 2010. https://www.govinfo.gov/content/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf
- 57.Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries' (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;2014(49):198–209. doi: 10.1093/jncimonographs/lgu018 [DOI] [PMC free article] [PubMed] [Google Scholar]