Abstract
There is increasing appreciation that non-neuronal cells contribute to the initiation, progression and pathology of diverse neurodegenerative disorders. This review focuses on the role of astrocytes in disorders including Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease and Amyotrophic Lateral Sclerosis. The important roles astrocytes have in supporting neuronal function in the healthy brain are considered, along with studies that have demonstrated how the physiological properties of astrocytes are altered in neurodegenerative disorders and may explain their contribution to neurodegeneration. Further, the question of whether in neurodegenerative disorders with specific genetic mutations these mutations directly impact on astrocyte function, and may suggest a driving role for astrocytes in disease initiation, is discussed. A summary of how astrocyte transcriptomic and proteomic signatures are altered during the progression of neurodegenerative disorders and may relate to functional changes is provided. Given the central role of astrocytes in neurodegenerative disorders, potential strategies to target these cells for future therapeutic avenues is discussed.
Keywords: Astrocyte, glia, neurodegeneration, synapse
1. Introduction
Astrocytes are a major glial cell-type in the central nervous system (CNS) and are well-established regulators of neural circuit function in the developing and adult brain1–4. In the developing brain astrocytes participate in circuit development through diverse mechanisms that induce the formation, elimination and maturation of neuronal synapses. This includes secreted proteins such as thrombospondins5 and glypicans6 that induce de novo synaptogenesis; phagocytic receptors including Mertk and Megf107 that enable synapse elimination; and proteins including Hevin8 and chordin like 19 that induce synapse maturation. In the adult brain astrocytes are essential for maintaining the neuronal environment and participate in processes such as neurotransmitter recycling from the synaptic cleft, maintenance of the blood-brain-barrier (BBB), and regulation of energy homeostasis10,11. Astrocytes can sense neurotransmitters and respond with increases in intracellular calcium, and they also have the ability to signal to neurons via release of gliotransmitters12.
Studies have identified that at the transcriptomic and functional level properties of astrocytes are altered both in the healthy aging brain13–15 and in neurodegenerative disorders (NDs)16–20. Dysregulation of astrocyte calcium activity, glutamate uptake and mitochondrial respiration have been reported in NDs21–23, which in turn can have downstream detrimental effects on neuronal health. For these reasons, astrocytes are now being considered as key players in NDs that are characterized by altered synaptic function, synapse loss and neuronal death24, including Alzheimer’s Disease (AD), Huntington’s Disease (HD), Amyotrophic Lateral Sclerosis (ALS) and Parkinson’s Disease (PD; Table 1). However, whether astrocytes contribute to disease progression, slow disease progression, or take on a combination of positive and negative roles are questions that require further investigation.
Table 1.
Clinical features of neurodegenerative disorders.
| Alzheimer’s disease (AD) | Amyotrophic Lateral Sclerosis (ALS) | Huntington’s disease (HD) | Parkinson’s disease (PD) |
|---|---|---|---|
| Symptoms - Progressive memory loss, disorientation, mood and behavior changes, deep confusion | Symptoms - Muscle weakness and atrophy followed by progressive deterioration of motor functions, ultimately leading to death mainly due to respiratory failure | Symptoms - Motor symptoms: hyperkinetic uncontrolled movements known as chorea; Non-motor symptoms: memory and cognitive impairments, neuropsychiatric disorders (anxiety, depression, compulsive behavior) |
Symptoms - Motor symptoms: tremors, bradykinesia, rigidity, postural instability Non-motor symptoms: apathy, anxiety, depression, psychosis, compulsive behavior |
| Hallmarks - Extracellular deposits of amyloid-beta (Aβ) plaques followed by intraneuronal accumulation of neurofibrillary tangles composed of hyperphosphorylated tau. Astrogliosis | Hallmarks - Insoluble protein inclusions in the soma of motor neurons containing TAR DNA binding protein-43 (TDP-43). Astrogliosis. | Hallmarks - Abnormal poly-glutamine tail cause oligomerization and aggregation of Htt into intra-neuronal inclusions. Astrogliosis. | Hallmarks - Formation of cytoplasmic inclusions called Lewy bodies, containing α-synuclein. Astrogliosis. |
| Brain regions affected - Entorhinal cortex and hippocampus are the primary affected brain regions followed by cerebral cortex. | Brain regions affected - Motor neurons in the brain, brainstem and spinal cord. | Brain regions affected - The dorsal striatum (basal ganglia) is primarily affected, followed by cortical involvement in all areas. | Brain regions affected - Loss of dopaminergic neurons of the substantia nigra pars compacta of basal ganglia. |
| Causes - More than 95% of cases are sporadic involving numerous environmental or genetic risk factors, while less than 5 % of cases are described as familial forms since they are due to inherited mutations resulting in more aggressive disease with earlier onset. These mutations have been observed on three genes: amyloid precursor protein (APP), presenilin 1 (PS1) and presenilin 2 (PS2). | Causes - 90% of cases occur without family history (sporadic) and 10% are inherited through autosomal dominant mutation (familial). The C9orf72 (40%), SOD1 (12–20%), TARDBP (4%), and FUS (5%) genes are the most commonly mutated genes observed in familial ALS. | Causes - Hereditary autosomal-dominant disease caused by mutation on huntingtin (Htt) gene. Expansion of repeating glutamines at the N-terminus. | Causes - Complex combination of environmental factors and genetic factors (e.g. mutations on SNCA, GBA1, LRRK2). |
| Risk factors - Aging, ApoEε4 allele, cardiovascular pathologies, diabetes, lack of sleep, sedentary lifestyle | Risk factors - Aging, sex (before age of 65 men are more affected than women), environmental toxin exposure (metals, pesticide), vigorous physical activity (athletes, military) | Risk factors - The number of glutamine repeats accounts for about 60% of the variation of the age of the onset of symptoms. 36 to 39 repeats result in a reduced-penetrance, with a later onset and slower progression of symptoms. More than 60 repeats result in onset below the age of 20, known as juvenile HD. | Risk factors - Exposure to pesticides, consumption of dairy products, traumatic brain injuries, MPTP |
|
Astrocyte-identified roles in human pathologies - astrogliosis with pro-inflammatory phenotype - ApoE predominantly expressed by astrocytes - glucose metabolism impaired - decreased expression of glutamate transporters - release of pro-inflammatory cytokines -alteration of potassium homeostasis |
Astrocyte-identified roles in human pathologies - astrogliosis with pro-inflammatory phenotype - decreased expression of glutamate transporter (EAAT2) - increased release of chemokines and cytokines |
Astrocyte-identified roles in human pathologies - astrogliosis with pro-inflammatory phenotype -altered expression of genes involved in calcium signaling and neurotransmitter recycling |
Astrocyte-identified roles in human pathologies - astrogliosis with pro-inflammatory phenotype - α-synuclein inclusions observed in astrocytes suggesting clearance defect |
| Main approved treatments*- Aducanumab (Aβ immunotherapy) |
Main approved treatments*- - Riluzole (glutamate antagonist) - Nuedexta (NMDAR antagonist) - Radicava (antioxidant) - Tiglutik (glutamate antagonist) |
Main approved treatments*- Deutetrabenazine (VMAT2 inhibitor) |
Main approved treatments* - Deep brain stimulation (thalamus, globus pallidus, or subthalamic nucleus) - Levodopa (dopamine precursor) |
| References 29,185–190 | References 29,191–195 | References 16,29,196 | References 29,197–200 |
No effective cure for disorders.
A common feature observed in NDs is the transition of astrocytes to a state of reactive astrogliosis25. This process is characterized by morphological, transcriptional, biochemical, metabolic, and physiological transformation of astrocytes in response to a pathological alteration to their microenvironment. It is now appreciated that astrocyte reactivity is a heterogeneous response, where the type of insult will induce different types of astrocyte reactivity. For example, reactive astrocytes can serve a neuroprotective role after stroke by stimulating angiogenesis and vascular remodeling. Reactive astrocyte ablation led to worsened motor recovery in a photothrombotic stroke mouse model26. Similarly, attenuation of astrocyte reactivity in a glial fibrillary acidic protein (Gfap) and vimentin (Vim) double knockout mouse model resulted in diminished axonal outgrowth after photothrombotic stroke and increased amyloid plaque deposition in an APP/PS1 mouse model of AD27,28. On the other hand, reactive astrocytes induced by an inflammatory stimulation in mice were suggested to take on a neurotoxic phenotype29,30. Advancements in single cell sequencing technology have permitted sensitive characterizations of a variety of reactive astrocyte subpopulations in different NDs (below), demonstrating that there is a broad spectrum of the astrocyte reactive state.
Increasing awareness of astrocyte heterogeneity extends beyond the reactive state. Astrocyte molecular signatures vary by brain region, indicating the utilization of region-specific astrocyte transcription factors (TFs)31. Additionally, there is mounting evidence that astrocytes are transcriptionally encoded to support the neurons that reside within their same anatomical region, and that astrocytes from different regions fail to compensate when neighboring astrocytes are injured or lost. For example, ventrally located spinal cord astrocytes fail to compensate after acute dorsal spinal cord injury, resulting in synaptic deficits on the dorsally located motor neurons32. An intriguing idea is that the distinct anatomical brain regions affected in different NDs (hippocampus in AD, striatum in HD, motor cortex and spinal cord in ALS, and substantia nigra in PD) may be due to molecular disturbances in the regional astrocytes, causing a failure to support the local neurons and ultimately progressing to neurodegeneration.
In this review we first ask how the physiological properties of astrocytes are altered in NDs and how this may explain their contribution to neurodegeneration. Second, we ask whether, in NDs with specific genetic mutations, these mutations either directly or indirectly impact on astrocyte function. Third, we ask how astrocyte transcriptomic and proteomic signatures are altered during the progression of neurodegeneration and how they may relate to functional changes observed. Given the central role of astrocytes in NDs, we end with a discussion of potential strategies to target these cells for future therapeutic avenues.
2. Astrocyte physiological changes
In this section we consider the impact that the pathology present in different NDs has on the functions of astrocytes, and how these alterations of astrocyte function may impact on neurons and neurodegeneration progression (Figure 1).
Figure 1: Astrocyte physiological alterations associated with neurodegeneration progression.
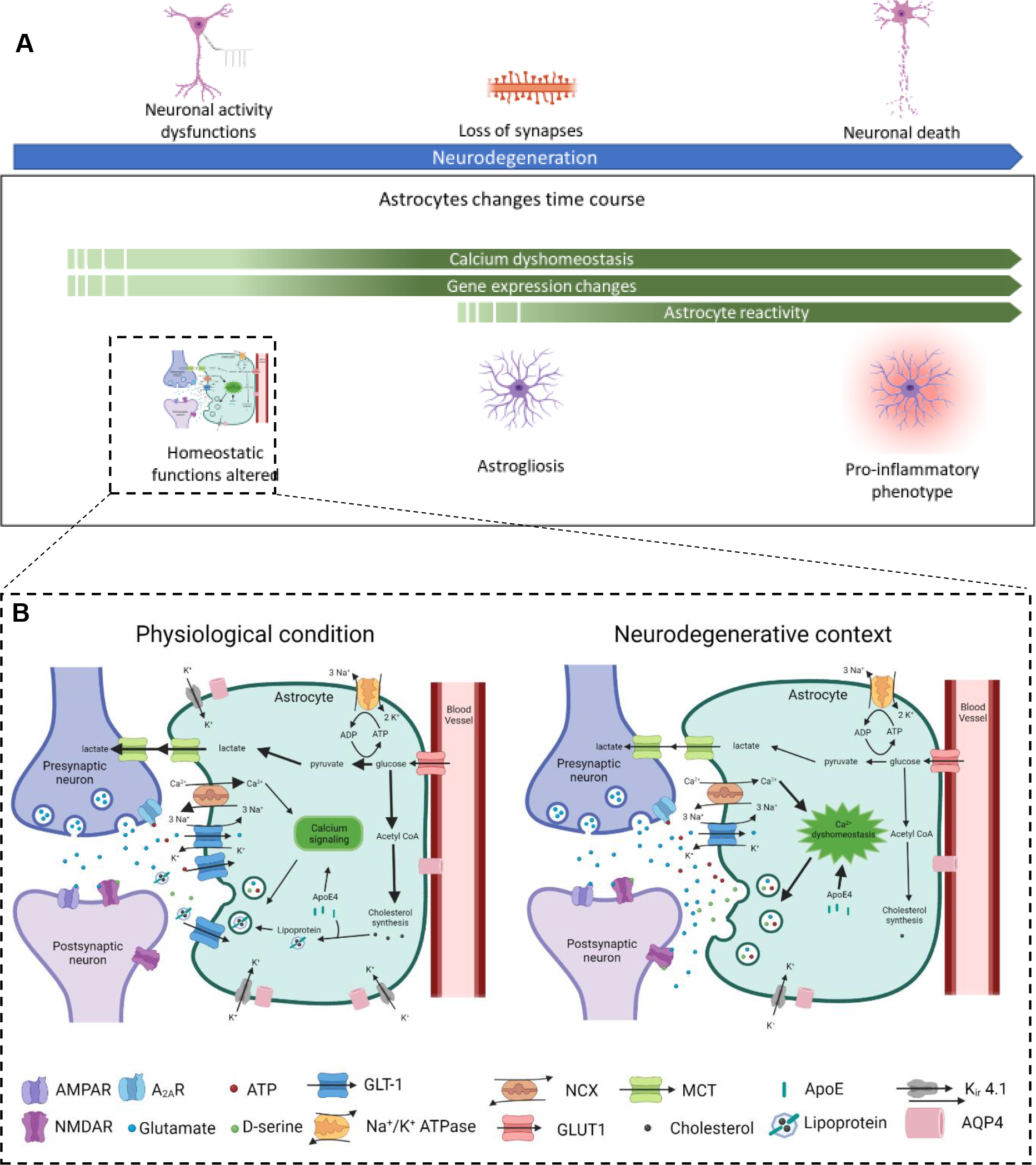
A. Astrocytes undergo physiological changes in conjunction with the progression of neurodegeneration. At early stages, astrocytes display calcium dyshomeostasis and changes in gene expression resulting in alteration of their homeostatic function at the synapse, which correlates with neuronal activity dysfunction. As neurodegeneration progresses astrocytes acquire a reactive phenotype (astrogliosis), often observed in parallel to loss of synapses. In late stage neurodegeneration astrocytes upregulate pathways that contribute to the pro-inflammatory environment and neuronal damage. B. Alteration of astrocyte homeostatic functions contributes to synaptic dysfunction in NDs. In physiological conditions astrocytes play a key role in maintaining synaptic homeostasis through numerous processes, many of which are regulated by intracellular calcium levels in astrocytes. These include the uptake and recycling of neurotransmitters, for example glutamate via GLT-1; uptake of glucose from the blood via GLUT1, which is converted to lactate and exported to neurons for energy production via MCTs; synthesis of lipids including cholesterol, which is packaged in ApoE lipoparticles and exported for uptake by neurons; maintenance of extracellular potassium levels via the potassium channel Kir4.1; release of gliotransmitters including ATP/adenosine, glutamate and D-serine, that interact with neuronal receptors and modulate synaptic transmission; maintenance of water balance via the channel AQP4, which also contributes to the glymphatic system. In neurodegeneration many of these physiological functions are altered, leading to dysfunction in synaptic communication. Intracellular calcium levels are dysregulated, in part due to altered activity of the sodium-calcium exchanger NCX. This impacts other cellular processes, including the release of gliotransmitters. Glutamate uptake is impacted by altered GLT-1 activity and level. The outcome of these changes is an increase in extracellular glutamate, which can contribute to excitotoxic damage. Reduced GLT-1 also impacts lactate supply to neurons, contributing to impairment of neuronal energy metabolism. Decreased expression of Kir4.1 is observed in many NDs, which impacts neuronal excitability. Astrocyte cholesterol synthesis is also impaired, with the E4 isoform of ApoE contributing to altered lipid metabolism.
2.1. Astrocytes and proteinopathies
A common pathological hallmark in many NDs is the accumulation of particular proteins that have the capacity to aggregate, causing proteinopathy. For example, AD, HD, PD and ALS are characterized by accumulation of amyloid beta (Aβ) and phosphorylated tau, mutated huntingtin, α-synuclein and TDP-43, respectively. In pathological conditions, these proteins undergo conformational changes and form oligomers that can form into bigger structures33. The relative toxicity of the different aggregated species for astrocytes remains under debate. In the case of ALS, TDP-43 aggregates can directly affect astrocyte survival due to subcellular mis-localization, as described for inducible pluripotent stem cell (iPSC) derived astrocytes carrying mutated TDP-4334. In the case of AD, amyloid plaques which contain aggregated Aβ have been considered as central to pathology and responsible for triggering astrocyte reactivity, as observations made in post-mortem tissue indicated a higher density of reactive astrocytes around plaques35. Recent work suggests that soluble oligomeric species of Aβ also impact astrocytes and can trigger astrocyte dysfunction before they become reactive36. It is however unclear if the continual aggregation of Aβ is driving astrocyte reactivity, or if astrocytes become reactive in order to constrain Aβ in plaques and neutralize the toxic properties of soluble oligomers37.
Astrocytes take part in a fluid-clearance pathway called the glymphatic system38. This pathway relies on a high level of aquaporin 4 (AQP4, water channel) in astrocytes that helps to create a flow from the arterial perivascular spaces to venous perivascular spaces, facilitating astrocyte clearance of factors from the brain interstitium towards the systemic circulation. AQP4 in astrocytes is also tightly colocalized with the potassium channel Kir4.1 (Kcjn10) and both play a synergistic role in osmolarity maintenance39. In AD, it has been estimated that 65% of Aβ peptide is removed from the brain through the glymphatic system38. The glymphatic system also plays an active role in the clearance of mutated huntingtin in HD mice40. Consequently, the downregulation of Kir4.1 that occurs in HD41 or the downregulation of AQP4 observed in AD42 can result in diminished clearance abilities and possibly contribute to protein accumulation. Indeed, the knock-out of Aqp4 in an amyloidosis APP/PS1 mouse model of AD increased Aβ accumulation within the brain resulting in exacerbation of cognitive deficits43. Furthermore, apolipoprotein E (ApoE) is involved in astrocyte clearance of Aβ and the ApoEe4 protein isoform fails to provide efficient clearance of the peptide (as reviewed in below )44. Astrocytes can also contribute to protein clearance through the lysosomal pathway by internalization of proteins. This pathway is reportedly disturbed in PD, where a mutation in Lrrk2 (reviewed below) affects the astrocytic lysosomal degradation of α-synuclein45. These findings suggest that astrocyte dysfunctions could contribute to or be affected by proteinopathy associated with multiple NDs.
2.2. Calcium dyshomeostasis
Under physiological conditions calcium signaling in astrocytes is associated with a broad range of processes including their interaction with synapses46. The complexity of astrocyte calcium signaling has been highlighted by a study that performed 3D calcium imaging of astrocytes in the cortex of awake mice47, showing that the majority of spontaneous calcium events are brief and localized at microdomains, thought to represent peri-synaptic astrocytic processes48. Consequently, dysregulation of astrocyte calcium signaling could result in deleterious consequences on synapses, leading to its study in multiple NDs.
An abnormal increase of intracellular calcium has been observed in the SOD1-G93A mouse model of ALS involving release of calcium from intracellular stores49,50. Altered Ca2+ homeostasis has been reported in the 6-hydroxydopamine-lesioned rodent model of PD51 and more recently in iPSC-derived astrocytes from PD patients22. In HD21 and AD36 alterations of spontaneous calcium activity in astrocytes have been observed in mouse models, with an upregulation in AD and downregulation in HD. In these studies, the calcium alterations occurred in microdomains in close proximity to synapses, and before the appearance of astrogliosis. This suggests that astrocyte calcium physiology is affected early in neurodegeneration progression, and could be involved in synaptic activity dysfunction of neighboring neurons. Acute exposure of hippocampal slices to Aβ oligomers failed to induce synaptic dysfunction when calcium elevation was blocked in astrocytes, suggesting not only involvement but a causal effect of astrocyte calcium dyshomeostasis in the triggering of synaptic dysfunction in AD52. Calcium is an essential second messenger for many functions in astrocytes, and global silencing of calcium dynamics is deleterious for basal synaptic transmission53. This means that a better understanding of molecular mechanisms underlying calcium dyshomeostasis in astrocytes in NDs is required to enable their specific targeting without altering the homeostatic roles of astrocytes.
A number of mechanisms have been proposed to underlie the alteration of astrocytic intracellular calcium levels in NDs, including activation of transmitter receptors and ion channels. These include two studies performed in the APP/PS1 model of AD. In the first, chronic intracerebroventricular infusion of an antagonist of the P2Y1 purinergic receptor that mediates astrocytic intracellular calcium release and astrocyte hyperactivity normalize astrocyte function, thus highlighting its involvement in calcium elevations in AD54. In the second study, blockade of the calcium channel TRPA1 was sufficient to normalize astrocytic activity, highlighting the involvement of calcium entry through membrane channels52. The two studies were performed at different stages of pathology progression, after and before the onset of astrogliosis respectively, and in both the chronic normalization of astrocyte calcium activity was sufficient to delay neuronal alterations and preserve cognitive function, identifying a critical role for astrocyte calcium signaling in the progression of pathology. This suggests that the trigger of calcium dyshomeostasis is not only disorder-specific but also stage-specific within a ND. However, little is known about mechanisms connecting the main pathological hallmarks of the different NDs (Table 1) and the triggering of aberrant calcium activity in astrocytes. Nevertheless it is clear that astrocyte calcium signaling is widely altered in the context of neurodegeneration, which could result in the impairment of essential functions that rely on calcium homeostasis and signaling.
2.3. Synaptic activity and ion homeostasis
An important role of astrocytes is neurotransmitter uptake and recycling. Glutamate is the main excitatory neurotransmitter in the brain and under physiological conditions it is estimated that 80% of glutamate released to the synaptic cleft is taken up by excitatory amino acid transporters 1 and 2 (GLAST and GLT-1) localized to astrocyte processes and recycled55. Decreased expression of GLT-1 is a common feature observed in neurodegenerative disorders56. For example there is an alteration of GLT-1 trafficking to the plasma membrane in a PD mouse model (LRRK2 G2019S, genetic risk factor for PD, see below), resulting in intracellular re-localization and degradation of the transporter57. Intriguingly, knock-down of GLT-1 in astrocytes in either the substantia nigra or striatum is sufficient to induce changes at the molecular and behavioral level that overlap with those seen in PD, including neuronal death and astrocyte reactivity58,59. This suggests alterations to glutamate transporter level, function or localization will alter the level of glutamate in the extracellular space, for example a decrease in transporter function may cause accumulation and spill-over of glutamate from the synaptic cleft, possibly contributing to excitotoxicity and neurodegeneration.
Excitotoxicity is neuronal death caused by excessive post-synaptic receptor activity, particularly at glutamatergic synapses60, and has been proposed to explain the pathogenesis of NDs61–64. However, in most NDs neuronal death is preceded by alterations at the level of synapses65, for example in AD, synapse loss has long been known to be the major correlate of cognitive impairment in human pathology66. Neuronal death is also absent in most amyloidosis mouse models of AD, although these models do demonstrate synapse loss and cognitive impairment phenotypes67. It is still unclear whether synapse loss is a direct cause of neuronal death or if they are two distinct mechanisms; studies investigating astrocyte contribution to synapse loss has been recently reviewed68. A long-standing hypothesis connecting glutamate dyshomeostasis and the loss of synapses observed in neurodegeneration is the overactivation of extra-synaptic glutamatergic NMDA (eNMDA) receptors which are deleterious for synaptic stability69,70 and thought to promote cell death in NDs71. These studies showed that eNMDA activation is a consequence of glutamate release from astrocytes after exposure to Aβ or α-synuclein oligomers. The release of glutamate from astrocytes is a process known as gliotransmission, that also includes the release of ATP, D-serine and GABA, giving the astrocyte the possibility to directly modulate synaptic transmission12. Similarly to the increased release of glutamate, the release of ATP72, D-serine52 and GABA73 is also increased in AD, contributing to altering synaptic transmission and/or cognitive performance.
Both the uptake and release of transmitters from astrocytes are linked to calcium levels in the cells, for example compartmentalized calcium activity in astrocyte microdomains is associated with release of gliotransmitters53,74. The activity of GLT-1 itself is not directly associated with calcium, but uptake of glutamate is co-dependent on sodium entry and the sodium gradient is regulated by the sodium calcium exchanger NCX which is colocalized with GLT-1 in synaptic microdomains75. Additionally potassium is extruded by GLT-1, and astrocytes regulate potassium homeostasis via the potassium channel Kir4.176. Altogether, this shows that these three molecular actors have an interconnected functional relationship that may be involved in neurodegeneration progression as a cause or a consequence of calcium dyshomeostasis, affecting not only synaptic transmission but also ion homeostasis. This is supported by different lines of evidence showing involvement of NCX and Kir4.1 in the ND context. Changes in the activity of NCX isoforms have been reported in ALS77 and AD78, and Kir4.1 is downregulated both in astrocytes derived from ALS patients with SOD1 mutations79 and in R6/2 and Q175 mouse models of HD41. Moreover, rescue of potassium channel expression in astrocytes can ameliorate aspects of neuronal dysfunction, prolong survival and attenuate some motor impairments in R6/2 HD mice41.
2.4. Energetics and lipid metabolism
There are several lines of evidence suggesting energetic metabolism impairments in AD, PD, ALS and HD, for example enhancement of glucose uptake and metabolism has neuroprotective effects in models of these NDs80. Astrocytes are considered at the forefront of these impairments as neurons are isolated from the systemic circulation by the BBB and rely on astrocytes to obtain metabolic substrates. A well described mechanism is the astrocyte–neuron–lactate shuttle (ANLS)81. This pathway couples glutamate transporter activity to the conversion of glucose into lactate, with lactate then exported to neurons for energy use. Astrocytic lactate is required for memory function and can modulate synaptic plasticity82,83, although it should be noted that the requirement for the ANLS in basal synaptic transmission is not fully established. This suggests decreased GLT-1 expression can indirectly affect the astrocyte–neuron metabolic relationship, as the activity of glutamate transporters is central to the ANLS. The involvement of astrocytic lactate in AD, PD and ALS has been recently reviewed84.
Cholesterol and unsaturated fatty acids are known to be enriched at the synaptic membrane, where they play an important role in membrane fluidity, vesicle formation and fusion, ion channel function and clustering, and stability of neurotransmitter receptors85. The BBB prevents most lipids from entering the brain and neurons are inefficient in lipid synthesis, so they rely on astrocytes to provide cholesterol, which is secreted as a lipoprotein complex containing ApoE, which is mainly produced by astrocytes in the brain86. Clinical data have shown that glucose metabolism is altered in human ApoEe4 carriers before pathological hallmarks and memory symptoms of AD appear (as reviewed below)87. A recent study also suggested that the different isoforms of ApoE exert different effects on lactate synthesis in astrocytes88. Moreover ApoE is also involved in increasing calcium excitability in astrocytes and altering intracellular cholesterol distribution89. Therefore, in the context of AD, variants of the astrocyte-enriched protein ApoE are at the intersection of cellular dysfunction where calcium signalling, energy metabolism and lipid metabolism are affected. Additionally, glucose metabolism is closely linked to lipid metabolism as astrocyte cholesterol can be synthetized from glucose-derived Acetyl CoA90, so a prediction of altered glucose metabolism in neurodegeneration is that this will also impact lipid metabolism. Besides AD, astrocytes display dysfunctional cholesterol metabolism in HD, and enhancing cholesterol biosynthesis in astrocytes preserves neuronal functions91,92. Altered levels of cholesterol metabolites are also found in blood, CSF, and brain tissue of ALS93 and PD94 patients. Therefore, neurons depend on astrocytes to provide them with both the energy substrates and cholesterol required for synaptic activity, and both these supplies are altered in neurodegeneration, suggesting a central role for astrocytes in these alterations.
3. Genetic risk for ND impacting astrocytes
Although they share many common features, NDs are a diverse set of maladies that are driven by unique genetic and environmental factors, affect different brain regions, and manifest with various physiological changes. Some diseases like HD and Alexander’s disease (AxD) are monogenic diseases caused by well-defined mutations in a single gene95,96. Other diseases such as AD have complex etiologies where the genetic heritability is divided among several genes and has yet to be fully elucidated97. The majority of early work to study these diseases focused on how these mutations drive neuronal dysfunction. However, as our understanding of these diseases improves, it is clear that these mutations have prominent effects on other cells, including astrocytes. In fact, in some cases it appears that these mutations initiate molecular events in astrocytes that serve as the primary drivers of disease onset and ultimately the dysfunction and loss of neurons. We consider the impact of genetic mutations in NDs in three broad categories: 1) mutations in genes enriched in astrocytes, 2) variants in genes that are expressed in astrocytes as well as other cell-types, and 3) astrocytes reacting to mutations in genes that are enriched in neurons (Figure 2).
Figure 2: Astrocyte and neuron expression of genes linked to risk for ND.
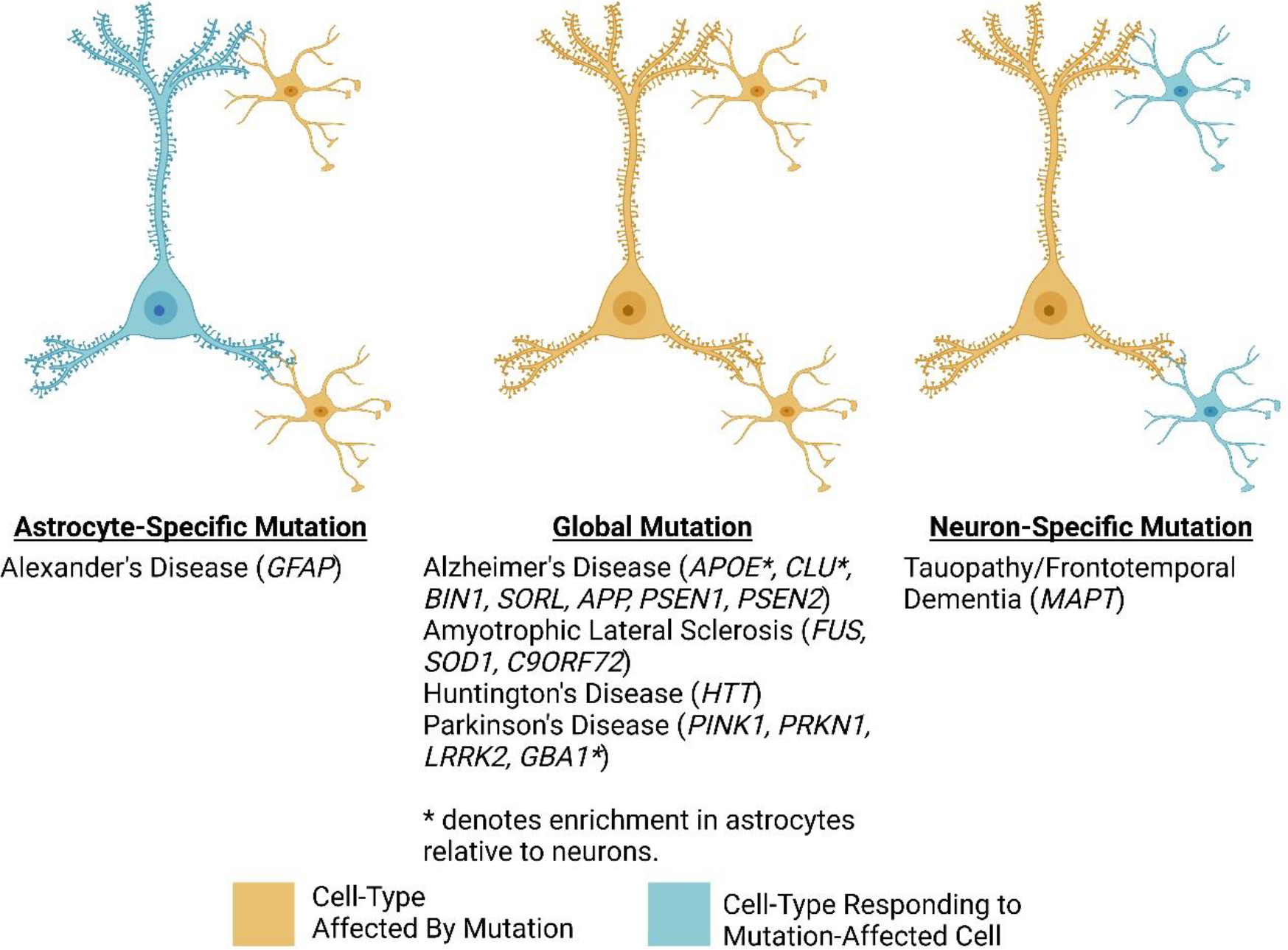
ND-associated mutations affect specific cell types depending on the disorder. This figure shows examples of astrocyte-specific, global, and neuron-specific mutations organized by disorder. For those mutations that affect neurons and astrocytes, some of these genes have enriched expression in astrocytes (indicated by an * next to the gene symbol).
3.1. Mutation in genes enriched in astrocytes
AxD is considered the first example of a ND that is primarily caused by variants in a gene enriched in astrocytes95. AxD is a rare leukodystrophy characterized by the destruction of the myelin sheath around axons, resulting in speech abnormalities, swallowing difficulties, seizures, and ataxia. Most cases appear before the age of two years and usually lead to death within the first decade of life95. AxD is caused by mutations in glial fibrillary acidic protein (Gfap), which encodes a cytoskeletal protein that is highly enriched in astrocytes and commonly used as an astrocytic marker in the brain. This leads to the deposition of cytoplasmic inclusions within astrocytes known as Rosenthal fibers95. Initially, AxD was described as an autosomal dominant genetic disorder95,98. However, more recent studies suggest that some patients carry recessive mutations in Gfap that can also lead to phenotypes consistent with AxD98. While the majority of AxD cases are caused by single nucleotide polymorphisms95,99, insertion and deletion events in the gene have also been described98. Interestingly mice lacking Gfap do not show the same phenotypes as those seen with disease-specific Gfap mutations100, so it is unclear what the molecular consequences of these Gfap mutations are, although one study found downregulation of the glutamate transporter GLT-1 in patients and mouse models of AxD, suggesting a link to excitotoxicity (discussed below)101. Most mouse models of AxD present a mild phenotype, and recently a rat model was developed that presents the main features of the human disease, including myelin pathology and motor impairments102. The introduction of the R239H mutation to Gfap, which is present in patients with early-onset and severe AxD, was sufficient to cause astrocyte dysfunction as well as non-cell-autonomous effects. Targeting Gfap using antisense oligonucleotides reversed the pathology, white matter deficits, and motor impairments. These findings support the hypothesis that in certain disorders genetic alterations to astrocytes can constitute the starting point of a neurodegenerative cascade.
3.2. Mutation in genes expressed by many cells
The most common category to consider is that where NDs are caused by mutations that occur in genes expressed by many cell types in the body, including astrocytes. One classic example is HD—a rare disorder caused by a CAG repeat expansion in exon 1 of the huntingtin (Htt) gene. In neurons, the Htt mutation has wide-ranging cellular consequences, including aberrations in synaptic function, ion homeostasis, autophagy, and metabolism103–106. However, HTT is a large scaffold protein required for diverse biological functions in many cell types105,107, including astrocytes108. Expression of mutant Htt in astrocytes is sufficient to drive changes in gene expression, as well as contribute to neurodegeneration108–110. For example, expression of mutant Htt specifically in striatal astrocytes causes motor function deficits in mice, which may be due to decreased expression of glutamate uptake transporters and potential excitotoxicity (discussed below)108. Conversely, selectively removing mutant Htt from astrocytes (retaining expression in other cells ), is sufficient to improve the pathological phenotype and attenuate disease progression110. Transplantation studies in mice demonstrated that human glial progenitor cells expressing mutant HTT were sufficient to induce HD-associated phenotypes and behaviors, whereas transplantation of non-diseased glial progenitors to an HD mouse model was sufficient to rescue some of the aberrant phenotypes, including restoration of potassium homeostasis109.
In PD, mutations in genes including PTEN induced kinase (Pink1), parkin 1 (Prkn1), and leucine-rich repeat kinase 2 (Lrrk2) are all able to induce a Parkinsonian phenotype, and some have been shown to affect astrocyte function in a cell-intrinsic manner22,111. For example, in astrocytes differentiated from patient iPSCs carrying mutations in Lrrk2 or glucocerebrosidase (Gba) displayed metabolic alterations, aberrant calcium signaling, and an increased inflammatory response22. A separate study found alteration in production of extracellular vesicles by Lrrk2 mutant astrocytes, leading to altered communication with dopaminergic neurons that normally internalize these vesicles, suggesting a failure in astrocyte-neuron communication112. As reviewed above, a mouse model with a patient-specific Lrrk2 mutation shows reduced functionality of the glutamate transporter GLT-1, which may contribute to excitotoxicity57.
Mutations in ALS are also acting in astrocytes as well as neurons. For example, primary motor neurons carrying Sod1 mutations show mild morphological alterations but are overall functional. Astrocytes from this mouse model, however, secrete factors that are toxic to both wildtype and Sod1 mutant neurons113. Non-cell autonomous toxicity exerted onto motor neurons has also been described with human iPSC astrocytes carrying a hexanucleotide expansion in the C9orf72 loci114. In vitro models revealed that when co-cultured with these mutant astrocytes, motor neurons show multiple functional changes including wide-spread alterations in gene expression, decreased electrophysiological output, and reduced viability115. A hexanucleotide repeat-expansion mutation in iPSC-derived astrocytes also drives increased release of reactive oxygen species and reduced ability to support motor neurons in culture116. Mutations in mutant fused in sarcoma (Fus) increase the release of toxic factors from astrocytes that drive inflammation-induced motor neuron degeneration, and also increase AMPA glutamate receptor expression that could contribute to hyperexcitability present in ALS117. A recent study identified a component of the toxic output of astrocytes in ALS is inorganic polyphosphate, a biopolymer, an effect seen in astrocytes from both mouse models and patients with different mutations118, providing a potential target for therapeutic intervention (see below).
Familial AD cases have been attributed to mutations in amyloid precursor protein (App), as well as the genes encoding subunits of the gamma-secretase complex responsible for processing amyloid protein, presenilin 1 (Psen1) and presenilin 2 (Psen2)119,120. Single-cell profiling of iPSC-derived astrocytes demonstrated that these genes are expressed by astrocytes, in addition to neurons, at higher levels than previously appreciated, suggesting astrocytes may contribute to amyloid burden and be important drivers of familial AD121. Genome-wide association studies (GWAS) have revealed some common heritable factors underlying AD, with ApoE among a few genetic loci that explain a substantial portion of heritability122,123. ApoE is a secreted lipid-carrying protein involved in cholesterol homeostasis and also localizes to amyloid plaques (see above)124. Although neurons express ApoE, the protein product is primarily produced and secreted from astrocytes and microglia125. The ApoE epsilon 4 variant (ApoEe4) in particular is most predictive of late onset AD126. In a transgenic tauopathy mouse model (Tau P301s), the introduction of the ApoEe4 variant greatly exacerbated pathology through a toxic gain of function127. Conversely, individuals who carry the epsilon 2 (ApoEe2) variant have lower risk for AD128. Astrocytes derived from ApoEe4 iPSCs show significant transcriptional differences, altered cholesterol metabolism, diminished capacity for amyloid uptake, and decreased neuronal support, demonstrating that ApoEe4 affects astrocyte biology directly129–131. Furthermore, the selective knock-out of ApoE in astrocytes improved spatial memory, reduced Aβ deposition and blocked astrogliosis in an APP/PS1 model of AD132.
In all, the association between ApoE and AD demonstrates that factors expressed by astrocytes play central roles in disease pathogenesis and can act as significant genetic risk factors. Variation at other loci involved in lipid metabolism such as clusterin (Clu), sortilin-related receptor (Sorl1), and bridging integrator 1(Bin1) have also been associated with late-onset AD120. In post-mortem prefrontal cortex samples from patients with AD, Bin1 isoforms specific to astrocytes were found to be associated with AD133. Astrocyte-specific, AAV-mediated overexpression of Clu in the 5xFAD mouse model of AD ameliorated amyloid plaque pathology and rescued associated deficits in synaptic performance, suggesting a protective role134. Although a mechanism is far from fully elucidated, together these studies suggest that lipid metabolism in astrocytes is part of a critical genetic network in AD pathology.
3.3. Mutation in genes enriched in neurons
The study of rare familial cases of neurodegeneration uncovered variants in microtubule associated protein tau (Mapt)135 in cases of frontotemporal dementia (FTD)136. Mapt expression is neuron-specific, and there are several mutations associated with FTD. Functionally, these mutations lead to hyperphosphorylation of tau protein that results in the formation of neurofibrillary tangles137. Astrocytes have a multitude of biological responses to filamentous or insoluble oligomers of tau. Transgenic mouse models that overexpress mutant human tau show a loss of astrocytic support of synapses138. Moreover, the treatment of healthy primary astrocytes with exogenous tau oligomers elicits immune responses and cytokine release139. Multiple studies have observed that hyperphosphorylated tau is engulfed by astrocytes140 which can induce changes to astrocyte shape. This pathology has been defined as aging-related tau astrogliopathy, and is observed not only as part of AD and FTD pathology, but also in other diseases such as PD141. Although pathogenic levels of tau exert their influence on astrocytes, it is the changes induced within astrocytes that can affect the progression of the disease in neurons. Based on the observation that immune activation increases with disease progression, the role of the innate immune system in tau pathogenesis has been investigated, showing modulation of complement signaling from astrocytes and microglia can alter the impact mutant tau has on neurons142.
4. Astrocyte transcriptomics and proteomics
Transcriptomics and proteomics studies have demonstrated that many NDs are characterized by changes in large networks of genes and proteins in multiple cell types. Early microarray and RNA-sequencing (RNA-seq) studies probing for transcriptional changes in NDs were conducted at the bulk tissue level and thus were mostly informative for neuronal gene changes such as synapse-associated genes and plasticity genes143. Advancements in technology progressed towards isolation of specific cell types from distinct brain regions at the bulk level (i.e. fluorescence activated cell sorting (FACS), antibody-conjugated magnetic bead enrichment, genetically encoded RiboTag). One such study used FACs of astrocytes and microglia and identified a proinflammatory transition that occurs in AD, along with a downregulation of neuronal support genes15. Sequencing technologies have further advanced to enable analysis of single cells, which has elucidated the complicated heterogeneity of pathological subpopulations of astrocytes in NDs17–20 (summarized in Table 2). Beyond RNA there is increased focus on proteomics approaches due to advancements in technologies allowing more sensitive detection of proteins, single cell analysis, and phosphoproteomics to evaluate protein changes at different levels of regulation144,145. Studies using meta-analysis of multiple datasets have harnessed the power of bioinformatics to elucidate new insight into mechanisms contributing to neurodegeneration, such as early endolysosome dysfunction in astrocytes146, and subregional heterogeneity of astrocyte transcriptional changes amongst different cortical layers in AD147. Given that there is often low concordance between changes at the RNA and protein level145, a focus should be placed on multi-omics data integration to allow for full interpretation of astrocyte molecular changes in NDs.
Table 2. Summary of advancements in RNA-Sequencing technologies.
RNA-Sequencing technology has advanced in recent years to allow for more sensitive analyses, with pros and cons to each advancement. References give examples of studies using different sequencing approaches and findings of the studies.
| Sequencing Method | Pros | Cons | References | Findings |
|---|---|---|---|---|
| Bulk Tissue | Inexpensive Can analyze multiple cell types Deep coverage of transcriptome High sensitivity for low expression genes mRNA from distal processes included |
Reads dominated by most abundant cell types Transcripts from rare cell types can be diluted out |
143 | Synapse-associated genes show progressive decline in AD, but bulk analysis misses important immune-related transcript upregulation in glia |
| Bulk Cell Type-Specific | Fairly inexpensive Specific to one cell type |
mRNA from distal processes may be excluded if enzymatic digestion of tissue is necessary Bulk analysis does not permit subpopulation analysis |
151 | FACS of microglia and astrocytes identifies immune-related transcript upregulation in AD from full cortex |
| Bulk Cell Type- and Region-Specific | Fairly inexpensive Specific to one cell type Permits analysis of regional heterogeneity |
mRNA from distal processes may be excluded if enzymatic digestion of tissue is necessary Bulk analysis does not permit subpopulation analysis |
13,14 | Astrocyte RiboTag from multiple brain regions reveals some brain regions more vulnerable to immune-related transcript upregulation than others |
| Single Cell/Single Nucleus | Allows for identification of subpopulation heterogeneity Can sequence rare cell populations without extensive purification or enrichment protocols |
Expensive mRNA from distal processes (and cytosol for nuclear sequencing) excluded after enzymatic digestion Poor sensitivity to detect low expression genes Data analysis requires more processing power and storage capacity than bulk analysis |
17–20 | Identification of pathological, disease-associated subpopulations of astrocytes in AD and HD |
4.1. Decreased synaptic support with aging
Although reactive astrogliosis is a hallmark of pathology in many NDs, it is important to note that astrocytes also show some markers of reactivity in physiological aging. In mouse studies using FACS or RiboTag to isolate astrocyte RNA for analysis, aged astrocytes were shown to upregulate genes related to immune signaling pathways15 and synapse elimination such as components of the complement cascade including C313,14,148. Decreased synaptic support with aging is predicted by downregulation of the transcript encoding the major rate-limiting enzyme for cholesterol synthesis, Hmgcr13. Conversely, transcripts associated with general astrocyte homeostatic functions such as Kcjn10 (Kir4.1 potassium channel) and Slc1a2 and Slc1a3 (GLT-1 and GLAST glutamate transporters) are unaltered in aged astrocytes13. This shows that aged astrocytes resemble reactive astrocytes in upregulation of genes related to proinflammatory signaling, antigen presentation and immune pathways13,14. There is an increased proportion of astrocytes in aged mice expressing reactive genes (Serpina3n and Cxcl10) after immune system insult with lipopolysaccharide (LPS) than astrocytes in young mice14, suggesting aged astrocytes are more sensitive to immune activation. These results suggest that in physiological aging astrocytes naturally become less supportive of neuronal synapses and more reactive, creating an environment that is poorly suited for synaptic function. However, there is less evidence that astrocytes undergo major alterations to their homeostatic functions in aging, such as neurotransmitter recycling, BBB maintenance or ion buffering in support of neuronal function (see above).
4.2. Decreased astrocyte homeostatic support
As noted, astrocytes upregulate complement cascade and immune components in physiological aging13,14, but this effect is exacerbated in NDs. For example, in human post-mortem tissue from AD, ALS, PD and HD the proportion of C3+ astrocytes is significantly increased in the prefrontal cortex, motor cortex, substantia nigra and caudate, respectively, compared to age-matched controls29. Increased astrocyte reactivity is particularly strong in AD, with an increase from 20% to 60% C3+ astrocytes in AD29. As described above, there are known astrocyte functional changes in neurodegeneration, including changes to intracellular calcium signaling that may modulate neuronal synaptic activity, neurotransmitter recycling, ion buffering, BBB maintenance and metabolic support, that may create an environment that is not conducive to synaptic function in NDs.
A single nucleus RNA-seq study on human post-mortem AD tissue (sNuc-Seq) found transcriptional changes suggesting functional impairments in the ability of astrocytes to support neuronal synapses. Notably, comparisons of different stages of pathology identified an early upregulation of the voltage-gated potassium channel Kcnip4 followed by a late stage downregulation, indicating a potential compensatory response to synaptic dysfunction early in disease, as well as a decrease in the metabotropic glutamate receptor Grm3 at late stages149. Upstream transcription factor (TF) analysis of a human AD dataset identified that TFEB, a transcriptional regulator of lysosomal function, was upstream of 10 GWAS loci in AD that were differentially expressed150, suggesting widespread alterations in protein homeostasis in astrocytes. Transcriptional analysis of acutely isolated astrocytes from the APP/PS1 mouse model of AD revealed a strong upregulation of transcripts associated with proinflammatory environments, suggesting a strong immune component in AD astrocytes151. Interestingly, it has been demonstrated with sNuc-Seq that astrocytes in AD upregulate more genes than they downregulate, whereas neurons are more likely to downregulate genes149, which suggests a gain of function phenotype for astrocytes in AD.
Similarly, a RiboTag study in the SOD1-G37R mouse model of ALS found a dominant trend of upregulated gene expression in mutant astrocytes, with 62% of 108 differentially expressed genes (DEGs) upregulated152. Transcripts encoding for inflammatory pathway and immune signaling components (Junb, Cebpd, Cebpb) increased, and downregulation of a transcriptional coactivator that modulates genes related to mitochondrial biogenesis (Ppargc1a) was observed152. Conversely, an astrocyte RiboTag study on two separate mouse models of HD (R6/2 and Q175) and post-mortem RNA-Seq of human striatal tissue demonstrated that 97% of 62 significantly changing transcripts shared between mice and humans were downregulated16. The authors propose these genes as a “core set” of consistent changes between two mouse models of HD differing in severity and pathology. Downregulated genes from the core set were related to calcium signaling (Camk4, Atp2b1, Itpr1, Cacna1e, Ryr3, Cacna2d3), GPCR signaling (Adcy5, Rgs9) and postsynaptic scaffolding (Shank3, Neto1, Dlg4)16, suggesting a widespread impairment of astrocyte modulation of synaptic activity. A study using iPSC-derived human astrocytes from PD patients showed reduced levels of the glutamate transporter Slc1a3, suggesting again impaired neurotransmitter recycling22. The PD astrocytes also had an upregulation of Ryr3 (calcium channel) and Itpr2 (receptor for calcium release from the endoplasmic reticulum), suggesting hyperactive calcium signaling in PD astrocytes (see Section 2.2)22.
Although homeostatic functions of astrocytes appear to be impaired in neurodegeneration, it is important to consider that not all changes to astrocytes during NDs are negative, and that some alterations may be an attempt to compensate for alterations in the brain environment. For example, there is an upregulation of components of the antioxidant system in astrocytes in AD, which may be protective against oxidative stress153. The Nrf2 transcription factor is a master regulator of the antioxidant response pathway and regulates many DEGs identified in the APP/PS1 and Tau P301s mouse models of amyloid and tau pathologies of AD154. Nrf2 upregulation in these mouse models decreases AD-related pathology, suggesting a protective role154. Manipulation of the JAK-STAT pathway to prevent astrocyte reactivity has had mixed results in AD mouse models. Blocking this pathway in APP/PS1 mice led to decreased amyloid plaque deposition and an improvement of synaptic deficits and spatial learning155,156, but failed to attenuate combined amyloid and tau pathology in 3xTg-AD mice157, which could indicate some differences and interactions between amyloid and tau pathologies in relation to astrocyte reactivity. In the SOD1-G93A mouse model of ALS genetic deletion of three cytokines that promote the formation of inflammatory reactive astrocytes (IL-1α, TNFα and C1q) extended their lifespan by 50%158. However, it should be noted that this was a global knockout and thus effects on non-astrocytic cells cannot be ruled out, and the SOD1-G93A mouse model does not recapitulate all features of the human disease, including TDP43 mis localization.
The transcriptional studies of astrocytes in different NDs suggest there may be common transcriptional changes across diseases (reduced neurotransmitter recycling/glutamate homeostasis and dysregulated calcium signaling) that correspond well with the known functional alterations described above. However, there are also distinct attributes of astrocyte transcriptional signatures across disorders, with prolonged inflammatory signaling and exacerbated immune modulation being more prominent in AD and ALS astrocytes compared to HD and PD. In addition, compensatory mechanisms may be more prominent at early disease stages than late pathology, and thus temporal dynamics of gene expression changes in NDs should be an important focus in the future.
4.3. Regional heterogeneity and reactivity
The notion that astrocytes from distinct brain regions are molecularly encoded to support neurons in that same region leads to a hypothesis that may explain why particular brain regions are disproportionately affected in different NDs. In the studies of aging mouse astrocytes discussed above it was found that although astrocytes from all brain regions upregulate genes related to immune pathways, they do so to different degrees. Cortical astrocytes showed the least changes with age, whereas those from the cerebellum, hippocampus and striatum, regions affected by different NDs, showed many more13,14. Importantly, these studies suggest that some brain regions may have greater vulnerability to immune insults and aging than others, something that should be considered in the context of NDs.
A sNuc-Seq study identified subpopulations of astrocytes in AD and mapped identified gene modules onto spatial transcriptomics datasets from mouse and human cortex. This showed a high degree of regional heterogeneity, with multiple astrocyte subclusters having differential expression in at least one cortical layer. For example, a subcluster in AD that showed upregulated transcripts associated with response to metal ions and downregulated axon-guidance pathways correlated with Layer 1 and white-matter astrocytes in the spatial transcriptomics dataset. A subcluster with upregulated immune pathway components did not show segregation and could be spatially localized to all cortical layers147. It should be noted that the spatial transcriptomics datasets used in these studies were from non-diseased humans and mice, and from mice exposed to acute LPS to cause an inflammatory response159,160. In the future, this same type of data integration study could be performed on AD spatial transcriptomics datasets as they become available.
In addition to regional heterogeneity, there is also a vast spectrum of astrocyte states in NDs that have been identified using sNuc-Seq. These correlate with both disease stage and proximity to pathology. Studies on human post-mortem tissue identified AD-specific pathological astrocyte subclusters, characterized by the highest levels of Gfap expression across all clusters, along with upregulation of other astrocyte reactive markers such as Cryab, Cd44 and Tnc and downregulation of glutamate transporters (Slc1a2, Slc1a3) and synaptic support molecules (Nrxn1 and Cadm2), including Sparcl1 and Ptn which encode for secreted proteins that regulate synapses in the healthy brain19,20. A pathological astrocyte subpopulation was also found in the hippocampus of 5xFAD mice and termed Disease Associated Astrocytes (DAAs)18. These DAAs had distinctively high levels of Gfap expression, along with upregulation of the reactive astrocyte marker Serpina3n, a lysosomal cysteine protease involved in APP processing (Ctsb), the intermediate filament protein Vim and the actin-depolymerizing protein Gsn. Spatial analysis of astrocytes positive for Gfap, Serpina3n and Vim demonstrated that DAAs are located near amyloid plaques. The DAAs upregulate more genes than they downregulate, again indicating acquisition of an altered transcriptional profile. DAAs appear in 5xFAD mice at 4 months, before any measurable cognitive decline, suggesting that early acquisition of a DAA phenotype may contribute to disease progression. It was also found that DAAs are present in wildtype mice at 13–14 months18, providing strong evidence that astrocytes in AD undergo an accelerated aging.
Pathological subpopulations of astrocytes have also been identified in other NDs. sNuc-Seq in human cingulate cortex identified four transcriptional states of astrocytes in HD17. The subpopulations were described as transitional states from homeostatic astrocytes to various degrees of astrocyte reactivity and were defined by changes in the expression of the glutamate transporter Slc1a2, the intermediate filament protein Gfap and a heavy metal ion binding protein with anti-oxidative properties Mt2a. A spatial transcriptomic analysis in the spinal cord of SOD1-G93A mouse model and post-mortem ALS patients identified an alteration in astrocyte transcriptional profiles early in the disease course, including an upregulation of reactive genes including Gfap, that was most pronounced near to the motor neurons that degenerate in ALS161.
The subpopulations of astrocytes detected in NDs are often characterized as reactive, which is often considered a negative trait. However as discussed above astrocyte reactivity can have both beneficial and detrimental effects depending on the context, in particular the stage of pathological progression of the disorder. Altogether, these studies suggest that homeostatic, pro-synaptogenic astrocytes are most prominent in the young brain (Figure 3A), but with aging there is a transition to a reactive state that creates an environment less supportive of neuronal synapses (Figure 3B). In NDs there are disease-associated subpopulations of astrocytes that have an exaggerated reactive state and are spatially localized near pathology (Figure 3C).
Figure 3. Astrocyte molecular phenotypes transition in aging and disease.
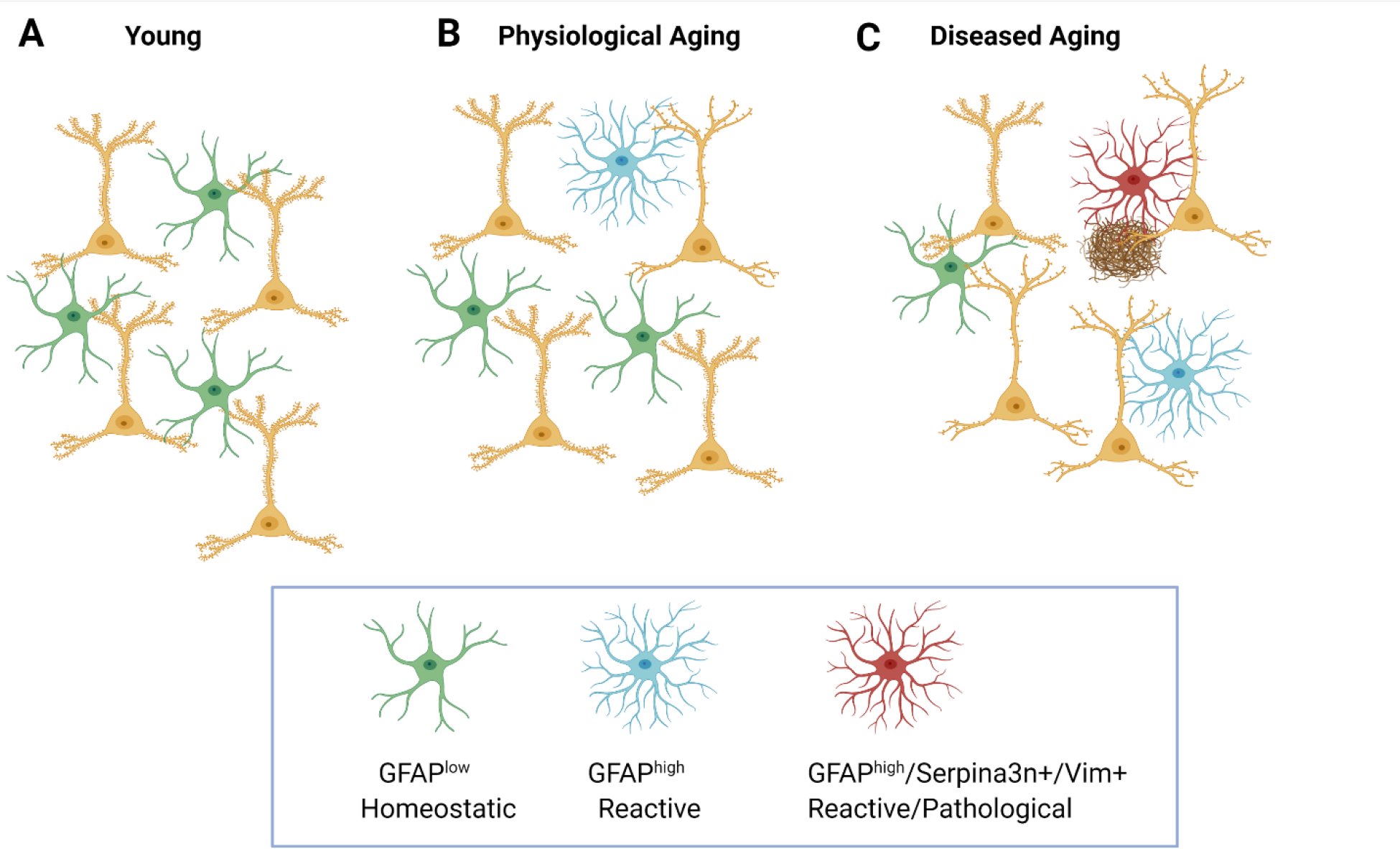
A.) In the young brain, neurons show normal spine numbers and astrocytes are predominantly homeostatic: they have low Gfap expression and are supportive of neurons and synapses. B.) Physiological aging is associated with a loss of spines from some neurons, and a subpopulation of astrocytes emerges in which Gfap expression is elevated; these astrocytes have a reduced ability to support neuronal synapses. C.) During diseased aging – such as neurodegeneration, a subpopulation of Serpina3n+ Vim+ astrocytes with elevated Gfap expression emerge that are spatially associated with neuronal pathology such as reduced spine number and pathological protein aggregates18.
4.4. Astrocyte proteomic and spatial analysis
Although RNA studies have provided insight into the molecular alterations that occur in neurodegeneration, they do not reflect changes that can occur at the post-transcriptional level, leading to an increased interest in proteomics approaches including in AD. Weighted gene correlation network analysis on a proteomics dataset from human AD patients yielded 13 protein coexpression modules that could be classified into 6 main regulatory processes: synapse, mitochondrial function, sugar metabolism, extracellular matrix, cytoskeleton and RNA binding/splicing. Interestingly, the sugar metabolism module (containing proteins involved in glucose and carbohydrate metabolism) was enriched for astrocyte and microglia-related proteins and had the strongest correlation with degree of cognitive impairment162. It was increased early in asymptomatic AD, highlighting this module as a window of opportunity for therapeutic intervention to mitigate progression to dementia. Another proteomics study on human brain samples investigated protein differences in AD as well as several other NDs with or without dementia as a primary characteristic. AD genetic risk analysis on the dataset showed strong correlation with a glial immune module that is enriched for astrocyte proteins and is upregulated early in the asymptomatic stage of AD - although this module did not show a significant correlation with NDs without dementia such as ALS and PD163. This could be an important difference between studies at the RNA and protein level because a transcriptional study on several NDs found that immune genes were commonly upregulated among NDs164. Beyond protein level, phosphoproteomics analysis has identified distinct temporal patterns for phosphorylation of proteins involved in splicing dysfunction and calcium regulation in AD, adding an additional level of complexity to protein regulation144.
Spatial localization of protein and RNA changes with respect to pathology, such as amyloid plaques, tau tangles, dystrophic neurite and cerebral angiopathies will be informative to dissect out heterogeneous astrocyte responses in neurodegeneration, and if these are dependent on the specific pathology. For example in 5xFAD mice, immunohistochemical labeling of Gfap+ and Serpina3n+ reactive astrocytes showed them in close proximity to amyloid plaques 18. Another study used proteomics analysis correlated with disease staging to identify a list of proteins most closely associated with Aβ accumulation in plaques165. Immunohistochemical analysis then verified that the two proteins with highest correlation with Aβ accumulation, MDK and NTN1, were localized to amyloid plaques. New technologies, such as GeoMX and imaging mass cytometry, will allow these types of analyses to be conducted on a larger scale compared to traditional immunofluorescence.
These studies emphasize the need to focus on post-transcriptional and post-translational regulation in NDs. A caveat, however, is that the majority of proteomic studies are not conducted in a cell-type specific-manner, and rather analyze bulk tissue and use correlation with RNA sequencing data to determine the cell-type most likely to contribute to the change. The application of emerging technologies to label proteins in a cell-type specific manner, such as non-canonical amino acid incorporation and proximity labeling with biotin, will enable such studies to be performed in animal models of ND166,167. In addition, most studies do not give information on the localization of proteins, for example if there are alterations in protein secretion or membrane trafficking, particularly important as astrocytes regulate synapses through the release of many different secreted proteins2. This suggests efforts should be made to perform sub-cellular analysis of protein localization in NDs. Combining these approaches with high-throughput proteomics, phosphoproteomics, lipidomics and metabolomics, as well as spatial analyses will provide important information to elucidate the pathological mechanisms of NDs.
5. Avenues for Therapeutic Intervention
Given the myriad ways that astrocytes are altered in NDs, they have become of increasing interest to target therapeutically in multiple disorders168. In this section we discuss existing therapeutic options, as well as consider novel approaches based on recent discoveries (Figure 4).
Figure 4: Therapeutic strategies to target astrocytes in NDs.
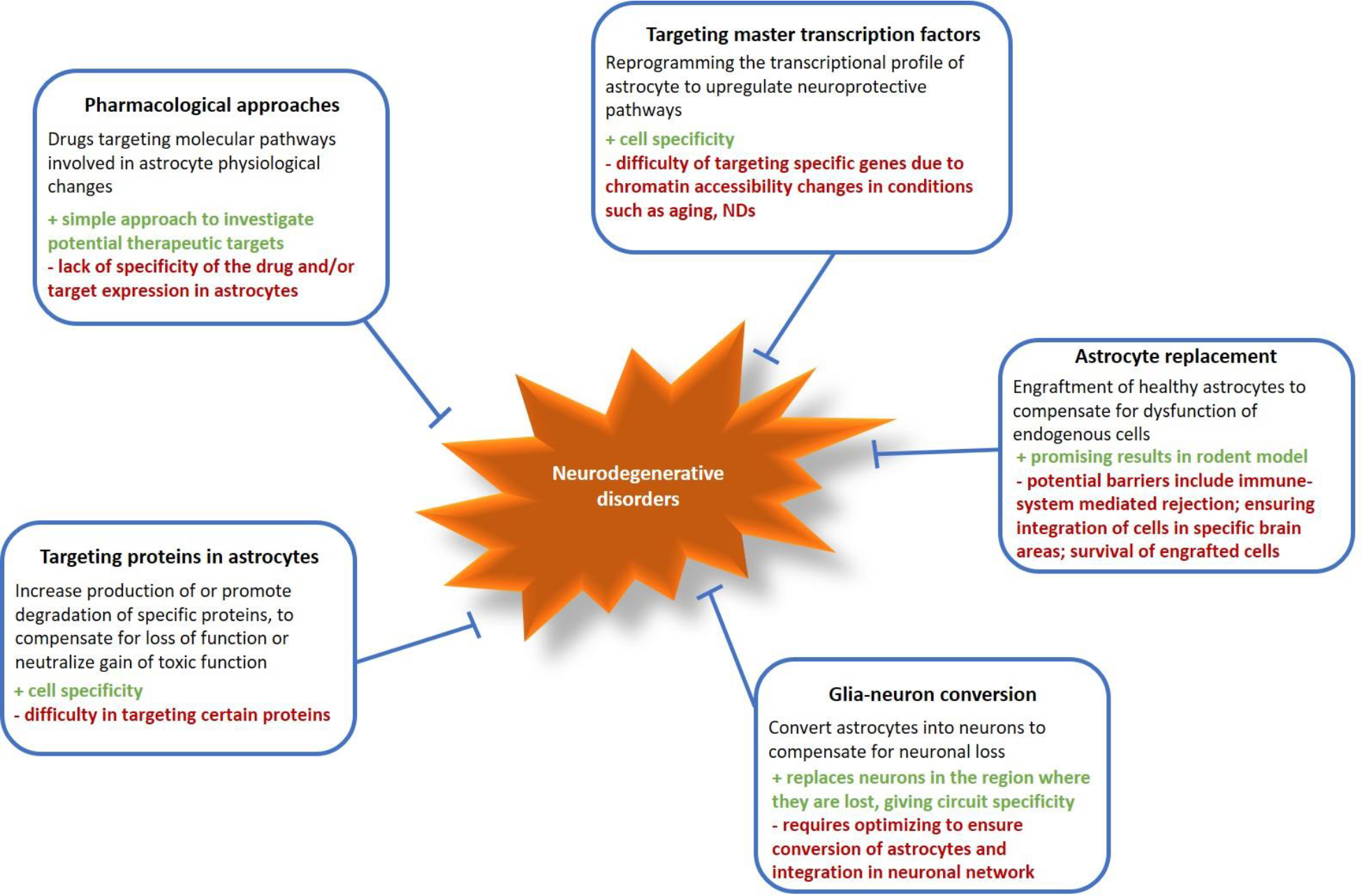
Multiple strategies to therapeutically target astrocytes in neurodegeneration have been proposed or tested in animal models, each with positive and negative aspects. These include pharmacological approaches using drugs to target pathways in astrocytes; modifying the expression of specific genes or proteins in astrocytes; transplanting healthy astrocytes into the region affected by neurodegeneration; converting reactive astrocytes into neurons to replace damaged cells.
5.1. Pharmacological Approaches
One way to modulate astrocytes is to use a pharmacological approach with drugs that act on astrocyte-enriched targets. For example, studies to restore astrocyte calcium signaling to a physiological range in an APP–PS1 mouse model used chronic inhibition of the TRPA1 channel with the inhibitor HC03003152, or long-term treatment with a P2Y1 receptor antagonist54, and showed promising improvements in the phenotype including decreasing neuronal hyperexcitability. In human AD, 22% to 54% of patients exhibit subclinical epileptiform activity169, and it has been shown that patients with AD and seizures show a faster decrease in cognitive function, so decreasing hyperexcitability may be beneficial170. A potential disadvantage of a pharmacological approach in humans is uncertainty around drug specificity, particularly if the target is expressed by cells besides astrocytes. For example, TRPA1 is also expressed in peripheral neurons involved in nociceptive pathways171 and P2Y1 is expressed by hypoglossal motor neurons that innervate muscles of the tongue and are important in maintaining airway patency172. Progress made in the characterization of transcriptomic and proteomic changes to astrocytes in relation to neurodegeneration will likely identify more astrocyte-specific drug targets enabling more therapies to be developed.
5.2. Targeting proteins in astrocytes
As outlined above, many of the mutations underlying NDs have cell-autonomous effects on astrocytes. Therefore, targeting the products of these variants should be sufficient to slow disease progression. A study used zinc-finger protein targeting of mutant Htt specifically in astrocytes, and this was sufficient to prevent the majority of the HD-associated transcriptional alterations16. Aside from directly targeting these neurotoxic proteins in the context of disease, other loci in the genome can be targeted to modify disease pathogenesis by altering astrocyte function (or dysfunction). Identifying these modifiers in astrocytes (and other cell types of the brain) is important not only for finding potential therapeutic targets but also because the same manipulation can have different effects in a cell-specific manner173. For example, in HD the over-production of sphingosine kinase 1 is detrimental in neurons but reverses pathogenesis in astrocytes by promoting autophagy and clearance of the mutant protein174.
5.3. Targeting master TFs
Given the widespread transcriptional and protein level changes that occur in astrocytes in NDs, a promising strategy is to modulate full networks of RNA and protein changes that are altered by targeting TFs. Research in the field of astrocyte heterogeneity has now begun to elucidate region-specific TFs in astrocytes, as well as master TFs that can modify vast transcriptional programs. NFIA is essential to drive production of reactive astrocytes in the subventricular zone after ischemic stroke, and these are thought to be protective and drive the plasticity that exists in the post-stroke recovery phase175. Furthermore, astrocytes in the spinal cord that lack NFIA display an inability to drive BBB remodeling after white matter injury and this contributes to a failure to remyelinate the white matter tracts175. Interestingly, NFIA is also expressed in hippocampal astrocytes. Knockout of NFIA in hippocampal astrocytes resulted in impaired synaptic plasticity and memory31. The particular vulnerability of the hippocampus in AD raises the question of whether NFIA transcriptional targeting may be dysregulated in AD. Another approach is to target master TFs that upregulate specific protective pathways. It was recently demonstrated that Nrf2 downstream targets are altered in both APP PS1 and Tau P301S mouse models of AD. Astrocyte-specific overexpression of Nrf2 ameliorated both amyloid and tau pathologies by upregulating antioxidant response and proteostasis genes154. One caveat to targeting astrocyte TFs is that chromatin remodeling occurs with aging176, and hence certain areas of the genome may be inaccessible with age. New variants of the Crispr-Cas9 system, such as Cas9-TV, which utilizes a transcriptional activator fused to the Cas9 protein, allow for more efficient gene activation in closed chromatin regions177. Cas9 fused to acetyltransferases has also proven effective for gene activation178. A concerted effort will need to be made to catalogue chromatin changes in astrocytes across development, in aging and in NDs using methods such as single cell ATAC-Seq. Databases of these chromatin changes will be useful to pinpoint areas of the genome that will require chromatin remodeling to target efficiently.
5.4. Astrocyte replacement
As an alternative to correcting changes in resident astrocytes, groups have introduced young astrocytes into brains affected by NDs to see if they are sufficient to correct the deficits. Human glial progenitor cells were grafted into brains of HD mice and a portion of these cells differentiated into astrocytes. This approach was sufficient to improve behavioral dysfunction and restore function in the striatal medium spiny neurons109. In a mouse model of amyloid-induced neurodegeneration, autologous implantation of astrocyte-like, enteric glial cells isolated from the gut halted disease progression179. Engraftment methods remain promising but present significant challenges such as issues with host integration, survival of the cells and immune rejection.
5.5. Glia-neuron conversion
A devastating effect of the progression of NDs is the death of neurons. Several groups have attempted glia-neuron conversion with a goal of producing new neurons that can integrate into neural circuits and form new synapses180,181. However, a lineage tracing study suggested that the viral targeting strategies used targeted endogenous neurons rather than converting astrocytes to neurons182.The strategy may still be promising if it can be harnessed to target the correct cell type with high efficiency183. For example, TF upregulation was successfully used to convert NG2 glia to neurons in models of cortical stab-wound injury184. Thus, it remains plausible to perform direct astrocyte-neuron conversion if the correct TFs and/or RNA-binding proteins are identified and their overexpression or downregulation is targeted specifically to astrocytes.
Although most of these studies were conducted in animal models, they highlight innovative strategies that can be harnessed to improve therapeutics for neurodegeneration.
6. Conclusion
NDs can no longer be considered as cell-autonomous disorders affecting neurons, and a holistic comprehension of these disorders should consider them as a disruption of the CNS environment. Because astrocytes are integral to maintaining CNS homeostasis, alterations to the functions of astrocytes will contribute to progression of pathology, or may even initiate it. Alterations to astrocytes encompass both toxic gain of function, as well as impairment of essential functions. Altogether, genetic mutations, RNA and protein level alterations and stressful changes in the environment result in alterations to astrocyte function that contribute to neurodegeneration.
Specific attention on astrocyte regional heterogeneity, the broad spectrum of the astrocyte response to neurodegeneration, and temporal dynamics of gene and protein expression changes in relation to pathological progression is needed moving forward. Outstanding questions remain as to why certain brain regions are more vulnerable to neurodegeneration than others, and why specific NDs affect distinct brain regions. Understanding astrocyte regional heterogeneity and region-specific astrocyte TFs may provide insight into these phenomena. Furthermore, it is clear that there is a broad spectrum of the astrocyte response to NDs. Studies focused on astrocyte states in a context-specific (acute injury vs. chronic disease) and disease-specific manner (differences between NDs) will be necessary to unravel the molecular underpinnings of the astrocyte response and shed light on whether this can be harnessed or manipulated to generate a positive outcome of neuroprotection in the context of neurodegeneration. Studies focused on identifying molecular changes in astrocytes at different stages of disease progression will aid in determining if astrocytes are initiating pathology or responding to disease-related changes in their environment, and how they contribute to the progression of neurodegeneration at different stages. Identification of if and how astrocytes are altered at early stages of neurodegeneration before overt reactivity is observed will highlight potential mechanisms that can be targeted with a goal of preventing disease progression.
Acknowledgements
Work in the lab of NJA is supported by the CZI Neurodegeneration Network. ANB is supported by NINDS 1F32NS117776-01A1. Figures were made with Biorender.
References
- 1.Allen NJ Role of glia in developmental synapse formation. Current opinion in neurobiology 23, 1027–1033 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Allen NJ & Eroglu C Cell Biology of Astrocyte-Synapse Interactions. Neuron 96, 697–708 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung W-S, Allen NJ & Eroglu C Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol 7, a020370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khakh BS & Sofroniew MV Diversity of astrocyte functions and phenotypes in neural circuits Nature Neuroscience 18, 942–952 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christopherson KS et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Allen NJ et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung W-S et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucukdereli H et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proceedings of the National Academy of Sciences 108, E440–E449 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco-Suarez E, Liu T-F, Kopelevich A & Allen NJ Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 100, 1116–1132.e1113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber B & Barros LF The Astrocyte: Powerhouse and Recycling Center. Cold Spring Harb Perspect Biol 7, a020396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Profaci CP, Munji RN, Pulido RS & Daneman R The blood-brain barrier in health and disease: Important unanswered questions. J Exp Med 217, e20190062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araque A et al. Gliotransmitters travel in time and space. Neuron 81, 728–739 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boisvert M, Erikson G, Shokhirev M & Allen N The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Reports 22, 269–285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke LE et al. Normal aging induces A1-like astrocyte reactivity. Proceedings of the National Academy of Sciences of the United States of America 115, E1896–E1905 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orre M et al. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiology of Aging 35, 1–14 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Diaz-castro B, Gangwani MR, Yu X, Coppola G & Khakh BS Astrocyte molecular signatures in Huntington’s disease. Science Translational Medicine 11, eaaw8546 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Al-Dalahmah O et al. Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathologica Communications 8, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habib N et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nature Neuroscience 23, 701–706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau S-F, Cao H, Fu AKY & Ip NY Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proceedings of the National Academy of Sciences 117, 25800–25809 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng K et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nature Neuroscience 24, 276–287 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang R, Diaz-Castro B, Looger LL & Khakh BS Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. Journal of Neuroscience 36, 3453–3470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonninen TM et al. Metabolic alterations in Parkinson ‘ s disease astrocytes. Scientific Reports 10, 14474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassina P et al. Mitochondrial Dysfunction in SOD1G93A-Bearing Astrocytes Promotes Motor Neuron Degeneration: Prevention by Mitochondrial-Targeted Antioxidants. The Journal of Neuroscience 28, 4115–4122 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae JR & Kim SH Synapses in neurodegenerative diseases. BMB Reports 50, 237–246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escartin C et al. Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience 24, 312–325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson MR, Fuertes CJA, Dunn AK, Drew MR & Jones TA Reactive astrocytes facilitate vascular repair and remodeling after stroke. Cell Reports 35, 109048 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 62, 2022–2033 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraft AW et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB Journal 27, 187–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamanian JL et al. Genomic Analysis of Reactive Astrogliosis. The Journal of neuroscience 32, 6391–6410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang AYS et al. Region-Specific Transcriptional Control of Astrocyte Function Oversees Local Circuit Activities. Neuron 106, 992–1008.e1009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai H. h. et al. Regional Astrocyte Allocation Regulates CNS Synaptogenesis and Repair. Science 337, 358–362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayer TA Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? European Neuropsychopharmacology 25, 713–724 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Serio A et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proceedings of the National Academy of Sciences 110, 4697–4702 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beach TG & McGeer EG Lamina-specific arrangement of astrocytic gliosis and senile plaques in Alzheimer’s disease visual cortex. Brain Research 463, 357–361 (1988). [DOI] [PubMed] [Google Scholar]
- 36.Bosson A et al. TRPA1 channels promote astrocytic Ca 2 + hyperactivity and synaptic dysfunction mediated by oligomeric forms of amyloid- β peptide. Molecular Neurodegeneration 12, 53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Nievas BG & Serrano-Pozo A Deciphering the Astrocyte Reaction in Alzheimer’s Disease. Frontiers in Aging Neuroscience 10, 114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliff JJ et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science Translational Medicine 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amiry-Moghaddam M & Ottersen OP The Molecular Basis of Water Transport in the Brain. Nature Reviews Neuroscience 4, 991–1001 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Caron NS et al. Mutant Huntingtin Is Cleared from the Brain via Active Mechanisms in Huntington Disease. J Neurosci 41, 780–796 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong X et al. Astrocyte Kir4 . 1 ion channel deficits contribute to neuronal dysfunction in Huntington ‘ s disease model mice. Nature Neuroscience 17, 694–703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoshi A et al. Characteristics of Aquaporin Expression Surrounding Senile Plaques and Cerebral Amyloid Angiopathy in Alzheimer Disease. Journal of Neuropathology & Experimental Neurology 71, 750–759 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Xu Z et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Molecular Neurodegeneration 10, 58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castellano JM et al. Human apoE Isoforms Differentially Regulate Brain Amyloid- b Peptide Clearance. Science Translational Medicine 3, 89ra57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streubel-Gallasch L et al. Parkinson’s Disease–Associated LRRK2 Interferes with Astrocyte-Mediated Alpha-Synuclein Clearance. Molecular Neurobiology 58, 3119–3140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verkhratsky A & Nedergaard M Physiology of astroglia. Physiological Reviews 98, 239–389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bindocci E et al. Three-dimensional Ca 2+ imaging advances understanding of astrocyte biology. Science 356, eaai8185 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Reichenbach A, Derouiche A & Kirchhoff F Morphology and dynamics of perisynaptic glia. Brain Research Reviews 63, 11–25 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Kawamata H et al. Abnormal Intracellular Calcium Signaling and SNARE- Dependent Exocytosis Contributes to SOD1G93A Astrocyte- Mediated Toxicity in Amyotrophic Lateral Sclerosis. The Journal of neuroscience 34, 2331–2348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martorana F, Brambilla L, Valori CF & Bergamaschi C The BH4 domain of Bcl-X L rescues astrocyte degeneration in amyotrophic lateral sclerosis by modulating intracellular calcium signals. Human Molecular Genetics 21, 826–840 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Bosson A, Boisseau S, Buisson A, Savasta M & Albrieux M Disruption of dopaminergic transmission remodels tripartite synapse morphology and astrocytic calcium activity within substantia nigra pars reticulata. Glia 63, 673–683 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Paumier A et al. Astrocyte–neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 145, 388–405 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Panatier A et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Reichenbach N et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer ‘ s disease model. Journal of Experimental Medicine 215, 1649–1663 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danbolt NC Glutamate uptake. Progress in Neurobiology 65, 1–105 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Pajarillo E, Rizor A, Lee J, Aschner M & Lee E The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders : Potential targets for neurotherapeutics. Neuropharmacology 161, 107559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iovino L et al. Trafficking of the glutamate transporter is impaired in LRRK2-related Parkinson’s disease. Acta Neuropathol 144, 81–106 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y et al. Generation of a Novel Mouse Model of Parkinson’s Disease via Targeted Knockdown of Glutamate Transporter GLT-1 in the Substantia Nigra. ACS Chemical Neuroscience 11, 406–417 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Ren C et al. Induction of Parkinsonian-Like Changes via Targeted Downregulation of Astrocytic Glutamate Transporter GLT-1 in the Striatum. Journal of Parkinson’s Disease 12, 295–314 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Olney JW Glutamate-induced Neuronal Necrosis in the Infant Mouse Hypothalamus. Journal of Neuropathology & Experimental Neurology 30, 75–90 (1971). [DOI] [PubMed] [Google Scholar]
- 61.Bogaert E, d’Ydewalle C & Van Den Bosch L Amyotrophic Lateral Sclerosis and Excitotoxicity : From Pathological Mechanism to Therapeutic Target. CNS Neurol Disord Drug Targets 9, 297–304 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Hynd MR, Scott HL & Dodd PR Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer ‘ s disease. Neurochemistry International 45, 583–595 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Iovino L, Tremblay ME & Civiero L Glutamate-induced excitotoxicity in Parkinson ‘ s disease : The role of glial cells. Journal of Pharmacological Science 144, 151–164 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Sepers MD & Raymond LA Mechanisms of synaptic dysfunction and excitotoxicity in Huntington ‘ s disease. Drug Discovery Today 19, 990–996 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Saura CA, Parra-Damas A & Enriquez-Barreto L Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Frontiers in Cellular Neuroscience 9, 318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terry RD et al. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology 30, 572–580 (1991). [DOI] [PubMed] [Google Scholar]
- 67.Esquerda-Canals G, Montoliu-Gaya L, Güell-Bosch J & Villegas S Mouse Models of Alzheimer’s Disease. Journal of Alzheimer’s Disease 57, 1171–1183 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Henstridge CM, Tzioras M & Paolicelli RC Glial Contribution to Excitatory and Inhibitory Synapse Loss in Neurodegeneration. Frontiers in Cellular Neuroscience 13, 63 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talantova M et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proceedings of the National Academy of Sciences of the United States of America 110, E2518–2527 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trudler D et al. α-Synuclein Oligomers Induce Glutamate Release from Astrocytes and Excessive Extrasynaptic NMDAR Activity in Neurons, Thus Contributing to Synapse Loss. The Journal of Neuroscience 41, 2264–2273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hardingham GE & Bading H Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11, 682–696 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shrivastava AN et al. b -Amyloid and ATP-Induced Diffusional Trapping of Astrocyte and Neuronal Metabotropic Glutamate Type-5 Receptors. Glia 61, 1673–1686 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Jo S et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nature medicine 20, 886–896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Castro MA et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nature Neuroscience 14, 1276–1284 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Minelli A et al. Cellular and subcellular localization of Na + – Ca 2 + exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium 41, 221–234 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Kucheryavykh YV et al. Downregulation of Kir4 . 1 Inward Rectifying Potassium Channel Subunits by RNAi Impairs Potassium Transfer and Glutamate Uptake by Cultured Cortical Astrocytes. Glia 55, 274–281 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Anzilotti S et al. Preconditioning, induced by sub-toxic dose of the neurotoxin L-BMAA, delays ALS progression in mice and prevents Na + / Ca 2 + exchanger 3 downregulation. Cell Death and Disease 9, 206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pannaccione A et al. A New Concept : AB1 – 42 Generates a Hyperfunctional Proteolytic NCX3 Fragment That Delays Caspase-12 Activation and Neuronal Death. The Journal of neuroscience 32, 10609–10617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelley KW et al. Kir4.1-Dependent Astrocyte-Fast Motor Neuron Interactions Are Required for Peak Strength. Neuron 98, 306–319.e307 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang BL Glucose, glycolysis, and neurodegenerative diseases. Journal of Cell Physiology 235, 7653–7662 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Bélanger M, Allaman I & Magistretti PJ Brain Energy Metabolism : Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metabolism 14, 724–738 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Suzuki A et al. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 144, 810–823 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proceedings of the National Academy of Sciences 111, 12228–12233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finsterwald C, Magistretti PJ & Lengacher S Astrocytes: New Targets for the Treatment of Neurodegenerative Diseases. Curr Pharm Des 21, 3570–3581 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Allen JA, Halverson-tamboli RA & Rasenick MM Lipid raft microdomains and neurotransmitter signalling. Nature Reviews Neuroscience 8, 128–140 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Pfrieger FW & Ungerer N Cholesterol metabolism in neurons and astrocytes. Progress in Lipid Research 50, 357–371 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Reiman EM et al. Correlations between apolipoprotein E4 gene dose and brain-imaging measurements of regional hypometabolism. Proceedings of the National Academy of Sciences 102, 8299–8302 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams HC et al. APOE alters glucose flux through central carbon pathways in astrocytes. Neurobiology of Disease 136, 104742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Larramona-Arcas R et al. Sex-dependent calcium hyperactivity due to lysosomal-related dysfunction in astrocytes from APOE4 versus APOE3 gene targeted replacement mice. Molecular Neurodegeneration 15, 35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong W, Lee H, Cho S & Seo J ApoE4-Induced Cholesterol Dysregulation and Its Brain Cell Type-Specific Implications in the Pathogenesis of Alzheimer ‘ s Disease. Molecules and cells 42, 739–746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valenza M et al. Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington ‘ s disease. Cell Death and Differentiation 22, 690–702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Birolini G et al. SREBP2 gene therapy targeting striatal astrocytes ameliorates Huntington’s disease phenotypes. Brain 144, 3175–3190 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Hartmann H, Ho WY, Chang J. c. & Ling S -c. Cholesterol dyshomeostasis in amyotrophic lateral sclerosis : cause, consequence, or epiphenomenon ? The FEBS Journal, 1–22 (2021). [DOI] [PubMed] [Google Scholar]
- 94.Jin U, Park SJ & Park SM Cholesterol metabolism in the brain and its association with Parkinson’s disease. Experimental Neurobiology 28, 554–567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brenner M et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nature genetics 27, 117–120 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Gusella J et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature 306, 234–238 (1983). [DOI] [PubMed] [Google Scholar]
- 97.Ridge PG, Mukherjee S, Crane PK & Kauwe JSK Alzheimer’s disease: Analyzing the missing heritability. PloS one 8, e79771 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu MH et al. Recessively-Inherited Adult-Onset Alexander Disease Caused by a Homozygous Mutation in the GFAP Gene. Movement Disorders 35, 1662–1667 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Ciammola A et al. A Novel Mutation of GFAP Causing Adult-Onset Alexander Disease. Frontiers in Neurology 10, 1124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomi H et al. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 14, 29–41 (1995). [DOI] [PubMed] [Google Scholar]
- 101.Tian R et al. Alexander disease mutant glial fibrillary acidic protein compromises glutamate transport in astrocytes. Journal of Neuropathology and Experimental Neurology 69, 335–345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hagemann TL et al. Antisense therapy in a rat model of Alexander disease reverses GFAP pathology, white matter deficits, and motor impairment. Science Translational Medicine 13, eabg4711 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Acuña AI et al. A failure in energy metabolism and antioxidant uptake precede symptoms of Huntington’s disease in mice. Nature Communications 4, 2917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Ramahi I et al. High-Throughput Functional Analysis Distinguishes Pathogenic, Nonpathogenic, and Compensatory Transcriptional Changes in Neurodegeneration. Cell Systems 7, 28–40.e24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ochaba J et al. Potential function for the Huntingtin protein as a scaffold for selective autophagy. Proceedings of the National Academy of Sciences of the United States of America 111, 16889–16894 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Virlogeux A et al. Reconstituting Corticostriatal Network on-a-Chip Reveals the Contribution of the Presynaptic Compartment to Huntington’s Disease. Cell Reports 22, 110–122 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Maiuri T et al. Huntingtin is a scaffolding protein in the ATM oxidative DNA damage response complex. Human Molecular Genetics 26, 395–406 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Bradford J et al. Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proceedings of the National Academy of Sciences of the United States of America 106, 22480–22485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benraiss A et al. Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nature Communications 7, 11758 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wood TE et al. Mutant huntingtin reduction in astrocytes slows disease progression in the BACHD conditional Huntington’s disease mouse model. Human Molecular Genetics 28, 487–500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choi I et al. PINK1 deficiency attenuates astrocyte proliferation through mitochondrial dysfunction, reduced AKT and increased p38 MAPK activation, and downregulation of EGFR. Glia 61, 800–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Rus Jacquet A et al. The LRRK2 G2019S mutation alters astrocyte-to-neuron communication via extracellular vesicles and induces neuron atrophy in a human iPSC-derived model of Parkinson’s disease. eLife 10, e73062 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nagai M et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nature Neuroscience 10, 615–622 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szebényi K et al. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nature Neuroscience 24, 1542–1554 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao C et al. Mutant C9orf72 human iPSC-derived astrocytes cause non-cell autonomous motor neuron pathophysiology. Glia 68, 1046–1064 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Birger A et al. Human iPSC-derived astrocytes from ALS patients with mutated C9ORF72 show increased oxidative stress and neurotoxicity. EBioMedicine 50, 274–289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kia A, McAvoy K, Krishnamurthy K, Trotti D & Pasinelli P Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia 66, 1016–1033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arredondo C et al. Excessive release of inorganic polyphosphate by ALS/FTD astrocytes causes non-cell-autonomous toxicity to motoneurons. Neuron 110, 1656–1670.e1612 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chavez-Gutiérrez L et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO Journal 31, 2261–2274 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van Cauwenberghe C, Van Broeckhoven C & Sleegers K The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genetics in Medicine 18, 421–430 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao MC et al. Single-cell detection of secreted Aβ and sAPPα from human IPSC-derived neurons and astrocytes. Journal of Neuroscience 36, 1730–1746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bertram L & Tanzi RE Genome-wide association studies in Alzheimer’s disease. Human Molecular Genetics 18, 137–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jansen IE et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nature genetics 51, 404–413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strittmatter WJ et al. Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America 90, 1977–1981 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fagan AM et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/−), and human apoE transgenic mice. Journal of Biological Chemistry 274, 30001–30007 (1999). [DOI] [PubMed] [Google Scholar]
- 126.Kim J, Basak JM & Holtzman DM The Role of Apolipoprotein E in Alzheimer’s Disease Neuron 63, 287–303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi Y et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shinohara M et al. Apoe2 is associated with longevity independent of alzheimer’s disease. eLife 9, e62199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin YT et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 98, 1141–1154.e1147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tcw J et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233.e2225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao J et al. APOE ϵ4/ϵ4 diminishes neurotrophic function of human iPSC-derived astrocytes. Human Molecular Genetics 26, 2690–2700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng J. y. et al. Selective deletion of apolipoprotein E in astrocytes ameliorates the spatial learning and memory deficits in Alzheimer’s disease (APP/PS1) mice by inhibiting TGF-β/Smad2/STAT3 signaling. Neurobiology of Aging 54, 112–132 (2017). [DOI] [PubMed] [Google Scholar]
- 133.Taga M et al. BIN1 protein isoforms are differentially expressed in astrocytes, neurons, and microglia: neuronal and astrocyte BIN1 are implicated in tau pathology. Molecular Neurodegeneration 15, 44 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen F et al. Clusterin secreted from astrocyte promotes excitatory synaptic transmission and ameliorates Alzheimer’s disease neuropathology. Molecular Neurodegeneration 16, 5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bugiani O et al. Frontotemporal Dementia and Corticobasal Degeneration in a Family with P301S Mutation in Tau. Journal of Neuropathology and Experimental Neurology 58, 667–677 (1991). [DOI] [PubMed] [Google Scholar]
- 136.Hutton M et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998). [DOI] [PubMed] [Google Scholar]
- 137.Alonso ADC, Mederlyova A, Novak M, Grundke-Iqbal I & Iqbal K Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. Journal of Biological Chemistry 279, 34873–34881 (2004). [DOI] [PubMed] [Google Scholar]
- 138.Sidoryk-Wegrzynowicz M et al. Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta neuropathologica communications 5, 89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang P & Ye Y Filamentous recombinant human Tau activates primary astrocytes via an integrin receptor complex. Nature Communications 12, 95 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perea JR et al. Extracellular Monomeric Tau Is Internalized by Astrocytes. Frontiers in Neuroscience 13, 442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kovacs GG Astroglia and Tau: New Perspectives. Frontiers in Aging Neuroscience 12, 96 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Litvinchuk A et al. Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer’s Disease. Neuron 100, 1337–1353.e1335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Berchtold NC et al. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobio Aging 34, 1653–1661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bai B et al. Deep Multilayer Brain Proteomics Identifies Molecular Networks in Alzheimer’s Disease Progression. Neuron 105, 975–991.e977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Johnson ECB et al. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nature Neuroscience 25, 213–225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Galea E et al. Multi-transcriptomic analysis points to early organelle dysfunction in human astrocytes in Alzheimer’s disease. Neurobiology of Disease 166, 105655 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sadick JS et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805.e1710 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stevens B et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 131, 1164–1178 (2007). [DOI] [PubMed] [Google Scholar]
- 149.Mathys H et al. Single-cell transcriptomic analysis of Alzheimer ‘ s disease. Nature 570, 332–337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grubman A et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nature Neuroscience 22, 2087–2097 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Orre M et al. Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiology of Aging 35, 2746–2760 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Sun S et al. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant sod1-mediated ALS. Proceedings of the National Academy of Sciences of the United States of America 112, E6993–E7002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.SantaCruz KS, Yazlovitskaya E, Collins J, Johnson J & DeCarli C Regional NAD(P)H:quinone oxidoreductase activity in Alzheimer’s disease. Neurobiology of Aging 25, 63–69 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Jiwaji Z et al. Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Aß pathology. Nat Commun 13, 135 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ceyzériat K et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta neuropathologica communications 6, 104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reichenbach N et al. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Molecular Medicine 11, e9665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guillemaud O et al. Complex roles for reactive astrocytes in the triple transgenic mouse model of Alzheimer disease. Neurobiology of Aging 90, 135–146 (2020). [DOI] [PubMed] [Google Scholar]
- 158.Guttenplan KA et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nature Communications 11, 3753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hasel P, Rose IVL, Sadick JS, Kim RD & Liddelow SA Neuroinflammatory astrocyte subtypes in the mouse brain. Nature Neuroscience 24, 1475–1487 (2021). [DOI] [PubMed] [Google Scholar]
- 160.Maynard KR et al. Transcriptome-scale spatial gene expression in the human dorsolateral prefrontal cortex. Nature Neuroscience 24, 425–436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maniatis S et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364, 89–93 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Johnson ECB et al. Large-scale Proteomic Analysis of Alzheimer’s Disease Brain and Cerebrospinal Fluid Reveals Early Changes in Energy Metabolism Associated with Microglia and Astrocyte Activation. Nature medicine 26, 769–780 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Swarup V et al. Identification of Conserved Proteomic Networks in Neurodegenerative Dementia. Cell Reports 31, 107807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wan YW et al. Meta-Analysis of the Alzheimer’s Disease Human Brain Transcriptome and Functional Dissection in Mouse Models. Cell Reports 32, 107908 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiong F, Ge W & Ma C Quantitative proteomics reveals distinct composition of amyloid plaques in Alzheimer’s disease. Alzheimer’s & Dementia 15, 429–440 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Hinz FI, Dieterich DC & Schuman EM Teaching old NCATs new tricks: using non-canonical amino acid tagging to study neuronal plasticity. Current Opinion in Chemical Biology 17, 738–746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qin W, Cho KF, Cavanagh PE & Ting AY Deciphering molecular interactions by proximity labeling. Nature Methods 18, 133–143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee H-G, Wheeler MA & Quintana FJ Function and therapeutic value of astrocytes in neurological diseases. Nature Reviews Drug Discovery 21, 339–358 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vossel KA et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol 80, 858–870 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Horvath AA et al. Subclinical epileptiform activity accelerates the progression of Alzheimer’s disease: A long-term EEG study. Clinical Neurophysiology 132, 1982–1989 (2021). [DOI] [PubMed] [Google Scholar]
- 171.Souza Monteiro de Araujo D, Nassini R, Geppetti P & De Logu F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 24, 997–1008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rajani V, Zhang Y, Revill AL & Funk GD The role of P2Y1 receptor signaling in central respiratory control. Respiratory Physiology & Neurobiology 226, 3–10 (2016). [DOI] [PubMed] [Google Scholar]
- 173.Onur TS et al. Downregulation of glial genes involved in synaptic function mitigates Huntington’s disease pathogenesis. eLife 10, e64564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moruno-Manchon JF et al. Sphingosine kinase 1-associated autophagy differs between neurons and astrocytes. Cell Death and Disease 9, 521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Laug D et al. Nuclear factor I-A regulates diverse reactive astrocyte responses after CNS injury. The Journal of Clinical Investigation 129, 4408–4418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chisholm NC et al. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 10, 142–152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu G, Yin K, Zhang Q, Gao C & Qiu JL Modulating chromatin accessibility by transactivation and targeting proximal dsgRNAs enhances Cas9 editing efficiency in vivo. Genome Biology 20, 145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hilton IB et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase Activates Genes From Promoters and Enhancers. Nature Biotechnology 33, 510–517 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Esposito G et al. Autologous transplantation of intestine-isolated glia cells improves neuropathology and restores cognitive deficits in β amyloid-induced neurodegeneration. Scientific Reports 6, 22605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Qian H et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 582, 550–556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou H et al. Glia-to-Neuron Conversion by CRISPR-CasRx Alleviates Symptoms of Neurological Disease in Mice. Cell 181, 590–603.e516 (2020). [DOI] [PubMed] [Google Scholar]
- 182.Wang LL et al. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 184, 5465–5481.e5416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bocchi R, Masserdotti G & Götz M Direct neuronal reprogramming: Fast forward from new concepts toward therapeutic approaches. Neuron 110, 366–393 (2022). [DOI] [PubMed] [Google Scholar]
- 184.Heinrich C et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports 3, 1000–1014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gitter BD, Cox LM, Rydel RE & May PC Amyloid beta peptide potentiates cytokine secretion by interleukin-1 beta-activated human astrocytoma cells. Proceedings of the National Academy of Sciences 92, 10738–10741 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Selkoe DJ Alzheimer’s Disease. Cold Spring Harb Perspect Biol 3, a004457 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Selkoe DJ Treatments for Alzheimer’s disease emerge. Science 373, 624–626 (2021). [DOI] [PubMed] [Google Scholar]
- 188.Simpson IA, Chundu KR, Davies-Hill T, Honer WG & Davies P Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Annals of Neurology 35, 546–551 (1994). [DOI] [PubMed] [Google Scholar]
- 189.Simpson JE et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiology of Aging 31, 578–590 (2010). [DOI] [PubMed] [Google Scholar]
- 190.Wilcock DM, Vitek MP & Colton CA Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer’s disease. Neuroscience 159, 1055–1069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Haidet-Phillips AM et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29, 824–828 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hardiman O et al. Amyotrophic lateral sclerosis. Nature Reviews Disease Primers 3, 17071 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Izrael M, Slutsky SG & Revel M Rising Stars : Astrocytes as a Therapeutic Target for ALS Disease. Frontiers in Neuroscience 14, 824 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rothstein JD, Martin LJ & Kuncl RW Decreased Glutamate Transport by the Brain and Spinal Cord in Amyotrophic Lateral Sclerosis. New England Journal of Medicine 326, 1464–1468 (1992). [DOI] [PubMed] [Google Scholar]
- 195.Schiffer D, Cordera S, Cavalla P & Migheli A Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. Journal of the Neurological Sciences 139, 27–33 (1996). [DOI] [PubMed] [Google Scholar]
- 196.Wyant KJ, Ridder AJ & Dayalu P Huntington’s Disease—Update on Treatments. Current Neurology and Neuroscience Reports 17, 33 (2017). [DOI] [PubMed] [Google Scholar]
- 197.Ascherio A & Schwarzschild MA The epidemiology of Parkinson’s disease: risk factors and prevention. The Lancet Neurology 15, 1257–1272 (2016). [DOI] [PubMed] [Google Scholar]
- 198.Miyazaki I & Asanuma M Neuron-Astrocyte Interactions in Parkinson’s Disease. Cells 9, 2623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tysnes O-B & Storstein A Epidemiology of Parkinson’s disease. Journal of neural transmission 124, 901–905 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H & Takahashi H NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathologica 99, 14–20 (2000). [DOI] [PubMed] [Google Scholar]


