Abstract
Background
Dyspnea and fatigue are characteristics of long SARS-CoV-2 (COVID)-19. Cardiopulmonary exercise testing (CPET) can be used to better evaluate such patients.
Research question
How significantly and by what mechanisms is exercise capacity impaired in patients with long COVID who are coming to a specialized clinic for evaluation?
Study design and methods
We performed a cohort study using the Mayo Clinic exercise testing database. Subjects included consecutive long COVID patients without prior history of heart or lung disease sent from the Post-COVID Care Clinic for CPET. They were compared to a historical group of non–COVID patients with undifferentiated dyspnea also without known cardiac or pulmonary disease. Statistical comparisons were performed by t-test or Pearson's chi2 test controlling for age, sex, and beta blocker use where appropriate.
Results
We found 77 patients with long COVID and 766 control patients. Long COVID patients were younger (47 ± 15 vs 50 ± 10 years, P < .01) and more likely female (70% vs 58%, P < .01). The most prominent difference on CPETs was lower percent predicted peak V̇O2 (73 ± 18 vs 85 ± 23%, p < .0001). Autonomic abnormalities (resting tachycardia, CNS changes, low systolic blood pressure) were seen during CPET more commonly in long COVID patients (34 vs 23%, P < .04), while mild pulmonary abnormalities (mild desaturation, limited breathing reserve, elevated V̇E/V̇CO2) during CPET were similar (19% in both groups) with only 1 long COVID patient showing severe impairment.
Interpretation
We identified severe exercise limitation among long COVID patients. Young women may be at higher risk for these complications. Though mild pulmonary and autonomic impairment were common in long COVID patients, marked limitations were uncommon. We hope our observations help to untangle the physiologic abnormalities responsible for the symptomatology of long COVID.
Keywords: Long COVID-19, Cardiopulmonary exercise testing, fatigue, dyspnea, autonomic abnormalities
After three years, SARS-CoV-2 (COVID-19) is still demonstrating new manifestations both in the acute phase and in the long term. The most common symptoms during the acute infection are fever, cough, shortness of breath, and myalgia, among others1. With each new variant there has been little change in these clinical features,2 , 3 though there are apparent differences among strains regarding the symptom onset and transmissibility.3., 4., 5.
Symptoms may persist more than 28 days, and new sequelae may appear after the acute infection. This phenomenon has been termed ‘post-acute sequelae of SARS-CoV-2 infection’ (PASC)6, 7, 8 – more commonly known as “long COVID”. A systematic review and meta-analysis published in 2021 reported more than 50 long COVID symptoms, among which fatigue, headache, attention disorders, hair loss, dyspnea, ageusia and anosmia were the most common.9 Clinical experience from our own Post COVID Care Clinic corroborates symptom prevalence with fatigue, dyspnea, brain fog, anxiety, unrefreshing sleep, and post exertional malaise being the most reported symptoms.10
Cardiopulmonary exercise testing (CPET) is widely used to evaluate the influence of cardiac, respiratory, musculoskeletal, and hematological systems in exercise tolerance.11, 12, 13 Several studies have described the results of CPET in patients with long COVID presenting with various symptoms including dyspnea,6 , 14, 15, 16, 17 although pulmonary function tests (PFT)15 , 16 , 18 and pulmonary14 , 18 and cardiac imaging14 have also been used. Parameters such as peak V̇O2 were found to be abnormal to varying degrees in patients included in these studies.6 , 14, 15, 16, 17 Data suggest that peripheral skeletal muscle abnormalities resulting in impaired tissue oxygen extraction is likely the cause of many of these manifestations,17 rather than pulmonary or cardiac dysfunction. However, similar changes could be present in patients with dyspnea without previous SARS-CoV-2 infection or any cardiopulmonary impairment. 11, 12, 13
This cohort study aims to compare CPET findings of long COVID patients with non-COVID patients with undifferentiated dyspnea on exertion seen at Mayo Clinic. Data gathered from the EMR (electronic medical record) will also serve to describe other symptoms presented as part of long COVID as well as other characteristics to contribute to the data already published by the studies cited above.
Study design and methods
We carried out a retrospective cohort study to report the cardiorespiratory fitness of patients with long COVID using CPET and compare their results to non-COVID patients with undifferentiated dyspnea on exertion.
Subjects
Our study group was composed of patients with long COVID presenting to Mayo Clinic's Post COVID Care Clinic between January 2021 and January 2022 for a post-COVID evaluation including CPET. All long COVID patients had PCR (polymerase chain reaction)- or antigen-confirmed SARS-CoV-2 infection during the acute illness. Patients were excluded if they had active cardiac or pulmonary disease pre-COVID. Controls were patients with undifferentiated dyspnea on exertion from a previously created database of CPETs performed between January 2011 and June 2016. Exclusion criterion for this group was a concurrent diagnosis of cardiac or pulmonary disease. After all criteria were applied, 77 long COVID patients were included in the study group and 766 patients in the control group. All study methods were approved by the Mayo Clinic IRB.
Study procedures
All long COVID patients underwent a standard evaluation in a specialized Post Covid Care Clinic. This included history and physical exam, chest X-ray, and laboratory panel. Echocardiography and pulmonary function tests were not a standard part of the order set but added liberally as clinically appropriate. Symptom-limited exercise testing with respiratory gas exchange analysis (MGC Diagnostics metabolic cart, St. Paul, Minnesota) was performed on a treadmill (GE, Milwaukee, WI) using an accelerated Naughton protocol (2-min workloads, 2 MET increments per workload).19 Most tests employed a 3-min active recovery at 1.7 MPH/% grade. Electrocardiograms were continuously monitored (GE CASE, Milwaukee, WI), and blood pressure was assessed the last 30 s of each 2-min workload using a stethoscope and aneroid with appropriately sized manually inflated cuff. Patients were encouraged to exercise to fatigue rating of perceived exertion (RPE) ≥ 17 on the standard Borg scale.20 Standard CPET variables, including peak V̇O2 and V̇E/V̇CO2 nadir, were calculated with BreezeSuite software provided MGC Diagnostics using 30 s averages updated every 10 s (“rolling 30s”). Calibration using gravimetric quality gases was performed before each test. Peak V̇O2 was the highest 30-s averaged V̇O2 during exercise and was expressed in mL/kg/min and as percent of age, sex, and weight predicted based on FRIEND data.21 V̇E/V̇CO2 nadir was taken as the lowest 30-s average during exercise. Quality of exercise effort was assessed by peak respiratory exchange ratio (RER = V̇CO2/V̇O2). Heart rate (HR) was taken from the continuously monitored ECG (GE CASE 8000, Milwaukee, WI). Rest HR was recorded after 5 min of sitting. Peak HR was based on a 10-s strip taken at peak exercise. HR recovery was defined as the difference between peak HR and HR at 1 min after peak exercise in active recovery. Oxygen saturation (O2 sat) was monitored via forehead oximetry using a Nellcor N-595 (Pleasonton, CA). Patients were asked about symptoms at each stage of the test; severity of responses were recorded on a 1 to 10 scale used throughout Mayo Clinic. Details of our CPET protocol have been previously published.22
Study variables
Variables of concern from the CPET included peak V̇O2 and percent predicted peak V̇O2, resting and peak HR, resting and peak systolic blood pressure (SBP), peak RER, maximum minute volume of expired ventilation (V̇E), breathing reserve (BR) – defined as 100% x (1 – maximal VE/40 x measured or estimated FEV1),23 minimum O2 saturation, and V̇E/V̇CO2 nadir (lowest value during exercise). Effort on the test was categorized as maximal if peak RER ≥ 1.15 was achieved, near-maximal if peak RER was 1.05–1.14, and sub-maximal if peak RER < 1.05.24, 25, 26
We also included patient-reported symptoms of general fatigue, dyspnea, chest pain, CNS (central nervous system) changes (which included brain fog, lightheadedness, dizziness, or pre-syncope), and neuromuscular or orthopedic limitations, and identified significant cardiac arrhythmias and ST-T changes during the test.
Other variables included in this study were sex, age, weight, body mass index (BMI), and use of beta blockers. COVID variables included acute illness management (hospitalization, use of oxygen therapy or ventilator, medications), reinfection, vaccination status, specific long COVID symptoms, and a standardized laboratory panel (Hb, ferritin, D-dimer, IL-6, CRP, ESR, ANA, CCP, RF, vitamin D, CK, TSH, AM cortisol). These data were abstracted from the EMR. PFTs and echocardiograms were performed based on clinical suspicion and were not part of the standard workup. All data were double-checked by two researchers (AM and LC). Patient confidentiality was protected by using a numerical identification number. There was no patient contact during the study.
Derived cardiac variables included the following: resting HR > 100 bpm, peak SBP < 10th percentile for age and sex,27 heart rate reserve (peak – resting HR), chronotropic incompetence defined as HR reserve <80% of the predicted value for age and sex,28 , 29 HR recovery (peak HR – HR at 1-min post-peak exercise), and abnormal HR recovery defined as <13 bpm during active recovery of <18 bpm if there was no active recovery. If there was ≥1. 0 mm ST deviation during exercise, regardless of the resting ST segments, the exercise ECG was considered abnormal. Premature ventricular contractions (PVCs) were categorized according to 4 levels: 1) no PVCs; 2) PVCs <5 per minute; 3) PVCs >5 per minute or in pairs; 4) ventricular tachycardia (VT). All VT episodes were reviewed for duration: 3–5 beats; > 5 beats but <30 s; ≥ 30 s.
Derived pulmonary responses included: BR < 15%, minimum O2 saturation < 93%, and V̇E/V̇CO2 nadir >2 standard deviations above the mean for age-sex based norms reported by Sun et al.30
Statistical procedures
To test for differences between long COVID and controls, Pearson's Chi square was used for categorical variables and t-tests for continuous variables. When necessary to adjust for covariates including age, sex, beta blocker, and effort according to peak RER where appropriate, testing was upgraded to logistic regression for categorical variables and ANOVA according to the general linear model was used for continuous variables. SAS was used for all statistical procedures. P < .05 was defined as the level of statistical significance.
Results
We identified 77 long COVID patients and 766 controls. Table 1 summarizes information about baseline characteristics of the long COVID group. Mean age was 47 ± 15 years, 55 (71%) were female, and mean BMI was 30.1 ± 7.3 kg/m2. During the acute illness, 14 (18%) were hospitalized, and only 2 (2.6%) required a ventilator. Most frequently reported long COVID symptoms were fatigue (92%) and dyspnea (71%) – meaning in general daily activity, not specifically on the CPET.
Table 1.
Post-COVID patients. Baseline characteristics.
| Anthropometrics | Sleep disturbances | ||
|---|---|---|---|
| Age (years) | 47 ± 15 | More sleep | 23 (30) |
| Female | 55 (71) | Less sleep | 10 (13) |
| Male | 22 (29) | Unrefreshing sleep | 9 (12) |
| Weight (kg) | 88 ± 24 | Weight change | 10 (13) |
| BMI (kg/m2) | 30 ± 7 | PFT | |
| Acute COVID | FEV1 | 3.1 ± 0.8 | |
| Hospitalized | 14 (18) | Predicted FEV1 (%) | 96 ± 16 |
| O2 requirement | 12 (16) | FVC | 4.0 ± 1.1 |
| Ventilator | 2 (2.6) | Predicted FVC (%) | 93 ± 16 |
| Any medication | 14 (18) | DLCOcSB | 22 ± 5 |
| Antiviral | 7 (50) | Pred DLCOcSB (%) | 93 ± 16 |
| Corticosteroid | 12 (86) | Labs | |
| Immunotherapy | 1 (7.1) | Hgb | 14 ± 1.5 |
| Antibiotics | 2 (14) | Ferritin | 100 ± 147 |
| Plasma | 2 (14) | D dimer | 433 ± 424 |
| Time post COVID (days) | 214 ± 116 | IL-6 | 2.1 ± 1 |
| Reinfection | 13 (17) | CRP | 5.2 ± 6 |
| Long COVID symptoms | ESR | 9.3 ± 9.9 | |
| Fatigue | 71 (92) | ANA | 11 (14) |
| Dyspnea | 55 (71) | CCP | 15 ± 2 |
| Anosmia/ageusia | 19 (25) | RF | 15 ± 1.4 |
| Myalgias/arthralgias | 47 (61) | CK | 116 ± 108 |
| Mental fog | 51 (66) | TSH | 2.4 ± 1.2 |
| Dizziness/syncope | 27 (35) | AM cortisol | 13 ± 5.8 |
Values are mean ± SD or n (%).
BMI = body mass index; PFT = pulmonary function test; FEV1 = forced expiratory volume in the first second; FVC = forced vital capacity; DLCOcSB = diffusion capacity of the lung for carbon monoxide corrected for hemoglobin single breath; Hgb = hemoglobin; IL-6 = interleukin-6; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; ANA = antinuclear antibody; CCP = citrullinated peptide; RF = rheumatoid factor; CK = creatine kinase; TSH = thyroid stimulating hormone.
Elective tests and other laboratory parameters were overall within the normal range.
Table 2 presents comparisons of the long COVID patients and controls. Long COVID patients were slightly younger and more likely female; BMI and use of beta blockers were similar. There were significant differences between the groups in percent predicted peak V̇O2, our principal study variable. Effort on the test as indicated by peak RER was similar in the two groups.
Table 2.
Comparison of long COVID and dyspnea controls.
| Long COVID |
Control subjects |
||
|---|---|---|---|
| N = 77 | N = 766 | P | |
| Age | 47 ± 15 | 50 ± 10 | 0.0064 |
| Female | 55 (71) | 441 (58) | 0.0329 |
| BMI (kg/m2) | 30.1 ± 7.3 | 29.4 ± 6.3 | 0.2814a |
| Use of B blockers | 12 (16) | 111 (15) | 0.8063 |
| Rest HR (bpm) | 78 ± 14 | 77 ± 14 | 0.8895a, b |
| Peak HR (bpm) | 156 ± 23 | 158 ± 20 | 0.0137a, b |
| HR recovery (bpm) | 18 ± 10 | 22 ± 13 | 0.0124 |
| Resting SBP (mmHg) | 120 ± 18 | 122 ± 18 | 0.9874a, b |
| Peak SBP mmHg | 164 ± 28 | 171 ± 28 | 0.2807a, b |
| Peak RER | 1.17 ± 0.13 | 1.15 ± 0.11 | 0.2 |
| Peak V̇O2 (mL/kg/min) | 21.9 ± 6.4 | 25.8 ± 7.4 | <0.0001a, b |
| Percent predicted peak V̇O2 | 73 ± 18 | 85 ± 23 | <0.0001 |
| Max V̇E (L/min BTPS) | 66.5 ± 21.7 | 74.9 ± 25.8 | 0.0307a, b |
| Breathing reserve (%) | 46 ± 15 | 44 ± 14 | 0.38a, b |
| Minimum O2 saturation (%) | 97 ± 2 | 98 ± 2 | 0.12 |
| V̇E/V̇CO2 nadir | 28 ± 5 | 29 ± 4 | 0.11a |
Values are mean ± SD or n (%).
Abbreviations: BMI = body mass index; FAC = functional aerobic capacity); HR = heart rate; SBP = systolic blood pressure; RER = respiratory exchange ratio; CPET = cardiopulmonary exercise test.
Adjusted for age and sex.
Adjusted for beta blocker use.
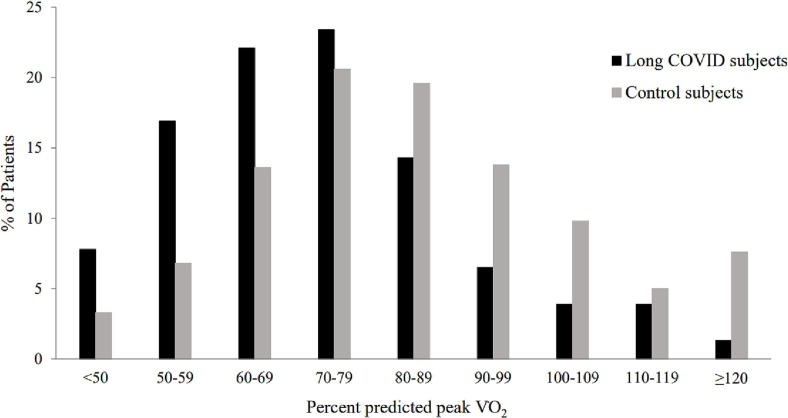
Figure 1 more closely examines the distribution of percent predicted peak V̇O2, which appears to be normally distributed in controls. In long COVID patients, however, the distribution is skewed to the left with significantly higher portions of patients in the percent predicted V̇O2 category below 80–89%. Only 5 (6.5%) long COVID patients achieved at least 100% predicted peak V̇O2.
Fig. 1.
Comparison of percent predicted peak V̇O2 in long COVID patients versus Control subjects.
Turning to Table 3 , which presents cardiac and pulmonary responses to exercise, long COVID patients achieved a slightly lower peak HR and a borderline lower HR reserve. There was more chronotropic incompetence and low peak SBP in the long COVID patients.
Table 3.
Comparison abnormal exercise test responses in long COVID and dyspnea control subjects.
| Long COVID |
Control |
||
|---|---|---|---|
| N = 77 | N = 766 | P | |
| Cardiac Responses | |||
| Resting HR ≥ 100 | 4 (5.2) | 45 (5.9) | 0.41a, b |
| Chronotropic incompetence | 34 (44) | 261 (34) | 0.05a, b, c |
| Abnormal HR recovery | 16 (21) | 157 (20) | 0.81a, b, c |
| Low Peak SBP | 16 (21) | 100 (13) | 0.046a, b, c |
| Exercise ST-T abnormalities | 4 (5.2) | 33 (4.3) | 0.78a, b, c |
| PVC Frequency | 0.38 | ||
| None | 47 (61) | 410 (54) | |
| < 5/min | 19 (25) | 263 (34) | |
| ≥ 5/min or pairs | 9 (12) | 80 (10) | |
| VT | 2 (2.6) | 13 (1.7) | |
| Symptoms of chest pain | 11 (14) | 108 (14) | 0.51 |
| Pulmonary Responses | |||
| Submaximal effort | 14 (18) | 134 (18) | 0.88 |
| Low breathing reserve <15% | 2 (2.6) | 17 (2.2) | 0.49a, b, c |
| Low O2 saturation < 93% | 3 (3.9) | 29 (3.8) | 0.84a, b, c |
| V̇E/V̇CO2 nadir | 28 ± 5 | 29 ± 4 | 0.32a, b, c |
| High V̇E/V̇CO2 | 11 (14) | 107 (14) | 0.80a, b, c |
| Symptoms of dyspnea | 44 (57) | 501 (66) | 0.09 |
Values are mean ± SD or n (%).
Abbreviations: HR = heart rate; SBP = systolic blood pressure.
Chronotropic incompetence = HR reserve <80% predicted for age and sex; Abnormal HR recovery = HR Recovery <13 bpm if active recovery or < 18 bpm if no active recovery; Low peak SBP = peak SBP < 10th percentile for age and sex; Submaximal effort = peak respiratory exchange ratio < 1.0; Low O2 saturation = minimum O2 saturation < 93%; V̇E/V̇CO2 nadir = lowest ratio of V̇E to V̇CO2 during exercise; High V̇E/V̇CO2 = V̇E/V̇CO2 nadir >2 standard deviations from mean for age and sex.
Adjusted for age and sex.
Adjusted for beta blocker use.
Adjusted for effort based on RER.
ST-T abnormalities were rare and not different in long COVID versus controls. The 4 long COVID patients with abnormal ST-T wave response to exercise all had baseline ST-T abnormalities, so exercise ECG responses were coded as non-diagnostic, as were 22 of the 33 controls with exercise ST-T abnormalities (the remaining 11 interpreted as “positive for ischemia”). PVC frequency was similar in both groups. All episodes of ventricular tachycardia (VT) in both groups were limited to 3–5 best runs.
In terms of pulmonary responses, peak V̇E was lower in long COVID patients versus controls – likely secondary to differences in proportion of fe’males, but breathing reserve was similar, as was minimum O2 saturation and V̇E/V̇CO2 nadir. The frequencies of limited breathing reserve, low O2 saturation, and high V̇E/V̇CO2 nadir were not different between the long COVID patients and controls.
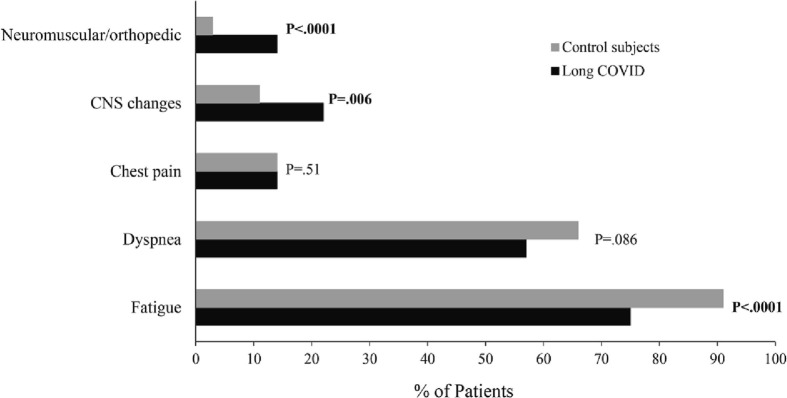
Figure 2 presents the symptom response to exercise. Chest pain was reported in 14% of patients of both groups. General fatigue was more commonly reported as a limiting symptom in controls, and there was a trend (P = .09) towards more common reporting of dyspnea. Dizziness/lightheadedness/presyncope and lower extremity neuromuscular complaints were conversely significantly more common in long COVID patients. While high resting HR, chronotropic incompetence, abnormal HR recovery, and low peak SBP were listed under cardiac responses, these could also to some degree represent autonomic abnormalities, reflecting the higher level of dizziness/lightheadedness/presyncope reported in the long COVID patients.
Fig. 2.
Symptoms during CPET in long COVID patients versus Control subjects.
Discussion
We have presented detailed CPET results on 77 patients with long COVID at a known time frame after carefully documented acute COVID infection and compared results to a large non-COVID control group of 766 patients evaluated for undifferentiated dyspnea. Our principal finding was a low percent predicted peak V̇O2 (73 ± 18%) in patients with long COVID, even in comparison to the controls complaining of dyspnea (85 ± 23%). Only 5/77 (6.5%) long COVID patients achieved at least 100% predicted peak V̇O2. Secondary findings were mild pulmonary abnormalities, similar to controls, and mild autonomic impairment including symptoms of dizziness/lightheadedness/presyncope (22%), chronotropic incompetence (44%), or low peak SBP (21%), all more frequently seen than in controls. Cardiac responses to exercise were largely normal with only 4 patients (5.2%) having non-diagnostic exercise ECGs and 11 patients (14%) with frequent PVCs, pairs, or short runs of VT – not different than controls.
To date there have been multiple reports of CPET results in patients with prior COVID infection.6 , 14, 15, 16, 17 , 31, 32, 33, 34 Peak V̇O2 ranged from 66% predicted15 to 91% predicted.16 Size of the cohorts ranged from only 10 patients15 , 17 up to 200 patients32. Our study with 77 patients and average peak V̇O2 of 73% predicted falls squarely within this range. Peak V̇O2 tended to be lower in studies of patients with long COVID syndrome6 , 14 , 15 , 17 , 31 , 34 versus those testing a cohort of post COVID patients at a fixed time point after acute presentation irrespective of persistent symptoms16 , 30, 31, 32. Durstenfeld et al. performed a systematic review and meta -analysis of the use of CPET after COVID-19 infection35. Their meta-analysis of 9 studies comparing patients either with (464 subjects) or without symptoms (359 subjects) found that the symptomatic subjects'peak V̇O2 was 4.9 ml/kg/min lower than asymptomatic subjects.
Singh et al. performed invasive CPET (right heart catheterization with upright cycle ergometer exercise) in 10 recovered COVID-19 subjects without cardiopulmonary disease and compared their responses to matched controls17. Percent predicted peak V̇O2 averaged 70% for COVID-19 subjects versus 131% for controls. For COVID-19 subjects, cardiac output during exercise was normal, however, tissue oxygen extraction was lower than for controls, indicating a peripheral, rather than central cardiac impairment..
A cardiac etiology for the reduced peak V̇O2 in our long COVID group was highly unlikely given the lack of any significant left ventricular dysfunction, ischemia, or arrhythmias based on close review of the resting and exercise ECGs, and available echocardiograms. Echocardiography was ordered liberally (58/77 = 75% of patients) on account of even minor abnormalities on the rest ECG, or clinical suspicion. Left ventricular ejection fraction (LVEF) ranged from 50 to 69% (mean 61 ± 4%). Minor valve abnormalities were listed as follows: mild-moderate tricuspid regurgitation 5/58 = 8.6%; mild-moderate mitral regurgitation 2/58 = 3.4%; moderate pulmonary regurgitation 1/58 = 1.7%; and mild aortic regurgitation 1/58 = 1.7%. On the rest ECG, the only abnormalities seen were T-wave inversion (4/77 = 5.2%), all with normal LVEF, and 1/77 = 1.3% with possible left ventricular hypertrophy (but normal echocardiogram). Almost all exercise ECGs were read as negative for ischemia (73/77 = 95%). Exercise ECG was called non-diagnostic in the 4 patients with baseline ST-T abnormalities. Minor arrythmias were present on 35 exercise tests (45%), mostly single premature atrial or ventricular complexes.
While there have been many case reports or case series documenting myocarditis during the acute phase of COVID-1936 persistent myocarditis with reduced LVEF has not been widely reported. Major markers of cardiac function such as LVEF (and most minor markers) were not different in long COVID patients 1-year after hospitalization versus controls.37 A second study looking at similar echocardiographic and Holter monitor variables only 3 months after hospitalization for long COVID reported mild right ventricular systolic and diastolic dysfunction and cardiac arrhythmias versus matched pre-pandemic controls, but none of these minor findings correlated with patient complaints of fatigue or dyspnea or the course of inpatient hospital care.38
Our long COVID patients exhibited a relatively low frequency of minor pulmonary abnormalities identical to the controls. The mild nature of the pulmonary findings was somewhat surprising, given the predilection of COVID for pulmonary tissues. Only one long COVID patient demonstrated severe pulmonary impairment with an O2 saturation of 83% and V̇E/V̇CO2 nadir of 50 during exercise. Other studies have described similarly mild pulmonary abnormalities in CPET in long COVID.31, 32, 33 On the other hand, Grist et al. performed hyperpolarized xenon magnetic resonance imaging on 9 patients with long COVID and found alveolar capillary diffusion limitations in all of them; patients did not undergo CPET, so no exercise data were presented.39
Other papers have addressed autonomic abnormalities and symptoms in long COVID. A review of 27 patients with history of confirmed COVID infection referred for autonomic testing at Mayo Clinic (Rochester and Jacksonville) found complaints of lightheadedness (93%), orthostatic headache (22%), syncope (11%), hyperhidrosis (11%), and “burning” (likely neurologic) pain (11%).40 We found all these symptoms – except actual syncope – reported in our long COVID patients. There have also been other reports of long COVID patients experiencing symptoms suggestive of postural orthostatic tachycardic syndrome, orthostatic cerebral hypoperfusion syndrome, and orthostatic hypotension.41, 42 Central nervous system changes, low peak SBP, and chronotropic incompetence were more common in our long COVID patients than controls. It is not clear – though unlikely – that autonomic impairment after COVID is in any substantive way different than after other viral infections.43
A publication by Aranyo et al.44 provides a more in-depth view of inappropriate sinus tachycardia (IST) in long COVID patients presenting to a specialized clinic. Among 200 study patients, 20% reported IST; one of the main underlying factors was autonomic instability. Also, resting and exercise ECG reports were reviewed looking for any significant arrhythmias, symptomatic drop in BP, angina with ST changes, or left bundle branch block. Only premature ventricular contractions (<5/min) and some T-wave inversions were found. Patients performed a 6 min walk test, but not a CPET.
In our long COVID group, resting sinus tachycardia had lower prevalence (4/77 = 5.2%), though it should be noted that 12/77 patients (16%) were taking beta blockers, thus potentially masking a higher rate of sinus tachycardia. Patients with autonomic abnormalities showed lower predicted peak V̇O2, both in long COVID (68 ± 16 vs. 82 ± 19% predicted peak V̇O2, P = .028) and in controls (76 ± 19 vs. 94 ± 22% predicted peak V̇O2, P < .0001).
Another possible reason for low peak V̇O2 is neuromuscular dysfunction. We found that roughly 1 in 7 (14%) of the long COVID patients reported neuromuscular symptoms specific to the lower extremities during their CPET on the treadmill – compared to only 2.9% of controls. A general reduction in physical activity, compounded by hospitalization, bed rest, and use of corticosteroids, likely explains these findings, though a review of the topic of neuromuscular complications of COVID by Suh and Amato45 leaves open the possibility that neuromuscular dysfunction may contribute to long COVID.
Strengths and limitations
Our study included patients with well-documented long COVID, referred clinically for careful assessment of exercise limitations by CPET in a laboratory with high volume and complexity of patients, extensive experience, and expertise. Cardiorespiratory fitness was directly measured as peak V̇O2.46 A relevant historical comparison group was available. In terms of limitations, we are not covering the entire spectrum of long COVID. Patients with mild symptoms, limited financial resources, or too sick to comfortably travel were less likely to come to Mayo Clinic for evaluation. Patients with pre-COVID cardiac, pulmonary, or neurologic diseases presenting to other specialty clinics within Mayo were not included in this cohort, though many of them referred for CPET. Our focus was on cardiopulmonary responses to exercise. The findings of relatively frequent autonomic impairment and neuromuscular complaints would be interesting to pursue in a separate, prospectively planned protocol.
Conclusions
Patients reporting symptoms consistent with long COVID have significantly impaired exercise tolerance with low peak V̇O2 and a variety of symptoms on the exercise test. Their limitations are greater than patients complaining of undifferentiated dyspnea on exertion, but we saw limited evidence of significant underlying cardiac or pulmonary impairment. Autonomic abnormalities were common, however – more frequent than in the controls, and exercise performance and peak V̇O2 were lower in the subset of patients with abnormal autonomic responses. Neuromuscular complaints were reported in 14% of long COVID patients – again, more common than in the controls. We hope that these observations contribute to the understanding of exercise impairment in long COVID, though much is still to be learned.
Prior abstract presentation
2022 Epi Lifestyle Sessions of the American Heart Associaion (AHA). March 1-4, Chicago, Illinois.
Declaration of Competing Interest
The authors report no conflicts of interest in this work.
Acknowledgments
Guarantor: The guarantor for all content of this manuscript is T Allison.
Author contributions: T. Allison and A. Meza, lead authors, had full access to all data in the study and contributed to the study design, data collection and interpretation, and writing of the manuscript. L. Cappelloni had full access to all data in the study and contributed to data collection and writing of the manuscript. D. Newman contributed to data interpretation and writing of the manuscript. M. Mueller and R. Ganesh provided us with the historical data base and contributed to the manuscript writing. A. Bonikowske contributed to data collection and study design. A. Niven, R. Squires contributed to the writing of the manuscript.
References
- 1.Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham M., Sudre C., May A., et al. Changes in symptomatology, reinfection and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021;6:e335–e345. doi: 10.1016/S2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) December. 2021. COVID-19: About the variants.https://www.cdc.gov/coronavirus/2019-ncov/variants/about-variants.html Accessed January 31, 2022. [Google Scholar]
- 4.NBC News . December. 2021. Omicron symptoms: what we know about illness caused by the new variant.https://www.nbcnews.com/health/health-news/omicron-symptoms-covid-what-to-know-rcna9469 Accessed January 31, 2022. [Google Scholar]
- 5.Healthline Omicron symptoms: How they Compare With Other Coronavirus Variants. 2021. https://www.healthline.com/health-news/omicron-symptoms-how-they-compare-with-other-coronavirus-variants Accessed January 31, 2022.
- 6.Mancini D.M., Brunjes D.L., Lala A., et al. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9(12):927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goertz Y., Van Herck M., Delbressine J., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:00542–02020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfi A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesh R., Grach S., Ghosh A., et al. The female predominant persistent immune dysregulation of the post COVID syndrome: A cohort study. Mayo Clin Proc. 2021;96 doi: 10.1016/j.mayocp.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta D., Normandin E., ZuWallack R. Cardiopulmonary exercise testing in the assessment of exertional dyspnea. Ann Thorac Med. 2015;10(2):77–86. doi: 10.4103/1817-1737.151438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guazzi M., Arena R., Halle M., et al. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016;133:e694–e711. doi: 10.1161/CIR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 13.Milani R., Lavie C., Mehra M. Cardiopulmonary exercise testing: how do we differentiate the cause of Dyspnea? Circulation. 2004;110:e27–e31. doi: 10.1161/01.CIR.0000136811.45524.2F. [DOI] [PubMed] [Google Scholar]
- 14.Mohr A., Dannerbeck L., Lange T.J., et al. Cardiopulmonary exercise pattern in patients with persistent dyspnea after recovery from COVID-19. Multidiscip Respir Med. 2021;16:732. doi: 10.4081/mrm.2021.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Chen R., Geng Q., et al. Cardiopulmonary exercise testing might be helpful for interpretation of impaired pulmonary function in recovered COVID-19 patients. Eur Respir J. 2021 Jan 28;57(1):2004265. doi: 10.1183/13993003.04265-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clavario P., De Marzo V., Lotti R., et al. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. MedRxiv. 2020 doi: 10.1101/2020.11.15.20231985. preprint. [DOI] [Google Scholar]
- 17.Singh I., Joseph P., Heerdt P.M., et al. Persistent exertional intolerance after COVID-19: Insights from invasive cardiopulmonary exercise testing. Chest. 2022;161(1):54–63. doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Shang Y., Song W., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daida H., Allison T.G., Johnson B.D., Squires R.W., Gau G.T. Comparison of peak exercise oxygen uptake in men versus women in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997 Jul 1;80(1):85–88. [PubMed] [Google Scholar]
- 20.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2) 92–98.35. [PubMed] [Google Scholar]
- 21.Kaminsky L.A., Arena R., Myers J., et al. Updated reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the Importance of Exercise National Database (FRIEND) Mayo Clin Proc. 2022 Feb;97(2):285–293. doi: 10.1016/j.mayocp.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Skalski J., Allison T., Miller T. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation. 2012;126:2465–2472. doi: 10.1161/CIRCULATIONAHA.112.110460. [DOI] [PubMed] [Google Scholar]
- 23.Kaminsky L.A., Harber M.P., Imboden M.T., Arena R., Myers J. Peak ventilation reference standards from exercise testing: from the FRIEND registry. Med Sci Sports Exerc. 2018 Dec;50(12):2603–2608. doi: 10.1249/MSS.0000000000001740. [DOI] [PubMed] [Google Scholar]
- 24.Howley E.T., Bassett D.R., Jr., Welch H.G. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27:1292–1301. [PubMed] [Google Scholar]
- 25.Issekutz B., Birkhead N.C., Rodahl K. The use of respiratory quotients in assessment of aerobic work capacity. J Appl Physiol. 1962;17:47–50. [Google Scholar]
- 26.Edvardsen E., Hem E., Anderssen S.A. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross-sectional study. PLoS One. 2014 Jan 14;9(1):e85276. doi: 10.1371/journal.pone.0085276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assaf Y., Barout A., Alhamid A., et al. Peak systolic blood pressure during the exercise test: reference values by sex and age and association with mortality. Hypertension. 2021 Jun;77(6):1906–1914. doi: 10.1161/HYPERTENSIONAHA.120.16570. [DOI] [PubMed] [Google Scholar]
- 28.Sydó N., Abdelmoneim S.S., Mulvagh S.L., Merkely B., Gulati M., Allison T.G. Relationship between exercise heart rate and age in men vs women. Mayo Clin Proc. 2014 Dec;89(12):1664–1672. doi: 10.1016/j.mayocp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Brubaker P.H., Kitzman D.W. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011 Mar 8;123(9):1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X.G., Hansen J.E., Garatachea N., Storer T.W., Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002 Dec 1;166(11):1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 31.Raman B., Cassar M.P., Tunnicliffe E.M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. E Clinical Medicine. 2021 Jan 7;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clavario P., De Marzo V., Lotti R., et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int J Cardiol. 2021;340:113–118. doi: 10.1016/j.ijcard.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skjørten I., Ankerstjerne O.A.W., Trebinjac D., et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021;58(2):2100996. doi: 10.1183/13993003.00996-2021. Published 2021 Aug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonbank K., Lehmann A., Bernitzky D., et al. Predictors of prolonged cardiopulmonary exercise impairment after COVID-19 infection: A prospective observational study. Front Med. 2021;8:773788. doi: 10.3389/fmed.2021.773788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durstenfeld M.S., Sun K., Tahir P., et al. Use of cardiopulmonary exercise testing to evaluate long COVID-19 symptoms in adults: A systematic review and meta analysis. JAMA Netw Open. 2022;5(10) doi: 10.1001/jamanetworkopen.2022.36057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oleszak F., Maryniak A., Botti E., et al. Myocarditis Associated With COVID-19. Am J Med Case Rep. 2020;8(12):498–502. [Google Scholar]
- 37.Luchian M., Motoc A., Lochy S., et al. Subclinical myocardial dysfunction in patients with persistent Dyspnea one year after COVID-19. Diagnostics. 2022;12(57) doi: 10.3390/diagnostics12010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ingul C., Grimsmo J., Mecinaj A., et al. Cardiac dysfunction and arrhythmias 3 months after hospitalization for COVID-19. J Am Heart Assoc. 2022;11(3) doi: 10.1161/JAHA.121.023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grist J., Chen M., Collier G., et al. Hyperpolarized 129Xe MRI abnormalities in DYSPNEIC patient 3 months after COVID-19 Pneumonia: preliminary results. Radiology. 2021;301:e353–e360. doi: 10.1148/radiol.2021210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shouman K., Vanichkachorn G., Cheshire W., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;16:1–10. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisaccia G., Ricci F., Recce V., et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: What do we know? J Cardiovasc Dev Dis. 2021;8(156) doi: 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dani M., Dirksen A., Taraborrelli P., et al. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin Med (Lond) 2021 Jan;21(1):e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carod-Artal F.J. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28(1):67–81. doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 44.Aranyo J., Bazan V., Llados G., et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci Rep. 2022;12:298. doi: 10.1038/s41598-021-03831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh J., Amato A.A. Neuromuscular complications of coronavirus disease-19. Curr Opin Neurol. 2021;34(5):669–674. doi: 10.1097/WCO.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross R., Blair S.N., Arena R., et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016 Dec 13;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]