Abstract
Immunocompromised patients still experience unpredictable courses of COVID-19, despite that effective vaccines and drugs against SARS-CoV-2 are now available. Antiviral combination regimens may have a role in SARS-CoV-2 infection in immunocompromised hosts, but current knowledge is still limited. We describe the case of a 73-year-old Italian man affected by follicular lymphoma with persistent SARS-CoV-2 infection who was successfully treated with co-administration of oral antivirals (10-day molnupiravir and nirmatrelvir/ritonavir). The therapy was well tolerated both from a clinical and biochemical standpoint, with no signs of toxicity. We also performed a scoping review, to sum up available knowledge on combined antiviral regimens including remdesivir, molnupiravir, or nirmatrelvir/ritonavir. Pending further studies on larger cohorts of patients, our report is consistent with available pre-clinical and clinical data, supporting the possible use of combination therapy in selected difficult-to-treat COVID-19 cases.
Keywords: COVID-19, Antiviral, Remdesivir, Relapsing, Association
Introduction
The development of vaccines and early treatments against SARS-CoV-2, including monoclonal antibodies, intravenous remdesivir, and orally administered molnupiravir and nirmatrelvir/ritonavir, have completely rewritten the course of COVID-19 for most patients during the early phase of the disease. In particular, both nirmatrelvir/ritonavir and molnupiravir, which act as protease inhibitors and competitive substrates of viral RNA-dependent-RNA-polymerase, respectively, demonstrated to be effective in reducing the risk of hospitalization or death among at-risk adults in phase III clinical trials [1]. However, while nirmatrelvir/ritonavir confirmed its performance under real-world conditions, post-marketing data on molnupiravir showed controversial results, leading the European Medicine Agency to refuse its market authorization [2].
Immunocompromised patients still experience an unpredictable course of the disease, often burdened with permanent sequelae, unfavorable outcomes, or long-lasting viral shedding [3]. Persistent infection may interfere with the management of the underlying disease in oncohematological patients, thus increasing the all-cause mortality in this population and acting as a potential driver of intra-host viral evolution [4]. This condition differs from the rebound phenomenon described in some patients after nirmatrelvir/ritonavir treatment, which is typically characterized by a short-term relapse of symptoms and resolves without additional COVID-19-directed therapy [5]. Although such situations are not infrequent, the knowledge about the role of antivirals in profoundly immunocompromised patients, including the potential role of combinations, is still limited, and based on granular experiences.
Case report
We report the case of a 73-year-old Italian man affected by follicular lymphoma with a persistent symptomatic SARS-CoV-2 infection. Lymphoma was diagnosed in 2014 and treated with rituximab and chlorambucil; for a first relapse (2019) the patient received rituximab and bendamustine with subsequent rituximab maintenance. In 2022, due to a new relapse, he started a third-line treatment with rituximab and lenalidomide.
The patient first tested positive for SARS-CoV-2 antigen on nasopharyngeal swab (NPS) in October 2022 and the following day (day 1) started early antiviral treatment with nirmatrelvir/ritonavir (300/100 mg q 12 hours for 5 days). Antigenic test on NPS yielded a negative result on day 9. However, due to the relapse of flu-like symptoms (cough, sore throat, rhinorrhea, and fever), the patient repeated an antigen test, which tested positive (day 25). Owing to his status of being immunocompromised, he received an intramuscular therapeutic dose (300/300 mg) of cilgavimab/tixagevimab (day 27), with no benefit. Due to the onset of type I respiratory failure, the patient was hospitalized on days 36-41 and administered methylprednisolone and ceftriaxone, but no antiviral drugs. After discharge, the cough and sore throat continued, while serial self-administered antigen testing on NPS yielded positive results (days 42-64). On day 64, a new NPS underwent a real-time polymerase chain reaction test which detected SARS-CoV-2 and subsequently, was subjected to whole genome sequencing and classified as lineage BA.5.2.23. Characteristic mutations of this lineage and other genetic mutations identified in the patient's sample are reported in Supplemental Data 1. No amino acids mutation previously associated with nirmatrelvir resistance (i.e., L50F, E166V, L167F) was revealed in the 3CL main protease (Mpro) from SARS-CoV-2.
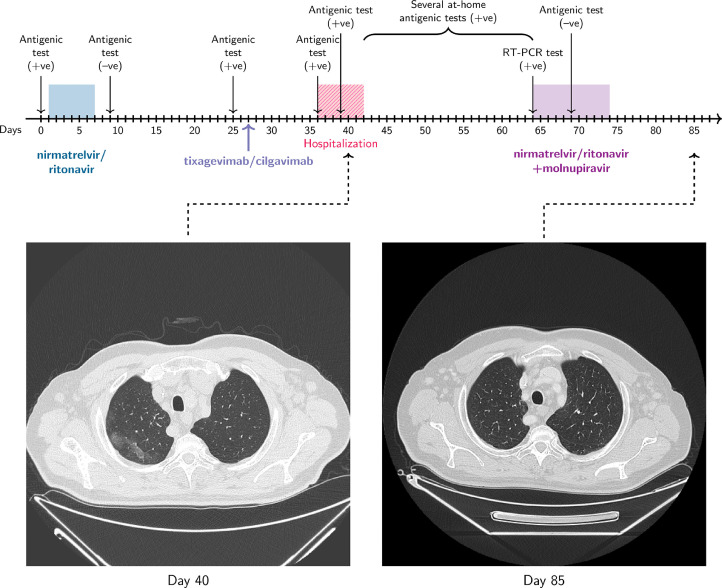
In virtue of pre-clinical studies suggesting a synergistic effect of antiviral combinations [6], [7], [8], [9], an off-label combination regimen of molnupiravir (800 mg q 12 hours) and nirmatrelvir/ritonavir (300/100 mg q 12 hours) was started (day 64). Five days later (day 69), antigenic testing on NPS resulted negative, nonetheless, the treatment was still carried out until day 73. At the same time, the patient reported a rapid and permanent regression of symptoms, confirmed at the follow-up visit performed on day 88. The combination therapy was well tolerated both from a clinical and biochemical standpoint, with no signs of toxicity (Supplemental Data 2). Clinical and virological response was corroborated by radiological findings at the chest computed tomography scans performed before (day 40) and after (day 85) antiviral combination therapy (Figure 1 ).
Figure 1.
Timeline showing the patient's history and the days elapsed from the first positivity (day 0). Chest computed tomography scans performed before (day 40) and after (day 85) antiviral combination therapy showed a complete resolution of the ground-glass areas consistent with interstitial pneumonia affecting the superior right lobe and, to a lesser extent, the lingula, middle lobe, and lower lobes. RT-PCR, real-time polymerase chain reaction.
Scoping review
We performed a scoping review aiming to retrieve clinical and pre-clinical data on the combination of the three antivirals actually licensed in Europe and the US for SARS-CoV-2 infection (molnupiravir, nirmatrelvir/ritonavir, remdesivir) from three peer-reviewed databases (PubMed, Embase, and Web of Science) and two preprint servers (medRxiv, bioRxiv), for studies published between November 24, 2021, and January 23, 2023, by using a predefined search string (Supplemental Data 3). Pertinent references from these articles were also evaluated, as well as gray literature from the web, including scientific congress proceedings, webinars, and/or workshops. No language filters were set. After duplicate exclusion, 1143 papers were identified and screened. Two authors (DM, RMA) independently screened the papers and jointly made the final decision about which papers to include. A third author (MS) supervised the whole selection process. Nine papers were finally included: three clinical reports (four patients treated), three animal studies, and three in vitro studies [10], [11], [12].
The first report described an immunocompromised person with prolonged, persistent, and severe COVID-19 (lineage BA.1.1) [10]. After multiple therapies had been tried without benefit (including monoclonal antibodies and IV remdesivir), the patient improved with a 10-day course of nirmatrelvir/ritonavir in combination with remdesivir and achieved a sustained clinical cure after a full 20 days of nirmatrelvir/ritonavir.
The second patient had previously received nirmatrelvir/ritonavir monotherapy, remdesivir monotherapy, and bebtelovimab with the persistence of symptoms and positive NPS. Remdesivir in combination with nirmatrelvir/ritonavir was started. The patient tested negative on NPS after 9 days of treatment and continued the dual therapy for a total of 20 days [11].
The third paper describes the cases of two immunocompromised patients, with COVID-19 pneumonia, on maintenance therapy with rituximab due to their underlying condition—non-Hodgkin lymphoma with autologous hematopoietic stem-cell transplantation, and eosinophilic granulomatosis with polyangiitis. Both were successfully treated with co-administration of sotrovimab (single infusion), remdesivir (7-day course), and nirmatrelvir/ritonavir (5-day course) [12].
In all four cases, there were no meaningful adverse effects or toxicity.
As for in vitro studies, molnupiravir-based combinations showed enhanced activity compared to molnupiravir tested alone. A synergistic effect of molnupiravir and nirmatrelvir was described on Vero E6 cells at 48 and 72 hours and on human airway organoids against both wild-type SARS-CoV-2 and the Omicron variant [8,9]. In an in vitro model of nasal epithelium, molnupiravir-based combinations boosted antiviral activity against SARS-CoV-2 compared to the single compound treatment as described [13].
Regarding in vivo models, the combination of molnupiravir plus nirmatrelvir was evaluated in two studies in mice and macaques, while one study evaluated molnupiravir in association with the parent nucleoside of remdesivir (GS-441524) in hamsters [6,7,14]. In macaques infected with the SARS-CoV-2 Delta variant, co-administration of molnupiravir and nirmatrelvir reduced viral shedding, virus replication in the lower respiratory tract, and viral antigen in respiratory tissues, but no definitive results were achieved regarding its safety and tolerability [6]. In mice infected with the SARS-CoV-2 Beta variant, the association of molnupiravir and nirmatrelvir reduced severity score, virus-induced tissue damage, and viral distribution when compared to the association of nirmatrelvir and remdesivir or either one of the antivirals administered alone [7].
Discussion
To the best of our knowledge, this is the first case reporting clinical, radiological, and microbiological success in an immunosuppressed oncohematological patient treated with a combination of two oral antivirals (nirmatrelvir/ritonavir plus molnupiravir) for prolonged symptomatic infection with SARS-CoV-2.
For combinations to be used in a clinical setting it is essential that antagonism is excluded by pre-clinical work. Once antagonism is excluded, an enhanced effect (additive or synergistic) could be expected by combinations, when compared with monotherapies. Regarding the combination of nirmatrelvir/ritonavir and molnupiravir, some authors raised concern, given that the former aims to prevent viral replication while the basis of the latter relies on direct interference with this process [15]. However, pre-clinical data on the combination of the three available antivirals against SARS-CoV-2 are promising, while clinical experience is still limited. The mechanism lying behind some synergistic effects is yet to be proven, but it is supposed that acting on different pathways enhances antiviral activity and prevents the emergence of drug resistance [16].
In our case, as well as in the other clinical report, combination therapy led to a quick clinical and virological recovery, allowing us to suppose a strong antiviral effect, against no concerns of tolerability. Unlike monoclonal antibodies, antiviral compounds are not affected by Spike protein mutations of SARS-CoV-2 variants of concern and retain activity against most recent Omicron subvariants similar to that against the ancestral SARS-CoV-2 strain [17]. However, sporadic cases of resistance to remdesivir have already emerged [18]. Oral antivirals are considered at low risk to induce resistance owing to the short period of treatment and the high drug dosage. However, the possibility that resistant strains emerge under the pressure of a single antiviral agent is real, especially in immunocompromised patients with long-lasting SARS-CoV-2 carriage. The lesson learned from the treatment of other viruses, such as HIV and hepatitis C, demonstrates that combinations of multiple antivirals are highly effective in preventing resistance.
The study has some limitations. For instance, whole genome sequencing was performed on a single sample (on day 64), thus reinfection with a different Omicron strain cannot be excluded. Actually, it was highly improbable, since the patient had only one negative NPS (on day 9), soon after the end of the first nirmatrelvir/ritonavir course, while COVID-related symptoms and viral shedding were steadily present between day 25-64 when a combination regimen was introduced.
Previous reports highlighted the importance of oral antiviral drugs beyond the early stage of COVID-19, for the treatment of persistent and/or relapsing COVID-19 in immunocompromised hosts [19]. Pending larger clinical studies to furtherly investigate the efficacy and safety of antiviral combination regimens in the treatment of SARS-CoV-2 infection, this possibility should be considered at least in selected cases, such as immunocompromised patients who fail to achieve clinical cure and viral clearance with conventional strategies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The study was performed in accordance with the ethical principles of the Declaration of Helsinki and with the International Conference on Harmonization Good Clinical Practice guidelines. The patient provided signed informed consent for publication.
Author contributions
Conceptualization: DM, RMA, LZ, GMR, AMV, AB, MS; Methodology/literature search: DM, RMA, MS; Clinical management: DM, MC, MP, LN, NS, LP, FM; Supervision: GMR, AMV, AB, MS; Writing - original draft preparation: DM, RMA, MC, MS; Writing – review and editing: all authors.
Declarations of competing interest
The authors have no competing interests to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.04.412.
Appendix. Supplementary materials
References
- 1.Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023 doi: 10.1038/s41573-023-00672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Medicines Agency . 2023. Refusal of the marketing authorisation for Lagevrio (molnupiravir) [Google Scholar]; https://www.ema.europa.eu/en/documents/smop-initial/questions-answers-refusal-marketing-authorisation-lagevrio-molnupiravir_en.pdf [accessed 26 April 2023].
- 3.Lodi L, Moriondo M, Pucci A, Pisano L, Ricci S, Indolfi G, et al. Chronic asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the immunocompromised patient: new challenges and urgent needs. Clin Infect Dis. 2022;74:553. doi: 10.1093/cid/ciab538. [DOI] [PubMed] [Google Scholar]
- 4.Spinicci M, Mazzoni A, Coppi M, Antonelli A, Salvati L, Maggi L, et al. Long-term SARS-CoV-2 asymptomatic carriage in an immunocompromised Host: Clinical, Immunological, and Virological Implications. J Clin Immunol. 2022;42:1371–1378. doi: 10.1007/s10875-022-01313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai CC, Hsueh PR. Coronavirus disease 2019 rebounds following nirmatrelvir/ritonavir treatment. J Med Virol. 2023;95:e28430. doi: 10.1002/jmv.28430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenke K, Lewis MC, Feldmann F, Bohrnsen E, Schwarz B, Okumura A, et al. Combined molnupiravir-nirmatrelvir treatment improves effect on SARS-CoV-2 in Macaques. JCI Insight. 2023;8 doi: 10.1172/jci.insight.166485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong JH, Chokkakula S, Min SC, Kim BK, Choi WS, Oh S, et al. Combination therapy with nirmatrelvir and molnupiravir improves the survival of SARS-CoV-2 infected mice. Antiviral Res. 2022;208 doi: 10.1016/j.antiviral.2022.105430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gidari A, Sabbatini S, Schiaroli E, Bastianelli S, Pierucci S, Busti C, et al. The combination of molnupiravir with nirmatrelvir or GC376 has a synergic role in the inhibition of SARS-CoV-2 replication in vitro. Microorganisms. 2022;10:1475. doi: 10.3390/microorganisms10071475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P, Wang Y, Lavrijsen M, Lamers MM, de Vries AC, Rottier RJ, et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ES, Simmons W, Karmarkar EN, Yoke LH, Braimah AB, Orozco JJ, et al. Successful treatment of prolonged, severe COVID-19 lower respiratory tract disease in a B-cell Acute Lymphoblastic Leukemia patient with an extended course of remdesivir and nirmatrelvir/ritonavir. Clin Infect Dis. 2023;76:926–929. doi: 10.1093/cid/ciac868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trottier CA, Wong B, Kohli R, Boomsma C, Magro F, Kher S, et al. Dual antiviral therapy for persistent COVID-19 and associated organizing pneumonia in an immunocompromised host. Clin Infect Dis. 2023;76:923–925. doi: 10.1093/cid/ciac847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldi F, Dentone C, Mikulska M, Fenoglio D, Mirabella M, Magnè F, et al. Case report: sotrovimab, remdesivir and nirmatrelvir/ritonavir combination as salvage treatment option in two immunocompromised patients hospitalized for COVID-19. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.1062450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsdottir HR, Siegrist D, Julien T, Padey B, Bouveret M, Terrier O, et al. Molnupiravir combined with different repurposed drugs further inhibits SARS-CoV-2 infection in human nasal epithelium in vitro. Biomed Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.113058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelnabi R, Maes P, de Jonghe S, Weynand B, Neyts J. Combination of the parent analogue of remdesivir (GS-441524) and molnupiravir results in a markedly potent antiviral effect in SARS-CoV-2 infected Syrian hamsters. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1072202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahmah L, Abarikwu SO, Arero AG, Essouma M, Jibril AT, Fal A, et al. Oral antiviral treatments for COVID-19: opportunities and challenges. Pharmacol Rep. 2022;74:1255–1278. doi: 10.1007/s43440-022-00388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagoner J, Herring S, Hsiang TY, Ianevski A, Biering SB, Xu S, et al. Combinations of host- and virus-targeting antiviral drugs confer synergistic suppression of SARS-CoV-2. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.03331-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, et al. Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N Engl J Med. 2022;387:468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi S, Klein J, Robertson AJ, Peña-Hernández MA, Lin MJ, Roychoudhury P, et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat Commun. 2022;13:1547. doi: 10.1038/s41467-022-29104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graziani L, Gori L, Manciulli T, Basile G, Campolmi I, Borchi B, et al. Successful use of nirmatrelvir/ritonavir in immunocompromised patients with persistent and/or relapsing COVID-19. J Antimicrob Chemother. 2023;78:555–558. doi: 10.1093/jac/dkac433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.