Abstract
Aims
Haematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for several malignant and non-malignant haematologic conditions. Patients undergoing HSCT are at an increased risk of developing atrial fibrillation (AF). We hypothesized that a diagnosis of AF would be associated with poor outcomes in patients undergoing HSCT.
Methods and results
The National Inpatient Sample (2016–19) was queried with ICD-10 codes to identify patients aged >50 years undergoing HSCT. Clinical outcomes were compared between patients with and without AF. A multivariable regression model adjusting for demographics and comorbidities was used to calculate the adjusted odds ratio (aOR) and regression coefficients with corresponding 95% confidence intervals and P-values. A total of 50 570 weighted hospitalizations for HSCT were identified, out of which 5820 (11.5%) had AF. Atrial fibrillation was found to be independently associated with higher inpatient mortality (aOR 2.75; 1.9–3.98; P < 0.001), cardiac arrest (aOR 2.86; 1.55–5.26; P = 0.001), acute kidney injury (aOR 1.89; 1.6–2.23; P < 0.001), acute heart failure exacerbation (aOR 5.01; 3.54–7.1; P < 0.001), cardiogenic shock (aOR 7.73; 3.17–18.8; P < 0.001), and acute respiratory failure (aOR 3.24; 2.56–4.1; P < 0.001) as well as higher mean length of stay (LOS) (+2.67; 1.79–3.55; P < 0.001) and cost of care (+67 529; 36 630–98 427; P < 0.001).
Conclusion
Among patients undergoing HSCT, AF was independently associated with poor in-hospital outcomes, higher LOS, and cost of care.
Keywords: Atrial fibrillation, Bone marrow transplantation, Haematopoietic stem cell transplantation, Mortality
Graphical Abstract
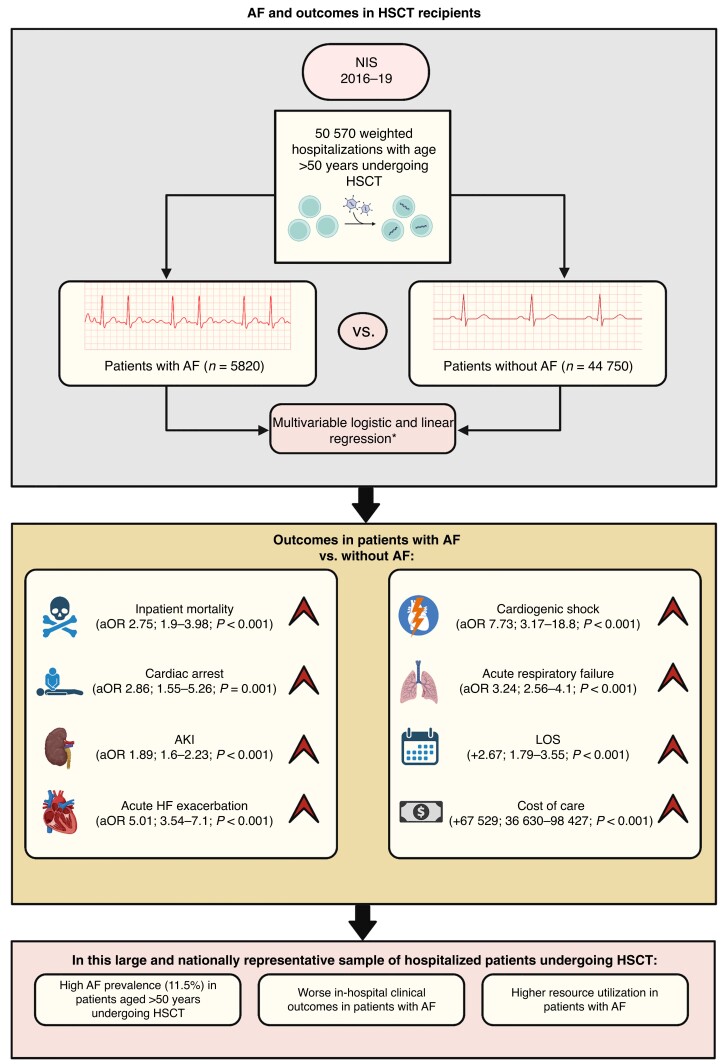
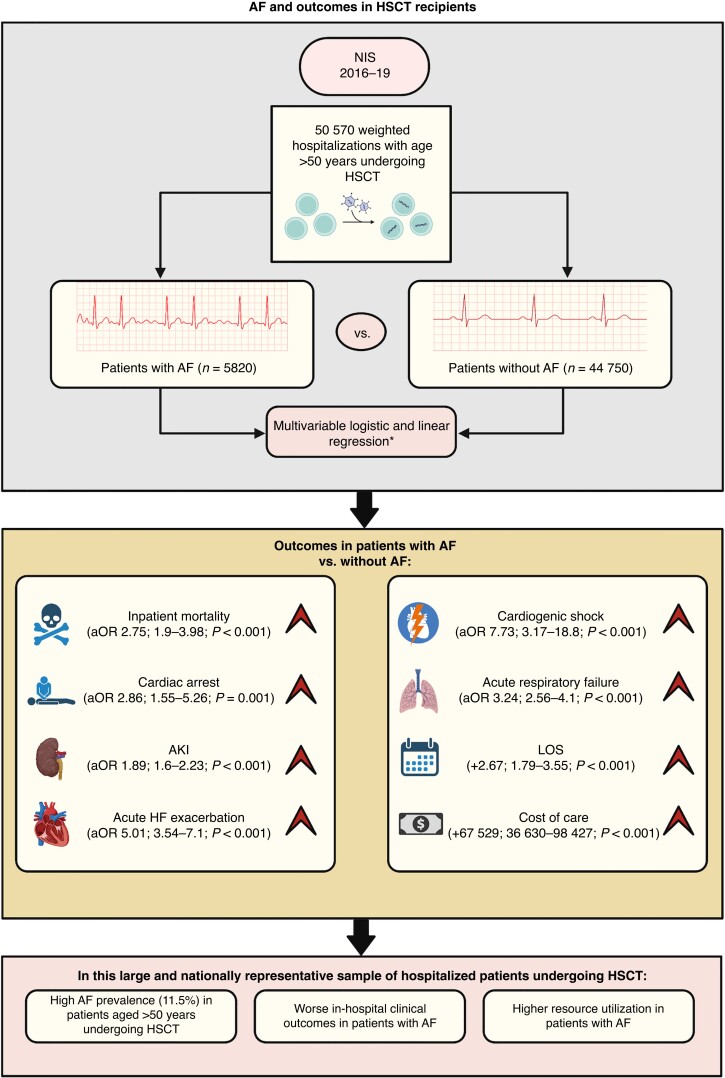
Graphical abstract.
What’s new?
Patients undergoing haematopoietic stem cell transplantation and >50 years have a high prevalence of atrial fibrillation (AF).
Haematopoietic stem cell transplant recipients with concomitant AF have worse in-hospital outcomes compared to those without AF.
Atrial fibrillation is associated with higher resource utilization in these patients.
Introduction
Haematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for several malignant and non-malignant haematologic and immune disorders.1 Patients undergoing HSCT are at an increased risk of developing HSCT-related cardiac complications, such as cardiomyopathy, myopericarditis, ischaemic heart disease, and cardiac arrhythmias.2–4 Previous studies have shown that supraventricular tachyarrhythmias (SVT), and particularly atrial tachyarrhythmias, are common in HSCT recipients.5–7
Prior studies have suggested that the presence of arrhythmias correlates with poor in-hospital and long-term outcomes in patients undergoing HSCT.6,8 While atrial fibrillation (AF) is common in patients undergoing HSCT, the impact of AF on clinical outcomes is uncertain in this patient population.6 Moreover, the current thrombo-embolic and bleeding risk scores for AF have not been validated in patients undergoing HSCT.9–11 In the current study, we sought to examine the association of AF with clinical outcomes and healthcare resource utilization in hospitalized patients undergoing HSCT.
Methods
Data source
We conducted a retrospective study using data from the National Inpatient Sample (NIS) for the years 2016–19. The NIS is part of a family of databases developed under the Healthcare Cost and Utilization Project (HCUP) through a Federal-State-Industry Partnership and sponsored by the Agency for Healthcare Research and Quality (AHRQ) and is the largest publicly available inpatient healthcare database. The NIS contains data on ∼7 million unweighted or 35 million weighted hospitalizations each year and can be used to compute national estimates of healthcare utilization, costs, and outcomes.12 Due to the de-identified nature of the NIS, the need for informed consent and Institutional Review Board (IRB) approval was waived. The NIS adheres to the 2013 Declaration of Helsinki for the conduct of human research.
Study population
Patients undergoing HSCT and those with a secondary diagnosis of AF were identified using International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes ‘30243A-’, ‘30243C-’, ‘30243G-’, ‘30243U-’, ‘30243X-’, ‘30243Y-’, ‘30233A-’, ‘30233C-’, ‘30233G-’, ‘30233U-’, ‘30233X-’, and ‘30233Y-’.
Clinical and demographic characteristics, inpatient clinical outcomes [inpatient mortality, cardiac arrest, acute respiratory failure, acute heart failure (HF) exacerbation, cardiogenic shock, stroke/transient ischaemic attack (TIA), acute kidney injury (AKI), and major bleeding], and resource utilization [hospital length of stay (LOS) expressed in days, cost of care in United States Dollars (USD), and discharge disposition] were compared between patients with and without AF. The cost of care was calculated and compared using cost-to-charge ratio files. We also analysed 4-year national trends for our outcomes of interest.
Statistical analyses
Descriptive statistics are presented as frequencies and percentages with corresponding 95% confidence intervals (CI) for categorical variables and as means with corresponding 95% CI for continuous variables. Baseline characteristics and unadjusted outcomes were compared using the Pearson χ2 test and univariable logistic regression for categorical variables and univariate linear regression for continuous variables. For assessment of the association of AF with outcomes, including inpatient mortality, cardiac arrest, acute respiratory failure, acute HF exacerbation, cardiogenic shock, stroke/TIA, AKI, and major bleeding, a multivariable logistic regression model was utilized to calculate adjusted odds ratio (aOR) with corresponding 95% CI and P-values. For the comparison of resource utilization, including LOS and cost of care, a multivariable linear regression model was utilized to calculate adjusted regression coefficients with corresponding 95% CI and P-values. The multivariable regression model was adjusted for demographics, including age, sex, race/ethnicity, insurance status, median household income quartile, and underlying comorbidities, including hypertension, complicated and uncomplicated diabetes mellitus (DM), coronary artery disease (CAD), peripheral artery disease (PAD), chronic HF, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cerebrovascular disease (CVD), obesity, cirrhosis, anaemia, thyroid dysfunction, alcohol use, drug use, haematologic malignancies (including Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma, acute and chronic myeloid leukaemia, acute and chronic lymphoid leukaemia, and monocytic leukaemia), and metastatic disease. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using the software Stata/BE version 17.0 (StataCorp).
Results
Our cohort was composed of a total of 50 570 hospitalizations for HSCT from 2016 to 2019. Among these hospitalizations, ∼5820 (11.5%) had AF as a secondary diagnosis. The prevalence of atrial flutter and other supraventricular tachycardias was 2.2 and 2.6%, respectively.
In the overall study cohort, the mean age was 62.5 years, 41.4% were females, and information on other clinical and demographic characteristics is available in Table 1. Patients with AF were older (64.9 vs. 62.2 years, P < 0.001) and more likely to be White (80.1 vs. 71.5%, P < 0.001). Patients without AF were more likely to be Black (12.6 vs. 6.3%, P < 0.001), Hispanic (7.8 vs. 5.7%, P < 0.001), and Asian (2.7 vs. 2.4%, P < 0.001). Patients with AF had a higher burden of comorbidities, including hypertension (62.1 vs. 54.3%, P < 0.001), DM (21.2 vs. 17.6%, P = 0.003), chronic HF (11.9 vs. 3.1%, P < 0.001), and CAD (15.5 vs. 8.3%, P < 0.001). Patients with AF were more likely to have Medicare insurance (49.0 vs. 35.6%, P < 0.001), and patients without AF were more likely to have Medicaid (7.2 vs. 3.8%, P < 0.001) and private insurance (56.3 vs. 46.8%, P < 0.001) and self-pay (0.9 vs. 0.4%, P < 0.001).
Table 1.
Clinical and demographic characteristics of patients undergoing BMT stratified by AF status
| AF (n = 5820) | No AF (44 750) | P-value | |
|---|---|---|---|
| Mean age | 64.9 (64.5–65.3) | 62.2 (62–62.3) | <0.0001 |
| Female (%) | 1940 (33.4%) | 1.9 × 104 (42.4%) | <0.0001 |
| Race (%) | <0.0001 | ||
| White | 4540 (80.1%) | 3.1 × 104 (71.5%) | — |
| Black | 355 (6.3%) | 5405 (12.6%) | — |
| Hispanic | 320 (5.7%) | 3335 (7.8%) | — |
| Asian or Pacific Islander | 135 (2.4%) | 1155 (2.7%) | — |
| Native American | 15 (0.3%) | 110 (0.3%) | — |
| Other | 250 (4.5%) | 2220 (5.2%) | — |
| Insurance status (%) | <0.0001 | ||
| Medicare | 2805 (49%) | 1.5 × 104 (35.6%) | — |
| Medicaid | 215 (3.8%) | 3110 (7.2%) | — |
| Private insurance | 2675 (46.8%) | 2.4 × 104 (56.3%) | — |
| Self-pay | 25 (0.4%) | 390 (0.9%) | — |
| Median household income quartile (%) | 0.82 | ||
| 0th–25th percentile | 1020 (17.7%) | 8230 (18.7%) | — |
| 26th–50th percentile (median) | 1335 (23.2%) | 1 × 104 (23.7%) | — |
| 51st–75th percentile | 1575 (27.4%) | 1.2 × 104 (26.6%) | — |
| 76th–100th percentile | 1820 (31.7%) | 1.4 × 104 (31%) | — |
| Hospital region (%) | 0.33 | ||
| Northeast | 740 (23.9%) | 5350 (23.8%) | — |
| Midwest | 900 (29.1%) | 5835 (26%) | — |
| South | 885 (28.6%) | 6995 (31.1%) | — |
| West | 570 (18.4%) | 4285 (19.1%) | — |
| Hypertension (%) | 3615 (62.1%) | 2.4 × 104 (54.3%) | <0.0001 |
| Diabetes (%) | 1235 (21.2%) | 7890 (17.6%) | 0.003 |
| Chronic HF (%) | 695 (11.9%) | 1395 (3.12%) | <0.0001 |
| CAD (%) | 900 (15.5%) | 3715 (8.3%) | <0.0001 |
| PAD (%) | 105 (1.8%) | 475 (1.1%) | 0.03 |
| COPD (%) | 60 (1.03%) | 375 (0.8%) | 0.48 |
| CKD (%) | 935 (16.1%) | 4705 (10.5%) | <0.0001 |
| CVD (%) | 140 (2.4%) | 585 (1.3%) | 0.004 |
| Obesity (%) | 635 (10.9%) | 3615 (0.8%) | 0.0003 |
| Cirrhosis (%) | 20 (0.3%) | 165 (0.4%) | 0.9 |
| Anaemia (%) | 4990 (85.7%) | 3.7 × 104 (83.3%) | 0.03 |
| Thyroid dysfunction (%) | 770 (13.2%) | 5640 (12.6%) | 0.49 |
| Coagulopathy (%) | 1200 (20.6%) | 6365 (14.2%) | <0.0001 |
| Alcohol use (%) | 25 (0.4%) | 325 (0.7%) | 0.23 |
| Drug abuse (%) | 300 (5.2%) | 2745 (6.1%) | 0.19 |
| Hodgkin’s lymphoma (%) | 15 (0.3%) | 90 (0.2%) | 0.69 |
| Non-Hodgkin’s lymphoma (%) | 220 (3.8%) | 1620 (3.6%) | 0.79 |
| Multiple myeloma (%) | 245 (4.2%) | 1655 (3.7%) | 0.41 |
| Lymphoid leukaemia (%) | |||
| Acute lymphoid leukaemia | <11 | 90 (0.2%) | 0.83 |
| Chronic lymphoid leukaemia | 40 (0.7%) | 225 (0.5%) | 0.38 |
| Myeloid leukaemia (%) | |||
| Acute myeloid leukaemia | 45 (0.8%) | 455 (1%) | 0.43 |
| Chronic myeloid leukaemia | <11 | 25 (0.06%) | 0.16 |
| Aplastic anaemia (%) | 4760 (81.8%) | 3.5 × 104 (77.4%) | 0.0007 |
| Chronic myeloproliferative neoplasm (%) | <11 | 45 (0.1%) | 0.32 |
For n < 11, the absolute numbers are not reported as per HCUP recommendations.
AF, atrial fibrillation; BMT, bone marrow transplantation; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; HCUP, Healthcare Cost and Utilization Project; HF, heart failure; PAD, peripheral artery disease.
Outcomes of patients with atrial fibrillation
Patients with concomitant AF had higher inpatient mortality (6.4 vs. 2.0%, P < 0.001), cardiac arrest (1.6 vs. 0.4%, P < 0.001), AKI (28.1 vs. 14.3%, P < 0.001), acute HF exacerbation (4.8 vs. 0.9%, P < 0.001), acute respiratory failure (14.4 vs. 4.1%, P < 0.001), and stroke/TIA (1.2 vs. 0.4%, P = 0.001). Atrial fibrillation patients also had longer mean LOS (24.4 vs. 21.3 days, P < 0.001) and increased cost of care (396 132 vs. 325 578 USD, P < 0.001) on unadjusted analyses. A higher proportion of AF patients were discharged to another facility (6.7 vs. 2.1%, P < 0.001) or required home health services (21.2 vs. 17.7%, P < 0.001) compared to those without AF. The distribution of other relevant outcomes is shown in Table 2.
Table 2.
Crude in-hospital outcomes and resource utilization of patients undergoing BMT stratified by AF status
| AF (n = 5820) | No AF (44 750) | P-value | |
|---|---|---|---|
| All-cause mortality (%) | 370 (6.4%) | 875 (2%) | <0.0001 |
| Cardiac arrest (%) | 90 (1.6%) | 170 (0.4%) | <0.0001 |
| AKI (%) | 1635 (28.1%) | 6405 (14.3%) | <0.0001 |
| Major bleeding (%) | 1210 (20.8%) | 8745 (19.5%) | 0.31 |
| Acute HF exacerbation (%) | 280 (4.8%) | 415 (0.9%) | <0.0001 |
| Acute respiratory failure (%) | 840 (14.4%) | 1845 (4.1%) | <0.0001 |
| Cardiogenic shock (%) | 65 (1.1%) | 45 (0.1%) | <0.0001 |
| Stroke/TIA (%) | 70 (1.2%) | 170 (0.4%) | 0.001 |
| LOS, days | 24.44 (23.5–25.4) | 21.27 (20.8–21.73) | <0.0001 |
| Total hospitalization expenses, USD | 396 287 (359 132–433 442) | 325 578 (307 974–343 183) | <0.0001 |
| Discharge disposition (%) | <0.0001 | ||
| Routine | 3795 (65.3%) | 3.5 × 104 (78%) | — |
| Short-term hospital | 25 (0.4%) | 95 (0.2%) | — |
| Another type of facility | 390 (6.7%) | 925 (2.1%) | — |
| Home healthcare | 1230 (21.2%) | 7935 (17.7%) | — |
| Against medical advice | 5 (0.02%) | 5 (0.01%) | — |
For n < 11, the absolute numbers are not reported as per HCUP recommendations.
AF, atrial fibrillation; AKI, acute kidney injury; BMT, bone marrow transplantation; HCUP, Healthcare Cost and Utilization Project; HF, heart failure; LOS, length of stay; TIA, transient ischaemic attack; USD, United States Dollars.
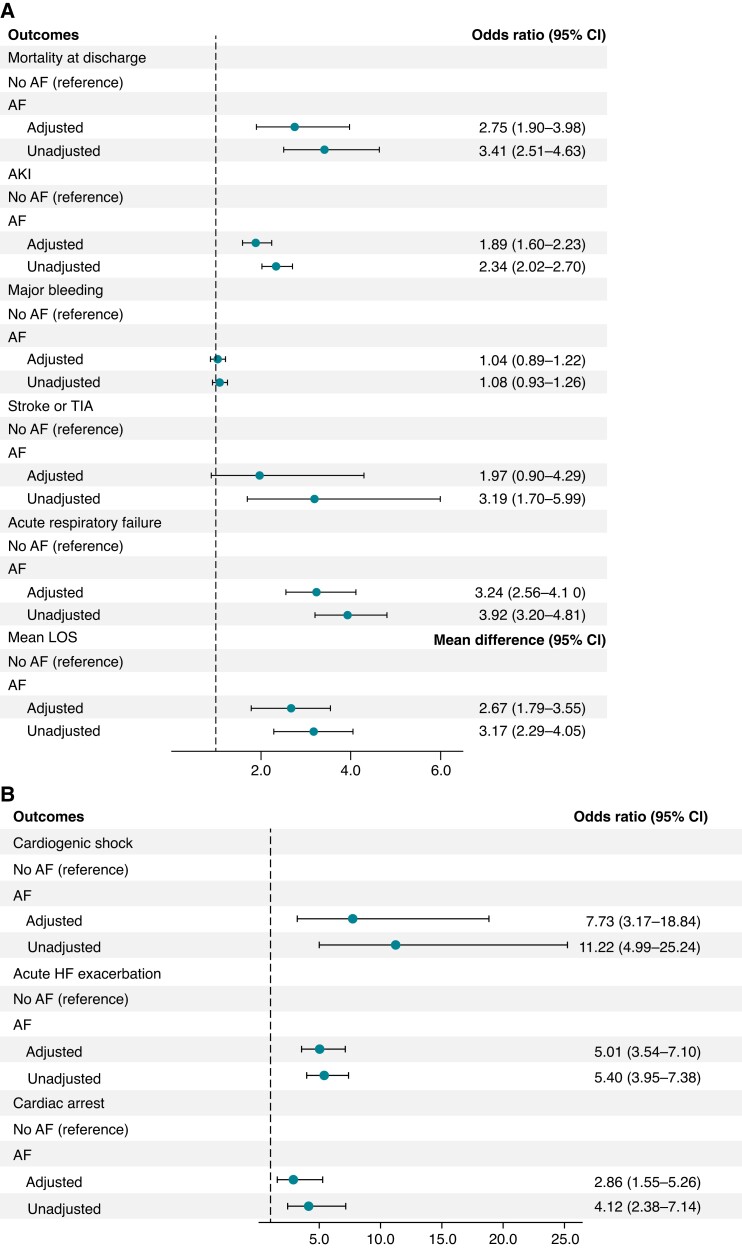
We assessed the association of AF with adverse outcomes and resource utilization using multivariable regression models, adjusted for potential confounders, such as age, sex, race, CAD, PAD, chronic HF, CVD, CKD, anaemia, coagulopathy, cirrhosis, and thyroid dysfunction. After adjusting for age, sex, race, insurance status, income status, and comorbidities, such as hypertension, diabetes, chronic HF, CAD, PAD, COPD, CKD, CVD, obesity, anaemia, thyroid dysfunction, cirrhosis, coagulopathy, and drug and alcohol abuse, AF was found to be associated with adverse outcomes, including higher inpatient mortality (aOR 2.75; 95% CI: 1.9–3.98; P < 0.001), cardiac arrest (aOR 2.86; 95% CI: 1.55–5.26; P = 0.001), AKI (aOR 1.89; 95% CI: 1.6–2.23; P < 0.001), acute HF exacerbation (aOR 5.01; 95% CI: 3.54–7.1; P < 0.001), cardiogenic shock (aOR 7.73; 95% CI: 3.17–18.8; P < 0.001), and acute respiratory failure (aOR 3.24; 95% CI: 2.56–4.1; P < 0.001) (Figure1A and B). Atrial fibrillation was also associated with longer mean LOS (+2.67; 95% CI: 1.79–3.55; P < 0.001) (Figure 1A) and higher cost of care (+67 529; 95% CI: 36 630–98 427; P < 0.001).
Figure 1.
(A) Adjusted and unadjusted association of AF with in-hospital clinical outcomes, including inpatient mortality, AKI, major bleeding, stroke/TIA, acute respiratory failure, and mean LOS. (B) Adjusted and unadjusted association of AF with in-hospital cardiovascular outcomes, including cardiogenic shock, acute HF exacerbation, and cardiac arrest. AF, atrial fibrillation; AKI, acute kidney injury; CI, confidence interval; HF, heart failure; LOS, length of stay; TIA, transient ischaemic attack.
Results demonstrating a comparison of clinical outcomes, including inpatient mortality, AKI, major bleeding, stroke/TIA, acute respiratory failure, and mean LOS, are shown in Figure 1A. Results demonstrating a comparison of cardiovascular outcomes, including acute HF exacerbation, cardiogenic shock, and cardiac arrest, are shown in Figure 1B. A comparison of the cost of care between AF and non-AF patients and a 4-year national trend of clinical outcomes are available in Supplementary material online.
Discussion
In this large and nationally representative sample of hospitalized patients undergoing HSCT, the main findings of our study are as follows (Figure 2):
Figure 2.
Central illustration depicting study design. *Logistic and linear regression analysis adjusted for age, female sex, race, insurance status, median household income, hypertension, diabetes, CAD, PAD, chronic HF, COPD, chronic kidney disease, obesity, cirrhosis, anaemia, coagulopathy, drug or alcohol use, and metastatic disease. AF, atrial fibrillation; AKI, acute kidney injury; aOR, adjusted odds ratio; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; HSCT, haematopoietic stem cell transplantation; LOS, length of stay; NIS, National Inpatient Sample; PAD, peripheral artery disease.
The overall prevalence of AF in patients aged >50 years undergoing HSCT was high (11.5%).
Atrial fibrillation was associated with worse outcomes, including inpatient mortality, cardiac arrest, AKI, acute HF exacerbation, cardiogenic shock, and acute respiratory failure. This association was independent of age, socioeconomic factors, and certain important comorbidities, including hypertension, diabetes, chronic HF, CAD, PAD, CKD, obesity, anaemia, and coagulopathy.
Atrial fibrillation was associated with longer LOS and higher cost of care.
Our findings confirm prior reports of high AF risk among patients undergoing HSCT. Our study cannot investigate the mechanisms of this association or suggest causation. The high prevalence of AF in HSCT patients could be due to several reasons. Haematopoietic stem cell transplantation-related cardiotoxicity is well known, and these effects may be contributed to by pre-HSCT conditioning therapy that can involve a combination of cardiotoxic chemotherapeutic agents, immunotherapy, and mediastinal radiation during the pre- or immediate post-transplant period.2,3,5 Conditioning therapy also leads to significant immunosuppression, predisposing patients to severe infections, sepsis, and subsequently AF.13–15 In our study, however, patients with AF were slightly older and in general had a higher comorbidity burden, including hypertension, DM, chronic HF, and CAD, compared to those without AF, and advanced age and certain comorbidities are well-established risk factors for the genesis of AF.16–18 Therefore, we are unable to determine the direct impact of underlying haematologic disease and HSCT therapy on AF risk in this population with high cardiovascular risk factors.
In our analyses, we observed higher rates of adverse clinical outcomes in patients with AF, including cardiogenic shock, acute HF exacerbation, and acute respiratory failure, which may possibly correspond to the use of specific AF treatment strategies in the setting of pre-existing HSCT-related cardiotoxicity. In our study, patients with AF did not appear to have higher rates of major bleeding as compared to those without AF, potentially due to the withholding of therapeutic anticoagulation due to underlying thrombocytopaenia.19,20 Notably, we found a significant association of AF with inpatient mortality and cardiac arrest, but we cannot determine whether AF directly contributed to these adverse outcomes or whether AF was just a marker of poor cardiovascular health.
The findings of this study are clinically significant, as AF has previously been shown to be associated with poor outcomes in patients undergoing inpatient chemotherapy and among patients undergoing chimeric antigen receptor T cell (CAR-T) therapy.21,22 It is important to note that there is a paucity of data on the prevention and management of AF in patients undergoing HSCT.10,11 Results from those studies, coupled with the current findings in HSCT patients, may indicate significant AF-related morbidity and mortality with therapeutic modalities currently available for malignant haematologic neoplasms. Current treatment guidelines for most cancers recommend baseline electrocardiogram (ECG) for patients prior to initiation of treatment, mainly to monitor QTc intervals.23,24 Whether these patients would benefit from longitudinal rhythm monitoring is debatable.25,26
In our study, the independent predictors of most adverse outcomes included CAD, chronic HF, CKD, CVD, and anaemia, which interestingly were also significant predictors of AF. Other predictors of AF were White ethnicity, hypertension, coagulopathy, and obesity. Whether AF or its drivers contributed to higher mortality is debatable. Hypertension, dyslipidaemia, and hyperglycaemia are known complications of HSCT and can be independent contributors to the high burden of cardiovascular disease in both adult and paediatric populations.27 Total body irradiation can predispose to the risk of developing dyslipidaemia and diabetes. The development of predictive models can be helpful in identifying high-risk patients for targeted surveillance in the peri-HSCT setting.28 Additionally, the adoption of specific management guidelines, such as the AF Better Care pathway with a primary focus on the management of comorbidities, should be studied.29–31 Advanced imaging techniques, such as coronary calcium scoring or cardiac computed tomography, can help identify HSCT patients at risk of developing CAD, and speckle tracking echocardiography or cardiac magnetic resonance imaging can allow for the assessment of early-stage left ventricular dysfunction.32 Therapeutic anticoagulation should be considered whenever considered feasible from a bleeding risk perspective.33
Regardless, comprehensive management of comorbidities and aggressive control of risk factors in the peri-HSCT setting may be considered while these patients undergo extensive evaluation prior to the decision to undergo HSCT. The development of AF in this population may be an indicator of poor prognosis and therefore may warrant clinicians to consider continuous inpatient rhythm monitoring for the detection of AF. Further research is needed to determine the clinical utility of these practices and outline specific treatment strategies and design management algorithms for AF in these patients.
Limitations
The results of this study should be interpreted within the context of the following limitations. First, the NIS is derived from administrative billing data that rely on ICD codes, which may be subject to error. However, it is worth noting that AHRQ utilizes robust quality control measures that ensure data integrity.12 Second, we are unable to distinguish the history of AF from new-onset AF because of the lack of distinct ICD-10 codes for the new-onset AF. Therefore, the current analysis’s temporal relationship between AF, HSCT, and outcomes is unclear. Third, the NIS censors data upon discharge from the facility; therefore, long-term or post-discharge follow-up outcomes cannot be ascertained from the database. Fourth, the NIS also censors medication administration data, thus precluding the analysis of chemotherapeutic agents used (for conditioning prior to HSCT) and specific rate or rhythm control strategies used to manage AF. Given the retrospective nature of the study, causation cannot be concluded, and our results merely suggest an association.
Conclusions
In this large and nationally representative cohort of hospitalized patients undergoing HSCT, the prevalence of AF is high, and it is associated with worse clinical outcomes. Collectively, these findings highlight the need for further studies examining specific AF prevention and management strategies in patients undergoing HSCT.
Supplementary Material
Contributor Information
Satyam Krishan, Department of Medicine, University of Oklahoma Health Sciences Center, 800 Stanton L. Young Blvd, AAT 5400, Oklahoma City, OK, 73104, USA.
Muhammad Bilal Munir, Department of Cardiovascular Medicine, Electrophysiology Section, University of California Davis, Davis, CA, USA.
Muhammad Zia Khan, Department of Medicine, West Virginia University, Morgantown, WV, USA.
Taha Al-Juhaishi, Department of Medicine, University of Oklahoma Health Sciences Center, 800 Stanton L. Young Blvd, AAT 5400, Oklahoma City, OK, 73104, USA.
Ryan Nipp, Department of Medicine, University of Oklahoma Health Sciences Center, 800 Stanton L. Young Blvd, AAT 5400, Oklahoma City, OK, 73104, USA.
Christopher V DeSimone, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Abhishek Deshmukh, Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA.
Stavros Stavrakis, Department of Medicine, University of Oklahoma Health Sciences Center, 800 Stanton L. Young Blvd, AAT 5400, Oklahoma City, OK, 73104, USA.
Ana Barac, Cardio Oncology Program, MedStar Heart and Vascular Institute, Georgetown University, Washington, DC, USA.
Zain Ul Abideen Asad, Department of Medicine, University of Oklahoma Health Sciences Center, 800 Stanton L. Young Blvd, AAT 5400, Oklahoma City, OK, 73104, USA.
Supplementary material
Supplementary material is available at Europace online.
Data availability
Data supporting the findings of this study are available from authors upon reasonable request.
References
- 1. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813–26. [DOI] [PubMed] [Google Scholar]
- 2. Tuzovic M, Mead M, Young PA, Schiller G, Yang EH. Cardiac complications in the adult bone marrow transplant patient. Curr Oncol Rep 2019;21:28. [DOI] [PubMed] [Google Scholar]
- 3. Fujimaki K, Maruta A, Yoshida M, Sakai R, Tanabe J, Koharazawa Het al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant 2001;27:307–10. [DOI] [PubMed] [Google Scholar]
- 4. Chiengthong K, Lertjitbanjong P, Thongprayoon C, Bathini T, Sharma K, Prasitlumkum Net al. Arrhythmias in hematopoietic stem cell transplantation: a systematic review and meta-analysis. Eur J Haematol 2019;103:564–72. [DOI] [PubMed] [Google Scholar]
- 5. Hidalgo JD, Krone R, Rich MW, Blum K, Adkins D, Fan M-Yet al. Supraventricular tachyarrhythmias after hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant 2004;34:615–9. [DOI] [PubMed] [Google Scholar]
- 6. Tonorezos ES, Stillwell EE, Calloway JJ, Glew T, Wessler JD, Rebolledo BJet al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transplant 2015;50:1212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olivieri A, Corvatta L, Montanari M, Brunori M, Offidani M, Ferretti GFet al. Paroxysmal atrial fibrillation after high-dose melphalan in five patients autotransplanted with blood progenitor cells. Bone Marrow Transplant 1998;21:1049–53. [DOI] [PubMed] [Google Scholar]
- 8. Chang EK, Chanson D, Teh JB, Iukuridze A, Peng K, Forman SJet al. Atrial fibrillation in patients undergoing allogeneic hematopoietic cell transplantation. J Clin Oncol 2021;39:902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998;158:229–34. [DOI] [PubMed] [Google Scholar]
- 10. Caine GJ, Stonelake PS, Lip GYH, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002;4:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. López-Fernández T, Martín-García A, Roldán Rabadán I, Mitroi C, Mazón Ramos P, Díez-Villanueva Pet al. Atrial fibrillation in active cancer patients: expert position paper and recommendations. Revista Española de Cardiología (English Edition) 2019;72:749–59. [DOI] [PubMed] [Google Scholar]
- 12. for Healthcare Research A, Quality. Overview of the National Inpatient Sample (NIS). AHRQ Rockville; 2020.
- 13. Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 2014;124:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steinberg I, Brogi E, Pratali L, Trunfio D, Giuliano G, Bignami Eet al. Atrial fibrillation in patients with septic shock: a one-year observational pilot study. Turk J Anaesthesiol Reanim 2019;47:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Induruwa I, Hennebry E, Hennebry J, Thakur M, Warburton EA, Khadjooi K. Sepsis-driven atrial fibrillation and ischaemic stroke. Is there enough evidence to recommend anticoagulation? Eur J Intern Med 2022;98:32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naser N, Dilic M, Durak A, Kulic M, Pepic E, Smajic Eet al. The impact of risk factors and comorbidities on the incidence of atrial fibrillation. Mater Sociomed 2017;29:231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Willeit K, Kiechl S. Atherosclerosis and atrial fibrillation—two closely intertwined diseases. Atherosclerosis 2014;233:679–81. [DOI] [PubMed] [Google Scholar]
- 18. Asad Z, Abbas M, Javed I, Korantzopoulos P, Stavrakis S. Obesity is associated with incident atrial fibrillation independent of gender: a meta-analysis. J Cardiovasc Electrophysiol 2018;29:725–32. [DOI] [PubMed] [Google Scholar]
- 19. Falanga A, Leader A, Ambaglio C, Bagoly Z, Castaman G, Elalamy Iet al. EHA guidelines on management of antithrombotic treatments in thrombocytopenic patients with cancer. Hemasphere 2022;6:e750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caro J, Navada S. Safety of anticoagulation in patients with atrial fibrillation and MDS/AML complicated by thrombocytopenia: an unresolved challenge: can they be managed? A report of three cases and literature review. Am J Hematol 2018;93:E112–4. [DOI] [PubMed] [Google Scholar]
- 21. Krishan S, Yalamanchili S, Atluri R, Khdeir O, Khan M, Munir Bet al. Atrial fibrillation predicts poor outcomes and higher cost of care in patients admitted for inpatient chemotherapy-insight from the National Inpatient Sample. J Am Coll Cardiol 2022;79:156. [Google Scholar]
- 22. Thotamgari SR, Grewal US, Nobel Bhuiyan MA, Abideen Asad ZU, Dominic P. The association of cardiac arrhythmias with chimeric antigen receptor T cell therapy in hospitalised patients: insights from National Inpatient Sample. Eur J Cancer 2022;174:131–3. [DOI] [PubMed] [Google Scholar]
- 23. Spînu Ș, Cismaru G, Boarescu P-M, Istratoaie S, Negru AG, Lazea Cet al. ECG markers of cardiovascular toxicity in adult and pediatric cancer treatment. Dis Markers 2021;2021:1–10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.http://www.survivorshipguidelines.org/pdf/2018/cog_ltfu_guidelines_v5.pdf survivorshipguidelines.org WW. For survivors of childhood, adolescent, and young adult cancers [Internet]. [cited 2023 Jan 26]. Available from:
- 25. Kilickap S, Barista I, Akgul E, Aytemir K, Aksoy S, Tekuzman G. Early and late arrhythmogenic effects of doxorubicin. South Med J 2007;100:262–5. [DOI] [PubMed] [Google Scholar]
- 26. Poręba M, Gać P, Usnarska-Zubkiewicz L, Pilecki W, Kuliczkowski K, Mazur Get al. The analysis of the parameters of 24-hr ECG Holter monitoring in patients with blood neoplasms undergoing high-dose chemotherapy and stem cell transplantation. Ann Noninvasive Electrocardiol 2018;23:e12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009;15:1100–7. [DOI] [PubMed] [Google Scholar]
- 28. Armenian SH, Sun C-L, Vase T, Ness KK, Blum E, Francisco Let al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood 2012;120:4505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vitolo M, Proietti M, Malavasi VL, Bonini N, Romiti GF, Imberti JFet al. Adherence to the “Atrial fibrillation Better Care” (ABC) pathway in patients with atrial fibrillation and cancer: a report from the ESC-EHRA EURObservational Research Programme in atrial fibrillation (EORP-AF) General Long-Term Registry. Eur J Intern Med 2022;105:54–62. [DOI] [PubMed] [Google Scholar]
- 30. Proietti M, Lip GYH, Laroche C, Fauchier L, Marin F, Nabauer Met al. Relation of outcomes to ABC (Atrial fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: an analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace 2021;23:174–83. [DOI] [PubMed] [Google Scholar]
- 31. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein Jet al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–361. [DOI] [PubMed] [Google Scholar]
- 32. Hemu M, Zimmerman A, Kalra D, Okwuosa T. Pretransplant cardiac evaluation using novel technology. J Clin Med Res 2019;8:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boriani G, Lee G, Parrini I, Lopez-Fernandez T, Lyon AR, Suter Tet al. Anticoagulation in patients with atrial fibrillation and active cancer: an international survey on patient management. Eur J Prev Cardiol 2021;28:611–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from authors upon reasonable request.