Abstract
Immune checkpoint inhibitors (ICIs), used to treat many advanced cancers, activate the immune system to elicit an antitumor response. ICIs can also cause immune-related adverse events (irAEs) when nontumor tissues are affected by excess inflammation and autoimmunity. Rheumatic irAEs include inflammatory arthritis, myositis, sicca syndrome, polymyalgia rheumatica, and several other rare phenotypes. Treating rheumatic irAEs requires balancing the desire to decrease off-target inflammation while not negatively impacting the antitumor immune response. In this review, treatment recommendations for rheumatic irAEs have been discussed. Pathogenesis of rheumatic irAEs has been briefly reviewed. Knowledge about the effects of corticosteroids and steroid-sparing agents on tumor responses has been detailed to give context for treatment decisions. Recommendations ultimately depend not only on the clinical presentation and severity of the irAE but also on the goals of cancer treatment. Finally, how to safely use ICI therapy in patients with preexisting autoimmune diseases is considered.
Keywords: Immune checkpoint inhibitors, Immune-related adverse events, Inflammation, Inflammatory arthritis, Myositis, Sicca syndrome
Introduction
Immune checkpoint inhibitors (ICIs) have changed the landscape of cancer therapy and are the standard of care for many different advanced malignancies given their survival benefits [1]. ICIs are monoclonal antibodies that antagonize negative regulatory proteins, such as cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligand PD-L1, leading to antitumor immunity. This block on natural immune checkpoints can lead to the development of a novel autoimmune disease or uncovering of an existing, subclinical autoimmune disease. The immune-mediated side effects of ICIs are called immune-related adverse events (irAEs). IrAEs have often challenged the ability to safely continue ICI therapy despite its clinical benefits.
IrAEs can vary in their presentation; they can involve multiple organs and are heterogeneous in timing, duration, and severity. In general, irAEs are more common with combination ICI therapy than ICI monotherapy. Graded by the Common Terminology Criteria for Adverse Events (CTCAE) severity scale, high-grade irAE (CTCAE grades 3–5) occur in more than half of the patients receiving combination ICI, a quarter of those on anti-CTLA-4 alone, and 10–20% on anti-PD(L)1 monotherapy [2,3] Cutaneous irAEs are the most common but are often low grade and manageable with topical therapies [4]. Checkpoint inhibitor-associated colitis (ICI-colitis) is the most common of high-grade toxicities, and ICI-myocarditis carries the highest case-mortality rate (up to 50%) [5].
Rheumatic irAEs include immune checkpoint inhibitor-induced inflammatory arthritis, polymyalgia rheumatica (PMR), myositis, sicca, and vasculitis. Noninflammatory musculoskeletal irAEs include arthralgia, myalgia, tendinopathy, or activated osteoarthritis; these noninflammatory syndromes will not be the focus of this review [6]. With an incidence of up to about 7%, ICI-inflammatory arthritis is the most common rheumatic irAE and, in particular, has been shown to persist beyond ICI cessation (Cappelli, 2017 27998041 [7–9]. ICI-myositis carries the highest mortality of all the rheumatic irAEs and can be associated with myocarditis or myasthenia gravis, or both; multisystem involvement has a higher mortality than ICI-myositis alone [10,11]. Acute-onset sicca syndrome has also been described in the literature, with incidence ranging up to 24% [7,12,13]. Sarcoidosis and sarcoid-like presentation, as well as less common cases of systemic sclerosis, eosinophilic fasciitis, and vasculitis, have also been reported [7,14]. Interestingly, cases of lupus are incredibly rare, with reports of systemic lupus erythematosus, cutaneous lupus, lupus-like syndrome, lupus nephritis, or central nervous system lupus comprising <1% of all rheumatic adverse events (AEs) [15].
Possible mechanisms for iraes
Given the heterogeneity in onset, response to therapy, and likelihood to become chronic among different types of irAEs, there are likely multiple pathways relevant to pathogenesis. Several possible mechanisms have been suggested by data from patients with irAEs. Cytokine-mediated inflammatory damage is likely relevant for several irAEs given the response to interleukin (IL)-6R and tumor necrosis factor-alpha (TNF-alpha) blockade. In colitis, higher levels of serum IL-17 before ICI treatment and after 6 weeks of therapy predicted the development of severe diseases [16]. A shift or expansion in particular subsets of T cells may also be responsible for irAE development. In inflammatory arthritis as an irAE, for example, Th-1 CD8+ T cells were expanded in both blood and synovial fluid (ref: distinct molecular and immune hallmarks). Shared antigen recognition by T cells, that is, recognition of tumor and nontumor tissue antigens, has been demonstrated in myocarditis and vitiligo [17–19].
There is some evidence that predisposition to developing irAEs overlaps with that of traditional autoimmune disease. Particular HLA haplotypes known to be associated with the risk for traditional autoimmune disease have also been associated with the development of irAEs in both insulin-dependent diabetes and inflammatory arthritis (PD1 type 1 diabetes mellitus; HLA-DRB1 shared epitope alleles). Although many patients are seronegative for traditional autoantibodies, a subset of patients may have a preclinical disease that is activated by ICI therapy as evidenced by serial autoantibody evaluation. Studies have shown the development of inflammatory arthritis in those with anti-CCP before ICI treatment and of myasthenia gravis in those with preexisting acetylcholine receptor antibodies [20,21].
Finally, alterations in the microbiome may lead to the development of irAEs. In colitis, having more Firmicutes bacteria in the intestinal microbiota was associated with developing colitis while an increase in Bacteroides species was not [22].
General irae management principles
Because of the lack of large, prospective randomized controlled trials to guide the management of rheumatic irAEs, immunosuppressive treatment and dosing are generally based on irAE severity, extent of organ involvement, and expert opinion. Multiple oncology organizations have put forth expert consensus-based treatment guidelines for all-type irAEs [23–28]. As mentioned earlier, checkpoint inhibitor toxicity severity has generally been graded by the CTCAE, a rubric that is commonly used by oncologists in clinical trials to standardize the quantification of drug toxicity such as ICI AEs, including rheumatic irAEs [2]. Although the CTCAE scale often fails to accurately reflect the severity of musculoskeletal or systemic manifestations in rheumatic diseases and efforts are underway to improve this for rheumatic irAEs, it has been used to grade rheumatic irAEs in previous studies [29].
Steroids are overall first-line treatment for most irAEs of grade 2 or higher severity. For steroid-refractory or steroid-dependent irAEs, steroid-sparing agent(s) need to be considered to improve symptomatic control and minimize potential toxicities from prolonged corticosteroid use. Beyond steroids, current guidelines primarily recommend expert-driven treatment plans for choice and duration of steroid-sparing agents [23–26]. Duration of treatment is mostly based on irAE response, patient tolerability, as well as cancer status.
One overarching principle to note is that rheumatic irAE incidence has been correlated to better cancer outcomes in multiple studies [30,31]. Therefore, many patients at the time of rheumatic irAE development will have a good tumor response, with the goal of sustaining this cancer outcome through irAE management. It is then vital to identify irAE treatment modalities that do not risk abrogation of antitumor immunity.
Steroids
Because of their quick and effective anti-inflammatory properties, steroids are the mainstay of acute therapy for irAEs. Apart from ICI-inflammatory arthritis where nonsteroidal anti-inflammatory drugs (NSAIDs) and/or articular corticosteroid injections (CSIs) may be considered as first-line treatment for low-grade presentation, first-line treatment for most rheumatic irAEs relies on systemic corticosteroids. Among the corticosteroids, glucocorticoids such as prednisone and methylprednisolone are used for most irAEs with mineralocorticoids used for endocrinopathies, resulting in adrenal insufficiency [23–27]. Steroid dosing is based on the severity of the disease, and duration is based on the disease response to the initial steroid course. While a case of ICI-PMR calls for doses of oral prednisone up to 15–20 mg per day, a case of ICI-myositis with cardiac involvement may require starting with high doses of prednisone up to 2 mg per kilogram or even a pulse of intravenous (IV) solumedrol [24]. Detailed recommended dosing of steroids for various rheumatic irAEs is delineated when discussing specific rheumatic irAEs in later sections.
Systemic corticosteroids, however, are not without risk. In a retrospective observational study, authors found that the infection rate for patients treated with ICI was 18%, although none of the patients were described to have severe or opportunistic infections [32]. While this study did not see an association with systemic steroid or immunosuppressant use, another retrospective review assessing patients treated with ICIs found steroids to be the only risk factor associated with serious infections (75% for patients who used steroids compared with about 30% for patients who did not use steroids, p = 0.0003) [33]. Similar to this finding, serious infections in a cohort of patients treated with ICIs occurred in about 7.3% of patients, and patients treated with systemic steroids had a 7.71 greater odds [95% confidence interval (CI) 3.71–16.18] of infection than those patients treated without systemic steroid therapy [34].
In addition to concern for known harms, such as infections, as well as adverse metabolic changes, including steroid-induced diabetes and osteopenia, there may also be concern regarding the potential abrogation of antitumor immunity. The risk of impacting tumor response with corticosteroids has been evaluated by various retrospective studies. Hence, while we have information on the potential effects of systemic steroids on tumor outcomes from a retrospective study, we do not yet have results from prospective trials to reliably address this concern. A 2015 study did not find a negative impact on survival with the use of steroids [35]. However, a 2018 study in patients with melanoma found that high doses of steroids used in the treatment of ICI-hypophysitis were associated with reduced survival [36]. Another retrospective assessment found a more deleterious impact of steroids on tumor outcomes with earlier (<2 months) and longer duration (>2 months) of corticosteroids [37]. A 2020 meta-analysis showed that, while there was an overall association with the use of steroids and worse cancer outcomes, this correlation was only true for patients who used steroids for metastatic disease or supportive care (HR 1.51, 95% CI 1.22–1.87, p-value <0.01 and 2.5, 95% CI 1.41–4.43, p-value <0.01, respectively) [38]. This correlation was not statistically significant for patients who used steroids for irAE treatment (1.08, 95% CI 0.79–1.49, p = 0.62) [38].
Steroid-sparing agents
A steroid-sparing agent or disease-modifying antirheumatic drug (DMARD) is overall considered when a patient has an irAE that is not fully responding to systemic steroids, worsens upon steroid taper, or recurs after steroid taper. The choice of DMARD is dependent on organ involvement, severity and grading of AE, comorbidities, and potentially whether or not a patient is on a clinical trial (most clinical trials often limit immunosuppression that can be used and still permit the patient to continue on trial). The DMARD is often chosen based on steroid-sparing treatment that has been found to be effective for the primary autoimmune disease that best resembles the irAE phenotype.
While it is critical to consider DMARD(s) that would be the most efficacious for treating the irAE, it is important to note the toxicity of the DMARD(s) as well, including the potential impact of DMARDs on cancer and antitumor immunity. Table 1 briefly summarizes the potential impacts of different DMARDs. A retrospective study examined 51 patients who had received ICI treatment and one or more steroid-sparing agents, 73% TNF-alpha inhibitor, and 20% mycophenolate mofetil [39]. The authors found that of those patients who died (13 of 51), 4 deaths were related to toxicity from immunesuppression [39]. Another retrospective study evaluating the risks and benefits of additional immunomodulators for steroid-refractory and steroid-resistant ICI-pneumonitis found that 3 patients (12% of deaths) died because of infections potentially associated with immunosuppression [40]. To date, because of the lack of any prospective randomized controlled trials to guide our choice of steroid-sparing agents, we rely on case reports, case series, retrospective studies, and mechanistically reasoned expert opinion.
Table 1.
Considerations for use of steroid-sparing agents to treat rheumatic irAEs.
| Drug type | Rheumatic irAE uses: Reported and theoretical | Potential of mitigating anti-tumor efficacy | Other DMARD toxicity to consider in context of ICI Treatment |
|---|---|---|---|
|
| |||
| csDMARDs | |||
| Hydroxychloroquine | Peripheral arthritis Sarcoid | Unlikely | None |
| Sulfasalazine | Peripheral arthritis | Unlikely | Skin rash |
| Methotrexate | Peripheral arthritis with/without psoriasis Myositis Sarcoidosis | Unlikely | Liver toxicity |
| Leflunomide | Peripheral arthritis Sarcoidosis | Possible | Diarrhea, liver toxicity |
| Mycophenolate mofetil | Myositis Sarcoidosis | Possible | Diarrhea, cytopenias |
| Azathioprine | Peripheral arthritis with/without colitis Myositis Vasculitis | Possible | GI symptoms, cytopenias |
| Apremilast | Peripheral arthritis with psoriasis | Unlikely | Diarrhea |
| Calcineurin inhibitors | Myositis/myocarditis | Possible | Nephrotoxicity |
| Biologics | |||
| TNF-alpha inhibitor | Axial or peripheral arthritis with/without psoriasis or colitis Sarcoidosis | Possible | Infection (opportunistic), rash, psoriasis |
| IL6 inhibitors | Peripheral arthritis with/without pneumonitis Polymyalgia rheumatica Giant cell arteritis | Unlikely | Intestinal perforation, hepatotoxicity |
| Abatacept | Severe myositis/myocarditis | Probable for patients on anti-CTLA-4, unclear for anti-PD-1/L1 | Pulmonary disease exacerbation |
| Rituximab | Peripheral arthritis Myositis Vasculitis | Unlikely | Systemic infusion reaction, Infection (opportunistic) |
| IL1 axis inhibitors | Peripheral arthritis | Unlikely | Skin rash |
| IL12/IL23 inhibitor | Axial or peripheral arthritis with/without psoriasis or colitis | Unlikely | |
| IL17 inhibitor | Axial or peripheral arthritis with/without psoriasis | Unlikely | Diarrhea |
| tsDMARD | |||
| JAK inhibitors | Axial or peripheral arthritis with/without psoriasis or colitis Myositis | Possible | Intestinal perforation, Infection (opportunistic, zoster) |
Because research has shown that patients who develop irAE or even rheumatic and musculoskeletal irAEs tend to have a better tumor response and/or overall survival (OS), the active immune pathway may be similar to that of the primary malignancy, as well as the irAEs [31,41,42]. More prospective translational studies are needed to clarify the true mechanism of rheumatic irAEs so that we can better identify targeted steroid-sparing agents and minimize potential abrogation of antitumor immunity.
Two main biologics that have been successful for irAE treatment are TNF-inhibitors and IL6R inhibitors [24]. For this reason, it is important to consider the data regarding these biological pathways and their relationship to cancer outcomes.
Tumor necrosis factor inhibition
Multiple retrospective cohort studies, systematic reviews, and meta-analyses have evaluated this risk for the development of cancer with the use of TNFi in different autoimmune disease groups and found that, while there is a concern for nonmelanoma skin cancers (NMSCs), this increase in cancer risk does not apply to risk of melanoma or that of solid tumor cancers [43–47]. In the past, there have been conflicting data on lymphoma risk and TNF-inhibition, but recent studies in rheumatoid arthritis (RA) have shown no increased risk over the background risk of lymphoma in RA.
A 2006 meta-analysis that assessed the risk of cancer for patients treated with infliximab or adalimumab or etanercept in randomized controlled trials found a higher incidence of lymphoma and NMSC in patients treated with TNFi versus those who were not [44]. In 2013, a retrospective study that included patients with various autoimmune conditions from multiple health insurance plans aimed to identify the risk of the most common cancers in the US in the group of patients treated with TNFi compared with patients treated with alternate therapy options with conventional DMARDs (patients with other biological DMARD uses were excluded). These authors found no significant increase in the risk of these common solid tumors for patients treated with TNFi [48]. This lack of association with TNFi and that of solid tumors has been seen consistently in other studies as well (REFs). There is concern, however, for the potential risk of NMSC as seen in a retrospective study that found this increased risk of NMSC in patients who had an addition of TNFi to methotrexate versus methotrexate alone for patients with RA or IBD [49], as well as a 2018 publication that systematically reviewed this risk for patients with psoriasis [50] and a meta-analysis that analyzed this risk for patients with RA [51]. Regarding the risk of melanoma, a 2013 cohort study identified a 50% increased risk of invasive melanoma for patients with RA treated with TNFi than those patients with RA treated without TNFi [52]. However, other cohort studies and meta-analyses have yielded contradictory results and no increased risk of melanoma for patients treated with TNFi compared with non-TNFi-treated patients [53–55].
While there is concern for NMSC with long-term TNF inhibition, we must acknowledge that the use of TNFis for irAE treatments cannot be equated to that of their use for primary autoimmune disease for reasons including difference in duration and cumulative dose of TNFi, as well as timing of TNFi administration regarding cancer diagnosis. While the use of TNFi for preexisting autoimmune diseases (pAIDs) can be decades long, the duration for TNFi in one study for ICI-arthritis was demonstrated to range from 3 to 16 months [56].
The use of TNFi and primarily infliximab is most studied in cases of ICI-colitis as this was one of the earliest and most prevalent high-grade toxicities recognized in patients treated with ICI therapy for metastatic melanoma [57]. A 2018 retrospective study for ICI-colitis showed that not only did immunosuppression for ICI-diarrhea or ICI-colitis not impact OS but the choice of treatment (corticosteroids alone versus corticosteroids with infliximab) also did not significantly impact OS [58]. A multicenter observational study found that TNFi used for high-grade ICI-colitis showed a progression-free survival (PFS) that was comparable to previously reported PFS in patients without TNFi treatment [59]. In contrast to these studies, a 2020 retrospective study that assessed data from the Dutch Melanoma Treatment Registry showed decreased median OS in patients treated with TNFi in addition to systemic steroid therapy versus steroid monotherapy; however, these authors did not seem to take into account immortal time bias that could have significantly impacted their comparison [60].
An observational study assessing the characteristics of ICI-arthritis reported that 7 of 30 patients with ICI-arthritis required TNFi treatment [56]. Of the TNFi-treated patients with ICI-arthritis, 4 had complete tumor response at the start of TNFi and continued to be in remission despite TNFi administration [56]. A multi-institutional, retrospective comparator study found that patients treated with TNFi ± methotrexate for ICI-arthritis had a shorter time to cancer progression than those patients treated with methotrexate alone [61]. In addition to these retrospective studies, a prospective phase 1b clinical trial (TICIMEL NCT03293784) studied the use of TNFi (certolizumab or infliximab) and also combination ICI therapy for advanced melanoma [62]. These authors found that all evaluable patients who received certolizumab demonstrated a high objective response rate (ORR), and half of the infliximab-treated patients demonstrated tumor response. The authors of this trial concluded that it was safe to administer TNFi for ICI-treated patients [62].
Interleukin 6 axis inhibition
IL-6 is a pleiotropic cytokine with proinflammatory function in many diseases such as rheumatic disease but also including cancer [63]. High-serum IL6 has been implicated in the pathogenesis of melanoma [64], ovarian cancer [65], and colorectal cancer [66]. Furthermore, prospective proteomic assays were done on patients who received checkpoint inhibitor therapy, and the authors found that higher levels of IL6 and C-reactive protein were associated with shorter OS [67].
Given the role of IL-6 in tumor pathogenesis, as well as its known role in autoimmune diseases, the question is whether blocking the IL6 pathway could enhance ICI antitumor immunity as well as abrogate potential autoimmunity from irAEs. A translational study led by providers at the MD Anderson Cancer Center demonstrated that IL-6R inhibition does decouple ICI antitumor immunity from ICI autoimmune toxicity [68]. In mouse models, these authors demonstrated that IL-6 blockade was associated with better tumor outcomes, and a combination of IL6-blockade with checkpoint inhibitor therapy showed better tumor control and better control of autoimmune encephalomyelitis than that with checkpoint inhibitor therapy alone [68]. The same authors conducted a retrospective study on patients with melanoma who received checkpoint inhibitor therapy and found that blocking the IL-6 pathway decreased irAEs without mitigating tumor response [68]. In 2021, Weber and colleagues evaluated the effectiveness and safety of combination checkpoint inhibitor therapy with that of tocilizumab, and in their preliminary findings, they showed an overall response rate and high-grade irAE rate that was favorable than a previous study that assessed the efficacy of same-dose ICI therapy in the same tumor type [69,70]. Most recently, a 2022 Danish feasibility study demonstrated the ability of tocilizumab 8 mg/kg monthly infusion to be able to improve ICI-arthritis or ICI-colitis by 1 CTCAE grade within 8 weeks for most patients [71]. In this study, tocilizumab was generally tolerated, and 6 of 20 (30%) patients experienced cancer progression within 24 weeks [71]. In addition, a multi-institutional study assessing the effectiveness and safety of certain DMARDs for ICI-arthritis compared with methotrexate demonstrated a shorter time to cancer progression for patients on tocilizumab than methotrexate [61].
The use of IL6-axis inhibitor with that of checkpoint inhibitor therapy has overall been described as a “win-win strategy” with the potential to abet ICI effectiveness all the while decreasing ICI toxicity [72], but large, prospective randomized controlled trials are needed to best evaluate the effectiveness and safety of medications such as tocilizumab in the context of ICI therapy.
Management principles for rheumatic iraes
Inflammatory arthritis
Epidemiology and clinical phenotypes
Of all the rheumatic irAEs described in the literature, ICI-arthritis is the most common, with a reported incidence of 1–7% in a systematic review and unique in its ability to present up to 2 years after ICI initiation or even after ICI cessation [7,9]. Notably, because ICI-arthralgia is common with prevalence as high as 43%, differentiating inflammatory versus noninflammatory arthropathy is important [7]. A phenomenon called ICI-associated-activated osteoarthritis has also been described in the literature; hence, it is important to consider the exacerbation of previous mechanical arthropathy that has resulted from degenerative arthropathy and/or trauma [73].
Once a diagnosis of inflammatory arthritis after ICI initiation has been determined, further evaluation to best characterize the clinical phenotype will influence the next steps for management. A 2020 systematic review of ICI-arthritis case series and case reports identified an RA-like joint pattern in 65% of cases, 21% with a PMR-like phenotype, psoriatic, or spondyloarthropathy-like presentations with asymmetrical large joint involvement in 13% of cases [74]. Of the cases with PMR-like presentation, about 20% had small joint involvement, along with typical girdle stiffness [74]. Serology positivity with RF and/or CCP was noted in about one-tenth of cases (9%), which was in concordance with other studies discussing autoantibody positivity with ICI-arthritis [74,75].
Arthritis severity
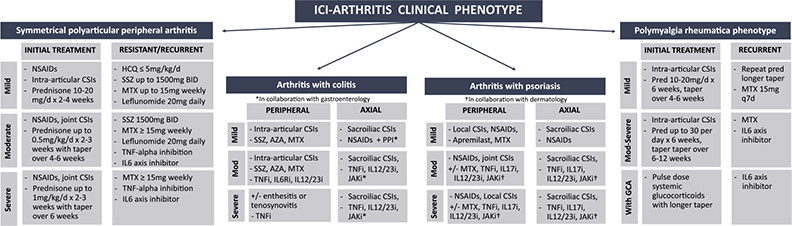
For patients with inflammatory arthritis, CTCAE grade 1 refers to mild joint pain with signs of inflammation at joints such as erythema or joint swelling [2]. CTCAE grade 2 is defined as moderate joint pain and signs of joint inflammation that are limiting instrumental activities of daily living (ADLs) while CTCAE grades 3 and 4 reflect severe joint pain and inflammation associated with irreversible joint damage that is limiting self-care ADLs. There has been discussion regarding the limitations of this CTCAE grading for that of ICI-arthritis, but further guidance is needed regarding the most appropriate and accurate grading scale to use for the various ICI-arthritis clinical phenotypes [76]. Given the heterogeneity in ICI-arthritis, we have depicted various treatment pathways for the different clinical phenotypes in Fig. 1.
Fig. 1.
ICI-arthritis treatment based on clinical presentation.
ICI: Immune checkpoint inhibitor; NSAIDs: nonsteroidal anti-inflammatory drugs; CSI: corticosteroid injections; HCQ: hydroxychloroquine; SSZ: sulfasalazine; MTX: methotrexate; TNF: tumor necrosis factor; IL6: interleukin 6; AZA: azathioprine; IL6Ri: interleukin 6 receptor inhibitor; IL12/23i: interleukin 12/interleukin 23 inhibitor; PPI: proton pump inhibitor, IL17i: interleukin 17 inhibitor; Pred: prednisone.
In most cases of grade 1 ICI-arthritis and sometimes grade 2 ICI-arthritis, ICI can be continued. However, one key consideration is whether the patient is a part of an immunotherapy clinical trial; the clinical trial may prevent the patient from receiving prednisone >10 mg per day while continuing in the trial. For CTCAE grade 2 ICI-arthritis, the recommendation is to hold ICI therapy until the arthritis returns to grade 1 or less severity. Similar to grade 1, the oncologist may want to continue the ICI despite grade 2 arthritis given its low-grade presentation and non-organ-threatening role. Inquiry regarding the desire to continue ICI therapy and/or to stay on an immunotherapy clinical trial (which may limit options for systemic immunosuppressive therapy) is key. Intra-articular CSIs are often allowed in ICI clinical trials and can be considered for moderate to large joint arthritis. For grade 3+ arthritis, the recommendation is to hold immunotherapy. Oncologists may consult with a rheumatologist to restart, or, if a patient was on combination ICI therapy, there may be a question regarding restarting single-agent ICI therapy with close monitoring for arthritis recurrence. These decisions are made on a case-by-case basis, according to patient preference, ICI effectiveness, presence of other irAEs, and options for alternative cancer therapies.
Initial approach
Peripheral polyarticular inflammatory arthritis.
For CTCAE grade 1 ICI-arthritis, patients are often managed by the primary treating oncologist without a rheumatology referral and the oncologists tend to continue ICI treatment. First-line therapy is still NSAIDs but consider glucocorticoids in cases where NSAIDs are not sufficient or contraindicated. For patients with limited moderate or large joint involvement, rheumatologists should consider local, intra-articular CSIs over systemic steroid treatment. However, for oligoarticular or polyarticular involvement and/or for small joint inflammation, systemic corticosteroids may have to be considered even for grade 1 arthritis. As shown in Fig. 1, for patients with grade 1 small joint peripheral arthritis, the recommendation is to consider prednisone 10–20 mg per day or equivalent for 2–4 weeks. However, if there is no resolution of arthritis, consider an immunomodulatory DMARD such as hydroxychloroquine or sulfasalazine for persistent low-grade ICI-arthritis.
For grade 2 arthritis, the NCCN guidelines recommend initial treatment with prednisone-equivalent 0.5 mg per kg per day over 2–3 weeks while ASCO and SITC guidelines recommend prednisone 10–20 mg per day or equivalent with a taper over 4–6 weeks [23–27]. As is done for primary inflammatory arthritis, the number and size of joints affected will also inform the initial starting dose of systemic steroids. For example, a patient with prolonged morning stiffness in hands, along with mild synovitis in two MCPs and four PIPs with minimal wrist symptoms, would likely require a different starting dose than a patient presenting with unilateral large knee joint effusion and moderate contralateral ankle swelling, along with some diarrhea. Again, similar to grade 1 ICI-arthritis, it is important to consider intra-articular CSIs if the patient has moderate to large joint involvement. If there is a lack of response to systemic steroids in 3–4 weeks after the initial dosing, the recommendation is to consider steroid-sparing agents as discussed below (Fig. 1).
For grade 3 arthritis that is severe in pain, limiting activities of daily living with or without irreversible joint damage or erosive changes seen on imaging, NCCN guidelines recommend starting with prednisone-equivalent 1 mg/kg per day while ASCO guidelines recommend prednisone 0.5–1 mg/kg per day, and, similarly, SITC guidelines recommend doses of prednisone-equivalent 40–60 mg may be needed for initial arthritis control [24,27]. If there is no improvement in symptoms or signs of arthritis after 1–2 weeks of steroids, guidelines agree to move forward with considering DMARD according to arthritis severity and clinical phenotype (Fig. 1).
Polymyalgia rheumatica phenotype
For patients with grade 1 PMR or PMR-like presentation with or without peripheral arthritis, NCCN guidelines recommend prednisone dose of 10–20 mg per day over 6 weeks with a taper over 4–6 weeks. In cases with only shoulder involvement, intra-articular CSIs in the glenohumeral joints may suffice in certain cases, potentially avoiding the need for systemic glucocorticoid use. For grade 2 or 3 PMR or PMR-like arthritis, there is a recommendation to consider prednisone doses up to 30 mg per day and to taper over 6–12 weeks, with a potentially longer taper and, if no resolution, to consider steroid-sparing agents.
Choice and dosing of DMARDs
Unfortunately, in about 33–45% of cases, treatment beyond steroids is needed [56,74]. ICI-arthritis has been shown to persist despite ICI cessation or even present as new diagnosis after ICI therapy has been stopped [9]. When considering steroid-sparing therapy for ICI-arthritis, the current recommendation is to guide the choice of DMARDs based on what primary inflammatory arthropathy the ICI-arthritis most phenotypically resembles (Fig. 1).
For low-severity, grade 1 arthritis, we recommend hydroxychloroquine up to 5 mg/kg/d or sulfasalazine up to 1500 mg BID. Sulfasalazine may be chosen over hydroxychloroquine if the patient has more of a seronegative spondyloarthropathy-like arthritis, potentially with associated diarrhea or colitis. Methotrexate 15 mg per week could also be considered for a slightly quicker onset of action than hydroxychloroquine or less pill burden than sulfasalazine. When starting oral medications, note that diarrhea can occur in up to 10% of patients with ICI monotherapy and >40% of patients with ICI combination therapy, and tend to present in the days or weeks following the ICI infusions [77]. In addition, for medications such as sulfasalazine, which is prone to cutaneous toxicities, it is important to note that ICI-dermatitis is the most common irAE, shown to occur in up to 41% of patients with combination ICI therapy [77].
If ICI-arthritis grade 2 has not improved after 3–4 weeks of the chosen initial steroid regimen or for ICI-grade 3 that has not improved after 1–2 weeks of initial steroid treatment, the guidelines recommend starting a steroid-sparing agent. If the patient’s arthritis is requiring steroids of prednisone >15 mg per day equivalent to maintain control, starting methotrexate 15 mg with quick uptitration or starting with a biologic may be necessary. If the patient is exhibiting moderate-to-severe signs and symptoms of symmetrical, small-to-medium joint peripheral polyarticular arthritis, regardless of seropositivity for rheumatoid factor or cyclic citrullinated peptide, steroid-sparing agents most effective for RA can be considered. Aside from hydroxychloroquine and sulfasalazine mostly used for low-grade ICI-arthritis, moderate-to-severe ICI-arthritis with a clinical phenotype similar to RA, the most commonly used DMARDs are methotrexate, infliximab, and tocilizumab [74]. In contrast, in a patient with phenotype most closely resembling that of primary IBD-associated arthritis, such as a patient with asymmetrical medium and large joint involvement and concern for ICI-colitis, steroid-sparing agents best for seronegative spondyloarthropathy may be considered. Given the notable success of infliximab 5 mg/kg IV, a TNF-alpha inhibitor can be considered in moderate-to-severe ICI-arthritis presenting with colitis resistant to steroid taper [78]. In addition, a 2022 open-label clinical trial found reliable safety for the use of tocilizumab for cases of ICI-arthritis and ICI-colitis; hence, interleukin 6 axis inhibitors could also potentially be considered for resistant or recurrent high-grade cases of ICI arthritis co-occurring with colitis [71].
For recurrent grade 1 ICI-PMR, a steroid-sparing agent such as methotrexate can be considered [79]. For steroid-taper-resistant moderate or severe (grade 2 or 3) ICI-PMR or recurrent moderate or severe symptoms after steroid taper, consider a biologic such as tocilizumab. In the rare cases where there is concern for associated giant cell arteritis with potential for visual compromise, the recommendation is for pulse dose methylprednisolone with prolonged taper and consideration for tocilizumab early on if there is an insufficient response to the initial steroid dosing [27,80].
For resistant or recurring ICI-arthritis with psoriasis, there are cases of treatment of low-grade symptoms with apremilast [81], but for moderate-to-severe presentations, higher doses of methotrexate (>15 mg per week) or biological treatment with TNF-alpha inhibition may be considered [27]. Successful use of IL17 inhibition with secukinumab and IL23 inhibition with guselkumab has been reported for psoriatic arthritis after ICI treatment [82,83].
Duration of treatment
Currently, there is no guidance on the duration of DMARD therapy. The primary goal is to get the patient to ICI-arthritis grade 1 or less with prednisone ≤10 mg equivalent or less per day. Once this goal is achieved and the patient’s arthritis symptoms are controlled, the first step would be to taper the patient off of systemic glucocorticoids. Then, if the patient is on conventional synthetic DMARD, dose decrease and discontinuation could be considered; for biological therapy, interval spacing can be considered. A large component regarding the next steps for the ICI-arthritis will depend on the potential next steps for cancer therapy if there is tumor persistence or progression. If there is a potential plan to restart checkpoint inhibitor therapy, continuation of steroid-sparing agents would need to be done in collaboration with the primary treating oncologist with close monitoring of ICI-arthritis recurrence or alternate irAE(s). Alternatively, if there are plans to start systemic chemotherapy, priority would need to be given to tapering off of biological therapy for the risk of severe immunosuppression.
Myositis
Epidemiology and clinical phenotypes
While myalgias after ICI are common (up to 20% incidence), ICI-myositis is rare, impacting up to 1.6% of patients treated with ICIs [7,10,84]. It is unique than the other rheumatic irAEs as it has been shown to co-occur with myasthenia gravis and/or myocarditis, both of which are associated with higher mortality and morbidity than ICI-myositis alone [85,86]. In a 2022 publication examining ICI-myositis from the WHO adverse drug reaction database, the authors found that about 95% of ICI-myositis cases were considered serious, requiring at least one hospitalization [84]. This study estimated that 11.3% of ICI-myositis cases were associated with myocarditis and 11.9% with myasthenia gravis [84]. In a retrospective analysis of a cohort with skin cancer, about one-third of cases with ICI-myositis were found to have myocarditis and about 5% with myasthenia gravis [87]. Fatality rate has been reported to be about 22–24% for ICI-myositis but up to 51–57% when there is co-occurrence with ICI-myocarditis or 27% with ICI-myasthenia gravis [10,84]. Most cases of ICI-myositis occur in the first month after ICI initiation; however, incidences later in the ICI course have also been reported [10,84].
ICI-myositis can present similarly to classic myositis with proximal muscle weakness but can also present with significant myalgia, which can closely mimic symptoms of PMR [27,29]. For this reason, it is important to assess muscle enzyme levels such as creatine kinase (CK) and aldolase in the context of clinical signs and symptoms [88] (Fig. 2). Note that myositis-specific antibodies have not been shown to be positive for cases of ICI-myositis and are not recommended as part of routine evaluation for ICI-myositis [29,87]. Because muscle enzymes are not commonly part of routine oncology bloodwork, one pearl is to have heightened suspicion for ICI-myositis with new-onset proximal muscle pain and weakness with elevated aspartate aminotransferase or alanine transaminase. Note that, however, lab abnormalities such as CK rise without symptoms may lead to overdiagnosis [89]. In a 2021 retrospective study, the authors showed that abnormal CK (rise of up to 400–800 UI/L) can be seen in almost 5% of patients treated with PD(L)1 antagonists; for those with CK rise ascertained to ICI therapy, about 40% of patients experienced neuromuscular or cardiac symptoms [89]. ICI-myositis is best diagnosed by considering patient symptoms, physical exam findings, as well as laboratory abnormalities and imaging or procedures when available (Fig. 2).
Fig. 2.
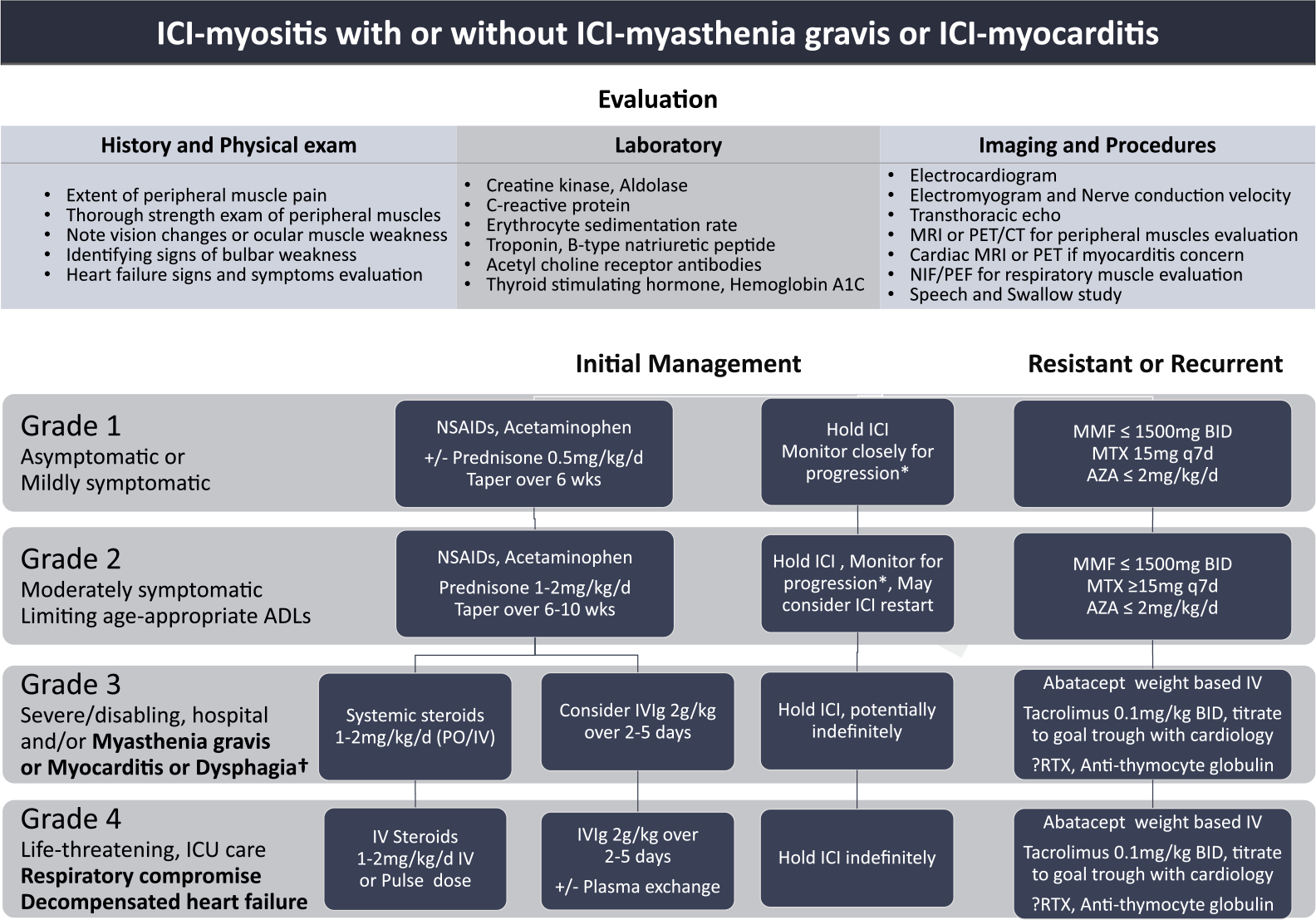
ICI-myositis evaluation and treatment.
*Frequent monitoring for progression to myocarditis and/or myasthenia gravis. For grade 2 ICI-myositis, may consider outpatient monitoring of symptoms and frequent labs accordingly. For grade 3 ICI-myositis, recommend inpatient care with at least daily monitoring of symptoms and associated labs, imaging, and procedures.
†For cardiac involvement, recommend cardiology consultation to assist with heart-directed therapies, and, for myasthenia gravis, recommend neurology consultation to help manage associated neurological manifestations.
ICI: Immune checkpoint inhibitor; NIF/PEF: negative inspiratory force/peak expiratory flow; NSAIDS: nonsteroidal anti-inflammatory drugs; MMF: mycophenolate mofetil; MTX: methotrexate; AZA: azathioprine; ADLs: activities of daily living; IVIg: intravenous immunoglobulin; BID: twice a day; ICU: intensive care unit; RTX: rituximab.
Initial approach
Treatment for ICI-myositis should not be delayed given its association with higher morbidity. For grade 1 ICI-myositis that may be characterized solely by lab abnormalities or mild symptoms of muscle pain and weakness, the recommendation is to start with NSAIDs or acetaminophen [27,28]. If the patient has notable CK elevation with mild muscle weakness, oral systemic steroids can be considered [28]. Grade 2 ICI-myositis is characterized by moderate symptoms of muscle weakness limiting age-appropriate activities of daily living; ASCO recommends systemic steroids 0.5–1 mg/kg/d while NCCN guidelines group moderate and severe together to recommend 1–2 mg/kg/d systemic steroids [27,28]. The guidelines are consistent in raising concern for disease progression, and SITC guidelines recommend that patients with grade 3 myositis should consider hospitalization with steroids starting at prednisone 1 mg/kg per day or equivalent. However, for patients with any signs of cardiac or respiratory muscle involvement and/or dysphagia, the SITC expert panel recommends 1–2 mg/kg per day IV steroids with consideration of pulse dose steroids as well as IV immunoglobulin (IVIg) or plasmapheresis [24]. For ICI-myositis with muscle weakness severely limiting mobility and/or cardiac or respiratory compromise, the recommendation is for high-dose IV steroids at 1–2 mg/kg per day but with strong consideration for pulse dose steroids with 500–1000 mg IV solumedrol per day for 3–5 days. For severe and/or rapidly progressing presentations, IVIg should be considered and is particularly effective for cases with myasthenia gravis [20,90].
Choice and dosing of steroid-sparing agents
In general, treatment beyond corticosteroids will be impacted by the severity and extent of neuromuscular involvement and/or cardiac involvement, as well as the acuity of the patient’s clinical status. For patients with persistent or recurrent symptoms that are mild and not requiring hospital admission, oral steroid-sparing immunosuppressants such as mycophenolate mofetil, methotrexate, or azathioprine can be considered [29,91,92]. For more severe cases with or without myocarditis or myasthenia gravis that are resistant to significant improvement from initial therapy by 24–48 h (closer monitoring particularly required in cases for concern of concurrent ICI-myocarditis), agents that have been used include mycophenolate mofetil, tacrolimus, and abatacept [85]. Tocilizumab, rituximab, and even infliximab have been used [85,91]. For cases with seropositivity, such as one or more of the myositis-specific antibodies or acetylcholine receptor antibody in cases of ICI-myositis with myasthenia gravis, B-cell-directed therapy such as rituximab can be particularly considered.
The management of ICI-myositis treatment overall will be informed by the severity of clinical presentation, the extent of organ systems involved, or the progression of the disease. Given its association with myocarditis and myasthenia gravis, it will be key to collaborate closely with cardiology in the setting of ICI-myocarditis to help monitor and manage cardiac instability and neurology for ICI-myasthenia gravis for assistance in the evaluation and management of myasthenia-specific tests and treatment.
Sicca syndrome
Epidemiology and clinical phenotypes
Sicca syndrome due to ICIs involves immune-mediated dry mouth and dry eyes. Sicca syndrome as an irAE typically affects the mouth more than the eyes. In two case series, well over half of the patients did not have any ocular dryness. Parotitis is rare and has only been reported once in the literature [7]. Most patients are seronegative for anti-Ro and anti-La antibodies (10% positive in one study, 20% in another [12,13]). The median time to develop sicca after ICI start was 70 days in one series [12]. Histology from minor salivary gland biopsies can show two patterns. First, a mild focal lymphocytic sialadenitis has been demonstrated. In severe sicca, however, a robust T-cell infiltrate distorting gland architecture can be seen with upregulated PD-1 and PD-L1 tissue expression.
Treatment
Symptomatic treatment is similar to that of primary Sjogren’s syndrome. For dry mouth, saliva substitutes can be used. Sialogogues such as pilocarpine and cevimeline are indicated if saliva substitutes are not helpful. For ocular dryness, referral to ophthalmology is appropriate. Artificial tears and punctal plugs can be helpful for dry eye symptoms. A key difference between treating primary Sjogren’s syndrome and sicca syndrome as an irAE is the role of prednisone. There is some evidence that a 4–6-week taper of prednisone for severe sicca may help restore some salivary function. Warner et al. suggest using prednisone in patients with grade 2 or higher sicca syndrome according to CTCAE, that is, those who need copious amounts of liquids to swallow, have to restrict or alter their diet, or need to use oral lubricants.
Managing preexisting autoimmune disease during ICI therapy
For patients with pAID, considering therapy that will exacerbate their immune system understandably raises concern. To date, no completed prospective checkpoint inhibitor clinical trial has allowed enrollment of patients with a pAID; hence, the safety and effectiveness of ICI therapy for patients with pAIDs with or without chronic immunomodulatory therapy are not truly known. Multiple observational studies and meta-analyses have investigated the safety and effectiveness of ICI therapy for patients with pAID (Table 2). Across other large-cohort studies (>100 patients) and reviews for patients with pAIDs who have received ICI treatment, 25–50% of patients may have a pAID flare and up to about 40% of patients may suffer from a de novo irAE (Table 2). Note that high-grade AEs (≥CTCAE grade 3) are less common, with a retrospective study estimating about 13% high-grade pAID flares and 16% high-grade de novo irAEs [93].
Table 2.
Evidence-based table for preexisting autoimmune diseases and ICI use.
| Study (First Author, Year), PMID | Cohort size | Tumor type | pAIDs studied (pAIDs ≥3 cases) | ICI | % Total pAID flare (% Grade ≥3) | % Total de novo irAE (% Grade ≥3) | % Total any AEs (AID flare and/or irAE) (% Grade ≥3) | % ICI discontinuation | Other Outcome measures | Tumor outcome |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Original Cohort Studies | ||||||||||
| Tison et al. Arthritis Rheumatol, 2019, PMID: 31379105 | 112 | Melanoma (66), NSCLC (40), Other (6) | PsO/PsA (28), RA (18), IBD (13), SLE (6), PMR/GCA 6), SpA (5), Others (25) | CTLA4i (13), PD(L)1i (85), Comb (3) | 47% (13%) | 42% (16%) | 71% (NA) | 21% | Any cancer ORR 49% Melanoma PFS: 13 months NSCLC PFS: 12 months |
|
| Kehl et al. Cancer Immunol Immunother, 2019, PMID: 30877325 | 179 | Lung cancer (87), Melanoma (42), Renal (20), Urothelial (7), Head and neck (8), Other (15) | IBD (70), RA (40), PsA (11), ITP (10), Sarcoid (8), MS (8), SLE (7), AS (7), PMR (4), vasculitis (4), vitiligo (3) | CTLA4i (18), PD(L)1i (154), Comb (7) | Not available | Not available | Not available | 35% all-cause hospitalization 11% hospitalization with probable any AE diagnosis 41% systemic steroids prescription at 3 months |
Not available | |
| Abu-Sbeih et al, J Clin Oncol, 2020., PMID: 31800340 | 102 | Melanoma (44), Lung (23), GI (17), GU (7), Others (10) | UC (48), CD (48), other IBD (4) | CTLA4i (7), PD(L)1i (83), Combi (10) | Only GI AEs reported | Only GI AEs reported | Only GI AEs reported | 23% | Any GI AE 41% Grade ≥3 GI AEs 21% |
ORR 48% |
| Chanza et al. J Immunother Cancer, 2020, PMID: 32217762 | 106 | RCC (58) UC (48) |
Psoriasis (24), thyroiditis (14), RA (12), polymyalgia rheumatica (8), IBD (6), systematic lupus erythematosus (4), MS (4), sarcoidosis (2), vasculitis (2) | PD(L)-1 (85), Comb (10) | 36% (6%) | 38% (12%) | 69% Either: 53% Both: 16% |
34% | RCC ORR: 31% UC ORR:40% |
|
| Bender, J Immunother Cancer, 2020. PMID: 33303578 | 124 | Lung cancer, Melanoma | Alopecia areata, AIHA, autoimmune hepatitis, CD, discoid lupus, Graves disease, Hashimoto’s thyroiditis, IPF, MS, MG, PMR, PsO/PsA, rheumatic fever, RA, SSc, seronegative arthritis, Sjogren’s syndrome, SLE, UC, vasculitis | CTLA4i (28), PD(L)1i (96) Comb (7) | Not available | Not available | Not available | Any cancer with any ICI regimen requiring systemic steroids: 31% | Not available | |
| Van der Kooij, Ann Intern Med, 2021, PMID: 33587686 | 415 pAIDs, 228 pAIDs with ICI(s) |
Melanoma | Rheumatic (227), Endocrine (143), IBD (55), or “other” (8) | CTLAi: 87/228 PD(L)1i: 187/228 Comb: 34/228 |
Not differentiated from de novo irAE | Not differentiated from pAID flares | Grade ≥3 only CTLAi: 30% PD(L)1: 17% Comb: 44% |
CTLA-4i 18% Anti-PD-1 17% Comb 29% |
All melanoma CTLA-4i ORR 10% PD-1i ORR 40% Comb ORR 39% |
|
| Tully, Am J Clin Oncol, 2021 PMID: 34081033 | 106 | Melanoma | RA (28), Pernicious gastritis (31), T1DM (16), but <11 patients not reported | CTLA4i, PD(L)li, Comb | Not available | Not available | 59% | Not available | Not available | |
| Tang, J Natl Cancer Inst, 2022. PMID: 35188215 | 17497 | Pulmonary (11079), Melanoma (3948), GI (3378), Urinary tract (3307) | IBD, RA, T1DM, MG, Vasculitis, SSc, PsA/PsO, AS, Dermatomyositis, SLE, Vitiligo, Celiac disease, Hashimoto disease, Graves disease, Mucositis, | PD(L)1i | Not available | Not available | Not available | No increased risk of mortality for patients with any pAID, RA, SLE, SSc, Vasculitis, dermatomyositis (all p > 0.05) | Not available | |
| Placais, Ann Rheum Dis, 2022. PMID 35788496 | 110 | Melanoma | Thyroiditis (47), psoriasis (18), RA (11), vtiligo (8), sarcoidosis (3), Raynaud disease (3), MS (3), spondylarthritis (3) | CTLA4i (15) PD(L)1i (86) Comb (9) | 30% | irAEs did not seem to specify “de novo” irAEs | 72% (57%) | Not available | OS: 65% at 24 months | |
| Fountzilas et al. Cancer Immunol Immunother, 2022. PMID: 34164709 | 123 | NSCLC (77), melanoma (18), SCLC (7), Head neck (6), others (15) | Rheumatic (54), Dermatologic (31), Endocrine (26), GI (10), Neurologic (3) | CTLA4i (4), PD(L)1i (116), Comb (3) | 25% | 35% (10%) | 60% | 9% | ORR 56% Median OS 41 months |
|
| Meta-analyses | ||||||||||
| Abdel-Wahab et al., 2018, PMID: 29297009 | 123 patients (49 studies) | Melanoma (103), lung (16), RCC (3), Merkel cell (1) | Pso/Psa (28), RA (20), IBD (13), Autoimmune Thyroid Disease (11), MS (6), Sarcoidosis (5), MG (4) |
CTLA4i (44), PD(L)1i (65), Comb (3) |
50% | 34% | 75% | 17% | ORR 47% | |
| Xie, Autoimmune Rev, 2020, PMID 33131688 | 619 patients (14 studies) | Any cancer | PsO/PsA, RA Inflammatory polyarthritis, SLE, MG, autoimmune thyroid disorder, IBD, MS, vitiligo, sarcoidosis, nonspecific terms for autoimmune disease also in search | CTLA4i, PD(L)1i, Comb | 35% | 33% | 60% | Not available | ORR 30% | |
| Yamaguchi, Support Care Cancer, 2021, 34164739 | 206 (6 studies) | Any cancer | PsO/PsA, RA, IBD, Sarcoidosis, Endocrine disorders, Dermatologic disorders | CTLA4i, PD(L)1i, Comb | Not differentiated from de novo irAEs | Not differentiated from pAID flares | 62% | Not available | Not available | |
| Meserve, Aliment Pharmacol Ther, 2021, PMID: 33314269 | 193 (12 studies) | Any cancer | IBD | CTLA4i, PD(L)1i, Comb | 40% | 35% | ||||
| Wu, Immunotherapy, 2021, PMID | 512 (52 studies) | Melanoma (278), NSCLC (106), Urologic (5), Others (10) | Rheumatologic (180), Dermatologic (129), Endocrine (111), GI/Hepatic (53), Neurologic (35), Others (23), Multiple sites (23) | CTLA4i (112), PD(L)1i (206), Comb (3) CTLA4i then PD1i (28) |
44% (10%) | 24% (8%) | 68% (18%) | Organ-specific flare of pAID: Derm: 47% GI/hepatic 43% Rheum 41% Endo: 42% |
ORR 34% Median PFS: 6.6 months Median OS 12.9 months |
|
| Gulave et al. ESMO Open, 2021, PMID 33887689 | 106 | Psoriasis (50), T1DM (13), RA (9), Hashimoto’s disease (5), Sarcoidosis (4), Autoimmune thyroiditis (3), others | Not specified for the population with pAIDs | Not available | Not available | 4%?? | ||||
| Zhang, Chest, 2022, 35026298 | 179 (10 studies) | NSCLC | ILD | PD(L)1i | 27% (14%) | irAEs did not seem to specify “de novo” irAEs | 57% (28%) | 16% | ORR: 35% Median PFS: 1.4–Smonths Median OS: 16–28 months |
|
| Yu, 2022. 35912183 | 191 (12 studies) | Any cancer | Psoriasis | 45% (11%) | 45% (17%) | 19% | ORR: 38.1% | |||
Identifying the factors associated with the risk of pAID flares and/or de novo irAEs can help guide conversation for patients with pAIDs considering ICI therapy. In a 2021 systematic literature review by Wu and colleagues, inactive AID than active AID at the time of ICI initiation experienced higher rates of high-grade pAID flares (50% versus 0%, respectively, p = 0.045) [94]. Furthermore, patients without immunosuppression at ICI start showed significantly higher rates of high-grade ICI AEs than those patients without immunosuppression at ICI start (70% versus 30%, respectively, p = 0.007) [94]; this was also demonstrated in another review where patients with immunosuppression at ICI initiation versus those without had less any-type AEs, pAID exacerbations, or de novo irAEs (67% versus 74%, 48% versus 50%, and 19% versus 34%, respectively) [95]. ICI regimen also impacts the rates of AEs. Anti-CTLA4 therapy showed a higher rate of de novo irAEs versus anti-PD(L)1s (42% versus 26%) (Wu, 2021, 33715386). In another review, more patients on combination therapy compared with ICI monotherapy witnessed de novo irAEs (67% in the combination group versus 42% in the CTLA4 inhibitor group and 26% in the PD(L)1 inhibitor group) [95].
In particular, patients with psoriasis or psoriatic arthritis are reported to be at the highest risk of ICI AEs, with pAID flares ranging from 43 to 79% and up to 89% suffering from any-type AEs (pAID flare or de novo irAE) [93,95,96]. For patients with RA, rates of RA flare can range from 50 to 65%, with about 9% experiencing severe flares [93,97,98]. Up to 79% of patients with preexisting RA can suffer from any-grade AEs after ICI therapy, with 57% reported to experience any-type high-grade AEs [98]. Cases of spondyloarthritis with ICI therapy are rare, but Tison et al. reported 2 of 5 patients experiencing an ICI AE and Abdel-Wahab found 2 of 2 patients with spondyloarthropathy and 1 of 1 patient with ankylosing spondylitis who suffered an ICI immunotoxicity [93,97]. In Tison et al.’s study, only 1 of 7 patients with lupus experienced a mild flare; and in Abdel-Wahab’s review, none of the 2 patients with lupus experienced an ICI toxicity [93,97].
Regarding the efficacy of ICIs for patients with pAIDs, a 2019 study with 85 patients with pAID found no statistically significant difference in ORR for patients with pAID (38–50%) versus those without pAID (35%) [99], but a 2022 multicenter, prospective study demonstrated that patients with pAIDs had an OS that was significantly higher than those patients without pAIDs (65% compared to 46%, p = 0.02) [98]. Smaller studies reported worse response rates and shorter progression-free survival for patients with pAID that are on baseline immunosuppressive therapy at the time of ICI initiation [93,100]. Future prospective study through randomized clinical trials of ICI therapy for patients with pAIDs is critical to truly appreciate the different factors that may contribute to ICI safety and effectiveness in this particular population (NCT03816345, NCT04805099, and NCT03140137). Meanwhile, it seems that checkpoint inhibitor therapy may be safe for patients with pAID and should not be withheld from patients with pAIDs, but providers and patients should monitor the AEs closely, particularly in the setting of combination ICI therapy.
Overarching considerations for rheum-onc multidisciplinary care
Rheumatology and oncology collaboration and close communication are key in the context of ICI therapy with or without pAID and the concern for, evaluation of, and management of ICI-associated rheumatic toxicities. Because of the correlation of rheumatic irAEs with that of favorable tumor response, the main priority is often to stay on ICI therapy [30,31]. Likewise, for patients enrolled in a clinical trial, considering irAE treatments that allow patients to continue a clinical trial is preferred. Most commonly, immunotherapy clinical trials allow local corticosteroid treatment, such as intra-articular CSIs and systemic glucocorticoids up to prednisone 10 mg daily equivalent [57,101–104]. Beyond these disease-related concerns, patient perception is important to note. ICI therapy is still predominantly used for advanced malignancies. Patients may have already gone through several cancer treatments with radiation, chemotherapy, or surgery before considering cancer immunotherapy. ICIs have offered considerable hope for cancer therapy, and ICI toxicities can cause significant stress to patients who may be worried about the cancer returning and/or development of another chronic condition such as a new autoimmune disease [105].
Conclusion
The advent of cancer immunotherapy has revolutionized the field of oncology, but immune-driven toxicities have often hindered the continuation of these life-saving therapies. Rheumatic irAEs are associated with significant mortality and morbidity, and prompt management by rheumatology can be key to recognize and effectively treat these conditions. Treatment of irAEs should be based on the extent and severity of the disease in the context of patients’ other comorbidities. Management plans should be made in collaboration with the oncologist and the patient with close monitoring for progression of the irAE after the start of initial treatment and/or recurrence upon tapering initial immunosuppression. Patients with pAID should not be excluded from receiving ICI therapy, but cancer immunotherapy in this at-risk population should be carefully dosed with regular supervision.
Practice points.
Rheumatic irAEs range widely in clinical presentation and severity.
Choosing appropriate treatment for a rheumatic irAE depends not only on the clinical phenotype and severity but also on the goals of cancer treatment.
Steroid-sparing agents such as TNF-inhibitors, IL-6R inhibitors, IVIg, and methotrexate may be used in patients who fail to respond to steroids, are unable to wean off steroids, or have a chronic course to their irAE.
Patients with pAID may have a flare or de novo irAE when treated with ICIs, but most patients do not require discontinuation of ICI therapy and can be managed with careful monitoring by the rheumatologist and oncologist.
Research agenda.
Further understanding of the underlying pathogenesis of rheumatic irAEs is needed so that more precisely targeted therapy can be used.
Prospective clinical trials comparing current treatment regimens are important to define the efficacy and safety of steroid-sparing agents for rheumatic irAEs.
Acknowledgment
The authors thank Arohi Singh, a University of Chicago undergraduate.
Footnotes
Declaration of competing interest
Pankti Reid has no conflicts of interest related to this article. She has a patent pending for tocilizumab use in infection-associated pulmonary hyperinflammation.
Laura Cappelli has research funding from Bristol-Myers Squibb.
References
- [1].Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27(4):450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Institute NIoHNNC. Common Terminology Criteria for Adverse Events (CTCAE). NIH; 2018. [Google Scholar]
- [3].Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Cancer Netw 2019;17(3):255–89. [DOI] [PubMed] [Google Scholar]
- [4].Thompson LL, Krasnow NA, Chang MS, et al. Patterns of cutaneous and noncutaneous immune-related adverse events among patients with advanced cancer. JAMA Dermatol 2021;157(5):577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Al-Kindi SG, Oliveira GH. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet 2018;392(10145):382–3. [DOI] [PubMed] [Google Scholar]
- [6].Roberts J, Ennis D, Hudson M, et al. Rheumatic immune-related adverse events associated with cancer immunotherapy: a nationwide multi-center cohort. Autoimmun Rev 2020;19(8):102595. [DOI] [PubMed] [Google Scholar]
- [7].Cappelli LC, Gutierrez AK, Bingham CO 3rd, et al. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken) 2017;69(11):1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buder-Bakhaya K, Benesova K, Schulz C, et al. Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother 2018;67(2):175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2020;79(3):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Allenbach Y, Anquetil C, Manouchehri A, et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev 2020;19(8):102586. [DOI] [PubMed] [Google Scholar]
- [11].Anquetil C, Salem JE, Lebrun-Vignes B, et al. Immune checkpoint inhibitor-associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation 2018;138(7):743–5. [DOI] [PubMed] [Google Scholar]
- [12].Warner BM, Baer AN, Lipson EJ, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. The oncologist 2019;24(9):1259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Prim 2020;6(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol 2018;14(10):569–79. [DOI] [PubMed] [Google Scholar]
- [15].Raschi E, Antonazzo IC, Poluzzi E, et al. Drug-induced systemic lupus erythematosus: should immune checkpoint inhibitors be added to the evolving list? Ann Rheum Dis 2021;80(7):e120. [DOI] [PubMed] [Google Scholar]
- [16].Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Canc 2015;3(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375(18):1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a phase II study. Cancer Sci 2017;108(6):1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wen X, Ding Y, Li J, et al. The experience of immune checkpoint inhibitors in Chinese patients with metastatic melanoma: a retrospective case series. Cancer Immunol Immunother 2017;66(9):1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Makarious D, Horwood K, Coward JIG. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer 2017;82:128–36. [DOI] [PubMed] [Google Scholar]
- [21].Belkhir R, Burel SL, Dunogeant L, et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 2017;76(10):1747–50. [DOI] [PubMed] [Google Scholar]
- [22].Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28(6):1368–79. [DOI] [PubMed] [Google Scholar]
- [23].Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36(17):1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Cancer Netw 2020;18(3):230–41. [DOI] [PubMed] [Google Scholar]
- [26].Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28(suppl_4):iv119–42. [DOI] [PubMed] [Google Scholar]
- [27].Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2022;20(4):387–405. [DOI] [PubMed] [Google Scholar]
- [28].Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 2021;39(36):4073–126. [DOI] [PubMed] [Google Scholar]
- [29].Kostine M, Finckh A, Bingham CO, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 2021;80(1):36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liew DFL, Leung JLY, Liu B, et al. Association of good oncological response to therapy with the development of rheumatic immune-related adverse events following PD-1 inhibitor therapy. Int J Rheum Dis 2019;22(2):297–302. [DOI] [PubMed] [Google Scholar]
- [31].Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77(3):393–8. [DOI] [PubMed] [Google Scholar]
- [32].Karam JD, Noel N, Voisin AL, et al. Infectious complications in patients treated with immune checkpoint inhibitors. Eur J Cancer 2020;141:137–42. [DOI] [PubMed] [Google Scholar]
- [33].Ross JA, Komoda K, Pal S, et al. Infectious complications of immune checkpoint inhibitors in solid organ malignancies. Cancer Med 2022;11(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Del Castillo M, Romero FA, Arguello E, et al. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis 2016;63(11):1490–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 2015;33(28):3193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124(18):3706–14. [DOI] [PubMed] [Google Scholar]
- [37].Maslov DV, Tawagi K, Kc M, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immun Therr Canc 2021;9(7):e002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers 2020;12(3):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luo J, Beattie JA, Fuentes P, et al. Beyond steroids: immunosuppressants in steroid-refractory or resistant immune-related adverse events. J Thorac Oncol : Off Publ Int Assoc Stud Canc 2021;16(10):1759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beattie J, Rizvi H, Fuentes P, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Adda L, Batteux B, Saidak Z, et al. Rheumatic and musculoskeletal disorders induced by immune checkpoint inhibitors: consequences on overall survival. Joint Bone Spine 2021;88(4):105168. [DOI] [PubMed] [Google Scholar]
- [43].Bongartz T, Warren FC, Mines D, et al. Etanercept therapy in rheumatoid arthritis and the risk of malignancies: a systematic review and individual patient data meta-analysis of randomised controlled trials. Ann Rheum Dis 2009;68(7):1177–83. [DOI] [PubMed] [Google Scholar]
- [44].Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295(19):2275–85. [DOI] [PubMed] [Google Scholar]
- [45].Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008;6(6):644–53. [DOI] [PubMed] [Google Scholar]
- [46].Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf 2011;20(2):119–30. [DOI] [PubMed] [Google Scholar]
- [47].Thompson AE, Rieder SW, Pope JE. Tumor necrosis factor therapy and the risk of serious infection and malignancy in patients with early rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2011;63(6):1479–85. [DOI] [PubMed] [Google Scholar]
- [48].Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor alpha inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum 2013;65(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016;152(2):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peleva E, Exton LS, Kelley K, et al. Risk of cancer in patients with psoriasis on biological therapies: a systematic review. Br J Dermatol 2018;178(1):103–13. [DOI] [PubMed] [Google Scholar]
- [51].Wang JL, Yin WJ, Zhou LY, et al. Risk of non-melanoma skin cancer for rheumatoid arthritis patients receiving TNF antagonist: a systematic review and meta-analysis. Clin Rheumatol 2020;39(3):769–78. [DOI] [PubMed] [Google Scholar]
- [52].Raaschou P, Simard JF, Holmqvist M, et al. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: nationwide population based prospective cohort study from Sweden. BMJ 2013;346:f1939. [DOI] [PubMed] [Google Scholar]
- [53].Lopez-Olivo MA, Tayar JH, Martinez-Lopez JA, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy: a meta-analysis. JAMA 2012;308(9):898–908. [DOI] [PubMed] [Google Scholar]
- [54].Hellgren K, Dreyer L, Arkema EV, et al. , Artis Study Group FtDSG. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis 2017;76(1):105–11. [DOI] [PubMed] [Google Scholar]
- [55].Mercer LK, Askling J, Raaschou P, et al. Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: results from a collaborative project of 11 European biologic registers. Ann Rheum Dis 2017;76(2):386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cappelli LC, Brahmer JR, Forde PM, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum 2018;48(3):553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 2018;6(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lesage C, Longvert C, Prey S, et al. Incidence and clinical impact of anti-TNFalpha treatment of severe immune checkpoint inhibitor-induced colitis in advanced melanoma: the mecolit survey. J Immunother 2019;42(5):175–9. [DOI] [PubMed] [Google Scholar]
- [60].Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the Dutch melanoma treatment Registry. Clin Cancer Res : Off J Am Assoc Canc Res 2020;26(9):2268–74. [DOI] [PubMed] [Google Scholar]
- [61].Bass AA-WN, Reid P, Sparks J, et al. Comparing the safety and effectiveness of methotrexate, TNF and IL6 inhibitors for the treatment of checkpoint inhibitor arthritis [abstract]. Arthritis Rheumatol 2022;74(suppl 9). [Google Scholar]
- [62].Montfort A, Filleron T, Virazels M, et al. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase ib clinical trial. Clin Cancer Res : Off J Am Assoc Canc Res 2021;27(4):1037–47. [DOI] [PubMed] [Google Scholar]
- [63].Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16(5):448–57. [DOI] [PubMed] [Google Scholar]
- [64].Hoejberg L, Bastholt L, Schmidt H. Interleukin-6 and melanoma. Melanoma Res 2012;22(5):327–33. [DOI] [PubMed] [Google Scholar]
- [65].Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol 2008;26(29):4820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J Colorectal Dis 2010;25(2):135–40. [DOI] [PubMed] [Google Scholar]
- [67].Laino AS, Woods D, Vassallo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hailemichael Y, Johnson DH, Abdel-Wahab N, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022;40(5):509–23. e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mehmi I, Hamid O, Hodi FS, et al. Ipilimumab, nivolumab and tocilizumab as first-line therapy for advanced melanoma. J Clin Oncol 2021;39(15_suppl):TPS9589. TPS9589. [Google Scholar]
- [70].Lebbe C, Meyer N, Mortier L, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 2019;37(11):867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Holmstroem RB, Nielsen OH, Jacobsen S, et al. COLAR: open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J Immunother Cancer 2022;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Delyon J, Lebbe C. IL-6 blockade in cancer patients treated with immune checkpoint blockade: a win-win strategy. Cancer Cell 2022;40(5):450–1. [DOI] [PubMed] [Google Scholar]
- [73].Reid PLD, Akruwala R, Bass AR, et al. Activated osteoarthritis following immune checkpoint inhibitor treatment: an observational study. J Immunother Cancer 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ghosh N, Tiongson MD, Stewart C, et al. Checkpoint inhibitor-associated arthritis: a systematic review of case reports and case series. J Clin Rheumatol 2021;27(8):e317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ghosh N, Chan KK, Jivanelli B, et al. Autoantibodies in patients with immune-related adverse events from checkpoint inhibitors: a systematic literature review. J Clin Rheumatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Naidoo J, Cappelli LC, Forde PM, et al. Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. The oncologist 2017;22(6):627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145(3):639–48. [DOI] [PubMed] [Google Scholar]
- [78].Alexander JL, Ibraheim H, Sheth B, et al. Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab. J Immunother Cancer 2021;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Calabrese C, Cappelli LC, Kostine M, et al. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open 2019;5(1):e000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377(4):317–28. [DOI] [PubMed] [Google Scholar]
- [81].Nigro O, Pinotti G, Gueli R, et al. Psoriatic arthritis induced by anti-PD1 and treated with apremilast: a case report and review of the literature. Immunotherapy 2020;12(8):549–54. [DOI] [PubMed] [Google Scholar]
- [82].Ma VT, Lao CD, Fecher LA, et al. Successful use of secukinumab in two melanoma patients with immune checkpoint inhibitor-induced inflammatory arthropathy. Immunotherapy 2022;14(8):593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Takeda K, Yanagitani N. Guselkumab for treating immune checkpoint inhibitor-induced psoriatic arthritis. Ann Rheum Dis 2022. [DOI] [PubMed] [Google Scholar]
- [84].Nguyen T, Maria ATJ, Ladhari C, et al. Rheumatic disorders associated with immune checkpoint inhibitors: what about myositis? An analysis of the WHO’s adverse drug reactions database. Ann Rheum Dis 2022;81(2):e32. [DOI] [PubMed] [Google Scholar]
- [85].Aldrich J, Pundole X, Tummala S, et al. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors. Arthritis Rheumatol 2021;73(5):866–74. [DOI] [PubMed] [Google Scholar]
- [86].Hamada N, Maeda A, Takase-Minegishi K, et al. Incidence and distinct features of immune checkpoint inhibitor-related myositis from idiopathic inflammatory myositis: a single-center experience with systematic literature review and meta-analysis. Front Immunol 2021;12:803410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Moreira A, Loquai C, Pfohler C, et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:12–23. [DOI] [PubMed] [Google Scholar]
- [88].Saygin D, Ghosh N, Reid P. Immune checkpoint inhibitor-associated myositis: a distinct form of inflammatory myopathy. J Clin Rheumatol 2022;28(7):367–73. [DOI] [PubMed] [Google Scholar]
- [89].Hajem S, Ederhy S, Champiat S, et al. Absence of significant clinical benefit for a systematic routine creatine phosphokinase measurement in asymptomatic patients treated with anti-programmed death protein (ligand) 1 immune checkpoint inhibitor to screen cardiac or neuromuscular immune-related toxicities. Eur J Cancer 2021;157:383–90. [DOI] [PubMed] [Google Scholar]
- [90].Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer 2019;7(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Solimando AG, Crudele L, Leone P, et al. Immune checkpoint inhibitor-related myositis: from biology to bedside. Int J Mol Sci 2020;21(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nakagomi Y, Tajiri K, Shimada S, et al. Immune checkpoint inhibitor-related myositis overlapping with myocarditis: an institutional case series and a systematic review of literature. Front Pharmacol 2022;13:884776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tison A, Quere G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol 2019;71(12):2100–11. [DOI] [PubMed] [Google Scholar]
- [94].Wu C, Zhong L, Wu Q, et al. The safety and efficacy of immune-checkpoint inhibitors in patients with cancer and preexisting autoimmune diseases. Immunotherapy 2021;13(6):527–39. [DOI] [PubMed] [Google Scholar]
- [95].Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 2018;168(2):121–30. [DOI] [PubMed] [Google Scholar]
- [96].Fountzilas E, Lampaki S, Koliou GA, et al. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother 2022;71(2):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann Intern Med 2018;169(2):133–4. [DOI] [PubMed] [Google Scholar]
- [98].Placais L, Dalle S, Dereure O, et al. Risk of irAEs in patients with autoimmune diseases treated by immune checkpoint inhibitors for stage III or IV melanoma: results from a matched case-control study. Ann Rheum Dis 2022;81(10):1445–52. [DOI] [PubMed] [Google Scholar]
- [99].Cortellini A, Buti S, Santini D, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. The oncologist 2019;24(6):e327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28(2):368–76. [DOI] [PubMed] [Google Scholar]
- [101].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384(14):1289–300. [DOI] [PubMed] [Google Scholar]
- [104].Kudo M Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr 2022;11(4):592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cappelli LC, Grieb SM, Shah AA, et al. Immune checkpoint inhibitor-induced inflammatory arthritis: a qualitative study identifying unmet patient needs and care gaps. BMC Rheumatol 2020;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]