Abstract
In order to expound upon the association between inflammatory biomarkers and magnetic resonance imaging (MRI) visible perivascular spaces (PVS), Framingham Heart Study participants with available inflammatory biomarkers and brain MRI were studied. PVS in the basal ganglia and centrum semiovale were categorized based on counts. A mixed score of high burden PVS in either or both regions was also evaluated. We analyzed a panel of 16 biomarkers representing various inflammatory mechanisms and related them to PVS burden using multivariable ordinal logistic regression analyses accounting for vascular risk factors and other MRI markers of cerebral small vessel disease.
Among 3604 participants (mean age 58±13 years, 47% males), significant associations were observed for intercellular adhesion molecule 1, fibrinogen, osteoprotegerin, and P-selectin in relation to basal ganglia PVS, P-selectin for centrum semiovale PVS, and tumor necrosis factor receptor 2, osteoprotegerin and cluster of differentiation 40 ligand for mixed topography PVS. Therefore, inflammation may have a role in the pathogenesis of cerebral small vessel disease and perivascular drainage dysfunction represented by PVS, with different and shared inflammatory biomarkers depending on PVS topography.
Keywords: MRI visible perivascular spaces, inflammatory biomarkers, basal ganglia, centrum semiovale, glymphatic function
Introduction
Perivascular spaces (PVS) visible on brain magnetic resonance imaging (MRI) are cerebrospinal fluid-filled spaces surrounding small penetrating cerebral blood vessels. They are considered to represent cerebral small vessel disease (CSVD) and possibly reflect a part of the brain glymphatic system (Doubal et al., 2010). PVS are seen on T2* weighted brain MRI, are observed in increasing numbers with advancing age, and have been associated with impaired cognitive function and stroke (Doubal et al., 2010). PVS are mainly observed in the basal ganglia (BG), midbrain, and centrum semiovale (CSO) regions, and their predominant topography may reflect different pathogenic mechanisms (Charidimou et al., 2017; Tsai et al., 2021). In the basal ganglia, PVS may be associated with hypertensive CSVD (Yang et al., 2017), while CSO PVS have been linked to cerebral amyloid angiopathy (CAA) (Tsai et al., 2021). However, advanced hypertensive CSVD has also been related to PVS across all brain regions (Charidimou et al., 2017).
PVS are considered as MRI markers of cerebral small vessel disease. However, their inciting or propagating mechanisms are unclear (Charidimou et al., 2017). Inflammation has been linked to the pathophysiology of various forms of cerebrovascular disease including CSVD, and clinical outcomes such as stroke and dementia (Gu et al., 2019). Inflammation may be involved in the most common forms of CSVD, including arteriolosclerosis (hypertensive vasculopathy) and CAA, and has been associated with brain MRI markers of CSVD such as covert infarcts and microbleeds (Shoamanesh et al., 2015). PVS have also been observed in close relation to active inflammatory lesions in neurological disorders strongly linked to an inflammatory response such as multiple sclerosis (Wuerfel et al., 2008).
Systemic inflammation is complex, invoking participation of several cell types and mechanisms of vascular injury such as endothelial dysfunction (Wardlaw et al., 2019), neutrophil activation (Karel et al., 2022), impaired vasodilatation, obstruction of perivascular flushing, and promotion of formation of amyloid-β (Aβ) plaques (Wardlaw et al., 2019). Notwithstanding the role of systemic inflammation in cerebral small vessel disease, little is known about the association between systemic and vascular inflammatory biomarkers and PVS in community dwelling individuals, or if there are any differences in the markers of inflammation according to PVS brain topography.
We hypothesized that inflammation is associated with MRI visible perivascular spaces and that specific markers may differ according to PVS topography. Thus, our aim was to characterize the relation between a comprehensive panel of inflammatory biomarkers reflecting systemic inflammation, vascular inflammation, and oxidative stress, and PVS, and to investigate whether there are any differences in the associations by PVS brain topography. Such knowledge may help elucidate the pathophysiology underlying PVS.
Methods
Study sample
The study included Framingham Heart Study (FHS) participants as previously documented (Lara et al., 2022) from the Offspring cohort (offspring of the Original cohort, recruited in 1971), Third Generation (participants with at least one parent in the Offspring cohort, recruited in 2002), New Offspring Spouse (NOS; spouses of Offspring participants who had not been enrolled in the FHS and had at least two biological children participate in the first exam of the Third Generation), and the OMNI 1 cohort (recruited to account for the increasing diversity of the Framingham, MA community starting in 1994). The institutional review board of Boston University Medical Center approved the study protocol and informed consent was obtained from all subjects.
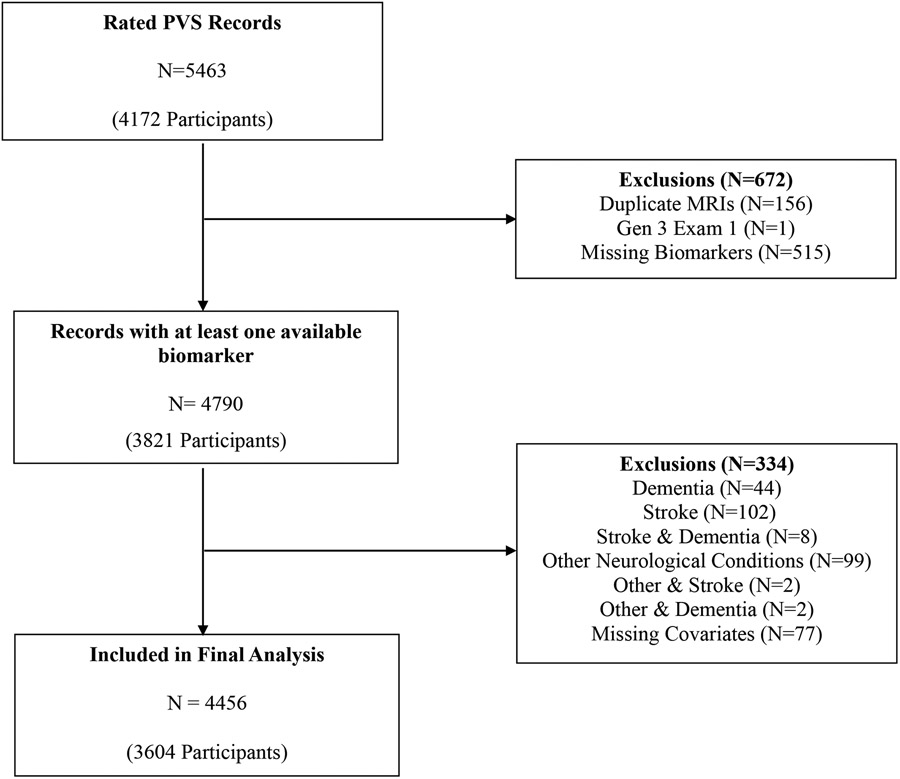
MRIs with PVS assessments and at least one biomarker assessment were included. We aimed to study community dwelling individuals without neurological disease, thus excluding four hundred and ninety-four scans with prevalent clinical stroke, dementia or other neurological diseases. Records were also excluded if there were missing covariate data. After these exclusions, 4456 records from 3604 participants were included in the final analysis. The sample selection flow chart is described in Figure 1.
Figure 1.
Sample selection.
MRI was done at examination cycles 7-9 for the Offspring cohort, examination cycle 2 for the Third Generation and NOS, and examination cycles 2-4 for OMNI 1. Fifteen participants in the New Offspring Spouse cohort were combined with the Third-Generation cohort for analysis because these cohorts had overlapping enrollment periods.
PVS Ratings
PVS were rated following the standards for research into small vessel disease (STRIVE) criteria (Wardlaw et al., 2013b). PVS appear as high signal intensities similar to cerebrospinal fluid on T2 sequences (Doubal et al., 2010) following perforating arteries as they course through the brain gray or white matter. They appear linear when the image is parallel to the course of the vessel and round with a diameter < 3mm when imaged perpendicular to the course of the vessel (Wardlaw et al., 2013a). They are most commonly seen in the basal ganglia, centrum semiovale and the midbrain and are differentiated from small lacunes by their small diameter and lack of T2-hyperintense rim around the fluid filled spaces on T2-weighted or FLAIR imaging unless they traverse an area of white matter hyperintensity (Doubal et al., 2010). MRI acquisition, measurement techniques, and interrater reliability have been described previously (Lara et al., 2022).
PVS ratings were performed by three investigators blinded to participants’ demographic and clinical information on axial or coronal views of T2 weighted MRI sequences using previously described methods (Lara et al., 2022; Potter et al., 2015). PVS burden was categorized using an ordinal scale into grades I-IV in each region based on PVS counts: grade I (1-10), grade II (11- 20), grade III (20-40) and grade IV (> 40). A mixed score was created to reflect high PVS burden (20 PVS counts or greater) in neither brain region, one region, or both brain regions: score of 0 (no high burden PVS in either the basal ganglia or centrum semiovale), score 1 (high burden in either basal ganglia or centrum semiovale), score 2 (high burden PVS in both the basal ganglia and centrum semiovale).
Volumetric analysis of white matter hyperintensity volumes has been previously reported. The MRI protocol in the FHS has been described previously (Massaro et al., 2004). Briefly, participants were imaged by MRI machines varying in field strength from one to three Tesla. Two sequences were used: a three-dimensional T1-weighted and two- or three-dimensional FLAIR imaging. All images were transferred to and processed by the University of California Davis Medical Center without knowledge of clinical information. Segmentation and quantification of brain volume measures were performed by automated procedures with quality control. Total cerebral cranial volume (TCV) was determined using a convolutional neural network method (Fletcher et al., 2021). Images were further segmented into four tissue types (gray matter, white matter, CSF, and WMH volumes) using previously published methods (Fletcher et al., 2012a; Fletcher et al., 2012b; Maillard et al., 2022). All MRI measures were corrected for head size by calculating the ratio of these volumes over TCV, multiplied by 100 (percent TCV). The percent of WMH/TCV was log-transformed for normality.
Inter-rater and intra-rater reliability measures for WMHV in the Framingham Heart Study were 0.94 and 0.98 respectively. Cerebral microbleeds were defined as rounded hypointense lesions less than 10mm on T2*GRE (Greenberg et al., 2009). Reliability measures for cerebral microbleeds have been previously documented (Romero et al., 2014).
Inflammation Biomarkers
We selected a set of 16 biomarkers representing various components of the inflammatory cascade, including systemic inflammation (high-sensitivity C-reactive protein [CRP, mg/L], interleukin 6 [IL-6, pg/mL], monocyte chemoattractant protein 1 [MCP-1, pg/mL], tumor necrosis factor α [TNFα, pg/mL], isoprostane [pg/ml], tumor necrosis factor receptor 2 [TNFR2, pg/mL], osteoprotegerin [OPG, pmol/L], and fibrinogen [mg/dl]), vascular inflammation/endothelial dysfunction (intercellular adhesion molecule 1 [ICAM-1, mg/L], cluster of differentiation 40 ligand [CD40L, ng/mL], P-selectin [ng/mL], lipoprotein-associated phospholipase A2 [Lp-PLA2] mass [ng/ml] and activity [nmol/min/ML], total homocysteine [μmol/L], and vascular endothelial growth factor A [VEGF, pg/ml]), and oxidative stress (myeloperoxidase [MPO, ng/mL]). Biomarkers were extracted from the same examination cycle as the PVS assessments.
Fasting morning samples were collected and plasma and serum aliquots were stored at −70° to −80°C. For some biomarkers (P-selectin, tumor necrosis factor receptor 2, TNFR2, lipoprotein-associated phospholipase A2 mass and activity, osteoprotegerin, and cluster of differentiation 40 ligand), the assessments were done using plasma samples while the rest of the biomarkers were assessed using serum samples. Several biomarkers were measured with commercially available ELISA kits as earlier described (Shoamanesh et al., 2015) from R&D Systems (Minneapolis, MN; ICAM-1, interleukin-6, monocyte chemoattractant protein-1, P-selectin, TNFR2, TNFα, vascular endothelial growth factor), Bender MedSystems (Vienna, Austria; CD40 ligand), and Oxis International Inc. (Beverly Hills CA; myeloperoxidase). C-reactive protein was measured using high sensitivity assay (BN 100 nephelometer; Dade Behring, Deerfield, IL), and fibrinogen by the Clauss method (Diagnostica Stago Inc., Parsippany, NJ). Lp-PLA2 activity was measured using a colorimetric activity method (diaDexus Inc., San Francisco, CA). Lp-PLA2 mass was measured using a commercially available sandwich enzyme immunoassay (diaDexus Inc.). Total homocysteine was measured with high-performance liquid chromatography with fluorometric detection. The intra-assay coefficient of variation for the biomarkers were as follows: CD40L 4.4%, IL-6 3.1%. ICAM-1 3.1%, MCP-1 4.1%, MPO 3.0%, OPG 3.7%, P-selectin 3.0%, TNFα 8.8%, TNFR2 2.3%, VEGF 3.9% (Jefferson et al., 2007; Pikula et al., 2013).
Other clinical characteristics
Demographic and clinical characteristics were extracted from the exam cycle closest to MRI assessment, either one year before or up to five years after. Systolic and diastolic blood pressures (mm Hg) were each taken as the average of the FHS clinic physician’s two measurements as previously documented (Shoamanesh et al., 2015). Readings were rounded to the nearest even number. Hypertension was defined using JNC-7 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) criteria as systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medications (Shoamanesh et al., 2015). We defined prevalent diabetes mellitus as a fasting blood glucose of ≥126 mg/dL or use of oral hypoglycemic agents or insulin. Current smoking status is self-reported smoking of at least one cigarette per day within the year preceding examination; use of statins and other medications was also self-reported (Shoamanesh et al., 2015).
Statistical Analysis
We obtained descriptive statistics for clinical and demographic characteristics, and inflammation biomarkers. Biomarkers were loge-transformed and standardized to mean zero and standard deviation of one for analysis. The three outcomes of interest were basal ganglia PVS (grades I-IV), centrum semiovale PVS (grades I-IV), and the high burden basal ganglia-centrum semiovale PVS mixed score (scores 0-2), all of which were treated as ordinal variables.
Multivariable ordinal logistic regression analyses were used to obtain odds ratios and 95% confidence intervals for the association between each biomarker and outcome and assumed proportionality of the odds. To account for the dependence of multiple observations per participant, robust standard error estimates were obtained. Our primary model adjusted for age at MRI, sex, FHS cohort, and time interval between biomarker ascertainment and MRI acquisition. A second model (model 2) additionally adjusted for vascular risk factors such as hypertension, diabetes, and lipid lowering medication. Model 3, in addition to the vascular risk factors, also adjusted for white matter hyperintensity volume using FLAIR and cerebral microbleeds in cases where those biomarkers were available. We evaluated if the MRI view used (coronal vs. axial) influenced results. We did not observe any differences and thus this variable was removed from the models.
Multivariable logistic regression was also used to explore associations between each biomarker and high burden (grades III-IV) PVS in strictly the centrum semiovale (excluding any participants with concurrent high burden in the basal ganglia) and high burden strictly in the basal ganglia (excluding any participants with concurrent high burden in the centrum semiovale) relative to low burden in the respective region. The same methods used to evaluate the ordinal outcomes were also used for these binary outcomes.
Lastly, we conducted a principal component analysis (PCA) to assess if some of the inflammatory biomarkers cluster together in their relation to PVS topography and burden, and to inform a potential “composite” assessment of inflammation in relation to PVS. This analysis was restricted to inflammatory markers with sufficient sample size (N=1500 or greater). As a sensitivity analysis, we included all biomarkers with sufficient sample sizes in one model to assess whether any particular biomarker was independently associated PVS, also adjusting for clinical covariates.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Forest plots were created in R using the meta package. A p-value < 0.05 was considered statistically significant.
Results
Our sample represents middle-aged to older participants (mean age 58.4 ± 13.1 years) with balanced inclusion of women and men and overall favorable vascular risk factor characteristics (Table 1). As previously reported in FHS participants (Lara et al., 2022), we observed higher mean age and prevalence of vascular risk as PVS burden increased in the basal ganglia and centrum semiovale regions. With a few exceptions, we mostly observed that levels of inflammatory markers increased with higher burden of enlarged periventricular spaces in both the basal ganglia and centrum semiovale. (Supplementary Tables 1 & 2). Nine biomarkers had sample sizes greater than 1500 (IL-6,OPG, P-selectin, CRP, ICAM-1, LpPLA2 activity and mass, MCP-1, and TNFR2).
Table 1.
Characteristics of the study sample.
| Clinical Characteristics | Study Sample N= 4456 |
Excluded N= 334 |
|
|---|---|---|---|
| Male, n (%) | 2087 (47) | 179 (54) | |
| Age at exam closest to baseline MRI, years, mean (SD) | 58.4 (13) | 65.2 (12.0) | |
| Age at MRI, years, mean (SD) | 60.2 (12.9) | 66.9 (11.8) | |
| APOE ε4+, n (%) | 1000 (23) | 91 (29) | |
| FHS Cohort, n (%) | |||
| Offspring | 2493 (56) | 240 (72) | |
| NOS/Third Generation | 1844 (41) | 60 (18) | |
| OMNI 1 | 119 (3) | 34 (10) | |
| Time between biomarker measurement and MRI, years, mean (SD) | 1.4 (1.0) | 1.2 (1.1) | |
| Vascular Risk Factors | |||
| Systolic blood pressure, mmHg, mean (SD) | 122.0 (16.0) | 128.0 (20.0) | |
| Diastolic blood pressure, mmHg, mean (SD) | 74.0 (10.0) | 72.0 (10.0) | |
| Total cholesterol, mg/dl, mean (SD) | 189.0 (36.0) | 182.0 (34.0) | |
| High density lipoprotein, mg/dl, mean (SD) | 59.0 (18.0) | 57.0 (18.0) | |
| Low density lipoprotein, mg/dl, mean (SD) | 107.0 (31.0) | 101.0 (29.0) | |
| Triglycerides, mg/dl, mean (SD) | 117.0 (71.0) | 125.0 (71.0) | |
| Fasting blood glucose, mg/dl, mean (SD) | 101.0 (22.0) | 106.0 (27.0) | |
| Hypertension,a n (%) | 1783 (40) | 191 (59) | |
| Current smoker, n (%) | 361 (8) | 31 (9) | |
| Diabetes mellitus, n (%) | 457 (10) | 49 (18) | |
| Antihypertensive use, n (%) | 1449 (33) | 163 (50) | |
| Lipid lowering medication, n (%) | 1295 (29) | 142 (43) | |
| Basal Ganglia PVS, n (%) | |||
| Grade I | 2303 (52) | 113 (34) | |
| Grade II | 1808 (41) | 160 (48) | |
| Grade III | 308 (7) | 51 (15) | |
| Grade IV | 37 (1) | 10 (3) | |
| Centrum Semiovale PVS, n (%) | |||
| Grade I | 1840 (41) | 79 (24) | |
| Grade II | 1924 (43) | 130 (39) | |
| Grade III | 575 (13) | 92 (28) | |
| Grade IV | 117 (3) | 33 (10) | |
| CSO-BG Mixed Scoreb, n (%) | |||
| None | 3624 (81) | 200 (60) | |
| One | 627 (14) | 82 (25) | |
| Both | 205 (5) | 52 (16) | |
|
Biomarkers, median (25
th
, 75
th
Percentile) |
N | ||
| Fibrinogen, mg/dl | 857 | 373.0 (331.0, 422.0) | |
| Interleukin 6, pg/mL | 1534 | 2.3 (1.5, 3.8) | |
| Homocysteine, μmol/L | 856 | 8.1 (6.7, 9.8) | |
| Osteoprotegerin, pmol/L | 1535 | 5.1 (4.2, 6.2) | |
| P-selectin, ng/mL | 1538 | 37.3 (30.0, 46.0) | |
| C-reactive protein, mg/L | 4440 | 1.5 (0.7, 3.3) | |
| Intercellular adhesion molecule 1, ng/L | 1524 | 260.1 (223.6, 316.3) | |
| Isoprostane, pg/mL | 1222 | 1094.1 (618.1, 1655.7) | |
| Lipoprotein-associated phospholipase A2 activity, nmol/mL/min | 1530 | 139.1 (116.8, 162.2) | |
| Lipoprotein-associated phospholipase A2 mass, ng/mL | 1530 | 231.2 (189.8, 301.7) | |
| Monocyte chemoattractant protein-1, pg/mL | 1508 | 344.9 (276.3, 422.8) | |
| Tumor necrosis factor receptor 2, pg/mL | 1508 | 2216.1 (1830.8, 2748.7) | |
| Tumor necrosis factor α, pg/mL | 567 | 1.3 (1.0, 1.6) | |
| Vascular endothelial growth factor A, pg/ml | 767 | 281.2 (161.8, 440.6) | |
| CD40 ligand, ng/mL | 856 | 1.2 (0.5, 3.6) | |
| Myeloperoxidase, ng/mL | 833 | 40.7 (27.3,61.4) |
BG = basal ganglia; CSO = centrum semiovale; FHS = Framingham Heart Study; MRI = magnetic resonance imaging; NOS = New Offspring Spouse; PVS = perivascular spaces; SD=standard deviation
Biomarkers are not shown for the excluded group due to large amounts of missing data
Hypertension is defined as SBP ≥140 mmHg or DBP ≥90 mmHg and/or use of antihypertensive medication
Number of CSO-BG regions with high PVS burden (0=none, 1=one region, or 2=both regions); high burden is defined as grade III or IV PVS
Isoprostane, CD40 ligand, myeloperoxidase, and lipoprotein-associated phospholipase A2 mass showed a U-shaped pattern across PVS burden categories in the BG (Supplementary Table 1). In the centrum semiovale, we observed that isoprostane, lipoprotein-associated phospholipase A2 activity, and myeloperoxidase showed a U–shaped pattern across PVS burden categories, while intercellular adhesion molecule, vascular endothelial growth factor A, and CD40 ligand showed an inverted U-shaped pattern with higher level of PVS burden (Supplementary Table 2).
Furthermore, we observed that levels of interleukin 6, homocysteine, osteoprotegerin, P-selectin, C-reactive protein, intracellular adhesion molecule 1, tumor necrosis factor receptor 2, and monocyte chemoattractant protein 1 increased with the number of regions with high PVS burden (Supplementary Table 3).
Spearman correlations of the nine inflammatory biomarkers with sufficient sample size (>1500) showed weak correlation between C-reactive protein and interleukin 6 (both markers of systemic inflammation) and between lipoprotein-associated phospholipase A2 mass and activity. The remaining biomarkers did not show substantial correlation (Supplementary Table 4).
Multivariable analyses
In multivariable adjusted analyses, we observed differing associations of systemic, vascular and oxidative stress biomarkers with PVS according to brain topography.
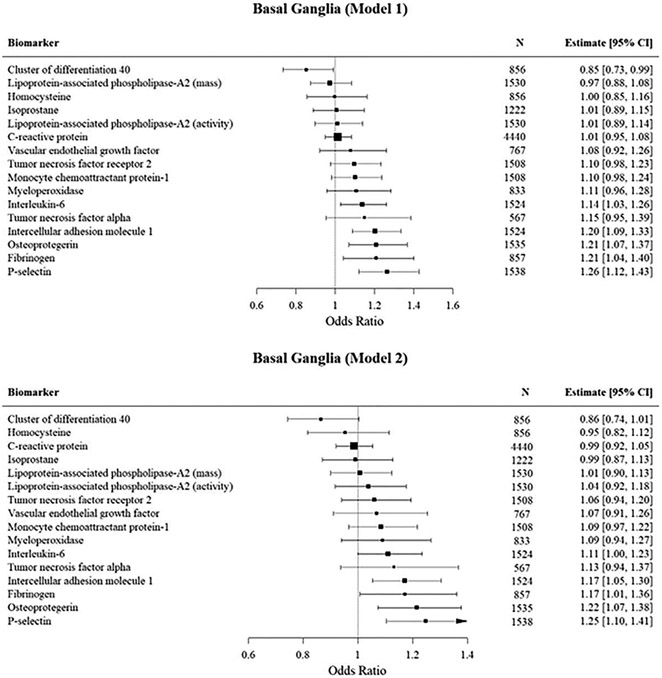
Basal Ganglia (Figure 2).
Figure 2. Mutivariable logistic regression analyses of the association of inflammatory biomarkers with PVS burden in the Basal Ganglia.
Estimates are for one unit increase in the standardized log transformed biomarker.
In our primary analysis (model 1) we observed that fibrinogen, IL-6, osteoprotegerin, P-selectin, and intercellular adhesion molecule 1 were associated with high PVS burden in the basal ganglia. After adjusting for vascular risk factors, the associations remained significant for fibrinogen, osteoprotegerin, P-selectin and intercellular adhesion molecule 1. In our subsequent model adjusting for white matter hyperintensity volume and cerebral microbleeds, we observed significant associations with osteoprotegerin and P-selectin while the effect of intercellular adhesion molecule 1 was attenuated. We had insufficient data to assess the effect of this adjustment on fibrinogen.
In contrast, higher CD40L levels were inversely associated with high PVS burden in the primary model though we did not observe a significant association after adjusting for vascular risk factors.
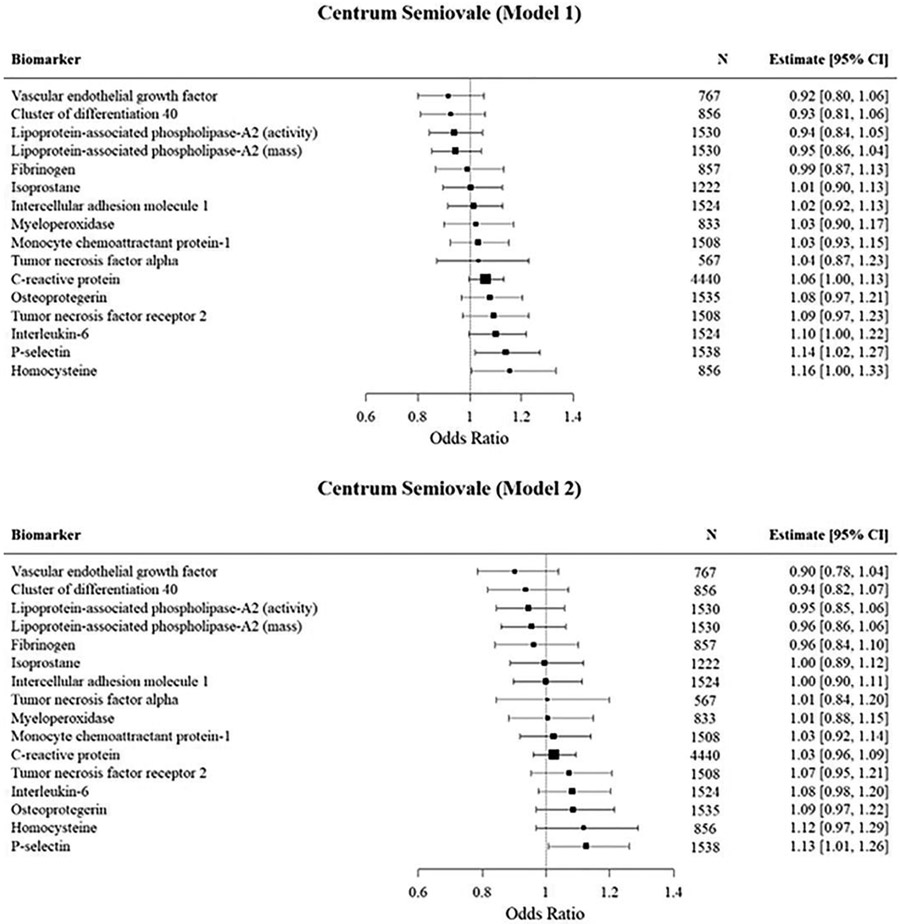
Centrum semiovale (Figure 3)
Figure 3:
Mutivariable logistic regression analyses of the association of inflammatory biomarkers with high PVS burden in the Centrum semiovale.
In our primary analysis (model 1) we observed significant associations of P-selectin and homocysteine with high PVS burden in the centrum semiovale. The associations were attenuated in model 2 but remained significant for P-selectin. However, the effect of P-selectin was attenuated in our model after adjusting for white matter hyperintensity volume and cerebral microbleeds.
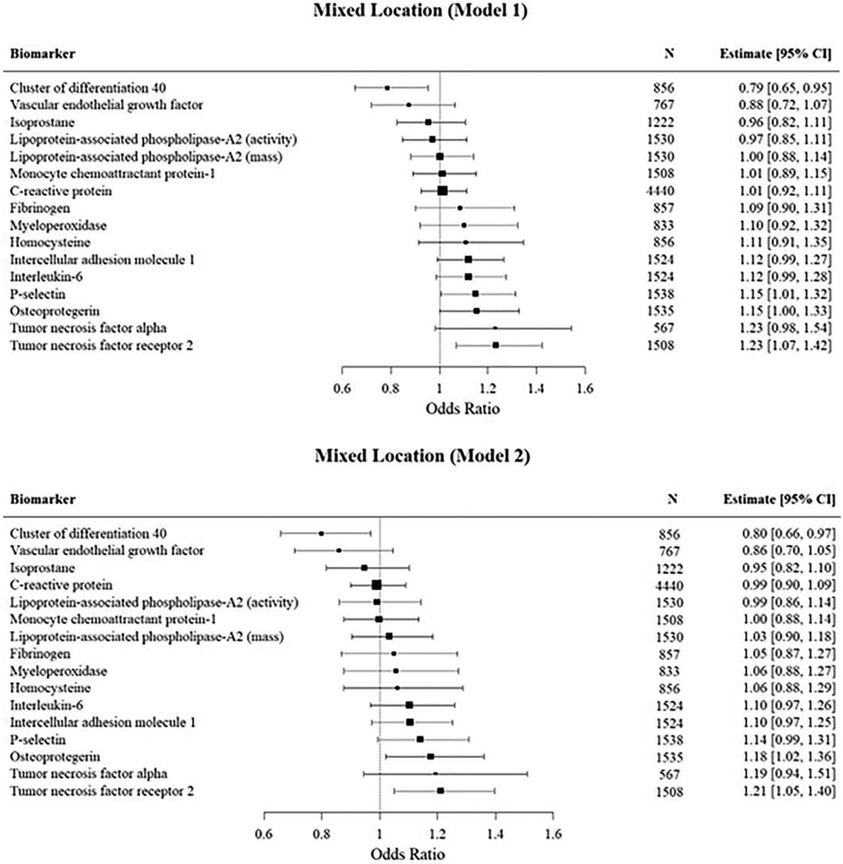
Mixed score high PVS burden (Figure 4)
Figure 4:
Mutivariable logistic regression analyses of the association of inflammatory biomarkers with mixed score PVS.
In our primary analysis (model 1) we observed significant associations with P-selectin, tumor necrosis factor receptor 2, and osteoprotegerin with mixed score high PVS burden. After adjusting for vascular risk factors, only tumor necrosis factor receptor 2 and osteoprotegerin remained significant. However, these associations were attenuated after adjusting for white matter hyperintensity volume and cerebral microbleeds. On the other hand, we observed an inverse association between CD40L and high PVS burden in both model 1 and model 2.
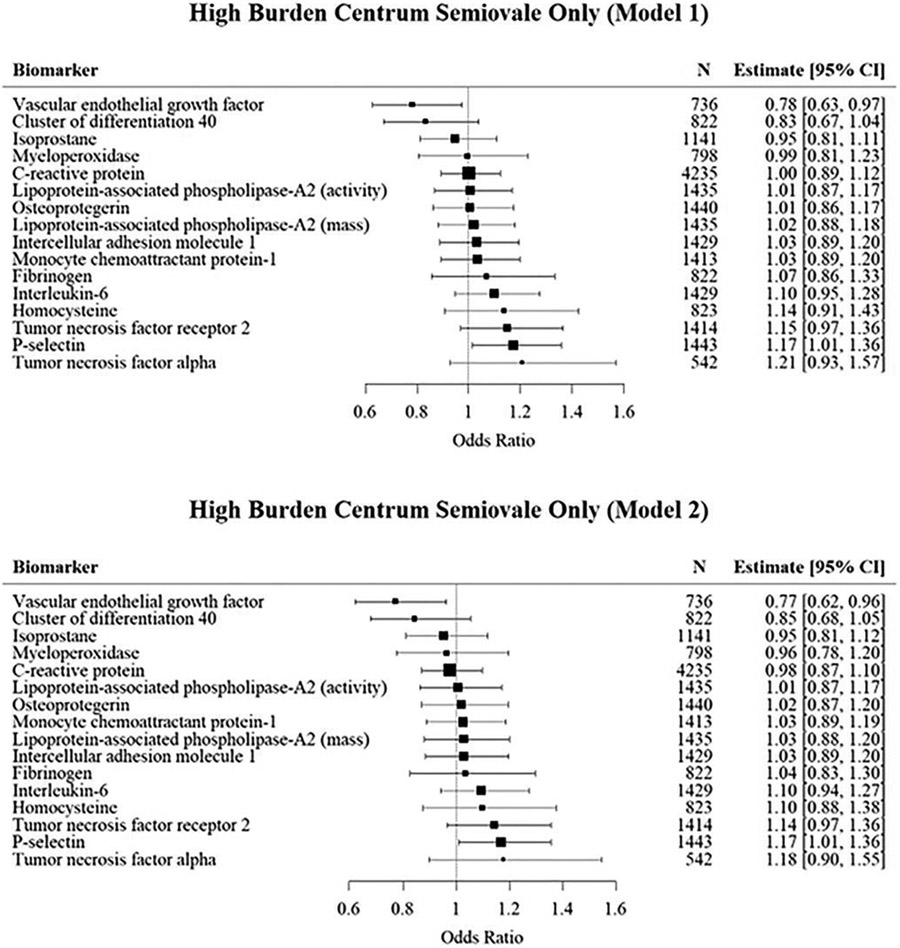
Strictly centrum semiovale high PVS burden (Figure 5)
Figure 5:
Mutivariable logistic regression analyses of the association of inflammatory biomarkers with high burden PVS in the centrum semiovale.
We separately assessed a subgroup of strictly high PVS burden in the centrum semioval that excluded participants with concurrent high PVS burden in the basal ganglia, which presumably reflects CAA (Kim et al., 2021) to evaluate if different relations of inflammatory markers with PVS were observed. In our primary analysis (model 1) we observed a borderline significant association with P-selectin, which was mildly attenuated in model 2. On the other hand, we observed an inverse association of vascular endothelial growth factor A with high burden PVS strictly in the CSO in both the primary and secondary models.
Due to the small number of records with strictly high PVS burden in the basal ganglia (N=140), meaningful associations with most biomarkers could not be evaluated.
Principal components analysis
Results from the principal component analysis included nine biomarkers with sample sizes greater than 1500. We retained three principal components (PC) with eigenvalues ≥1 which explained 55% of the total variability in the biomarkers. The biomarkers with high loadings on the PC were as follows: Factor 1: IL-6, OPG, and CRP; Factor 2: P-Selectin, ICAM-1, MCP-1, and TNFR2; and Factor 3: LpPLA2 activity and mass. Factor 1 and factor 2 were significantly associated with basal ganglia PVS, but only factor 2 remained significant after adjusting for vascular risk factors, while the association of factor 1 was borderline significant. These results were consistent with our main findings where P-Selectin, OPG, ICAM-1 were significant in Model 2 (IL-6 was borderline significant).
Neither of the three factors was significant in the centrum semiovale, which was also consistent with our main findings, where only P-Selectin was associated with CSO PVS in both models 1 and 2.
In the mixed CS-BG regions, factor 1 and 2 were significant. After vascular risk factor adjustment, only factor 2 remained significant while factor 1 showed a borderline association. Again, these results were consistent with the main findings where we observed a significant association with TNFR2, OPG, P-Selectin.
Sensitivity analysis
P-selectin, OPG, and ICAM were independently associated with PVS in the basal ganglia in both the primary and secondary models. In the centrum semiovale, only P-selectin was significantly associated with PVS after adjustment for the other biomarkers and vascular risk factors. No biomarkers were independently associated with PVS in the mixed regions.
These results were consistent with our main findings where P-Selectin, OPG, and ICAM were associated with BG PVS and where only P-selectin was associated in the CSO. We observed a significant association with TNFR2 in our main findings in the mixed regions but after adjustment for the other biomarkers, the association was borderline significant (p=0.08).
Discussion
In our community-based study of stroke-free and dementia-free participants, we observed that several plasma inflammatory markers were associated with high PVS burden. We found both differing and shared associations of biomarkers with PVS burden based on brain topography. While some of these associations were attenuated after adjustment for vascular risk factors and other markers of cerebral small vessel disease (white matter hyperintensity volume and cerebral microbleeds), some remained significant, suggesting a relation of inflammatory markers through mechanisms other than common vascular risk factors. Given the attributed role of PVS as markers of cerebral small vessel disease and possibly perivascular drainage dysfunction, our results suggest a role of inflammation in these processes.
Our study expands knowledge in the field by examining the relation between a comprehensive panel of inflammatory biomarkers and PVS burden in a large community dwelling cohort of middle-aged adults. Previous population-based studies have examined the relation of inflammatory biomarkers and markers of cerebral small vessel disease. For instance, population studies have examined the relation of IL6 and CRP supporting their associations with white matter hyperintensities and brain infarcts. In the Rotterdam study, high levels of CRP were associated with more severe periventricular and subcortical white matter lesions, progression of white matter disease, and development of new lacunar infarcts (van Dijk et al., 2005). In the 3C-Dijon Study, higher levels of IL-6 were independently associated with higher total and periventricular white matter hyperintensities and low gray matter volumes, while the effect of CRP was attenuated with adjustments for vascular risk factors (Satizabal et al., 2012). The Cardiovascular Health Study reported independent associations of circulating IL6 and CRP with white matter lesions and brain infarcts (Fornage et al., 2008).
In contrast to the Rotterdam study (van Dijk et al., 2005), our sample was younger, thus extending results to involve inflammation’s role to middle adult age. Similar to other population-based studies (van Dijk et al., 2005; Fornage et al., 2008; Satizabal et al., 2012), we observed that IL-6 was associated with CSVD as evidenced by PVS in the basal ganglia, though this effect was attenuated after adjustments for vascular risk factors unlike the previous studies. The comprehensive panel of inflammatory markers we studied also represent key aspects of the pathophysiology of vascular disease including endothelial dysfunction, systemic inflammation and thrombogenesis.
Prior reports suggest that the topographic distribution of high PVS burden may reflect a different underlying type of CSVD: hypertensive arteriopathy in relation to basal ganglia PVS, cerebral amyloid angiopathy in relation to centrum semi ovale PVS, and advanced hypertensive arteriopathy in both brain regions (Charidimou et al., 2013). In the basal ganglia, markers of endothelial dysfunction, systemic inflammation and thrombogenesis were related to PVS, which are aspects of vascular injury associated with hypertensive arteriopathy (Charidimou et al., 2013; Hadi et al., 2005; Iadecola and Davisson, 2008). Endothelial dysfunction associated with systemic inflammation and hypertension has been related to disruption of the blood brain barrier with subsequent leakage of plasma proteins into the perivascular space, impaired vasodilation, impaired myelination and myelin repair, and obstruction of perivascular fluid drainage (Wardlaw et al., 2019). Our observation of the association of biomarkers representing endothelial dysfunction and systemic inflammation with PVS in the basal ganglia even after adjustments for vascular risk factors and in our composite analysis supports earlier findings that the pathogenesis of cerebral small vessel disease is complex and may occur despite hypertension (Wardlaw et al., 2019).
Recently, markers of neutrophil activation-like myeloperoxidase have been implicated in the pathogenesis of cerebral small vessel disease (Karel et al., 2022). Neutrophils and nuclear DNA expelled by neutrophils have inflammatory compounds that induce endothelial dysfunction (Karel et al., 2022). Our study showed higher mean levels of myeloperoxidase with high grade PVS in the basal ganglia although the associations did not reach statistical significance. The small sample size of participants with high grade PVS in the basal ganglia may have affected these findings.
We observed an inverse relation of CD40L with PVS burden in the basal ganglia, an apparent paradoxical protective association given that CD40L promotes amyloid β deposition (Chen et al., 2006) and is considered to promote deleterious inflammatory effects. A potential factor in this result is the higher prevalence of smoking in participants with lower PVS basal ganglia burden (i.e., relative lower risk in high burden PVS) or residual confounding (Liang et al., 2018).
In the centrum semiovale, our finding of high mean value of homocysteine and IL-6 with centrum semiovale PVS in the unadjusted model support earlier findings (Guthikonda and Haynes, 2006). Homocysteine causes oxidative stress (Guthikonda and Haynes, 2006), neuroinflammation, may accelerate neurodegeneration, and impairs vasodilatation (Smith, 2018) with resultant deposition of Aβ in perivascular spaces (Kumar-Singh, 2008).
Higher concentrations of P-selectin were associated with PVS burden in both the basal ganglia and centrum semiovale regions in multivariable adjusted analysis and in our composite analysis suggesting diffuse brain effects of P-selectin. P-selectin is considered a marker of endothelial dysfunction (Wang et al., 2012), thus implicating endothelial dysfunction in PVS in both brain regions independent of vascular risk factors.
Analyses of strictly high burden PVS in the centrum semiovale, presumably reflecting cerebral amyloid angiopathy (Kim et al., 2021), showed an inverse association of VEGF with high PVS burden in the centrum semiovale. Despite mixed evidence of the role of VEGF in the context of Alzheimer’s disease and dementia (Chiappelli et al., 2006; Tarkowski et al., 2002), our findings align with growing evidence of a possible neuroprotective effect (Mahoney et al., 2021). This is similar to findings by Hohman and colleagues, who observed that higher baseline cerebrospinal fluid VEGF levels were associated with slower rates of hippocampal atrophy and cognitive decline (Hohman et al., 2015). We were unable to explore the effects of biomarkers strictly in the basal ganglia due to the small number of participants in this category.
Our observation of a protective effect of CD40L in the unadjusted model mirrors our findings in the basal ganglia, as previously discussed. In addition, high mean level of tumor necrosis factor receptor 2 in this region may suggest a generalized proinflammatory effect of TNR2 (Medler and Wajant, 2019).
Clinical relevance
Chronic inflammation has been described as an underlying mechanism in several neurological disorders (Lee et al., 2012; Perkins et al., 2019). Our results support the association of inflammation with high perivascular space burden detected on brain MRI. PVS as marker of CSVD and possibly perivascular drainage dysfunction may be an attractive marker in the pathway to clinical stroke and dementia. Several of the inflammatory markers we report may be potential treatment targets for cerebral small vessel disease. For instance, early expression of P-selectin during endothelial activation makes it a potent biomarker for early pathologic changes and to monitor disease treatment (Perkins et al., 2019). Animal studies show beneficial effects of antibodies against P-selectin in preventing endothelial dysfunction and visceral fat inflammation in mice models with metabolic syndrome (Lee et al., 2012; Wang et al., 2012). Our observation of P-selectin as a common biomarker associated with PVS in both the basal ganglia and centrum semiovale calls for further confirmatory studies, which may provide a compelling rationale for consideration of P-selectin inhibitors as disease modifying agents against PVS related disorders and clinical outcomes.
Strengths and limitations
Among the strengths of our study are the evaluation of a comprehensive panel of inflammatory biomarkers, use of reliable PVS ratings performed blinded to all other data, acquisition of exposure and outcomes blinded to each other, the large sample included and detailed prospective assessment of Framingham Heart Study participants.
Due to the cross-sectional and observational design of the study, we are unable to establish the causal role of the biomarkers with PVS. FHS participants attend exam cycles at a time when they are free of acute illnesses, and we excluded individuals with conditions known to be associated with prominent inflammation such as multiple sclerosis and history of encephalitis. Given that most of the inflammatory biomarkers in our study with significant association to PVS showed modest associations, our study should be considered as hypothesis generating as we did not adjust for multiple comparisons. Thus, further studies are required to replicate our findings.In addition, our analyses were limited by the availability of data for each biomarker. For example, we only had 567 records with TNFα data, in contrast to the 4440 records with CRP data. This may introduce bias arising from sub setting our sample due to the missing data. Another limitation is the measurement of inflammatory biomarkers was not simultaneous with MRI acquisition. We adjusted our analysis for the time interval between both assessments, but further studies are needed to assess longitudinal changes in the relation of inflammatory biomarkers and PVS including measurements at the time of MRI acquisition. Lastly, our study participants were mainly of white European descent, thus limiting generalizability of our findings to other racial or ethnic groups.
Conclusions
Our study supports that inflammation is associated with PVS as marker of cerebral small vessel disease and possibly glymphatic dysfunction. Several associations were significant after adjustment for vascular risk factors suggesting that inflammatory markers may also relate to PVS through other mechanisms. In addition, there are shared and differing predominant inflammatory biomarkers in relation to basal ganglia and centrum semiovale PVS burden. Future studies will clarify the potential role of PVS as mediators in the pathway from inflammation to adverse cognitive outcomes and stroke. Our study may spur further longitudinal studies to assess the role of these biomarkers of inflammation as treatment targets for prevention of CSVD and related outcomes.
Supplementary Material
Highlights.
Inflammation is associated with PVS regardless of brain topography.
Inflammatory biomarkers may be related to PVS independent of vascular risk factors.
Some inflammatory biomarkers related to PVS may vary depending on brain topography
Other inflammatory biomarkers may be related to PVS regardless of their topography.
Inflammatory biomarkers could represent potential treatment targets for CSVD
Funding
This work (design and conduct of the study, collection and management of the data) was supported by the Framingham Heart Study’s National Heart, Lung, and Blood Institute contract (N01-HC-25195; HHSN268201500001I) and by grants from the National Institute of Neurological Disorders and Stroke (R01-NS017950-37; UF1 NS125513), the National Institute on Aging (R01 AG059725; AG008122; AG054076; K23AG038444; R03 AG048180-01A1; AG033193; AG054076; AG049607; RF1 AG059421; RF1 AG063507); NIH grant (P30 AG010129; P30 AG066546). Dr. Benjamin is funded by 1RO1 HL64753; R01 HL076784; 1 R01 AG028321; R01HL092577.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interest
The authors do not have any potential conflict of interest to disclose.
References
- Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, Ayres A, Schwab KM, Martinez-Ramirez S, Goldstein JN, Rosand J, Viswanathan A, Greenberg SM, Gurol ME, 2017. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 88(12), 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, Baron JC, Jager HR, Werring DJ, 2013. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry 84(6), 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Gong W, Zhang L, Yu P, Wang JM, 2006. CD40/CD40L dyad in the inflammatory and immune responses in the central nervous system. Cell Mol Immunol 3(3), 163–169. [PubMed] [Google Scholar]
- Chiappelli M, Borroni B, Archetti S, Calabrese E, Corsi MM, Franceschi M, Padovani A, Licastro F, 2006. VEGF gene and phenotype relation with Alzheimer's disease and mild cognitive impairment. Rejuvenation Res 9(4), 485–493. [DOI] [PubMed] [Google Scholar]
- Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM, 2010. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41(3), 450–454. [DOI] [PubMed] [Google Scholar]
- Fletcher E, Carmichael O, Decarli C, 2012a. MRI non-uniformity correction through interleaved bias estimation and B-spline deformation with a template. Conf Proc IEEE Eng Med Biol Soc 2012, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, DeCarli C, Fan AP, Knaack A, 2021. Convolutional Neural Net Learning Can Achieve Production-Level Brain Segmentation in Structural Magnetic Resonance Imaging. Front Neurosci 15, 683426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Singh B, Harvey D, Carmichael O, Decarli C, 2012b. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf Proc IEEE Eng Med Biol Soc 2012, 5319–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, Tracy RP, Longstreth WT Jr., 2008. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain: The Cardiovascular Health Study. Stroke 39(7), 1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM, Microbleed Study G, 2009. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8(2), 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Gutierrez J, Meier IB, Guzman VA, Manly JJ, Schupf N, Brickman AM, Mayeux R, 2019. Circulating inflammatory biomarkers are related to cerebrovascular disease in older adults. Neurol Neuroimmunol Neuroinflamm 6(1), e521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthikonda S, Haynes WG, 2006. Homocysteine: role and implications in atherosclerosis. Curr Atheroscler Rep 8(2), 100–106. [DOI] [PubMed] [Google Scholar]
- Hadi HA, Carr CS, Al Suwaidi J, 2005. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1(3), 183–198. [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Bell SP, Jefferson AL, Alzheimer's Disease Neuroimaging, I., 2015. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol 72(5), 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL, 2008. Hypertension and cerebrovascular dysfunction. Cell Metab 7(6), 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, Larson MG, Meigs JB, Keaney JF Jr., Lipinska I, Kathiresan S, Benjamin EJ, DeCarli C, 2007. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology 68(13), 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karel MFA, Roosen M, Tullemans BME, Zhang CE, Staals J, Cosemans J, Koenen RR, 2022. Characterization of cerebral small vessel disease by neutrophil and platelet activation markers using artificial intelligence. J Neuroimmunol 367, 577863. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Cho H, Park M, Kim JW, Ahn SJ, Lyoo CH, Suh SH, Ryu YH, 2021. MRI-Visible Perivascular Spaces in the Centrum Semiovale Are Associated with Brain Amyloid Deposition in Patients with Alzheimer Disease-Related Cognitive Impairment. AJNR Am J Neuroradiol 42(7), 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, 2008. Cerebral amyloid angiopathy: pathogenetic mechanisms and link to dense amyloid plaques. Genes Brain Behav 7 Suppl 1, 67–82. [DOI] [PubMed] [Google Scholar]
- Lara FR, Scruton AL, Pinheiro A, Demissie S, Parva P, Charidimou A, Francis M, Himali JJ, DeCarli C, Beiser A, Seshadri S, Romero JR, 2022. Aging, prevalence and risk factors of MRI-visible enlarged perivascular spaces. Aging (Albany NY) 14(undefined). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Chait A, Kim F, 2012. P-selectin glycoprotein ligand-1: a cellular link between perivascular adipose inflammation and endothelial dysfunction. Diabetes 61(12), 3070–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Shen Y, Kuang L, Zhou G, Zhang L, Zhong X, Zhang J, Liu J, 2018. Cigarette smoke exposure promotes differentiation of CD4(+) T cells toward Th17 cells by CD40-CD40L costimulatory pathway in mice. Int J Chron Obstruct Pulmon Dis 13, 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney ER, Dumitrescu L, Moore AM, Cambronero FE, De Jager PL, Koran MEI, Petyuk VA, Robinson RAS, Goyal S, Schneider JA, Bennett DA, Jefferson AL, Hohman TJ, 2021. Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer's disease. Mol Psychiatry 26(3), 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Lu H, Arfanakis K, Gold BT, Bauer CE, Zachariou V, Stables L, Wang DJJ, Jann K, Seshadri S, Duering M, Hillmer LJ, Rosenberg GA, Snoussi H, Sepehrband F, Habes M, Singh B, Kramer JH, Corriveau RA, Singh H, Schwab K, Helmer KG, Greenberg SM, Caprihan A, DeCarli C, Satizabal CL, Mark VC, 2022. Instrumental validation of free water, peak-width of skeletonized mean diffusivity, and white matter hyperintensities: MarkVCID neuroimaging kits. Alzheimers Dement (Amst) 14(1), e12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro JM, D'Agostino RB Sr., Sullivan LM, Beiser A, DeCarli C, Au R, Elias MF, Wolf PA, 2004. Managing and analysing data from a large-scale study on Framingham Offspring relating brain structure to cognitive function. Stat Med 23(2), 351–367. [DOI] [PubMed] [Google Scholar]
- Medler J, Wajant H, 2019. Tumor necrosis factor receptor-2 (TNFR2): an overview of an emerging drug target. Expert Opin Ther Targets 23(4), 295–307. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Anderson CJ, Novelli EM, 2019. Targeting P-Selectin Adhesion Molecule in Molecular Imaging: P-Selectin Expression as a Valuable Imaging Biomarker of Inflammation in Cardiovascular Disease. J Nucl Med 60(12), 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikula A, Beiser AS, Chen TC, Preis SR, Vorgias D, DeCarli C, Au R, Kelly-Hayes M, Kase CS, Wolf PA, Vasan RS, Seshadri S, 2013. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke 44(10), 2768–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM, 2015. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke 10(3), 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, Kase CS, Wolf PA, Seshadri S, 2014. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 45(5), 1492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C, 2012. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 78(10), 720–727. [DOI] [PubMed] [Google Scholar]
- Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, Wolf PA, DeCarli C, Romero JR, Seshadri S, 2015. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology 84(8), 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, 2018. Cerebral amyloid angiopathy as a cause of neurodegeneration. J Neurochem 144(5), 651–658. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P, 2002. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer's disease and vascular dementia. Neurobiol Aging 23(2), 237–243. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Pasi M, Tsai LK, Huang CC, Chen YF, Lee BC, Yen RF, Gurol ME, Jeng JS, 2021. Centrum Semiovale Perivascular Space and Amyloid Deposition in Spontaneous Intracerebral Hemorrhage. Stroke 52(7), 2356–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ,Breteler MM, 2005. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 112(6), 900–905. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo W, Wang J, Guo C, Wang X, Wolffe SL, Bodary PF, Eitzman DT, 2012. Obesity-induced endothelial dysfunction is prevented by deficiency of P-selectin glycoprotein ligand-1. Diabetes 61(12), 3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith C, Dichgans M, 2013a. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12(5), 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith C, Dichgans M, 2019. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 18(7), 684–696. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M, nEuroimaging, S.T.f.R.V.c.o., 2013b. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12(8), 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, Zipp F, Paul F, 2008. Perivascular spaces--MRI marker of inflammatory activity in the brain? Brain 131(Pt 9), 2332–2340. [DOI] [PubMed] [Google Scholar]
- Yang S, Yuan J, Zhang X, Fan H, Li Y, Yin J, Hu W, 2017. Higher ambulatory systolic blood pressure independently associated with enlarged perivascular spaces in basal ganglia. Neurol Res 39(9), 787–794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.