Abstract
Background
Systemic postnatal corticosteroid use in extremely preterm infants poses a risk of adverse neurodevelopmental outcomes. This study explores their use beyond seven days of age with early neurodevelopmental assessments during the fidgety period (9–20 weeks postterm age).
Methods
This retrospective single-center cohort study included inborn extremely preterm infants from 1 January 2014 to 31 December 2018. Outborn infants, those with congenital or genetic abnormalities, and those who received postnatal corticosteroids for nonrespiratory reasons were excluded. The cohort was dichotomized based on the status of corticosteroid receipt. Early neurodevelopmental outcomes were reported using Prechtl’s General Movements Assessment.
Results
Of the 282 infants, 67 (23.75%) received corticosteroids. Of these, 34 (50.75%) received them for dependency on invasive ventilation (intermittent positive-pressure ventilation), and the remainder received them for dependency on non-invasive ventilation continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP). Abnormal or absent fidgety movements were observed in 13% of infants (7/54) who received corticosteroids compared to 2% of infants (3/146) who did not. An increased odds for an abnormal general movements assessment from corticosteroid use after adjusting for gestational age [adjusted odds ratio (aOR) = 5.5, 95% confidence interval (CI) = 1.14–26.56] was observed. The motor optimality scores differed between the two groups [corticosteroid group: 25.5 (23–26) versus no-corticosteroid group: 26 (24–28); z = − 2.02]. A motor optimality score < 20 was observed in 14.8% of infants (8/54) in the corticosteroid group compared to 2% of infants (3/146) in the noncorticosteroid group. This difference was significant after adjustment for gestational age (aOR 5.96, 95% CI 1.28–27.74).
Conclusions
Abnormal early neurodevelopment was observed in infants who received systemic postnatal corticosteroids. The relationship between these findings and other factors influencing early neurodevelopment needs further exploration.
Keywords: Corticosteroids, Early neurodevelopment, General movement assessment, Preterm infants
Introduction
Bronchopulmonary dysplasia (BPD) is the most prevalent morbidity in convalescing preterm infants [1–3]. In Australia and New Zealand, it affects approximately 50% of extremely preterm infants (born < 28 weeks gestational age) [1, 4–8]. For this study, the Australian and New Zealand Neonatal Network's definition for BPD was used. BPD was defined as the ongoing need for supplemental oxygen and/or respiratory support at or beyond 36 weeks postmenstrual age (PMA) [1]. Increased survival of extremely preterm infants and provision of active care to infants as young as 22 weeks at birth may have increased its prevalence [1, 7, 9, 10]. While BPD is complex and multifactorial in origin, inflammation of the lungs plays a key role in its pathogenesis [4, 7, 11–14]. Postnatal corticosteroids (PNC), including dexamethasone, are used in the management of BPD due to their anti-inflammatory properties. However, there is controversy regarding their impact on long-term neurodevelopment [3, 4, 7, 10, 12, 15–17].
While the American Academy of Pediatrics and other organizations recommend using a course of low-dose dexamethasone for ventilator-dependent preterm infants, there are no formal recommendations for its use in preterm infants with evolving BPD who are dependent on noninvasive respiratory support [5, 9, 10, 12, 18, 20]. Using PNC in the first seven days of life is associated with short- and long-term adverse effects, such as gastrointestinal perforation and cerebral palsy (CP) [11, 21, 22]. Prechtl’s General Movements Assessment (GMA), conducted during the fidgety period of development (9–20 weeks postterm age), is a simple method of assessing infant neurodevelopment. It is a sensitive early marker used to identify infants at risk of developing CP [23–29]. Reports exploring the association between PNC use and GMAs during the fidgety period of development are scarce.
Our unit’s current practice is to administer systemic PNC to a select group of extremely preterm infants after seven days of age either to facilitate extubation or prevent reintubation on the background of evolving BPD. Using a shorter course and a lower dose of PNC beyond seven days of life may reduce long-term adverse effects [4–8, 11, 12, 16, 30, 31]. In our unit, low-dose dexamethasone (a cumulative dose of 0.89 mg/kg over 10 days) is used as per the Dexamethasone: a Randomized Trial (DART) protocol [32, 33]. At our institute, betamethasone is used as an antenatal corticosteroid and is administered to all women at risk of preterm birth (up to 34 weeks of gestational age). For infants with respiratory distress syndrome, poractant alfa is used for exogenous surfactant replacement as an early rescue therapy. The aim of this study was to explore the relationship between systemic PNC use and early neurodevelopment using Prechtl’s GMA. Based on concerns raised in the literature regarding a potential link between using PNC and adverse neurodevelopmental outcomes, we hypothesized that the neurodevelopmental outcomes for preterm infants who did not receive PNC would be better than for those who had received PNC [3, 4, 7, 10, 12, 15–17]. This has implications for improving practice in the use of PNC.
Methods
Study design
A single-center retrospective cohort study was conducted at a tertiary neonatal intensive care unit (NICU). All inborn preterm infants born before 29 weeks gestation and admitted to the NICU between 1 January 2014 and 31 December 2018 were identified from a prospectively maintained and verified Neonatal Intensive Care Units’ (NICUS) database and were included in the study. Infants with congenital or genetic abnormalities, those who received systemic steroids for nonrespiratory indications such as hypotension, and outborn infants were excluded. The Human Research Ethics Committee granted ethical approval (2108–15 QA). Obtaining informed consent from all research individuals was not required because this study retrospectively reviewed medical records. This database is a prospective data collection system of preterm infants (< 32 weeks) admitted to all tertiary NICUs in New South Wales (NSW) and the Australian Capital Territory (ACT) that collates maternal, perinatal and neonatal clinical data. These data are collected and verified by designated audit officers following standardized definitions for clinical outcomes. Clinical data were extracted from this database.
We dichotomized the cohort based on whether they had received PNC or not. Other variables potentially influencing neurodevelopmental outcomes, such as gestational age, small for gestational age (birth weight < 10th centile), major intraventricular hemorrhage (Grade III and IV based on Papile’s classification), histopathology-proven chorioamnionitis, antenatal steroids and antepartum magnesium sulphate administration, were included in the regression analysis [34].
Our unit's standard practice is to perform the GMA during the fidgety period of infant development (9–20 weeks postterm equivalent age). The infant’s general movements were captured by a short (2–3 minutes) video recording in the high-risk follow-up clinic. All videos were rated according to Prechtl’s method by two to four raters who had advanced training in GMAs. The raters were blinded to the infants’ PNC status and perinatal course. In case of disagreement, the rating was determined by the New South Wales General Movements Rater Network [24]. General movements were rated as normal, abnormal (exaggerated), or absent/sporadic fidgety. In addition, the infant’s motor repertoire was scored in detail using the motor optimality score revised (MOS-R). The quality of movement (observed movement patterns), repertoire (age-adequate movement repertoire), posture (observed postural patterns) and movement character were scored using the MOS-R. A motor optimality score (MOS) was then generated, which had a maximum score of 28 (the best possible) and a minimum score of 5. MOS-R is reported to be a good predictor of adverse neurodevelopmental outcomes [27, 28]. A MOS of 20–28 was considered an optimal score, and scores below 20 were considered suboptimal [27]. For analysis, GMAs were dichotomized to either normal or abnormal. An abnormal GMA result included abnormal or absent fidgety movements or a MOS score < 20. MOS was further classified as optimal range (20–28), suboptimal (9–19) and indicative of severe impairment (≤ 8).
Data analysis
Descriptive statistics were used to summarize the study cohort characteristics, using the mean (standard deviation) for symmetrically distributed continuous variables and the median (interquartile range) for asymmetrically distributed variables. Numbers (percentages) were used to describe categorical data. Data were analyzed using Stata 17 (StataCorp, College Station, TX, USA). Differences between the groups for continuous data were tested using Student’s t test for symmetrically distributed data and the Mann-Whitney U test for asymmetrically distributed data. A chi-square test was used to report between-group differences in categorical variables. Binary multivariable logistic models were then created using backward stepwise selection to investigate the association for univariable models with a P value of < 0.2. The results from these models are reported with odds ratios and 95% confidence intervals. A two-tailed P value < 0.05 was considered significant. No adjustments have been made for multiple comparisons.
Results
Group characteristics
Between 1 January 2014 and 31 December 2018, we identified 282 eligible infants. Of these, 67 (23.75%) received PNC. PNC was administered for dependency on invasive ventilation (intermittent positive-pressure ventilation) in 34 infants (50.75%). Among the 33 infants (49.25%) received them for dependency on non-invasive ventilation continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP), of which two infants were concurrently being weaned off noninvasive ventilation. Of the 67 infants who received PNC, 37 infants had a single course over 10 days, 21 had two courses, six had three courses and three had four courses. The first course of PNC was administered at a median age of 22 days [interquantile range (IQR) 13–34]. For the first course of PNC, the median age for infants dependent on invasive respiratory support was 16 days (IQR 10–24), compared to 23 days (14–25) for infants dependent on BiPAP and 39 days (25–61) for infants dependent on CPAP.
Demographic and baseline clinical characteristics of the cohort are shown in Table 1. Infants in the PNC recipient group were more premature (by two weeks) and were lighter at birth. In addition, this group had more small-for-gestational-age infants, histopathology-proven chorioamnionitis, infants who were intubated and received surfactant, and BPD. They also required both invasive and noninvasive ventilation for longer periods of time.
Table 1.
Mother-infant dyad demographics and baseline clinical characteristics based on PNC status
| Variables | PNC received group (n = 67) | No-PNC group (n = 215) | P |
|---|---|---|---|
| GA, wk, median (IQR) | 25 (24–26) | 27 (26–28) | < 0.001‡ |
| BW, g, mean (SD) | 778.6 (165) | 977.8 (218) | < 0.001‡ |
| Male sex, n (%) | 42 (62.7) | 109 (50.7) | 0.085 |
| Multiple births, n (%) | 12 (17.9) | 54 (25.1) | 0.224 |
| Maternal age, y, mean (SD) | 31.6 (5.4) | 31.8 (5.8) | 0.802 |
| GDM, n (%) | 6 (9.0) | 33 (15.4) | 0.186 |
| HDP, n (%) | 16 (23.9) | 42 (19.5) | 0.442 |
| SGA (BW < 10th centile), n (%) | 11 (16.4) | 11 (5.1) | 0.003† |
| ANS, n (%) | |||
| Complete | 27 (40.3) | 77 (35.8) | 0.849 |
| Incomplete | 21 (31.3) | 72 (33.5) | |
| None | 4 (6.0) | 10 (4.7) | |
| MgSO4, n (%) | 49 (73.1) | 140 (65.1) | 0.223 |
| Spontaneous preterm labour, n (%) | 27 (40.3) | 91 (42.3) | 0.769 |
| Maternal ABx, n (%) | 42 (62.7) | 131 (61.0) | 0.797 |
| Histopathological chorioamnionitis, n (%) | 42 (62.7) | 100 (46.5) | 0.021* |
| Caesarean births, n (%) | 36 (53.73%) | 126 (58.6%) | 0.480 |
| Intubated at delivery for continuation of mechanical ventilation, n (%) | 55 (82.1) | 138 (64.2) | 0.006† |
| Apgar score at 5 min, median (IQR) | 7 (6–8) | 7 (6–8) | 0.863 |
| Invasive ventilation, d, median (IQR) | 9.2 (4.95–19.5) | 0.7 (0.3–3.5) | < 0.001‡ |
| Non-invasive ventilation, d, median (IQR)a | 70.4 (57.2–91.1) | 42.8 (24.6–57) | < 0.0001‡ |
| Received surfactant, n (%) | 65 (97) | 184 (85.6) | 0.011* |
| Deaths prior to discharge, n (%) | 4 (6.0) | 16 (7.4) | 0.682 |
| BPD, n (%) | 55 (82.1) | 70 (32.6) | < 0.001‡ |
| Major IVH (Grade III/IV)b, n (%) | 3 (4.5) | 12 (5.6) | 0.725 |
| PVL, n (%) | 3 (4.5) | 2 (0.9) | 0.055 |
| Major surgery during hospital stay, n (%)c | 28 (41.8) | 24 (11.2) | < 0.001‡ |
PNC postnatal corticosteroid, GA gestational age, BW birth weight, g grams, IQR interquartile range, SD standard deviation, GDM gestational diabetes, HDP hypertensive disease of pregnancy, SGA small for gestational age, ANS antenatal steroids, MgSO4 magnesium sulfate, ABx antibiotics, BPD bronchopulmonary dysplasia, IVH intraventricular hemorrhage, PVL periventricular leukomalacia. *P ≥ 0.01 and < 0.05, †P ≥ 0.001 and < 0.01, ‡P < 0.001
aNon-invasive ventilation days include infants discharged home on CPAP and oxygen
bMajor IVH (Grade III or IV) as per Papile's classification [34]
cMajor surgery during hospital stay such as ligation of patent ductus arteriosus, laparotomy for necrotizing enterocolitis
General movements assessment at 9–20 weeks post-term equivalent age
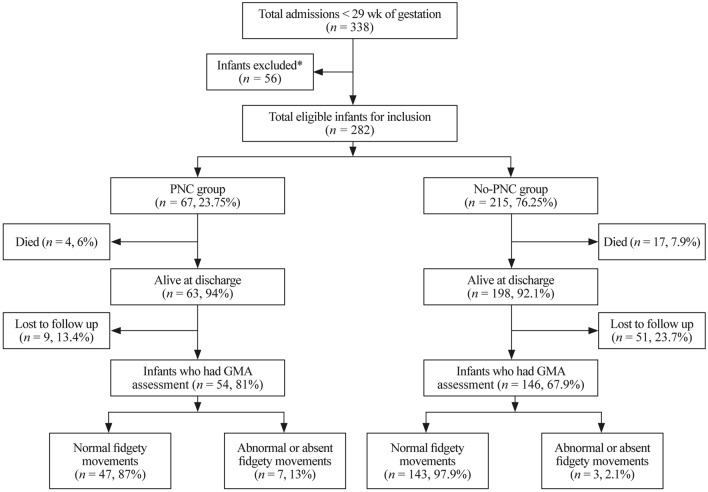
Of the 67 infants in the PNC recipient group, 54 (80.5%) infants had GMAs during the fidgety period. Of the 215 infants in the no-PNC group, 146 (67.9%) infants had GMAs during the fidgety period. Figure 1 provides details on GMA outcomes and loss to follow-up based on corticosteroid status.
Fig. 1.
GMA outcomes and loss to follow-up based on corticosteroid status. *Infants met the study exclusion criteria. PNC postnatal corticosteroids, GMA General Movements Assessment
Seven infants in the PNC recipient group (13%) had an abnormal GMA, while only three infants in the no-PNC group (2%) were abnormal [odds ratio (OR) 7.09, 95% confidence interval (CI) 1.76–28.56 and adjusted odds ratio (aOR) 5.5, 95% CI 1.14–26.56, P = 0.03] after adjusting for gestational age. The difference in the median MOS between the PNC recipient group and the no-PNC group was significant [25.5 (23–26) versus 26 (24–28); P = 0.043, Z = −2.02]. Eight out of 54 infants (14.8%) in the PNC group had MOS < 20, while 3 out of 146 infants (2%) in the no-PNC group had MOS < 20; this difference was significant after adjustment for gestational age in logistic regression analysis (coefficient 1.78, 95% CI 0.24–3.32, P = 0.02). There were clear differences in MOS between the groups. In the PNC group, three (5.6%) infants fell into the range of severe impairment (MOS ≤ 8), and five infants (9.3%) fell into the suboptimal range (MOS 9–19), as opposed to no infants and three (2.1%) infants, respectively, in the no-PNC group. This difference was significant after adjustment for gestational age (aOR 5.96, 95% CI 1.28–27.74, P = 0.02).
Movements were further characterized and scored as summarized in Table 2. For quality of movement, there were four (7.4%) infants with more atypical movements (A) than normal movements (N) in the PNC group and four (2.7%) in the no-PNC group. The expected repertoire of movements was absent for 19 (35.2%) infants in the PNC group, as opposed to 33 (22.6%) in the no-PNC group. There was a higher proportion of infants in the PNC group with N < A for posture [6 (11.1%) versus 6 (4.1%)]. The only infant with cramped-synchronized movements was in the PNC group.
Table 2.
Motor optimality score (MOS) components from General Movements Assessment (GMA) based on PNC status
| Variables | PNC received group (n = 54) | No-PNC group (n = 146) |
|---|---|---|
| Abnormal or absent fidgety movements | 7 (13%) | 3 (2%) |
| MOS < 20 | 8 (14.8%) | 3 (2%) |
| Quality of movement | ||
| N > A | 48 (88.9%) | 136 (93.2%) |
| N = A | 2 (3.7%) | 6 (4.1%) |
| N < A | 4 (7.4%) | 4 (2.7%) |
| Movement repertoire | ||
| Adequate | 19 (35.2%) | 52 (35.6%) |
| Reduced | 16 (29.6%) | 61 (41.8%) |
| Absent | 19 (35.2%) | 33 (22.6%) |
| Posture | ||
| N > A | 43 (76.9%) | 131 (89.7%) |
| N = A | 5 (9.3%) | 9 (6.2%) |
| N < A | 6 (11.1%) | 6 (4.1%) |
| Movement characters | ||
| Normal | 25 (46.3%) | 95 (65.1%) |
| Abnormal but not cramped-synchronized | 28 (51.9%) | 51 (34.9%) |
| Cramped-synchronized | 1 (1.9%) | 0 |
PNC postnatal corticosteroid, N normal, A atypical
For infants in the PNC recipient group, GMA outcomes were similar for single versus multiple courses of PNC. The median MOS for the group receiving multiple courses was 25.5 (IQR 23–26) versus 25 (IQR 21–26) for the single course group. There were no differences in the fidgety movements, repertoire or posture between the two groups. There were more movements of atypical quality in infants who received multiple courses of PNC [3 (12%) versus 1 (3%)]. One infant in the multiple course group and none from the single course group had cramped-synchronized movements.
We explored the effects of PNC use on GMAs for infants dependent on invasive versus noninvasive respiratory support within the PNC recipient group. Overall, PNC had similar effects on GMA, irrespective of the type of respiratory support. The median MOS for the group with invasive ventilation was 24 (IQR 21–26) versus 26 (IQR 23–28) for the noninvasive group; the difference was not significant. There were some differences in the quality of movements, with more atypical movements observed in the invasive ventilation group for fidgety movements [four (16%) versus three (10%)], quality of movement [three (12%) versus one (3%)], repertoire [10 (40%) versus 9 (31%)] and posture [four (14%) versus two (8%)]. The one infant with cramped-synchronized movement belonged to the invasive ventilation group.
Relationship between postnatal corticosteroids status, clinical characteristics, and fidgety movement outcomes
The relationship between clinical characteristics and fidgety movement outcomes at GMA is shown in Table 3. On univariable analysis, the perinatal factors associated with increased odds for abnormal or absent fidgety movements were PNC administration (OR 7.1, 95% CI 1.77–28.56, P = 0.006), gestational age (OR 0.63, 95% CI 0.40–0.99, P = 0.047), birth weight (OR 0.99, 95% CI 0.99–0.99, P = 0.004), BPD (OR 10.88, 95% CI 1.35–87.61, P = 0.025), major intraventricular hemorrhage (OR 9.25, 95% CI 1.55–55.18, P = 0.015), periventricular leucomalacia (OR 15.58, 95% CI 2.28–106.72, P = 0.005) and major surgery (OR 4.28, 95% CI 1.18–15.57, P = 0.027). Of the infants with normal fidgety movements in the PNC group, 26 (55.32%) received one course of dexamethasone, 16 (34.04%) received two courses, three (6.38%) received three courses and two (4.26%) received four courses. For infants with abnormal or absent fidgety movements, four (57.14%) received one course of dexamethasone, and three (42.86%) received two courses. No infants in this group received three or four courses of dexamethasone. The multivariable logistic regression model is shown in Table 3. Although the adjusted odds ratio of abnormal or absent fidgety movements was higher in the PNC recipient group, this was not statistically significant. The presence of PVL significantly increased the odds of abnormal or absent fidgety movements.
Table 3.
Relationship between perinatal variables and outcomes of fidgety movements from binary univariable and multivariable logistic regression analysis
| Clinical characteristics | Univariable OR (95% CI) | P value | Multivariable aOR (95% CI) | P value |
|---|---|---|---|---|
| Received PNC | 7.1 (1.76–28.56) | 0.006† | 3.980 (0.77–20.44) | 0.098 |
| GA (median, wk) | 0.63 (0.40–0.99) | 0.047* | NA | NA |
| BW (median, g) | 0.99 (0.99–0.99) | 0.004† | 0.996 (0.991–1.000) | 0.052 |
| SGA | 3.14 (0.60–16.23) | 0.172 | NA | NA |
| Female gender | 1.02 (0.28–3.64) | 0.974 | NA | NA |
| Multiples | 2.21 (0.59–8.19) | 0.235 | NA | NA |
| Chorioamnionitis | 0.532 (0.41–5.60) | 0.519 | NA | NA |
| Received antenatal steroids (complete) | 1.329 (0.21–8.21) | 0.760 | NA | NA |
| MgSO4 | 1.213 (0.30–4.84) | 0.784 | NA | NA |
| Spontaneous labour onset | 1.235 (0.34–4.40) | 0.745 | NA | NA |
| Antibiotics in labour | 1.00 (0.27–3.66) | 1 | NA | NA |
| Apgar score at 5 min (median) | 0.800 (0.57–1.12) | 0.193 | NA | NA |
| Apgar score < 6 at 5 min | 1.000 (0.12–8.32) | 1 | NA | NA |
| Intubated at birth for continuation of mechanical ventilation | 4.571 (0.56–36.87) | 0.154 | NA | NA |
| ECM at birth | 2.905 (0.32–26.20) | 0.342 | NA | NA |
| BPD | 10.884 (1.35–87.61) | 0.025* | NA | NA |
| Major IVH (Grade III/IV) | 9.250 (1.55–55.18) | 0.015* | 5.758 (0.693–47.868) | 0.105 |
| PVL | 15.583 (2.27–106.72) | 0.005† | 12.020 (1.111–130.086) | 0.041* |
| Died before discharge | 10.444 (0.86–126.21) | 0.065 | NA | NA |
| Major surgery during hospital stay | 4.278 (1.17–15.565) | 0.027* | NA | NA |
PNC postnatal corticosteroids, OR unadjusted odds ratio from univariable model, aOR adjusted odds ratio from multivariable model, GA gestational age, BW birth weight, g grams, SGA small for gestational age, MgSO4 magnesium sulphate, ECM external cardiac massage for cardiopulmonary resuscitation, min minutes, BPD bronchopulmonary dysplasia, IVH intraventricular hemorrhage, PVL periventricular leukomalacia, NA variables excluded from multivariable regression analysis as P ≥ 0.2.
*P ≥ 0.01 and < 0.05
†P ≥ 0.001 and < 0.01
Discussion
We explored the relationship between systemic PNC use and early neurodevelopmental assessment at 9–20 weeks postterm equivalent age in extremely preterm infants born < 29 weeks gestation. We observed that using PNC was associated with higher adjusted odds (adjusted for gestational age at birth) for an abnormal GMA in a univariable logistic regression but not in a multivariable regression model. Overall, GMA outcomes were similar for single versus multiple courses of systemic PNC, as well as for infants dependent on invasive versus noninvasive ventilation. A modest increase in the frequency of abnormal movements was observed for infants dependent on invasive ventilation.
Overall, about one-third of infants < 29 weeks gestation received systemic PNC for dependency on respiratory support. While the proportion of infants who received PNC for dependency on invasive ventilation was similar to regional and international practices [1, 22], there was considerable use of systemic PNC for dependency on noninvasive respiratory support. This practice might reflect the lack of evidence for PNC use in infants dependent on noninvasive ventilation. Doyle et al. described the relationship between the risk of BPD and death or CP [35]. Systemic PNC increased the risk of death or CP when the risk of BPD was below 35% and decreased its risk when the risk of BPD exceeded 65% [35]. The meta-regression analysis in Doyle’s study suggests reserving the use of systemic PNC for ventilator-dependent infants with a predicted risk of BPD ≥ 50%, which is supported in the recent literature [2, 6, 16, 22].
Our results support findings in previous studies in relation to risk factors for BPD [2, 3, 6, 7, 11, 13, 30, 36]. Interestingly, although perhaps reflective of the small sample size and dissimilar to previous studies, male sex was not found to be a significant risk factor for BPD [2, 13, 14, 37]. Gestational age has the strongest association with BPD, so it could be extrapolated that the lower the gestational age, the more likely it is that PNC is prescribed [2, 13].
The strongest predictors of a later diagnosis of CP on the MOS are absent fidgety movements and cramped-synchronized character of movements [27, 28, 31, 38]. An important observation from our study was the association between abnormal GMAs and PNC use. Even though our study was limited by a small sample size and small number of abnormal GMAs, this difference was significant after adjusting for gestational age, albeit only for univariable analysis. Likewise, quality of movement, repertoire, posture, and movement character differed between the PNC and no-PNC groups, suggesting that PNC may have a negative effect on early neurodevelopment. A larger multisite sample size may demonstrate a more significant effect to support this finding.
Our study reported a lower median MOS and a higher frequency of abnormal assessments in the PNC recipient group than in the no-PNC group, although this difference was not statistically significant. A small sample size may have accounted for this finding. However, this observation was consistent with the study hypothesis, and there were more infants with MOS within the severely impaired and suboptimal ranges for the PNC group than for the no-PNC group. Hitzert et al. investigated the effect of hydrocortisone versus dexamethasone on GMAs at three months post-term [25, 31]. They found that MOS scores were lower for dexamethasone, suggesting that neurological functioning was poor with dexamethasone [25, 31]. Univariable analysis from this study reports several variables, including PNC, that may contribute to abnormal GMA results; however, only the presence of PVL was associated with abnormal GMAs on multivariable analysis. Further studies are needed to explore the relationship between PNC and long-term neurodevelopmental outcomes, although it is difficult to draw causation when infants more likely to receive PNC are those who are born earlier and smaller, which already puts these infants at higher risk of poorer neurodevelopmental outcomes.
There are limited studies using Prechtl’s GMA during the fidgety period to predict neurodevelopmental outcomes following the use of PNC. There are some studies with small sample sizes that link high-dose PNC to abnormal GMAs as early as 24 hours post-administration and at three months corrected age [39, 40]. A more recent study by Hitzert et al. reported higher MOSs when low-dose and later dexamethasone was used for a shorter duration of mechanical ventilation [31]. Another study, also by Hitzert et al., reported a higher rate of abnormal GMAs for infants treated with low-dose dexamethasone and for those treated with hydrocortisone after seven days of life, with a greater rate of abnormality in the dexamethasone group [25]. It is important to note the limitations, differences in the study cohort and the methodology between this and our study. In the context of very limited previous evidence, our findings suggest an ongoing link between PNC use and abnormal neurodevelopment that warrants further exploration.
Our study provides new information on the relationship between GMAs and systemic PNC in infants dependent on invasive and noninvasive respiratory support. Blinding raters to the infant's PNC status and their perinatal course as well using a recognized process for resolution of disagreement between raters strengthens the study methodology and the generalizability of the results. We acknowledge certain limitations. This was a single-center study with a small sample size. The proportion of loss to follow-up was higher in the no-PNC group, which may also have influenced the study findings.
In conclusion, abnormal early neurodevelopment, as indicated by abnormal GMAs, was observed in infants who received PNC. In this study, the influence of other adverse perinatal conditions or complications contributing to abnormal early neurodevelopment remains unclear. Nevertheless, the effect of PNC on early neurodevelopment needs further exploration. Until further evidence is available, the use of systemic PNC is best reserved as per the current international recommendations.
Acknowledgements
We acknowledge the assistance of SuperScript Writing and Editing for professional proofreading.
Author contributions
EE, GTA and JP: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing of original draft, writing–reviewing and editing. LG, BJ, MR, DD, LM, and SD: data curation, project administration, visualization, writing–reviewing and editing. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
This study protocol was reviewed and approved by The Western Sydney Local Health District’s Human Research Ethics Committee (Approval number: 2108–15 QA). Written consent for publication of the case details together with imaging or videos have been obtained from participants (or their parent or legal guardian in the case of children under 16) should be written in this section—not applicable.
Informed consent
Obtaining informed consent from all research individuals was not required as this study retrospectively reviewed medical records.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chow SSW, Creighton P, Chambers GM, Lui K. Report of the Australian and New Zealand Neonatal Network 2019. 2021. https://npesu.unsw.edu.au/sites/default/files/npesu/surveillances/Report%20of%20the%20Australian%20and%20New%20Zealand%20Neonatal%20Network%202019.pdf. Accessed 10 Feb 2023.
- 2.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer SE, Schneider L, Lynch SK, Malleske DT, Shepherd EG, Nelin LD. Factors associated with neurodevelopmental impairment in bronchopulmonary dysplasia. J Pediatr. 2020;218:22–27. doi: 10.1016/j.jpeds.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Cheong JLY, Doyle LW. Long-term effects of postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia: balancing the risks and benefits. Semin Fetal Neonatal Med. 2019;24:197–201. doi: 10.1016/j.siny.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;11:Cd001145. [DOI] [PMC free article] [PubMed]
- 6.Doyle LW. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Neonatology. 2021;118:244–251. doi: 10.1159/000515950. [DOI] [PubMed] [Google Scholar]
- 7.Wang SH, Tsao PN. Phenotypes of bronchopulmonary dysplasia. Int J Mol Sci. 2020;21:6112. doi: 10.3390/ijms21176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ertuğrul S, Darakci SM, Kaplan İ, Yolbaş İ, Deger İ, Tanrıverdi Yilmaz S, et al. The contribution of postnatal steroid administration to early brain damage in preterm babies with bronchopulmonary dysplasia. Turk J Med Sci. 2021;51:1917–1923. doi: 10.3906/sag-2101-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zayegh AM, Davis PG. BPD treatments: the never-ending smorgasbord. Semin Fetal Neonatal Med. 2021;26:101223. doi: 10.1016/j.siny.2021.101223. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Zhang Y, Gao S, Wang X, He N, Zhang D, et al. Hydrocortisone to treat early bronchopulmonary dysplasia in very preterm infants: study protocol for a randomized controlled trial. Trials. 2020;21:1–7. doi: 10.1186/s13063-020-04698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Htun ZT, Schulz EV, Desai RK, Marasch JL, McPherson CC, Mastrandrea LD, et al. Postnatal steroid management in preterm infants with evolving bronchopulmonary dysplasia. J Perinatol. 2021;41:1783–1796. doi: 10.1038/s41372-021-01083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramaswamy VV, Bandyopadhyay T, Nanda D, Bandiya P, Ahmed J, Garg A, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA Pediatr. 2021;175:e206826. doi: 10.1001/jamapediatrics.2020.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019;5:1–23. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson SJ, Hall GL, Wilson AC. Lung function following very preterm birth in the era of 'new' bronchopulmonary dysplasia. Respirology. 2015;20:535–540. doi: 10.1111/resp.12503. [DOI] [PubMed] [Google Scholar]
- 15.Onland W, De Jaegere AP, Offringa M, van Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;1:Cd010941. [DOI] [PMC free article] [PubMed]
- 16.Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18–22 months' adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123:e430–e437. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin G, Lo JW, Marlow N, Calvert SA, Greenough A, Peacock JL. Postnatal dexamethasone, respiratory and neurodevelopmental outcomes at two years in babies born extremely preterm. PLoS One. 2017;12:e0181176. doi: 10.1371/journal.pone.0181176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watterberg KL. Policy statement–postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126:800–808. doi: 10.1542/peds.2010-1534. [DOI] [PubMed] [Google Scholar]
- 19.Halliday HL. Update on postnatal steroids. Neonatology. 2017;111:415–422. doi: 10.1159/000458460. [DOI] [PubMed] [Google Scholar]
- 20.Lemyre B, Dunn M, Thebaud B. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia in preterm infants. Paediatr Child Health. 2020;25:322–331. doi: 10.1093/pch/pxaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Early (≤ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;10:Cd001146. [DOI] [PMC free article] [PubMed]
- 22.Olaloko O, Mohammed R, Ojha U. Evaluating the use of corticosteroids in preventing and treating bronchopulmonary dysplasia in preterm neonates. Int J Gen Med. 2018;11:265–274. doi: 10.2147/IJGM.S158184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 2017;171:897–907. doi: 10.1001/jamapediatrics.2017.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan C, Crowle C, Goyen TA, Hardman C, Jackman M, Novak I, et al. Sensitivity and specificity of general movements assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J Paediatr Child Health. 2016;52:54–59. doi: 10.1111/jpc.12995. [DOI] [PubMed] [Google Scholar]
- 25.Hitzert MM, Benders MJ, Roescher AM, van Bel F, de Vries LS, Bos AF. Hydrocortisone vs. dexamethasone treatment for bronchopulmonary dysplasia and their effects on general movements in preterm infants. Pediatr Res. 2012;71:100–6. [DOI] [PubMed]
- 26.Kwong AKL, Fitzgerald TL, Doyle LW, Cheong JLY, Spittle AJ. Predictive validity of spontaneous early infant movement for later cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60:480–489. doi: 10.1111/dmcn.13697. [DOI] [PubMed] [Google Scholar]
- 27.Einspieler C, Bos AF, Krieber-Tomantschger M, Alvarado E, Barbosa VM, Bertoncelli N, et al. Cerebral palsy: early markers of clinical phenotype and functional outcome. J Clin Med. 2019;8:1616. doi: 10.3390/jcm8101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott C, Alexander C, Salt A, Spittle AJ, Boyd RN, Badawi N, et al. Early moves: a protocol for a population-based prospective cohort study to establish general movements as an early biomarker of cognitive impairment in infants. BMJ Open. 2021;11:e041695. doi: 10.1136/bmjopen-2020-041695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyton C, Einspieler C. General movements: a behavioral biomarker of later motor and cognitive dysfunction in NICU graduates. Pediatr Ann. 2018;47:e159–e164. doi: 10.3928/19382359-20180325-01. [DOI] [PubMed] [Google Scholar]
- 30.Parikh NA, Sharma P, He L, Li H, Altaye M, Priyanka Illapani VS. Perinatal risk and protective factors in the development of diffuse white matter abnormality on term-equivalent age magnetic resonance imaging in infants born very preterm. J Pediatr. 2021;233:58–65. doi: 10.1016/j.jpeds.2020.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hitzert MM, Roescher AM, Bos AF. The quality of general movements after treatment with low-dose dexamethasone in preterm infants at risk of bronchopulmonary dysplasia. Neonatology. 2014;106:222–228. doi: 10.1159/000362919. [DOI] [PubMed] [Google Scholar]
- 32.Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117:75–83. doi: 10.1542/peds.2004-2843. [DOI] [PubMed] [Google Scholar]
- 33.Bolisetty S, Osborn, D, Phad, N. Australian Neonatal Medicines Formulary. 2022. https://www.anmfonline.org/wp-content/uploads/2022/05/Dexamethasone_ANMFv6.0_20220506.pdf. Accessed 6 Oct 2022.
- 34.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 35.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115:655–661. doi: 10.1542/peds.2004-1238. [DOI] [PubMed] [Google Scholar]
- 36.Villamor-Martinez E, Álvarez-Fuente M, Ghazi AMT, Degraeuwe P, Zimmermann LJI, Kramer BW, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Netw Open. 2019;2:e1914611. doi: 10.1001/jamanetworkopen.2019.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies) BMJ. 2012;345:e7976. doi: 10.1136/bmj.e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari F, Cioni G, Einspieler C, Roversi MF, Bos AF, Paolicelli PB, et al. Cramped synchronized general movements in preterm infants as an early marker for cerebral palsy. Arch Pediatr Adolesc Med. 2002;156:460–467. doi: 10.1001/archpedi.156.5.460. [DOI] [PubMed] [Google Scholar]
- 39.Bos AF, Martijn A, van Asperen RM, Hadders-Algra M, Okken A, Prechtl HF. Qualitative assessment of general movements in high-risk preterm infants with chronic lung disease requiring dexamethasone therapy. J Pediatr. 1998;132:300–306. doi: 10.1016/S0022-3476(98)70449-4. [DOI] [PubMed] [Google Scholar]
- 40.Bos AF, Dibiasi J, Tiessen AH, Bergman KA. Treating preterm infants at risk for chronic lung disease with dexamethasone leads to an impaired quality of general movements. Biol Neonate. 2002;82:155–158. doi: 10.1159/000063612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.