Abstract
Purpose
Bevacizumab, ranibizumab, and aflibercept are commonly used to treat neovascular age-related macular degeneration (nAMD). The results of various interventional, mostly randomized head-to-head studies, indicate statistical non-inferiority of these three drugs. The results of these studies are often interpreted as the three drugs being freely interchangeable, resulting in some health systems to pressure ophthalmologists to preferentially use the less expensive bevacizumab. This study analyzes switching from aflibercept or ranibizumab to bevacizumab and back under real-world conditions in order to investigate the assumption of interchangeability of the drugs.
Methods
Treatment data of IVT patients with diagnosed nAMD were extracted from the clinical Berlin Macular Registry database. Patients who underwent a drug switch from aflibercept or ranibizumab to bevacizumab were subject of this study. Statistical comparisons were pre-planned for best corrected visual acuity, central retinal thickness, macular volume, and length of injection interval. Additional endpoints were analyzed descriptively.
Results
Mean visual acuity decreased from 0.57 ± 0.05 under aflibercept/ranibizumab to 0.68 ± 0.06 logMAR after the switch (P = 0.001; N = 63). CRT increased from 308 ± 11 µm to 336 ± 16 µm (P = 0.011; N = 63). About half of the subjects were switched back: visual acuity increased from 0.69 ± 0.08 logMAR to 0.58 ± 0.09 logMAR (N = 26). CRT decreased from 396 ± 28 to 337 ± 20 µm (N = 28).
Conclusion
The data provides real-world evidence that there is loss of visual acuity and an increase in retinal edema after switching to bevacizumab. Thus, the assumption of free interchangeability cannot be confirmed in this cohort.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00417-022-05952-8.
Keywords: Aflibercept, Bevacizumab, Ranibizumab, VEGF switch, Vascular endothelial growth factor, Exudative age-related macular degeneration
Introduction
Vascular endothelial growth factor (VEGF) inhibition through intravitreal injections has revolutionized the treatment of neovascular AMD (nAMD). While subjects suffering from nAMD inevitably went blind in the pre-anti-VEGF-era, intravitreal VEGF inhibition not only maintained vision in nAMD, but even improved visual function in a majority of subjects [1–3].
Over the past two decades, four VEGF-inhibitors have been approved for the treatment of neovascular AMD: pegaptanib, ranibizumab, aflibercept, and, as of lately, brolucizumab and faricimab. Of these, Ranibizumab pioneered the field, with proven superiority to the past standard-of-care, i.e., photodynamic therapy(PDT) for predominantly classic choroidal neovascularization (CNV) (ANCHOR study) and observation for occult CNV (MARINA study) [4, 5]. However, to achieve maximum efficacy, ranibizumab was required to be administered monthly, which proves to be challenging, if not infeasible, in clinical routine [6]. Hence, flexible, individualized dosing regimens were established to reduce the number of injections [7, 8]. Parallel to ranibizumab, the cancer drug bevacizumab gained importance as a cost-effective off-label alternative. While a formal pivotal study program for bevacizumab and regulatory approval is lacking, there is broad evidence from multiple studies suggesting non-inferior efficacy compared to ranibizumab [8–11]. Hence, off-label bevacizumab is an integral component of present standard-of-care globally. A few years later, aflibercept was demonstrated to be clinically equivalent efficacy to ranibizumab if administered every second month after an initiation phase of three-monthly injections (VIEW-studies) [12, 13]. The latest addition to the armamentarium of treatment options are brolucizumab and faricimab. The pivotal studies of brolucizumab indicated an option for an early extension of the treatment interval to three months in about half of the patients, while the remaining subset of patients can be treated on a two-month interval [14]. Faricimab was also recently approved and the pivotal program suggests interval extensions out to four months in 45% of subjects [15].
According to these pivotal study programs, the treatment interval is the main differentiator between the approved drugs.
Yet, despite these great advances in therapy, nAMD continues to have its challenges. The invasive administration route through intravitreal (IVT) injections continues to result in a desire to minimize the number treatments. While the pivotal studies mainly employed fixed dosing schemes, real-world treatments are being performed with flexible dosing schemes [16, 17]. If executed rigorously, flexible schemes, particularly treat-and-extend schemes, lead to comparable outcomes to fixed dosing schemes [6, 18, 19]. However, the key risk of any flexible scheme is undertreatment for various reasons such as missed or delayed visits [16, 17]. Therefore, visual function may deteriorate despite IVT treatments.
Tachyphylaxis is another often presumed reason for the deterioration of visual function [20, 21]. Since the available anti-VEGF drugs showed comparable efficacy, they are considered freely interchangeable. Hence, several Ophthalmology societies, such as the German Ophthalmological Society, recommend the switch to another anti-VEGF drug in their guidelines, if an insufficient therapeutic effect is observed despite consistent therapy [22]. This is supported by studies, indicating an improvement in structural outcomes after switch of recalcitrant patients to another anti-VEGF drug [23–28].
The mechanisms of improved efficacy after switching between two anti-VEGF drugs can be explained by different molecule sizes and associated transport through the retina and into the subretinal space (ranibizumab compared with bevacizumab) or different binding characteristics [29].
Thus, the main motivation for a switch is to tackle the unsatisfactory treatment response of recalcitrant patients to ranibizumab or bevacizumab [30–32]. The results of most of these studies showed an anatomical benefit after the switch in terms of central retinal thickness and pigment epithelium detachment characteristics, whereas functional outcomes were variable [30, 31].
The potential interchangeability of the drugs leads to a second motivation to change between anti-VEGF drugs. There is a general debate about whether the higher cost of approved drugs is justified or whether patients should be switched to the less expensive and off-label bevacizumab [33–35]. Drug costs of off-label bevacizumab are by roughly a factor of 20 lower than the costs for the two approved drugs [36]. Hence, drug costs are one of the most dominant topics since the advent of this class of drugs in ophthalmology. It has led to the initiation of large scale randomized clinical trials such as the pioneering CATT and IVAN trials [9–11]. CATT, IVAN, and others suggested equivalent efficacy and safety of bevacizumab compared to ranibizumab.
As this is a highly relevant question from a health-economic standpoint, this point is repeatedly raised by sick funds, physicians and patients [37]. In an increasingly aging society, cost efficiency is of great importance for the stability of the social security system. Thus, these studies influenced political decisions to incentivize or even mandate the use of off-label bevacizumab [38–40]. Several countries, such as France, adjusted their legislation in order to enable and facilitate off-label use of bevacizumab to reduce costs [39]. This occurs under the assumption of clinical equivalence of all available anti-VEGF compounds and in particular the equivalence of off-label bevacizumab. In addition to the influence of the discussion at the political level, this topic, which is discussed in both public and professional circles, also has an influence on drug selection by the treating physician. While there is plenty of data on switches from bevacizumab to ranibizumab or aflibercept, there is a data gap for switches into the other direction. Therefore, this work investigates real-world-experiences with drug switches from ranibizumab or aflibercept to bevacizumab and back.
Methods
This study was designed as a retrospective, monocentric, real-world study. The source data is archived in the Electronic Medical Record (EMR) system of the Charité Universitätsmedizin Berlin (i.s.h.med, Cerner, München, Germany). Imaging data is stored in the Heidelberg Eye Explorer (Heidelberg Engineering, Heidelberg, Germany). For the present study, data was extracted into a separate clinical study database, in the following referred to as Berlin Macula Registry. The primary data capture was done in the REDCap electronic Case Report From (eCRF) system. For analysis, data was extracted from REDCap and transferred into appropriate statistical analysis software. All data handling was done within the safeguarded Charité IT environment to comply with data protection law. The study was approved by the ethics committee of Charité (reference number: EA1/085/20) and has been reviewed by the data protection committee of Charité. Consent for use of data was obtained from each patient.
Eligibility
All patients, who underwent treatment for nAMD between 01-JUL-2017 and 31-JAN-2020, were reviewed. Subjects, who underwent a switch from aflibercept or ranibizumab to bevacizumab between 01-JUL-2017 and 31-JAN-2020, were included into the analysis set for this study. Detailed inclusion criteria were as follows: patient age > 50 years, drug switch from either aflibercept or ranibizumab to bevacizumab, at least three injections with the same drug before and after switch, interval between last injection of aflibercept or ranibizumab, and first injection of bevacizumab < 5 months. Exclusion criteria involved any type of ocular surgery between the first out of three IVT injections before switch and last out of three IVT injections after switch as well as additional presence of long-term anti-VEGF-indicating disease in the study eye other than nAMD. If both eyes of one patient were eligible, the eye receiving more IVT injections in total was selected.
IVT injections
All IVT injections took place at one of the Charité IVT injection centers. The treatment algorithm and the IVT injection procedure itself were done in accordance with recommendations of the German Ophthalmological Society [22]. In brief, it includes an upload phase of three injections at a 4-week interval followed by maintenance therapy using the Treat and Extend algorithm [22]. In the latter, the treatment interval is adjusted stepwise by 2-week increments/decrements depending on disease activity. If no disease activity is seen at an injection interval of 12 weeks or longer, cessation of therapy is considered.
Drugs and choice of drug
The choice of the drug was at the discretion of the treating physician after considering patient preferences as well as reimbursement conditions of the patient’s health insurance. Commercially available presentations used for therapy were ranibizumab (Lucentis®, Novartis Pharma AG, Basel, Switzerland) and aflibercept (Eylea®, Bayer Pharma AG, Berlin, Germany). Off-label Bevacizumab (Avastin®, F. Hoffmann-La Roche AG, Basel Switzerland) was prepared for intravitreal use by the pharmacy of Charité under sterile conditions.
Objectives
The objective of the study was to investigate the impact of the switch on functional outcomes (primary objective), anatomic outcomes, and treatment interval.
Endpoints
Visual acuity and OCT measurements were recorded for each visit as available. Visit and injection dates were collected and analyzed to describe the treatment interval.
The main analysis was focused on functional and morphological outcomes immediately before and after the switch from aflibercept or ranibizumab to bevacizumab. The primary endpoint was the change in visual acuity (logMAR). Secondary endpoints were the change in central retinal thickness (central ETDRS subfield), the change in macular volume, as well as the treatment interval. The treatment interval was defined as the last observed treatment interval on the respective drug. This definition was chosen in order to capture a treatment interval under steady-state conditions.
Furthermore, a qualitative fluid compartment analysis of all OCT images was performed [41]. Criteria for the compartment analysis are shown in Table S1 (Supplemental Digital Content 1). The results of the compartment analysis were summarized descriptively.
To determine robustness of the results, several sensitivity analyses for all endpoints were performed. The definitions of these sensitivity analyses are reported along with their results in the supplemental material.
Second switch
Some subjects experienced a second switch, i.e., a switch from bevacizumab back to aflibercept or ranibizumab. This second switch was analyzed descriptively using the same metrics and systematics as for the first switch. An informal comparison with the outcomes in those subjects staying on bevacizumab for the entire observation period was performed.
Statistical analysis
Unless stated otherwise, data is displayed as mean ± standard error of means. To analyze switch results, statistical tests on the following four variables were pre-planned and performed (before first switch vs. after first switch): (1) best corrected visual acuity, (2) central macular thickness, (3) macular volume, and (4) treatment interval. These four Wilcoxon tests were done only for the primary switch from ranibizumab or aflibercept to bevacizumab. The significance level (alpha) was chosen to be 0.05. To correct for multiplicity, Bonferroni adjustment was done. Consequently, P < 0.0125 was regarded to be statistically significant. As this is an exploratory real-world-study, no formal sample size calculation was done. A 95% confidence interval (CI) of the mean is reported for the four variables. All further analyses provided are purely descriptive.
Data was stored in REDCap (Vanderbilt, Nashville, USA). Statistical tables were created by using SQL-functions of Microsoft Access (Version 2008; Microsoft, Seattle, USA). Statistical analysis was performed with SPSS Version 25 (IBM, Armonk, USA). Descriptive statistics were performed with Microsoft Excel Version 16.45 (Microsoft, Seattle, USA). Figures were produced using PrismGraph (GraphPad, San Diego, USA).
Results
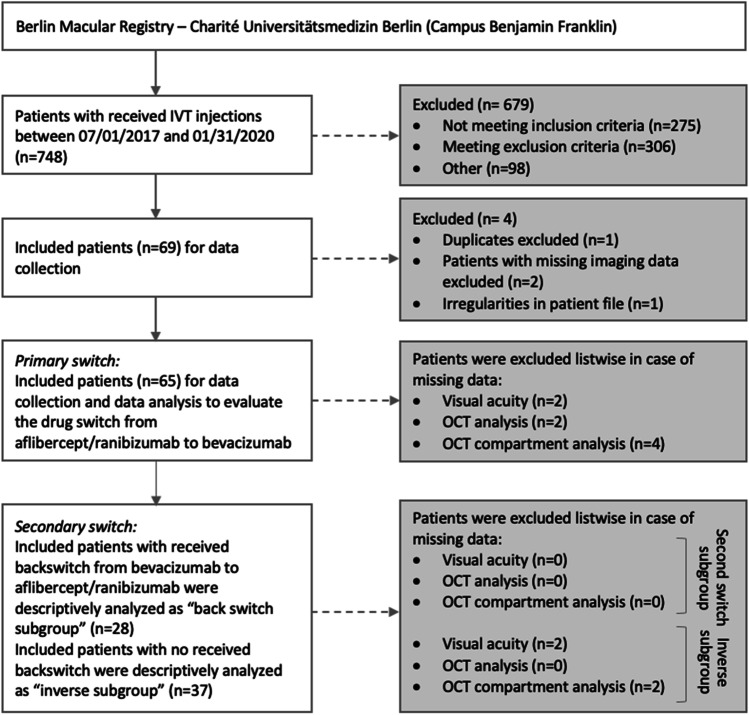
A total of 748 patient files has been reviewed, 69 of which qualified for this analysis. Two patients were excluded, because of insufficiently available follow-up data. One dataset turned out to be a duplicate and was excluded. One further patient was excluded due to a lack of imaging in the patient´s file. This results in a final number of 65 subjects being eligible for the analysis (CONSORT chart in Fig. 1).
Fig. 1.
Recruitment process
The mean age of the eligible subjects was 80.8 ± 1.1 years. 61.5% (n = 40) of the patients were female and 38.5% (n = 25) male. There were 29 (44.6%) right study eyes and 36 (55.4%) left study eyes included. Demographic and clinical characteristics of the patients are described in Table 1. Concomitant diagnoses and surgical history on the study eye are shown in supplemental material (Table S2; Supplemental Digital Content 2).
Table 1.
Demographic and clinical characteristics of study population
| Total group (N = 65) | ||
|---|---|---|
| Sex | Male | 25 (40%) |
| Female | 40 (60%) | |
| Age [in years]a | Mean | 78.9 ± 1.1 |
| Median | 77.8 (62.1; 98.1) | |
| Study eye | Right Eye | 29 (44.6%) |
| Left Eye | 36 (55.4%) | |
|
Visual acuitya [in LogMAR] |
Mean | 0.57 ± 0,05 |
| Median | 0.4 (0;1,5) | |
|
Intraocular pressurea [in mmHg] |
Mean | 13.9 ± 0.3 |
| Median | 14 (9;20) | |
| CRT [in µm]a | Mean | 310.1 ± 11.0 |
| Median | 293 (129;619) | |
|
Macular volumea [in mm3] |
Mean | 8.1 ± 0.14 |
| Median | 8.1(5.2;11.4) | |
|
Treatment time [in years] |
Mean | 4.8 ± 0.3 |
| Median | 4.6 (0.6;9.8) | |
| IVT injections | Mean | 29.5 ± 1.7 |
| Median | 28 (6;64) | |
aBaseline = date of the last IVT injection before switch to bevacizumab
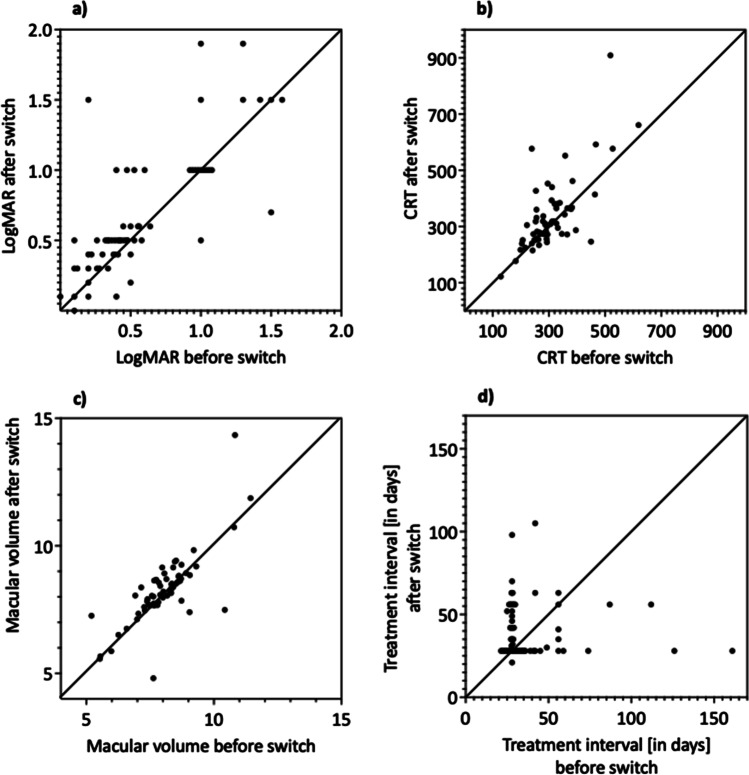
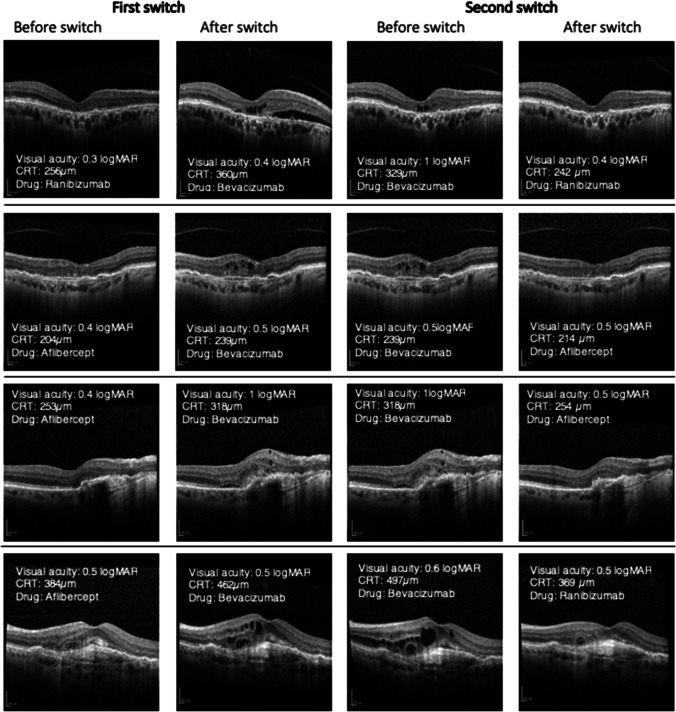
When switched from aflibercept or ranibizumab to bevacizumab, best-corrected visual acuity decreased significantly from 0.57 ± 0.05 (20/74.2; CI [0.48, 0.68]) to 0.68 ± 0.06 (20/95.7; CI [0.57, 0.79]) logMAR (P = 0.001; N = 63). Two patients had to be excluded due to missing data. This vision decrease was accompanied by morphological deterioration. CRT increased significantly from 307.59 ± 10.7 (CI [286.18, 329]) µm to 335.95 ± 15.84 (CI [304.3, 367.61]) µm (P = 0.011; N = 63) and the macular volume from 8.03 ± 0.14 (CI [7.74, 8.32]) mm3 to 8.24 ± 0.14 (CI [7.9, 8.58]) mm3 (P < 0.001; N = 63). Two patients had to be excluded due to missing data. The treatment interval was shortened close to the minimum of 4 weeks (39 ± 3.1 days to 32.8 ± 2.3 days) and, initially, was then gradually extended and reached 39 days 39 ± 3.1 (CI [32.9, 45.1]) days before and 39.8 ± 2.1 (CI [35.6, 44]) days after the switch (P = 0.323; N = 65). These results are shown in Fig. 2. The OCT-compartment analysis confirmed the trend toward anatomic worsening after the switch to bevacizumab (Table 2; representative OCT images in Fig. 3). The results of the sensitivity analyses are in line with the main analyses (Table S3; Supplemental Digital Content 3).
Fig. 2.
Results of primary switch
Table 2.
Qualitative OCT changes
| OCT criteria | Available before switch | Available after switch | Difference | |
|---|---|---|---|---|
| Primary switch | ||||
|
Total group - N = 61 |
Foveal depression | 51 (83.6%) | 48 (78.7%) | -4.92% |
| Macular edema | 34 (55.7%) | 49 (80.3%) | + 24.6% | |
| Intraretinal edema | 19 (31.2%) | 33 (54.1%) | + 22.9% | |
| Subretinal edema | 18 (29.5%) | 29 (47.5%) | + 18.0% | |
| RPEa detachment | 14 (23.0%) | 15 (24.6%) | + 1.6% | |
| Secondary switch | ||||
|
Back switch subgroup - First switch - N = 28 |
Foveal depression | 23 (82.1%) | 22 (78.6%) | -3.5% |
| Macular edema | 19 (67.9%) | 25 (89.3%) | + 21.4% | |
| Intraretinal edema | 8 (28.6%) | 16 (57.1%) | + 28.5% | |
| Subretinal edema | 12(42.9%) | 15 (53.6%) | + 10.7% | |
| RPEadetachment | 5 (17.9%) | 6 (21.4%) | + 3.5% | |
|
Back switch subgroup - Second switch - N = 26 |
Foveal depression | 21 (80.8%) | 23 (88.5%) | + 7.7% |
| Macular edema | 25 (96.2%) | 18 (69.2%) | -27.0% | |
| Intraretinal edema | 16 (61.5%) | 11 (42.3%) | -19.2% | |
| Subretinal edema | 20 (76.9%) | 8 (30.8%) | -46.1% | |
| RPEa detachment | 8 (30.8%) | 4 (15.4%) | -15.4% | |
|
Inverse subgroup - First switch - N = 33 |
Foveal depression | 28 (84.9%) | 26 (78.8%) | + 6.1% |
| Macular edema | 15 (45.5%) | 24 (72.7%) | + 27.2% | |
| Intraretinal edema | 11 (33.3%) | 17 (51.2%) | + 17.9% | |
| Subretinal edema | 6 (18.2%) | 14 (42.4%) | + 24.2% | |
| RPEa detachment | 9 (27.3%) | 9 (27.3%) | ± 0.0% | |
aRPE, retinal pigment epithelial
Fig. 3.
Representative OCT images
Subgroup analyses: second switch vs. no second switch
Close to half of the subjects (43.1%; N = 28) were switched back from bevacizumab to aflibercept or ranibizumab. The mean age of this group (77.6 ± 1.4 years) and gender ratio (F/M = 16/12) was similar to the overall group and the inverse sub-group, i.e., those not undergoing a second switch. Detailed demographics and disposition of the two subgroups are shown in Table S4 (Supplemental Digital Content 4).
As in the overall population, best-corrected visual acuity dropped in this subgroup during the first switch (0.47 ± 0.06 (20/95) vs. 0.6 ± 0.07 logMAR (20/79)). The CRT and macular volume deteriorated (329.4 ± 18.7 vs. 401.0 ± 29.3 µm and 8.2 ± 0.2 vs. 8.7 ± 0.3 mm3, resp.). The IVT injection interval remained constant (33.9 ± 2.2 vs. 33.9 ± 2.0 days).
This worsening in three out of four key metrics used in this study was reversible, when treatment with bevacizumab was replaced by treatment with ranibizumab or aflibercept. BCVA increased from 0.69 ± 0.08 (20/98) to 0.58 ± 0.09 logMAR (20/76) (N = 26). At the same time, there was a decrease in CRT from 396.3 ± 28.3 to 337.3 ± 19.8 µm and the macular volume from 8.7 ± 0.3 to 8.1 ± 0.2 mm3 (N = 28). The IVT injection interval increased from 33.9 ± 2.0 to 49.5 ± 6.0 days (N = 28). The trend toward improvement was confirmed by OCT compartment analyses (Table 2 and Fig. 3).
In the inverse sub-group, i.e., those subjects staying on bevacizumab for the entire follow-up period, best-corrected visual acuity and OCT compartment analyses showed deterioration at a similar magnitude than in the overall population subsequent to the switch from aflibercept or ranibizumab to bevacizumab. At the same time, CRT and macular volume decreased (Tables 2 and 3). Interestingly and despite functional worsening, the treatment interval was not shortened and in fact on average even slightly extended (42.8 ± 5.1 to 44.2 ± 3.3 days) in this group.
Table 3.
BCVA and quantitative OC`T-findings in inverse subgroup
| Inverse subgroup (N = 37) | Before switch | After switch |
|---|---|---|
| Visual acuity [in LogMAR] | 0.61 ± 0.07 | 0.76 ± 0.09 |
| CRT [in µm] | 290.1 ± 12.1 | 283.9 ± 11.0 |
| Macular volume [in mm3] | 7.9 ± 0.18 | 7.86 ± 0.2 |
Additional sensitivity analyses on the subgroups (Tables S5 and S6) including analysis by primary drug (Table S7) are summarized in the supplementary material. The results of these sensitivity analyses corroborate the findings of the main analyses.
Discussion
This real-world study found–consistent through all analyses–a worsening of functional and morphological outcomes in subjects switched from aflibercept or ranibizumab to bevacizumab. In a sub-set of subjects experiencing a switch back from bevacizumab to aflibercept or ranibizumab, the trend towards worsening of functional and morphological outcomes could be reversed.
Switching drugs in IVT treatment of neovascular AMD is an highly relevant topic gaining a lot of attention. Several interventional and real-world studies have been published on this matter. Most studies deal with switches from ranibizumab or bevacizumab to aflibercept and originate from the time, when aflibercept was introduced to the market as second widely used approved VEGF-inhibitor for nAMD.
The main motivation for switching in these studies was unsatisfactory response to treatment with ranibizumab or bevacizumab in recalcitrant patients [30–32].
A second motivation for switching may be cost-saving reasons, which are of such relevance that different studies have already influenced policy decisions promoting or even mandating the use of bevacizumab as off-label [38–40].
In contrast to the quite broad availability of comparative data from RCTs for bevacizumab vs. ranibizumab, only limited real-world data on switches from aflibercept or ranibizumab to bevacizumab is available. A retrospective study by Pinheiro-Costa et al. in which a switch from ranibizumab to bevacizumab was performed in 110 patients with nAMD, and which was performed due to an institutional decision based on economic reasoning, showed a significant decrease in visual acuity as well as a trend towards increase of CRT due to the switch [42]. The reason for the CRT increase is mainly caused by intraretinal fluid and subretinal fluid, which is in line with our results [42].
Two other smaller retrospective studies analyzing the switch from ranibizumab to bevacizumab were largely in line with our results.
Andreoli et al. did not show a significant change but a trend towards better visual acuity and IVT injection intervals under treatment with ranibizumab compared to bevacizumab [43].
In a case series by Yamada et al., 7 patients were switched after three monthly ranibizumab injections to six weekly bevacizumab injections. This study found a non-significant decrease in visual acuity after 6 weeks but a significant reduction in foveal retinal thickness (FRT) after 6 months of therapy with bevacizumab [44].
Comparability of the afore mentioned studies to our study is reduced because the switch from aflibercept to bevacizumab was not included in their studied cohort.
The present study from the Berlin Macula Registry aims to close this gap. In the health-economic environment in Germany, there is occasionally a gentle push towards yet no enforcement of off-label prescriptions of bevacizumab, which also have influenced drug choice for the subjects in this study. While physicians are encouraged to consider health-economic aspects, they still remain free in their ultimate decision. In our study, population consistent deterioration of functional and morphological outcomes was observed. This suggests that RCT results implying clinical equivalence of bevacizumab do not translate into real-world practice outcomes. This finding is in line with limited previous study data from different geographies. Different to previous studies, the present study was not limited to ranibizumab as primary drug and also included aflibercept. This is a relevant differentiator to previous work, as aflibercept is an important pillar of the present standard-of-care.
Besides the main objective of analyzing the outcomes of a switch to bevacizumab, the study disclosed additional interesting aspects of the treatment reality of neovascular AMD subjects. Looking at the sub-group that was not switched back to aflibercept or ranibizumab, it is striking that the functional and anatomic outcomes are as such that the response to the treatment with bevacizumab must be regarded as unsatisfactorily. In hindsight, one would have expected that also this population would have been switched back or that the treatment interval would have at least been shortened. In some cases, this may be due to development of atrophy as indicated by reduction of mean CRT. However, others may have benefitted from a switch-back. This illustrates the main risk of any flexible treatment regimens: Subtle signs of worsening may be overlooked leading to under-treatment.
Of course, the present study also has its limitations, mainly those inherent to secondary data collection of real-world data. The treatment did not follow a prospectively developed protocol, which, however, is the nature of patient care in a real-world setting. The monocentric approach ensures homogeneity of the data and reduces confounders through different clinical practice across centers. The lack of a control group is mitigated by the analyses of second switch back to aflibercept or ranibizumab. With all caution as the number of cases in the switch-back sub-group is limited, these analyses demonstrate that deterioration of functional and anatomic outcomes is reversible. This observation fits well into the overall picture generated from the totality of data of this study. It corroborates the primary result and strongly suggests that the observed effects be truly attributable to bevacizumab.
The additional sensitivity analyses constitute a further measure to mitigate the shortcomings of a secondary data collection. The combination of data quality and quantity of data points per subject and the large number of additional sensitivity and sub-group analyses allow a robust conclusion on the study cohort. This is supported by the fact that all primary analyses, sensitivity analyses and sub-group analyses show a consistent picture so that the observed effects can be regarded as robust and assumed to be real.
Summary
This study shows that bevacizumab leads to outcomes inferior to those with aflibercept or ranibizumab under real-world-conditions. It shows that conclusions from RCTs suggesting that drugs are interchangeable do not translate into clinical practice. Given that economics are the sole driving force behind the usage of bevacizumab, this is an important information. This may also be of relevance once decisions on real-world equivalence of biosimiliars have to be made. The observation of this study should be considered by reimbursement decision makers.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- IVT
Intravitreal
- nAMD
Neovascular age-related macular degeneration
- CRT
Central retinal thickness
- SEM
Standard error of mean
- VEGF
Vascular endothelial growth factor
- PDT
Photodynamic therapy
- CNV
Choroidal neovascularization
- EMR
Electronic medical record
- eCRF
Electronic case report from
- CI
Confidence interval
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Ethical approval
All data handling was done within the safeguarded Charité IT environment to comply with data protection law. The study was approved by the ethics committee of Charité (reference number: EA1/085/20) and has been reviewed by the data protection committee of Charité.
Informed consent
Consent for use of data was obtained from each patient.
Conflict of interest
Oliver Zeitz received personal fees from Bayer AG, grants from Novartis, personal fees from Boehringer Ingelheim, outside the submitted work.
Antonia M. Joussen received consulting fees and speaking Honoria from Bayer AG, Novartis, Roche and Böhringer Ingelheim.
Alexander Böker received speaking honoria from Novartis.
Anne Rübsam received speaking honoria from Bayer Healthcare and Novartis and served as consultant for Novartis.
Saskia Rau received speaking honoria from Bayer Healthcare, Novartis and Allergan/AbbVie.
Footnotes
The original version of this article was revised. The author names is now corrected.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/16/2023
A Correction to this paper has been published: 10.1007/s00417-023-06011-6
References
- 1.Bourne RR, Jonas JB, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98:629–638. doi: 10.1136/bjophthalmol-2013-304033. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmol. 2009;116:57–65.e55. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138:244–250. doi: 10.1001/jamaophthalmol.2019.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58.e41. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmol. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmol. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmol. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmol. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmol. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–740. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 16.Khanani AM, Skelly A, Bezlyak V, et al. SIERRA-AMD: a retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4:122–133. doi: 10.1016/j.oret.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Mehta H, Kim LN, Mathis T, et al. Trends in real-world neovascular AMD treatment outcomes in the UK. Clin Ophthalmol. 2020;14:3331–3342. doi: 10.2147/OPTH.S275977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avery RL. Re: Berg et al.: Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol (Ophthalmology 2015;122:146–52) Ophthalmol. 2016;123:e14–e16. doi: 10.1016/j.ophtha.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Ohji M, Takahashi K, Okada AA, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR : a randomized controlled trial. Adv Ther. 2020;37:1173–1187. doi: 10.1007/s12325-020-01236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binder S. Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol. 2012;96:1–2. doi: 10.1136/bjophthalmol-2011-301236. [DOI] [PubMed] [Google Scholar]
- 21.Dirani A, Mantel I. Ranibizumab treatment history as predictor of the switch-response to aflibercept: evidence for drug tolerance. Clin Ophthalmol. 2018;12:593–600. doi: 10.2147/OPTH.S160367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutsche Ophthalmologische Gesellschaft (2020) Stellungnahme der Deutschen Ophthalmologischen Gesellschaft, der Retinologischen Gesellschaft und des Berufsverbandes der Augenärzte Deutschlands Anti-VEGF-Therapie bei der neovaskulären altersabhängigen Makuladegeneration. https://www.retinologie.org/fileadmin/Dateien/RG_Behandlungsempfehlungen/2020_Stellungnahme_AMD.pdf Accessed 10 March 2021
- 23.De Massougnes S, Dirani A, Ambresin A, et al. Pigment epithelial detachment response to aflibercept in neovascular age-related macular degeneration refractory to ranibizumab: Time Course and Drug Effects. Retina. 2016;36:881–888. doi: 10.1097/IAE.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 24.Despreaux R, Cohen SY, Semoun O, et al. Short-term results of switchback from aflibercept to ranibizumab in neovascular age-related macular degeneration in clinical practice. Graefes Arch Clin Exp Ophthalmol. 2016;254:639–644. doi: 10.1007/s00417-015-3084-1. [DOI] [PubMed] [Google Scholar]
- 25.Kumar N, Marsiglia M, Mrejen S, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33:1605–1612. doi: 10.1097/IAE.0b013e31828e8551. [DOI] [PubMed] [Google Scholar]
- 26.Sarao V, Parravano M, Veritti D, et al. Intravitreal aflibercept for choroidal neovascularization due to age-related macular degeneration unresponsive to ranibizumab therapy. Retina. 2016;36:770–777. doi: 10.1097/IAE.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 27.Schachat AP. Switching anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156:1–2.e1. doi: 10.1016/j.ajo.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Slean GR, Hemarat K, Khurana RN, et al. Conversion back to bevacizumab or ranibizumab for recurrent neovascular activity with aflibercept in age-related macular degeneration: a case series. Int J Retina Vitreous. 2016;2:2. doi: 10.1186/s40942-016-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Empeslidis T, Storey M, Giannopoulos T, et al. How successful is switching from bevacizumab or ranibizumab to aflibercept in age-related macular degeneration? A systematic overview. Adv Ther. 2019;36:1532–1548. doi: 10.1007/s12325-019-00971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamid MA, Abdelfattah NS, Salamzadeh J, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Int J Retina Vitreous. 2021;7:26. doi: 10.1186/s40942-021-00299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh RP, Srivastava SK, Ehlers JP, et al. A single-arm, investigator-initiated study of the efficacy, safety, and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration previously treated with ranibizumab or bevacizumab (ASSESS study): 12-month analysis. Clin Ophthalmol. 2015;9:1759–1766. doi: 10.2147/OPTH.S87043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dakin HA, Wordsworth S, Rogers CA, et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open. 2014;4:e005094. doi: 10.1136/bmjopen-2014-005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein JD, Newman-Casey PA, Mrinalini T, et al. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmol. 2014;121:936–945. doi: 10.1016/j.ophtha.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbrook R. The price of sight–ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355:1409–1412. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- 36.Van Asten F, Michels CTJ, Hoyng CB, et al. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-a cost-effectiveness analysis from a societal perspective. PLoS ONE. 2018;13:e0197670. doi: 10.1371/journal.pone.0197670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jhaveri CD, Glassman AR, Ferris FL, 3rd, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387:692–703. doi: 10.1056/NEJMoa2204225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agenzia Italiana del Farmaco (2014) AIFA: precisazioni regolatorie su Avastin e Lucentis. https://www.aifa.gov.it/-/aifa-precisazioni-regolatorie-su-avastin-e-lucent-1 Accessed 28 October 2021
- 39.Ampnews (2015) Inscription au remboursement d'Avastin* dans la DMLA. https://www.apmnews.com/story.php?uid=0&objet=264595 Accessed 28 October 2021
- 40.Rivasi M Written question P-002767/14 Michèle Rivasi (Verts/ALE) to the Commission. Collusion between Novartis and Roche in connection with the marketing of the drugs Lucentis and Avastin. Official Journal of the European Union C 355:p. 299–300 (EN, FR)
- 41.Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24. doi: 10.1016/j.preteyeres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Pinheiro-Costa J, Freitas-Da-Costa P, Falcão MS, et al. Switch from intravitreal ranibizumab to bevacizumab for the treatment of neovascular age-related macular degeneration: clinical comparison. Ophthalmologica. 2014;232:149–155. doi: 10.1159/000363422. [DOI] [PubMed] [Google Scholar]
- 43.Andreoli MT, Pinnolis M, Kieser T, et al. Feasibility and efficacy of a mass switch from ranibizumab (Lucentis) to bevacizumab (Avastin) for treatment of neovascular age-related macular degeneration. Digit J Ophthalmol. 2015;21:1–17. doi: 10.5693/djo.01.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada K, Kimoto K, Kono H, et al. Switching from intravitreal ranibizumab to bevacizumab for age-related macular degeneration. ISRN Ophthalmol. 2011;2011:916789. doi: 10.5402/2011/916789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.