Abstract
Objective:
To examine changes in body weight and fat in Black and White women during the first year postpartum, and to determine whether there is preferential retention of fat mass and abdominal fat.
Methods:
Body composition was quantified by dual-energy X-ray absorptiometry in Black (n=49) and White (n=85) women at 6–8 weeks, 6 months, and 12 months after delivery of a singleton infant.
Results:
Weight, fat mass, percent body fat, and fat in the trunk, android, gynoid, and limbs decreased from 6–8 weeks to 12 months in White, but not in Black women [fat mass: 29.6 (1.3) to 26.9 (1.3) kg in White and 34.5 (1.5) to 36.8 (1.8) kg in Black women, adjusted mean (SE)]. In the entire sample, fat mass was higher at 6 months than at 6–8 weeks independent of weight change; visceral fat was higher at 12 months [686 (45) gm] than at 6–8 weeks [611 (42) gm] and 6 months [626 (43) gm]), and android fat were higher at 12 months than 6 months, independent of fat changes.
Conclusions:
Black women were less likely than White women to lose weight and fat postpartum. There was preferential retention of fat in the abdominal area.
Keywords: visceral fat, body composition, fat distribution, longitudinal, physical activity
INTRODUCTION
Obesity is significantly associated with several serious diseases.1,2 Women’s body composition changes in pregnancy, and postpartum weight retention is common. At 6 months postpartum, 73% of women in a large cohort were on average 3.5 kg heavier than pre-pregnancy,3 and the average weight at 12 months postpartum ranges from 1 to 5% greater than pre-pregnancy weight.4 Previous research has shown that Black women are more prone to postpartum weight retention than White women.5,6 It has also been shown that women who retain weight at 6 or 12 months postpartum are more likely to gain more weight several years later.7,8 Thus, the literature suggests an elevated weight trajectory after pregnancy and postpartum, and more so in Black than White women.
There are limited data on body fat changes in the first year postpartum. Some suggest a reduction in fat mass or percent body fat during the first year postpartum; however, results vary depending on the period examined and the population. For example, percent body fat reduced by 1% from 2 to 6 months postpartum in 23 Swedish women,9 and adipose tissue volume was lower at 12 months than 5–10 days postpartum in 15 Swedish women.10 However, fat mass and percent body fat did not change from 2 weeks to 6 weeks and 27 weeks postpartum in a sample of predominantly White (77% of 63 total) women.11 A study in India found a lower percent body fat at 12 months, but not 6 months, than <7 days postpartum in 42 women.12 To our knowledge, no study has specifically examined body fat in Black women. Given the differences between Black and White women in weight retention,5,6 it is important to examine racial differences in body fat changes postpartum.
Central adiposity, especially excess intra-abdominal fat (visceral adipose tissue, VAT), is known to be more strongly associated with risks for metabolic abnormalities than overall amount of fat.13 Janumala et al. found that VAT (quantified by magnetic resonance imaging, MRI) increased by ~30% at 59 weeks postpartum compared to baseline (15 weeks gestation) despite no weight retention.14 Although the study enrolled a diverse population (24% of 210 being Black), the racial composition of the 68 women who completed the MRI scan at both baseline and 59 weeks postpartum was not reported. There may be an increase in VAT beyond the change of weight after the first year postpartum; however, due to the lack of data on body fat in Black women, it is unknown whether Black and White women experience similar changes in VAT.
The purpose of this study was to examine changes in body weight and body fat measures quantified by Dual-energy X-ray absorptiometry (DXA), in a sample of Black and White women during the first year postpartum. We hypothesized that Black women had less loss of weight and fat than White women up to 12 months postpartum. We also determined whether fat retention was independent of weight change, and whether there was preferential retention of fat in the central and abdominal area compared to other areas.
METHODS
Participants
Women were recruited from Columbia, South Carolina during October 2018 to January 2020. Those who were interested were screened by phone or survey between 4–6 weeks postpartum and scheduled a first study visit if eligible. Women must have reported to be either Black or White (no exclusion of mixed race), age ≥ 18 years, and have delivered a singleton at ≥ 37 weeks of gestation. Exclusion criteria included self-reported diseases or medications that might affect body weight (e.g., thyroid diseases, pre-pregnancy diabetes, pregnancy-induced hypertension that did not resolve at enrollment, clinical depression, and use of contraceptive Depo-Provera). Those who became pregnant again within the 12 months postpartum were excluded at the time of known pregnancy. The study visits occurred at 6–8 weeks and 6 and 12 months after delivery. The study was approved by the University of South Carolina Institutional Review Board (Pro00076434) and all participants signed an informed consent form.
Anthropometry and body composition
Women reported their pre-pregnancy weight. Height and postpartum weights were obtained using a calibrated digital scale while women were in standard scrubs and without shoes or outer garments. Waist and hip circumferences were measured to the nearest 0.1 cm using a tape ruler over light clothing. Waist circumference was taken around the abdomen midway between the lowest rib and the top of the iliac crest. Hip circumference was taken around the widest portion of the buttocks, with the tape parallel to the floor. Duplicate measures were performed and considered reproducible if their difference was less than 0.4 cm. Additional measurements were taken as necessary.
Body composition was determined using the DXA (enCORE, GE Healthcare model 8743, Waukesha, WI). Women were instructed not to exercise or consume a meal within 2 hours, and to limit fluid intake to <250 ml within an hour of their visit. The imaging and positioning protocols were provided by GE Healthcare, which were consistent with previous recommendations15 and the positioning protocol used in the National Health and Nutrition Examination Survey.16 Fat mass, lean mass, bone mass, and percent body fat in the whole body and specific body segments (trunk, arm, leg, android, and gynoid) were determined, and VAT was estimated by the CoreScan™ software (GE Healthcare©). The software automatically defined the regions of interest and the technician manually adjusted the lines according to the manufacturer’s protocol. The android region is between the lowest ribs and the upper part of iliac wings around the waist. The gynoid region includes the gluteo-femoral region. VAT was estimated within the android region. VAT estimated by DXA using this method has been shown to be precise and interchangeable with that quantified by computed tomography (CT) and MRI.17,18 The same technician who completed the enCORE™ Operator Training performed all the scans and measurement protocols. The test-retest reliability determined from reanalysis of 10 scans by this technician was 0.998. The machine was calibrated using a phantom per manufacturer instructions. Of the total 317 DXA scans, 25 did not provide VAT (10, 9, and 6 at each time point) because the abdomen was considered not in range.
Covariates
Women were asked to report their date and mode of delivery, and number of pregnancies and live births during screening. At each time point, women were asked to complete several questionnaires. A sociodemographic questionnaire included questions regarding demographics, socioeconomic information, cigarette smoking, and breastfeeding (see Table 1 for categories). Breastfeeding status was categorized to exclusively breastfeeding (including pumping milk; infant does not use formula or other source of nutrients), some (infant also uses other source of nutrients), or not breastfeeding currently. Other questionnaires included the Perceived Stress Scale,19 Edinburgh Postnatal Depression Scale,20 Rosenberg Self-Esteem Scale,21 and Pittsburgh Sleep Quality Index.22 The summary scores for these questionnaires were calculated according to their respective instructions. A higher score from these scales indicates greater stress, more depressive symptoms, higher self-esteem, and worse overall sleep quality, respectively.
Table 1.
Participant characteristics at 6–8 weeks postpartum
| Characteristics | Black N = 49 | White N = 85 | P values |
|---|---|---|---|
|
| |||
| Age, year | 28.0 ± 5.1 | 31.7 ± 4.1 | < 0.001 |
| Height, cm | 164.0 ± 5.0 | 164.5 ± 5.6 | 0.641 |
| *Pre-pregnancy weight, kg | 82.1 ± 26.0 | 71.7 ± 16.3 | 0.014 |
| †Pre-pregnancy BMI, kg•m−2 | 30.3 ± 8.6 | 26.5 ± 6.1 | 0.008 |
| ‡Weight retention, kg | 2.5 ± 7.2 | 6.0 ± 9.7 | 0.017 |
| Education, n (%) ≤ 12th grade or high school graduate Some college Graduate school |
7 (14.3) 33 (67.4) 9 (18.4) |
6 (7.1) 49 (57.7) 30 (35.3) |
0.074 |
| Family income, n (%) < $40,000 $40,000–60,000 $60,000–80,000 ≥ 80,000 |
21 (42.9) 12 (24.5) 9 (18.4) 7 (14.3) |
19 (22.35) 14 (16.5) 11 (12.9) 41 (48.2) |
0.001 |
| Currently not working, n (%) | 32 (65.3) | 63 (74.1) | 0.557 |
| Living with spouse, n (%) | 36 (73.5) | 82 (96.5) | <0.001 |
| Smoking status, n (%) Never History of smoking Currently smoking |
35 (71.4) 10 (20.4) 4 (8.2) |
67 (78.8) 14 (16.5) 4 (4.7) |
0.653 |
| Primipara, n (%) | 18 (36.7) | 34 (40.0) | 0.709 |
| Vaginal delivery, n (%) | 34 (69.4) | 61 (75.3) | 0.461 |
| Breastfeeding, n (%) Exclusively Some No |
25 (51.0) 7 (14.3) 17 (34.7) |
68 (80.0) 8 (9.4) 9 (10.6) |
0.001 |
| Physical activity, counts per minute | 325 ± 112 | 463 ± 131 | <0.001 |
| Daily servings of fruits and vegetables | 4.3 ± 4.6 | 2.6 ± 1.7 | 0.019 |
| Perceived Stress Scale score | 13.1 ± 7.8 | 10.9 ± 6.3 | 0.072 |
| Edinburgh Postnatal Depression Scale score | 5.2 ± 4.2 | 3.3 ± 3.0 | 0.008 |
| Rosenberg Self-Esteem Scale score | 34.2 ± 4.9 | 33.5 ± 4.2 | 0.375 |
| Pittsburgh Sleep Quality Index score | 9.2 ± 3.4 | 7.3 ± 2.9 | 0.001 |
Data are mean ± SD or n (%). BMI, body mass index. P values are for comparison between Black and White women using t-test or chi-square test.
, self-reported.
, pre-pregnancy BMI was calculated using self-reported pre-pregnancy weight and measured height at 6–8 weeks after delivery.
, weight retention was calculated using weight at 6–8 weeks minus self-reported pre-pregnancy weight.
Women were also asked to complete the National Cancer Institute’s All-Day screener, which includes questions on frequency and portion sizes for 9 fruit and vegetable items.23 The total daily number of servings of fruits and vegetables was the sum across all food groups. This screener was considered useful to estimate intakes of fruit and vegetable servings in the US population, although not recommended for assessing precise food intake.24
Additionally, women were instructed to wear a triaxial accelerometer (GT3X+, ActiGraph LLC, Pensacola, FL) on the hip for 7 days during waking hours to assess physical activity at each time point. The manufacturer-provided software ActiLife was used to set up the monitor and download and process data. The parameter “counts per minute” combines the magnitude of acceleration from all three axes and was used as a metric of total physical activity.
Statistical analysis
Differences in continuous or categorical measures between races at baseline (6–8 weeks postpartum) were determined using t-tests or chi-square tests, as appropriate, respectively. Mixed-effects linear models including a race by time interaction were performed to examine whether the trajectories of anthropometry and body composition variables between Black and White women differed after adjusting for confounders. A random intercept was used to allow for various baseline values among individuals, and account for correlation within women’s measurements. When a significant race by time interaction was found, analyses stratified by race were conducted. Because we were interested in differences in outcome variables between each pair of time points, no adjustment for multiple testing was used for these comparisons. Separate models were run for each outcome of interest.
The potential confounders that were considered included covariates obtained at baseline which did not change or had little change to 12 months postpartum (age at delivery, pre-pregnancy weight, parity, and household income, education, whether living with a spouse, and smoking status), and time-varying covariates, which included breastfeeding, physical activity, working status, daily servings of fruits and vegetables, and summary scores of stress, depressive symptoms, self-esteem, and sleep quality. Bivariate associations were first performed to determine associations of these variables with the outcome variables at each time point. All variables with moderate or stronger associations (p < 0.15) were adjusted in the final mixed-effects models. Analyses were conducted using the SAS software (9.4. SAS Institute, Cary, NC). Statistical significance was defined as p < 0.05.
We planned to retain > 96 women (equal racial distribution) in the study. That would provide > 80% power to determine whether race significantly interacts with time, based on Monte Carlo simulation of the linear mixed model.
RESULTS
Participants
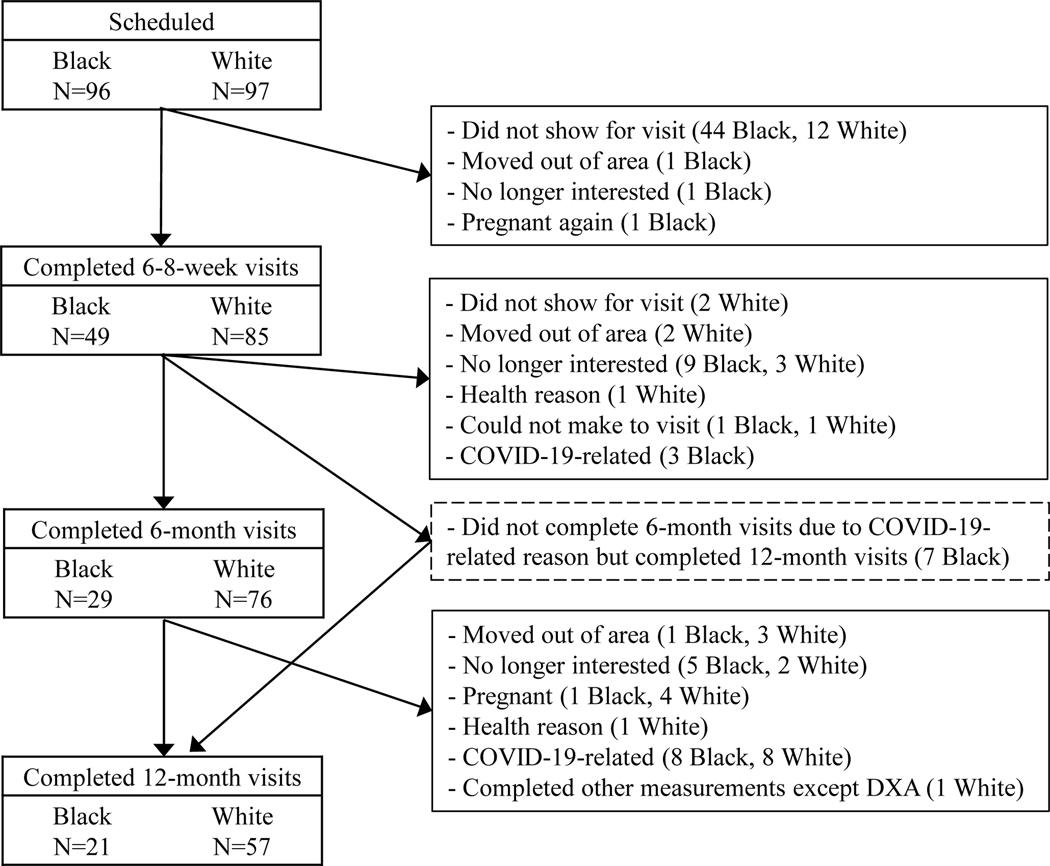
A total of 193 women were eligible and scheduled for the first visit. Of them, 134 (49 Black and 85 White) completed the baseline measurements (Figure 1). Of note, 11 Black and 8 White women dropped out of the study due to COVID-19 related issues (i.e., university campus closure, childcare, concerns, etc.), and 7 additional Black women missed their 6-month visits but completed the 12-month visits.
Figure 1.
Sample size flowchart. The 6-month and 12-month visits were allowed to be within 4 weeks after 6 and 12 months postpartum.
As shown in Table 1, compared to White participants, Black participants were younger, had a greater proportion with lower family income, and had a smaller proportion living with spouse. Before pregnancy, Black participants were heavier and had higher BMI. Their weight retention at baseline, however, was less than White participants. They also had a lower breastfeeding rate, lower physical activity, more depressive symptoms, worse overall sleep quality, but more servings of fruits and vegetables at baseline than White participants. The two racial groups were not different in education level, smoking status, and the percentages of first-time giving birth and vaginal delivery.
Anthropometry and body composition changes
The trajectories of body weight and waist and hip circumferences over time were different between Black and White participants (p values for time by race interaction < 0.05 for all). As shown in Table 2, in White women, body weight decreased from baseline to 6 months and further decreased until 12 months postpartum. Waist and hip circumferences both decreased from baseline to 6 months. Hip circumference at 12 months continued to be lower than at baseline, but not different than 6 months. Waist circumference at 12 months was not different from either baseline or 6 months. In Black women, however, body weight and hip circumference did not change over the period from baseline to 12 months postpartum. Waist circumference increased and it was higher at 12 months than baseline.
Table 2.
Body weight and circumferences during the first year postpartum by race
| Black |
White |
|||||
|---|---|---|---|---|---|---|
| Mean (SE) | P values, overall trend | P values, compared with the previous time point | Mean (SE) | P values, overall trend | P values, compared with the previous time point | |
|
| ||||||
| Body weight, kg | 0.234 | <0.001 | ||||
| 6–8 weeks | 85.8 (1.2) | 76.7 (1.0) | ||||
| 6 months | 86.0 (1.4) | 0.878 | 74.6 (1.0) | <0.001 | ||
| 12 months | 88.1 (1.5) | 0.153 | 73.4 (1.1)a | 0.044 | ||
| Waist circumference, cm | 0.015 | 0.032 | ||||
| 6–8 weeks | 93.2 (1.9) | 89.2 (1.8) | ||||
| 6 months | 95.9 (2.0) | 0.055 | 87.2 (1.8) | 0.009 | ||
| 12 months | 97.9 (2.2)b | 0.204 | 87.8 (1.9) | 0.487 | ||
| Hip circumference, cm | 0.149 | <0.001 | ||||
| 6–8 weeks | 111.5 (1.2) | 110.0 (1.6) | ||||
| 6 months | 111.8 (1.3) | 0.781 | 107.6 (1.6) | <0.001 | ||
| 12 months | 113.8 (1.5) | 0.107 | 106.6 (1.7)a | 0.138 | ||
Means are least squares means from mixed-effects models. Pre-pregnancy weight, weight retention at 6–8 weeks postpartum (weight at 6–8 weeks minus pre-pregnancy weight), parity, household income, education, marital status, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity were adjusted in the models. The visits at 6 and 12 months were within 4 weeks after the respective postpartum time point.
, p<0.001,
, p=0.005 compared to 6–8 weeks.
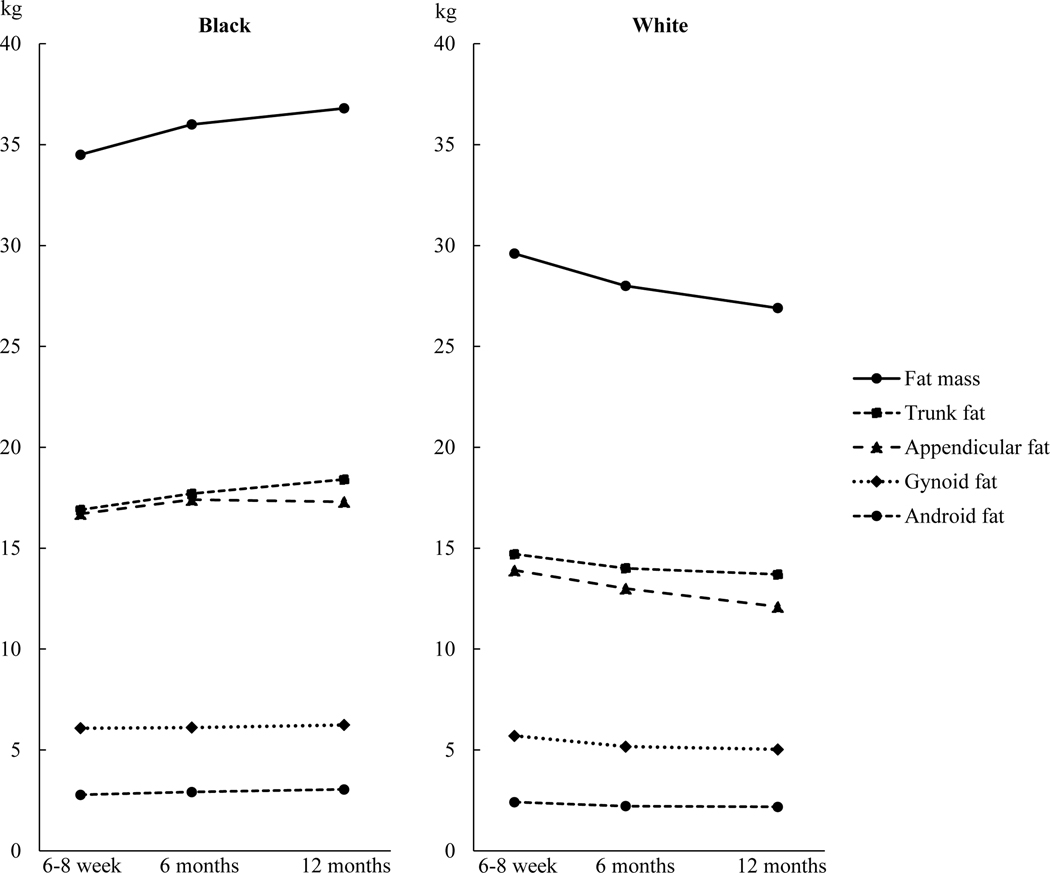
Table 3 and Figure 2 present body composition variables from the DXA scan. The trajectories of the fat measures were different between Black and White participants (p values for time by race interaction < 0.05 for all). In White women, fat mass, percent body fat, and appendicular fat continued to decrease from baseline to 6 and 12 months postpartum. Trunk, android, and gynoid fat decreased, and the decrease mostly occurred between baseline to 6 months; at 12 months, they remain to be lower than at baseline. However, VAT did not change over time.
Table 3.
Body composition determined by dual-energy X-ray absorptiometry during the first year postpartum by race
| Black |
White |
|||||
|---|---|---|---|---|---|---|
| Mean (SE) | P values, overall trend | P values, compared with the previous time point | Mean (SE) | P values, overall trend | P values, compared with the previous time point | |
|
| ||||||
| Fat mass, kg | 0.196 | <0.001 | ||||
| 6–8 weeks | 34.5 (1.5) | 29.6 (1.3) | ||||
| 6 months | 36.0 (1.6) | 0.197 | 28.0 (1.3) | <0.001 | ||
| 12 months | 36.8 (1.8) | 0.534 | 26.9 (1.3)a | 0.034 | ||
| Lean mass, kg | 0.332 | <0.001 | ||||
| 6–8 weeks | 48.5 (1.0) | 44.5 (1.0) | ||||
| 6 months | 48.0 (1.1) | 0.267 | 43.8 (1.0) | <0.001 | ||
| 12 months | 48.6 (1.1) | 0.190 | 43.5 (1.0)a | 0.121 | ||
| Body fat, % | 0.674 | <0.001 | ||||
| 6–8 weeks | 38.7 (1.2) | 38.3 (1.4) | ||||
| 6 months | 39.3 (1.3) | 0.420 | 37.0 (1.4) | <0.001 | ||
| 12 months | 39.3 (1.4) | 0.987 | 36.1 (1.5)a | 0.025 | ||
| Appendicular fat, kg | 0.442 | <0.001 | ||||
| 6–8 weeks | 16.7 (0.9) | 13.9 (0.7) | ||||
| 6 months | 17.4 (1.0) | 0.229 | 13.0 (0.7) | <0.001 | ||
| 12 months | 17.3 (1.0) | 0.923 | 12.1 (0.7)a | <0.001 | ||
| Trunk fat, kg | 0.138 | 0.012 | ||||
| 6–8 weeks | 16.9 (0.9) | 14.7 (0.8) | ||||
| 6 months | 17.7 (1.0) | 0.242 | 14.0 (0.8) | 0.008 | ||
| 12 months | 18.4 (1.1)c | 0.308 | 13.7 (0.8)b | 0.411 | ||
| Android fat, gm | 0.169 | <0.001 | ||||
| 6–8 weeks | 2774 (192) | 2411 (176) | ||||
| 6 months | 2912 (202) | 0.259 | 2217 (175) | <0.001 | ||
| 12 months | 3040 (192) | 0.337 | 2177 (180)b | 0.502 | ||
| Gynoid fat, gm | 0.814 | <0.001 | ||||
| 6–8 weeks | 6077 (325) | 5695 (282) | ||||
| 6 months | 6108 (345) | 0.893 | 5166 (281) | <0.001 | ||
| 12 months | 6238 (372) | 0.606 | 5023 (290)a | 0.146 | ||
| *Visceral adipose tissue, gm | 0.025 | 0.467 | ||||
| 6–8 weeks | 667 (62) | 558 (82) | ||||
| 6 months | 694 (65) | 0.507 | 528 (82) | 0.219 | ||
| 12 months | 803 (72)b | 0.025 | 532 (84) | 0.896 | ||
Means are least squares means from mixed-effects models. Pre-pregnancy weight, weight retention at 6–8 weeks postpartum (weight at 6–8 weeks minus pre-pregnancy weight), parity, household income, education, marital status, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity were adjusted in the models. The visits at 6 and 12 months were within 4 weeks after the respective postpartum time point.
, Sample size for visceral adipose tissue (VAT): 43, 26, and 18 Black women, and 81, 70, and 54 White women, at 6–8 weeks, 6 months, and 12 months, respectively.
, p ≤0.0001;
, p < 0.01;
, p < 0.05, compared to 6–8 weeks.
Figure 2.
Fat mass (kg) in the whole body and body segments at 6–8 weeks, 6 months, and 12 months postpartum by race. Data are least squares means from mixed-effects models, with pre-pregnancy weight, weight retention at 6–8 weeks postpartum (weight at 6–8 weeks minus pre-pregnancy weight), parity, household income, education, spouse, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity adjusted.
In Black women, fat mass, percent body fat, and appendicular, android, and gynoid fat did not change and were not different between any two time points. Trunk fat, however, was higher at 12 months than baseline. VAT increased and the increase mostly occurred between 6 and 12 months, with it higher at 12 months than both 6–8 weeks and 6 months.
Lean mass in Black and White women also changed differently (p for time by race interaction = 0.029). As shown in Table 3, in White women, lean mass decreased and the decrease mostly occurred from 6–8 weeks to 6 month. In Black women, no significant differences were found between any two time points.
Additional analyses were conducted and modifications to the abovementioned adjusted models include: 1) to adjust for weight at 6–8 weeks postpartum instead of self-reported pre-pregnancy weight and weight retention at baseline, and 2) to adjust for self-reported pre-pregnancy weight and without adjusting for weight retention at 6–8 weeks postpartum. Results are very similar to the main results (Tables S1 and S2, respectively).
Relative changes of fat
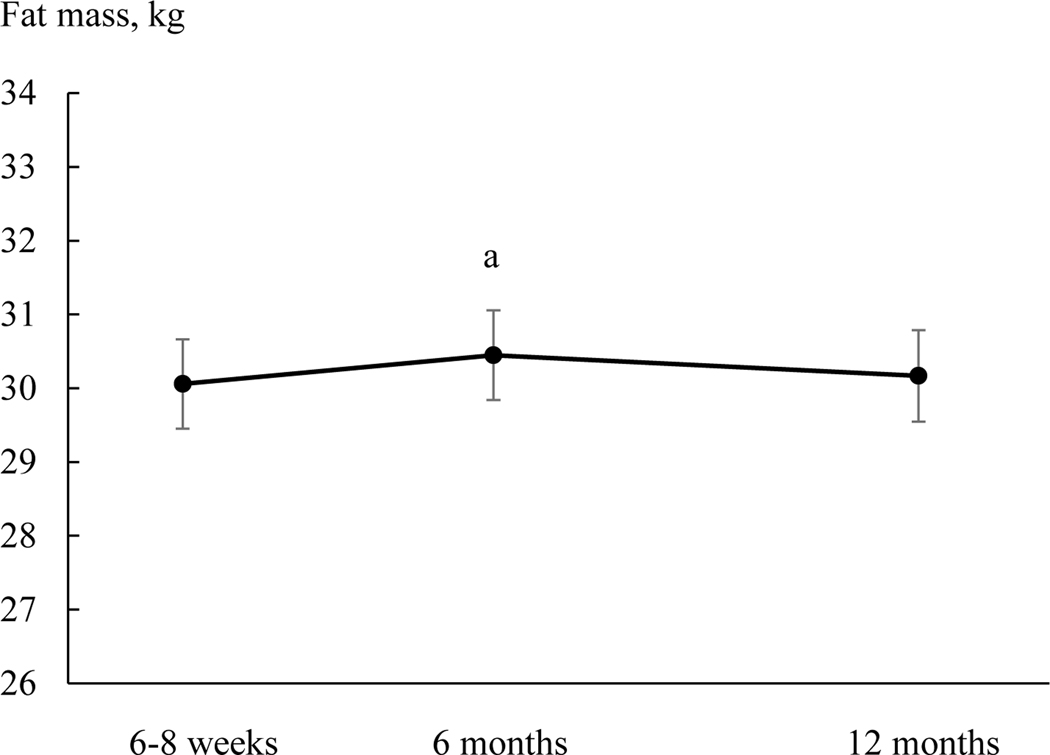
To examine changes in fat mass relative to body weight, weight was added to the mixed-effects model as a time-varying variable. Fat mass no longer changed differently between Black and White participants (p for time by race interaction = 0.115). In the overall sample, fat mass at 6-month [30.4 (0.6) kg] was higher than at 6–8 weeks [30.1 (0.6) kg] (Figure 3).
Figure 3.
Total fat mass at 6–8 weeks, 6 months, and 12 months postpartum in the overall sample. Means are least squares means and error bars are SE from mixed-effects models with adjustment of body weight, parity, household income, education, spouse, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity. a, p = 0.028 compared to 6–8 weeks.
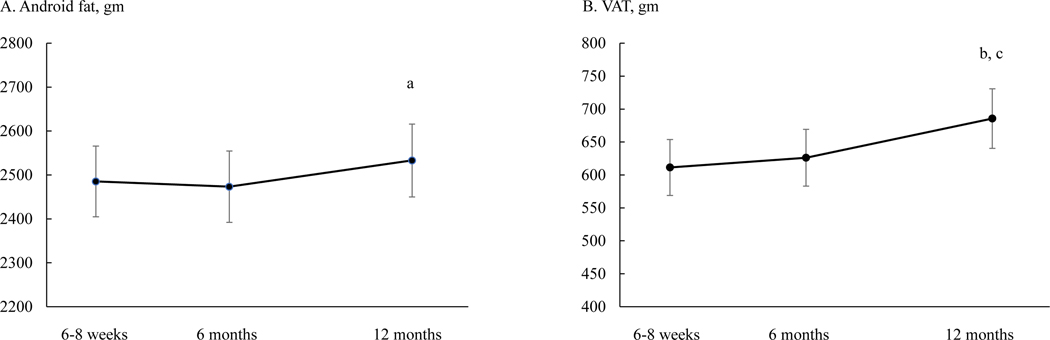
Similarly, to examine changes in central adiposity (android fat and VAT) relative to total fat, fat mass was added to the mixed-effects models as a time-varying variable. Both android fat and VAT no longer changed differently between Black and White participants (p for time by race interaction = 0.547 and 0.409, respectively). In the overall sample, android fat at 12 months [2533 (83) gm] was higher than at 6 months [2473 (81) gm] (Figure 4A). VAT at 12 months [686 (45) gm] was higher than both 6–8 weeks [611 (42) gm] and 6 months [626 (43) gm] (Figure 4B).
Figure 4.
Android fat (panel A) and visceral adipose tissue (VAT, panel B) at 6–8 weeks, 6 months, and 12 months postpartum in the overall sample. Means are least squares means and error bars are SE from mixed-effects models with adjustment of total fat mass, parity, household income, education, spouse, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity. a, p = 0.034 compared to 6 months. b, p = 0.002 compared to 6–8 weeks; c, p = 0.005 compared to 6 months.
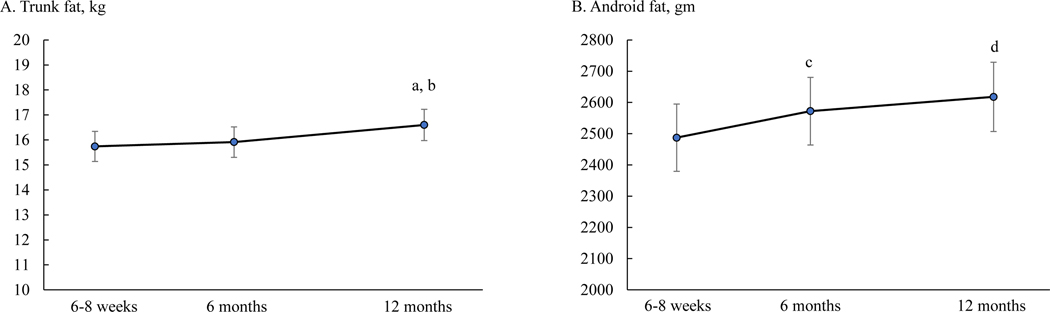
To further examine changes in fat distribution, appendicular fat was added to the mixed-effects model for trunk fat as a time-varying variable, and gynoid fat was added to the model for android fat. Both trunk fat and android fat no longer changed differently between Black and White participants (p = 0.259 and 0.301, respectively). In the overall sample, trunk fat at 12 months [16.6 (0.6) kg] was greater than at 6–8 weeks [15.7 [0.6] kg] and 6 months [15.9 (0.6) kg]; android fat at 12 months [2618 (111) gm] and 6 months [2572 (108) gm] were both greater than at 6–8 weeks [2487 (108) gm] (Figure 5).
Figure 5.
Trunk fat (panel A) and android fat (panel B) at 6–8 weeks, 6 months, and 12 months postpartum in the overall sample. Means are least squares means and error bars are SE from mixed-effects models with adjustment of appendicular fat (for trunk fat), gynoid fat (for android fat), parity, household income, education, spouse, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity. a, p = 0.003 compared to 6–8 weeks; b, p = 0.008 compared to 6 months; c, p = 0.017 compared to 6–8 weeks; d, p = 0.003 compared to 6–8 weeks.
Covariates of weight and body composition changes
In the adjusted mixed-effects models, pre-pregnancy weight was a positive predictor of postpartum weight, waist and hip circumferences, all fat measures, and lean mass in both Black and White women (p < 0.001 for all). Weight retention as baseline was a positive predictor for all the outcome variables (p < 0.05) except for VAT in Black women (p = 0.120). Other covariates (parity, household income, education, spouse, Pittsburgh Sleep Quality Index score, breastfeeding status, smoking status, and physical activity), however, were associated with different outcome variables between Black and White women (Tables S3 and S4). For example, greater physical activity was associated with lower waist and hip circumferences and less percent body fat in Black women, but greater lean mass in White women.
DISCUSSION
To our knowledge, this is among the first studies examining body fat determined by DXA along with body weight longitudinally in the postpartum period (from early postpartum to12 months after delivery) in Black and White women. We found that weight and fat measures changed differently between Black and White women, such that they (except VAT) decreased in White but did not decrease (trunk fat and VAT even increased) in Black women. Other important findings include: in the entire sample, fat mass increased from 6–8 weeks to 6 months with adjustment for weight; android fat increased from 6 to 12 months and VAT also increased from 6–8 weeks and 6 months to 12 months, with adjustment for fat mass; trunk fat increased with adjustment for appendicular fat; and android fat increased with adjustment for gynoid fat.
Few studies have quantified fat multiple times and up to 12 months, and the period being examined influenced the results. For example, Swedish women were found to have a continuous reduction in adipose tissue volume that became significantly different at 12 months from 5–10 days postpartum,10 and in a sample of predominantly White (77% of 63 total) women in the US, total fat mass and percent body fat did not change significantly from 2 weeks to 6 weeks and 27 weeks postpartum.11 In the White women in our study, continuous decreases in total fat mass and percent body fat occurred over the observation period.
In White women, fat mass decreased in the whole body, and appendicular, trunk, android, and gynoid regions. Interestingly, the amount of fat in the trunk, android, and gynoid region only decreased from 6–8 weeks to 6 months, but appendicular fat continued to decrease from 6 months to 12 months. Previous studies also showed reductions in trunk and leg fat in the postpartum period,25 and fat mobilization from the thighs being the most consistent and complete in all women.10 Our results indicate different patterns of change for fat in the trunk region compared to appendicular fat.
Our data in Black women are unique, as we are not aware of other studies that have specifically examined body fat in the postpartum period longitudinally in Black women. Our results indicate that the changes in weight, waist and hip circumferences, fat measures and lean mass contrasted with White women; they did not decrease, and waist circumference, trunk fat and VAT even increased, with adjustment for covariates. It is previously recognized that Black women are more likely to retain weight after pregnancy than White women. Our results demonstrated that racial differences also exist for fat mass; Black women did not lose fat in the whole body or any segment that we have evaluated. These racial differences were independent of several covariates. Examining energy intake and energy expenditure may help explain the racial differences. We also suspect that there may be differential metabolic changes in pregnancy between Black and White women. In support of this notion, after having gestational diabetes, Black women have higher risk for diabetes than White women after adjusting for covariates.26 Future research is needed to examine why racial differences in postpartum body composition changes exist.
Previous studies suggest an increase in VAT due to pregnancy. A cross-sectional study found higher VAT (by DXA) in women with higher parity independent of percent body fat.27 Another study found women who had given birth once during a 5-year follow-up had a 3-fold greater increase in VAT (by CT) and greater increase in waist-to-hip ratio compared to women who had not given birth during a 5-year period, independent of weight change.28,29 More recently, Janumala et al. found that VAT (by MRI) increased by ~30% at 59 weeks postpartum compared to 15 weeks gestation, despite no weight retention.14 Due to differences in study design, we cannot directly compare with these studies, and cannot compare to pre-pregnancy level. However, we found that with adjustment of fat mass, VAT was significantly higher at 12 months than 6–8 weeks and 6 months, and android fat was higher at 12 months than 6 months, therefore supporting previous studies’ findings. We also found that trunk fat increased with appendicular fat adjusted, and android fat increased with gynoid fat adjusted. Interestingly, with body weight adjusted, total fat mass was higher at 6 months than 6–8 weeks; but at 12 months, fat mass was not different from either 6–8 weeks or 6 months. Taken together, there was disproportionate changes in fat in different regions and depots. As a result, at 12 months postpartum, there was more fat being stored in the trunk, android, and intra-abdominal visceral area relative to other regions of the body. Previous studies have shown DXA-derived central adiposity measures (trunk fat, android fat, and VAT) have strong associations with cardiometabolic risk factors.30–32 Thus, these changes in fat distribution could have critical implications for women’s long-term cardiometabolic health.
In our study, behavioral and lifestyle factors, including physical activity, breastfeeding, sleep, and smoking, were found to be correlated with several body composition variables. Consistent with findings from a previous study,27 currently smoking was associated with greater abdominal fat in both Black and White women. Other associations appear to be different between Black and White women. In Black women, total physical activity was correlated with whole body fat and waist and hip circumferences. Lower sleep quality was correlated with higher abdominal fat. In White women, physical activity was correlated with lean mass only, and exclusive breastfeeding was correlated with lower fat in the gynoid region and limbs. Together, these results suggest that these behavioral and lifestyle factors could influence postpartum body composition differently for women with different races.
The strengths of this study include that a cohort of Black and White women was longitudinally followed from 6–8 weeks to 12 months postpartum, and total and regional body composition was quantified using the DXA three times in this period, which allowed comparison of postpartum body composition changes between Black and White women. Also, several covariates were examined and controlled for in analyses. However, due to costs and logistics, we used DXA rather than MRI or CT to quantify VAT. Our findings cannot be generalized to other racial and ethnic groups. We only examined intake of fruits and vegetables; thus, our findings may subject to residual confounding due to limited dietary information. Furthermore, we did not measure weight or body composition before pregnancy, and did not have information on gestational weight gain. However, our results suggest that the racial differences in the changes in weight and body composition after 6–8 weeks postpartum are likely not fully attributed to differences in gestational weight gain.
Fewer Black than White women completed the first study visit. Due to the limit of the funding period and longer time to find effective ways to recruit Black women, we were not able to recruit additional Black women. Furthermore, due to the COVID-19 pandemic, we were not able to conduct DXA scans for almost 4 months. Many of the Black women missed the timing of their 6- or 12-month body composition measurements, while most White women already completed the 6-month visit. This is reflected by the large reduction of sample size by 6 and 12 months for Black women, and only by 12 months for White women. A few Black women who missed the 6-month visit were able to complete the 12-month visit. The mixed-effects model we used in the analysis included all available data, regardless of participants completing some or all visits.
CONCLUSION
Our study showed that Black and White women differ in the trajectories of anthropometry, and body fat measures and lean mass determined by DXA, from 6–8 weeks to 6 and 12 months postpartum. With confounders adjusted, White women demonstrated decreased body fat in the whole body and segments of the body, while Black women did not show any decrease. VAT and android fat increased when fat mass was adjusted; trunk fat increased when appendicular fat was adjusted; and android fat increased when gynoid fat was adjusted. These results suggesting preferential retention of fat in the trunk and abdominal area. Behavioral and lifestyle factors might influence body composition in the postpartum period. Future studies are recommended to further examine the contribution of these factors to body composition in greater detail and whether intervening on these factors could modify postpartum body composition.
Supplementary Material
STUDY IMPORTANCE.
What is already known about this subject?
Postpartum weight retention is common, and Black women tend to retain more weight than White women.
There is increased abdominal visceral adipose tissue due to pregnancy independent of weight change.
What are the new findings in your manuscript?
From 6–8 weeks to 12 months postpartum, fat mass, percent body fat, and fat in appendicular, trunk, android, and gynoid regions decreased in White, but not in Black women, after adjustment for covariates. Visceral fat did not change in White, but increased in Black women.
Fat mass increased from 6–8 weeks to 6 months postpartum, independent of weight. Android fat increased from 6 to 12 months and visceral fat increased from 6–8 weeks and 6 months to 12 months, independent of fat mass.
How might your results change the direction of research or the focus of clinical practice?
The mechanisms for the differences between Black and White women in body composition changes postpartum need to be investigated.
Future research is needed to further examine whether and how behavioral and lifestyle factors may influence postpartum body composition differently in Black and White women.
Acknowledgements
We thank the research assistants, clinic staff and administrators for their assistance in recruitment of participants and data collection. We also thank the participants for participating in the study.
Funding:
This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R21MD012740. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Bray GA, Kim KK, Wilding JPH, on behalf of the World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obesity Reviews. 2017;18(7):715–723. [DOI] [PubMed] [Google Scholar]
- 2.Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a Disease: The Obesity Society 2018 Position Statement. Obesity (Silver Spring, Md). 2019;27(1):7–9. [DOI] [PubMed] [Google Scholar]
- 3.Hollis JL, Crozier SR, Inskip HM, et al. Modifiable risk factors of maternal postpartum weight retention: an analysis of their combined impact and potential opportunities for prevention. Int J Obes. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity - results of a systematic review and meta-analysis on the natural history of postpartum weight retention. International Journal Of Obesity (2005). 2007;31(11):1642–1651. [DOI] [PubMed] [Google Scholar]
- 5.Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. American Journal Of Public Health. 1993;83(8):1100–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker JD, Abrams B. Differences in postpartum weight retention between black and white mothers. Obstetrics and gynecology. 1993;81(5 ( Pt 1)):768–774. [PubMed] [Google Scholar]
- 7.Kirkegaard H, Stovring H, Rasmussen KM, Abrams B, Sørensen TI, Nohr EA. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. The American Journal of Clinical Nutrition. 2014;99(2):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linné Y, Dye L, Barkeling B, Rössner S. Long-Term Weight Development in Women: A 15-Year Follow-up of the Effects of Pregnancy. Obesity Research. 2004;12(7):1166–1178. [DOI] [PubMed] [Google Scholar]
- 9.Sadurskis A, Kabir N, Wager J, Forsum E. Energy metabolism, body composition, and milk production in healthy Swedish women during lactation. The American journal of clinical nutrition. 1988;48(1):44–49. [DOI] [PubMed] [Google Scholar]
- 10.Sohlström A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. The American Journal of Clinical Nutrition. 1995;61(2):287–295. [DOI] [PubMed] [Google Scholar]
- 11.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EOB. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. American Journal of Obstetrics and Gynecology. 2003;189(5):1423–1432. [DOI] [PubMed] [Google Scholar]
- 12.Kajale NA, Khadilkar VV, Mughal Z, Chiplonkar SA, Khadilkar AV. Changes in body composition of Indian lactating women: a longitudinal study. Asia Pacific journal of clinical nutrition. 2016;25(3):556–562. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MD. Role of Body Fat Distribution and the Metabolic Complications of Obesity. The Journal of clinical endocrinology and metabolism. 2008;93(11 Suppl 1):S57–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janumala I, Toro-Ramos T, Widen E, et al. Increased Visceral Adipose Tissue Without Weight Retention at 59 Weeks Postpartum. Obesity. 2020;28(3):552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watts NB. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA). Osteoporos Int. 2004;15(11):847–854. [DOI] [PubMed] [Google Scholar]
- 16.National Health and Nutrition Examination Survey (NHANES). Centers for Disease Control and Prevention. wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf.
- 17.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). 2012;20(6):1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree CD, LaFountain RA, Hyde PN, et al. Quantification of Human Central Adipose Tissue Depots: An Anatomically Matched Comparison Between DXA and MRI. Tomography. 2019;5(4):358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 20.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150(6):782. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg M.Society and the adolescent self-image. Princeton, NJ: University Press; 1965. [Google Scholar]
- 22.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 23.National Institute of Cancer. Scoring the All-Day Screener. https://epi.grants.cancer.gov/diet/screeners/fruitveg/scoring/allday.html. Accessed October 25, 2021.
- 24.Thompson FE, Subar AF, Smith AF, et al. Fruit and vegetable assessment: performance of 2 new short instruments and a food frequency questionnaire. J Am Diet Assoc. 2002;102(12):1764–1772. [DOI] [PubMed] [Google Scholar]
- 25.Butte NF, Hopkinson JM. Body Composition Changes during Lactation Are Highly Variable among Women. The Journal of Nutrition. 1998;128(2):381S–385S. [DOI] [PubMed] [Google Scholar]
- 26.Bower JK, Butler BN, Bose-Brill S, Kue J, Wassel CL. Racial/Ethnic Differences in Diabetes Screening and Hyperglycemia Among US Women After Gestational Diabetes. Prev Chronic Dis. 2019;16:E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaudeau TE, Hunter GR, Sirikul B. Intra-abdominal adipose tissue deposition and parity. Int J Obes (Lond). 2006;30(7):1119–1124. [DOI] [PubMed] [Google Scholar]
- 28.Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing May Increase Visceral Adipose Tissue Independent of Overall Increase in Body Fat. Obesity. 2008;16(5):1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. Jama. 1994;271(22):1747–1751. [PubMed] [Google Scholar]
- 30.Zhu K, Walsh JP, Murray K, Hunter M, Hui J, Hung J. DXA-Derived vs Standard Anthropometric Measures for Predicting Cardiometabolic Risk in Middle-Aged Australian Men and Women. J Clin Densitom. 2022;25(3):299–307. [DOI] [PubMed] [Google Scholar]
- 31.Rothney MP, Catapano AL, Xia J, et al. Abdominal visceral fat measurement using dual-energy X-ray: association with cardiometabolic risk factors. Obesity (Silver Spring). 2013;21(9):1798–1802. [DOI] [PubMed] [Google Scholar]
- 32.Konieczna J, Abete I, Galmés AM, et al. Body adiposity indicators and cardiometabolic risk: Cross-sectional analysis in participants from the PREDIMED-Plus trial. Clin Nutr. 2019;38(4):1883–1891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.