Abstract
Introduction:
Diabetic kidney disease (DKD) is a leading cause of mortality in people with type 2 diabetes (T2D) and over 50% of individuals with youth-onset T2D will develop DKD as a young adult. Diagnosis of early-onset DKD remains a challenge in young persons with T2D secondary to a lack of available biomarkers for early DKD while the injuries may still be reversible. Furthermore, multiple barriers exist to initiate timely prevention and treatment strategies for DKD including a lack of Food and Drug Administration approval of medications in pediatrics; provider comfort with medication prescription, titration, and monitoring; and medication adherence.
Areas Covered:
Therapies that have promise for slowing DKD progression in youth with T2D include metformin, renin-angiotensin-aldosterone system inhibitors, glucagon-like peptide-1 receptor agonists, sodium glucose co-transporter 2 inhibitors, thiazolidinediones, sulfonylureas, endothelin receptor agonists, and mineralocorticoid antagonists. Novel agents are also in development to act synergistically on the kidneys with the aforementioned medications. We comprehensively review the available pharmacologic strategies for DKD in youth-onset T2D including mechanisms of action, potential adverse effects, and kidney-specific effects, with an emphasis on published pediatric and adult trials.
Expert Opinion:
Large clinical trials evaluating pharmacologic interventions targeting treatment of DKD in youth-onset T2D are strongly needed.
Keywords: Diabetic kidney disease, pediatrics, therapies, treatments, youth-onset type 2 diabetes
1. Introduction
Global rates of type 2 diabetes (T2D) are persistently rising and remain closely tied to rising rates of metabolic syndrome features including insulin resistance and associated glucose intolerance, central obesity, hyperlipidemia, and hypertension [1]. In comparison to adult-onset T2D, youth-onset T2D exhibits a more severe and progressive phenotype with increased β-cell destruction and insulin resistance [2, 3], which not only results in a decreased life expectancy but also a more significant risk for micro- and macro-vascular complications. Diabetic kidney disease (DKD) develops from a complex interplay of pathophysiologic factors in T2D including upregulation of intracellular and metabolic pathways, increased production of reactive oxygen species, inflammatory and epigenetic changes, dysregulated autophagy, and end-organ hypoxia which culminate in increased intraglomerular pressure and hyperfiltration in the early phases of DKD and subsequently lead to disease progression [4]. DKD is strongly associated with cardiovascular disease and both conditions serve as the leading causes for morbidity and mortality in T2D [5], with metabolic, morphologic, and molecular features occurring early in the disease course, long before laboratory evidence such as albuminuria or changes in glomerular filtration rate (GFR) are evident. In the Treatment Options for Type 2 Diabetes in Adolescents and Youth Follow-Up Study (TODAY2), DKD exhibited a 15-year cumulative incidence of greater than 50% [5-9] in youth-onset T2D, direct evidence of a significantly underrecognized and undertreated complication. Yet, recommendations for the evaluation and treatment of early DKD in youth with T2D are limited by a paucity of dedicated pediatric trials (Table 1) and are formulated based on data that are inferred from studies completed in adults. In this review, we comprehensively appraise the currently available pharmacologic management strategies for DKD in youth and adult-onset T2D, with an emphasis on therapies that are approved by the United States Food and Drug Administration (FDA) for use in youth with T2D, as well as therapies that are currently being used off label or are in development.
Table 1.
Published Pediatric Clinical Trials in Youth
| Medication class |
Trial (Reference) |
Agent | Number of participants |
Condition | Phase | Design | Duration of treatment (weeks) |
Outcomes |
|---|---|---|---|---|---|---|---|---|
|
Metformin
TZD |
NCT00081328 [10] | Metformin Rosiglitazone |
699 | T2D | 3 | Randomized, parallel assignment, quadruple blinded | 6 months | Loss of glycemic control |
| GLP-1RA | NCT01541215 [11] | Liraglutide | 135 | T2D | 3 | RCT, double blind | 26 weeks and 26 weeks open extension | Changes in hemoglobin A1c, fasting blood glucose |
|
NCT00943501 NCT00993304 [12, 13] |
Liraglutide | 21 | T2D | 1 | RCT, double blind | 5 weeks and 3 weeks adverse event follow up | Pharmacokinetics | |
| NCT00254354 [14] | Exenatide | 13 | T2D | 2 | RCT, single blind | 1 day x 3 | Pharmacokinetics | |
| SGLT2i | NCT01525238 [15] | Dapagliflozin | 20 | T2D | 1 | Randomized open label | 2 days | Pharmacokinetics, safety, glycemia |
| NCT01498185 [16] | Dapagliflozin | 62 | T1D | 2 | RCT, double blind | 14 days | Glycemia, pharmacokinetics | |
| [17] | Dapagliflozin | 9 | Heart failure | N/A | Observational, retrospective analysis | 30 days | Safety | |
| NCT02121483 [18, 19] | Empagliflozin | 27 | T2D | 1 | Randomized open label | 3 days | Pharmacokinetics, fasting plasma glucose, urinary glucose excretion |
Key: GLP-1RA – glucagon-like peptide-1 receptor agonist; RCT – randomized controlled trial; SGLT2i – sodium glucose co-transporter 2 inhibitors; T2D – type 2 diabetes; T1D – type 1 diabetes; T2D – type 2 diabetes; TZD – thiazolidinedione.
NOTE: All pediatric trials have been conducted pursuant to the pediatric rule which is a mandate by the Food and Drug Administration that states that all medications approved for use in adults must undergo safety and efficacy testing in pediatrics.
2. Therapeutic Agents for Management of DKD in T2D
2.1. Metformin
Metformin, a dimethylbiguanide, is an antihyperglycemic agent first synthesized in the 1950’s that remains a first line therapy for T2D in nearly all adult and pediatric guidelines.
2.1.A. Mechanism and Clinical Effects
Metformin exerts its insulin sensitizing and blood glucose lowering effects primarily through inhibition of gluconeogenesis and opposition of glucagon-mediated signaling in the liver [20]. It inhibits the liver mitochondrial respiratory chain and enhances insulin sensitivity through modulation of lipid metabolism, as well as reduction of gluconeogenic enzymes [21] and improvements in skeletal muscle glucose uptake [20]. Metformin may also exert renoprotective effects through a variety of mechanisms including attenuation of inflammation and oxidative stress [22, 23], apoptosis [24], tubular injury [25], and fibrosis [26], and inducing autophagy [27], lipid availability [28], glucagon-like peptide 1 (GLP-1) receptor activation [29], and urinary sodium excretion [30]. The positive effects of metformin are complex and require further study, particularly in individuals across the age spectrum with T2D.
2.1.B. Adverse Effects
Metformin is frequently associated with gastrointestinal side effects including abdominal discomfort, nausea, vomiting, and diarrhea, particularly with the use of immediate-release formulations [31]. Approximately 50% of metformin remains unabsorbed in the gut and accumulates in the distal small intestine mucosa, with concentrations that exceed 30 to 300 times that achieved in the plasma [31], thereby contributing to the high rates of side effects. Proposed mechanisms for the gastrointestinal side effects of metformin include delays in intestinal glucose absorption [32], effects on bile acid metabolism or the intestinal microbiome [33, 34], augmentation of enterocyte lactate production [32], or enhancements of GLP-1 secretion [35]. Side effects are attenuated through a combination of a month-long gradual titration to goal dose and the use of extended-release formulations.
Metformin use in the setting of chronic conditions such as kidney or liver disease must also be considered. In conditions that disrupt the processes of lactate production or clearance, metformin is associated with increased plasma concentrations of lactate secondary to inhibition of liver mitochondrial respiration [36], a process called “metformin-associated lactic acidosis (MALA)”. MALA carries an almost 50% mortality rate and thus metformin has been classically contraindicated in the setting of moderate to severe kidney impairment. However, there is a growing movement to liberalize the use of metformin in individuals with kidney disease, as the incidence of MALA remains exceedingly low (i.e., <10 cases per 100,000 patient-years) and the known benefits of treatment with metformin in people with T2D are high [37].
2.1.C. Metformin and DKD
As the most frequently prescribed therapy in T2D, metformin has been shown to have positive effects on glycemia, cardiovascular disease risk, and possibly mortality risk in people with T2D, although this finding has not been extensively corroborated. In the “United Kingdom Prospective Diabetes Study (UKPDS)”, overweight people with new onset T2D were randomized to treatment with metformin vs. conventional therapy with diet alone and these individuals demonstrated significant risk reductions in any diabetes-related endpoint, diabetes-related death, and all-cause mortality as a result of metformin therapy (all p<0.05) [38]. Notably, metformin’s therapeutic effects persisted 10 years post study completion despite differences in hemoglobin A1c being lost between groups after the first year [39]. While these positive effects may be attributed to a short period of glycemic lowering due to metformin use, other potential physiologic effects warrant further investigation including changes in insulin sensitivity and/or alterations of kidney physiology. No differences in microvascular disease relative risk (a composite score of vitreous hemorrhage, retinal photocoagulation, or kidney failure) were seen between the metformin and conventional therapy groups after 10 years [39].
Despite widespread use of metformin in T2D, large-scale clinical trials with DKD-specific primary endpoints are lacking and current data are mixed. In a trial of 51 normotensive adults less than 65 years of age with T2D and nephropathy who received 12-weeks of either the sulfonylurea glibenclamide or metformin, metformin was associated with a decrease in urine albumin excretion (mean reduction of 24.2 mg/day, p=0.008) [40]. In contrast, the “Hyperinsulinemia: The Outcome of its Metabolic Effects (HOME)” study found no significant difference in urine albumin excretion with metformin therapy vs. placebo with insulin; however, endothelial function was significantly improved and this effect was unrelated to changes in glycemia or generalized inflammation [41]. Additionally, in the “A Diabetes Outcomes Prevention Trial (ADOPT)”, 4,351 recently diagnosed, drug naïve adults with T2D were randomized to treatment with metformin, thiazolidinedione rosiglitazone, or sulfonylurea glyburide for 4 years and urine albumin to creatinine ratio was noted to slowly rise over the course of the study with metformin therapy. Relative to treatment with either rosiglitazone or glyburide, metformin was associated with the highest change in urine albumin excretion from baseline over time (+20.9% [95% CI: 13.3-28.9%] with metformin, +2.1% [95% CI: −4.2-8.8%] with rosiglitazone, and +6.1% [95% CI: −1.2-14.0%] with glyburide, p<0.001 for metformin vs. rosiglitazone). Changes in estimated GFR via the Modification of Diet in Renal Disease (MDRD) Study equation from baseline were +1.4% [95% CI: 0.0-2.9%] with metformin, +5.1% [95% CI: 3.6-6.7%] for rosiglitazone, and −0.4% [95% CI: −2.0-1.2%] for glyburide (p=0.0005 for metformin vs. rosiglitazone). There were no differences between groups in incident microalbuminuria, hypertension, or GFR <60 mL/min/1.73m2 [42]. It is evident that our knowledge of metformin’s effects on kidney function in adults with T2D is currently inconclusive and this necessitates future study with DKD-specific primary endpoints.
Furthermore, studies evaluating treatment specific DKD outcomes in youth with T2D are even more limited. In the “Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY)” study, incidence rates of microalbuminuria increased across all treatment groups (metformin vs. metformin plus lifestyle vs. metformin plus rosiglitazone) over time (overall incidence rate of 6.3% at baseline to 16.6% at study completion) [43]. Interestingly, hemoglobin A1c as a time-dependent covariate was the only factor which associated with microalbuminuria risk (p=0.03), with a 17% increased risk of microalbuminuria for every 1% increase in hemoglobin A1c [43]. Further studies of kidney specific outcomes in youth-onset T2D are also necessary.
2.2. Renin-angiotensin-aldosterone system (RAAS) inhibitors
There are two primary categories of RAAS inhibitors, angiotensin converting enzyme inhibitors (ACE-is) and angiotensin receptor blockers (ARBs). ACE-is and ARBs are largely equivalent in their clinical effects and efficacy. Both classes of medications have been studied comprehensively in DKD and represent a mainstay treatment for those with active DKD. Ample evidence in pediatric and adult DKD exists demonstrating their utility and limitations [44, 45]. Extensive reviews outlining their general mechanisms and clinical effects have been published previously [46].
2.2.A. Clinical Evidence of ACE-is/ARBs in DKD
Evidence for efficacy of ACE-is/ARBs is strongest for individuals with macroalbuminuria related to DKD [47]. Treatment with ACE-is/ARBs reduces progression of DKD in both T2D and T1D [48, 49]. For persons with DKD without macroalbuminuria, ACE-is/ARBs reduce worsening of albuminuria [44]. However, pre-emptive treatment with ACE-is/ARBs in the absence of albuminuria or DKD does not appear effective in reducing risk of developing DKD [50]. Both ACE-is and ARBs are teratogenic, so women of childbearing potential should be counseled accordingly, and contraception advised. Rare side effects of ACE-is include angioedema and cough. If individuals receiving treatment with an ACE-i present with a chronic dry cough or swelling while on therapy, they should be switched to an ARB which is not associated with this side effect. Given that ACE-is/ARBs are well tolerated and have demonstrated efficacy for improving cardiovascular and kidney outcomes across a wide spectrum of diseases, they should be a first consideration for young people with diabetes presenting with albuminuria and/or hypertension.
2.2.B. Mechanisms and Intermediate Effects of ACE-is/ARBs
Glomerular hyperfiltration is a manifestation of intraglomerular hemodynamic dysfunction in individuals with diabetes, even without overt DKD. Studies suggest that hyperfiltration may be the result of inappropriate and chronic activation of RAAS, and a potential mediator of albuminuria and DKD development [51]. Treatment with ACE-is/ARBs both targets direct hormonal effects and attenuates glomerular hyperfiltration through relaxation of the efferent arteriole [46]. In simplest terms, reduced intraglomerular pressure secondary to treatment with ACE-is/ARBs improves glomerular size selectivity and reduces filtered albumin. While long term implications of improving or preventing worsening albuminuria remains under investigation, at this point it remains a valid indication for use of ACE-is/ARBs in young persons with diabetes.
2.3. Glucagon-like peptide-1 receptor agonists (GLP-1RAs)
GLP-1RAs are a burgeoning area of study for the treatment of T2D in youth and are currently available in once daily injections, once weekly injections, and once daily pills. At present, there are a total of ten GLP-1RAs that are approved for the treatment of T2D in adults including albiglutide (once weekly injection, “TANZEUM”, GlaxoSmithKline), beinaglutide (three times daily injection, Benemae Pharmaceuticals), dulaglutide (once weekly injection, “TRULICITY”, Eli Lilly), exenatide (twice daily injections, “BYETTA”; and once-weekly injections, “BYDUREON”, both Amylin & Eli Lilly), liraglutide (once daily injection, “VICTOZA”, Novo Nordisk), lixisenatide (once daily injection, “LIXUMIA”, Sanofi), PEG-ioxenatide (once weekly injection, “FU LAIMEI”, Hansoh Pharmaceuticals), and semaglutide (once weekly injection, “OZEMPIC”; and once daily oral pill, “RYBELSUS”, both Novo Nordisk). To date, the FDA has approved only two GLP-1RAs for the treatment of T2D in youth aged 10 to 17 years including liraglutide 1.8 mg/day and exenatide extended-release injections.
2.3.A. Mechanism and Clinical Effects
Glucagon-like peptide-1 (GLP-1) is a peptide hormone that is released from the L-cells of the distal ileum following an oral glucose load that subsequently causes post-prandial insulin secretion by the incretin effect [52]. GLP-1 receptor agonists (RAs) act at the level of the 7-transmembrane G-protein coupled GLP-1 receptor to activate adenylate cyclase and stimulate a cascade resulting in the post-prandial release of insulin as well as numerous additional downstream effects including increased β-cell proliferation, somatostatin release, natriuresis and diuresis, glucose uptake in the muscle and adipose tissue, skeletal muscle perfusion, lipolysis, and satiety, and decreased β-cell apoptosis, glucagon secretion, gastric emptying, gastrointestinal motility, gluconeogenesis, steatosis, and generalized inflammation [53]. Post-prandial insulin secretion due to the incretin effect is significantly attenuated in T2D [54] while the glucagonostatic effects of GLP-1 remain largely intact [55]. Postulated mechanisms for the modulation of DKD risk in T2D include improvements in insulin resistance, hyperglycemia, blood pressure, obesity, dyslipidemia, inflammation, and potentially renal hypoxia [56]. Notably, the 2022 American Diabetes Association Standards of Care for Children and Adolescents with Diabetes also recommends consideration of GLP-1RAs (i.e., high dose liraglutide) as adjunctive therapy to lifestyle modification for weight loss in adolescents with T2D and obesity [57].
2.3.B. Adverse Effects
The most commonly reported adverse effects of GLP-1RAs are gastrointestinal in nature and include nausea, vomiting, and diarrhea [58]. Rarely, hemodynamic side effects due to excessive fluid losses and dehydration may result in pre-renal acute kidney injury, particularly in the setting of treatment with exenatide [59]. Kidney biopsies in adults with T2D and concern for exenatide-associated kidney failure and have identified ischemic glomeruli along with tubular atrophy, moderate to severe interstitial fibrosis, and early diabetic nephropathy [59]. In combination with insulin sensitizing agents such as metformin and/or thiazolidinediones, one unique benefit of GLP-1RAs is a lack of reported hypoglycemia [60, 61]. When combined with a sulfonylurea and/or insulin, use of GLP-1RAs may necessitate a decrease in dosage of either the concomitant sulfonylurea or insulin due to an increased risk of hypoglycemia [60]. Other common side effects include headaches, injection site reactions, particularly with extended-release formulations, and/or nasopharyngitis; however, these side effects are typically mild, often do not result in therapy discontinuation, and improve over time.
2.3.C. Effects on the Kidney
GLP-1RAs undergo renal clearance; thus, kidney effects of GLP-1RAs remain difficult to evaluate. Preliminary results are promising; however, studies are largely limited to soft kidney function outcomes including changes in estimated GFR (eGFR) or urinary albumin to creatinine ratio [62-64], new onset macroalbuminuria [65, 66], time to new onset macroalbuminuria [67], or persistent macroalbuminuria [68] in lieu of gold standard measures. Additionally, most kidney outcomes data in GLP-1RA studies comes from large, placebo-controlled safety and cardiovascular outcome trials in adults with T2D and pre-existing cardiovascular disease or risk, a population that only allows for pediatric inferences.
In the “Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with T2D (SUSTAIN-6)”, 3,297 individuals with T2D and cardiovascular disease or risk were randomized to semaglutide 0.5 mg or 1 mg once weekly vs. placebo and semaglutide was associated with improvement in glycemia, body weight, and risk for new or worsening nephropathy (HR=0.64 [95% CI: 0.46-0.88], p=0.005) after 2 years of therapy [68]. Additionally, the “Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER)” trial also showed a significant difference in HbA1c, weight, and worsening nephropathy (−22%, HR=0.74 [95% CI: 0.60-0.91]) in participants with T2D on liraglutide vs. placebo for a median of 3.8 years [69]. However, both the SUSTAIN-6 and LEADER trials utilized a pre-specified composite kidney outcome [68, 69] rather than direct assessments of kidney function. The “Exenatide Study of Cardiovascular Event Lowering (EXSCEL)” trial also used a kidney function composite score and found significant improvement in kidney outcomes with extended release exenatide when compared to placebo in adults with T2D (HR 0.85 [95% CI: 0.73-0.98, p=0.027). Dedicated kidney outcomes trials, particularly in individuals with known kidney disease, are limited but support the positive effects of GLP-1RAs seen in safety and efficacy trials. In the “Study Comparing Dulaglutide with Insulin Glargine on Glycemic Control in Participants with Type 2 Diabetes and Moderate or Severe Chronic Kidney Disease (AWARD-7)”, dulaglutide reduced albuminuria by 39% (10-69%) and attenuated eGFR decline vs. glargine alone in adults with T2D and moderate to severe chronic kidney disease [63].
GLP-1RA therapy remains a promising avenue for the prevention and treatment of DKD; however, gold-standard assessments of kidney function are lacking and have exhibited mixed results to date. In a study of 10 overweight men, an infusion of exenatide was associated with an acute increase in measured GFR by inulin clearance, renal plasma flow by p-aminohippurate clearance, and estimated glomerular pressure, as well as decreased renal vascular resistance [70]. In a cohort of 52 overweight men and post-menopausal women with T2D, an exenatide infusion was not associated with changes in intraglomerular hemodynamic function but it did increase proximal tubule sodium excretion [71]. No studies evaluating the effects of GLP-1RAs in youth with T2D using gold standard methods have yet been published.
GLP-1RA treatment effects on kidney oxygen bioavailability must also be considered. Diabetes-induced kidney microvascular injury has been shown to result in excessive energy expenditure due to hyperfiltration, hypoxia, inflammatory changes with deposition of excess extracellular matrix, and late stage fibrosis [72]. Treatment with GLP-1RAs in obese rat models has resulted in restoration of kidney energy homeostasis, as well as a reduction in reactive oxygen species, inflammation, and fatty kidney [73]. Further evaluation of the effects of GLP-1RAs on intraglomerular hemodynamic function and kidney oxygen availability in youth and adults with T2D is necessary to help attenuate DKD, particularly in the early stages of disease.
2.3.D. Combination GLP-1 and glucagon-dependent insulinotropic polypeptide (GIP) RAs
Dual GLP-1/GIP RAs further expand on the armamentarium of potential targetable receptors within the body for the treatment of T2D and integrate the downstream actions of incretin hormones GLP-1 and GIP to improve glycemia and potentially modify kidney function. In the Study of Tirzepatide (LY3298176) Once a Week Versus Insulin Glargine Once a Day in Participants with Type 2 Diabetes and Increased Cardiovascular Risk (SURPASS-4), a randomized, open-label, parallel group, multi-site Phase 3 study in adults with T2D, a BMI ≥25 kg/m2, and either high risk or known CVD, a median duration of 85 weeks of Tirzepatide was associated with a slowed rate of eGFR decline (−1.4 [74] mL/min/1.73 m2 vs. −3.6 mL/min/1.73 m2) and a reduced UACR versus treatment with the insulin glargine (36.9% [74] vs. −6.8% [95 CI: −14.1 to 1.1%] [74]. While direct comparisons between GLP-1RAs and dual GLP-1/GIP RAs have yet to be done to evaluate primary kidney outcomes, dual GLP-1/GIP RAs remain a promising avenue for future treatment of DKD associated with T2D.
2.4. Sodium Glucose Co-Transporter 2 Inhibitors (SGLT2is)
SGLT2is have transformed cardiovascular and kidney outcomes in adults with T2D [75, 76]. SGLT2is that have achieved FDA approval for the treatment of T2D in adults include canagliflozin (“INVOKANA”, Janssen), dapagliflozin (“FARXIGA”, AstraZeneca), and empagliflozin (“JARDIANCE”, Lilly/Boehringer Ingelheim Pharmaceuticals). There appears to be a class-effect in terms of efficacy and safety of SGLT2is [77, 78]; yet currently no SGLT2is are approved by the FDA for use in persons younger than 18 years of age.
2.4.A. Mechanism and Clinical Effects
SGLT2 is almost exclusively expressed in the proximal tubule of the kidney. Glucose is freely filtered at the glomerulus and 90% of filtered glucose is reabsorbed in the apical membrane of the proximal tubule by SGLT2 [79, 80]. The clinical effects and tolerability of SGLT2is were demonstrated in families with autosomal recessive familial glycosuria related to mutations in SGLT2 [81]. SGLT2is have only modest efficacy for lowering hemoglobin A1c. Accordingly, the positive benefit of SGLT2 inhibition likely derives from mechanisms outside of tighter glycemic control [82].
Treatment with SGLT2is induces favorable metabolic effects. At the tissue level, treatment with SGLT2is results in diminished adiposity and reduced intracellular lipid accumulation [83]. Shifts in cellular metabolism observed with inhibition of SGLT2 results from a combination of macro-effects (i.e., negative total-body glucose flux) and direct cellular effects in cells that express SGLT2 [83]. SGLT2is shift metabolism away from glucose oxidation and towards ketone and fatty acid oxidation, increase the efficiency of oxygen utilization, and reduce free radical generation [84-86].
While SGLT2i is almost exclusively expressed in the proximal tubule, cellular effects of SGLT2i are more widespread in the kidney. In a recent study by Schaub et al., metabolomic profiling performed on human kidney biopsy tubular cells in youth with T2D and healthy controls showed that T2D was associated with perturbations in mechanistic target of rapamycin complex 1 (mTORC1) signaling and altered metabolomics in kidney tubular cells from all segments [87]. Individuals with T2D treated with SGLT2is demonstrated metabolic profiles that were more similar to healthy controls. Interestingly, SGLT2i use was strongly associated with reduction in mTORC1 signaling in both the proximal and distal tubular cells. Further research is needed to understand how SGLT2is induce metabolic shifts in tubular cells that do and do not express SGLT2.
SGLT2i may also stimulate erythropoietin production in the kidney [88], thereby improving anemia, a well-established risk factor for progression of CKD and cardiovascular disease [89, 90]. Erythrocytosis and increases in red blood cell counts have been noted in studies of SGLT2is [91], but the exact cellular mechanisms remain under debate. SGLT2is may exert either direct or indirect effects on hypoxia-inducible factors (HIF), HIF-1α and HIF-2α [92].
The hemodynamic and natriuretic effects of SGLT2 inhibition are also postulated as protective mechanisms. Treatment with SGLT2is results in decreases in blood pressure, weight, and N-terminal of prohormone of brain natriuretic peptide (NT-proBNP) [93, 94]. Kidney-glomerular hyperfiltration is common in people with diabetes and is postulated to contribute to DKD [95]. SGLT2is induce a drop in the GFR, primarily by reactivating tubuloglomerular feedback mechanism [95]. Participants randomized to SGLT2is in all large clinical trials experienced an acute drop in GFR [96]. Despite this, it is well documented that SGLT2is slow the long-term decline in eGFR associated with DKD [97]. Relatedly, SGLT2 inhibition reduces proteinuria and protects against incident or worsening albuminuria.
In sum, the potential of SGLT2is to greatly improve long-term kidney outcomes further supports their use in youth with diabetes, a population with a significant lifetime risk of developing progressive kidney disease.
2.4.B. Adverse Effects
Overall, extensive research demonstrates SGLT2is have an outstanding safety profile. Nonetheless, there are several considerations with their use in pediatric populations. Initial trials in populations with diabetes have raised the concern that SGLT2i-induced glucosuria increases the risk for urinary tract infections (UTIs). However, subsequent analyses have not supported an increased risk of UTIs with SGLT2i use [98, 99]. The incidence of mycotic genitourinary infections is increased with SGLT2 inhibition, so patients must be monitored and treated accordingly [100].
Another important safety consideration with SGLT2is is the risk for diabetic ketoacidosis. Euglycemic ketosis is a hallmark of SGLT2 inhibition, and in low concentrations is not necessarily harmful [101]. However, development of ketoacidosis, a dangerous and potentially life-threatening complication if severe, was found to occur in 0-1% of participants randomized to treatment with SGLT2is in major clinical trials [102, 103]. The risk for ketoacidosis is highest in persons with T1D and the cumulative incidence of ketoacidosis in persons with T1D treated with SGLT2is in major clinical trials was 3-6%, leading the FDA to not extend approval of SGLT2is to T1D [104]. Several risk factors in persons with T1D increase the relative risk of developing ketoacidosis with SGLT2is, including relative insulin deficiency, dehydration, and/or illness [102].
2.4.C. Landmark Trials in Adults
Multiple large, randomized controlled trials have demonstrated the efficacy of SGLT2is to improve kidney outcomes in adults with T2D and/or DKD. The “Empagliflozin, Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)” trial enrolled 7,020 participants greater than 18 years of age with T2D and eGFR >30mL/min/1.73m2 to assess the cardiovascular safety of empagliflozin [105]. A secondary analysis of trial data noted that individuals randomized to treatment with empagliflozin had a 39% reduction in their risk for worsening nephropathy (incident macroalbuminuria, doubling of serum creatinine, or need for dialysis/transplant) [106]. The “Canagliflozin Cardiovascular Assessment Study (CANVAS)” and “Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE)” trials demonstrated similar efficacy of canagliflozin, and enrolled adults with T2D and an eGFR >30mL/min/1.73m2 [107, 108]. Of note, CREDENCE excluded persons with an eGFR >90mL/min/1.73m2. Both trials demonstrated a 30-40% reduction in risk for most adverse kidney outcomes including worsening albuminuria, greater than 40% decline in eGFR, and dialysis dependence. “Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD)” was the only trial not to exclusively enroll participants with diabetic nephropathy [109]. Nonetheless, this trial demonstrated the efficacy of dapagliflozin to improve kidney outcomes for those with CKD with or without T2D. The “Empagliflozin in Patients with Chronic Kidney Disease (EMPA-KIDNEY)” shared a similar study design, and nearly identical results, to DAPA-CKD [110]. Taken together, these trials demonstrate the class-effect of SGLT2is for treatment of DKD and non-diabetic CKD.
2.4.D. Studies in Pediatrics
Evidence on the tolerability and efficacy of SGLT2is in pediatric populations is limited. However, the positive efficacy and safety results demonstrated in adults has led to increasing off-label use of SGLT2is in pediatrics and this will lead to new opportunities for research. A recent study of 9 young individuals with heart failure (mean age 14.2 years) receiving dapagliflozin did not observe any adverse effects, including UTIs, ketoacidosis, fracture, or hypovolemia after a mean treatment duration of 30 days with SGLT2is [17].
There has also been several mechanistic studies of SGLT2is in youth with diabetes. Laffel et. al performed a pharmacokinetic study of empagliflozin by administering a single dose to youth aged 10-17 years with T2D [18]. There was a dose-dependent increase in urinary glucose excretion. Importantly, the pharmacokinetics were similar to those observed in studies of adults. A secondary analysis revealed that a single dose of empagliflozin resulted in an increase in the fractional excretion of sodium and a modest decrease in eGFR (−5.5 mL/min/1.73m2) [19]. The drop in eGFR was most significant in those with hyperfiltration at baseline.
There is growing interest to expand the benefits of SGLT2is to young persons with T1D [111]. The “Adolescent Type 1 Diabetes Treatment with SGLT2i for Hyperglycemia and Hyperfiltration (ATTEMPT)” trial (Clinicaltrials.gov identifier: NCT04333823) is actively recruiting to study the efficacy of dapagliflozin versus placebo in modulating glomerular hyperfiltration and metabolic parameters in adolescents with T1D. These results will add a pediatric perspective to recent studies of SGLT2is in adults with T1D [16, 112]. SGLT2 inhibition may demonstrate future efficacy and feasibility in youth with T1D, albeit with an informed approach and implemented strategies to reduce the risk for ketoacidosis, such as continuous ketone monitoring.
3. Other drugs
3.1. Thiazolidinediones
Thiazolidinediones (TZDs) contribute to glycemic control by activating the peroxisome proliferator-activated receptor gamma (PPARγ). PPARγ activation is linked to improvements in peripheral insulin sensitivity and β-cell function [113, 114]. TZDs are not approved for youth with T2D but are often used off-label due to their beneficial effects on insulin sensitivity and glycemic control. Indeed, in the TODAY study, glycemic failure rates decreased by 13% in adolescents with T2D when adding rosiglitazone, a TZD, to metformin compared to metformin alone [10]. However, rates of albuminuria did not differ among the metformin plus rosiglitazone, metformin plus lifestyle, or metformin alone groups in TODAY.
Although TZD therapy has been associated with adverse events including fractures, fluid retention, and heart failure in adults [115], these side effects were not observed in the TODAY study. In adults with T1D and T2D, the TZD pioglitazone has been used in combination with SGLT2is, as the diuretic properties of SGLT2is are thought to mitigate the fluid retention conferred by the TZD. Additionally, murine models suggest that combination therapy of an ACEi plus rosiglitazone provides synergy in attenuating diabetic kidney injury that is not simply additive of either monotherapy alone [116].
3.2. Sulfonylureas
Sulfonylureas are among the oldest class of hypoglycemic agents, and work by stimulating insulin secretion by closing ATP-sensitive K+ channels in the pancreatic β-cell plasma membrane. There are few studies on sulfonylureas in youth with T2D. One study showed that the sulfonylurea glimepiride reduced HbA1c similarly to metformin in youth with T2D, but larger weight gain and a higher rate of hypoglycemia were documented in the glimepiride group [117]. To our knowledge, there are no studies in youth with T2D that have evaluated the effects on diabetic kidney disease. Accordingly, sulfonylureas are rarely used in adolescents with T2D.
3.3. Endothelin receptor agonists
Endothelin receptor antagonists (ERA) primarily target the endothelin A (ETA) receptor, which have demonstrated kidney protective effects. The ERA atrasentan attenuated DKD progression in adults with T2D in the “Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR)” trial [118]. Unlike SGLT2 inhibitors, ERAs increase sodium and fluid retention, leading to increased body weight and hemodilution (decreased hematocrit and hemoglobin), as well as edema and a higher risk of developing heart failure [119, 120]. This has limited the development and widespread use of agents in this class, especially in people who are predisposed to volume overload, including individuals with DKD. Indeed, a large randomized controlled trial in people with T2D and DKD using a relatively non-selective ERA avosentan was terminated early because of an increased frequency of heart failure [119]. In addition, despite the precautionary approach taken in the SONAR trial with atrasentan, including the use of diuretics and exclusion of people with heart failure or high BNP concentrations, there was a higher rate (3.5% versus 2.6%) of heart failure hospitalizations with atrasentan [118]. There are no ERA trial data in young persons with T2D. Although ERA holds promise as a therapy for T2D and DKD, it is unclear whether benefits would outweigh risks, and it is likely best used in combination with a natriuretic or diuretic agent.
3.4. Mineralocorticoid antagonists
T2D has been linked to inappropriate mineralocorticoid receptor (MR) activation with resultant inflammation and fibrosis [121]. Indeed, murine MR knockout models document protection against kidney inflammation and fibrosis [122]. In the “Chronic Renal Insufficiency Cohort (CRIC)”, higher serum aldosterone concentrations independently predicted greater risk of chronic kidney disease progression in adults with and without T2D [123]. These data provide mechanistic support for mineralocorticoid antagonism in mitigating CKD progression. In the “FInerenone reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease (FIDELIO-DKD)” and “FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease (FIGARO-DKD)” clinical trials, finerenone, a nonsteroidal MR antagonist (MRA), attenuated risk of DKD progression and kidney failure in adults with T2D and DKD [124]. Albuminuria lowering was also observed with esaxerenone, another nonsteroidal MRA, in Japanese people with T2D. In these trials, tolerability of MRA was reassuring, but hyperkalemia was found to be more common compared to placebo. Thus, combination with a potassium lowering agent such as an SGLT2i holds promise and warrants further study. There are no data yet in young persons with T2D, but it is plausible that these agents will be used off-label in combination with SGLT2is in individuals with DKD not responsive to monotherapy.
3.5. Novel agents
Other therapeutic interventions that may hold promise to mitigate DKD risk in young persons with T2D include serine/threonine kinase, apoptosis signal–regulating kinase 1 (ASK1) inhibitors, and JAK-STAT inhibitors that attenuate inflammation, apoptosis, and fibrosis [125, 126]. Soluble guanylyl cyclase (sGC) activators have also been proposed to mitigate DKD as low cyclic guanosine monophosphate (cGMP) concentrations have been implicated in DKD progression [127]. However, a randomized, placebo-controlled trial in 156 adults with T2D, impaired GFR, and albuminuria ≥200 mg/g, failed to demonstrate attenuation of albuminuria following 12 weeks of praliciguat treatment [128]. Finally, interventions that improve renal substrate metabolism such as mitochondrial peptides and bioavailable-small molecule activators of AMPK and mTORC1 inhibitors offer promise as even small enhancements in fuel utilization may translate into large improvements in kidney function and ultimately clinical outcomes [90, 129-134].
4. Conclusion
DKD is common and occurs early in the course of youth-onset T2D. Therapeutic options to mitigate DKD risk in youth with T2D are currently limited to insulin, metformin, GLP-1RAs and RAASis. Other agents such as SGLT2is, MRAs and ERAs hold promise, but have limited data in youth with T2D and are considered off label. To address the scarce therapeutic options to prevent and treat DKD in youth with T2D, we need more dedicated pediatric trials.
5. Expert Opinion
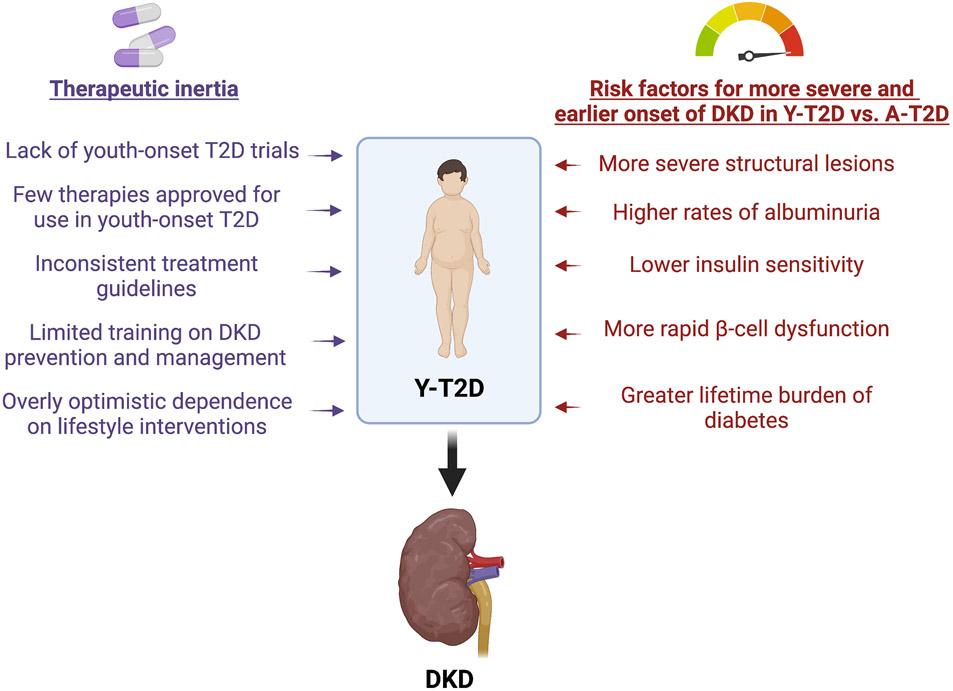
Current projections predict that 50-70% of people with youth-onset T2D will develop DKD during adolescence and young adulthood. Despite these grave projections, pharmacological interventions are scarce, which is ascribed to a paucity of trial data in youth-onset T2D (Figure 1). Accordingly, recommendations are formulated based on data extrapolated from trials performed in adults with T2D, which may not be effective in youth. Additionally, due to limited efficacy and safety data, several drugs available to adults with T2D are not approved for the treatment of youth with T2D. Indeed, the only medications for the treatment of youth with T2D approved by the United States Food and Drug Administration, the European Medicines Agency, and Health Canada are metformin and insulin, with the recent addition of a GLP-1-RA.
Figure 1. Challenges for the Timely Management of Diabetic Kidney Disease in Youth with Type 2 Diabetes.
Key: A-T2D – adult-onset type 2 diabetes; DKD – diabetic kidney disease; T2D – type 2 diabetes; Y-T2D – youth-onset type 2 diabetes.
In addition to the constrained armamentarium of approved medications, we also contend that the management of DKD in youth-onset T2D is challenged by therapeutic inertia. The reluctance to prescribe these medications during adolescence may be ascribed to the unfamiliarity of pediatric providers with newer adjunctive medications to mitigate the risk of DKD, and concerns about poor medication adherence and safety. A misguided dependence on lifestyle modifications prior to starting pharmacotherapy, and primarily focusing on glycemic control, in lieu of starting kidney protective therapies early and in parallel with targeting glycemia, also contributes to a lack of therapeutic inertia. Additionally, the typical busy clinician may find it difficult to allocate sufficient time for DKD risk management in part due to their lack of training and in part due to unwillingness of the adolescent to accept the initiation of additional therapies. Finally, reluctance to follow existing guidelines may relate to the limited number of trials in youth with T2D and the inferential nature of the evidence from adult studies.
To address the limited therapeutic options to prevent and treat DKD in youth with T2D, we need more dedicated pediatric trials, and to address the therapeutic inertia, professional societies must work together to train clinicians and reach consensus on the guidelines to minimize conflicting recommendations.
Article Highlights.
Diabetic kidney disease (DKD) and strongly associated cardiovascular disease are the leading causes for morbidity and mortality in youth-onset type 2 diabetes (T2D), with metabolic, morphologic, and molecular features occurring early in the disease course, long before laboratory evidence such as albuminuria or changes in glomerular filtration rate are evident.
Therapeutic options to mitigate DKD risk in youth with T2D are currently limited to insulin, metformin, glucagon-like peptide-1 receptor agonists (GLP-1RAs) and renin-angiotensin-aldosterone system inhibitors (RAASis).
Other agents such as sodium-glucose co-transporter 2 inhibitors (SGLT2is), mineralocorticoid receptor antagonists (MRAs) and endothelin receptor antagonists (ERAs) hold promise but have limited data in youth with T2D and are considered off label.
Limitations in approved medications for the treatment of DKD in youth-onset T2D have directly inhibited therapeutic inertia for clinicians which could possibly be addressed through coordination of professional societies to reach consensus on the guidelines and thereby minimize conflicting recommendations.
Additionally, to address the limited therapeutic options to prevent and treat DKD in youth with T2D, we need more dedicated pediatric trials.
Financial contributions:
K.L.T. receives salary and research support from the NIH/NHLBI (K23 HL159292), Children’s Hospital Colorado Research Institute Research Scholar Award, NIH/NIDDK (P30 DK116073), Ludeman Family Center for Women’s Health Research at the University of Colorado, ISPAD-JDRF Research Fellowship, and the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine. AJK receives salary and research support from the Department of Pediatrics, Division of Pediatric Nephrology, Ann and Robert H. Lurie Children’s Hospital of Chicago and Northwestern University Feinberg School of Medicine. P.B. receives salary and research support from NIDDK (R01 DK129211, R01 DK132399, R21 DK129720, K23 DK116720, UC2 DK114886), NHLBI (R01HL165433), JDRF (3-SRA-2022-1097-M-B, 3-SRA-2022-1243-M-B, 3-SRA-2022-1230-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Ludeman Family Center for Women’s Health Research at the University of Colorado, the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Declaration of Interests: K.L.T. and A.J.K have no relevant disclosures to report. P.B. reports serving as a consultant for AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, LG Chemistry, Sanofi, Novo Nordisk, and Horizon Pharma. P.B. also serves on the advisory boards and/or steering committees of AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and XORTX. No potential conflicts of interest relevant to this article were reported from any of the authors.
References
- 1.Shin JA, et al. , Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig, 2013. 4(4): p. 334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Consortium, R. and R.C. Investigators, Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on beta-Cell Function: Comparison of Responses In Youth And Adults. Diabetes, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Consortium, R., Impact of Insulin and Metformin Versus Metformin Alone on beta-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care, 2018. 41(8): p. 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugahara M, et al. , Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology (Carlton), 2021. 26(6): p. 491–500. [DOI] [PubMed] [Google Scholar]
- 5.Bjornstad P, et al. , Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis, 2018. 71(1): p. 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjornstad P, et al. , Incidence of Complications and Comorbidities in Young People with Type 2 Diabetes. N Engl J Med, 2021. In-press. [Google Scholar]
- 7.Bjornstad P, et al. , Elevated Serum Uric Acid Is Associated With Greater Risk for Hypertension and Diabetic Kidney Diseases in Obese Adolescents With Type 2 Diabetes: An Observational Analysis From the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. Diabetes Care, 2019. 42(6): p. 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornstad P, et al. , Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes Care, 2014. 37(11): p. 3033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seegmiller JC, et al. , Tubular Secretion Markers, Glomerular Filtration Rate, Effective Renal Plasma Flow, and Filtration Fraction in Healthy Adolescents. Kidney Medicine, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group, T.S., et al. , A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med, 2012. 366(24): p. 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamborlane WV, et al. , Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med, 2019. 381(7): p. 637–646. [DOI] [PubMed] [Google Scholar]
- 12.Petri KC, Jacobsen LV, and Klein DJ, Comparable liraglutide pharmacokinetics in pediatric and adult populations with type 2 diabetes: a population pharmacokinetic analysis. Clin Pharmacokinet, 2015. 54(6): p. 663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein DJ, et al. , Liraglutide's safety, tolerability, pharmacokinetics, and pharmacodynamics in pediatric type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Technol Ther, 2014. 16(10): p. 679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malloy J, et al. , Pharmacology and tolerability of a single dose of exenatide in adolescent patients with type 2 diabetes mellitus being treated with metformin: a randomized, placebo-controlled, single-blind, dose-escalation, crossover study. Clin Ther, 2009. 31(4): p. 806–15. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson J, et al. , Comparison of the exposure-response relationship of dapagliflozin in adult and paediatric patients with type 2 diabetes mellitus. Diabetes Obes Metab, 2016. 18(7): p. 685–92. [DOI] [PubMed] [Google Scholar]
- 16.Henry RR, et al. , Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care, 2015. 38(3): p. 412–9. [DOI] [PubMed] [Google Scholar]
- 17.Newland DM, et al. , Safety of Dapagliflozin in Children with Heart Failure. The Journal of Heart and Lung Transplantation, 2021. 40(4, Supplement): p. S280. [Google Scholar]
- 18.Laffel LMB, et al. , Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: a randomized trial. Diabetic medicine : a journal of the British Diabetic Association, 2018. 35(8): p. 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornstad P, et al. , Acute Effect of Empagliflozin on Fractional Excretion of Sodium and eGFR in Youth With Type 2 Diabetes. Diabetes Care, 2018. 41(8): p. e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pernicova I and Korbonits M, Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol, 2014. 10(3): p. 143–56. [DOI] [PubMed] [Google Scholar]
- 21.Rena G, Hardie DG, and Pearson ER, The mechanisms of action of metformin. Diabetologia, 2017. 60(9): p. 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, et al. , Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp Ther Med, 2017. 14(1): p. 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen M, et al. , Metformin modulates immune cell infiltration into the kidney during unilateral ureteral obstruction in mice. Physiol Rep, 2019. 7(13): p. e14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polianskyte-Prause Z, et al. , Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J, 2019. 33(2): p. 2858–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takiyama Y, et al. , Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1alpha expression and oxygen metabolism. Diabetes, 2011. 60(3): p. 981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, et al. , Metformin attenuates renal fibrosis in both AMPKalpha2-dependent and independent manners. Clin Exp Pharmacol Physiol, 2017. 44(6): p. 648–655. [DOI] [PubMed] [Google Scholar]
- 27.Ren H, et al. , Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol, 2020. 500: p. 110628. [DOI] [PubMed] [Google Scholar]
- 28.Lee M, et al. , Phosphorylation of Acetyl-CoA Carboxylase by AMPK Reduces Renal Fibrosis and Is Essential for the Anti-Fibrotic Effect of Metformin. J Am Soc Nephrol, 2018. 29(9): p. 2326–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DI, et al. , Metformin ameliorates lipotoxicity-induced mesangial cell apoptosis partly via upregulation of glucagon like peptide-1 receptor (GLP-1R). Arch Biochem Biophys, 2015. 584: p. 90–7. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto H, et al. , Metformin increases urinary sodium excretion by reducing phosphorylation of the sodium-chloride cotransporter. Metabolism, 2018. 85: p. 23–31. [DOI] [PubMed] [Google Scholar]
- 31.Bailey CJ, Wilcock C, and Scarpello JH, Metformin and the intestine. Diabetologia, 2008. 51(8): p. 1552–3. [DOI] [PubMed] [Google Scholar]
- 32.Bailey CJ and Turner RC, Metformin. N Engl J Med, 1996. 334(9): p. 574–9. [DOI] [PubMed] [Google Scholar]
- 33.Lien F, et al. , Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest, 2014. 124(3): p. 1037–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H and Ko G, Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol, 2014. 80(19): p. 5935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulherin AJ, et al. , Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology, 2011. 152(12): p. 4610–9. [DOI] [PubMed] [Google Scholar]
- 36.Inzucchi SE, et al. , Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA, 2014. 312(24): p. 2668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eppenga WL, et al. , Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care, 2014. 37(8): p. 2218–24. [DOI] [PubMed] [Google Scholar]
- 38.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet, 1998. 352(9131): p. 854–65. [PubMed] [Google Scholar]
- 39.Holman RR, et al. , 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med, 2008. 359(15): p. 1577–89. [DOI] [PubMed] [Google Scholar]
- 40.Amador-Licona N, et al. , The short-term effect of a switch from glibenclamide to metformin on blood pressure and microalbuminuria in patients with type 2 diabetes mellitus. Arch Med Res, 2000. 31(6): p. 571–5. [DOI] [PubMed] [Google Scholar]
- 41.De Jager J, et al. , Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med, 2005. 257(1): p. 100–9. [DOI] [PubMed] [Google Scholar]
- 42.Lachin JM, et al. , Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol, 2011. 6(5): p. 1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Group, T.S., Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care, 2013. 36(6): p. 1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang JL, et al. , Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care, 2014. 37(7): p. 2034–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parving H-H, et al. , Long-term beneficial effect of ACE inhibition on diabetic nephropathy in normotensive type 1 diabetic patients. Kidney International, 2001. 60(1): p. 228–234. [DOI] [PubMed] [Google Scholar]
- 46.Ruggenenti P, Cravedi P, and Remuzzi G, The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nature Reviews Nephrology, 2010. 6(6): p. 319–330. [DOI] [PubMed] [Google Scholar]
- 47.Parving H-H, et al. , The Effect of Irbesartan on the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes. New England Journal of Medicine, 2001. 345(12): p. 870–878. [DOI] [PubMed] [Google Scholar]
- 48.Lewis EJ, et al. , The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. New England Journal of Medicine, 1993. 329(20): p. 1456–1462. [DOI] [PubMed] [Google Scholar]
- 49.Brenner BM, et al. , Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med, 2001. 345(12): p. 861–9. [DOI] [PubMed] [Google Scholar]
- 50.Patel A, Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. The Lancet, 2007. 370(9590): p. 829–840. [DOI] [PubMed] [Google Scholar]
- 51.Lovshin JA, et al. , Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight, 2018. 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anandhakrishnan A and Korbonits M, Glucagon-like peptide 1 in the pathophysiology and pharmacotherapy of clinical obesity. World J Diabetes, 2016. 7(20): p. 572–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalra S, et al. , Consensus Recommendations on GLP-1 RA Use in the Management of Type 2 Diabetes Mellitus: South Asian Task Force. Diabetes Ther, 2019. 10(5): p. 1645–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holst JJ, Vilsbøll T, and Deacon CF, The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol, 2009. 297(1-2): p. 127–36. [DOI] [PubMed] [Google Scholar]
- 55.Nauck MA, et al. , Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest, 1993. 91(1): p. 301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosterd CM, Bjornstad P, and van Raalte DH, Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol, 2020. 33(5): p. 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Diabetes Association Professional Practice, C., et al. , 14. Children and Adolescents: Standards of Medical Care in Diabetes-2022. Diabetes Care, 2022. 45(Suppl 1): p. S208–S231. [DOI] [PubMed] [Google Scholar]
- 58.Sun F, et al. , Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Technol Ther, 2015. 17(1): p. 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weise WJ, et al. , Exenatide-associated ischemic renal failure. Diabetes Care, 2009. 32(2): p. e22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marre M, et al. , Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med, 2009. 26(3): p. 268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reusch J, et al. , Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes Obes Metab, 2014. 16(12): p. 1257–64. [DOI] [PubMed] [Google Scholar]
- 62.Davies MJ, et al. , Efficacy and Safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients With Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): A Randomized Clinical Trial. Diabetes Care, 2016. 39(2): p. 222–30. [DOI] [PubMed] [Google Scholar]
- 63.Tuttle KR, et al. , Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol, 2018. 6(8): p. 605–617. [DOI] [PubMed] [Google Scholar]
- 64.Muskiet MHA, et al. , Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol, 2018. 6(11): p. 859–869. [DOI] [PubMed] [Google Scholar]
- 65.Bethel MA, et al. , Microvascular and Cardiovascular Outcomes According to Renal Function in Patients Treated With Once-Weekly Exenatide: Insights From the EXSCEL Trial. Diabetes Care, 2020. 43(2): p. 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerstein HC, et al. , Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet, 2019. 394(10193): p. 121–130. [DOI] [PubMed] [Google Scholar]
- 67.Mann JFE, Ørsted DD, and Buse JB, Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med, 2017. 377(22): p. 2197–2198. [DOI] [PubMed] [Google Scholar]
- 68.Marso SP, et al. , Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med, 2016. 375(19): p. 1834–1844. [DOI] [PubMed] [Google Scholar]
- 69.Marso SP, et al. , Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med, 2016. 375(4): p. 311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muskiet MH, et al. , Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab, 2016. 18(2): p. 178–85. [DOI] [PubMed] [Google Scholar]
- 71.Tonneijck L, et al. , Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia, 2016. 59(7): p. 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fine LG and Norman JT, Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int, 2008. 74(7): p. 867–72. [DOI] [PubMed] [Google Scholar]
- 73.Wang C, et al. , GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS One, 2018. 13(3): p. e0193473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heerspink HJL, et al. , Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol, 2022. 10(11): p. 774–785. [DOI] [PubMed] [Google Scholar]
- 75.de Boer IH and Kahn SE, SGLT2 Inhibitors—Sweet Success for Diabetic Kidney Disease? Journal of the American Society of Nephrology, 2017. 28(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braunwald E, SGLT2 inhibitors: the statins of the 21st century. European Heart Journal, 2021: p. ehab765. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt DW, Argyropoulos C, and Singh N, Are the Protective Effects of SGLT2 Inhibitors a “Class-Effect” or Are There Differences between Agents? Kidney360, 2021. 2(5): p. 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Donnan JR, et al. , Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ open, 2019. 9(1): p. e022577–e022577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wright EM, et al. , Surprising Versatility of Na+-Glucose Cotransporters: SLC5. Physiology, 2004. 19(6): p. 370–376. [DOI] [PubMed] [Google Scholar]
- 80.Wright EM, Loo DDF, and Hirayama BA, Biology of Human Sodium Glucose Transporters. Physiological Reviews, 2011. 91(2): p. 733–794. [DOI] [PubMed] [Google Scholar]
- 81.Santer R and Calado J, Familial Renal Glucosuria and SGLT2: From a Mendelian Trait to a Therapeutic Target. Clinical Journal of the American Society of Nephrology, 2010. 5(1): p. 133–141. [DOI] [PubMed] [Google Scholar]
- 82.Bakris GL, et al. , Renal sodium–glucose transport: role in diabetes mellitus and potential clinical implications. Kidney International, 2009. 75(12): p. 1272–1277. [DOI] [PubMed] [Google Scholar]
- 83.Heerspink HJL, et al. , Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney International, 2018. 94(1): p. 26–39. [DOI] [PubMed] [Google Scholar]
- 84.Cai T, et al. , Sodium–glucose cotransporter 2 inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death & Disease, 2020. 11(5): p. 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowie MR and Fisher M, SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nature Reviews Cardiology, 2020. 17(12): p. 761–772. [DOI] [PubMed] [Google Scholar]
- 86.Packer M, SGLT2 Inhibitors Produce Cardiorenal Benefits by Promoting Adaptive Cellular Reprogramming to Induce a State of Fasting Mimicry: A Paradigm Shift in Understanding Their Mechanism of Action. Diabetes Care, 2020. 43(3): p. 508. [DOI] [PubMed] [Google Scholar]
- 87.Schaub JA, et al. , SGLT2 inhibition mitigates perturbations in nephron segment-specific metabolic transcripts and mTOR pathway activity in kidneys of young persons with type 2 diabetes. medRxiv, 2022: p. 2022.07.23.22277943. [Google Scholar]
- 88.Mazer CD, et al. , Effect of Empagliflozin on Erythropoietin Levels, Iron Stores, and Red Blood Cell Morphology in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease. Circulation, 2020. 141(8): p. 704–707. [DOI] [PubMed] [Google Scholar]
- 89.Atkinson MA and Furth SL, Anemia in children with chronic kidney disease. Nature Reviews Nephrology, 2011. 7(11): p. 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hesp AC, et al. , The role of renal hypoxia in the pathogenesis of diabetic kidney disease: a promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int, 2020. 98(3): p. 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sano M and Goto S, Possible Mechanism of Hematocrit Elevation by Sodium Glucose Cotransporter 2 Inhibitors and Associated Beneficial Renal and Cardiovascular Effects. Circulation, 2019. 139(17): p. 1985–1987. [DOI] [PubMed] [Google Scholar]
- 92.Packer M, Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics. American Journal of Kidney Diseases, 2021. 77(2): p. 280–286. [DOI] [PubMed] [Google Scholar]
- 93.Griffin M, et al. , Empagliflozin in Heart Failure. Circulation, 2020. 142(11): p. 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmieder R, et al. , OS 12-03 SGLT-2-INHIBITION WITH DAPAGLIFLOZIN REDUCES TISSUE SODIUM CONTENT. Journal of Hypertension, 2016. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J, et al. , Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration during Hyperglycemia. Journal of the American Society of Nephrology, 2019. 30(4): p. 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heerspink HJL and Cherney DZI, Clinical Implications of an Acute Dip in eGFR after SGLT2 Inhibitor Initiation. Clinical Journal of the American Society of Nephrology, 2021. 16(8): p. 1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meraz-Muñoz AY, Weinstein J, and Wald R, eGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney360, 2021. 2(6): p. 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilding J, SGLT2 inhibitors and urinary tract infections. Nature Reviews Endocrinology, 2019. 15(12): p. 687–688. [DOI] [PubMed] [Google Scholar]
- 99.Dave CV, et al. , Sodium–Glucose Cotransporter-2 Inhibitors and the Risk for Severe Urinary Tract Infections. Annals of Internal Medicine, 2019. 171(4): p. 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fadini GP, et al. , Pharmacovigilance assessment of the association between Fournier’s gangrene and other severe genital adverse events with SGLT-2 inhibitors. BMJ Open Diabetes Research & Care, 2019. 7(1): p. e000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qiu H, Novikov A, and Vallon V, Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes/Metabolism Research and Reviews, 2017. 33(5): p. e2886. [DOI] [PubMed] [Google Scholar]
- 102.Palmer BF and Clegg DJ, Euglycemic Ketoacidosis as a Complication of SGLT2 Inhibitor Therapy. Clinical Journal of the American Society of Nephrology, 2021: p. CJN.17621120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baigent C, et al. , Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. The Lancet, 2022. 400(10365): p. 1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peters AL, et al. , Diabetic Ketoacidosis With Canagliflozin, a Sodium–Glucose Cotransporter 2 Inhibitor, in Patients With Type 1 Diabetes. Diabetes Care, 2016. 39(4): p. 532. [DOI] [PubMed] [Google Scholar]
- 105.Zinman B, et al. , Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine, 2015. 373(22): p. 2117–2128. [DOI] [PubMed] [Google Scholar]
- 106.Wanner C, et al. , Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. New England Journal of Medicine, 2016. 375(4): p. 323–334. [DOI] [PubMed] [Google Scholar]
- 107.Neal B, et al. , Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine, 2017. 377(7): p. 644–657. [DOI] [PubMed] [Google Scholar]
- 108.Perkovic V, et al. , Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine, 2019. 380(24): p. 2295–2306. [DOI] [PubMed] [Google Scholar]
- 109.Heerspink HJL, et al. , Dapagliflozin in Patients with Chronic Kidney Disease. New England Journal of Medicine, 2020. 383(15): p. 1436–1446. [DOI] [PubMed] [Google Scholar]
- 110.Empagliflozin in Patients with Chronic Kidney Disease. New England Journal of Medicine, 2022. [Google Scholar]
- 111.Taylor SI, et al. , SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. The Lancet Diabetes & Endocrinology, 2019. 7(12): p. 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cherney DZI, et al. , Renal Hemodynamic Effect of Sodium-Glucose Cotransporter 2 Inhibition in Patients With Type 1 Diabetes Mellitus. Circulation, 2014. 129(5): p. 587–597. [DOI] [PubMed] [Google Scholar]
- 113.Lebovitz HE, Thiazolidinediones: the Forgotten Diabetes Medications. Curr Diab Rep, 2019. 19(12): p. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DeFronzo RA, Eldor R, and Abdul-Ghani M, Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care, 2013. 36 Suppl 2: p. S127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Singh S, Loke YK, and Furberg CD, Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care, 2007. 30(8): p. 2148–53. [DOI] [PubMed] [Google Scholar]
- 116.Wu H, et al. , Mapping the single-cell transcriptomic response of murine diabetic kidney disease to therapies. Cell Metabolism, 2022. 34(7): p. 1064–1078.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gottschalk M, et al. , Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care, 2007. 30(4): p. 790–4. [DOI] [PubMed] [Google Scholar]
- 118.Heerspink HJL, et al. , Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet (London, England), 2019. 393(10184): p. 1937–1947. [DOI] [PubMed] [Google Scholar]
- 119.Mann JF, et al. , Avosentan for overt diabetic nephropathy. J Am Soc Nephrol, 2011. 21(3): p. 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoekman J, et al. , Predictors of congestive heart failure after treatment with an endothelin receptor antagonist. Clin J Am Soc Nephrol, 2013. 9(3): p. 490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagase M and Fujita T, Mineralocorticoid receptor activation in obesity hypertension. Hypertens Res, 2009. 32(8): p. 649–57. [DOI] [PubMed] [Google Scholar]
- 122.Barrera-Chimal J, et al. , Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat Rev Nephrol, 2022. 18(1): p. 56–70. [DOI] [PubMed] [Google Scholar]
- 123.Verma S, et al. , Aldosterone in chronic kidney disease and renal outcomes. Eur Heart J, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pitt B, et al. , Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med, 2021. 385(24): p. 2252–2263. [DOI] [PubMed] [Google Scholar]
- 125.Liles JT, et al. , ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J Clin Invest, 2018. 128(10): p. 4485–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tuttle KR, et al. , JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant, 2018. 33(11): p. 1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krishnan SM, et al. , The Impact of the Nitric Oxide (NO)/Soluble Guanylyl Cyclase (sGC) Signaling Cascade on Kidney Health and Disease: A Preclinical Perspective. Int J Mol Sci, 2018. 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hanrahan JP, et al. , Effects of the Soluble Guanylate Cyclase Stimulator Praliciguat in Diabetic Kidney Disease: A Randomized Placebo-Controlled Clinical Trial. Clin J Am Soc Nephrol, 2020. 16(1): p. 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Juszczak F, et al. , Critical Role for AMPK in Metabolic Disease-Induced Chronic Kidney Disease. Int J Mol Sci, 2020. 21(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yasuda-Yamahara M, Kume S, and Maegawa H, Roles of mTOR in Diabetic Kidney Disease. Antioxidants (Basel), 2021. 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Birk AV, et al. , Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol, 2014. 171(8): p. 2017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szeto HH, et al. , Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol, 2011. 22(6): p. 1041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herzig S and Shaw RJ, AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol, 2018. 19(2): p. 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morita M, et al. , mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol Cell, 2017. 67(6): p. 922–935 e5. [DOI] [PubMed] [Google Scholar]