Abstract
Background:
Chemicals used or emitted by unconventional oil and gas development (UOGD) include reproductive/developmental toxicants. Associations between UOGD and certain birth defects were reported in a few studies, with none conducted in Ohio, which experienced a thirty-fold increase in natural gas production between 2010 and 2020.
Methods:
We conducted a registry-based cohort study of 965,236 live births in Ohio from 2010–2017. Birth defects were identified in 4,653 individuals using state birth records and a state surveillance system. We assigned UOGD exposure based on maternal residential proximity at birth to active UOG wells and a metric specific to the drinking-water exposure pathway that identified UOG wells hydrologically connected to a residence (“upgradient UOG wells”). We estimated odds ratios (ORs) and 95% confidence intervals (CIs) for all structural birth defects combined and specific birth defect types using binary exposure metrics (presence/absence of any UOG well and presence/absence of an upgradient UOG well within 10 km), adjusting for confounders. Additionally, we conducted analyses stratified by urbanicity, infant sex, and social vulnerability.
Results:
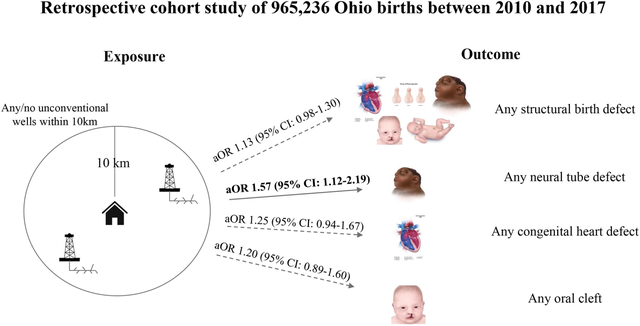
The odds of any structural defect were 1.13 times higher in children born to mothers living within 10 km of UOGD than those born to unexposed mothers (95%CI: 0.98–1.30). Odds were elevated for neural tube defects (OR: 1.57, 95%CI: 1.12–2.19), limb reduction defects (OR: 1.99, 95%CI: 1.18–3.35), and spina bifida (OR 1.93; 95%CI 1.25–2.98). Hypospadias (males only) was inversely related to UOGD exposure (OR: 0.62, 95%CI: 0.43–0.91). Odds of any structural defect were greater in magnitude but less precise in analyses using the hydrological-specific metric (OR: 1.30; 95%CI: 0.85–1.90), in areas with high social vulnerability (OR: 1.27, 95%CI: 0.99–1.60), and among female offspring (OR: 1.28, 95%CI: 1.06–1.53).
Conclusions:
Our results suggest a positive association between UOGD and certain birth defects, and findings for neural tube defects corroborate results from prior studies.
Keywords: oil and gas, birth defects, fracking, congenital malformations, congenital anomalies, epidemiology, Ohio, hydraulic fracturing
Graphical Abstract
1. Introduction
Unconventional oil and gas development (UOGD) refers to the extraction of oil and gas from previously inaccessible reservoirs through the use of directional drilling and high-volume hydraulic fracturing.1, 2 High-volume hydraulic fracturing uses the injection of large quantities of fluids to liberate oil and natural gas from low permeability rock, such as shale.1, 2 Widespread application of these techniques transformed the United States (U.S.) from a net importer to net exporter of natural gas.3–5 The state of Ohio, which is situated above the Marcellus and Utica Shales and ranks seventh in the nation with regard to natural gas production, experienced a thirty-fold increase in the volume of natural gas produced between 2010 and 2020.6, 7
In addition to the U.S. “shale boom,” widespread exploration and/or development of UOG reserves is occurring in numerous countries and on every continent except Antarctica.8–15 Proponents of UOGD support natural gas as a “transition fuel” that will enable a shift from coal to renewable energy, create jobs, and decrease reliance on foreign countries for energy needs.5, 16–18 Others contend that economic benefits have been overstated19,20, 21 investments in new natural gas infrastructure will prolong the production of greenhouse gas emitting fossil fuel resources22, 23 and that energy independence would be more safely and reliably achieved through more expeditious adoption of renewable energy sources.24–26 Finally, there is growing evidence of the negative health implications associated with living in proximity to UOGD, particularly with regard to children’s health, including birth defects.5, 27–30
Annually, an estimated 5 million babies are born with a birth defect worldwide.31 Birth defects, also known as congenital anomalies, are defined as a change to an organ or body part that can negatively impact a baby’s survival, health status, and/or ability to thrive developmentally.32 Approximately 3% of infants in the U.S. are born with a defect, and birth defects are the leading cause of infant mortality in the U.S.32 Globally, birth defects result in an estimated 400,000 deaths in children under five years.31 Children born with a defect may experience a lifelong disability and have specialized healthcare and social needs.31, 33, 34 The etiology of birth defects is multifactorial and as many as 60% of birth defects have an unknown cause.35–38 Established risk factors include genetics, maternal age, alcohol use, and medication use; however, certain involuntary and modifiable environmental exposures are also potential risk factors (e.g., radiation, air pollution, chemical toxicants), likely in combination with genetic determinants of vulnerability, but evidence remains limited.34, 39–41
UOGD can release air pollutants, water contaminants, and other stressors that could result in an increased risk of adverse birth outcomes for those living in proximity to these sites.29 Hydraulic fracturing fluids and UOGD wastewater contain numerous known and suspected reproductive and developmental toxicants including metals (e.g., arsenic, cadmium, lead and mercury), polycyclic aromatic hydrocarbons, and volatile organic compounds (e.g. benzene and toluene).36, 42–45 Although management of these wastewaters is intended to reduce human exposure, surface spills, leaks, or containment failure could lead to migration of chemicals into groundwater or surface water.46–48 Increased vehicle emissions and diesel equipment emissions near well sites may also expose pregnant women to environmental teratogens such as fine particulate matter, nitrous oxides, and other airborne pollutants.49–53 UOGD can also lead to increased noise exposure, particularly at night,54 which can activate the sympathetic nervous system and potentially contribute to sleep disturbance, cardiovascular disease, and adverse birth outcomes, although evidence for birth defects specifically is limited.55, 56 UOGD has also been associated with increased psychosocial stress in proximal communities,49, 57, 58 another potential risk factor for birth defects.59–63 Hypothesized mechanistic pathways connecting UOGD hazards with birth defects include oxidative stress,36, 64, 65 fetal hypoxia,66 insulin resistance,60 inflammation,67 and endocrine disruption.68
To our knowledge, eight studies have evaluated associations between UOGD exposure and risk of structural birth defects. All studies reported evidence of a relationship, although the direction and statistical significance of associations vary by birth defect and study (Table 1).69–75 Studies have been conducted in Colorado, Texas, Pennsylvania, Oklahoma, and Alberta, Canada. The most common associations reported were with congenital heart and neural tube defects. These studies primarily applied aggregate UOG proximity metrics, while McKenzie et al. 2019 applied an intensity-adjusted inverse distance-weighted metric that reflected additional detail about air pollution-emitting activities at the UOG well site.74 A study conducted in Texas by Willis et al. 2023 used a variety of metrics specific to the volume of oil, gas, and waste water produced within 5 km of maternal address.19 To help clarify the relationship between UOGD exposure and risk of a range of structural birth defects in a less studied region, we conducted a registry- and population-based study in the state of Ohio and applied a new UOGD metric specific to the drinking water exposure pathway. The study protocol was approved by the Institutional Review Board of Yale University (HIC #2000021809) and by the Ohio Department of Health.
Table 1.
Characteristics and results of studies of exposure to UOGD and birth defects.
| Lead author (year) | State/ region | Study design | Exposure metric | Study population | Covariates | Findings |
|---|---|---|---|---|---|---|
| McKenzie (2014) | Colorado | Retrospective cohort | IDW well count (10 mi buffer) | 124,842 births between 1996 and 2009 | Maternal age, ethnicity, tobacco use, alcohol use, parity at time of pregnancy, education, elevation of residence, and infant sex |
CHDs ↑ (aOR: 1.3, 95% CI: 1.2–1.5)
NTDs ↑ (aOR: 2.0, 95% CI: 1.0–3.9) Oral clefts ↘ (aOR: 0.82, 95% CI: 0.55–1.2) |
| Ma (2016) | Pennsylvania | Semiecological | Presence of 1+ well in zip code | 1,401,813 births between 2003 and 2012 | Maternal age, maternal highest education level, self-designated race, maternal pre-pregnancy BMI, primary payer for delivery, mother receiving WIC assistance, maternal pre and during pregnancy diabetes status, maternal hypertension, maternal smoking, and maternal infection during pregnancy reported in birth registry | Zip codes with UOGD: Any birth defects ↑ (OR: 1.22, 95% CI: 1.13–1.32) Structural birth defects ↑ (OR: 1.21, 95% CI: 1.111.32) Functional or developmental birth defects: ↗ (OR:1.23, 95% CI: 1.06–1.43) Increase in UOG well density per square kilometer: Any birth defects prevalence ↘ (OR: 0.93, 95% CI: 0.851.01) Structural birth defects prevalence ↘ (OR: 0.95, 95% CI: 0.86–1.04) Functional or developmental birth defects prevalence ↘ (OR: 0.90, 95% CI: 0.76–1.07) |
| Hill (2018) | Pennsylvania | Difference-indifference | Nearest UOG well Buffers (2km, 2.5km, 3km, 3.5km, 4km, 4.5km, 5km), Well density at 2.5 km | 1,098,884 births between 2003 and 2010 | Race, education, age, marital status, WIC status, insurance type, previous risky pregnancy, whether the mother smoked during her pregnancy, month of birth, year of birth, month/year interaction, and gender of the child | Any congenital anomaly ↔ |
| Janitz (2019) | Oklahoma | Retrospective cohort | IDW well count (2, 5 and 10 mi buffers) | 476,000 births between 1997 and 2009 | Birth year, infant sex, race/ethnicity, gestational age, birth weight, urban/rural status of census block, maternal age, marital status, prenatal care, parity, maternal tobacco use during pregnancy, maternal education | At the 2-mile buffer: NTD ↗ (PPR: 1.20, 95% CI: 0.82–1.75) Oral clefts ↔ (PPR: 1.03, 95% CI: 0.82–1.29) CCHD ↘ (PPR: 0.91, 95% CI: 0.75–1.11) Exposure to any natural gas activity compared to none, at the 2-mile buffer: Mix of insignificant positive and negative associations for a variety of specific congenital malformations (common truncus, etc.) ↔ |
| McKenzie (2019) | Colorado | Nested case-control | Intensity adjusted inverse distance weighted well count (10 mi buffer) during second month of pregnancy | 469 cases, 2860 controls born between 2005 and 2011 | IDW count of other oil and gas facilities, IA-IDW count of non oil and gas air pollution sources, maternal age, socioeconomic status index, parity, infant sex | Overall: Any CHD ↑ (OR: 1.7, 95% CI: 1.1–2.6); Insignificant positive association with various specific heart defects Rural: Any CHD ↑ (OR: 2.47, 95% CI: 1.3–4.4); Conotruncal defects ↑ (OR: 4.07, 95% CI: 1.4–12); Insignificant positive associations with various specific heart defects ↗ Urban:- Any CHD ↘ (OR: 0.95, 95% CI: 0.47, 1.9); Insignificant positive and negative associations with various specific heart defects |
| Tang (2020) | Texas | Case-control | Well count (1, 3, and 7.5 km buffers) | 52,955 cases and 642,399 controls between 1999 and 2011 | - Maternal Characteristics: smoking status, plurality of birth, maternal age, race/ethnicity, and education status - Neighborhood Characteristics: median household income at maternal address block group, urbanicity in 2010, and average daily vehicle miles traveled for all trucks by county |
Within 1 km of maternal address: Anencephaly ↑ (aOR: 2.44, 95% CI: 1.55–3.86) Spina bifida ↑ (aOR: 2.09, 95% CI: 1.47–2.99) Gastrochisis (older mothers) ↑ (aOR: 3.19, 95% CI: 1.77–5.73) Atrial septal defect (1.66, 95% CI: 1.54–1.79) Aortic valve stenosis ↑ (aOR: 1.90, 95% CI: 1.33–2.71) Hypoplastic left heart syndrome ↑ (aOR: 2.00, 95% CI: 1.39–2.86) Pulmonary valve atresia/stenosis ↑ (aOR: 1.36, 95% CI: 1.10–1.66) Orofacial clefts: no effect |
| Cairncross (2022) | Alberta, Canada | Retrospective cohort | Presence of 1+ wells within 10 km | 34,873 births between 2013 and 2018 | Parental age at delivery, multiple births (ie, twins, triplets), infant sex, obstetric comorbidities, and area-level socioeconomic status (Pampalon Index) | Major congenital anomalies (aRR: 1.31; 95% CI, 1.01–1.69) |
| Willis (2023) | Texas | Retrospective cohort | Tertiles of inverse distance-squared weighting within 5 km for drilling site count, gas production, oil production, and produced water | 2,234,138 births between 1999 and 2009 | -Individual Characteristics infant sex, gestational age, birth weight, maternal age, maternal race and ethnicity, maternal education, maternal smoking, maternal alcohol usage, prenatal care, distance to nearest highways, birth year, county -Neighborhood Characteristics: unemployment, % White population, median household income |
Temporal comparison, inverse distance-squared well count within 5km: All defects ↑ (aOR: 1.25, CI: 1.21–1.30) > 1 site ↑ (aOR: 1.19, CI: 1.10–1.33) Cardiac and circulatory ↑ (aOR: 1.20, CI: 1.13–1.28) Central nervous system ↔ (aOR: 1.00, CI: 0.82–1.21) Eye and ear ↑ (aOR: 1.53, CI: 1.10–2.12) Gastrointestinal ↗ (aOR: 1.10, CI: 0.97–1.24) Genitourinary ↑ (aOR: 1.12, CI: 1.03–1.21) Musculoskeletal ↗ (aOR: 1.11, CI: 0.96–1.29) Oral clefts ↔ (aOR: 1.02, CI: 0.85–1.22) Respiratory ↔ (aOR: 0.96, CI: 0.69–1.35) Chromosomal ↑ (aOR: 1.32, CI: 1.10–1.59) |
| Current study | Ohio | Retrospective | Presence of 1+ wells within 10 km, presence of upgradient UOG well within 10 km, IDW well count | 965,236 births between 2010 and 2017 | Year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration | Any UOG wells within 10km: Any structural defect ↗ (aOR: 1.11, 95% CI: 0.99–1.25) Any CHD ↗ (aOR: 1.16, 95% CI: 0.94–1.44) Any NTD ↑ (aOR: 1.45, 95% CI: 1.07–1.97) Oral clefts ↗ (aOR: 1.18, 95% CI: 0.99–1.81) Limb reduction ↑ (aOR: 2.13, 95% CI: 1.30–3.49) Hypospadias ↓ (aOR: 0.65, 95% CI: 0.45–0.93) |
IDW—inverse distance-weighted; OR—odds ratio; CI—confidence interval; UOG—unconventional oil and gas; CHD—congenital heart defect; NTD—neural tube defect; aOR—adjusted odds ratio; aRR—absolute risk reduction; CCHD—critical congenital heart defects; PPR—prevalence proportion ratios ↑ = significant increase; ↓ = significant decrease; ↗ = non-significant increase; ↘ = non-significant decrease; ↔ = non-significant direction not reported or zero effect
2. Methods
2.1. Study Population
The source study population included all live singleton births in Ohio from 2010–2017, obtained from birth records from the Ohio Department of Health (n=1,029,682) (Figure 1). We geocoded the maternal addresses at birth using SAS 9.4 PROC GEOCODE. Addresses unable to be geocoded to street level were excluded (n=60,070; 5.8%). In addition, records were excluded if they were missing infant sex (n=11; 0.0011%), had unknown or implausible values for term birth weight (<500 g or >5500 g) or gestational age (<18 weeks or >47 weeks) (n=4329; 0.42%), or had geocoded birth addresses outside of Ohio (n=35; 0.0025%). Application of exclusion criteria yielded a final cohort of 965,236 births.
Figure 1.
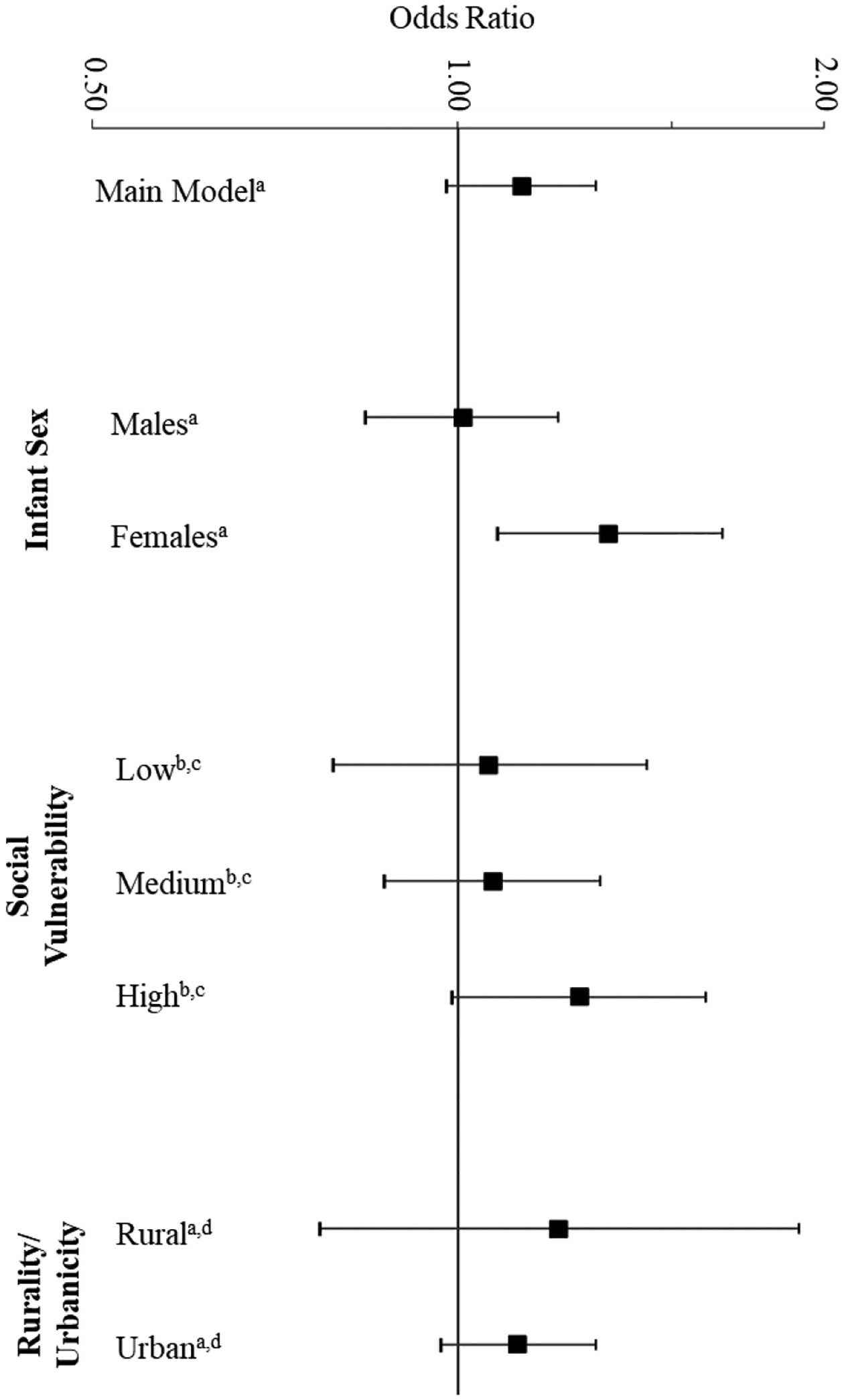
Stratified analyses of odds of any structural defect in relation to presence of unconventional oil and gas well within 10 km of maternal residence at birth (2010–2017).
aOdds Ratios and confidence intervals are for logistic regression including the following covariates: year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration.
bNeighborhoods with “low,” “moderate,” and “high” social vulnerability are defined as those census tracts which had a Social Vulnerability Index in the bottom 33%, the middle 33%, or the top 33% compared to other census tracts across the state, as ranked in the Center for Disease Control 2014 Social Vulnerability Index dataset
cOdds Ratios and confidence intervals are for logistic regression including the following covariates: year of birth, parity, maternal race, maternal smoking, use of WIC, and ambient PM2.5 concentration.
dRural” and “urban” are defined in accordance with 2010 Rural-Urban Commuting Area (RUCA) codes from the United States Department of Agriculture. Individuals living in a census tract with a RUCA code of 1–7 are considered “urban”; individuals living in a census tract with a RUCA code of 8–10 are considered “rural.”
2.2. Outcome Assessment
Birth defects can be categorized into “structural birth defects” which primarily affect the structure of body parts, and “functional birth defects,”31, 76 which primarily affect the development and function of whole-body systems. In this study, we focus on structural birth defects because they are more likely to be accurately identified and diagnosed at birth, while functional abnormalities require a longer follow up and more sensitive outcome assessment methods to ascertain.77
Information on structural birth defect outcomes was obtained from two sources: (i) “congenital anomalies” identified on the Ohio Department of Public Health birth records (obtained for 2010–2017), and (ii) the Ohio Connections for Children with Special Needs (OCCSN) birth defects surveillance system (available at time of data acquisition for 2012–2017). The birth records were available for all live births and for more years than the surveillance data and therefore the state birth records were used in the primary analyses. However, because these are typically based on a visual examination, defects not overtly manifested at birth may be missed.78 The OCCSN is a passive surveillance system in which all hospitals, physicians, and freestanding birthing centers in Ohio are required to report cases of children from birth to 5 years of age with specific birth defects via file upload to a secure website.35 The OCCSN data was available for fewer years than the state birth records, but provided the data for more specific birth defect subtypes.
The birth record data included diagnoses for nine types of structural birth defects: anencephaly, meningomyocele/spina bifida, cyanotic congenital heart disease, gastroschisis, omphalocele, diaphragmatic hernia, limb reduction defects, cleft lip with or without cleft palate, and cleft palate alone. The surveillance data included another ten diagnoses, seven of which fall within the category of “cyanotic congenital heart disease”—common truncus, transposition of the great arteries, tetralogy of Fallot, pulmonary valve atresia, tricuspid valve atresia and stenosis, hypoplastic left heart syndrome, and total anomalous pulmonary venous connection—as well as aortic valve stenosis and coarctation of the aorta, which are only cyanotic if critical, and encephalocele. Individuals with multiple diagnoses were selected as cases for each diagnosis present and considered a single case for the aggregate “any structural birth defect” outcome.
2.3. Exposure Assessment
We retrieved oil and gas well location and production datasets from the Ohio Department of Natural Resources Division of Oil and Gas Resources Risk Based Data Management System to identify all active wells, defined as having been drilled or producing as confirmed by having a reported spud date or production report.79, 80 The data were cleaned and quality checked to address missing data, remove duplicates, and harmonize variables that changed over time. For example, for wells reporting gas, oil, or brine production from Marcellus and Utica formations but a missing spud date, we assigned a spud date equal to the first day of the earliest producing production reporting period minus 251 days (the median number of days between the spud date and the first reported production period). Wells with a missing spud date and no production reporting periods were considered inactive and excluded. The final Ohio UOGD well dataset included 2,290 ever active Marcellus and Utica coalbed methane, gas, and oil wells, with spud dates between January 21, 2008, and December 31, 2017.
We applied three UOG proximity-based metrics used in prior studies: (i) a binary metric for presence or absence of any active UOG wells within the buffer distance, (ii) an inverse-distance-square-weighted (ID2W) metric capturing the density of active UOG wells within the buffer, which was categorized into tertiles, and (iii) a hydrological metric that is specific to the drinking water exposure pathway (IDups). Due to the low prevalence in UOGD exposure, we were unable to apply our exposure metrics with more than three categories or use them continuously. The ID2W metric uses the following formula:
| [1] |
where d is the distance between the ith UOG well and a residence, and n the number of active UOG wells. This metric accounts for all UOG wells within a buffer zone while weighting closer wells more heavily than distant wells. Variations of the inverse-distance-weighted metric have been used in several previous health studies (Table 1).29
The details and programming code for the IDups metric was previously presented; this metric considers only the closest active UOG well that could be hydrologically connected to a residence.81–83 This exposure metric assumes that UOG wells that are located upgradient of a residence contribute more to exposure than downgradient wells.
The IDups metric is expressed as
| [2] |
where u is the distance to the nearest topographically upgradient UOG well, as determined by the D-infinity algorithm in TauDEM.84 IDups was found to be a highly informative predictor in physics-informed models of groundwater vulnerability in regions of hill-and-valley topography where groundwater tends to flow in the downhill direction, parallel to the local topographic gradient. The IDups metric was applied in one previous exposure study and one previous epidemiologic analysis of children’s health.85, 86 In using the IDups metric, we implicitly assume that consumption or contact with groundwater from domestic wells is an important exposure source.83 In Ohio, more than 40% of the population is served by groundwater87, and approximately 15–20% of residences utilize a domestic well as a source of drinking water;88, 89 this percentage is likely higher in the rural northeast of the state, where UOGD is most prevalent.
Buffer distances of 2, 5, and 10 km were used based on plausible dispersion of air and water pollutants and to facilitate comparisons to prior literature. With respect to exposure timing, we examined two etiologically important exposure windows for our UOGD exposure metrics: (i) the year prior to birth, called the “primary window,” and (ii) the first trimester only, as this is a particularly vulnerable time in fetal development with regard to teratogen exposure.90 The time window corresponding to the first trimester was calculated using the date of birth and the obstetric estimate of gestation.
2.4. Covariates
A list of candidate individual and community-level factors was compiled a priori based on the published literature including known or suspected risk factors for birth defects and covariates used in prior studies of UOGD and birth defects. These included the following individual-level demographic, socioeconomic, health, and lifestyle factors obtained from state birth records: infant sex, birth year, season of birth, maternal age, maternal race, maternal ethnicity, maternal educational attainment, maternal marital status, maternal smoking status during pregnancy, maternal alcohol use during pregnancy, parity (nulliparious, one or more previous live births), primary payer for delivery (Medicaid, private insurance), use of federal Women Infants and Children (WIC) program, pre-pregnancy body mass index (BMI), whether a mother received prenatal care, and maternal hypertension or diabetes.
We also obtained community-level variables such as urbanicity/rurality as captured by the 2010 Rural Urban Commuting Area (RUCA) codes which classify U.S. census tracts into 10 categories based on population density, urbanization, and daily commuting91 and the 2014 Social Vulnerability Index (SVI) developed by the U.S. Center for Disease Control (CDC), which combines 15 U.S. Census variables into a percentile ranking capturing the likelihood of experiencing disproportionate harm from environmental disasters with a higher percentile indicating greater vulnerability.92 The SVI has been associated with preterm birth and other health outcomes.93–95 We used agricultural data from the Cropland Data Layer of the USDA CropScape tool (USDA 2009–2016) to classify individuals with a maternal geocoded residence with any/none cropland within 500 m as a proxy for the potential for pesticide exposures.96, 97 In addition, each birth was linked to the mean average daily fine particulate matter (PM2.5) concentration in their census tract during the first trimester of pregnancy, a previously identified sensitive time window, using data from the U.S. Environmental Protection Agency98–100 Both PM2.5 and SVI were modeled as tertiles. We considered including SVI as a time-varying factor, but none of the tracts changed tertiles across 2010, 2014, and 2018.
2.5. Statistical Analysis
We used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for all structural birth defects combined, categories of birth defects (e.g., neural tube defects), and individual birth defects (e.g., anencephaly). Our primary analyses used defects identified from the state birth records for the years 2010–2017. The exposure metric used in our main model was presence or absence of a UOG well within 10 km, informed by the overall low exposure prevalence and rarity of birth defects. Our main model included the following potential confounding factors as covariates, selected based on the published literature, evidence of associations with the exposure or outcome, sufficient variability in the distribution of subjects across categories, and to avoid overadjustment and unnecessary adjustment:101 year of birth, parity, maternal race, maternal smoking, use of WIC, SVI, and ambient PM2.5 concentrations. A directed acyclic graph (Supplemental Figure 1) illustrates how we have conceptualized the relationship between covariates included in the models. The main model was run separately for each birth outcome data source (state birth records and surveillance system) and for several variations on the exposure metrics (different time windows, buffer sizes, tertiles of inverse-distance weighted well count, water-pathway specific metric). Models for hypospadias were restricted to males only.
In addition, we conducted several sensitivity analyses to test the robustness of our main findings to inclusion of other covariates, as some of the relationships depicted in our directed acyclic graph could be debated. Additional analyses included (a) main model + additional sociodemographic (infant sex, maternal ethnicity, maternal marital status), (b) main model + lifestyle factors (maternal alcohol consumption during pregnancy), (c) main model + maternal health (hypertension, diabetes, previous risk pregnancy), (d) main model + socioeconomic factors (maternal education, use of WIC), and (e) main model + other environmental factors (season of birth percent cropland). In stratified analyses, we ran our main model separately for urban (RUCA codes 1–7) and rural census tracts (RUCA codes 8–10), male and female infants, and three SVI categories (<33rd percentile, 33–66th percentile, and >66th percentile). In addition, we considered whether ambient PM2.5 concentrations could be on the causal pathway between UOGD and birth defects, although we assumed these data would reflect general, regional air pollution and would not be sensitive enough to detect intermittent emissions from oil and gas well pads. However, we reran our models with and without PM2.5. All statistical analyses were conducted within the R Statistical Environment (http://www.R-project.org/).
3. Results
There were 4,112 individuals in our 2010–2017 cohort with at least one structural defect recorded in state birth records and 2,321 identified from surveillance data (2012–2017). Population characteristics for the full population and those identified as having birth defects from either state birth records or the surveillance system are presented in Table 2. The most prevalent birth defect categories based on state birth records were hypospadias (n=943), followed by congenital heart defects (n=904), and oral clefts (n=874). These categories were also the most prevalent based on the surveillance data (Table 3). Between 2010 and 2017, there were 41,152 births to mothers who resided within 10 km of an UOGD well during pregnancy (4.3% of the total cohort) (Table 4).
Table 2.
Population characteristics by outcome data source.
| Maternal or infant characteristics | Full cohort (N = 965,236) | Individuals with structural birth defects identified on birth record (N = 3,976) | Individuals with structural birth defects identified in surveillance database (N = 2,246) |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Birth year | |||
| 2010 | 115011 (11.9) | 374 (9.1) | 0 (0) |
| 2011 | 114324 (11.8) | 354 (8.6) | 0 (0) |
| 2012 | 122019 (12.6) | 441 (10.7) | 327 (14.1) |
| 2013 | 122683 (12.7) | 515 (12.5) | 417 (18) |
| 2014 | 123395 (12.8) | 555 (13.5) | 383 (16.5) |
| 2015 | 123759 (12.8) | 591 (14.4) | 438 (18.9) |
| 2016 | 122395 (12.7) | 656 (16) | 419 (18.1) |
| 2017 | 121651 (12.6) | 626 (15.2) | 337 (14.5) |
| Parity | |||
| 0 | 372287 (38.6) | 1693 (41.2) | 917 (39.5) |
| 1+ | 586729 (60.8) | 2404 (58.5) | 1396 (60.1) |
| Unknown | 6221 (0.6) | 15 (0.4) | 8 (0.3) |
| Maternal race | |||
| Black | 168437 (17.5) | 710 (17.3) | 322 (13.9) |
| White | 735025 (76.1) | 3173 (77.2) | 1862 (80.2) |
| Other | 31352 (3.2) | 112 (2.7) | 60 (2.6) |
| Unknown | 30423 (3.2) | 117 (2.8) | 77 (3.3) |
| Maternal smoking | |||
| Yes | 151065 (15.7) | 757 (18.4) | 385 (16.6) |
| No | 810522 (84) | 3335 (81.1) | 1921 (82.8) |
| Unknown | 3650 (0.4) | 20 (0.5) | 15 (0.6) |
| Use of WIC | |||
| Yes | 371497 (38.5) | 1672 (40.7) | 919 (39.6) |
| No | 586685 (60.8) | 2400 (58.4) | 1381 (59.5) |
| Unknown | 7055 (0.7) | 40 (1) | 21 (0.9) |
| Social vulnerability index | |||
| Least vulnerable (Tertile 1) | 314335 (32.6) | 1194 (29) | 728 (31.4) |
| Moderately vulnerable (Tertile 2) | 318778 (33) | 1442 (35.1) | 803 (34.6) |
| Most vulnerable (Tertile 3) | 332124 (34.4) | 1476 (35.9) | 790 (34) |
| Ambient PM2.5 Concentrations During First Trimester | |||
| Low (Tertile 1) | 319730 (33.1) | 1575 (38.3) | 942 (40.6) |
| Medium (Tertile 2) | 322256 (33.4) | 1264 (30.7) | 760 (32.7) |
| High (Tertile 3) | 323251 (33.5) | 1273 (31) | 619 (26.7) |
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) of structural birth defects for infants exposed to unconventional oil and gas development as defined as having an active UOGD well within 10 km of the maternal residence at birthcompared to unexposed for outcomes based on birth records alone and birth records or surveillance data.
| Birth defect type | Defect recorded in certificate data-2010–2017 cohort (n = 965,236) | Defect recorded in either certificate or surveillance data 2012–2017 cohort (n = 735,901) | ||||
|---|---|---|---|---|---|---|
| Exposed Cases/ All Cases | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | Exposed Cases/ All Cases | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) | |
| Any structural birth defect | 216/4112 | 1.25 (1.09–1.43) | 1.13 (0.98–1.3) | 287/4824 | 1.1 (0.98–1.24) | 1.06 (0.94–1.2) |
| Neural tube defect | ||||||
| Any | 38/545 | 1.68 (1.21–2.34) | 1.57 (1.12–2.19) | 45/584 | 1.45 (1.07–1.97) | 1.42 (1.05–1.93) |
| Anencephaly | 16/294 | 1.29 (0.78–2.14) | 1.28 (0.77–2.13) | 17/238 | 1.34 (0.82–2.19) | 1.41 (0.86–2.32) |
| Spina bifida | 23/256 | 2.22 (1.44–3.4) | 1.93 (1.25–2.98) | 25/313 | 1.51 (1.00–2.27) | 1.4 (0.93–2.12) |
| Congenital heart defect | ||||||
| Any | 50/904 | 1.32 (0.99–1.75) | 1.25 (0.94–1.67) | 52/984 | 0.97 (0.73–1.28) | 0.96 (0.73–1.27) |
| Cyanotic congenital heart disease | 50/904 | 1.32 (0.99–1.75) | 1.25 (0.94–1.67) | 74/1281 | 1.07 (0.84–1.35) | 1.09 (0.86–1.38) |
| Coarctation of aorta | N/A | * | * | 21/311 | 1.26 (0.81–1.96) | 1.21 (0.77–1.89) |
| Tetralogy of Fallot | N/A | * | * | 14/232 | 1.12 (0.65–1.92) | 1.17 (0.68–2.03) |
| Oral clefts | ||||||
| Any | 49/874 | 1.33 (1–1.78) | 1.2 (0.89–1.6) | 79/1240 | 1.18 (0.94–1.49) | 1.08 (0.86–1.36) |
| Cleft lip | 33/576 | 1.36 (0.96–1.94) | 1.24 (0.87–1.77) | 46/643 | 1.34 (0.99–1.81) | 1.23 (0.91–1.67) |
| Cleft palate | 16/298 | 1.27 (0.77–2.11) | 1.12 (0.67–1.85) | 34/624 | 1 (0.71–1.42) | 0.91 (0.64–1.29) |
| Other | ||||||
| Gastroschisis | 21/408 | 1.22 (0.79–1.89) | 1.03 (0.66–1.61) | 20/331 | 1.12 (0.71–1.76) | 0.99 (0.63–1.57) |
| Diaphragmatic hernia | 11/197 | 1.33 (0.72–2.44) | 1.24 (0.67–2.29) | 11/187 | 1.09 (0.59–2) | 1.13 (0.61–2.08) |
| Limb reduction defects | 16/174 | 2.27 (1.36–3.8) | 1.99 (1.18–3.35) | 17/155 | 2.14 (1.30–3.55) | 2.02 (1.21–3.37) |
| Hypospadias | 28/943 | 0.69 (0.47–1) | 0.62 (0.43–0.91) | 30/879 | 0.61 (0.43–0.88) | 0.62 (0.43–0.89) |
Adjusted odds ratios included the following covariates: year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentrations.
““N/A”” identifies those birth defects for which there was no available record in a given data source. Cells are occupied with a ““*”” when they correspond to models for which there were insufficient exposed cases to calculate a meaningful odds ratio (< 10 cases).
Table 4.
Sensitivity analyses of odds of any structural defect in relation to unconventional oil and gas development (2010–2017) using different exposure metrics.
| Model description | Buffer size | Exposure time window | Type of exposure metric | N exposed (cases/total) | ORa (95% CI) |
|---|---|---|---|---|---|
| Main model | 10 km | Year prior to birth | Presence of UOG well | 216/41152 | 1.13 (0.98–1.30) |
| Smaller buffer size | 5 km | Year prior to birth | Presence of UOG well | 63/11959 | 1.12 (0.87–1.44) |
| Narrow time window | 10 km | First trimester | Presence of UOG well within buffer | 193/35467 | 1.14 (0.99–1.32) |
| Inverse distance squared (ref: ID2W=0) | 10 km | Year prior to birth | Inverse distance-squared-weighted: Tertile 1 | 66/13717 | 1.07 (0.83–1.36) |
| Inverse distance squared (ref: ID2W=0) | 10 km | Year prior to birth | Inverse distance-squared-weighted: Tertile 2 | 75/13717 | 1.18 (0.94–1.48) |
| Inverse distance squared (ref: ID2W=0) | 10 km | Year prior to birth | Inverse distance-squared-weighted: Tertile 3 | 75/13718 | 1.14 (0.90–1.43) |
| Water-specific metric (IDups) | 10 km | Year prior to birth | Presence of upgradient UOG well | 22/3564 | 1.30 (0.85–1.97) |
Odds ratios (ORs) adjusted for the following covariates: year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentrations.
In our main model using outcome data from state birth records only (2010–2017), we observed an elevated odds ratio for any structural defect among those living within 10 km of a UOGD well (OR 1.13; 95% CI 0.98–1.30) (Table 3). UOGD exposure based on the binary metric was positively associated with several categories of birth defects, including neural tube defects (OR 1.57; 95% CI 1.12–2.19), spina bifida (OR 1.93; 95% CI 1.25–2.98), and limb reduction defects (OR 1.99; 95% CI 1.18–3.35) (Table 3). In contrast, an inverse association was observed for hypospadias (OR 0.62; 95% CI 0.43–0.91).
Table 4 presents the magnitude of observed ORs for all structural defects combined for variations in the exposure assessment including buffer size (5 km versus 10 km), exposure time window (year prior to birth versus first trimester only), and exposure metric (binary, inverse distance squared weighted, and IDups) (Table 4). Restricting the buffer distance to 5 km and limiting the exposure window to the first trimester each yielded ORs of larger magnitudes and wider confidence intervals. The ORs across tertiles of the inverse-distance-squared-weighted metric were similar to those observed with the binary metric. The largest magnitude OR was observed for IDups, the water-pathway specific metric (OR: 1.30, 95% CI: 0.85–1.97).
In stratified analyses, the OR for structural defects in relation to UOGD exposure were higher among individuals living in Census tracts with greatest neighborhood social vulnerability (OR: 1.26, 95% CI: 0.99–1.60), compared to the OR for the lowest tertile of neighborhood social vulnerability (OR: 1.06, 95% CI: 0.79–1.43) (Figure 1). Sex stratified analyses yielded stronger associations between UOGD exposure and structural birth defects among female offspring (OR: 1.33, 95% CI: 1.08–1.65), but not males (OR: 1.01, 95% CI: 0.84–1.21) (Figure 1). ORs did not differ by rural/urban designation (Figure 1). Results from sensitivity analyses in which additional covariates were added to the main model were consistent with the findings from the main model (Table 5). Excluding ambient PM2.5 concentrations had negligible impact on the results (Supplemental Table 2).
Table 5.
Sensitivity analyses of odds of any structural defect in relation to presence of unconventional oil and gas well within 10 km of maternal residence at birth (2010–2017).
| Model | Variables | OR (95% CI)* |
|---|---|---|
| Main Model | year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration | 1.13 (0.98–1.30) |
| Model A (Main model + additional sociodemographic factors) | year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, ambient PM2.5 concentration, infant sex, maternal ethnicity, maternal marital status | 1.13 (0.98–1.29) |
| Model B (Main model + lifestyle factors) | year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration, maternal alcohol consumption | 1.11 (0.97–1.28) |
| Model C (Main model + maternal health factors) | year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration, hypertension, diabetes, previous risky pregnancy | 1.14 (0.99–1.30) |
| Model D (Main model + socioeconomic factors) | year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration, maternal education, primary payer for delivery | 1.12 (0.97–1.29) |
| Model E (Main model + environmental factors) | year of birth, parity, maternal race, maternal smoking, use of WIC, social vulnerability index, and ambient PM2.5 concentration, season of birth, percent cropland | 1.12 (0.98–1.29) |
4. Discussion
In our study of birth defects in Ohio, we observed elevated ORs of several types of structural birth defects in relation to residential proximity to UOGD, with positive associations observed for neural tube defects and the specific anomalies of spina bifida and limb reduction defects. Hypospadias was inversely associated with potential UOGD exposure. These results add to the current limited body of evidence with analyses from a different state and application of a new water-specific exposure metric.
The positive associations observed in our analysis are generally consistent with the eight existing studies of UOGD and birth defects, with some variations in findings (Table 1). Of the four other studies that assessed neural tube defects, two also observed a positive association with UOGD exposure in the principal metric.19, 73, 75 We found elevated but not statistically significant ORs for congenital heart defects, consistent with findings from four of the five prior studies examining this endpoint.73–75 Our study reports results for limb reduction defects, which was positively associated with UOGD exposure, and for hypospadias, which was inversely associated with the exposure. The unexpected inverse association with hypospadias could be due to chance, or could be indicative of the possibility that endocrine disruptors can produce effect estimates in different directions.102–104 For example, hypospadias is linked to exposure to hormone disruptors,102, 105 and both androgenic and anti-androgenic chemicals have been detected in hydraulic fracturing fluid and wastewater.68, 106–108 Another reason for the inverse relationship could be there are other dominant sources of endocrine disruptors that we did not assess that may have confounded for this endpoint. Of the eight birth defect studies previously mentioned, none reported results for hypospadias specifically. One reported marginally elevated odds of being born with a genitourinary defect in association with maternal residential proximity to oil and gas.19
Ours is one of the few health studies on oil and gas conducted in Ohio, and the only study regarding unconventional oil and gas and birth defects in this state. It is valuable to conduct similar studies in different states, where regulations, geology, demographics, and covariates may vary, to facilitate cross-cohort comparisons, triangulate evidence, and enhance generalizability of findings.109 For example, Ohio has among the shortest allowable setback distances (the allowable distance between a directionally drilled well and a sensitive receptor such as a residence, drinking water well), ranging from 50 to 200 feet depending on the circumstance.110, 111 State-specific studies can hold more weight for policy makers seeking to update state policies.
The low exposure prevalence in conjunction with the rarity of birth defects prevented more refined exposures assessments and posed challenges in terms of statistical power, common issues in research of rare outcomes.78 Our study included 4,653 cases, which is comparable to 2014 and 2019 studies done in Colorado but substantially fewer than a 2020 study conducted in the larger and more populous state of Texas, which included over 50,000 cases.73–75 We grouped specific diagnoses with potentially different etiologies into broader categories to increase sample size, which may have biased results towards the null. Similarly, the IDups metric, which offers more detail and specificity for the drinking water pathway, was only able to be evaluated in a binary fashion and in relation to “any birth defects”, our most aggregate outcome. A study with a larger population size could increase statistical power and enable more refined exposure assessments. We focused on UOGD, which is deep, water-intensive, has had documented well integrity issues, and has been linked to numerous increased health risks in children.1, 28, 29, 112 However, Ohio also has conventional oil and gas development, which emits several similar pollutants113 and future work could expand this analysis to include both conventional and unconventional oil and gas wells.
Although outcome misclassification is possible with administrative birth defects datasets, we used two data sources and observed similar results, lending confidence to the findings. Ohio follows a passive rather than active surveillance system, which means the overall number of birth defects are likely underreported.78, 114 Studies in states with active surveillance systems or in which researchers themselves abstract hospital records would be expected to yield more complete case ascertainment; the 2020 study done by Tang et al. in Texas is an example of this.75, 78, 114 On the other hand, the passive surveillance offers an advantage in terms of providing a lower likelihood of false positives.
Other possible limitations include the use of maternal address at birth to estimate prenatal residential exposure does not account for residential mobility during pregnancy; however, this has been shown to have minimal impact on exposure misclassification.115–117 Finally, live birth bias could be an issue and may underestimate the effect if UOG exposure increases the risk for fetal or neonatal death, especially among those with or susceptible to birth defects.118, 119
5. Conclusions
In this Ohio study, we observed associations between residential proximity to UOGD and neural tube defects, corroborating prior findings. We presented new findings of relationships with the specific anomalies of limb reduction and spina bifida and presented results using a newly developed metric specific to the drinking water exposure pathway. These results, in conjunction with the broader literature, underscore the need to consider impacts to children’s health specifically when developing or improving public health protections around UOGD.
Supplementary Material
Highlights:
Higher odds of neural tube defects in infants born near oil and gas development
Higher odds of limb reduction defects in infants born near oil and gas development
Higher odds of spina bifida in infants born near oil and gas development
Lower odds of hypospadias in infants born near oil and gas development
Greater risk in areas with high neighborhood social vulnerability
Funding:
This research was supported in part by National Priority Research Project under Assistance Agreement No. CR839249 awarded by the U.S. EPA to Yale University. The publication has not been formally reviewed by the U.S. EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the U.S. EPA. In addition, the U.S. EPA does not endorse any products or commercial services mentioned in this publication. C.J.C. was also supported by the National Institute of Environmental Health Sciences under the National Institutes of Health (NIH; F31ES031441), the Yale Cancer Center (T32CA250803). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no competing interests to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.U.S. Environmental Protection Agency. Hydraulic Fracturing for Oil and Gas: Impacts from the Hydraulic Fracturing Water Cycle on Drinking Water Resources in the United States (Final Report). 2016. https://cfpub.epa.gov/ncea/hfstudy/recordisplay.cfm?deid=332990
- 2.U.S. Department of Energy. How is Shale Gas Produced?. Accessed June 29, 2022. https://www.energy.gov/sites/prod/files/2013/04/f0/how_is_shale_gas_produced.pdf
- 3.Fukui R, Greenfield C, Pogue K, van der Zwaan B. Experience curve for natural gas production by hydraulic fracturing. Energy Policy. 2017/06/01/ 2017;105:263–268. doi: 10.1016/j.enpol.2017.02.027 [DOI] [Google Scholar]
- 4.U.S. Energy Information Administration. Natural gas explained. Accessed April 11, 2022. https://www.eia.gov/energyexplained/natural-gas/where-our-natural-gas-comes-from.php
- 5.Wang Q, Chen X, Jha AN, Rogers H. Natural gas from shale formation – The evolution, evidences and challenges of shale gas revolution in United States. Renewable and Sustainable Energy Reviews. 2014/02/01/2014;30:1–28. doi: 10.1016/j.rser.2013.08.065 [DOI] [Google Scholar]
- 6.U.S. Energy Information Administration. Natural Gas Gross Withdrawals and Production. https://www.eia.gov/dnav/ng/NG_PROD_SUM_A_EPG0_FPD_MMCF_A.htm
- 7.U.S. Energy Information Administration. Ohio Dry Natural Gas Production. https://www.eia.gov/dnav/ng/hist/na1160_soh_2a.htm
- 8.Abdollahi R, Motahhari SM, Esfandyari H. Integrated Technical and Economical Methodology for Assessment of Undeveloped Shale Gas Prospects: Applying in the Lurestan Shale Gas, Iran. Mathematical Problems in Engineering. 2021/07/08 2021;2021:7919264. doi: 10.1155/2021/7919264 [DOI] [Google Scholar]
- 9.Jacobs T Unconventional Reservoirs: Australian Exploration Experts Weigh In on Rosy Reserves Estimates. Journal of Petroleum Technology. https://jpt.spe.org/unconventional-reservoirs-australian-exploration-experts-weigh-in-on-rosy-reserves-estimates [Google Scholar]
- 10.Mu L, Chen Y, Xu A, Wang R. Technological progress and development directions of PetroChina overseas oil and gas field production. Petroleum Exploration and Development. 2020/02/01/ 2020;47(1):124–133. doi: 10.1016/S1876-3804(20)60011-8 [DOI] [Google Scholar]
- 11.Perreault T Energy, extractivism and hydrocarbon geographies in contemporary Latin America. Journal of Latin American Geography 2018;17(3):235–252. doi:doi: 10.1353/lag.2018.0048 [DOI] [Google Scholar]
- 12.Tan Y, Hu J, Zhang H, et al. Hydraulic Fracturing Induced Seismicity in the Southern Sichuan Basin Due to Fluid Diffusion Inferred From Seismic and Injection Data Analysis. Geophysical Research Letters. 2020;47(4):e2019GL084885. doi: 10.1029/2019GL084885 [DOI] [Google Scholar]
- 13.McGranahan DA, Kirkman KP. Be proactive on energy sprawl: South Africa must anticipate surface impacts of fracking in rural areas. Resources Policy. 2021/08/01/ 2021;72:102081. doi: 10.1016/j.resourpol.2021.102081 [DOI] [Google Scholar]
- 14.Stephan HR. The discursive politics of unconventional gas in Scotland: Drifting towards precaution? Energy Research & Social Science. 2017/01/01/ 2017;23:159–168. doi: 10.1016/j.erss.2016.09.006 [DOI] [Google Scholar]
- 15.Castro-Alvarez F, Marsters P, Ponce de León Barido D, Kammen DM. Sustainability lessons from shale development in the United States for Mexico and other emerging unconventional oil and gas developers. Renewable and Sustainable Energy Reviews. 2018/02/01/ 2018;82:1320–1332. doi: 10.1016/j.rser.2017.08.082 [DOI] [Google Scholar]
- 16.U.S. Department of Energy. Economic and National Security Impacts under a Hydraulic Fracturing Ban. 2021. https://www.energy.gov/sites/prod/files/2021/01/f82/economic-and-national-security-impacts-under-a-hydraulic-fracturing-ban.pdf
- 17.U.S. Congressional Budget Office. The Economic and Budgetary Effects of Producing Oil and Natural Gas From Shale. 2014. https://www.cbo.gov/publication/49815
- 18.Zakeri B, Paulavets K, Barreto-Gomez L, et al. Pandemic, War, and Global Energy Transitions. Energies. 2022;15(17):6114. [Google Scholar]
- 19.Willis MD, Carozza SE, Hystad P. Congenital anomalies associated with oil and gas development and resource extraction: a population-based retrospective cohort study in Texas. Journal of Exposure Science & Environmental Epidemiology. 2023;33(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black KJ, Boslett AJ, Hill EL, Ma L, McCoy SJ. Economic, environmental, and health impacts of the fracking boom. Annual Review of Resource Economics. 2021;13:311–334. [Google Scholar]
- 21.Sovacool BK. Cornucopia or curse? Reviewing the costs and benefits of shale gas hydraulic fracturing (fracking). Renewable and Sustainable Energy Reviews. 2014;37:249–264. [Google Scholar]
- 22.Stephenson E, Doukas A, Shaw K. “Greenwashing gas: Might a ‘transition fuel’ label legitimize carbon-intensive natural gas development?”. Energy Policy. 2012/07/01/ 2012;46:452–459. doi: 10.1016/j.enpol.2012.04.010 [DOI] [Google Scholar]
- 23.Acemoglu D, Hemous D, Barrage L, Aghion P. Climate Change, Directed Innovation, and Energy Transition: The Long-run Consequences of the Shale Gas Revolution. 2019. https://ideas.repec.org/p/red/sed019/1302.html
- 24.Howarth RW, Santoro R, Ingraffea A. Venting and leaking of methane from shale gas development: response to Cathles et al. Climatic Change. 2012/07/01 2012;113(2):537–549. doi: 10.1007/s10584-012-0401-0 [DOI] [Google Scholar]
- 25.Meyer R America is Facing an Energy-Security Crisis. The Atlantic; 2022. [Google Scholar]
- 26.Twomey DF, Twomey RF, Farias C, Farias G, Harris DL. FRACKING: BLASTING THE BEDROCK OF BUSINESS. Journal of Competitiveness Studies. 2016 2016;24(3):107–127. [Google Scholar]
- 27.Bushong A, McKeon T, Regina Boland M, Field J. Publicly available data reveals association between asthma hospitalizations and unconventional natural gas development in Pennsylvania. Report. PLoS ONE. 2022/03/31/ // 2022;17:e0265513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deziel NC, Brokovich E, Grotto I, et al. Unconventional oil and gas development and health outcomes: A scoping review of the epidemiological research. Environmental Research. 2020/03/01/ 2020;182:109124. doi: 10.1016/j.envres.2020.109124 [DOI] [PubMed] [Google Scholar]
- 29.Deziel NC, Clark CJ, Casey JA, Bell ML, Plata DL, Saiers JE. Assessing Exposure to Unconventional Oil and Gas Development: Strengths, Challenges, and Implications for Epidemiologic Research. Current Environmental Health Reports. 2022/05/06 2022;doi: 10.1007/s40572-022-00358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuele H, Baum CF, Landrigan PJ, Hawkins SS. Associations between proximity to gas production activity in counties and birth outcomes across the US. Prev Med Rep. Dec 2022;30:102007. doi: 10.1016/j.pmedr.2022.102007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Birth defects fact sheet. Updated 28 February 2022. https://www.who.int/news-room/fact-sheets/detail/birth-defects
- 32.March of Dimes. Birth Defects. https://www.marchofdimes.org/peristats/data?reg=99&top=16&lev=0&slev=4&sreg=39
- 33.Christianson A, Howson CP, Modelle B. Global Report on Birth Defects: The Hidden Toll of Dying and Disabled Children. 2006. https://www.marchofdimes.org/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-full-report.pdf
- 34.Weinhold B Environmental Factors in Birth Defects: What We Need to Know. Environmental Health Perspectives. 2009;117(10):A440–A447. [Google Scholar]
- 35.Ohio Department of Health. Ohio Connections for Children with Special Needs 2019 Annual Report. 2019. https://odh.ohio.gov/know-our-programs/birth-defects/resources/ohio-connections-for-children-with-special-needs-(occsn)-annual-report-2019
- 36.Feldkamp ML, Botto LD, Carey JC. Reflections on the etiology of structural birth defects: Established teratogens and risk factors. Birth Defects Research Part A: Clinical and Molecular Teratology. 2015;103(8):652–655. doi: 10.1002/bdra.23392 [DOI] [PubMed] [Google Scholar]
- 37.Harris BS, Bishop KC, Kemeny HR, Walker JS, Rhee E, Kuller JA. Risk Factors for Birth Defects. Obstet Gynecol Surv. Feb 2017;72(2):123–135. doi: 10.1097/ogx.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 38.Brent RL. Environmental causes of human congenital malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics. Apr 2004;113(4 Suppl):957–68. [PubMed] [Google Scholar]
- 39.Abebe S, Gebru G, Amenu D, Mekonnen Z, Dube L. Risk factors associated with congenital anomalies among newborns in southwestern Ethiopia: A case-control study. PLoS One. 2021;16(1):e0245915. doi: 10.1371/journal.pone.0245915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beames TG, Lipinski RJ. Gene-environment interactions: aligning birth defects research with complex etiology. Development. 2020;147(21)doi: 10.1242/dev.191064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright JM, Evans A, Kaufman JA, Rivera-Núñez Z, Narotsky MG. Disinfection By-Product Exposures and the Risk of Specific Cardiac Birth Defects. Environmental Health Perspectives. 2017;125(2):269–277. doi:doi: 10.1289/EHP103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott EG, Ettinger AS, Leaderer BP, Bracken MB, Deziel NC. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity. Journal of Exposure Science & Environmental Epidemiology. 2017/01/01 2017;27(1):90–99. doi: 10.1038/jes.2015.81 [DOI] [PubMed] [Google Scholar]
- 43.Warner NR, Christie CA, Jackson RB, Vengosh A. Impacts of shale gas wastewater disposal on water quality in western Pennsylvania. Environmental science & technology. 2013;47(20):11849–11857. [DOI] [PubMed] [Google Scholar]
- 44.Getzinger GJ, O’Connor MP, Hoelzer K, et al. Natural gas residual fluids: sources, endpoints, and organic chemical composition after centralized waste treatment in Pennsylvania. Environmental science & technology. 2015;49(14):8347–8355. [DOI] [PubMed] [Google Scholar]
- 45.Parker KM, Zeng T, Harkness J, Vengosh A, Mitch WA. Enhanced formation of disinfection byproducts in shale gas wastewater-impacted drinking water supplies. Environmental science & technology. 2014;48(19):11161–11169. [DOI] [PubMed] [Google Scholar]
- 46.Martin N, Nicholaides K, Southard P. Enhanced Water Resources Risk from Collocation of Disposal Wells and Legacy Oil and Gas Exploration and Production Regions in Texas. JAWRA Journal of the American Water Resources Association. 2022;n/a(n/a)doi: 10.1111/1752-1688.13048 [DOI] [Google Scholar]
- 47.Drollette B, Hoelzer K, Warner N, et al. Elevated levels of diesel range organic compounds in groundwater near Marcellus gas operations are derived from surface activities. PNAS. 2015;doi: 10.1073/pnas.1511474112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maloney KO, Baruch-Mordo S, Patterson LA, et al. Unconventional oil and gas spills: Materials, volumes, and risks to surface waters in four states of the U.S. Science of The Total Environment. 2017/03/01/ 2017;581–582:369–377. doi: 10.1016/j.scitotenv.2016.12.142 [DOI] [PubMed] [Google Scholar]
- 49.Allshouse WB, McKenzie LM, Barton K, Brindley S, Adgate JL. Community Noise and Air Pollution Exposure During the Development of a Multi-Well Oil and Gas Pad. Environ Sci Technol. Jun 18 2019;53(12):7126–7135. doi: 10.1021/acs.est.9b00052 [DOI] [PubMed] [Google Scholar]
- 50.Macey GP, Breech R, Chernaik M, et al. Air concentrations of volatile compounds near oil and gas production: a community-based exploratory study. Environmental Health. 2014/10/30 2014;13(1):82. doi: 10.1186/1476-069X-13-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKenzie LM, Blair B, Hughes J, et al. Ambient Nonmethane Hydrocarbon Levels Along Colorado’s Northern Front Range: Acute and Chronic Health Risks. Environ Sci Technol. Apr 17 2018;52(8):4514–4525. doi: 10.1021/acs.est.7b05983 [DOI] [PubMed] [Google Scholar]
- 52.Field RA, Soltis J, Murphy S. Air quality concerns of unconventional oil and natural gas production. 10.1039/C4EM00081A. Environmental Science: Processes & Impacts. 2014;16(5):954–969. doi: 10.1039/C4EM00081A [DOI] [PubMed] [Google Scholar]
- 53.Colborn T, Kwiatkowski C, Schultz K, Bachran M. Natural Gas Operations from a Public Health Perspective. Human and Ecological Risk Assessment: An International Journal. 2011/09/01 2011;17(5):1039–1056. doi: 10.1080/10807039.2011.605662 [DOI] [Google Scholar]
- 54.Hays J, McCawley M, Shonkoff SBC. Public health implications of environmental noise associated with unconventional oil and gas development. Science of The Total Environment. 2017/02/15/ 2017;580:448–456. doi: 10.1016/j.scitotenv.2016.11.118 [DOI] [PubMed] [Google Scholar]
- 55.Basner M, Babisch W, Davis A, et al. Auditory and non-auditory effects of noise on health. The Lancet. 2014 Apr 12 2014;383(9925):1325–1332. doi: 10.1016/S0140-6736(13)61613-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ristovska G, Laszlo HE, Hansell AL. Reproductive Outcomes Associated with Noise Exposure — A Systematic Review of the Literature. International Journal of Environmental Research and Public Health. 2014;11(8):7931–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sangaramoorthy T, Jamison AM, Boyle MD, et al. Place-based perceptions of the impacts of fracking along the Marcellus Shale. Social Science & Medicine. 2016/02/01/ 2016;151:27–37. doi: 10.1016/j.socscimed.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 58.Casey JA, Goin DE, Rudolph KE, et al. Unconventional natural gas development and adverse birth outcomes in Pennsylvania: The potential mediating role of antenatal anxiety and depression. Environ Res. Oct 2019;177:108598. doi: 10.1016/j.envres.2019.108598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmichael SL, Ma C, Tinker S, Rasmussen SA, Shaw GM, Study NBDP. Maternal Stressors and Social Support as Risks for Delivering Babies with Structural Birth Defects. Paediatric and Perinatal Epidemiology. 2014;28(4):338–344. doi: 10.1111/ppe.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carmichael SL, Shaw GM, Yang W, Abrams B, Lammer EJ. Maternal stressful life events and risks of birth defects. Epidemiology. 2007;18(3):356–361. doi: 10.1097/01.ede.0000259986.85239.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Govindaraj S, Shanmuganathan A, Rajan R. Maternal psychological stress-induced developmental disability, neonatal mortality and stillbirth in the offspring of Wistar albino rats. PLoS One. 2017;12(2):e0171089. doi: 10.1371/journal.pone.0171089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Z, Zhang L, Li H, Ye R, Liu J, Ren A. Maternal severe stressful life events and risk of neural tube defects among rural Chinese. Birth Defects Res A Clin Mol Teratol. Feb 2013;97(2):109–14. doi: 10.1002/bdra.23108 [DOI] [PubMed] [Google Scholar]
- 63.Suarez L, Cardarelli K, Hendricks K. Maternal stress, social support, and risk of neural tube defects among Mexican Americans. Epidemiology. Sep 2003;14(5):612–6. doi: 10.1097/01.ede.0000073270.39780.e9 [DOI] [PubMed] [Google Scholar]
- 64.Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient Air Pollution and Risk of Birth Defects in Southern California. American Journal of Epidemiology. 2002;155(1):17–25. doi: 10.1093/aje/155.1.17 [DOI] [PubMed] [Google Scholar]
- 65.Song G, Wang R, Cui Y, Hao CJ, Xia HF, Ma X. Oxidative stress response associates with the teratogenic effects of benzyl butyl phthalate (BBP). Toxicol Res (Camb). Jun 2020;9(3):222–229. doi: 10.1093/toxres/tfaa022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fajersztajn L, Veras MM. Hypoxia: From Placental Development to Fetal Programming. Birth Defects Res. Oct 16 2017;109(17):1377–1385. doi: 10.1002/bdr2.1142 [DOI] [PubMed] [Google Scholar]
- 67.Hashemi F, Hamidinejad FS, Hoepner L, Rafiee A, Abbasi A, Hoseini M. BTEX exposure of pregnant women and associations with pro-inflammatory cytokines (IL-6 and TNF-α). Air Quality, Atmosphere & Health. 2022/04/01 2022;15(4):707–719. doi: 10.1007/s11869-021-01122-7 [DOI] [Google Scholar]
- 68.Kassotis CD, Klemp KC, Vu DC, et al. Endocrine-Disrupting Activity of Hydraulic Fracturing Chemicals and Adverse Health Outcomes After Prenatal Exposure in Male Mice. Endocrinology. Dec 2015;156(12):4458–73. doi: 10.1210/en.2015-1375 [DOI] [PubMed] [Google Scholar]
- 69.Cairncross ZF, Couloigner I, Ryan MC, et al. Association Between Residential Proximity to Hydraulic Fracturing Sites and Adverse Birth Outcomes. JAMA Pediatrics. 2022;doi: 10.1001/jamapediatrics.2022.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hill EL. Shale gas development and infant health: Evidence from Pennsylvania. J Health Econ. Sep 2018;61:134–150. doi: 10.1016/j.jhealeco.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janitz AE, Dao HD, Campbell JE, Stoner JA, Peck JD. The association between natural gas well activity and specific congenital anomalies in Oklahoma, 1997–2009. Environment International. 2019/01/01/ 2019;122:381–388. doi: 10.1016/j.envint.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Z-Q. Time Series Evaluation of Birth Defects in Areas with and without Unconventional Natural Gas Development. Journal of Epidemiology and Public Health Reviews. 01/01 2016;1 doi: 10.16966/2471-8211.107 [DOI] [Google Scholar]
- 73.McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. Birth Outcomes and Maternal Residential Proximity to Natural Gas Development in Rural Colorado. Environmental Health Perspectives. 2014;122(4):412–417. doi:doi: 10.1289/ehp.1306722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKenzie LM, Allshouse W, Daniels S. Congenital heart defects and intensity of oil and gas well site activities in early pregnancy. Environment International. 2019/11/01/ 2019;132:104949. doi: 10.1016/j.envint.2019.104949 [DOI] [PubMed] [Google Scholar]
- 75.Tang IW, Langlois PH, Vieira VM. Birth defects and unconventional natural gas developments in Texas, 1999–2011. Environmental Research. 2021/03/01/ 2021;194:110511. doi: 10.1016/j.envres.2020.110511 [DOI] [PubMed] [Google Scholar]
- 76.National Institute of Health. What are the types of birth defects? Accessed 3/3/2022, https://www.nichd.nih.gov/health/topics/birthdefects/conditioninfo/types
- 77.Tinker SC, Gilboa S, Reefhuis J, Jenkins MM, Schaeffer M, Moore CA. Challenges in Studying Modifiable Risk Factors for Birth Defects. Current Epidemiology Reports. 2015/03/01 2015;2(1):23–30. doi: 10.1007/s40471-014-0028-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tinker SC, Gilboa S, Reefhuis J, Jenkins MM, Schaeffer M, Moore CA. Challenges in Studying Modifiable Risk Factors for Birth Defects. Curr Epidemiol Rep. Mar 2015;2(1):23–30. doi: 10.1007/s40471-014-0028-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohio Department of Natural Resources. Risk Based Data Management System. Accessed April 11, 2020. https://ohiodnr.gov/discover-and-learn/safety-conservation/about-odnr/oil-gas/oil-gas-resources/featured-content-3
- 80.Ohio Department of Natural Resources. Marcellus & Utica Shale Permitting Reports. Accessed June 5, 2020. https://ohiodnr.gov/wps/portal/gov/odnr/business-and-industry/energy-resources/oil-and-gas-wells/horizontal-wells
- 81.Soriano MA, Siegel HG, Johnson NP, et al. Assessment of groundwater well vulnerability to contamination through physics-informed machine learning. Environmental Research Letters. 2021/07/22 2021;16(8):084013. doi: 10.1088/1748-9326/ac10e0 [DOI] [Google Scholar]
- 82.Soriano MA Jr., Deziel NC, Saiers JE. Regional Scale Assessment of Shallow Groundwater Vulnerability to Contamination from Unconventional Hydrocarbon Extraction. Environmental Science & Technology. 2022/09/06 2022;56(17):12126–12136. doi: 10.1021/acs.est.2c00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soriano MA Jr., Siegel HG, Gutchess KM, et al. Evaluating Domestic Well Vulnerability to Contamination From Unconventional Oil and Gas Development Sites. Water Resources Research. 2020;56(10):e2020WR028005. doi: 10.1029/2020WR028005 [DOI] [Google Scholar]
- 84.Tarboton DG. Terrain analysis using digital elevation models (TauDEM). Utah State University, Logan. 2005;3012:2018. [Google Scholar]
- 85.Clark CJ, Johnson NP, Soriano M, et al. Unconventional Oil and Gas Development Exposure and Risk of Childhood Acute Lymphoblastic Leukemia: A Case–Control Study in Pennsylvania, 2009–2017. Environmental Health Perspectives. 2022;130(8):087001. doi:doi: 10.1289/EHP11092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark CJ, Xiong B, Soriano MA, et al. Assessing Unconventional Oil and Gas Exposure in the Appalachian Basin: Comparison of Exposure Surrogates and Residential Drinking Water Measurements. Environmental Science & Technology. 2022/01/18 2022;56(2):1091–1103. doi: 10.1021/acs.est.1c05081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agency OEP. https://ohiowatersheds.osu.edu/sites/own/files/imce/GroundWtr%20in%20Ohio%20factsht.pdf.
- 88.Murray A, Hall A, Weaver J, Kremer F. Methods for Estimating Locations of Housing Units Served by Private Domestic Wells in the United States Applied to 2010. JAWRA Journal of the American Water Resources Association. 2021;57(5):828–843. doi: 10.1111/1752-1688.12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson TD, Belitz K, Lombard MA. Estimating domestic well locations and populations served in the contiguous U.S. for years 2000 and 2010. Science of The Total Environment. 2019/10/15/ 2019;687:1261–1273. doi: 10.1016/j.scitotenv.2019.06.036 [DOI] [PubMed] [Google Scholar]
- 90.Alwan S, Chambers CD. Identifying Human Teratogens: An Update. J Pediatr Genet. Jun 2015;4(2):39–41. doi: 10.1055/s-0035-1556745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.USDA. Data from: 2010 Rural-Urban Commuting Area Codes. 2019.
- 92.CDC/ATSDR. Data from: SVI Documentation 2014. 2021.
- 93.Chau PH, Gusmano MK, Cheng JOY, Cheung SH, Woo J. Social Vulnerability Index for the Older People—Hong Kong and New York City as Examples. Journal of Urban Health. 2014/12/01 2014;91(6):1048–1064. doi: 10.1007/s11524-014-9901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring Community Vulnerability to Natural and Anthropogenic Hazards: The Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health. 2018;80(10):34–36. [PMC free article] [PubMed] [Google Scholar]
- 95.Givens M, Teal EN, Patel V, Manuck TA. Preterm birth among pregnant women living in areas with high social vulnerability. American Journal of Obstetrics & Gynecology MFM. 2021/09/01/ 2021;3(5):100414. doi: 10.1016/j.ajogmf.2021.100414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Warren JL, Luben TJ, Sanders AP, Brownstein NC, Herring AH, Meyer RE. An evaluation of metrics for assessing maternal exposure to agricultural pesticides. Journal of Exposure Science & Environmental Epidemiology. 2014/09/01 2014;24(5):497–503. doi: 10.1038/jes.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.U.S. Department of Agriculture. CropScape — Cropland Data Layer. Accessed October 22, 2021. https://nassgeodata.gmu.edu/CropScape/
- 98.U.S. Environmental Protection Agency. Data from: Fused Air Quality Surface Using Downscaling (FAQSD) Files. Downscaling Output Files – PM 2.5 Daily Average, 2002– 2017.
- 99.Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environmental Research. 2018/11/01/ 2018;167:144–159. doi: 10.1016/j.envres.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 100.Ravindra K, Chanana N, Mor S. Exposure to air pollutants and risk of congenital anomalies: A systematic review and metaanalysis. Science of The Total Environment. 2021/04/15/ 2021;765:142772. doi: 10.1016/j.scitotenv.2020.142772 [DOI] [PubMed] [Google Scholar]
- 101.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. Jul 2009;20(4):488–95. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raghavan R, Romano ME, Karagas MR, Penna FJ. Pharmacologic and Environmental Endocrine Disruptors in the Pathogenesis of Hypospadias: a Review. Current Environmental Health Reports. 2018/12/01 2018;5(4):499–511. doi: 10.1007/s40572-018-0214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kjersgaard CL, Arendt LH, Ernst A, et al. Lifestyle in Pregnancy and Hypospadias in Sons: A Study of 85,923 Mother-Son Pairs from Two Danish Pregnancy Cohorts. Clin Epidemiol. 2022;14:149–157. doi: 10.2147/clep.S335877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. Sep-Oct 2011;17(5):589–604. doi: 10.1093/humupd/dmr022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bouty A, Ayers KL, Pask A, Heloury Y, Sinclair AH. The Genetic and Environmental Factors Underlying Hypospadias. Report. Sexual Development. 2016/02/01/ // 2016;9:239+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He Y, Zhang Y, Martin JW, Alessi DS, Giesy JP, Goss GG. In vitro assessment of endocrine disrupting potential of organic fractions extracted from hydraulic fracturing flowback and produced water (HF-FPW). Environment International. 2018/12/01/ 2018;121:824–831. doi: 10.1016/j.envint.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 107.Kassotis CD, Tillitt DE, Davis JW, Hormann AM, Nagel SC. Estrogen and Androgen Receptor Activities of Hydraulic Fracturing Chemicals and Surface and Ground Water in a Drilling-Dense Region. Endocrinology. 2014;155(3):897–907. doi: 10.1210/en.2013-1697 [DOI] [PubMed] [Google Scholar]
- 108.Sapouckey SA, Kassotis CD, Nagel SC, Vandenberg LN. Prenatal Exposure to Unconventional Oil and Gas Operation Chemical Mixtures Altered Mammary Gland Development in Adult Female Mice. Report. Endocrinology. 2018/03// // 2018;159:1277+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richmond RC, Al-Amin A, Davey Smith G, Relton CL. Approaches for drawing causal inferences from epidemiological birth cohorts: A review. Early Human Development. 2014/11/01/ 2014;90(11):769–780. doi: 10.1016/j.earlhumdev.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ohio Administrative Code, (2019). https://codes.ohio.gov/ohio-administrative-code/rule-1501:9-1-04
- 111.Ohio Revised Code, (2011). https://codes.ohio.gov/ohio-revised-code/section-1509.021
- 112.Ingraffea AR, Wells MT, Santoro RL, Shonkoff SB. Assessment and risk analysis of casing and cement impairment in oil and gas wells in Pennsylvania, 2000–2012. Proceedings of the National Academy of Sciences. 2014;111(30):10955–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Czolowski ED, Santoro RL, Srebotnjak T, Shonkoff SB. Toward consistent methodology to quantify populations in proximity to oil and gas development: a national spatial analysis and review. Environmental health perspectives. 2017;125(8):086004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reichard A, McDermott S, Ruttenber M, et al. Testing the Feasibility of a Passive and Active Case Ascertainment System for Multiple Rare Conditions Simultaneously: The Experience in Three US States. JMIR Public Health Surveill. Aug 29 2016;2(2):e151. doi: 10.2196/publichealth.5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. Journal of Exposure Science & Environmental Epidemiology. 2006/11/01 2006;16(6):538–543. doi: 10.1038/sj.jes.7500501 [DOI] [PubMed] [Google Scholar]
- 116.Khoury MJ, Stewart W, Weinstein A, Panny S, Lindsay P, Eisenberg M. Residential mobility during pregnancy: implications for environmental teratogenesis. J Clin Epidemiol. 1988;41(1):15–20. doi: 10.1016/0895-4356(88)90004-2 [DOI] [PubMed] [Google Scholar]
- 117.Shaw GM, Malcoe LH. Residential mobility during pregnancy for mothers of infants with or without congenital cardiac anomalies: a reprint. Arch Environ Health. May-Jun 1992;47(3):236–8. doi: 10.1080/00039896.1992.9938355 [DOI] [PubMed] [Google Scholar]
- 118.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol. Feb 2015;44(1):345–54. doi: 10.1093/ije/dyu249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leung M, Kioumourtzoglou M-A, Raz R, Weisskopf MG. Bias due to selection on live births in studies of environmental exposures during pregnancy: a simulation study. Environmental health perspectives. 2021;129(4):047001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


