Abstract
Introduction:
People with serious mental illness (SMI) are more likely to smoke and less likely to receive tobacco treatment. Implementation strategies may address clinician and organizational barriers to treating tobacco in mental healthcare.
Methods:
A cluster-randomized trial (Clinic N=13, Client N=610, Staff N=222) tested two models to promote tobacco treatment in community mental healthcare: standard didactic training vs. Addressing Tobacco Through Organizational Change (ATTOC), an organizational model that provides clinician and leadership training and addresses system barriers to tobacco treatment. Primary outcomes were changes in tobacco treatment from clients, staff, and medical records. Secondary outcomes were changes in smoking, mental health, and quality of life (QOL), and staff skills and barriers to treat tobacco.
Results:
Clients at ATTOC sites reported a significant increase in receiving tobacco treatment from clinician at weeks 12 and 24 (ps<0.05) and tobacco treatments and policies from clinics at weeks 12, 24, 36, and 52 (ps<0.05), vs. standard sites. ATTOC staff reported a significant increase in skills to treat tobacco at week 36 (p=0.05), vs. standard sites. For both models, tobacco use medications, from clients (week 52) and medical records (week 36), increased (ps<0.05), while perceived barriers decreased at weeks 24 and 52 (ps<0.05); 4.3% of clients quit smoking which was not associated with model. QOL and mental health improved over 24 weeks for both models (ps<0.05).
Conclusions:
Standard training and ATTOC improve use of evidence-based tobacco treatments in community mental healthcare without worsening mental health, but ATTOC may more effectively address this practice gap.
Keywords: Tobacco Treatment, Mental Health, Implementation, Training
1. Introduction
The use of US Food and Drug Administration (FDA)-approved medications and guideline-based behavioral interventions for smoking has led to a significant decline in the US adult smoking rate, from 50% in the 1960s to 13% today (Cornelius et al., 2022). Unfortunately, this rate of decline has plateaued and about 31 million Americans smoke regularly. An impediment to further reductions in the rate of smoking in the US is the low rate of use of evidence-based medications and behavioral interventions for smoking. Data from Medicaid, (Ku et al., 2016), Medicare (Jarlenski et al., 2016), outpatient medical settings (Jamal et al., 2005), and primary care (Huang et al., 2013) show that only 25% of those interested in quitting smoking use evidence-based tobacco treatments in their quit attempts.
This reality is worse for people with severe mental illness (SMI). While evidence-based medications and behavioral interventions are safe and effective for smokers with SMI (Anthenelli et al., 2016; Hawes et al., 2021), only about 10% of these smokers receive evidence-based tobacco treatments in clinical settings (Himelhoch and Daumit, 2003; Montoya et al.,2005; Rogers et al., 2014; Rojewski et al., 2019). Not surprisingly, the rates of smoking among those with SMI are 2–4-fold higher vs. the general population, resulting in severe health outcomes disparities (Cook et al., 2014; Streck et al., 2020). Yet, smokers with SMI are just as motivated to quit and just as interested in evidence-based tobacco treatments as the general population (Kalkhoran et al., 2019).
Clinicians who work with populations with SMI cite barriers to the provision of tobacco treatment. Greater knowledge about how to treat tobacco use has been associated with greater rates of tobacco treatment (Knudsen, 2017; Siegel et al., 2020), indicating that didactic training can help clinicians working within mental health clinics to address patient smoking. Training clinicians in guidelines for treating tobacco use in community mental health centers has increased the provision of tobacco treatment (Chen et al., 2018; Japuntich et al., 2020). However, additional barriers associated with the provision of tobacco treatments in community mental healthcare have been identified, including believing that smoking is less harmful than the assumed negative consequences of cessation (e.g., decompensation, self-injurious behavior), treating tobacco use is not the organizations’ responsibility, and the lack of organizational systems and leadership endorsement to ensure that client tobacco use is identified and managed (Prochaska, 2010; Himelhoch et al., 2014; Pagano et al., 2016; Richter et al., 2017). Thus, there have been calls to evaluate organizational-level interventions to address tobacco use in mental healthcare settings (McGinty et al., 2016; Samaha et al., 2017).
The Addressing Tobacco Through Organizational Change (ATTOC) model is an organization-level model designed to address system-level and cultural barriers that undermine tobacco use treatment (Ziedonis et al., 2003). ATTOC assumes that effective organizational change requires staff didactic training and the application of organizational theory to address system barriers and promote a culture in which tobacco use is not accepted (Ziedonis et al., 2007; Ziedonis et al., 2022). ATTOC has been implemented in behavioral health treatment settings (e.g., Veteran’s Affairs settings). The most rigorous evaluation of ATTOC was conducted within residential substance use disorder treatment settings and used a non-randomized, single-arm, prospective design (Guydish et al., 2011). Compared to baseline, clinician attitudes towards treating patient smoking and the provision of tobacco treatment increased over time, as did patient use of tobacco treatments. However, evaluations of the model’s impact have been limited to program evaluations without randomization and, thus, there is a paucity of rigorous data upon which to determine the impact of organizational models to promote the implementation of tobacco treatment in mental healthcare (Guillaumier et al., 2020; Brown et al., 2021).
This study sought to address this gap by comparing two models for promoting evidence-based treatment of tobacco among people with SMI. The standard didactic approach trained clinics in evidence-based tobacco treatments, consistent with training and educating stakeholders as an implementation strategy (Powell et al., 2015; Waltz et al., 2015). The ATTOC model included didactic instruction in treating tobacco, but also addressed organizational climate (i.e., beliefs established by leaders), culture (i.e., assumptions, values, and norms), and capacity (i.e., resources to meet goals; Cummings & Worley, 2008; Boonstra, 2008). ATTOC strategies included assessing organizational readiness and barriers to change, assessing ongoing changes with agency feedback related to goals; establishing and preparing local champions for leading change and providing ongoing training; implementing environmental policy changes to support tobacco treatment; and formulating and messaging leadership endorsement for change, strategies consistent with using evaluative and iterative strategies, developing stakeholder interrelationships, supporting clinicians, and changing infrastructure approaches (Powell et al., 2015; Waltz et al., 2015).
2. Methods
2.1. Overview
Community mental health clinics (CMHCs) in Philadelphia were randomized to standard training (N=6) or ATTOC (N=7) (ClinicalTrials.gov ID: NCT02849652). Sites were eligible if they had an electronic health record, provided access to tobacco medication data, and could enroll staff to the trial (Flitter et al., 2019). A total of 15 sites were assessed for eligibility. One site refused participation prior to enrollment and one site was unable to complete the study because of COVID-19. The study was conducted September 2016 until December 2021.
2.2. Participants
At each site, we recruited clients and staff (Figure 1). For clients, inclusion criteria were age >18, reporting daily average of 5 cigarettes/day for the past 6 months, have a documented DSM diagnosis, and ability to communicate in English and provide informed consent. Clients who reported only e-cigarette use were ineligible (dual users were permitted). For staff, inclusion criteria were age >18, have clinical, administrative/leadership, or supervisory duties, and ability to communicate in English and provide informed consent. We recruited 610 clients (standard=266; ATTOC=344; mean clients/site=47; range= 30–74 clients/site) and 222 staff (91 from standard and 131 from ATTOC sites; mean staff/site=17.1; range= 11–25 staff/site). Sites contributed 30–74 clients and 11–25 staff.
Figure 1.
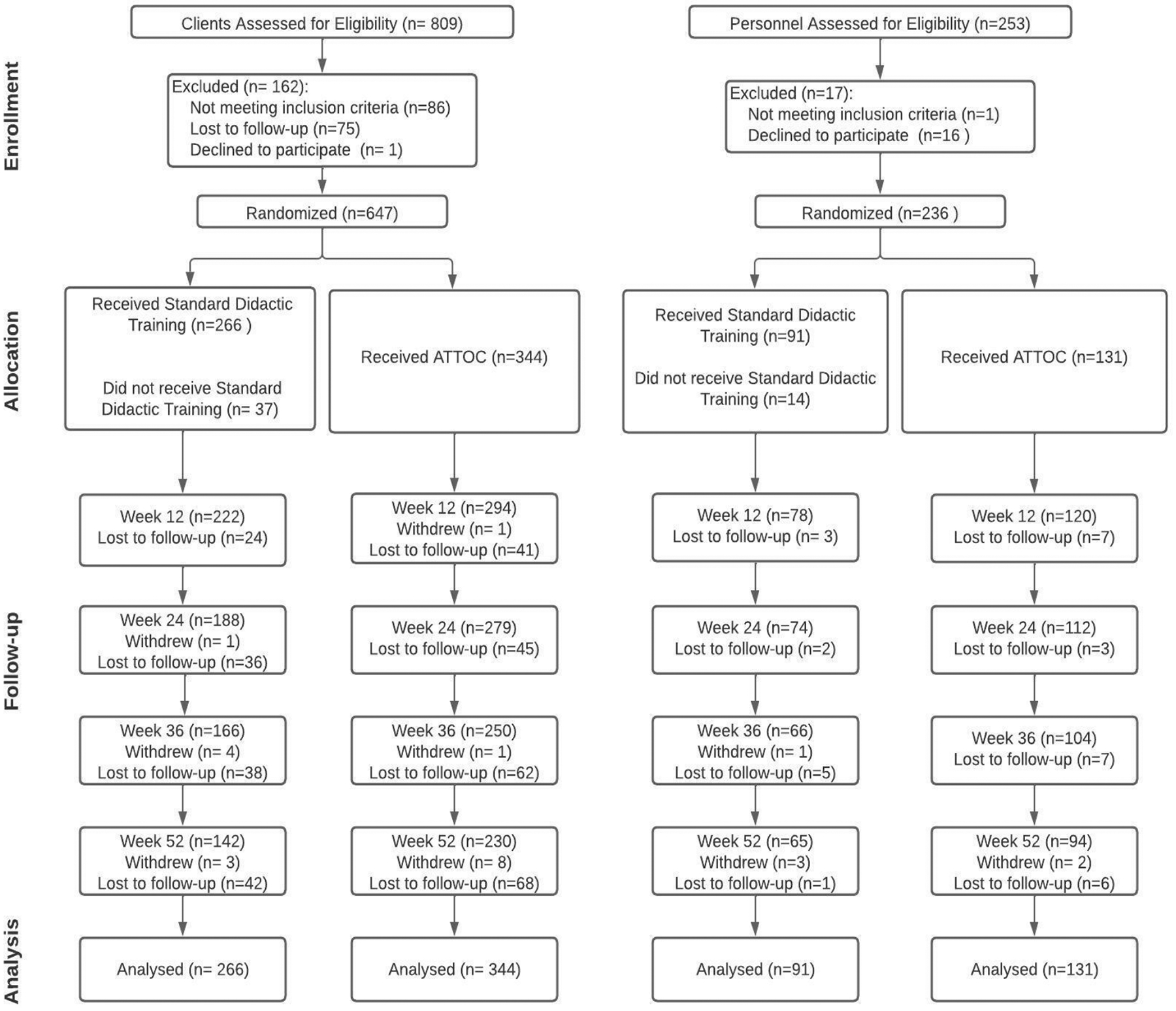
CONSORT Diagram
2.3. Procedures
Institutional Review Boards at the University of Pennsylvania and the City of Philadelphia approved the study. We coordinated recruitment with the Alliance of Community Service Providers and the Mental Health Partnerships, which support Philadelphia CMHCs. Direct outreach to clinic CEOs and directors occurred. Interested sites completed an eligibility and interest assessment and were randomized using a computer-generated procedure from our statistician. Research personnel attended clinics for 3–5 weeks to enroll participants. Clients were approached in the waiting area and screened for eligibility. Staff were recruited and screened during site meetings. After providing informed consent, participants completed a baseline assessment. Site training was scheduled and implemented over 36 weeks. Assessments were conducted at Weeks 12, 24, 36, and 52.
2.4. Implementation Strategies
Standard Didactic Training.
Two senior staff (RS, FL) provided 2-day didactic training involving formal instruction and case study review. Topics included program review and rationale for treating nicotine dependence in mental healthcare, an introduction to nicotine dependence, a review of guidelines for the treatment of nicotine dependence that included methods to identify smokers, and the provision of behavioral interventions and guidelines for the medical management of tobacco use among those with an SMI. Case studies were presented, and collaborative problem-solving sessions were led. An open-ended, question-and-answer period closed the training.
ATTOC.
Staff and leadership were guided through cultural change and training in the implementation of evidence-based treatment of tobacco use by a senior author (DZ). ATTOC accommodated unique needs, barriers, resources, and goals of each agency. ATTOC started with a baseline organizational readiness assessment. Seven core strategies were used: 1) Meetings, calls, and video-conferences to prepare for and implement the intervention; 2) On-site consultation and technical assistance, including a baseline and repeated environmental scan (i.e., determination of current patient assessment and treatment; staff smoking, training, and attitudes and beliefs; and evaluation of indoor and outdoor agency spaces for evidence of tobacco use and tobacco-related policies); 3) Formation of the agency’s tobacco champion/leadership to support culture and practice change, including the use of a “dashboard” assessment to provide staff with performance feedback; 4) Implementation of the agency’s change plan to achieve staff and agency goals (e.g., initiation of tobacco treatment training, methods of tobacco use assessment, and documentation of treatment plans); 5) Formal training in treating tobacco use with monitoring, feedback, and coaching by champions; 6) Sustained consultations, including the use of the dashboard assessment to monitor organizational change and provide feedback; and 7) Web-based support. ATTOC used a monthly self-report dashboard and a quarterly environmental scan tool that tracked progress of changes during the intervention with feedback to the agency (for more details on ATTOC, see Flitter et al., 2019 and Ziedonis et al. 2007). ATTOC was implemented over 36 weeks via two in-person/on-site and eight video/teleconference sessions. Sites completed an average of 7.3 training sessions (range 4–9).
2.5. Measures
Demographic, Mental Health, and Employment Characteristics.
Demographic information was collected from clients and staff. From clients, and from staff who smoke, we collected smoking history and the Fagerström Test for Cigarette Dependence (FTCD) (Heatherton et al., 1991). Clients provided information on their psychiatric diagnoses. Employment characteristics of staff were collected, including type of position, years of experience, and number of hours/week worked, as well as number of clients and number of patient hours.
Client Reports of Tobacco Treatment.
The Smoking Knowledge, Attitudes, and Services (S-KAS) instrument was administered to clients. The S-KAS items evaluate attitudes and knowledge about smoking cessation, using a Likert-type scale, and reported receiving of tobacco treatment services. using a “yes” or “no” format (Guydish et al., 2013). Specific questions assess asking about smoking status, being advised to quit smoking, whether staff and clients smoke together, whether clients are provided with behavioral smoking cessation counseling or referral for smoking cessation treatment, and whether smoking cessation treatment is a requirement at the clinic or provided as routine care. Three subscales were formed: 1) receiving tobacco medications (e.g., NRT, varenicline); 2) receiving tobacco treatments from clinicians (e.g., advice, counseling, referrals); and 3) program-level tobacco treatment services and policies (e.g., routine assessment of tobacco use, provision of educational material, integration of tobacco treatments with services).
Staff Reports of Tobacco Treatment.
The Smoking Knowledge, Attitudes, and Practices Instrument (S-KAP)38 is composed of 44 self-report Likert-type items. The S-KAP was developed with staff working in substance use and HIV care settings; therefore, minor changes were made to adapt the instrument for this study (e.g., changing references to “using drugs” to “psychiatric illness”) (Delucchi et al., 2009). Further, based on a previous evaluation of the factor structure of the S-KAP16, we used subscales that reflected: 1) clinician practices (e.g., asking about tobacco use, advising to quit, and providing behavioral counseling and/or medication), 2) clinician skills (e.g., have the ability to treat tobacco use), and 3) clinician barriers (e.g., lack of time or reimbursement).
Tobacco Medication Electronic Health Record (EHR) Data.
Sites provided EHR tobacco medication data given to participants (NRTs, bupropion, varenicline) at baseline and at weeks 36 and 52. We assessed overall medications and NRTs and varenicline or bupropion separately.
Client Smoking.
Smoking was assessed at weeks 12, 24, 36, and 52 by self-report since this was not a cessation clinical trial. Missing data were coded as smoking.
Client Mental Health and Quality of Life (QOL).
The Revised Behavior and Symptom Identification Scale (BASIS-R), a 24-item survey of mental health functioning, was administered at baseline and at weeks 12, 24, 36, and 52 (Eisen et al., 2004). A total overall score was used. The Short-Form Health Survey (SF-12) assessed physical and emotional QOL; higher scores equal lower QOL (Ware et al., 1996).
2.6. Analyses
Descriptive statistics characterized the sample; chi-square for categorical variables (e.g., race) and ANOVA for continuous variables (e.g., age) compared the study arms. Variables that were significantly different across arms were included as covariates in modeling of outcomes. We used generalized estimating equations (GEEs) to examine changes over time (from baseline to weeks 12, 24, 36, and 52) and between arms in: 1) client reports of receiving tobacco medications, receiving tobacco treatments, and program level tobacco services and policies; 2) staff reports of providing tobacco treatment and staff skills and barriers; 3) EHR tobacco medication data; 4) the rate of client smoking; and 5) mental health functioning and QOL. The primary analyses focused on the interaction effects for study arm (standard vs. ATTOC) and time. If the interaction effect was not significant, main effects of study arm and time were assessed. Models and predictors were evaluated using coefficients, 95% confidence intervals, and probabilities.
3. Results
3.1. Sample Characteristics/Covariates
Table 1 shows sample characteristics. The client sample was mostly male and Black or African American, almost 90% earned <$20,000/year, and 42% had a comorbid psychotic disorder and 48% had a comorbid substance use disorder. The staff sample was comprised of mostly women (78%) and were 50% Black or African American. Of note, the staff reported an average of 42 clients and 22.5% currently smoked. Clients at ATTOC sites were older, had higher baseline carbon monoxide (CO), were less likely to be female, more likely to have a comorbid psychotic disorder, and less likely to have a comorbid substance use disorder (p’s<0.02). Staff at ATTOC sites were older and were more likely to be minorities (p’s<0.05). These variables were included as covariates in the GEE models (Supplementary Table 2).
Table 1.
Sample Characteristics
| Clients | Staff | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Standard (N=266) | ATTOC (N=344) | Overall (N=610) | Characteristic | Standard (N=91) | ATTOC (N=131) | Overall (N=222) |
| % or Mean (SD) | % or Mean (SD) | % or Mean (SD) | % or Mean (SD) | % or Mean (SD) | % or Mean (SD) | ||
| Age* | 45.2 (11.8) | 48.3 (11.8) | 46.9 (11.9) | Age* | 38.8 (15.6) | 45.5 (13.4) | 42.7 (13.8) |
| Gender(% Female)* | 49.2 | 40.7 | 44.4 | Gender (% Female) | 82.4 | 75.6 | 78.4 |
| Race (% Minority) | 67.4 | 68.6 | 68.1 | Race (% Minority)* | 53.9 | 67.9 | 62.3 |
| Cigarettes/Day | 12.9 (8.0) | 13.7 (9.5) | 13.3 (9.0) | Tobacco Use (% Yes) | 20.9 | 23.7 | 22.5 |
| Education (% < HS) | 33.2 | 34.6 | 34.0 | Education (% < College) | 9.9 | 18.3 | 14.9 |
| FTND | 5.2 (2.1) | 5.2 (2.1) | 5.2 (2.1) | No. of Active Clients | 42.2 (66.3) | 41.9 (61.5) | 42.0 (63.4) |
| Years Smoked | 31.3 (60.9) | 33.0 (53.9) | 32.3 (57.0) | Years at Agency | 5.4 (6.9) | 6.6 (7.3) | 6.1 (7.2) |
| CO (ppm)* | 14.1 (8.2) | 17.1 (11.7) | 15.9 (10.5) | Years in Current Job | 4.0 (5.3) | 5.4 (6.3) | 4.8 (5.9) |
| Income (% < 20k) | 86.2 | 88.6 | 87.5 | Hours/Week | 37.4 (10.5) | 38.5 (8.3) | 38.0 (9.3) |
| Employed (% Yes) | 13.2 | 14.5 | 13.9 | Patient Hours/Week | 21.5 (11.5) | 25.2 (15.1) | 23.6 (13.8) |
| No. Psychiatric Dx | 2.4 (0.97) | 2.3 (1.1) | 2.3 (1.0) | ||||
| Psychotic Dx (% Yes)* | 30.8 | 50.9 | 42.1 | Clinician Supervisor Other |
80.2 18.7 1.1 |
67.2 30.5 2.3 |
72.5 25.7 1.8 |
| Subs. Use Dx (% Yes)* | 53.0 | 43.3 | 47.5 | ||||
Note.
denotes p < .05;
CO = Carbon Monoxide; ppm = parts per million; No. = number; Dx = diagnosis; subs = substance.
3.2. Client Reported Tobacco Treatment
Results are shown in Table 3. The study arm and time interaction effect was not significant for client reported use of tobacco medications (p=0.44). In the main effects model, there was an effect of study arm (β=1.69, 95% CI:1.10 to 2.62, p=0.02), with higher medication rates in ATTOC sites. There was also an effect for time: for both arms, client-reported use of tobacco medication increased significantly from baseline to week 52 (β=1.53, 95% CI:1.18 to 1.99, p=0.001). There was a significant study arm by time interaction for client reported receiving of tobacco treatments from personnel (χ2[4]=11.59, p=0.02). Clients at ATTOC sites reported a significant increase in reports of tobacco treatment from clinicians at week 12 (β=1.43, 95% CI:0.33 to 2.53, p=0.01) and 24 (β=2.38, 95% CI:1.24 to 3.53, p<0.01), vs. clients at standard sites. Likewise, there was a significant study arm by time interaction for client reported receiving of program-level tobacco treatment services and policies (χ2[4]=22.67, p=0.001). ATTOC clients reported a significant increase in program-level tobacco treatment services and policies at week 12 (β=0.61, 95% CI:0.03 to 1.20, p=0.04), 24 (β=1.23, 95% CI:0.62 to 1.84, p<0.001), 36 (β=1.49, 9.5% CI:0.86 to 2.12, p=<0.001), and 52 (β=1.26, 95% CI:0.59 to 1.93, p=.01), vs clients at standard sites.
Table 3.
Client and Staff Reported and EHR Outcomes
| Standard | ATTOC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BL | W12 | W24 | W36 | W52 | BL | W12 | W24 | W36 | W52 | |
| Client Reported Tobacco Medications (No., %) | 27 (10.1) | 28 (10.5) | 23 (8.6) | 28 (10.5) | 24 (9) | 61 (17.7) | 59 (17.2) | 55 (16) | 48 (14) | 62 (18) |
| Client Reported Tobacco Treatment from Staff (M, SD) | 14.8 (5.1) | 15.7 (5.7) | 15.1 (5.4) | 15.9 (5.9) | 15.5 (6) | 16.2 (5.5) | 17.4 (6.2) | 17.7 (7) | 16.7 (6.2) | 16.3 (6.5) |
| Client Reported Tobacco Services and Policies (M, SD) | 6.3 (3.2) | 6.4 (3.7) | 5.7 (3.4) | 5.5 (3.2) | 5.6 (3.6) | 6.5 (2.9) | 6.9 (3.1) | 6.7 (3.2) | 6.8 (3.6) | 6.5 (3.5) |
| Personnel Reported Tobacco Treatment (M, SD) | 8 (6.1) | 9.6 (6.4) | 10.5 (7.9) | 10.2 (7) | 11.6 (7.4) | 7.7 (6.5) | 10.9 (7.2) | 11.6 (7.7) | 12.3 (8.1) | 12.8 (8.6) |
| Personnel Reported Skills to Treat Tobacco (M, SD) | 11.2 (3.3) | 13 (2.6) | 13.2 (2.8) | 12.9 (3) | 13.5 (2.6) | 11 (2.7) | 13.4 (2.7) | 13.9 (2.7) | 14 (2.6) | 14 (2.7) |
| Personnel Reported Barriers to Treat Tobacco (M, SD) | 8.1 (2.5) | 8.2 (2.4) | 7.9 (2.4) | 8.1 (2.5) | 7.7 (2.6) | 8.9 (2.2) | 8.5 (2.8) | 7.9 (2.9) | 8.1 (2.8) | 8 (2.4) |
| EHR Tobacco Medication, Total (No. %) | 15 (5.6) | N/A | N/A | 33 (12.4) | 21 (7.9) | 1 (0.3) | N/A | N/A | 20 (5.8) | 20 (5.8) |
| EHR Tobacco Medication, NRT (No. %) | 3 (1.1) | N/A | N/A | 13 (4.9) | 6 (2.3) | 0 | N/A | N/A | 16 (4.7) | 13 (3.8) |
| EHR Tobacco Medication, Varenicline/Bupropion (No. %) | 12 (4.5) | N/A | N/A | 23 (8.6) | 18 (6.8) | 1 (0.3) | N/A | N/A | 5 (1.5) | 8 (2.3) |
| Client Smoking Rate (No. %) | 266 (100) | 261 (98.1) | 261 (98.1) | 262 (98.4) | 257 (96.6) | 344 (100) | 335 (97.4) | 332 (96.5) | 333 (96.8) | 327 (95) |
| Mental Health Functioning (M, SD) | 36.2 (23.9) | 44.9 (33.0) | 41.9 (33.9) | 31.8 (24.4) | 30.9 (25.0) | 30.7 (21.5) | 41.8 (30.6) | 41.7 (32.1) | 27.3 (21.5) | 28.7 (23.6) |
| Emotional QoL (M, SD) | 19.7 (4.0) | 18.5 (4.1) | 18.8 (4.4) | 18.5 (4.3) | 18.8 (4) | 18.3 (4.0) | 18.3 (4.1) | 18.2 (4.2) | 18.6 (3.8) | 17.9 (4.2) |
| Physical QoL (M, SD) | 9.9 (2.7) | 9.8 (2.7) | 9.6 (2.8) | 9.8 (2.9) | 10.1 (2.7) | 9.6 (2.7) | 9.7 (2.7) | 9.6 (2.8) | 9.5 (2.8) | 9.6 (2.7) |
Note. No = Number, M = Mean, SD = Standard Deviation, QoL = Quality of Life; N/A = Not applicable since data were not collected at that time-point.
3.3. Staff Reported Tobacco Treatment
Results are shown in Table 3. The interaction of study arm and time was not significant for staff reported provision of tobacco treatments (p=0.11). In the main effects model, there was no effect of study arm (p=0.48) but there was an effect for time: for both arms, staff reported provision of tobacco treatments increased significantly from baseline to week 12 (β=2.95, 95% CI:2.08 to 3.81, p<0.001), 24 (β=3.57, 95% CI:2.69 to 4.45, p<0.001), 36 (β=4.24, 95% CI:3.32 to 5.16, p<0.001), and 52 (β=4.77, 95% CI:3.83 to 5.70, p<0.001). There was a significant study arm by time interaction for staff reported skills to treat tobacco (χ2[4]=9.68, p=0.05). Staff at ATTOC sites reported a significant increase in their skills to treat tobacco at week 36 (β=0.83, 95% CI:0.01 to 1.64, p=0.05), vs. standard sites. The interaction effect for study arm and time was not significant for staff reported barriers for treating tobacco (p=0.72). In the main effects model, there was no effect of study arm on staff barriers to treat tobacco (p=0.51) but there was an effect for time: for both arms, staff reported a significant decrease in barriers to treat tobacco from baseline to week 24 (β=−0.53, 95% CI:−0.53 to −1.93, p<0.009) and 52 (β=−0.58, 95% CI:−1.00 to −0.15, p<0.007).
3.4. EHR Tobacco Medication Data
Results are shown in Table 3. The study arm and time interaction was significant for tobacco medications (χ2[2]=7.97, p=0.02). While both sites reported an increase in medication use from baseline to week 36 (β=2.42, 95% CI:1.4 to 4.19, p=0.002), this increase was greater for standard sites (β=0.44, 95% CI:0.24 to 0.82, p<0.009). Notably, standard sites had higher baseline tobacco medications use vs. ATTOC (p=0.04), and the increase in tobacco medication among ATTOC sites from baseline to week 36 was sustained at week 52 but week 52 rates returned to baseline for standard sites.
The study arm-time interaction effect was not significant for NRT (p=0.26) and varenicline or bupropion (p=0.17). For NRT, there was no study arm main effect (p=0.84). But, across both arms, NRT use increased from baseline to week 36 (β=10.43, 95% CI:3.45 to 31.47, p<0.001) and 52 (β=6.63, 95% CI:2.18 to 20.21, p=0.001). For varenicline or bupropion, there was a main effect for study arm, with higher rates at standard sites, vs. ATTOC (β=0.25, 95% CI:0.11 to −0.53, p<0.001). However, standard sites showed significantly higher varenicline and bupropion use at baseline (p=0.001). For both arms, varenicline and bupropion use increased from baseline to week 36 (β=2.26, 95% CI:1.29 to 4.0, p=0.005) and 52 (β=2.08, 95% CI:1.18 to 3.68, p=0.01).
3.5. Tobacco Use Rates
The interaction effect for study arm and time was not significant for smoking rate (p=0.66). In the main effects model, there was no effect of study arm (p=0.10). Across both study arms, there was a significant reduction in the proportion of clients reporting current smoking (Table 3; β=0.43, 95% CI:0.21 to 0.83, p=0.012). At week 52, 26 clients across both groups (4.3%) reported quitting smoking.
3.6. Mental Health Functioning and QOL
The study arm-time interaction effect was not significant for mental health functioning (p=0.06). There was no main effect for study arm (p=0.10). But, across both arms, mental health functioning improved from baseline to week 12 (β=10.6, 95% CI:8.66 to 12.55, p<0.001) and 24 (β=9.35, 95% CI:7.50 to 11.3, p<0.001; Table 3), and then decreased at weeks 36 and 52. The time by study arm interaction effect was significant for emotional QOL (χ2[4]=11.77, p=0.02), although this was from a significant difference between arms at baseline only (β=−1.14, 95% CI:−1.88 to −0.41, p=0.001). Over time, emotional QOL improved for participants in both arms (p’ <0.05; Table 3). The interaction effect for study arm and time was not significant for physical QOL (p=0.84) and there were no significant main effects.
4. Discussion
We compared two implementation approaches for increasing tobacco treatment for individuals with SMI at community treatment centers. Both approaches improved use of tobacco medications over time (client reports and EHR data), with little indication that mental health functioning or QOL worsened. ATTOC clinics, however, demonstrated increased client reports of receiving tobacco treatments from their clinicians, while the availability of tobacco treatments at ATTOC clinics and enhanced tobacco cessation policies increased significantly over time vs. standard sites. Further, ATTOC staff reported a significant increase in their skills to treat tobacco, vs. standard sites.
The client reports of increased tobacco treatment, on a clinician and system level, at ATTOC sites indicates that a system-level implementation approach can increase utilization of evidence-based tobacco treatment in community mental healthcare vs. didactic models. This finding from the first randomized study of ATTOC supports prior findings from a single-arm evaluation of ATTOC (Guydish et al., 2011). This study shows for the first time that important indicators of evidence-based treatment for tobacco use among those with SMI can be significantly improved using this organizational implementation approach. Enhancing staff perceptions of their skill to treat tobacco use may be a critical factor since ATTOC staff reported a significant increase in perceived ability to treat tobacco relative to standard training staff, which has been identified as a pre-implementation driver of tobacco treatment in mental health settings (Fokuo et al., 2022).
The client and EHR data also indicate that both implementation approaches improved use of tobacco medications. The use of tobacco medications in standard sites doubled from baseline to week 36 and went from ~0 to 6% in ATTOC sites by weeks 36 and 52. The increase in medication rates in ATTOC was sustained at week 52. While these results converge with past similar studies, the rate of use of these medications remains very low (Brown et al., 2021). These data converge with a recent study that used national data from the United Kingdom on prescription medications, showing that 3.9% and 2% of smokers with a mental health disorder received NRT or varenicline (Taylor et al., 2020). As such, additional efforts are needed to ensure adequate treatment of smokers with FDA-approved medications for tobacco in community mental healthcare.
Likewise, both implementation models yielded a significant increase over time in staff reported provision of tobacco treatment, a significant decrease over time in staff perceived barriers to treat client smoking, and a significant decrease in reported smoking. Similar benefits from organizational interventions and didactic training have been reported (Brown et al., 2021; Lappin et al., 2020). Given that perceived barriers, including insufficient training, time, and client interest are frequently cited predictors of the provision of tobacco treatment, these effects may be affected by the impact of the implementation approaches on clinician perceptions of treatment barriers (Brown et al., 2015; Koch and Breland, 2017; Chen et al., 2017). Nevertheless, with an overall quit rate of <5% in this study, additional approaches are needed to address tobacco use in community mental health. Providing evidence-based tobacco treatments, consistently, to this population likely takes longer than the study time frame to see larger improvements in quit rates.
Lastly, this study found little evidence that the introduction of either ATTOC or standard training within community mental healthcare jeopardizes client safety. In fact, mental health functioning improved for all sites from baseline to week 24 before returning to baseline at weeks 36 and 52; the decrease in mental health functioning after week 24 may have been from COVID-19 since several sites were undergoing week 36 and 52 assessments during 2020 and 2021. Likewise, emotional QOL improved over time across all sites. These results lend further support to the growing recognition that it is safe to treat tobacco use among those with SMI and should encourage broader implementation of tobacco treatments within these settings (Taylor et al., 2020; Peckham et al., 2015; Evins et al., 2015). Of note, mental health treatment culture has had a belief that tobacco-free buildings or campuses or treatment might increase hostility, which was not evident in this study.
Despite the diverse racial sample composition and our multiple sources of data collection, this study is limited. First, while randomizing by clinic was necessary to minimize potential contamination and is consistent with the gold-standard approach in implementation science, this design also yielded baseline differences across study arms, including client gender, age, and the rate of substance abuse or psychotic disorder comorbidity. These variables can predict outcomes and the higher rate of psychotic disorder at ATTOC sites, for example, could have minimized the potential for study arm differences. Further, the rate of tobacco medication use reported by clients at baseline was significantly higher for ATTOC sites vs. standard training, making it more challenging to find intervention effects. Thus, while necessary, the cluster RCT design may have made it more challenging to find significant differences between the implementation approaches. Second, this trial overlapped with the COVID-19 pandemic. This led to a loss of one standard training site, affected data collection from several additional sites, and reduced clinic visits and opportunities for tobacco treatment. Further, standard training did not match for time and attention. Third, both implementation approaches focused only on either clinician-level implementation strategies (standard training) or clinician- and organization-level implementation strategies (ATTOC). Client attitudes about smoking (e.g., as an effective coping strategy), medications (e.g., safety), their ability to quit, and potential adverse effects of cessation (e.g., worsening mental health) likely influence engagement with tobacco treatment (Gobarani et al., 2022). The absence of direct patient-level implementation strategies and the amount of time needed to change treatment culture in mental health settings likely mitigated the impact of both implementation approaches tested in this study. Fourth, use of the inclusion criterion of five cigarettes per day and the failure to consider e-cigarettes should be noted as a limitation. Future work in this area should address all tobacco use, even non-daily combustible tobacco and novel tobacco products. Finally, the failure to detect more consistent differences across the models may have emerged from the standard didactic training being too impactful and not representing the limited experience in treating tobacco that most community behavioral health clinics have.
Nevertheless, this study shows that integrating implementation strategies that target clinician-level barriers to the provision of tobacco treatment can improve the use of evidence-based tobacco treatments in community mental healthcare and that the inclusion of efforts to address organization-level barriers yields additional benefits that may be sustained. Moreover, these benefits can be realized without jeopardizing client safety. Future studies are needed, however, given the modest impact on the use of tobacco medications and quit rates. Subsequent work in this area could focus on testing patient-level implementation strategies such as motivation-based counseling and/or the leveraging of external resources to engage patients in treatment such as national quit-lines approaches to address the rate of tobacco treatment in mental healthcare settings.
Supplementary Material
Table 2.
GEE Analyses of Tobacco Treatment Activities, Cessation, and Mental Health Functioning over Time and Between Standard and ATTOC
| Client Reported Tobacco Medications | β | 95% CI | p |
|---|---|---|---|
| Constant | 0.07 | 0.03 to 0.17 | <0.001 |
| Age | 1.0 | 0.99 to 1.02 | 0.97 |
| Sex | 1.22 | 0.83 to 1.8 | 0.31 |
| CO | 1.0 | 0.99 to 1.0 | 0.76 |
| Psychotic Dx | 2.27 | 1.53 to 3.35 | <0.001 |
| Substance Abuse Dx | 0.80 | 0.54 to 1.18 | 0.26 |
| Time (Baseline vs. Week 12) | 1.19 | 0.74 to 1.91 | 0.47 |
| Time (Baseline vs. Week 24) | 1.03 | 0.62 to 1.74 | 0.90 |
| Time (Baseline vs. Week 36) | 1.56 | 0.96 to 2.55 | 0.08 |
| Time (Baseline vs. Week 52) | 1.47 | 0.87 to 0.34 | 0.18 |
| Treatment Arm at Baseline | 1.80 | 1.03 to 3.14 | 0.04 |
| Treatment Arm at Week 12 | 1.73 | 0.99 to 3.02 | 0.54 |
| Treatment Arm at Week 24 | 1.93 | 1.05 to 3.56 | 0.03 |
| Treatment Arm at Week 36 | 1.17 | 0.66 to 2.07 | 0.59 |
| Treatment Arm at Week 52 | 1.91 | 1.05 to 3.44 | 0.03 |
| Client Reported Tobacco Treatments from Personnel | |||
| Constant | 14.82 | 13.00 to 16.64 | <0.001 |
| Age | −0.02 | −0.06 to 0.01 | 0.19 |
| Sex | 1.04 | 0.24 to 1.83 | 0.01 |
| CO | −0.001 | −0.04 to 0.04 | 0.96 |
| Psychotic Dx | 1.89 | 1.07 to 2.7 | <0.001 |
| Substance Abuse Dx | −1.06 | −1.85 to −0.26 | 0.009 |
| Time (Baseline vs. Week 12) | 0.92 | 0.07 to 1.77 | 0.03 |
| Time (Baseline vs. Week 24) | 0.24 | −0.67 to 1.14 | 0.61 |
| Time (Baseline vs. Week 36) | 1.12 | 0.18 to 2.07 | 0.02 |
| Time (Baseline vs. Week 52) | 0.57 | −0.44 to 1.58 | 0.27 |
| Treatment Arm at Baseline | 0.92 | −0.11 to 1.96 | 0.08 |
| Treatment Arm at Week 12 | 1.43 | 0.33 to 2.53 | 0.01 |
| Treatment Arm at Week 24 | 2.38 | 1.24 to 3.53 | <0.001 |
| Treatment Arm at Week 36 | 0.54 | −0.65 to 1.73 | 0.37 |
| Treatment Arm at Week 52 | 0.63 | −0.63 to 1.88 | 0.33 |
| Client Reported Tobacco Treatments from Clinic | |||
| Constant | 5.55 | 4.55 to 6.55 | <0.001 |
| Age | −0.01 | −0.02 to 0.01 | 0.55 |
| Sex | 0.59 | 0.15 to 1.03 | 0.009 |
| CO | 0.008 | −0.12 to 0.03 | 0.43 |
| Psychotic Dx | 1.02 | 0.57 to 1.48 | <0.001 |
| Substance Abuse Dx | −0.84 | 0.57 to 1.47 | <0.001 |
| Time (Baseline vs. Week 12) | 0.23 | −0.20 to 0.66 | 0.29 |
| Time (Baseline vs. Week 24) | −0.54 | −1.0 to −0.09 | 0.02 |
| Time (Baseline vs. Week 36) | −0.68 | −1.16 to −0.21 | 0.005 |
| Time (Baseline vs. Week 52) | −0.74 | −1.25 to −0.22 | 0.005 |
| Treatment Arm at Baseline | 0.26 | −0.29 to 0.81 | 0.36 |
| Treatment Arm at Week 12 | 0.61 | 0.03 to 1.20 | 0.04 |
| Treatment Arm at Week 24 | 1.23 | 0.62 to 1.84 | <0.001 |
| Treatment Arm at Week 36 | 1.49 | 0.86 to 2.12 | <0.001 |
| Treatment Arm at Week 52 | 1.26 | 0.59 to 1.93 | <0.001 |
| Personnel Reported Tobacco Treatments | β | 95% CI | p |
| Constant | 5.83 | 2.56 to 9.11 | <0.001 |
| Age | 0.04 | −0.04 to 0.11 | 0.31 |
| Race | 0.61 | −1.15 to 2.36 | 0.50 |
| Time (Baseline vs. Week 12) | 1.90 | 0.53 to 3.26 | 0.006 |
| Time (Baseline vs. Week 24) | 2.85 | 1.46 to 4.24 | <0.001 |
| Time (Baseline vs. Week 36) | 2.73 | 1.23 to 4.18 | <0.001 |
| Time (Baseline vs. Week 52) | 3.98 | 2.51 to 5.44 | <0.001 |
| Treatment Arm at Baseline | −0.53 | −2.06 to 1.54 | 0.62 |
| Treatment Arm at Week 12 | 1.21 | −0.92 to 3.36 | 0.27 |
| Treatment Arm at Week 24 | 0.67 | −1.49 to 2.84 | 0.54 |
| Treatment Arm at Week 36 | 1.97 | −0.26 to 4.20 | 0.08 |
| Treatment Arm at Week 52 | 0.78 | −1.48 to 3.04 | 0.50 |
| Personnel Reported Perceived Skills to Treat Tobacco Use | β | 95% CI | p |
| Constant | 10.11 | 8.96 to 11.28 | <0.001 |
| Age | 0.02 | −0.005 to 0.05 | 0.11 |
| Race | 0.69 | 0.07 to 1.30 | 0.03 |
| Time (Baseline vs. Week 12) | 1.84 | 1.30 to 2.38 | <0.001 |
| Time (Baseline vs. Week 24) | 2.11 | 1.56 to 2.66 | <0.001 |
| Time (Baseline vs. Week 36) | 1.71 | 1.13 to 2.28 | <0.001 |
| Time (Baseline vs. Week 52) | 2.37 | 1.79 to 2.94 | <0.001 |
| Treatment Arm at Baseline | −0.33 | −1.08 to 0.42 | 0.39 |
| Treatment Arm at Week 12 | 0.30 | −0.48 to 1.08 | 0.45 |
| Treatment Arm at Week 24 | 0.27 | −0.53 to 1.06 | 0.51 |
| Treatment Arm at Week 36 | 0.83 | 0.01 to 1.64 | 0.05 |
| Treatment Arm at Week 52 | 0.25 | −0.58 to 1.07 | 0.56 |
| Personnel Reported Perceived Barriers to Treat Tobacco Use | |||
| Constant | 9.28 | 8.32 to 10.26 | <0.001 |
| Age | −0.01 | −0.03 to 0.01 | 0.33 |
| Race | −1.66 | −2.15 to −1.17 | <0.001 |
| Time (Baseline vs. Week 12) | 0.04 | −0.58 to 0.66 | 0.90 |
| Time (Baseline vs. Week 24) | −0,26 | −0.89 to 0.36 | 0.42 |
| Time (Baseline vs. Week 36) | −0.02 | −0.68 to 0.65 | 0.96 |
| Time (Baseline vs. Week 52) | −0.51 | −1.17 to 0.15 | 0.13 |
| Treatment Arm at Baseline | 0.41 | −0.28 to 1.10 | 0.25 |
| Treatment Arm at Week 12 | 0.20 | −0.52 to 0.93 | 0.58 |
| Treatment Arm at Week 24 | −0.05 | −0.79 to 0.70 | 0.90 |
| Treatment Arm at Week 36 | −0.11 | −0.89 to 0.67 | 0.78 |
| Treatment Arm at Week 52 | 0.29 | −0.50 to 1.08 | 0.47 |
| Tobacco Medication Use (Overall) from EHR | |||
| Constant | 0.04 | 0.01 to 0.12 | <0.001 |
| Age | 1.01 | 0.99 to 1.03 | 0.41 |
| Sex | 1.11 | 0.68 to 1.81 | 0.67 |
| CO | 1.01 | 0.99 to 1.03 | 0.42 |
| Psychotic Dx | 1.50 | 0.90 to 2.47 | 0.12 |
| Substance Abuse Dx | 0.78 | 0.48 to 1.28 | 0.12 |
| Time (Baseline vs. Week 36) | 2.42 | 1.40 to 4.19 | 0.002 |
| Time (Baseline vs. Week 52) | 1.44 | 0.80 to 2.59 | 0.22 |
| Treatment Arm at Baseline | 0.04 | 0.01 to 0.38 | 0.004 |
| Treatment Arm at Week 36 | 0.44 | 0.24 to 0.82 | 0.009 |
| Treatment Arm at Week 52 | 0.74 | 0.38 to 1.44 | 0.37 |
| Tobacco Medication Use (NRT) from EHR | β | 95% CI | p |
| Constant | 0.01 | 0.001 to 0.04 | <0.001 |
| Age | 1.0 | 0.98 to 1.04 | 0.53 |
| Sex | 1.44 | 0.70 to 3.0 | 0.32 |
| CO | 1.01 | 0.98 to 1.04 | 0.53 |
| Psychotic Dx | 2.46 | 1.17 to 5.16 | 0.02 |
| Substance Abuse Dx | 0.91 | 0.45 to 1.82 | 0.78 |
| Time (Baseline vs. Week 36) | 4.59 | 1.56 to 13.54 | 0.006 |
| Time (Baseline vs. Week 52) | 2.03 | 0.65 to 6.38 | 0.22 |
| Treatment Arm at Baseline | 1.0 | ||
| Treatment Arm at Week 36 | 0.86 | 0.38 to 1.91 | 0.71 |
| Treatment Arm at Week 52 | 1.54 | 0.56 to 4.28 | 0.40 |
| Tobacco Medication Use (Varenicline/Bupropion) from EHR | |||
| Constant | 0.04 | 0.01 to 0.18 | <0.001 |
| Age | 1.0 | 0.98 to 1.03 | 0.58 |
| Sex | 0.81 | 0.43 to 1.51 | 0.50 |
| CO | 1.01 | 0.97 to 1.04 | 0.64 |
| Psychotic Dx | 1.04 | 0.54 to 2.04 | 0.90 |
| Substance Abuse Dx | 0.85 | 0.45 to 1.56 | 0.61 |
| Time (Baseline vs. Week 36) | 2.02 | 1.09 to 3.75 | 0.03 |
| Time (Baseline vs. Week 52) | 2.54 | 0.81 to 2.92 | 0.18 |
| Treatment Arm at Baseline | 0.07 | 0.01 to 0.56 | 0.01 |
| Treatment Arm at Week 36 | 0.18 | 0.07 to 0.49 | 0.001 |
| Treatment Arm at Week 52 | 0.38 | 0.16 to 0.92 | 0.03 |
| Tobacco Use Rates | |||
| Constant | 11.06 | 2.4 to 50.89 | 0.002 |
| Age | 1.0 | 0.98 to 1.03 | 0.89 |
| Sex | 1.83 | 0.97 to 3.45 | 0.06 |
| CO | 1.10 | 1.05 to 1.15 | <0.001 |
| Psychotic Dx | 0.74 | 0.39 to 1.38 | 0.34 |
| Substance Abuse Dx | 0.85 | 0.45 to 1.57 | 0.60 |
| Time (Baseline vs. Week 12) | 1.31 | 0.41 to 4.17 | 0.65 |
| Time (Baseline vs. Week 24) | 1.07 | 0.33 to 3.44 | 0.91 |
| Time (Baseline vs. Week 36) | 1.36 | 0.37 to 4.97 | 0.64 |
| Time (Baseline vs. Week 52) | 0.78 | 0.24 to 2.59 | 0.69 |
| Treatment Arm at Baseline | 1.08 | 1.35 to 3.32 | 0.90 |
| Treatment Arm at Week 12 | 0.65 | 0.19 to 2.24 | 0.50 |
| Treatment Arm at Week 24 | 0.54 | 0.17 to 1.75 | 0.30 |
| Treatment Arm at Week 36 | 0.36 | 0.10 to 1.33 | 0.13 |
| Treatment Arm at Week 52 | 0.43 | 0.14 to 1.34 | 0.15 |
| Mental Health Functioning | β | 95% CI | p |
| Constant | 37.13 | 27.99 to 46.28 | <0.001 |
| Age | 0.02 | −0.14 to 0.19 | 0.78 |
| Sex | −3.59 | −7.65 to 0.45 | 0.08 |
| CO | 0.001 | −0.19 to 0.20 | 0.92 |
| Psychotic Dx | 0.35 | −2.76 to 5.32 | 0.53 |
| Substance Abuse Dx | 1.28 | −2.76 to 0.45 | 0.08 |
| Time (Baseline vs. Week 12) | 9.12 | 6.01 to 12.22 | <0.001 |
| Time (Baseline vs. Week 24) | 6.10 | 3.00 to 9.21 | <0.001 |
| Time (Baseline vs. Week 36) | −6.90 | −10.36 to −3.44 | <0.001 |
| Time (Baseline vs. Week 52) | −7.35 | −11.07 to −3.64 | <0.001 |
| Treatment Arm at Baseline | −6.35 | −11.14 to −1.56 | 0.009 |
| Treatment Arm at Week 12 | −3.90 | −9.0 to 1.09 | 0.13 |
| Treatment Arm at Week 24 | −1.01 | −6.02 to 3.97 | 0.69 |
| Treatment Arm at Week 36 | −2.86 | −8.16 to 2.44 | 0.29 |
| Treatment Arm at Week 52 | −0.81 | −6.32 to 4.69 | 0.77 |
| Quality of Life (Emotional) | |||
| Constant | 19.07 | 17.77 to 20.38 | <0.001 |
| Age | 0.02 | 0.02 to 0.05 | 0.04 |
| Sex | −1.50 | −2.07 to −0.93 | <0.001 |
| CO | 0.007 | −0.02 to 0.03 | 0.60 |
| Psychotic Dx | −0.64 | −1.22 to −0.06 | 0.03 |
| Substance Abuse Dx | 0.37 | −0.19 to 0.95 | 0.19 |
| Time (Baseline vs. Week 12) | −0.96 | −1.52 to −0.40 | 0.001 |
| Time (Baseline vs. Week 24) | −0.74 | −1.34 to −0.15 | 0.02 |
| Time (Baseline vs. Week 36) | −1.03 | −1.64 to −0.41 | 0.001 |
| Time (Baseline vs. Week 52) | −0.91 | −1.57 to −0.26 | 0.006 |
| Treatment Arm at Baseline | −1.14 | −1.88 to −0.40 | 0.002 |
| Treatment Arm at Week 12 | −0.11 | −0.86 to 0.64 | 0.77 |
| Treatment Arm at Week 24 | −0.38 | −1.14 to 0.40 | 0.84 |
| Treatment Arm at Week 36 | 0.08 | −0.72 to 0.88 | 0.84 |
| Treatment Arm at Week 52 | −0.38 | −1.22 to 0.46 | 0.38 |
| Quality of Life (Physical) | |||
| Constant | 7.19 | 6.34 to 8.04 | <0.001 |
| Age | 0.07 | 0.05 to 0.08 | <0.001 |
| Sex | −0.88 | −1.25 to −0.50 | <0.001 |
| CO | −0.01 | −0.03 to 0.08 | 0.24 |
| Psychotic Dx | −0.40 | −0.78 to −0.01 | 0.04 |
| Substance Abuse Dx | 0.55 | 0.17 to 0.92 | 0.004 |
| Time (Baseline vs. Week 12) | −0.09 | −0.40 to 0.20 | 0.53 |
| Time (Baseline vs. Week 24) | −0.08 | −0.40 to 0.24 | 0.64 |
| Time (Baseline vs. Week 36) | −0.18 | −0.51 to 0.15 | 0.28 |
| Time (Baseline vs. Week 52) | 0.12 | −0.24 to 0.47 | 0.53 |
| Treatment Arm at Baseline | −0.15 | −0.60 to 0.29 | 0.50 |
| Treatment Arm at Week 12 | −0.12 | −0.59 to 0.35 | 0.62 |
| Treatment Arm at Week 24 | −0.19 | −0.68 to 0.29 | 0.43 |
| Treatment Arm at Week 36 | −0.12 | −0.62 to 0.37 | 0.62 |
| Treatment Arm at Week 52 | −0.37 | −0.88 to 0.15 | 0.16 |
Note. CO = carbon monoxide; Dx = diagnosis.
Highlights.
First randomized trial of Addressing Tobacco through Organizational Change
Tested implementation strategies to improve tobacco treatment in mental healthcare
Tobacco treatment for those with mental illness can be improved safely
Organizational treatment may improve tobacco treatment more than didactic training
Acknowledgments:
The authors thank Cherie Brummans, Michael Brody, Dr. Geoff Neimark, Dr. Matthew Hurford, and Jessica Griffith for assistance with the study.
Funding
This work was supported by grants from the National Cancer Institute (R01 CA202699) and the National Institute on Drug Abuse (K24 DA045244).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests:
Dr. Schnoll has received medication and placebo free from Pfizer and has provided consultation to Pfizer, GlaxoSmithKline, and Curaleaf. Dr. Beidas is principal at Implementation Science & Practice, LLC. She receives royalties from Oxford University Press, consulting fees from United Behavioral Health and OptumLabs, and serves on the advisory boards for Optum Behavioral Health, AIM Youth Mental Health Foundation, and the Klingenstein Third Generation Foundation outside of the submitted work.
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are not publicly available due the risk of identifying participants, but extracts are available from the corresponding author on reasonable request. Training materials used in the implementation models
REFERENCES
- 1.Cornelius ME, Loretan CG, Wang TW, Jamal A, Homa DM. Tobacco product use among adults-United States, 2020. Morbidity and Mortality Weekly Report. 2022;71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ku L, Bruen BK, Steinmetz E, Bysshe T. Medicaid tobacco cessation: Big gaps remain in efforts to get smokers to quit. Health Aff (Millwood). 2016;35(1):62–70. doi: 10.1377/hlthaff.2015.0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarlenski M, Hyon Baik S, Zhang Y. Trends in use of medications for smoking cessation in Medicare, 2007–2012. Am J Prev Med. 2016;51(3):301–308. doi: 10.1016/j.amepre.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamal A, Dube SR, King BA. Peer Reveiwed: Tobacco Use Screening and Counseling During Hospital Outpatient Visits Among US Adults, 2005–2010. Preventing Chronic Disease. 2005;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Britton J, Hubbard R, Lewis S. Who receives prescriptions for smoking cessation medications? An association rule mining analysis using a large primary care database. Tob Control. 2013;22(4):274–279. doi: 10.1136/tobaccocontrol-2011-050124 [DOI] [PubMed] [Google Scholar]
- 6.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. doi: 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 7.Hawes MR, Roth KB, Cabassa LJ. Systematic Review of psychosocial smoking cessation interventions for people with serious mental illness. J Dual Diagn. 2021;17(3):216–235. doi: 10.1080/15504263.2021.1944712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228–2230. doi: 10.1176/appi.ajp.160.12.2228 [DOI] [PubMed] [Google Scholar]
- 9.Montoya ID, Herbeck DM, Svikis DS, Pincus HA. Identification and treatment of patients with nicotine problems in routine clinical psychiatry practice. Am J Addict. 2005;14(5):441–454. doi: 10.1080/10550490500247123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers E, Sherman S. Tobacco use screening and treatment by outpatient psychiatrists before and after release of the American Psychiatric Association treatment guidelines for nicotine dependence. Am J Public Health. 2014;104(1):90–95. doi: 10.2105/AJPH.2013.301584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojewski AM, Bailey SR, Bernstein SL, et al. Considering systemic barriers to treating tobacco use in clinical settings in the United States. Nicotine and Tobacco Research. 2019;21:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014;311(2):172–182. doi: 10.1001/jama.2013.284985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streck JM, Weinberger AH, Pacek LR, Gbedemah M, Goodwin RD. Cigarette smoking quit rates among persons with serious psychological distress in the United States from 2008 to 2016: Are mental health disparities in cigarette use increasing? Nicotine Tob Res. 2020;22(1):130–134. doi: 10.1093/ntr/nty227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkhoran S, Thorndike AN, Rigotti NA, Fung V, Baggett TP. Cigarette smoking and quitting-related factors among US adult health center patients with serious mental illness. J Gen Intern Med. 2019;34(6):986–991. doi: 10.1007/s11606-019-04857-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen HK. Implementation of smoking cessation treatment in substance use disorder treatment settings: a review. Am J Drug Alcohol Abuse. 2017;43(2):215–225. doi: 10.1080/00952990.2016.1183019 [DOI] [PubMed] [Google Scholar]
- 16.Siegel SD, Laurenceau JP, Hill N, et al. Assessing barriers to providing tobacco use disorder treatment in community mental health settings with a revised version of the Smoking Knowledge, Attitudes, and Practices (S-KAP) instrument. Addict Behav. 2021;114(106735):106735. doi: 10.1016/j.addbeh.2020.106735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LS, Baker TB, Korpecki JM, et al. Low-burden strategies to promote smoking cessation treatment among patients with serious mental illness. Psychiatr Serv. 2018;69(8):849–851. doi: 10.1176/appi.ps.201700399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japuntich SJ, Dunne EM, Krieger NH, et al. Proactive tobacco treatment in a behavioral health home. Community Ment Health J. 2020;56(2):328–332. doi: 10.1007/s10597-019-00458-w [DOI] [PubMed] [Google Scholar]
- 19.Prochaska JJ. Failure to treat tobacco use in mental health and addiction treatment settings: a form of harm reduction? Drug Alcohol Depend. 2010;110(3):177–182. doi: 10.1016/j.drugalcdep.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himelhoch S, Riddle J, Goldman HH. Barriers to implementing evidence-based smoking cessation practices in nine community mental health sites. Psychiatr Serv. 2014;65(1):75–80. doi: 10.1176/appi.ps.201200247 [DOI] [PubMed] [Google Scholar]
- 21.Pagano A, Tajima B, Guydish J. Barriers and facilitators to tobacco cessation in a nationwide sample of addiction treatment programs. J Subst Abuse Treat. 2016;67:22–29. doi: 10.1016/j.jsat.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter KP, Hunt JJ, Cupertino AP, et al. Commitment and capacity for providing evidence-based tobacco treatment in US drug treatment facilities. Subst Abus. 2017;38(1):35–39. doi: 10.1080/08897077.2016.1265039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: A comprehensive review. Schizophr Bull. 2016;42(1):96–124. doi: 10.1093/schbul/sbv101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samaha HL, Correa-Fernández V, Lam C, et al. Addressing tobacco use among consumers and staff at behavioral health treatment facilities through comprehensive workplace programming. Health Promot Pract. 2017;18(4):561–570. doi: 10.1177/1524839917696713 [DOI] [PubMed] [Google Scholar]
- 25.Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci. 2003;326(4):223–230. doi: 10.1097/00000441-200310000-00014 [DOI] [PubMed] [Google Scholar]
- 26.Ziedonis DM, Zammarelli L, Seward G, et al. Addressing tobacco use through organizational change: a case study of an addiction treatment organization. J Psychoactive Drugs. 2007;39(4):451–459. doi: 10.1080/02791072.2007.10399884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziedonis D, Das S, Larkin C. Tobacco Use Disorder and Treatment: New Challenges and Opportunities. Dialogues in Clinical Neuroscience.; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guydish J, Ziedonis D, Tajima B, et al. Addressing Tobacco Through Organizational Change (ATTOC) in residential addiction treatment settings. Drug Alcohol Depend. 2012;121(1–2):30–37. doi: 10.1016/j.drugalcdep.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillaumier A, Skelton E, Shakeshaft A, et al. Effect of increasing the delivery of smoking cessation care in alcohol and other drug treatment centres: a cluster-randomized controlled trial. Addiction. 2020;115(7):1345–1355. doi: 10.1111/add.14911 [DOI] [PubMed] [Google Scholar]
- 30.Brown RA, Minami H, Hecht J, et al. Sustained care smoking cessation intervention for individuals hospitalized for psychiatric disorders: The Helping HAND 3 randomized clinical trial: The helping HAND 3 randomized clinical trial. JAMA Psychiatry. 2021;78(8):839–847. doi: 10.1001/jamapsychiatry.2021.0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):21. doi: 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waltz TJ, Powell BJ, Matthieu MM, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10(1):109. doi: 10.1186/s13012-015-0295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings TG, Worley CG. Dynamics of Organizational Change and Learning. (Boonstra J, ed.). John Wiley & Sons; 2008. [Google Scholar]
- 34.Boonstra J, ed. Dynamics of Organizational Change and Learning. John Wiley & Sons; 2008. [Google Scholar]
- 35.Flitter AS, Lubitz SF, Ziedonis D, et al. A cluster-randomized clinical trial testing the effectiveness of the Addressing Tobacco Through Organizational Change model for improving the treatment of tobacco use in community mental health care: Preliminary study feasibility and baseline findings. Nicotine Tob Res. 2019;21(5):559–567. doi: 10.1093/ntr/nty239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 37.Guydish J, Tajima B, Chan M, Delucchi KL, Ziedonis D. Measuring smoking knowledge, attitudes and services (S-KAS) among clients in addiction treatment. Drug Alcohol Depend. 2011;114(2–3):237–241. doi: 10.1016/j.drugalcdep.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delucchi KL, Tajima B, Guydish J. Development of the smoking knowledge, attitudes, and practices (S-KAP) instrument. J Drug Issues. 2009;39(2):347–364. doi: 10.1177/002204260903900207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisen SV, Normand SL, Belanger AJ, Spiro A 3rd, Esch D. The Revised Behavior and Symptom Identification Scale (BASIS-R): reliability and validity. Med Care. 2004;42(12):1230–1241. doi: 10.1097/00005650-200412000-00010 [DOI] [PubMed] [Google Scholar]
- 40.Jr W, Kosinski JE, Keller M. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. Published online 1996:220–233. [DOI] [PubMed] [Google Scholar]
- 41.Fokuo JK, McCuistian CL, Masson CL, et al. Pre-implementation assessment of tobacco cessation interventions in substance use disorder residential programs in California. Subst Use Misuse. 2022;57(9):1345–1355. doi: 10.1080/10826084.2022.2079139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor GMJ, Itani T, Thomas KH, et al. Prescribing prevalence, effectiveness, and mental health safety of smoking cessation medicines in patients with mental disorders. Nicotine Tob Res. 2020;22(1):48–57. doi: 10.1093/ntr/ntz072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lappin JM, Thomas D, Curtis J, et al. Targeted intervention to reduce smoking among people with severe mental illness: Implementation of a smoking cessation intervention in an inpatient mental health setting. Medicina (Kaunas). 2020;56(4):204. doi: 10.3390/medicina56040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown CH, Medoff D, Dickerson FB, et al. Factors influencing implementation of smoking cessation treatment within community mental health centers. J Dual Diagn. 2015;11(2):145–150. doi: 10.1080/15504263.2015.1025025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koch JR, Breland A. Behavioral healthcare staff attitudes and practices regarding consumer tobacco cessation services. J Behav Health Serv Res. 2017;44(3):399–413. doi: 10.1007/s11414-015-9477-4 [DOI] [PubMed] [Google Scholar]
- 46.Chen LS, Baker T, Brownson RC, et al. Smoking cessation and electronic cigarettes in Community Mental Health Centers: Patient and provider perspectives. Community Ment Health J. 2017;53(6):695–702. doi: 10.1007/s10597-016-0065-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peckham E, Man MS, Mitchell N, et al. Smoking Cessation Intervention for severe Mental Ill Health Trial (SCIMITAR): a pilot randomised control trial of the clinical effectiveness and cost-effectiveness of a bespoke smoking cessation service. Health Technol Assess. 2015;19(25):1–148, v–vi. doi: 10.3310/hta19250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evins AE, Cather C, Laffer A. Treatment of tobacco use disorders in smokers with serious mental illness: toward clinical best practices: Toward clinical best practices. Harv Rev Psychiatry. 2015;23(2):90–98. doi: 10.1097/HRP.0000000000000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gobarani RK, Weeks GR, Abramson MJ, Bonevski B, Liau SJ, George J. Experiences of hospitalized smokers initiated on varenicline as part of a pragmatic smoking cessation trial. J Addict Dis. Published online 2022:1–9. doi: 10.1080/10550887.2022.2101339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due the risk of identifying participants, but extracts are available from the corresponding author on reasonable request. Training materials used in the implementation models


