Abstract
This study assessed the contribution of five genes previously known to be involved in cholestatic liver disease in British Bangladeshi and Pakistani people. Five genes (ABCB4, ABCB11, ATP8B1, NR1H4, TJP2) were interrogated by exome sequencing data of 5236 volunteers. Included were non-synonymous or loss of function (LoF) variants with a minor allele frequency < 5%. Variants were filtered, and annotated to perform rare variant burden analysis, protein structure, and modelling analysis in-silico. Out of 314 non-synonymous variants, 180 fulfilled the inclusion criteria and were mostly heterozygous unless specified. 90 were novel and of those variants, 22 were considered likely pathogenic and 9 pathogenic. We identified variants in volunteers with gallstone disease (n = 31), intrahepatic cholestasis of pregnancy (ICP, n = 16), cholangiocarcinoma and cirrhosis (n = 2). Fourteen novel LoF variants were identified: 7 frameshift, 5 introduction of premature stop codon and 2 splice acceptor variants. The rare variant burden was significantly increased in ABCB11. Protein modelling demonstrated variants that appeared to likely cause significant structural alterations. This study highlights the significant genetic burden contributing to cholestatic liver disease. Novel likely pathogenic and pathogenic variants were identified addressing the underrepresentation of diverse ancestry groups in genomic research.
Subject terms: Disease genetics, Cholestasis, Protein function predictions, Risk factors
Introduction
Cholestatic liver disease encompass a broad range of diagnoses that can present with fatigue, pruritus or jaundice1. Several genes are implicated, including the ABCB4 gene coding for the canalicular phosphatidylcholine floppase ABCB4, and the ABCB11 gene coding for the bile salt export pump (BSEP). Homozygous mutations in ABCB4 and ABCB11 cause a spectrum of disease from mild cholestasis to severe progressive familial intrahepatic cholestasis (PFIC), PFIC3 and PFIC2 respectively2,3. ABCB4 variants also increase the risk of developing drug-induced intrahepatic cholestasis, gallstone disease, gallbladder and bile duct carcinoma, liver cirrhosis and abnormal liver function tests4. Other canalicular transporters and their regulators are implicated in the pathogenesis of cholestatic liver disease e.g. ATP8B1 (a P-type ATPase that flips phospholipids into the cytoplasmic leaflet of the membrane)3, NR1H4 (farnesoid X receptor (FXR)) gene5, and TJP2 (tight junction protein 2)6. While homozygous mutations of these genes are implicated in rare cases of severe familial cholestasis, the evidence base for a role of heterozygous mutations in milder forms of liver disease is limited. ABCB4 and ABCB11 are involved in 20% of patients with severe intrahepatic cholestasis of pregnancy (ICP). Heterozygous ABCB4 variants have also been reported in ICP7–13. ICP is the commonest gestational liver disease14 and may be complicated by preterm birth, meconium-stained amniotic fluid and stillbirth, in association with maternal serum bile acid concentrations ≥ 40 µmol and the timing of its occurrence during pregnancy15–17. In Europeans the incidence is 0.62% versus 1.24% in women of Indian and 1.46% of Pakistani origin18. Despite the increased prevalence of ICP and other liver conditions like non-alcoholic fatty liver disease in South Asian populations they often remain undiagnosed and under-investigated19,20.
Genes and Health is a long-term population-based cohort that assesses the health and disease in British Bangladeshi and British Pakistani people. Using the Genes & Health genomics (whole exome sequencing (WES)) and data linkage to electronic health records (EHR)21, this study investigated rare variation in a unique British Bangladeshi and Pakistani cohort around 5 candidate loci (ABCB4, ABCB11, ATP8B1, NR1H4, and TJP2) implicated in cholestatic liver disease.
Results
Genotype to phenotype analysis
Screening of the 5 candidate genes identified 300 variants; and 180 (166 non-synonymous and 14 loss of function (LoF) variants), were included in the analysis (Table 1). Most variants identified were heterozygous unless otherwise specified. A small number of volunteers carried more than one variant, and this is summarised in Supplementary Table 1. None of these volunteers displayed a disease phenotype. Further variant interpretation including scoring details of individual in-silico predictors, the impact of the coding substitution on disease propensity and conservation information for all variants are presented in Supplementary Table 2.
Table 1.
Overall summary of mutational burden discovered in the Genes and Health cohort for all five gene candidates.
| Gene | Overall summary of variants (n) | ||||
|---|---|---|---|---|---|
| ABCB4 | ABCB11 | ATP8B1 | NR1H4 | TJP2 | |
| NSV | 68 | 77 | 50 | 22 | 83 |
| After inclusion criteria | |||||
| Total | 41 | 48 | 31 | 9 | 37 |
| Pathogenicity | |||||
| LP | 22 | 25 | 3 | 0 | 0 |
| VUS | 13 | 18 | 23 | 8 | 35 |
| Benign | 6 | 4 | 5 | 1 | 2 |
| Phenotypes | |||||
| ICP | 5 | 5 | 1 | 2 | 3 |
| GD | 7 | 10 | 6 | 8 | |
| Other | 1 (cholangiocarcinoma) | 1 (cirrhosis), 1 (cirrhosis with secondary malignant neoplasm of liver and bile duct, and gallstone disease) | |||
Inclusion criteria (< 5% MAF), NSV and at least any of the following: 1. Include all variants with a phenotype 2. Include all variants known in the literature 3. Include all variants with no GnomAD allele frequency but ELGH allele frequency 4. Include all variants with in silico prediction of 7.
GD, gallstone disease, ICP intrahepatic cholestasis of pregnancy, LP likely pathogenic, MAF minor allele frequency, NSV non-synonymous variants, VUS variant of unknown significance.
Phenotype to genotype analysis
We were able to validate variants of interest in 15 cases reporting ICP, most of whom had documented raised BA concentrations (Table 2). Further, variants discovered in 10 cases with raised BA but an uncertain or no diagnosis of ICP based on their EHR (Supplementary Table 3). This secondary analysis missed 2 individuals from the primary analysis with a diagnosis of ICP as there were no bile acid concentrations available for them. This analysis demonstrated a pragmatic approach to identifying disease causing variants and demonstrates the value of large genomic cohorts linked to electronic health data records.
Table 2.
Variants identified and TSBA concentration in volunteers with a diagnosis of ICP based on electronic health records.
| Volunteers | All variants in volunteers with raised BA and diagnosis of ICP | Highest BA (umol/L) | |||
|---|---|---|---|---|---|
| Gene | Variants | Zygosity | Type | ||
| 3 | ABCB4 | G1254S/G1261S | het | Non-synonymous | 80.1 |
| 3 | ABCB11 | N591S | het | Non-synonymous | |
| 4 | ABCB4 | P1050S | het | Non-synonymous | 53 |
| 5 | TJP2 | Q105K | hom | Non-synonymous | 15 |
| 6 | ABCB11 | N591S | het | Non-synonymous | 17 |
| 13 | TJP2 | Q105K | het | Non-synonymous | 25 |
| 15 | ATP8B1 | R384H | het | Non-synonymous | 31.6 |
| 15 | TJP2 | Q105K | hom | Non-synonymous | |
| 18 | ABCB11 | N591S | het | Non-synonymous | 115 |
| 18 | TJP2 | R21H | het | Non-synonymous | |
| 20 | ABCB11 | N591S | het | Non-synonymous | 18 |
| 20 | NH1R4 | M173T | het | Non-synonymous | |
| 21 | ABCB11 | M677V | het | Non-synonymous | 14 |
| 21 | NH1R4 | N358H | het | Non-synonymous | |
| 22 | ABCB11 | N591S | het | Non-synonymous | 14 |
| 25 | ABCB4 | S99x | het | Frameshift | 55 |
| 26 | ABCB11 | V284A | het | Non-synonymous | 35 |
| 26 | ABCB4 | N510S | het | Non-synonymous | |
| 31 | ABCB4 | T175A | het | Non-synonymous | 32 |
| 32 | ABCB11 | D1284N | het | Non-synonymous | |
| 32 | ABCB11 | N591S | het | Non-synonymous | |
| 38 | ABCB11 | N591S | het | Non-synonymous | 45.2 |
TSBA total serum bile acid concentrations, ICP intrahepatic cholestasis of pregnancy.
Rare variant burden analysis
We observed in cases versus controls a significant enrichment of rare variants in ABCB11 (p = 0.00247). There was no enrichment in ABCB4 (p = 0.39138), ATP8B1 (p = 0.57957), TJP2 (p = 0.17390), or NR1H4 (p = 0.70232). A further Fisher’s exact analysis compared percentage of rare variants in ICP cases in Genes & Health compared to Genomics England demonstrating that the rare genetic burden was significantly increased in tier 1 gene candidates in British Pakistani and Bangladeshi (ABCB4, p = 0.0191; ABCB11, p = 0.0191) but not in ATP8B1 (p = 0.4857) NR1H4 (p = 0.2286) or TJP2 (p = 0.1039).
ABCB4 variants
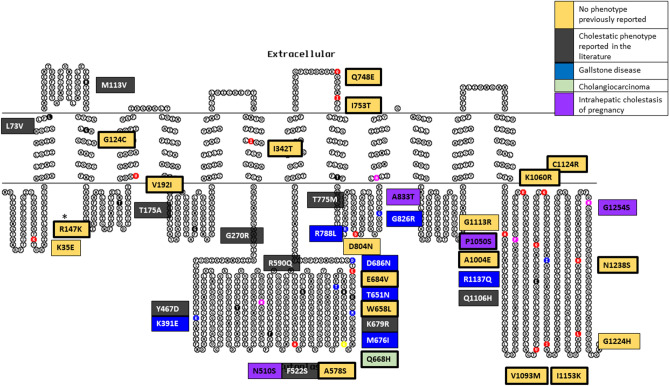
There was a total of 68 ABCB4 variants identified (Table 1 and Fig. 1). 41 variants fulfilled the inclusion criteria. Variants were identified in people with cholestatic liver disease phenotypes: ICP (n = 5 out of 88 women in the analysis), gallstone disease (n = 7), and cholangiocarcinoma (n = 1) (Table 3). For some identified variants a known cholestatic phenotype had previously been reported in the literature (n = 9) whilst others had no phenotype reported (n = 19) (Supplementary Table 4). We identified novel LoF variants (n = 4): three frameshift (one associated with an ICP phenotype) and one introduction of a premature stop codon (no associated phenotype) (Table 4).
Figure 1.
ABCB4 variant summary in a 2-dimensional illustration. 41 variants are divided into their phenotypic presentation and coloured by: No phenotype previously reported (n = 19), cholestatic phenotype reported in the literature (n = 9), gallstone disease (n = 7), cholangiocarcinoma (n = 1), and intrahepatic cholestasis of pregnancy (n = 5). Bold border represents variants that are unique to the Genes & Health cohort. Topo2 software (Johns S.J., TOPO2, Transmembrane protein display software, http://www.sacs.ucsf.edu/TOPO2) was used for illustration95.
Table 3.
Non-synonymous variants identified in the five gene candidates associated with a cholestatic phenotype in the Genes and Health cohort.
| Gene | Phenotype | Transcript | Protein change | dbSNP | gnomAD AF | G&H AF^ | ACMG-AMP | ACMG-AMP criteria | Clinvar | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| ABCB4 | Intrahepatic cholestasis of pregnancy | ENSP00000496956.1:p.Gly1254Ser | G1254S* | rs781315185 | 0.00003656 | 0.00028843 | LP | PM1, PM2, PP2, PP3 | 22 | |
| ENSP00000497931.1:p.Asp1284Asn | P1050S* | 0.00019146 | LP | PM1, PM2, PP2, PP3 | ||||||
| ENSP00000496956.1:p.Ala833Thr | A833T* | 0.00009638 | LP | PM1, PM2, PP2, PP3 | ||||||
| ENSP00000496956.1:p.Asn510Ser | N510S | rs375315619 | 0.00019110 | 0.00057394 | LP | PM1, PM2, PP2, PP3, PP5 | LP | 23–30 | ||
| ENSP00000496956.1:p.Thr175Ala | T175A1 | rs58238559 | 0.01155000 | 0.01315030 | LB | PM1, PP2, PP3, BS1, BS2, BP6 | Benign/likely benign | 3,12,23,25,26,28–43 | ||
| Gallstone disease | ENSP00000496956.1:p.Arg1137Gln | R1137Q | rs780738927 | 0.00003250 | 0.00028846 | LP | PM1, PM2, PP2, PP3 | |||
| ENSP00000496956.1:p.Gly826Arg | G826R* | 0.00028681 | LP | PM1, PM2, PP2, PP3 | ||||||
| ENSP00000496956.1:p.Arg788Leu | R788L | rs8187801 | 0.00000813 | 0.00009579 | LP | PM1, PM2, PP2, PP3 | Benign | |||
| ENSP00000496956.1:p.Asp686Asn | D686N | rs78653500 | 0.00009586 | VUS | PM2, PP2, BP4 | |||||
| ENSP00000496956.1:p.Met676Ile | M676I | rs376702091 | 0.00002033 | 0.00038278 | VUS | PM2, PP2, BP4 | ||||
| ENSP00000496956.1:p.Thr651Asn | T651N | rs45476795 | 0.0005776 | 0.0006719 | LB | PM2, PP2, BP4, BP6 | conflicting | |||
| ENSP00000496956.1:p.Lys391Glu | K391E | rs781347049 | 0.00002440 | 0.00009582 | LB | PM2, PP2, BP4, BP6 | ||||
| Cholangiocarcinoma | ENSP00000496956.1:p.Gln668His | Q668H* | 0.00009586 | LB | PM2, PP2, BP4 | |||||
| ABCB11 | Intrahepatic cholestasis of pregnancy | ENSP00000497931.1:p.Asp1284Asn | D1284N | rs766784155 | 0.00001228 | 0.00028780 | LP | PM1, PM2, PP2, PP3 | ||
| ENSP00000497931.1:p.Arg1050His | R1050H | rs72549398 | 0.00000421 | 0.00019135 | VUS | PM2, PP2, BP4 | ||||
| ENSP00000497931.1:p.Met677Val | M677V2 | rs11568364 | 0.02364000 | 0.01005750 | Benign | PP2, BA1, BS3, BP6, BP4 | Benign | 12,43–52 | ||
| ENSP00000497931.1:p.Asn591Ser | N591S3 | rs11568367 | 0.01436000 | 0.12647200 | Benign | PM1, PP2, BA1, BP6 | Benign | 3,12,28,36,46,52–61 | ||
| ENSP00000497931.1:p.Val284Ala | V284A | rs200739891 | 0.00026040 | 0.00009558 | LP | PM1, PM2, PM5, PP2, PP3, BP6 | Conflicting | 31,48,50 | ||
| Gallstone disease | ENSP00000497931.1:p.Ala1260Pro | A1260P | rs772097949 | 0.00001641 | 0.00028153 | LP | PM1, PM2, PP2, PP3 | VUS | ||
| ENSP00000497931.1:p.Gln976Arg | Q976R | rs199940188 | 0.00054840 | 0.00066883 | VUS | PM1, PM2, PP2, BP4 | Conflicting | |||
| ENSP00000497931.1:p.Ala926Ser | A926S* | 0.00040667 | LP | PM1, PM2, PM5, PP2,PP3 | ||||||
| ENSP00000497931.1:p.Ala679Val | A679V | rs200912109 | 0.00045560 | 0.00143761 | VUS | PM2, PP2, BP4 | Conflicting | |||
| ENSP00000497931.1:p.Asn539Asp | N539D* | 0.00022604 | VUS | PM1, PM2, PP2, BP4 | ||||||
| ENSP00000497931.1:p.Arg487Cys | R487C | rs770693935 | 0.00002043 | 0.00009549 | LP | PM1, PM2, PP2, PP3 | 62 | |||
| ENSP00000497931.1:p.Ala311Thr | A311T | rs200509511 | 0.00004073 | 0.00028969 | LP | PM1, PM2, PP2, PP3 | ||||
| ENSP00000497931.1:p.Val95Ile | V95I | rs201735739 | 0.00009766 | 0.00028708 | Benign | PM1, PM2, PP2, BP4 | ||||
| ENSP00000497931.1:p.Asp94Asn | D94N | rs760920706 | 0.00010170 | 0.00095621 | LP | PM1, PM2, PP2 | Conflicting | |||
| ENSP00000497931.1:p.Lys12Arg | K12R* | 0.00010378 | LP | PM1, PM2, PP2, PP3 | ||||||
| ATP8B1 | Intrahepatic cholestasis of pregnancy | ENSP00000497896.1:p.Arg384His | R384H# | rs2271260 | 0.00026400 | 0.00048040 | VUS | PM2, PP2, BP6 | 3 | |
| Gallstone disease | ||||||||||
| Gallstone disease | ENSP00000497896.1:p.Val1161Ala | V1161A | rs1255793857 | 0.00000406 | 0.00009549 | VUS | PM2, PP2 | |||
| ENSP00000497896.1:p.Thr1092Ile | T1092I | rs780425796 | 0.00001220 | 0.00030581 | VUS | PM2, PP2, PP3 | ||||
| ENSP00000497896.1:p.Met674Thr | M674T+4 | rs35470719 | 0.00456300 | 0.00632063 | Benign | PP2, BA1, BP4, BP6 | Benign/Likely benign | 52,63–68 | ||
| ENSP00000497896.1:p.Ile577Val | I577V+5 | rs3745078 | 0.00467800 | 0.00628992 | Benign | PP2, BA1, BP6 | Benign | 52,63–66,68 | ||
| ENSP00000497896.1:p.His78Gln | H78Q+6 | rs3745079 | 0.00421800 | 0.00495751 | Benign | PP2, BP4,BP6, BS1, BS2 | Benign | 52,63–66,68 | ||
| ENSP00000497896.1:p.Asp14Tyr | D14Y* | 0.00009560 | VUS | PM1, PM2, PP2, BP4 | ||||||
| Cirrhosis | ENSP00000497896.1:p.Ile513Thr | I513T | rs772028343 | 0.00008531 | 0.00066973 | VUS | PM2, PP2 | |||
| Cirrhosis, secondary malignant neoplasm of liver and bile duct, gallstone disease | ENSP00000497896.1:p.Asp70Asn | D70N7 | rs34719006 | 0.00313900 | 0.00302678 | VUS | PM2, PP2, PP3 | Conflicting | 24,64,69–75 | |
| NR1H4 | Intrahepatic cholestasis of pregnancy | ENSP00000496908.1:p.Asn358His | N358H | rs149287629 | 0.00041020 | 0.00038307 | VUS | PM2 | VUS | |
| ENSP00000496908.1:p.Met173Thr | M173T | rs61755050 | 0.00374800 | 0.00267482 | Likely benign | PM1, PP3, BS1, BS2, BP6 | likely benign | 6,76 | ||
| TJP2 | Intrahepatic cholestasis of pregnancy | ENSP00000497787.1:p.Thr377Ala | T377A | rs766748789 | 0.00000406 | 0.00047765 | VUS | PM2, BP4 | ||
| ENSP00000496791.1:p.Gln105Lys | Q105K8 | rs41305539 | 0.05150000 | 0.12031400 | Benign | BA1, BP4, BP6, | benign | 44,77,78 | ||
| ENSP00000497861.1:p.Arg21His | R21H9 | rs4493966 | 0.07416000 | 0.04555170 | Benign | BA1, BP4, BP6, | benign | |||
| Gallstone disease | ENSP00000496791.1:p.Gln8Arg | Q8R* | 0.00009553 | VUS | PM2, PP3 | |||||
| ENSP00000496791.1:p.Thr68Asn | T68N* | 0.00019150 | VUS | PM1, PM2 | ||||||
| ENSP00000496791.1:p.Pro152Leu | P152L | rs754300892 | 0.00007876 | 0.00046685 | VUS | BP4 | ||||
| ENSP00000497787.1:p.Arg178Cys | R178C | rs199761505 | 0.00043060 | 0.00223305 | VUS | PP3 | ||||
| ENSP00000497787.1:p.Arg255His | R255H | rs532438219 | 0.00012990 | 0.00066947 | VUS | |||||
| ENSP00000497787.1:p.Arg461Pro | R461P | rs748523814 | 0.00009746 | 0.00078125 | VUS | PP3 | ||||
| ENSP00000496791.1:p.Thr902Met | T902M | rs774198938 | 0.00010970 | 0.00010449 | VUS | PP3 | ||||
| ENSP00000496791.1:p.Arg1070Lys | R1070K* | 0.00009564 | VUS | PM2, BP4 | VUS |
AF allele frequency, ACMG-AMP American College of Medical Genetics and Genomics and the Association for Molecular Pathology, BP benign supporting, PM pathogenic moderate, PP pathogenic supporting, G&H Genes & Health, LP likely pathogenic, Ref references, VUS variant of unknown significance.
*South Asian specific variant.
#multiple phenotype.
+Linkage disequilibrium.
^Allele frequency specific to East London Genes & Health cohort.
1T175A, Hom (n) 6, Het (n) 126.
2M677V, Hom (n) 3, Het (n) 99.
3N591S, Hom (n) 99, Het (n) 760.
4M674T, Hom (n) 2, Het (n) 67.
5I577V, Hom (n) 2, Het (n) 61.
6H78Q, Hom (n) 1, Het (n) 47.
7D70N, Hom (n) 1, Het (n) 24.
8Q105K, Hom (n) 76, Het (n) 711.
9R21H, Hom (n) 16, Het (n) 443.
Table 4.
Loss of function variants identified in the five gene candidates.
| Phenotype | Gene | Transcript | Protein change | Info | gnomAD AF | G&H AF^ | ACMG-AMP | ACMG-AMP criteria | Clinvar |
|---|---|---|---|---|---|---|---|---|---|
| ICP | ABCB4 | ENSP00000395716.1:p.Ser99LeufsTer11 | S99x | Frameshift | 0.00039970 | 0.00336538 | P | PVS1, PM2, PP3 | |
| ENSP00000392983.1:p.Leu759TyrfsTer38 | F758x | Frameshift | 0.00009610 | LP | PVS1, PM2 | ||||
| ENSP00000392983.1:p.Lys30GlyfsTer7 | Lys30Glyfster7 | Frameshift | 0.00000408 | 0.00020243 | LP | PVS1, PM2 | |||
| ENSP00000392983.1:p.Arg595Ter | R595* | Stop-gained | 0.00001627 | 0.00009593 | P | PVS1, PM2, PP3, PP5 | Pathogenic | ||
| Gallstone disease | ABCB11 | ENSP00000497931.1:p.Ala1044LeufsTer53 | A1044x | Frameshift | 0.00009562 | P | PVS1, PM2, PP3 | ||
| ENST00000263817.7:c.2611-2A > T | c.2611-2A > T | Splice-acceptor-variant | 0.00000407 | 0.00057870 | P | PVS1, PM2, PP3 | |||
| ENSP00000497931.1:p.Trp239Ter | W239x | Stop-gained | 0.00009566 | P | PVS1, PM2, PP3 | ||||
| ATP8B1 | ENSP00000283684.4:p.Gln1179GlufsTer56 | IQ1178-1179IX | Frameshift_variant & splice_region_variant | 0.00193798 | |||||
| ENSP00000283684.4:p.Pro792HisfsTer8 | F791X | frameshift_variant | 0.00019069 | P | PVS1, PM2, PP3 | ||||
| Gallstone disease | ENST00000283684.9:c.182-4_183del | ?-61 | Splice_acceptor_variant & coding_sequence_variant & intron_variant | 0.00048956 | |||||
| ENSP00000283684.4:p.Glu20Ter | E20* | Stop_gained | 0.00009566 | P | PVS1, PM2, PP3 | ||||
| NR1H4 | ENSP00000446760.1:p.Lys4Ter | K4* | Stop_gained | 0.00014188 | P | PVS1, PM2, PP3 | |||
| TJP2 | ENSP00000438262.1:p.Glu44Ter | E44* | Stop_gained | 0.00009604 | LP | PVS1, PM2 | |||
| ENSP00000345893.4:p.Gly5ArgfsTer26 | M1MPVX | Frameshift_variant & start_lost | 0.00001343 | 0.00029768 | P | PVS1, PM2, PP3 |
AF allele frequency, ACMG-AMP American College of Medical Genetics and Genomics and the Association for Molecular Pathology, BP benign supporting, PM pathogenic moderate, PP pathogenic supporting, G&H Genes & Health, LP likely pathogenic, P pathogenic.
^Allele frequency specific to East London Genes & Health cohort.
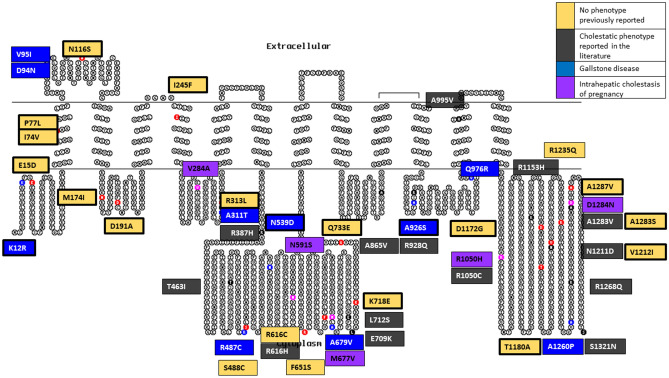
ABCB11 variants
There were 77 ABCB11 variants identified of which 48 were included in the analysis (Table 1 and Fig. 2). The associated cholestatic liver disease phenotypes identified were: ICP (n = 5 out of 83 women in the analysis), and gallstone disease (n = 10) (Table 3). Some were linked to cholestatic phenotypes previously reported in the literature (n = 14), whilst for 19, no phenotype had been previously reported (n = 19) (Supplementary Table 5). Likely novel LoF variants were identified (n = 3): a frameshift, a splice-acceptor and an introduction of premature stop codon variant. These variants were not associated with a known disease phenotype (Table 4).
Figure 2.
ABCB11 variant summary in a 2-dimensional illustration. 48 variants are divided into their phenotypic presentation and coloured by: No phenotype previously reported (n = 19), cholestatic phenotype reported in the literature (n = 14), gallstone disease (n = 10), and intrahepatic cholestasis of pregnancy (n = 5). Bold border represents variants that are unique to the Genes & Health cohort. Topo2 software (Johns S.J., TOPO2, Transmembrane protein display software, http://www.sacs.ucsf.edu/TOPO2) was used for illustration95.
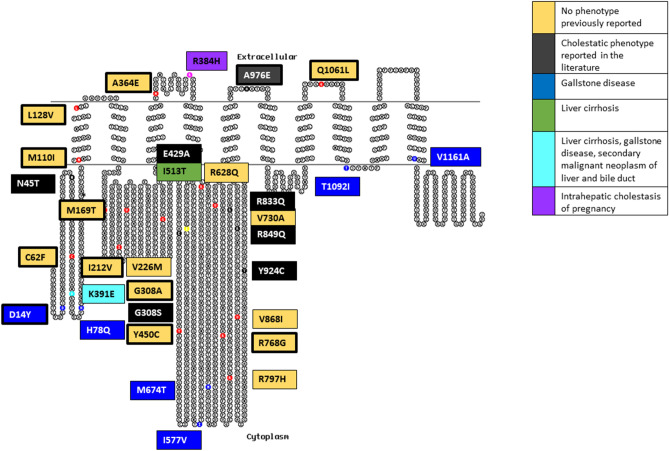
ATP8B1 variants
We identified a total of 50 ATP8B1 variants and 31 were included in the final analysis (Table 1 and Fig. 3). There were 7 variants associated with gallstone disease; three appeared to be in linkage disequilibrium (LD) noted in three volunteers: M674T, I577V, and H78Q. The R384H variant was associated with gallstone disease and with an ICP phenotype (in separate individuals, n = 2 out of 33 women in the analysis). A further variant was associated with hepatitis-induced liver cirrhosis (I513T), and a final variant (D70N) was associated with liver cirrhosis, secondary malignant neoplasm of liver and bile duct, and gallstone disease (Table 3). In addition, previously reported cholestatic liver disease phenotypes (n = 7) and variants with no previous reported phenotype were seen (n = 15) (Supplementary Table 6). Finally, we identified 4 novel LoF variants: a frameshift/splice region, frameshift, splice-acceptor/coding-sequence, and stop-gain variant. The latter variant was associated with a gallstone disease phenotype (Table 4).
Figure 3.
ATP8B1 variant summary in a 2-dimensional illustration. 31 variants are divided into their phenotypic presentation and coloured by: No phenotype previously reported (n = 15), cholestatic phenotype reported in the literature (n = 7), gallstone disease (n = 7), liver cirrhosis (n = 1), Liver cirrhosis and multiple cholestatic phenotype (n = 1), and intrahepatic cholestasis of pregnancy (n = 2). Bold border represents variants that are unique to the Genes & Health cohort. Topo2 software (Johns S.J., TOPO2, Transmembrane protein display software, http://www.sacs.ucsf.edu/TOPO2) was used for illustration95.
NR1H4 variants
There were 22 NR1H4 variants in the Genes & Health cohort and 9 variants in the final analysis (Table 1 and Supplementary Fig. 2). We only identified an ICP phenotype (n = 2 out of 33 women in the analysis) (Table 3) and otherwise novel variants that had no previous phenotype reported (n = 7) (Supplementary Table 7). Furthermore, one novel LoF variant was identified without demonstrating a phenotype (Table 4).
TJP2 variants
There were 83 TJP2 variants identified of which 37 were analysed (Table 1). People with TJP2 variants had ICP (n = 3 out of 103 women in the analysis), gallstone disease (n = 8), previously reported cholestatic liver disease phenotype (n = 4), and 22 did not have a previously reported phenotype (Table 3, Supplementary Table 8). There were two novel LoF variants without a clinical phenotype (Table 4).
Protein structure and molecular modelling
A flow chart illustrating the variants included in this analysis is shown in Supplementary Fig. 3. Results of the protein structure and molecular modelling software tools are presented in Supplementary Table 9.
Some novel variants are in regions of transporters for which we can hypothesis a mechanistic impact. Of particular interest are Q1106H in ABCB4 and D191A in ABCB11. These ABC B-family transporters share 48% amino acid identity and are very likely have a common mechanism of action. The two amino acids are conserved in both proteins, and we propose that they are involved in energy transduction through the transporter in order to couple the substrate efflux cycle to the ATP binding and catalysis cycle.
In ABCB4 and ABCB11, two transmembrane domains (TMDs) bind the transport substrates (phosphatidylcholine (PC) and bile acids), respectively. The conformational changes required for substrate transport are driven by ATP hydrolysis at the interface between two nucleotide binding domains (NBDs). The TMDs and NBDs must therefore be intimately coupled, and this is achieved via four ‘coupling helices’ (CH) located at the base of the long intracellular loops extending from the transmembrane alpha helices of the TMDs (Supplementary Fig. 4A).
Q1106 (ABCB4) and D191 (ABCB11) are particularly interesting because they are located at this interface that is conserved in both ABCB4 and ABCB11. Q1106 is in a groove on the surface of NBD2 where it interacts with CH2 (Supplementary Fig. 4B).
In the PC-bound conformation of ABCB4, Q1106 forms a weak electrostatic interaction with the peptide bond of G270. In the ATP-bound conformation (from which PC has most likely been released), Q1106 now interacts with Q272 which illustrates the movement of CH2 and its changing juxtaposition with the NBD during the transport cycle; essentially, a hinge region. The geometry of these interactions will not be preserved if Q1106 is replaced by histidine. In ABCB11, this triad is preserved in Q1150 and G295, with E297 providing a conservative change for the glutamine in CH2 (with respect to formation of an equivalent electrostatic bond with Q1150).
In the sole structural model that we have for ABCB11, D191 is in CH1 where it interacts with Y472 in a surface groove of NBD1 and also, intriguingly, with R946 which is in CH4, suggesting that CH1 and CH4 likely work together in energy transduction through the transporter (Supplementary Fig. 4C).
These electrostatic bonds will not be possible if D191 is replaced with alanine. This triad and its bond architecture is perfectly conserved in ABCB4 in the ATP bound conformation through amino acids D166, Y446 and R902. However, in ABCB4 there is also an additional electrostatic interaction between the carbonyl of the D166 peptide bond and the side chain of Q1171. Q1171 (which is conserved in ABCB11) is adjacent to the ABC signature motif 1172LSGGQ1176 which is involved in coordination of ATP and provides a direct mechanism for how CH1 influences, and responds to, the ATP catalytic cycle of these transporters.
Discussion
This study identified novel variants implicated in the aetiopathogenesis of cholestatic liver disease that occur uniquely in this British Bangladeshi and Pakistani cohort36,79–81. There have not been any other studies of this magnitude analysing the burden of mutational variation in cholestatic liver disease in a large South Asian cohort. Using a genotype to phenotype approach we discovered novel likely pathogenic variants that appear to be unique to this cohort. We then employed a phenotype to genotype analysis using the ICP phenotype as an exemplar, which offered a pragmatic interrogation of electronic health records to identify rare genetic variants that are likely pathogenic. Thus, this study improves representation of this distinct population especially as prevalence of cholestatic liver disease is increased in the Genes & Health cohort, e.g. 1.54% are affected by ICP compared to white Europeans (0.62%). This study demonstrates the importance of multi-ancestry genomic research and offers the potential of tailored treatment for this population.
In the Genes & Health cohort, out of 194 variants meeting inclusion criteria we identified 53 that had a cholestatic liver disease phenotype reported in their linked EHR. Of those, 16 are unique to this British Bangladeshi and Pakistani population and a large number were predicted to be likely pathogenic or known pathogenic based on in-silico prediction tools. In addition, there were 35 variants that were previously reported in the literature with a cholestatic phenotype. However, 87 variants had no previously reported phenotype; 67 were novel (34% of all variants analysed in this study) as they were also not previously reported in the GnomAD population database. Despite that, 9 were considered likely pathologic and 5 known pathogenic. It is important to consider that heterozygosity as noted in most cases means that they are likely rescued by the wild-type allele but at higher risk of disease in later life or during times of liver stress, e.g. during pregnancy.
Our findings reflect the difficulty with interpretation of rare variants in clinically important genes when there is no previous evidence in the literature or functional data to interpret them further. The ACMG rare variant interpretation guideline30 provides a standardised analysis pathway. However, it relies in part on the interpretation of the variant in the context of the literature and does not account for specific genes and diseases. It also may not be robust for flexible membrane proteins which do not work by lock and key mechanism. For example, the ABCB11 variant V444A, considered as benign by the ACMG criteria, has been reported to increase the risk of ICP, hepatitis C disease progression, and drug-induced liver injury although the exact functional mechanisms are not clear yet55,82.
By employing computational protein modelling software tools, we were able to identify variants that likely have a significant impact on the conformation of the protein and could therefore be of clinical significance. It is important to bear in mind that all these tools have inherent flaws and are beyond the scope of this paper to discuss in detail. By taking ICP as a cholestatic liver disease example we were able to highlight further difficulties with rare variant interpretation in gestational syndromes as the inherent transient nature of the disease makes variant interpretation challenging. However, ICP is a clinically relevant example as the gestational disease consequences are not just relevant to their current pregnancy but also can result in later hepatobiliary disorders such as cancer, immune-mediated and cardiovascular diseases83. In addition, they have a higher gallstone-related morbidity and a strong positive association between ICP and hepatitis C exists as well84.
Limitations
The use of electronic health records to determine phenotype is extremely useful but dependent upon appropriate information having been coded. Participants with at-risk variants may not have presented yet with symptoms of disease but still be at high risk of developing complications at a later stage in their life, particularly given that the median age of volunteers in this study was around 45 years. It demonstrates the difficulty with interpreting variants when recalling the genotype first.
Conclusions
In this study we provide the first comprehensive evaluation of gene candidates associated with cholestatic liver diseases in a unique cohort of British Bangladeshi and Pakistani origin demonstrating the importance of characterising genetic disease in diverse ethnic groups. Our findings have demonstrated the increased mutational burden of cholestatic liver disease in British Bangladeshi and Pakistani people who thus far remain understudied despite their distinct genetic background and increased risk of developing ICP in comparison to other populations. We were able to identify novel variants that have not been previously identified and are likely implicated in disease. We demonstrated the ability to identify participants at risk both by a phenotype or genotype first approach. This demonstrates the importance of providing more personalised care in a clinical setting as identification of high-risk individuals and their family members enables early intervention to prevent adverse outcomes, for example hepato-protective drugs such as UDCA, in addition to hepatic surveillance. Furthermore, it provides the necessary foundation for improved therapy and drug development.
Methods
Study population
A detailed description of the Genes & Health cohort has been described by Finer et al.21. Ethical approval for the study was provided by the South East London National Research Ethics Committee (14/LO/1240) including consent for publishing http://www.genesandhealth.org/volunteer-information22. All Genes & Health volunteers consented to lifelong EHR linkage, DNA extraction and genetic tests. All research was conducted in accordance with NHS Health Research Authority guidelines and regulations. An individual application to support data access for this study was granted by Genes & Health (reference S00037) taking into consideration community prioritisation, acceptability and scientific merit.
Exome sequencing samples from 5236 Genes & Health volunteers reporting parental relatedness were available for analysis in variant call format files. For the initial analysis a genotype to phenotype approach was employed interrogating 5 gene candidate loci (Table 5). For the rare burden analysis female volunteers without ICP served as controls (n = 3048). In a secondary analysis, a phenotype to genotype analysis was used to validate these findings, using ICP as the exemplar. For this approach, electronic health records allowed total serum bile acid concentrations ≥ 10 µmol to be retrieved from a network of acute hospitals that provide maternity care to (n = 15,500) women per year living in east London to identify patients with a diagnosis of liver disease in pregnancy (ICD 10 diagnosis code O26.6), see Supplementary Fig. 1. Maternal health records were screened by an experienced clinician to verify a diagnosis of ICP.
Table 5.
Description of the five gene candidates.
| Genes | Chr | Gene product | Omim ID | Exons | Length | Associated disease |
|---|---|---|---|---|---|---|
| ABCB4 | 7 (7q21.1) | 171,060 | 27 | 81 kb | PFIC-3 | |
| ABCB11 | 2 (2q31.1) | BSEP | 603,201 | 28 | 115 kb | PFIC-2 |
| ATP8B1 | 18 (18q21.31) | FIC1 | 602,397 | 28 | 157 kb | PFIC-1 |
| TJP2 | 9 (9q21.11) | 607,709 | 25 | 153 kb | PFIC-4 | |
| NR1H4 | 12 (12q23.1) | FXR | 603,826 | 11 | 90 kb | PFIC-5 |
Chr chromosomes.
Exome sequencing & bioinformatic pipeline
Low/mid exome sequencing was performed as previously described85. The exome sequencing data is being held under a data access agreement at the European Genotype-phenome Archive (www.ebi.ac.uk/ega) under accession numbers EGAD00001005469. Minor allele frequency (MAF) was set at < 5% to include rare and low-frequency genetic variants to allow for a comprehensive evaluation.
Variant annotation
All protein-altering missense, non-sense, frameshift indels or splice site variants identified in the candidate gene set underwent the same processing as described below. Synonymous variants were excluded from further analysis. Variants were filtered and annotated if they met any of the following inclusion criteria (MAF < 5%): 1. associated with a phenotype; 2. known in the literature; 3. no recorded GnomAD allele frequency; 4. predicted to be likely pathogenic (LP) based on all 7 in-silico predictors. To assess the likelihood of functional impact of variants a variety of in-silico tools were employed, including Polyphen86, SIFT87, CADD88, Revel89, MetaLR90, MetaSVM90 and M_CAP91. Open-source databases (Leiden Open Variation Database—LoVD, and ClinVar) including a commercial database (Mastermind) were interrogated to assess whether variants were reported previously in the literature. The American College of Medical Genetics/Association for Molecular Pathology (ACMG/AMP) guidelines for rare variant interpretation was used to assess variant pathogenicity. The guidelines consider a variant to be LP if there is a > 90% certainty it being disease-causing, but below a higher “pathogenic” threshold92.
Rare variant burden analysis
To assess the significance of any rare variant burden of SNPs in all 5 gene candidates the exactCMC function in RVTESTS93 was used. The burden was calculated as the proportion of all ICP cases versus control in the Genes & Health cohort and who had at least one alternate allele. Variants with an allele frequency of 0.01 or less were included. ICP cases (n = 18) from the Genomics England database (Project ID 747)—a predominantly European genetic cohort—were accessed to serve as a direct comparison to rare variant burden in Genes & Health.
Protein structure and modelling analysis
Inclusion criteria for further protein structure and modelling analysis required variants to have an associated phenotype, and/or be predicted to be known pathogenic based on all 7 in-silico tools. VarMap was used to assess protein sequence variants94. 2D representations were designed using the open source tool TOPO295. Variants that were LP or associated with a phenotype underwent further analysis using: (1) Dynamut96 (2) CUPSAT97 (3) SNPMuSiC98. 3D structural representations were generated using PyMOL software (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.) using ABCB4 with phosphatidylcholine (PDB ID: 7NIV)99, ABCB11 open structure (PDB ID: 6LR0)100, ATP8B1 (PDB ID: 7PY4)101, and NR1H4 (PDB ID: 1OSH)102. There is no 3D protein structure available for TJP2.
Supplementary Information
Acknowledgements
Genes & Health has recently been core-funded by Wellcome (WT102627, WT210561), the Medical Research Council (UK) (M009017, MR/X009777/1), Higher Education Funding Council for England Catalyst, Barts Charity (845/1796), Health Data Research UK (for London substantive site), and research delivery support from the NHS National Institute for Health Research Clinical Research Network (North Thames). Genes & Health has recently been funded by Alnylam Pharmaceuticals, Genomics PLC; and a Life Sciences Industry Consortium of Astra Zeneca PLC, Bristol-Myers Squibb Company, GlaxoSmithKline Research and Development Limited, Maze Therapeutics Inc, Merck Sharp & Dohme LLC, Novo Nordisk A/S, Pfizer Inc, Takeda Development Centre Americas Inc. We thank Social Action for Health, Centre of The Cell, members of our Community Advisory Group, and staff who have recruited and collected data from volunteers. We thank the NIHR National Biosample Centre (UK Biocentre), the Social Genetic & Developmental Psychiatry Centre (King's College London), Wellcome Sanger Institute, and Broad Institute for sample processing, genotyping, sequencing and variant annotation. We thank: Barts Health NHS Trust, NHS Clinical Commissioning Groups (City and Hackney, Waltham Forest, Tower Hamlets, Newham, Redbridge, Havering, Barking and Dagenham), East London NHS Foundation Trust, Bradford Teaching Hospitals NHS Foundation Trust, Public Health England (especially David Wyllie), Discovery Data Service/Endeavour Health Charitable Trust (especially David Stables), NHS Digital—for GDPR-compliant data sharing backed by individual written informed consent. Most of all we thank all of the volunteers participating in Genes & Health.
Author contributions
C.W. and P.D. conceived the idea of the study and applied for data access. J.Z. accessed, extracted, analysed, and verified the data. S.F. and D.H. helped with clinical data extraction. J.Z., C.W., P.D. and K.L. contributed to data interpretation. K.L. and J.Z. lead on protein modelling and interpretation. S.F. and D.H. helped with clinical phenotype data interpretation and extractions. All authors gave final approval of the final draft to be published and have contributed to the manuscript.
Funding
Disclose funding sources for the publication: CW is funded by a National Institute of Health and Care Research (NIHR) Senior Investigator grant (NIHR200254). The views expressed in this Article are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data availability
The imputed genotype data from Genes & Heath are available on EGA (www.ega-archive. org; study accession number: EGAS00001005373). The individual-level phenotypic data will be made available to researchers by completing an application to Genes & Health, following their open access policy described on https://www.genesandhealth.org/research/scientists-using-genes-health-scientific-research.
Competing interests
SF receives research funding for Genes & Health from MRC, NIHR, Alnylam Pharmaceuticals, Takeda, Glaxo Smith Kline, Merck, Pfizer, NovoNordisk, Maze Pharmaceuticals, Bristol Myers Squibb. DvH receives research funding for Genes & Health from EU-H2020, NIH, MRC, HEFCE-Catalyst, Wellcome, HDR-UK, Alnylam Pharmaceuticals, Takeda, Glaxo Smith Kline, Merck, Pfizer, NovoNordisk, Maze Pharmaceuticals, Bristol Myers Squibb. CW consults for Mirum and GSK. JZ, PD, and KL have none to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Catherine Williamson, Email: catherine.williamson@kcl.ac.uk.
Genes and Health Research Team:
Shaheen Akhtar, Mohammad Anwar, Elena Arciero, Samina Ashraf, Saeed Bidi, Gerome Breen, James Broster, Raymond Chung, David Collier, Charles J. Curtis, Shabana Chaudhary, Megan Clinch, Grainne Colligan, Panos Deloukas, Ceri Durham, Faiza Durrani, Fabiola Eto, Sarah Finer, Joseph Gafton, Ana Angel Garcia, Chris Griffiths, Joanne Harvey, Teng Heng, Sam Hodgson, Qin Qin Huang, Matt Hurles, Karen A. Hunt, Shapna Hussain, Kamrul Islam, Vivek Iyer, Ben Jacobs, Ahsan Khan, Cath Lavery, Sang Hyuck Lee, Robin Lerner, Daniel MacArthur, Daniel Malawsky, Hilary Martin, Dan Mason, Rohini Mathur, Mohammed Bodrul Mazid, John McDermott, Caroline Morton, Bill Newman, Elizabeth Owor, Asma Qureshi, Samiha Rahman, Shwetha Ramachandrappa, Mehru Reza, Jessry Russell, Nishat Safa, Miriam Samuel, Michael Simpson, John Solly, Marie Spreckley, Daniel Stow, Michael Taylor, Richard C. Trembath, Karen Tricker, Nasir Uddin, David A. van Heel, Klaudia Walter, Caroline Winckley, Suzanne Wood, John Wright, and Julia Zöllner
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-33391-w.
References
- 1.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J. Hepatol.51, 237-67 (2009). [DOI] [PubMed]
- 2.Sticova E, Jirsa M. ABCB4 disease: Many faces of one gene deficiency. Ann. Hepatol. 2020;19:126–133. doi: 10.1016/j.aohep.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Huynh MT, et al. Clinical characteristics and genetic profiles of young and adult patients with cholestatic liver disease. Rev. Esp. Enferm. Dig. 2019;111:775–788. doi: 10.17235/reed.2019.6168/2019. [DOI] [PubMed] [Google Scholar]
- 4.Stättermayer AF, Halilbasic E, Wrba F, Ferenci P, Trauner M. Variants in ABCB4 (MDR3) across the spectrum of cholestatic liver diseases in adults. J. Hepatol. 2020;73:651–663. doi: 10.1016/j.jhep.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Davit-Spraul A, Gonzales E, Jacquemin E. NR1H4 analysis in patients with progressive familial intrahepatic cholestasis, drug-induced cholestasis or intrahepatic cholestasis of pregnancy unrelated to ATP8B1, ABCB11 and ABCB4 mutations. Clin. Res. Hepatol. Gastroenterol. 2012;36:569–573. doi: 10.1016/j.clinre.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Sambrotta M, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat. Genet. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vree JM, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl. Acad. Sci. U. S. A. 1998;95:282–7. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353:210–211. doi: 10.1016/S0140-6736(05)77221-4. [DOI] [PubMed] [Google Scholar]
- 9.Dixon PH, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: Evidence for a defect in protein trafficking. Hum. Mol. Genet. 2000;9:1209–1217. doi: 10.1093/hmg/9.8.1209. [DOI] [PubMed] [Google Scholar]
- 10.Lucena J-F, et al. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology. 2003;124:1037–1042. doi: 10.1053/gast.2003.50144. [DOI] [PubMed] [Google Scholar]
- 11.Müllenbach R, et al. ABCB4 gene sequence variation in women with intrahepatic cholestasis of pregnancy. J. Med. Genet. 2003;40:e70–e70. doi: 10.1136/jmg.40.5.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauli-Magnus C, et al. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistance p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91–102. doi: 10.1097/00008571-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Floreani A, et al. Intrahepatic cholestasis of pregnancy: Three novel MDR3 gene mutations. APT. 2006;23:1649–1653. doi: 10.1111/j.1365-2036.2006.02869.x. [DOI] [PubMed] [Google Scholar]
- 14.Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstet. Gynecol. 2014;124:120–133. doi: 10.1097/AOG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 15.Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–474. doi: 10.1002/hep.20336. [DOI] [PubMed] [Google Scholar]
- 16.Geenes V, et al. Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: A prospective population-based case-control study. Hepatology. 2014;59:1482–1491. doi: 10.1002/hep.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estiú MC, et al. Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid. PLoS ONE. 2017;12:e0176504. doi: 10.1371/journal.pone.0176504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J. Gastroenterol. 2009;15:2049–2066. doi: 10.3748/wjg.15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alazawi W, et al. Ethnicity and the diagnosis gap in liver disease: A population-based study. BJGP. 2014;64:e694–e702. doi: 10.3399/bjgp14X682273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szanto KB, Li J, Cordero P, Oben JA. Ethnic differences and heterogeneity in genetic and metabolic makeup contributing to nonalcoholic fatty liver disease. Diabet. Metab. Synd. Ob. 2019;12:357–367. doi: 10.2147/DMSO.S182331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finer S, et al. Cohort profile: East London Genes & Health (ELGH), a community-based population genomics and health study in British Bangladeshi and British Pakistani people. Int. J. Epidemiol. 2019;49:20–21i. doi: 10.1093/ije/dyz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeap SP, et al. Biliary transporter gene mutations in severe intrahepatic cholestasis of pregnancy: Diagnostic and management implications. J. Gastroenterol. Hepatol. 2019;34:425–435. doi: 10.1111/jgh.14376. [DOI] [PubMed] [Google Scholar]
- 23.Delaunay J-L, et al. A functional classification of ABCB4 variations causing progressive familial intrahepatic cholestasis type 3. Hepatology. 2016;63:1620–1631. doi: 10.1002/hep.28300. [DOI] [PubMed] [Google Scholar]
- 24.Aydın GA, Özgen G, Görükmez O. The role of genetic mutations in intrahepatic cholestasis of pregnancy. Taiwan. J. Obstet. Gynecol. 2020;59:706–710. doi: 10.1016/j.tjog.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Poupon R, et al. Genotype-phenotype relationships in the low-phospholipid-associated cholelithiasis syndrome: A study of 156 consecutive patients. Hepatology. 2013;58:1105–1110. doi: 10.1002/hep.26424. [DOI] [PubMed] [Google Scholar]
- 26.Anzivino C, et al. ABCB4 and ABCB11 mutations in intrahepatic cholestasis of pregnancy in an Italian population. Dig. Liver Dis. 2013;45:226–232. doi: 10.1016/j.dld.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Condat B, et al. Prevalence of low phospholipid-associated cholelithiasis in young female patients. Dig. Liver Dis. 2013;45:915–919. doi: 10.1016/j.dld.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Stalke A, et al. Diagnosis of monogenic liver diseases in childhood by next-generation sequencing. Clin. Genet. 2018;93:665–670. doi: 10.1111/cge.13120. [DOI] [PubMed] [Google Scholar]
- 29.Gudbjartsson DF, et al. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 2015;47:435–444. doi: 10.1038/ng.3247. [DOI] [PubMed] [Google Scholar]
- 30.Degiorgio D, et al. ABCB4 mutations in adult patients with cholestatic liver disease: Impact and phenotypic expression. J. Gastroenterol. 2016;51:271–280. doi: 10.1007/s00535-015-1110-z. [DOI] [PubMed] [Google Scholar]
- 31.Jirsa M, et al. ABCB4 mutations underlie hormonal cholestasis but not pediatric idiopathic gallstones. World J. Gastroenterol. 2014;20:5867–5874. doi: 10.3748/wjg.v20.i19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bronský J, Jirsa M, Nevoral J, Hrebícek M. Role of common canalicular transporter gene variations in aetiology of idiopathic gallstones in childhood. Folia Biol. (Praha) 2010;56:9–13. [PubMed] [Google Scholar]
- 33.Schatz SB, et al. Phenotypic spectrum and diagnostic pitfalls of ABCB4 deficiency depending on age of onset. Hepatol. Commun. 2018;2:504–514. doi: 10.1002/hep4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordo-Gilart R, et al. Functional analysis of <em>ABCB4</em> mutations relates clinical outcomes of progressive familial intrahepatic cholestasis type 3 to the degree of MDR3 floppase activity. Gut. 2015;64:147–155. doi: 10.1136/gutjnl-2014-306896. [DOI] [PubMed] [Google Scholar]
- 35.Krawczyk M, et al. The ABCB4 p.T175A variant as potential modulator of hepatic fibrosis in patients with chronic liver diseases: Looking beyond the cholestatic realm. Hepatology. 2017;66:666–667. doi: 10.1002/hep.29100. [DOI] [PubMed] [Google Scholar]
- 36.Dixon PH, et al. An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci. Rep. 2017;7:11823. doi: 10.1038/s41598-017-11626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakken KE, et al. ABCB4 sequence variations in young adults with cholesterol gallstone disease. Liver Int. 2009;29:743–747. doi: 10.1111/j.1478-3231.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 38.Denk GU, et al. ABCB4 deficiency: A family saga of early onset cholelithiasis, sclerosing cholangitis and cirrhosis and a novel mutation in the ABCB4 gene. Hepatol. Res. 2010;40:937–941. doi: 10.1111/j.1872-034X.2010.00698.x. [DOI] [PubMed] [Google Scholar]
- 39.Wendum D, et al. Aspects of liver pathology in adult patients with MDR3/ABCB4 gene mutations. Virchows Arch. 2012;460:291–298. doi: 10.1007/s00428-012-1202-6. [DOI] [PubMed] [Google Scholar]
- 40.Tomaiuolo R, et al. An MBL2 haplotype and ABCB4 variants modulate the risk of liver disease in cystic fibrosis patients: A multicentre study. Dig. Liver Dis. 2009;41:817–822. doi: 10.1016/j.dld.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Degiorgio D, et al. Molecular characterization and structural implications of 25 new ABCB4 mutations in progressive familial intrahepatic cholestasis type 3 (PFIC3) Eur. J. Hum. Genet. 2007;15:1230–1238. doi: 10.1038/sj.ejhg.5201908. [DOI] [PubMed] [Google Scholar]
- 42.Ziol M, et al. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology. 2008;135:131–141. doi: 10.1053/j.gastro.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 43.Lang C, et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet. Genome. 2007;17:47–60. doi: 10.1097/01.fpc.0000230418.28091.76. [DOI] [PubMed] [Google Scholar]
- 44.Vitale G, et al. Cryptogenic cholestasis in young and adults: ATP8B1, ABCB11, ABCB4, and TJP2 gene variants analysis by high-throughput sequencing. J. Gastroenterol. 2018;53:945–958. doi: 10.1007/s00535-017-1423-1. [DOI] [PubMed] [Google Scholar]
- 45.Davit-Spraul A, et al. Liver transcript analysis reveals aberrant splicing due to silent and intronic variations in the ABCB11 gene. Mol. Genet. Metab. 2014;113:225–229. doi: 10.1016/j.ymgme.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Ho RH, et al. Polymorphic variants in the human bile salt export pump (BSEP; ABCB11): Functional characterization and interindividual variability. Pharmacogenet. Genome. 2010;20:45–57. doi: 10.1097/FPC.0b013e3283349eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meier Y, et al. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62–74. doi: 10.1002/hep.21214. [DOI] [PubMed] [Google Scholar]
- 48.Lang T, et al. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11) Drug Metab. Dispos. 2006;34:1582–1599. doi: 10.1124/dmd.105.008854. [DOI] [PubMed] [Google Scholar]
- 49.Davit-Spraul A, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): Phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–1655. doi: 10.1002/hep.23539. [DOI] [PubMed] [Google Scholar]
- 50.Pauli-Magnus C, et al. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779–791. doi: 10.1002/hep.20159. [DOI] [PubMed] [Google Scholar]
- 51.Kim SR, et al. Genetic variations of the ABC transporter gene ABCB11 encoding the human bile salt export pump (BSEP) in a Japanese population. Drug Metab. Pharmacokinet. 2009;24:277–281. doi: 10.2133/dmpk.24.277. [DOI] [PubMed] [Google Scholar]
- 52.Dröge C, et al. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J. Hepatol. 2017;67:1253–1264. doi: 10.1016/j.jhep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Mitra S, Das A, Thapa B, KumarVasishta R. Phenotype-genotype correlation of north indian progressive familial intrahepatic cholestasis type2 children shows p.Val444Ala and p.Asn591Ser variants and retained BSEP expression. Fetal Pediatr. Pathol. 2020;39:107–123. doi: 10.1080/15513815.2019.1641860. [DOI] [PubMed] [Google Scholar]
- 54.Lam P, et al. Levels of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am. J. Physiol. Cell Physiol. 2007;293:C1709–C1716. doi: 10.1152/ajpcell.00327.2007. [DOI] [PubMed] [Google Scholar]
- 55.Dixon PH, et al. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut. 2009;58:537–544. doi: 10.1136/gut.2008.159541. [DOI] [PubMed] [Google Scholar]
- 56.Byrne JA, et al. Missense mutations and single nucleotide polymorphisms in ABCB11 impair bile salt export pump processing and function or disrupt pre-messenger RNA splicing. Hepatology. 2009;49:553–567. doi: 10.1002/hep.22683. [DOI] [PubMed] [Google Scholar]
- 57.Chong CP, et al. Bile acid-CoA ligase deficiency–a new inborn error of bile acid metabolism. J. Inherit. Metab. Dis. 2012;35:521–530. doi: 10.1007/s10545-011-9416-3. [DOI] [PubMed] [Google Scholar]
- 58.Sharma A, et al. Spectrum of genomic variations in Indian patients with progressive familial intrahepatic cholestasis. BMC Gastroenterol. 2018;18:107. doi: 10.1186/s12876-018-0835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X-Q, Wang L-L, Shan Q-W, Tang Q, Lian S-J. Multidrug resistance protein 3 R652G may reduce susceptibility to idiopathic infant cholestasis. World J. Gastroenterol. 2009;15:5855–5858. doi: 10.3748/wjg.15.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varma S, et al. Retargeting of bile salt export pump and favorable outcome in children with progressive familial intrahepatic cholestasis type 2. Hepatology. 2015;62:198–206. doi: 10.1002/hep.27834. [DOI] [PubMed] [Google Scholar]
- 61.Roustit M, et al. CYP2C9, SLCO1B1, SLCO1B3, and ABCB11 polymorphisms in patients with bosentan-induced liver toxicity. Clin. Pharmacol. Ther. 2014;95:583–585. doi: 10.1038/clpt.2014.42. [DOI] [PubMed] [Google Scholar]
- 62.Liu T, et al. Changes in plasma bile acid profiles after partial internal biliary diversion in PFIC2 patients. Ann. Transl. Med. 2020;8:185. doi: 10.21037/atm.2020.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abuduxikuer K, et al. Novel methionyl-tRNA synthetase gene variants/phenotypes in interstitial lung and liver disease: A case report and review of literature. World J. Gastroenterol. 2018;24:4208–4216. doi: 10.3748/wjg.v24.i36.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Woerd WL, et al. Mutational analysis of ATP8B1 in patients with chronic pancreatitis. PLoS ONE. 2013;8:e80553. doi: 10.1371/journal.pone.0080553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hasegawa Y, et al. Intractable itch relieved by 4-phenylbutyrate therapy in patients with progressive familial intrahepatic cholestasis type 1. Orphanet J. Rare Dis. 2014;9:89. doi: 10.1186/1750-1172-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayashi H, et al. Assessment of ATP8B1 deficiency in pediatric patients with cholestasis using peripheral blood monocyte-derived macrophages. EBioMedicine. 2018;27:187–199. doi: 10.1016/j.ebiom.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagasaka H, et al. Effects of bezafibrate on dyslipidemia with cholestasis in children with familial intrahepatic cholestasis-1 deficiency manifesting progressive familial intrahepatic cholestasis. Metabolism. 2009;58:48–54. doi: 10.1016/j.metabol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Qiu Y-L, et al. Defects in myosin VB are associated with a spectrum of previously undiagnosed low γ-glutamyltransferase cholestasis. Hepatology. 2017;65:1655–1669. doi: 10.1002/hep.29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klomp LWJ, et al. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27–38. doi: 10.1002/hep.20285. [DOI] [PubMed] [Google Scholar]
- 70.Müllenbach R, et al. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut. 2005;54:829. doi: 10.1136/gut.2004.058115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Folmer DE, van der Mark VA, Ho-Mok KS, Oude Elferink RP, Paulusma CC. Differential effects of progressive familial intrahepatic cholestasis type 1 and benign recurrent intrahepatic cholestasis type 1 mutations on canalicular localization of ATP8B1. Hepatology. 2009;50:1597–1605. doi: 10.1002/hep.23158. [DOI] [PubMed] [Google Scholar]
- 72.Stolz A, et al. Severe and protracted cholestasis in 44 young men taking bodybuilding supplements: Assessment of genetic, clinical and chemical risk factors. APT. 2019;49:1195–1204. doi: 10.1111/apt.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takatsu H, et al. Phospholipid flippase activities and substrate specificities of human type IV P-type ATPases localized to the plasma membrane. J. Biol. Chem. 2014;289:33543–33556. doi: 10.1074/jbc.M114.593012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giovannoni I, et al. Genetics and molecular modeling of new mutations of familial intrahepatic cholestasis in a single Italian center. PLoS ONE. 2015;10:e0145021. doi: 10.1371/journal.pone.0145021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKay K, et al. Mutation detection in cholestatic patients using microarray resequencing of ATP8B1 and ABCB11 [version 2; peer review: 2 approved, 1 approved with reservations] F1000Research. 2013;2:32. doi: 10.12688/f1000research.2-32.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Mil SW, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Kim M-A, et al. Genetic analysis of genes related to tight junction function in the Korean population with non-syndromic hearing loss. PLoS ONE. 2014;9:e95646. doi: 10.1371/journal.pone.0095646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, et al. Evaluation of 12 myopia-associated genes in chinese patients with high myopia. Invest. Ophthalmol. Vis. Sci. 2015;56:722–729. doi: 10.1167/iovs.14-14880. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, et al. Whole-exome sequencing identifies novel mutations in ABC transporter genes associated with intrahepatic cholestasis of pregnancy disease: A case-control study. BMC Pregnancy Childbirth. 2021;21:110–110. doi: 10.1186/s12884-021-03595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avena A, et al. ABCB4 variants in adult patients with cholestatic disease are frequent and underdiagnosed. Dig. Liver Dis. 2021;53:329–344. doi: 10.1016/j.dld.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Wright CF, et al. Assessing the pathogenicity, penetrance, and expressivity of putative disease-causing variants in a population setting. Am. J. Hum. Genet. 2019;104:275–286. doi: 10.1016/j.ajhg.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ali I, Khalid S, Stieger B, Brouwer KLR. Effect of a common genetic variant (p.V444A) in the bile salt export pump on the inhibition of bile acid transport by cholestatic medications. Mol. Pharm. 2019;16:1406–1411. doi: 10.1021/acs.molpharmaceut.8b01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wikström Shemer E, Marschall HU, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated adverse pregnancy and fetal outcomes: A 12-year population-based cohort study. BJOG. 2013;120:717–723. doi: 10.1111/1471-0528.12174. [DOI] [PubMed] [Google Scholar]
- 84.Marschall H-U, Wikström Shemer E, Ludvigsson JF, Stephansson O. Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease: A population-based cohort study. Hepatology. 2013;58:1385–1391. doi: 10.1002/hep.26444. [DOI] [PubMed] [Google Scholar]
- 85.Narasimhan VM, et al. Health and population effects of rare gene knockouts in adult humans with related parents. Science. 2016;352:474–477. doi: 10.1126/science.aac8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sim N-L, et al. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ioannidis NM, et al. REVEL: An ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 2016;99:877–885. doi: 10.1016/j.ajhg.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Li C, Mou C, Dong Y, Tu Y. dbNSFP v4: A comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med. 2020;12:103. doi: 10.1186/s13073-020-00803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jagadeesh KA, et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat. Genet. 2016;48:1581–1586. doi: 10.1038/ng.3703. [DOI] [PubMed] [Google Scholar]
- 92.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ. RVTESTS: An efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics. 2016;32:1423–1426. doi: 10.1093/bioinformatics/btw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stephenson JD, Laskowski RA, Nightingale A, Hurles ME, Thornton JM. VarMap: A web tool for mapping genomic coordinates to protein sequence and structure and retrieving protein structural annotations. Bioinformatics. 2019;35:4854–4856. doi: 10.1093/bioinformatics/btz482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johns, S.J. TOPO2. Transmembrame protein display software.
- 96.Rodrigues CH, Pires DE, Ascher DB. DynaMut: Predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018;46:W350–W355. doi: 10.1093/nar/gky300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parthiban V, Gromiha MM, Schomburg D. CUPSAT: Prediction of protein stability upon point mutations. Nucleic Acids Res. 2006;34:W239–W242. doi: 10.1093/nar/gkl190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ancien F, Pucci F, Godfroid M, Rooman M. Prediction and interpretation of deleterious coding variants in terms of protein structural stability. Sci. Rep. 2018;8:4480. doi: 10.1038/s41598-018-22531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nosol K, et al. Structures of ABCB4 provide insight into phosphatidylcholine translocation. Proc. Natl. Acad. Sci. U. S. A. 2021 doi: 10.1073/pnas.2106702118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L, et al. Cryo-EM structure of human bile salts exporter ABCB11. Cell Res. 2020;30:623–625. doi: 10.1038/s41422-020-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He Y, Xu J, Wu X, Li L. Structures of a P4-ATPase lipid flippase in lipid bilayers. Protein Cell. 2020;11:458–463. doi: 10.1007/s13238-020-00712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Downes M, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol. Cell. 2003;11:1079–1092. doi: 10.1016/S1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The imputed genotype data from Genes & Heath are available on EGA (www.ega-archive. org; study accession number: EGAS00001005373). The individual-level phenotypic data will be made available to researchers by completing an application to Genes & Health, following their open access policy described on https://www.genesandhealth.org/research/scientists-using-genes-health-scientific-research.