Abstract
During respiratory glucose dissimilation, eukaryotes produce cytosolic NADH via glycolysis. This NADH has to be reoxidized outside the mitochondria, because the mitochondrial inner membrane is impermeable to NADH. In Saccharomyces cerevisiae, this may involve external NADH dehydrogenases (Nde1p or Nde2p) and/or a glycerol-3-phosphate shuttle consisting of soluble (Gpd1p or Gpd2p) and membrane-bound (Gut2p) glycerol-3-phosphate dehydrogenases. This study addresses the physiological relevance of these mechanisms and the possible involvement of alternative routes for mitochondrial oxidation of cytosolic NADH. Aerobic, glucose-limited chemostat cultures of a gut2Δ mutant exhibited fully respiratory growth at low specific growth rates. Alcoholic fermentation set in at the same specific growth rate as in wild-type cultures (0.3 h−1). Apparently, the glycerol-3-phosphate shuttle is not essential for respiratory glucose dissimilation. An nde1Δ nde2Δ mutant already produced glycerol at specific growth rates of 0.10 h−1 and above, indicating a requirement for external NADH dehydrogenase to sustain fully respiratory growth. An nde1Δ nde2Δ gut2Δ mutant produced even larger amounts of glycerol at specific growth rates ranging from 0.05 to 0.15 h−1. Apparently, even at a low glycolytic flux, alternative mechanisms could not fully replace the external NADH dehydrogenases and glycerol-3-phosphate shuttle. However, at low dilution rates, the nde1Δ nde2Δ gut2Δ mutant did not produce ethanol. Since glycerol production could not account for all glycolytic NADH, another NADH-oxidizing system has to be present. Two alternative mechanisms for reoxidizing cytosolic NADH are discussed: (i) cytosolic production of ethanol followed by its intramitochondrial oxidation and (ii) a redox shuttle linking cytosolic NADH oxidation to the internal NADH dehydrogenase.
As in other eukaryotes, respiratory dissimilation of sugars by Saccharomyces cerevisiae leads to the reduction of NAD+ to NADH in separate cellular compartments. Cytosolic NADH is produced by the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase, as well as in assimilatory reactions (1, 30). In the mitochondrial matrix, NADH is formed by the tricarboxylic acid cycle and the pyruvate-dehydrogenase complex. Under anaerobic conditions, glucose dissimilation occurs exclusively via alcoholic fermentation, which is a redox-neutral process. The additional NADH originating from biomass production can be reoxidized via glycerol production (30). Under aerobic conditions, glycerol production is not necessary, because the reoxidation of cytosolic NADH can be coupled to the mitochondrial respiratory chain.
Although the outer mitochondrial membrane is permeable to NADH (17), the inner membrane is not (35). Therefore, coupling of NADH reoxidation to the respiratory chain has to occur on both sides of the mitochondrial inner membrane. In plant mitochondria, cytosolic NADH can be oxidized either by an external NADH dehydrogenase or by a redox shuttle (14), whereas in the mitochondrial matrix, NADH can be oxidized by the proton-translocating complex I or by an alternative, nontranslocating, internal NADH dehydrogenase (9). Mammalian mitochondria lack an external NADH dehydrogenase and therefore rely on redox shuttle mechanisms to couple the oxidation of cytosolic NADH to complex I (5).
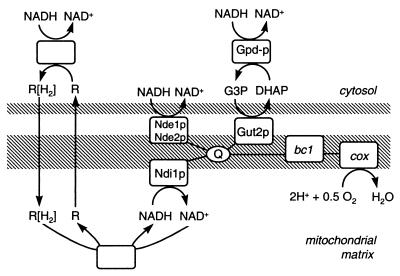
In S. cerevisiae, the NADH in the mitochondrial matrix can be oxidized by an NADH:ubiquinone oxidoreductase (35). This enzyme is located in the inner mitochondrial membrane, and its active site faces the mitochondrial matrix. In contrast to the classical complex I of higher eukaryotes, the S. cerevisiae internal NADH dehydrogenase (Ndi1p [Fig. 1]) does not translocate protons (7, 22). The enzyme is encoded by a single nuclear gene, NDI1 (8). Mitochondria isolated from ndi1Δ null mutants do not oxidize intramitochondrial NADH (20).
FIG. 1.
Overview of possible mechanisms of oxidation of cytosolic NADH by mitochondria of S. cerevisiae. Abbreviations: R[H2], reduced metabolite; R, oxidized metabolite; Gpd, cytosolic glycerol-3-phosphate dehydrogenase; Gut2, membrane-bound mitochondrial glycerol-3-phosphate dehydrogenase; Nde, external NADH dehydrogenase; Ndi, internal NADH dehydrogenase; Q, ubiquinon pool; bc1, cytochrome bc1 complex; cox, cytochrome c oxidase.
Several mechanisms have been proposed for reoxidation of cytosolic NADH by S. cerevisiae mitochondria (Fig. 1). First, it can be oxidized by the external NADH dehydrogenases Nde1p and Nde2p. These dehydrogenases are homologous to Ndi1p, but their active sites face the lumen between the membranes (19, 29). Similar to the internal NADH dehydrogenase, they directly couple the oxidation of NADH to the respiratory chain. Another mechanism for oxidation of cytosolic NADH is the glycerol-3-phosphate shuttle (Fig. 1), all of whose essential enzymes are present in S. cerevisiae (28). A recent study indicates that although the glycerol-3-phosphate shuttle is functional in this yeast, it is not essential for respiratory growth on ethanol (16). Other proposed shuttles include the ethanol-acetaldehyde shuttle (35), the malate-oxaloacetate shuttle (3), and the malate-aspartate shuttle (3). All these shuttles operate according to a similar principle (Fig. 1) but with different substrates. However, it is unknown whether these shuttles operate in vivo in S. cerevisiae.
As discussed above, the relative importance of the various proposed systems for respiratory oxidation of cytosolic NADH by S. cerevisiae mitochondria is unclear. Since these systems are coupling the oxidation of cytosolic NADH to the respiratory chain, their function must be studied under respiratory growth conditions. However, S. cerevisiae has a strong tendency toward alcoholic fermentation. Even under fully aerobic conditions, a mixed respirofermentative metabolism is observed when the sugar concentration in the growth medium exceeds a threshold value (typically ca. 1 mM [34]) or when the specific growth rate is high (usually higher than two-thirds of the maximal growth rate [25, 26]). Instead of studying the NADH-oxidizing systems in shake flask cultures, in which sugar metabolism is predominantly fermentative (15), it is essential to use aerobic, glucose-limited chemostat cultures for this purpose. In glucose-limited chemostat cultures the glucose concentration is very low and the specific growth rate, and hence the rate of glycolysis, can be controlled by varying the dilution rate (25). In this way, glucose catabolite repression of respiratory enzymes (11) and alcoholic fermentation can be avoided.
The aim of the present study is to assess the physiological significance of the external NADH dehydrogenases, the glycerol-3-phosphate shuttle, and possible other mechanisms in the oxidation of cytosolic NADH by S. cerevisiae mitochondria. To investigate the relative importance of the external NADH dehydrogenases and the glycerol-3-phosphate shuttle, the physiology of an nde1Δ nde2Δ and a gut2Δ mutant was studied while varying the specific growth rate of the culture and thereby the turnover of cytosolic NADH. Furthermore, to investigate whether other enzymes are involved in the respiration of cytosolic NADH, the physiology of an nde1Δ nde2Δ gut2Δ mutant was studied.
MATERIALS AND METHODS
Yeast strains and maintenance.
The S. cerevisiae strains used in this study (Table 1) were maintained by cultivating them in shake flasks on complex medium containing 1% (wt/vol) Bacto yeast extract, 2% (wt/vol) peptone, and 2% (wt/vol) glucose (YPD medium). When stationary phase was reached, 30% (vol/vol) sterile glycerol was added and 2-ml aliquots were stored in sterile vials at −80°C. These stock cultures were used subsequently as the inoculum for precultures from which all experiments were started.
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotypea |
|---|---|
| CEN.PK113-7D | MATaURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 |
| CEN.PK152 | MATaURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 nde1(41–1659)::loxP-kanMX4-loxP |
| CEN.PK163 | MATαURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 nde2(51–100)::loxP-kanMX4-loxP |
| CEN.PK167-2B | MATaURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 nde1(41–1659)::loxP-kanMX4-loxP nde2(51–100)::loxP-kanMX4-loxP |
| CEN.PK225-2C | MATaURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 gut2(41–2010)::loxP-kanMX4-loxP |
| CEN.PK225-1B | MATαURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 gut2(41–2010)::loxP-kanMX4-loxP |
| CEN.PK239-1C | MATaURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 nde1(41–1659)::loxP-kanMX4-loxP gut2(41–2010)::loxP-kanMX4-loxP |
| CEN.PK240-1C | MATαURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 nde2(51–100)::loxP-kanMX4-loxP gut2(41–2010)::loxP-kanMX4-loxP |
| CEN.PK263-5D | MATaURA3 HIS3 LEU2 TRP1 MAL2-8cSUC2 nde1(41–1659)::loxP-kanMX4-loxP nde2(51–100)::loxP-kanMX4-loxP gut2(41–2010)::loxP-kanMX4-loxP |
The numbers in parentheses indicate the deleted nucleotides (ATG = 1 to 3) of the corresponding genes.
Construction of null mutants.
Standard techniques and media for genetic manipulation of S. cerevisiae were used (2). Deletion in GUT2 (YIL155c) was obtained by the short flanking homology method (36) using plasmid pUG6 as the template (12). PCR primers were used for amplification of the loxP-kanMX-loxP cassette and for verification of the correct gene deletion (Table 2). PCR amplifications, yeast transformation, and verification of the correct gene deletion as well as determination of the mating type were carried out as described by Luttik et al. (19). To obtain a strain with GUT2, NDE1 (YMR145c) and NDE2 (YDL085w) deleted, strains were crossed as follows. Strain CEN.PK225-1B (gut2Δ) was crossed with CEN.PK152 (nde1Δ) and was also crossed with CEN.PK163 (nde2Δ), resulting in strains CEN.PK239-1C (nde1Δ gut2Δ) and CEN.PK240-1C (nde2Δ gut2Δ), respectively. These double-mutant strains were subsequently crossed again to construct strain CEN.PK263-5D (nde1Δ nde2Δ gut2Δ). In all cases, strains were analyzed by tetrad analysis and spores showing the nonparental ditype for the KanMX marker were subsequently verified by diagnostic PCR to confirm the correct deletion of the corresponding genes.
TABLE 2.
Oligonucleotides used for construction of disruption cassettes (S1/S2) and as primers for analytical PCR of disruptants (A1/K1) and (A4/K2)
| Open reading frame | Oligonucleotide | Sequencea |
|---|---|---|
| GUT2 | S1 | 5′-ATG TTT TCG GTA ACG AGA AGA AGA GCT GCC GGT GCA GCT GCA GCT GAA GCT TCG TAC GC-3′ |
| GUT2 | S2 | 5′-TTA GAC ACC AAA CGT CTT GAT GAA GTT CAC AGT TTT TTC AGC ATA GGC CAC TAG TGG ATC TG-3′ |
| GUT2 | A1 | 5′-CGA CGG CGA CGG CGA CG-3′ |
| GUT2 | A4 | 5′-GCC ATT ACA CGT CAC TGG TG-3′ |
| KanMX | K1 | 5′-GGA TGT ATG GGC TAA ATG TAC G-3′ |
| KanMX | K2 | 5′-GTT TCA TTT GAT GCT CGA TGA G-3′ |
Sequences complementary to the multiple-cloning site of pUG6 are underlined.
Isolation of mitochondria.
The procedure for the isolation of mitochondria, which involved enzymatic degradation of the cell wall, controlled lysis of the spheroplasts, and harvesting of mitochondria by differential centrifugation, has been described previously (19). The protein content of mitochondrial preparations was determined by the method of Lowry et al. (18) with bovine serum albumin (fatty acid free; Sigma) as a standard. Protein contents were corrected for the bovine serum albumin added to the buffer in which the mitochondria were resuspended.
Oxygen consumption by isolated mitochondria.
Specific rates of oxygen consumption were measured in a 4-ml thermostatted vessel (30°C) with a Clark-type oxygen electrode. Experiments were performed in a buffer (pH 7.0) consisting of 25 mM potassium phosphate, 5 mM MgCl2, and 0.65 M sorbitol. Substrates were added at a final concentration of 5 mM. Since commercially available NADH is contaminated with ethanol (24), pure NADH was generated in situ using Bacillus megaterium glucose dehydrogenase (19). Each substrate was tested at two different dilutions of the mitochondrial preparation. Oxygen consumption rates were calculated based on a dissolved-oxygen concentration of 236 μM in air-saturated water at 30°C. Respiratory-control values were determined by adding 0.25 mM ADP (4).
Chemostat cultivation.
S. cerevisiae wild-type and mutant strains were cultivated in a laboratory fermentor as described previously (19). This reference also describes the gas analysis and the determination of the culture dry weight. The culture medium used was a defined mineral medium, with vitamins prepared as described by Verduyn et al. (33), containing 7.5 g of glucose per liter as the carbon source. Dilution rate ranges were started a dilution rate of 0.05 h−1. After a steady state was reached, the dilution rate was increased in steps of 0.05 h−1 or, when close to the point where ethanol started to appear, in steps of 0.025 h−1. For each dilution rate, a steady state was reached before the dilution rate was increased further.
Metabolite measurements.
The glucose concentration in the medium was determined by enzymatic analysis with a hexokinase and glucose-6-phosphate dehydrogenase kit (Boehringer Mannheim). The concentrations of glycerol, ethanol, and acetate in culture supernatants were determined by high-pressure liquid chromatography and confirmed by enzymatic analysis (27). The protein content of whole cells was determined by a modified biuret method (32). The calculated carbon recoveries were between 95 and 103% for all cultures, with an assumed carbon content of biomass of 48%.
RESULTS
Oxygen consumption by isolated mitochondria.
To confirm the absence of the gene products (and any isoenzymes) in the deletion mutants, substrate-dependent oxygen consumption by isolated mitochondria was measured in wild-type S. cerevisiae and in isogenic gut2Δ, nde1Δ nde2Δ, and nde1Δ nde2Δ gut2Δ strains (Table 3). External NADH dehydrogenase activity was completely abolished in the nde1Δ nde2Δ, and nde1Δ nde2Δ gut2Δ mutants. An earlier report (29) that mitochondria of an nde1Δ nde2Δ mutant exhibited residual oxidation of NADH can be explained by the use of commercial NADH (19). This is contaminated with ethanol (24), which is readily oxidized by S. cerevisiae mitochondria. Glycerol-3-phosphate dehydrogenase activity was completely abolished in gut2Δ mutants.
TABLE 3.
Specific rates of substrate-dependent oxygen consumption by isolated mitochondriaa
| Substrate | Wild typeb
|
nde1Δ nde2Δb
|
gut2Δ
|
nde1Δ nde2Δ gut2Δ
|
||||
|---|---|---|---|---|---|---|---|---|
| Respiratory rate (μmol of O2/min/mg of protein) | RC | Respiratory rate (μmol of O2/min/mg of protein) | RC | Respiratory rate (μmol of O2/min/mg of protein) | RC | Respiratory rate (μmol of O2/min/mg of protein) | RC | |
| NADH | 0.22 ± 0.06 | 3.0 ± 0.3 | <0.01 | 0.27 ± 0.03 | 2.9 ± 0.1 | <0.01 | ||
| Ethanol | 0.10 ± 0.02 | 1.4 ± 0.1 | 0.15 ± 0.04 | 1.6 ± 0.1 | 0.12 ± 0.01 | 1.3 ± 0.1 | 0.09 ± 0.2 | 1.4 ± 0.2 |
| Glycerol-3-phosphate | 0.18 ± 0.04 | 2.3 ± 0.1 | 0.26 ± 0.04 | 2.6 ± 0.1 | <0.01 | <0.01 | ||
| Succinate | 0.10 ± 0.02 | 1.6 ± 0.4 | 0.17 ± 0.05 | 3.0 ± 0.2 | 0.14 ± 0.01 | 2.1 ± 0.3 | 0.11 ± 0.01 | 3.9 ± 0.9 |
| Malate + pyruvate | 0.11 ± 0.01 | 1.7 ± 0.1 | 0.15 ± 0.03 | 2.0 ± 0.1 | 0.15 ± 0.02 | 1.7 ± 0.1 | 0.12 ± 0.01 | 1.8 ± 0.3 |
Mitochondria were isolated from S. cerevisiae strains cultivated in aerobic, glucose-limited chemostat cultures at a dilution rate of 0.10 h−1. The rates presented in the table were measured in the presence of 0.25 mM ADP; the RC value represents the rate in the presence of ADP divided by the rate in the absence of ADP. All values are averages of at least two independent experiments (with independent chemostat cultures) ± standard deviation (ςn−1).
Data for the wild-type and nde1Δ nde2Δ strains are from reference 19.
To investigate whether deletion of the NDE and/or GUT genes affected other mitochondrial dehydrogenase systems, oxidation of some other substrates was tested (Table 3). Metabolism of a mixture of malate and pyruvate or of ethanol generates intramitochondrial NADH, which can be oxidized by the internal NADH dehydrogenase. Succinate is oxidized by an internal flavin adenine dinucleotide-dependent succinate dehydrogenase. These substrates were oxidized by mitochondria from wild-type as well as mutant strains. Whether the observed small quantitative differences (Table 3) are due to biological differences or to variations in the quality of the mitochondrial preparations is not clear.
Physiology of wild-type S. cerevisiae in aerobic, glucose-limited chemostat cultures.
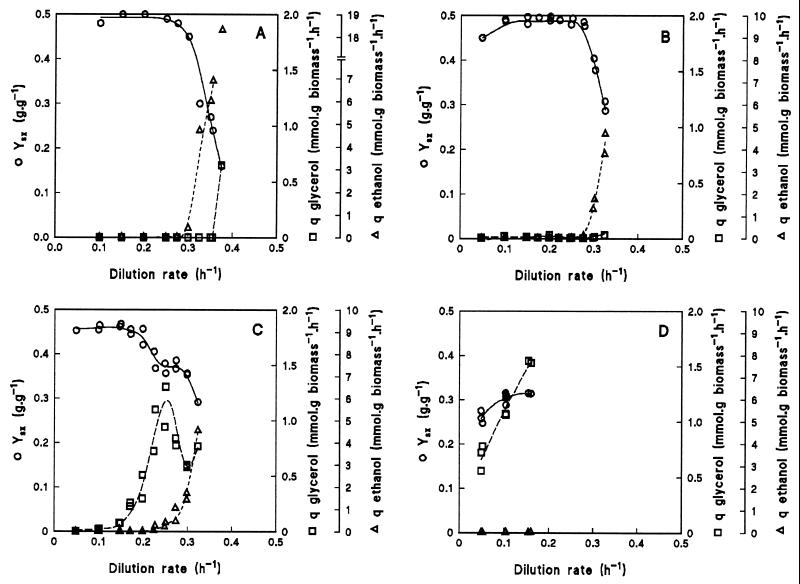
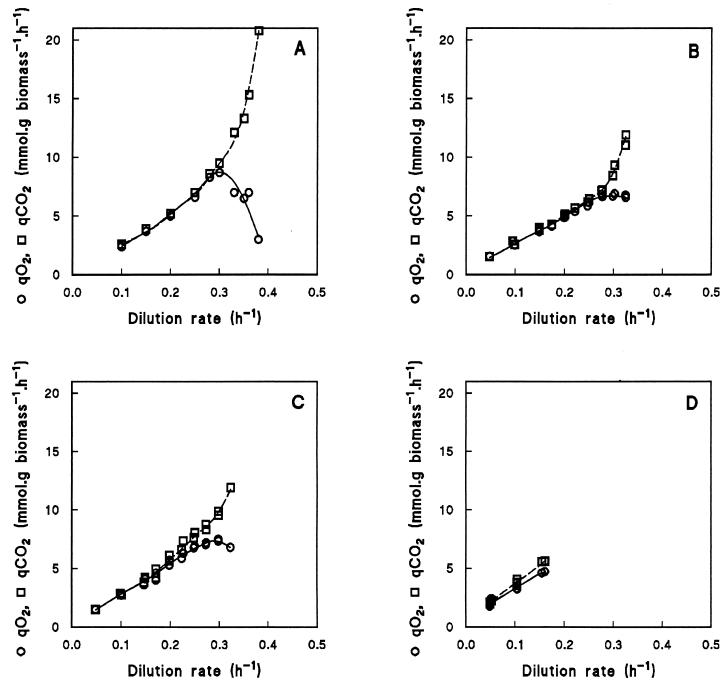
The physiological consequences of the different deletions were studied by growing wild-type and mutant S. cerevisiae strains in aerobic, glucose-limited chemostats at dilution rates varying from 0.05 to 0.38 h−1 (Fig. 2). If a chemostat culture is at steady state, the dilution rate is by definition equal to the specific growth rate. At specific growth rates below 0.30 h−1, the wild-type strain CEN.PK113-7D (Fig. 2A) had a high biomass yield (0.5 g of biomass g of glucose−1) and produced neither ethanol nor glycerol. Completely respiratory growth was also evident from the respiratory quotient (the specific CO2 production rate, qCO2, divided by the specific O2 consumption rate, qO2), which was 1.0 at specific growth rates below 0.30 h−1 (Fig. 3A). Above a dilution rate of 0.30 h−1, ethanol was produced and the respiratory quotient increased to values above 1.0 as the qCO2 increased and the qO2 decreased (Fig. 2A and 3A). Concomitantly, the biomass yield decreased sharply. Glycerol production was observed only at the highest dilution rate applied. The highest dilution rate at which wild-type cultures reached a steady state was 0.38 h−1. Above this dilution rate, the wild-type strain washed out of the chemostat.
FIG. 2.
Effects of dilution rate in an aerobic, glucose-limited chemostat culture on biomass yield (YXS) and specific production rate of ethanol and glycerol (respectively qethanol and qglycerol) for wild-type S. cerevisiae CEN.PK113-7D (data taken from reference 31) (A), gut2Δ mutant CEN.PK225-2C (B), nde1Δ nde2Δ mutant CEN.PK167-2B (C), and nde1Δ nde2Δ gut2Δ mutant CEN.PK263-5D (D). Apparently filled-in symbols are a result of overlapping data points from independent chemostat cultures.
FIG. 3.
Effects of dilution rate in an aerobic, glucose-limited chemostat culture on the specific production rate of CO2 (qCO2) and the specific consumption rate of O2 (qO2) for wild-type S. cerevisiae CEN.PK113-7D (data taken from reference 31) (A), gut2Δ mutant CEN.PK225-2C (B), nde1Δ nde2Δ mutant CEN.PK167-2B (C), and nde1Δ nde2Δ gut2Δ mutant CEN.PK263-5D (D). Apparently filled-in symbols are a result of overlapping data points from independent chemostat cultures.
Physiology of a gut2Δ mutant.
In S. cerevisiae the glycerol-3-phosphate shuttle can, in principle, couple the oxidation of cytosolic NADH to the respiratory chain. To test whether its contribution is essential, the GUT2 gene, encoding the mitochondrial membrane-bound glycerol-3-phosphate dehydrogenase, was deleted. When the gut2Δ mutant was cultivated over a range of dilution rates, the measured metabolite production rates were essentially the same as those of the wild type (Fig. 2B and 3B). Aerobic fermentation set in at the same dilution rate as in the wild type (0.30 h−1), but washout of biomass occurred at a lower dilution rate (0.33 versus 0.38 h−1). Apparently, elimination of the mitochondrial membrane-bound glycerol-3-phosphate dehydrogenase hardly affected the respiratory capacity during aerobic glucose-limited growth. Thus, even if the glycerol-3-phosphate shuttle were active in a wild-type strain, in the mutant its contribution to the oxidation of cytosolic NADH could be taken over completely by other NADH-oxidizing systems.
Physiology of an nde1Δ nde2Δ mutant.
Cytosolic NADH can also be oxidized by the external NDE1- and NDE2-encoded NADH dehydrogenases. In contrast to the gut2Δ mutant, the physiology of an nde1Δ nde2Δ mutant in aerobic, glucose-limited chemostat cultures differed substantially from that of the wild-type strain (Fig. 2C and 3C). The nde1Δ nde2Δ mutant produced glycerol at all specific growth rates tested, albeit in small quantities at low dilution rates, whereas the wild-type strain produced glycerol only at a dilution rate of 0.38 h−1. No ethanol production was observed up to a dilution rate of 0.23 h−1. Above this dilution rate, ethanol was produced and, concomitantly, the specific glycerol production rate decreased, suggesting a decreased need for a cytosolic redox sink under these conditions. At the highest applied dilution rate, the glycerol production increased again. The respiratory quotient was 1.0 at low dilution rates but increased as production of glycerol and ethanol became apparent (Fig. 3C). Washout of biomass occurred at dilution rates above 0.33 h−1. At low dilution rates, the biomass yield was almost as high as that of the wild type, but it decreased when glycerol and ethanol were being produced. Apparently, other NADH-oxidizing systems, such as the glycerol-3-phosphate shuttle, could not completely replace the external NADH dehydrogenases but had a sufficiently high capacity to prevent alcoholic fermentation at low dilution rates.
Physiology of an nde1Δ nde2Δ gut2Δ mutant.
To investigate whether, in addition to the glycerol-3-phosphate shuttle and the external NADH dehydrogenases, other enzymes contribute to the respiration of cytosolic NADH, an nde1Δ nde2Δ gut2Δ mutant was grown in aerobic, glucose-limited chemostat cultures. At dilution rates between 0.05 and 0.15 h−1 (Fig. 2D and 3D), this mutant produced no ethanol but its specific glycerol production rate was high. The biomass yield of the nde1Δ nde2Δ gut2Δ mutant (0.3 g g−1) was significantly lower than that of the wild type (0.5 g g−1).
At dilution rates above 0.15 h−1, no reproducible steady-state data were obtained: it took a long time (sometimes more than 50 volume changes) before cultures of the nde1Δ nde2Δ gut2Δ mutant became steady. Moreover, in multiple experiments at the same dilution rate, different apparent steady states were obtained with different biomass yields and different specific production rates of ethanol and glycerol. However, the calculated carbon recoveries were always within 95 to 102%. Qualitatively, ethanol was produced at dilution rates of 0.20 h−1 and higher and the specific rate of glycerol production decreased. If the specific ethanol production rate was higher, the specific glycerol production rate was lower. This inverse relationship was also observed with the nde1Δ nde2Δ mutant (Fig. 2C).
As was evident from the glycerol production, the simultaneous deletion of the NDE and GUT genes resulted in a situation where reoxidation of cytosolic NADH could no longer occur exclusively via respiration. At low dilution rates, however, the nde1Δ nde2Δ gut2Δ mutant could still grow without alcoholic fermentation. The fact that the observed glycerol production is not large enough to oxidize all cytosolic NADH (see below) indicates that in addition to the external NADH dehydrogenases and the glycerol-3-phosphate shuttle, alternative mechanisms must exist that can couple the reoxidation of cytosolic NADH to the mitochondrial respiratory chain.
DISCUSSION
Physiological significance of Nde1/2p and Gut2p.
The production of glycerol by aerobic, glucose-limited chemostat cultures of the nde1Δ nde2Δ mutant (Fig. 2C) is indicative of redox stress. This observation shows that external NADH dehydrogenase is involved in respiratory glucose dissimilation by wild-type S. cerevisiae. A gut2Δ mutant did not exhibit such a phenotype (Fig. 2B). Apparently, either this system is less important in wild-type cells or its role can be taken over by the external NADH dehydrogenases and/or alternative systems. The observation that respiratory growth on glucose was more severely impaired in an nde1Δ nde2Δ gut2Δ mutant than in an nde1Δ nde2Δ mutant confirms the earlier conclusion that the glycerol-3-phosphate shuttle can operate in S. cerevisiae (16).
Surprisingly, even at low specific growth rates, aerobic glucose-limited chemostat cultures of the nde1Δ nde2Δ gut2Δ mutant produced substantial amounts of glycerol. At a dilution rate of 0.10 h−1, the glycerol yield on glucose was 0.63 mol mol−1. This is higher than the glycerol yields reached with the classical sulfite process (ca. 0.5 mol mol−1 [10]), in which NADH reoxidation via alcoholic fermentation is prevented by trapping acetaldehyde with sulfite. At low dilution rates, glycerol production by the nde1Δ nde2Δ gut2Δ strain was not accompanied by alcoholic fermentation. This was unexpected, since earlier studies on wild-type S. cerevisiae (32, 38) indicated that as the oxygen feed to glucose-limited cultures is reduced, alcoholic fermentation replaces respiration as the preferred mode of glycolytic NADH reoxidation (38). In such cultures, glycerol formation occurred only when the oxygen feed became too low to reoxidize the NADH that was formed in biosynthetic reactions. Indeed, the specific rate of glycerol production in anaerobic glucose-limited cultures of wild-type S. cerevisiae matches the theoretical rate required for reoxidation of “biosynthetic” NADH, while oxidation of glycolytic NADH can be accounted for by ethanol production (1, 30, 32).
Alternative mechanisms for oxidation of cytosolic NADH.
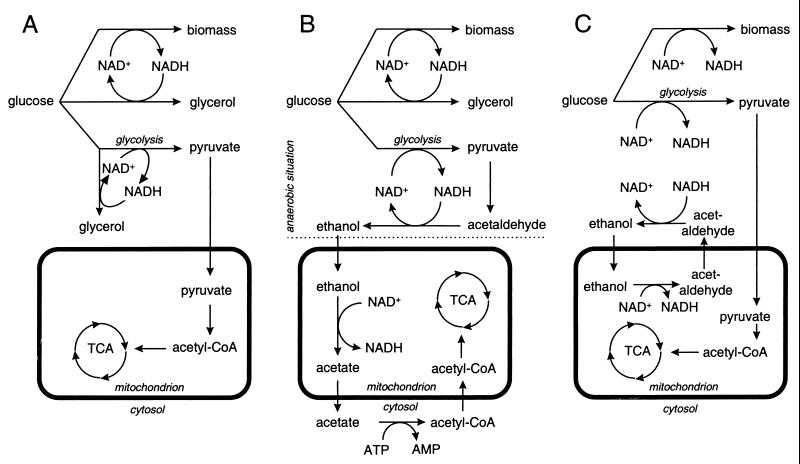
One explanation for the lack of ethanol production by the nde1Δ nde2Δ gut2Δ mutant at low dilution rates is cytosolic conversion of glucose to equimolar amounts of pyruvate and glycerol (Fig. 4A). This results in a closed cytosolic redox balance, because each molecule of NADH formed in the production of pyruvate is reoxidized in the production of glycerol from glucose. Pyruvate can be oxidized further in the mitochondria, leading to the production of 1 mol of glycerol and 3 mol of CO2 for each dissimilated mole of glucose. At a dilution rate of 0.1 h−1, the specific production rate of glycerol in the nde1Δ nde2Δ gut2Δ mutant was 1.2 mmol of glycerol g of biomass−1 h−1. The amount of glycerol formed according to the scheme depicted in Fig. 4A should correspond to the sum of the NADH formed during biomass synthesis and the pyruvate produced in glycolysis, and it can be calculated as follows. Assuming a constant biomass composition, the rate of glycerol production as a result of biomass formation in the mutant equals the value observed in anaerobic wild-type cultures: ca. 0.9 mmol of glycerol g of biomass−1 h−1 (32, 37). The amount of pyruvate formed during glucose catabolism in the mutant can be approximated from the rate of CO2 production, since mitochondrial oxidation of 1 mol of pyruvate yields 3 mol of CO2. The rate of CO2 production by the nde1Δ nde2Δ gut2Δ mutant at a dilution rate of 0.1 h−1 equaled 3.9 mmol g−1 h−1 (Fig. 3D), corresponding to approximately 1.3 mmol of pyruvate g−1 h−1. Thus, the total rate of glycerol production expected according to Fig. 4A would amount to 0.9 + 1.3 = 2.2 mmol g−1 h−1. Since the observed value was only 1.2 mmol g−1 h−1, glycerol production cannot be solely responsible for the oxidation of cytosolic NADH in the nde1Δ nde2Δ gut2Δ mutant. This signifies that in addition to the external NADH dehydrogenases and the glycerol-3-phosphate shuttle, at least one other mechanism must exist which can oxidize cytosolic NADH.
FIG. 4.
Schematic representation of proposed metabolic pathways for the oxidation of cytosolic NADH in an S. cerevisiae nde1Δ nde2Δ gut2Δ mutant, grown in an aerobic, glucose-limited chemostat culture at low dilution rates. (A) Conversion of dissimilatory glucose into equimolar amounts of pyruvate and glycerol; (B) consumption of ethanol produced in cytosol by mitochondria; (C) ethanol-acetaldehyde shuttle.
An alternative explanation for the phenotype of the nde1Δ nde2Δ gut2Δ mutant at low specific growth rates is alcoholic fermentation in the cytosol followed by respiratory ethanol dissimilation via intramitochondrial dehydrogenases (Fig. 4B). For complete oxidation, the intermediate acetate first has to be exported to the cytosol, where it can be converted to acetyl coenzyme A (acetyl-CoA) by cytosolic acetyl-CoA synthase (6). Subsequently, acetyl-CoA should be transported back into the mitochondrion for its further oxidation via the tricarboxylic acid cycle (Fig. 4B). This mechanism, by which reducing equivalents from glycolytic NADH are transported into the mitochondrion via ethanol, cannot account for oxidation of the cytosolic NADH from biomass production, because cytosolic conversion of glucose into ethanol is redox neutral. Thus, the need to reoxidize the NADH arising from biomass synthesis might explain the glycerol production by the mutant. Consistent with this model, the glycerol production rate by the mutant at a dilution rate of 0.10 h−1 (1.2 mmol of glycerol g−1 h−1) corresponded well to that of anaerobic glucose-limited chemostat cultures of wild-type S. cerevisiae (0.9 mmol g−1 h−1) (32, 37). Although Fig. 4B adequately describes the phenotype of the mutant at low specific growth rates, it cannot explain the reduced glycerol yields found at higher specific growth rates.
A third explanation for the lack of ethanol production by the nde1Δ nde2Δ gut2Δ mutant at low dilution rates is the presence of a redox shuttle. An interesting option is the involvement of an ethanol-acetaldehyde shuttle (Fig. 4C) as originally proposed by von Jagow and Klingenberg (35). The enzymes required for this shuttle mechanism are the same as in the mechanism proposed above (Fig. 4B), but in a shuttle mechanism, catalytic amounts of ethanol and acetaldehyde suffice to transfer all cytosolic redox equivalents into the mitochondria. Consequently, operation of this shuttle also allows oxidation of the NADH from biomass production. When involvement of a redox shuttle is assumed, the observed glycerol production might be explained from an insufficient capacity of the shuttle to oxidize all cytosolic NADH.
Significance of mitochondrial redox shuttles.
In vivo, mitochondrial ethanol consumption (Fig. 4B) and the ethanol-acetaldehyde shuttle (Fig. 4C) may occur simultaneously: in the latter mechanism, acetaldehyde returns to the cytosol, while in the former, it is oxidized to acetate inside the mitochondria. The contribution of the two mechanisms may depend on activities of key enzymes (in particular mitochondrial acetaldehyde dehydrogenase, which withdraws acetaldehyde from the shuttle cycle), as well as on the intracellular concentrations of acetaldehyde and ethanol. It is conceivable that at low dilution rates (≤0.15 h−1), reoxidation of glycolytic NADH in the nde1Δ nde2Δ gut2Δ mutant involves ethanol consumption by the mitochondria, whereas at dilution rates of ≥0.20 h−1, where ethanol appears outside the cells, the yeast gradually shifts to the ethanol-acetaldehyde shuttle. The reduced glycerol production by the nde1Δ nde2Δ gut2Δ mutant at dilution rates above 0.15 h−1, which coincided with an increased rate of ethanol production (data not shown), is consistent with a need for the presence of ethanol to obtain a functional shuttle. The nde1Δ nde2Δ mutant displayed similar behavior at dilution rates above 0.25 h−1 (Fig. 2C).
A role of ethanol and acetaldehyde as redox carriers reconciles the observed phenotype of the nde1Δ nde2Δ gut2Δ strain with the previous observation that alcoholic fermentation is the preferred mode of glycolytic NADH reoxidation by S. cerevisiae under conditions where respiration is compromised (38). However, involvement of alternative redox shuttle mechanisms (Fig. 1) cannot be entirely excluded. S. cerevisiae has long been assumed to lack a malate-aspartate shuttle, because mitochondrial aspartate aminotransferase was thought to be absent (7, 13). Recently, a gene homologous to other aspartate aminotransferase genes and with a mitochondrial targeting sequence was found in S. cerevisiae (21), indicating that a malate-aspartate shuttle may exist in this yeast. A recent study on mutants lacking the cytosolic (Mdh2p) or the mitochondrial (Mdh1p) malate dehydrogenases (29) does not provide conclusive evidence for in vivo activity of the malate-aspartate shuttle, since both malate dehydrogenases have other functions in the cell. Moreover, in the ethanol-grown cultures of the nde1Δ mdh1Δ and the nde1Δ mdh2Δ mutant studied in these experiments (29), the glycerol-3-phosphate shuttle is likely to contribute to the oxidation of cytosolic NADH (16). Another possible shuttle is the malate-oxaloacetate shuttle (3, 39). Recently, a mitochondrial oxaloacetate transporter has been found in S. cerevisiae (23). Whether this transporter works in vivo in the direction necessary for the shuttle remains to be elucidated.
The proposed involvement of mitochondrial alcohol dehydrogenase, either via an ethanol-acetaldehyde shuttle (Fig. 4C) or via ethanol-consuming mitochondria (Fig. 4B), can be tested by deleting the structural gene(s) encoding mitochondrial alcohol dehydrogenase in the nde1Δ nde2Δ gut2Δ mutant. In such a strain, neither of the two mechanisms should be possible. While exploring this line of research, we obtained evidence that ADH3 is not the sole S. cerevisiae gene encoding mitochondrial alcohol dehydrogenase (B. M. Bakker et al., unpublished results). Experimental verification of an ethanol-acetaldehyde shuttle will therefore have to await the identification of the additional structural gene(s) encoding mitochondrial alcohol dehydrogenases.
Our study illustrates that quantitative physiological analysis of defined mutants in chemostat cultures is a useful approach for studies on the compartmentation of redox metabolism in S. cerevisiae. S. cerevisiae is very easily amenable to genetic modification and isolation of functional mitochondria is relatively straightforward. Therefore, the nde1Δ nde2Δ gut2Δ mutant described in this study offers an attractive system for in vivo and in vitro functional analysis of structural genes encoding corresponding redox enzymes from other organisms.
ACKNOWLEDGMENTS
This work was financially supported by the Delft University DIOC-6 program “Mastering the Molecules of Manufacturing” and by the Dutch Ministry of Economic Affairs (EET program).
We are grateful to Bird Engineering BV, Schiedam, The Netherlands, for a gift of alcohol oxidase used in colorimetric alcohol assays.
REFERENCES
- 1.Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol. 1996;62:3187–3195. doi: 10.1128/aem.62.9.3187-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 3.Borst P. Hydrogen transport and transport metabolites. In: Karson P, editor. Funktionelle und morphologischen Organisation der Zelle. Heidelberg, Germany: Springer-Verlag KG; 1963. pp. 137–162. [Google Scholar]
- 4.Chance B, Williams G R. The respiratory chain and oxidative phosphorylation. Adv Enzymol. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 5.Dawson A G. Oxidation of cytosolic NADH formed during aerobic metabolism in mammalian cells. Trends Biochem Sci. 1979;4:171–176. [Google Scholar]
- 6.de Jong-Gubbels P. Metabolic fluxes at the interface of glycolysis and TCA cycle in Saccharomyces cerevisiae. Ph.D. thesis. Delft, The Netherlands: Delft University of Technology; 1998. [Google Scholar]
- 7.de Vries S, Marres C A. The mitochondrial respiratory chain of yeast. Structure and biosynthesis and the role in cellular metabolism. Biochim Biophys Acta. 1987;895:205–239. doi: 10.1016/s0304-4173(87)80003-4. [DOI] [PubMed] [Google Scholar]
- 8.de Vries S, van Witzenburg R, Grivell L A, Marres C A. Primary structure and import pathway of the rotenone-insensitive NADH-ubiquinone oxidoreductase of mitochondria from Saccharomyces cerevisiae. Eur J Biochem. 1992;203:587–592. doi: 10.1111/j.1432-1033.1992.tb16587.x. [DOI] [PubMed] [Google Scholar]
- 9.Douce R, Neuburger M. The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:371–414. [Google Scholar]
- 10.Freeman G G, Donald G M S. Fermentation processes leading to glycerol. I. The influence of certain variables on glycerol formation in the presence of sulfites. Appl Microbiol. 1957;5:197–210. doi: 10.1128/am.5.4.197-210.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gancedo J M. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Güldener U, Heck S, Fiedler T, Beinhauer J, Hegemann J H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollenberg C P, Riks W F, Borst P. The glutamate dehydrogenases of yeast: extra-mitochondrial enzymes. Biochim Biophys Acta. 1970;201:13–19. doi: 10.1016/0304-4165(70)90004-8. [DOI] [PubMed] [Google Scholar]
- 14.Krömer S, Heldt H W. Respiration of pea leaf mitochondria and redox transfer between the mitochondrial and extramitochondrial compartment. Biochim Biophys Acta. 1991;1057:42–50. [Google Scholar]
- 15.Lagunas R. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast. 1986;2:221–228. doi: 10.1002/yea.320020403. [DOI] [PubMed] [Google Scholar]
- 16.Larsson C, Påhlman I L, Ansell R, Rigoulet M, Adler L, Gustafsson L. The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast. 1998;14:347–357. doi: 10.1002/(SICI)1097-0061(19980315)14:4<347::AID-YEA226>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee A C, Xu X, Blachly-Dyson E, Forte M, Colombini M. The role of yeast VDAC genes on the permeability of the mitochondrial outer membrane. J Membr Biol. 1998;161:173–181. doi: 10.1007/s002329900324. [DOI] [PubMed] [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Luttik M A H, Overkamp K M, Kötter P, de Vries S, van Dijken J P, Pronk J T. The Saccharomyces cerevisiae NDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J Biol Chem. 1998;273:24529–24534. doi: 10.1074/jbc.273.38.24529. [DOI] [PubMed] [Google Scholar]
- 20.Marres C A, de Vries S, Grivell L A. Isolation and inactivation of the nuclear gene encoding the rotenone-insensitive internal NADH:ubiquinone oxidoreductase of mitochondria from Saccharomyces cerevisiae. Eur J Biochem. 1991;195:857–862. doi: 10.1111/j.1432-1033.1991.tb15775.x. [DOI] [PubMed] [Google Scholar]
- 21.Morin P J, Subramanian G S, Gilmore T D. AAT1, a gene encoding a mitochondrial aspartate aminotransferase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1992;1171:211–214. doi: 10.1016/0167-4781(92)90124-i. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973;301:105–128. [PubMed] [Google Scholar]
- 23.Palmieri L, Vozza A, Agrimi G, de Marco V, Runswick M J, Palmieri F, Walker J E. Identification of the yeast mitochondrial transporter for oxaloacetate and sulfate. J Biol Chem. 1999;274:22184–22190. doi: 10.1074/jbc.274.32.22184. [DOI] [PubMed] [Google Scholar]
- 24.Patchett R A, Jones C W. The apparent oxidation of NADH by whole cells of the methylotrophic bacterium Methylophilus methylothropus: a cautionary tale. Antonie Leeuwenhoek. 1986;52:387–392. doi: 10.1007/BF00393466. [DOI] [PubMed] [Google Scholar]
- 25.Petrik M, Käppeli O, Fiechter A. An expanded concept for the glucose effect in the yeast Saccharomyces cerevisiae: involvement of short- and long-term regulation. J Gen Microbiol. 1983;129:43–49. [Google Scholar]
- 26.Postma E, Verduyn C, Scheffers W A, van Dijken J P. Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pronk J T, Wenzel T J, Luttik M A H, Klaassen C C, Scheffers W A, Steensma H Y, van Dijken J P. Energetic aspects of glucose metabolism in a pyruvate-dehydrogenase-negative mutant of Saccharomyces cerevisiae. Microbiology. 1994;140:601–610. doi: 10.1099/00221287-140-3-601. [DOI] [PubMed] [Google Scholar]
- 28.Rønnow B, Kielland-Brandt M C. Yeast sequencing reports. IX. GUT2, a gene for mitochondrial glycerol 3-phosphate dehydrogenase of Saccharomyces cerevisiae. Yeast. 1993;9:1121–1130. doi: 10.1002/yea.320091013. [DOI] [PubMed] [Google Scholar]
- 29.Small W C, McAlister-Henn L. Identification of a cytosolically directed NADH dehydrogenase in mitochondria of Saccharomyces cerevisiae. J Bacteriol. 1998;180:4051–4055. doi: 10.1128/jb.180.16.4051-4055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Dijken J P, Scheffers W A. Redox balances in the metabolism of sugars by yeast. FEMS Microbiol Rev. 1986;32:199–224. [Google Scholar]
- 31.van Hoek P, Flikweert M T, van der Aart Q J M, Steensma H Y, van Dijken J P, Pronk J T. Effects of pyruvate decarboxylase overproduction on flux distribution at the pyruvate branch point in Saccharomyces cerevisiae. Appl Environ Microbiol. 1998;64:2133–2140. doi: 10.1128/aem.64.6.2133-2140.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verduyn C, Postma E, Scheffers W A, van Dijken J P. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol. 1990;136:395–403. doi: 10.1099/00221287-136-3-395. [DOI] [PubMed] [Google Scholar]
- 33.Verduyn C, Postma E, Scheffers W A, van Dijken J P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 34.Verduyn C, Zomerdijk T P L, van Dijken J P, Scheffers W A. Continuous measurement of ethanol production by aerobic yeast suspensions with an enzyme electrode. Appl Microbiol Biotechnol. 1984;19:181–185. [Google Scholar]
- 35.von Jagow G, Klingenberg M. Pathways of hydrogen in mitochondria of Saccharomyces carlsbergensis. Eur J Biochem. 1970;12:583–592. doi: 10.1111/j.1432-1033.1970.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 36.Wach A, Brachat A, Poehlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 37.Weusthuis R A, Adams H, Scheffers W A, van Dijken J P. Energetics and kinetics of maltose transport in Saccharomyces cerevisiae—a continuous-culture study. Appl Environ Microbiol. 1993;59:3102–3109. doi: 10.1128/aem.59.9.3102-3109.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weusthuis R A, Visser W, Pronk J T, Scheffers W A, van Dijken J P. Effects of oxygen limitation on kinetics of sugar metabolism in yeasts. Microbiology. 1994;140:703–715. doi: 10.1099/00221287-140-4-703. [DOI] [PubMed] [Google Scholar]
- 39.Wills C, Benhaim P, Martin T. Effect of mutants and inhibitors on mitochondrial transport systems in vivo in yeast. Biochim Biophys Acta. 1984;778:57–66. doi: 10.1016/0005-2736(84)90447-4. [DOI] [PubMed] [Google Scholar]