Abstract
Background
Current diagnostic criteria for hypoxic–ischemic encephalopathy in the early hours lack objective measurement tools. Therefore, this systematic review aims to identify putative molecules that can be used in diagnosis in daily clinical practice (PROSPERO ID: CRD42021272610).
Data sources
Searches were performed in PubMed, Web of Science, and Science Direct databases until November 2020. English original papers analyzing samples from newborns > 36 weeks that met at least two American College of Obstetricians and Gynecologists diagnostic criteria and/or imaging evidence of cerebral damage were included. Bias was assessed by the Newcastle–Ottawa Scale. The search and data extraction were verified by two authors separately.
Results
From 373 papers, 30 met the inclusion criteria. Data from samples collected in the first 72 hours were extracted, and increased serum levels of neuron-specific enolase and S100-calcium-binding protein-B were associated with a worse prognosis in newborns that suffered an episode of perinatal asphyxia. In addition, the levels of glial fibrillary acidic protein, ubiquitin carboxyl terminal hydrolase isozyme-L1, glutamic pyruvic transaminase-2, lactate, and glucose were elevated in newborns diagnosed with hypoxic–ischemic encephalopathy. Moreover, pathway analysis revealed insulin-like growth factor signaling and alanine, aspartate and glutamate metabolism to be involved in the early molecular response to insult.
Conclusions
Neuron-specific enolase and S100-calcium-binding protein-B are potential biomarkers, since they are correlated with an unfavorable outcome of hypoxic–ischemic encephalopathy newborns. However, more studies are required to determine the sensitivity and specificity of this approach to be validated for clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-023-00698-7.
Keywords: Biomarker, Hypoxic–ischemic encephalopathy, Neonatal brain injury, Newborn, Neuron-specific enolase, S100-calcium-binding protein-B
Introduction
Perinatal asphyxia (PA) can lead to severe brain injury and is the most frequent cause of hypoxic–ischemic encephalopathy (HIE), occurring in 1–8/1000 live births [1]. Several conditions might lead to an interruption of the blood flow to the brain, resulting in an insufficient supply of oxygen and nutrients to the brain required to maintain the high energy demands of this organ [2]. Briefly, after the initial insult, energy failure results in an impairment of active membrane transport and, consequently, membrane depolarization and glutamate release. Its accumulation in the synaptic cleft leads to increased excitotoxicity, culminating in cytotoxic edema, activation of inflammatory and apoptotic pathways, and finally neuronal death [3]. These events may lead to permanent sequelae in the neonatal brain, namely, epilepsy, cerebral palsy, mental disability, motor and sensorial impairment, or even death [4].
There are no accurate and objective tools with high sensitivity and specificity to diagnose newborns suffering from HIE. Some complementary blood tests have been proposed to evaluate liver and renal function and support the diagnosis, but they lack neuronal specificity [5]. According to the American College of Obstetricians and Gynecologists (ACOG) [6], if more than one of the following criteria is met, the newborn is more likely to suffer from a peripartum hypoxic–ischemic event: (1) appearance, pulse, grimace, activity, respiration (APGAR) score below 5 at 5 and 10 minutes; (2) fetal umbilical artery pH less than 7.0 and/or base deficit equal to or greater than 12 mmol/L; (3) neuroimaging evidence of acute brain injury seen on magnetic resonance imaging (MRI) or magnetic resonance spectroscopy (MRS), and (4) presence of multiorgan failure. However, imaging is usually performed in the first two weeks of life and is not suitable for a rapid diagnosis [5].
The therapeutic window to treat HIE is limited to the first 6 hours of life, before the beginning of inflammatory and apoptotic pathways [7]. Presently, therapeutic hypothermia (TH) is the treatment standard for moderate to severe cases of HIE, consisting of cooling either the newborn’s whole-body temperature (keeping it between 32 °C and 34 °C) or selectively the head for up to 72 hours. This approach aims to slow the metabolic rate and the accumulation of inflammatory cytokines, lowering the activation of intracellular pathways leading to programmed cell death. Furthermore, innovative treatments are emerging, including drugs, such as topiramate, erythropoietin, and stem cells, which are not yet used as standard guideline treatments [8]. The lack of a definitive test to diagnose HIE might lead to a misdiagnosis and a lack of proper treatment choices that can have an irretrievable impact on these neonates’ future.
Several studies have been published in recent decades proposing hypothetical biomarkers for HIE [5, 9]. Nevertheless, to our knowledge, there is no review on the literature that collects all these data. Therefore, this systematic review proposes to critically assess potential biomarkers for the diagnosis of term newborns who have been diagnosed with HIE in accordance with ACOG criteria and/or MRI brain injury evidence.
Methods
The study design was registered on PROSPERO on 1st October 2021 (ID: CRD42021272610) [10]. In addition, this review was written in accordance with PRISMA guidelines [11]. The search strategy, study eligibility, and quality assessment were performed by IC and MC, while IC and MR performed data extraction. The evaluation was performed independently, and disagreements were resolved by consensus.
Search strategy
An article search was conducted in three distinct databases until November 11, 2020: PubMed, Web of Science, Science Direct, and OpenGrey. Since HIE terminology is not consensual, four different terms were used: “neonatal brain injury”, “neonatal encephalopathy”, “hypoxic–ischemic encephalopathy” and “neonatal hypoxic–ischemic encephalopathy”. These terms were combined with the preposition and with the terms “biomarker*”, “proteomic*”, “metabolomic*”, restricting it to the title and abstract fields. The methods are described in detail in the supplementary material.
Study eligibility
Selected articles were subjected to abstract evaluation and three selection phases. The first approach aimed to categorize the results by document type, language, species of the samples studied, and biomarker type. Only original English papers in which the research was focused on biochemical HIE biomarkers in human samples were selected for method evaluation. The studies were then classified according to study type, sample size, sample type, association with other pathologies, gestational age (GA) or age, disease, sample collection time, therapy, outcome assessment, and association of the biomarkers with multiorgan failure. Only studies regarding term newborns collected in the first 72 hours of life were selected for a diagnostic criteria analysis. Moreover, studies analyzing cerebrospinal fluid (CSF) were excluded, since its collection from newborns may be considered unethical in many countries.
Since diagnosis criteria for HIE are not standardized in all studies, to analyze a homogenous population, the diagnostic criteria applied were assessed in each study: APGAR score, fetal acidemia, MRI and multiorgan failure. Studies that matched at least two ACOG diagnostic criteria or had neuroimaging evidence of brain injury were selected for quality assessment and data extraction.
Quality assessment
The quality of each study was evaluated by the Newcastle–Ottawa Scale (NOS) [12]. In accordance with the authors’ guidelines, different scales were applied depending on the study type (cohort or case–control). The scores for selection, comparability, and outcomes are presented separately.
Data extraction
Population characteristics were analyzed to infer the homogeneity of the populations being reviewed in this manuscript. Information about the study location, type of study, gestational age and/or birth weight, diagnosis criteria, HIE severity assessment, complementary diagnostic exams, therapeutic hypothermia, sample size, sample type, and the biomarker described in the study was extracted. In addition, information about the biomarker, namely, the biomarker type, the technique used to analyze the biomarker, the sample size of each group, collection time, and P value (when available), was also extracted. The extracted data is available in Tables 1-3 and in the supplementary material.
Data analysis
Venn diagrams were generated on InteractiVenn [13]. A UniProt accession number, Human Metabolome Database (HMBD) or GeneCard code was manually attributed to identified proteins, metabolites, or genes, respectively. Proteins and metabolites identified in serum and plasma samples were subjected to further analysis. Protein gene ontology (GO) analysis was performed on the DAVID Bioinformatic Database [14], and images were generated using the ggplot2 R package [15]. Metabolite pathway and mixomic analyses were performed on MetaboAnalyst [16].
Results
Literature search and study selection
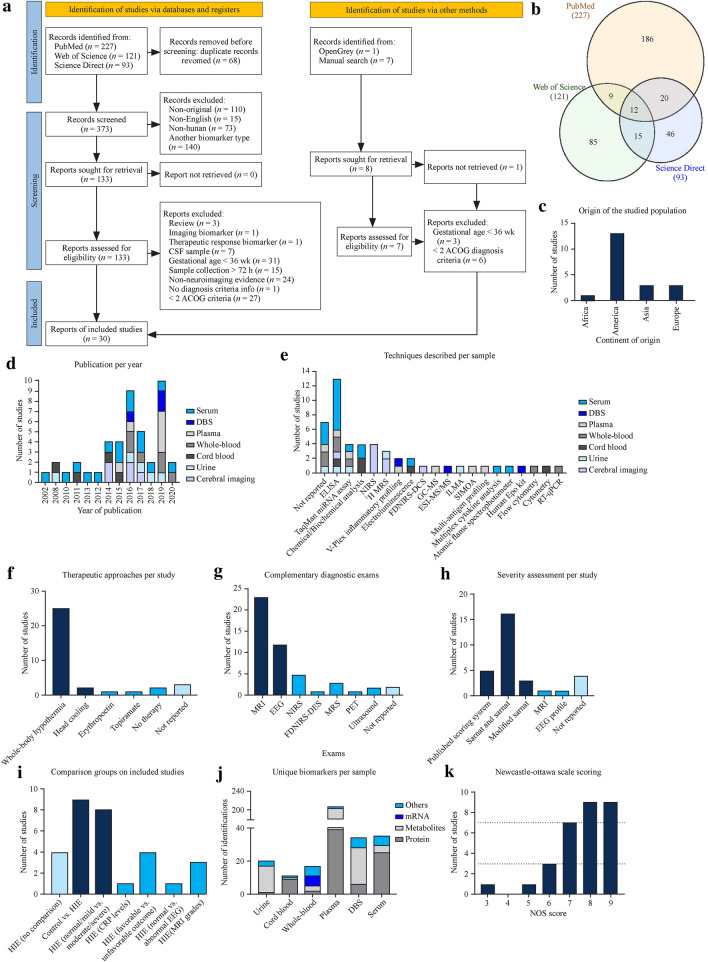
The PubMed search resulted in 279 hits and 227 unique records. The Web of Science search resulted in 233 hits and 121 unique records. The Science Direct search resulted in 145 hits and 93 unique records. After duplicate removal, a list of 373 unique records was obtained (Fig. 1a, b). From these, a total of 240 records were excluded considering nonoriginal records (n = 110), non-English records (n = 15), nonhuman studies (n = 73) and nonbiochemical, therapeutic response, genetic, pH, or associated with other disease biomarker studies (n = 140), resulting in 133 records for evaluation of the methods. During this selection phase, three articles were reclassified as review articles, one was reclassified as an imaging biomarker article, and four focused on therapeutic response biomarkers. Then, studies analyzing CSF samples were excluded (n = 7), because the collection of this fluid is not a common clinical practice in newborns. Works studying children, adults, and newborns < 36 week GA were also excluded (n = 31), since this review focuses on HIE biomarkers for term newborns. Finally, articles in which sample collection was not within the first 72 hours after birth (n = 15) were excluded, as this review focuses on identifying biomarkers of HIE to be used as an early diagnostic tool. These steps identified 81 records, of which diagnostic criteria were analyzed. It was not possible to obtain diagnosis criteria information in one of the reports; therefore, it was excluded. Studies that matched at least two ACOG diagnostic criteria or had neuroimaging evidence of acute brain injury by MRI or MRS (n = 29) were selected. Regarding the manual search, one study matched all eligibility criteria and was considered for further analysis. In conclusion, 30 studies were included in this systematic review [17–46] (Fig. 1a).
Fig. 1.
Database search results and summary of population characteristics. a PRISMA 2020 flow diagram. Only English manuscripts that analyzed human samples collected within 72 h (excluding CSF) and studied biochemical biomarkers associated with HIE were included. In addition, selected studies had to match at least two ACOG diagnosis criteria or present neuroimaging evidence of acute brain ischemia. b Common articles between PubMed, Web of Science, and Science Direct. Only 12 articles were common between the three databases. c and d Concerning population characteristics, the majority of selected studies were performed in Europe and America and published after 2014. f, g Almost all studies applied whole-body hypothermia and had MRI data available. h Studies lacked uniformity in comparison groups, but the severity was preferably assessed by the Sarnat scoring system. e, j Technique most prevalently used is ELISA, while plasma was the fluid with more identifications. k Summary of NOS scoring. CSF cerebrospinal fluid, ACOG American College of Obstetricians and Gynecologists, DBS dried blood spots, ELISA enzyme linked immunosorbent assay, miRNA microRNA, NIRS near-infrared spectroscopy, MRS magnetic resonance spectroscopy, FDNIRS–DCS frequency-domain near-infrared spectroscopy–diffuse correlation spectroscopy, MS mass spectrometer, GC–MS gas chromatography–mass spectrometer, ESI–MS electro spray ionization MS, ILMA immunoluminometric assay, SIMOA single molecular array, Epo erythropoietin, RT–PCR reverse transcription–polymerase chain reaction, MRI magnetic resonance imaging, EEG electroencephalogram, PET positron emission computed tomography, CRP C-reactive protein, NOS Newcastle–Ottawa scale
Quality assessment
Considering the NOS, no case–control study was considered to be at risk of bias (Supplementary Tables 1–2). Regarding cohort studies, one article was considered to be at high risk of bias (score ≤ 3), and four were at medium risk (score < 7). Five articles were identified as being potentially biased (Fig. 1k). These articles were not excluded from further analysis.
Studies and population characteristics
The population characteristics are summarized in Table 1, and the studies included were published between 2002 and 2020, despite the majority being released after 2014 (Fig. 1d). In addition, most studies took place in Europe and America (Fig. 1c) and are prospective studies. Regarding population characteristics, all studies included newborns older than 36 weeks of gestation that matched at least two ACOG diagnosis criteria or neuroimaging evidence of brain injury to allow studying a more homogeneous population. Moreover, almost all studies used whole-body hypothermia as a therapeutic approach and MRI as a complementary exam (Fig. 1f, g).
Table 1.
Summary of studies and population characteristics
| References | Year of publication | Study location | Study type | Gestational age/birth weight | Diagnosis criteria | Severity evaluation | Complementary exams | Therapeutic approaches | Sample size | Sample type | Biomarker | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akamatsu, Sugiyama et al. 2019 | [19] | 2019 | Tokyo, Japan | Prospective | ≥ 36 wk and birth weight ≥ 1800 g | (1) APGAR score ≤ 5 at 10 min; (2) pH ≤ 7 or base deficit ≥ 16 mmol/L in any blood within 1 h of birth; (3) need for resuscitation or assisted ventilation ≥ 10 min after birth | Modified Sarnat | MRI within 3–4 weeks | Whole-body hypothermia | 78 | Plasma | sLOX-1 |
| Alshweki, Perez-Munuzuri et al. 2017 | [35] | 2017 | Santiago de Compostela, Spain | Prospective | ≥ 36 wk and birth weight ≥ 1800 g | (1) APGAR score < 5 at 5 min; (2) pH ≤ 7 on arterial cord blood; (3) need for prolonged major resuscitation | Sarnat and Sarnat | MRI; EEG; PET | Whole-body hypothermia | 31 | Serum | NSE, S100B |
| Urine | S100B | |||||||||||
| Balada, Tebe et al. 2020 | [20] | 2020 | Barcelona, Spain | Prospective | ≥ 36 wk and birth weight ≥ 1800 g | (1) APGAR score ≤ 5 at 10 min; (2) pH ≤ 7 or base deficit ≥ 16 mmol/L in umbilical cord blood or arterial, venous or capillary blood within 1 h of birth; (3) need for resuscitation for more than 10 min after birth; (4) neurological dysfunction manifested by subnormal level of unconsciousness with or without seizures or palmary hyperexcitability, tremor, overactive myotatic reflexes, hypersensitivity to stimulation or startle responses | Scoring system available on supplementary data | MRI; EEG; MODs (3 d); GMFCS, BFMF and BSITD-III at 24 mon | Whole-body hypothermia | 58 | Whole blood | MMP9; IL-8; HSPA1A; CCR5; PPARG; TLR8 |
| Bale, Mitra et al. 2014 | [41] | 2014 | London, England | Prospective | ≥ 36 wk | (1) pH ≤ 7 or base deficit ≥ 16 mmol/L in cord blood any blood sample in 1 h; (2) APGAR score ≤ 5 at 10 min; (3) need for resuscitation or mask ventilation at 10 min | Sarnat and Sarnat | MRI (up to 7 d); EEG; NIRS; 1H MRS | Whole-body hypothermia | 6 | Cerebral imaging | ΔSpO2 (systemic oxygen saturation), Δ[HbD] (= oxygenated ‒ deoxygenated haemoglobin), Δ[HbT] (= oxygenated + deoxygenated haemoglobin), Δ[oxCCO] (oxidation state of cytochrome-c oxidase), Lac/NAA ratio |
| Bersani, Ferrari et al. 2019 | [44] | 2019 | Alessandria, Modena, Rome, Italy; Warsaw, Poland; Cairo, Egypt | Retrospective case–control | > 36 wk | (1) pH < 7 in umbilical artery or base excess ≤ − 12 mmol/L in cord blood or venous blood within 1 h; (2) APGAR score < 3 at 5 min; (3) occurrence of multiorgan failure; (4) need for resuscitation and/or positive pressure ventilation for more than 3 min | Sarnat and Sarnat | EEG; neurological examination by Prechtl | Whole-body hypothermia and no therapy | 108 | Plasma | Glucose |
| Whole blood | Creatinine; Urea | |||||||||||
| Urine | S100B | |||||||||||
| Chalak, Sánchez et al. 2014 | [18] | 2014 | Dallas, Texas, USA | Prospective | ≥ 36 wk and birth weight ≥ 1800 g | (1) pH ≤ 7 or base deficit ≥ 16 mEq/L in umbilical arterial cord plasma; (2) if history of acute perinatal event or blood pH between 7.01–7.15 or base deficit between 10–15.9 mmol/L: combination with (a) APGAR score ≤ 5 at 10 min or (b) need for assisted ventilation at 10 min after birth | Sarnat and Sarnat | MRI at 7–14 d; BSID-III at 15–18 mon | Whole-body hypothermia | 27 | Umbilical cord arterial serum | GFAP; UCHL1; IL-1; IL-6; IL-8; VEGF; IFN-γ; TNF, RANTES |
| Umbilical cord arterial plasma | GFAP; UCHL1 | |||||||||||
| Chouthai, Sobczak et al. 2015 | [46] | 2015 | Michigan, USA | Retrospective case–control | ≥ 36 wk | (1) base deficit ≥ 16 mmol/L or pH ≤ 7.0 in cord blood or any blood sample, within the first hour of life; (2) if blood analysis revealed pH between 7.01–7.15 or base deficit between 10–15.9 mmol/L or not available, additional criteria requested (a) existence of perinatal data compatible with perinatal asphyxia, as cord prolapse or (b) APGAR score ≤ 5 at 10 min or need for assisted ventilation at 10 min after birth | Moderate/severe disability criteria (https://doi.org/10.1056/NEJMcps050929) | MRI | Whole-body hypothermia or no therapy | 56 | Serum | Glucose |
| Dehaes, Aggarwal et al. 2014 | [30] | 2014 | Massachusetts, USA | Prospective | ≥ 36 wk and birth weight ≥ 2000 g | (1) APGAR score ≤ 5 at 10 min; (2) pH ≤ 7 or base deficit ≥ 16 mEq/L within 1 h of birth; (3) evidence of neonatal encephalopathy by physical exam; (4) abnormal cerebral function monitor evidenced by seizures or amplitude-EEG; (5) need for ventilation for at least 10 min after birth | – | MRI within 6 d; FDNIRS–DCS; GMFCS and MDI at 18 mon | Whole-body hypothermia | 27 | Cerebral imaging | CMRO2; CBF; CBV; SO2 |
| Douglas-Escobar, Yang et al. 2010 | [22] | 2010 | Florida, USA | Prospective | ≥ 38 wk | (1) APGAR score < 3 at 5 min; (2) presence of multiorgan failure; (3); neurological symptoms, as seizures, coma or hypotonia | Sarnat and Sarnat | MRI (1 to 5 d) | Whole-body hypothermia | 28 | Serum | pNF-H; UCHL1 |
| El-Mazary, Abdel-Aziz et al. 2015 | [40] | 2015 | Minia, Egypt | Prospective | ≥ 37 wk | (1) pH ≤ 7 in umbilical artery or base deficit ≥ 16 mmol/L; (2) APGAR score ≤ 3 for more than 10 min; (3) disturbed conscious level, abnormal neuromuscular control or abnormalities in complex reflexes or autonomic function; (4) presence of seizures | Sarnat and Sarnat | – | – | 80 | Serum | Selenium, sodium, potassium, calcium, urea, creatinine, hemoglobin, ALT, AST |
| Whole blood | Platelet: WBC | |||||||||||
| Ennen, Huisman et al. 2011 | [17] | 2011 | Baltimore, USA | Prospective | ≥ 36 wk | (1) pH < 7 in umbilical artery or base deficit > 12 mM; (2) APGAR score < 7 at 5 min | Sarnat and Sarnat | MRI | Whole-body hypothermia | 46 | Cord blood | GFAP |
| Serum | GFAP | |||||||||||
| Ezgu, Atalay et al. 2002 | [39] | 2002 | Ankara, Turkey | Prospective | ≥ 37 wk | (1) pH ≤ 7.2 in cord blood; (2) APGAR score ≤ 6 at 5 min | Sarnat and Sarnat | MRI (up to 3 d); EEG Griffiths’ developmental scales (1 y) | – | 26 | Serum | NSE |
| CSF | NSE | |||||||||||
| Fredly, Nygaard et al. 2016 | [37] | 2016 | Oslo, Norway | Prospective | ≥ 36 wk | (1) an Apgar score ≤ 5 at 10 min; (2) a need for respiratory support at 10 min following birth (3) pH ≤ 7.00 or a base deficit ≥ 16 mmol/L obtained via either umbilical arterial blood or any blood samples taken within 60 min of birth | Sarnat and Sarnat | MRI; NIRS | Whole-body hypothermia | 28 | Serum | Lactate; CRP; cerebral oxygenation |
| Haiju, Suyuan et al. 2008 | [45] | 2008 | Shandong, China | Prospective | ≥ 37 wk | (1) APGAR < 3 at 5 min; (2) pH < 7.0 in first arterial blood; (3) presence of encephalopathy at least one: hypotonia, abnormal reflexes, absent or weak suck, or clinical seizures; (4) multiple organ failure | Sarnat and Sarnat | EEG (in case of seizures); neuroimaging; BSID-II at 4, 8, 12, 18–24 mon | – | 63 | Cord blood | NRBC/per 100WBC; lactate |
| Jain, Pagano et al. 2017 | [27] | 2017 | St. Louis, Missouri, and Nashville, Tennessee, USA | Prospective | > 36 wk | (1) APGAR < 5 at 10 min; (2) base deficit > 16 mmol/L or pH < 7.0 in cord blood or any blood sample within the first hour of life; (3) requiring assisted ventilation for at least 10 min | Sarnat | NIRS (for 48 h); MRI (second week); BSID at 18 to 24 mon | Whole-body hypothermia; Head cooling (n = 7) | 21 | Cerebral imaging | CrSO2 |
| Jones, Heep et al. 2018 | [34] | 2017 | North Bristol, England | Retrospective | ≥ 36 wk | (1) APGAR ≤ 5 at 10 min; (2) base deficit > 16 mmol/L or pH < 7.0 in cord blood or any blood sample within first hour of life; (3) requiring assisted ventilation at 10 min of life | EEG profile | EEG | Whole-body hypothermia | 79 | Serum | Lactate, Glucose, Troponin T, CK, LDH, ALT, AST, GGT, CRP, Alk Phos |
| Locci, Noto et al. 2018 | [43] | 2018 | Cuneo, Italy | Prospective | ≥ 36 wk and birth weight ≥ 2500 g | (1) APGAR ≤ 5 at 10 min; (2) base deficit ≥ 12 mmol/L or pH < 7.0 in cord blood or any blood sample within first hour of life; (3) required resuscitation at 10 min | Sarnat | MRI (1 wk–1 mon); EEG; Head ultrasound (130 d) | Whole-body hypothermia | 26 | Urine | Lactate, myoinositol, betaine, taurine, arginine, acetate, N-Ac-Groups, pyruvate, succinate, glutamine, acetone, citrate, DMA, α-ketoglutarate |
| Lopez-Suarez, Concheiro-Guisan et al. 2019 | [21] | 2019 | Galicia, Spain | Retrospective | ≥ 37 wk and birth weight ≥ 2500 g | (1) pH ≤ 7.0 in cord blood; (2) APGAR score < 5 at 5 min; (3) prolonged major resuscitation; (4) sentinel events of fetal distress | Sarnat | MRI; EEG | Whole-body hypothermia | 894 | Serum | NSE |
| DBS | Acylcarnitine profile | |||||||||||
| Maggiotto, Sondhi et al. 2019 | [29] | 2019 | California, San Francisco, USA | Prospective | > 36 wk and birth weight > 2000 g | (1) base deficit > 12 mEq/L or pH ≤ 7.0 in cord blood gas or any blood sample in the first hour of life; (2) APGAR score ≤ 5 at 10 min; (3) abnormal neurological examination; (4) seizures | Neurological score | MRS and MRI at 4–13 d; EEG; BSID-III at 6, 12, 18 and/or 24 mon | Whole-body hypothermia | 19 | Whole blood | RBC(GLUT1); WBC(GLUT3); |
| Plasma | NSE; GFAP | |||||||||||
| Massaro, Chang et al. 2014 | [36] | 2014 | Washington DC, USA | Prospective | > 36 wk and > 1800 g | (1) signs of moderate or severe encephalopathy by EEG; (2) base deficit > 16 mmol/L or pH < 7.0 in cord blood or any blood sample in the first hour of life | modified Sarnat | EEG; BSID-II at 15 mon | Whole-body hypothermia | 83 | Serum | NSE; S100B |
| Massaro, Chang et al. 2012 | [28] | 2012 | California, San Francisco, USA (multicentered) | Prospective | > 36 wk and > 1800 g | (1) base deficit ≥ 16 mmol/L or pH ≤ 7.0 in cord blood or any blood sample, within the first hour of life; (2) if blood analysis revealed pH between 7.01–7.15 or base deficit between 10–15.9 mmol/L or not available, additional criteria requested (a) existence of perinatal data compatible with perinatal asphyxia, as cord prolapse or (b) APGAR score ≤ 5 at 10 min or need for assisted ventilation at 10 min after birth | MRI (basal ganglia score and cortical/watershed score) | MRI (7–10 d); Amiel-Tison neurological assessment (14th day) | Whole-body hypothermia | 75 | Serum | NSE; S100B |
| Massaro, Jeromin et al. 2013 | [25] | 2013 | California, San Francisco, (multicentered) | Prospective | > 36 wk and > 1800 g | (1) base deficit ≥ 16 mmol/L or pH ≤ 7.0 in cord blood or any blood sample, within the first hour of life; (2) if blood analysis revealed pH between 7.01–7.15 or base deficit between 10–15.9 mmol/L or not available, additional criteria requested (a) existence of perinatal data compatible with perinatal asphyxia, as cord prolapse or (b) APGAR score ≤ 5 at 10 min or need for assisted ventilation at 10 min after birth | Moderate/severe disability (https://doi.org/10.1056/NEJMcps050929) | NIRS; EEG from admission to 12 h after rewarming; MRI at 5–14 d of life | Whole-body hypothermia | 20 | Serum | UCHL1; GFAP |
| Massaro, Wu et al. 2019 | [32] | 2019 | California, San Francisco (multicentered) | Prospective | ≥ 36 wk and birth weight ≥ 1800 g | (1) APGAR ≤ 5 at 10 min; (2) base deficit ≥ 15 mmol/L or pH < 7.0 in cord blood or any blood sample within the first hour of life; (3) required resuscitation at 10 min | Modified Sarnat | MRI 4–7 d of age; AIMS and WIDEA at 12 mon | Whole-body hypothermia or head cooling + erythropoietin | 50 | DBS | S100B; TNF-α; IL-1β; IL-6; IL-8; Epo |
| Plasma | S100B; TNF-α; IL-1β; IL-6; IL-8; Epo | |||||||||||
| Mitra, Bale et al. 2016 | [26] | 2016 | London, England | Prospective | ≥ 36 wk | MRI | – | NIRS; 1H MRS | Whole-body hypothermia | 14 | Perri cerebral imaging | Δ oxCCO; ΔHbD |
| Lac/NAA | ||||||||||||
| Oh, Perritt et al. 2008 | [23] | 2008 | USA (multicentered) | Prospective | ≥ 36 wk and > 2000 g | (1) base deficit ≥ 16 mmol/L or pH ≤ 7.0 in cord blood or any blood sample, within first hour of life; (2) if blood analysis revealed pH between 7.01 and 7.15 or base deficit between 10–15.9 mmol/L or not available, additional criteria requested (a) existence of perinatal data compatible with perinatal asphyxia, as cord prolapse or (b) APGAR score ≤ 5 at 10 min or need for assisted ventilation at 10 min after birth | Moderate/severe disability criteria (https://doi.org/10.1056/NEJMcps050929) | – | Whole-body hypothermia | 58 | Urine | Lactate/creatinine ratio |
| Pineiro-Ramos, Nunez-Ramiro et al. 2020 | [24] | 2020 | Valencia, Spain (multicentered) | Prospective | ≥ 36 wk and > 2000 g | (1) base deficit ≥ 16 mmol/L and/or pH ≤ 7.0 in cord blood or any blood sample, within the first hour of life; (2) if blood analysis revealed pH between 7.01–7.15 or base deficit between 10 and 15.9 mmol/L or not available, additional criteria requested (a) existence of perinatal data compatible with perinatal asphyxia, as cord prolapse or (b) APGAR score ≤ 5 at 10 min or need for assisted ventilation at 10 min after birth | Sarnat and Sarnat | MRI 4–8 d after birth | Whole-body hypothermia + topiramate/placebo | 62 | Plasma | Pyruvate; lactate |
| Alanine, aspartate, and glutamate metabolism; arginine and proline metabolism; caffeine metabolism; D-glutamine and D-glutamate metabolism; limonene and pinene degradation; lysine biosynthesis; lysine degradation; nitrogen metabolism; phenylalanine metabolism; seleno amino acid metabolism; steroid hormone biosynthesis | ||||||||||||
| Ponnusamy, Kapellou et al. 2016 | [31] | 2016 | London, England | Prospective | ≥ 36 wk and ≥ 2000 g | (1) APGAR ≤ 5 at 10 min; (2) continued need for resuscitation at 10 min after birth; (3) base deficit ≥ 16 mmol/L or pH ≤ 7.0 in cord blood or any blood sample, within the first hour of life | – | MRI 5–35 d of life | Whole-body hypothermia | 30 | Plasma | RNU6B; Let7b; miR-21 |
| EDTA–blood | RNU6B; Let7b; miR-21 | |||||||||||
| Urine | RNU6B; Let7b; miR-21 | |||||||||||
| DBS | RNU6B; Let7b; miR-21; miR-29b; miR-124; miR-155 | |||||||||||
| Saito, Shibasaki et al. 2016 | [33] | 2016 | Yokohama, Japan | Prospective | ≥ 36 wk and ≥ 2000 g | (1) APGAR ≤ 5 at 10 min; (2) continued need for resuscitation at 10 min after birth; (3) base deficit ≥ 16 mmol/L or pH ≤ 7.0 in cord blood or any blood sample, within the first hour of life | Sarnat and Sarnat | MRI | Whole-body cooling | 22 | Serum | CRP; IL-6; PCT |
| Whole blood | WBCC; neutrophil count; platelet count | |||||||||||
| Shaikh, Boudes et al. 2015 | [42] | 2015 | Monteral, Canada | Prospective | ≥ 36 wk and ≥ 2000 g | (1) base deficit ≥ 16 mmEq/L or pH ≤ 7.0 in postnatal blood within the first hour of life; (2) APGAR score ≤ at 5 and 10 min; (3) need for ventilation at least 10 min; (4) evidence of moderate/severe NE by neurological exam or EEG | – | MRI 9–13 d of life | Whole-body hypothermia | 16 | Plasma | Ang-2; Cathepsin D; Hepsin; MMP-3; MMP-7; MMP-9; MMP-10; Ang; AXL; Endoglin; EGFR; FABP-4; Galectin-3; G6PI; HB-EGF; HER-2; IGFBP-2; IGFBP-3; ICAM-1; KLK-5; MSP; NP-1; ErbB3; SCF; SOD-1; TN-C; TNFR2; VEGF-C; VEGFR-2; VEGFR-3; YKL-40; BDNF; NSE; Nr-CAM; cFib; Collagen IV; TIE-2; Endostatin; Fib-1C; HE4; IGFBP-1; IGFBP4; IGFBP5; IGFBP-6; TIMP-1; FasL; FasR; Sortilin; TRAIL-R3 |
| Sweetman, Onwuneme et al. 2017 | [38] | 2017 | Dublin, Ireland | Prospective | ≥ 36 wk and ≥ 2000 g | 2 of 3: (1) evidence or suspicion of HIE based on the history of fetal distress; (2) need for resuscitation after birth (3) base deficit > 15 mmol/l or pH < 7.2 in cord blood or admission arterial sample | Sarnat and Sarnat | Cranial ultrasound (up to 24 h); MRI (up tp 7 d) | Whole-body hypothermia | 94 | Serum | VEGF; Epo |
| 34 | CSF | VEGF; Epo | ||||||||||
APGAR appearance, pulse, grimace, activity, respiration, EEG electroencephalogram, MRI magnetic resonance imaging, PET positron emission computed tomography, GMFCS the gross motor function classification system, BFMF bimanual fine motor function, BSITD-III Bayley scale of infant and toddler development, CSF cerebrospinal fluid, DBS dried blood spots, EDTA ethylene diamine tetraacetic acid, sLOX-1 soluble lectin-like oxidized low density lipoprotein receptor-1, NSE neuron-specific enolase, S100B S100-calcium-binding protein-B, MMP matrix metalloprotein, IL interleukin, HSPA1A heat shock protein family A member 1A, CCR5 C–C motif chemokine receptor 5, PPARG peroxisome proliferator activated receptor gamma, TLR8 toll like receptor 8, GFAP glial fibrillary acidic protein, UCHL1 ubiquitin C-terminal hydrolase L1, VEGF vascular endothelial growth factor, IFN-γ interferon γ, TNF tumor necrosis factor, RANTES reduced upon activation, nornal T cell expressed and secreted, CMRO2 cerebral oxygen metabolism index, CBF cerebral blood flow index, CBV cerebral blood volume, SO2 hemoglobin oxygen saturation, WBC white blood cell, NRBC nucleated red blood cell, CRP C-reactive protein, CrSO2 cerebral regional oxygen saturation, CK creatine kinase, LDH lactate dehydrogenase, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT γ-glutamyl transpeptidase, Alk Phos alkaline phosphatase, DMA dynamic muscle activation, GLUT glucose transporter, Epo erythropoietin, RNU6B U6 small nuclear 2, PCT procalcitonin, Ang angiotensin, EGFR epidermal growth factor receptor, FABP fatty acid-binding protein, G6PI glucose-6-phosphate isomerase, HB-EGF heparin-binding epidermal growth factor, HER-2 human epidermal growth factor receptor 2, IGFBP insulin-like growth factor binding protein, ICAM-1 intercellular cell adhesion molecule-1, KLK-5 kallikrein 5, MSP macrophage stimulating protein, NP-1 alpha-defensin-1, SCF Stem cell factor, SOD-1 Superoxide dismutase, TN-C Tenascin-C, TNFR2 tumor necrosis factor receptor-2, VEGFR VEGF receptor, YKL-40 chitinase-3-like protein 1, BDNF brain-derived neurotrophic factor, Nr-CAM neuronal cell adhesion molecule, cFib Cellular Fibronectin, TIE-2 tyrosine–protein kinase receptor, Fib-1C Fibulin-1C, HE4 human epididymis protein 4, TIMP-1 tissue inhibitor of metalloproteinase-1, FasL Fas Ligand, FasR Fas receptor, TRAIL-R3 tumor necrosis factor-related apoptosis-inducing ligand receptor 3
Potential biomarkers were identified in the cord blood, plasma, serum, whole blood, dried blood spots (DBS), and urine (Tables 2, 3 and Supplementary Tables 4–7). Interestingly, some studies used advanced cerebral imaging techniques to address the behavior of specific molecules during TH, while enzyme linked immunosorbent assay (ELISA) was the most commonly used technique (Fig. 1e). Although metabolites have a high number of identifications (due to the use of high throughput techniques) (Fig. 1j), most studies have focused their attention on proteins. Nevertheless, recent reports indicate that RNA and microRNA (miRNA) are emerging as possible diagnostic targets.
Table 2.
Summary of findings of biomarkers present in plasma
| References | Sample type | Biomarker | Code | Technique | Group | Collection time | Results | Summary | Observations | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteins | |||||||||||
| Akamatsu, Sugiyama et al. 2019 | [19] | Plasma | sLOX-1 | P78380 | ELISA | Control | < 6 h, 24 h, 48 h–96 h, 120 h–216 h | ||||
| Mild HIE | |||||||||||
| Moderate HIE | Increased levels compared to control (P < 0.01) | ↑ at first 6 h | |||||||||
| Levels decreased significantly at 48 h–216 h | |||||||||||
| Severe HIE | Increased levels compared to control (P < 0.01) | ↑↑ at first 6 h | Increased levels of moderate and severe HIE compared to mild HIE (P < 0.01) | ||||||||
| Increased levels compared to mild HIE (P < 0.05) | |||||||||||
| Maggiotto, Sondhi et al. 2019 | [29] | Plasma | NSE | P09104 | SIMOA | Control | 6 h–76 h | ||||
| HIE | Pre-TH; during TH; rewarming; pos-TH | Increased levels at pre-TH compared to control groups (P < 0.05) | ↑ at pre-TH | ||||||||
| Levels decreased during, rewarming and pos TH (P < 0.01) | |||||||||||
| GFAP | P14136 | SIMOA | Control | 6 h–76 h | |||||||
| HIE | Pre-TH; during TH; rewarming; pos-TH | No significant differences | = on all timepoints | ||||||||
| Massaro, Wu et al. 2019 | [32] | Plasma | S100B | P04271 | ELISA | HIE | < 24 h, 120 h | No comparisons between groups were performed | |||
| IL-1β | P01584 | V-PLEX proinflammatory panel | |||||||||
| IL-6 | P05231 | ||||||||||
| IL-8 | P10145 | ||||||||||
| TNF-α | P01375 | ||||||||||
| Erythropoietin | P01588 | Human EPO base kit | |||||||||
| Shaikh, Boudes et al. 2015 | [42] | Plasma | MMP-9 | P14780 | Multi-analyte profiling antigen analysis | Control | 24 h | ||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | ||||||||
| FABP4 | P15090 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Increased levels compared to healthy controls (P < 0.05) | ↑ at 24 h | ||||||||
| Galectin-3 | P17931 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Increased levels compared to healthy controls (P < 0.05) | ↑ at 24 h | ||||||||
| KLK-5 | Q9Y337 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | ||||||||
| VEGF-C | P49767 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | ||||||||
| BDNF | P23560 | Control | 24 h | ||||||||
| HIE | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | |||||||||
| Fib-1C | P23142 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | ||||||||
| IGFBP-6 | P24592 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | ||||||||
| HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 72 h | ||||||||
| ↓ at 96 h | |||||||||||
| FasL | P48023 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to healthy controls (P < 0.05) | ↓ at 24 h | ||||||||
| HIE (with brain injury) | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 72 h | |||||||||
| FasR | P25445 | Control | 24 h | ||||||||
| HIE | 6 h, 24 h, 48 h, 72 h, 96 h | Increased levels compared to healthy controls (P < 0.05) | ↑ at 24 h | ||||||||
| Ang-2 | O15123 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓ ↓ at 96 h | |||||||||||
| Hepsin | P05981 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 6 h | ||||||
| ↓ at 24 h | |||||||||||
| HB-EGF | Q99075 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓ at 48 h | |||||||||||
| NP-1 | O14786 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓ at 48 h | |||||||||||
| ↓ at 96 h | |||||||||||
| ErbB3 | P21860 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓ at 48 h | |||||||||||
| YKL-40 | P36222 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓↓ at 24 h | ||||||
| ↓↓ at 48 h | |||||||||||
| IGFBP-1 | P08833 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓↓ at 48 h | |||||||||||
| IGFBP-4 | P22692 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓ at 48 h | |||||||||||
| IGFBP-5 | P24593 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 6 h | ||||||
| ↓ at 24 h | |||||||||||
| ↓ at 48 h | |||||||||||
| Sortilin | Q99523 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 24 h | ||||||
| ↓ at 48 h | |||||||||||
| MMP-3 | P08254 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓↓ at 72 h | ||||||
| Cathepsin-D | P07339 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 72 h | ||||||
| Endoglin | P17813 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 96 h | ||||||
| ICAM-1 | P05362 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 96 h | ||||||
| MSP | P26927 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 6 h | ||||||
| TNFR-2 | P20333 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 48 h | ||||||
| ↓ at 72 h | |||||||||||
| NrCAM | Q92823 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 96 h | ||||||
| Fibronectin | P02751 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓↓ at 48 h | ||||||
| ↓↓ at 72 h | |||||||||||
| TIMP-1 | P01033 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE without brain injury (P < 0.05) | ↓ at 48 h | ||||||
| ↓ at 72 h | |||||||||||
| ↓ at 96 h | |||||||||||
| TRAIL-R3 | O14798 | HIE (with brain injury) | 6 h, 24 h, 48 h, 72 h, 96 h | Increased levels compared to HIE without brain injury (P < 0.05) | ↑↑ at 6 h | ||||||
| Metabolites | |||||||||||
| Bersani, Ferrari et al. 2019 | [44] | Plasma | Glucose | HMDB0304632 | – | Moderate HIE (with hypothermia) | At birth | ||||
| Severe HIE (with hypothermia) | At birth | No statistical differences (P > 0.05) | = on all groups | ||||||||
| Pineiro-Ramos, Nunez-Ramiro et al. 2020 | [24] | Plasma | Lactate | HMDB0000190; HMDB0001311 | GC–MS | HIE (normal MRI) | Pre-TH, 24 h, 48 h, 72 h | ||||
| HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Increased levels compared to HIE with normal MRI (P < 0.05) | ↑ at 24 h | ||||||||
| ↑ at 48 h | |||||||||||
| Pyruvate | HMDB0000243 | HIE (normal MRI) | Pre-TH, 24 h, 48 h, 72 h | ||||||||
| HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Increased levels compared to HIE with normal MRI (P < 0.05) | ↑ at 72 h | ||||||||
| Lactate/pyruvate ratio | - | HIE (normal MRI) | Pre-TH, 24 h, 48 h, 72 h | ||||||||
| HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | No statistical differences/no clear trend | |||||||||
| Alanine, aspartate, and glutamate metabolism | HMDB0000812; HMDB0060350; HMDB0000191; HMDB0000168 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 72 h | ||||||
| HMDB0006483; HMDB0000052 | |||||||||||
| HMDB0000161; HMDB0000208 | |||||||||||
| HMDB0000641; HMDB0000148 | |||||||||||
| HMDB0000112; HMDB0001552 | |||||||||||
| HMDB0001301; HMDB0000254 | |||||||||||
| HMDB0001254; HMDB0001128 | |||||||||||
| Arginine and proline metabolism | HMDB0002104; HMDB0001369 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 48 h and 72 h | ||||||
| HMDB0000641; HMDB0000214 | |||||||||||
| HMDB0000191; HMDB0000052 | |||||||||||
| HMDB0000148; HMDB0001138 | |||||||||||
| HMDB0006456; HMDB0006488 | |||||||||||
| HMDB0003357; HMDB0000162 | |||||||||||
| METPA0228; HMDB0003411 | |||||||||||
| HMDB0006875; HMDB0000725 | |||||||||||
| METPA0212; HMDB0000064 | |||||||||||
| HMDB0012265; HMDB0000562 | |||||||||||
| HMDB0004225; METPA0313 | |||||||||||
| HMDB0003464; HMDB0000112 | |||||||||||
| METPA0359; HMDB0033458 | |||||||||||
| METPA0384; HMDB0001199 | |||||||||||
| HMDB0001301; HMDB0001080 | |||||||||||
| HMDB0000988; HMDB0001185 | |||||||||||
| HMDB0002064; HMDB0003681 | |||||||||||
| HMDB0060460; HMDB0002234 | |||||||||||
| HMDB0000134; HMDB0006272 | |||||||||||
| HMDB0003355; METPA0471 | |||||||||||
| HMDB0000271; HMDB0000745 | |||||||||||
| HMDB0003705 | |||||||||||
| Caffeine metabolism | HMDB0000299; HMDB0000292 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at pre-TH timepoint | ||||||
| D-glutamine and D-glutamate metabolism | HMDB0003423; HMDB0003339 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 48 and 72 h | ||||||
| METPA0146; HMDB0000148 | |||||||||||
| HMDB0000641; HMDB0000805 | |||||||||||
| HMDB0000208 | |||||||||||
| Limonene and pinene degradation | HMDB0003667; METPA0496 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 72 h | ||||||
| HMDB0003647; HMDB0035089 | |||||||||||
| HMDB0003450; HMDB0003634 | |||||||||||
| HMDB0004321 | |||||||||||
| Lysine biosynthesis | HMDB0001370; HMDB0000510 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 48 h and 72 h | ||||||
| HMDB0000191; HMDB0012250 | |||||||||||
| HMDB0000719; HMDB0012249 | |||||||||||
| HMDB0012289; METPA0439 | |||||||||||
| HMDB0012267; METPA0493 | |||||||||||
| HMDB0001370; HMDB0000279 | |||||||||||
| HMDB0003518; HMDB0000208 | |||||||||||
| HMDB0000225; HMDB0012247 | |||||||||||
| HMDB0012266; HMDB0001263 | |||||||||||
| HMDB0060320 | |||||||||||
| Lysine degradation | HMDB0003405; HMDB0000182 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 72 h | ||||||
| HMDB0001084; HMDB0000279 | |||||||||||
| HMDB0001345; HMDB0001325 | |||||||||||
| HMDB0062259; HMDB0000070 | |||||||||||
| HMDB0000206; HMDB0001263 | |||||||||||
| HMDB0000510; HMDB0000225 | |||||||||||
| HMDB0001339; HMDB0000661 | |||||||||||
| HMDB0012233; HMDB0012176 | |||||||||||
| HMDB0012175; HMDB0012114 | |||||||||||
| HMDB0012115; METPA0449 | |||||||||||
| METPA0112; METPA0456 | |||||||||||
| HMDB0012151; HMDB0062496 | |||||||||||
| HMDB0059600; HMDB0012130 | |||||||||||
| HMDB0003355; HMDB0012131 | |||||||||||
| Nitrogen metabolism | HMDB0000159; HMDB0000158 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 72 h | ||||||
| HMDB0000929; METPA0417 | |||||||||||
| HMDB0000191; HMDB0000168 | |||||||||||
| HMDB0000148; HMDB0000641 | |||||||||||
| HMDB0001123; HMDB0000099 | |||||||||||
| HMDB0000742; HMDB0000455 | |||||||||||
| HMDB0000177; HMDB0000045 | |||||||||||
| Phenylalanine metabolism | C03589; HMDB0000159; HMDB0006236; METPA0060 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at 24 h, 48 h and 72 h | ||||||
| HMDB0134042; HMDB0000205 | |||||||||||
| METPA0264; HMDB0010715 | |||||||||||
| C00423; HMDB0033752 | |||||||||||
| HMDB0013677; HMDB0000714 | |||||||||||
| HMDB0002641; HMDB0002035 | |||||||||||
| HMDB0001587; HMDB0006344 | |||||||||||
| HMDB0000254; HMDB0000134 | |||||||||||
| HMDB0012225; HMDB0000512 | |||||||||||
| HMDB0000500 | |||||||||||
| HMDB0000020; HMDB0001895 | |||||||||||
| HMDB0000158 | |||||||||||
| Seleno amino acid metabolism | HMDB0003011; HMDB0000161 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at pre-TH timepoint | ||||||
| Steroid hormone biosynthesis | HMDB0000063; HMDB0000037 | HIE (pathological MRI) | Pre-TH, 24 h, 48 h, 72 h | Altered pathway associated with pathological MRI outcome | Altered at pre-TH, 24 h, 48 h, 72 h | ||||||
| HMDB0000319; HMDB0000067 | |||||||||||
| HMDB0000253; HMDB0000077 | |||||||||||
| HMDB0003818; HMDB0000016 | |||||||||||
| HMDB0001547; HMDB0001830 | |||||||||||
| HMDB0003759; | |||||||||||
| HMDB0000374 | |||||||||||
| HMDB0002802; HMDB0000234 | |||||||||||
| HMDB0000053; HMDB0003769 | |||||||||||
| HMDB0000490; HMDB0000899 | |||||||||||
| HMDB0000031; HMDB0000145 | |||||||||||
| HMDB0002961; HMDB0001425 | |||||||||||
| HMDB0000920; HMDB0003069 | |||||||||||
| HMDB0060437; METPA0446 | |||||||||||
| miRNA | |||||||||||
| Ponnusamy, Kapellou et al. 2016 | [31] | Plasma | EDTA-blood | RNU6B | GC10P013220 | TaqMan miRNA assay | HIE | 18 h–19 h | No comparisons between groups were performed | ||
| Let7b | GC22P046119 | ||||||||||
| miR-21 | GC17P059841 | ||||||||||
sLOX-1 soluble lectin-like oxidized low density lipoprotein receptor-1, NSE neuron-specific enolase, S100B S100-calcium-binding protein-B, MMP matrix metalloprotein, IL interleukin, GFAP glial fibrillary acidic protein, TNF tumor necrosis factor, FABP4 fatty-acid-binding protein 4, KLK-5 kallikrein 5, VEGF vascular endothelial growth factor, BDNF brain-derived neurotrophic factor, Fib-1C Fibulin-1C, IGFBP insulin-like growth factor binding protein, FasL Fas Ligand, FasR Fas receptor, Ang angiotensin, HB-EGF heparin-binding epidermal growth factor, NP-1 alpha-defensin-1, YKL-40 chitinase-3-like protein 1, ICAM-1 intercellular cell adhesion molecule-1, MSP macrophage stimulating protein, TNFR-2 TNF receptor 2, NrCAM neuron cell adhesion molecule, TIMP-1 tissue inhibitor of metalloproteinase-1, TRAIL-R3 tumor necrosis factor-related apoptosis-inducing ligand receptor 3, EDTA ethylene diamine tetraacetic acid, SIMOA single molecular array, ELISA enzyme linked immunosorbent assay, Epo erythropoietin, GC–MS gas chromatography–mass spectrometer, HIE hypoxic–ischemic encephalopathy, MRI magnetic resonance imaging, miRNA microRNA, TH Therapeutic hypothermia
Table 3.
Summary of major findings related to biomarkers present in serum
| References | Sample type | Biomarker | Code | Technique | Group | Collection time | Results | Summary | Observations | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteins | |||||||||||
| Ennen, Huisman et al. 2011 | [17] | Serum | GFAP | P14136 | Electrochemiluminescence, Sandwich Immunoassay | Control | < 6 h, 24 h, 48 h, 72 h, 96 h | ||||
| HIE | < 6 h, 24 h, 48 h, 72 h, 96 h | Increased levels compared to controls at 6 h (P = 0.032), 24 h (P = 0.013), 48 h, 72 h (P = 0.013), 96 h (P = 0.003) | ↑ at 6 h | ||||||||
| ↑ at 24 h | |||||||||||
| ↑ at 72 h | |||||||||||
| ↑ at 96 h | |||||||||||
| HIE (abnormal MRI) | < 6 h, 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, 168 h | Increased levels compared to controls at 24 h (P = 0.02), 48 h (P = 0.007), 96 h (P < 0.001), 120 h (P < 0.001), 144 h (P = 0.007), 168 h (P < 0.05) | ↑ at 24 h | ||||||||
| ↑ at 48 h | |||||||||||
| ↑ at 96 h | |||||||||||
| ↑ at 120 h | |||||||||||
| ↑ at 144 h | |||||||||||
| ↑ at 168 h | |||||||||||
| Lopez-Suarez, Concheiro-Guisan et al. 2019 | [21] | Serum | NSE | P09104 | ELISA | HIE | < 6 h, 48 h, 72 h | High on 3 days of hypothermia | - | Positive correlation with carnitine C4 | |
| Correlation with an unfavorable outcome (P = 0.029) | |||||||||||
| Douglas-Escobar, Yang et al. 2010 | [22] | Serum | pNF-H | P12036 | ELISA | Control | < 6 h | Increased levels compared to control (P = 0.051) | ↑ at 6 h | ||
| HIE | < 6 h, 7 h–12 h, 13 h–24 h, 25 h–48 h, 49 h–72 h | Tendency to higher levels on HIE with abnormal MRI, when compared to normal MRI, | |||||||||
| Higher levels on newborns with basal ganglia/hippocampus/thalamus injury | |||||||||||
| UCHL1 | P09936 | Control | < 6 h | No significant differences | = at 6 h | ||||||
| HIE | < 6 h, 7 h–12 h, 13 h–24 h, 25 h–48 h, 49 h–72 h | ||||||||||
| Massaro, Jeromin et al. 2013 | [25] | Serum | UCHL1 | P09936 | ELISA | HIE with no/mild MRI injury | Initiation, 12 h, 24 h, 72 h | ||||
| HIE with severe MRI injury or died | Initiation, 12 h, 24 h, 72 h | Increased levels compared to HIE without brain injury at initiation (P = 0.005) and 72 h (P = 0.039) | ↑ at 6 h | ||||||||
| ↑ at 72 h | |||||||||||
| GFAP | P14136 | HIE with no/mild MRI injury | Initiation, 12 h, 24 h, 72 h | ||||||||
| HIE with severe MRI injury or died | Initiation, 12 h, 24 h, 72 h | Increased levels compared to HIE without brain injury at 24 h (P = 0.003) and 72 h (P = 0.002) | ↑ at 24 h | ||||||||
| ↑ at 72 h | |||||||||||
| Massaro, Chang et al. 2012 | [28] | Serum | NSE | P09104 | ELISA | HIE (good outcome) | Initiation, 12 h, 24 h, 72 h | Baseline values provide discrimination of death or severe MRI-brain injury, at 72 h, levels were good predictors of death or neurological deficit | |||
| HIE (bad outcome) | Initiation, 12 h, 24 h, 72 h | Higher levels on adverse outcomes (death, severe MRI injury or neurological deficit) | |||||||||
| S100B | P04271 | HIE (good outcome) | Initiation, 12 h, 24 h, 72 h | Baseline values provide discrimination of death or severe MRI-brain injury | |||||||
| HIE (bad outcome) | Initiation, 12 h, 24 h, 72 h | Higher levels on adverse outcomes (death, severe MRI injury or neurological deficit) | |||||||||
| Saito, Shibasaki et al. 2016 | [34] | Serum | CRP | P02741 | Chemical analysis | HIE | 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, 168 h | Increased levels at 48 h, peaking at 96 h | |||
| IL-6 | P05231 | HIE | 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, 168 h | Increased levels at 24 h, peaking at 48 h | |||||||
| PCT | P01258 | HIE | 24 h, 48 h, 72 h, 96 h, 120 h, 144 h, 168 h | Levels peaked at 48 h | |||||||
| Jones, Heep et al. 2018 | [34] | Serum | Troponin-T | P45379 | - | non-HIE (normal EEG) | < 6 h | ||||
| HIE (abnormal EEG) | Increased levels compared to non-HIE (P = 0.004) | ↑ at < 6 h | |||||||||
| CK | P12277 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | No significant differences (P = 0.157) | ||||||||||
| CRP | P02741 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | No significant differences (P = 0.846) | ||||||||||
| LDH | P07195 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | No significant differences (P = 0.225) | ||||||||||
| ALT | Q8TD30 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | Increased levels compared to non-HIE (P = 0.004) | ↑ at < 6 h | |||||||||
| AST | P17174 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | No significant differences (P = 0.291) | ||||||||||
| GGT | Q9UJ14 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | No significant differences (P = 0.480) | ||||||||||
| Alk Phos | P05186 | non-HIE (normal EEG) | < 6 h | ||||||||
| HIE (abnormal EEG) | No significant differences (P = 0.102) | ||||||||||
| Alshweki, Perez-Munuzuri et al. 2017 | [35] | Serum | S100B | P04271 | ELISA | HIE (favorable outcome) | 24 h, 48 h, 72 h | ||||
| HIE (unfavorable outcome) | 24 h, 48 h, 72 h | No significant differences | = on all timepoints | ||||||||
| HIE (deceased) | 24 h, 48 h, 72 h | Higher levels compared to alive at 24 h (P = 0.032) | ↑ at 24 h | ||||||||
| NSE | P09104 | HIE (favorable outcome) | 24 h, 48 h, 72 h | ||||||||
| HIE (unfavorable outcome) | 24 h, 48 h, 72 h | Higher levels compared to the favorable outcome at 48 h (P = 0.019) | ↑ at < 48 h | Higher levels on infants with abnormal PET (P = 0.015) | |||||||
| HIE (deceased) | 24 h, 48 h, 72 h | Higher levels compared to alive at 24 h (P = 0.001) and 72 h (P = 0.006) | ↑ at < 24 h | ||||||||
| ↑ at < 72 h | |||||||||||
| Massaro, Chang et al. 2014 | [36] | Serum | S100B | P04271 | ELISA | Normal HIE (MDI and PSD > 85) | Initiation, 12 h, 24 h, 72 h | ||||
| Mild/moderate HIE (MDI and PSD 70–85) | Initiation, 12 h, 24 h, 72 h | ||||||||||
| Severe HIE (MDI and PSD < 70) | Initiation, 12 h, 24 h, 72 h | Higher levels associated with worse cognitive (P = 0.07) and motor (P = 0.012) outcomes at 72 h | |||||||||
| HIE (dead) | Initiation, 12 h, 24 h, 72 h | ↑ at < 72 h | ROC curve predicts death or BSID-II MDI or PDI < 70 | ||||||||
| NSE | P09104 | Normal HIE (MDI and PSD > 85) | Initiation, 12 h, 24 h, 72 h | ||||||||
| Mild/moderate HIE (MDI and PSD 70–85) | Initiation, 12 h, 24 h, 72 h | ||||||||||
| Severe HIE (MDI and PSD < 70) | Initiation, 12 h, 24 h, 72 h | Higher levels associated to worse cognitive (P = 0.010) and motor (P = 0.010) outcomes at 72 h | |||||||||
| HIE (dead) | Initiation, 12 h, 24 h, 72 h | ↑ at < 72 h | ROC curve predicts death or BSID-II MDI or PDI < 70 | ||||||||
| Fredly, Nygaard et al. 2016 | [37] | Serum | CRP | P02741 | - | HIE with low CRP levels | Initiation, 12 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, 120 h, 132 h, 144 h, 156 h, 168 h | ||||
| HIE with high CRP levels | High levels in 82% of the infants studied | ||||||||||
| Sweetman, Onwuneme et al. 2017 | [38] | Serum | VEGF | P49767 | Multiplex Cytokine Analysis | Controls | 24 h, 48 h, 72 h, 96 h | ||||
| HIE with no/mild injury | 24 h, 48 h, 72 h, 96 h | ||||||||||
| HIE with moderate/severe injury | 24 h, 48 h, 72 h, 96 h | Decreased levels compared to HIE with no/mild injury at 24 h (P = 0.035) | ↓ at 24 h | Decreased levels at 24 h associated to death (P = 0.03) | |||||||
| Erythropoietin | P01588 | Controls | 24 h, 48 h, 72 h, 96 h | ||||||||
| HIE with no/mild injury | 24 h, 48 h, 72 h, 96 h | ||||||||||
| HIE with moderate/severe injury | 24 h, 48 h, 72 h, 96 h | Increased levels compared to HIE with no/mild injury at 48 h (P = 0.006) | ↑ at 48 h | Increased levels at 72 h associated to death (P = 0.02) | |||||||
| Ezgu, Atalay et al. 2002 | [39] | Serum | NSE | P09104 | ELISA | No HIE | < 72 h | No significant differences | = at < 72 h | ||
| HIE with mild injury | |||||||||||
| HIE with moderate injury | |||||||||||
| HIE with severe injury | |||||||||||
| El-Mazary, Abdel-Aziz et al. 2015 | [40] | Serum | Hemoglobin | P69891 | Chemical analysis | Control | < 48 h | ||||
| HIE | Decreased levels compared to control (P = 0.001) | ↓ at 48 h | |||||||||
| ALT | Q8TD30 | Control | < 48 h | ||||||||
| HIE | Increased levels compared to control (P = 0.001) | ↑ at 48 h | |||||||||
| AST | P17174 | Control | < 48 h | ||||||||
| HIE | Increased levels compared to control (P = 0.001) | ↑ at 48 h | |||||||||
| Metabolites | |||||||||||
| El-Mazary, Abdel-Aziz et al. 2015 | [40] | Serum | Urea | HMDB0000294 | Chemical analysis | Control | < 48 h | ||||
| HIE | Increased levels compared to control (P = 0.001) | ↑ at < 48 h | |||||||||
| Creatinine | HMDB0000562 | Control | < 48 h | ||||||||
| HIE | Increased levels compared to control (P = 0.001) | ↑ at < 48 h | |||||||||
| Chouthai, Sobczak et al. 2015 | [46] | Serum | Glucose | HMDB0304632 | – | HIE (no TH) | Admission, < 24 h, 24 h–48 h, 48 h–72 h, 72 h–96 h | ||||
| HIE (with TH) | Increased levels compared to HIE without TH (P = 0.025) | ↑ at < 24 h | |||||||||
| HIE with moderate/severe injury | Increased levels compared to HIE with mild or normal outcomes (P = 0.005) | ↑ at < 24 h | High levels (> 200) associated with abnormal neuroimaging or death (P = 0.025) | ||||||||
| Jones, Heep et al. 2018 | [34] | Serum | Lactate | HMDB0000190; HMDB0001311 | – | non-HIE (normal EEG) | < 6 h | ||||
| HIE (abnormal EEG) | No significant differences (P = 0.07) | = at 6 h | |||||||||
| Glucose | HMDB0304632 | non-HIE (normal EEG) | < 6 h | First glucose measurement | |||||||
| HIE (abnormal EEG) | Higher levels compared to non-HIE (P = 0.02) | ↑ at 6 h | |||||||||
| Fredly, Nygaard et al. 2016 | [37] | Serum | Lactate | HMDB0000190; HMDB0001311 | HIE with low CRP levels | Initiation, 12 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, 120 h, 132 h, 144 h, 156 h, 168 h | |||||
| HIE with high CRP levels | High levels compared to low-CRP group at 72 h (P = 0.004) and 96 h (P = 0.046) | ↑ at 72 h | |||||||||
| ↑ at 96 h | |||||||||||
| Electrolytes | |||||||||||
| El-Mazary, Abdel-Aziz et al. 2015 | [40] | Serum | Sodium | – | Chemical analysis | Control | < 48 h | ||||
| HIE | Decreased levels compared to control (P = 0.001) | ↓ at < 48 h | |||||||||
| Potassium | – | Control | < 48 h | ||||||||
| HIE | Increased levels compared to control (P = 0.001) | ↑ at < 48 h | |||||||||
| Calcium | – | Control | < 48 h | ||||||||
| HIE | Decreased levels compared to control (P = 0.001) | ↓ at < 48 h | |||||||||
| Selenium | – | Atomic flame spectrophotometer | Control | < 48 h | |||||||
| HIE | Decreased levels compared to control (P = 0.001) | ↓ at < 48 h | |||||||||
| HIE with mild injury | < 48 h | No statistical differences (P = 0.05) | |||||||||
| HIE with moderate injury | < 48 h | Decreased levels compared to control (P = 0.001) | ↓ at < 48 h | ||||||||
| HIE with severe injury | < 48 h | Decreased levels compared to control (P = 0.001) | ↓ ↓ at < 48 h | ||||||||
GFAP glial fibrillary acidic protein, NSE neuron-specific enolase, pNF-H phosphorylated neurofilament heavy chain, UCHL1 ubiquitin C-terminal hydrolase L1, S100B S100-calcium-binding protein-B, CRP C-reactive protein, PCT procalcitonin, IL interleukin, CK creatine kinase, LDH lactate dehydrogenase, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT γ-glutamyl transpeptidase, VEGF vascular endothelial growth factor, ELISA enzyme linked immunosorbent assay, HIE hypoxic–ischemic encephalopathy, MRI magnetic resonance imaging, EEG electroencephalogram, PSD particle size distribution, MDI metered dose inhaler, ROC receiver operating characteristic, BSID-II Bayley scales of infant development II
Advanced cerebral imaging techniques are emerging as a therapeutic response monitoring approach
A small number of studies have evaluated the therapeutic response of newborns to hypothermia by evaluating the redox state of cytochrome oxidase [26, 41] or hemoglobin oxygenation [26, 30, 41] using near-infrared spectroscopy (NIRS) (Supplementary Table 3). In addition, the lactate/N-acetylaspartate ratio (assessed by 1H MRS) was suggested as a promising severity predictor for HIE [26, 41]. However, additional studies are needed for more robust conclusions.
Several proteins as candidates for the diagnosis of hypoxic–ischemic encephalopathy are altered in various body fluids
The proteins identified in more than one body fluid and/or cited in more than one study are summarized in Fig. 2a. While serum appears to be the most studied sample type, in which eleven different proteins were identified as potential biomarkers, only one protein was identified in urine—S100-calcium-binding protein-B (S100B). This protein was reported to be elevated in newborns' urine, presenting an unfavorable outcome in the first 48 hours of life [35] and severe cases in the first 24 hours of life [44]. Accordingly, higher S100B serum levels during hypothermia (at 72 hours) were associated with a worse prognosis [28, 36] and even death [28, 35, 36]. For glial fibrillary acidic protein (GFAP), data available from the umbilical cord are not consensual: one study reported no differences between control and HIE [17], while another describes that elevated GFAP levels are significantly different in infants who developed severe brain injury compared to mild HIE [18]. Although no differences were found between control and HIE on plasma samples during TH and on rewarming [29], GFAP serum levels were described to be significantly higher in infants with abnormal MRI during the first 24 hours [17, 25]. In severe cases of HIE, ubiquitin C-terminal hydrolase L1 (UCHL-1) levels were described to be increased in umbilical cord plasma, but no differences were found when comparing moderate and severe HIE groups with the mild HIE group [18]. It is also unclear if serum levels of this protein are associated with a worse prognosis, since no differences were found between the HIE and control groups [22], but the levels were described to be elevated at 6 hours and 72 hours in newborns with severe MRI brain injury when compared with those who did not develop or developed a mild brain injury [25]. Interestingly, newborns with HIE showed higher levels of plasma neuron-specific enolase (NSE) before TH than controls [29]. Increased serum levels of NSE in the first 72 hours were also found to be positively correlated with an unfavorable outcome [28, 35, 36], whereas one study did not find differences between newborns with different severity grades [39], and another did not have a control group [21].
Fig. 2.
Proteins and metabolites identified as potential biomarkers. a Representation of biomarkers that were present in more than one fluid and/or more than one study. Green, red and yellow triangles represent studies with evidence of significantly increased, decreased, or altered levels of the molecule in the HIE group. Blue circles represent molecules without significant differences, and on gray squares, no comparison was performed. Plasma and serum samples were then combined for pathway analysis. b, c Gene ontology analysis of proteins, namely, biological process and molecular function. Altered pathways were identified using a metabolite enrichment analysis (d) and a combined approach of protein and metabolite analysis (mixomics approach) (e). f Alterations in the alanine, aspartate and glutamate pathways were identified, where metabolites are highlighted in green and proteins in blue. Balls represent metabolites, and squares represent proteins. S100B S100-calcium-binding protein-B, DBS dried blood spots, GFAP glial fibrillary acidic protein, UCHL-1 ubiquitin C-terminal hydrolase L1, NSE neuron-specific enolase, IL interleukin, CRP C-reactive protein, VEGF vascular endothelial growth factor, ALT alanine aminotransferase, AST aspartate aminotransferase, TNF-α tumor necrosis factor α, GO Gene Ontology
Concerning the identified cytokines, no significant differences were found either in the umbilical cord serum [18] or the DBS [32]. Particularly for IL-6 serum levels, no control group was available in the study [33]. No comparisons were performed on erythropoietin levels in plasma or DBS [32]. However, a study reported that erythropoietin was increased in serum at 48 hours in newborns with moderate/severe HIE when compared to those who did not develop or developed mild HIE [38]. In addition, this study associated increased levels of erythropoietin at 72 hours with death [38]. C-reactive protein (CRP) serum levels and their correlation to an outcome are unclear, since one study did not find significant differences [34], and other studies did not have control groups to compare [33], or their levels were associated with microcirculatory issues [37]. Vascular endothelial growth factor (VEGF) serum levels were decreased in newborns with moderate/severe injury at 24 hours [38], a tendency also verified in plasma samples, where VEGF-C was decreased at 24 hours compared to controls [42]. Levels of VEGF in umbilical cord serum did not vary significantly [18].
Finally, alanine aminotransferase (ALT) serum levels were increased in HIE newborns with an abnormal electroencephalogram (EEG) in the first 6 hours of life [34] and continued to increase at 48 hours compared to the control group [40]. Aspartate aminotransferase (AST) levels were also reported to be elevated in newborns with HIE at 48 hours [40], but no difference was found in the first 6 hours of life [34]. However, it should be taken into consideration that they lack neurological specificity [47].
Together, these data suggest that additional studies are still required to corroborate the findings presented in this review regarding GFAP, UCHL-1, ALT, and VEGF. Concerning urinary S100B, receiver operating characteristic (ROC) curves show high sensitivity and specificity to predict death and short outcomes [35]. In addition, S100B and NSE serum levels also showed good predictive power for short- [28] and long-term outcomes [36]. Therefore, these two proteins should be validated to provide further support in the diagnosis of HIE.
Metabolites are promising candidates for the diagnosis of hypoxic–ischemic encephalopathy but require further studies. The metabolites identified and altered in more than one body fluid are summarized in Fig. 2a. Plasma is the sample type that reported more biomarkers, while umbilical cord blood metabolites were only studied by one research group. The most studied metabolite was lactate, which was reported to be increased in urine in the first 6 hours of life on HIE compared to controls [43]. In addition, HIE newborns with a severe phenotype or pathological MRI showed increased lactate levels in the cord blood [45] and plasma [24] at birth, respectively. However, the levels of this metabolite were reported not to change significantly in whole blood [27] or serum [34] in the first hours of life. Creatinine and urea are reported to be elevated in serum samples of the HIE group at 48 hours [40] but not in whole blood at birth [44]. In plasma, creatinine was reported to be significantly altered [24].
Interestingly, glucose levels were reported to be significantly increased in the serum of newborns in the first hours of life with severe injury or abnormal EEG [34, 46], but Bersani and his team did not find differences in plasma levels [44]. Last, glutamine, succinate, pyruvate and α-ketoglutarate urine levels were found to be decreased in HIE patients in the first 6 hours of life [43], whereas in plasma, glutamine and α-ketoglutarate were reported to be altered at 48 hours and 72 hours, and succinate was altered at 24 hours, 48 hours, and 72 hours [24]. Regarding pyruvate, its levels were elevated at 72 hours in newborns who presented a pathological MRI [24]. Furthermore, there are studies that propose ratios of metabolites as putative biomarkers. In urine, the lactate/creatinine ratio was described to be significantly elevated in the first 24 hours of life [23], but no differences were found in the lactate/pyruvate ratio [24] in plasma or the free/total carnitine ratio in DBS [21]. Inconsistencies and the reduced number of studies impaired the identification of any metabolite as a putative biomarker. Nevertheless, lactate is a candidate that should be explored in future studies along with new in-depth screenings, considering that it is a systemic severity marker but not neuronal-specific [48].
New approaches to identify other classes of biomarkers
MicroRNAs (miRNAs) were studied as potential biomarkers [31]. However, no differences were found between HIE patients with favorable and unfavorable outcomes in Let7b, miR-21, miR-29b, miR-124, and mir-155 levels in DBS. Alterations in the number of blood cells were also evaluated as an approach to identify biomarkers. Nucleated red blood cells in the cord blood were increased in newborns with moderate/severe HIE [45], but no differences were found in neutrophils or white blood cells in the whole blood [33, 40]. Platelet levels did not show significant differences when comparing newborns with high CRP levels [33] but were decreased in the HIE group compared to the control group [40]. Furthermore, electrolyte serum levels were also studied. One study showed that sodium, calcium, and selenium levels were decreased in the HIE group, while potassium levels were increased [40]. Finally, studies concerning alterations in the abundance of RNA for several inflammation markers were also reported. Proliferator-activated receptor gamma (PPARG), matrix metallopeptidase 9 (MMP-9), interleukin (IL)-8, heat shock protein family A (Hsp70) member 1A (HSPA1A), and toll-like receptor 8 (TLR8) were found to be increased in the whole blood of the HIE group, while C–C motif chemokine receptor 5 (CCR5) was decreased [20]. Since these approaches to identify biomarkers are recent, due to the lack of corroborating evidence from different authors, no objective conclusion can be drawn about the use of the aforementioned analytes as potential biomarkers.
Plasma and serum potential biomarkers showed altered pathways in hypoxic–ischemic encephalopathy
Pieces of evidence from plasma and serum were combined for further pathway analysis to highlight potential mechanisms. Gene Ontology (GO) of extracted proteins showed several biological processes and molecular functions associated with inflammatory responses, as well as insulin-growth factor pathways (Fig. 2b, c). Specifically, the majority of the proteins associated with these ontologies were decreased in HIE newborns [42]. Concerning metabolite pathways, arginine and proline metabolism, urea cycle and lysine degradation were the most significantly altered (Fig. 2d). Finally, integrating protein and metabolite data, two pathways were identified as being modified in HIE: alanine, aspartate and glutamate metabolism and arginine and proline metabolism (Fig. 2e, f).
Discussion
Presently, no accurate tools are available to diagnose HIE immediately after birth and quickly define the best therapeutic approach. In addition, the guidelines for diagnosing HIE are not standardized among all pediatric centers. While verifying the eligibility criteria of each study, we found huge discrepancies between the parameters applied to diagnose HIE, assessment of severity, and time of sample collection. Together, this reinforces the need to establish standard diagnostic criteria worldwide. In this review, to reduce bias, the population was homogenized by (1) matching at least two ACOG diagnosis criteria and (2) having neuroimaging evidence of brain injury, since these data are unequivocal proof of brain damage. In addition, potentially biased studies were not excluded, since they did not focus on the major findings of this review.
This systematic review summarized the potential biomarkers for HIE. Reported results lack high-throughput screenings, which hampers the identification of a larger number of putative biomarkers. Briefly, serum is the most cited fluid, and proteins are the major candidates with more consistent results among the different studies. In particular, NSE and S100B were identified as potential biomarkers for HIE. Interestingly, not only these proteins but also UCHL-1 and GFAP were described as potential biomarkers for traumatic brain injury in adults [49], since they are also involved in brain damage mechanisms. NSE is a brain and peripheral neuroendocrine-specific enolase that is highly expressed in neurons [50]. The postinsult collapse of the plasma membrane, as after a perinatal asphyxia event, could cause the release of this protein to peripheral fluids. In particular, after ischemic stroke, NSE protein levels were positively correlated with the extent of brain damage [51]. However, it should be considered that altered levels of this protein might also be associated with the diagnosis of small cell lung cancer, among others [52]. S100B is a calcium-binding protein expressed by glia, especially astrocytes [53]. This protein is associated with intracellular structures, but it is also secreted, playing a role in cell survival (in nanomolar concentrations), apoptosis, lipid peroxidation (in micromolar concentrations) and cytokine production [53, 54]. In addition, a highlighted pathway in GO analysis was the insulin-like growth factor signaling pathway (Fig. 2b, c). Although a single research group [42] studied the identified proteins, this is a promising target, since Insulin-like growth factor 1 (IGF-1) has already been tested as a therapeutic approach, exhibiting good outcomes in a rat HIE model [55].
Regarding the direct inflammatory response, no differences were found in the analysis of a panel of cytokines at the protein level [18, 32]. However, when analyzing the RNA levels of inflammatory markers, five were increased, and one was decreased [33]. GO analysis also identified alterations in several inflammatory pathways (Fig. 2b, c). However, some proteins have opposite tendencies, reinforcing the need to clarify the relevance of these molecules in the diagnosis of HIE. Nevertheless, it should be taken into consideration that the elevation of IL-6, for example, has been described to be associated with neonatal sepsis [56], which could lead to a misleading diagnosis.
The alanine, aspartate, and glutamate pathways were found to be altered in newborns with HIE. These metabolites were already described to be increased in brain tissue in a rat model of traumatic brain injury [57]. However, it should be taken into consideration that dysregulation of alanine transaminase was associated with liver dysfunction in HIE [58], enhancing the need to consider systemic biomarkers. Glutamate and alanine were also described to be elevated in the CSF in an HIE piglet model [58], while an excitotoxicity mouse model identified proline and arginine as players in response to injury [59]. Based on these pieces of evidence, future studies should focus on the characterization of these pathways in HIE.
Studies using advanced imaging techniques, such as NIRS and 1H MRS, are emerging as promising noninvasive approaches to monitor newborns’ response to TH [26, 27, 30, 37, 41]. However, they refer to a low number of publications, focusing on a low number of patients and without healthy controls, and were classified as potentially biased according to the NOS scale. Although these techniques can provide more information about the metabolic state of the newborns, access to these specialized techniques might be easier at reference centers to treat HIE but not in all maternities due to financial and logistical reasons.
One of the weak points of this review is the lack of studies with healthy newborn controls (Fig. 1i). Due to ethical reasons, it is not possible to obtain samples from healthy newborns at several timepoints. As an alternative, studies use non-neurological brain-injured newborns or newborns who have suffered an episode of perinatal asphyxia but did not develop/or developed a mild brain injury. In either case, using these populations as controls can bias the conclusions. Another limitation is the lack of uniformization of the groups between the studies (Fig. 1i), which makes it more difficult to compare studies. Likewise, the lack of uniformity of sample collection time, which might be influenced by hypothermia, hindered drawing conclusions. Unfortunately, some of the studies analyzed lack transparency on the methodologies and the availability of raw data, which compromised data extraction and further analysis of the published data.
It should be noted that only a small number of studies performed screenings, which limits the amount of information extracted from the samples and, therefore, reduces the chances of identifying a biomarker. In addition, the lack of raw data available (even after direct request) impaired a more detailed analysis to determine the sensitivity and specificity of the identified biomarkers and assess their predictive value to diagnose HIE and/or predict its severity. Therefore, future studies should present a higher consistency in the diagnosis criteria, establishment of groups, preferably using healthy controls, and sample collection time, so that data presented in this manuscript can be corroborated and finally get to a routine clinical application.
In conclusion, elevated serum levels of NSE and S100B correlated with a worse prognosis in newborns suffering from HIE. Nevertheless, future studies should focus on determining the sensitivity and specificity of these molecules before entering clinical practice. In addition, since other molecules were identified as potential biomarkers, such as GFAP, UCHL1, ALT, glutamate and lactate, we suggest that future studies focus on identifying a panel of biomarkers instead of a standalone biomarker.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Inês Caramelo and Margarida Coelho contributed equally to this paper. Inês Caramelo wrote the manuscript and participated in the database search, articles selection, data extraction, quality assessment, and data analysis. Margarida Coelho participated in the database search, articles selection, quality assessment, data analysis and critically reviewed the article. Miguel Rosado participated in the data extraction of the final articles. Alexandra Dinis established clinically relevant criteria for articles selection and critically reviewed the manuscript. Carla Cardoso critically reviewed the manuscript. Carlos B. Duarte critically reviewed the manuscript. Mário Grãos critically reviewed the manuscript. Bruno Manadas supervised database search, article selection, data extraction and quality assessment, and critically reviewed the manuscript.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was financed by the European Regional Development Fund (ERDF), through the COMPETE 2020—Operational Programme for Competitiveness and Internationalization and Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia, under projects POCI-01–0145-FEDER-029311, POCI-01–0247-FEDER-045311, UIDB/04539/2020 and UIDP/04539/2020, and individual Ph.D. fellowships PD/BD/135178/2017 (Margarida Coelho), SFRH/BD/143442/2019 (Inês Caramelo), and 2020.07749.BD (Miguel Rosado).
Data Availability
All the data is provided on the tables of the manuscript, as well as in the supplementary data.
Declarations
Conflict of interest
The authors have no conflicts of interest to disclose. No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article."
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169:397–403. doi: 10.1001/jamapediatrics.2014.3269. [DOI] [PubMed] [Google Scholar]
- 3.Gunn AJ, Thoresen M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb Clin Neurol. 2019;162:217–237. doi: 10.1016/B978-0-444-64029-1.00010-2. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. NEJM. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasineni GK, Panigrahy N, Rath SN, Chinnaboina M, Konanki R, Chirla DK, et al. Diagnostic and therapeutic roles of the "Omics" in hypoxic-ischemic encephalopathy in neonates. Bioengineering (Basel, Switzerland) 2022;9:498. doi: 10.3390/bioengineering9100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Task Force onNeonatal Encephalopathy. TheAmerican College of Obstetricians and Gynecologists Neonatal encephalopathy and neurologic outcome, second edition. Pediatrics. 2014;133:e1482–e1488. doi: 10.1542/peds.2014-0724. [DOI] [Google Scholar]
- 7.Gunn AJ, Laptook AR, Robertson NJ, Barks JD, Thoresen M, Wassink G, et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr Res. 2017;81:202–209. doi: 10.1038/pr.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair J, Kumar VHS. Current and emerging therapies in the management of hypoxic ischemic encephalopathy in neonates. Children (Basel, Switzerland) 2018;5:99. doi: 10.3390/children5070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas-Escobar M, Weiss M. Biomarkers of hypoxic-ischemic encephalopathy in newborns. Front Neurol. 2012;3:144. doi: 10.3389/fneur.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caramelo I, Coelho M, Rosado M, Cardoso C, Dinis A, Duarte CB, et al. Biomarkers for the diagnosis of hypoxic-ischemic encephalopathy: a systematic review: PROSPERO 2021 CRD42021272610. 2021. Available: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=272610. Accessed 8 Nov 2021.
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: Ottawa Hospital Research Institute. 2021. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 3 Dec 2021.
- 13.Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R. InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015;16:169. doi: 10.1186/s12859-015-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 15.Bonnot T, Gillard MB, Nagel DH. A simple protocol for informative visualization of enriched Gene Ontology terms. Bioprotocol. 2019;9:e3429. [Google Scholar]
- 16.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ennen CS, Huisman TA, Savage WJ, Northington FJ, Jennings JM, Everett AD, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol. 2011;205(251):e1–7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalak LF, Sánchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for Severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014;164:468–74.e1. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akamatsu T, Sugiyama T, Aoki Y, Kawabata K, Shimizu M, Okazaki K, et al. A pilot study of soluble form of LOX-1 as a novel biomarker for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2019;206:49–55.e3. doi: 10.1016/j.jpeds.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Balada R, Tebe C, Leon M, Arca G, Alsina M, Castells AA, et al. Enquiring beneath the surface: can a gene expression assay shed light into the heterogeneity among newborns with neonatal encephalopathy? Pediatr Res. 2020;88:451–458. doi: 10.1038/s41390-020-0764-2. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Suarez O, Concheiro-Guisan A, Sanchez-Pintos P, Cocho JA, Fernandez Lorenzo JR, Couce ML. Acylcarnitine profile in neonatal hypoxic-ischemic encephalopathy: the value of butyrylcarnitine as a prognostic marker. Medicine (Baltimore) 2019;98:e15221. doi: 10.1097/MD.0000000000015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas-Escobar M, Yang C, Bennett J, Shuster J, Theriaque D, Leibovici A, et al. A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatr Res. 2010;68:531–536. doi: 10.1203/PDR.0b013e3181f85a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh W, Perritt R, Shankaran S, Merritts M, Donovan EF, Ehrenkranz RA, et al. Association between urinary lactate to creatinine ratio and neurodevelopmental outcome in term infants with hypoxic-ischemic encephalopathy. J Pediatr. 2008;153:375–378. doi: 10.1016/j.jpeds.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pineiro-Ramos JD, Nunez-Ramiro A, Llorens-Salvador R, Parra-Llorca A, Sanchez-Illana A, Quintas G, et al. Metabolic phenotypes of hypoxic-ischemic encephalopathy with normal vs. pathologic magnetic resonance imaging outcomes. Metabolites. 2020;10:109. doi: 10.3390/metabo10030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massaro AN, Jeromin A, Kadom N, Vezina G, Hayes RL, Wang KK, et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr Crit Care Med. 2013;14:310–317. doi: 10.1097/PCC.0b013e3182720642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra S, Bale G, Meek J, Uria-Avellanal C, Robertson NJ, Tachtsidis I. Relationship between cerebral oxygenation and metabolism during rewarming in newborn infants after therapeutic hypothermia following hypoxic-ischemic brain injury. Adv Exp Med Biol. 2016;923:245–251. doi: 10.1007/978-3-319-38810-6_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain SV, Pagano L, Gillam-Krakauer M, Slaughter JC, Pruthi S, Engelhardt B. Cerebral regional oxygen saturation trends in infants with hypoxic-ischemic encephalopathy. Early Hum Dev. 2017;113:55–61. doi: 10.1016/j.earlhumdev.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Massaro AN, Chang T, Kadom N, Tsuchida T, Scafidi J, Glass P, et al. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. J Pediatr. 2012;161:434–440. doi: 10.1016/j.jpeds.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggiotto LV, Sondhi M, Shin BC, Garg M, Devaskar SU. Circulating blood cellular glucose transporters - surrogate biomarkers for neonatal hypoxic-ischemic encephalopathy assessed by novel scoring systems. Mol Genet Metab. 2019;127:166–173. doi: 10.1016/j.ymgme.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehaes M, Aggarwal A, Lin PY, Rosa Fortuno C, Fenoglio A, Roche-Labarbe N, et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J Cereb Blood Flow Metab. 2014;34:87–94. doi: 10.1038/jcbfm.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponnusamy V, Kapellou O, Yip E, Evanson J, Wong LF, Michael-Titus A, et al. A study of microRNAs from dried blood spots in newborns after perinatal asphyxia: a simple and feasible biosampling method. Pediatr Res. 2016;79:799–805. doi: 10.1038/pr.2015.276. [DOI] [PubMed] [Google Scholar]
- 32.Massaro AN, Wu YW, Bammler TK, MacDonald JW, Mathur A, Chang T, et al. Dried blood spot compared to plasma measurements of blood-based biomarkers of brain injury in neonatal encephalopathy. Pediatr Res. 2019;85:655–661. doi: 10.1038/s41390-019-0298-7. [DOI] [PubMed] [Google Scholar]
- 33.Saito J, Shibasaki J, Shimokaze T, Kishigami M, Ohyama M, Hoshino R, et al. Temporal relationship between serum levels of interleukin-6 and c-reactive protein in therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. Am J Perinatol. 2016;33:1401–1406. doi: 10.1055/s-0036-1583192. [DOI] [PubMed] [Google Scholar]
- 34.Jones R, Heep A, Odd D. Biochemical and clinical predictors of hypoxic-ischemic encephalopathy after perinatal asphyxia. J Matern Fetal Neonatal Med. 2018;31:791–796. doi: 10.1080/14767058.2017.1297790. [DOI] [PubMed] [Google Scholar]
- 35.Alshweki A, Perez-Munuzuri A, Lopez-Suarez O, Bana A, Couce ML. Relevance of urinary S100B protein levels as a short-term prognostic biomarker in asphyxiated infants treated with hypothermia. Medicine (Baltimore) 2017;96:e8453. doi: 10.1097/MD.0000000000008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massaro AN, Chang T, Baumgart S, McCarter R, Nelson KB, Glass P. Biomarkers S100B and neuron-specific enolase predict outcome in hypothermia-treated encephalopathic newborns*. Pediatr Crit Care Med. 2014;15:615–622. doi: 10.1097/PCC.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredly S, Nygaard CS, Skranes JH, Stiris T, Fugelseth D. Cooling effect on skin microcirculation in asphyxiated newborn infants with increased c-reactive protein. Neonatology. 2016;110:270–276. doi: 10.1159/000446763. [DOI] [PubMed] [Google Scholar]
- 38.Sweetman DU, Onwuneme C, Watson WR, Murphy JF, Molloy EJ. Perinatal asphyxia and erythropoietin and VEGF: serial serum and cerebrospinal fluid responses. Neonatology. 2017;111:253–259. doi: 10.1159/000448702. [DOI] [PubMed] [Google Scholar]
- 39.Ezgu FS, Atalay Y, Gucuyener K, Tunc S, Koc E, Ergenekon E, et al. Neuron-specific enolase levels and neuroimaging in asphyxiated term newborns. J Child Neurol. 2002;17:824–829. doi: 10.1177/08830738020170111301. [DOI] [PubMed] [Google Scholar]
- 40.El-Mazary AA, Abdel-Aziz RA, Mahmoud RA, El-Said MA, Mohammed NR. Correlations between maternal and neonatal serum selenium levels in full term neonates with hypoxic ischemic encephalopathy. Ital J Pediatr. 2015;41:83. doi: 10.1186/s13052-015-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bale G, Mitra S, Meek J, Robertson N, Tachtsidis I. A new broadband near-infrared spectroscopy system for in vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed Opt Express. 2014;5:3450–3466. doi: 10.1364/BOE.5.003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaikh H, Boudes E, Khoja Z, Shevell M, Wintermark P. Angiogenesis dysregulation in term asphyxiated newborns treated with hypothermia. PLoS ONE. 2015;10:e0128028. doi: 10.1371/journal.pone.0128028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Locci E, Noto A, Puddu M, Pomero G, Demontis R, Dalmazzo C, et al. A longitudinal 1H-NMR metabolomics analysis of urine from newborns with hypoxic-ischemic encephalopathy undergoing hypothermia therapy. Clinical and medical legal insights. PLoS ONE. 2018;13:e0194267. doi: 10.1371/journal.pone.0194267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersani I, Ferrari F, Lugli L, Ivani G, Conio A, Moataza B, et al. Monitoring the effectiveness of hypothermia in perinatal asphyxia infants by urinary S100B levels. Clin Chem Lab Med. 2019;57:1017–1025. doi: 10.1515/cclm-2018-1094. [DOI] [PubMed] [Google Scholar]
- 45.Haiju Z, Suyuan H, Xiufang F, Lu Y, Sun R. The combined detection of umbilical cord nucleated red blood cells and lactate: early prediction of neonatal hypoxic ischemic encephalopathy. J Perinat Med. 2008;36:240–247. doi: 10.1515/JPM.2008.035. [DOI] [PubMed] [Google Scholar]
- 46.Chouthai NS, Sobczak H, Khan R, Subramanian D, Raman S, Rao R. Hyperglycemia is associated with poor outcome in newborn infants undergoing therapeutic hypothermia for hypoxic ischemic encephalopathy. J Neonatal Perinatal Med. 2015;8:125–131. doi: 10.3233/NPM-15814075. [DOI] [PubMed] [Google Scholar]
- 47.Choudhary M, Sharma D, Dabi D, Lamba M, Pandita A, Shastri S. Hepatic dysfunction in asphyxiated neonates: prospective case-controlled study. Clin Med Insights Pediatr. 2015;9:1–6. doi: 10.4137/CMPed.S21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu YF, Wu PM, Yu WH, Li CI, Wu CL, Kang L, et al. Lactate predicts neurological outcomes after perinatal asphyxia in post-hypothermia era: a prospective cohort study. Life (Basel) 2021;11:1193. doi: 10.3390/life11111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amoo M, Henry J, O'Halloran PJ, Brennan P, Husien MB, Campbell M, et al. S100B, GFAP, UCH-L1 and NSE as predictors of abnormalities on CT imaging following mild traumatic brain injury: a systematic review and meta-analysis of diagnostic test accuracy. Neurosurg Rev. 2022;45:1171–1193. doi: 10.1007/s10143-021-01678-z. [DOI] [PubMed] [Google Scholar]
- 50.Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–295. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- 51.Oh SH, Lee JG, Na SJ, Park JH, Choi YC, Kim WJ. Prediction of early clinical severity and extent of neuronal damage in anterior-circulation infarction using the initial serum neuron-specific enolase level. Arch Neurol. 2003;60:37–41. doi: 10.1001/archneur.60.1.37. [DOI] [PubMed] [Google Scholar]
- 52.Isgro MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125–143. doi: 10.1007/978-94-017-7215-0_9. [DOI] [PubMed] [Google Scholar]
- 53.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, et al. S100B's double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793:1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 55.Peeples ES, Dafferner A, Jiang J, Lyden E, Punsoni M, Agrawal DK. Combined treatment with insulin-like growth factor 1 and amd3100 improves motor outcome in a murine model of neonatal hypoxic-ischemic encephalopathy. Dev Neurosci. 2019;41:255–262. doi: 10.1159/000505264. [DOI] [PubMed] [Google Scholar]
- 56.Qiu X, Zhang L, Tong Y, Qu Y, Wang H, Mu D. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of the membranes: a meta-analysis. Medicine (Baltimore) 2018;97:e13146. doi: 10.1097/MD.0000000000013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amorini AM, Lazzarino G, Di Pietro V, Signoretti S, Lazzarino G, Belli A, et al. Severity of experimental traumatic brain injury modulates changes in concentrations of cerebral free amino acids. J Cell Mol Med. 2017;21:530–542. doi: 10.1111/jcmm.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusaka T, Matsuura S, Fujikawa Y, Okubo K, Kawada K, Namba M, et al. Relationship between cerebral interstitial levels of amino acids and phosphorylation potential during secondary energy failure in hypoxic-ischemic newborn piglets. Pediatr Res. 2004;55:273–279. doi: 10.1203/01.PDR.0000102702.39608.82. [DOI] [PubMed] [Google Scholar]
- 59.Blaise BJ, Schwendimann L, Chhor V, Degos V, Hodson MP, Dallmann G, et al. Persistently altered metabolic phenotype following perinatal excitotoxic brain injury. Dev Neurosci. 2017;39:182–191. doi: 10.1159/000464131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data is provided on the tables of the manuscript, as well as in the supplementary data.