Abstract
Background
Incomplete antiretroviral therapy (ART) adherence has been linked to deleterious immunologic, inflammatory, and clinical consequences, even among virally suppressed (<50 copies/mL) persons with human immunodeficiency virus (PWH). The impact of improving adherence in the risk of severe non-AIDS events (SNAEs) and death in this population is unknown.
Methods
We estimated the reduction in the risk of SNAEs or death resulting from an increase in ART adherence by (1) applying existing data on the association between adherence with high residual inflammation/coagulopathy in virally suppressed PWH, and (2) using a Cox proportional hazards model derived from changes in plasma interleukin 6 (IL-6) and D-dimer from 3 randomized clinical trials. Comparatively, assuming 100% ART adherence in a PWH who achieves viral suppression, we estimated the number of persons in whom a decrease in adherence to <100% would need to be observed for an additional SNAE or death event to occur during 3- and 5-year follow-up.
Results
Increasing ART adherence to 100% in PWH who are suppressed on ART despite imperfect adherence translated into a 6%–37% reduction in the risk of SNAEs or death. Comparatively, based on an anticipated 12% increase in IL-6, 254 and 165 PWH would need to decrease their adherence from 100% to <100% for an additional event to occur over 3- and 5-year follow-up, respectively.
Conclusions
Modest gains in ART adherence could have clinical benefits beyond virologic suppression. Increasing ART adherence (eg, via an intervention or switch to long-acting ART) in PWH who remain virally suppressed despite incomplete adherence should be evaluated.
Keywords: adherence, coagulopathy, HIV, inflammation, viral suppression
In the early days of antiretroviral therapy (ART), high adherence (ie, ≥95%) was typically required to achieve and sustain viral suppression, mainly due to the unfavorable pharmacokinetics of older antiretrovirals [1]. However, with advances in modern ART, adherence as low as 80%–85% (or even lower) [2–6] is likely sufficient to achieve this goal, prevent human immunodeficiency virus (HIV)–related disease progression, and inhibit HIV transmission. This pharmacologic “forgiveness” is advantageous because it allows for missed doses without penalty on viral suppression, aligns with the adherence barriers encountered in life (eg, competing priorities, pill fatigue, structural barriers), and supports the premise of “Undetectable = Untransmittable [7]. Yet, the clinical consequences of achieving viral suppression (typically defined as achieving a viral load <20 or <50 copies/mL in plasma) with imperfect adherence, which potentially allows low-level viral replication [8–10], remain poorly understood.
Evidence is emerging that incomplete (ie, <100%) adherence to ART, even if sufficient to achieve and sustain viral suppression through modern clinical cutoffs, is associated with deleterious immunologic and clinical consequences, including higher residual inflammation [11–15], immune activation [13, 16], coagulopathy [13, 15–17], non–cardiovascular disease (CVD) mortality [17], and progression of coronary stenosis [18]. However, the absolute risk reduction in clinical events for each quantifiable change in treatment adherence is unknown. To address this gap, we combined findings from clinical studies of ART adherence and biomarkers of inflammation and coagulopathy (ie, IL-6 and D-dimer) with data that translate changes in these biomarkers with clinical events; our goal was to estimate the effect that a change in ART adherence would have on serious non-AIDS events (SNAEs) and death in persons with HIV (PWH) who are virologically suppressed.
METHODS
Literature Review
To encompass all data available in the topic, we first performed a literature review of studies that reported an association between ART adherence and biomarkers of inflammation, immune activation, or coagulopathy. This was not intended to be a systematic review, but rather a literature search of published literature in the topic to include all studies that reported plasma concentrations of inflammatory biomarkers according to different ART adherence thresholds limited to PWH with viral suppression (to reduce the known effect of viremia on these biomarkers) [19]. This literature search was conducted on 24 August 2022 in PubMed and Google Scholar and included the following terms: HIV, human immunodeficiency virus, adherence, inflammation, immune activation, coagulopathy, interleukin 6 (IL-6), and D-dimer. Only studies that quantified IL-6 and/or D-dimer were included because these biomarkers were utilized in a previously published Cox proportional hazard model by Grund et al [20], which modeled data from the control arms from 3 landmark studies (SMART [Strategies for Management of Antiretroviral Therapy] [21]; ESPRIT [International Study to Evaluate Recombinant Interleukin-2 in HIV Positive Patients Taking Antiretroviral Therapy] [22]; and SILCAAT [Interleukin-2 Plus Antiretroviral Therapy for HIV-Infected Patients With Low CD4+ Counts] [22]), and established a strong association between these biomarkers and SNAEs or death among PWH with suppressed virus.
Statistical Analysis
Data Synthesis
We graphically combined findings from the Cox model by Grund et al [20] with estimated percentage differences in IL-6 and D-dimer between PWH with high (ie, 100%) and low (ie, <100%) adherence [11–15, 23] to illustrate the impact that increasing ART adherence may have in the risk of SNAEs or death (CVD, end-stage renal disease, decompensated liver cirrhosis, non-AIDS cancer, and death due to any cause) [20]. Percentage reductions assume that the estimated decrease in IL-6 and D-dimer are relative to a baseline value.
Statistical Modeling
Conversely to our estimation on the risk reduction on SNAE or death driven by an increase in adherence, we also aimed to estimate the number of PWH who would need to reduce adherence before an additional SNAE or death were to be observed. This approach was selected as we assumed that most PWH who achieve viral suppression are highly adherent to ART at some point during the course of their treatment (eg, early stage of treatment initiation) [24, 25], but that a decrease in adherence—without the development of viremia—is expected in some patients [2, 3]. To achieve this, we used a simplified version of the Markov model developed by Serrano-Villar et al [26], where we inputted the change in biomarkers available in the literature to estimate the number of PWH who would need to reduce adherence before an additional SNAE or death is observed. This model relied on previously published Kaplan-Meier curves illustrating the cumulative number of participants with an event by IL-6 quartile [20], with separate Weibull distributions fit to each quartile specific curve, from which in-year probabilities of a SNAE or death were derived. In addition to the model by Serrano-Villar et al [26], we utilized least squares optimization to fit gamma distributions to IL-6 quartiles published by Grund et al [20], and subsequent movement between quartiles was based on previously reported elevated plasma IL-6 levels for those with <100% ART adherence. We assumed a one-time increase in plasma IL-6 with subsequent events predicted over a 3- or 5-year follow-up.
RESULTS
Data on the Association Between Suboptimal ART Adherence, Inflammation, and Coagulopathy
Based on our literature review, we identified several studies in which the association between suboptimal ART adherence and high residual inflammation was observed. In the Multicenter AIDS Cohort Study (MACS), data from 921 men with HIV who were virologically suppressed on ART to <50 copies/mL were analyzed [11]. ART adherence was quantified by self-report in the preceding 4 days and assessed whether this pattern was typical in the period between study visits (6 months). In this analysis, <100% ART adherence was associated with higher biomarkers of inflammation in the range of 11%–21% relative to 100% adherence after multivariate adjustment [11]. In particular, men who reported <100% ART adherence had 12% higher plasma IL-6 compared to those with 100% adherence (D-dimer was not evaluated) (Table 1) [11], and the association with interferon-γ was more pronounced among men taking a nonnucleoside reverse transcriptase inhibitor–based regimen when compared to men taking a ritonavir-boosted protease inhibitor–based regimen [11]. When <100% adherence was dichotomized as <85% versus 85%–99%, these associations were similar overall but driven mostly by <85% adherence (16% higher concentrations in IL-6 in men who reported <85% adherence compared to those with 100% adherence) [11]. In a follow-up analysis in this same cohort, using data from 1,469 men (including men with HIV who reported <100% ART adherence and men without HIV), <100% ART adherence was associated with 17% higher plasma concentrations of IL-6 when compared to men without HIV (Table 1) [23]. Interestingly, in this additional analysis, there was no significant difference in the concentrations of IL-6 in men with HIV who reported 100% adherence when compared to men without HIV (6% [95% confidence interval {CI}, −.9% to 13%]), suggesting that optimal adherence may minimize residual immune activation in HIV to modest or negligible levels [23].
Table 1.
Summary of Available Data Demonstrating an Association Between Suboptimal Antiretroviral Adherence and Inflammation/Coagulopathy in Persons With HIV Who Are Virally Suppressed While on Antiretroviral Therapy
| Cohort | Adherence Measure |
No. | Population Compared | Viral Suppression Threshold, Copies/mL |
IL-6, % Difference (95% CI) |
D-dimer, % Difference (95% CI) |
Reference |
|---|---|---|---|---|---|---|---|
| MACS | Self-report (4-day) | 921 | Men reporting <85% vs 100% adherence |
<50 | 12% higher (2%–22%) |
Not assessed | [11] |
| MACS | Self-report (4-day) | 1469 | Men reporting <100% adherence vs men without HIV | <50 | 17% higher (6%–29%) |
Not assessed | [23] |
| SMART | Self-report (7-day) | 3056 | PWH reporting <100% vs 100% adherence |
<200 | 9% higher (1%–18%) |
11% higher (11%–22%) |
[15] |
| START | Self-report | 1627 | PWH reporting <100% vs 100% adherence |
<50 | 12% higher (0–26%) |
4% lower (ns) (−13% to 7%) |
[12] |
| UARTO | MEMS caps | 282 | Change for every 10% increase in ART adherence | <400 | 15% decrease (−8% to −21%) |
11% decrease (−2% to −18%) |
[13] |
| UARTO | Adherence interruptions | 282 | Change for every 9 days on a treatment interruption | <400 | 14% increase (4%–26%) |
10% increase (3%–18%) |
[16] |
| META (early stage) |
Electronic monitoring (Wisepill) | 488 | Change for every 10% increase in ART adherence | <400 | 6% decrease (−2% to −10%) |
4% decrease (ns) (−10% to 1%) |
[14] |
| META (late stage) |
Electronic monitoring (Wisepill) | 488 | Change for every 10% increase in ART adherence | <400 | 4% decrease (−7% to −.2%) |
3% decrease (ns) (−8% to 2%) |
[14] |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IL-6, interleukin 6; MACS, Multicenter AIDS Cohort Study; MEMS, Medication Event Monitoring System; META, Monitoring of Early Treatment Adherence; ns, not significant; PWH, persons with human immunodeficiency virus; SMART, Strategies for Management of Antiretroviral Therapy; START, Strategic Timing of Antiretroviral Therapy; UARTO, Uganda AIDS Rural Treatment Outcomes.
Following the observations in MACS, the association between suboptimal adherence and increased inflammation was confirmed in PWH enrolled in 2 large randomized clinical trials where adherence was measured by 7-day self-report. Using baseline data from 3,056 participants enrolled in the SMART study who were virologically suppressed to <200 copies/mL, plasma concentrations of IL-6 and D-dimer were 9% and 11% higher in participants who reported suboptimal versus 100% adherence, respectively, after adjustment (Table 1) [15]. This was followed by an analysis of 1,627 participants who initiated ART as part of the Strategic Timing of Antiretroviral Treatment (START) study and who achieved virologic suppression (<50 copies/mL) at the 8-month visit. In that analysis, and after adjusting for covariates, plasma concentrations of IL-6 were 12% higher in participants reporting <100% versus 100% adherence, without any significant differences observed in D-dimer [12].
The relationship between ART adherence with residual inflammation and coagulopathy has also been evaluated using more objective adherence measures, such as electronic adherence monitoring. In the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort, data were analyzed from 282 treatment-naive PWH who achieved viral suppression (<400 copies/mL) within 6 months after treatment initiation and in whom adherence was measured using Medication Event Monitoring System electronic pill bottles [13]. In this study, for every 10% increase in average ART adherence, there was a 15% and 11% decrease in IL-6 and D-dimer, respectively, after adjustment for covariates (Table 1) [13]. A subsequent analysis in this cohort found a 14% and 10% increase in IL-6 and D-dimer, respectively, for every 9 days spent in an ART adherence interruption (Table 1) [16]. A similar analysis was conducted in the Monitoring Early Treatment Adherence (META) study, which enrolled treatment-naive PWH who initiated ART in Uganda and South Africa during early-stage (CD4+ T-cell count >350 cells/µL) or late-stage (CD4+ T-cell count <200 cells/µL) disease and utilized a real-time electronic monitoring pill container to measure adherence (Wisepill) [14]. Among individuals in the META study population who achieved and sustained viral suppression (<400 copies/mL) at 6 and 12 months (n = 488), a 10% higher average ART adherence was associated with a 3% lower IL-6 at the 12-month visit in the adjusted analysis (Table 1). This finding was consistent in PWH who initiated treatment both in early-stage (6% lower) and late-stage (4% lower) disease. No significant associations with D-dimer were observed (Table 1) [14].
While the data supporting the association between suboptimal ART adherence with high residual activation and coagulopathy in the setting of viral suppression are robust, the clinical translation of this relationship is poorly understood and has only been evaluated in 2 studies. Within the Swiss HIV Cohort study, an analysis in 6,971 PWH who were virally suppressed (<50 copies/mL) demonstrated that individuals who reported suboptimal adherence had a significantly increased risk of non-CVD-related mortality in an adjusted competing risk model, with a hazard ratio of 1.44 and 2.21 if they reported missing ≥1 and ≥2 ART doses in the preceding month, respectively [17]. In this analysis, PWH with suboptimal adherence also exhibited an increased risk of CVD events, although this difference was not statistically significant (hazard ratio, 1.23 [95% CI, .85–1.8]) [17]. Recently, an analysis in the MACS demonstrated that viremia was associated with greater progression of coronary stenosis in men with HIV using serial computed tomography angiography [18]. Interestingly, when limiting this analysis to the 212 men with HIV who were virologically suppressed (<50 copies/mL), imperfect ART adherence was found to be associated with disease progression; within this group, men who reported <100% adherence had greater progression of coronary stenosis (relative risk, 1.91) compared with men who reported 100% adherence [18].
Data Synthesis of the Risk of SNAEs and Death With Percentage Change in IL-6 and D-Dimer
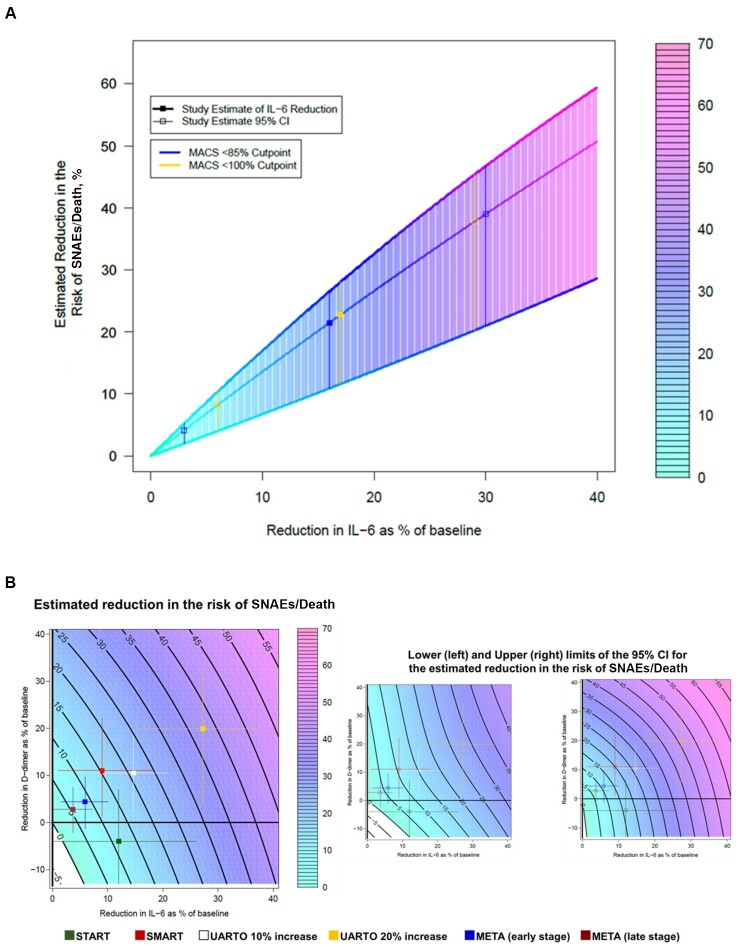
Based on the previously published model by Grund et al [20], we initially utilized the point estimates observed in the MACS (which only quantified IL-6) [11] and estimated that increasing ART adherence from <100% to 100% and from <85% to 100% could reduce the risk of SNAE or death by approximately 21%–23%, respectively (Table 2 and Figure 1A). By comparison, using results from SMART [15] and START [12], we found that achieving 100% adherence would reduce the risk of an event by approximately 15% and 11%, respectively (Table 2 and Figure 1B). Based on the results from META (early- and late-disease-stage groups) [14], an increase in 10% adherence (eg, from 90% to 100%) would translate into a 9% and 6% risk reduction, respectively (Table 2 and Figure 1B). Finally, using results from UARTO [13], a 10% increase in adherence (from 90% to 100%) would result in a reduction of approximately 21% in the risk of SNAEs or death, whereas an adherence increase in 20% (from 80% to 100%) could reduce this risk by approximately 37% (Table 2 and Figure 1B).
Table 2.
Modeled Reduction in the Risk of a Severe Non-AIDS Event and/or Death Based on the Change in Plasma Interleukin 6 and D-Dimer for Persons With HIV Who Are Virally Suppressed and Increase Their Antiretroviral Adherence
| Cohort | Increase in ART Adherence | Reduction in the Risk of SNAE or Death (95% CI) |
|---|---|---|
| UARTO | 20% | 37% (28%–46%) |
| MACS | <100%–100% | 23% (12%–28%) |
| MACS | <85%–100% | 21% (11%–27%) |
| UARTO | 10% | 21% (15%–26%) |
| SMART | <100%–100% | 15% (11%–20%) |
| START | <100%–100% | 11% (6%–16%) |
| META (early stage) | 10% | 9% (6%–11%) |
| META (late stage) | 10% | 6% (4%–8%) |
Source: Based on a Cox proportional hazard model by Grund et al [20].
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; MACS, Multicenter AIDS Cohort Study; META, Monitoring of Early Treatment Adherence; SMART, Strategies for Management of Antiretroviral Therapy; SNAE, severe non-AIDS event; START, Strategic Timing of Antiretroviral Therapy; UARTO, Uganda AIDS Rural Treatment Outcomes.
Figure 1.
Modeled (95% confidence interval [CI]) reduction in the risk of a severe non-AIDS event or death as a result of an increase in antiretroviral therapy (ART) adherence (ie, intervention) based on a Cox proportional hazard model by Grund et al [20]. A, Risk reduction based on data from the Multicenter AIDS Cohort Study that used self-reported adherence. B, Risk reduction based on data from observational cohorts in Africa and interventional clinical trials that used self-reported adherence and electronic adherence monitoring. Solid black lines represent predicted risk reductions (eg, 10%, 20%). Filled squares represent the estimate, and the colored lines represent the 95% CI for each estimate. Open squares represent the 95% CI upper and lower limits of the estimated risk reduction with their corresponding 95% CI. Uganda Aids Rural Treatment Outcomes (UARTO) 10% and UARTO 20% increase represent a 10% and 20% increase in ART adherence, respectively. Adapted from Grund et al [20]. Abbreviations: CI, confidence interval; IL-6, interleukin 6; MACS, Multicenter AIDS Cohort Study; META, Monitoring of Early Treatment Adherence; SMART, Strategies for Management of Antiretroviral Therapy; SNAE, severe non-AIDS event; START, Strategic Timing of Antiretroviral Therapy; UARTO, Uganda Aids Rural Treatment Outcomes.
Estimated Number of PWH Who Need to Reduce ART Adherence for an Additional SNAE or Death to Occur
In the MACS, men who reported <100% ART adherence had 12% (95% CI, 2%–22%) higher plasma IL-6 concentrations compared to those with 100% adherence [11]. Assuming an initial average increase of 12% in plasma concentrations of IL-6—and comparing SNAEs or death to a population with no change—a reduction in adherence would need to be witnessed in 165 PWH for an additional SNAE or death event to occur over a 5-year follow-up (Table 3). When considering a 3-year follow-up, an additional event is estimated to occur after 254 PWH decrease their adherence. Comparatively, assuming a 22% increase in plasma concentrations of IL-6 (ie, higher estimate of the CI [11]), 1 additional SNAE or death would be observed after 146 and 95 PWH decreased their adherence to <100% over a 3-year and 5-year follow-up period, respectively (Table 3). The additional predicted numbers of individuals corresponding to a lower range in the increase in plasma concentrations of IL-6 observed in the MACS [11] are shown in Table 3.
Table 3.
Estimated a Number of Virally Suppressed Persons With HIV on Antiretroviral Therapy (ART) Who Would Need to Reduce ART Adherence From 100% to <100% to Observe an Additional Severe Non-AIDS Event or Death Over 3 and 5 Years Based on the Change in Plasma Interleukin 6
| Change in IL-6 Driven by a Decrease in ART Adherence | 3-Year Follow-up | 5-Year Follow-up |
|---|---|---|
| 2% increase | 1,001 PWH | 637 PWH |
| 12% increase | 254 PWH | 165 PWH |
| 22% increase | 146 PWH | 95 PWH |
Changes in plasma IL-6 represent the model-estimated 95% confidence interval levels from the Multicenter AIDS Cohort Study [11].
Abbreviations: ART, antiretroviral therapy; IL-6, interleukin 6; PWH, people with human immunodeficiency virus.
Assumes that PWH who achieve viral suppression are 100% adherent to ART but that a decrease in adherence—without the development of viremia—is expected in some patients.
DISCUSSION
Extrapolating data that relate imperfect ART adherence to increased inflammation, and high inflammation to adverse outcomes, we demonstrate that achieving optimal ART adherence among PWH who are virally suppressed could have substantial benefits in terms of reductions in SNAEs and death. For example, our model estimates that increasing adherence from 90% to 100% could lead to an estimated reduction in the composite outcome of SNAE or death of 6% (META) to 21% (UARTO), which could further increase up to a 37% reduction for a 20% increase in adherence (UARTO). Comparatively, considering that most (if not all) PWH who achieve viral suppression are 100% adherent to ART, as few as 95 PWH would need to decrease their adherence to observe 1 new SNAE or death. These data provide a strong rationale for interventions to further improve adherence to ART in a patient population in whom it would have been otherwise been overlooked (ie, PWH with an undetectable HIV RNA load).
While wide, the potential reduction in the risk of SNAE or death in the range of 6%–37% gained through improved adherence is comparable to that of statins (20% lower risk of cardiovascular death) [27] or aspirin (22% reduction in nonfatal myocardial infarction) [28] in the general population. It is likely that this broad range of risk reduction was due to the diverse studies utilized in this modeling, which included distinct patient populations on different antiretrovirals. Our results are also similar to data on adherence to other cardiovascular preventive therapies, which also show a dose response in terms of reductions in events [29]. Nonetheless, clinical data directly linking improved adherence to clinical outcomes, or a potential clinical trial using an intervention to improve adherence in PWH with imperfect adherence in the setting of suppression, are needed to better support these modeled estimates. Outside of the need for an interventional trial, studies of surrogate endpoints (eg, biomarkers of inflammation and preclinical disease) could assess the impact of adherence beyond viral suppression. In fact, several recent randomized studies, including the QUATUOR and BREATHER studies, have established noninferiority of less-than-daily ART dosing in well-controlled PWH for maintenance of viral suppression, without an increase in biomarkers of inflammation, immune activation, or coagulopathy in the non-daily ART arm [4, 30]. However, the study populations in these trials were followed for short periods of time and were highly selected for long-standing suppression with specific ART regimens, which may not represent the majority of ART-treated PWH. In comparison, a recent modeling study projected that 2-drug ART regimens, which are theoretically less potent, are associated with a long-term increase in inflammation (IL-6) [31]. Further studies to assess the benefits of improving ART adherence beyond viral suppression, including the switching of PWH who are virally suppressed but <100% adherent to long-acting injectables, are needed.
Reducing residual inflammation, coagulopathy, and the risk of SNAEs and death is of growing importance for PWH, particularly because SNAEs are increasingly the most common cause of morbidity among PWH and interventions (eg, statins, anti-inflammatories, aspirin) aimed at reducing them have been mostly unsuccessful [32]. Moreover, increasing ART adherence could be theoretically achieved without introducing new medications, reducing the risk for polypharmacy (which could further decrease ART adherence [33]), and without anticipating additional toxicity. Health education about the positive secondary effects or improving adherence could serve as a framework for clinicians and patients to address imperfect adherence, in a similar fashion that the 10-year cardiovascular risk assessment does when discussing smoking cessation or blood pressure control [34], or to discuss initiation of long-acting antiretroviral therapy when imperfect adherence is identified, even if viral suppression is sustained. For patients, understanding the risk reduction associated with high adherence could increase their awareness of the health implications of missed doses, even if they remain undetectable.
While our model provides a clinically relevant translation for improving ART adherence in PWH who are virally suppressed, limitations to this anticipated effect must be outlined. First, we modeled 2 sets of correlated data on adherence and inflammation, and 1 separate set of data on specific inflammatory biomarkers on clinical outcomes. In particular, it is possible that referenced studies could have underestimated adherence (and the observed associations) since they mostly used short-term self-report as their adherence measure. Similarly, the limited use of integrase strand transfer inhibitors in these reports would limit the interpretability of these data in particular, given their high antiviral potency. To date, data directly linking adherence with clinical outcomes among PWH who are virologically suppressed, or studies focusing on other biomarkers (eg, soluble CD163 or soluble tumor necrosis factor receptors) and modern ART regimens (ie, integrase inhibitor–based ART), are lacking. Second, confounders could also explain these relationships. For example, higher ART adherence could be a surrogate marker of positive health behaviors (ie, no smoking, frequent exercise, healthy diet) or higher adherence to other pharmacologic interventions, such as aspirin or statins. In addition, we did not assess the effect of sex in our findings, as most of the data used were derived from men. This is of particular importance given recent findings demonstrating that residual inflammation may be higher in women and that their relationship with clinical outcomes may be stronger [35]. Third, our proposed reduction in SNAEs or death requires mechanistic confirmation to demonstrate that high adherence can lead to lower inflammation via less cumulative viral replication in multiple tissues (including time between viral load assessments) and, in turn, to a reduced risk of events. Further research to elucidate the mechanisms behind these associations is needed.
In conclusion, suboptimal ART adherence, even if sufficient to achieve and sustain viral suppression, is associated with high residual inflammation and coagulopathy, which are in turn associated with a higher risk of SNAEs and death. We estimate that increasing ART adherence from <100% to 100% could lead to a reduction of up to 37% in the risk of SNAEs or death based on its impact on IL-6 and D-dimer, without the need for any additional medications or potential drug toxicity, and could impact a significant number of otherwise people with well-controlled HIV. This finding makes improving adherence an attractive intervention to reduce inflammation, improve coagulopathy, and ultimately, reduce morbidity and mortality in PWH who are <100% adherent. Future studies are needed to assess whether adherence interventions, or if switching to long-acting ART to achieve high and durable drug exposure, in PWH with low adherence and virologic suppression can lead to clinical benefit.
Contributor Information
Jose R Castillo-Mancilla, Department of Medicine, Division of Infectious Diseases, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Mary Morrow, Department of Biostatistics & Informatics, Colorado School of Public Health, Aurora, Colorado, USA.
Peter W Hunt, Division of Infectious Diseases, University of California, San Francisco, San Francisco, California, USA.
Samuel R Schnittman, Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA.
Andrew N Phillips, Institute of Global Health, University College London, London, United Kingdom.
Jason V Baker, Division of Infectious Diseases, Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA; Division of Infectious Diseases, University of Minnesota, Minneapolis, Minnesota, USA.
Jessica E Haberer, Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA.
Maria Joao Janeiro, Maple Health Group, New York, New York, USA.
Filipa Aragao, Incremental Action, Lisbon, Portugal; Public Health Research Centre, NOVA National School of Public Health, Universidade NOVA de Lisboa, Lisbon, Portugal.
Cal Cohen, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Nicholas Musinguzi, Department of Medicine, Mbarara University of Science and Technology–Massachusetts General Hospital Global Health Collaborative, Mbarara, Uganda.
Todd T Brown, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Matthias Cavassini, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Tracy R Glass, Department of Medicine, University of Basel, Basel, Switzerland; Swiss Tropical and Public Health Institute, Basel, Switzerland.
Sergio Serrano-Villar, Department of Infectious Diseases, Hospital Universitario Ramon y Cajal, Instituto Ramón y Cajal de Investigación Sanitaria, and Centro de Investigación Biomédica en Red Enfermedades Infecciosas, Madrid, Spain.
Samantha Mawhinney, Department of Biostatistics & Informatics, Colorado School of Public Health, Aurora, Colorado, USA.
Mark Siedner, Division of Infectious Diseases, Massachusetts General Hospital, Boston, Massachusetts, USA.
Notes
Author contributions. J. R. C.-M. conceptualized the research idea, performed the literature search, led all aspects regarding data interpretation, wrote the first manuscript draft, and performed all the edits for all the subsequent drafts. M. M. contributed to the research idea, performed data and statistical analysis and interpretation, generated figures and tables, and made substantial edits and critical revisions of the manuscript. P. W. H., S. R. S., A. N. P., J. V. B., J. E. H., N. M., T. T. B., M. C., and T. R. G. provided critical input for the research idea and made substantial edits and critical revisions to the manuscript. M. J. J., F. A., C. C., and S. S.-V. contributed to the Markov model and made substantial edits and critical revisions of the manuscript. S. M. contributed to the research idea and to statistical analysis and interpretation, generated figures and tables, and made substantial edits and critical revisions to the manuscript. M. S. co- conceptualized the research idea and made substantial edits and critical revisions to the manuscript.
Patient consent. All studies evaluated have been previously published; thus, no patient consent was required for our analysis.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant numbers R01AI145453 to J. R. C.-M. and T32AI007387 to S. R. S.).
References
- 1. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 2. Viswanathan S, Detels R, Mehta SH, Macatangay BJ, Kirk GD, Jacobson LP. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015; 19:601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrd KK, Hou JG, Hazen R, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr 2019; 82:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Landman R, de Truchis P, Assoumou L, et al. A 4-days-on and 3-days-off maintenance treatment strategy for adults with HIV-1 (ANRS 170 QUATUOR): a randomised, open-label, multicentre, parallel, non-inferiority trial. Lancet HIV 2022; 9:e79–90. [DOI] [PubMed] [Google Scholar]
- 5. Bellagamba R, Giancola ML, Tommasi C, et al. Randomized clinical trial on efficacy of fixed-dose efavirenz/tenofovir/emtricitabine on alternate days versus continuous treatment. AIDS 2019; 33:493–502. [DOI] [PubMed] [Google Scholar]
- 6. Musinguzi N, Mocello RA, Boum Y, et al. Duration of viral suppression and risk of rebound viremia with first-line antiretroviral therapy in rural Uganda. AIDS Behav 2017; 21:1735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA 2019; 321:451–2. [DOI] [PubMed] [Google Scholar]
- 8. Konstantopoulos C, Ribaudo H, Ragland K, Bangsberg DR, Li JZ. Antiretroviral regimen and suboptimal medication adherence are associated with low-level human immunodeficiency virus viremia. Open Forum Infect Dis 2015; 2:ofu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li JZ, Gallien S, Ribaudo H, Heisey A, Bangsberg DR, Kuritzkes DR. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014; 28:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Low-level viremia is associated with cumulative adherence to antiretroviral therapy in persons with HIV. Open Forum Infect Dis 2021; 8:ofab463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Int AIDS Soc 2019; 22:e25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castillo-Mancilla JR, Morrow M, Yap B, et al. Higher ART adherence is associated with lower systemic inflammation in treatment-naive Ugandans who achieve virologic suppression. J Acquir Immune Defic Syndr. 2018; 77:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castillo-Mancilla JR, Musinguzi N, Asiimwe S, et al. High residual inflammation despite HIV viral suppression: lessons learned from real-time adherence monitoring among people with HIV in Africa. HIV Med 2022; 23:465–73. [DOI] [PubMed] [Google Scholar]
- 15. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. Association of suboptimal antiretroviral therapy adherence with inflammation in virologically suppressed individuals enrolled in the SMART study. Open Forum Infect Dis 2018; 5:ofx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Musinguzi N, Jose C-M, Morrow M, et al. Antiretroviral therapy adherence interruptions are associated with systemic inflammation among Ugandans who achieved viral suppression. J Acquir Immune Defic Syndr. 2019; 82:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castillo-Mancilla JR, Cavassini M, Schneider MP, et al. Association of incomplete adherence to antiretroviral therapy with cardiovascular events and mortality in virologically suppressed persons with HIV: the Swiss HIV cohort study. Open Forum Infect Dis 2021; 8:ofab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Post WS, Haberlen SA, Witt MD, et al. Suboptimal HIV suppression is associated with progression of coronary artery stenosis: the Multicenter AIDS Cohort Study (MACS) longitudinal coronary CT angiography study. Atherosclerosis 2022; 353:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grund B, Baker JV, Deeks SG, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016; 11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strategies for Management of Antiretroviral Therapy (SMART) Study Group; El-Sadr WM, Lundgren JD, et al. CD4+ . count–guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 22. INSIGHT-ESPRIT Study Group; SILCAAT Scientific Committee; Abrams D, Lévy Y, Losso MH, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361:1548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castillo-Mancilla JR, Brown TT, Palella FJ Jr, et al. Partial normalization of biomarkers of inflammation and immune activation among virally suppressed men with HIV infection and high ART adherence. Open Forum Infect Dis 2020; 7:ofaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mody A, Eshun-Wilson I, Sikombe K, et al. Longitudinal engagement trajectories and risk of death among new ART starters in Zambia: a group-based multi-trajectory analysis. PLoS Med 2019; 16:e1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elbur AI, Ghebremichael M, Konkle-Parker D, et al. Dual trajectories of antiretroviral therapy adherence and polypharmacy in women with HIV in the United States. Research Square [Preprint]. Posted online 23 January 2023. doi: 10.21203/rs.3.rs-2443973/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serrano-Villar S, Cohen C, Baker JV, et al. Translating the observed differences in interleukin-6 levels between some antiretroviral regimens into potential long-term risk of serious non-AIDS events: a modeling study. Front Immunol 2022; 13:976564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orkaby AR, Driver JA, Ho Y-L, et al. Association of statin use with all-cause and cardiovascular mortality in US veterans 75 years and older. JAMA 2020; 324:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guirguis-Blake JM, Evans CV, Senger CA, O’Connor EA, Whitlock EP. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164:804–13. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2019; 4:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. BREATHER (PENTA 16) Trial Group . Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents, and young adults (BREATHER): a randomised, open-label, non-inferiority, phase 2/3 trial. Lancet HIV 2016; 3:e421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serrano-Villar S, López-Huertas MR, Jiménez D, et al. Long-term changes of inflammatory biomarkers in individuals on suppressive three-drug or two-drug antiretroviral regimens. Front Immunol 2022; 13:848630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Titanji B, Gavegnano C, Hsue P, Schinazi R, Marconi VC. Targeting inflammation to reduce atherosclerotic cardiovascular risk in people with HIV infection. J Am Heart Assoc 2020; 9:e014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Back D, Marzolini C. The challenge of HIV treatment in an era of polypharmacy. J Int AIDS Soc 2020; 23:e25449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–53. [DOI] [PubMed] [Google Scholar]
- 35. Schnittman S, Beck-Engeser G, Shigenaga J, Ahn H. Sex modifies the association between inflammation and vascular events in treated HIV. In: Conference on Retroviruses and Opportunistic Infections, Virtual, 6–10 March 2021. [Google Scholar]