Abstract
Background
Solid organ transplant (SOT) recipients are at risk for severe coronavirus disease 2019 (COVID-19), despite vaccination. Our study aimed to elucidate COVID-19 vaccine immunogenicity and evaluate adverse events such as hospitalization, rejection, and breakthrough infection in a SOT cohort.
Methods
We performed a prospective, observational study on 539 adult SOT recipients (age ≥18 years old) recruited from 7 Canadian transplant centers. Demographics including transplant characteristics, vaccine types, and immunosuppression and events such as hospitalization, infection, and rejection were recorded. Follow ups occurred every 4–6 weeks postvaccination and at 6 and 12 months from first dose. Serum was processed from whole blood to measure anti-receptor binding domain (RBD) antibodies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein to assess immunogenicity.
Results
The COVID-19 vaccines were found to be safe in SOT recipients with low rates of rejection requiring therapy (0.7%). Immunogenicity improved after the third vaccine dose, yet 21% developed no anti-RBD response. Factors such as older age, lung transplantation, chronic kidney disease, and shorter duration from transplant were associated with decreased immunogenicity. Patients with at least 3 doses were protected from hospitalization when experiencing breakthrough infections. Significantly increased anti-RBD levels were observed in patients who received 3 doses and had breakthrough infection.
Conclusions
Three or four doses of COVID-19 vaccines were safe, increased immunogenicity, and protected against severe disease requiring hospitalization. Infection paired with multiple vaccinations significantly increased anti-RBD response. However, SOT populations should continue to practice infection prevention measures, and they should be prioritized for SARS-CoV-2 pre-exposure prophylactics and early therapeutics.
Keywords: SARS-CoV-2, solid organ transplant, vaccines
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in solid organ transplant (SOT) recipients is associated with increased risk of severe coronavirus disease 2019 (COVID-19) and death [1–3]. Reduced humoral and T-cell responses after 2 doses of COVID-19 vaccines and breakthrough infections with severe disease have been reported in SOT recipients [4–13]. The blunted response to vaccines improves with administration of a third dose of SARS-CoV-2 mRNA vaccine, although effectiveness varies depending on the study [14–20]. Older age, lung transplantation, and the use of certain immunosuppressive agents such as mycophenolate and belatacept have been associated with poor response to vaccines [4, 21–24].
The primary series for transplant recipients is 3 doses of mRNA vaccines followed by a booster (fourth dose). A positive humoral response after 3 doses of mRNA vaccine ranges from 50% to 70%. The majority of these are single-center studies with limited follow up. Limited data are available on the immunogenicity of booster vaccination in this population. In addition, the safety of mRNA vaccines including the potential for graft rejection in a large SOT cohort and factors associated with suboptimal vaccine response require further investigation. Moreover, the outcomes of breakthrough infection after vaccination have not been well documented.
In this study, we performed a longitudinal and observational multicenter Canadian study in adult SOT recipients who received up to 4 doses of COVID-19 vaccines. We aimed to assess the safety of COVID-19 vaccination, determine antibody response after each dose of vaccine, and identify factors associated with low response to vaccine. In addition, we looked at outcome of breakthrough infection after vaccination.
METHODS
Patient Enrollment and Study Design
This is a prospective, multicenter study of adult solid organ transplant recipients (age ≥18 years old) recruited from 7 Canadian tertiary care transplant programs. Patients were enrolled in April 2021 and observed until May 2022. We excluded patients with known previous SARS-CoV-2 infection before vaccination, active cytomegalovirus (CMV) viremia at time of enrollment, use of rituximab in the past 6 months before enrollment, and those who received intravenous immunoglobulin in the past 30 days. We excluded patients with CMV viremia because CMV can exert an immunosuppressive effect in solid organ transplant recipients; moreover, CMV may impair response to vaccines in the elderly, where both T cell- and B cell-mediated effector functions can be affected [25, 26]. Patients were recruited after either their first, second, or third COVID-19 vaccine dose. Baseline demographics, transplant characteristics, vaccine types, and immunization dates were recorded. The follow-up period was up to 12 months from first dose of vaccine.
Patients completed a vaccine diary describing local and systemic side effects after each vaccine. In addition, study team members performed a chart review for rejection and hospitalization episodes as well as the development of SARS-CoV-2 infection.
Whole blood was collected for anti-SARS-CoV-2 spike (S) receptor binding domain (RBD) antibodies 4–6 weeks after each vaccination and 4–6 weeks after documented SARS-CoV-2 infection. Evusheld (tixagevimab and cilgavimab), a long-acting antibody combination, was approved and marketed in Canada on April 19, 2022 for prevention of COVID-19. The results of anti-RBD in this study are not affected by Evusheld, because the collection of all anti-RBD postvaccinations occurred before April 7, 2022.
Patient Consent Statement
The patient's written consent was obtained from all participates. The design was approved by the institutional ethics of each study site, and all institutions followed a shared protocol.
Antibody Responses
Whole blood was collected in a single red-top BD Vacutainer tube and allowed to clot for 30 minutes before processing. Blood tubes were centrifuged (2000 relative centrifugal force for 10 minutes at room temperature) and serum was collected with a pipette. Serum aliquots were cryopreserved at −80°C for batch processing. Total anti-SARS-CoV-2 spike RBD antibodies in sera were measured using the Roche Elecsys anti-SARS-CoV-2 S electrochemiluminescence immunoassay according to the manufacturer's instructions [27]. The lower limit of detection (nonreactive) and medical decision point (reactive) is defined by the product manufacturer at 0.4 U/mL and ≥0.8 U/mL, respectively. When RBD values were >250 U/mL, the laboratory performed an on-board dilution using the manufacturer recommended diluent. If the value was >2500 U/mL, they performed a manual dilution and load onto the instrument. Results were multiplied by the dilution factor to obtain the corrected final value. Results were accepted if they fell within the linear range of the assay. All testing was performed at a central laboratory at the University Health Network, Toronto.
Statistical Analysis
Each center entered data into a central REDCap form. Statistics were calculated with SPSS statistical package (Chicago, IL) version 24 and data were plotted using Graph Pad Prism 9.4.1 (San Diego, CA). Categorical variables were summarized as percentages. Continuous variables were summarized as median and interquartile range (IQR). The primary outcome was the proportion of patients with a positive anti-RBD response after the third vaccine dose. A positive anti-RBD response was defined as ≥0.8 U/mL. Vaccination titers were compared using nonparametric Kruskal-Wallis test and Mann-Whitney U test (titers <0.4 were given a value of 0.2 for statistical analyses). Logistic regression analysis was performed to identify independent variables associated with a positive anti-RBD ≥0.8 U/mL. Variables with P < .05 in the univariate analysis were used in the multivariate analysis. A P < .05 was considered statistically significant.
RESULTS
Patient Characteristics
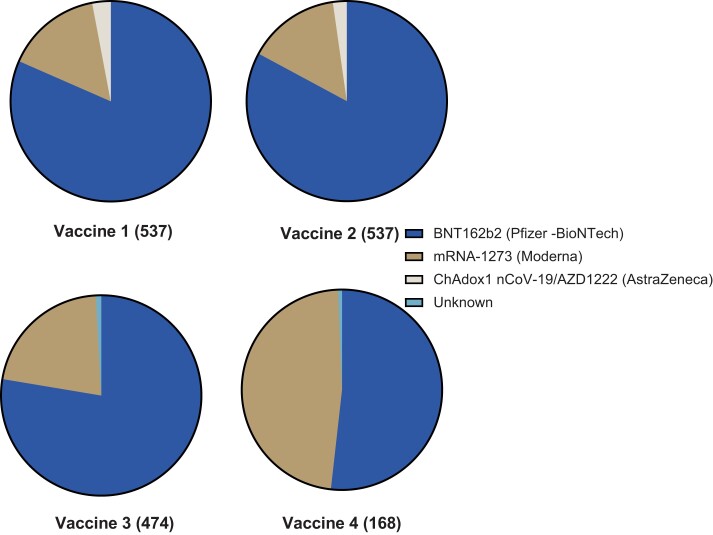
In total, 539 SOT recipients were enrolled. Two were excluded from final analysis (1 had infection before first vaccine and 1 had vaccination before transplant). Baseline characteristics are summarized in Table 1. The median age was 57 years (IQR, 46–65) and 13.8% were within the first year of transplant. Kidney transplant (48%) was the most common organ transplant, followed by liver (22.3%), lung (14.9%), heart (10.8%), and multiorgan (3.9%). The most common vaccine received for the primary series was monovalent BNT162b2 (Pfizer-BioNTech) followed by monovalent mRNA-1273 (Moderna) and ChAdOx1 nCoV-19/AZD1222 (AstraZeneca) vaccines (Figure 1).
Table 1.
Patient Characteristics
| Characteristics | All (n = 537) |
|---|---|
| Age, median in years (IQR) | 57 (46–65) |
| Male sex, n (%) | 323 (60.1) |
| Type of Transplant, n (%) Kidney Liver Lung Heart Kidney-pancreas or kidney-islet Others |
258 (48) 120 (22.3) 80 (14.9) 58 (10.8) 15 (2.8) 6 (1.1) |
| Time From Transplant to First Dose of Vaccine in Years, Median (IQR) | 4 (1–11) |
| Within 1 year of transplant, n (%) | 74 (13.8) |
| Rejection in preceding 3 months, n (%) | 10 (1.9) |
| CKD eGFR <30, n (%) | 68 (12.7) |
| Immunosuppression, n (%) Prednisone Tacrolimus Cyclosporin Mycophenolate Azathioprine Sirolimus |
352 (65.8) 443 (83) 44 (8.2) 369 (68.8) 45 (8.4) 46 (8.6) |
| Vaccine Brand by Dose (%) 1st BNT162b2/mRNA-1273/AZD1222 2nd BNT162b2/mRNA-1273/AZD1222 3rd BNT162b2/mRNA-1273/AZD1222 |
438/83/16 (81.6/15.5/3) 445/80/12 (82.9/14.9/2.2) 368/102/0 (77.6/21.5/0) |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Figure 1.
Type of vaccine received with each dose.
Antireceptor Binding Domain Response After Vaccination
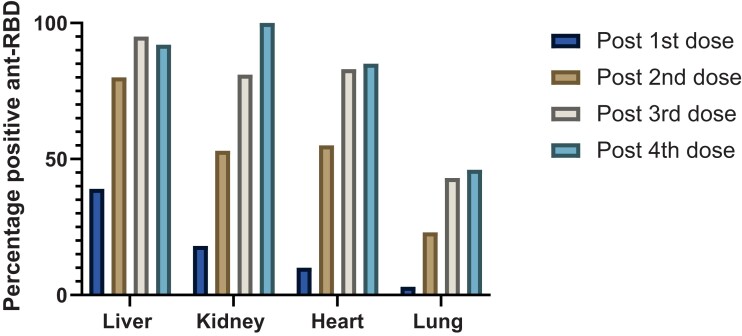
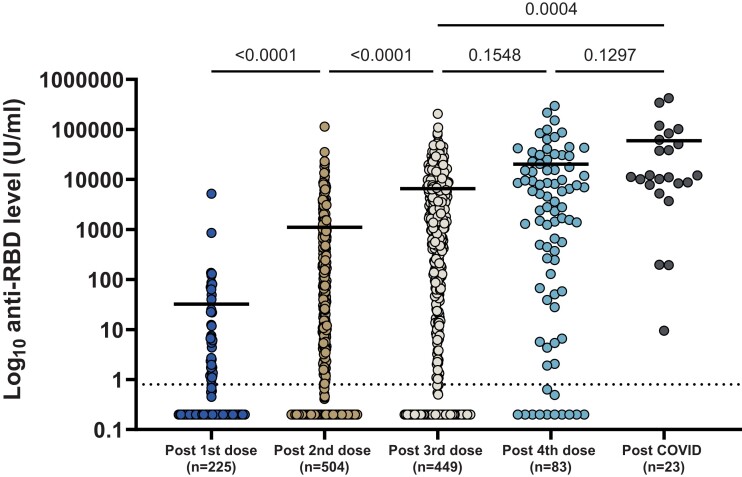
Of the 537 participants, anti-RBD was available after first, second, third, and fourth dose in 225, 504, 449, and 83 SOT recipients, respectively. The percentage of patients positive after each dose was as follows, respectively: 39%, 80.2%, 95.9%, and 100% in liver; 18.7%, 53%, 81.2%, and 92.9% in kidney; 10.7%, 55.3%, 83.7%, and 85.7% in heart; and 3.7%, 23.9%, 43.3%, and 46.2% in lung transplant recipients (Figure 2). The median anti-RBD titers after first, second, third, and fourth dose were 0.2 U/mL (IQR, 0.2–0.2), 2.08 U/mL (IQR, 0.2–223.9), 909.4 U/mL (IQR, 6.16–6784), and 2800 U/mL (IQR, 50.75–17 735), respectively, P < .001 (Figure 3). Because our main analysis was based on the primary series of 3 doses, we analyzed factors associated with positive anti-RBD after third dose of vaccine (Table 2).
Figure 2.
Percentage positive anti-receptor binding domain (RBD) after each dose of vaccine.
Figure 3.
Dot plot of anti-receptor binding domain (RBD) response after each vaccine dose. Horizontal line denotes median responses. Dotted line is the cutoff value for positivity of the assay (0.8 U/mL). Note that post-coronavirus disease (COVID) patients are not included in the postvaccination plots.
Table 2.
Univariate and Multivariate Analysis of Factors Associated With Anti-RBD Positivity After Third Dose of Vaccine
| Characteristics | No. (%) Anti-RBD Response After 3 Doses |
Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Negative 94 |
Positive 355 |
P Value | aOR | 95% CI | P Value | |
| Age, median years | 60 | 57 | .04 | 0.968 | .948–.988 | .002 |
| Time Tx to first dose vaccine (median years) | 2 | 5 | <.001 | 1.069 | 1.025–1.114 | .002 |
| Sex male | 56 (60) | 218 (61.4) | .788 | … | … | … |
| Organ -Lung -Heart -Liver -Kidney -Others |
38 (40.4) 8 (8.5) 4 (4.3) 41 (43.6) 3 (3.2) |
29 (8.2) 41 (11.5) 94 (26.5) 177 (49.9) 14 (3.9) |
<.001 | 0.173 5.924 |
.092–.325 2.024–17.341 |
<.001 |
| Retransplant | 8 (8.5) | 23 (6.5) | .490 | … | … | … |
| Vaccine Type BNT162b2 mRNA-1273 |
68 (72.3) 26 (27.7 |
282 (79.4) 73 (20.6) |
.140 | … | … | … |
| CKD with GFR <30 | 19 (20) | 27 (7.6) | <.001 | .402 | .190–.852 | .017 |
| Other immunosuppressive condition | 3 (3.2) | 13 (13.7) | 1 | … | … | … |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; RBD, receptor binding domain; Tx, treatment.
Older age, shorter time from transplantation, chronic kidney disease defined as estimated glomerular filtration rate <30, and having a lung transplant were associated with a negative anti-RBD in the multivariate analysis. Liver transplantation was associated with a positive response. We did not include immunosuppressive drugs in our analysis to avoid collinearity because more lung and kidney transplant patients were on mycophenolate compared to liver transplant (lung 80%, kidney 79.6% to 36, 7% liver; P < .001). However, within each organ group, treatment with mycophenolate was associated with lower rate of positive anti-RBD after third dose of vaccine (mycophenolate vs no mycophenolate: kidney, 133 of 172 [77.3%] vs 45 of 48 [93.8%], P = .010; lungs, 24 of 56 [42.9%] vs 5 of 11 [45.5%], P = 1; heart, 29 of 37 [78.4%] vs 12 of 12 [100%], P = .173; and liver, 34 of 37 [92%] vs 60 of 61 [98.4%], P = .294).
Severe Acute Respiratory Syndrome Coronavirus 2 Infection After Vaccination
During the study period, 96 of 537 (17.9%) participants developed SARS-CoV-2 infection. Diagnosis of COVID-19 occurred after receiving 1 (n = 2, 2%), 2 (n = 11, 11.5%), 3 (n = 66, 68.8%), or 4 (n = 17 17.7%) doses vaccines. Early therapy was administered in 50: sotrovimab in 43 and remdesivir in 7. Twenty-seven did not receive early therapy and 19 had missing information for early therapy. Eighteen required hospitalization, 2 of whom died of COVID-19. The 2 individuals who died, both lung transplant recipients, received 2 and 3 doses of vaccine, respectively, before infection. All infections occurred from December 2021 to May 2022 at which time the Omicron variant predominated [28]. Using a Kaplan-Meier survival analysis, with time period between the third and fourth dose of vaccine, the cumulative proportion of getting infection was 4% at 90 days, 13% at 120 days, 35.3% at 181 days, and 65.5% at 199 days.
Solid organ transplant recipients with fewer than 3 doses of vaccine before infection had increased hospitalization or death (≤2 vaccines, 5 of 11 [45.5%] vs ≥3 vaccine doses, 13 of 79 [15.5%]; P = .039). In addition, the median anti-RBD level after the third vaccine (n = 79) was lower in those infected and requiring hospitalization (hospitalized, 0.2 U/mL [IQR, 0.2–121.875] vs not hospitalized, 1500.30 U/mL [IQR, 11.785–7685]; P = <.001). Anti-RBD was assessed in 23 patients after COVID-19; the antibody response was significantly greater compared to vaccinated patients without infection after dose 3 with a median titer of 11 225 U/mL (IQR, 7813–62 170) postinfection versus 909.4 U/mL (IQR, 6.16–6784) postthird dose without infection, P < .001 (Figure 3).
Safety Analysis
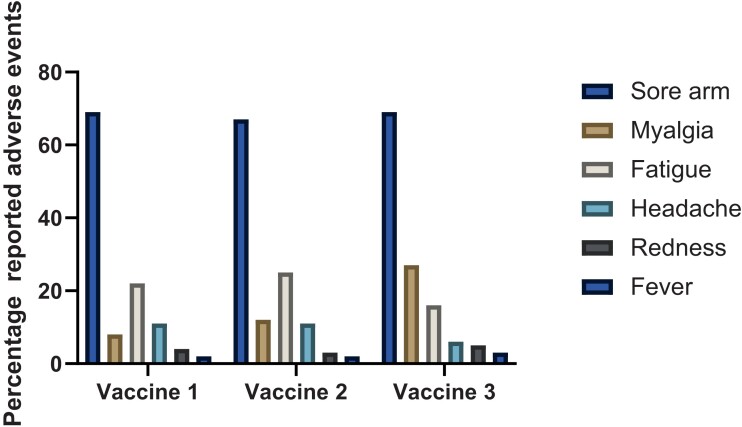
The most common reported side effects were local tenderness (>60% after each dose), followed by fatigue (16%–25%), myalgia (8%–27%), and headache (6%–11%) (Figure 4). The majority (>90%) were able to continue with their usual daily activities.
Figure 4.
Percentage of patients with adverse effects of vaccination after each vaccine dose.
Neurological adverse effects reported included paresthesia in the leg (n = 1) and burning pain from herpetic whitlow (n = 1) (after first dose), acute transient quadriparesis (n = 1) and Bell's palsy ([BP] n = 1) treated with steroids (after second dose), and seizure (n = 1) 1 month after third dose. Other recorded events possibly related to vaccine include 1 kidney transplant recipient developing thrombocytopenia, anemia, and epistaxis after the first dose of BNT162b2 and 1 heart transplant recipient developing pulmonary infiltrates requiring treatment with steroids after the second dose of vaccine.
During the study period, 6 patients developed a biopsy-proven acute rejection episode (Table 3). One heart transplant recipient had mild cellular rejection, 27 days after his first vaccine dose. Another heart transplant recipient, in his first year posttransplant, had treated moderate cellular rejection before his first vaccine dose and developed another episode of moderate cellular rejection requiring treatment after his second dose. Two liver transplant recipients had rejection after second vaccine dose (1 mild cellular rejection 3 months postvaccine, 1 chronic ductopenic rejection >2 months postvaccination requiring increase in immunosuppression). Two kidney transplant recipients developed acute humoral rejection after second dose of vaccines requiring treatment.
Table 3.
Rejection Episodes Postvaccination
| Organ | Within 1st Year of Transplant | Previous Rejection in the Past 12 Months | Time (Days) Last Vaccine to Rejection |
Number of Vaccine Doses- Type of Vaccine | Type of Rejection | Therapy of Rejection |
|---|---|---|---|---|---|---|
| Heart | No | No | 27 | 1- BNT162b2 | Mild ACR | No |
| Heart | Yes | Yes | 44 | 2- BNT162b2 | Moderate ACR | Yes |
| Kidney | No | No | 90 | 2- BNT162b2 | AMR | Yes |
| Kidney | Yes | Yes | 70 | 2- BNT162b2 | AMR | Yes |
| Liver | No | No | 123 | 2- BNT162b2 | Mild ACR | No |
| Liver | No | No | 77 | 2- BNT162b2 | Chronic ductopenic rejection | Yes |
Abbreviations: ACR, acute cellular rejection; AMR, antibody-mediated rejection.
DISCUSSION
We performed a multicenter observational study assessing the anti-RBD response and safety of COVID-19 vaccination in a large multicenter cohort of transplant recipients and described the severity of illness with breakthrough infections. The main findings of the study include the following: (1) a 79% rate of anti-RBD response after the primary vaccine series; (2) factors associated with low anti-RBD response include older age, lung transplantation, chronic kidney disease, and shorter duration from transplant; (3) having at least 3 doses of vaccine was protective against hospitalizations in patients with breakthrough COVID-19; (4) hybrid immunity (vaccination followed by infection) seemed to provide the most robust humoral response; and (5) vaccines were generally safe in SOT with low rate of rejection (0.7%) requiring therapy in our cohort.
Similar to other studies that examined the side effects of COVID-19 vaccines in SOT, we mainly found local side effects at the site of injection and, less commonly, systemic reactions such as fever, fatigue, myalgia, and arthralgia [5, 8, 14, 16, 29, 30]. We had no cases of myocarditis postvaccination; however, there were several neurologic side effects, including BP and herpetic whitlow that have been described in other cohorts [8, 31, 32]. In the general population, Tamaki et al [32] found BP at higher rates in patients with COVID-19, and this incidence exceeds the reported incidence of BP in those who received a COVID-19 vaccine. We had 4 (0.7%) patients with acute graft rejection requiring therapy in our cohort. This is similar to baseline rejection rates in the transplant population. Although rejection has been rarely documented after vaccination in studies of SOT recipients, a few case reports have described acute cellular and antibody rejection in kidney and heart transplant like ours [8, 29, 33, 34, 35]. Similar to other respiratory viruses (eg, influenza), the rate of rejection post-COVID-19 seems to exceed cases that have been reported postvaccines [1, 36, 37].
Our findings related to anti-RBD response and risk factors related to response are generally in agreement with other studies. Studies measuring antibody responses after 3 vaccine doses all showed improvement after the third dose; however, the response varied according to type of transplant. Lung transplants had the poorest response and liver transplant had the most robust response [17, 18, 23, 38, 39]. The liver has better immune tolerance in general, with a low incidence of rejection, which is in sharp contrast to other solid organ transplants and hence generally requires less immunosuppressive medications. Similar to others, we found a poor response with shorter time from transplantation. This is likely a result of more profound immunosuppression early posttransplant and higher chance of graft dysfunction and rejection, and this been described in other studies. Older age is associated with immune senescence and decreased humoral and cellular response to vaccines [40]. Finally, we found humoral response to be much higher postinfection compared with vaccination [8, 41]. This is consistent with studies of hybrid immunity. A novel aspect of our study was to examine the first booster (or fourth dose) of vaccine, which significantly increased antibody levels and decreased breakthrough infections [42, 43].
In our cohort, breakthrough infection with severe disease requiring hospitalization was more common in patients who had 2 or fewer doses of vaccine. In a large US National COVID Cohort, comparing people with and without immune dysfunction, SOT had the highest rate of breakthrough infections [44]. Data on the severity of illness after vaccination in SOT are variable. Hall et al [45] found that disease severity in medically attended SOT who had breakthrough infection after 1 or 2 doses of vaccination was similar to unvaccinated SOT recipients. In a large SOT cohort of patients who received 2 doses of vaccine, breakthrough was seen in 3.34% with 53% developing severe disease [46]. Hamm et al [47] found that receiving 3 vaccine doses decreased the risk of hospital admission by 58%. Kwon et al [48] found that vaccine efficacy against COVID-19 hospitalization was substantially higher after 3 mRNA vaccine doses (77%) than after 2 doses (29%). We also found lower antibody in those who were hospitalized with COVID-19. In a recent study with patients infected with SARS-CoV-2, low antispike antibody levels correlated with poor outcomes in COVID-19 breakthrough hospitalizations [49]. Because there is no threshold currently that predicts protection in the general population, and multiple antibody assays with different ranges and limit of detection are used in different studies, we did not calculate a threshold of protection.
Strengths of our study include a multicenter approach that allowed for a large cohort and analysis of a variety of solid organ transplants. Patients were prospectively followed for 1 year, which allowed us to conduct detailed safety analyses including for allograft rejection as well as at the incidence and nature of breakthrough infections.
Our study has some limitations. We did not assess the development of neutralizing antibodies or cell-mediated immunity induced by vaccination. Although we assessed the rate of rejection postvaccination, we did not systematically measure anti-human leukocyte antigen (HLA) antibodies in our cohort. However, rejection as an outcome is more robust than the surrogate of HLA antibodies, which in any case would not detect cellular rejection. Finally, data on outpatient treatment of COVID-19 with monoclonal antibodies or antivirals was lacking on some patients and could have influenced the outcome of COVID-19.
CONCLUSIONS
In summary, in our large SOT cohort, the primary series of COVID-19 vaccine was safe, increased anti-RBD response, and offered protection against severe COVID-19 requiring hospitalization compared to 1 or 2 doses of vaccine. Booster doses provided additional anti-RBD humoral immunity. However, with waning immunity and emergence of highly transmissible variants and poor neutralization versus Omicron even with a fourth dose [50], transplant recipients should continue to practice infection prevention measures, and they should be prioritized for pre-exposure monoclonal antibodies and early therapies for SARS-CoV-2 infection.
Acknowledgments
We thank the PreVenT COVID group: Sasan Hosseini-Moghaddam (UHN), Dr. Zineb Khrifi (CHUM), Dr. Julie Turgeon (CHUM), Dr. Karina Top (Dalhousie University), Dr. Gaston DeSerres (INSPQ, Public Health), Dr. Megan Levings (UBC), Dr. Allison Mah (UBC), Dr. Alissa Wright (UBC), Dr. Robert Wright (UBC), Dr. Robert Levy (UBC), Dr. Cindy Luo (UBC), Dr. Matthew Kadatz (UBC), Dr. James Lan (UBC), Dr. Trana Hussaini (UBC), Dr. Vladimir Marquez-Azalgara (UBC), Dr. Manish Sadarangani (UBC), Dr. Theodore Steiner, Dr. Marc Cloutier (Héma-Québec), Dr. Renée Bazin (Héma-Québec), Dr. Christopher Lemieux (Université Laval), France Samson (Université Laval), Maryse Desjardins (ICM), Hélène Brown (ICM), Johanne Doiron (ICM), Dr. Patricia Gongal (CDTRP), Kristian Stephens (CDTRP) Taylor Toth (LHSC), Grant Luke (LHSC), Taylor Toth (LHSC) and Gabriela Offerni (LHSC). We also thank the PreVenT COVID group Coordinators: Julie Turgeon (CHUM), Gale Ladua (UBC), Cadence Baker (LHSC), Natalia Pinzon (UHN), Kimberly Robertson (UofA), Heather Mangan (UofA).
Author contributions. DKa, DKu, AH, MD, M-JH, and HC contributed to study design. DMY, VHF, VK, MI, SS, SB, and VT contributed to data collection. DKa and DKu contributed to data analysis. All authors contributed to manuscript writing.
Financial support. This study was funded Public Health Agency of Canada through the COVID-19 Immunity Task Force and Vaccine Surveillance Reference Group. This work was also supported by a Fonds de recherche du Québec -Santé (FRQS) Grant Number 308941. The study was coordinated by the Canadian Donation and Transplantation Research Program.
Contributor Information
Dima Kabbani, Division of Infectious Diseases, Department of Medicine, University of Alberta, Edmonton, Alberta, Canada.
Demitra M Yotis, Canadian Donation and Transplantation Research Program (CDTRP), Edmonton, Alberta, Canada.
Victor H Ferreira, Transplant Infectious Diseases and Ajmera Transplant Centre, University Health Network, Toronto, Ontario, Canada.
Sarah Shalhoub, Division of Infectious Diseases, Department of Medicine, Western University, London, Ontario, Canada.
Sara Belga, Division of Infectious Diseases, Department of Medicine, University of British Columbia, and Vancouver Coastal Health Research Institute, Vancouver, British Columbia, Canada.
Varalika Tyagi, Division of Infectious Diseases, Department of Medicine, University of Alberta, Edmonton, Alberta, Canada.
Matthew Ierullo, Transplant Infectious Diseases and Ajmera Transplant Centre, University Health Network, Toronto, Ontario, Canada.
Vathany Kulasingam, Laboratory Medicine Program, University Health Network, University Health Network, University of Toronto, Ontario, Canada.
Marie-Josée Hébert, Canadian Donation and Transplantation Research Program (CDTRP), Edmonton, Alberta, Canada; Department of Medicine, Centre Hospitalier de l’Université de Montréal, Faculté de Médecine, Université de Montréal, Quebec, Canada.
Lori West, Canadian Donation and Transplantation Research Program (CDTRP), Edmonton, Alberta, Canada; Pediatric Cardiac Transplantation Program, Stollery Children's Hospital, University of Alberta, Edmonton, Alberta, Canada; Alberta Transplant Institute, University of Alberta, Edmonton, Alberta, Canada.
Jean-Sébastien Delisle, Canadian Donation and Transplantation Research Program (CDTRP), Edmonton, Alberta, Canada; Centre de Recherche de l’Hôpital Maisonneuve-Rosemoent, Montréal, Quebec, Canada; Department of Medicine, Université de Montréal, Montréal, Quebec, Canada.
Normand Racine, Institut de Cardiologie de Montréal, Faculté de Médecine, Université de Montréal, Montréal, Quebec, Canada.
Sacha A De Serres, Transplantation Unit, Renal Division, Department of Medicine, University Health Center of Quebec, Faculty of Medicine, Laval University, Québec, Québec, Canada.
Héloïse Cardinal, Centre de Recherche de l’Hôpital Maisonneuve-Rosemoent, Montréal, Quebec, Canada.
Mélanie Dieudé, Canadian Donation and Transplantation Research Program (CDTRP), Edmonton, Alberta, Canada; Héma-Québec, Montréal, Québec, Canada; Microbiology, Infectiology and Immunology Department, Faculty of Medicine, Université de Montréal, Montréal, Québec, Canada; Research Center, Centre Hospitalier de L’Université de Montréal (CHUM), Montréal, Québec, Canada.
Atul Humar, Transplant Infectious Diseases and Ajmera Transplant Centre, University Health Network, Toronto, Ontario, Canada.
Deepali Kumar, Transplant Infectious Diseases and Ajmera Transplant Centre, University Health Network, Toronto, Ontario, Canada.
References
- 1. Kates OS, Haydel BM, Florman SS, et al. . COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis 2021; 73:e4090–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marinelli T, Ferreira VH, Ierullo M, et al. . Prospective clinical, virologic, and immunologic assessment of COVID-19 in transplant recipients. Transplantation 2021; 105:2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation 2021; 105:1365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hallett AM, Greenberg RS, Boyarsky BJ, et al. . SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant 2021; 40:1579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazzola A, Todesco E, Drouin S, et al. . Poor antibody response after two doses of SARS-CoV-2 vaccine in transplant recipients. Clin Infect Dis 2022; 74:1093–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miele M, Busà R, Russelli G, et al. . Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant 2021; 21:2919–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peled Y, Ram E, Lavee J, et al. . BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant 2021; 40:759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall VG, Ferreira VH, Ierullo M, et al. . Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant 2021; 21:3980–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyarsky BJ, Werbel WA, Avery RK, et al. . Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant 2021; 21:3496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shostak Y, Shafran N, Heching M, et al. . Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med 2021; 9:e52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haidar G, Agha M, Bilderback A, et al. . Prospective evaluation of coronavirus disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 vaccination in the immunocompromised study (COVICS). Clin Infect Dis 2022; 75:e630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin CX, Moore LW, Anjan S, et al. . Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation 2021; 105:e265–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall VG, Ferreira VH, Ku T, et al. . Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peled Y, Ram E, Lavee J, et al. . Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant 2021; 40:759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benotmane I, Gautier G, Perrin P, et al. . Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021; 326:1063–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peled Y, Ram E, Lavee J, et al. . Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant 2022; 41:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alejo JL, Ruck JM, Chiang TPY, et al. . Antibody response to a third dose of SARS-CoV-2 vaccine in heart and lung transplant recipients. Clin Transplant 2022; 36:e14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naylor KL, Kim SJ, Smith G, et al. . Effectiveness of first, second, and third COVID-19 vaccine doses in solid organ transplant recipients: a population-based cohort study from Canada. Am J Transplant 2022; 22:2228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chavarot N, Morel A, Leruez-Ville M, et al. . Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am J Transplant 2021; 21:4043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bae S, Alejo JL, Chiang TPY, et al. . mTOR inhibitors, mycophenolates, and other immunosuppression regimens on antibody response to SARS-CoV-2 mRNA vaccines in solid organ transplant recipients. Am J Transplant 2022; 22:3137–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davidov Y, Indenbaum V, Tsaraf K, et al. . A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J Hepatol 2022; 77:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manothummetha K, Chuleerarux N, Sanguankeo A, et al. . Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: a systematic review and meta-analysis. JAMA Netw Open 2022; 5:e226822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merani S, Pawelec G, Kuchel GA, McElhaney JE. Impact of aging and cytomegalovirus on immunological response to influenza vaccination and infection. Front Immunol 2017; 8:784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13512. [DOI] [PubMed] [Google Scholar]
- 27. Jochum S, Kirste I, Hortsch S, et al. . Clinical utility of elecsys anti-SARS-CoV-2 S assay in COVID-19 vaccination: an exploratory analysis of the mRNA-1273 phase 1 trial. Front Immunol 2021; 12:798117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Public health Agency of Canada. PHAC. Available at: https://health-infobase.canada.ca/covid-19/. Accessed February 9 2023.
- 29. Villavicencio A, Ebisu Y, Raja M, Sanchez-Covarrubias AP, Reynolds JM, Natori Y. Adverse events after SARS-CoV-2 vaccination in solid organ transplant recipients: a systematic review. Transpl Infect Dis 2022; 24:e13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boyarsky BJ, Barbur I, Chiang TP, et al. . SARS-CoV-2 messenger RNA vaccine immunogenicity in solid organ transplant recipients with prior COVID-19. Transplantation 2021; 105:e270–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wan EYF, Chui CSL, Lai FTT, et al. . Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis 2022; 22:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tamaki A, Cabrera CI, Li S, et al. . Incidence of bell palsy in patients with COVID-19. JAMA Otolaryngol Head Neck Surg 2021; 147:767–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Werbel WA, Boyarsky BJ, Ou MT, et al. . Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021; 174:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bau JT, Churchill L, Pandher M, Benediktsson H, Tibbles LA, Gill S. Acute kidney allograft rejection following coronavirus mRNA vaccination: a case report. Transplant Direct 2022; 8:e1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jang HW, Bae S, Ko Y, et al. . Acute T cell-mediated rejection after administration of the BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient: a case report. Korean J Transplant 2021; 35:253–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vinson AJ, Agarwal G, Dai R, et al. . COVID-19 in solid organ transplantation: results of the national COVID cohort collaborative. Transplant Direct 2021; 7:e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinson AJ, Dai R, Agarwal G, et al. . Sex and organ-specific risk of major adverse renal or cardiac events in solid organ transplant recipients with COVID-19. Am J Transplant 2022; 22:245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balsby D, Nilsson AC, Möller S, et al. . Determinants of antibody response to a third SARS-CoV-2 mRNA vaccine dose in solid organ transplant recipients: results from the prospective cohort study COVAC-tx. Vaccines (Basel) 2022; 10:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Havlin J, Skotnicova A, Dvorackova E, et al. . Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein-specific T cells in lung transplant recipients. Transplantation 2022; 106:e183–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murasko DM, Bernstein ED, Gardner EM, et al. . Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol 2002; 37:427–39. [DOI] [PubMed] [Google Scholar]
- 41. Havlin J, Svorcova M, Dvorackova E, et al. . Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant 2021; 40:754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alejo JL, Mitchell J, Chiang TP, et al. . Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation 2021; 105:e280–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caillard S, Thaunat O, Benotmane I, Masset C, Blancho G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med 2022; 175:455–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun J, Zheng Q, Madhira V, et al. . Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022; 182:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hall VG, Al-Alahmadi G, Solera JT, et al. . Outcomes of SARS-CoV-2 infection in unvaccinated compared with vaccinated solid organ transplant recipients: a propensity matched cohort study. Transplantation 2022; 106:1622–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marinaki S, Xagas E, Tsoutsoura P, Katsaros D, Korogiannou M, Boletis IN. Occurrence of severe SARS-CoV-2 infection in fully vaccinated solid organ transplant recipients. Transplant Proc 2022; 54:1405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamm SR, Rezahosseini O, Møller DL, et al. . Incidence and severity of SARS-CoV-2 infections in liver and kidney transplant recipients in the post-vaccination era: real-life data from Denmark. Am J Transplant 2022; 22:2637–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwon JH, Tenforde MW, Gaglani M, et al. . mRNA vaccine effectiveness against coronavirus disease 2019 hospitalization among solid organ transplant recipients. J Infect Dis 2022; 226:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanghavi DK, Bhakta S, Wadei HM, et al. . Low antispike antibody levels correlate with poor outcomes in COVID-19 breakthrough hospitalizations. J Intern Med 2022; 292:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karaba AH, Johnston TS, Aytenfisu TY, et al. . A fourth dose of COVID-19 vaccine does not induce neutralization of the omicron variant among solid organ transplant recipients with suboptimal vaccine response. Transplantation 2022; 106:1440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]