Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) BA.2/BA.2.12.1 and BA.4/BA.5 subvariants have mutations associated with increased capacity to evade immunity when compared with prior variants. We evaluated mRNA monovalent booster dose effectiveness among persons ≥5 years old during BA.2/BA.2.12.1 and BA.4/BA.5 predominance.
Methods
A test-negative, case-control analysis included data from 12 148 pharmacy SARS-CoV-2 testing sites nationwide for persons aged ≥5 years with ≥1 coronavirus disease-2019 (COVID-19)-like symptoms and a SARS-CoV-2 nucleic acid amplification test from April 2 to August 31, 2022. Relative vaccine effectiveness (rVE) was estimated comparing 3 doses of COVID-19 mRNA monovalent vaccine to 2 doses; for tests among persons ≥50 years, rVE estimates also compared 4 doses to 3 doses (≥4 months since third dose).
Results
A total of 760 986 test-positive cases and 817 876 test-negative controls were included. Among individuals ≥12 years, rVE of 3 versus 2 doses ranged by age group from 45% to 74% at 1-month post vaccination and waned to 0% by 5–7 months post vaccination during the BA.4/BA.5 period.
Adults aged ≥50 years (fourth dose eligible) who received 4 doses were less likely to have symptomatic SARS-CoV-2 infection compared with those with 3 doses; this rVE remained >0% through at least 3 months since last dose. For those aged ≥65 years, rVE of 4 versus 3 doses 1-month post vaccination was higher during BA.2/BA.2.12.1 (rVE = 49%; 95% confidence interval [CI], 43%–53%) than BA.4/BA.5 (rVE = 40%; 95% CI, 36%–44%). In 50- to 64-year-olds, rVE estimates were similar.
Conclusions
Monovalent mRNA booster doses provided additional protection against symptomatic SARS-CoV-2 infection during BA.2/BA.2.12.1 and BA.4/BA.5 subvariant circulation, but protection waned over time.
Keywords: COVID-19, infection, monovalent boosters, SARS-CoV-2
Individuals with 1 or 2 monovalent mRNA COVID-19 booster doses were less likely to have symptomatic SARS-CoV-2 infection compared to those without booster doses, during the BA.2/BA.2.12.1 and BA.4/BA.5 predominant subvariant periods.
In the United States, a monovalent coronavirus disease-2019 (COVID-19) mRNA vaccine booster (third dose) was recommended by the Advisory Committee on Immunization Practices at least 5 months after the last primary dose for older adults ≥65 years old (September 23, 2021), adults 18–64 (November 21, 2021), young adults 16–17 (December 9, 2021), and adolescents 12–15 (January 5, 2022) [1]. To address waning from this first booster dose, on March 29, 2022 the US Food and Drug Administration (FDA) authorized a second booster (fourth dose) of monovalent vaccine to be given at least 4 months after receipt of a third dose for immunocompetent adults aged ≥50 years, who are at increased risk of severe COVID-19. On May 19, 2022, a first booster (third dose) of a monovalent vaccine was recommended for children aged 5–11 years.
Observational studies have demonstrated effectiveness of monovalent COVID-19 vaccine booster doses against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant and Omicron BA.1 subvariant for a variety of outcomes and populations [2–11]. However, Omicron BA.2/BA.2.12.1 subvariants accounted for ≥75% of sequenced isolates in the United States by April 11, 2022. The BA.2/BA.2.12.1 and BA.4/BA.5 subvariants have additional mutations associated with increased capacity to evade neutralizing antibodies generated in response to monovalent booster doses or BA.1 infection, suggesting monovalent vaccines may be less effective in preventing infection against these subvariants [12–15].
To address waning monovalent vaccine effectiveness (VE) in the setting of Omicron variants, on September 1, 2022, the FDA authorized bivalent COVID-19 mRNA vaccines for any persons who have completed a primary series, regardless of number of doses, at least 2 months prior. Monitoring of monovalent COVID-19 VE against SARS-CoV-2 infection during periods of BA.2/BA.2.12.1 and BA.4/BA.5 subvariant predominance is crucial to inform vaccine policy by setting a baseline to understand how well newly authorized bivalent vaccines may work. We analyzed data from a national, pharmacy-based SARS-CoV-2 testing program, Increasing Community Access to Testing (ICATT) [16], to estimate age group-specific effectiveness of 1 or 2 monovalent mRNA vaccine booster doses among persons aged ≥5 years during periods of Omicron BA.2/BA.2.12.1 and BA.4/BA.5 subvariant predominance.
METHODS
Data Source
ICATT is a Department of Health and Human Services program that contracts with pharmacy chains and laboratories to provide no-cost, drive-through SARS-CoV-2 testing at selected sites nationwide [7–9, 16]. The ICATT program seeks to address COVID-19 health disparities by selecting SARS-CoV-2 testing sites located in (1) racially and ethnically diverse communities, small towns, and rural communities as assessed using Rural-Urban Commuting Area (RUCA) codes [17] and (2) areas with moderate to high social vulnerability based on Centers for Disease Control and Prevention (CDC)/Agency for Toxic Substances and Disease Registry (ATSDR) 2018 Social Vulnerability Index (SVI) data [18, 19].
ICATT data were analyzed from SARS-CoV-2 nucleic acid amplification tests (NAATs) performed during BA.2/BA.2.12.1 (April 2–June 11, 2022) and BA.4/BA.5 (July 2–August 31, 2022) Omicron subvariant predominance, based on when each subvariant accounted for ≥75% of all sequenced isolates in the United States [20]. Because neither set of subvariants accounted for at least 75% of sequenced isolates between June 12 and July 1, 2022, tests performed during this period were excluded from analyses. Analytic start dates for age groups varied based on vaccine product and timing of product authorization (Supplementary Table 1).
During BA.2/BA.2.12.1 predominance, all 4 pharmacy chains with data were included in the analysis. During BA.4/BA.5 predominance, 1 pharmacy chain no longer contributed data, whereas 2 pharmacy chains (1 new and 1 old comprising of only 0.2% of the total tests during this period) began using a test registration form that gathered vaccination history differently, leaving this analysis with 2 chains with complete data during this period. Self-reported information was collected from individuals registering for testing online, including vaccination history, gender, race, ethnicity, age, state of residence, symptom status, prior SARS-CoV-2 infections, and underlying conditions. Severe acute respiratory syndrome coronavirus 2 NAAT date of collection, assay type, and results were reported directly from testing sites. For detailed information on how vaccination history was collected, see Supplementary Information (Vaccination History Ascertainment).
Study Design
A test-negative design was used to evaluate relative and absolute effectiveness against symptomatic SARS-CoV-2 infection of 1 booster (3 doses) compared to 2 doses or unvaccinated, and effectiveness of 2 booster doses (4 doses) compared to 3 doses or unvaccinated. At registration, persons were asked whether they had an immunocompromising condition using the following examples: immunocompromising medications, solid organ or blood stem cell transplant, human immunodeficiency virus (HIV), or other immunocompromising conditions. The respondents were excluded if they reported an immunocompromising condition. The unit of analysis was tests: among individuals with ≥1 COVID-19-like illness symptoms, cases were defined by a positive NAAT result and controls were defined by a negative NAAT result. Individuals reporting any prior SARS-CoV-2 infection were excluded from analysis.
Exposures
The main exposures were receipt of 1 booster dose for individuals aged 5 to 49 years and receipt of 1 or 2 booster doses for individuals aged 50 years and older, with last dose received ≥2 weeks before test dates. During the analysis time frame, bivalent mRNA vaccines had not yet been authorized; thus, reported mRNA vaccine doses were assumed to be monovalent. Individuals were excluded if they (1) were aged 5 to 49 years and received >3 doses or were aged ≥50 years and received >4 doses, (2) received a booster dose earlier (<5 months) than the recommended interval, (3) received any doses of a non-mRNA vaccine, (4) received only 1 dose of vaccine, or (5) were missing vaccination information or covariables of interest.
For estimating relative VE (rVE) comparing 3 versus 2 doses, the reference group consisted of individuals who had received 2 doses and were eligible for a third dose (ie, ≥5 months since second dose). Similarly, for estimating rVE comparing 4 versus 3 doses, the reference group consisted of individuals who had received 3 doses and were eligible for a fourth dose (ie, ≥4 months since third dose). For absolute VE (aVE) analyses, the reference group for all exposure categories were unvaccinated individuals who had not received any doses of COVID-19 vaccine. Absolute VE estimates are presented for time frames at which the next dose was recommended (ie, 5 or 4 months after the last dose and eligible for a first or second booster).
Statistical Analysis
For rVE, we estimated associations between SARS-CoV-2 infection and prior mRNA vaccination status using multivariable logistic regression. In secondary analyses, we estimated aVE by comparing odds of vaccination with 2, 3, or 4 doses of an mRNA vaccine versus being unvaccinated among cases and test-negative controls using multivariable logistic regression. All VE estimates were expressed as a percentage and calculated from odds ratios (ORs) by the formula (1 − OR) × 100.
Models were adjusted for calendar day of test (modeled as a continuous linear variable), age, gender, race, ethnicity, pharmacy site census tract SVI, underlying conditions (presence vs absence) (list of conditions in Supplementary Table 2), state of residence, pharmacy chain, and 7-day average of cases per 100 000 by site zip code (local incidence: modeled as a continuous linear variable). Both calendar day of test and local incidence were evaluated for presence of a nonlinear relationship by plotting the quartiles of each continuous variable against the probability of the log odds of having a positive NAAT. Calendar day of test had a continuous linear relationship, whereas local incidence had a slight logarithmic relationship with the outcome. Sensitivity analyses including the log of local incidence produced no differences in the main effect. When race or ethnicity were not reported, they were classified as “unknown” race/ethnicity and included as a separate category in analyses (Supplementary Table 2).
Adjusted VE estimates were stratified by age group with month since last vaccine dose included as a categorical variable based on analytic start and stop dates for each age group (Supplementary Table 1). Age group-specific models estimated rVE for 4 versus 3 doses (50–64 years, ≥65 years) and for 3 versus 2 doses (12–15 years, 16–49 years, 50–64 years, ≥65 years). For children ages 5 to 11 years, rVE estimates for 3 versus 2 doses were calculated overall, rather than by month since third dose, due to the short time frame when the booster was recommended. Absolute VE 2 doses versus unvaccinated (all age groups), 3 doses versus unvaccinated (age groups ≥12 years), and 4 doses versus unvaccinated (50–64 years, ≥65 years) were also estimated.
Two of the 4 pharmacy chains collected month and year of vaccination (without day) during the study period. Two pharmacy chains collected exact day of vaccination. Months since last dose was calculated as the interval between month of testing and month of the last dose. Because the interval of 2 weeks was required between the last dose and testing date, and only month of vaccination was available for 2 pharmacy chains, we designated intervals as follows: 0 month (ie, testing and vaccination in the same month), 14 to 30 days; 1 month, 14 to 60 days; 2 months, 30 to 90 days; 3 months, 60 to 120 days, and so on (assuming 30-day month intervals). For example, person A was vaccinated on June 1, 2022 and tested on July 31, 2022 (month interval, 1; days, 60). Person B was vaccinated on June 14, 2022 and tested on August 15, 2022 (month interval, 2; days, 60). A simulation study found VE results utilizing months since vaccination was similar to using days since vaccination [9]. Statistical analyses were performed in R (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Patient Consent Statement
This activity was reviewed by the CDC and was conducted consistent with applicable federal law and CDC policy (see 45 C.F.R. part 46; 21 C.F.R. part 56; 42 U.S.C. §241(d), 5 U.S.C. §552a, 44 U.S.C. §3501 et seq). It was determined to be public health surveillance and was not submitted for institutional review board approval and informed consent was not needed.
RESULTS
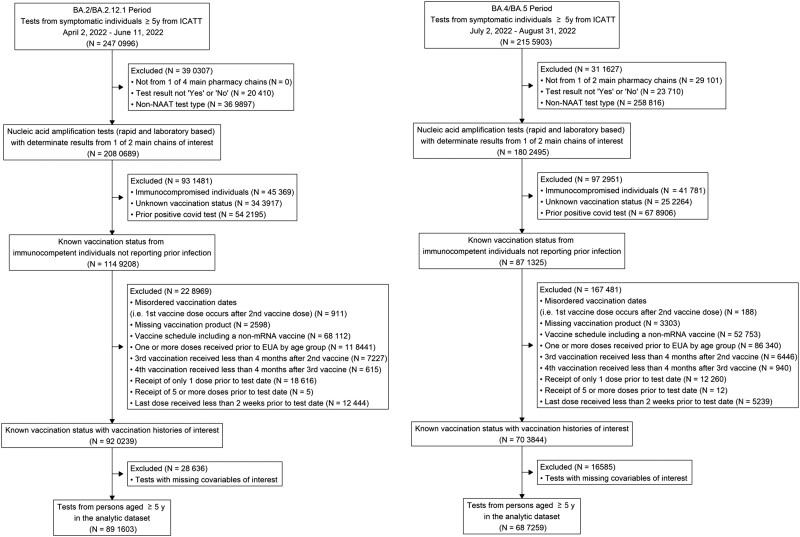
A total of 1 578 862 tests for SARS-CoV-2 from 12 148 sites across 49 states, Puerto Rico, and Washington, DC met inclusion criteria (Figure 1), including 760 986 cases (395 196 during Omicron BA.2/BA.2.12.1 and 365 790 Omicron BA.4/BA.5 periods) and 817 876 test-negative controls (496 407 Omicron BA.2/BA.2.12.1 and 321 469 Omicron BA.4/BA.5) (Table 1). Sixty-three percent of tests were laboratory-based NAAT (37% with rapid NAAT). Approximately 62% of tests included were from persons aged 16–49 years, 923 007 were female (58%), 873 969 were White (55%), and 324 185 were Hispanic/Latino (21%). Among tests included in analyses, 41% were from persons who reported receiving 3 doses (range, 3% of 5-11 age group to 49% of ≥65 age group) and 34% from persons who received 2 doses (range, 19% of ≥65 age group to 45% of 12–15 age group) of an mRNA vaccine. The proportion of the study population reporting 1 of more underlying conditions ranged from 4% (5–11 years) to 52% (≥65 years). Age distributions and frequencies of underlying conditions by age group are reported in Supplementary Table 2.
Figure 1.
Inclusion criteria for analysis of association of booster doses of an mRNA COVID-19 vaccine with symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children, adolescents, and adults during BA.2/BA.2.12.1 (April 2, 2022–June 11, 2022) and BA.4/BA.5 (July 2, 2022–August 31, 2022) predominant periods. EUA, Emergency Use Authorization; ICATT, Increasing Community Access to Testing; NAAT, nucleic acid amplification test; y, years.
Table 1.
Distribution of Characteristics for Symptomatic SARS-CoV-2 Cases and Symptomatic Test-Negative Controls Without Self-Reported Prior SARS-CoV-2 Infection During BA.2/BA.2.12.1a and BA.4/BA.5b Predominant Periods by Age Group
| … | Cases (SARS-CoV-2 Positive) No. (%) |
Controls (SARS-CoV-2 Negative) No. (%) |
… | Doses of mRNA COVID-19 Vaccine No. (%) | … | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | BA.2/BA.2.12.1 | BA.4/BA.5 | BA.2/BA.2.12.1 | BA.4/BA.5 | Unvaccinated | 2 | 3 | 4 | Overall |
| 5–11 Years Old | |||||||||
| Vaccination Historyc | … | … | … | … | … | … | … | … | … |
| Unvaccinated | 8335 (53) | 8298 (64) | 20 307 (52) | 11 682 (57) | 48 622 (100) | 0 (0) | 0 (0) | 0 (NA) | 48 622 (55) |
| 2 Doses | 7403 (47) | 4209 (32) | 18 811 (48) | 7119 (34) | 0 (0) | 37 542 (100) | 0 (0) | 0 (NA) | 37 542 (42) |
| 3 Doses | 12 (0.08) | 505 (4) | 90 (0.2) | 1859 (9) | 0 (0) | 0 (0) | 2466 (100) | 0 (NA) | 2466 (3) |
| 4 Doses | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (NA) | 0 (0) |
| SARS-CoV-2 Positive | … | … | … | … | … | … | … | … | … |
| BA.2/BA2.12.1 | 15 750 (100) | 0 (0) | 0 (0) | 0 (0) | 8335 (17) | 7403 (20) | 12 (0.5) | 0 (NA) | 15 750 (18) |
| BA.4/BA.5 | 0 (0) | 13 012 (100) | 0 (0) | 0 (0) | 8298 (17) | 4209 (11) | 505 (20) | 0 (NA) | 13 012 (15) |
| SARS-CoV-2 Negative | … | … | … | … | … | … | … | … | … |
| BA.2/BA.2.12.1 | 0 (0) | 0 (0) | 39 208 (100) | 0 (0) | 20 307 (42) | 18 811 (50) | 90 (4) | 0 (NA) | 39 208 (44) |
| BA.4/BA.5 | 0 (0) | 0 (0) | 0 (0) | 20 660 (100) | 11 682 (24) | 7119 (19) | 1859 (75) | 0 (NA) | 20 660 (23) |
| SARS-CoV-2 Test Type, NAAT Only | … | … | … | … | … | … | … | … | … |
| Rapidd | 5849 (37) | 5150 (40) | 17 761 (45) | 9604 (46) | 22 165 (46) | 15 315 (41) | 884 (36) | 0 (NA) | 38 364 (43) |
| Laboratory-basede | 9901 (63) | 7862 (60) | 21 447 (55) | 11 056 (54) | 26 457 (54) | 22 227 (59) | 1582 (64) | 0 (NA) | 50 266 (57) |
| Underlying chronic conditionsf, any (%) | 524 (3) | 492 (4) | 1320 (3) | 804 (4) | 1630 (3) | 1397 (4) | 113 (5) | 0 (NA) | 3140 (4) |
| 12–15 Years Old | |||||||||
| Vaccination Historyc | … | … | … | … | … | … | … | … | … |
| Unvaccinated | 3545 (33) | 3566 (40) | 6383 (34) | 4114 (33) | 17 608 (100) | 0 (0) | 0 (0) | 0 (NA) | 17 608 (34) |
| 2 Doses | 5343 (49) | 3762 (42) | 8433 (44) | 5409 (44) | 0 (0) | 22 947 (100) | 0 (0) | 0 (NA) | 22 947 (45) |
| 3 Doses | 1926 (18) | 1581 (18) | 4187 (22) | 2853 (23) | 0 (0) | 0 (0) | 10 547 (100) | 0 (NA) | 10 547 (21) |
| 4 Doses | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (NA) | 0 (0) |
| SARS-CoV-2 Positive | … | … | … | … | … | … | … | … | … |
| BA.2/BA2.12.1 | 10 814 (100) | 0 (0) | 0 (0) | 0 (0) | 3545 (20) | 5343 (23) | 1926 (18) | 0 (NA) | 10 814 (21) |
| BA.4/BA.5 | 0 (0) | 8909 (100) | 0 (0) | 0 (0) | 3566 (20) | 3762 (16) | 1581 (15) | 0 (NA) | 8909 (17) |
| SARS-CoV-2 Negative | … | … | … | … | … | … | … | … | … |
| BA.2/BA.2.12.1 | 0 (0) | 0 (0) | 19 003 (100) | 0 (0) | 6383 (36) | 8433 (37) | 4187 (40) | 0 (NA) | 19 003 (37) |
| BA.4/BA.5 | 0 (0) | 0 (0) | 0 (0) | 12 376 (100) | 4114 (23) | 5409 (24) | 2853 (27) | 0 (NA) | 12 376 (24) |
| SARS-CoV-2 Test Type, NAAT Only | … | … | … | … | … | … | … | … | … |
| Rapidd | 4095 (38) | 3395 (38) | 8644 (45) | 5948 (48) | 7904 (45) | 10 487 (46) | 3691 (35) | 0 (NA) | 22 082 (43) |
| Laboratory-basede | 6719 (62) | 5514 (62) | 10 359 (55) | 6428 (52) | 9704 (55) | 12 460 (54) | 6856 (65) | 0 (NA) | 29 020 (57) |
| Underlying chronic conditionsf, any (%) | 504 (5) | 492 (6) | 1068 (6) | 760 (6) | 904 (5) | 1271 (6) | 649 (6) | 0 (NA) | 2824 (6) |
| 16–49 Years Old | |||||||||
| Vaccination Historyc | … | … | … | … | … | … | … | … | … |
| Unvaccinated | 47 383 (19) | 55 070 (24) | 67 460 (22) | 47 171 (24) | 217 084 (100) | 0 (0) | 0 (0) | 0 (NA) | 217 084 (22) |
| 2 Doses | 91 712 (37) | 89 960 (39) | 98 626 (33) | 74 942 (38) | 0 (0) | 355 240 (100) | 0 (0) | 0 (NA) | 355 240 (36) |
| 3 Doses | 106 149 (43) | 84 893 (37) | 135 206 (45) | 76 508 (39) | 0 (0) | 0 (0) | 402 756 (100) | 0 (NA) | 402 756 (41) |
| 4 Doses | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (NA) | 0 (0) |
| SARS-CoV-2 Positive | … | … | … | … | … | … | … | … | … |
| BA.2/BA2.12.1 | 245 244 (100) | 0 (0) | 0 (0) | 0 (0) | 47 383 (22) | 91 712 (26) | 106 149 (26) | 0 (NA) | 245 244 (25) |
| BA.4/BA.5 | 0 (0) | 229 923 (100) | 0 (0) | 0 (0) | 55 070 (25) | 89 960 (25) | 84 893 (21) | 0 (NA) | 229 923 (24) |
| SARS-CoV-2 Negative | … | … | … | … | … | … | … | … | … |
| BA.2/BA.2.12.1 | 0 (0) | 0 (0) | 301 292 (100) | 0 (0) | 67 460 (31) | 98 626 (28) | 135 206 (34) | 0 (NA) | 301 292 (31) |
| BA.4/BA.5 | 0 (0) | 0 (0) | 0 (0) | 198 621 (100) | 47 171 (22) | 74 942 (21) | 76 508 (19) | 0 (NA) | 198 621 (20) |
| SARS-CoV-2 Test Type, NAAT Only | … | … | … | … | … | … | … | … | … |
| Rapidd | 89 303 (36) | 83 912 (36) | 114 487 (38) | 80 574 (41) | 90 379 (42) | 149 267 (42) | 128 630 (32) | 0 (NA) | 368 276 (38) |
| Laboratory-basede | 155 941 (64) | 146 011 (64) | 186 805 (62) | 118 047 (59) | 126 705 (58) | 205 973 (58) | 274 126 (68) | 0 (NA) | 606 804 (62) |
| Underlying chronic conditionsf, any (%) | 41 453 (17) | 39 310 (17) | 56 114 (19) | 36 666 (18) | 34 756 (16) | 61 899 (17) | 76 888 (19) | 0 (NA) | 173 543 (18) |
| 50–64 Years Old | |||||||||
| Vaccination Historyc | … | … | … | … | … | … | … | … | … |
| Unvaccinated | 10 376 (13) | 9526 (13) | 11 193 (14) | 6906 (13) | 38 001 (100) | 0 (0) | 0 (0) | 0 (0) | 38 001 (13) |
| 2 Doses | 25 633 (33) | 25 415 (34) | 22 712 (28) | 17 681 (33) | 0 (0) | 91 441 (100) | 0 (0) | 0 (0) | 91 441 (32) |
| 3 Doses | 40 378 (51) | 32 282 (44) | 42 960 (53) | 21 795 (40) | 0 (0) | 0 (0) | 137 415 (100) | 0 (0) | 137 415 (48) |
| 4 Doses | 2466 (3) | 6712 (9) | 3876 (5) | 7851 (14) | 0 (0) | 0 (0) | 0 (0) | 20 905 (100) | 20 905 (7) |
| SARS-CoV-2 Positive | … | … | … | … | … | … | … | … | … |
| BA.2/BA2.12.1 | 78 853 (100) | 0 (0) | 0 (0) | 0 (0) | 10 376 (27) | 25 633 (28) | 40 378 (29) | 2466 (12) | 78 853 (27) |
| BA.4/BA.5 | 0 (0) | 73 935 (100) | 0 (0) | 0 (0) | 9526 (25) | 25 415 (28) | 32 282 (23) | 6712 (32) | 73 935 (26) |
| SARS-CoV-2 Negative | … | … | … | … | … | … | … | … | … |
| BA.2/BA.2.12.1 | 0 (0) | 0 (0) | 80 741 (100) | 0 (0) | 11 193 (29) | 22 712 (25) | 42 960 (31) | 3876 (19) | 80 741 (28) |
| BA.4/BA.5 | 0 (0) | 0 (0) | 0 (0) | 54 233 (100) | 6906 (18) | 17 681 (19) | 21 795 (16) | 7851 (38) | 54 233 (19) |
| SARS-CoV-2 Test Type, NAAT Only | … | … | … | … | … | … | … | … | … |
| Rapidd | 30 363 (39) | 26 123 (35) | 31 372 (39) | 20 950 (39) | 15 489 (41) | 38 267 (42) | 48 871 (36) | 6181 (30) | 108 808 (38) |
| Laboratory-basede | 48 490 (61) | 47 812 (65) | 49 369 (61) | 33 283 (61) | 22 512 (59) | 53 174 (58) | 88 544 (64) | 14 724 (70) | 178 954 (62) |
| Underlying chronic conditionsf, any (%) | 28 686 (36) | 28 448 (38) | 31 303 (39) | 21 748 (40) | 13 402 (35) | 35 344 (39) | 52 916 (39) | 8523 (41) | 110 185 (38) |
| ≥65 Years Old | |||||||||
| Vaccination Historyc | … | … | … | … | … | … | … | … | … |
| Unvaccinated | 3661 (8) | 2948 (7) | 4560 (8) | 2508 (7) | 13 677 (100) | 0 (0) | 0 (0) | 0 (0) | 13 677 (8) |
| 2 Doses | 8723 (20) | 8805 (22) | 8971 (16) | 6704 (19) | 0 (0) | 33 203 (100) | 0 (0) | 0 (0) | 33 203 (19) |
| 3 Doses | 26 181 (59) | 16 527 (41) | 31 790 (57) | 12 247 (34) | 0 (0) | 0 (0) | 86 745 (100) | 0 (0) | 86 745 (49) |
| 4 Doses | 5970 (13) | 11 731 (29) | 10 842 (19) | 14 120 (40) | 0 (0) | 0 (0) | 0 (0) | 42 663 (100) | 42 663 (24) |
| SARS-CoV-2 Positive | … | … | … | … | … | … | … | … | … |
| BA.2/BA2.12.1 | 44 535 (100) | 0 (0) | 0 (0) | 0 (0) | 3661 (27) | 8723 (26) | 26 181 (30) | 5970 (14) | 44 535 (25) |
| BA.4/BA.5 | 0 (0) | 40 011 (100) | 0 (0) | 0 (0) | 2948 (22) | 8805 (27) | 16 527 (19) | 11 731 (27) | 40 011 (23) |
| SARS-CoV-2 Negative | … | … | … | … | … | … | … | … | … |
| BA.2/BA.2.12.1 | 0 (0) | 0 (0) | 56 163 (100) | 0 (0) | 4560 (33) | 8971 (27) | 31 790 (37) | 10 842 (25) | 56 163 (32) |
| BA.4/BA.5 | 0 (0) | 0 (0) | 0 (0) | 35 579 (100) | 2508 (18) | 6704 (20) | 12 247 (14) | 14 120 (33) | 35 579 (20) |
| SARS-CoV-2 Test Type, NAAT Only | … | … | … | … | … | … | … | … | … |
| Rapidd | 18 896 (42) | 14 612 (37) | 22 846 (41) | 13 433 (38) | 6067 (44) | 14 602 (44) | 34 655 (40) | 14 463 (34) | 69 787 (40) |
| Laboratory-basede | 25 639 (58) | 25 399 (63) | 33 317 (59) | 22 146 (62) | 7610 (56) | 18 601 (56) | 52 090 (60) | 28 200 (66) | 106 501 (60) |
| Underlying chronic conditionsf, any (%) | 22 528 (51) | 21 051 (53) | 28 937 (52) | 19 349 (54) | 5776 (42) | 17 311 (52) | 45 804 (53) | 22 974 (54) | 91 865 (52) |
Abbreviations: COVID-19, coronavirus disease 2019; NA, not applicable; NAAT, nucleic acid amplification test; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
BA.2/BA.2.12.1: April 2, 2022–June 11, 2022.
BA.4/BA.5: July 2, 2022–August 31, 2022.
Only month and year of receipt were reported for each vaccination dose from some participating pharmacies; therefore, the number of months between a vaccine dose and testing is a whole number calculated as the difference between the month and year of testing and the month and year of the vaccine dose. Persons reporting a non-mRNA vaccination were excluded from analyses. For doses received in the same month or the month before SARS-CoV-2 testing, an additional question was asked to specify whether the dose was received ≥2 weeks before testing, and only doses received ≥2 weeks before testing were included.
Rapid NAAT was performed onsite on self-collected nasal swabs using ID Now (Abbott Diagnostics Scarborough Inc.) and Accula (Thermo Fisher Scientific).
Laboratory-based NAAT was performed on self-collected nasal swabs at contracted laboratories using a variety of testing platforms.
Underlying conditions included on the survey were as follows: heart conditions, high blood pressure, overweight or obesity, diabetes, current or former smoker, kidney failure or end-stage renal disease, cirrhosis of the liver, chronic lung disease (such as chronic obstructive pulmonary disease, moderate to severe asthma, cystic fibrosis, or pulmonary embolism).
Relative Vaccine Effectiveness
For children aged 5–11 years, rVE of 3 versus 2 doses of mRNA vaccine against infection was 77% (95% confidence interval [CI], 54%–88%; maximum time since last dose, <1 month) during BA.2/BA.2.12.1 predominance and 56% (95% CI, 50%–60%; maximum time, 3.5 months) during BA.4/BA.5 predominance (Supplementary Table 3).
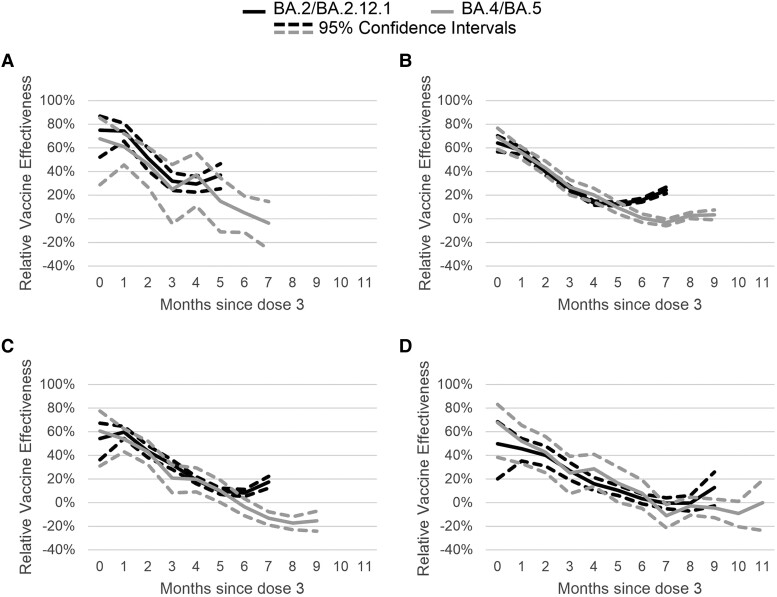
Estimates of rVE for 3 versus 2 doses of mRNA vaccine for children aged 12–15 years at 1 month post third dose was 74% (95% CI, 65%–81%) during BA.2/BA.2.12.1 and 62% (95% CI, 51%–70%) during BA.4/BA.5. At 5 months post third dose receipt, VE was 38% (95% CI, 26%–47%) during BA.2/BA.2.12.1 and 11% (95% CI, −8% to 26%) during BA.4/BA.5 (Figure 2; Supplementary Table 3).
Figure 2.
Adjusted mRNA COVID-19 relative vaccine effectiveness of 3 doses compared to 2 doses against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during BA.2/BA.2.12.1 (April 2, 2022–June 11, 2022) and BA.4/BA.5 (July 2, 2022–August 31, 2022) periods by time since third dose receipt. Multivariable logistic regression models controlling for calendar day of test (continuous), age, gender, race, ethnicity, 2018 Centers for Disease Control and Prevention census tract social vulnerability index, underlying conditions (presence vs absence—included on the survey were heart conditions, high blood pressure, overweight or obesity, diabetes, current or former smoker, kidney failure or end-stage renal disease, cirrhosis of the liver, chronic lung disease [such as chronic obstructive pulmonary disease, moderate to severe asthma, cystic fibrosis, or pulmonary embolism]), individual state of residence, pharmacy chain, and average cases per 100 000 by site zip code (over the last 7 days) were used to estimate vaccine effectiveness. Tests included in the reference group were from persons receiving 2 doses and eligible for a third dose (ie, if 5 months had passed since second dose). (A) Children aged 12–15 years. (B) Young adults aged 16–49 years. (C) Adults aged 50–64 years. (D) Adults aged 65 years and older.
Adults aged 16–49 years at 1 month post third dose receipt had a rVE for 3 versus 2 doses of 58% (95% CI, 55%–61%) during BA.2/BA.2.12.1 and 56% (95% CI, 52%–60%) during BA.4/BA.5. Relative VE was lower by 5 months after third dose receipt for BA.2/BA.2.12.1 predominance (VE, 14%; 95% CI, 13%–16%) and BA.4/BA.5 predominance (VE, 13%; 95% CI, 9%–17%) (Figure 2; Supplementary Table 3).
rVE for 3 versus 2 doses among those aged 50–64 years at 1 month after third dose receipt was 60% (95% CI, 54%–65%) during BA.2/BA.2.12.1 and 57% (95% CI, 50%–64%) during BA.4/BA.5. At 5 months post third dose receipt, rVE decreased to 11% during BA.2/BA.2.12.1 (95% CI, 8%–13%) and 12% during BA.4/BA.5 (95% CI, 5%–19%) (Figure 2; Supplementary Table 3).
Relative VE of 3 versus 2 doses among adults ≥65 years at 1 month after receipt of third dose was 45% (95% CI, 35%–54%) during BA.2/BA.2.12.1 and 50% (95% CI, 37%–61%) during BA.4/BA.5. By 5 months post third dose receipt, rVE declined to 11% (95% CI, 6%–15%) during BA.2/BA.2.12.1 and 13% (95% CI, 0%–24%) during BA.4/BA.5 (Figure 2; Supplementary Table 3).
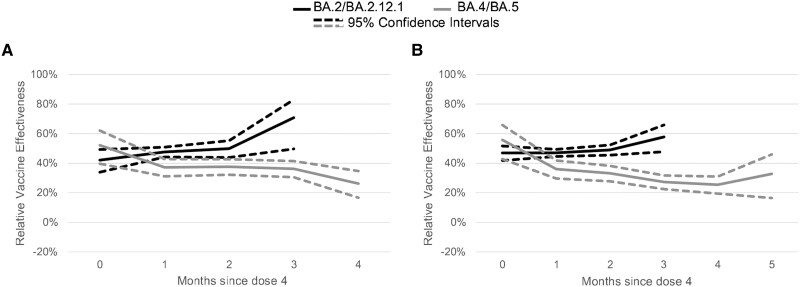
At 1 month after receipt of a fourth dose, rVE for 4 versus 3 doses among adults aged 50–64 years was 48% (95% CI, 44%–51%) during BA.2/BA.2.12.1 and 45% (95% CI, 41%–48%) during BA.4/BA.5. In the third month since receipt of the fourth dose, rVE was 74% (95% CI, 54%–85%) during BA.2/BA.2.12.1 and 38% (95% CI, 33%–41%) during BA.4/BA.5. Among adults ages ≥65 years at 1 month after receipt of a fourth dose, rVE for 4 versus 3 doses was 49% (95% CI, 46%–51%) during BA.2/BA.2.12.1 and 40% (95% CI, 36%–44%) during BA.4/BA.5. In the third month since receipt of the fourth dose, rVE was 58% (95% CI, 47%–66%) during BA.2/BA.2.12.1 and 33% (95% CI, 30%–36%) during BA.4/BA.5 (Figure 3; Supplementary Table 3).
Figure 3.
Adjusted mRNA COVID-19 relative vaccine effectiveness of 4 doses compared to 3 doses against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during BA.2/BA.2.12.1 (April 2, 2022–June 11, 2022) and BA.4/BA.5 (July 2, 2022–August 31, 2022) periods by time since fourth dose receipt. Multivariable logistic regression models controlling for calendar day of test (continuous), age, gender, race, ethnicity, 2018 Centers for Disease Control and Prevention census tract social vulnerability index, underlying conditions (presence vs absence—included on the survey were heart conditions, high blood pressure, overweight or obesity, diabetes, current or former smoker, kidney failure or end-stage renal disease, cirrhosis of the liver, chronic lung disease [such as chronic obstructive pulmonary disease, moderate to severe asthma, cystic fibrosis, or pulmonary embolism]), individual state of residence, pharmacy chain, and average cases per 100 000 by site zip code (over the last 7 days) were used to estimate vaccine effectiveness. Tests included in the reference group were from persons receiving 3 doses and eligible for a fourth dose (ie, if 5 months had passed since third dose). (A) Adults aged 50–64 years. (B) Adults aged 65 years or older.
Absolute Vaccine Effectiveness
Estimates of aVE for 2, 3, and 4 doses compared to no doses (unvaccinated) during BA.2/BA.2.12.1 and BA.4/BA.5 predominance were similar across all age groups and declined by time since last dose receipt (SupplementaryFigures 1–3). In aVE models, 2 doses were no longer effective by 6 months after receipt of the second dose for all age groups and during both subvariant periods except for children aged 5–11 years where 2 doses remained effective throughout the BA.2/BA.2.12.1 study period. Three doses were no longer effective by 6 months after receipt of the third dose for those ≥16 years.
DISCUSSION
In this analysis of national pharmacy testing data, monovalent mRNA boosters provided additional protection against symptomatic SARS-CoV-2 infection among adults, adolescents, and children across BA.2/BA.2.12.1 and BA.4/BA.5 predominant periods. During BA.4/BA.5, protection among adults ≥50 years declined moderately and remained above zero 4 months after receipt of the second booster dose. Among adolescents 12–15 years old, the first booster (dose 3) was no longer effective by 5 months after receipt, and by 6 months after receipt among those 16–49 years old.
In March 2022, the FDA authorized a fourth monovalent vaccine dose for all adults aged 50 years and older 4 months after receiving their third dose. As of September 21, 2022, a minority of adults ≥50 years (35.5%) and ≥65 years (42.8%) had received a second booster (fourth dose) [21]. However, the current study demonstrates added benefit of the fourth dose against infection, particularly 3–5 months after vaccination. These results are consistent with immunogenicity studies demonstrating an increase in antibodies from a fourth dose after almost a 10-fold antibody decrease due to waning after the third dose, and studies evaluating effectiveness of 4 versus 3 doses of mRNA vaccine [22–26].
Relative VE of a third mRNA dose over a second waned with time after receipt of the third dose. Similar waning was seen during both Omicron periods in this analysis. During pre-Omicron, waning of rVE for 3 versus 2 doses has been observed among adults against infection but not against more severe outcomes [27]. Previous analyses from this ICATT platform have noted waning of aVE against infection among adolescents aged 12–15 years [9] and adults [8] during the early Omicron period; our results suggest continued waning occurred during this study period after receipt of 3 doses of an mRNA vaccine. A similar magnitude of waning was observed across all age groups.
To contextualize interpretation of our primary analysis of rVE, we evaluated aVE for 4, 3, and 2 doses versus no doses, and we found similar patterns of waning after receipt of 2 or 3 doses. Absolute VE estimates for 2 and 3 doses at 5 months post receipt were zero (ie, no protection), indicating rVE likely approximates aVE. Significantly negative rVE estimates observed among adults aged ≥50 years during BA.4/BA.5 predominance 7–9 months post receipt of a third dose compared to those with 2 doses and higher aVE by time since fourth dose may represent residual confounding, potentially due to underreported prior SARS-CoV-2 infection.
A recent study by Petrie et al [25] reported similar findings with modest to high protection after the third dose (or first booster) compared to a primary series that waned to null by 6 months after receipt of the third dose. Petrie et al [25] found no significant VE after the second booster dose when compared to a single booster dose, whereas an earlier study from Israel confirmed similar VE to our study with a relative VE of 52% (95% CI, 49%–54%) in the first month after the fourth dose [28]. Other recent findings showing that 4 doses are effective against severe COVID-19 disease [29–31], and have an increase in antibody levels and neutralizing activity [26] underscore the continued need for people of all ages to remain current with their COVID-19 vaccinations. Despite the emergence of new subvariants, mRNA vaccines continue to demonstrate at least short-term effectiveness against infection and longer term protection against more severe outcomes. Recently approved COVID-19 bivalent mRNA vaccines, more closely matching currently circulating variants, appear to provide increased protection [32–34].
Limitations
There are several important limitations of this study. First, these data are from pharmacies selected based on RUCA codes [17] and SVI [19] to better reach underserved communities; therefore, the population included may not be representative of the general US population, or even geographic areas where reporting pharmacy sites are located. Second, the population included is based on persons accessing pharmacies for SARS-CoV-2 testing. Changes in individual testing practices and increased use of home tests [35], by presence of symptoms or vaccination status, could introduce selection bias. Third, we did not have information on community or household-level exposures, individual prevention behaviors (eg, mask use), or other confounders [36]. Those who remain unvaccinated may be fundamentally different from vaccinated individuals. Fourth, vaccination status and prior infection were self-reported and subject to recall bias; therefore, there is a potential for misclassification. As of June 2022, 58.7% of the US population had infection-induced seroprevalence; however, only 21% (during BA.2/BA.2.12.1) and 31% (during BA.4/BA.5) of the initial sample reported prior infection [37], indicating underreporting of prior infection. Given the poor characterization of prior infection in this sample, the present study included only individuals who indicated no prior infection.
CONCLUSIONS
Although the goal of the US COVID-19 vaccination program is prevention of severe disease [38], VE against symptomatic infection can provide important insight to help understand how vaccines are working in the real world, especially in the context of emerging variants and before estimates against more severe outcomes are available. Continued monitoring of COVID-19 VE is crucial to inform and guide future vaccine policy decisions.
Our study highlights how VE against SARS-CoV-2 infection changes over time, which can signal reduced effectiveness against new variants, waning vaccine protection, or both. We found booster doses provide added protection against SARS-CoV-2 infection among immunocompetent persons ages 5 years and older during BA.2/BA.2.12.1 and BA.4/BA.5 predominance. However, protection waned in the months after vaccination; although little to no waning was observed after the fourth dose in older adults, long-term follow up was not available. Recently introduced bivalent COVID-19 mRNA vaccine doses have the potential to restore protection against circulating new Omicron subvariants. Our study supports the need to stay up to date on recommended doses of COVID-19 vaccines.
Supplementary Material
Acknowledgments
We thank Stephanie J. Schrag (Centers for Disease Control and Prevention [CDC]).
Contributor Information
Allison Avrich Ciesla, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Eagle Health Analytics, San Antonio, Texas, USA.
Ryan E Wiegand, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Zachary R Smith, Division of Research and Methodology, National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, Maryland, USA.
Amadea Britton, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine E Fleming-Dutra, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Joseph Miller, Center for Preparedness and Response, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Emma K Accorsi, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Jennifer R Verani, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; US Public Health Service Commissioned Corps, Rockville, Maryland, USA.
Nong Shang, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Gordana Derado, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Tamara Pilishvili, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Ruth Link-Gelles, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; US Public Health Service Commissioned Corps, Rockville, Maryland, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Author contributions. AAC and REW had full access to the data used in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AAC, RL-G, AB, KEF-D, JRV, TP, and REW contributed to concept and design. All authors contributed to acquisition, analysis, or interpretation of data. AAC, RL-G, TP, and REW drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. AAC, NS, GD, and REW contributed to statistical analysis. JM obtained funding. ZRS, AB, KEF-D, JM, JRV, TP, and RL-G contributed to administrative, technical, or material support. JM, TP, RL-G, and REW supervised the work.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support . Funding for the Increasing Community Access to Testing platform is provided by the US Department of Health and Human Services. Funding for this analysis was provided by the CDC.
Role of the funder/sponsor. The CDC was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The CDC controlled publication decisions.
References
- 1. Centers for Disease Control and Prevention . Interim Clinical Considerations for Use of COVID-19 Vaccines: Appendices, References, and Previous Updates. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us-appendix.html. Accessed 19 October 2022.
- 2. Chenchula S, Karunakaran P, Sharma S, Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review. J Med Virol 2022; 94:2969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrews N, Stowe J, Kirsebom F, et al. . Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monge S, Rojas-Benedicto A, Olmedo C, et al. . Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis 2022; 22:1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen CH, Schelde AB, Moustsen-Helm IR, et al. . Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study [preprint]. medRxiv 2021. 10.1101/2021.12.20.21267966 [DOI] [Google Scholar]
- 6. Yoon SK, Hegmann KT, Thiese MS, et al. . Protection with a third dose of mRNA vaccine against SARS-CoV-2 variants in frontline workers. N Engl J Med 2022; 386:1855–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Accorsi EK, Britton A, Shang N, et al. . Effectiveness of homologous and heterologous Covid-19 boosters against Omicron. N Engl J Med 2022; 386:2433–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Accorsi EK, Britton A, Fleming-Dutra KE, et al. . Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and delta variants. JAMA 2022; 327:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming-Dutra KE, Britton A, Shang N, et al. . Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA 2022; 327:2210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Butt AA, Talisa VB, Shaikh OS, Omer SB, Mayr FB. Relative vaccine effectiveness of a severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine booster dose against the omicron variant. Clin Infect Dis 2022; 75:2161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prasad N, Derado G, Nanduri SA, et al. . Effectiveness of a COVID-19 additional primary or booster vaccine dose in preventing SARS-CoV-2 infection among nursing home residents during widespread circulation of the Omicron variant - United States, February 14-March 27, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao Y, Yisimayi A, Jian F, et al. . BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022; 608:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khan K, Karim F, Ganga Y, et al. . Omicron sub-lineages BA.4/BA.5 escape BA.1 infection elicited neutralizing immunity. Nat Commun 2022; 13:4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arora P, Kempf A, Nehlmeier I, et al. . Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. Lancet Infect Dis 2022; 22:1117–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Kainulainen MH, Jiang N, et al. . Differential neutralization and inhibition of SARS-CoV-2 variants by antibodies elicited by COVID-19 mRNA vaccines. Nat Commun 2022; 13:4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Britton A, Fleming-Dutra KE, Shang N, et al. . Association of COVID-19 vaccination with symptomatic SARS-CoV-2 infection by time since vaccination and delta variant predominance. JAMA 2022; 327:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. US Department of Agriculture . Rural-Urban Commuting Area Codes. Available at: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx. Accessed 19 October 2022.
- 18. Flanagan BE, Gregory EW, Hallisey EJ, Heitgerd JL, Lewis B. A social vulnerability index for disaster management. J Homel Secur Emerg Manag 2011; 8:1–22. [Google Scholar]
- 19. U.S. Centers for Disease Control and Prevention . CDC/ATSDR Social Vulnerability Index (2018). Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html. Accessed 19 October 2022.
- 20. Centers for Disease Control and Prevention . SARS-CoV-2 Variant Proportions. Available at: https://data.cdc.gov/Laboratory-Surveillance/SARS-CoV-2-Variant-Proportions/jr58-6ysp. Accessed 28 July 2022.
- 21. Centers for Disease Control and Prevention . COVID-19 Vaccinations in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-additional-dose-totalpop. Accessed 27 September 2022.
- 22. Bar-On YM, Goldberg Y, Mandel M, et al. . Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med 2022; 386:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regev-Yochay G, Gonen T, Gilboa M, et al. . Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. N Engl J Med 2022; 386:1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eliakim-Raz N, Stemmer A, Ghantous N, et al. . Antibody titers after a third and fourth SARS-CoV-2 BNT162b2 vaccine dose in older adults. JAMA Network Open 2022; 5:e2223090-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrie JG, King JP, McClure DL, et al. . Effectiveness of first and second COVID-19 mRNA vaccine monovalent booster doses during a period of circulation of Omicron variant sublineages: December 2021-July 2022. Influenza Other Respir Viruses 2023; 17:e13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nugent C, Abul Y, White E, et al. . Second monovalent SARS-CoV-2 mRNA booster restores Omicron-specific neutralizing activity in both nursing home residents and health care workers [preprint]. medRxiv 2023. 10.1101/2023.01.22.23284881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tartof SY, Slezak JM, Puzniak L, et al. . Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am 2022; 9:100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magen O, Waxman JG, Makov-Assif M, et al. . Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2022; 386:1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Link-Gelles R, Levy ME, Gaglani M, et al. . Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated - VISION network, 10 states, December 2021-June 2022. MMWR Morb Mortal Wkly Rep 2022; 71:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatzilena A, Hyams C, Challen R, et al. . Relative vaccine effectiveness (rVE) of mRNA COVID-19 boosters in the UK vaccination programme, during the spring-summer (monovalent vaccine) and autumn-winter 2022 (bivalent vaccine) booster campaigns: a prospective test negative case-control study [preprint]. medRxiv 2023. 10.1101/2023.03.16.23287360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grewal R, Nguyen L, Buchan SA, et al. . Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat Commun 2023; 14:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention . Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. Accessed 19 October 2022.
- 33. Surie D, DeCuir J, Zhu Y, et al. . Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19–associated hospitalization among immunocompetent adults aged ≥65 years - IVY network, 18 states, September 8–November 30, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tenforde MW, Weber ZA, Natarajan K, et al. . Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19–associated emergency department or urgent care encounters and hospitalizations among immunocompetent adults—vISION network, nine states, September–November 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rader B, Gertz A, Iuliano AD, et al. . Use of at-home COVID-19 tests - United States, August 23, 2021-March 12, 2022. MMWR Morbidity Mortal Wkly Rep 2022; 71:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Westreich D, Hudgens MG. Invited commentary: beware the test-negative design. Am J Epidemiol 2016; 184:354–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention . 2022 Nationwide COVID-19 Infection- and Vaccination-Induced Antibody Seroprevalence (Blood donations). Available at: https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence-2022. Accessed 23 March 2023.
- 38. Rosenblum HG, Wallace M, Godfrey M, et al. . Interim recommendations from the Advisory Committee on Immunization Practices for the use of bivalent booster doses of COVID-19 vaccines—United States, October 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.