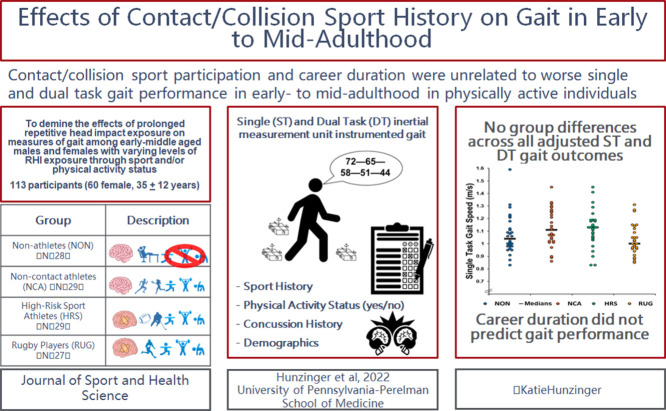
Graphical abstract
Keywords: Exercise, Neurodegenerative disease, Postural control, Rugby, Subconcussive impacts
Abstract
Background
To determine the effect of contact/collision sport participation on measures of single-task (ST) and dual-task (DT) gait among early- to middle-aged adults.
Methods
The study recruited 113 adults (34.88 ± 11.80 years, (mean ± SD); 53.0% female) representing 4 groups. Groups included (a) former non-contact/collision athletes and non-athletes who are not physically active (n = 28); (b) former non-contact/collision athletes who are physically active (n = 29); (c) former contact/collision sport athletes who participated in high-risk sports and are physically active (n = 29); and (d) former rugby players with prolonged repetitive head impact exposure history who are physically active (n = 27). Gait parameters were collected using inertial measurement units during ST and DT gait. DT cost was calculated for all gait parameters (double support, gait speed, and stride length). Groups were compared first using one-way analysis of covariance. Then a multiple regression was performed for participants in the high-risk sport athletes and repetitive head impact exposure athletes groups only to predict gait outcomes from contact/collision sport career duration.
Results
There were no significant differences between groups on any ST, DT, or DT cost outcomes (p > 0.05). Contact/collision sport duration did not predict any ST, DT, or DT cost gait outcomes.
Conclusion
Years and history of contact/collision sport participation does not appear to negatively affect or predict neurobehavioral function in early- to mid-adulthood among physically active individuals.
1. Introduction
Repetitive head impacts (RHI) are a form of neurotrauma that occur through normal participation in contact/collision sports (i.e., American football, football/soccer, ice hockey, boxing, rugby, lacrosse, and wrestling).1,2 It is theorized that RHI accumulated over years of contact/collision sports participation may have adverse effects on both short- and long-term neurobehavioral functioning and health outcomes, yet many of the existing studies are limited in generalizability and overall effect sizes of significant findings.3 Moreover, controversy remains as to whether RHI exposure leads to a higher risk of neurodegenerative diseases later in life.4, 5, 6 Since retrospective RHI exposure information is not available for many patients seeking evaluation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NINDS) traumatic encephalopathy syndrome consensus statement endorsed the use of contact/collision sport career duration as a proxy measure.7 However, the homogenous data that informed the traumatic encephalopathy syndrome criteria were derived mostly from male college- or middle-aged former collision sport athletes to the exclusion of females and other sport/cohort groups.3,7
Rugby is the most popular collision sport worldwide and the only collision sport where the laws are the same for both males and females,8,9 thus indicating similar RHI exposure.10 While most contact/collision sport athletes typically stop their careers after high school or college, many Americans only begin playing rugby at this time. Therefore, they continue to experience RHI through adulthood and often exceed the traumatic encephalopathy syndrome threshold for exposure.7,11,12 Growing evidence links rugby participation and long-term physical and mental health dysfunction; however, sports participation during adulthood is associated with increased physical activity, a known positive modifier of long-term health outcomes.13, 14, 15, 16 Thus, the negative effects of RHI and the positive effects of physical activity on mid- and later-life neurobehavioral health (e.g., gait) remain to be elucidated.15,17,18
Previous research on the long-term effects of RHI exposure has relied on self-report or on simplistic clinical measures with little to no inquiry into more objective and sensitive measures of neurobehavioral health in aging/post-collegiate populations.3,19, 20, 21, 22 Gait has been referred to as the 6th vital sign since it is an objective measure of neurological health, reflects quality of life, and is sensitive to age- and neurological impairment-related changes, with decreased performance (i.e., conservative gait strategies) in older and neurologically compromised populations.23, 24, 25 The addition of a cognitive challenge during gait, termed dual-task (DT) gait, has been utilized to identify differences between neurologically impaired populations and healthy controls.26 Further, DT gait can be used to identify post-concussion and persistent subclinical deficits in executive function as well as neurophysiological impairments by calculating the DT cost (i.e., the change from single task (ST) to DT).26 Yet, because most investigations into gait abnormalities due to RHI exposure/neurotrauma have been limited to younger (e.g., college or high school) male collision sport athletes or male and female contact/collision sport athletes and have failed to account for potential confounders (e.g., body mass index (BMI), age, concussion history, and sex), the long-term effects of RHI on gait remain inconclusive.19,20,22
Contact/collision sport-related RHI have been associated with poorer long-term health outcomes,7,17 but increased physical activity is known to improve many health outcomes.15,27 Thus, the purpose of this study was to determine the relationship between contact/collision sport career duration on ST and DT gait in physically active early- to middle-aged adults. We hypothesized that individuals with a history of RHI exposure would have a conservative gait strategy, evidenced by slower gait speed, a longer percentage of time in double support, and shorter stride length compared to individuals without RHI exposure. Secondly, we hypothesized that longer career duration would be associated with a conservative gait strategy (i.e., decreased gait speed and stride length, and increased double support) and worse DT cost in collision sport athletes.
2. Methods
2.1. Participants
Power analyses have been historically underutilized in neurotrauma and gait research.28 As such, a power analysis (G*Power 3.1; https://link.springer.com/content/pdf/10.3758/BF03193146.pdf) was conducted based on the ST gait speed of 13 total subjects across the 4 groups. (The ability of ST gait to discriminate healthy from neurologically impaired individuals has been shown in previous research.29,30) Results indicated that 22 participants were needed per group to achieve 80% power for a medium effect size (Cohen's f = 0.25);31 overall, 113 adults were enrolled (Table 1) (Fig. 1). Groups differed significantly in terms of BMI and concussion history, and thus these variables were accounted for in adjusted models. The inclusion and exclusion criteria for the 4 groups (non-athletes (NON), non-contact/collision athletes (NCA), high-risk sport athletes (HRS), and rugby/prolonged RHI exposure athletes (RUG)) are described below (Table 2). Consistent with previous studies, contact/collision sports included those with potential and/or routine RHI exposure (i.e., American football, soccer, ice hockey, boxing, rugby, lacrosse, and wrestling).2,11 Briefly, participants were recruited from 4 groups: (a) NON: individuals with no previous contact/collision sport experience who are not currently physically active (n = 28); (b) NCA: non-contact/collision sport athletes/individuals with no previous contact/collision sport experience who are physically active (n = 29); (c) HRS: former contact/collision sport athletes who are physically active (n = 29); and (d) RUG: current/former rugby players with a history of playing rugby after age 22 who are physically active (n = 27). Of note, none of the RUG group participants had actively participated in contact rugby in the 6 months prior to the study due to coronavirus disease 2019 (COVID-19) related shutdowns (years since last participating in contact/collision rugby: 8.5 ± 9.8 years; range: 0.5–36 years). Participants were recruited via word of mouth, social media, and flyers posted at various sports venues, and they were compensated for their participation in the form of an Amazon.com gift card. All participants provided oral and written informed consent in accordance with the University of Delaware's Institutional Review Board.
Table 1.
Participant demographics by group.
| Group 1 (NON) | Group 2 (NCA) | Group 3 (HRS) | Group 4 (RUG) | Overall | ANOVA F-value/Mann–Whitney U-value | p | Effect size (η2) | |
|---|---|---|---|---|---|---|---|---|
| n | 28 | 29 | 29 | 27 | 113 | N/A | N/A | N/A |
| Age (year) | 35.43 ± 14.17 (Range: 18–67) (95%CI: 29.93–40.92) | 33.90 ± 10.79 (Range: 23–67) (95%CI: 29.87–37.93) | 33.29 ± 8.39 (Range: 22–58) (95%CI: 29.03–35.54) | 38.07 ± 12.98 (Range: 22–67) (95%CI: 32.94–43.21) | 34.88 ± 11.80 (Range: 18–67) (95%CI: 32.68–37.07) | 1.185 | 0.319 | 0.032 |
| Sex (M/F) | 9/19 | 11/19 | 17/11 | 16/11 | 53/60 | 2.269 | 0.085 | 0.059 |
| BMI (kg/m2) | 26.90 ± 6.56 (Range: 17.85–44.63) (95%CI: 24.35–29.44) | 24.46 ± 3.47(Range: 19.97–37.92) (95%CI: 23.17–25.76) | 26.04 ± 4.38 (Range: 19.20–41.09) (95%CI: 24.33–27.73) | 29.31 ± 5.01# (Range: 22.46–40.18) (95%CI: 27.33–31.30) | 26.61 ± 5.20 (Range: 17.85–44.63) (95%CI: 25.64–27.58) | 4.508 | 0.005 | 0.110 |
| Concussion history (Y/N) | 7/21 | 11/18 | 18/11* | 19/8* | 55/58 | 5.472 | 0.002 | 0.131 |
| Number of concussions | 0 (Range: 0–2) (IQR: 0,0) | 0 (Range: 0–4) (IQR: 0,1) | 1 (Range: 0–7) (IQR: 0,2) | 2 (Range: 0–18) (IQR: 1,3) | 0 (Range: 0–18) (IQR: 0,2) | 8.310 | <0.001 | 0.186 |
| Contact/collision career duration (years) | N/A | N/A | 14.48 ± 9.30 | 16.33 ± 11.98 | 15.37 ± 10.62 | 363.500 | 0.646 | 2.961 |
Note: Data are presented as mean ± SD, except for number of concussions, which is reported as median and interquartile range due to the skew of zero concussions.
Abbreviations: 95% CI = 95% confidence interval; ANOVA = analysis of variance; BMI = body mass index; F = females; HRS = high-risk sport athletes; IQR = interquartile range; M = males; N = no; N/A = not applicable; NCA = non-contact athletes; NON = non-athletes; RUG = rugby/prolonged RHI exposure athletes; Y = yes.
Significant difference from NON.
Significant difference from NCA.
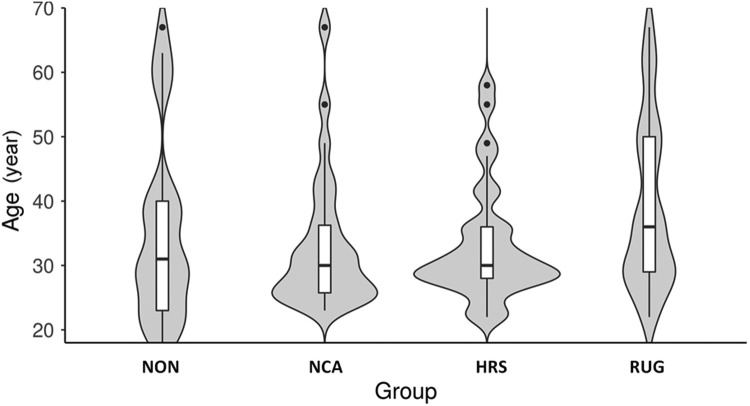
Fig. 1.
Violin plot of age distribution by group with box and whisker plot overlay. Groups were not significantly different by age (p = 0.319). HRS = high-risk sport athletes; NCA = non-contact athletes; NON = non-athletes; RUG = rugby/prolonged RHI exposure athletes.
Table 2.
Inclusion and exclusion criteria by group.
| Group ID | Description | Inclusion criteria | Exclusion criteria |
|---|---|---|---|
| NON | Former non-contact athletes or non-athletes who are not physically active (no RHI exposure) | Never played organized contact/collision sports Do not self-report currently meeting the ACSM physical activity guidelines (150 min of moderate or 60 min of vigorous physical activity per week)32 |
Self-report: Current pregnancy Any acute or chronic impairment that would interfere with normal gait and balance (e.g., vestibular disorders) Lower-extremity musculoskeletal injury at the time of testing Concussion within 6 months of the test date Any pre-existing neurological, balance, hearing, vestibular, or ocular disorders History of stroke or neurodegenerative disease Unstable cardiac or pulmonary disease |
| NCA | Former non-contact athletes who are physically active (no RHI exposure) | Never played organized contact/collision sports Self-report meeting ACSM physical activity guidelines |
|
| HRS | Former contact/collision sport athletes who participated in sports with a high risk for RHI (i.e., boxing, football, ice hockey, lacrosse, soccer, wrestling) who are physically active (previous RHI exposure) | History of organized contact/collision sport participation but ceased by age 22 Self-report meeting ACSM physical activity guidelines |
|
| RUG | Current and former rugby players with a history of playing rugby after the age of 22 (i.e., RHI exposure into adulthood after the age at which most collision sport participation ceases) (prolonged RHI exposure) | Older than 22 years of age with a history of playing at least 1 year of full contact (i.e., tackle) rugby after the age of 22 Self-report meeting ACSM physical activity guidelines |
Abbreviations: ACSM = American College of Sports Medicine; HRS = high-risk sport athletes; NCA = non-contact athletes; NON = non-athletes; RHI = repetitive head impacts; RUG = rugby/prolonged RHI exposure athletes.
2.2. Procedures
Participants completed online questionnaires to ascertain relevant demographic information, physical activity status (yes/no meeting American College of Sports Medicine guidelines of 150 min/week of moderate or 60 min/week of vigorous physical activity32), sport history, and career duration of contact/collision sports via Qualtrics (Qualtrics, Provo, UT, USA). Consistent with traumatic encephalopathy syndrome guidelines, career duration was calculated for each participant as the sum of each year played in each contact/collision sport (e.g., 2 years of ice hockey and 2 years of American football = 4 years career duration).7
Participants completed 5 ST walking trials and 5 DT walking trials with a cognitive task (i.e., spelling 5-letter words backwards, serial 6 s/7 s, or naming the months in reverse order) while wearing 3 inertial measurement units on the dorsal surface of each foot and L5 vertebrae (Opal Sensor-V1, APDM, Portland, OR, USA).33,34 Data were collected at 128 Hz and analyzed using Mobility Lab software (APDM).35 Walking trials consisted of the participant traversing a 7-m walkway at a self-selected pace. Dependent variables were double support (% of gait cycle), gait speed (m/s), and stride length (m) for ST, DT, and the associated DT cost for each. These variables were chosen because they differ the most between neurologically impaired populations and healthy controls, with negative changes being indicative of a conservative gait.26 DT cost was calculated as a percentage change between ST and DT conditions: (DT–ST)/(ST) × 100%.36
2.3. Statistical analysis
Group demographics were compared using either a one-way analysis of variance when comparing all 4 groups or a Mann–Whitney U-test when comparing the collision groups only (i.e., HRS and RUG), due to the violation of normality.
Unadjusted models can be found in Supplementary Table 1. To determine the effect of group on gait outcomes, groups were compared using an analysis of covariance adjusted for known covariates (i.e., age, sex, concussion history, and BMI)26,37 that may affect gait performance. All test assumptions of linearity, outliers, and homogeneity were met. Post hoc analyses were performed for significant outcomes, with a Bonferroni test for multiple comparisons. Effect sizes are reported as η2 and interpreted as small = 0.01−<0.06; medium =0.06−<0.14; large = ≥0.14.31
To investigate the relationship between contact/collision career duration (years) and gait outcomes, we performed a linear regression using the enter method to predict ST, DT, and DT cost for gait outcomes (i.e., gait speed, stride length, double support) in collision sport athletes (i.e., HRS and RUG groups) (Supplementary Table 2). We also used a multiple linear regression with Bonferroni adjustment for multiple comparisons to adjust analyses for sex (M/F), concussion history, age, and BMI (kg/m2). All assumptions of linearity, independence of residuals, homoscedasticity, and multicollinearity were met. All analyses were run using SPSS Version 26 (SPSS, IBM, Armonk, NY, USA).
3. Results
3.1. Instrumented gait parameters by group
Means, standard errors, range, and 95% confidence intervals (95%CIs) for each gait variable of interest (i.e., gait speed, stride length, double support) are presented in Table 3 along with their associated DT cost outcomes. There were no significant differences between groups in adjusted models for all gait outcomes and DT costs for each variable (p > 0.05) (Table 3). Of note, none of the groups differed by the clinically meaningful change for ST gait speed (0.09 m/s) in adjusted models, further indicating no clinical significance.38
Table 3.
Adjusted single- and dual-task gait outcomes by groups.
| Measure | Group 1 (NON) | Group 2 (NCA) | Group 3 (HRS) | Group 4 (RUG) | F | p | ηp2 |
|---|---|---|---|---|---|---|---|
| ST gait speed (m/s) | 1.07 ± 0.03 (95%CI: 1.01‒1.12) | 1.12 ± 0.03 (95%CI: 1.07‒1.17) | 1.11 ± 0.03(95%CI: 1.06‒1.16) | 1.04 ± 0.03(95%CI: 0.99‒1.10) | 1.979 | 0.122 | 0.054 |
| DT gait speed (m/s) | 0.87 ± 0.03 (95%CI: 0.81‒0.94) | 0.94 ± 0.03 (95%CI: 0.88‒1.00) | 0.96 ± 0.03(95%CI: 0.90‒1.02) | 0.88 ± 0.03(95%CI: 0.81‒0.95) | 1.779 | 0.156 | 0.049 |
| DT cost gait speed (%) | ‒16.75 ± 2.16(95%CI: ‒21.03 to ‒12.46) | ‒15.14 ± 2.05(95%CI: ‒19.21 to ‒11.07) | ‒12.26 ± 2.05(95%CI: ‒16.33 to ‒8.19) | ‒14.34 ± 2.20(95%CI: ‒18.71 to ‒9.98) | 0.753 | 0.523 | 0.021 |
| ST stride length (m) | 1.15 ± 0.02(95%CI: 1.11‒1.18) | 1.16 ± 0.02(95%CI: 1.13‒1.20) | 1.15 ± 0.02(95%CI: 1.11‒1.18) | 1.10 ± 0.02(95%CI: 1.07‒1.14) | 1.912 | 0.132 | 0.052 |
| DT stride length (m) | 1.05 ± 0.02(95%CI: 1.01‒1.10) | 1.07 ± 0.02(95%CI: 1.03‒1.11) | 1.06 ± 0.02(95%CI: 1.02‒1.10) | 1.02 ± 0.02(95%CI: 0.97‒1.06) | 1.071 | 0.365 | 0.030 |
| DT cost stride length (%) | ‒7.85 ± 1.20(95%CI: ‒10.24 to ‒5.47) | ‒8.49 ± 1.14(95%CI: ‒10.76 to ‒6.22) | ‒7.47 ± 1.14(95%CI: ‒9.74 to ‒5.20) | ‒8.00 ± 1.22(95%CI: ‒10.43 to ‒5.57) | 0.139 | 0.936 | 0.004 |
| ST double support (%) | 21.25 ± 0.53(95%CI: 20.19‒22.30) | 20.71 ± 0.50(95%CI: 19.71‒21.71) | 20.55 ± 0.50(95%CI: 19.55‒21.55) | 21.81 ± 0.54(95%CI: 20.74‒22.88) | 1.163 | 0.327 | 0.032 |
| DT double support (%) | 24.22 ± 0.61(95%CI: 23.00‒25.43) | 23.65 ± 0.58(95%CI: 22.50‒24.80) | 22.42 ± 0.58(95%CI: 21.27‒23.57) | 24.38 ± 0.62(95%CI: 23.15‒25.61) | 2.250 | 0.087 | 0.061 |
| DT cost double support (%) | 14.85 ± 2.18(95%CI: 10.52‒19.18) | 15.23 ± 2.07(95%CI: 11.12‒19.34) | 11.85 ± 2.07(95%CI: 7.74‒17.10) | 12.70 ± 2.22(95%CI: 8.29‒17.10) | 0.576 | 0.633 | 0.016 |
Notes: Data reported as adjusted mean ± SE. Models are adjusted for concussion history, age, sex, and BMI. For DT cost outcomes, a negative value indicates slower gait speed, less time in double support, and shorter strides in DT conditions. There were significant differences between groups in adjusted models.
Abbreviations: 95%CI = 95% confidence interval; BMI = body mass index; DT = dual task; HRS = high‒risk sport athletes; NCA = non-contact athletes; NON = non-athletes; RHI = repetitive head impacts; RUG = rugby/prolonged RHI exposure athletes; ST = single task.
3.2. Career duration and gait parameters
Only the adjusted model for ST double support was significant (p = 0.009); however, career duration did not add significantly to this model (p = 0.058) (Table 4). None of the other models significantly predicted any of the gait outcomes in contact/collision sport athletes (i.e., HRS and RUG groups) (p > 0.05) (Table 4).
Table 4.
Adjusted multiple linear regression: Collision sport athletes and gait.
| Outcomes | F | p | R2 | Adjusted R2 |
|---|---|---|---|---|
| ST gait speed | 1.222 | 0.313 | 0.109 | 0.020 |
| DT gait speed | 0.732 | 0.603 | 0.068 | ‒0.025 |
| DT cost gait speed | 0.641 | 0.669 | 0.060 | ‒0.034 |
| ST double support | 3.485 | 0.009* | 0.258 | 0.184 |
| DT double support | 1.735 | 0.144 | 0.248 | 0.063 |
| DT cost double support | 1.223 | 0.312 | 0.109 | 0.050 |
| ST stride length | 2.083 | 0.083 | 0.172 | 0.090 |
| DT stride length | 1.796 | 0.131 | 0.152 | 0.067 |
| DT cost stride length | 1.356 | 0.257 | 0.119 | 0.031 |
Notes: Career duration was the predictor variable for all models. Adjusted model covariates included concussion history, age, sex, body mass index, and career duration.
Abbreviations: DT = dual task; ST = single task.
Significant at p < 0.01.
4. Discussion
RHI exposure may put individuals at risk for subtle acute and chronic neurophysiological deficits;3,19,20 however, prior studies have focused primarily on current collegiate or former professional American football players.3,19,20,39,40 We addressed this limitation by including male and female athletes from a variety of sports, with a special emphasis on rugby—a sport representative of prolonged RHI exposure into adulthood across the sexes. Contrary to our hypothesis, the primary finding of this study was that participation in contact/collision sports, including prolonged rugby participation into early adulthood and middle age, was not associated with impaired postural control. Further, career duration of contact/collision sport was unrelated to postural control outcomes in physically active contact/collision sport athletes. Collectively, these findings suggest that previous and/or prolonged RHI exposure may not negatively impact gait in early- to mid-adulthood in individuals who report being physically active.
Gait was not impaired in early- to mid-adulthood in self-reported physically active individuals with a history of sports-related RHI exposure compared to both physically active and inactive individuals without a history of RHI. This is consistent with a 2021 review of current adolescent and collegiate athletes that identified limited effects of acute RHI on gait across a variety of measures.19 Similarly, recent investigations of middle-aged former American football players and American amateur rugby players (male and female) reported that career duration was unrelated to later-life or mid-life health conditions and dysfunction.11,39,41 Taken together, data among physically active early- to mid-adult collision sport athletes (i.e., aged 22–50 years) suggest that RHI exposure does not adversely affect objective measures of neurobehavioral health;3 however, the later-life effects (i.e., older adults aged >65 years) and the effects in physically inactive individuals with histories of prolonged RHI exposure (e.g., former adult rugby players who are inactive) remain unknown.
The lack of differences between the collision sport athletes and the non-contact/collision sport athletes herein may be the result of the neuroprotective effects of exercising outweighing the potential negative consequences of RHI exposure on postural control.42, 43, 44 Rugby is a multi-activity sport consisting of repeated high-intensity sprint efforts separated by variable rest durations, jogging, and dynamic exertion (e.g., scrum, ruck, or tackle); moreover, the average rugby player covers between 4200–6500 m per 80-min match and spends 42% of the match above 85% of their maximum heart rate.45 As such, physical activity from contact/collision sport participation provides players with improved cardiovascular and physical health in later life, reducing risk of chronic disease and increasing lifespan compared to the general population, which may be a result of the cumulative effects of increased levels of lifetime physical activity.15,46, 47, 48 This may partially explain our lack of group differences. Since the HRS and RUG groups were physically active, the chronic benefits of exercise may outweigh the negative consequences, if any, of RHI. Of note, these participants were generally young (∼35 years old; 87% were under 50 years old) compared to other cohorts of middle-aged adults, meaning it is possible that the negative consequences of RHI may not have manifested yet among the HRS and RUG groups. Indeed, self-reported head trauma is related to worse DT gait in middle-aged former professional American football players, independent of career duration, age, and BMI.49 Furthermore, physical inactivity, which usually indicates low levels of cardiorespiratory fitness, is a risk factor for many diseases.43,50,51 Indeed, among former professional American football players, declines in physical activity were related to increased neuropsychiatric dysfunction,52 which has been linked to worse gait performance.53 However, many of these chronic conditions result from chronic physical inactivity and poor lifestyle choices, which, again, may not yet have manifested negative consequences in our young cohort. In addition, our measurement of physical activity was based upon current physical activity status and did not account for previous regular physical activity throughout one's lifespan. Taken together, these findings suggest that RHI history and physical activity status in early- to mid-adulthood are unrelated to gait performance.
A secondary finding of this study, contrary to our hypothesis, was that contact/collision sport career duration was unrelated to gait performance in current and former contact/collision sport athletes (HRS and RUG groups). Again, gait—particularly DT gait—is an important marker of neurobehavioral health that is sensitive to changes in neurological function and aging.23,25 To our knowledge, this was the first study to investigate the relationship between RHI and gait in early- to mid-life (i.e., aged 30–50 years) across a variety of RHI exposure history.19 Concussion leads to a more conservative gait strategy acutely post-injury, with DT gait highlighting impairments, yet the literature on the effects of RHI on gait remain mixed.19,26 Herein, our data suggest that neither career duration, sex, age, BMI, nor concussion history added significantly to the prediction of ST and DT gait performance. This is consistent with a recent finding by Oldham et al.21 whereby career duration in collision and contact sports was unrelated to ST, DT, and DT cost for gait speed or stride length in a younger cohort of collegiate athletes with shorter career duration. Further, the finding that career duration, age, and BMI were unrelated to gait performance is consistent with Manor et al.’s49 finding that these factors did not influence the relationship between self-reported symptomatic head trauma and walking performance in middle-aged American football players. This finding could also be the result of any negative consequences not yet having manifested. Additionally, it could be that the RHI experienced by these individuals were not sufficient to warrant any changes in gait. Thus, consistent with previous data among contact/collision sport athletes in college, our data suggest that RHI history is unrelated to gait performance in early- to mid-adulthood.19,21
A primary limitation of this study was that this heterogeneous sample had a relatively small proportion of older adults, which may contribute additional confounders that were not accounted for and limit extrapolation to older cohorts. Our sample may have been susceptible to survivor or respondent bias as individuals with neurophysiological dysfunction may have removed themselves from participation in contact/collision sports or failed to volunteer for the study while healthy and resilient individuals continued to participate in sport and subsequently volunteered for study participation. However, it should be noted that our study is different from previous research that limited itself by enrolling only symptomatic RHI-exposed individuals, which potentially biased the results.3 Furthermore, there were considerable differences in the sex composition of each group, which although not statistically significant, may have introduced further bias into our analyses. We addressed this difference in sex composition (which may be a result of the varying availability of contact/collision sports for females compared to males2) by accounting for sex in all adjusted models. Another limitation was the use of career duration as a metric for RHI exposure, which may not be sensitive to the effects of playing style, culture, and competition levels. However, this method was chosen based on the recommendation of the NINDS traumatic encephalopathy syndrome consensus statement and has been previously utilized in other research as a measure of RHI exposure because it permits a rough estimation in the absence of biomechanical data, which is not feasible to collect across the lifespan.3,7,11 Lastly, this study relied on self-reported measures of physical activity as a dichotomous outcome (yes/no for meeting American College of Sports Medicine physical activity guidelines). Although self-reported measures of physical activity have been used in previous research,54 they may fail to capture the accuracy and precision needed to detect the true effects of exercise (i.e., frequency, intensity, type, time) on the included outcomes. Furthermore, our cohort did not have an HRS or RUG group that was not currently physically active, thereby preventing analysis of the effects of physical activity and RHI on gait. Future studies should further explore both current and prior exercise habits (i.e., the chronicity of regular physical activity) across the sporting spectrum.
5. Conclusion
Prolonged participation in contact/collision sports in physically active individuals is unrelated to postural control in early- to mid-adulthood. Further, RHI exposure, assessed by career duration, does not appear to negatively affect postural control in early- to mid-adulthood. These findings add to the growing body of literature showing that exposure to RHI through routine contact/collision sport participation, assessed by career duration, is unrelated to neurological dysfunction in early- to mid-adulthood. This study fills a gap in the literature among female and male collision sport athletes in early- to mid-adulthood who participated in prolonged contact/collision sports. However, consistent with the NINDS traumatic encephalopathy syndrome call to action, future research should seek to expand on these findings by conducting more research in older adults and utilizing more specific measures of RHI exposure and physical activity.
Acknowledgments
Acknowledgments
The authors thank all the participants who participated in this study and all the collaborators and friends who helped share our study flyers to promote participant recruitment. This study and publication were made possible and funded in part by the University of Delaware Unidel Distinguished Graduate Scholars Fellowship and Department of Kinesiology and Applied Physiology Doctoral Research Fund. Additionally, Dr. Hunzinger acknowledges funding support in part by the Department of Defense grant W81XWH-21-1-0590, the Penn Injury Science Center, and National Institutes of Health/National Institute of Neurological Disorders and Stroke brain injury training grant T32 NS043126. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Authors’ contributions
KJH conceived the study and study design, recruited all participants, performed statistical analysis, and drafted the manuscript; JBC, RM, WPM, and JFH participated in study design, assisted in statistical analysis, and reviewed manuscript drafts; CBS and TAB assisted with study design, study resource allocation, assisted with data interpretation, and reviewed all manuscript drafts. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Dr. Hunzinger is an independent contractor with USA Rugby as a World Rugby Educator for the Strength and Conditioning and Referee Strands. Dr. Meehan receives royalties from (1) ABC-Clio publishing for the sale of his books, Kids, sports, and concussion: A guide for coaches and parents, and concussions; (2) Springer International for the book Head and neck injuries in young athlete, and (3) Wolters Kluwer for working as an author for UpToDate. His research is funded, in part, by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament, and a grant from the National Football League. All the supporting entities had no involvement in the study design and writing of the manuscript or the decision to submit it for publication. The other authors have nothing to disclose.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.12.004.
Supplementary materials
References
- 1.Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg. 2013;119:1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- 2.Rice SG, Small EW, McCambridge TM, et al. Medical conditions affecting sports participation. Pediatrics. 2008;121:841–848. doi: 10.1542/peds.2008-0080. [DOI] [PubMed] [Google Scholar]
- 3.Iverson GL, Büttner F, Caccese JB. Age of first exposure to contact and collision sports and later in life brain health: A narrative review. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.727089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales JS, Valenzuela PL, Saco-Ledo G, et al. Mortality risk from neurodegenerative disease in sports associated with repetitive head impacts: Preliminary findings from a systematic review and meta-analysis. Sport Med. 2021;52:835–846. doi: 10.1007/s40279-021-01580-0. [DOI] [PubMed] [Google Scholar]
- 5.Savica R, Parisi JE, Wold LE, Josephs KA, Ahlskog JE. High school football and risk of neurodegeneration: A community-based study. Mayo Clin Proc. 2012;87:335–340. doi: 10.1016/j.mayocp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mez J, Daneshvar DH, Abdolmohammadi BA, et al. Duration of American football play and chronic traumatic encephalopathy. Ann Neurol. 2020;87:116–131. doi: 10.1002/ana.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz DI, Bernick C, Dodick DW, et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for traumatic encephalopathy syndrome. Neurology. 2021;96:848–863. doi: 10.1212/WNL.0000000000011850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller CW, Taylor A, Raftery M. Epidemiology of concussion in men's elite Rugby-7s (Sevens World Series) and Rugby-15s (Rugby World Cup, Junior World Championship and Rugby Trophy, Pacific Nations Cup and English Premiership) Br J Sports Med. 2015;49:478–483. doi: 10.1136/bjsports-2013-093381. [DOI] [PubMed] [Google Scholar]
- 9.World Rugby. World Rugby year in review 2017. Available at: http://publications.worldrugby.org/yearinreview2017/en/0-1.[accessed 21.04.2020]
- 10.Paul L, Naughton M, Jones B, et al. Quantifying collision frequency and intensity in Rugby Union and Rugby Sevens: A systematic review. Sport Med Open. 2022;8:12. doi: 10.1186/s40798-021-00398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunzinger KJ, Caccese JB, Costantini KM, Swanik CB, Buckley TA. Age of first exposure to collision sports does not affect patient reported outcomes in women and men community rugby players. Med Sci Sport Exerc. 2021;53:1895–1902. doi: 10.1249/MSS.0000000000002657. [DOI] [PubMed] [Google Scholar]
- 12.Hunzinger KJ, Costantini KM, Swanik CB, Buckley TA. Diagnosed concussion is associated with increased risk for lower extremity injury in community rugby players. J Sci Med Sport. 2020;24:368–372. doi: 10.1016/j.jsams.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Yan T, Chu JMT, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Invest. 2019;99:943–957. doi: 10.1038/s41374-019-0232-y. [DOI] [PubMed] [Google Scholar]
- 14.Fritz S, Lusardi M. White paper: “Walking speed: The sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–49. [PubMed] [Google Scholar]
- 15.Griffin SA, Panagodage Perera NK, Murray A, et al. The relationships between rugby union, and health and well-being: A scoping review. Br J Sports Med. 2021;55:319–326. doi: 10.1136/bjsports-2020-102085. [DOI] [PubMed] [Google Scholar]
- 16.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: Results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hind K, Konerth N, Entwistle I, et al. Mental health and wellbeing of retired elite and amateur rugby players and non-contact athletes and associations with sports-related concussion: The UK Rugby Health Project. Sport Med. 2022;52:1419–1431. doi: 10.1007/s40279-021-01594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owens TS, Calverley TA, Stacey BS, et al. Concussion history in rugby union players is associated with depressed cerebrovascular reactivity and cognition. Scand J Med Sci Sports. 2021;31:2291–2299. doi: 10.1111/sms.14046. [DOI] [PubMed] [Google Scholar]
- 19.Bonke EM, Southard J, Buckley TA, Reinsberger C, Koerte IK, Howell DR. The effects of repetitive head impacts on postural control: A systematic review. J Sci Med Sport. 2021;24:247–257. doi: 10.1016/j.jsams.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belanger HG, Vanderploeg RD, McAllister T. Subconcussive blows to the head: A formative review of short-term clinical outcomes. J Head Trauma Rehabil. 2016;31:159–166. doi: 10.1097/HTR.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 21.Oldham JR, Lanois CJ, Caccese JB, et al. Association between collision sport career duration and gait performance in male collegiate student-athletes. Am J Sports Med. 2022;50:2526–2533. doi: 10.1177/03635465221104685. [DOI] [PubMed] [Google Scholar]
- 22.Buckley TA, Oldham JR, Watson DJ, Murray NG, Munkasy BA, Evans KM. Repetitive head impacts in football do not impair dynamic postural control. Med Sci Sports Exerc. 2019;51:132–140. doi: 10.1249/MSS.0000000000001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko SU, Hausdorff JM, Ferrucci L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: Results from the Baltimore Longitudinal Study of Ageing. Age Ageing. 2010;39:688–694. doi: 10.1093/ageing/afq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 26.Fino PC, Parrington L, Pitt W, et al. Detecting gait abnormalities after concussion or mild traumatic brain injury: A systematic review of single-task, dual-task, and complex gait. Gait Posture. 2018;62:157–166. doi: 10.1016/j.gaitpost.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity–A systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi: 10.1186/1471-2458-13-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood TA, Hsieh KL, An R, Ballard RA, Sosnoff JJ. Balance and gait alterations observed more than 2 weeks after concussion: A systematic review and meta-analysis. Am J Phys Med Rehabil. 2019;98:566–576. doi: 10.1097/PHM.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 29.D'Silva L, Chalise P, Rippee M, Devos H. Challenging the vestibular system affects gait speed and cognitive workload in chronic mild traumatic brain injury and healthy adults. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.819169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fino PC, Parrington L, Walls M, et al. Abnormal turning and its association with self-reported symptoms in chronic mild traumatic brain injury. J Neurotrauma. 2018;35:1167–1177. doi: 10.1089/neu.2017.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J. Routledge Academic; New York, NY: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 32.American College of Sports Medicine (ACSM). Physical activityguidelines. Available at: https://www.acsm.org/read-research/trending-topics-resource-pages/physical-activity-guidelines. [accessed 12.02.2021].
- 33.Mancini M, Chiari L, Holmstrom L, Salarian A, Horak FB. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture. 2016;43:125–131. doi: 10.1016/j.gaitpost.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howell D, Osternig L, Chou LS. Monitoring recovery of gait balance control following concussion using an accelerometer. J Biomech. 2015;48:3364–3368. doi: 10.1016/j.jbiomech.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 35.King LA, Horak FB, Mancini M, et al. Instrumenting the Balance Error Scoring System for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95:353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell DR, Oldham JR, Meehan WP, Difabio MS, Buckley TA. Dual-task tandem gait and average walking speed in healthy collegiate athletes. Clin J Sport Med. 2019;29:238–244. doi: 10.1097/JSM.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 37.Hamacher D, Liebl D, Hödl C. Gait stability and its influencing factors in older adults. Front Physiol. 2019;9:1955. doi: 10.3389/fphys.2018.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wellons RD, Duhe SE, MacDowell SG, Hodge A, Oxborough S, Levitzky EE. Estimating the minimal clinically important difference for balance and gait outcome measures in individuals with vestibular disorders. J Vestib Res. 2022;32:223–233. doi: 10.3233/VES-201630. [DOI] [PubMed] [Google Scholar]
- 39.Iverson GL, Merz ZC, Terry DP. High school football and midlife brain health problems. Clin J Sport Med. 2022;32:86–94. doi: 10.1097/JSM.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell ER, Mackay DF, Stewart K, MacLean JA, Pell JP, Stewart W. Association of field position and career length with risk of neurodegenerative disease in male former professional soccer players. JAMA Neurol. 2021;78:1057–1063. doi: 10.1001/jamaneurol.2021.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iverson GL, Terry DP, Caccese JB, Büttner F, Merz ZC. Age of first exposure to football is not associated with midlife brain health problems. J Neurotrauma. 2021;38:538–545. doi: 10.1089/neu.2020.7041. [DOI] [PubMed] [Google Scholar]
- 42.Wahl D, Cavalier AN, LaRocca TJ. Novel strategies for healthy brain aging. Exerc Sport Sci Rev. 2021;49:115–125. doi: 10.1249/JES.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deslandes A, Moraes H, Ferreira C, et al. Exercise and mental health: Many reasons to move. Neuropsychobiology. 2009;59:191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- 44.Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubois R, Paillard T, Lyons M, McGrath D, Maurelli O, Prioux J. Running and metabolic demands of elite rugby union assessed using traditional, metabolic power, and heart rate monitoring methods. J Sport Sci Med. 2017;16:84–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Brett BL, Kerr ZY, Aggarwal NT, et al. Cumulative concussion and odds of stroke in former national football league players. Stroke. 2021;53:e5–e8. doi: 10.1161/STROKEAHA.121.035607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackay DF, Russell ER, Stewart K, Maclean JA, Pell JP, Stewart W. Neurodegenerative disease mortality among former professional soccer players. N Engl J Med. 2019;381:1801–1808. doi: 10.1056/NEJMoa1908483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manor B, Zhou J, Lo OY, et al. Self-reported head trauma predicts poor dual task gait in retired national football league players. Ann Neurol. 2020;87:75–83. doi: 10.1002/ana.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair SN. Physical inactivity: The biggest public health problem of the 21st century. Br J Sports Med. 2005;43:1–2. [PubMed] [Google Scholar]
- 51.Stephen R, Hongisto K, Solomon A, Lönnroos E. Physical activity and Alzheimer's disease: A systematic review. J Gerontol A Biol Sci Med Sci. 2017;72:733–739. doi: 10.1093/gerona/glw251. [DOI] [PubMed] [Google Scholar]
- 52.Brett BL, Kerr ZY, Walton SR, et al. Longitudinal trajectory of depression symptom severity and the influence of concussion history and physical function over a 19-year period among former National Football League (NFL) players: An NFL-LONG Study. J Neurol Neurosurg Psychiatry. 2022;93:272–279. doi: 10.1136/jnnp-2021-326602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemke M, Wendorff T, Mieth B, Buhl K, Linnemann M. Spatiotemporal gait patterns during overground locomotion in major depression compared with healthy controls. J Psychiatr Res. 2000;34:277–283. doi: 10.1016/s0022-3956(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 54.Evenson K, Chasan-Taber L, Symons Downs D, Pearce E. Review of self-reported physical activity assessments for pregnancy: Summary of the evidence for validity and reliability. Paediatr Perinat Epidemiol. 2013;26:479–494. doi: 10.1111/j.1365-3016.2012.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.