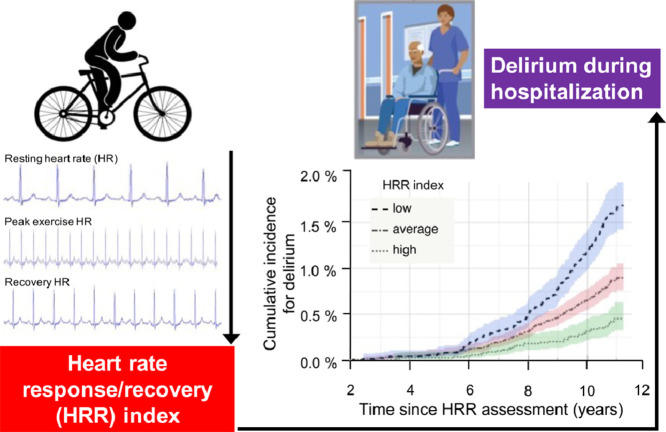
Highlights
-
•
Heart rate response/recovery (HRR) to submaximal exercise predicted future delirium risk during hospitalization.
-
•
Lowest quartile for a HRR index was equivalent to being 6 years older, a current smoker, or having 3 or more additional cardiovascular risks compared to those in the highest quartile.
-
•
Findings were robust in subsets by age, sex, physical activity level, rate-control medications, but the risk from low HRR appeared greater in those with better baseline cognition.
-
•
Results were also consistent when only postoperative delirium was considered, and when dementia related cases were excluded.
Keywords: Brain health, Delirium, Exercise stress test, UK Biobank
Abstract
Background
Delirium is a neurocognitive disorder characterized by an abrupt decline in attention, awareness, and cognition after surgical/illness-induced stressors on the brain. There is now an increasing focus on how cardiovascular health interacts with neurocognitive disorders given their overlapping risk factors and links to subsequent dementia and mortality. One common indicator for cardiovascular health is the heart rate response/recovery (HRR) to exercise, but how this relates to future delirium is unknown.
Methods
Electrocardiogram data were examined in 38,740 middle- to older-aged UK Biobank participants (mean age = 58.1 years, range: 40–72 years; 47.3% males) who completed a standardized submaximal exercise stress test (15-s baseline, 6-min exercise, and 1-min recovery) and required hospitalization during follow-up. An HRR index was derived as the product of the heart rate (HR) responses during exercise (peak/resting HRs) and recovery (peak/recovery HRs) and categorized into low/average/high groups as the bottom quartile/middle 2 quartiles/top quartile, respectively. Associations between 3 HRR groups and new-onset delirium were investigated using Cox proportional hazards models and a 2-year landmark analysis to minimize reverse causation. Sociodemographic factors, lifestyle factors/physical activity, cardiovascular risk, comorbidities, cognition, and maximal workload achieved were included as covariates.
Results
During a median follow-up period of 11 years, 348 participants (9/1000) newly developed delirium. Compared with the high HRR group (16/1000), the risk for delirium was almost doubled in those with low HRR (hazard ratio = 1.90, 95% confidence interval (95%CI): 1.30–2.79, p = 0.001) and average HRR (hazard ratio = 1.54, 95%CI: 1.07–2.22, p = 0.020)). Low HRR was equivalent to being 6 years older, a current smoker, or ≥3 additional cardiovascular disease risks. Results were robust in sensitivity analysis, but the risk appeared larger in those with better cognition and when only postoperative delirium was considered (n = 147; hazard ratio = 2.66, 95%CI: 1.46–4.85, p = 0.001).
Conclusion
HRR during submaximal exercise is associated with future risk for delirium. Given that HRR is potentially modifiable, it may prove useful for neurological risk stratification alongside traditional cardiovascular risk factors.
Graphical abstract
1. Introduction
Delirium is a distressing and complex neurocognitive syndrome characterized by an acute change in attention, awareness, and cognition.1 The incidence dramatically increases with aging and, even after recovery, is linked to increased risks for morbidity, mortality,2 and Alzheimer's disease3 leading to substantially higher healthcare costs.4 The etiology of delirium is complex, such that a multimodal approach for risk stratification in earlier life is desperately needed.5 “Stressors” such as acute medical illness, drug use or withdrawal trauma, or surgery/anesthesia are the usual circumstances that typically trigger an episode of delirium.1 Previous studies have often centered on the predictive value of cognitive impairment or medical comorbidities, but advances in our understanding of the role of cardiovascular health factors have shown them to be closely associated with brain health.6 Given that many of these factors are also risk factors for neurocognitive disorders, and are modifiable, there is an active drive to focus public health attention on understanding how cardiovascular health is linked to specific neurocognitive disorders such as delirium.6
One way of assessing cardiovascular health has been exercise stress testing, which is commonly used for evaluating cardiopulmonary diseases, particularly prior to surgery, or in the management of ischemic heart disease.7 The response and recovery from submaximal and peak exercise involves the pulmonary, cardiovascular, hematopoietic, neuropsychological, and skeletal muscle systems.7 Disorders in many of these systems are thought to be implicated in the etiology of delirium.5 Specifically, the heart rate (HR) response to exercise is thought to reflect chronotropic competence,8 whereas recovery after exercise is more specific to parasympathetic reactivation9 after the sympathetic surge. Both chronotropic incompetence and muted parasympathetic reactivation have been associated with increased morbidity and mortality.10,11 For example, in a small study of 313 patients, a 6-min walk test was found to be a potential predictor of delirium following elective cardiac surgery.12 However, to date, no large prospective study has examined the link between heart rate response/recovery (HRR) to exercise and future delirium risk. This is in part due to testing being more commonly done in middle age,13 when delirium incidence is relatively low,14 thus requiring many years of follow-up before events occur.
To address these gaps in the literature, we analyzed results from a large community sample of 38,740 participants from the UK Biobank who took part in a 7-min submaximal exercise stress test. We hypothesized that acute cardiovascular adaptation to exercise (via HRR) might predict future delirium onset. We derived an HRR index and tested whether it, and its individual components, predicted incident delirium during 11 years of follow-up.
2. Materials and methods
2.1. Study population and data source
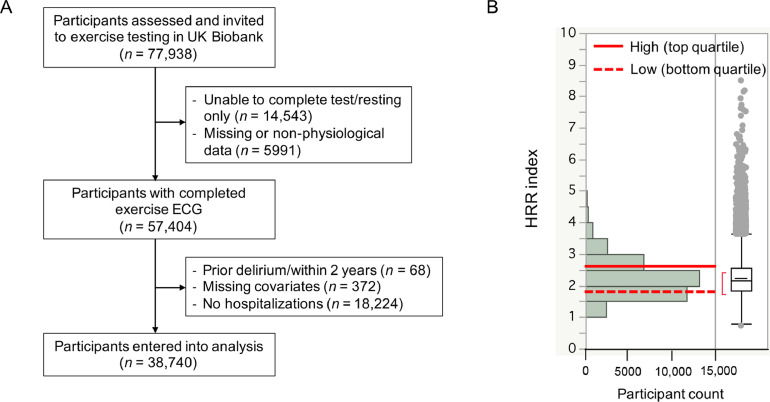
Between August 2009 and December 2010, community-based participants from across the United Kingdom were recruited to participate in an exercise stress test as part of baseline recruitment to the UK Biobank.15,16 Total 38,740 participants (mean age = 58.1 years, range: 40–72 years; 47.3% males) entered our analysis (Fig. 1A). Participants completed extensive questionnaires on demographics, lifestyle choices, and medical conditions. Prospective data on the cohort were analyzed in February 2021, after participants had been followed up for a median time period of 11 years using reported procedures.17
Fig. 1.
Flowchart of participant selection and distribution of HRR. (A) Flowchart of participants included for analysis of incident delirium; (B) Distribution of HRR index and categorization into high (top quartile >2.55), low (bottom quartile <1.83), and average (middle 2 quartiles: 1.83−2.55, which are corresponding to right panel box plot for IQR and outliers whiskers as 1.5 × IQR). ECG = electrocardiogram; HRR = heart rate response/recovery; IQR = interquartile range.
2.2. Standard protocol approvals, registrations, and patient consents
The UK Biobank received National Health Service (NHS) Research Ethics Committee Approval, and participants gave written informed consent. This study was conducted under the terms of UK Biobank access Number 40556 and Mass General Brigham institutional review board approval (2020P002097) and in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki, 1964 and Declaration of Tokyo, 1975, as revised in 1983).
2.3. Protocol
The UK Biobank assigned participants to individualized, submaximal bicycle protocols; this was done to increase the number of participants with exposure information and reduce the risk of adverse health events during exercise testing. The best test for assessing aerobic capacity, i.e., the maximal oxygen uptake measured during a graded exercise test calibrated to reach exhaustion in about 10 min,18 was considered unfeasible in this large epidemiological study involving individuals with a wide range of abilities.19
The testing protocol and maximal desired participant effort were calculated from the risk category (determined from cardiovascular disease (CVD) risk factors), and the predicted maximal workload was determined from age, height, resting HR, and sex. Exercise testing was performed using an incremental ramp cycle ergometer test. A 4-lead electrocardiogram (ECG) was performed during a seated pre-exercise rest period (15 s), throughout exercise (6 min), and during recovery (1 min). The 6-min exercise period started with a 2-min phase at constant workload (30 watts (W) for women and 40 W for men) and was followed by a 4-min ramp phase with graded increases in power (in 10–30 W increments up to every 30 s) up until their individually assigned peak power (or cycling at constant workload if chest pain with physical activity), ending with a 1-min rest phase; subjects were instructed to maintain cadence at 60 revolutions per minute (rpm) throughout. If the participant achieved their desired maximal workload during the test, then the exercise test was terminated. To investigate the relationships between HRR and delirium risk, independent of participant effort, we controlled for the achieved maximal oxygen uptake during exercise as estimated from the age, height, weight, resting HR, sex, and the maximal HR of each participant. The maximal oxygen uptake was expressed in metabolic equivalent of task (MET), previously well-defined in the UK Biobank cohort as a measure of cardiorespiratory fitness (CRF).19, 20, 21, 22
Out of 77,938 UK Biobank participants invited for an exercise test, 14,543 (19%) were unable to complete the exercise test or had a resting ECG assessment only. We further excluded participants with missing information on HR and/or workload/METs or participants with resting or recovery HRs < 40 beats per minute (bpm) or > 200 bpm as unlikely to be physiological; those who were unable to generate an HR higher than the resting HR (i.e., response ratio (= peak HR achieved/resting HR) < 1) were also excluded. A total of 57,404 (74% of the invited participants) had useable exercise test data (see Fig. 1A for flowchart of participant selection).
2.4. Assessment of HRR index
To assess HR, normal R-waves were detected from ECG recordings using the modified Aristotle algorithm of QRS detection23,24 and were consistent with our prior study.25 Mean resting HR was determined from pre-exercise value, peak HR during exercise was the maximal rate achieved, and the recovery HR was determined at 1-min from cessation of exercise. In order to gauge a participant's HR responses from rest to peak and subsequent recovery, regardless of preset workload, we determined: (1) response ratio = peak HR achieved/resting HR, (2) recovery ratio = peak HR /recovered HR (lowest HR in final rest phase), and (3) their combined product, HRR index = response ratio × recovery ratio. Higher HRR product represented greater response and/or recovery. Low HRR index was defined as bottom quartile, average HRR index was the middle 2 quartiles, and high HRR index was the top quartile. In summary, predictors included in this study were HRR index and its individual components (resting HR, recovery HR, response ratio, and recovery ratio).
2.5. Assessment of incident delirium, postoperative delirium (POD), and non-dementia-related delirium
The UK Biobank has access to hospitalization records linked to study participants during NHS follow-up. Incident delirium diagnosis was derived as the first date of occurrence of the International Classification of Disease-Tenth Version-10 Code F05 included in hospital admissions health records during follow-up, which is in keeping with the practices of similar studies using this data.26,27 We further searched for operation codes prior to delirium for each participant, and if an operation was performed within 3 days, we recorded this as POD28 and tested this group separately. Finally, we identified a non-dementia-related group after exclusion of those who had “delirium superimposed on dementia” (F05.1; n = 54). We excluded 61 cases where delirium pre-dated HRR assessment.
2.6. Assessment of covariates
We assessed participant medical history at the baseline assessment through a combination of self-reported questionnaire data, nurse-led interview data, and/or health record data including medication use. Covariates were grouped into demographics (age, sex, ethnicity, education, and deprivation), lifestyle factors (body mass index, physical activity, smoking, and alcohol), and CVD risk, HR controlling medications (beta-blockers and non-dihydropyridine calcium channel blockers), morbidity, and cognitive function. Age at exercise assessment was calculated in years based on date of birth. Sex (male/female) and ethnicity (European and non-European, given that the majority (91%) self-identified as of British or Caucasian European descent) were self-reported. Education was college-level (yes/no). Townsend deprivation index, a score based on national geographic census data immediately prior to participant enrollment, was dichotomized by the median into higher vs. lower groups. Body mass index was calculated as weight (kg) divided by height squared (m2); physical activity was the summed metabolic equivalent minutes (MET-min) per week for all activities; smoking included never, former, and current; and alcohol use was <4 drinks vs. ≥4 drinks per week. Significant CVD risk included a risk score (0–5) based on the presence of hypertension, high cholesterol, diabetes, ischemic heart disease, and peripheral vascular disease. We derived a morbidity burden based on the summed presence (yes/no) of cancer (in response to “Has a doctor ever told you that you have had cancer?”) or of any respiratory/neurological/gastrointestinal/renal/hematological/endocrine/musculoskeletal/connective tissue/infectious diseases/disorders by self-report; the burden was classified as none (without any condition), low (1 condition), or high (2 or more conditions). Both HRR and delirium risk may be affected by drugs that specifically target HR, so we coded for and derived an HR-influencing drug (yes/no) based on answers self-reported to a nurse. Participants also completed a cognitive test of timed symbol matching, similar to the common card game “Snap”; scores for this task reflected the mean response time in milliseconds across trials that consisted of matching pairs.29 Cronbach's α for this task and consistency with other cognitive domains has been reported as high as 0.85.30
2.7. Statistical analysis
Descriptive characteristics are presented for the whole study group, by 3 HRR groups, with means and standard deviations (SDs) for quantitative variables that were normally distributed, and as medians with interquartile range for those that were nonnormally distributed. Categorical variables are presented as frequencies and percentages. Associations between exercise HRR measures and delirium incidence were investigated using Cox proportional hazard models and reported as hazards ratios and corresponding 95% confidence intervals (95%CIs).
In Model 1, the core model controlled for demographics. Model 2 further adjusted for lifestyle factors. Model 3 additionally accounted for CVD risk, HR-control medications, morbidity, and cognition. In Model 4, a final adjustment for the maximal METs achieved was added to Model 3 to account for the confounding effects of effort and individualized protocols. Sensitivity analyses were performed to examine the relationship between exercise HRR and delirium by setting (i.e., postoperative) and after exclusion of dementia-related cases. In addition, we explored the interaction effects of HRR and other covariates, including age (<65 years/≥65 years), sex, CVD risk (none/any), physical activity (lower/higher), maximal METs achieved (lower/higher), any HR-control drugs taken (yes/no), and mental reaction time (slower/faster), in which HRR was included as a continuous variable and continuous covariate were dichotomized (e.g., “lower/higher” and “slower/faster”) based on the median values. We included number of hospitalization episodes in all models.
Time-to-event was calculated as the time interval in years between date of HRR assessment and date of delirium. For those who remained delirium-free, we censored participant follow-up in February 2021, the latest date of available records. To minimize possible reverse causality, all analyses were done using a 2-year landmark analysis, where participants who experienced events prior to or within 2 years of exercise testing were excluded (n = 7). The proportional hazards assumption was assessed using the global χ2 test in R-package cox.zph (survival package; R Core Team, Vienna, Austria) incorporating methods described by Grambsch and Therneau.31 All other statistical analyses were performed using John's Mcintosh Project Pro (Version 15.0; SAS Institute, Cary, NC, USA). A p value < 0.05 was used for statistical significance.
2.8. Data availability
Data are available from the UK Biobank upon submission of application (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The syntax for conducting the analysis will be provided to UK Biobank and made freely available upon request.
3. Results
3.1. Participant characteristics
This prospective study included 38,740 participants (age = 58.1 ± 8.0 years (mean ± SD), range: 40–72 years; 47.3% males) who had data available for HRR assessment, were free from prior delirium before the assessment, were hospitalized at least once after the assessment, and had covariates available (Fig. 1A). The cohort was followed for a median period of 10.8 years (interquartile range: 10.7–11.0 years) after HRR assessment upon conclusion of the 2-year landmark period. During this period, 348 participants (9/1000) newly developed delirium.
The HRR index (2.24 ± 0.60) was categorized into groups (high: top quartile > 2.55; average: middle 2 quartiles: 1.83–2.55; and low: bottom quartile < 1.83; Fig. 1B). Cohort characteristics by HRR categories are shown in Table 1. Compared to participants with high HRR index, those with low HRR index were more likely to be older, female, deprived, European, and less likely to have attended college. For lifestyle factors, low HRR index participants had higher body mass index, drank alcohol more frequently, and reported lower weekly physical activity. They were also more likely to have high morbidity burden and CVD risks, to be taking HR-control medications, and to score slower on reaction times than individuals with high index. Last, when compared to individuals with high or average HRR index, those with low index had higher resting and recovery HRs but lower METs and lower peak HRs achieved. This translated into lower response and recovery ratios in those with low index (Table 1).
Table 1.
Baseline characteristics by HRR index.
| All | High HRR index | Average HRR index | Low HRR index | p | |
|---|---|---|---|---|---|
| (n = 38,740) | (n = 9054) | (n = 19,575) | (n = 10,111) | ||
| Demographics | |||||
| Age at baseline (year) | 58.1 ± 8.0 | 55.7 ± 8.0 | 58.2 ± 8.0 | 60.0 ± 7.0 | <0.001 |
| Male | 47.3% | 42.9% | 46.7% | 52.6% | <0.001 |
| College attendance | 33.6% | 36.6% | 34.0% | 30.2% | <0.001 |
| Ethnic background (European) | 90.9% | 88.5% | 91.0% | 92.9% | <0.001 |
| Deprivation index (higher) | 49.5% | 50.0% | 48.7% | 50.4% | 0.007 |
| BMI and lifestyle | |||||
| BMI (kg/m2) | 27.5 ± 4.4 | 26.0 ± 3.9 | 27.5 ± 4.3 | 28.7 ± 5.0 | <0.001 |
| Alcohol (≥4 drinks/week) | 47.2% | 45.7% | 47.4% | 48.0% | 0.005 |
| Physical activity level (MET-min)a | 2719 ± 2688 | 2911 ± 2768 | 2712 ± 2685 | 2535 ± 2613 | <0.001 |
| Cardiovascular risk and comorbidity | |||||
| Morbidity burden (high) | 26.9% | 24.8% | 26.7% | 29.3% | <0.001 |
| CVD risk scoreb | 0.55 ± 0.80 | 0.35 ± 0.70 | 0.52 ± 0.80 | 0.77 ± 0.90 | <0.001 |
| Reaction time (ms) | 568 ± 121 | 560 ± 118 | 568 ± 120 | 575 ± 123 | <0.001 |
| HR-control drugsc | 13.9% | 8.9% | 13.2% | 19.7% | <0.001 |
| Exercise metrics | |||||
| Resting HR (bpm) | 71 ± 10 | 62 ± 8 | 71 ± 9 | 80 ± 12 | <0.001 |
| Peak exercise HR (bpm) | 111 ± 15 | 116 ± 15 | 111 ± 14 | 107 ± 16 | <0.001 |
| Recovery HR (bpm)d | 81 ± 13 | 71 ± 13 | 81 ± 13 | 89 ± 15 | <0.001 |
| Peak exercise METse | 3.4 ± 0.6 | 3.5 ± 0.6 | 3.4 ± 0.5 | 3.2 ± 0.6 | <0.001 |
| Response ratio | 1.6 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.1 | 1.3 ± 0.1 | <0.001 |
| Recovery ratio | 1.4 ± 0.1 | 1.7 ± 0.2 | 1.4 ± 0.1 | 1.2 ± 0.1 | <0.001 |
| HRR index | 2.2 ± 0.1 | 3.1 ± 0.6 | 2.2 ± 0.2 | 1.6 ± 0.2 | <0.001 |
Note: UK Biobank participants selected for the exercise electrocardiogram characteristics at baseline expressed as mean ± SD or percentage of total for each quartile of HRR index for categorical variables.
Abbreviations: BMI = body mass index; bpm = beat per minute; CVD = cardiovascular disease; HR = heart rate; HRR = heart rate response/recovery; MET = metabolic equivalent of task.
Physical activity level: Summed MET minutes per week for all activities.
CVD risk score: Presence of hypertension, cholesterol, diabetes mellitus, smoking status, or ischemic heart disease.
HR controlling medications (beta-blockers and non-dihydropyridine calcium channel blockers).
HR at 1 min from cessation of exercise.
Maximal oxygen uptake during exercise as estimated from the age, height, weight, resting HR, sex, and the maximal HR of each participant.
3.2. HRR index predicts higher incidence of delirium
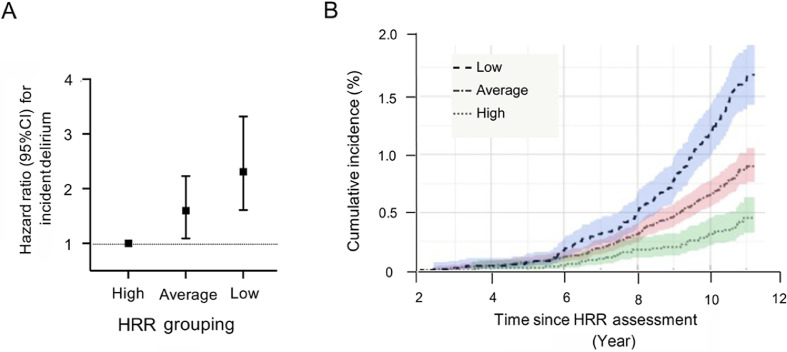
The associations between HRR groups and delirium were estimated using Cox proportional hazard models (Table 2). In Model 1, adjusting for demographics (age, sex, education, ethnic background, and deprivation), individuals with average (hazard ratio = 1.60, 95%CI: 1.09–2.23, p = 0.010) and low (hazard ratio = 2.24, 95%CI: 1.56–3.22, p < 0.001) HRR indexes had significantly higher risk for delirium compared with high HRR index individuals. These graded increases in risk from high (reference group) to average or to low HRR are shown in Fig. 2A and are demonstrated to have resulted in higher cumulative incidence of delirium over time (Fig. 2B). These associations were slightly attenuated if adjusted for lifestyle factors (1% decrease in hazards for average HRR index and 2% decrease for low HRR index; Model 2 in Table 2). Once CVD risk, HR-control medications, comorbidities, and reaction time were included, average HRR index individuals remained at a similarly elevated risk (2% decrease; hazard ratio = 1.57, 95%CI: 1.09–2.26, p = 0.010) when compared with high index individuals, but there was more attenuation for the low HRR individuals (17% decrease; hazard ratio = 2.05, 95%CI: 1.41–2.98, p < 0.001; Model 3). Finally, after adjusting for individual protocol and effort achieved via the peak exercise METs, average HRR index individuals still had a 54% higher risk (hazard ratio = 1.54, 95%CI: 1.07–2.22, p = 0.020), while low HRR individuals had a 90% higher risk (15% decrease; hazard ratio = 1.90, 95%CI: 1.30–2.79, p = 0.001; Model 4). The latter (hazard ratio = 1.90) is equivalent to the risk from being 6.2 years older, being a current smoker, or having 3 additional CVD risk factors (by comparing log estimates from the full model in Supplementary Table 1).
Table 2.
Associations between exercise HRR group and delirium incidence.
| Average HRR index (n = 19,575) |
Low HRR index (n = 10,111) |
|||||
|---|---|---|---|---|---|---|
| Hazard ratio (95%CI) | p | Hazard differencea | Hazard ratio (95%CI) | p | Hazard differencea | |
| Delirium incidence, all settings (n = 348) | ||||||
| Model 1 | 1.60 (1.09 – 2.23) | 0.01 | — | 2.24 (1.56 – 3.22) | <0.001 | — |
| Model 2 | 1.59 (1.11 – 2.29) | 0.01 | –0.01 | 2.22 (1.53 – 3.24) | <0.001 | –0.02 |
| Model 3 | 1.57 (1.09 – 2.26) | 0.01 | –0.02 | 2.05 (1.41 – 2.98) | <0.001 | –0.17 |
| Model 4 | 1.54 (1.07 – 2.22) | 0.02 | –0.03 | 1.90 (1.30 – 2.79) | 0.001 | –0.15 |
| Postoperative deliriumb(n = 147) | ||||||
| Model 1 | 1.53 (0.81 – 2.52) | 0.21 | — | 2.87 (1.64 – 5.02) | <0.001 | — |
| Model 2 | 1.53 (0.85 – 2.74) | 0.15 | 0 | 2.89 (1.61 – 5.20) | <0.001 | +0.02 |
| Model 3 | 1.53 (0.85 – 2.75) | 0.15 | 0 | 2.75 (1.53 – 4.96) | <0.001 | –0.14 |
| Model 4 | 1.52 (0.84 – 2.73) | 0.16 | –0.01 | 2.66 (1.46 – 4.85) | 0.001 | –0.09 |
| Non-dementia-related deliriumc(n = 294) | ||||||
| Model 1 | 1.56 (1.06 – 2.29) | 0.02 | — | 2.29 (1.55 – 3.38) | <0.001 | — |
| Model 2 | 1.54 (1.04 – 2.26) | 0.02 | –0.02 | 2.13 (1.43 – 3.18) | <0.001 | –0.16 |
| Model 3 | 1.51 (1.03 – 2.22) | 0.03 | –0.03 | 1.97 (1.32 – 2.95) | <0.001 | –0.16 |
| Model 4 | 1.49 (1.01 – 2.19) | 0.04 | –0.02 | 1.87 (1.25 – 2.82) | 0.002 | –0.10 |
Notes: Delirium incidence was estimated using hospitalization data. Participants in the top quartile (high) HRR index were used as reference. All analyses were done using a 2-year landmark analysis, excluding participants who experienced events prior to or within the first 2 years of follow-up (n = 69). Model 1 included sociodemographic variables (age, sex, education, ethnicity, and deprivation). Model 2 further included lifestyle factors (physical activity, body mass index, smoking, and alcohol intake). Model 3 further included CVD risk score (high blood pressure, cholesterol, diabetes, ischemic heart disease, and peripheral vascular disease), HR-control medications, morbidity burden, and reaction time at baseline. Model 4 included the final adjustment made for maximal METs achieved.
Abbreviations: 95%CI = 95% confidence interval; CVD = cardiovascular disease; HRR = heart rate response/recovery; MET = metabolic equivalent of task.
Risk difference from prior model.
Operation occurring within 3 days prior.
After exclusion of delirium superimposed on dementia.
Fig. 2.
HRR quartiles risk for incident delirium over time. (A) Hazard ratios (±95%CIs) by HRR index groups (average and low) and risk for delirium compared to participants with high HRR as referenced using Cox proportional hazards regression models adjusted for age, sex, education, ethnicity, and socioeconomic deprivation. (B) Cumulative incidence plot (±95%CI shaded) showing percentage of cohort with a first diagnosis of delirium over time in each of the 3 HRR index groups. 95%CI = 95% confidence interval; HRR = heart rate response/recovery.
3.2. HRR index in POD
In the subset of participants who experienced POD within 3 days of an operation (42%, n = 147), those with average HRR index at baseline had a similar magnitude hazard ratio for delirium when compared to the full cohort, but this did not reach significance (hazard ratio = 1.52, 95%CI: 0.84–2.73, p = 0.160; Model 4 in Table 2). However, participants with low HRR index remained at higher risk (hazard ratio = 2.66, 95%CI: 1.46–4.85, p = 0.001; Model 4 in Table 2) compared to those with high HRR index.
3.3. HRR index in non-dementia-related delirium
After exclusion of 54 delirium cases associated with dementia, (leaving 84%, n = 294), participants with average HRR index (hazard ratio = 1.49, 95%CI: 1.01–2.19, p = 0.040) and low HRR index (hazard ratio = 1.87, 95%CI: 1.25–2.82, p = 0.002) still experienced higher delirium risks compared to those with high HRR (Table 2).
3.4. Relationship between individual components of HRR index and incident delirium
In multivariate analyses, resting HR (hazard ratio = 1.12, 95%CI: 1.03–1.22, p = 0.009), recovery HR (hazard ratio = 1.10, 95%CI: 1.02–1.18, p = 0.010), response ratio (hazard ratio = 1.29, 95%CI: 1.14–1.47, p < 0.001), and recovery ratio (hazard ratio = 1.37, 95%CI: 1.18–1.59, p < 0.001) were each associated with delirium in models adjusted for demographic factors (Model 1 in Table 3; hazard ratios relate to a 1-SD difference in exposure at baseline). However, only the response ratio (hazard ratio = 1.21, 95%CI: 1.05–1.38, p = 0.007) and recovery ratio (hazard ratio = 1.25, 95%CI: 1.07–1.45, p = 0.004) remained associated with delirium in the fully-adjusted model (Model 4 in Table 3). Interestingly, peak HR achieved was not associated with delirium even in Model 1 (hazard ratio = 1.00, 95%CI: 0.99–1.01, p = 0.610). Maximal METs achieved was associated with delirium in Models 1–3, but the association was no longer significant when HRR index was included (hazard ratio = 1.19, 95%CI: 0.99–1.42, p = 0.060; Model 4 in Table 3).
Table 3.
Individual components of exercise HRR, maximal METs, and their association with delirium incidence.
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95%CI) | p | Hazard ratio (95%CI) | p | Hazard ratio (95%CI) | p | Hazard ratio (95%CI) | p | |
| Resting HRa | 1.12 (1.03–1.22) | 0.009 | 1.10 (1.01–1.20) | 0.030 | 1.10 (1.01–1.20) | 0.040 | 1.07 (0.98–1.18) | 0.120 |
| Peak HRb | 1.00 (0.99–1.01) | 0.610 | 1.00 (1.00–1.01) | 0.470 | 1.00 (0.99–1.01) | 0.700 | 1.00 (0.99–1.01) | 0.680 |
| Recovery HRa | 1.10 (1.02–1.18) | 0.010 | 1.08 (1.00–1.16) | 0.050 | 1.07 (0.99–1.15) | 0.060 | 1.07 (0.99–1.14) | 0.110 |
| Response ratiob | 1.29 (1.14–1.47) | <0.001 | 1.27 (1.11–1.45) | <0.001 | 1.24 (1.09–1.41) | 0.001 | 1.21 (1.05–1.38) | 0.007 |
| Recovery ratiob | 1.37 (1.18–1.59) | <0.001 | 1.32 (1.14–1.54) | 0.001 | 1.27 (1.10–1.48) | 0.001 | 1.25 (1.07–1.45) | 0.004 |
| Maximal METsb | 1.43 (1.21–1.69) | <0.001 | 1.38 (1.17–1.57) | <0.001 | 1.29 (1.09–1.53) | 0.004 | 1.19 (0.99–1.42)c | 0.060 |
Notes: Delirium incidence was estimated using hospitalization data. All analyses were done using a 2-year landmark analysis, excluding participants who experienced events prior to or within the first 2 years of follow-up (n = 69). Model 1 included sociodemographic variables (age, sex, education, ethnicity, and deprivation). Model 2 further included lifestyle factors (physical activity, body mass index, smoking, and alcohol intake). Model 3 further included CVD risk score (high blood pressure, cholesterol, diabetes, ischemic heart disease, and peripheral vascular disease), HR-control medications, morbidity burden, and reaction time at baseline. Model 4 included the final adjustment made for maximal METs achieved (except for Maximal METs, which included the HRR index).
Abbreviations: 95%CI = 95% confidence interval; CVD = cardiovascular disease; HR = heart rate; HRR = heart rate response/recovery; MET = metabolic equivalent of task.
Per 1-SD increase.
Per 1-SD decrease.
HRR index was included.
As expected, HRR index was positively correlated with the response (R2 = 0.750, p < 0.001) and recovery ratios (R2 = 0.760, p < 0.001). It was also weakly correlated with peak HR (R2 = 0.310, p < 0.001) and maximal METs achieved (R2 = 0.025, p < 0.001), while negatively correlated with resting HR (R2 = 0.310, p < 0.001) and recovery HR (R2 = 0.220, p < 0.001) (Supplementary Fig. 1).
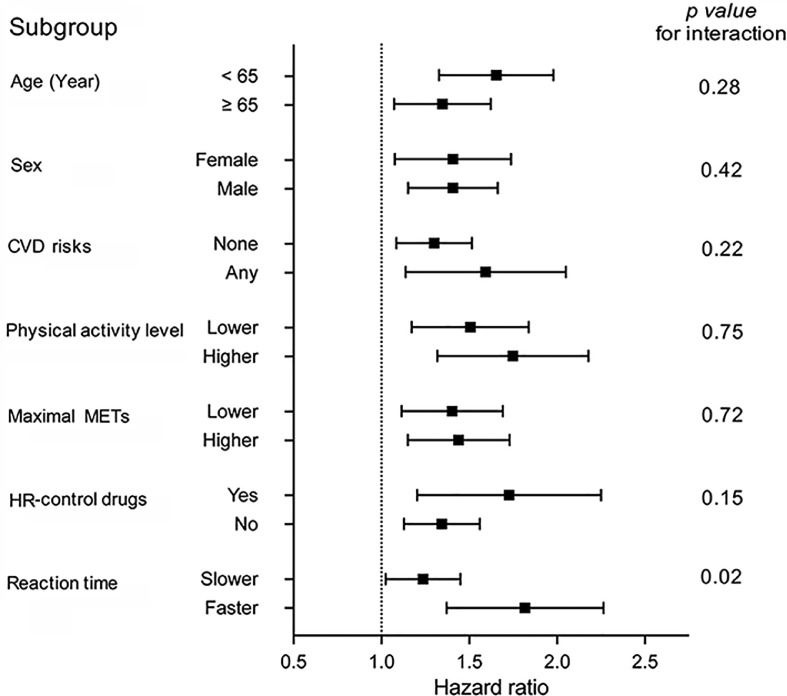
3.5. Incident delirium risk by subgroups
We further examined the HRR-associated risk for delirium by subgroups in Fig. 3 and found that decreased HRR index (per 1-SD) was equally predictive by age, sex, CVD risk, self-reported physical activity level, use of HR-control drugs, maximal METs, and morbidity burden. However, the association appeared stronger in those with faster (below median; hazard ratio = 1.78, 95%CI: 1.39–2.28) compared to those with slower (below median; hazard ratio = 1.22, 95%CI: 1.03–1.46) baseline reaction times (p for interaction = 0.020).
Fig. 3.
Subgroup analyses of delirium risk with HRR index. Forrest plot of hazard ratios with 95% confidence intervals for each 1-SD decrease in HRR index predicting incident delirium based on subgroups of patients by age, sex, CVD risk, physical activity level, maximal METs achieved during exercise, use of HR-control drugs, and reaction time. “Lower/Higher” and “Slower/Faster” were dichotomized based on the median values. CVD = cardiovascular disease; HR = heart rate; HRR = heart rate response/recovery; MET = metabolic equivalent of task.
4. Discussion
To the best of our knowledge, this is the first large-scale prospective study examining the link between HRR during exercise and incident delirium in middle and older age over almost 11 years of follow-up. The key novel finding from the current study is that dynamic changes in HRs from resting to peak exercise and recovery, rather than the average rates themselves, are most important in assessing future vulnerability to delirium. In fully adjusted models, we found a 90% higher risk for those with low HRR index compared with those with high HRR index; the magnitude of association was equivalent to being 6 years older, a current smoker, or having 3 or more additional CVD risk factors. These effects appeared larger in those with faster reaction times and may represent a feasible addition to neurocognitive screening within an aging population.
In prior studies, HRR after exercise (i.e., the change from resting HR and peak exercise HR, respectively) have been shown to be predictors of morbidity and mortality.10,32 While there is some evidence linking irregularity of heart beats and delirium,33, 34, 35, 36 there is a paucity of studies examining the prognostic value of HRR during exercise stress testing. These results show that the HRR index may be able to stratify risk many years before delirium. The HRR index is a dynamic measure that can be easily calculated from the product of both response and recovery ratios. These ratios were individually predictive of delirium in the final models, but they do not fully capture the body's adaption to exercise (each accounted for 75% of the variation in HRR; Supplementary Fig. 1). HR response to exercise and recovery from exercise provide complementary information about cardiac adaptability. A robust HR response during exercise does not guarantee a swift recovery of HR. For example, when participants achieved a doubling of their resting HR during exercise (response ratio ≥ 2.0), there remained great variation in their recovery ratio (from 1.0 to 3.4) as well as maximal achieved METs (Supplementary Fig. 1). Therefore, the HRR index was proposed to capture the effects of both HR measures.
The HRR index also appeared to be a better indicator of delirium risk than the absolute HRs. For example, we did not see an association between the peak HR and delirium risk. The resting HRs (12% increased risk per 1-SD increase) and recovery HRs (10% increased risk per 1-SD) were initially predictive in the core model but became non-significant in the final model once exercise capacity was adjusted for. This was similarly observed in the POD subset (models not shown) and is consistent with prior observations linking higher static measures of resting HRs and perioperative myocardial injury;37, 38, 39 however, no prior study had examined links to POD. Interestingly, those in the low HRR index group were nearly 3 times more likely to experience delirium within 3 days of surgery, which is consistent with work by Ogawa et al.,12 linking preoperative exercise capacity and POD.
Whether these results point to a causal role of cardiac dysfunction in developing delirium or an unmasking of neurodegeneration-related cognitive vulnerability is unclear. Given some evidence linking HR indices to dementia,40,41 early pre-clinical cognitive decline may have confounded our results. However, this cohort is relatively young (mean age = 58.1 years), and even in the younger group (<65 years), when there is a lower likelihood of underlying neurodegenerative diseases, observations are robust (Fig. 3). Results were also independent of a domain of cognition (reaction time) and may even have been stronger in those with better cognition (Fig. 3; faster reaction time, p for interaction = 0.02). Finally, the association was robust when underlying dementia was excluded. Thus, the relationship between HRR index and delirium is unlikely to be fully accounted for by concomitant or undiagnosed neurogenerative pathology. Delirium etiology is likely complex, and other potential pathways include autonomic nervous system dysfunction, cardiometabolic disorders, and neuropsychiatric changes, which can start in early adulthood42 and may be reflected in low HRR index during exercise years before delirium.
Low HR response to exercise (meaning the peak HR is barely above the resting HR in a response ratio close to 1) has been described as chronotropic incompetence and is thought to reflect beta-adrenoreceptor dysfunction.8 On the other hand, attenuated HR recovery after exercise (meaning the lowest recovery HR remained close to the peak HR, resulting in a recovery ratio close to 1) is thought to be a marker of reduced parasympathetic reactivation and autonomic nervous system imbalance.9 Tian et al.43 recently used Mendelian Randomization to show causal evidence between autonomic function and white matter intensity that has been associated with delirium.44 It is conceivable that both play a role in the inability to augment cardiac output during illness or surgical stress,45 leading to subclinical cerebral vascular events or white matter demyelination.46 For example, stroke after surgery is relatively rare,47 but cerebral ischemia has been seen in up to 10% of older surgical patients, which more than doubles the risk of delirium.48 The caveat to this is that submaximal exercise testing, while much more convenient in large and older samples, is considerably distinct from maximal exercise testing. However, Ogawa et al.12 did find that even a 6-min walk test performance was protective, albeit modestly (odds ratio = 0.98, p = 0.02), from POD. Considering the fact that absolute value of peak HR matters very little, the risk difference between low and high HRR index individuals would probably have been enhanced had subjects been pushed further. Though future studies are required to formally test this possibility, our finding that HRR from a “modest” exercise is able to predict risk (whereas other studies have required maximal exercise for links to mortality/CVD) adds clinical relevance for risk stratification in a disease such as delirium since most at-risk subjects are older than those tested for mortality/CVD risk populations.
Reduced parasympathetic reactivation may be a marker of undiagnosed cardiopulmonary and/or autonomic impairment associated with cardiometabolic disorders, such as subclinical heart failure, diabetes, or metabolic syndrome.49 This may result in a chronic imbalance between oxygen supply/demand and subclinical myocardial injuries over time. For example, some have recently observed that impaired pupillary constriction (governed by the parasympathetic system) preceded delirium.50,51 Despite controlling for CVD risks, low HRR index remains predictive, which supports the hypothesis that HRR may be a proxy for CVD risks that were yet to clinically manifest. Whether low HRR index predisposes individuals to worsening neurological insults and delirium risk merits further investigation.
In relation to the putative mechanisms above, this work complements a growing body of literature using HR variability (HRV) measures (a surrogate indication of autonomic nervous system activity, predominantly parasympathetic) during exercise,52,53 psychopathology,54,55 and CVD.49,56,57 There is now emerging evidence for the effect on HRV from POD following hip fractures/orthopedic surgery34,58 as well as intensive care delirium.36 However, few have used HRV for the prediction of delirium. Recently, a pilot study of 30 patients by Echizen et al.59 did find lower preoperative high frequency power, a surrogate for parasympathetic activity, predictive of delirium following esophageal cancer surgery. However, maximal HR is essentially unaffected by aerobic or CRF, while submaximal HR response is strongly influenced by CRF.60 Thus, HRR during an incremental submaximal exercise protocol is likely a combination of both CRF and autonomic modulation, in which CRF predominates. Future work should compare and/or combine HRR and HRV indices with measures of CRF in order to determine their optimal contributions to delirium risk.
Exercise training has been shown to increase resting parasympathetic tone and decrease sympathetic tone in both humans and animals,61, 62, 63 which has translated into improved HR response64 and recovery.65 More specifically, neurobiological studies have suggested that exercise can influence the brain via angiogenesis, neurogenesis, and synaptogenesis.66 Others have shown that exercise and physical activity decreases white matter hyperintensities67 and may slow the accumulation of Aβ and tau burden.68 Unfortunately, this has yet to translate into proven reductions in delirium risk. A recent randomized controlled trial comprised of enhanced exercise and a cognitive program did not appear to reduce incident delirium in hospitalized patients.69 But, in this case, it may be that the intervention came too late, as patients were already in the hospital. In addition, those with low HRR index also self-reported lower physical activity levels (Table 1), but interestingly, physical activity level was not independently predictive of future delirium risk after adjustment for age and other lifestyle factors (Supplementary Table 1). This finding is somewhat consistent with a recent study by Wu et al.70 showing that accelerometry-derived physical activity was not causally related to common neurodegenerative diseases. This points to the importance of an objective and dynamic HRR assessment that could be used to guide future trial interventions. Whether earlier-life optimization of HRR as part of a multicomponent risk prevention strategy71 (e.g., sleep/circadian health72,73 or muscle strength/motor function74) can decrease risk for neurodegenerative diseases such as delirium remains to be seen.
Strengths of this study included the large sample size and prospective design with nearly 11 years of follow-up data available. Despite these strengths, several limitations must be acknowledged. UK Biobank participants are mostly Caucasians of European descent and, therefore, the results may have limited applicability to other ethnic groups. The UK Biobank cohort is generally healthier than the background population, but associations between risk factors and phenotypes have been shown to be generalizable.75 In addition, those selected for exercise testing were slightly healthier still (Supplementary Table 2). The recruitment criterion for the exercise protocol, coupled with the average age of 58.1 years, meant that the population-wide incidence for delirium was relatively low. While this may limit the generalizability and clinical relevance of these results at this point in time, our observations were robust in the higher and lower physical activity groups as well as the higher and lower maximal MET groups (Fig. 3), and they were consistent when the delirium setting/etiology was limited to POD. By stratifying different subgroups, we were able to control for a wide range of potential confounders, but residual confounding in likely given the complex nature of cardiovascular resilience and the heterogeneity of delirium.
In addition, some factors could be on the causal pathway between HRR index and delirium rather than being confounders. For example, cognition could decline faster in those with low HRR index and then go on to influence delirium outcomes. This might have implications for the interpretation of results, particularly the attenuation of risks in those who are in the slower half of reaction times, where the true effects of HRR are potentially underestimated. Furthermore, HRR was assessed only at baseline and could have changed over time. Although we were able to adjust our model for 1 cognitive test, UK Biobank does not have other cognitive measurements, such as the MiniMental State Examination or the Instrumental Activities of Daily Living. Therefore, residual confounding might have occurred due to baseline cognitive ability, which could overestimate the association.
Another major limitation is that the availability of clinical data in the large UK Biobank cohort was limited to International Classification of Disease coding. Many have used this approach for delirium26,27 and a variety of other diseases76, 77, 78 within this cohort. While this method is highly specific (up to 96%) for delirium,79 the sensitivity was only 53%–64% in recent studies.79,80 Thus, we are likely missing cases, particularly milder or hypoactive forms. However, our novel findings remain valuable as a starting point for bridging research in cardiovascular resilience and delirium as long as they are interpreted with caution. Similarly, we made the assumption that in cases where an operation took place within 3 days prior to the onset of delirium, the delirium was likely postoperative. It is, however, conceivable that these occurrences were coincidental and that another illness unrelated to the operation led to hospitalization. Additionally, we note that we may have limited sufficient power for extensive delirium subtype (hypoactive/hyperactive) analysis at this point. Further work is needed to better classify delirium subtypes, especially as the cohort ages and more cases accumulate, before conclusions can be drawn for pre-hospitalization screening using HRR index. Finally, this is an observational study and should not be interpreted as causal. Although we adopted a 2-year landmark analysis, which excluded participants who experienced events in the first 2 years following HRR assessment, reverse causality remains a possibility.
Finally, to estimate overall cardiac regulation, we used the product of HR response and HR recovery ratios. There are other alternative approaches. For instance, Duarte et al.,81 developed another approach whereby participant response and recovery ratios were divided into quintiles (1–5) and summed into a dimensionless score (2–10) known as the Exercise Heart Rate Gradient.82 Reassuringly, the performance of the Exercise Heart Rate Gradient was comparable to HRR in this cohort (Supplementary Fig. 2). Future work ought to compare the relative merits of both methods, particularly in the context of delirium, which has yet to be extensively explored with exercise-related measures of HR.
5. Conclusion
Low HRR during exercise is associated with future risk for delirium during hospitalization. These associations appear larger in POD (not significant due to power limitations) and in those with better cognition. Furthermore, among the individual components, it was the dynamic changes in HR rather than the mean HRs that contributed most. Given that HRR to exercise may be a modifiable and non-cognitive proxy for resilience to inciting stressors prior to delirium, it may prove useful for neurological risk stratification alongside traditional cardiovascular risk factors.
Acknowledgments
This research has been conducted using the UK Biobank Resource under Application Number 40556. LG is funded by National Institutes of Health (NIH) Grant R03AG067985 and the Foundation for Anesthesia Education and Research. PL is funded by the BrightFocus Foundation Alzheimer's Disease Research Program (A2020886S). KH is funded by NIH Grants RF1AG059867 and RF1AG064312. FAJLS is funded by NIH Grant R01HL140574. The organizations mentioned above had no direct input on any aspect of this work.
Authors' contributions
LG contributed to the conception and design of the study, organized the database, performed the statistical analysis, and wrote the first draft of the manuscript; AG organized the database and performed the statistical analysis; PL contributed to the conception and design of the study, performed the statistical analysis, interpreted the data, and drafted the manuscript; RS, FAJLS, OA, and MKR interpreted the data and drafted the manuscript; KH contributed to the conception and design of the study, supervised the study, performed the statistical analysis, interpreted the data, and drafted the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
FAJLS has received lecture fees from Bayer HealthCare 2016, Sentara HealthCare 2017, Philips 2017, Vanda Pharmaceuticals 2018, and Pfizer Pharmaceuticals 2018. MKR has received consulting fees and speaker fees from NovoNordisk and consulting fees from Cell Catapult. The authors declare that they have no other competing interests.
Footnotes
Peer review under the responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.12.002.
Supplementary materials
References
- 1.Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primers. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162:457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 3.Fong TG, Vasunilashorn SM, Libermann T, Marcantonio ER, Inouye SK. Delirium and Alzheimer disease: A proposed model for shared pathophysiology. Int J Geriatr Psychiatry. 2019;34:781–789. doi: 10.1002/gps.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gou RY, Hshieh TT, Marcantonio ER, et al. One-year Medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg. 2021;156:430–442. doi: 10.1001/jamasurg.2020.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman MW, O'Dwyer LC, Rosenthal L. Predicting delirium: A review of risk-stratification models. Gen Hosp Psychiatry. 2015;37:408–413. doi: 10.1016/j.genhosppsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: A presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Thoracic Society; American College of Chest Physicians ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 8.Brubaker PH, Kitzman DW. Chronotropic incompetence: Causes, consequences, and management. Circulation. 2011;123:1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai Y, Saul JP, Albrecht P, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256:H132–H141. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 10.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 11.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa M, Izawa KP, Satomi-Kobayashi S, et al. Preoperative exercise capacity is associated with the prevalence of postoperative delirium in elective cardiac surgery. Aging Clin Exp Res. 2018;30:27–34. doi: 10.1007/s40520-017-0736-5. [DOI] [PubMed] [Google Scholar]
- 13.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 14.Davis DH, Barnes LE, Stephan BC, et al. The descriptive epidemiology of delirium symptoms in a large population-based cohort study: Results from the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) BMC Geriatr. 2014;14:87. doi: 10.1186/1471-2318-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Littlejohns TJ, Sudlow C, Allen NE, Biobank Collins R.UK. Opportunities for cardiovascular research. Eur Heart J. 2019;40:1158–1166. doi: 10.1093/eurheartj/ehx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen N, Sudlow C, Downey P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy and Technology. 2012;1:123–126. [Google Scholar]
- 18.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- 19.Orini M, Tinker A, Munroe PB, Lambiase PD. Long-term intra-individual reproducibility of heart rate dynamics during exercise and recovery in the UK Biobank cohort. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: Evidence from 498,135 UK-Biobank participants. Eur Heart J. 2017;38:116–122. doi: 10.1093/eurheartj/ehw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laukkanen JA, Kunutsor SK, Yates T, et al. Prognostic relevance of cardiorespiratory fitness as assessed by submaximal exercise testing for all-cause mortality: A UK Biobank prospective study. Mayo Clin Proc. 2020;95:867–878. doi: 10.1016/j.mayocp.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Tarp J, Grøntved A, Sanchez-Lastra MA, Dalene KE, Ding D, Ekelund U. Fitness, fatness, and mortality in men and women from the UK Biobank: Prospective cohort study. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moody G, Mark RG. Development and evaluation of a 2-lead ECG analysis program. Comput Cardiol. 1982;1:39–44. [Google Scholar]
- 24.Friesen GM, Jannett TC, Jadallah MA, Yates SL, Quint SR, Nagle HT. A comparison of the noise sensitivity of nine QRS detection algorithms. IEEE Trans Biomed Eng. 1990;37:85–98. doi: 10.1109/10.43620. [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Gaba A, Cui L, et al. Resting heartbeat complexity predicts all-cause and cardiorespiratory mortality in middle- to older-aged adults from the UK Biobank. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowman K, Jones L, Pilling LC, et al. Vitamin D levels and risk of delirium: A mendelian randomization study in the UK Biobank. Neurology. 2019;92:e1387–e1394. doi: 10.1212/WNL.0000000000007136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilling LC, Jones LC, Masoli JAH, et al. Low Vitamin D levels and risk of incident delirium in 351,000 older UK Biobank participants. J Am Geriatr Soc. 2021;69:365–372. doi: 10.1111/jgs.16853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011;77:448–456. [PMC free article] [PubMed] [Google Scholar]
- 29.Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagenaars SP, Harris SE, Davies G, et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (n = 112,151) and 24 GWAS consortia. Mol Psychiatry. 2016;21:1624–1632. doi: 10.1038/mp.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 32.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: The St. James Women Take Heart Project. Circulation. 2010;122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 33.Jooyoung Oh, Dongrae Cho, Jongin Kim, et al. Changes in heart rate variability of patients with delirium in intensive care unit. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:3118–3121. doi: 10.1109/EMBC.2017.8037517. [DOI] [PubMed] [Google Scholar]
- 34.Oh J, Cho D, Park J, et al. Prediction and early detection of delirium in the intensive care unit by using heart rate variability and machine learning. Physiol Meas. 2018;39 doi: 10.1088/1361-6579/aaab07. [DOI] [PubMed] [Google Scholar]
- 35.Neerland BE, Wyller TB, Wyller VBB. Autonomic cardiovascular control in older patients with acute infection and delirium: A pilot study of orthostatic stress responses. BMC Geriatr. 2019;19:23. doi: 10.1186/s12877-019-1035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Zhang Q, Lin B, et al. Association between postoperative long-term heart rate variability and postoperative delirium in elderly patients undergoing orthopedic surgery: A prospective cohort study. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.646253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladha KS, Beattie WS, Tait G, Wijeysundera DN. Association between preoperative ambulatory heart rate and postoperative myocardial injury: A retrospective cohort study. Br J Anaesth. 2018;121:722–729. doi: 10.1016/j.bja.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Abbott TEF, Ackland GL, Archbold RA, et al. Preoperative heart rate and myocardial injury after non-cardiac surgery: Results of a predefined secondary analysis of the VISION study. Br J Anaesth. 2016;117:172–181. doi: 10.1093/bja/aew182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbott TEF, Pearse RM, Beattie WS, et al. Chronotropic incompetence and myocardial injury after noncardiac surgery: Planned secondary analysis of a prospective observational international cohort study. Br J Anaesth. 2019;123:17–26. doi: 10.1016/j.bja.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed RM, Landin-Romero R, Collet T-H, et al. Energy expenditure in frontotemporal dementia: A behavioural and imaging study. Brain. 2017;140:171–183. doi: 10.1093/brain/aww263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Silva VP, Ramalho Oliveira BR, Tavares Mello RG, Moraes H, Deslandes AC, Laks J. Heart rate variability indexes in dementia: A systematic review with a quantitative analysis. Curr Alzheimer Res. 2018;15:80–88. doi: 10.2174/1567205014666170531082352. [DOI] [PubMed] [Google Scholar]
- 42.Gao L, Li P, Hu C, et al. Nocturnal heart rate variability moderates the association between sleep–wake regularity and mood in young adults. Sleep. 2019;42:zsz034. doi: 10.1093/sleep/zsz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian D, Zhang L, Zhuang Z, Huang T, Fan D. A two-sample Mendelian randomization analysis of heart rate variability and cerebral small vessel disease. J Clin Hypertens (Greenwich) 2021;23:1608–1614. doi: 10.1111/jch.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvani A, Calandra-Buonaura G, Dampney RAL, Cortelli P. Brain–heart interactions: Physiology and clinical implications. Philos Trans A Math Phys Eng Sci. 2016;374 doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- 46.Jin Z, Hu J, Ma D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 47.Mrkobrada M, Hill MD, Chan MTV, et al. Covert stroke after non-cardiac surgery: A prospective cohort study. Br J Anaesth. 2016;117:191–197. doi: 10.1093/bja/aew179. [DOI] [PubMed] [Google Scholar]
- 48.Mrkobrada M, Chan MTV, Cowan D, et al. Perioperative covert stroke in patients undergoing non-cardiac surgery (NeuroVISION): A prospective cohort study. The Lancet. 2019;394:1022–1029. doi: 10.1016/S0140-6736(19)31795-7. [DOI] [PubMed] [Google Scholar]
- 49.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 50.Favre E, Bernini A, Morelli P, et al. Neuromonitoring of delirium with quantitative pupillometry in sedated mechanically ventilated critically ill patients. Crit Care. 2020;24:66. doi: 10.1186/s13054-020-2796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang E, Kreuzer M, Hesse S, Davari P, Lee SC, García PS. Infrared pupillometry helps to detect and predict delirium in the post-anesthesia care unit. J Clin Monit Comput. 2018;32:359–368. doi: 10.1007/s10877-017-0009-z. [DOI] [PubMed] [Google Scholar]
- 52.Bellenger CR, Fuller JT, Thomson RL, Davison K, Robertson EY, Buckley JD. Monitoring athletic training status through autonomic heart rate regulation: A systematic review and meta-analysis. Sports Med. 2016;46:1461–1486. doi: 10.1007/s40279-016-0484-2. [DOI] [PubMed] [Google Scholar]
- 53.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 54.Beauchaine TP, Thayer JF. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol. 2015;98:338–350. doi: 10.1016/j.ijpsycho.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Thayer JF, Mather M, Koenig J. Stress and aging: A neurovisceral integration perspective. Psychophysiology. 2021;58:e13804. doi: 10.1111/psyp.13804. [DOI] [PubMed] [Google Scholar]
- 56.Kors JA, Swenne CA, Greiser KH. Cardiovascular disease, risk factors, and heart rate variability in the general population. J Electrocardiol. 2007;40(Suppl. 1):S19–S21. [Google Scholar]
- 57.Huikuri HV, Stein PK. Heart rate variability in risk stratification of cardiac patients. Prog Cardiovasc Dis. 2013;56:153–159. doi: 10.1016/j.pcad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Ernst G, Watne LO, Rostrup M, Neerland BE. Delirium in patients with hip fracture is associated with increased heart rate variability. Aging Clin Exp Res. 2020;32:2311–2318. doi: 10.1007/s40520-019-01447-5. [DOI] [PubMed] [Google Scholar]
- 59.Echizen M, Satomoto M, Miyajima M, Adachi Y, Matsushima E. Preoperative heart rate variability analysis is as a potential simple and easy measure for predicting perioperative delirium in esophageal surgery. Ann Med Surg (Lond) 2021;70 doi: 10.1016/j.amsu.2021.102856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berglund IJ, Sørås SE, Relling BE, Lundgren KM, Kiel IA, Moholdt T. The relationship between maximum heart rate in a cardiorespiratory fitness test and in a maximum heart rate test. J Sci Med Sport. 2019;22:607–610. doi: 10.1016/j.jsams.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 61.Goldsmith RL, Bloomfield DM, Rosenwinkel ET. Exercise and autonomic function. Coron Artery Dis. 2000;11:129–135. doi: 10.1097/00019501-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Convertino VA, Adams WC. Enhanced vagal baroreflex response during 24 h after acute exercise. Am J Physiol. 1991;260:R570–R575. doi: 10.1152/ajpregu.1991.260.3.R570. [DOI] [PubMed] [Google Scholar]
- 63.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 64.Ciolac EG, Greve JMD. Exercise-induced improvements in cardiorespiratory fitness and heart rate response to exercise are impaired in overweight/obese postmenopausal women. Clinics (Sao Paulo) 2011;66:583–589. doi: 10.1590/S1807-59322011000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jolly MA, Brennan DM, Cho L. Impact of exercise on heart rate recovery. Circulation. 2011;124:1520–1526. doi: 10.1161/CIRCULATIONAHA.110.005009. [DOI] [PubMed] [Google Scholar]
- 66.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013 doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: Exploratory analysis of a 12-month randomized controlled trial. J Am Geriatr Soc. 2015;63:2052–2060. doi: 10.1111/jgs.13644. [DOI] [PubMed] [Google Scholar]
- 68.Rabin JS, Klein H, Kirn DR, et al. Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 2019;76:1203–1210. doi: 10.1001/jamaneurol.2019.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeffs KJ, Berlowitz DJ, Grant S, et al. An enhanced exercise and cognitive programme does not appear to reduce incident delirium in hospitalised patients: A randomised controlled trial. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu PF, Lu H, Zhou X, et al. Assessment of causal effects of physical activity on neurodegenerative diseases: A Mendelian randomization study. J Sport Health Sci. 2021;10:454–461. doi: 10.1016/j.jshs.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gual N, García-Salmones M, Brítez L, et al. The role of physical exercise and rehabilitation in delirium. Eur Geriatr Med. 2020;11:83–93. doi: 10.1007/s41999-020-00290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li P, Gao L, Gaba A, et al. Circadian disturbances in Alzheimer's disease progression: A prospective observational cohort study of community-based older adults. Lancet Healthy Longev. 2020;1:e96–105. doi: 10.1016/s2666-7568(20)30015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu K, Li P, Gao L. Sleep, rest-activity rhythms and aging: A complex web in Alzheimer's disease? Neurobiol Aging. 2021;104:102–103. doi: 10.1016/j.neurobiolaging.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao L, Li P, Gaba A, Musiek E, Ju Y-ES, Hu K. Fractal motor activity regulation and sex differences in preclinical Alzheimer's disease pathology. Alzheimers Dement (Amst) 2021;13:e12211. doi: 10.1002/dad2.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: Prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385,292 UK biobank participants. Eur Heart J. 2020;41:1182–1189. doi: 10.1093/eurheartj/ehz849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wyse CA, Morales CAC, Graham N, et al. Adverse metabolic and mental health outcomes associated with shiftwork in a population-based study of 277,168 workers in UK biobank. Ann Med. 2017;49:411–420. doi: 10.1080/07853890.2017.1292045. [DOI] [PubMed] [Google Scholar]
- 78.Li P, Zheng X, Ulsa MC, et al. Poor sleep behavior burden and risk of COVID-19 mortality and hospitalization. Sleep. 2021;44:zsab138. doi: 10.1093/sleep/zsab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sepulveda E, Franco JG, Trzepacz PT, et al. Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: Diagnostic accuracy study. BMC Psychiatry. 2016;16:167. doi: 10.1186/s12888-016-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casey P, Cross W, Mart MW-S, Baldwin C, Riddell K, Dārziņš P. Hospital discharge data under-reports delirium occurrence: Results from a point prevalence survey of delirium in a major Australian health service. Intern Med J. 2019;49:338–344. doi: 10.1111/imj.14066. [DOI] [PubMed] [Google Scholar]
- 81.Duarte CV, Myers J, de Araújo CGS. Exercise heart rate gradient: A novel index to predict all-cause mortality. Eur J Prev Cardiol. 2015;22:629–635. doi: 10.1177/2047487314520784. [DOI] [PubMed] [Google Scholar]
- 82.Wang S, Müller J, Goeder D, Araujo CG, Silva CG de SE, Myers J. Effect of beta-blocker use on exercise heart rate gradient and reclassification of mortality risk in patients referred for exercise testing. Am J Cardiol. 2020;130:152–156. doi: 10.1016/j.amjcard.2020.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the UK Biobank upon submission of application (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). The syntax for conducting the analysis will be provided to UK Biobank and made freely available upon request.