Abstract
One of the known important functions of hair is protection from extensive sunlight. This protection is accomplished in large part due to natural hair pigmentation which is known to reflect the number of melanin granules (melanosomes) in the hair shaft, and melanin variants. Melanin takes in excessive light energy and converts it to heat in a process called absorption; heat is then dissipated into the environment as infrared radiation, thereby protecting the underlying skin. We used transmission electron microscopy (TEM) to visualize the melanosome counts in samples of human hair, and used thermal microscopy to measure the temperature changes of the samples when exposed to green and blue light lasers. In our experiments green light conversion to heat was highly correlated to the number of melanosomes, whereas blue light conversion to heat was less correlated, which may be because the reddish melanosomes it contains are less effective in absorbing energy from the blue spectrum of light. Anyway, we have shown the metals accumulation in the melanin can be easily visualized with TEM. We confirmed that the amount of melanin granules in human hair defines the conversion to heat of light energy in the visible spectrum.
Keywords: hair, melanin, melanosomes, light absorption
Melanosomes in hair shaft accumulate metals which make them visible under electron microscope (TEM) without additional staining, at high magnification. We used TEM to visualize the melanosome counts in samples of human hair, and used thermal microscopy to measure the temperature changes of the samples when exposed to green and blue light lasers. It was shown that green light conversion to heat was highly correlated to the number of melanosomes, whereas blue light conversion to heat was less correlated, which may be because the reddish melanin it contains is less effective in absorbing energy from the blue spectrum of light.
INTRODUCTION
Historically, hair phenotypes have played an essential evolutionary role in the advancement of the human species. Hair color is known to be an important factor in social communication as it provides much information regarding an individual’s race, ethnicity, gender, age and health status, as well as physical and sexual attractiveness (1). In addition, hair pigmentation and growth are important for camouflage and trauma protection, as well as for protection from light and general thermoregulation. It has also been elucidated that melanin variants and combinations determine hair color (2–5). Melanin is concentrated inside melanosomes, which are specialized pigment-producing organelles. These melanosomes are first created by melanocytes, then transferred to keratinocytes in hair follicles, and later relocated to the hair shaft/cortex (6, 7).
In many mammals, the skin or fur on the upper part of the body, usually facing the sun, is darker than on the lower surface. This may be because the more melanin-rich upper surfaces are more protected from ultraviolet (UV) radiation and help to get more heat from the sun (8–10). It is also possible that darker upper surfaces enhance visual camouflage. Currently most researchers agree that all three factors were important in the evolutionary development of melanin-containing tissues in mammals (8, 9, 11).
The biochemistry of the effects of visible light and UV light on melanin is quite complex and needs further research (8). Although they have been shown to lighten hair through different mechanisms, both lighten the melanin granules (8). At the same time, UV, unlike visible light, damages almost all hair tissues (8).
Besides melanin, melanosomes contain various proteins and lipids, while some chemicals, including metals, may bind to melanin and be retained in the tissues for long periods, although the physiological significance of this process remains partly unclear. Melanin in human hair is present in two main types: eumelanin (black melanin), which is mainly composed of oligomers of dihydroxy indole and its derivatives, and pheomelanin (orange-red melanin), which is derived from benzothiazine units (12). Each of the melanin types is present within its distinctive melanosome. Black and reddish melanosomes of relatively similar shape and size in hair can be found mainly in the middle layer of the hair shaft, or cortex (1, 13). Interestingly, significant amounts of diverse metal ions are usually bound to the two types of melanosomes. Cu(II) and Zn(II) are present in similar amounts, while Fe(III) content is four times higher in the reddish melanosomes, and may reach 1.6 mmol/g or more (14, 15). Heavy metals like Cu(II) and Fe(III) bind tightly to melanin, and practically become sequestered by melanosomes. Meanwhile, Ca(II) or Zn(II) have less affinity and can be accumulated and eliminated if necessary. Melanosomes can be either metal reservoirs or metal sinks depending on the specific type of metal (15).
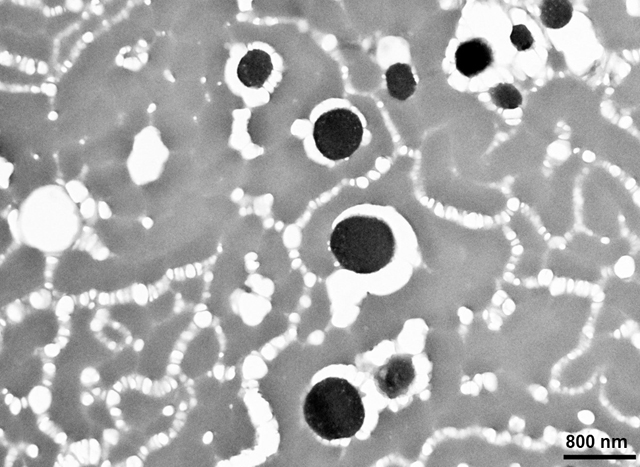
Various methods may be used to recognize the metals that accumulate in melanosomes bound to melanin molecules. For example, metals in melanin can be detected by magnetic resonance (16). In transmission electron microscopy (TEM), biological material is usually stained with heavy metals to see cellular components, because heavy metals deflect the electron beam and thus make cellular structures visible. Since melanosomes already contain metals, we hypothesized that it would be possible to see melanosomes in the hair shaft with TEM without staining with external heavy metals. We also hypothesized that resulting contrast images would contain mainly melanosomes, and that their quantity could be easily calculated using standard fiches of imaging software.
Under irradiation by visible light, its energy may be partially reflected and partially absorbed. When visible light is absorbed by an object, the object converts the short wavelength light into heat that can be measured because it emits long-wavelength infrared radiation (IR) (17). The same happens when light illuminates hair: the illuminated zone temperature grows, while heat transfer by hair keratin is low (about 0.1 W/m∙K compared to 400 W/m∙K for copper)(18). Thus, the heat in the illuminated zone is not transferred by the keratin in the hair shaft to the skin but rather dissipates into the environment, the hair working as a protective device against light.
Since any melanin contains a certain amount of heavy metals accumulated during the life of the organism, in this work we attempted to leverage this phenomenon by using TEM without additional staining with heavy metals to visualize melanosomes in three different variants of human hair color and to determine the relative number of melanosomes in the three variants. Hair samples were also irradiated by visual light, using lasers with specific wavelengths. We measured the temperature of the hair shaft in the point of irradiation and determined the difference between it and the temperature of a non-affected point of the shaft. This allowed us to estimate the light absorption by different hair types at different visible spectra. Also, we measured the relative quantity of melanosomes in different hair types and determined if melanosome quantity is correlated to light absorbance.
MATERIALS AND METHODS
The hair samples considered in this study were collected in a haircut studio, in San Juan, Puerto Rico. Hairdresser experts helped us to determine that the samples were not altered with dyes or by other means and represented natural hair coloration. Samples of black and brown hair, as well as a blond hair were used in our experiments. For this study of human samples without personal identification, we obtained exemption from the Institutional Review Board of the Universidad Central del Caribe School of Medicine, Bayamon, Puerto Rico, Protocol 2022–27.
Transmission Electron Microscopy (TEM) of hair slices.
In order to visualize melanosomes with high contrast in the hair slices, we used no external staining with heavy metals because both eumelanin and pheomelanin are prone to adsorb/accumulate cations of heavy metals in vivo (15). Under TEM, melanin with adsorbed metal ions became visible as black spots with high contrast in non-stained hair slices (see Fig. 1 A, B, C). For hair slice preparation, dehydration was carried out through an ascending series of ethanol–water solutions (50, 70, 90, and 100% ethanol) for 10 minutes each, and then incubation in 100% ethanol was repeated three times for 10 minutes to ensure complete dehydration. The 100% ethanol was replaced with a 1:1 mixture of 100% ethanol and Epon/Spurr EMbed 812 EMS embedding medium (Electron Microscopy Sciences, Hatfield, PA, USA) and left to infiltrate overnight. Next, this ethanol:Epon/Spurr mixture was replaced with pure Epon/Spurr medium and left to infiltrate for 2 hours, replaced again with a fresh solution and left for another 2 hours, before being replaced with fresh solution and left to infiltrate overnight. Finally, the hair trunks were oriented and polymerized at 60°C for 48 hours. Ultrathin (60-nm) sections were made using an LKB Ultratome (LKB-Produkter, Bromma, Sweden) and examined using a JEM-100B electron microscope (JEOL Ltd., Tokyo, Japan). High-contrast TEM images were analyzed using standard Particle analysis fiche of ImageJ 1.51k (http://imagej.nih.gov/ij).
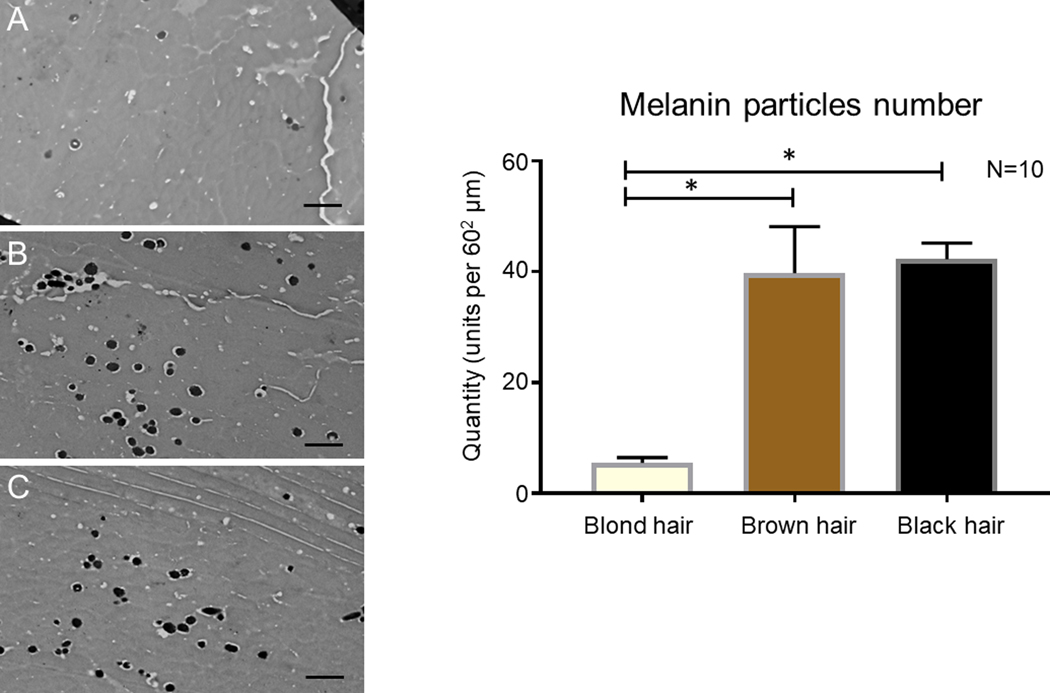
Figure 1.
Left panel: TEM Imaging of Melanosomes in Hair Shaft Slices: representative images of middle layer of hair shaft slices of: A–blond hair, B–brown hair, C–black hair. Right panel: Graph representing the quantity of melanosomes on the standard 2900X TEM slices from samples of blond, brown and black hair. Scale bar for TEM: 1 µm.
Temperature reading.
To read the temperatures, we used an OPTRIS S-107 Infrared Thermal Microscope (Optris GmbH, Berlin, Germany) with 10 X objective. Two metal prongs were positioned side by side on the platform of the microscope. The prongs were secured to the base of the microscope using adhesive tape. Each end of the hair sample was placed on the tips of the prongs, to allow clear visualization of the sample temperature through the OPTRIS PI Connect (Rel.2.16.22270) infrared thermometer program (Fig. 2 upper panel). Increases in temperature for blond, brown and black hair samples after exposure to green (532 nm) or blue (441 nm) light lasers (5 mW Sparkle Magic 4.0 Laser, Yard Illumination, San Marcos, CA, USA) were recorded as follows:
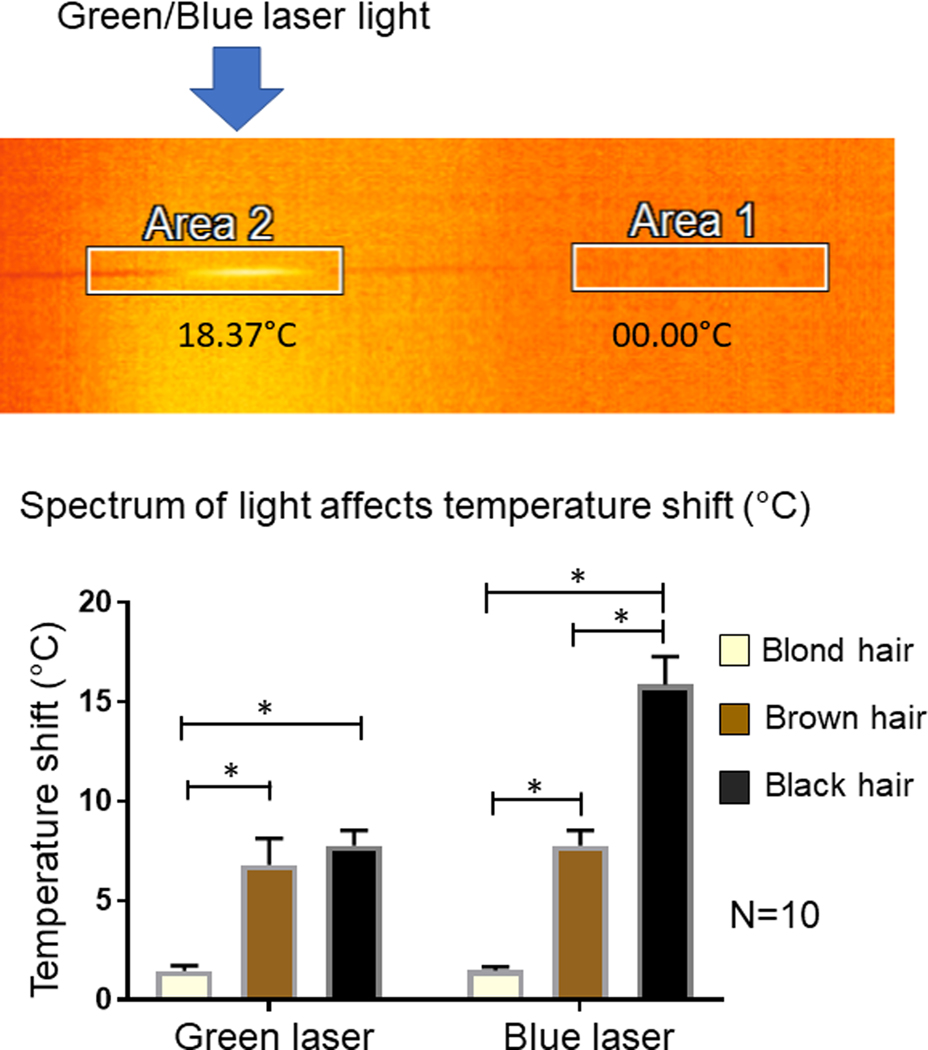
Figure 2.
Hair shaft temperature measurements. Upper panel: Representative example of hair shaft temperature image under IR Thermal Microscope. Area 2 in this example is illuminated with green or blue laser and its temperature recorded. Lower panel: The graph of a temperature shift of hair shaft of illuminated area under one minute of green or blue laser illumination. * asterisk represent the statistically significant difference.
Blue or green light lasers were pointed at three distinct areas along the length of the hair shaft for 1 minute, while simultaneously recording real-time temperature increase readings for each area. The maximum temperature reached in each area along the length of the hair sample was recorded and averaged per run, 10 runs for each hair sample. There was no visible spread of temperature from radiated to un-radiated areas, and un-radiated shaft temperature was the same as room temperature, which was 25°C.
Statistics and measurements.
GRAPHPAD PRISM 7.03 (GraphPad Software, Inc., La Jolla, CA, USA) were used for data calculations. Paired T-test was used to analyze differences between variables. Values were determined to be significantly different if the two-tailed P-value was <0.05.
RESULTS
Amount of melanin granules (melanosomes) in hair samples of blond, brown and black hair.
Transmission electron microscopy of blond (Fig. 1A), brown (Fig. 1B) and black (Fig. 1C) hair samples revealed that melanosomes, which looked like ellipsoidal black particles, are present in the middle layer of the hair shaft and distributed stochastically. Blond hair samples have relatively low quantities of melanosomes (5.5 ± 0.97) in the middle layer of the hair shaft, compared to other samples (t=38.41, df=18, P<0.05), while brown (39.8 ± 8.36) and black (42.3 ± 2.87) samples have a statistically undistinguishable number of melanosomes. We have not distinguished between red and black melanosomes in the hair because, under TEM, all the melanosomes appeared the same color (Fig. 1 A, B, C).
Effects of light irradiation on hair temperature.
Hair shafts were positioned as discussed (see Methods) and were subsequently illuminated by the green or blue lasers in three different areas along the shaft for one minute. Temperature readings were determined using infrared Thermal Microscope (Fig.. 2, Upper panel) and then averaged (see Methods). The illuminated area of the hair shaft adsorbed light and the light energy was converted to heat, elevating the temperature of the shaft. The area with elevated temperature emitted IR light visible under the IR Thermal Microscope (Fig. 2, Upper panel) as a shining area of the shaft. We found that hair temperature was elevated in the illuminated area of the hair shaft in all samples; however, blond hair temperature was affected only marginally, while brown and black hair temperatures were elevated significantly more (for brown hair t=25.09, df=9, P<0.05; for black hair t=30.09, df=9, P<0.05) by both green and blue light (Fig. 2, Lower panel). Interestingly, green light elevated the hair shaft temperature of brown and black samples similarly (no statistic difference, P=0.15), while blue light elevated the temperature of black hair samples significantly more strongly (t=14.24, df=9, P<0.05).
We also calculated the correlation of the quantity of melanosomes in the samples to the temperature elevation under green and blue light illumination of the hair shafts. Under green illumination, there was clear direct correlation between these numbers (Pearson r=0.9966, P<0.05), but for blue illumination the correlation was still relatively big but less significant (Pearson r=0.8597, P=0.34).
DISCUSSION
In this study, we investigated whether the number of melanosomes in the shafts of blond, brown and black human hair samples correlated to the blue (441 nm) and green (534 nm) light conversion to the heat. To visualize melanosomes in hair samples, we used TEM without additional staining with heavy metals and determined the relative amount of melanosomes in three different hair variants. The methods we used does not allow us to distinguish between reddish and black melanosomes (black eumelanosome vs redish pheomelanosome), since they only allow us to determine the size and shape of melanosomes. Although some authors mention that reddish melanosomes may be generally smaller than black ones, and that clustered melanosomes are usually smaller than solitary ones (19, 20), melanosomes’ sizes overlap and the types cannot be distinguished by shape and size alone. The actual ratio of reddish and black melanin in human hair depends on age and its exact value can only be determined by chemical analysis (21–23). The amount of melanosomes in blond hair was significantly lower than the amounts in brown or black hair; brown and black hair have similar amount of melanosomes, although some of the melanosomes in brown hair were reddish instead of black. This data is consistent with earlier findings. Previously, it was reported that the amount of melanosomes depends on the lightness of hair color; for example, the yield of melanin granules derived from light-brown hair was 3.95% (+/− 0.3%) of the total hair weight, while the yield from untreated black hair was 4.8% (+/− 0.25%) (3, 19). It may happen that the amount of melanin can be reduced by long irradiation, especially by UV; however, this is not the case in our experiments since we only used very mild-powered and small-time intervals of irradiation.
We also irradiated hair samples with visual light, using lasers with specific green and blue wavelengths, and measured the difference between the temperatures at the point of irradiation and at a non-affected portion of the shaft. This allowed us to estimate the dependence of light energy absorption and its conversion to heat by different hair types at different visible spectrum wavelength. Brown hair contains both black and reddish melanosomes with different melanin mixtures (2). It has been shown that typical in vitro absorption spectra of the eumelanin and pheomelanin depends very much on the method of extraction (e.g., acid-base, enzymatic) or on the method of synthesis for synthetic variants (24).
In most of the visible spectrum, eumelanin (black) absorbs light better than pheomelanin (reddish brown). This effect is especially pronounced in the longer wavelength of the visible spectrum (21, 24–29). However, the absorption spectrum can be different because of the participation of other components within melanosomes besides melanin, as well as because of Mie scattering by the melanosomes themselves (largely wavelength independent), allowing bulk absorption rather than surface absorption of light energy (30). Our data measuring light energy absorption of the hair shafts of different hair variants thus are very interesting, because our results point to a bigger difference in light energy absorption in black hair samples in blue rather than green light, which is counterintuitive if only melanin-type adsorption is taken into consideration. Also, eumelanin absorbs light better than pheomelanin at the low wavelength part of the visible light spectrum; yet, we observed practically no difference at the green wavelength for brown hair samples, which contain both reddish and black melanosomes. Our data suggest that absorption of light energy may be dependent on the number of melanosomes rather than only on the melanin content, because the correlation between melanosome quantity and the absorption and conversion to heat of light energy was very clear, especially for green light (Pearson correlation coefficient r=0.9966). A similar but less clear correlation (r=0.8597) was found for blue light absorption. These data confirm the importance of other components of absorption besides melanin.
Our results have intriguing implications as far as a connection between the accumulation of metals in melanin and Parkinson’s disease (PD), which is a neurodegenerative disease characterized by the loss of dopaminergic neurons containing neuromelanin (NM) in the substantia nigra (SN)(31). NM is a mixture of pheomelanin and eumelanin, the two major forms corresponding to hair phenotypes (32). Neuromelanin thus also accumulates metals, including iron (16); with the disappearance of NM-containing neurons, these metals must also disappear. Nevertheless, iron levels are increased significantly in the PD brain, not only in SN but in other parts as well, leading some authors to suggest these iron deposits may be a possible cause of PD (33, 34). It has been demonstrated that earlier age at onset of graying of hair, greater proneness to sunburn, difficulty with tanning, and sebum dysregulation are more common in patients with PD (35). It is also known that lighter hair and skin color correlate to a higher risk of developing PD (36). This correlation is more pronounced in the early onset of PD (37). The role of hair melanocytes in the development of pathological processes may also be associated with the elimination of excess metal ions, first of all iron, from the body (38).
Finally, we have shown the metals accumulation in the melanin can be easily visualized with transmission electron microscopy (TEM), paving the way to study with TEM melanin-containing cells. As a conclusion from our in situ experiments, we suggest that the amount of melanin granules in human hair defines the absorption and conversion to heat of light energy in the visible spectrum.
ACKNOWLEDGEMENTS:
The authors appreciate Dr. E.C. Lennox close reading of the manuscript and Ms. Alexandra Elbakyan for help in obtaining references. This work was sponsored by the National Institute of General Medical Science (NIGMS) of the National Institute of Health (NIH) under awards U54GM133807 and SC3GM143983. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
REFERENCES
- 1.Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology [Internet]. 2009. Jul [cited 2022 Aug 31];1(2):83–93. Available from: https://pubmed.ncbi.nlm.nih.gov/20927229/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Césarini JP. Hair Melanin and Hair Color. In: Hair and Hair Diseases [Internet]. Berlin, Heidelberg: Springer, Berlin, Heidelberg; 1990. [cited 2022 Aug 31]. p. 165–97. Available from: 10.1007/978-3-642-74612-3_8 [DOI] [Google Scholar]
- 3.Ortonne J-P, Prota G. Hair melanins and hair color: ultrastructural and biochemical aspects. J Invest Dermatol [Internet]. 1993. Jul [cited 2022 Aug 31];101(1 Suppl):82S-89S. Available from: https://pubmed.ncbi.nlm.nih.gov/8326157/ [DOI] [PubMed] [Google Scholar]
- 4.Riley PA. Melanin. Int J Biochem Cell Biol [Internet]. 1997. [cited 2022 Aug 31];29(11):1235–9. Available from: https://pubmed.ncbi.nlm.nih.gov/9451820/ [DOI] [PubMed] [Google Scholar]
- 5.Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment cell Res [Internet]. 2003. Oct [cited 2022 Aug 31];16(5):523–31. Available from: https://pubmed.ncbi.nlm.nih.gov/12950732/ [DOI] [PubMed] [Google Scholar]
- 6.Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol [Internet]. 2003. [cited 2022 Aug 31];12 Suppl 2(2):5–12. Available from: https://pubmed.ncbi.nlm.nih.gov/14756517/ [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Hammer JA. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol [Internet]. 2014. [cited 2022 Aug 31];29(1):1–7. Available from: https://pubmed.ncbi.nlm.nih.gov/24662021/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtt EH. The Adaptiveness of Animal Colors. Bioscience. 2011;31(10):723–9. [Google Scholar]
- 9.Penacchio O, Cuthill IC, Lovell PG, Ruxton GD, Harris JM. Orientation to the sun by animals and its interaction with crypsis. Funct Ecol. 2015;29(9):1165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noonan FP, Zaidi MR, Wolnicka-Glubisz A, Anver MR, Bahn J, Wielgus A, et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat Commun 2012. 31 [Internet]. 2012 Jun 6 [cited 2022 Nov 9];3(1):1–10. Available from: https://www.nature.com/articles/ncomms1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imsland F, McGowan K, Rubin CJ, Henegar C, Sundström E, Berglund J, et al. Regulatory mutations in TBX3 disrupt asymmetric hair pigmentation that underlies Dun camouflage color in horses. Nat Genet. 2016;48(2):152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micillo R, Panzella L, Koike K, Monfrecola G, Napolitano A, D’Ischia M. “Fifty Shades” of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties. Int J Mol Sci [Internet]. 2016. May 1 [cited 2022 Aug 31];17(5). Available from: https://pubmed.ncbi.nlm.nih.gov/27196900/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol [Internet]. 2005. [cited 2022 Aug 31];124(1):13–21. Available from: https://pubmed.ncbi.nlm.nih.gov/15654948/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Hong L, Wakamatsu K, Ito S, Adhyaru B, Cheng C-Y, et al. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem Photobiol [Internet]. 2005. [cited 2022 Aug 31];81(1):135. Available from: https://pubmed.ncbi.nlm.nih.gov/15504086/ [DOI] [PubMed] [Google Scholar]
- 15.Hong L, Simon JD. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J Phys Chem B [Internet]. 2007. Jul 19 [cited 2022 Aug 31];111(28):7938–47. Available from: https://pubmed.ncbi.nlm.nih.gov/17580858/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP. The “swallow tail” appearance of the healthy nigrosome - a new accurate test of Parkinson’s disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One [Internet]. 2014. Apr 7 [cited 2022 Sep 7];9(4). Available from: https://pubmed.ncbi.nlm.nih.gov/24710392/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole RK, Kalnenieks U. Introduction to light absorption: visible and ultraviolet spectra. Spectrophotometry Spectrofluorim [Internet]. 2000. May 4 [cited 2022 Aug 31]; Available from: https://academic.oup.com/book/41698/chapter/353943288 [Google Scholar]
- 18.Xue Y, Lofland S, Hu X. Thermal Conductivity of Protein-Based Materials: A Review. Polym 2019, Vol 11, Page 456 [Internet]. 2019 Mar 11 [cited 2022 Aug 31];11(3):456. Available from: https://www.mdpi.com/2073-4360/11/3/456/htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höcker EHZ. Photochemical alterations in human hair. Part II: Analysis of melanin. Vol. 46. 1995. [Google Scholar]
- 20.D’Alba L, Shawkey MD. Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol Rev [Internet]. 2019. Jan 1 [cited 2022 Aug 31];99(1):1–19. Available from: https://pubmed.ncbi.nlm.nih.gov/30255724/ [DOI] [PubMed] [Google Scholar]
- 21.Ozeki H, Ito S, Wakamatsu K, Thody AJ. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment cell Res [Internet]. 1996. [cited 2022 Nov 9];9(5):265–70. Available from: https://pubmed.ncbi.nlm.nih.gov/9014213/ [DOI] [PubMed] [Google Scholar]
- 22.Ito S, Nakanishi Y, Valenzuela RK, Brilliant MH, Kolbe L, Wakamatsu K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res [Internet]. 2011. Aug [cited 2022 Nov 9];24(4):605–13. Available from: https://pubmed.ncbi.nlm.nih.gov/21535429/ [DOI] [PubMed] [Google Scholar]
- 23.Itou T, Ito S, Wakamatsu K. Effects of Aging on Hair Color, Melanosome Morphology, and Melanin Composition in Japanese Females. Int J Mol Sci [Internet]. 2019. Aug 1 [cited 2022 Nov 9];20(15). Available from: https://pubmed.ncbi.nlm.nih.gov/31370161/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brian Nofsinger J, Forest SE, Simon JD. Explanation for the Disparity among Absorption and Action Spectra of Eumelanin. J Phys Chem B [Internet]. 1999. Dec 23 [cited 2022 Aug 31];103(51):11428–32. Available from: 10.1021/jp992640y [DOI] [Google Scholar]
- 25.D’Ischia M, Wakamatsu K, Napolitano A, Briganti S, Garcia-Borron JC, Kovacs D, et al. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res [Internet]. 2013. Sep [cited 2022 Nov 9];26(5):616–33. Available from: https://pubmed.ncbi.nlm.nih.gov/23710556/ [DOI] [PubMed] [Google Scholar]
- 26.Ye T, Simon JD, Sarna T. Ultrafast Energy Transfer from Bound Tetra(4-N,N,N,N-trimethylanilinium)porphyrin to Synthetic Dopa and Cysteinyldopa Melanins¶. Photochem Photobiol [Internet]. 2003. Jan 1 [cited 2022 Aug 31];77(1):1–4. Available from: 10.1562/0031-8655(2003)0770001UETFBT2.0.CO2 [DOI] [PubMed] [Google Scholar]
- 27.Zonios G, Dimou A, Bassukas I, Galaris D, Tsolakidis A, Kaxiras E. Melanin absorption spectroscopy: new method for noninvasive skin investigation and melanoma detection. J Biomed Opt [Internet]. 2008. [cited 2022 Aug 31];13(1):014017. Available from: https://pubmed.ncbi.nlm.nih.gov/18315375/ [DOI] [PubMed] [Google Scholar]
- 28.Kollias N, Baqer AH. Absorption mechanisms of human melanin in the visible, 400–720 nm. J Invest Dermatol [Internet]. 1987. [cited 2022 Sep 1];89(4):384–8. Available from: https://pubmed.ncbi.nlm.nih.gov/3668281/ [DOI] [PubMed] [Google Scholar]
- 29.Simon JD, Hong L, Peles DN. Insights into melanosomes and melanin from some interesting spatial and temporal properties. J Phys Chem B [Internet]. 2008. Oct 23 [cited 2022 Nov 9];112(42):13201–17. Available from: https://pubmed.ncbi.nlm.nih.gov/18817437/ [DOI] [PubMed] [Google Scholar]
- 30.Wolbarsht ML, Walsh AW, George G. Melanin, a unique biological absorber. Appl Opt [Internet]. 1981. Jul 1 [cited 2022 Aug 31];20(13):2184. Available from: https://pubmed.ncbi.nlm.nih.gov/20332914/ [DOI] [PubMed] [Google Scholar]
- 31.Zecca L, Bellei C, Costi P, Albertini A, Monzani E, Casella L, et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc Natl Acad Sci U S A [Internet]. 2008. Nov 11 [cited 2022 Nov 9];105(45):17567. Available from: /pmc/articles/PMC2582310/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haining RL, Achat-Mendes C. Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res [Internet]. 2017. Mar 1 [cited 2022 Sep 7];12(3):372–5. Available from: https://pubmed.ncbi.nlm.nih.gov/28469642/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Y, Gong T, Wu J, Li J, Chen Y, Wang Y, et al. Subtypes evaluation of motor dysfunction in Parkinson’s disease using neuromelanin-sensitive magnetic resonance imaging. Neurosci Lett [Internet]. 2017. Jan 18 [cited 2022 Sep 7];638:145–50. Available from: https://pubmed.ncbi.nlm.nih.gov/27993708/ [DOI] [PubMed] [Google Scholar]
- 34.Mochizuki H, Choong CJ, Baba K. Parkinson’s disease and iron. J Neural Transm [Internet]. 2020. Feb 1 [cited 2022 Sep 7];127(2):181–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32025811/ [DOI] [PubMed] [Google Scholar]
- 35.Jucevičiūtė N, Banaitytė I, Vaitkus A, Balnytė R. Preclinical signs of Parkinson’s disease: A possible association of Parkinson’s disease with skin and hair features. Med Hypotheses. 2019;127(April):100–4. [DOI] [PubMed] [Google Scholar]
- 36.Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Genetic determinants of hair color and Parkinson’s disease risk. Ann Neurol [Internet]. 2009. Jan [cited 2022 Nov 9];65(1):76–82. Available from: https://pubmed.ncbi.nlm.nih.gov/19194882/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disse M, Reich H, Lee PK, Schram SS. A Review of the Association Between Parkinson Disease and Malignant Melanoma. Dermatol Surg [Internet]. 2016. [cited 2022 Nov 9];42(2):141–6. Available from: https://pubmed.ncbi.nlm.nih.gov/26771684/ [DOI] [PubMed] [Google Scholar]
- 38.Tolleson WH. Human melanocyte biology, toxicology, and pathology. J Environ Sci Heal - Part C Environ Carcinog Ecotoxicol Rev. 2005;23(2):105–61. [DOI] [PubMed] [Google Scholar]