Abstract
Disruptions in circadian rhythms can occur in healthy aging; however, these changes are more severe and pervasive in individuals with age-related and neurodegenerative diseases, such as dementia. Circadian rhythm alterations are also present in preclinical stages of dementia, e.g., in patients with mild cognitive impairments (MCI), thus providing a unique window of opportunity for early intervention in neurodegenerative disorders. Nonetheless, there is a lack of studies examining the association between relevant changes in circadian rhythms and their relationship with cognitive dysfunctions in MCI individuals. In this review, we examine circadian system alterations occurring in MCI patients compared to healthy aging individuals while also considering their association with MCI neurocognitive alterations. Our main findings are that abnormal circadian changes in rest-activity, core body temperature, melatonin, and cortisol rhythms appear in the MCI stage and that these circadian rhythm disruptions are associated with some of the neurocognitive deficits observed in MCI patients. Also, preliminary evidence indicates that interventions aimed at restoring regular circadian rhythms may prevent or halt the progress of neurodegenerative diseases and mitigate their related cognitive impairments. Future longitudinal studies with repeated follow-up assessments are needed to establish the translational potential of these findings in clinical practice.
Keywords: Circadian rhythm, Mild cognitive impairment, Neurocognitive function, Young adults, older adults
Graphical Abstract
This article provides a narrative review of the most relevant findings of circadian rhythm changes/disruptions in four domains, which involve rest-activity rhythm (RAR), core body temperature (CBT), melatonin, and cortisol in patients with mild cognitive impairment (MCI) relative to healthy aging individuals. We also examine the relationships between normal aging and MCI-related changes in circadian rhythms relative to cognitive functions.
1. Introduction
With the rapid growth of the world population, it is estimated that between 2010 to 2050, the proportion of adults over 65 years will increase from 8% to 16%.[1] Aging affects virtually all human physiological processes, including circadian rhythms. Although the underlying mechanisms of aging are complex and have yet to be fully understood, an increasing number of studies indicate that changes in circadian rhythms across the lifespan significantly affect age-related modifications in brain physiology and related behaviors.[2-14]
Circadian rhythm changes in the elderly (≥65 years) affect temperature regulation, perception, information processing, as well as general cognitive abilities. Many of these changes are part of the normal aging process, but some can be caused by pathophysiological mechanisms underlying neurodegenerative and neurocognitive disorders.[2] Understanding how to distinguish the above-mentioned processes can provide an opportunity to intervene and improve the quality of life of patients suffering from neurodegenerative disorders, including Alzheimer's disease (AD) and Lewy Body dementia (LBD), since pathological disruptions in circadian rhythms may represent an early sign of these disorders.[4, 15-28] Specifically, circadian rhythm changes that deviate from a healthy aging trajectory are likely to begin when the functional and cognitive decline leading to AD and other major neurodegenerative disorders first occur, thus providing a unique window of opportunity for early interventions aimed at rectifying this abnormal trajectory.
Mild cognitive impairment (MCI) is defined as a greater cognitive decline compared to the average expected decline for an individual's age and education level, which occurs without significant changes in daily routine activities (Figure 1.a).[29, 30] Based on the DSM-5 criteria, MCI is a condition characterized by memory complaints and abnormal memory for the age that does not meet the criteria for dementia, in the context of retained ability to perform activities of daily living independently and preserved general cognitive functioning.[31, 32] MCI has been identified as a high-risk condition for more severe neurological disorders, including AD and LBD, and it is considered a prodromal state of variable duration between normal aging and dementia.[33-37] It is reported that MCI affects up to 42% of people over 60 years of age.[38-40] Within this subgroup, approximately 70% of individuals will develop AD or some other forms of dementia within 5 years.[29, 41] Although it is still unclear whether circadian rhythm changes/disruptions precede the onset neurodegenerative disorders, thus being causally implicated in their development, biological and environmental disruptions to these systems do seem to worsen with the appearance and progression of these disorders.[9] Thus, although prospective, longitudinal studies are needed to establish whether circadian rhythm alterations are risk factor for MCI and dementia.[42-44], comprehensively characterizing these alterations in MCI patients can help identify early disruption in circadian rhythmicity in individuals at higher risk for neurocognitive disorders. However, circadian rhythm dysregulations in MCI individuals have not been thoroughly described.
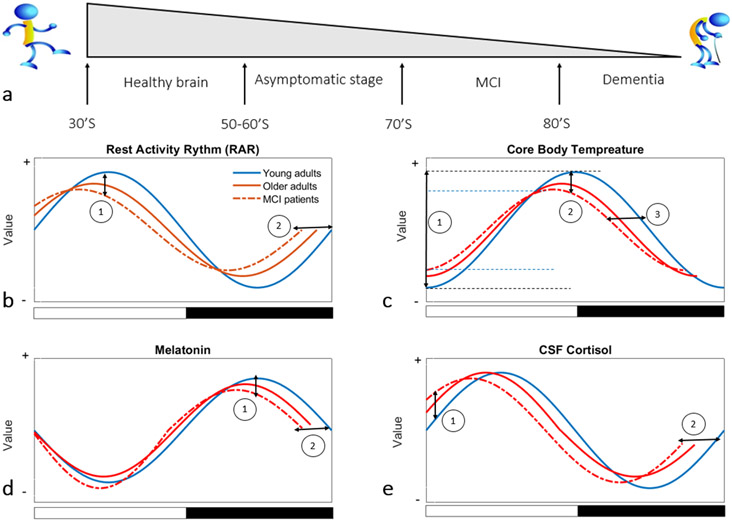
Figure 1.
A general illustration of circadian rhythms in young healthy, older healthy adults and MCI patients: a) presentation of different stages from young, healthy adulthood to dementia, with the gray-shaded triangle reflecting the progressive decrease in life expectancy across the lifespan; b) sketching of rest-activity rhythm in young, elderly, and MCI indicates: (1) a reduction in RAR amplitude in older adults compared to young subjects and of MCI individuals compared to both young and older adults, and (2) a RAR phase advance in older adults compared to young adults and MCI patients compared to other groups; c) sketching of core body temperature (CBT) changes in three groups depicts: (1) a lower peak-to-trough CBT amplitude in MCI compared to young and older healthy adults, (2) a decreased CBT amplitude of rhythm in older adults and MCI compared to young adults, and (3) a strong CBT phase advances in older adults compared to young adults; d) sketching of melatonin rhythm displays: (1) a decrease in melatonin with increasing age and in MCI compared to healthy groups, and (2) a phase advance in melatonin rhythm in older adults and MCI compared to young adults; e) sketching of cortisol rhythm changes in three groups shows: (1) elevation of morning cortisol in MCI patients compared to older and young healthy adults, and (2) phase advance in cortisol rhythm with increasing age. (Note: The X-axis and Y-axis values are on arbitrary units).
In this article, we reviewed key findings from the extant literature on circadian rhythm changes in normally aging elderly subjects and MCI patients, including the main established differences in circadian patterns between MCI and control groups. We also investigated the association between circadian rhythm disruptions and altered cognitive function in MCI relative to age-matched healthy individuals. Finally, we discussed how this body of evidence may inform prognosis and lead to the development of targeted, timely treatment interventions in MCI aimed at preventing worse clinical outcomes and/or progression towards major neurocognitive disorders.
2. Literature search
To infer circadian rhythm changes in healthy aging compared to mild cognitive impairment, we searched the PubMed and Web of Science databases for the "(MCI OR Mild Cognitive Impairment OR Healthy Brain Aging OR Normal Brain Aging) AND (circadian OR diurnal OR actigraph* OR actimet* OR accelerometer) AND (cognitive function)" terms. After removing duplicates, one author (AK) screened titles and abstracts and, where relevant, the full text of the studies to assess their eligibility. Because we wanted to specifically assess circadian rhythmicity in healthy aging and MCI, sleep parameters were not examined, and studies exclusively assessing these parameters were excluded.
3. Circadian rhythm changes in healthy aging and MCI
Based on the findings from our literature search we decided to report on the four main aspects of circadian rhythm changes: rest-activity rhythm (RAR), core body temperature (CBT), cortisol, and melatonin release in healthy aging and MCI: (Table 1, Figure 1).
Table 1.
Studies of circadian rhythm and neurocognitive changes in patients with MCI and healthy adults.
| Title | Authors (Year) |
Type | Circadian rhythm/Assessment tool |
Diagnosed (Subjects) | Key findings |
|---|---|---|---|---|---|
| The Characterization of Biological Rhythms in Mild Cognitive Impairment | Ortiz-Tudela E. et al., (2014) | Original | RAR, CBT, Melatonin, recording wrist skin temperature, motor activity, body position, and the integrated variable TAP (including temperature, activity, and position) for one week. | 40 subjects; 21 MCI (74.1 ± 1.5 y) and 19 healthy subjects (71.7 ± 1.4 y) |
|
| Rest-Activity Pattern and Circadian Phase Alterations Across the Alzheimer’s Disease Clinical Spectrum | Hyun W. R. et al., (2021) | Review | RAR, actigraphy | Alzheimer’s disease clinical spectrum including MCI |
|
| Circadian disturbances in Alzheimer’s disease progression: a prospective observational cohort study of community-based older adults | Peng L., et al., (2020) | Original | RAR, annual assessments of cognition (with a battery of 21 cognitive performance tests) and motor activities (with actigraphy) | 1401 healthy older adults (81·8 [76·3–85·7] y), prospective observational cohort study (followed up for up to 15 years), |
|
| Sleep-Wake Patterns and Cognition of Older Adults with Amnestic Mild Cognitive Impairment (aMCI): A Comparison with Cognitively Healthy Adults and Moderate Alzheimer's Disease (AD) Patients | Wams E. J. et al., (2017) | Review | RAR, Mini-Mental-State-Examination and five computerized tests (CANTABeclipse™), Jupiter Sleep Questionnaire and Pittsburgh Sleep Quality Index, wrist-worn actigraphy | Cognitively healthy adults, amnestic mild cognitive impairment (aMCI) and moderate AD |
|
| A population-based prospective study on rest-activity rhythm and mild cognitive impairment among Hong Kong healthy community-dwelling older adults | Yi Lee P.M., et al., (2021) | Original | RAR, wrist actigraphy, Montreal Cognitive Assessment (MoCA) | 174 Hong Kong healthy adults aged ≥65 years (36 males vs. 138 females) (Normal cognition [n=123, 74.9 ± 6.8 y], MCI [n=51, 77.5 ± 7.1 y], Followed up them for 12 months |
|
| Circadian Activity Rhythms and Risk of Incident Dementia and Mild Cognitive Impairment in Older Women | Tranah G.S., et al., (2011) | Original | RAR, wrist actigraphy for a minimum of three 24-hour periods. Each participant completed a neuropsychological test battery and had clinical cognitive status (dementia, MCI, normal) assessed by an expert panel approximately 5 years later. | 1,282 healthy community dwelling women (mean age 83 years) |
|
| Associations of actigraphic sleep and circadian rest/activity rhythms with cognition in the early phase of Alzheimer’s disease | Alfini A., et al., (2021) | Original | RAR, standard and novel actigraphic metrics | 179 older individuals (72.6 ± 8.4 y) with normal cognition (n = 153, 71.8±8.3 y) and MCI (n = 26, 77.3 ± 7.9 y) |
|
| Circadian Misalignment and Sleep Disruption in Mild Cognitive Impairment | Naismith S.L., et al., (2014) | Original | RAR, psychiatric, medical, and neuropsychological assessment, followed by overnight polysomnography and dim light melatonin onset assessment, performing episodic memory task | 30 patients with MCI and 28 age-matched controls (> 50 y) |
|
| Multicenter Study on Sleep and Circadian Alterations as Objective Markers of Mild Cognitive Impairment and Alzheimer’s Disease Reveals Sex Differences | Guarnieri B. et al., (2020) | Original | RAR, wearable activity trackers data, actigraphic sleep parameters | 158 subjects (86 females and 72 males), 42 AD (64.83±13.36), 28 MCI (80.57 ± 5.71 y), and 88 controls (75.55 ± 7.89 y) |
|
| Association of Circadian Rhythm with Mild Cognitive Impairment Among Pneumoconiosis Workers in Hong Kong: a Cross-sectional Study | Huang B. et al., (2022) | Original | RAR, A wrist actigraphy for 168 hours, a face-to-face questionnaire containing information on sociodemographic, lifestyle behavior, and anthropometric measurements | 186 male pneumoconiosis patients and 208 age-matched healthy community men (71.3 ± 7.8 y), Cross sectional |
|
| Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy | Kuhlmei A. et al., (2013) | Original | RAR, actigraphy, rating scales for apathy (AES) and depression (Beck Depression Inventory, BDI) | Subjects had a mean ± standard deviation age of 81 ± 7 (Controls [n= 23]: 78 ± 7, subjects with MCI with/without apathy [n=21] 79 ± 4/86 ± 4, participants with dementia [n=32] without/with apathy 80 ± 2/82 ± 4) |
|
| Unique Sleep and Circadian Rhythm Dysfunction Neuroinflammatory and Immune Profiles in Alzheimer's Disease with Mild Cognitive Impairment | Pillai J. A., et al., (2021) | Original | RAR, a wrist accelerometer (Motion logger Micro Watch by Ambulatory Monitoring, Inc®) | MCI-AD subjects (69.5 [min=64, max=79] y), Cohort |
|
| Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study | Cochrane A. et al. (2012) | Original | RAR, a battery of neuropsychological tests and completed sleep diaries and 6 days of actigraphy | 26 healthy community-dwelling older adults (intact [n=16], 71.94 ± 2.53 y, declined[n=10], 70.90 ± 1.5 y) |
|
| The circadian rest-activity pattern predicts cognitive decline among mild-moderate Alzheimer’s disease patients | Targa A. D., et al., (2021) | Original | RAR, use of actigraphy for 14 days, cerebrospinal fluid, 12 months of follow-up, neuropsychological evaluation (MMSE) | The cohort included 100 individuals (mean, 76.0 [73.0; 80.0] median [p25; p75]) y) with mild-moderate AD. |
|
| Rest-activity rhythms and cognitive impairment and dementia in older women: Results from the Women's Health Initiative | Xiao Q. et al., (2022) | Original | RAR, accelerometry-based rest-activity parameters, telephone Interview for Cognitive Status-modified (TICS-m), East Boston Memory Test, Oral Trail Making Test, Verbal Fluency-Animals test, and Digit Span Test | Cognitively unimpaired women ages 65–79 years at baseline, 193 women developed MCI (n=120) or probable dementia (n=73) after average of 4.5 years |
|
| Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: a critically appraised topic | Covell G.E.S. et al., (2012) | Review | RAR, wrist actigraphy | A prospective cohort study of 1282 cognitively normal women [65 years or older, with a mean age of 83 years] |
|
| Association between circadian disruption and diseases: A narrative review | Hou, Y. et al., (2020) | Review | RAR, Melatonin, CBT, Cortisol | Variety of diseases |
|
| The clocks that time us'—circadian rhythms in neurodegenerative disorders | Videnovi A. et al., (2014) | Review | RAR, Melatonin, Cortisol, CBT SCN | Neurodegenerative disorders |
|
| Association between circadian rhythms and neurodegenerative diseases | Leng Y. et al., (2019) | Review | RAR, Melatonin, Cortisol, CBT SCN | MCI, Alzheimer’s disease and related dementias, Parkinson’s disease |
|
| Body Temperature Is Associated with Cognitive Performance in Older Adults with and Without Mild Cognitive Impairment: A Cross-sectional Analysis | Eggenberger P. et al., (2021) | Original | CBT, Skin temperatures at the rib cage and the scapula were measured in the laboratory, Neuropsychological tests | 80 older adults (74.6 ± 6.0 y), 54 participants were cognitively healthy and 26 participants met the criteria for MCI, Cross-sectional study |
|
| Misaligned core body temperature rhythms impact cognitive performance of hospital shift work nurses | Molzof H. E. et al., (2019) | Original | CBT, Mini-International Neuropsychiatry Interview (MINI), Vital Sense monitoring device | Day shift (n = 14) and night shift (n = 14), 31.2 years (with a range of 22 to 56 years) |
|
| Increase in Core Body Temperature of Alzheimer’s Disease Patients as a Possible Indicator of Chronic Neuroinflammation: A Meta-Analysis | Klegeris A. et al., (2007) | Meta-analysis | CBT | Alzheimer’s Disease |
|
| Trough Melatonin Levels Differ between Early and Late Phases of Alzheimer Disease | Lin C.H. et al., (2021) | Original | Melatonin, Clinical Dementia Rating (CDR) and Mini-Mental State Examination (MMSE), Enzyme-linked immunosorbent assay (ELISA) | 270 elder individuals including 73 Healthy controls (66.3 ± 9.0 y), 47 aMCI (66.1 ± 5.0 y), 98 Mild AD (75.2 ± 7.1 y), 52 Moderate to severe AD (82.5 ± 7.0 y), Cohort study |
|
| Plasma 8-isoPGF2α and serum melatonin levels in patients with minimal cognitive impairment and Alzheimer disease | Sirin F. B. et al., (2015) | Original | Melatonin, serum melatonin levels, Mini-mental state examination (MMSE) | AD group (n = 20, 79.05 ± 8.93 y), MCI group (n = 21, 73.95 ± 7.19 y), and control group (n = 22, 71.59 ± 6.65 y) |
|
| Therapeutic application of melatonin in mild cognitive impairment | Cardinali D.P. et al., (2012) | Original | Melatonin, MMSE, cognitive subscale of the Alzheimer’s disease Assessment Scale, neuropsychological battery comprising a Mattis´ test, Digit-symbol test, Trail A and B tasks and the Rey´s verbal test | MCI- Melatonin (n= 61, 69.2 ± 9.03 y), MCI-Non-Melatonin (n= 35, 72.0 ± 9.78 y) |
|
| Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study | Furio A.M. et al., (2007) | Original | Melatonin, MMSE, a battery of neuropsychological tests including Mattis’ test, Digit-symbol test, Trail A and B tasks and the Rey’s verbal test | MCI- Melatonin (n= 25, 72.2 ± 1.02 y), MCI-Non-Melatonin (n= 25, 72.0 ± 0.78 y) |
|
| Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment | Jean-Louis G et al., (1998) | Original | Melatonin, Alzheimer’s Disease Assessment Scale, Digit Span, Digit Symbol Substitution, Finger Tapping, Mini Mental Status, Self-reported sleep-wake disturbances | 10 elderly MCI individuals (68.8 ± 15.8 y) |
|
| Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition | Waller K.L. et al., (2016) | Original | Melatonin, Cortisol, Saliva samples | 24 Cognitively high-functioning men (57.5±0.45 y), 26 cognitively impaired men (57.3±0.45 y) |
|
| Melatonin levels in the Alzheimer’s disease continuum: a systematic review | Nous A. et al., (2021) | Review | Melatonin, CSF, blood, saliva and urine melatonin | Alzheimer’s disease continuum including MCI |
|
| Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia | Csernansky J. G. et al., (2006) | Original | Cortisol, plasma cortisol, continuous cortisol, changes in multiple cognitive tests | AD (n=10, 76 ± 4.6 y) MCI (n=23, 74.2 ± 7.5), Healthy control (n= 21, 77.6 ± 9.7 y), Follow up for 4 years |
|
| Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer's type | Popp J. et al., (2015) | Original | Cortisol, cerebrospinal fluid cortisol, changes in multiple cognitive tests | MCI-AD (n=102, 67.35 ± 7.92 y), MCI-O (n= 45, 66.22 ± 8.16 y), AD (n= 105, 72.95 ± 7.42 y), Healthy control (n= 37, 64.35 ± 8.08 y) |
|
| Lower morning to evening cortisol ratio is associated with cognitive impairment in men but not women: An analysis of 733 older subjects of the cross-sectional KORA-Age study | Johar H et al., (2015) | Original | Cortisol, salivary cortisol level, Cognitive function (determined by telephone interview for cognitive status-modified, TICS-m) | 733 study participants (65-90 years old, mean age=74.9), Probable dementia (n= 33, 77.8 ± 6.8 y), MCI (n=101, 77.5 ± 5.6 y), Healthy (n= 599, 74.4 ± 6.1 y), cross-sectional study |
|
| Increased saliva cortisol awakening response in patients with mild cognitive impairment | Lind K. et al., (2007) | Original | Cortisol, saliva samplings, MMSE | 27 patients with MCI (61 ± 6 y) and 15 healthy controls (69 ± 5 y), case-control study |
|
| Exercise, the diurnal cycle of cortisol and cognitive impairment in older adults | Tortosa-Martínez J. et al., (2018) | Review | Cortisol | - |
|
| Salivary cortisol day profiles in elderly with mild cognitive impairment | Wolf O.T. et al., (2002) | Original | Cortisol, salivary cortisol levels | MCI (n=16, 70.9±2.0 y), young control group (n= 14, 27.0±2.1 y), and normal elderly group (n=28, 68.6±1.2 y) |
|
| Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment | Souza-Talarico J.N. et al., (2010) | Original | Cortisol, brief Cognitive Screening Battery | Controls (n = 40, 72.2 ± 6.3 y), MCI (n = 31, 74.7 ± 5.8 y), and AD (n=40, 80.1 ± 6.0 y) |
|
| Hair cortisol and cognitive performance in healthy older people | Pulopulos M.M. et al., (2014) | Original | Cortisol, hair and diurnal salivary cortisol levels, Trail-making Test A and B, Digit Span Forward and Backward, word list-RAVLT and Stories subtest of the Rivermead | 57 healthy older people (64.75 ± 4.17 y) |
|
| Cortisol hypersecretion and the risk of Alzheimer's disease: A systematic review and meta-analysis | Zheng B. et al., (2020) | Review and Meta-analysis | Cortisol, blood samples cortisol, Saliva cortisol, CSF cortisol | Alzheimer's disease continuum including MCI |
|
| Plasma cortisol levels, brain volumes and cognition in healthy elderly men | MacLullich A.M. et al., (2005) | Original | Cortisol, plasma cortisol levels, cognitive testing and MRI | 97 healthy men aged 65–70 y |
|
| Sex differences in cortisol and memory following acute social stress in amnestic mild cognitive impairment, | Murphy K.J. et al., (2020) | Original | Cortisol, salivary cortisol, tests of episodic, associative, and spatial working memory with psychosocial stressor (TSST) | age-normal cognition (n= 15, 75.3 ± 8.7 y), amnestic MCI (n=16, 74.6 ± 8.0 y) |
|
| Intact circadian rhythm despite cortisol hypersecretion in Alzheimer’s disease: A meta-analysis | Saelzler U.G., et al., (2021) | Meta-analysis | Cortisol, cortisol indices like concentration or circadian indices, Mini Mental Status Examination (MMSE) | Alzheimer's disease continuum including MCI |
|
3.1. Rest activity rhythm (RAR)
Age has a significant effect on the rest-activity rhythm (RAR, also described as the sleep/wake cycle). The shift in preference from eveningness to morningness is consistently associated with age-related circadian rhythm changes.[2-4, 7] Specifically, finding from healthy aging human studies indicate that adults in their 60s and 70s commonly tend to rise from and retire to bed earlier than adults in their 20s and 30s.[2, 3, 45-49] Several studies have also reported that the amplitude of circadian rhythms is progressively blunted in healthy aging.[3, 50, 51] Taken together, weakening amplitude and phase advance in the sleep/wake cycle chronotype appear to be a reliable pattern occurring in healthy aging (Figure 1.b). Compared to healthy, age-matched control groups, MCI patients tend to show a further phase advance in rest-activity, light exposure, and position.[15, 52] Findings also suggest greater wake after sleep onset and increased sleep latency in these patients.[53] Other studies on MCI have highlighted a disruption in circadian rhythm measures, including weakened circadian activity rhythm,[54] significant fragmented and weakened circadian rest-activity/sleep-wake rhythm,[55] a less robust rhythm, lower amplitude, and delayed timing of peak activity based on actigraphy.[56] Patients with MCI have also greater nighttime activity and less activity in the morning compared to aged matched controls (Figure 1.b),[57] although one study found no differences in circadian phase or rest-activity patterns in cognitively intact older adults compared with patients with MCI.[58] Several recent studies have also confirmed that advanced acrophase (representing the rest-activity phase, timing of peak activity) and increased activity fragmentation, as reflected by increased intra-daily variability, are the most consistent alterations in MCI relative to age-matched healthy comparison groups.[23, 35, 59]
3.2. Core body temperature (CBT) rhythm
The regulation of core body temperature (CBT) is one of the most critical functions of the nervous system, which is controlled by the thermoregulatory system. Two types of mechanisms, physiologic and behavioral, regulate body temperature. Physiologic effectors are involuntary and mostly autonomic responses that generate or dissipate heat. Brown adipose tissue (BAT) thermogenesis and skeletal muscle shivering are primary physiologic responses to cold exposure, which generate heat, and the constriction of blood vessels via vasoconstriction to prevent heat loss. Warmth exposure triggers a complementary set of autonomic responses, including suppression of thermogenesis and facilitation of heat loss through water evaporation, that can be achieved via sweating and/or vasodilation.[60] Three other physiological aspects that define the CBT variation include the molecules and cells that measure body temperature in the periphery, the neural pathways that communicate this information to the brain, and the central circuits that coordinate the homeostatic response. Behavioral mechanisms are also involved in body temperature control. Thermoregulatory behaviors are motivated, meaning that they are goal-oriented actions that are learned by reinforcement and driven by the expectation of reward. For example, the most basic thermoregulatory behaviors are cold and warm seeking that regulate human behaviors like wearing clothing or using air-conditioning respectively. The engagement of specific thermoregulatory mechanisms is hierarchical, meaning that different effectors become activated at different temperature thresholds and, in turn, affect the shape (e.g., amplitude and duration, etc.) of the CBT rhythm.[60, 63] In young adults (mid-20s), the peak of the core body temperature rhythm is early in the evening, and the minimum core body temperature (i.e., the trough of the rhythm) occurs early in the morning. The period of the rhythm remains stable in older adults (late 60s), when compared with healthy young subjects under carefully controlled lighting conditions.[64-67] However, in older adults (between 60 to 80 years old), the amplitude of this rhythm decreases by about 20-40%, such that the peak and the trough of the core body temperature do not rise as high and fall as low, respectively, as in young adults (Figure 1.c). Furthermore, compared to younger adults in their 20s-30s, a 1-2 hours phase advance in the rhythm has been observed in older adults in their 60s to 80s.[67, 68] MCI patients also show phase advances in temperature rhythms and rest-activity[52] and higher median body temperature and lower peak-to-trough body temperature compared to healthy older adults (Figure 1. c).[69] Review and meta-analysis studies have confirmed these findings in MCI and AD patients, thus suggesting that core body temperature is a potential marker for MCI and major neurocognitive disorders.[15, 70]
3.3. Melatonin rhythm
Melatonin can directly influence the activity of the suprachiasmatic nucleus (SCN), a key regulator of circadian rhythmicity that is considered the “master circadian pacemaker” or “master clock”.[2, 3, 71-76] Melatonin release helps to promote sleep onset, modulating the activity of intrinsically photosensitive retinal ganglion cells that, in turn, provide timekeeping signals to the SCN and regulate core body temperature. [72, 74, 77] However, the complexity of melatonin's actions/function extends to the multiplicity of target cells and signal transduction mechanisms, which are more diverse than originally believed. In mammals, two melatonin-binding GPCRs exist, MT1 and MT2, while in humans, only MT1 has been discovered and MT2 seems to be absent or expressed only at a very low level. Outside of the SCN, MT1 receptors have been detected in numerous tissues, including the kidney, cerebral arteries, and immune system cells. Moreover, several recent publications have reported melatonin antioxidant actions in numerous tissues that can be activated at physiological melatonin concentrations. Declines in melatonin seem to be, in many cases, the consequence of SCN degeneration, such as tissue destruction in the SCN or in the pineal gland, which leads to reduced melatonin secretion and results in circadian and sleep disturbances. [78] Notably, total melatonin secretion starts decreasing from the third decade of life (Figure 1.d).[71, 72, 74, 79, 80] However, some studies reported that the melatonin rhythm was preserved for healthy older adults, and only the peak amplitude of melatonin was reduced for elderly individuals.[81, 82] This discrepancy (i.e., preserved of the melatonin rhythm in older adults, which however shows a change of the melatonin’s peak amplitude in comparison to younger individuals) suggests that the reduction in melatonin is not a strong feature of the aging process. Several articles have explored melatonin activity in MCI.[15, 23, 29, 52-54, 83-92] Nonetheless, only a handful of these studies reported melatonin changes in MCI compared to the healthy age-matched control group. A couple of these studies reported phase advance in melatonin secretion in patients with MCI relative to healthy comparison subjects (Figure 1.d),[52, 53] although the levels of melatonin secreted did not differ [53] or were not compared across groups.[52] One study comparing serum melatonin levels between patients with AD, MCI, and healthy controls found significantly lower levels in AD compared to MCI patients and no differences between the MCI and the control groups.[93] Another study demonstrated that the trough melatonin levels in the peripheral blood were decreased in the MCI group, but it was elevated in the mild and moderate to severe AD groups.[94] Overall, these inconsistent melatonin findings could be caused by differences in study design and demographic/baseline characteristics of subjects in both studies, including age and severity of cognitive impairment.
3.4. Cortisol rhythm
One of the key hormones in human physiology is cortisol, which targets nearly all cells of the body. Cortisol facilitates the transmission of circadian messages from the SCN to the peripheral tissues. For example, as the body perceives stress, adrenal glands release the hormone cortisol into the bloodstream to regulate the body's stress response. The release rhythm of cortisol is under the control of the SCN. [76, 95] Peak of cortisol after waking can play a specific role in synchronizing the body to both the RAR and light-dark cycle. [96] Furthermore, it is reported that the cortisol circadian rhythm transcribes the message of the time of day to the immune system. Cortisol coordination is essential for having good physical and mental well-being, and disruption in this rhythm is correlated with a variety of negative physiological, psychological, and clinical implications. [96] However, the patterns of cortisol rhythm changes during the lifetime and age-related changes in cortisol rhythmicity include phase advance of the peak of cortisol to earlier in the morning and a decrease in peak amplitude due to higher cortisol secretion at night during healthy aging (Figure 1.e). [97-99] Cortisol rhythm disruptions in older individuals may reflect the progression of neurodegeneration, although not all studies support this assumption.[100-103] An association between MCI and cortisol rhythm changes has been reported by several studies,[15, 21, 102-125] some of which compared MCI patients with control groups.[114, 115, 123, 124] For example, a mild elevation of morning cortisol was observed in cerebrospinal fluids from patients with MCI compared to healthy, age-matched individuals.[114] In contrast, a meta-analysis of five studies showed no difference in blood cortisol levels between MCI patients and cognitively intact individuals.[114] Furthermore, a meta-analysis of ten studies reported no differences in salivary cortisol levels between MCI patients and healthy controls (Figure 1.e), although MCI patients showed a moderate elevation in central cortisol (i.e., CSF) with minimum heterogeneity. [114] Additionally, a cross-sectional study in probable dementia, MCI, and healthy individuals found that lower morning to evening cortisol ratio was associated with cognitive impairment in men, but not women.[126] Another study showed an increased saliva cortisol awakening response in patients with mild cognitive impairment relative to healthy control subjects.[117]
4. Circadian rhythm and gene expression in aging and MCI
In humans, the SCN is the main clock of the circadian system that interacts (i.e., by afferent and efferent connections) with other cerebral and peripheral tissues, which also contain their own autonomous circadian clocks (Figure 2). The interplay and synchronization between SCN inputs and the clocks of other brain and body regions can therefore affect a wide range of functions, including cognition, psychological functions, and behavior. At the molecular level, up to 80% of the mammalian protein-coding genes show circadian rhythmicity in expression levels; a set of clock genes, which regulates the rhythmic expression of the genome also controls these circadian rhythms. The core clock genes include PER1, PER2 and PER3 which encode the period circadian protein homologues, the cryptochromes (i.e., CRY1 and CRY2), as well as CLOCK and ARNTL, also known as BMAL1. These latter genes create a 24-hour synchronized period with a complex, interlocking transcription-translation feedback loop. Other genes and proteins involved in the feedback loop include nuclear receptor subfamily 1 group D members 1 and 2 (NR1D1 and NR1D2), which play an important role in encoding the REV-ERB nuclear receptors, the nuclear receptor ROR-α (RORA), the D site-binding protein (DBP), nuclear receptor ROR-β (RORB), and the nuclear factor interleukin 3-regulated protein (NFIL3).[22] Evidence from major neurodegenerative disorders, including Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington Disease (HD), and animal models of such diseases indicate abnormal expression rhythms of these genes, especially BMAL1 and PER2. Specifically, in Alzheimer’s disease, complex changes in pattern of BMAL1 mRNA expression was observed.[19, 127] In peripheral tissues and several brain regions of AD patients, BMAL1 mRNA expression remains rhythmic, but the temporal phase relationships among these tissues differ compared with healthy control. It was also reported that in the pineal gland, the rhythms of BMAL1, PER1 and CRY1 mRNA are lost in these patients. Furthermore, circadian distributions during MCI are associated with aberrant cycles of DNA methylation in BMAL1.[19, 127]
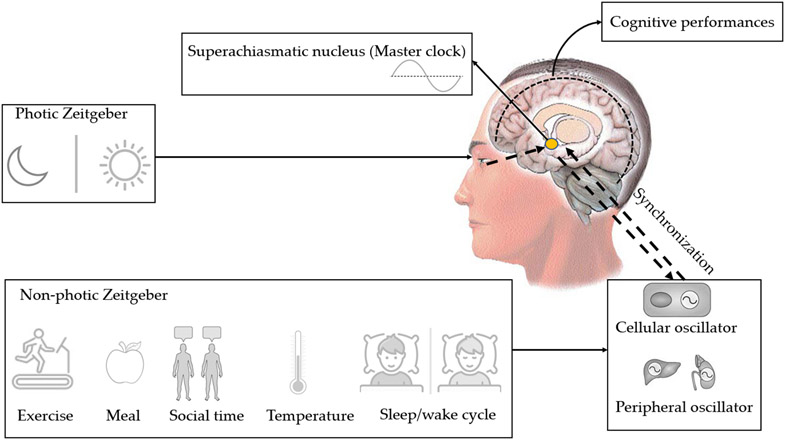
Figure 2.
Organization of human circadian rhythms. The superchiasmatic nucleus (SCN) is the master clock of the circadian rhythms. Photic Zeitgeber and non-photic Zeitgebers synchronize the SCN. All cerebral regions and peripheral tissues have their own internal clocks, where the final synchronization of the rhythms is regulated by SCN via humoral and neural connections.
5. Circadian rhythm and cognitive functioning in healthy aging and MCI
Numerous studies have investigated the effects of circadian rhythmicity on brain cognitive functioning and performance.[8, 128-133] The relationship between circadian rhythms and cognition was examined using primarily cognitive tasks that require considerable top-down executive control,[132] including attention and working memory. Generally, it was reported that circadian rhythm can influence cognitive processes in the brain. Cognitive performance tends to fluctuate throughout the day, likely because of the underlying brain regions that play a role in these cognitive functions (e.g., frontal cortex, dorsolateral prefrontal cortex, hippocampus, anterior medial frontal, and posterior cingulate regions), which have a deep relationship with the fluctuation of the circadian rhythm.[132, 134] However, not all cognitive functions are equally affected. For example, worse performances in attention and working memory has been reported in the morning, while better performances were observed in the afternoon.[8, 132] It is also important to point out that cognitive functions are affected by the level of arousal.[132, 136] Age-related decline in working, declarative, spatial, and other forms of memory is well documented.[137-139]. Of note, an in-depth characterization of the effects of circadian rhythms on the different domains of cognition in aging, although important, is beyond the scope of this review that focuses on changes in circadian rhythms in healthy aging and MCI. We do think, however, that it is important to present and discuss how alterations in these four main circadian rhythms affect cognitive function in MCI vs. control by specifying, whenever possible, the cognitive domain affected. Thus, in what follows we discuss findings relative to cognitive dysfunctions and changes in these circadian rhythms in patients with MCI.
5.1. RAR and cognitive functioning
Compared to healthy aging, in MCI patients daytime activity was reported to be negatively correlated with memory deficits,[140] and altered circadian RARs measures have been found to be associated with worse cognitive performance in these patients.[57] Another study suggested that very subtle changes in circadian rhythm are detected in older adults (e.g., amplitude change, mean activity level around which the rhythm oscillates (MESOR), or percentage of variance in activity explained by the 24h cosine wave) that are related to preclinical changes in cognitive performance,[141] while a prospective study confirmed significant changes between delayed acrophase were associated with worse cognition in Hong Kong healthy community-dwelling older adults.[58] Furthermore, a couple of recent studies showed that the reduced amplitude and overall rhythmicity of RAR were associated with adverse cognitive outcomes and that individuals with RAR alterations exhibited a faster cognitive decline during follow-up assessments compared with subjects with intact RAR.[34, 142]
5.2. CBT rhythm and cognitive functioning
Despite a dearth of work examining the association between neurocognitive decline and core body temperature, one study reported that misaligned core body temperature rhythms have a negative impact on the cognitive performance of hospital shift work nurses at the end of their shift.[143] Furthermore, a cognitively healthy control group showed lower median body temperature and higher peak-to-trough amplitude temperature compared to MCI individuals.[69] Overall, these studies demonstrated that higher peak-to-trough body temperature amplitude and lower median body temperature tend to be associated with better cognitive performance.
5.3. Melatonin rhythm and cognitive functioning
Misalignment in melatonin rhythm (i.e., dim light melatonin onset) has shown different effects on cognition in MCI patients and aged-matched control. For example, earlier dim light melatonin onset was associated with poorer memory performance in MCI patients, but not in age matched healthy controls. [53] One study evaluating the trough melatonin levels in early (i.e., MCI stage) and late phases of Alzheimer's reported that melatonin levels in the peripheral blood decreased in MCI, while they increased with AD severity.[53] Review, meta-analysis, and retrospective studies have shown that melatonin administration in patients with MCI but not with AD had positive effects on cognitive status.[87, 125, 144, 145] Other studies showed that melatonin could safely improve some cognitive aspects of sleep, memory, and mood in the elderly following short-term use.[83] Furthermore, positive correlations between MMSE score and melatonin levels suggest a protective effect on cognition, [93] although additional studies are needed to fully elucidate the effects of this hormone levels on cognitive function. However, additional studies with melatonin administration in patients with MCI, or even in patients with preclinical AD, accounting for pre-existing sleep disturbances, are needed.
5.4. Cortisol rhythm and cognitive functioning
Several studies have shown that cortisol plays a critical role in regulating cognition in both healthy aging and MCI individuals.[103, 108, 111, 112, 146] One study reported that visual recognition memory deficits were associated with atypical high evening salivary cortisol levels in healthy subjects,[147] while another study concluded that higher levels of plasma cortisol were associated with worse age-related cognitive changes, but with no age-related brain volume atrophy.[148] Regarding sex covariant differences, it has been reported that high cortisol predicted an impaired performance on episodic memory only in amnestic MCI males.[149] Furthermore, a study examining the association between cortisol levels and visual memory performance in individuals presenting mild MCI or AD compared to healthy control found that higher levels of cortisol were associated with better memory performance in the control group, while higher cortisol levels were correlated with poorer memory performance in MCI patients, but not in the AD group.[112] The effects of the diurnal cycle of cortisol on cognitive function and physical activity in older adults with and without cognitive impairment have been more extensively reported in a recent review:[108] available evidence from this work supports the notion that cognitive improvement is associated with exercise, which could be partially mediated by changes in the diurnal cortisol secretion pattern and/or its related dynamic profile. Other meta-analysis and review studies have suggested that higher morning cortisol accelerates cognitive decline in mild AD or MCI patients, but the results in cognitively healthy adults were inconsistent.[114, 115]
6. Discussion
In this review, we explored four aspects of circadian rhythm disruption: RARs, core body temperature, melatonin, and cortisol rhythms in normal aging and the preclinical/prodromal stage of Alzheimer’s disease (i.e., patients with MCI). Overall, behavioral circadian rhythm disruption parameters (e.g., RAR) have been examined more than biological measures of circadian rhythmicity, such as melatonin or cortisol secretion rhythms and core body temperature. Circadian rhythm disruption is consistently present in MCI patients and often presented with earlier, more severe disruptions than those typically observed in normal aging.[6, 9, 15, 22, 25] Unlike healthy older adults, who usually have reduced circadian amplitude and advanced circadian phase, patients with MCI tend to have circadian rhythms that are less robust, more fragmented, and severely reduced in amplitude (Figure 1. b, c, d, and e).
6.1. Intervention strategies on circadian rhythms and their relevance to cognition
Based on aforementioned findings, in what follows we will discuss preliminary but promising treatment interventions aimed at restoring regular circadian rhythms that may prevent or halt the progress of neurodegenerative diseases in MCI individuals as well as mitigate their related cognitive deficits.
Bright light therapy (BLT) has been found to be an efficient method to improve the main parameters of circadian rhythms in mild/moderate cognitive impairment. For example, ninety minutes of BLT (for five days) appeared to achieve a significant improvement in cognitive factors (general cognitive capabilities), circadian rhythms, and general health.[150] BLT is an affordable, effective, fast-acting therapy for age-related disturbances, with many advantages over pharmacological alternatives, and a recent review exploring the connections between circadian sleep disorders, cognition, and neurodegenerative disease showed that intermittent light stimuli improved sleep and cognition in patients with AD and MCI.[151] However, some studies have not confirmed the positive effects of bright light on cognition (e.g., in patients with diagnosed dementia, sleep disruption, and agitated behavior).[15, 152] This discrepancy may be related to differences in BLT parameters, such as exposure duration and intensity of light, which are important for older adults who have reduced circadian system response to light.[15, 22, 150, 153]
Melatonin therapy, used as an add-on treatment, has beneficial effects on MCI and Alzheimer's patients with sleep disorders by improving their sleep quality and their RARs.[154] A recent review study has proposed that melatonin should be prescribed as early as possible and for long periods of time, at a dose between 2 and 10 mg.[154] This review also reported that melatonin may have a beneficial effect on cognitive function in MCI, but showed no effects in moderate to severe Alzheimer's disease.[154] Another study proposing a higher dose of melatonin (i.e., 25 mg) showed that this compound could act as an antioxidant in the MCI stage to reduce progression to dementia.[29] Overall, these studies indicate that melatonin has positive effects in MCI patients[29, 83, 154, 155] while also reporting no significant side effects.[154, 155]
Regular physical activity, which is associated with cortical rhythm, has been proposed as a strategy to mitigate disruptions of circadian rhythms in normal older adults and patients with MCI, which in turn could reduce/halt their cognitive decline.[106, 108, 120, 156-160] Consistent with this assumption, physical activity and exercise has been found to enhance memory formation and memory consolidation in patients with MCI.[160] A review in older adults with and without cognitive impairment reported that exercise could reduce the risk of developing MCI and AD as well as improve cognition in both healthy and cognitively impaired individuals, possibly through the regulation of the diurnal cycle of cortisol.[120] Another study showed that physical, as well as cognitive activity, had a protective effect on cognitive impairment, even when one or the other was lowly engaged.[158] Furthermore, increasing the overall physical amount by decreasing sedentary helped prevent older adults from developing dementia.[159] Again, the observations that physical activity has protective effects on cognitive decline and cognitive status may differ depending on the goal and type of physical activity intervention in healthy aging, MCI, and AD individuals.[161] In this regard, objective measurements have confirmed that greater levels of physical activity were associated with decreased risk of a future diagnosis of MCI or AD; [157] additionally, findings in MCI patients have shown that, compared with a control condition, exercise interventions successfully increased fitness and resulted in a greater fall in cortisol concentration from peak to midday while also enhancing indices of executive function. [157]
6.2. Limitations of existing research and future directions
Several questions and remaining issues should be addressed before these promising findings may be applied in the clinical practice. For example, we should take into consideration the large between-individual differences (i.e., differing chronotypes) and other non-photic zeitgebers like exercise, food, and caffeine consumption that affect the peripheral clocks and their feedback to the central circadian clock (Figure 2).[15, 162-165] Also, despite underlying pathways, including immune and inflammatory function and alterations of protein homeostasis, have been suggested, the mechanistic connections between the progress of neurodegeneration and altered circadian rhythms is still not fully understood .[15] Currently, most of the evidence linking brain disorders and circadian dysfunction is only correlational; thus, whether and what type of causal relationships may exist between circadian rhythm changes and cognitive decline remain undetermined.[9] Design future research toward an in-depth understanding of the links between circadian disruption and MCI by focusing on the interaction between biological rhythms and cognitive assessments (behavioral aspects) should therefore be undertaken. It would also be important to combine circadian rhythm with neuroimaging assessments, including structural positron emission tomography (PET) and/or magnetic resonance imaging (MRI) scans, to further clarify the contribution of circadian rhythm disruption to functional/anatomical changes in the brain. This work will help establish whether circadian rhythm disruption may represent a biomarker for mild cognitive impairment. Additionally, longitudinal studies with long-term follow-up periods are needed to confirm the effects of circadian rhythm disruption on subsequent cognitive decline and the risk of developing Alzheimer’s disease and related dementias in asymptomatic and MCI stages. Relatedly, understanding the relationship and directionality between circadian rhythm alterations and neurodegenerative disorders will help to draw causal inferences and choose suitable therapeutic strategies in the early stage of these disorders. Eventually, it would be important to employ interventions strengthening circadian rhythmicity at the MCI stage and see whether this can help prevent the progression to AD and related major neurodegenerative diseases.
7. Conclusion
In this article, we examined circadian changes for RAR, CBT, melatonin, and cortisol rhythms and their associations with cognitive function in patients with MCI compared to healthy adults. There is evidence from the reviewed literature that circadian changes are present in the early stage of neurodegenerative diseases. However, future longitudinal studies are needed to further investigate these alterations and to clarify their relationships with cognition, which in turn may lead to novel, early treatment interventions for patients affected by neurocognitive and neurodegenerative disorders.
8. Acknowledgment
This research was funded by the National Institute of Mental Health (NIMH), grant number R01 MH113827, awarded to Fabio Ferrarelli.
Biography

Dr. Ferrarelli earned his MD and Ph.D. in psychiatry at the Catholic University of the Sacred Heart in Rome. At the University of Wisconsin, and while completing his residency at Western Psychiatric Institute and Clinic, he studied the alterations in neuronal circuits contributing to altered sleep architecture in schizophrenia. The primary research interests of his lab focus on the utilization of hd-EEG, MRI, actigraphy, and Transcranial Magnetic Stimulation (TMS) to better understand neurological disorders and to identify potential treatment targets.
9. References
- [1].Suzman R and Beard J, "Global health and aging," NIH Publ, vol. 1, no. 4, pp. 273–277, 2011. [Google Scholar]
- [2].Hood S and Amir S, "The aging clock: circadian rhythms and later life," The Journal of clinical investigation, vol. 127, no. 2, pp. 437–446, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duffy JF, Zitting K-M, and Chinoy ED, "Aging and circadian rhythms," Sleep medicine clinics, vol. 10, no. 4, pp. 423–434, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mattis J and Sehgal A, "Circadian rhythms, sleep, and disorders of aging," Trends in Endocrinology & Metabolism, vol. 27, no. 4, pp. 192–203, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Froy O, "Circadian rhythms, aging, and life span in mammals," Physiology, vol. 26, no. 4, pp. 225–235, 2011. [DOI] [PubMed] [Google Scholar]
- [6].Stallings DT, Lach HW, and Lorenz RA, "Circadian rhythm and quality of life in older adults," Applied Nursing Research, p. 151457, 2021. [DOI] [PubMed] [Google Scholar]
- [7].Garbarino S, Lanteri P, Prada V, Falkenstein M, and Sannita WG, "Circadian rhythms, sleep, and aging," Journal of Psychophysiology, vol. 35, no. 3, p. 129, 2021. [Google Scholar]
- [8].Valdez P, "Focus: Attention Science: Circadian Rhythms in Attention," The Yale journal of biology and medicine, vol. 92, no. 1, p. 81, 2019. [PMC free article] [PubMed] [Google Scholar]
- [9].Logan RW and McClung CA, "Rhythms of life: circadian disruption and brain disorders across the lifespan," Nature Reviews Neuroscience, vol. 20, no. 1, pp. 49–65, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Froy O, "Circadian rhythms, nutrition and implications for longevity in urban environments," Proceedings of the Nutrition Society, vol. 77, no. 3, pp. 216–222, 2018. [DOI] [PubMed] [Google Scholar]
- [11].Zhao J, Warman GR, and Cheeseman JF, "The functional changes of the circadian system organization in aging," Ageing Research Reviews, vol. 52, pp. 64–71, 2019. [DOI] [PubMed] [Google Scholar]
- [12].De Nobrega AK and Lyons LC, "Aging and the clock: Perspective from flies to humans," European Journal of Neuroscience, vol. 51, no. 1, pp. 454–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Farhud D and Aryan Z, "Circadian rhythm, lifestyle and health: a narrative review," Iranian journal of public health, vol. 47, no. 8, p. 1068, 2018. [PMC free article] [PubMed] [Google Scholar]
- [14].Reddy S, Reddy V, and Sharma S, "Physiology, circadian rhythm," 2018. [PubMed] [Google Scholar]
- [15].Leng Y, Musiek ES, Hu K, Cappuccio FP, and Yaffe K, "Association between circadian rhythms and neurodegenerative diseases," The Lancet Neurology, vol. 18, no. 3, pp. 307–318, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wulff K, Gatti S, Wettstein JG, and Foster RG, "Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease," Nature Reviews Neuroscience, vol. 11, no. 8, pp. 589–599, 2010. [DOI] [PubMed] [Google Scholar]
- [17].Steele TA, St Louis EK, Videnovic A, and Auger RR, "Circadian rhythm sleep–wake disorders: a contemporary review of neurobiology, treatment, and dysregulation in neurodegenerative disease," Neurotherapeutics, vol. 18, no. 1, pp. 53–74, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hastings MH and Goedert M, "Circadian clocks and neurodegenerative diseases: time to aggregate?," Current opinion in neurobiology, vol. 23, no. 5, pp. 880–887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hood S and Amir S, "Neurodegeneration and the circadian clock," Frontiers in aging neuroscience, vol. 9, p. 170, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Musiek ES, "Circadian clock disruption in neurodegenerative diseases: cause and effect?," Frontiers in pharmacology, vol. 6, p. 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Colwell CS, "Defining circadian disruption in neurodegenerative disorders," Journal of Clinical Investigation, vol. 131, no. 19, p. e148288, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nassan M and Videnovic A, "Circadian rhythms in neurodegenerative disorders," Nature Reviews Neurology, vol. 18, no. 1, pp. 7–24, 2022. [DOI] [PubMed] [Google Scholar]
- [23].Videnovic A, Lazar AS, Barker RA, and Overeem S, "'The clocks that time us'—circadian rhythms in neurodegenerative disorders," Nature Reviews Neurology, vol. 10, no. 12, pp. 683–693, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fifel K and Videnovic A, "Circadian and sleep dysfunctions in neurodegenerative disorders—An update," Frontiers in Neuroscience, vol. 14, p. 627330, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Werdann M and Zhang Y, "Circadian rhythm and neurodegenerative disorders," Brain Science Advances, vol. 6, no. 2, pp. 71–80, 2020. [Google Scholar]
- [26].Hou Y, Liu L, Chen X, Li Q, and Li J, "Association between circadian disruption and diseases: A narrative review," Life Sciences, vol. 262, p. 118512, 2020. [DOI] [PubMed] [Google Scholar]
- [27].Simpson C, "The Relationship Between Circadian Rhythms and Neurodegenerative Disease," 2022. [Google Scholar]
- [28].Xie Y et al. , "New insights into the circadian rhythm and its related diseases," Frontiers in physiology, p. 682, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schrire ZM et al. , "Feasibility of 3-month melatonin supplementation for brain oxidative stress and sleep in mild cognitive impairment: protocol for a randomised, placebo-controlled study," BMJ open, vol. 11, no. 2, p. e041500, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jessen F et al. , "AD dementia risk in late MCI, in early MCI, and in subjective memory impairment," Alzheimer's & Dementia, vol. 10, no. 1, pp. 76–83, 2014. [DOI] [PubMed] [Google Scholar]
- [31].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, and Kokmen E, "Mild cognitive impairment: clinical characterization and outcome," Archives of neurology, vol. 56, no. 3, pp. 303–308, 1999. [DOI] [PubMed] [Google Scholar]
- [32].Sachs-Ericsson N and Blazer DG, "The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment," Aging & mental health, vol. 19, no. 1, pp. 2–12, 2015. [DOI] [PubMed] [Google Scholar]
- [33].Guarnieri B et al. , "Multicenter study on sleep and circadian alterations as objective markers of mild cognitive impairment and Alzheimer’s disease reveals sex differences," Journal of Alzheimer's Disease, vol. 78, no. 4, pp. 1707–1719, 2020. [DOI] [PubMed] [Google Scholar]
- [34].Targa AD et al. , "The circadian rest-activity pattern predicts cognitive decline among mild-moderate Alzheimer’s disease patients," Alzheimer's research & therapy, vol. 13, no. 1, pp. 1–10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li P et al. , "Circadian disturbances in Alzheimer's disease progression: a prospective observational cohort study of community-based older adults," The Lancet Healthy Longevity, vol. 1, no. 3, pp. e96–e105, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Smith GE and Bondi MW, Mild cognitive impairment and dementia: Definitions, diagnosis, and treatment. Oxford University Press, 2013. [Google Scholar]
- [37].Petersen RC, "Mild cognitive impairment as a diagnostic entity," Journal of internal medicine, vol. 256, no. 3, pp. 183–194, 2004. [DOI] [PubMed] [Google Scholar]
- [38].Lu Y et al. , "Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: a meta-analysis and systematic review," BMC geriatrics, vol. 21, no. 1, pp. 1–16, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu C, Yu D, Sun X, Zhang M, Wang L, and Qin H, "The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis," International psychogeriatrics, vol. 29, no. 10, pp. 1595–1608, 2017. [DOI] [PubMed] [Google Scholar]
- [40].Sachdev PS et al. , "The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration," PloS one, vol. 10, no. 11, p. e0142388, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Buchhave P, Minthon L, Zetterberg H, Wallin ÅK, Blennow K, and Hansson O, "Cerebrospinal fluid levels ofβ-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia," Archives of general psychiatry, vol. 69, no. 1, pp. 98–106, 2012. [DOI] [PubMed] [Google Scholar]
- [42].Walsh CM et al. , "Weaker circadian activity rhythms are associated with poorer executive function in older women," Sleep, vol. 37, no. 12, pp. 2009–2016, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tranah GJ et al. , "Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women," Annals of neurology, vol. 70, no. 5, pp. 722–732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rogers-Soeder TS et al. , "Rest-activity rhythms and cognitive decline in older men: the osteoporotic fractures in men sleep study," Journal of the American Geriatrics Society, vol. 66, no. 11, pp. 2136–2143, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Roenneberg T, Wirz-Justice A, and Merrow M, "Life between clocks: daily temporal patterns of human chronotypes," Journal of biological rhythms, vol. 18, no. 1, pp. 80–90, 2003. [DOI] [PubMed] [Google Scholar]
- [46].Carrier J, Monk TH, Buysse DJ, and Kupfer DJ, "Sleep and morningness-eveningness in the ‘middle’years of life (20–59y)," Journal of sleep research, vol. 6, no. 4, pp. 230–237, 1997. [DOI] [PubMed] [Google Scholar]
- [47].Gordijn M and Merrow M, "Epidemiology of the human circadian clock," Sleep Med Rev, vol. 11, p. 429438Romejin, 2007. [DOI] [PubMed] [Google Scholar]
- [48].Yoon C, May CP, and Hasher L, "Aging, circadian arousal patterns, and cognition," in Cognition, aging and self-reports: Psychology Press, 1998, pp. 113–136. [Google Scholar]
- [49].Broms U et al. , "Long-term consistency of diurnal-type preferences among men," Chronobiology international, vol. 31, no. 2, pp. 182–188, 2014. [DOI] [PubMed] [Google Scholar]
- [50].Hofman MA and Swaab DF, "Living by the clock: the circadian pacemaker in older people," Ageing research reviews, vol. 5, no. 1, pp. 33–51, 2006. [DOI] [PubMed] [Google Scholar]
- [51].Popa-Wagner A, Buga A-M, Dumitrascu DI, Uzoni A, Thome J, and Coogan AN, "How does healthy aging impact on the circadian clock?," Journal of Neural Transmission, vol. 124, no. 1, pp. 89–97, 2017. [DOI] [PubMed] [Google Scholar]
- [52].Ortiz-Tudela E et al. , "The characterization of biological rhythms in mild cognitive impairment," BioMed Research International, vol. 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Naismith SL et al. , "Circadian misalignment and sleep disruption in mild cognitive impairment," Journal of Alzheimer's Disease, vol. 38, no. 4, pp. 857–866, 2014. [DOI] [PubMed] [Google Scholar]
- [54].Huang B et al. , "Association of Circadian Rhythm With Mild Cognitive Impairment Among Pneumoconiosis Workers in Hong Kong: a Cross-sectional Study," 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang Q, XU F, and XU N, "Studies on circadian rest-activity and sleep-wake rhythm patterns in patients with mild cognitive impairment," Medical Journal of Chinese People's Liberation Army, 2001. [Google Scholar]
- [56].Covell GES et al. , "Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: a critically appraised topic," The neurologist, vol. 18, no. 6, pp. 426–429, 2012. [DOI] [PubMed] [Google Scholar]
- [57].Alfini A et al. , "Associations of actigraphic sleep and circadian rest/activity rhythms with cognition in the early phase of Alzheimer’s disease," Sleep Advances, vol. 2, no. 1, p. zpab007, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee PMY, Kwok BHL, Ma JYT, and Tse LA, "A population-based prospective study on rest-activity rhythm and mild cognitive impairment among Hong Kong healthy community-dwelling older adults," Neurobiology of Sleep and Circadian Rhythms, vol. 10, p. 100065, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Roh HW and Son SJ, "Rest-Activity Pattern and Circadian Phase Alterations Across the Alzheimer’s Disease Clinical Spectrum," Chronobiology in Medicine, vol. 3, no. 4, pp. 137–141, 2021. [Google Scholar]
- [60].Tan CL and Knight ZA, "Regulation of body temperature by the nervous system," Neuron, vol. 98, no. 1, pp. 31–48, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jessen C, "Thermal afferents in the control of body temperature," Pharmacology & therapeutics, vol. 28, no. 1, pp. 107–134, 1985. [DOI] [PubMed] [Google Scholar]
- [62].Romanovsky AA et al. , "The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not," Pharmacological reviews, vol. 61, no. 3, pp. 228–261, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Terrien J, Perret M, and Aujard F, "Behavioral thermoregulation in mammals: a review," Frontiers in Bioscience-Landmark, vol. 16, no. 4, pp. 1428–1444, 2011. [DOI] [PubMed] [Google Scholar]
- [64].Refinetti R and Menaker M, "The circadian rhythm of body temperature," Physiology & behavior, vol. 51, no. 3, pp. 613–637, 1992. [DOI] [PubMed] [Google Scholar]
- [65].Dijk D-J, Duffy JF, and Czeisler CA, "Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep," Chronobiology international, vol. 17, no. 3, pp. 285–311, 2000. [DOI] [PubMed] [Google Scholar]
- [66].Czeisler CA et al. , "Stability, precision, and near-24-hour period of the human circadian pacemaker," Science, vol. 284, no. 5423, pp. 2177–2181, 1999. [DOI] [PubMed] [Google Scholar]
- [67].Czeisler CA et al. , "Association of sleep-wake habits in older people with changes in output of circadian pacemaker," The lancet, vol. 340, no. 8825, pp. 933–936, 1992. [DOI] [PubMed] [Google Scholar]
- [68].Duffy JF, Dijk D-J, Klerman EB, and Czeisler CA, "Later endogenous circadian temperature nadir relative to an earlier wake time in older people," American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, vol. 275, no. 5, pp. R1478–R1487, 1998. [DOI] [PubMed] [Google Scholar]
- [69].Eggenberger P, Bürgisser M, Rossi RM, and Annaheim S, "Body temperature is associated with cognitive performance in older adults with and without mild cognitive impairment: a cross-sectional analysis," Frontiers in Aging Neuroscience, vol. 13, p. 585904, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Klegeris A, Schulzer M, Harper DG, and McGeer PL, "Increase in core body temperature of Alzheimer’s disease patients as a possible indicator of chronic neuroinflammation: a meta-analysis," Gerontology, vol. 53, no. 1, pp. 7–11, 2007. [DOI] [PubMed] [Google Scholar]
- [71].Kennaway DJ and Wright H, "Melatonin and circadian rhythms," Current topics in medicinal chemistry, vol. 2, no. 2, pp. 199–209, 2002. [DOI] [PubMed] [Google Scholar]
- [72].Arendt J, "Melatonin and human rhythms," Chronobiology international, vol. 23, no. 1-2, pp. 21–37, 2006. [DOI] [PubMed] [Google Scholar]
- [73].Touitou Y, "Human aging and melatonin. Clinical relevance," Experimental Gerontology, vol. 36, no. 7, pp. 1083–1100, 2001. [DOI] [PubMed] [Google Scholar]
- [74].Cardinali DP, "Melatonin and healthy aging," Vitamins and Hormones, vol. 115, pp. 67–88, 2021. [DOI] [PubMed] [Google Scholar]
- [75].Gursoy AY, Kiseli M, and Caglar G, "Melatonin in aging women," Climacteric, vol. 18, no. 6, pp. 790–796, 2015. [DOI] [PubMed] [Google Scholar]
- [76].Heaney JL, Phillips AC, and Carroll D, "Aging, health behaviors, and the diurnal rhythm and awakening response of salivary cortisol," Experimental aging research, vol. 38, no. 3, pp. 295–314, 2012. [DOI] [PubMed] [Google Scholar]
- [77].Pack W, Hill D, and Wong KY, "Melatonin modulates M4-type ganglion-cell photoreceptors," Neuroscience, vol. 303, pp. 178–188, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hardeland R, "Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction," The Scientific World Journal, vol. 2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kennaway DJ, Lushington K, Dawson D, Lack L, Van Den Heuvel C, and Rogers N, "Urinary 6-sulfatoxymelatonin excretion and aging: new results and a critical review of the literature," Journal of pineal research, vol. 27, no. 4, pp. 210–220, 1999. [DOI] [PubMed] [Google Scholar]
- [80].Zhao Z-Y, Xie Y, Fu Y-R, Bogdan A, and Touitou Y, "Aging and the circadian rhythm of melatonin: a cross-sectional study of Chinese subjects 30–110 yr of age," Chronobiology international, vol. 19, no. 6, pp. 1171–1182, 2002. [DOI] [PubMed] [Google Scholar]
- [81].Zeitzer JM et al. , "Do plasma melatonin concentrations decline with age?," The American journal of medicine, vol. 107, no. 5, pp. 432–436, 1999. [DOI] [PubMed] [Google Scholar]
- [82].Ng Ying Kin N, Nair N, Schwartz G, Thavundayil J, and Annable L, "Secretion of melatonin in healthy elderly subjects: a longitudinal study," Annals of the New York Academy of Sciences, vol. 1019, no. 1, pp. 326–329, 2004. [DOI] [PubMed] [Google Scholar]
- [83].Jean-Louis G, von Gizycki H, and Zizi F, "Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment," Journal of pineal research, vol. 25, no. 3, pp. 177–183, 1998. [DOI] [PubMed] [Google Scholar]
- [84].Cardinali DP, "Melatonin: clinical perspectives in neurodegeneration," Frontiers in endocrinology, vol. 10, p. 480, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang J, Lu J, Zhu H, Zhou X, Wei X, and Gu M, "Association of Serum Melatonin Level with Mild Cognitive Impairment in Type 2 Diabetic Patients: A Cross-Sectional Study," International Journal of Endocrinology, vol. 2021, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gr JK, "Melatonin: therapeutic intervention in mild cognitive impairment and Alzheimer disease," Journal of Neurology & Neurophysiology, vol. 4, no. 2, pp. 1–6, 2013. [Google Scholar]
- [87].Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, and Brusco LI, "Therapeutic application of melatonin in mild cognitive impairment," American journal of neurodegenerative disease, vol. 1, no. 3, p. 280, 2012. [PMC free article] [PubMed] [Google Scholar]
- [88].Onaolapo OJ and Onaolapo AY, "Melatonin and major neurocognitive disorders: beyond the management of sleep and circadian rhythm dysfunction," Sleep Hypn, vol. 21, no. 1, pp. 73–96, 2018. [Google Scholar]
- [89].Sroykham W and Wongsawat Y, "Correlation of morning salivary cortisol-melatonin ratio with qeeg and delayed recall in aging," ACTA NEUROPSYCHOLOGICA, 16 (2), pp. 177–188, 2018. [Google Scholar]
- [90].Cardinali DP and Karasek M, "Melatonin, aging, and Alzheimer’s disease," Principles and practice of geriatric sleep medicine, pp. 97–107, 2010. [Google Scholar]
- [91].Falck RS et al. , "Buying time: a proof-of-concept randomized controlled trial to improve sleep quality and cognitive function among older adults with mild cognitive impairment," Trials, vol. 19, no. 1, pp. 1–9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wu Y-H et al. , "Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages," The Journal of clinical endocrinology & metabolism, vol. 88, no. 12, pp. 5898–5906, 2003. [DOI] [PubMed] [Google Scholar]
- [93].ŞİRİN FB et al. , "Plasma 8-isoPGF2? and serum melatonin levels in patients with minimal cognitive impairment and Alzheimer disease," Turkish Journal of Medical Sciences, vol. 45, no. 5, pp. 1073–1077, 2015. [DOI] [PubMed] [Google Scholar]
- [94].Lin C-H, Chiu C-C, and Lane H-Y, "Trough melatonin levels differ between early and late phases of Alzheimer disease," Clinical Psychopharmacology and Neuroscience, vol. 19, no. 1, p. 135, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Oster H et al. , "The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock," Cell metabolism, vol. 4, no. 2, pp. 163–173, 2006. [DOI] [PubMed] [Google Scholar]
- [96].Mohd Azmi NAS et al. , "Cortisol on circadian rhythm and its effect on cardiovascular system," International journal of environmental research and public health, vol. 18, no. 2, p. 676, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Touitou Y et al. , "Adrenal circadian system in young and elderly human subjects: a comparative study," Journal of Endocrinology, vol. 93, no. 2, pp. 201–210, 1982. [DOI] [PubMed] [Google Scholar]
- [98].Van Cauter E, Leproult R, and Kupfer DJ, "Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol," The Journal of Clinical Endocrinology & Metabolism, vol. 81, no. 7, pp. 2468–2473, 1996. [DOI] [PubMed] [Google Scholar]
- [99].Sherman B, WYSHAM W, and PFOH B, "Age-related changes in the circadian rhythm of plasma cortisol in man," The Journal of Clinical Endocrinology & Metabolism, vol. 61, no. 3, pp. 439–443, 1985. [DOI] [PubMed] [Google Scholar]
- [100].Breen DP et al. , "Sleep and circadian rhythm regulation in early Parkinson disease," JAMA neurology, vol. 71, no. 5, pp. 589–595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, and Heuser I, "Twenty-four hour cortisol release profiles in patients with Alzheimer’s and Parkinson’s disease compared to normal controls: ultradian secretory pulsatility and diurnal variation," Neurobiology of aging, vol. 18, no. 3, pp. 285–289, 1997. [DOI] [PubMed] [Google Scholar]
- [102].Hatfield CF, Herbert J, Van Someren EJ, Hodges J, and Hastings M, "Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia," Brain, vol. 127, no. 5, pp. 1061–1074, 2004. [DOI] [PubMed] [Google Scholar]
- [103].Waller KL et al. , "Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition," Nature and science of sleep, vol. 8, p. 47, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Arsenault-Lapierre G, Lupien S, and Chertkow H, "P1-152: Cortisol levels are increased in mild cognitive impairment and Alzheimer's disease compared to normal elderly subjects when effects of season are taken into account," Alzheimer's & Dementia, vol. 4, pp. T251–T251, 2008. [Google Scholar]
- [105].Basta M et al. , "Basal Cortisol Levels Are Increased in Patients with Mild Cognitive Impairment: Role of Insomnia and Short Sleep Duration," Journal of Alzheimer's Disease, no. Preprint, pp. 1–12, 2022. [DOI] [PubMed] [Google Scholar]
- [106].Dijckmans B et al. , "Does the diurnal cycle of cortisol explain the relationship between physical performance and cognitive function in older adults?," European Review of Aging and Physical Activity, vol. 14, no. 1, pp. 1–10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lara VP et al. , "High cortisol levels are associated with cognitive impairment no-dementia (CIND) and dementia," Clinica chimica acta, vol. 423, pp. 18–22, 2013. [DOI] [PubMed] [Google Scholar]
- [108].Tortosa-Martínez J, Manchado C, Cortell-Tormo JM, and Chulvi-Medrano I, "Exercise, the diurnal cycle of cortisol and cognitive impairment in older adults," Neurobiology of stress, vol. 9, pp. 40–47, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ouanes S and Popp J, "High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature," Frontiers in aging neuroscience, vol. 11, p. 43, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ho RT et al. , "Diurnal cortisol slope mediates the association between affect and memory retrieval in older adults with mild cognitive impairment: a path-analytical study," Frontiers in Aging Neuroscience, vol. 12, p. 35, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wolf OT, Convit A, Thorn E, and de Leon MJ, "Salivary cortisol day profiles in elderly with mild cognitive impairment," Psychoneuroendocrinology, vol. 27, no. 7, pp. 777–789, 2002. [DOI] [PubMed] [Google Scholar]
- [112].Souza-Talarico JN, Chaves EC, Lupien SJ, Nitrini R, and Caramelli P, "Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment: evidence from healthy elderly, mild cognitive impairment and Alzheimer's disease subjects," Journal of Alzheimer's disease, vol. 19, no. 3, pp. 839–848, 2010. [DOI] [PubMed] [Google Scholar]
- [113].Souza-Talarico J. N. d., Marin M-F, Sindi S, and Lupien SJ, "Effects of stress hormones on the brain and cognition: Evidence from normal to pathological aging," Dementia & Neuropsychologia, vol. 5, pp. 8–16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zheng B, Tal R, Yang Z, Middleton L, and Udeh-Momoh C, "Cortisol hypersecretion and the risk of Alzheimer’s disease: A systematic review and meta-analysis," Ageing Research Reviews, vol. 64, p. 101171, 2020. [DOI] [PubMed] [Google Scholar]
- [115].Saelzler UG, Verhaeghen P, Panizzon MS, and Moffat SD, "Intact circadian rhythm despite cortisol hypersecretion in Alzheimer’s disease: A meta-analysis," Psychoneuroendocrinology, vol. 132, p. 105367, 2021. [DOI] [PubMed] [Google Scholar]
- [116].Dhikav V et al. , "Basal serum cortisol levels, depression and medial temporal lobe atrophy in patients with mild cognitive impairment and Alzheimer's disease," J Depress Ther, vol. 1, no. 1, pp. 25–31, 2016. [Google Scholar]
- [117].Lind K, Edman Å, Nordlund A, Olsson T, and Wallin A, "Increased saliva cortisol awakening response in patients with mild cognitive impairment," Dementia and geriatric cognitive disorders, vol. 24, no. 5, pp. 389–395, 2007. [DOI] [PubMed] [Google Scholar]
- [118].Johar HB, "Cortisol secretion patterns in the elderly: in the perspectives of frailty and cognitive function and sleep disturbances as risk factors of cognitive decline," Dissertation, München, Ludwig-Maximilians-Universität, 2016, 2016. [Google Scholar]
- [119].Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, and Ju Y-ES, "Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease," JAMA neurology, vol. 75, no. 5, pp. 582–590, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Tortosa-Martínez J, Clow A, Caus-Pertegaz N, González-Caballero G, Abellán-Miralles I, and Saenz MJ, "Exercise increases the dynamics of diurnal cortisol secretion and executive functionin people wiht MCI," Journal of Aging and Physical Activity, vol. 23, no. 4, pp. 550–558, 2015. [DOI] [PubMed] [Google Scholar]
- [121].Kalafatakis K et al. , "Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man," Proceedings of the National Academy of Sciences, vol. 115, no. 17, pp. E4091–E4100, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Venero C et al. , "Increased morning salivary cortisol levels in older adults with nonamnestic and multidomain mild cognitive impairment," Psychoneuroendocrinology, vol. 38, no. 4, pp. 488–498, 2013. [DOI] [PubMed] [Google Scholar]
- [123].Popp J et al. , "Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer's type," Neurobiology of aging, vol. 36, no. 2, pp. 601–607, 2015. [DOI] [PubMed] [Google Scholar]
- [124].Csernansky JG et al. , "Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia," American Journal of Psychiatry, vol. 163, no. 12, pp. 2164–2169, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Nous A, Engelborghs S, and Smolders I, "Melatonin levels in the Alzheimer’s disease continuum: A systematic review," Alzheimer's research & therapy, vol. 13, no. 1, pp. 1–12, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Johar H et al. , "Lower morning to evening cortisol ratio is associated with cognitive impairment in men but not women: An analysis of 733 older subjects of the cross-sectional KORA-Age study," Psychoneuroendocrinology, vol. 51, pp. 296–306, 2015. [DOI] [PubMed] [Google Scholar]
- [127].Cronin P et al. , "Circadian alterations during early stages of Alzheimer's disease are associated with aberrant cycles of DNA methylation in BMAL1," Alzheimer's & Dementia, vol. 13, no. 6, pp. 689–700, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Schmidt C, Collette F, Cajochen C, and Peigneux P, "A time to think: circadian rhythms in human cognition," Cognitive neuropsychology, vol. 24, no. 7, pp. 755–789, 2007. [DOI] [PubMed] [Google Scholar]
- [129].Blatter K and Cajochen C, "Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings," Physiology & behavior, vol. 90, no. 2-3, pp. 196–208, 2007. [DOI] [PubMed] [Google Scholar]
- [130].Valdez P, Ramírez C, García A, Talamantes J, Armijo P, and Borrani J, "Circadian rhythms in components of attention," Biological rhythm research, vol. 36, no. 1-2, pp. 57–65, 2005. [Google Scholar]
- [131].Burke TM, Scheer FA, Ronda JM, Czeisler CA, and Wright KP Jr, "Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions," Journal of sleep research, vol. 24, no. 4, pp. 364–371, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Xu S, Akioma M, and Yuan Z, "Relationship between circadian rhythm and brain cognitive functions," Frontiers of Optoelectronics, vol. 14, no. 3, pp. 278–287, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Schneider AC, "Changes in cognition during middle-aged and older adulthood," The University of Iowa, 2020. [Google Scholar]
- [134].Sherman SM, Mumford JA, and Schnyer DM, "Hippocampal activity mediates the relationship between circadian activity rhythms and memory in older adults," Neuropsychologia, vol. 75, pp. 617–625, 2015. [DOI] [PubMed] [Google Scholar]
- [135].West R, Murphy KJ, Armilio ML, Craik FI, and Stuss DT, "Effects of time of day on age differences in working memory," The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, vol. 57, no. 1, pp. P3–P10, 2002. [DOI] [PubMed] [Google Scholar]
- [136].Schmidt C, Peigneux P, and Cajochen C, "Age-related changes in sleep and circadian rhythms: impact on cognitive performance and underlying neuroanatomical networks," Frontiers in neurology, vol. 3, p. 118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kaup AR, Mirzakhanian H, Jeste DV, and Eyler LT, "A review of the brain structure correlates of successful cognitive aging," The Journal of neuropsychiatry and clinical neurosciences, vol. 23, no. 1, pp. 6–15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Merrill DA and Small GW, "Prevention in psychiatry: effects of healthy lifestyle on cognition," Psychiatric Clinics, vol. 34, no. 1, pp. 249–261, 2011. [DOI] [PubMed] [Google Scholar]
- [139].Pace-Schott EF and Spencer RM, "Age-related changes in the cognitive function of sleep," Progress in brain research, vol. 191, pp. 75–89, 2011. [DOI] [PubMed] [Google Scholar]
- [140].Kuhlmei A, Walther B, Becker T, Müller U, and Nikolaus T, "Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy," European psychiatry, vol. 28, no. 2, pp. 94–97, 2013. [DOI] [PubMed] [Google Scholar]
- [141].Cochrane A, Robertson IH, and Coogan AN, "Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study," Journal of neural transmission, vol. 119, no. 10, pp. 1233–1239, 2012. [DOI] [PubMed] [Google Scholar]
- [142].Xiao Q et al. , "Rest-activity rhythms and cognitive impairment and dementia in older women: Results from the Women's Health Initiative," Journal of the American Geriatrics Society, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Molzof HE, Prapanjaroensin A, Patel VH, Mokashi MV, Gamble KL, and Patrician PA, "Misaligned core body temperature rhythms impact cognitive performance of hospital shift work nurses," Neurobiology of learning and memory, vol. 160, pp. 151–159, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Furio AM, Brusco LI, and Cardinali DP, "Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study," Journal of pineal research, vol. 43, no. 4, pp. 404–409, 2007. [DOI] [PubMed] [Google Scholar]
- [145].Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, and Xiang YT, "Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer's disease," International Journal of Geriatric Psychiatry, vol. 32, no. 1, pp. 50–57, 2017. [DOI] [PubMed] [Google Scholar]
- [146].Pulopulos MM, Hidalgo V, Almela M, Puig-Perez S, Villada C, and Salvador A, "Hair cortisol and cognitive performance in healthy older people," Psychoneuroendocrinology, vol. 44, pp. 100–111, 2014. [DOI] [PubMed] [Google Scholar]
- [147].Gilpin H, Whitcomb D, and Cho K, "Atypical evening cortisol profile induces visual recognition memory deficit in healthy human subjects," Molecular Brain, vol. 1, no. 1, pp. 1–7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].MacLullich AM, Deary IJ, Starr JM, Ferguson KJ, Wardlaw JM, and Seckl JR, "Plasma cortisol levels, brain volumes and cognition in healthy elderly men," Psychoneuroendocrinology, vol. 30, no. 5, pp. 505–515, 2005. [DOI] [PubMed] [Google Scholar]
- [149].Murphy KJ, Hodges TE, Sheppard PA, Troyer AK, Hampson E, and Galea LA, "Sex differences in cortisol and memory following acute social stress in amnestic mild cognitive impairment," Journal of Clinical and Experimental Neuropsychology, vol. 42, no. 9, pp. 881–901, 2020. [DOI] [PubMed] [Google Scholar]
- [150].Rubiño JA, Gamundí A, Akaarir M, Canellas F, Rial R, and Nicolau MC, "Bright light therapy and circadian cycles in institutionalized elders," Frontiers in Neuroscience, vol. 14, p. 359, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Figueiro MG and Leggett S, "Intermittent light exposures in humans: a case for dual entrainment in the treatment of Alzheimer's disease," Frontiers in Neurology, vol. 12, p. 625698, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Burns A, Allen H, Tomenson B, Duignan D, and Byrne J, "Bright light therapy for agitation in dementia: a randomized controlled trial," International Psychogeriatrics, vol. 21, no. 4, pp. 711–721, 2009. [DOI] [PubMed] [Google Scholar]
- [153].Figueiro MG, "Light, sleep and circadian rhythms in older adults with Alzheimer's disease and related dementias," Neurodegenerative Disease Management, vol. 7, no. 2, pp. 119–145, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Vecchierini M-F, Kilic-Huck U, and Quera-Salva M, "Melatonin (MEL) and its use in neurological diseases and insomnia: Recommendations of the French Medical and Research Sleep Society (SFRMS)," Revue Neurologique, vol. 177, no. 3, pp. 245–259, 2021. [DOI] [PubMed] [Google Scholar]
- [155].P Cardinali D, M Furio A, and I Brusco L, "Clinical aspects of melatonin intervention in Alzheimer's disease progression," Current Neuropharmacology, vol. 8, no. 3, pp. 218–227, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wanigatunga AA et al. , "Daily Physical Activity Patterns as a Window on Cognitive Diagnosis in the Baltimore Longitudinal Study of Aging (BLSA)," Journal of Alzheimer's Disease, no. Preprint, pp. 1–11. [DOI] [PubMed] [Google Scholar]
- [157].Covell GES et al. , "Physical activity level and future risk of mild cognitive impairment or dementia: a critically appraised topic," The Neurologist, vol. 19, no. 3, pp. 89–91, 2015. [DOI] [PubMed] [Google Scholar]
- [158].Kurita S et al. , "Association of physical and/or cognitive activity with cognitive impairment in older adults," Geriatrics & Gerontology International, vol. 20, no. 1, pp. 31–35, 2020. [DOI] [PubMed] [Google Scholar]
- [159].Suh M, "Influences of Autonomic Function, Salivary Cortisol and Physical Activity on Cognitive Functions in Institutionalized Older Adults with Mild Cognitive Impairment: Based on Neurovisceral Integration Model," Journal of Korean Academy of Nursing, vol. 51, no. 3, pp. 294–304, 2021. [DOI] [PubMed] [Google Scholar]
- [160].Chang Y-T, "Physical activity and cognitive function in mild cognitive impairment," ASN neuro, vol. 12, p. 1759091419901182, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lu Z, Harris TB, Shiroma EJ, Leung J, and Kwok T, "Patterns of physical activity and sedentary behavior for older adults with Alzheimer’s disease, mild cognitive impairment, and cognitively normal in Hong Kong," Journal of Alzheimer's Disease, vol. 66, no. 4, pp. 1453–1462, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Schibler U et al. , "Clock-talk: interactions between central and peripheral circadian oscillators in mammals," in Cold Spring Harbor symposia on quantitative biology, 2015, vol. 80: Cold Spring Harbor Laboratory Press, pp. 223–232. [DOI] [PubMed] [Google Scholar]
- [163].Yoshizaki T et al. , "Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels," European journal of applied physiology, vol. 113, no. 10, pp. 2603–2611, 2013. [DOI] [PubMed] [Google Scholar]
- [164].Burke TM et al. , "Effects of caffeine on the human circadian clock in vivo and in vitro," Science translational medicine, vol. 7, no. 305, pp. 305ra146–305ra146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Youngstedt SD, Kline CE, Elliott JA, Zielinski MR, Devlin TM, and Moore TA, "Circadian phase-shifting effects of bright light, exercise, and bright light+ exercise," Journal of circadian rhythms, vol. 14, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]