Abstract
Late embryogenesis abundant (LEA) proteins comprise a large family that plays important roles in the regulation of abiotic stress, however, no in-depth analysis of LEA genes has been performed in grapevine to date. In this study, we analyzed a total of 52 putative LEA genes in grapevine at the genomic and transcriptomic level, compiled expression profiles of four selected (V. amurensis) VamLEA genes under cold and osmotic stresses, and studied the potential function of the V. amurensis DEHYDRIN3 (VamDHN3) gene in grapevine callus. The 52 LEA proteins were classified into seven phylogenetic groups. RNA-seq and quantitative real-time PCR results demonstrated that a total of 16 and 23 VamLEA genes were upregulated under cold and osmotic stresses, respectively. In addition, overexpression of VamDHN3 enhanced the stability of the cell membrane in grapevine callus, suggesting that VamDHN3 is involved in osmotic regulation. These results provide fundamental knowledge for the further analysis of the biological roles of grapevine LEA genes in adaption to abiotic stress.
Keywords: Cold and osmotic stresses, Grapevine LEA genes, Overexpression of VamDHN3 gene, Phylogenetic analysis, Transcriptomic analysis
Introduction
Late embryogenesis abundant (LEA) proteins accumulate in the late stages of seed maturation (Artur et al. 2019). The first LEA protein was purified and characterized from cotton seeds in 1983 (Galau et al. 1983). Since then, LEA homologs have been identified from several plant species, such as wheat (Ried and Walker-Simmons 1993), soybean (Hsing et al. 1995), maize (Li and Cao 2016), Arabidopsis thaliana (Hundertmark et al. 2011) and cucumber (Zhou et al. 2017). In addition, LEA homologs have been found in bacteria (Mtwisha et al. 1998, Stacy and Aalen 1998) and animals (Denekamp et al. 2010, Warner et al. 2010, Wu et al. 2011). The LEA protein family consists of large and highly diverse polypeptides, which were primitively grouped into six groups on the basis of specific domains (Dure et al. 1989), but then more recently divided into nine groups (İbrahime et al. 2019). The various classifications of the LEA family members based on sequence similarity and domains are not consistent (Tunnacliffe and Wise 2007, Battaglia et al. 2008, Bies-Etheve et al. 2008, Hundertmark and Hincha 2008, Shih et al. 2008, Battaglia and Covarrubias 2013). However, most studies suggest that the LEA protein family can be classified into eight groups [LEA_1, LEA_2, LEA_3, LEA_4, LEA_5, LEA_6, dehydrin (DHN) and seed maturation protein (SMP)] (Finn et al. 2008, Du et al. 2013, Liang et al. 2016, Artur et al. 2019).
LEA proteins are hydrophilic, contain a high percentage of glycine or other small amino acids (alanine, serine and threonine) and lack or contain small amounts of tryptophan and cysteine residues (Battaglia et al. 2008). Plant LEA genes are considered to play important roles in protecting cells from abiotic stress. The overexpression (OE) of Cot_AD24498 showed that the LEA2 gene was involved in promoting root growth and in turn conferred drought stress tolerance (Magwanga et al. 2018). The OE of AtLEA33 confers tolerance to cold stress in Escherichia coli and provided enhanced osmotic stress tolerance and ABA sensitivity in A. thaliana (Zhao et al. 2011). The overexpressed CsLEA11 gene of cucumber enhanced cell viability and conferred tolerance to heat and cold stresses in E. coli (Zhou et al. 2017). DHN is a group within the LEA gene family and plays a critical role during cold, drought, heat or salinity stress. For example, four members of DHNs were cloned from V itis vinifera and wild V itis yeshanensis and expression analysis showed that the DHN1 gene is stress responsive (Yang et al. 2012). Another study identified two DNH genes from wild and cultivated grapevines and found that DHN1 responds to low temperature, drought and ABA (Xiao and Nassuth 2006). The OE of ShDHN in cultivated tomato increased tolerance to cold and drought stresses and improved seedling growth under salt and osmotic stresses (Liu et al. 2015). A DHN gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants (Chiappetta et al. 2015).
Grapevine is widely cultivated under diverse climate and soil conditions and contributes significantly to each region’s economy. Abiotic stresses, such as drought, cold, heat and salinity, are the main factors affecting the yield and quality of grapevine. Therefore, there is considerable interest in identifying genes to improve grapevine’s stress tolerance. LEA genes from different plant species contribute to stress tolerance, but the transcriptomic analysis of the grapevine LEA gene family in response to osmotic and cold stresses and the functional analyses of DHN genes in grapevine were lacking. Vitis amurensis is a wild grapevine species with remarkable cold and drought tolerance and therefore widely used as the experimental material for studying the resistance of cold and drought stresses in grapevine (Su et al. 2015, Zhao et al. 2017).
In this study, we conducted a genome-wide analysis of LEA proteins and their encoding genes in V . vinifera, measured LEA genes transcript abundance in grapevine in response to cold or osmotic stresses, and determined the expression profiles of selected LEA genes under various cold and osmotic stresses. Furthermore, a DHN gene was selected to verify its function in grapevine by the transgene OE strategy. The results provide baseline knowledge of an important gene family in Vitis species that will form the basis for further functional analyses and for breeding stress-tolerant grapevines.
Results
Identification and phylogenetic analysis of LEA genes in grapevine
A total of 52 LEA genes were identified in V. vinifera by combining HMM (e-value <0.01) scan, BLAST (e-value <1e−5) search and Conserved Domain Data (CDD) search method. The 52 LEA genes were named according to the grapevine gene nomenclature system (Grimplet et al. 2014). The accession numbers of all 52 LEA genes are found in GenBank. A huge difference in gene length was apparent among the 52 LEA genes in grapevine, ranging from 454 bp (VviLEA6-2) to 9,729 bp (VviLEA2-1). The length of coding sequence (CDS) ranged from 228 nt (VviLEA1-5) to 1,170 nt (VviLEA6-1) (Table 1).
Table 1.
Features of LEA gene family in V. vinifera
| Gene locus ID | Gene symbol | Accession number | Chromosome location | Group | Start site | End site | Gene length (bp) | CDS (bp) | Protein length | Strand |
|---|---|---|---|---|---|---|---|---|---|---|
| VIT_202s0012g00940 | VviLEA1-1 | XP_002281237.1 | 2 | LEA_1 | 6868929 | 6869682 | 754 | 387 | 128 | − |
| VIT_202s0025g00530 | VviLEA2-1 | XP_002272642.1 | 2 | LEA_2 | 640979 | 650707 | 9,729 | 642 | 213 | + |
| VIT_202s0025g00540 | VviLEA2-2 | – | 2 | LEA_2 | 647453 | 649381 | 1,929 | 582 | 193 | + |
| VIT_202s0025g01600 | VviLEA2-3 | XP_002268138.1 | 2 | LEA_2 | 1539437 | 1540047 | 611 | 585 | 194 | − |
| VIT_203s0038g03545 | VviDHN1 | XP_010646029.1 | 3 | Dehydrin | 2566856 | 2567955 | 1,100 | 501 | 166 | + |
| VIT_203s0038g04390 | VviDHN2 | XP_002283605.1 | 3 | Dehydrin | 3186151 | 3187565 | 1,415 | 576 | 191 | − |
| VIT_203s0091g00490 | VviLEA3-1 | XP_002262812.1 | 3 | LEA_3 | 6859799 | 6860427 | 629 | 309 | 102 | − |
| VIT_203s0091g00500 | VviLEA3-2 | XP_010647936.1 | 3 | LEA_3 | 6871728 | 6872458 | 731 | 318 | 105 | − |
| VIT_203s0091g00510 | VviLEA3-3 | XP_010647938.1 | 3 | LEA_3 | 6894959 | 6895636 | 678 | 309 | 102 | − |
| VIT_204s0008g01230 | VviLEA2-4 | XP_002279306.1 | 4 | LEA_2 | 1005251 | 1006469 | 1,219 | 798 | 265 | − |
| VIT_204s0023g02480 | VviDHN3 | AAW58106.1 | 4 | Dehydrin | 19034554 | 19035405 | 852 | 393 | 130 | + |
| VIT_204s0069g01010 | VviLEA2-5 | XP_002263161.1 | 4 | LEA_2 | 9458009 | 9459027 | 1,019 | 756 | 251 | + |
| VIT_205s0020g03530 | VviSMP1 | XP_002282590.1 | 5 | SMP | 5290176 | 5291777 | 1,602 | 777 | 258 | − |
| VIT_205s0094g01520 | VviLEA2-6 | XP_010650759.1 | 5 | LEA_2 | 24796845 | 24802836 | 5,992 | 954 | 317 | + |
| VIT_206s0009g01620 | VviLEA2-7 | XP_002273485.1 | 6 | LEA_2 | 13573148 | 13574097 | 950 | 804 | 267 | − |
| VIT_206s0061g00850 | VviLEA2-8 | CBI17121.3 | 6 | LEA_2 | 18386525 | 18387540 | 1,016 | 789 | 262 | + |
| VIT_206s0080g01200 | VviLEA2-9 | XP_002270691.1 | 6 | LEA_2 | 21361847 | 21362572 | 726 | 627 | 208 | − |
| VIT_206s0080g01220 | VviLEA2-10 | – | 6 | LEA_2 | 21376191 | 21380935 | 4,745 | 771 | 256 | − |
| VIT_207s0005g00660 | VviLEA3-4 | XP_002270639.1 | 7 | LEA_3 | 3320933 | 3322368 | 1,436 | 294 | 97 | + |
| VIT_207s0129g00510 | VviLEA6-1 | – | 7 | LEA_6 | 15737695 | 15740375 | 2,681 | 1170 | 389 | + |
| VIT_207s0151g00840 | VviSMP2 | XP_010651983.1 | 7 | SMP | 1087451 | 1089038 | 1,588 | 780 | 259 | + |
| VIT_208s0007g02350 | VviLEA2-11 | XP_002280566.1 | 8 | LEA_2 | 16424245 | 16425159 | 915 | 624 | 207 | + |
| VIT_208s0007g02360 | VviLEA2-12 | – | 8 | LEA_2 | 16430898 | 16431875 | 978 | 621 | 206 | + |
| VIT_208s0007g06420 | VviLEA1-2 | XP_002279196.1 | 8 | LEA_1 | 20198585 | 20199194 | 610 | 420 | 139 | − |
| VIT_208s0007g06430 | VviLEA1-3 | XP_002279220.1 | 8 | LEA_1 | 20200948 | 20201666 | 719 | 396 | 131 | − |
| VIT_208s0007g06440 | VviLEA1-4 | XP_010653892.1 | 8 | LEA_1 | 20203229 | 20204364 | 1,136 | 615 | 204 | − |
| VIT_208s0056g00250 | VviLEA2-13 | CBI31144.3 | 8 | LEA_2 | 391491 | 393755 | 2,265 | 567 | 188 | + |
| VIT_208s0056g00260 | VviLEA2-14 | XP_010653100.1 | 8 | LEA_2 | 395846 | 398213 | 2,368 | 648 | 215 | + |
| VIT_208s0105g00600 | VviLEA5-1 | – | 8 | LEA_5 | 7969437 | 7970216 | 780 | 396 | 131 | − |
| VIT_209s0002g00410 | VviLEA3-5 | – | 9 | LEA_3 | 323916 | 324504 | 589 | 282 | 93 | − |
| VIT_209s0002g00481 | VviLEA2-15 | – | 9 | LEA_2 | 361694 | 362842 | 1,149 | 747 | 248 | + |
| VIT_209s0002g02230 | VviLEA2-16 | XP_010654527.1 | 9 | LEA_2 | 2047331 | 2048290 | 960 | 762 | 253 | + |
| VIT_209s0002g04610 | VviLEA2-17 | XP_002279706.1 | 9 | LEA_3 | 4178471 | 4181992 | 3,522 | 714 | 237 | + |
| VIT_210s0116g00300 | VviLEA2-18 | XP_002265341.1 | 10 | LEA_2 | 132655 | 133472 | 818 | 648 | 215 | − |
| VIT_211s0016g04040 | VviLEA2-19 | CBI28084.3 | 11 | LEA_2 | 3289439 | 3295150 | 5,712 | 639 | 212 | + |
| VIT_212s0055g00370 | VviLEA2-20 | XP_002265790.1 | 12 | LEA_2 | 13112125 | 13115305 | 3,181 | 732 | 243 | + |
| VIT_213s0067g01240 | VviLEA5-2 | CBI25460.3 | 13 | LEA_5 | 713095 | 713742 | 648 | 288 | 95 | − |
| VIT_213s0067g01250 | VviLEA5-3 | XP_002274326.1 | 13 | LEA_5 | 714451 | 715151 | 701 | 282 | 93 | − |
| VIT_214s0030g01470 | VviSMP3 | XP_003633664.1 | 14 | SMP | 6071198 | 6072331 | 1,134 | 792 | 263 | + |
| VIT_215s0045g01150 | VviLEA1-5 | XP_002264509.1 | 15 | LEA_1 | 6533110 | 6533750 | 641 | 228 | 75 | − |
| VIT_215s0046g00430 | VviLEA2-21 | XP_002263581.1 | 15 | LEA_2 | 17405666 | 17406259 | 594 | 585 | 194 | − |
| VIT_215s0046g01410 | VviLEA2-22 | XP_010661876.1 | 15 | LEA_2 | 18396678 | 18399356 | 2,679 | 741 | 246 | − |
| VIT_215s0048g02530 | VviLEA2-23 | CBI38717.3 | 15 | LEA_2 | 16684350 | 16685973 | 1,624 | 609 | 219 | − |
| VIT_216s0039g00340 | VviLEA2-24 | XP_002263732.2 | 16 | LEA_2 | 175127 | 175993 | 867 | 867 | 288 | + |
| VIT_216s0050g01650 | VviLEA2-25 | XP_010662664.1 | 16 | LEA_2 | 18545611 | 18546522 | 912 | 561 | 186 | + |
| VIT_216s0050g01750 | VviLEA2-26 | – | 16 | LEA_2 | 18692093 | 18696266 | 4,174 | 582 | 193 | + |
| VIT_216s0050g01760 | VviLEA2-27 | – | 16 | LEA_2 | 18698075 | 18698731 | 657 | 645 | 214 | + |
| VIT_216s0050g01770 | VviLEA2-28 | XP_010662657.1 | 16 | LEA_2 | 18721720 | 18722435 | 716 | 636 | 211 | + |
| VIT_216s0098g00890 | VviLEA2-29 | – | 16 | LEA_2 | 21228114 | 21228963 | 850 | 633 | 210 | − |
| VIT_218s0001g00360 | VviDHN4 | XP_002285919.1 | 18 | Dehydrin | 1297211 | 1299363 | 2,153 | 621 | 206 | + |
| VIT_218s0001g05305 | VviLEA2-30 | – | 18 | LEA_2 | 4265173 | 4266355 | 1,183 | 1,092 | 363 | − |
| VIT_218s0122g00820 | VviLEA6-2 | XP_010663997.1 | 18 | LEA_6 | 597440 | 597893 | 454 | 282 | 93 | − |
The phylogenetic tree of 52 VviLEA members was constructed by MEGA7.0 (Fig. 1). The VviLEA genes were classified into seven groups (LEA_1, LEA_2, LEA_3, LEA_5, LEA_6, DHN, SMP) based on their conserved domain structures, in which the LEA_2 group is the largest group containing 30 members and the LEA_6 is the smallest group with only two members. Unfortunately, the member of LEA_4 group was not identified in genome of V. vinifera.
Fig. 1.
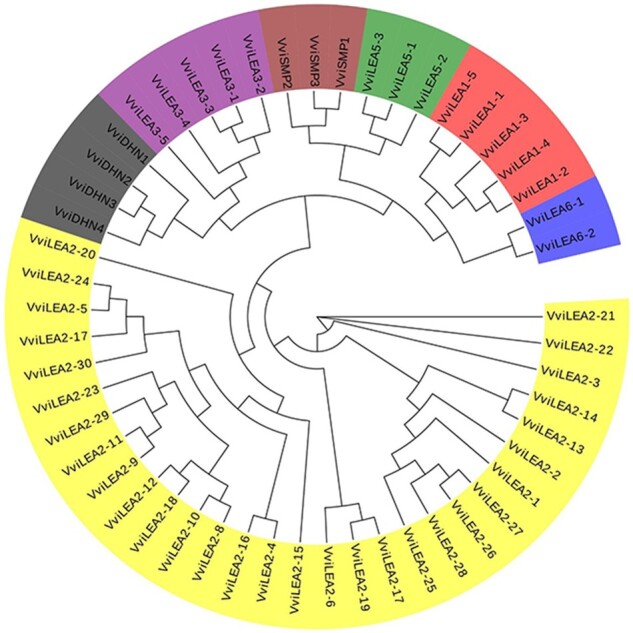
Phylogenetic analysis of the LEA genes in V. vinifera. LEA gene groups are distinguished by different colors. The unrooted tree was generated by ClustalW in MEGA7 using the conserved amino acid sequences of the 52 V. vinifera LEA proteins.
Exon–intron organization of VviLEA genes
The exon–intron construction of 52 VviLEA genes was obtained by Gene Structure Display Server 2.0 (http://gsds.cbi.pku.edu.cn/) . Exon–intron organizations were known to play crucial roles in the evolution of multiple gene families (Hu et al. 2015). Exon–intron organization of VviLEA genes showed that two exons and one intron existed in the members of LEA_1, LEA_3, LEA_5, LEA_6 and DHN groups except for VviLEA3-5 and VviLEA6-2 genes (one exon and none intron), three exons and two introns existed in the members of SMP group and 1–3 exons and 0–3 introns existed in the members of LEA_2 group (Supplementary Fig. S1). In the LEA_2 group, 20 VviLEA genes contain one exon, 9 VviLEA genes contain 2 exons and 1 VviLEA genes contain 3 exons and 16 VviLEA genes contain none intron, 8 VviLEA genes contain 1 intron, 8 VviLEA genes contain 2 introns and 1 VvLEA gene contain 3 introns (Supplementary Fig. S1).
Chromosome localization and duplication analysis of VviLEA genes
The 52 VvLEA genes were distributed on 16 chromosomes except for chromosomes 1, 17 and 19 (Supplementary Fig. S2, Table 1). Chromosomes 8 and 16 contained six VvLEA genes, and chromosomes 10, 11, 12 and 14 only contained one member of VvLEA gene. The results of duplication analysis of VviLEA genes indicated that 20 VviLEAs were involved in 15 segmental duplication events, and these genes were group members in LEA_2, LEA_5, DHN and SMP, but group members in LEA_1, LEA_3 and LEA_6 did not show significant evidence about segment duplication. Besides segmental duplication, 15 genes were found involved in 6 tandem duplication events, including LEA_1, LEA_2, LEA_3 and LEA_5. Five members (VviLEA2-11, VviLEA2-1, VviLEA2-27, VviLEA2-26 and VviLEA5-2) were involved in both segmental and tandem duplication, and 22 members of LEA genes in grapevine were not detected in the duplication events.
Expression profiles of VviLEA genes at different developmental stages in specific tissues/organs in grapevine
Based on microarray data of grapevine gene expression atlas (GSE62744) (Fasoli et al. 2012), expression patterns of the VviLEA gene family in different developmental stages of organs/tissues were assessed (Supplementary Table S2). The VviLEA gene family has a broad expression pattern across different organs/tissues in V. vinifera, but the expression of different VviLEA genes has significant difference in different organs/tissues, which indicates that VviLEA genes exhibit a tissue-specific expression (Supplementary Fig. S3). For example, VviSMP1, VviSMP2 and VviSMP3 were expressed at high levels in seed, but VviLEA6-2 and VviLEA1-5 expression levels were particularly high in flower.
In addition, we carefully examined the expression profiles of VviLEA genes in the various developmental stages of organs/tissues. Most of VviLEA genes have a higher expression level in the late stage of development than in early stage (e.g. VviLEA2-12, VviLEA1-2, Vvi2-23, VviLEA2-5, VviLEA2-9, VviLEA5-1, VviDHN2, VviSMP2, VviLEA5-2, VviLEA1-1, VvLEA1-3, VviLEA5-3, VviSMP1, VviSMP3 and VviLEA 1 - 4 in seed) (Supplementary Fig. S3), which indicated that most of VviLEA genes have a positive response on maturation.
Transcriptomic analysis of VamLEA genes in response to cold and osmotic stresses
Due to better resistance to cold and drought stresses, V. amurensis was selected as experimental materials for expressing analysis of LEA genes in grapevine. To obtain insights into the potential roles of all 52 VamLEA genes in stress responses, 6-week-old micropropagated plantlets of V. amurensis were exposed to cold (4°C for 12 h) or osmotic (8% PEG-6000 for 12 h) stress and their expression was monitored using RNA-seq (RNA sequencing). The results indicated that cold stress treatment resulted in a wide variety of VamLEA gene expression profiles (Fig. 2). A total of 16 VamLEA genes showed significant upregulation by cold treatment, while 11 VamLEA genes were significantly downregulated. However, the expression profiles resulted from osmotic treatments were distinct from those modulated by cold. Most of VamLEA genes, a total of 23, were upregulated by osmotic and 7 VamLEA genes were downregulated. Interestingly, there are 7 commonly upregulated VamLEA genes and 4 downregulated VamLEA genes under both cold and osmotic stresses, which indicated that some VamLEA genes are involved in response to both cold and osmotic stresses in grapevine.
Fig. 2.

Expression profiles of the VamLEA genes under cold and osmotic stresses. The results of RNA-seq were analyzed using the Tophat and Cufflinks software. The color intensity represents relative expression levels, with red as increased transcript abundance and blue as decreased transcript abundance. (A) Expression profiles of VamLEA genes under no osmatic stress. (B) Expression profiles of VamLEA genes under osmotic stress. (C) Expression profiles of VamLEA genes under no cold stress. (D) Expression profiles of VamLEA genes under cold stress.
To verify the RNA-seq data, four LEA genes that were upregulated by osmotic stress (VamLEA2-5, VamLEA2-12, VamDHN3 and VamSMP2) and cold stress (VamLEA2-4, VamLEA2-12, VamLEA3-4 and VamDHN3) were selected for further analysis by using quantitative real-time PCR (qRT-PCR). The qRT-PCR results were in accordance with RNA-seq data (Figs. 2–4). It is interesting that VamDHN3 gene was significantly upregulated in both cold and osmotic stresses. Especially, it was upregulated more than hundreds fold under the osmotic stress. Thus, we cloned and overexpressed VamDHN3 gene in grapevine embryogenic cell suspension to investigate its potential function.
Fig. 3.
Real-time quantitative PCR assays of four selected VamLEA genes under cold stress. (A) The relative expression levels of VamLEA2-4 gene after different cold treatment. (B) The relative expression levels of VamDHN3 gene after different cold treatment. (C)The relative expression of VamLEA3-4 gene after different cold treatment. (D)The relative expression levels of VamLEA2-12 gene after different cold treatment. The expression levels were normalized. Mean values and SDs were obtained from three biological and three technical replicates. Asterisks indicate the corresponding genes significantly up- or downregulated under cold treatment by t-test (*P < 0.05, **P < 0.01).
Fig. 4.
Real-time quantitative PCR assays of four selected VamLEA genes under osmotic stress. (A) The relative expression levels of VamDHN3 gene after different PEG treatment. (B)The relative expression levels of VamLEA2-5 gene after different PEG treatment. (C) The relative expression levels of VamSMP2 gene after different PEG treatment. (D) The relative expression levels of VamLEA2-12 gene after different PEG treatment. The expression levels were normalized. Mean values and SDs were obtained from three biological and three technical replicates. Asterisks indicate the corresponding genes significantly up- or downregulated under osmotic treatment by t-test (*P < 0.05, **P < 0.01).
OE of VamDHN3 gene improves tolerance to cold and osmic stresses
We performed qRT-PCR to measure the relative expression level of VamDHN3 gene in transgenic embryogenic cell suspension (Fig. 5D). The qRT-PCR assays showed that the expression of VamDHN3 was upregulated in the transgenic embryogenic callus, with 2.5-fold more abundant than in embryogenic callus transformed with an empty vector (EV). The cold tolerance of VamDHN3-overexpressing embryogenic callus was evaluated by the low temperature exotherms (LTEs) as described by Sun et al. (2018) and Sun et al. (2019), and the LTEs of VamDHN3 OE embryogenic callus (−9.73°C) were significantly lower than the EV transgenic embryogenic callus (−7.85°C) (Fig. 5E).
Fig. 5.
OE of VamDHN3 gene enhances cold tolerance in transgenic grapevine callus. (A) Schematic presentation of binary recombinant plasmid for transformation. (B) Transgenic grapevine callus introduced with EV and pSAK277-VamDHN3. (C) Detection of VamDHN3 gene at the DNA and mRNA levels in the transgenic grapevine callus was obtained and verified for DNA insertion and transcript expression of VamDHN3 gene. (D) VamDHN3 gene expression in transgenic grapevine callus. (E) The LTEs of transgenic grapevine callus. Data are mean values ± SE of nine biological replicates. Asterisks indicate significant differences compared with the EV at **P < 0.01 and *P < 0.05 (Student’s t-test).
We then measured the relative electrolyte leakage (EL) rate, superoxide dismutase (SOD) and peroxidase (POD) to evaluate tolerance to cold and osmotic stresses (Fig. 6). The results indicated that the relative EL of the VamDHN3 OE embryogenic callus under cold and osmotic stresses was significantly lower than that in the EV transgenic callus (P < 0.01) (Fig. 6A, D), which indicated that the OE of VamDHN3 genes enhanced the ability of cell membrane in tolerating cold and osmotic stresses. In addition, SOD and POD activities of VamDHN3 OE embryogenic callus were significantly higher than the EV transgenic embryogenic callus under cold and osmotic treatments (Fig. 6B, C, E, F). These results supported the hypothesis that the VamDHN3 gene is involved in grapevine’s tolerance to cold and osmotic stresses.
Fig. 6.
OE of VamDHN3 gene prevents membrane and oxidative damage under cold and osmotic stresses. (A) The EL rate of VamDHN3 OE grapevine callus and EV callus under cold stress. (B) The SOD activity of VamDHN3 OE grape callus and EV callus under cold stress. (C) The POD activity of VamDHN3 OE grape callus and EV callus under cold stress. (D) The EL rate of VamDHN3 OE grapevine callus and EV callus under osmotic stress. (E) The SOD activity of VamDHN3 OE grape callus and EV callus under osmotic stress. (F) The POD activity of VamDHN3 OE grapevine callus and EV callus under osmotic stress. Asterisks indicate significant differences compared with the EV at **P < 0.01 and *P < 0.05 (Student’s t-test).
Discussion
In this study, we identified the VviLEA genes in grapevine genome and examined the expression profiles of VamLEA genes under cold and osmotic stress conditions. Furthermore, a DHN gene (VamDHN3) was selected and its function was verified by the transgene OE approach. The acquired results provide an overview of the LEA gene family in grapevine and lay the foundation for future functional analyses of individual LEA genes in grapevine.
There are 52 members of LEA protein in the genome of V. vinifera; the results did not agree with a recent study that 60 members of LEA protein were identified in the grapevine genome (İbrahime et al. 2019). The difference might result from the different classification methods of LEA family and the source of LEA genes. According to the nomenclature of the different LEA protein groups in the Pfam database (Finn et al. 2014), the LEA family was divided into eight groups in this study, but nine groups were used in the previous study. Simultaneously, the difference might result from the different obtained approach of LEA genes, too. In this study, all of the LEA genes obtained from the V. vinifera reference genome (12×), but the candidate genes obtained from the NCBI Refseq Protein database in the previous study.
The number of LEA genes in V. vinifera was intermediate, which was similar in A . thaliana (51 members) (Hundertmark and Hincha 2008) and poplar (Populus trichocarpa) (53 members) (Liu et al. 2012). Grapevine genome encodes more LEA proteins than Chinese plum (Prunus mume) (30 members) (Du et al. 2013), potato (Solanum tuberosum) (29 members) (Charfeddine et al. 2015), tomato (Solanum lycopersicum) (27 members) (Cao and Li 2015) and maize (32 members) (Li and Cao 2016) but less than Brassica napus (108 members) (Liang et al. 2016) and cucumber (79 members) (Celik Altunoglu et al. 2016). In comparison with other species, the number of members in different LEA groups showed a large difference. For example, 30 members of LEA_2 group in grapevine genome were identified, accounting for 57.7% of all numbers, but only 3 and 10 members were identified in Arabidopsis (accounting for 5.9% of all numbers) (Hundertmark and Hincha 2008) and B . napus (accounting for 9.3% of all numbers) (Liang et al. 2016), respectively. These results demonstrated that the number of LEA gene family members in different plant species showed a large difference. On the basis of former studies, the difference of number of LEA genes might be related to the species and ploidy of plants. Interestingly, seven LEA groups (LEA_1, LEA_2, LEA_3, LEA_5, LEA_6, DHN and SMP) were identified in this study and LEA_4 group was absent in the V. vinifera reference genome based on a phylogenetic tree. We deduced that LEA_4 gene group was lost in the course of grapevine evolution.
Gene duplication events are the main cause for the expansion of gene families, which includes segmental and tandem duplication (Cannon et al. 2004). According to the whole genome duplication and tandem repeat analysis, 20 VviLEAs were involved in 15 segmental duplication events and 15 VviLEAs were involved in tandem duplication events. A total of 30 VviLEAs were identified involved in duplication events, implying that the duplication significantly contributed to this family expansion. The expansion of VviLEA_2 group was especially achieved by the segmental duplication, and this duplication maybe led to VviLEA_2 group, which contains the most members in the LEA family of grapevine. Tandem repeats also gave rise to the appearance of 28.85% of members. The segmental and tandem duplication analysis have thus provided an insight into the origin and expansion of the VviLEA family.
A large number of studies have shown that LEA genes play a protective role under abiotic stress (e.g. cold, osmotic, salt and drought stresses) (Acosta-García et al. 2015, Zhang et al. 2016, Rodriguez-Salazar et al. 2017, Zeng et al. 2018). Since gene expression profiles can provide important clues for gene function, we examined the expression profiles of the VamLEA family genes under cold and osmotic stress conditions using RNA-seq. A total of 12 and 23 of the 52 VamLEA genes were upregulated under cold and osmotic stress conditions, respectively. Moreover, four different VamLEA genes were selected for verifying their expression patterns by qRT-PCR and the results indicated that all four selected VamLEA genes were upregulated under cold and osmotic stress conditions. Obviously, VamLEA expression is influenced by a broad range of abiotic stresses, indicating that these genes may play different roles in the regulation of plant responses to various abiotic stresses. Thus, highly or differentially expressed VamLEA genes may play a regulatory role in stress tolerance.
One group of the LEA protein family, DHN, plays an important role in response to abiotic challenges (Zhou et al. 2017, Lv et al. 2018). It had been proved that DHN genes were regulated by various abiotic stresses including drought and cold stresses (Bao et al. 2017, Guo et al. 2017). Over the past few years, numerous DHN genes have been identified in various plant species (Garcia-Bañuelos et al. 2009, Liu et al. 2012, Jing et al. 2016). Yang et al. (2012) identified four DHN genes in the V. vinifera genome, which was consistent with our analysis result based on the genome-wide identification in V. vinifera; VamDHN3 was one of the isolated DHN genes in V. amurensis. Expression of VamDHN3 was induced by osmotic and cold treatments, implying a positive correlation between VamDHN3 gene expression and tolerance to cold or osmotic stress.
Several recent studies investigated the heterogenous OE of DHN genes to reveal their possible functions under stress conditions. For example, heterogenous expression of a DHN-like protein gene AmCIP from Ammopiptanthus mongolicus conferred cold stress tolerance in E. coli (Shi et al. 2016). The OE of DHN gene CsLEA11 from cucumber (Cucumis sativus) in E. coli resulted in an increased tolerance to heat and cold stresses (Zhou et al. 2017). A recent study reported that tobacco plants expressing P. mume dehydrin genes showed enhanced viability and tolerance under drought and cold stresses (Bao et al. 2017). Another report also revealed that OE of SiDHN from Saussurea involucrata Kar. et Kir. enhanced cold and drought tolerance of tobacco plants (Guo et al. 2017). In this study, to verify the function of the VamDHN3 gene, OE of VamDHN3 gene in grapevine callus was performed and physiological indexes were measured in transgenic grapevine callus. Various physiological indexes are often used to evaluate the tolerance of transgenic and non-transgenic plants to abiotic stresses. For example, relative EL was used frequently to assess damage to the cell membrane in plants upon treatments with cold and osmotic stresses (Morsy et al. 2005, Munir et al. 2016). Earlier studies indicated that there was a negative correlation between relative EL and membrane injury induced by abiotic stress (Sun et al. 2018). In the present study, relative EL was significantly lower (P < 0.01) in VamDHN3-overexpressing grapevine callus under cold and osmotic stresses than in EV transgenic callus (Fig. 6A, D), indicating that the VamDHN3-overexpressing grapevine callus suffered less membrane injury than the EV transgenic callus under cold and osmotic stresses.
Reactive oxygen species (ROS) induce membrane lipid peroxidation (Apel and Hirt 2004). Plants contain efficient ROS-scavenging defense mechanisms using enzymes such as SOD, POD and catalase (Jaillon et al.) to protect from this oxidative stress-induced cell damage (Choudhury et al. 2013). The SOD and POD have been reported to protect the oxidative stress-related membrane damage in tobacco (Allen 1995), and there was a positive correlation between SOD and POD concentration and membrane injury induced by abiotic stress. VamDHN3 transgenic callus showed higher SOD and POD activities than EV transgenic callus under cold and osmotic stress conditions. The ROS-scavenging mechanisms decreased the accumulation of ROS in VamDHN3 transgenic callus than in EV transgenic callus under cold and osmotic stresses. Indeed, the VamDHN3 transgenic callus showed higher abiotic stress tolerance than the EV transgenic callus, probably due to ROS-scavenging protein to limit the lipid peroxidative cell membrane damage under cold and osmotic stresses. Therefore, based on these results, we can draw a preliminary conclusion that VamDHN3 gene in grapevine might participate in adaptive responses to cold and osmotic stresses by enhancing the stability of the cell membrane.
Materials and Methods
Identification of LEA genes in V . vinifera
LEA proteins were identified by eight Hidden Markov Models (HMM, e-value <0.01) (PF03760.14, PF03168.12, PF03242.12, PF02987.15, PF00477.16, PF10714.8, PF00257.18 and PF04927.11) in the Pfam protein family database (http://xfam.rog/) and by BLAST (e-value <1e−5) search against the proteins of the grapevine reference genome (V2.1, http://genomes.cribi.unipd.it/DATA/) based on 51, 29, 32 and 79 LEA gene sequences from A . th aliana (Hundertmark and Hincha 2008), tomato (S . lycopersicum) (Cao and Li 2015), maize (Li and Cao 2016) and cucumber (C . sativus L.) (Celik Altunoglu et al. 2016). The candidate members were finally confirmed by NCBI-CDD search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Length of sequences, molecular weights and isoelectric points of deduced polypeptides were calculated by the methods provided at the ExPasy website (ProtParam, available online: http://web.expasy.org/protparam/) (Zhu et al. 2017).
Phylogenetic and structure analyses of LEA genes in V . vinifera
Phylogenetic analyses of amino acid sequences were performed by MEGA7.0. Protein sequences were aligned by MUSCLE. A phylogenetic tree was constructed using the neighbor-joining statistical method with 1,000 bootstrap replications (Di et al. 2018). Domain analysis was performed by SMART (http://smart.embl-heidelberg.de/) (Liu et al. 2014). The genes structure was constructed by Gene Structure Display Server 2.0 (GSDS) (http://gsds.cbi.pku.edu.cn/) (Cheng et al. 2018). Introns and exons of VviLEA genes were obtained from the annotated grapevine genome.
Gene location and duplication analysis of LEA genes in V . vinifera
The locations of the LEA genes were obtained from the gene model annotation files (v2.1). They were exhibited on a circle chromosome map by CIRCOS (Krzywinski et al. 2009). Duplication analyses were processed by all vs. all BLAST of the whole protein sequences, the duplications were identified by MCScanX (Wang et al. 2012), with the e-value of 1e−5, and blocks contained more than five genes were selected. The duplication events related to VviLEA genes were selected and exhibited by CIRCOS (Krzywinski et al. 2009). The tandem duplication was also identified by the MCScanX and all the tandem repeat was identified by e-value <1e−5 and on the adjacent gene loci. Each tandem duplication events was also exhibited on the CIRCOS map with same color dots.
Plants and treatments
Vitis amurensis plantlets were grown under in vitro conditions on solid medium (half-strength B5 (Duchefa Biochemie, Haarlem, Niederlande) solid medium supplemented with 0.2 mg l−1 IBA (Phyto Tech, Lenexa, Kansas, USA), 30 g l−1 sucrose (Beijing Chemical Works, Beijing, China) 0.6% agar (BD, Franklin Lakes, NJ, USA), pH 5.8) and liquid medium (half-strength B5 solid medium supplemented with 0.2 mg l−1 IBA, 30 g l−1 sucrose, pH 5.8) in culture flasks (300 ml) at 25°C under a 16-h light/8-h dark photoperiod and 100 μmol m−2 s−1 light intensity. Six-week-old V . amurensis plantlets were then exposed to solid medium at 4°C for cold stress treatment and liquid medium supplemented with 8% PEG-6000 (BioRular, Danbury, CT, USA) for osmotic stress treatment. The same plantlets with no treatments were used as control. There were three biological replicates in different treatments.
Microarray data analysis
To understand the spatial and temporal expression patterns of VviLEA genes in grapevine, a published microarray data (GSE62744) (Fasoli et al. 2012) was used for further analysis. In this work, 19 samples, including different tissues and organs (e.g. seed, bud, flower, berry, root, stem and leaf) at different developmental stages (Supplementary Table S2), were selected to survey the expression profiles of VviLEA genes. A heatmap was constructed using the pheatmap package of R.
RNA extraction, RNA-seq library construction and sequencing
One fully developed leaf of 6-week-old V. amurensis plantlets with cold and osmotic stress treatments for 12 h was sampled for RNA extraction. Total RNA was extracted from the leaves using TRIzol® Reagent (Plant RNA Purification Reagent for plant tissue) according the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and genomic DNA was removed using DNase I (TaKara, Dalian,Liaoning, China). Then, RNA quality was determined by 2100 Bioanalyser (Agilent, Santa Clara, CA, USA) and quantified using the ND-2000 (NanoDrop Technologies, Wilmington, DE, USA). Only high-quality RNA sample (RIN ≥ 6.5) was used to construct sequencing library. Three biological replicates were employed in different samples, and samples without stress treatment were used as controls.
RNA-seq library was prepared following TruSeq™ RNA Sample Preparation Kit from Illumina (Illumina, San Diego, CA, USA) using 1 μg of total RNA. Shortly, messenger RNA was isolated according to polyA selection method by oligo(dT) beads and then fragmented. Double-stranded cDNA was synthesized using a SuperScript double-stranded cDNA synthesis kit (Invitrogen) with random hexamer primers (Illumina). The synthesized cDNA was subjected to end-repair, phosphorylation and ‘A’ base addition according to Illumina’s library construction protocol. Libraries were size selected for cDNA target fragments of 200–300 bp on 2% Low Range Ultra Agarose followed by PCR amplified using Phusion DNA polymerase (NEB, Rockville, Maryland, USA) for 15 cycles. After quantified by TBS380, paired-end RNA-seq sequencing library was sequenced with the Illumina HiSeq 4000 system (2 × 150-bp read length) from Shanghai Majorbio Bio-pharm Biotechnology Co., Ltd. (Majorbio, Shanghai, China) (Wu et al. 2017).
Data processing and expression analysis
Trimmomatic v0.36 (http://www.usadellab.org/cms/index.php?page=trimmomatic) was used to remove low-quality reads, adapter sequences and reads with ambiguous bases (N) (Bolger et al. 2014). Some parameters were set for high-quality reads, in which the value of LEADING is 3, that of TRALING is 3, that of SLIDINGWINDOW is 4:15 and that of MINLEN is 90. All clean reads were mapped to the V. vinifera reference genome (12X) (http://genomes.cribi.unipd.it/grape/) (Jaillon et al. 2007) using the TopHat (v2.1.1) (Kim et al. 2013), which allowed no more than a two-nucleotide mismatch. Gene annotation and expression quantification were performed using the software Cufflinks (v2.2.1) (Trapnell et al. 2012), and the FPKM (fragments per kilobase of exon per million fragments) method was used to identify DEGs based on the false discovery rate (FDR) of <0.05 and estimated absolute log2(fold change) > 1. The statistical analysis was performed with R.
The original RNA-seq data of cold and osmotic have been deposited in NCBI Sequence Read Archive under Bioproject PRJNA549981. An expression heatmap was constructed using the pheatmap package of R.
qRT-PCR analysis
To verify the expression of LEA genes in V . amurensis, different VamLEA genes (VamLEA2-4, VamLEA2-12, VamLEA3-4, VamDHN3) under cold stress treatment and (VamLEA2-5, VamLEA2-12, VamSMP2, VamDHN3) under osmotic stress treatment were selected for qRT-PCR analysis according to the RNA-seq results. RNA was extracted from one fully developed leaf of 6-week-old V. amurensis plantlets with cold or osmotic stress treatment at 0, 2, 4, 8, 12 and 24 h, and three biological replicates were carried out. The primer of each gene (listed in Supplementary Table S1) was designed through Primer 5, the cDNA used for qRT-PCR analysis was synthesized through the reverse transcription of obtained total RNA using HiScript® II Reverse Transcriptase (Vazyme, nanjing, Jiangsu, China) and the PCRs were performed using AceQ qPCR SYBR Green Master Mix (without ROX) (Vazyme) with the following thermal cycling profile: 95°C for 10 min, 40 cycles of 95°C for 10 s and 58°C for 30 s. In qRT-PCR, actin gene (accession number: EC969944) was used as reference gene.
Cloning of VamDHN3 gene in V. amurensis
Genomic DNA fragment of VamDHN3 gene in V . amurensis was amplified using the primers (VamDHN3F: CCGGAATTCTTGTTGCCGCTTTCATAC; VamDHN3R: CCCAAGCTTCTATGACCTATGGACCTAG) designed based on its homologous gene sequence (VIT_204s0023g02480) in ‘Pinot Noir’ (PN40024) 12Xv1 genome accession. The PCR was performed with DNA polymerase I-5™ 2× High-Fidelity Master Mix (MCLAB, Beijing, China) in a total volume of 50 μl at 98°C for 2 min; 34 cycles of 98°C for 10 s, 58°C for 15 s and 72°C for 15 s; 72°C for 5 min. The PCR product was cloned into pLB-Simple vector (TIANGEN, Beijing, China), and five clones were sequenced. After verifying its sequence, the PCR fragment was ligated into the linear expression vector pSAK277 that was digested by EcoRI and HindIII and treated by T4 DNA ligase (NEB).
Transformation of 41B (V . vinifera × V itis berlandieri) embryogenic callus and generation of transgenic callus
The full-length ORF of grapevine VamDHN3 was ligated into the pSAK277 vector under the control of the 35S promoter and transformed into 41B (V . vinifera × V . berlandieri) embryogenic callus by Agrobacterium tumefaciens EHA105. As described by Ren et al. (2016) with some modifications. 41B suspension cells were cultured in GM liquid medium (GM medium supplemented with 1 mg l−1 NOA (Phyto Tech, Lenexa, Kansas, USA), 30 g l−1 sucrose, pH 5.8), and the transgenic cells were selected in selective medium (GM liquid medium supplemented with 200 mg l−1 timentin (Caisson Lab, Smithfield, UT, USA) and 5 mg l−1 Kanamycin (Phyto Tech, Lenexa, Kansas, USA)). To confirm transgenic embryogenic callus, the specific primers (Supplementary Table S1) for grapevine VamDHN3 gene were used for PCR and RT-PCR. The overexpressed embryogenic callus was selected for further physiological analyses.
Cold tolerance evaluation of VamDHN3-overexpressing grapevine callus
The method for differential thermal analysis that was adopted from Sun et al. (2018) with slightly modifications was used to evaluate the cold tolerance of grapevine callus. The LTEs were directly measured in both of VamDHN3 OE grapevine callus and the EV transgenic callus on GM solid medium (GM liquid medium supplemented with 0.7% agar) for 15 d using the Keithley Multimeter Data Acquisition System (model 2700-DAQ-40) (Keithley Multimeter, Cleveland, Ohio, USA) and were used to evaluate the cold tolerance, and nine biological replicates were performed.
Measurement of EL, SOD and POD in VamDHN3-overexpressing grapevine callus after cold and osmotic treatments
VamDHN3-overexpressing 41B embryogenic callus that was exposed to GM solid medium at 4°C or GM liquid medium with 15% PEG-6000 for 7 d was collected to measure EL, SOD and POD. The EV transgenic callus was used as control. EL was measured by the method described by Su et al. (2015) with modification, and 0.1 g of embryogenic callus was incubated in 5 ml of distilled water. After shaking at 0.5 g and 25°C for 3 h, initial conductivity (C1) was measured with FE30 ( Mettler Toledo, Zurich, Switzerland). The samples were then boiled for 20 min. The conductivity was remeasured after cooling to room temperature as C2. EL was calculated using the equation EL (%) = C1/C2 × 100. The SOD and POD isolation kits ( Solarbio, Beijing, China) were used to measure SOD and POD activities according to the manufacturer’s instructions. Three technical and three biological replicates were performed.
Supplementary Data
Supplementary data are available at PCP online.
Funding
Grape breeding project of Ningxia, China (NXNYYZ201502); major science and technology program of Ningxia Hui Autonomous region, China (2016BZ06).
Supplementary Material
Acknowledgment
We thank Dr. Wenping Qiu at Missouri State University for polishing the article.
Footnotes: The cold and osmotic stress RNA-seq data reported in this article have been deposited in NCBI Sequence Read Archive under Bioproject.
Disclosures
The authors have no conflicts of interest to declare.
Contributor Information
Meilong Xu, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China; State Key Laboratory of the Seedling Bioengineering, Yinchuan 750004, China.
Qian Tong, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Yi Wang, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Zemin Wang, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Guangzhao Xu, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Gathunga Kirabi Elias, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Shaohua Li, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Zhenchang Liang, Beijing Key Laboratory of Grape Science and Enology, CAS Key Laboratory of Plant Resources, Institute of Botany, Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100093, China; Sino-Africa Joint Research Center, Chinese Academy of Sciences, Wuhan 430074, China.
References
- Acosta-García G., Chapa-Oliver A.M., Millán-Almaraz J.R., Guevara-González R.G., Cortez-Baheza E., Rangel-Cano R.M., et al. (2015) CaLEA 73 gene from Capsicum annuum L. enhances drought and osmotic tolerance modulating transpiration rate in transgenic Arabidopsis thaliana. Can. J. Plant Sci. 95: 227–235. [Google Scholar]
- Allen R.D. (1995) Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 107: 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55: 373–399. [DOI] [PubMed] [Google Scholar]
- Artur M.A.S., Zhao T., Ligterink W., Schranz E., Hilhorst H.W.M. (2019) Dissecting the genomic diversification of late embryogenesis abundant (LEA) protein gene families in plants. Genome Biol. Evol. 11: 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F., Du D., An Y., Yang W., Wang J., Cheng T., et al. (2017) Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Front. Plant Sci. 8: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Covarrubias A.A. (2013) Late embryogenesis abundant (LEA) proteins in legumes. Front. Plant Sci. 4: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Olvera-Carrillo Y., Garciarrubio A., Campos F., Covarrubias A.A. (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 148: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bies-Etheve N., Gaubier-Comella P., Debures A., Lasserre E., Jobet E., Raynal M., et al. (2008) Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol. Biol. 67: 107–124. [DOI] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S.B., Mitra A., Baumgarten A., Young N.D., May G. (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Li X. (2015) Identification and phylogenetic analysis of late embryogenesis abundant proteins family in tomato (Solanum lycopersicum). Planta 241: 757–772. [DOI] [PubMed] [Google Scholar]
- Celik Altunoglu Y., Baloglu P., Yer E.N., Pekol S., Baloglu M.C. (2016) Identification and expression analysis of LEA gene family members in cucumber genome. Plant Growth Regul. 80: 225–241. [Google Scholar]
- Charfeddine S., Saidi M.N., Charfeddine M., Gargouri-Bouzid R. (2015) Genome-wide identification and expression profiling of the late embryogenesis abundant genes in potato with emphasis on dehydrins. Mol. Biol. Rep. 42: 1163–1174. [DOI] [PubMed] [Google Scholar]
- Cheng C., Wang Y., Chai F., Li S., Xin H., Liang Z. (2018) Genome-wide identification and characterization of the 14-3-3 family in Vitis vinifera L. during berry development and cold- and heat-stress response. BMC Genomics 19: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappetta A., Muto A., Bruno L., Woloszynska M., Van Lijsebettens M., Bitonti M.B. (2015) A dehydrin gene isolated from feral olive enhances drought tolerance in Arabidopsis transgenic plants. Front. Plant Sci. 6: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S., Panda P., Sahoo L., Panda S.K. (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 8: e23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp N.Y., Reinhardt R., Kube M., Lubzens E. (2010) Late embryogenesis abundant (LEA) proteins in nondesiccated, encysted, and diapausing embryos of rotifers. Biol. Reprod. 82: 714–724. [DOI] [PubMed] [Google Scholar]
- Di F., Jian H., Wang T., Chen X., Ding Y., Du H., et al. (2018) Genome-wide analysis of the PYL gene family and identification of PYL genes that respond to abiotic stress in Brassica napus. Genes 9: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D., Zhang Q., Cheng T., Pan H., Yang W., Sun L. (2013) Genome-wide identification and analysis of late embryogenesis abundant (LEA) genes in Prunus mume. Mol. Biol. Rep. 40: 1937–1946. [DOI] [PubMed] [Google Scholar]
- Dure L., Crouch M., Harada J., Ho T.-H.D., Mundy H., Quatrano R., et al. (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 12: 475–486. [DOI] [PubMed] [Google Scholar]
- Fasoli M., Dal Santo S., Zenoni S., Tornielli G.B., Farina L., Zamboni A., et al. (2012) The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 24: 3489–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., et al. (2014) Pfam: the protein families database. Nucleic Acids Res. 42: D222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R.D., Tate J., Mistry J., Coggill P.C., Sammut S.J., Hotz H.R., et al. (2008) The Pfam protein families database. Nucleic Acids Res. 36: D281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G.A., Chlan C.A., Dure L. (1983) Developmental biochemistry of cottonseed embryogenesis and germination. Plant Mol. Biol. 2: 189–198. [DOI] [PubMed] [Google Scholar]
- Garcia-Bañuelos M.L., Gardea A.A., Winzerling J.J., Vazquez-Moreno L. (2009) Characterization of a midwinter-expressed dehydrin (DHN) gene from apple trees (Malus domestica). Plant Mol. Biol. Rep. 27: 476–487. [Google Scholar]
- Grimplet J., Adam-Blondon A.-F., Bert P.-F., Bitz O., Cantu D., Davies C., et al. (2014) The grapevine gene nomenclature system. BMC Genomics 15: 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zhang L., Zhu J., Liu H., Wang A. (2017) Cloning and characterization of SiDHN, a novel dehydrin gene from Saussurea involucrata Kar. et Kir. that enhances cold and drought tolerance in tobacco. Plant Sci. 256: 160–169. [DOI] [PubMed] [Google Scholar]
- Hsing Y-iC., Chen Z-y., Shih M-D., Hsieh J-S., Chow R-y. (1995) Unusual sequences of group 3 LEA mRNA inducible by maturation or drying in soybean seeds. Plant Mol. Biol. 29: 863–868. [DOI] [PubMed] [Google Scholar]
- Hu B., Jin J., Guo A.Y., Zhang H., Luo J., Gao G. (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31: 1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundertmark M., Dimova R., Lengefeld J., Seckler R., Hincha D.K. (2011) The intrinsically disordered late embryogenesis abundant protein LEA18 from Arabidopsis thaliana modulates membrane stability through binding and folding. Biochim. Biophys. Acta 1808: 446–453. [DOI] [PubMed] [Google Scholar]
- Hundertmark M., Hincha D.K. (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İbrahime M., Kibar U., Kazan K., Yüksel Özmen C., Mutaf F., Demirel Aşçı S., et al. (2019) Genome-wide identification of the LEA protein gene family in grapevine (Vitis vinifera L.). Tree Genet. Genomes 15: 55. [Google Scholar]
- Jaillon O., Aury J.M., Noel B., Policriti A., Clepet C., Casagrande A., et al. (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449: 463–467. [DOI] [PubMed] [Google Scholar]
- Jing H., Li C., Ma F., Ma J.H., Khan A., Wang X., et al. (2016) Genome-wide identification, expression diversication of dehydrin gene family and characterization of CaDHN3 in pepper (Capsicum annuum L.). PLoS One 11: e0161073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., et al. (2009) Circos: an information aesthetic for comparative genomics. Genome Res. 19: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Cao J. (2016) Late embryogenesis abundant (LEA) gene family in maize: identification, evolution, and expression profiles. Plant Mol. Biol. Rep. 34: 15–28. [Google Scholar]
- Liang Y., Xiong Z., Zheng J., Xu D., Zhu Z., Xiang J., et al. (2016) Genome-wide identification, structural analysis and new insights into late embryogenesis abundant (LEA) gene family formation pattern in Brassica napus. Sci. Rep. 6: 24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-C., Li C.-M., Liu B.-G., Ge S.-J., Dong X.-M., Li W., et al. (2012) Genome-wide identification and characterization of a dehydrin gene family in poplar (Populus trichocarpa). Plant Mol. Biol. Rep. 30: 848–859. [Google Scholar]
- Liu H., Yu C., Li H., Ouyang B., Wang T., Zhang J., et al. (2015) Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomato. Plant Sci. 231: 198–211. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen N., Chen F., Cai B., Santo S.D., Tornielli G.B., et al. (2014) Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genomics 15: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv A., Su L., Liu X., Xing Q., Huang B., An Y., et al. (2018) Characterization of dehydrin protein, CdDHN4-L and CdDHN4-S, and their differential protective roles against abiotic stress in vitro. BMC Plant Biol. 18: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwanga R.O., Lu P., Kirungu J.N., Dong Q., Hu Y., Zhou Z., et al. (2018) Cotton late embryogenesis abundant (LEA2) genes promote root growth and confer drought stress tolerance in transgenic Arabidopsis thaliana. G3 (Bethesda) 8: 2781–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy M.R., Almutairi A.M., Gibbons J., Yun S.J., de Los Reyes B.G. (2005) The OsLti6 genes encoding low-molecular-weight membrane proteins are differentially expressed in rice cultivars with contrasting sensitivity to low temperature. Gene 344: 171–180. [DOI] [PubMed] [Google Scholar]
- Mtwisha L., Brandt W., McCready S., Lindsey G.G. (1998) HSP 12 is a LEA-like protein in Saccharomyces cerevisiae. Plant Molecular Biology 37: 513–521. [DOI] [PubMed] [Google Scholar]
- Munir S., Liu H., Xing Y., Hussain S., Ouyang B., Zhang Y., et al. (2016) Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 6: 31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., Liu X., Zhang Z., Wang Y., Duan W., Li S., et al. (2016) CRISPR/Cas9-mediated efficient targeted mutagenesis in chardonnay (Vitis vinifera L.). Sci. Rep. 6: 32289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried J.L., Walker-Simmons M.K. (1993) Group 3 late embryogenesis abundant proteins in desiccation-tolerant seedlings of wheat (Triticum aestivum L.). Plant Physiol. 102: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Salazar J., Moreno S., Espin G. (2017) LEA proteins are involved in cyst desiccation resistance and other abiotic stresses in Azotobacter vinelandii. Cell Stress Chaperones 22: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Liu M., Chen Y., Wang J., Lu C. (2016) Heterologous expression of the dehydrin-like protein gene AmCIP from Ammopiptanthus mongolicus enhances viability of Escherichia coli and tobacco under cold stress. Plant Growth Regul. 79: 71–80. [Google Scholar]
- Shih M.-D., Hoekstra F.A., Hsing Y.-I.C. (2008) Late embryogenesis abundant proteins. Adv. Bot. Res. 48: 211–255. [Google Scholar]
- Stacy R.A.P., Aalen R.B. (1998) Identification of sequence homology between the internal hydrophilic repeated motifs of group 1 late embryogenesis abundant proteins in plants and hydrophilic repeats of the general stress protein GsiB of Bacillus subtilis. Planta 206: 476–478. [DOI] [PubMed] [Google Scholar]
- Su L., Dai Z., Li S., Xin H. (2015) A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 15: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Shen Y., Yin K., Guo Y., Cai X., Yang J., et al. (2019) A late embryogenesis abundant protein GsPM30 interacts with a receptor like cytoplasmic kinase GsCBRLK and regulates environmental stress responses. Plant Sci. 283: 70–82. [DOI] [PubMed] [Google Scholar]
- Sun X., Matus J.T., Wong D.C.J., Wang Z., Chai F., Zhang L., et al. (2018) The GARP/MYB-related grape transcription factor AQUILO improves cold tolerance and promotes the accumulation of raffinose family oligosaccharides. J. Exp. Bot. 69: 1749–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., et al. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnacliffe A., Wise M.J. (2007) The continuing conundrum of the LEA proteins. Naturwissenschaften 94: 791–812. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., et al. (2012) MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40: e49–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A.H., Miroshnychenko O., Kozarova A., Vacratsis P.O., MacRae T.H., Kim J., et al. (2010) Evidence for multiple group 1 late embryogenesis abundant proteins in encysted embryos of Artemia and their organelles. J. Biochem. 148: 581–592. [DOI] [PubMed] [Google Scholar]
- Wu G., Zhang H., Sun J., Liu F., Ge X., Chen W.H., et al. (2011) Diverse LEA (late embryogenesis abundant) and LEA-like genes and their responses to hypersaline stress in post-diapause embryonic development of Artemia franciscana. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 160: 32–39. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang M., Zhang B., Zhang X., Guo L., Qi T., et al. (2017) Genome-wide comparative transcriptome analysis of CMS-D2 and its maintainer and restorer lines in upland cotton. BMC Genomics 18: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Nassuth A. (2006) Stress- and development-induced expression of spliced and unspliced transcripts from two highly similar dehydrin 1 genes in V. riparia and V. vinifera. Plant Cell Rep. 25: 968–977. [DOI] [PubMed] [Google Scholar]
- Yang Y., He M., Zhu Z., Li S., Xu Y., Zhang C., et al. (2012) Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 12: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Ling H., Yang J., Li Y., Guo S. (2018) LEA proteins from Gastrodia elata enhance tolerance to low temperature stress in Escherichia coli. Gene 646: 136–142. [DOI] [PubMed] [Google Scholar]
- Zhang J., Duan Z., Zhang D., Zhang J., Di H., Wu F., et al. (2016) Co-transforming bar and CsLEA enhanced tolerance to drought and salt stress in transgenic alfalfa (Medicago sativa L.). Biochem. Biophys. Res. Commun. 472: 75–82. [DOI] [PubMed] [Google Scholar]
- Zhao P., Liu F., Ma M., Gong J., Wang Q., Jia P., et al. (2011) Overexpression of AtLEA3-3 confers resistance to cold stress in Escherichia coli and provides enhanced osmotic stress tolerance and ABA sensitivity in Arabidopsis thaliana. Mol. Biol. 45: 785–796. [PubMed] [Google Scholar]
- Zhao T., Wang Z., Su L., Sun X., Cheng J., Zhang L., et al. (2017) An efficient method for transgenic callus induction from Vitis amurensis petiole. PLoS One 12: e0179730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., He P., Xu Y., Liu Q., Yang Y., Liu S. (2017) Overexpression of CsLEA11, a Y3SK2-type dehydrin gene from cucumber (Cucumis sativus), enhances tolerance to heat and cold in Escherichia coli. AMB Expr. 7: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang M., Li X., Jiu S., Wang C., Fang J. (2017) Genome-wide analysis of the sucrose synthase gene family in grape (Vitis vinifera): structure, evolution, and expression profiles. Genes 8: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






