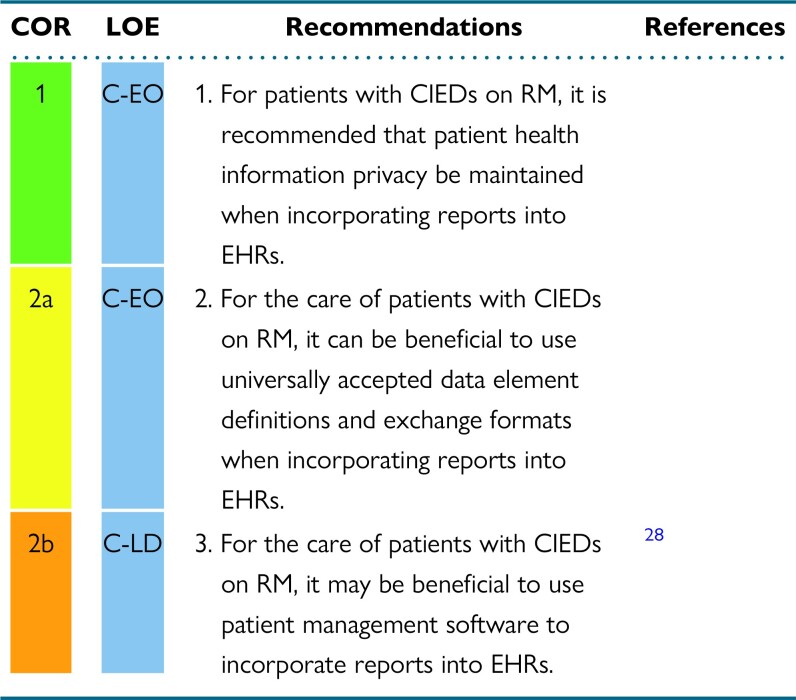
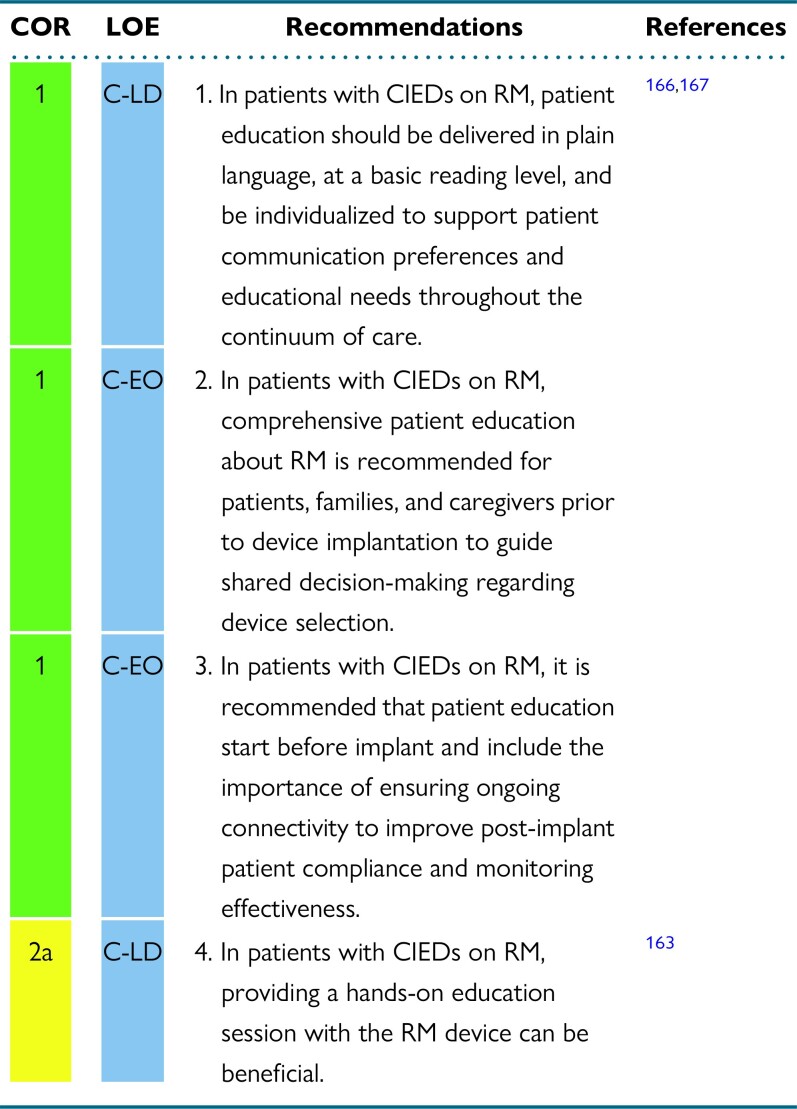
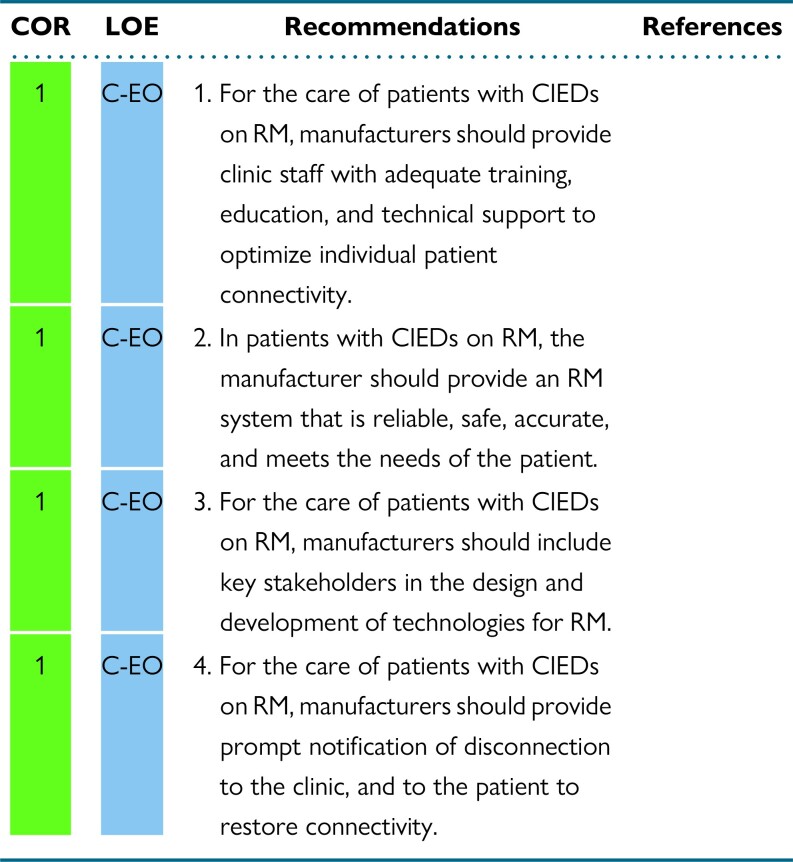
Abstract
Remote monitoring is beneficial for the management of patients with cardiovascular implantable electronic devices by impacting morbidity and mortality. With increasing numbers of patients using remote monitoring, keeping up with higher volume of remote monitoring transmissions creates challenges for device clinic staff. This international multidisciplinary document is intended to guide cardiac electrophysiologists, allied professionals, and hospital administrators in managing remote monitoring clinics. This includes guidance for remote monitoring clinic staffing, appropriate clinic workflows, patient education, and alert management. This expert consensus statement also addresses other topics such as communication of transmission results, use of third-party resources, manufacturer responsibilities, and programming concerns. The goal is to provide evidence-based recommendations impacting all aspects of remote monitoring services. Gaps in current knowledge and guidance for future research directions are also identified.
Keywords: Alerts, Cardiovascular implantable electronic device, CIED, Connectivity, Device clinic, Programming, Remote monitoring
Table of Contents Take-Home Messages
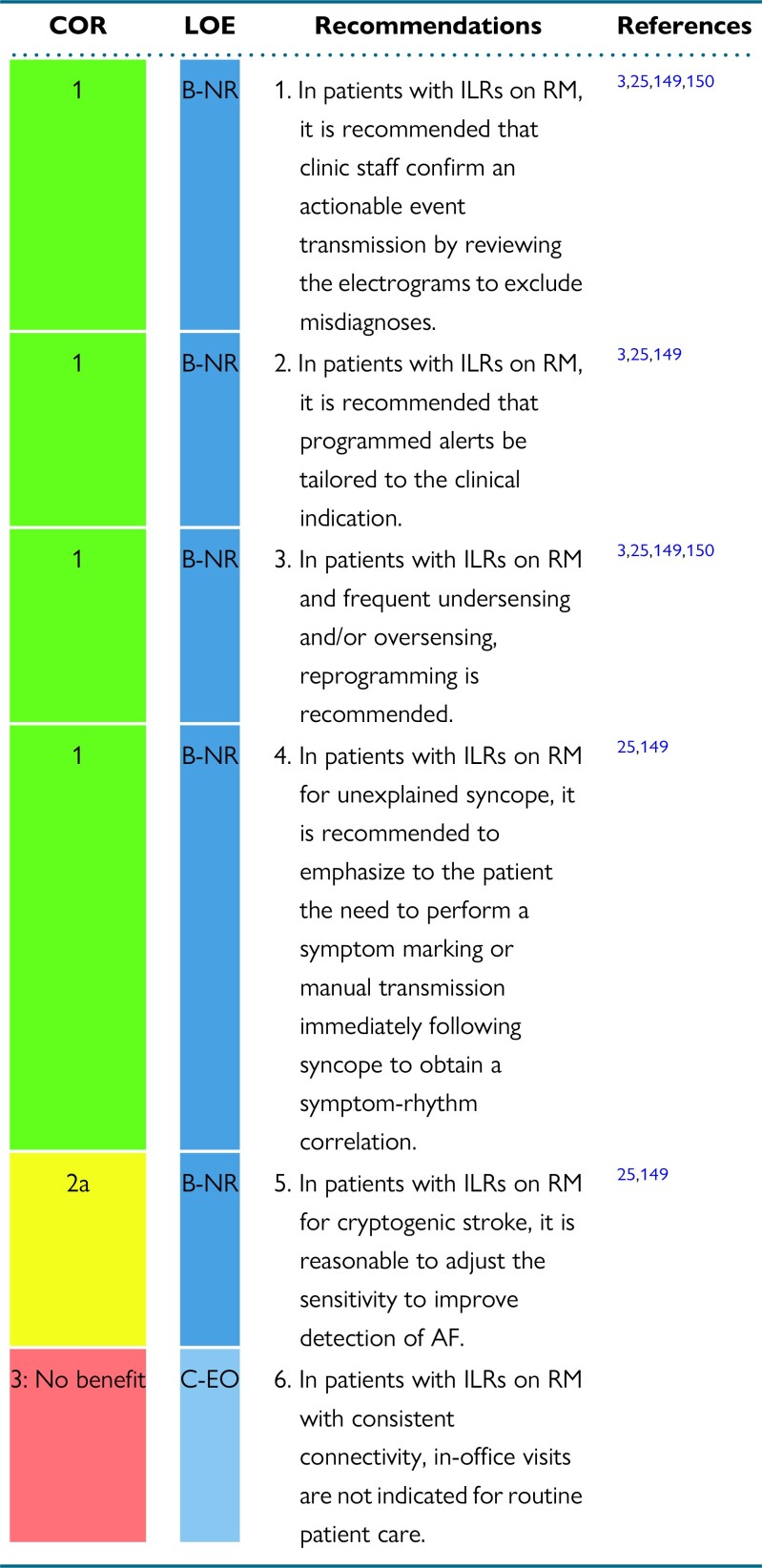
For patients with cardiovascular implantable electronic devices (CIED), remote monitoring (RM) is the standard of care.
Prompt patient enrollment and maintenance of regular connectivity with long-term adherence to RM accomplished by individualized patient and caregiver education is essential to an effective RM program.
Adequate staffing using both clinical and nonclinical personnel with appropriate patient-to-staff ratios and dedicated time to perform defined roles and responsibilities are essential for managing RM clinic workflows.
Clinical staff in the RM clinic should be appropriately educated and/or certified and participate in ongoing quality assurance and improvement programs.
Programming alerts specific to device type and indication with established mechanisms for promptly dealing with high-priority alerts can moderate increasing data volume and workload for RM programs.
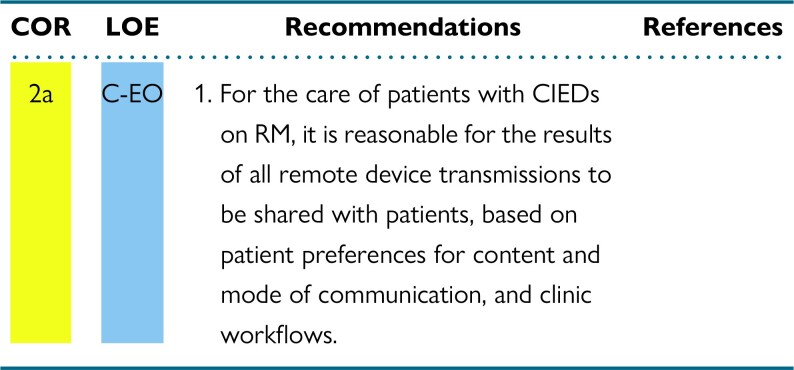
Communicating RM device results with patients, their health care providers, and the patient electronic medical record in a secure and confidential manner should be accomplished according to individual device clinic workflows.
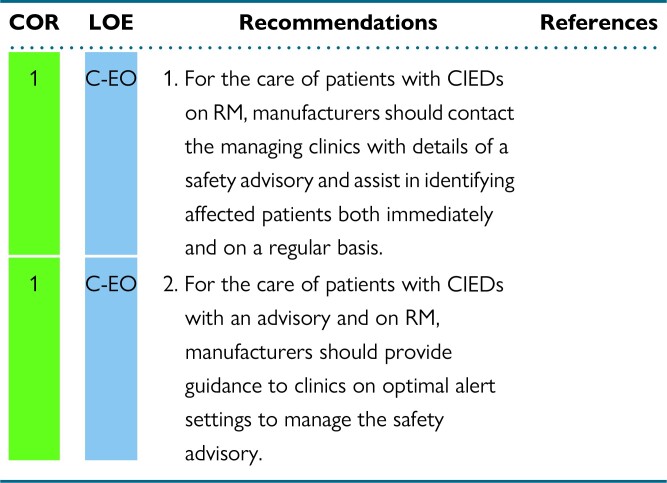
A relationship between RM clinics and device manufacturers for bidirectional exchange of ideas for staff training, patient education, patient care services, and management of safety advisories and recalls is imperative.
Use of third-party resources may offer financial and practical benefits for dealing with increased device clinic volume.
Pediatric patients with CIEDs on RM require scheduling similar to that for RM of adult patients but may have special needs requiring additional considerations.
Implantable loop recorders require immediate connectivity to RM with special programming needs based on the patient's clinical indication for the implantable loop recorder.
Alert-based RM that relies on continuous connectivity allowing for extended time intervals between in-office device interrogations.
1. Introduction
1.1. Preamble
The Heart Rhythm Society (HRS) has developed scientific and clinical documents that have guided clinical care in the management of cardiac arrhythmias since 1996. This HRS-led expert consensus statement was developed in partnership with the European Heart Rhythm Association (EHRA), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS), and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the Pediatric and Congenital Electrophysiology Society (PACES), and the International Society for Holter and Noninvasive Electrocardiology (ISHNE). This international expert consensus statement is intended to provide comprehensive guidance to cardiac electrophysiologists, allied professionals, and other supportive health care technicians and administrative professionals who participate in the management of cardiovascular implantable electronic device (CIED) remote monitoring (RM) programs.
1.2. Document scope and rationale
The years since the publication of 2015 HRS Expert Consensus Statement on Remote Interrogation and Monitoring of Cardiovascular Implantable Electronic Devices (2015 HRS Expert Consensus Statement on RM)1 have seen several key factors that have had direct and lasting impact on RM and RM management. The number of CIEDs implanted on an annual basis has grown to approximately 1.7 million worldwide.2 The number of patients followed remotely has increased significantly. Possible drivers of this increase in RM have been the class 1 recommendation in the 2015 HRS Expert Consensus Statement on RM to use RM for CIED patients as standard of care and the use of RM for staff safety during the COVID-19 pandemic. Also, implantable loop recorders (ILRs), which are designed for alert-based management, have added significantly to the daily volume of data generated for RM clinic workflow.3
The overarching goal of this document is to provide evidence-based and expert consensus recommendations on how to effectively operate an RM clinic, whether hospital or nonhospital based. This takes a joint effort from RM clinic staff―which includes clinical and nonclinical personnel―hospital and health system administrators, payers, manufacturers, and regulators. Many topics considered for this document were identified through a survey of RM device clinic staff. RM is an international issue, but different jurisdictions face very different challenges related to RM availability and reimbursement.
Although RM is beneficial, its increased use can place an extra strain on already limited device clinic resources. This additional workload magnifies preexisting challenges associated with CIED RM. Some of the issues identified by RM clinic stakeholders include managing differences unique to each CIED manufacturer (eg, monitoring hardware, connectivity, programmability, nomenclature, accessibility, and web-based platforms) as well as the dynamic evolution and complexity of new devices and technology. There are other issues specific to the needs of individual RM clinics, which include the coordination of patient enrollment, scheduling, reporting, billing, and interfacing with electronic medical records. Adequate staffing with both clinical and nonclinical personnel is required for an effective and efficient RM program. Appropriate staffing roles, ratios, and credentialing are discussed. Third-party services have emerged that allow for outsourcing some or all RM services. Some of the advantages, challenges, and costs that can come with these third-party services are presented. While RM is available around the world, some regions and jurisdictions face challenges in RM availability, uptake, and reimbursement. The barriers that lead to this disparity in RM use are explained.
Patients and their caregivers are central to the RM process. Education is key to maintaining patient adherence and connectivity. Concepts related to patient and caregiver engagement are suggested for guiding RM clinics in maintaining their interest and understanding the value of the benefits of RM. The pediatric section reviews specific needs of the pediatric patient with a CIED as it relates to RM. Although the pediatric RM recommendations are similar to the adult recommendations, it is recognized that the needs of pediatric patients may be different in specific circumstances. Industry partnership is essential for updating key stakeholders to maintain quality initiatives related to ever-emerging new technology and the potential need to coordinate safety notifications and advisories. Some ideas presented in this document may be a “wish list” of ideas with the hope that manufacturers can provide the means to accomplish certain goals as a collaborative team inclusive of patients and their caregivers.
The document finishes with a discussion of future research and goals for improving RM. Knowledge gaps are evident, and it is only through the ongoing process of acquiring evidence through research that these gaps in knowledge can be addressed.
1.3. Editorial independence
This expert consensus statement is sponsored by the HRS and was developed without commercial support. All writing committee members volunteered their time to the writing and review efforts.
1.4. Organization of the writing committee
The writing committee consisted of internationally recognized experts from 11 countries in the fields of clinical electrophysiology, cardiology, pediatric cardiology, and heart failure (HF) nominated by the partnering and collaborating organizations. HRS strives to ensure that the writing committee contains both requisite expertise and diverse representation from the broader medical community. This is achieved by selecting participants from a wide range of backgrounds representing different geographic regions, genders, races, ethnicities, intellectual perspectives, and scopes of clinical practice and by inviting organizations and professional societies with related interests and expertise to participate as partners or collaborators. In addition, three patient partners were included in the writing committee to ensure a focus on delivering optimal patient care that is in alignment with patients’ wants, needs, and preferences.
HRS has rigorous policies and methods to ensure that documents are developed without bias or improper influence. The HRS policy on relationships with industry (RWI) and other entities can be found the HRS Code of Ethics and Professionalism: Appendix C and in the HRS Clinical Document Development Methodology Manual and Policies. A majority of the writing committee was free of relevant RWI throughout the development of the document and sections with recommendations were written by the writing committee members who were free of relevant RWI. For full transparency, Appendix 1 is a comprehensive list of RWI (both relevant and nonrelevant to the document topic) disclosed by the writing committee members. Appendix 2 is a comprehensive list of RWI disclosed by the peer reviewers.
1.5. Evidence review and formulation of recommendations
This expert consensus statement was developed in accordance with the clinical practice methodology processes detailed in the HRS Clinical Document Development Methodology Manual and Policies: Executive Summary,4 and with the standards issued in 2011 by the Institute of Medicine (now National Academy of Medicine).5
The writing committee reviewed evidence gathered by electronic literature searches (MEDLINE, PubMed, Embase, Cochrane Library, Ovid). No specific year was chosen for the oldest literature. The asterisk (*) was used for truncation to search for all forms of a word, the plus (+) symbol was used to search for plural and singular forms of a word, and the pound symbol (#) was used as a wildcard to search for variant spellings or hyphenation of a word. Search terms included but were not limited to the following: 3rd party, action*, active transm*, adher*, agree*, AICD*, alert, alert#burden, alert#driven, allied professional, arrhythmi*, artifact*, artificial, cardiac, cardiac implantable electronic device*, cardiac resyn* therap*, cardiovert*, care utilization, child*, CIED, clinic*, clinical outcomes, communic*, complian*, comply*, connecti*, consistent, continu*, contract*, cost effective*, CRT-device*, customiz*, defibrillator*, devic*, disparit*, economic impact, economic model, economic outcomes, educat*, efficient*, EHR, electronic health record*, electronic#device*, enrol*, event*, geograph*, heart failure, HF, home monitor*, ICD, implant*, implantable loop recorder, in#office visit, in#person, inclus*, industry, inform*, initiati*, insertable cardia monitor, instruct*, interrog*, leadless, letter*, loop record*, manage*, manufactur*, mode*, monitor*, noise*, nurse, optimiz*, organization*, organizational model, outsource*, pacemaker*, passive transm*, patient compli*, patient educat*, patient monitor*, patient portal, patient#driven, pediatri*, personnel, physiologic* monitoring, program*, randomized controlled trial, RCT, reimburs*, remote monitor*, remote*, report, reportable results, resource, responsibility*, routine results, schedul*, socio#econom*, staff*, subcutaneous cardiac monitor, surveil*, task*, techn*, technical overview, telehealth, telemetry, telemonitor*, third-party, tim*, transm*, utility analysis, utilization, variabil*, website, work#flow*, workforce, workload. Literature searches focused whenever possible on randomized controlled trials, but systematic reviews, nonrandomized and registry studies, cohort studies, and case series were included. Case reports were not used to support recommendations. Evidence tables are included in Appendix 3 and summarize the evidence used by the writing committee to formulate recommendations. References are representative of the totality of data and are not meant to be all-inclusive. Limitations of the evidence base are discussed in individual sections.
To assess consensus after discussions, the writing committee members participated in surveys. A predefined threshold of 70% approval for each recommendation was required, with a minimum quorum of two-thirds of the writing committee. An initial failure to reach consensus was resolved by subsequent discussions, revisions as needed, and re-voting. Writing committee members with RWI did not vote on recommendations concerning relevant topics. The final mean consensus over all recommendations was 98.9%, with 46 of 59 recommendations reaching 100% consensus.
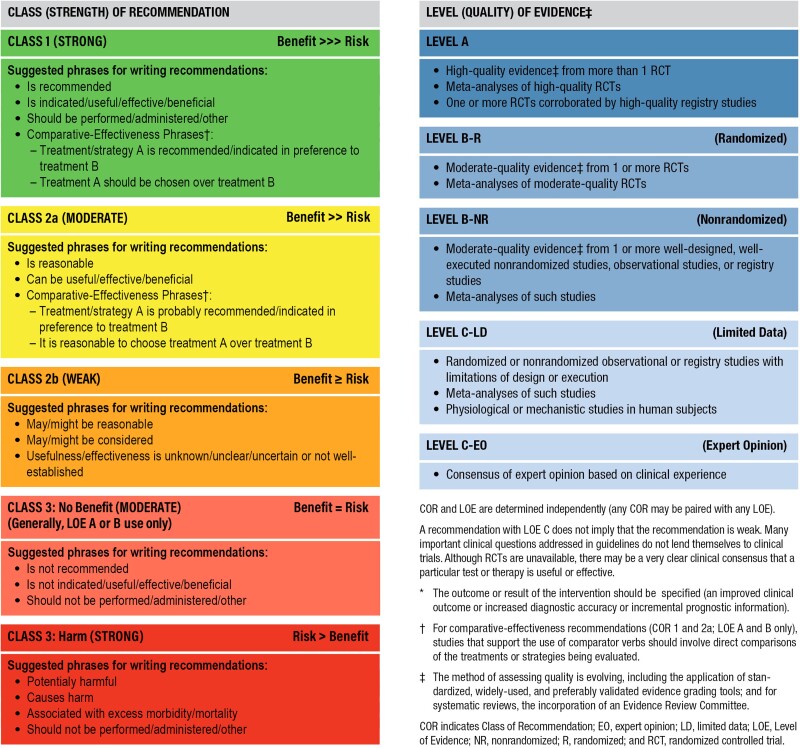
1.6. Class of recommendation and level of evidence
Recommendations in this expert consensus statement are designated with both a class of recommendation (COR) and a level of evidence (LOE). The COR denotes the strength of the recommendation based on the assessment of the magnitude and certainty of the benefits in proportion to the risks. The LOE reflects the quality of the evidence that supports the recommendation based on type, quantity, and consistency of data from clinical trials and other sources (Table 1).6
Table 1.
ACC/AHA recommendation system: Applying class of recommendation and level of evidence to clinical strategies, interventions, treatments, and diagnostic testing in patient care (updated May 2019)∗
Adapted with permission from the American College of Cardiology (ACC) and the American Heart Association (AHA).
For clarity and usefulness, each recommendation is linked to the supportive evidence through the specific references from the literature used to justify the LOE rating, which are also summarized in their evidence tables (Appendix 3). Each recommendation is accompanied by explanatory text. Flow diagrams and appropriate tables provide a summary of the recommendations, intended to assist clinicians at the point of care.
1.7. Document review and approval
The HRS invites public and stakeholder involvement in document development. In addition to patient representation in the writing committee, draft recommendations were posted for public comment, and contribution was solicited from regulatory agencies and patient organizations.
This expert consensus statement was approved by the writing committee and underwent internal review by the HRS Scientific and Clinical Documents Committee. The document underwent external peer review by reviewers appointed by HRS and each of the collaborating societies, and revisions were made by the chairs. A record of writing committee response to reviewer comments and rationale is maintained by the HRS.
1.8. Document updates
The HRS Scientific and Clinical Documents Committee reviews each clinical practice document for currency at least every 5 years, or earlier in the event of newly published data. The literature is routinely monitored to evaluate the continued validity of recommendations.
1.9. Relevant clinical practice documents
Table 2 lists pertinent guidelines and expert consensus statements that the writing committee considered for this document. The included documents contain relevant information for the practical management of the remote device clinic.
Table 2.
Relevant clinical practice documents
| Title | Publication Year |
|---|---|
| 2021 ISHNE/HRS/EHRA/APHRS Collaborative Statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals7 | 2021 |
| 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy8 | 2021 |
| 2021 PACES Expert Consensus Statement on the Indications and Management of Cardiovascular Implantable Electronic Devices in Pediatric Patients9 | 2021 |
| Guidance for Cardiac Electrophysiology During the COVID-19 Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology10 | 2020 |
| HRS/EHRA/APHRS/LAHRS/ACC/AHA Worldwide Practice Update for Telehealth and Arrhythmia Monitoring During and After a Pandemic11 | 2020 |
| EHRA/HRS/APHRS/LAHRS Expert Consensus on Risk Assessment in Cardiac Arrhythmias: Use the Right Tool for the Right Outcome, in the Right Population12 | 2020 |
| HRS White Paper on Interoperability of Data From Cardiac Implantable Electronic Devices13 | 2019 |
| Transparent Sharing of Digital Health Data: A Call to Action14 | 2019 |
| HRS Expert Consensus Statement on Remote Interrogation and Monitoring for Cardiovascular Implantable Electronic Devices1 | 2015 |
| ISHNE/EHRA Expert Consensus on Remote Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs)15 | 2012 |
| HRS/EHRA Expert Consensus on the Monitoring of Cardiovascular Implantable Electronic Devices (CIEDs): Description of Techniques, Indications, Personnel, Frequency and Ethical Considerations16 | 2008 |
| Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE)17 | 2006 |
2. General concepts
Studies since 2015 have continued to show the value of RM and its potential positive effects on morbidity and mortality, cementing RM as an essential part of CIED patient care.18–23 This has led to a deluge of patients on RM,24 resulting in large amounts of RM data and an increase in RM-related workload.25–28 While the 2015 HRS Expert Consensus Statement on RM1 provides recommendations on the benefits of RM and the importance of integrating RM into CIED patient care, it does not account for the challenges related to RM that have been realized in the intervening years. These include the need for organizational RM infrastructure, staffing, and workflow to handle RM data and RM-related work. It also includes ensuring that patients with CIEDs on RM remain connected and at the center of RM programs. There is also a need for developing an improved RM reimbursement structure. The RM device clinic includes a multidisciplinary team involved with the monitoring of CIEDs. The increasing number of CIEDs implanted as well as unexpected challenges such as the COVID-19 pandemic have resulted in high demands on in-person services and a shift toward virtual outpatient clinics.11,29
2.1. Definitions
To standardize the terminology used in the description of RM, terms used in this expert consensus statement are defined in Table 3.
Table 3.
Definitions
| Term | Definition |
|---|---|
| Programmer | A manufacturer-specific device designed to receive and transmit information from CIEDs and allow temporary and permanent programming of CIEDs. |
| Device interrogation | Data transmission from the CIED to the programmer, including device settings and data stored in the CIED memory. The data can be viewed and stored directly on the programmer or transformed to a report that can be exported to a computer, dedicated CIED follow-up software, and internet servers. |
| Device programming | Bidirectional telemetry allowing the programmer operator to assess CIED function, select CIED settings, and optimize system performance tailored to the individual patient's condition in a noninvasive and reversible manner. |
| Home monitor | Remote telemetry device, either a strategically positioned device in the proximity of the patient or a smartphone-based application, able to communicate with the CIED, which serves as a substation to transmit the encrypted data to dedicated servers. |
| Remote monitoring (RM) | Automated remote transmissions of predefined alerts related to clinical events (eg, ICD therapies) or related to device functioning (eg, lead integrity alerts). |
|
RM where the manufacturer-specific transmitter is assigned to an individual patient at enrollment. |
|
RM where the manufacturer-specific transmitter is assigned to a specific site and could be used to collect device data for many individual patients (even if they are not individually enrolled). |
| RM platform | Manufacturer-specific remote web-based communication system allowing access to the encrypted data transmission from the home monitor to individual clinic and/or third-party resources. |
| Third-party resources | External services available using manufacturer-specific RM systems to collect and communicate patient data. This could be software based, which collates data, or personnel based, which can outsource some of the clinics' work. |
| Scheduled transmission | Programmable scheduled transmissions during which routine CIED parameters are collected remotely from the RM platform by members of the remote device clinic team in a format like that obtained during a routine in-person clinic visit. |
| Nonscheduled transmission | |
|
Nonscheduled data transmission initiated by the patient due to experiencing real or perceived clinical events, for which the patient is seeking expert evaluation. |
|
Nonscheduled data transmission initiated by predefined programmed parameters for alerting the clinic of a potentially actionable event. |
| Actionable event | Device-related or clinical event that requires intervention prior to the next scheduled in-person clinic visit. |
| Continuous connectivity | Continuous data collection within the device with automatic transmission using manufacturer-specific transmission frequency, which often occurs once daily. While the data collection is continuous, the transmissions and monitoring are not continuous. |
| Noncontinuous monitoring | Noncontinuous data collection requiring manual transmission using manufacturer-specific transmission either scheduled by the clinic or initiated by the patient. |
CIED = cardiovascular implantable electrical device; ICD = implantable cardioverter-defibrillator; RM = remote monitoring.
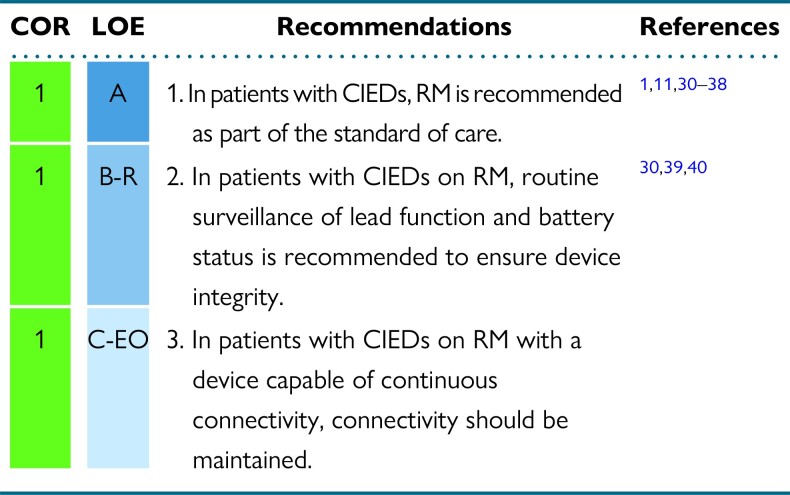
2.2. Remote monitoring considerations
Since the 2015 HRS Expert Consensus Statement on RM1, more recent studies have strengthened the evidence for the organizational benefits of RM and have offered new insights into the impact of RM on patient outcome, particularly in those with HF. RM as first-line strategy for CIED follow-up has been established by the 2020 HRS/EHRA/APHRS/LAHRS/ACC/AHA Worldwide Practice Update for Telehealth and Arrhythmia Monitoring During and After a Pandemic11 and in 2021 ESC Guidelines on Cardiac Pacing and Resynchronization Therapy.8
Recommendations for RM considerations
Synopsis
In patients with CIEDs, RM is recommended as standard of care in the 2015 HRS Expert Consensus Statement on RM.1 Several large, randomized studies as well as large registries and observational studies consistently demonstrated major organizational benefits, such as follow-up optimization, and clinical benefits, with improved patient management and clinical outcome associated with RM.
Recommendation-specific supportive text
-
RM reduces the number of health care visits and increases follow-up adherence and patient retention. It provides earlier detection of actionable events such as atrial and ventricular arrhythmias without compromising safety.30–38 It has been demonstrated to be useful in reducing inappropriate implantable cardioverter-defibrillator (ICD) shocks by early detection of atrial fibrillation (AF) with rapid ventricular response rates,41 T-wave oversensing, electromagnetic interference, and device malfunction. No study to date has shown a reduction in appropriate ICD shocks with RM. RM can facilitate early detection and quantification of AF episodes and arrhythmia burden that may prompt clinical reaction, preventing adverse events such as stroke, shock therapy, and HF. Continuous connectivity allows individualized patient treatment and continuous updating of therapeutic strategy. Observational studies,42–44 subanalysis of randomized trials,37 and metanalysis20 suggest potential benefits of RM in preventing stroke; these findings have yet to be confirmed by randomized studies.45
The ability of RM to prevent disease progression and improve outcomes with HF is still controversial. Modern implantable devices continuously provide diagnostic information to monitor for HF decompensation, creating opportunities for early intervention prior to deterioration and hospitalization. Some trials demonstrated significant benefits of RM46 in reducing hospitalizations and mortality,47,48 as corroborated by real-world large registries.49 Continuous connectivity50,51 and prompt and structured reaction to alerts23,52,53 may be key to improving patient outcomes. Automatic multiparameter monitoring53–55 seems promising in prevention of HF exacerbation. Analysis of mega-cohorts22,56 showed improved survival in patients followed by RM, with high connectivity being the greatest benefit. This is consistent with the pooled analysis of 3 trials50 in which RM reduced all-cause mortality and the composite endpoint of all-cause mortality or worsening HF hospitalization. The similar magnitudes of absolute risk reductions for worsening HF and cardiovascular endpoints suggest that the benefit of RM is driven by the prevention of HF exacerbation.
RM is generally regarded as cost-effective, depending on the health care model and items assessed,57 as it results in reduction of in-hospital scheduled and emergency visits, reduction of diagnostic test burden, and reduction of follow-up duration and physician and nurse time.58–60 RM also reduces patient costs for travel to in-person visits, time off from work, and interruption of daily activities of patients and accompanying persons.61
Conflicting results do exist regarding the impact of RM on patient acceptance and quality of life. Several studies have reported a high rate of patient satisfaction for diverse aspects such as the patient's perceived relationship with their health care providers, ease of use, psychological impact, and the ability to maintain follow-up compliance.35,62–65 Other studies observed neutral effects.66–68
RM allows effective and safe surveillance of device functioning with alerts for battery depletion, circuit disruption, and lead failure, ensuring device function and integrity. Early detection of malfunctions when the patient is still asymptomatic may prevent catastrophic consequences, particularly in cases of lead or device advisory.21,39,69–71 RM also allows for continuous connectivity of pacing thresholds, allowing optimization of battery longevity.72–74
For patients with continuous connectivity, consistent connectivity depends on appropriate functioning of the RM home device as well as on telecommunication system availability and patient adherence to the follow-up plan. Many manufacturers currently provide mobile smartphone applications75 that can facilitate CIED RM transmission and alert patients to the status of RM connectivity, encouraging patient engagement and partnership vital to maintaining RM.76–78 Consistent connectivity is critical to maximize RM benefits by early detection of actionable events, allowing for early intervention for arrhythmias and HF decompensation, with potential to improve overall patient outcomes.19,21,22,56,69–72 Timely reaction to implanted system technical failure as well as to changes in clinical status may impact patient outcomes.73–75
2.3. Remote monitoring payment/reimbursement models
Recommendations for RM payment/reimbursement models
Synopsis
There are an increasing number of economic studies that report the cost-effectiveness58 (ie, increased clinical benefits for additional costs that fall within country-specific, societally accepted thresholds for health care value) or cost savings of RM compared to conventional in-clinic visits.57,59,60,62,66,79–85,87,90,91 Possible mechanisms of economic benefit include fewer clinic visits without clinically actionable events,92 reduction in hospitalizations or emergency department visits due to earlier detection of clinical deterioration,35,93 or a reduction in patient- and caregiver-borne costs related to travel and missed work.59 It is important to note that these prior studies describe the economic outcomes associated with the RM of ICDs, cardiac resynchronization therapy (CRT) devices, and pacemakers (PMs), but not ILRs, for which the evidence of clinical benefit is less certain. Lack of reimbursement is frequently cited as a barrier to widespread adoption of RM86,94 that varies widely by country,15 and within country by health jurisdiction.95
Recommendation-specific supportive text
Given the fundamental differences in the health care financing across health systems, a single prototypic reimbursement model is likely unsuitable for all settings. More generally, however, implementation or reform of existing reimbursement should consider several cost categories: (a) costs associated with the RM system itself, such as hardware, software, and industry service reimbursement; (b) physician fees for RM data interpretation; and (c) hospital- and nonhospital-based clinic overhead costs including those for allied health professionals (AHPs) and administrative and nonclinical personnel. In particular, reimbursement models should account for the effort required to coordinate care (for instance, between device clinics and HF clinics) and the added indirect workload when managing an RM clinic that is not reflected by in-clinic patient evaluations. This additional work may include triaging and reviewing frequent remote transmissions, and timely management of alerts.3 Reimbursement will also need to be adaptable to the potentially evolving landscape of industry charges and ongoing expenses for RM infrastructure, data servers, and technical support personnel. Ideally, reimbursement models should be aligned with the broader goals of the health care system, which may include access, sustainability, quality, and equity. RM could decrease health care costs by reducing and shortening hospital stays if implemented properly.83 Innovative models may be required to facilitate the goals of access and equity, particularly among patients without cell phone coverage or internet access.96 Without focused policy efforts, there is a risk of exacerbating care disparities and excluding vulnerable patients from the potential benefits of RM.
3. Administrative and nonclinical staff
As device clinics are burdened with the increased volume of remote transmissions sent from patient with CIEDs on RM, there is an opportunity to review responsibilities that could be completed by administrative and nonclinical staff to assist in optimizing prompt patient enrollment, patient follow-up, and workflow efficiencies. This could include but is not limited to tasks such as assisting with patient enrollment, handling missed appointment notices, managing patient connectivity, ordering monitors, handling patient transfer requests, scheduling, and maintaining patient information on manufacturer web-based platforms. It is important to define the scope of practice when evaluating appropriate duties for administrative and nonclinical staff.
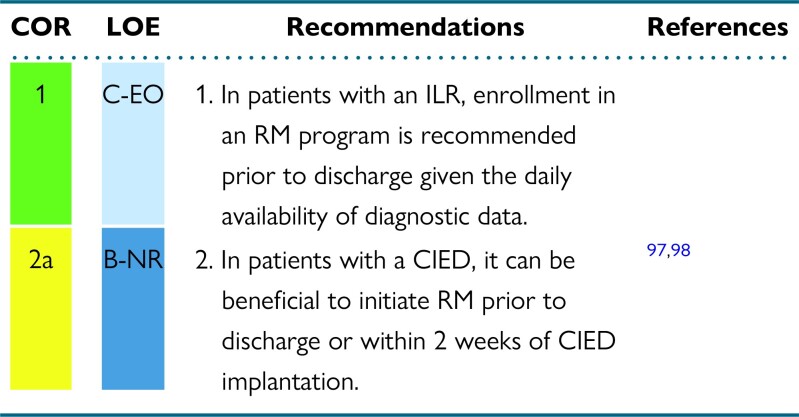
3.1. Patient enrollment techniques
Recommendations for patient enrollment techniques
Synopsis
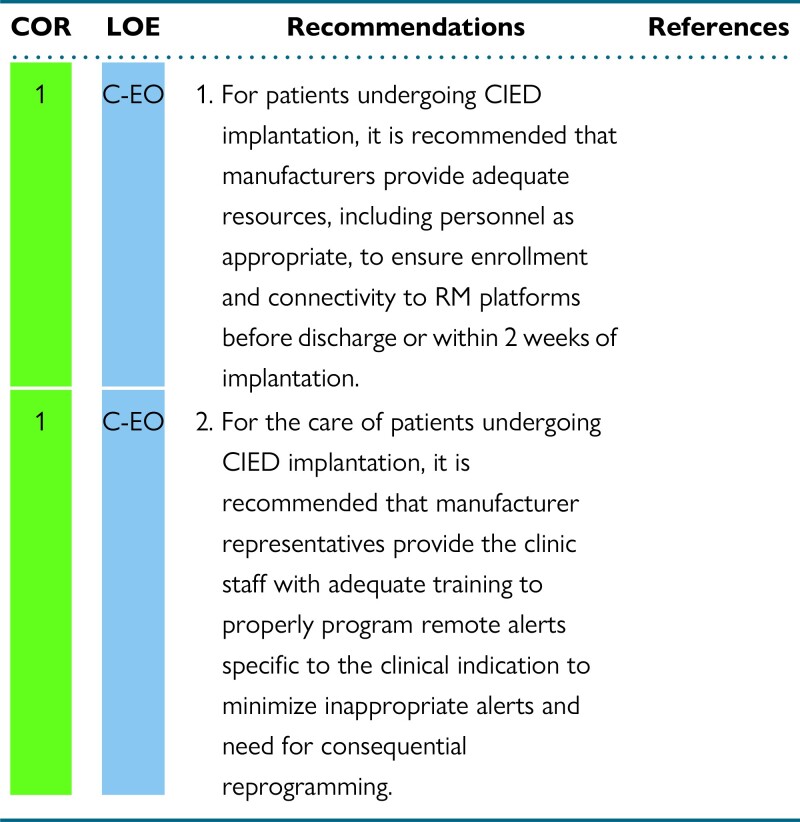
The concept of RM should be discussed as part of patient education before CIED implantation, allowing assessment of the preferred connection method that may affect device selection in certain circumstances. For ILRs, diagnostic data might be available very soon after discharge. It is important that RM enrollment occurs prior to discharge from the hospital or clinic. For non-ILR CIEDs, there is significant variability in practice regarding the timing of patient enrollment in RM. Ideally, patients would be enrolled prior to discharge, with chosen RM equipment. For same-day discharge, this would assure additional safety by providing immediate remote surveillance, replacing what was previously hospital-based surveillance. There are challenges and limitations to this model. Patients may need time to process the life change a CIED implementation could represent. Technical limitations (eg, lack of hardware availability) and patient characteristics (eg, absence of primary caregivers) could also limit the opportunity to initiate RM prior to discharge. In these circumstances, patients should be enrolled virtually or at the first post-implantation in-office visit. Both enrollment options have been used in clinical trials without direct comparison for any clinical outcome. As up to 50% of patients fail to activate their RM receiver,22,99 the use and confirmation of a successful “handshake transmission” can minimize the proportion of patients who fail to activate RM. In-office setup with pairing of the CIED and the RM receiver has been shown to be feasible and to increase the proportion of patients with a successful first RM transmission after discharge.100
Recommendation-specific supportive text
ILRs have daily diagnostic data available, and their entire raison d’être is to provide diagnostic information. Important diagnostic information could occur in the days immediately following ILR implantation. To avoid missing this information, it is important that RM be initiated prior to discharge. In this way, symptom-rhythm correlation will not be lost when the patient is no longer monitored in the hospital.101
It can be beneficial to enroll patients with a CIED in an RM program within 2 weeks of CIED implantation, and ideally prior to discharge if feasible. In a randomized trial comparing RM with conventional follow-up, enrollment in RM before discharge was associated with earlier detection of actionable events without increasing unnecessary in-patient evaluations.98 RM enrollment within 3 months of implant was associated with improved survival in all CIED types, but the survival benefit was greatest in patients with cardiac resynchronization therapy defibrillators (CRT-Ds).97
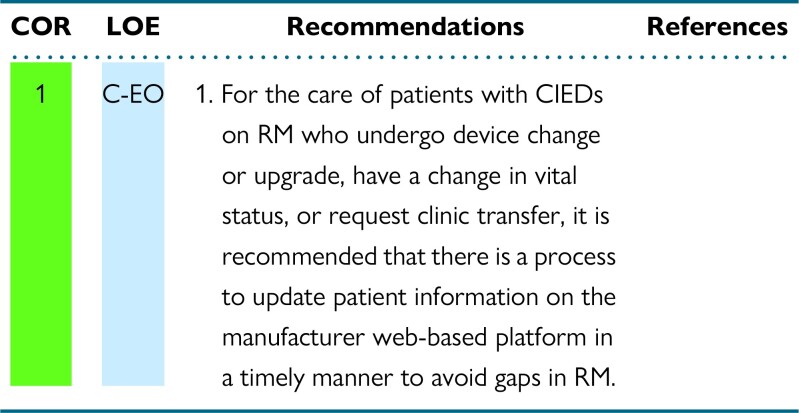
3.2. Managing and updating manufacturer websites
Recommendations for managing and updating manufacturer websites
Synopsis
In patients with CIEDs on RM, timely updates of the device manufacturer's web-based platform are needed to avoid gaps when a patient undergoes device change or upgrade, dies, or requests clinic transfer, or when there are other equipment changes. For continuous optimal care of patients with CIEDs on RM with these circumstances, updating baseline device/demographic information on the manufacturer web-based platform is essential to avoid clinical or demographical gaps with ongoing use of RM. This information-updating process also contributes to maintaining a more accurate roster of patients being followed by device clinic staff, thus improving workflow efficiency.
Recommendation-specific supportive text
RM staff in clinic- or hospital-based programs need to update patient information on the manufacturer web-based platform for those who undergo device change or upgrade before discharge to ensure ongoing monitoring and compatibility of new device with existing RM equipment. These updates on the manufacturer website are also required in the case of a change in vital status (such as patient death), a change in patient's contact information (telephone number or address), or a patient transfer from or to another clinic. Workflows should be established to address whether administrative or clinical staff should address these updates.
3.3. Techniques to optimize patient connectivity
Recommendations for techniques to optimize patient connectivity
Synopsis
Established processes for overcoming challenges with connectivity increase efficiency, thereby reducing response time necessary to address patients’ concerns as well as minimizing time that patients remain disconnected.
Recommendation-specific supportive text
Patient connectivity to RM is critical for the success of an RM program and most importantly for the patient to realize the known benefits of RM (see Section 2.2). Rapid management of disconnected patients is imperative. This responsibility falls on the patient and the device clinic. It is important that every reasonable effort be made by the clinic to reach the disconnected patient. Manufacturers as collaborative partners can assist by providing a notification directly to the patient about a disconnection and can provide technical support if needed. This time-intensive process that includes contacting the patient and troubleshooting the issue(s) can be accomplished by adequately trained nonclinical or clinical staff with adequate time budgeted for this important task.
4. Staffing of remote monitoring clinics
The 2015 HRS Expert Consensus Statement on RM identified the roles and responsibilities of the RM team members.1 The document identified the following as members of the team: physician, advanced practice provider, allied professional, and ancillary staff. In addition, the original document clearly stipulated that an event detected by RM can trigger a full interrogation, office visit, or even an emergency department evaluation, each of which would be associated with the appropriate communication with the patient's additional health care providers. Inherent to RM is the work effort needed to consistently operate and maintain an effective and efficient RM clinic. Furthermore, several important developments have transpired since the 2015 HRS Expert Consensus Statement on RM was published. These include an increase in the number of patients on RM, the availability of ILRs that transmit data daily for years at a time, the continued absence of a national coverage determination that provides a uniform reimbursement model for RM, and the proliferation of multiple operational models to conduct an RM program. These developments require us to reconsider the appropriate staffing requirements for RM clinics.
4.1. Recommended staffing requirements for remote monitoring
Recommendations for staffing requirements for RM
Synopsis
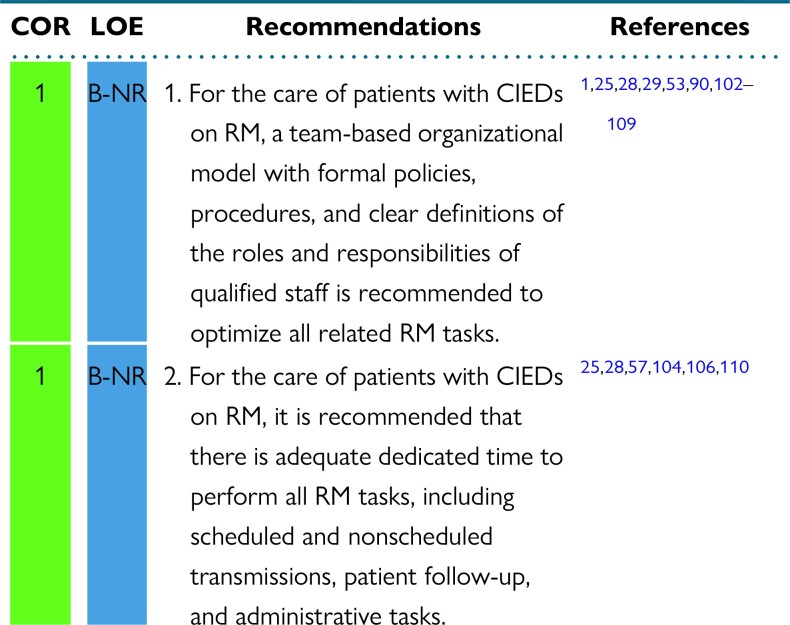
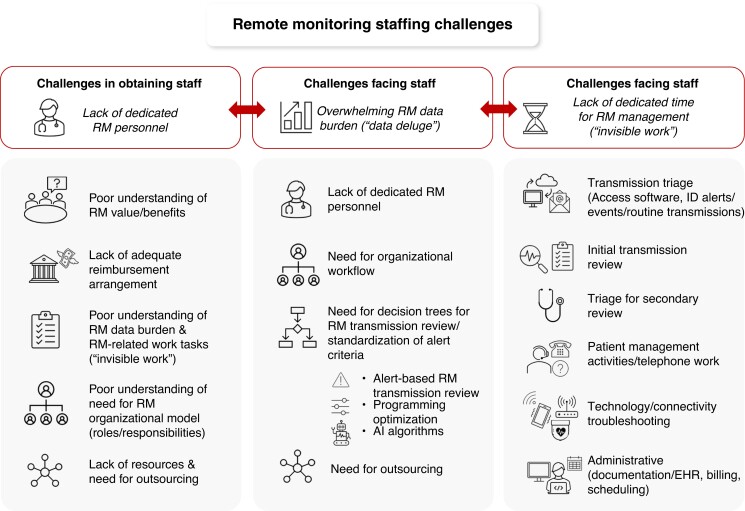
The 2015 HRS Expert Consensus Statement on RM assigned a class 1 recommendation to RM in all CIED patients.1 The adoption of RM was further facilitated by the COVID-19 pandemic, which prompted adoption of digital and virtual technologies to provide safe, uninterrupted monitoring and care for CIED patients.10,11,24,113 However, staffing challenges are multifaceted, interrelated, and continue to persist (Figure 1). The value of RM and its benefits may not be widely known or accepted, which can affect resourcing, reimbursement, and ultimately allocation of staffing for RM monitoring within an institution.28,95,114 CIED RM work hours are incorporated into a “virtual” space; while the patient may not be physically in the clinic, the work burden related to managing CIED RM patients still exists on multiple levels.28 The success of CIED RM programs is directly related to the ability to absorb and complete this workload in an efficient manner. This requires organizational models and infrastructure, with policies and procedures to govern operations and workflow and dedicated time, space, and equipment.25,28,29,90,102–104,108 Critical to this organizational model is a team of CIED RM personnel with clearly defined roles―physicians and advanced allied professionals, nurses and/or cardiac physiologists, technicians, and administrative support staff.1,25,28,29,102,103,107
Figure 1.
Staffing challenges with remote monitoring. AI = artificial intelligence; EHR = electronic health record; RM = remote monitoring.
Recommendation-specific supportive text
The continued uptake of RM has led to a deluge of data from scheduled and unscheduled RM transmissions. Some institutions report receiving > 100,000 transmissions annually.3,103 Although RM transmission volume can be extremely high, RM transmission review can be highly efficient, as long as staff, workflow, and decision trees are in place.29,103,107,111 The Italian HomeGuide Registry structured organizational model of a primary nurse and physician team to review RM transmissions and manage RM patients was found to be highly effective, safe, and efficient.29,107 Subsequent observational studies have corroborated the use of structured organizational models with dedicated staff, workflows, and decision trees, showing improvements in patient management, timely detection of actionable events, and gains in clinic efficiencies.90,103,108,109 Structured workflow with dedicated RM staff (a central monitoring unit) and duties (RM transmission review with forwarding of events to clinical teams) may have contributed to improved outcomes in patients on RM in the implant-based multiparameter telemonitoring of patients with HF randomized controlled trial (IN-TIME).53 These data led to a position paper from the Italian Association of Arrhythmology and Cardiac Pacing calling for RM organizational model standardization and formally recommending the use of dedicated, trained teams to manage RM transmissions in CIED patients.102
The growth of RM and RM transmissions has been accompanied by an exponential increase in workload.3,25,28,103 In addition to reviewing RM transmissions, other RM-related tasks must be completed to appropriately manage CIED patients on RM.28,57,103,105,106,110,112 While review of RM transmissions is rapid, the total RM-related work burden is high.110,112 The EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients) trial showed that total staff time required to manage home monitoring “on” vs home monitoring “off” groups was similar (176 vs 178 min, P = NS).57 In 2021, an international study of RM time-motion workflow28 found that for each RM transmission, at least 15 tasks needed completion, including transmission review and diagnosis, patient communication and clinical action, and electronic charting and billing. Furthermore, there were 17 additional tasks, including triage and scheduling, technology and connectivity troubleshooting, and telephone work, to completely manage a CIED patient on RM.28 Without investment in infrastructure and personnel with dedicated time for RM, the benefits of RM on clinic efficiencies, on patient adherence, satisfaction, and quality of life, and, most importantly, on patient morbidity and mortality cannot be realized, and systems become overwhelmed.3,25,28,90,95,104–106 Lack of formalized policies to perform the “invisible work” of RM prevents personnel from performing at the top of their license, especially if also tasked with other non-RM responsibilities. This leads to job dissatisfaction, burnout, and high staff turnover in under-resourced teams.3,25,28,90,95,104–106
Clinical trials have highlighted the efficiencies and time saved by an RM scheduled follow-up vs an in-clinic follow-up.107,112 However, many patients will have unscheduled transmissions in addition to their scheduled follow-ups and each of these requires triage, data review, and documentation with an associated time cost.3 Unscheduled remote transmissions comprise 27–40% of clinic workload, and as such, sufficient staff resources will need to be provided to review this data.111 Additionally unscheduled transmissions have more actionable events that require longer time for clinical management; this also needs to be taken into consideration when calculating the number of staff required.28,92 When integrating this evidence into clinical practice, the actual remote clinic workload may be underestimated, and thus staffing has become an important issue for many clinics as the number of patients followed by RM continues to increase.
-
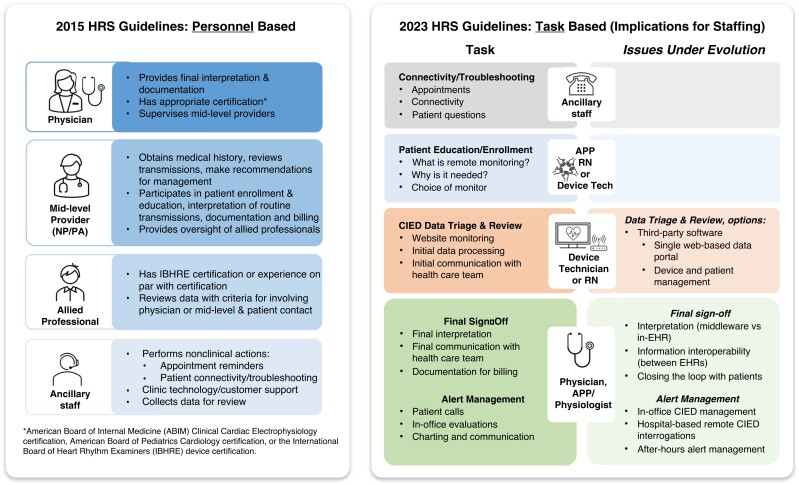
CIED RM comprises multiple tasks; these include patient education and enrollment, connectivity and troubleshooting, data triage and review, alert management, and final sign-off, which includes documentation, communication, and billing. Figure 2 shows task-based responsibilities and implications for staffing as compared to prior guidelines that were personnel based. Requirements for documentation and communication vary extensively from region to region. Each of these tasks is best performed by a different member of the RM team, which includes physicians, advanced practice providers, registered nurses, physiologists, device technicians, and ancillary staff. It is important to understand how many staff members are required to manage RM, keeping in mind that RM does not exist in a vacuum, but rather adds to the non-RM workload inherent to ongoing in-person device evaluations. Future research is needed to establish specific models balancing in-office vs RM roles.
A recent analysis attempted to quantify the mean cumulative staff time required per remote transmission and in-person clinic visit, in a population of clinics using guideline-recommended RM in combination with in-person device follow-up for their patients.28 The analysis determined the average staff time required to review these transmissions, both scheduled and unscheduled, stratifying data for device type (PM, ICD, CRT, and ILR) and location (United States vs Europe). The combined workload of RM transmission review and in-person device follow-up varied based on device type, ranging in the United States from 2.1 hours per patient per year with a PM to 9.3 hours per patient per year with an ILR. Another analysis of a large multicenter cohort of patients undergoing RM and using proprietary patient management software reported the following breakdown of devices: 46.7% PMs, 18.8% non-CRT ICDs, 18.7% CRT-Ds, and 15.7% ILRs.3 Based on these two data sets, assuming a 40-hour work week, an estimated 53.5 hours a week (equivalent to 1.34 full-time equivalent [FTEs]) in Europe and 64.2 hours a week (equivalent to 1.61 FTEs) in the United States are required to manage 1000 CIED patients followed via a combination of RM and in-clinic visits. As the proportion of patients with ILRs monitored remotely increases, the associated workload increases in a disproportionate manner.3,25 Modeling the aforementioned data with an increase in proportion of ILRs to 30% increases the staffing needs to 2.3 FTEs.115 This also did not account for the workload from additional RM-related tasks shown in Figure 2. Furthermore, institutions need to account for time away from the office and the potential need to monitor on weekends. Thus, we estimated that 3.0 FTEs are required to support the care of 1000 CIED patients managed with a combination RM and in-clinic visits with varying proportions of the type of personnel (clinical vs nonclinical) depending on individual clinic workflows and mix of devices being followed. This staffing ratio will differ in practices using third-party staffing resources and may also vary based on other local circumstances.
Figure 2.
2015 HRS Expert Consensus Statement on RM1 vs 2023 HRS Expert Consensus Statement. APP = advanced practice provider; CIED = cardiovascular implantable electronic device; EHR = electronic health record; HRS = Heart Rhythm Society; NP = nurse practitioner; PA = physician assistant; RN = registered nurse; Tech = technician.
4.2. Staff credentialing and qualifications for remote monitoring
Recommendations for staff credentialing and qualifications for RM
Synopsis
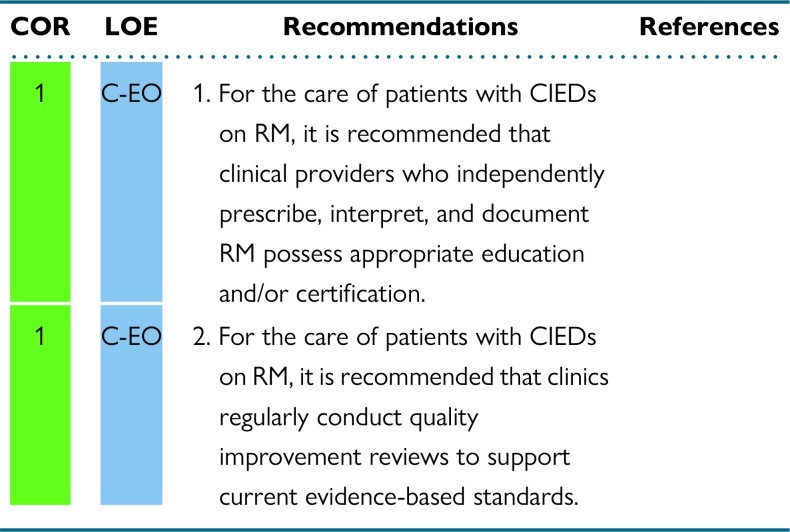
Similar to the 2015 HRS Expert Consensus Statement on RM, this document upholds the recommendation that providers who oversee or independently review, manage, or document and bill for CIED RM demonstrate specific expertise in CIED management by holding appropriate education and/or certification.1,9 Certification and education should be supported by the institute/center of employment. Quality improvement review is essential for maintaining high-quality care. All members of the RM team should receive training and continuing education specific to RM. All staff/personnel involved with CIED RM should engage in quality improvement review to support current evidence-based standards. All related complications should be reviewed at these meetings, and a process should be in place for reporting outcomes and complications with a goal of continuous improvement.
Recommendation-specific supportive text
The International Board of Heart Rhythm Examiners (IBHRE), Certified Cardiac Device Specialist (CCDS) or Cardiac Device Remote Monitoring Specialist (CDRMS), or American Board of Internal Medicine (ABIM), are currently recognized options for certification of CIED clinic personnel.1,9 For AHPs performing initial review and/or triage of RM who do not possess appropriate certification, final remote interpretation should be completed by an appropriately trained professional with such certification. AHPs are eligible for the IBHRE CCDS certification, which focuses on comprehensive clinical knowledge pertinent to CIED management, or CDRMS certification, which focuses specifically on RM technology and interpretation of remote CIED transmissions. Additional details and eligibility requirements for these examinations are listed on the IBHRE website (www.ibhre.org). The 2021 HRS Educational Framework for Clinical Cardiac Electrophysiology recommends continuing education for both physicians and AHPs who provide clinical care for heart rhythm patients.116 It provides a topical framework for education for all professionals delivering heart rhythm care and can be used to structure existing or new education through the HRS. The IBHRE supports continuing education through IBHRE-C3―a program providing up-to-date accredited continuing education (ACE) options for maintenance of certification. Additional options for RM continuing education are available through various entities such as the HRS's online learning platform, HRS 365 (heartrhythm365.org), or the annual Heart Rhythm conference.
Quality improvement is an important part of health care delivery and has been the focus of many international and multidisciplinary collaborations such as the IMPACT registry (Improving Pediatric and Adult Congenital Treatments)117 and Pediatric Cardiac Critical Care Consortium (PC4).118 These registries support the review and transparency of internal data, which then can be compared to other similar programs with the goal of improving care. The Intersocietal Accreditation Commission (IAC) accredits facilities meeting high standards of process and now has accreditation for the CIED clinic that focuses on postprocedural onsite and longitudinal RM of implantable cardiac devices. IAC accreditation requires programs to perform regular quality improvement review.119
5. Technical considerations in remote monitoring
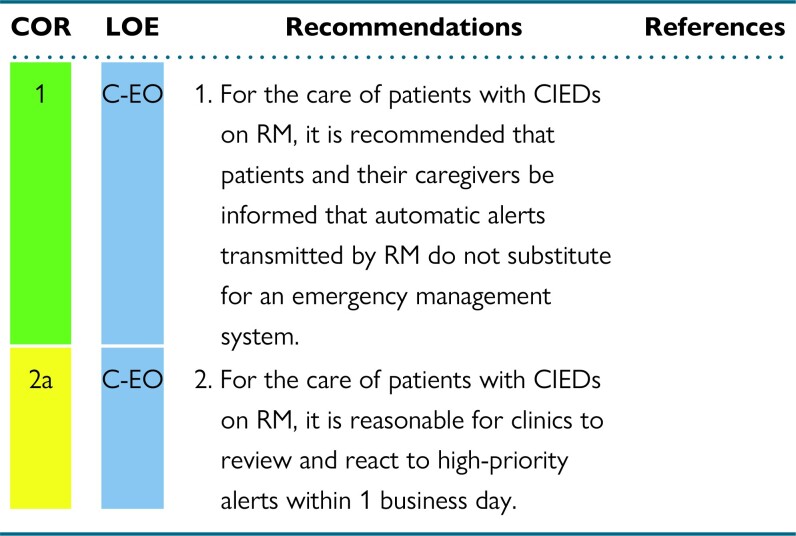
RM technology differs widely by manufacturer. Some RM technologies offer continuous connectivity capabilities, and others offer noncontinuous monitoring capabilities. Continuous connectivity involves continuous data collection within the device with automatic transmission using manufacturer-specific transmission frequency, which often occurs once daily. This assures ongoing surveillance of device and lead parameters with the potential of rapid communication when there is a problem. These monitors are not transmitting on a minute-by-minute, or even hourly, basis. This transmission frequency should be communicated to patients, their caregivers, and their other health care providers. They are not substitutes for an emergency medical system. Noncontinuous monitoring involves noncontinuous data collection and requires manual efforts for transmission to occur. This can be either scheduled by the clinic or initiated by the patient.
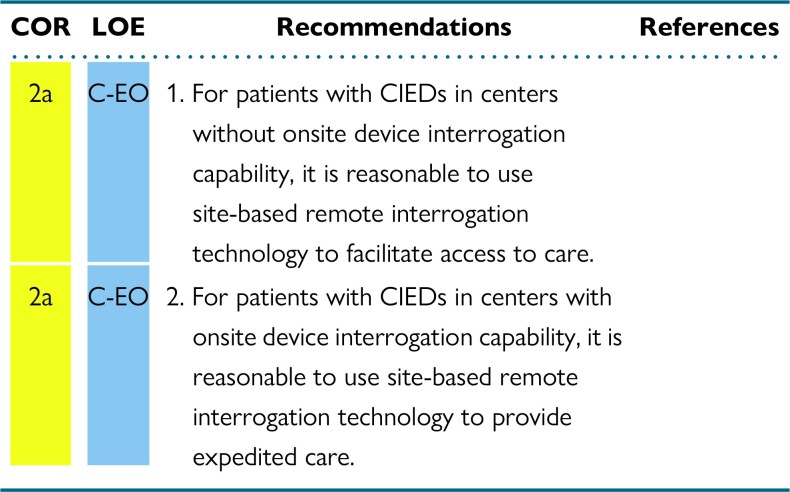
Whereas continuous connectivity may be preferred as it expedites transmission of actionable events, intermittent monitoring should at least meet the recommended frequency of in-person device interrogation. Some centers may be without on-site device interrogation capabilities but still have a need for acute device surveillance. In these instances, site-based RM and an established workflow to connect with device experts may help to reduce time to getting diagnostic information from the device.
5.1. Devices with noncontinuous remote monitoring
Recommendations for devices with noncontinuous RM
Synopsis
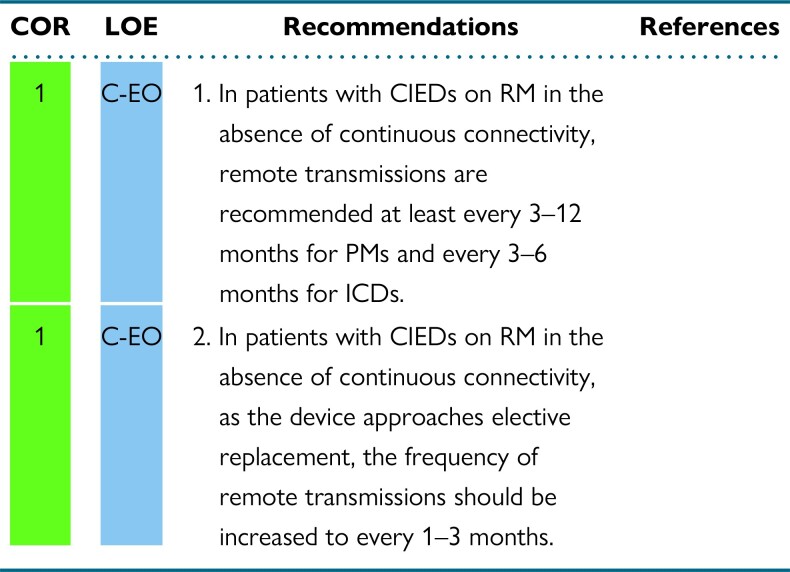
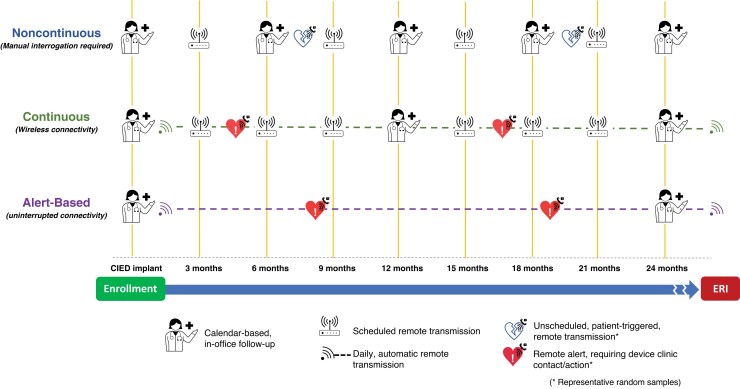
Remote device management may consist of multiple types of transmissions. The first, full remote device interrogation at scheduled intervals, mimics in-office visits. The second, automatic unscheduled RM transmission, consists of continuous connectivity with ongoing, real-time assessment of device function following predefined alert events. In the final type, patients can initiate a remote transmission when they experience an event (Figure 3).8 Evidence regarding the frequency of remote follow-up interrogations and transmissions is lacking. In general, transmissions for ICDs should be more frequent than for PMs due to the increased complexity of their function as well as the in general, sicker population. There will be some circumstances (eg, if a patient is PM dependent) where the transmission frequency for PMs may match or exceed that for some ICDs. In most cases, a CRT-D could be treated like an ICD and a cardiac resynchronization therapy pacemaker (CRT-P) could be treated like a PM for RM follow-up.
Figure 3.
Example of a timeline for patients with cardiovascular implantable electronic devices on remote monitoring. CIED = cardiovascular implantable electronic device; ERI = elective replacement indicator.
Recommendation-specific supportive text
In patients without continuous connectivity, the frequency of routine remote device transmissions should be based on the recommendations for in-office visits of devices that are not monitored remotely. This approach uses a remote platform to mimic traditional in-office visits and does not offer ongoing monitoring and timely communication of any potential problem between visits. We have not altered the previously recommended interval between visits for these patients based on the prior guidelines and expert consensus statements.1,8,15,16 Patients may need to be evaluated more frequently in specific circumstances. These circumstances could include patients who are PM dependent, those whose device is under safety advisory, or those who have other medical conditions that warrant closer assessment.
The 1–3 month frequency of transmissions for devices on RM that approach elective replacement due to battery depletion, and that do not have continuous connectivity, match the indicated frequency of follow-up of cardiac devices without RM.1,8,16 As the CIED approaches end of life and the battery depletes, more frequent monitoring is needed due to the unpredictable risk of rapid falls in battery voltage. As the device gets closer to its elective replacement indicator, or beyond it, monthly monitoring will likely be needed, as the expected operational longevity of the device is only 90 days from that point. This recommendation is not substantively different than those from prior guidelines and expert consensus statements.1,8,16
5.2. Site-based remote monitoring
Recommendations for site-based RM
Synopsis
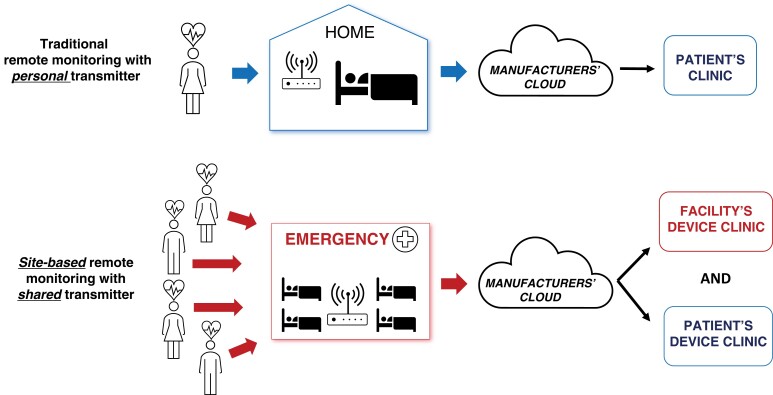
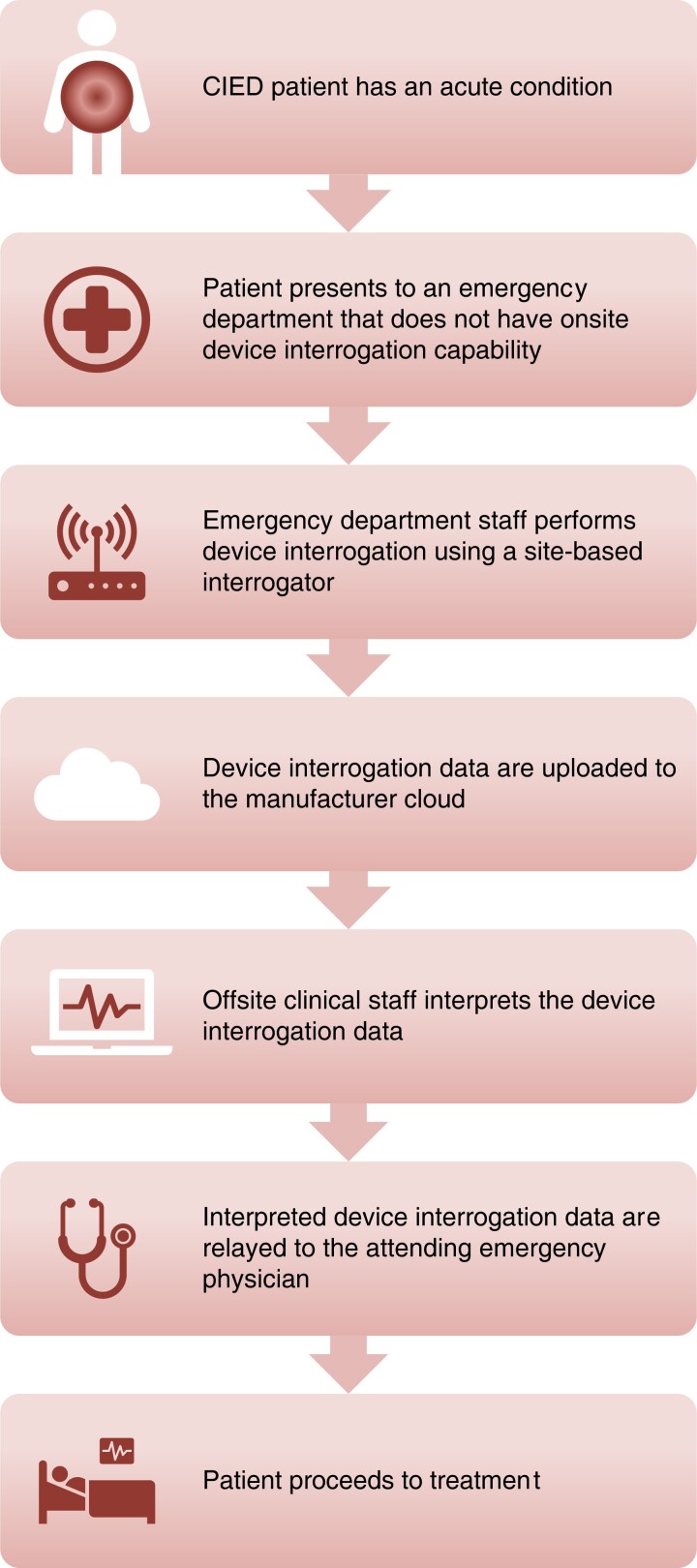
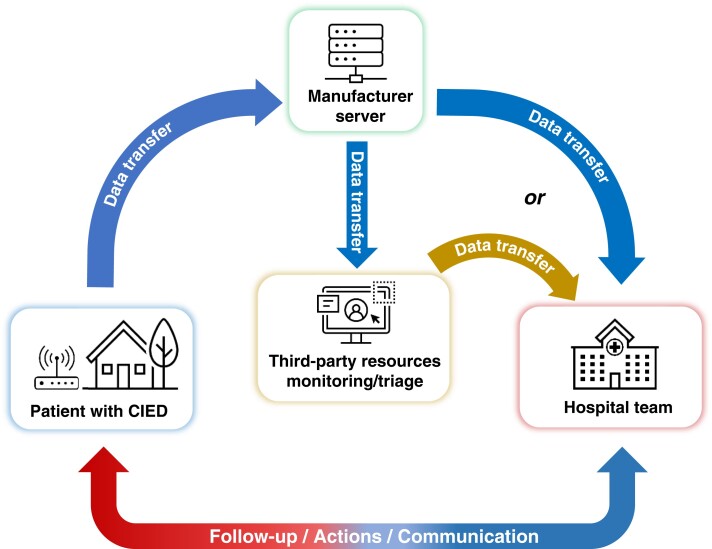
CIED patients frequently encounter situations whereby an immediate, unscheduled device interrogation is clinically necessary. The most common settings for these encounters are the emergency department or perioperative areas where the patient may have presented with cardiac or CIED-related symptoms such as perceived shocks, or unrelated conditions, for urgent surgical interventions, magnetic resonance imaging (MRI) scans, or unplanned hospitalizations. In the past, a device physician, a trained AHP, or a manufacturer representative would be notified. The person notified would then travel to perform the interrogation and discuss the findings with the attending clinician or implanting electrophysiologist. This arrangement is costly, time-consuming, and associated with significant delays to clinical decision-making. A more recent alternative is site-based RM. In this type of RM, a special manufacturer-specific transmitter is provided to a clinical site and can be used to interrogate CIEDs belonging to the associated manufacturer, even if the patient is not individually enrolled in RM. Figure 4 depicts the difference between traditional and site-based RM. These transmitters have no ability to reprogram the device. This tool can be used to expedite CIED device interrogation and patient care when onsite CIED interrogation is not immediately available.
Figure 4.
Traditional personal 1:1 vs site-based remote monitoring. With traditional personal 1:1 remote monitoring (RM), each patient is individually enrolled into the RM program and the RM data is routed to both the facility and the patient's device clinic. With site-based RM, multiple patients can use the system, even if they are not individually enrolled into the RM program, and the RM data is shared with that facility's clinic in addition to the patient's home clinic.
Recommendation-specific supportive text
To leverage the capabilities of RM, most device manufacturers have developed site-based (rather than individual-based) remote transmission systems. These can be placed in clinical areas with the largest need for unscheduled interrogations, including from patients not enrolled in an RM system.120,121 This could include hospital and urgent care centers without on-site device interrogation capabilities. These transmitters can perform manual download of device data onto the manufacturers’ proprietary web portal. They can be downloaded by trained technical staff through a CIED clinic or a third-party monitoring service for review and interpretation by an expert device clinician. (Figure 5.) These systems could be used to extend the reach of RM into rural, isolated, inaccessible, or other underserved areas. Device reprogramming is not possible using these monitoring devices.
Using the Medtronic CareLink Express system to handle 7044 transmissions from the emergency department and operating room, time to device interrogation/interpretation was reduced by 78% to a mean of 22 ± 14 minutes, compared to calling for the local device representative to physically attend the patient's location.120 Only 9.1% of interrogations were clinically actionable. In the overwhelming majority of cases, the device was functioning normally, no device or arrhythmia concerns were found after an expert technical review of the transmitted data, and the attending clinician could be notified and provided with a report of the interrogation. In the minority of cases where there are concerns about device function or reprogramming is required, an in-person evaluation by trained electrophysiology staff with a programmer can be arranged immediately or nonurgently when the clinic reopens. Similarly, using a Boston Scientific LATITUDE Consult installed in 42 hospital facilities to evaluate 509 discreet unscheduled transmissions, device evaluation was completed in less than 15 minutes for 89% of cases and only 10% of transmissions were classified as urgent.121 These site-based RM workflows provide a time-efficient and cost-effective strategy to manage unscheduled device interrogations, even when there are on-site device interrogation capabilities.
Figure 5.
Illustrative example of unscheduled cardiovascular implantable electronic device interrogation using a site-based remote monitoring transmitter. CIED = cardiovascular implantable electronic device.
6. Alert-based remote monitoring
The follow-up and management of increasing numbers of patients with CIEDs is generating larger workloads for clinical staff. Advances in telecommunication technologies can minimize this burden by monitoring chronic conditions during ambulatory care, thus creating more efficient health care systems. In the 2015 HRS Expert Consensus Statement on RM, the recommendation was to interrogate CIEDs every 3 months, either in-person or remotely. In clinical practice, this regimen requires significant effort from both patients and clinic staff. These scheduled visits miss interim events until the next scheduled visit, delaying treatment of actionable alerts. RM systems are evolving to continuous connectivity, where device and disease-related alerts are generated as and when they occur and transmitted often within 1 day.122 Continuous connectivity may facilitate the implementation of alert-based RM, which is a combination of continuous connectivity with clinic visits that are prompted only by the detection of actionable events.
Recommendations for alert-based RM
Synopsis
The implementation of continuous connectivity extends remote patient management beyond periodic calendar-based follow-up (see Figure 3, Continuous).122 In randomized clinical trials, RM was associated with a reduction of hospital use and staff workload and a shorter time to clinical decisions.31,32,35,92,125,126 “Alert-based RM” was increasingly used during the COVID-19 pandemic out of necessity.11 The practice was effective and yielded a positive experience. This form of remote management has the potential to replace structured intermittent device follow-up (whether in-person or remote).92 This could minimize low-value effort, optimize clinic visits for actionable events, and decrease health care costs.89 For alert-based RM to be effective, there must be near-perfect connectivity, robust systems to assure connectivity from the manufacturers, and excellent patient compliance.
Recommendation-specific supportive text
During the last few decades, the number of safety advisories for CIED components has increased due to the increasing complexity of the technology.127,128 Monitoring compromised CIED system integrity is challenging due to the unpredictability of CIED malfunction and the need for immediate action. The addition of continuous connectivity to regularly scheduled remote or in-person follow-up has been shown to allow for more rapid detection of and response to actionable events, including system malfunction.18,29,31,32,35,39,40,42,71,123,124,128
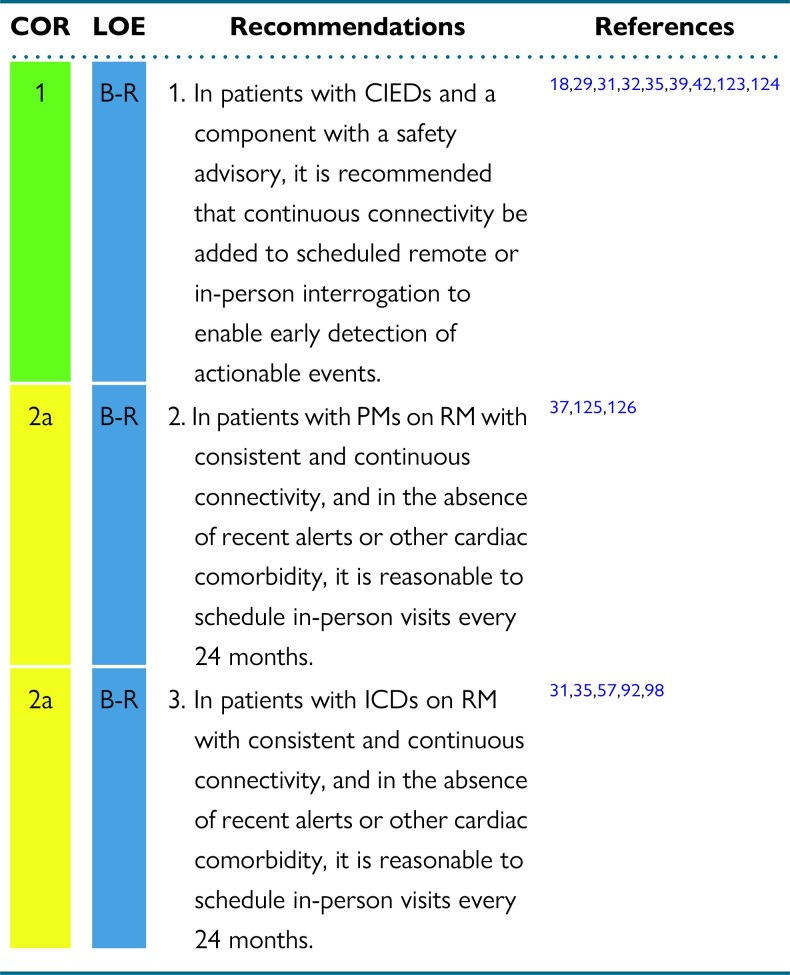
In randomized trials, alert-based RM in patients with a PMs was safe, cost-effective, and an efficient substitute for conventional follow-up, reducing hospital visits and staff workload and facilitating early detection of actionable events. If, however, continuous connectivity is not present, the connectivity is inconsistent for any reason, there have been CIED alerts, or there are concomitant comorbidities, then more frequent in-person visits might be necessary.37,125,126 If shorter follow-up intervals are necessary due to cardiac comorbidities, the most recent RM data may be referenced and an in-person device check may not be necessary.
Randomized trials comparing alert-based RM with conventional follow-up in patients with ICDs on continuous connectivity have shown reduced in-person visits, lower staff workload, almost immediate detection of actionable events, and also improved patient retention, follow-up, and quality of life.31–33,35,57,92,98 If there are inconsistent and noncontinuous connectivity issues, recent CIED alerts, or concomitant cardiac comorbidities, more frequent in-person visits may be necessary.31,35,57,92,98 If shorter follow-up intervals are necessary due to cardiac comorbidities, the most recent RM data may be referenced and an in-person device check may not be necessary.
7. Programming considerations for optimal remote monitoring
RM of CIEDs has facilitated effective surveillance of device function as well as follow-up for arrhythmic events that require clinical intervention, regardless of CIED type.1 RM significantly reduces the volume of in-person evaluations and can decrease the delay from arrhythmia onset to clinical decision, without undermining safety concerns.31,32,35,46 To optimize the efficient use of RM, both optimal device programming and an infrastructure of trained clinicians, who can interpret massive information derived from RM, are required. Although the programming details might vary by platform, preferred programming strategies are those that enable the most accurate detection of arrhythmia or problems, enable earlier detection of arrhythmia or problems, and facilitate subsequent therapeutic measures. All types of CIEDs should be programmed to alert for intrinsic changes of device function that need attention. CIEDs that are capable of monitoring atrial arrhythmia should be programmed to improve detection rate of sustained atrial arrhythmia and its burden. Since CIEDs are utilized by different patient populations with distinct cardiovascular needs, RM should be programmed and stratified according to the indications for the CIED. Patients with ICD or CRT often have underlying HF, which necessitates specific monitoring for signs of HF decompensation. In contrast, monitoring for right ventricular (RV) pacing burden may be of interest in patients without CRT pacing. Programing can reduce nonactionable alerts (see Section 8.2) for patients that have known clinical conditions, such as sinus tachycardia especially during exercise (especially in younger patients), chronic AF, or complete heart block with 100% RV pacing. CIEDs that are indicated for diagnostic purpose rather than therapeutic indication (such as ILRs) should be programmed to optimize diagnostic accuracy and reduce false-positive events caused by undersensing or oversensing.
7.1. Manufacturer and device-specific knowledge
Recommendations for manufacturer and device-specific knowledge
Synopsis
Proper management of patients with CIEDs on RM essentially depends on specific knowledge of the system in use. This knowledge includes the differences in layout and presentation of the various information displayed, but, more importantly, relates to the programmability of parameters and alerts. It is essential that the team that will remotely monitor the patient has a full understanding about the specific system that will be used. This should be considered even before implanting the device, since specific device-related differences may make one CIED/RM system preferable to another system for a particular patient. Manufacturers’ support for training staff about their systems is imperative.
Recommendation-specific supportive text
Although the different RM systems share common principles, they differ significantly in philosophy and practical application, the type and number of programmable alerts, and some proprietary algorithms. The programming and information display screen itself differs considerably among different manufacturers.129 The capabilities and limitations of the different RM systems should be understood when considering the best CIED system for an individual patient. Some examples of these differences between manufacturers are outlined in Table 4.
Table 4.
Remote monitoring system differences between manufacturers
| Manufacturer | Abbott | Biotronik | Boston Scientific | Medtronic | MicroPort |
|---|---|---|---|---|---|
| RM system | Merlin.net | Home Monitoring | LATITUDE | CareLink | SmartView |
| Home monitor | Merlin@home | CardioMessenger II CardioMessenger II-S CardioMessenger Smart |
LATITUDE NXT Remote Patient Management System | MyCareLink Relay home communicator MyCareLink monitor |
SmartView SmartView Connect (Bluetooth-enabled CIED) |
| Smartphone-based RM applications | myMerlinPulse mobile app (ICD and CRT-D) myMerlin mobile app (ILR) |
No | No | MyCareLink Heart mobile app (Bluetooth ILR, IPG, CRT-P, ICD, CRT-D) MyCareLink Smart (IPG, including leadless IPG) |
Yes; limited to a dedicated smartphone delivered to the patient |
| Patient smartphone applications without RM | No | Biotronik patient app (Biomonitor III or IIIm) | MyLATITUDE Patient App | No | No |
| Transmitter | Stationary or mobile | Stationary or mobile | Stationary | Stationary or mobile | Stationary or mobile |
| Connectivity | Bluetooth; mobile network; Wi-Fi; analog phoneline; RF; inductive telemetry | Mobile network; analog phoneline | Mobile network; Wi-Fi; ethernet; analog phoneline | Bluetooth; mobile network; Wi-Fi; analog phoneline | Bluetooth; mobile network |
| Frequency of transmissions | Scheduled FU; daily FU; alert events | Scheduled FU; daily FU; alert events | Scheduled FU; daily FU; alert events | Scheduled FU; daily FU; alert events | Scheduled FU; daily FU; alert events |
| Programmability of frequency of transmissions | Yes | No | Yes | Yes | Yes |
| Programmability of alerts and parameters | Alerts fully configurable online (settings, such as alert notifications, report settings, and data export settings can be done online; adjustments to the patient''s device settings must be done in person). | Alerts can be customized by users into high, medium, and low priorities according to their preferences. Some alerts and parameters can be programmed online. Certain life-threatening alerts cannot be changed as a safety feature. |
RM alerts and parameters can be programmed online through the LATITUDE website. | BlueSync devices: parameters and alerts are configurable via in-clinic programming; notifications for alerts are remotely configurable. BlueSync device (LINQ II only): parameters, alerts, and notifications are configurable remotely. Conexus devices: parameters and alerts are configurable via in-clinic programming; notifications for alerts are configurable remotely; alerts may be manually reset remotely (awaiting approval). |
Alerts can be programmed. |
| Patient-initiated transmission | Yes | No | Yes | Yes | Yes |
| Recommended distance from transmitter | <2 meters | <2 meters | <3 meters | <3 meters | <2 meters |
| Real-time IEGM at remote follow-up | 30 seconds | 30 seconds | 10 seconds | 10 seconds | 7 seconds |
| IEGM of arrhythmic episodes | All memorized | All memorized | All memorized | All memorized | All memorized |
| Provider communication method | E-mail, SMS, fax | E-mail, SMS, fax | E-mail, SMS, fax | E-mail, SMS, website | E-mail, SMS, pager, voicemail, mobile app |
| FDA and CE Mark approved | Yes | Yes | Yes | Yes | Yes |
| Additional features | Electronic health record export compatibility; patient callback feature; CorVue fluid status alert; integrated heart failure website for patients with both CardioMEMS (pulmonary artery pressures) and Abbott CIED. | Electronic health record export compatibility; patient callback feature; HeartInsight heart failure monitoring. | Electronic health record export compatibility; HeartLogic heart failure monitoring; optional Bluetooth weight scales and blood pressure cuffs; configurable data transmission to associated caregivers. | Electronic health record export compatibility; OptiVol thoracic impedance alert; Cardiac Compass HF report; TriageHF integrated HF risk assessment tool. |
Electronic health record export compatibility. |
This is current as of the publication date of this document and is subject to change over time. App = application; CE = Conformité Européene; CIED = cardiovascular implantable electronic device; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy defibrillator; CRT-P = cardiac resynchronization therapy pacemaker; FDA = Food and Drug Administration; FU = follow-up; ICD = implantable cardioverter-defibrillator; IEGM = intracardiac electrogram; ILR = implantable loop recorder; IPG = implantable pulse generator; PM = pacemaker; RF = radio frequency; RM = remote monitoring; SMS = Short Message Service.
7.2. Programming for clinical indications with different types of cardiovascular implantable electronic devices
Recommendations for programming for clinical indications with different types of CIEDs
Synopsis
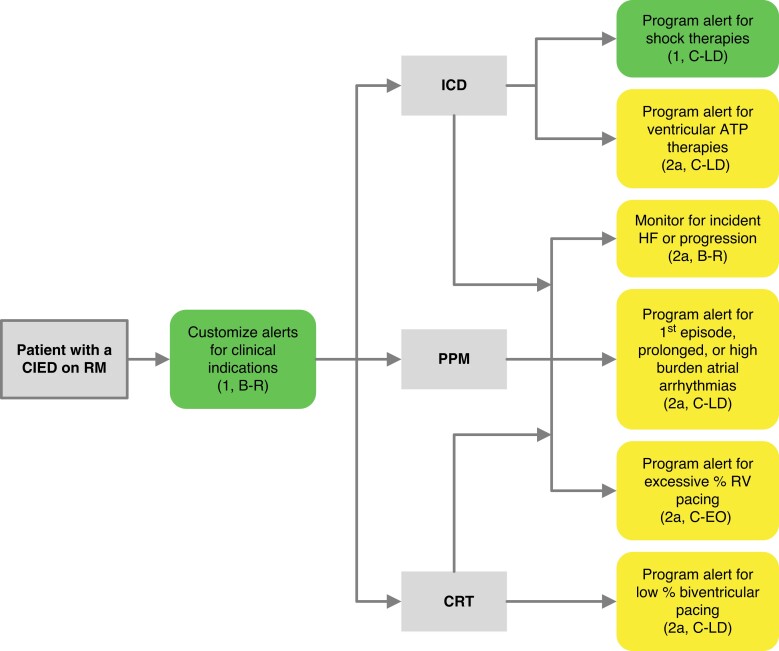
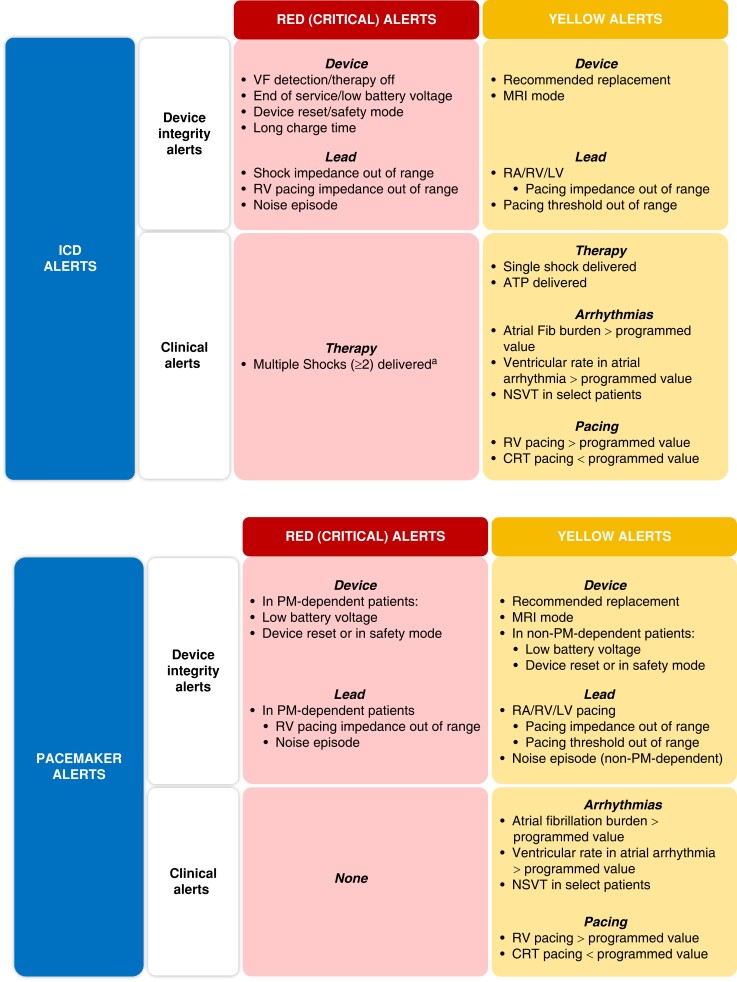
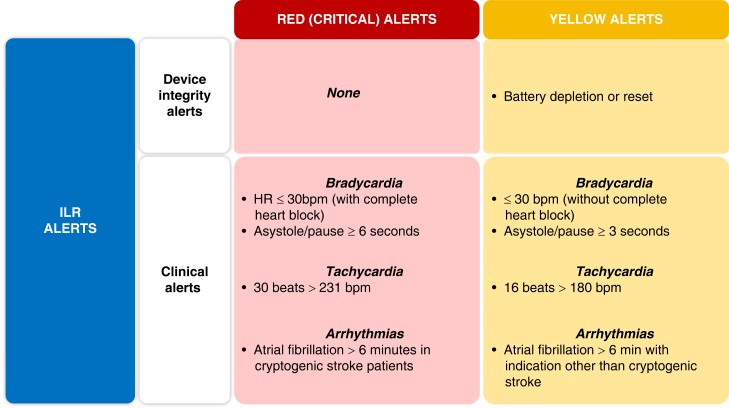
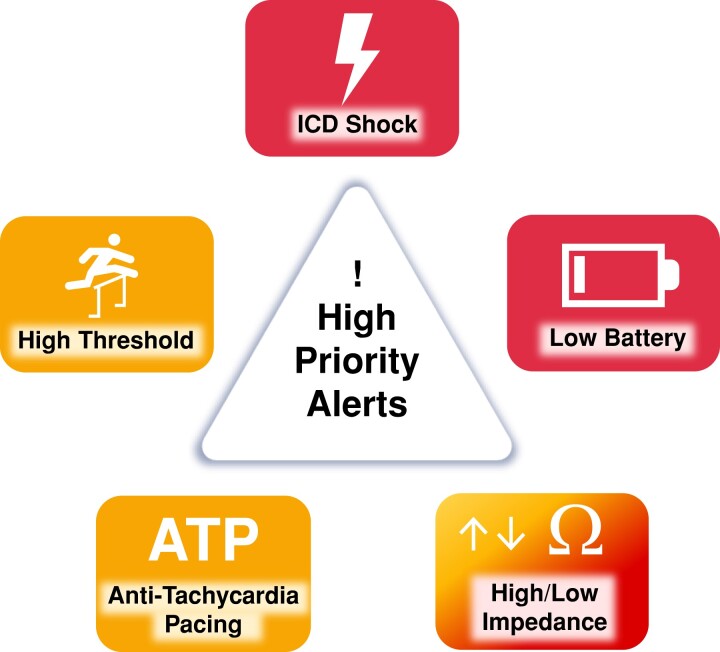
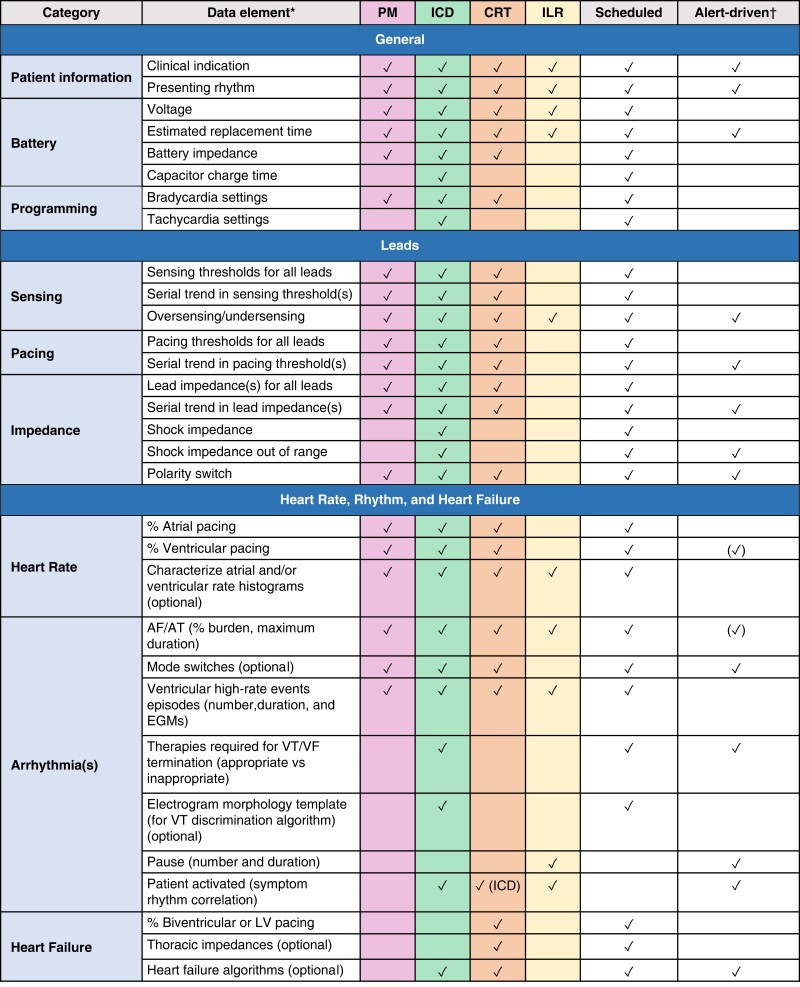
The programming of the devices that will be remotely monitored must be customized based on the capabilities of the system and according to the type of device itself, the clinical characteristics of each patient, and the expectation of the occurrence of clinically relevant outcomes. Some information is important regardless of device type, such as lead integrity, battery longevity, and AF occurrence. In contrast, diagnostics related to HF, risk of life-threatening ventricular arrhythmias triggering shock oranti-tachycardia pacing (ATP) therapies, and percentage of ventricular or biventricular pacing are parameters that will not be relevant for all patients. (Figure 6.)
Figure 6.
Alert recommendations by device type. Color corresponds to the class of recommendation (COR) in Table 1. ATP = anti-tachycardia pacing; CRT = cardiac resynchronization therapy; HF = heart failure; ICD = implantable cardioverter defibrillator; PM = pacemaker; RV = right ventricular.
Recommendation-specific supportive text
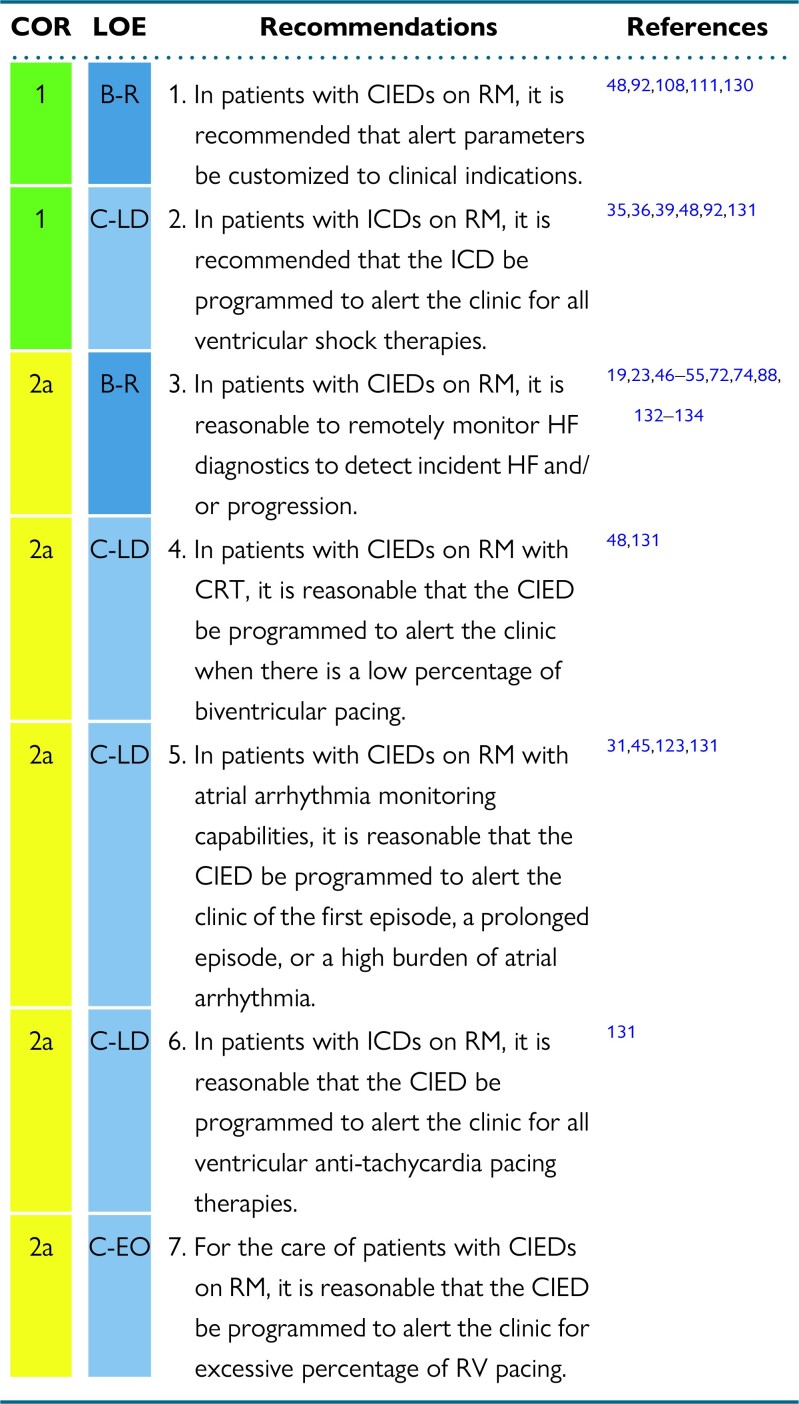
RM alerts should be programmed at a minimum to monitor battery/lead status, lead integrity, and arrhythmic events in virtually all scenarios. Beyond those basic parameters, the patient's clinical profile and needs will drive a customized pool of programming. Examples include the use of LV/biventricular pacing in a patient with a CRT device.
The clinic needs to quickly know about significant CIED events that may indicate the necessity of reprogramming or system revision (eg, battery status; increasing pacing threshold; AF and ventricular tachycardia [VT]/ventricular fibrillation [VF] detection and shock therapy). Shock therapy is usually related to a high-risk event, or a device sensing problem, and the cause of the shock discharge should be checked and appropriately managed. Hemodynamically destabilizing rhythms (VT/VF), unnecessary therapy (nonsustained ventricular tachycardia), inappropriate therapy (AF, oversensing, sinus tachycardia), and noise interference (lead failure) all can result in harm to the patient. Likewise, electrical storm and repeat device discharges can result in adverse physical and psychological effect, in addition to draining the battery.
CIEDs are currently able to monitor several parameters such as heart rate and rhythm, daily activity, and transthoracic impedance for estimating fluid status that can help to identify the patient's clinical status. RM-based risk stratification of HF patients can indicate the possibility of clinical decompensation. A recent meta-analysis of three randomized controlled trials (TRUST [Lumos-T Safely Reduces Routine Office Device Follow-Up], ECOST [Effectiveness and Cost of ICD Follow-up Schedule With Telecardiology], and IN-TIME [Influence of Home Monitoring on the Clinical Status of Heart Failure Patients]) demonstrated improved survival and a reduced composite endpoint of all-cause mortality or HF hospitalizations with RM.50
One important reason for CRT nonresponse is inadequate biventricular pacing. A direct correlation between CRT response and maintenance of high percentage of biventricular pacing (CRT%) has been well proven. The relation between optimal CRT% and clinical outcomes has been studied on different cut-off values (from > 80% to > 98.47%). RM seems to be the best tool for early identification of those patients at risk for CRT% loss and the cut-off > 95% should be the target. Using alert-based RM strategy for CRT% makes it possible to restore optimal biventricular pacing as quickly as possible. 48,56,135,136
Early detection of AF may help to reduce clinical complications, such as preventing inappropriate ICD therapies (the ECOST trial showed a 74% reduction in the number of inappropriate shocks related to supraventricular tachycardia in the RM arm compared with standard follow-up).137 AF may trigger hemodynamic instability and worsen congestive HF, both directly and via the loss of adequate CRT%. The IN-TIME study showed more favorable outcomes and survival in patients with HF and RM of their ICD ([1] patients with a history of AF benefited more from RM than did the patients without AF, and [2] AF was the RM alert that most often led to patient contact).137 Early detection of AF may lead to initiation of anticoagulation therapy after appropriate risk stratification. A large proportion of AF episodes are asymptomatic, and RM shortens the time to its detection (1 to 5 months earlier). Furthermore, an electrogram of an AF episode that has been initially stored in the device, but not yet transmitted, may be absent from the ICD records if overwritten by more recent episodes.29,35,39 If the patient is known to have a high burden of AF where additional transmissions documenting atrial arrhythmias will not alter management, then these alerts can be turned off (see Section 8.2).
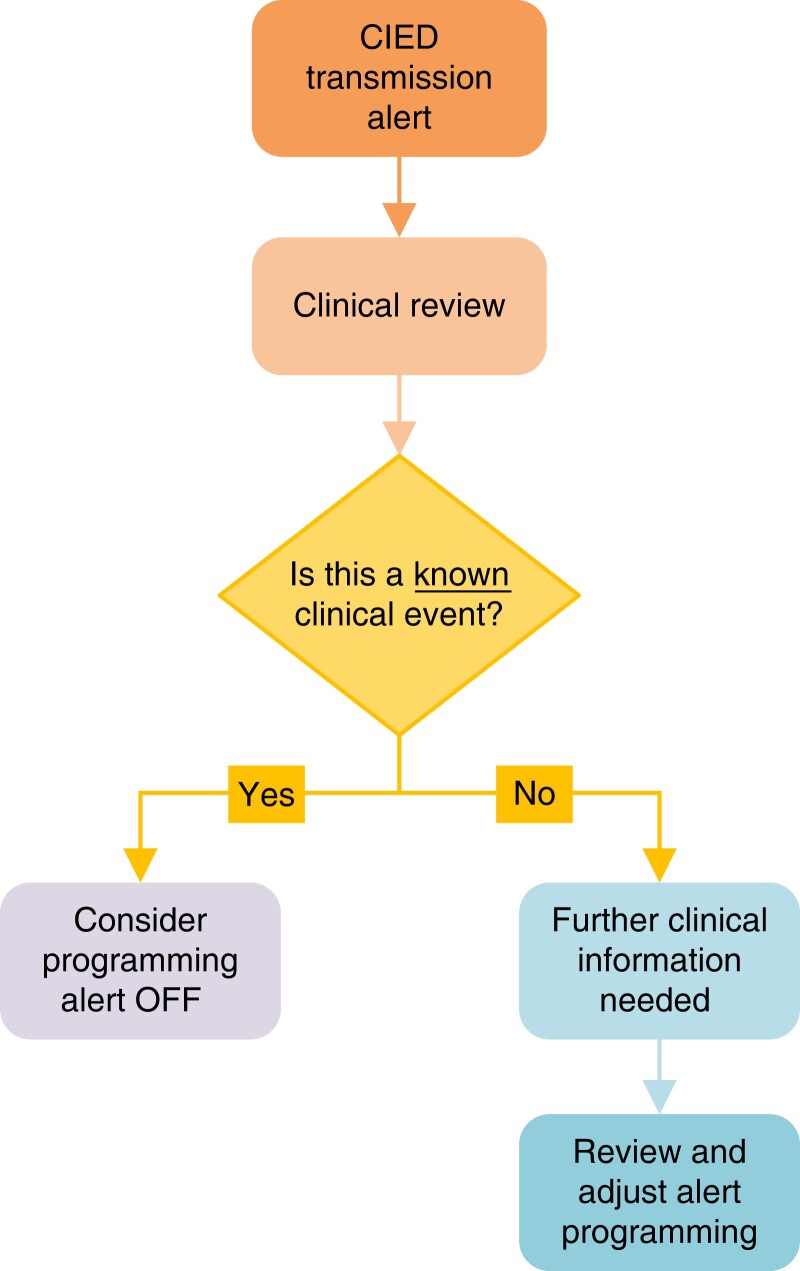
Nonshocked ventricular therapy episode alerts allow reduction in time to medical evaluation for VT and VF events, as shown in the TRUST trial.31 Such episodes may relate to supraventricular tachycardia, P/R/T-wave oversensing, noise oversensing, or lead dysfunction. RM systems that generate alerts following ATP delivery could reduce emergency presentations for ICD shock by 24%.138 Asymptomatic cancelled shock therapy (whether for actual VT or noise) may reduce battery longevity. Their early identification provides an opportunity for prevention of therapy and battery preservation. Early RM notification of ventricular arrhythmia episodes enables preemptive action to avoid further inappropriate shock therapy and/or aborted shocks.73,129,138,130,139
Conventional pacing from the right ventricle results in altered electromechanical ventricular activation that can have detrimental effects on myocardial perfusion and metabolism.140 This can lead to progressive ventricular remodeling, function deterioration, and HF (pacing-induced cardiomyopathy).141 Even though only a subset of patients with RV pacing develop cardiomyopathy (9–19.5%),142–145 the MOST (Dual-Chamber and VVI Implantable Defibrillator) and DAVID (Mode Selection Trial in Sinus-Node Dysfunction) trials suggested a threshold of RV pacing of more than 40% for the development of pacing-induced cardiomyopathy.146 Another study identified a RV pacing burden > 33% as a risk factor.147 Early knowledge of high RV pacing burdens could lead to mitigation strategies that could lower the rate of RV pacing.147 Conduction system pacing (His area or left bundle branch area), with its advantage of minimizing or eliminating electromechanical dyssynchrony, is emerging as an attractive alternative and this concern may not apply.148 If the clinic is aware that the patient is chronically paced in the right ventricle 100% of the time, then this alert is not required.
7.3. Special programming considerations for implantable loop recorders
Recommendations for special programming considerations for ILRs
Synopsis
ILRs can have some unique challenges when implementing RM. These include a high burden of transmissions, frequent misdiagnoses, and the need for a symptom-rhythm correlation in some cases. Due to the relatively high false-positive rate for both AF and sinus pauses with ILRs,25,149,150 clinical staff must confirm putative dysrhythmias by manually reviewing electrograms of individual events to exclude misdiagnosis.3 The sensitivity of detection of atrial arrhythmias could be increased in patients with cryptogenic stroke, to improve detection of symptomatic or asymptomatic AF. Conversely, this might not be desired when the ILR is implanted for unexplained syncope. In this group, educating the patient about the importance of a manual activation at the time of syncope is critical to obtain a symptom-rhythm correlation.
Recommendation-specific supportive text
In 2021, Geneva Remote Technicians archived 20% of all ILR transmissions due to oversensing or undersensing. The most common cause of an archived false alert was misidentification of AF (54%), followed by Pause (33%).151 This analysis demonstrates the burden of false arrhythmic events in remote ILR data management and suggests the need for improved arrhythmia detection algorithms and greater attention to ILR programming of sensitivity and blanking periods. Factors for false-positives of bradycardic episodes in ILRs may include undersensing of small R-wave amplitude, variable signal amplitude, nonphysiologic flatline from loss of electrode contact, and/or saturated sense amplifiers. The incidence of false-positive detections of AF during RM has been reported in 46–86% patients,149 although this might improve with newer devices and newer algorithms.150 When monitoring for tachycardia episodes, low detection and false-positives from undersensing and oversensing due to noise may cause frequent false-positives. To avoid misdiagnosis and potential errors in clinical management, device clinic staff need to confirm an actionable event transmission by reviewing the electrograms.3,25,149
Programming of alert setting on RM should be optimized for different clinical indications.3,149 In unexplained syncope patients, alert transmission is essential for the patient's symptom-rhythm correlation. In patients with symptomatic AF, programmed alert transmission should be set based on rate, frequency, duration, or AF burden. In cryptogenic stroke patients, it important to detect correctly for symptomatic or asymptomatic AF. Careful and tailored programming will help maximize the diagnostic benefit of ILRs.25,149
Primary causes of false-positives in ILR transmissions are signal dropout, undersensing, and atrial and ventricular ectopy.3,149 More recent ILR algorithms use a combination of R-R variability, beat variation, ventricular scatter, heart rate density index, P-wave morphology, artificial intelligence (AI), and filters that evaluate QRS morphology, noise discrimination, and/or pattern detection to aid in rejecting false-positives.150 This advancement of technologies may reduce the false-positive transmissions with ILRs and may help to avoid misdiagnosis. Clinical staff still need to confirm each transmission by reviewing the electrogram for accuracy. If the presence of a false-positive transmission is confirmed after review, reprogramming the ILR could help to minimize future false-positive transmissions.149
The purpose of an ILR in the setting of unexplained syncope is to determine if the syncope is due to an arrhythmia. Many patients can have an arrhythmia that is incidental to their syncope. A symptom-rhythm correlation is critical to establish that the arrhythmia is truly causing syncope. The patient needs to be instructed to initiate a symptom marking or manual transmission directly following the event to communicate the symptoms to the clinic.3,25
ILRs allow clinics to alter their sensitivity to make it more likely, or less likely, to detect AF. For patients in whom the ILR was implanted for cryptogenic stroke, it is of paramount importance that diagnostic episodes of AF are not missed.149 In these patients, the sensitivity of the ILR for AF should be maximized, even if this is at the cost of reduced specificity.25 Device clinic staff need to confirm the AF transmission by reviewing the electrograms and clinically determining if anticoagulation is appropriate.25,49,99
After ILR insertion, if ILR alerts are appropriately programmed for device indication, a wound check has been satisfactorily completed in a timely manner, and continuous RM connectivity is confirmed, future routine in-office visits are not indicated until battery depletion.
8. Managing alerts
RM of CIEDs allows both scheduled remote follow-up and automatic unscheduled transmission of data for predefined alerts. There is clear evidence of a significant reduction in the time to diagnosis and clinical decision-making for unscheduled actionable events, compared to in-clinic follow-up alone.31,35 Early notification of actionable events is associated with improved outcomes and reductions in hospitalizations and health care costs.36,59,87,138 A significant reduction in all-cause mortality for systems with daily RM has been reported in systematic reviews and meta-analyses.50,51
Unscheduled transmissions generate a significant workload for clinics, since all transmissions require triage, review, and documentation.3 The number of alert transmissions received by clinics will vary depending on the programming practices of each individual clinic, device indication, and patient engagement.28 Some manufacturers provide auditory or vibratory alerts to the patient directly, and the use of RM of these alerts can allow the clinics to advise the patients as to the nature of the alert. Given that there are fundamental differences between manufacturers in the number and type of programmable alerts, and how and when these are communicated, this introduces further complexity for clinics in RM alert management.129,152,153 Remote clinics will require robust organizational models and processes in place to safely manage alerts and workload.29,90,108
8.1. Defining high-priority alerts
Recommendations for defining high-priority alerts
Synopsis
The definition of high-priority alerts, and of the response to them, is crucial for organization of care pathways, prioritization of review of alerts, and definition of acceptable response timelines. A significant percentage, if not the majority, of alerts transmitted from remotely monitored CIED are nonactionable alerts (such as detection of known AF)3,27 and concern events that do not require immediate action. In contrast, and as demonstrated in randomized studies, reaction to alerts concerning battery capacity, lead integrity, and therapies delivered by ICDs for ventricular tachyarrhythmias has been shown to reduce adverse outcomes.39,138,139 Arrhythmic events such as shock therapies or ATP therapies delivered by the ICD not only indicate an increased risk for subsequent therapies, but may also indicate lead integrity issues.154 Reaction to these alerts may reduce adverse clinical events.
Recommendation-specific supportive text
Alerts related to lead impedance or pacing threshold may indicate lead failure leading to adverse clinical events, similar to alerts related to battery capacity, as shown in randomized trials.39,48 These alerts should be considered high-priority alerts. “Red alerts” are “critical alerts” that could potentially leave the patient without device therapy, whereas “yellow alerts” are important alerts that do not rise to the level of a critical alert but do warrant review or investigation. Figure 7 indicates what constitutes CIED red and yellow alerts. Shock therapies may indicate clinical deterioration requiring corrective action but may also indicate lead failure.39,48,154 The capability to differentiate between effective and ineffective shocks is not currently available, and therefore multiple shocks delivered ( > 2) should be programmed as a red alert. Evidence for the high-priority character of ATP therapies delivered by the ICD is weaker than for shock therapies. Nevertheless, observational studies indicate that review of alerts related to ATP may be associated with reduced consequent adverse events such as shocks and emergency presentations.138,139 Therefore, it is reasonable to consider such alerts high-priority alerts (Figure 8). Regarding ICD alerts, one set of alerts was tested in a randomized controlled trial and found to be safe during alert-based monitoring.155
Figure 7.
Red and yellow alerts for pacemakers and implantable cardioverter-defibrillators. Red alerts are defined as critical alerts requiring urgent review. Yellow alerts are those that, with early review, may lead to an action that impacts patient outcomes. aMultiple shocks could demonstrate clinical deterioration or be ineffective. ATP = anti-tachycardia pacing; bpm = beats per minute; CRT = cardiac resynchronization therapy; LV = left ventricular; ICD = implantable cardioverter-defibrillator; ILR = implantable loop recorder; MRI = magnetic resonance imaging; NSVT = nonsustained ventricular tachycardia; PM = pacemaker; RA = right atrial; RV = right ventricular; VF = ventricular fibrillation.
Figure 8.
Remote monitoring alerts that should be considered “high-priority.” Red corresponds to red alerts, and yellow corresponds to yellow alerts. High/low impedance can be considered a red or yellow alert. ICD = implantable cardioverter-defibrillator.
8.2. Programming considerations to minimize inappropriate alerts
Recommendations for programming considerations to minimize inappropriate alerts
Synopsis
Unscheduled alert transmissions and the associated workload are an ongoing concern for RM clinics.3 Alerts for arrhythmias that are already known, and where further alerts will not lead to any clinical action, can contribute to this workload.138 In an observational study, two-thirds of transmissions were reported to have shown at least one abnormal event, with the majority requiring no clinical action.156 Most nonactionable alerts occur for known arrhythmias. Individualizing RM alerts to suit patients’ individual clinical circumstances can improve clinic efficiency.24 Ideally, optimized alert programming would occur at the time of implantation based on individual clinical circumstances.
Recommendation-specific supportive text
Once sufficient information has been gathered regarding a particular alert and there is no further requirement to receive this information, it is recommended that RM settings should be adjusted to receive only those alerts that will result in a clinical action and minimize further nonactionable alerts (Figure 9). The ongoing triage and review of unscheduled alerts has a significant impact on clinic workload and productivity. In one study, many unscheduled alert transmissions were not clinically relevant, as the information was already known and action had already been taken.157 Alert transmissions occur more frequently in patients with known AF. Alert settings could be adjusted based on the patient's clinical situation to minimize unnecessary alerts.111 In addition, Morimoto et al. describe a high abnormal event rate (66.7%) in remotely monitored patients, but a low “critical event” rate (4.1%).156 One method to reduce the volume of incoming transmissions is by reprograming alert criteria from the CIED default settings to allow only critical events to be received.108 This may include turning off alerts for a high burden of ventricular pacing in patients with known AV block or turning off AF alerts in patients known to have a high burden of chronic AF.
Figure 9.
Minimizing alerts for nonactionable events. Each cardiovascular implantable electrical device transmission alert is critically reviewed by a credentialed clinician. If the event is a known clinical event that has been previously addressed, the specific alert may be programmed OFF and it is no longer considered an actionable event. If this is not a known clinical event, more information is needed about the patient's status. Based on the review of additional information, the alert may be adjusted or programmed OFF. CIED = cardiovascular implantable electronic device.
8.3. Timeline recommendations for alert management
Recommendations for timeline recommendations for alert management
Synopsis
Management of alerts is a crucial part of the workflow in each remote device clinic. Reactions to critical alerts and noncritical alerts need to be tailored to the individual patient. Reactions to alerts transmitted during nonbusiness hours are frequently a particular concern for RM clinics. Concerns include potential liability for nonimmediate response to incoming high-priority alerts. Nevertheless, there is no evidence for the need of an immediate response to alerts outside of the working hours. Most RM sites are not able to provide an immediate response. For this reason, it is crucial that patients and their care providers realize that RM should not be misinterpreted as a replacement for an emergency system.
Recommendation-specific supportive text
For logistical and organizational reasons, the vast majority of RM sites operate during normal working hours158 and are not able to provide review and response to these alerts outside of their normal business hours. The benefit of reactions to alerts outside of the normal business hours has not been investigated. It is important that patients and their caregivers have an emergency management plan for a device problem in addition to RM.
Timely review and appropriate reaction to high-priority alerts are considered crucial. The definition of the term “timely” regarding RM is unclear, as there are no direct comparisons of clinical outcomes based on differing reaction times to critical alerts. Prompt review of alerts is important. Indirect evidence shows that daily transmissions have better patient outcomes than less frequent transmissions.51 Most device clinics are not designed for 7 days per week and/or 24-hour per day monitoring. Alerts should be addressed within 1 business day, with red alerts prioritized. A workflow based on review of transmissions within 1 business day was highly effective for detection and management of clinical events without overwhelming staff and resources.29 It should be emphasized to patients that RM is not an emergency management system.
9. Remote monitoring reporting
A report of the results of RM must offer detailed information on device functioning and clinical status of the patient. RM device transmissions include continuous or noncontinuous, alert-based, and patient-activated manual transmissions. Each transmission includes comprehensive data on device technical functioning and data related to arrhythmias and HF. All data should be stored in the medical record.
9.1. Communication of the remote monitoring report to patients
Recommendations for communication of the RM report to patients
Synopsis
Patient awareness of RM transmissions is critical to improve compliance and maximize clinical benefits. Sharing results of patient transmissions should take into account patient preference, considering culture and psychological and social status, as well as clinic workflow to avoid work overload. Modes of communication must be consistent with local regulations.
Recommendation-specific supportive text
Timing and mode of communication of transmission results to patients depend on clinical relevance and actionability of detected events. Actionable events should be promptly communicated, and clinical reaction performed with a timely plan. Routine transmission and nonactionable event reports, as well as billing, may be delivered periodically. According to patient preference and clinic workflow, reports may be delivered by mail, secure e-mail, patient portal, or directly during in-person visits. Patient health information privacy should be emphasized. Incorporating device reports into the electronic health record (EHR) is crucial for data availability to all hospital services. This may include routinely to the primary care provider or urgently during emergency needs, to maintain optimal patient care.
9.2. Components of a comprehensive report
Similar to reports generated from in-clinic device visits, the content of an RM report will depend on clinical and technical factors as well as the type of CIED.1,16 An additional consideration for RM is the context of remote transmission (ie, scheduled vs automatic/alert-driven). Suggestions for the components of a comprehensive remote follow-up/interrogation report are provided in Figure 10.
Figure 10.
Suggested components of remote monitoring report. *Additional manufacturer-specific features can be added if these data will influence patient care/management and be used by the local device clinic (eg, activity monitor, heart rate variability, heart failure algorithms). Listed data elements (✓) would be considered the mandatory minimal data set for a remote monitoring report, unless otherwise denoted. †Availability of alerts are manufacturer specific. These may include but are not limited to RV lead integrity alert, RV lead noise, lead impedance out of range, AT/AF daily burden (as per user set threshold), excessive charge time, and low battery voltage. Alert programming should balance patient safety and actionable clinical information with the burden of nonactionable alerts that device clinics may encounter with undiscerning programming. AF = atrial fibrillation; AT = atrial tachycardia; CIED = cardiovascular implantable electronic device; CRT = cardiac resynchronization therapy; EGM = electrogram; ICD = implantable cardioverter-defibrillator; ILR = implantable loop recorder; LV = left ventricular; PM = pacemaker; RV = right ventricular; VT = ventricular tachycardia; VF = ventricular fibrillation.
9.3. Techniques for incorporating reports into electronic health records
Recommendations for techniques for incorporating reports into EHRs
Synopsis
RM information must be private but also should be available in the patient's health record. The element definitions and report formats should be universally accepted, regardless of the manufacturer, to be compatible with management software that can be incorporated into EHRs. Manual or automatic capability for populating databases is essential both for the regular follow-up and for unscheduled transmission including site-based interrogations that can occur in emergency departments, intensive care units, or even long-distance service.
Recommendation-specific supportive text
Solutions for data management continue to evolve. The goal is to be able to access data stored in CIEDs in a timely fashion, review the data for clinically valuable information, and present this information in a contextual and relevant format in the EHR system for the physician following the patient with a CIED. Device data downloads need to be accessible to, for example, operating rooms and emergency departments for interpretation by trained technical staff. With accessibility, however, comes challenges to maintaining the privacy of patient health information as well as potential issues related to liability when using RM-related services.159
Patient device data is regarded as a part of the patient file and should be stored in the hospital information system. The manufacturer's web-based platform provides data in a protected environment that are suitable for incorporation into the hospital information system. However, the diversity and incompatibility of sources for current device data is a barrier to high-quality patient care. Universally accepted data element definitions and exchange formats facilitate accurate and efficient data transfer (regardless of manufacturer) from RM servers and programmers to EHRs and other data repositories, thereby increasing clinical and administrative efficiency, patient safety, regulatory post-marketing surveillance, product advisory recall management, and clinical research.1,25,160,161
For both remote transmission review and in-person clinic visits, time-saving protocols are driven by steps for documentation in EHRs. The staff time required per remote and in-person device check is less when a vendor-neutral CIED management software is used. A recent publication demonstrated that sites using management software reduced, on average, the total staff time to review a remote transmission by 2.1 minutes (11.5 vs 13.6 minutes) and an in-clinic visit by 2.2 minutes (50.4 vs 52.6 minutes). When extrapolated to an average clinic size of 5758 patients, the use of such software was associated with an estimated 10.1 cumulative staff hours saved during a clinic day (50.7 hours per week) based on 171 weekly clinic visits and 1335 weekly remote transmissions. Annually, this translates to 2639 hours of staff time saved, equivalent to 1.4 annual FTEs.28
10. Patient education for remote monitoring
Device implantation represents the beginning of a lifelong relationship between patients and their device clinic care providers. It requires communication and trust between patients and providers. Anticipating patients' concerns before and after device implantation as well as adapting to their changing needs over time increases the likelihood that patients understand and adhere to the quality-improving and life-saving implications of RM.104,162,163 Evidence to support specific algorithms for timing and methodologies of patient education is sparse. Available evidence is confounded by technology complexity, including different transmission platforms supported by the various device companies.162 In-person instruction after device implantation is optimal, especially in the early follow-up period, to establish personal relationships with device clinic staff.164,165 This is often not possible based on a patient's geographic distance from a device clinic. Also, patients may be overwhelmed by the experience of device implantation, so their retention of in-hospital education may be suboptimal. There are different phases of patient education on RM (Figure 11); (1) BEFORE device implantation that promotes shared decision-making, (2) SHORTLY AFTER device implantation that increases patient satisfaction and adherence,92,164,165 and (3) ONGOING education that adapts to the changing needs of the patient over time. The communication should be transparent, with clarity about the emergency action plan. An engaged patient is critical to the success of this partnership. Finally, the specific needs of the patient will vary with their technological skills, educational attainment, and facility with language.
Figure 11.
Patient as a team member in remote monitoring.
10.1. Patient education for participation and compliance
Recommendations for patient education for participation and compliance
Synopsis
High-quality patient education is delivered when the patient's level of comprehension and dynamic communication preferences are considered. To promote maximal engagement of the patient in decision-making, family members should be included, and ideally the educational process should begin prior to device implantation. An initial in-person hands-on education session can promote trust and engagement.
Recommendation-specific supportive text
In a study that assessed the readability of patient education materials on ICDs from a variety of sources (including industry, hospital resources, and patient support organizations), it was determined that 95% of the materials exceeded the recommended 8th grade reading level. Accordingly, this may explain the acknowledged disparity that patients with a preference for RM tend to have higher educational attainment.167 As part of identifying patients' individual needs for optimal comprehension, information should be adapted to address personal and cultural preferences that will optimize communication. Beyond patients' reading skills, additional factors that may limit their comprehension should be considered when personalizing education including age, language barriers, learning preferences for written vs auditory information, sociodemographic factors, and physical limitations such as visual or auditory impairment. It is also relevant to acknowledge that an individual's educational preferences may change over time as individuals age.
Shared decision-making, especially for nonurgent procedures, ensures patient understanding and that their choices align with their goals and values.168 Sensitivity to this topic is essential in acknowledging the impact this may have on a patient's decision to proceed with a CIED implantation. With a focus on patient-centered care, the Centers for Medicare and Medicaid Services (CMS) mandated documentation of an evidence-based patient decision aid for individuals receiving primary prevention ICDs for systolic HF.163 The engagement of patients' family members and caregivers supports this process to promote the highest level of understanding possible. An additional factor regarding shared decision-making for a CIED implant can be the choice of manufacturer. What best aligns with an individual patient's preferences for RM interaction, such as app-based software, should be considered.
Formalized education on RM is an opportunity to enhance the understanding of practical device function and alert management.168 For nonurgent device implants, patients may be overwhelmed by hospital events surrounding the device implantation procedure. It is recommended that these educational efforts begin prior to implantation, preferably during the discussion for indication of the device. As ongoing connectivity is paramount for appropriate monitoring, the importance of its maintenance should be included in standardized education.
Survey-based studies have demonstrated that patients feel they receive less information and interact less with providers when they are remotely monitored than if they had in-person care.165,169 Doing an in-person, hands-on demonstration of the manufacturer-specific transmitter or the software that the patient will use for RM can provide an opportunity to answer all questions about the system. While this is not feasible for all patients all of the time, it should be considered as part of the initial educational process to establish trust. Increased knowledge and understanding may improve patient adherence and connectivity to RM.
10.2. Patient education of clinic-specific policies
Recommendations for patient education of clinic-specific policies
Synopsis
The monitoring of patients with a CIED is a partnership between patients and their device clinic staff. When providing patient education, dialogue on clinic-specific policies sets clear expectations for patients who will be remotely monitored by that device clinic.
Recommendation-specific supportive text
Clinic-specific policies should be established and presented to patients. In addition to verbal communication, this could also include an educational brochure or a patient/clinic agreement form. This information may include hours of clinic operation, remote scheduling, billing information, communication preference for device transmission report, use of third-party resources (see Section 12), and commitment to maintaining follow-up. The importance of updated and current contact information for the patient should be emphasized. It is critical to clarify that remote transmissions do not replace emergent care. Patients should develop an emergency plan in advance of a crisis situation. Instructions will be provided to the patient regarding who to contact for home monitoring troubleshooting including the manufacturers' technology service phone numbers.
11. Manufacturer responsibilities with remote monitoring
Manufacturers have a central role in the development of technology and in ensuring its safety and effectiveness. Responsibilities include (1) informing clinic staff and patients about any disruption in the RM service, (2) communication about recalls and advisories to CIED clinic providers and patients in a transparent and timely manner, and (3) refraining from direct patient care (either within the clinic or at home). Although manufacturer representatives can play an important role in training clinic staff, it is not their role to perform, collect, or triage data on behalf of the clinic staff or be used as a staffing resource in lieu of local qualified personnel. It is the responsibility of industry to ensure RM systems function across varying geographies. This includes a need to overcome problems related to the telephone network, communication evolution, and the impossibility of using the electromagnetic spectrum band of the CIED already assigned for other uses. As the data reside on servers owned and managed by the manufacturers, the onus lies with the manufacturers to maintain the servers in a secure and encrypted environment. Privacy is of paramount consideration. The minimal standard is to maintain privacy in accordance with local and national laws. The data pooled from the servers are important to industry for quality assurance purposes (eg, tracking device performance and watching for early signs of device trouble) and for making improvements to the CIED technology. The data are also of value to individual CIED programs for improving the quality of their processes. Finally, these data are essential to investigators, independent of industry, and so it is important for manufacturers to have in place a procedure to process requests for an independent scientific review.
11.1. Manufacturers' role to optimize individual patient care
Recommendations for manufacturers’ role to optimize individual patient care
Synopsis
Successful and ongoing connectivity of individual patients is associated with challenges for RM clinics.28 Industry plays an important role in supporting clinics to provide and maintain this connectivity. This starts with the design and development of RM technology and continues through providing education to clinic staff and to patients directly. Patient care can benefit from the early notification of critical events, and this requires ongoing compliance with RM.
Including patient partners and clinic staff in the development and design of RM products will ensure that the technology will continue to evolve and meet stakeholder needs from the perspectives of ease of use, flexibility, and reliability.22,121 Systems designed to clearly indicate, to both patients and the clinics, when connectivity has been interrupted will allow for the timely resumption of monitoring. Recent studies have highlighted the large workloads for RM clinics associated with maintaining connectivity.28 Technology that empowers patients to independently troubleshoot their RM systems would be optimal.
Recommendation-specific supportive text
Ensuring optimal connectivity for remotely monitored CIED patients enhances patient safety and can aid in clinical decision-making. Manufacturer representatives are important partners in the training and education of RM clinic staff. This may take the form of onsite support (such as enrolling patients into an RM platform) or suggesting manufacturer-specific programming settings for alert parameters. This manufacturer support also extends to providing online, telephone, or in-person (in the clinic) technical help when troubleshooting connectivity concerns of individual patients.
As new devices and RM platforms are developed and upgraded, it is important to remember that no single RM transmitter technology will be suitable for all patients. Fraiche et al. recently highlighted a lack of understanding by patients about how RM works.162 Timmermans et al. found that patients with negative RM experiences will opt for in-clinic follow-up.170 Manufacturer partners should consider having alternate systems available for those patients who have limited mobile connectivity or digital literacy. As technology upgrades can place a financial burden on a patient, mobile device applications should allow compatibility with all smartphone manufacturers. Ideally, the RM transmitter will match the lifetime of the implanted device. Given the international mobility of patients, the RM systems should be designed to work in different jurisdictions. Security concerns, including cybersecurity, are important to ensure both patient safety and the safety of their personal health information.
There are workload implications for clinics when there are high volumes of alerts and calls with patients to troubleshoot connectivity.3,28 The involvement of key stakeholders, including patients and clinic staff, in the development of RM technology will ensure that ongoing product designs will meet needs as technology rapidly evolves and changes. In addition, this collaborative approach with industry will lead to increased efficiency and satisfaction for stakeholders.
Studies have demonstrated that patient outcomes are directly correlated to RM adherence.22,97 Clinics need to be notified about disconnected patients in a prompt manner. Manufacturer monitors, or mobile applications, should provide a clear indication to patients about transmission status and should provide easy-to-follow instructions to re-establish connection when connectivity is lost. Dechert et al. have found that when patients perceive a lack of device feedback (lack of recognition that the device is transmitting), patient-initiated transmissions increase, resulting in more extraneous data for the RM teams to review.171 If patients are able to troubleshoot and resolve connectivity issues unassisted, patient will spend less time disconnected, clinic work load will decrease, and clinical care will improve.28
11.2. Manufacturers' role in the management of patient safety advisories via remote monitoring
As the implantation of CIEDs has become more commonplace, there have been more device hardware and software errors or failure, leading to manufacturer safety advisory or recalls. Data from as early as the 1990s and early 2000s found escalating numbers of recalls and advisories,172 with a subsequent review of U.S. Food and Drug Administration (FDA) Enforcement Reports from 2000–2008 revealing a 26.4% recall alert of either device generator or leads.173 Though there were no major complications attributed to these alerts, the patient burden related to extra visits or procedures did lead to an increased cost burden. Additional studies confirmed the high volume of patients affected, though there continued to be no evidence of associated of increased mortality.174 Acknowledging these challenges, in 2005 the HRS and the FDA convened a conference on PM and ICD performance. The resulting taskforce called for improved communication from manufacturers to physicians and patients regarding recalls and advisories, and generally more cooperation among industry, the FDA, and the physician community.17,175 Industry was asked to use the Patient Device Advisory Notification letter format to communicate with physicians and use patient registration information found at the time of implant to communicate with patients. The HRS Task Force on Lead Performance Policies and Guidelines further recommended that manufacturers should develop and adapt RM technology to monitor longitudinal lead performance.176 The increased number of advisories have had a significant impact on provider workflow, financial costs, patient anxiety, and patient safety. Patients can experience a range of emotions, including outrage, if such information is learned through media outlets and not from their clinical team.177 The clinical team requires early access to recall and advisory information to preserve trust in the patient-provider relationship.
Recommendations for manufacturers’ role in the management of patient safety advisories via RM
Synopsis
Manufacturers and industry representatives play a vital role in the management of patients affected by CIED safety advisories by providing timely patient reports to the clinical team when devices meet advisory conditions. To help navigate vendor-specific nuances, manufacturer guidance is critical for the clinical team to best manage the advisory through device reprogramming or reprogramming alert settings. Manufacturers provide ongoing support to the clinical team with updated patient lists and safety advisory details, and they should continue to do so.
Recommendation-specific supportive text
It is important that manufacturers directly contact the clinical team as soon as device safety advisories or recalls are issued. Providing the clinical team with a list of affected patients and specific system components involved in the advisory could help enable a timely response. Industry representatives should continue to contact clinical providers using different modalities (email, certified mail, or in-person communication) until confirmation of communication is received. Updates to the safety advisories should be ongoing.
Many safety advisory and recall communications deal with issues that are particular to the system of the involved manufacturer. Therefore, it is important that manufacturers or industry representatives provide detailed guidance about how to manage the safety advisory through device reprogramming or alert settings. This may include changing the frequency of remote transmission or modifying details of critical alerts, or the development of novel advisory-specific alerts. Manufacturer representatives should also share planned or suggested long-term solutions to the safety advisory such as software updates or potential lead or device removal.
11.3. Support surrounding implantation from manufacturers
Recommendations for support surrounding implantation from manufacturers
Synopsis
RM platforms and device programming across manufacturers continue to have many distinct differences between manufacturers. Each manufacturer has a different RM interface that processes and reports alerts differently.3 Manufacturers vary in what is considered a red vs yellow alert and in when these data get transmitted to the clinics.178 For example, some devices provide continuous connectivity, while others provide only noncontinuous (or intermittent) connectivity. Some device RM systems are connected to a mobile transmitter, while others utilize a stationary transmitter. This impacts the speed and frequency with which information can be shared with the clinical team. Each manufacturer uses unique CIEDs algorithms and software (and sometimes multiple algorithms/systems within a manufacturer), which can make it challenging for an implanter to recall the nuances of each system and device. Guidance on optimal manufacturer-specific alert parameters relevant to a clinical scenario can minimize inappropriate alerts and need for immediate future reprogramming.
Recommendation-specific supportive text
Manufacturers should provide personnel as appropriate (in-person manufacturer representatives), adequate support of clinic staff through education, or “on-demand” off-site support to ensure that patients are properly enrolled into the manufacturer's RM platform. This should ideally be accomplished prior to discharge, but no later than 2 weeks after discharge. This includes confirming that the chosen RM interface matches the patient's technology literacy and ability. For example, connecting a patient living in a rural area with poor internet or cellular service to a cellular-based/wireless device may prevent successful remote transmissions and result in poor patient care.
CIED manufacturer-specific algorithms and software can pose challenges for implanters or clinic staff related to the nuances of each system and device. For example, criteria for a red (critical) alert can differ between manufacturers. Industry support during and immediately after implant can help ensure optimal device programming to meet the clinical indications. This guidance on optimal manufacturer-specific alert parameters relevant to a clinical scenario can minimize inappropriate alerts. Industry support during and immediately after implant can help ensure timely and optimal device programming.
12. Third-party resources for remote monitoring
There is a high volume of data captured through RM systems. The amount of data can make it challenging to manage these data without adequate clinical staff and administrative support. To address this challenge, some hospitals and clinics have turned to third-party resources to aid in RM (Figure 12). Third-party resources refer to hiring an outside service to help with any of the tasks described later in this section. The use of third-party resources has potential clinical, financial, and workflow benefits but may also have drawbacks. The goal is to ease the workload on overwhelmed staff in order to improve timeliness of remote transmission review and enhance patient communication. Risks of using third-party resources include exposing private patient data to maleficence (hacking). Adoption of third-party resources into an RM program requires careful thought and consideration to ensure patient safety and optimize communication between the patient and medical team.
Figure 12.
Remote monitoring outsourcing to third-party resources. CIED = cardiovascular implantable electrical device.
Third-party resources may be task specific (eg, only reviewing CIED alerts) or encompass the entire RM process. Third-party resources should not include professional decision-making. Some third-party providers act as “middleware services,” centralizing data from multiple manufacturers. Others offer comprehensive services that include facilitating patient enrollment on RM, assessing and addressing compliance to transmission, providing review of routine and acute CIED transmissions with accompanying report, notifying the medical team of actionable data, generating a service charge, and integrating report data with the electronic medical record to support patient communication. They can assist with troubleshooting, patient feedback, and billing.179 These third parties may use either cloud-based or server-based services to store patient data.
12.1. Use of third-party resources in remote monitoring
Recommendations for use of third-party resources in RM
Synopsis
As stated above, there are many benefits associated with utilizing third-party resources with device clinics. In some cases, they can help to increase revenues and, in other cases, could assist programs with undersized staffing resources to meet the standards of care expected for an efficient RM program. The use of third-party resources poses many challenges, including cybersecurity risks, dependency on data processors to share clinical information, and potential financial burden. Third-party personnel should be well trained in device interrogation management and ideally have credentials that verify this training. Many third-party resources are cloud based and rely on cloud data storage. This can introduce a risk for data breach or loss, which may affect patient data privacy and safety. It is important for patients to be informed that the institution utilizes third-party services. Furthermore, institutions can become dependent upon the third-party resources to initially review and triage patient clinical information in an accurate and timely manner. Third-party resource workforce shortages or novice employees may risk missing actionable event transmissions, thus affecting patient care and safety.179,180 The costs of such third-party resources can become a financial burden in some cases.160 One less technical concern is that the use of a third-party service may change the patient's perception of the patient-medical team relationship. The loss of a more personal connection with the clinic team may decrease patient compliance and satisfaction.
Recommendation-specific supportive text
Third-party resources have created the infrastructure to manage high-volume data. Offloading administrative tasks from clinical personnel (nurses, advanced practitioners, and physicians) can improve staff efficiency with resulting financial benefits. Furthermore, redistribution of administrative tasks can help alleviate burnout associated with such burdensome tasks.181 Quality of care and communication between providers and patients may improve when outsourcing to third parties if more data can be reviewed and results communicated in a timely manner.179,180
RM is a communication process that transmits patient data between data controllers and data processors.182 Institutions have traditionally been the data controllers, with manufacturers or third-party resources acting as data processors. Using third-party resources for their monitoring or reporting services adds an additional layer between the processor and controller (Figure 12). Each additional interface between the processor and controller introduces the opportunity for potential maleficence (hacking). Traditional data processors (device manufacturers) either use their own secure servers or engage the hosts' servers through a virtual private network connection. The legal and regulatory implications of outsourcing patient data must also be considered.182 For example, the General Data Protection Regulation (GDPR) in the European Union was implemented in 2018 and provides a legal framework for participating countries. This requires patient consent for the transfer of data to a third party. Other countries across the globe are working to emulate this landmark regulation. CIED patients need to be aware of the use of third-party services in order to properly provide their informed consent for enrolling in an RM program.
13. Pediatric considerations with remote monitoring
As in the adult population, RM in pediatric patients (defined as those < 18 years of age or followed by a pediatric provider) has significant benefit allowing the medical team to detect and intervene on CIED issues, such as battery depletion, lead or device malfunction, or arrhythmic issues.1,9,183–185 RM can be useful to identify acute lead malfunction after new implants.184 Younger age was associated with an increase in lead/device malfunction.184 Although the overall likelihood of an actionable event in pediatric patients is low, RM is recommended for early detection and management of device or arrhythmia concerns, as the patient may be asymptomatic.183–185 Tachyarrhythmia is the most common abnormality found on RM transmissions in younger patients.184 A pediatric study showed the median time between RM interrogations was every 91 days and the median time between last follow-up and occurrence of actionable events was 46 days.185 Pediatric studies support interrogations every 3 to 12 months RM for PM and every 3 to 6 months for ICDs.183–185 Frequency of RM noncontinuous device interrogations should be increased as the device reaches elective replacement, as device replacement is an actionable event and needs clinical attention.184
The recommendations outlined in this document for the adult population are applicable to the pediatric population. Recommendations detailing indications, management, and timing/frequency for pediatric patients with CIEDs on RM were outlined in the 2021 PACES Expert Consensus Statement on the Indications and Management of Cardiovascular Implantable Electronic Devices in Pediatric Patients.9 Additional considerations for pediatric patients include the importance of engaging the patient early in their life to promote independence, and compliance as they reach adulthood. Transition, defined as the active process that focuses on the medical, psychosocial, and educational/vocational needs of adolescents as they move toward adulthood, to adult CIED care should be the eventual goal. The transition process is dynamic, and duration of transition can vary from patient to patient. Shared medical decision-making that promotes open dialogue and exchange of medical information between the child, parents, and provider can also help to support compliance, transition to adult care, and better outcomes. Implementation of focused patient education to adolescent patients has been shown to increase self-management and knowledge of their medical condition in a recent clinical trial. At each follow-up visit, pediatric CIED providers should evaluate transition readiness and provide targeted education on device care and the importance of RM beginning in the young adolescence period.
14. Geographic differences with remote monitoring practices
Despite near global availability of RM by a limited number of manufacturers, there is significant geographic variability in the uptake of RM, both within countries and between countries and regions.22,186 Significant variability also exists as to how RM is conducted, including the frequency of scheduled transmissions, enrollment criteria for RM, and the technologies used for RM.187,188 Disparity exists due to a multitude of barriers that include insufficient reimbursement for patients and care teams, a lack of personnel resources, and inadequate infrastructure for RM.3,86
14.1. Availability of remote monitoring
Recommendations for availability of RM
Synopsis
RM has been developed to address limitations with in-office follow-up, such as late detection of clinical or technical actionable events, by providing need-based care and continuous surveillance of CIED devices. In 2013, an update on the use of RM in the Asia-Pacific region reported a global availability of RM, but the actual use of RM was highly variable.189 Although the use of RM was very limited in South East Asia, India, and Hong Kong, up to 50% of patients with an ICD or CRT in Japan were provided with RM. Availability is a necessary prerequisite, but it does not necessarily lead to adequate use and patient compliance. Real-world data from 2014 on the RM of ICDs and CRT-Ds illustrated large geographical variability in the compliance of RM within the United States, with noncompliance rates ranging between 8.5% and 19.5%.190
Recommendation-specific supportive text
There is very limited data available on geographical disparities in RM availability, use, and compliance. Future research should focus on identifying barriers to RM adoption and compliance as well as their respective solutions. Examples of these barriers include geographical and socioeconomic disparities. The optimal approach to this research would involve a collaboration between patients, health systems, and RM providers.
15. Knowledge gaps and future needs
The innovations in RM technologies over the last few decades have greatly contributed to the advancement in the “standard of care” for patients with CIEDs. The vast potential of these technologies is just starting to be harnessed. Advances are still needed on many fronts―technology development, policy leadership, payment reform, and patient-centered communications. Seven important challenges related to remote care of patients are enumerated below. If these can be adequately addressed in the coming years, there is great potential to improve the quality of care able to be delivered to patients.
15.1. Remote monitoring can “shorten” large geographic distances
While some countries are geographically small with a high population density, other countries are large with areas of very low population density. Examples of the latter in North America might be in parts of the central regions of the United States or in the Canadian North. Patients may need to travel great distances in order to receive CIED care. RM might be able to address this problem. It is likely not feasible to establish CIED clinics with trained staff in every rurality. However, site-based RM in smaller towns with transmission interpretation performed by off-site, trained CIED staff could be a solution. A network of site-based remote monitors could be deployed that could decrease the financial, time, and travel burden to patients as well as infrastructure and personnel costs for local municipalities. If the CIED interrogation is reassuring, then no further action would be required.
15.2. Remote programming
One of the most notable benefits of RM is the ability to provide high-quality care to patients who are not near a CIED clinic (or a programmer). Currently, these benefits accrue primarily to those patients in whom the programmed settings are optimal. If the remote interrogation provides actionable information necessitating reprogramming, the patient needs to travel to a CIED clinic (or at least to a physical CIED programmer) for care. Fortunately, this applies only to a minority of interrogations.
Remote programming has recently become available for newer-generation ILRs. The next frontier will encompass not only RM of patients with CIEDs, but true “remote programming.”191 In the future, care needs to be delivered to the patient where the patient lives―ideally in the patient's home. More recently, remote programming has been reported in a case series from China,192 in the context of reprogramming a CIED before and after an MRI scan,193 and a small feasibility study from Bordeaux, France, with ongoing testing in a larger cohort.194 Each of these have used a model of remote programming that requires a programmer near the patient, and remote control of that programmer to be performed by an off-site clinician. These small forays into remote programming need to be expanded to the point that they do not require the patient to be in a health care facility. The engineering challenges are likely easily s3urmountable. It is critical that this technology be deployed in a manner that instills confidence in both patients and providers about the safety and security of RM. Certain “high risk” features (eg, noninvasive programmed stimulation) should probably not be available to be programmed remotely. Conversely, the reprogramming of RM alert features should be easily accessible.
15.3. Inequitable access to cardiovascular implantable electronic device remote monitoring
RM was strongly recommended for most CIED patients in the 2015 HRS Expert Consensus Statement on RM. Across the world in 2022, most CIED patients did not have access to RM. There are significant disparities in the use of RM both between and within counties, with differing reasons for these disparities. In some cases, they relate to the cost burden on some national health systems. In other cases, the telecommunications infrastructure might not be able to provide a stable support for RM. In some smaller city-states, the value proposition has not been clear to the payers given the short distances between the citizens and the CIED clinics. Within the United States, patients are often required to make a recurring co-payment for their RM. Without obvious benefits that are tangible to the patient, many patients decline further RM.
There are many different problems leading to this variability in access to RM, so there need to be many different solutions. In some cases, the solutions are changes to government policy; in other cases, technological solutions are needed; and in some cases, novel reimbursement models are required. Efforts will be required on multiple fronts to decrease these disparities.
15.4. Reimbursement reform for remote monitoring
There are currently many challenges with the reimbursement model for RM. First, there is a large variability in what the Medicare program pays for RM visits across the United States. This variability makes RM financially challenging in some parts of the United States. This makes little sense, especially since the background structural costs of RM are likely similar for central monitoring services.
Second, the co-payments required for RM (mentioned above) serve as a barrier to optimal patient care. In the United States, while co-payments are often required for diagnostic care and for treatments, co-payments can be waived for preventative care and screening tests. Most CIED RM could be considered a form of preventative care. The remote visits are to ascertain whether there is a problem that might be treated, even in the absence of symptoms, to prevent progression to more serious and potentially life-threatening problems.
Third, there is significant worldwide heterogeneity in the reimbursement for RM.86,189,195 In Europe, many clinics receive no reimbursement for RM, and this has been identified as a major barrier to the use and expansion of RM.86,195 Uptake of RM has slowed in parts of the Asia-Pacific region, with a lack of reimbursement identified as a barrier in many locales.189
Finally, a larger shift may be needed in how we think about RM of CIEDs. Currently, most remote visits are scheduled in advance at a prespecified interval. This is a relic from the CIED clinic visits that were used exclusively prior to RM. Most of these visits (both remote and in person) conclude that the CIED is working properly and that no further action is needed. One could argue that those visits are of “low value” to the patients, but they still require a significant effort from trained clinic staff (with the related costs). While clinic reimbursements are tied to these visits, this “low-value care” will continue. To shift efforts to an “alert-based” model of care that focuses on clinic visits for an actionable event will require a restructuring of CEID clinics and their reimbursement models. The reimbursement would need to shift from the current model of reimbursement on a per visit basis to a model of reimbursement for remote and in-person care over a window of time (eg, annually). We need a model where the system of reimbursement is designed to match the optimal care for the patient, instead of the care of the patient being designed to match the system of reimbursement. Such a change will require the involvement of a myriad of stakeholders, including patients, physicians, clinics/offices, and payers, to determine the optimal models and time windows.
15.5. Better information, not more information
RM has generated a lot of information. CIED providers are already suffering from “information overload” with frequent transmissions, especially from ILRs. The increasing use of wearable technologies with transmission capabilities will only make this problem worse.
The problem is that while some of these data are valuable, most of these data are not useful. It can be very labor intensive and costly to manually review all these data to find clinically actionable events or trends. Here, AI might prove to be particularly valuable. There have already been some early publications about AI models improving the classification accuracy of diagnoses by ILRs.196 There are also studies assessing whether AI could be used to predict ventricular arrhythmia events and ICD therapies.197 If these algorithms could be used to enrich the quality of the data that requires manual review by staff, this would enhance patient care.
A critical first step in this regard is standardizing the nomenclature used by the devices and RM systems across manufacturers and implementing new technical standards. This will make it easier for the transfer of information from the manufacturers' proprietary systems to EHRs or middleware providers. This is a necessary step to accelerate the ability to use AI-based analytical approaches at scale. There is currently an HRS working group that is in the process of creating such standards.
15.6. Direct patient access to device information
Some patients want to know about all their CIED parameters, while other patients just want to know that everything is okay. Both types of patients are entitled to information about the function of their CIED in their desired manner. This must be approached from the viewpoint that the patient owns their health information. Some jurisdictions have laws in place mandating this, such as the European Union regulation 2016/679 (General Data Protection Regulation) Article 20: Right to Data Portability198 and the United States 21st Century Cures Act.199 Whether or not it is the local law, taking a patient-centric perspective is the correct approach. The challenge is in presenting this information in a way that conveys information effectively and efficiently to the patient, in accordance with their preferences. Manufacturers need to provide clinics with better tools to aid in this communication. Further discussions among stakeholders are needed to determine if this communication to the patient is best kept via the clinics or whether there should be communication to the patient directly from the manufacturers. This is critical if our goal is to care for the patient and not just care for the device.
15.7. Summary: Further research about remote monitoring
RM technologies are evolving quickly, and there has been a need to adapt, even without clear data on outcomes and best practices. Many of the recommendations in this document are based on expert opinion. This is a stopgap solution. Moving forward, research studies should ideally be performed to determine optimal models of RM clinics, including workflows, staffing, and management of alerts. Value can be defined in terms of patient satisfaction, costs, efficiencies, and improved patient outcomes. These benefits need to be better studied and quantified. It will be important to assess whether RM can impact or improve important patient outcomes such as decreasing the rates of stroke or mortality.
15.8. Summary: Past, present, and future
RM has already enhanced the care of CIED patients, which is why the use of RM is a class I recommendation in the 2015 HRS Expert Consensus Statement on RM.1 The early models of care with RM followed the same pattern as the prior clinic visits, with the sessions scheduled and planned. This is still the pattern with noncontinuous monitoring. Most of these visits confirm that the device is working appropriately and do not require any intervention from the clinic staff. The current schedule of RM visits is often driven by the reimbursement schedule for RM visits. For example, if reimbursement for RM is provided every 91 days, then clinics are incentivized to schedule these RM visits every 91 days.
Increasingly, we are seeing RM platforms with continuous connectivity of devices that can transmit to the clinic information about the lead function, device function, and the patient's clinical status (eg, development of AF) shortly after problems develop. This could allow for the transition to alert-based care. In this model, there would be fewer routinely scheduled “low-value visits” (and perhaps eventually none), with visits scheduled based on device alerts suggesting that device reprogramming or other intervention is needed for patient care (“high-value visits”). We can move to a model of care driven by optimizing care for the patient.
For this to happen, all stakeholders need to participate. Manufacturers need to transition more completely to RM platforms that offer reliable continuous connectivity. Health care facilities need to staff their clinics properly to address both the volume of RM transmissions and the unpredictable nature of alert-based RM. Payers need to develop novel payment schemes that provide payment to clinics for managing the patient with CIED for a duration of time (eg, annually), and not only for a visit.
This paradigm shift has the potential to reduce the requisite clinic resources, to decrease health care costs, to save time and money for patients, and to improve patient satisfaction.
Supplementary data
Supplementary data (Appendix 3) associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2023.03.1525.
Supplementary Material
Abbreviations
- AF
atrial fibrillation
- AHP
allied health professional
- AI
artificial intelligence
- ATP
anti-tachycardia pacing
- CIED
cardiovascular implantable electronic device
- COR
class of recommendation
- CRT
cardiac resynchronization therapy
- CRT-D
cardiac resynchronization therapy defibrillator
- CRT-P
cardiac resynchronization therapy pacemaker
- EHR
electronic health record
- HF
heart failure
- ICD
implantable cardioverter-defibrillator
- IEGM
intracardiac electrogram
- ILR
implantable loop recorder
- LOE
level of evidence
- LV
left ventricular
- MRI
magnetic resonance imaging
- PM
pacemaker
- RM
remote monitoring
- RV
right ventricular
- RWI
relationships with industry
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Appendix
Appendix 1.
Writing committee member disclosure of relationships with industry and other entities
| Writing committee member | Employment | Honoraria/speaking/ consulting |
Speakers' bureau | Research* | Fellowship support* | Ownership/ partnership/ principal/ majority stockholder |
Stock or stock options | Intellectual property/ royalties |
Other |
|---|---|---|---|---|---|---|---|---|---|
| Aileen M. Ferrick, PhD, ACNP, RN, FHRS (Co-Chair) |
White Plains Hospital, White Plains, New York | None | None | None | None | None | None | None | None |
| Satish R. Raj, MD, MSCI, FHRS (Co-Chair) |
University of Calgary, Calgary, Alberta, Canada | 0: Arena Pharmaceuticals 1: Amneal Pharmaceuticals 1: Argenx 1: Elsevier 1: Regeneron 1: Servier 1: Spire Learning |
None | 0: Theravance 3: Stand Up To POTS 4: Heart & Stroke 5: CIHR 5: Dysautonomia International |
None | None | None | None | 0: AAS 0: CCS 0: Dysautonomia International |
| Thomas Deneke, MD, PhD, FHRS (EHRA Vice-Chair) | Heart Center Bad Neustadt, Bad Neustadt, Germany | 1: Abbott 1: Biotronik 1: Farapulse 1: Galaxy Medical, Inc. 1: Imricor |
None | None | None | None | None | None | 1: Boston Scientific |
| Pipin Kojodjojo, MBBS, PhD, FHRS (APHRS Vice-Chair) | Asian Heart & Vascular Centre, Singapore | None | None | None | None | None | None | None | None |
| Nestor Lopez-Cabanillas, MD (LAHRS Vice-Chair) | Adventist Cardiovascular Institute, Buenos Aires, Argentina | None | None | None | None | None | None | None | None |
| Haruhiko Abe, MD, PhD | University of Occupational and Environmental Health Hospital, Kitakyushu, Japan | 1: Abbott Japan | 1: Boston Scientific Japan 1: Japan Medtronic, Inc. |
None | None | None | None | None | None |
| Felix A. Ayala-Paredes, MD† | Universite de Sherbrooke Faculty Sherbrooke, Quebec, Canada | None | None | None | None | None | None | None | None |
| Serge Boveda, MD, PhD, FEHRA, FESC | Clinique Pasteur, Toulouse, France | 1: Boston Scientific 1: Microport CRM 1: Zoll Medical Corporation 2: Medtronic |
None | None | None | None | None | None | None |
| Derek S. Chew, MD, MSc, FHRS | University of Calgary, Calgary, Alberta, Canada | 1: UTI Limited Partnership (Innovate Calgary) | None | 4: CIHR | None | None | None | None | None |
| Jong-Il Choi, MD, PhD, MHS | Korea University Medical Center, Seoul, Korea | 0: Abbott 0: Medtronic 1: Boehringer Ingelheim 1: Chong Kun Dang Pharmaceutical Corp 1: Daewoong Pharm 1: Daiichi Sankyo 1: Johnson & Johnson 1: Menarini Group 1: Novartis 1: Sanofi 1: Samjin Pharmaceutical Co, Ltd |
None | 0: Sanofi 5: Chong Kun Dang Pharmaceutical Corp |
None | None | None | None | None |
| Nikolaos Dagres, MD | Heart Center Leipzig at the University of Leipzig, Leipzig, Germany | None | None | None | None | None | None | None | 0: EHRA |
| Aarti S. Dalal, DO, FACC, FHRS, CEPS-P | Vanderbilt University Medical Center, Nashville, Tennessee | 1: Medtronic | None | None | None | None | None | None | None |
| Brynn E. Dechert, APN, FHRS, CCDS | C.S. Mott Children's Hospital, Ann Arbor, Michigan | None | None | None | None | None | None | None | None |
| Camille G. Frazier-Mills, MD, MHS, CCDS | Duke University Medical Center, Durham, North Carolina | 1: Boston Scientific 1: Johnson & Johnson 2: Medtronic |
None | None | None | None | None | None | None |
| Olivia Gilbert, MD, MSc, FACC | Wake Forest Baptist Medical Center, Winston-Salem, North Carolina | None | None | None | None | None | None | None | None |
| Janet K. Han, MD, FACC, FHRS | VA Greater Los Angeles Healthcare System, Los Angeles, California | 1: Medtronic | None | None | None | None | None | None | None |
| Sherri Hewit, PharmD | None | None | None | None | None | None | None | None | |
| Christine Kneeland, BSN | Mayo Clinic, Rochester, Minnesota | None | None | None | None | None | None | None | None |
| Starr DeEllen Mirza | None | None | None | None | None | None | None | None | |
| Suneet Mittal, MD, FHRS | The Valley Hospital, Ridgewood, New Jersey | 1: Abbott 1: AltaThera Pharmaceuticals 1: ARCA Biopharma, Inc. 1: AtriCure, Inc. 1: Baylis Medical Company 1: Biosense Webster, Inc. 1: BMS / Pfizer Alliance 1: CathVision 1: CVRx Inc. 1: Haemonetics 1: Implicity 1: Impulse Dynamics USA 1: Octagos 2: Catawba 2: Philips 3: Boston Scientific 4: Medtronic |
None | None | None | None | None | None | None |
| Renato Pietro Ricci, MD | Cardio Arrhythmology Center, Rome, Italy | 0: Abbott 1: Dompé Farmaceutici S.p.A. |
None | None | None | None | None | None | None |
| Mary Runte, PhD | University of Lethbridge, Lethbridge, Alberta, Canada | None | None | None | None | None | None | None | None |
| Susan Sinclair, NZCS, PGDHSc | Auckland City Hospital, Auckland, New Zealand | None | None | None | None | None | None | None | None |
| Ricardo Alkmim-Teixeira, MD, PhD | Hospital Renascentista, Pouso Alegre, Minas Gerais, Brazil | 1: Abbott 1: Bayer Healthcare Pharmaceuticals 1: Daiichi Sankyo 1: Spectranetics Corporation 1: Wyeth |
None | None | None | None | None | None | None |
| Bert Vandenberk, MD, PhD | University of Calgary, Calgary, Alberta, Canada Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium |
None | None | None | 4: Frans Van de Werf Fund for Clinical Cardiovascular Research | None | None | None | None |
| Niraj Varma, MA, MD, PhD | Cleveland Clinic, Cleveland, Ohio | 0: Biotronik 0: Boston Scientific 0: Pacemate 1: EP Solutions 1: Implicity 1: Medtronic 2: Abbott 3: Impulse Dynamics USA |
None | None | None | None | None | None | None |
Number value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = > $100,000.
This table is a comprehensive list of the relationships with industry and other entities (RWI)―regardless of relevance to the document topic―disclosed by each writing committee member for the 12 months prior to the initial meeting of the writing committee and up through the completion of the document. The table does not necessarily reflect the RWI of the writing committee members at the time of publication. Please refer to the HRS Code of Ethics and Professionalism for definitions of disclosure categories or additional information about the HRS policy on the disclosure of relationships with industry and other entities. To mitigate potential bias and conflict of interest, the recommendations and supportive text were written by writing committee members who were free of relevant RWI.*Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers.
†Dr. Ayala-Paredes stepped down from the writing committee in July 2022 when he developed a new role in industry, which precluded him from participation in the development of the document. Dr. Ayala-Paredes was one of the authors for Section 9, Remote Monitoring Reporting. He left the writing committee before recommendations were developed in this section. After his departure, this section was re-reviewed by the section lead/authors and the document chairs, and the evidence for this section was re-reviewed by the document methodologists.
AAS = American Autonomic Society; CCS = Canadian Cardiovascular Society; CIHR = Canadian Institute of Health Research; EHRA = European Heart Rhythm Society.
Appendix 2.
Reviewer disclosure of relationships with industry and other entities
| Peer Reviewer | Employment | Honoraria/ speaking/ consulting |
Speakers' bureau | Research* | Fellowship support* | Ownership/ partnership/ principal/ majority stockholder |
Stock or stock options | Intellectual property/ royalties |
Other |
|---|---|---|---|---|---|---|---|---|---|
| Elizabeth Davenport, MSN, CNML | Sanger Heart & Vascular Institute - Atrium Health, Charlotte, North Carolina | 1: Biotronik 1: Boston Scientific 1: Rhythm Management Group Corp |
None | None | None | None | None | None | None |
| Vicki Freedenberg, PhD, RN, MSN | Children's National Hospital, Washington, DC | None | None | None | None | None | None | None | None |
| Taya V. Glotzer, MD | Hackensack University Medical Center, Hackensack, New Jersey | 1: Abbott 1: Mayo Clinic 1: Medtronic |
None | None | None | None | None | None | None |
| Jin-Long Huang, MD, PhD | Taichung Veterans General Hospital, Taichung City, Taiwan | None | None | None | None | None | None | None | None |
| Takanori Ikeda, MD, PhD, FACC, FESC, FJCS | Toho University Faculty of Medicine/ Medical Center, Tokyo, Japan | None | None | None | None | None | None | None | None |
| Daniel B. Kramer, MD, FACC | Beth Israel Deaconess Medical Center, Boston, Massachusetts | 1: Firefly Health 1: HeartCoR, LLC |
None | 0: Greenwall Foundation 0: NIH |
None | None | None | None | None |
| David Lin, MD, FHRS, FACC | Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania | 1: Abbott 1: BioTelemetry 1: Philips |
None | None | None | None | None | None | None |
| Ulises Rojel-Martínez, MD, FHRS | South Medical Center, Puebla, México | None | None | None | None | None | None | None | None |
| Markus Stühlinger, MD, FACC, FEHRA | Medical University of Innsbruck, Innsbruck, Austria | 1: Biotronik 1: Daiichi Sankyo 1: Medtronic |
None | None | None | None | None | None | None |
| Paul D. Varosy, MD | VA Eastern Colorado Health Care System, Aurora, Colorado | None | None | 4: NIH | None | None | 2: HRCRS/ 3PH Alliance | None | 0: ACC 0: NCDR |
Number value: 0 = $0; 1 = ≤ $10,000; 2 = > $10,000 to ≤ $25,000; 3 = > $25,000 to ≤ $50,000; 4 = > $50,000 to ≤ $100,000; 5 = > $100,000.
This table is a comprehensive list of the relationships with industry and other entities (RWI)―regardless of relevance to the document topic―disclosed by the reviewers at the time the document was under review. The table does not necessarily reflect the RWI of the reviewers at the time of publication. Please refer to the HRS Code of Ethics and Professionalism for definitions of disclosure categories or additional information about the HRS policy on the disclosure of relationships with industry and other entities. *Research and fellowship support are classed as programmatic support. Sources of programmatic support are disclosed but are not regarded as a relevant relationship with industry for writing group members or reviewers.
ACC = American College of Cardiology; HRCRS = Heart Rhythm Clinical Research Solutions; NCRD = National Cardiovascular Data Registry; NIH = National Institutes of Health.
Contributor Information
Aileen M Ferrick, White Plains Hospital, White Plains, New York.
Satish R Raj, University of Calgary, Calgary, Alberta, Canada.
Thomas Deneke, Heart Center Bad Neustadt, Bad Neustadt, Germany.
Pipin Kojodjojo, Asian Heart & Vascular Centre, Singapore.
Nestor Lopez-Cabanillas, Adventist Cardiovascular Institute, Buenos Aires, Argentina.
Haruhiko Abe, University of Occupational and Environmental Health Hospital, Kitakyushu, Japan.
Serge Boveda, Clinique Pasteur, Toulouse, France.
Derek S Chew, University of Calgary, Calgary, Alberta, Canada.
Jong-Il Choi, Korea University Medical Center, Seoul, Korea.
Nikolaos Dagres, Heart Center Leipzig at the University of Leipzig, Leipzig, Germany.
Aarti S Dalal, Vanderbilt University Medical Center, Nashville, Tennessee.
Brynn E Dechert, C.S. Mott Children’s Hospital, Ann Arbor, Michigan.
Camille G Frazier-Mills, Duke University Medical Center, Durham, North Carolina.
Olivia Gilbert, Wake Forest Baptist Medical Center, Winston-Salem, North Carolina.
Janet K Han, VA Greater Los Angeles Healthcare System, Los Angeles, California.
Christine Kneeland, Mayo Clinic, Rochester, Minnesota.
Suneet Mittal, The Valley Hospital, Ridgewood, New Jersey.
Renato Pietro Ricci, Cardio Arrhythmology Center, Rome, Italy.
Mary Runte, University of Lethbridge, Lethbridge, Alberta, Canada.
Susan Sinclair, Auckland City Hospital, Auckland, New Zealand.
Ricardo Alkmim-Teixeira, Hospital Renascentista, Pouso Alegre, Minas Gerais, Brazil.
Bert Vandenberk, University of Calgary, Calgary, Alberta, Canada; Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Niraj Varma, Cleveland Clinic, Cleveland, Ohio.
References
- 1. Slotwiner D, Varma N, Akar JG, et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015;12(7):e69–e100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 2. Akinyele B, Marine JE, Love C, et al. Unregulated online sales of cardiac implantable electronic devices in the United States: A six-month assessment. Heart Rhythm O2 2020;1(4):235–238. doi: 10.1016/j.hroo.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Shea CJ, Middeldorp ME, Hendriks JM, et al. Remote monitoring alert burden: An analysis of transmission in > 26,000 patients. JACC Clin Electrophysiol 2021;7(2):226–234. doi: 10.1016/j.jacep.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 4. Indik JH, Patton KK, Beardsall M, et al. HRS clinical document development methodology manual and policies: Executive summary. Heart Rhythm 2017;14(10):e495–e500. doi: 10.1016/j.hrthm.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 5. Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice Guidelines . Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, eds. Clinical Practice Guidelines We Can Trust. National Academies Press; 2011. [PubMed] [Google Scholar]
- 6. Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;67(13):1572–1574. doi: 10.1016/j.jacc.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 7. Varma N, Cygankiewicz I, Turakhia M, et al. 2021. ISHNE/ HRS/ EHRA/ APHRS collaborative statement on mHealth in arrhythmia management: Digital medical tools for heart rhythm professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society. Ann Noninvasive Electrocardiol 2021;26(2):e12795. doi: 10.1111/anec.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glikson M, Nielsen JC, Kronborg MB, et al. 2021. ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42(35):3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 9. Shah MJ, Silka MJ, Silva JNA, et al. 2021. PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Heart Rhythm 2021;18(11):1888–1924. doi: 10.1016/j.hrthm.2021.07.038. [DOI] [PubMed] [Google Scholar]
- 10. Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm 2020;17(9):e233–e241. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varma N, Marrouche NF, Aguinaga L, et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Heart Rhythm 2020;17(9):e255–e268. doi: 10.1016/j.hrthm.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielsen JC, Lin YJ, de Oliveira Figueiredo MJ, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on risk assessment in cardiac arrhythmias: Use the right tool for the right outcome, in the right population. Heart Rhythm 2020;17(9):e269–e316. doi: 10.1016/j.hrthm.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 13. Slotwiner DJ, Abraham RL, Al-Khatib SM, et al. HRS white paper on interoperability of data from cardiac implantable electronic devices (CIEDs). Heart Rhythm 2019;16(9):e107–e127. doi: 10.1016/j.hrthm.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 14. Slotwiner DJ, Tarakji KG, Al-Khatib SM, et al. Transparent sharing of digital health data: A call to action. Heart Rhythm 2019;16(9):e95–e106. doi: 10.1016/j.hrthm.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 15. Dubner S, Auricchio A, Steinberg JS, et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace 2012;14(2):278–293. doi: 10.1093/europace/eur303. [DOI] [PubMed] [Google Scholar]
- 16. Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm 2008;5(6):907–925. doi: 10.1016/j.hrthm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 17. Carlson MD, Wilkoff BL, Maisel WH, et al. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE). Heart Rhythm 2006;3(10):1250–1273. doi: 10.1016/j.hrthm.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 18. Chew DS, Li Z, Steinberg BA, et al. Arrhythmic burden and the risk of cardiovascular outcomes in patients with paroxysmal atrial fibrillation and cardiac implanted electronic devices. Circ Arrhythm Electrophysiol 2022;15(2):e010304. doi: 10.1161/circep.121.010304. [DOI] [PubMed] [Google Scholar]
- 19. D'Onofrio A, Solimene F, Calò L, et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: Results from the SELENE HF study. Europace 2022;24(2):234–244. doi: 10.1093/europace/euab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jang J-P, Lin H-T, Chen Y-J, Hsieh M-H, Huang Y-C. Role of remote monitoring in detection of atrial arrhythmia, stroke reduction, and use of anticoagulation therapy: A systematic review and meta-analysis. Circ J 2020;84(11):1922–1930. doi: 10.1253/circj.CJ-20-0633. [DOI] [PubMed] [Google Scholar]
- 21. Poole JE, Swerdlow CD, Tarakji KG, et al. Clinical performance of implantable cardioverter-defibrillator lead monitoring diagnostics. Heart Rhythm 2022;19(3):363–371. doi: 10.1016/j.hrthm.2021.10.032. [DOI] [PubMed] [Google Scholar]
- 22. Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The relationship between level of adherence to automatic wireless remote monitoring and survival in pacemaker and defibrillator patients. J Am Coll Cardiol 2015;65(24):2601–2610. doi: 10.1016/j.jacc.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 23. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: Results from the OptiLink HF study. Circ Arrhythm Electrophysiol 2021;14(1):e008693. doi: 10.1161/circep.120.008693. [DOI] [PubMed] [Google Scholar]
- 24. Maines M, Palmisano P, Del Greco M, et al. Impact of COVID-19 pandemic on remote monitoring of cardiac implantable electronic devices in Italy: Results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). J Clin Med 2021;10(18):4086. doi: 10.3390/jcm10184086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Afzal MR, Nadkarni A, Niemet L, et al. Resource use and economic implications of remote monitoring with subcutaneous cardiac rhythm monitors. JACC Clin Electrophysiol 2021;7(6):745–754. doi: 10.1016/j.jacep.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 26. Holtzman JN, Wadhera RK, Choi E, et al. Trends in utilization and spending on remote monitoring of pacemakers and implantable cardioverter–defibrillators among Medicare beneficiaries. Heart Rhythm 2020;17(11):1917–1921. doi: 10.1016/j.hrthm.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 27. O'Shea CJ, Middeldorp ME, Hendriks JM, et al. Remote monitoring of implantable loop recorders: False-positive alert episode burden. Circ Arrhythm Electrophysiol 2021;14(11):e009635. doi: 10.1161/CIRCEP.121.009635. [DOI] [PubMed] [Google Scholar]
- 28. Seiler A, Biundo E, Bacco MD, et al. Clinic time required for remote and in-person management of patients with cardiac devices: Time and motion workflow evaluation. JMIR Cardio 2021;5(2):e27720. doi: 10.2196/27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ricci RP, Morichelli L, D'Onofrio A, et al. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: The HomeGuide Registry. Europace 2013;15(7):970–977. doi: 10.1093/europace/eus440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crossley GH, Chen J, Choucair W, et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol 2009;54(22):2012–2019. doi: 10.1016/j.jacc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 31. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010;122(4):325–332. doi: 10.1161/circulationaha.110.937409. [DOI] [PubMed] [Google Scholar]
- 32. Varma N, Michalski J, Stambler B, Pavri BB. Superiority of automatic remote monitoring compared with in-person evaluation for scheduled ICD follow-up in the TRUST trial - testing execution of the recommendations. Eur Heart J 2014;35(20):1345–1352. doi: 10.1093/eurheartj/ehu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hindricks G, Elsner C, Piorkowski C, et al. Quarterly vs yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: Results of the REFORM trial. Eur Heart J 2014;35(2):98–105. doi: 10.1093/eurheartj/eht207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boriani G, Da Costa A, Ricci RP, et al. The MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: Phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res 2013;15(8):e167. doi: 10.2196/jmir.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: The value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol 2011;57(10):1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 36. Guédon-Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: Safety and efficacy report of the ECOST trial. Eur Heart J 2013;34(8):605–614. doi: 10.1093/eurheartj/ehs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mabo P, Victor F, Bazin P, et al. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur Heart J 2012;33(9):1105–1111. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landolina M, Perego GB, Lunati M, et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: The evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012;125(24):2985–2992. doi: 10.1161/circulationaha.111.088971. [DOI] [PubMed] [Google Scholar]
- 39. Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: The Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol 2010;3(5):428–436. doi: 10.1161/circep.110.951962. [DOI] [PubMed] [Google Scholar]
- 40. Varma N, Pavri BB, Stambler B, Michalski J. Same-day discovery of implantable cardioverter defibrillator dysfunction in the TRUST remote monitoring trial: Influence of contrasting messaging systems. Europace 2013;15(5):697–703. doi: 10.1093/europace/eus410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ricci RP, Pignalberi C, Landolina M, et al. Ventricular rate monitoring as a tool to predict and prevent atrial fibrillation-related inappropriate shocks in heart failure patients treated with cardiac resynchronisation therapy defibrillators. Heart 2014;100(11):848–854. doi: 10.1136/heartjnl-2013-305259. [DOI] [PubMed] [Google Scholar]
- 42. Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace 2009;11(1):54–61. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 43. Ricci RP, Vaccari D, Morichelli L, et al. Stroke incidence in patients with cardiac implantable electronic devices remotely controlled with automatic alerts of atrial fibrillation: A sub-analysis of the HomeGuide study. Int J Cardiol 2016;219:251–256. doi: 10.1016/j.ijcard.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 44. Ricci RP, Morichelli L, Gargaro A, Laudadio MT, Santini M. Home monitoring in patients with implantable cardiac devices: Is there a potential reduction of stroke risk? Results from a computer model tested through monte carlo simulations. J Cardiovasc Electrophysiol 2009;20(11):1244–1251. doi: 10.1111/j.1540-8167.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 45. Martin DT, Bersohn MM, Waldo AL, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J 2015;36(26):1660–1668. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 46. Chiu CSL, Timmermans I, Versteeg H, et al. Effect of remote monitoring on clinical outcomes in European heart failure patients with an implantable cardioverter-defibrillator: Secondary results of the REMOTE-CIED randomized trial. Europace 2022;24(2):256–267. doi: 10.1093/europace/euab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011;377(9766):658–666. doi: 10.1016/s0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 48. Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014;384(9943):583–590. doi: 10.1016/s0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 49. Kurek A, Tajstra M, Gadula-Gacek E, et al. Impact of remote monitoring on long-term prognosis in heart failure patients in a real-world cohort: Results from all-comers COMMIT-HF trial. J Cardiovasc Electrophysiol 2017;28(4):425–431. doi: 10.1111/jce.13174. [DOI] [PubMed] [Google Scholar]
- 50. Hindricks G, Varma N, Kacet S, et al. Daily remote monitoring of implantable cardioverter-defibrillators: Insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J 2017;38(22):1749–1755. doi: 10.1093/eurheartj/ehx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parthiban N, Esterman A, Mahajan R, et al. Remote monitoring of implantable aardioverter-defibrillators: A systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol 2015;65(24):2591–2600. doi: 10.1016/j.jacc.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 52. Geller JC, Lewalter T, Bruun NE, et al. Implant-based multi-parameter telemonitoring of patients with heart failure and a defibrillator with vs without cardiac resynchronization therapy option: A subanalysis of the IN-TIME trial. Clin Res Cardiol 2019;108(10):1117–1127. doi: 10.1007/s00392-019-01447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Husser D, Christoph Geller J, Taborsky M, et al. Remote monitoring and clinical outcomes: Details on information flow and workflow in the IN-TIME study. Eur Heart J Qual Care Clin Outcomes 2019;5(2):136–144. doi: 10.1093/ehjqcco/qcy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: Results from the MultiSENSE study. JACC Heart Fail 2017;5(3):216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 55. Burri H, da Costa A, Quesada A, et al. Risk stratification of cardiovascular and heart failure hospitalizations using integrated device diagnostics in patients with a cardiac resynchronization therapy defibrillator. Europace 2018;20(5):e69–e77. doi: 10.1093/europace/eux206. [DOI] [PubMed] [Google Scholar]
- 56. Saxon LA, Hayes DL, Gilliam FR, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: The ALTITUDE survival study. Circulation 2010;122(23):2359–2367. doi: 10.1161/circulationaha.110.960633. [DOI] [PubMed] [Google Scholar]
- 57. Heidbuchel H, Hindricks G, Broadhurst P, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): A provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J 2015;36(3):158–169. doi: 10.1093/eurheartj/ehu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Health Quality Ontario . Remote monitoring of implantable cardioverter-defibrillators, cardiac resynchronization therapy and permanent pacemakers: A health technology assessment. Ont Health Technol Assess Ser 2018;18(7):1–199. [PMC free article] [PubMed] [Google Scholar]
- 59. Ricci RP, Vicentini A, D'Onofrio A, et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: Results of the Health Economics Evaluation Registry for Remote Follow-up (TARIFF) study. Heart Rhythm 2017;14(1):50–57. doi: 10.1016/j.hrthm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 60. Sequeira S, Jarvis CI, Benchouche A, Seymour J, Tadmouri A. Cost-effectiveness of remote monitoring of implantable cardioverter-defibrillators in France: A meta-analysis and an integrated economic model derived from randomized controlled trials. Europace 2020;22(7):1071–1082. doi: 10.1093/europace/euaa082. [DOI] [PubMed] [Google Scholar]
- 61. Ricci RP, Vicentini A, D'Onofrio A, et al. Impact of in-clinic follow-up visits in patients with implantable cardioverter defibrillators: Demographic and socioeconomic analysis of the TARIFF study population. J Interv Card Electrophysiol 2013;38(2):101–106. doi: 10.1007/s10840-013-9823-5. [DOI] [PubMed] [Google Scholar]
- 62. Burri H, Sticherling C, Wright D, Makino K, Smala A, Tilden D. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace 2013;15(11):1601–1608. doi: 10.1093/europace/eut070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Petersen HH, Larsen MC, Nielsen OW, Kensing F, Svendsen JH. Patient satisfaction and suggestions for improvement of remote ICD monitoring. J Interv Card Electrophysiol 2012;34(3):317–324. doi: 10.1007/s10840-012-9675-4. [DOI] [PubMed] [Google Scholar]
- 64. Ricci RP, Morichelli L, Quarta L, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace 2010;12(5):674–679. doi: 10.1093/europace/euq046. [DOI] [PubMed] [Google Scholar]
- 65. Zanaboni P, Landolina M, Marzegalli M, et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: Randomized controlled trial. J Med Internet Res 2013;15(5):e106. doi: 10.2196/jmir.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guedon-Moreau L, Lacroix D, Sadoul N, et al. Costs of remote monitoring vs ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace 2014;16(8):1181–1188. doi: 10.1093/europace/euu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leppert F, Siebermair J, Wesemann U, et al. The INFluence of Remote monitoring on Anxiety/depRession, quality of lifE, and Device acceptance in ICD patients: A prospective, randomized, controlled, single-center trial. Clin Res Cardiol 2021;110(6):789–800. doi: 10.1007/s00392-020-01667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Versteeg H, Timmermans I, Widdershoven J, et al. Effect of remote monitoring on patient-reported outcomes in European heart failure patients with an implantable cardioverter-defibrillator: Primary results of the REMOTE-CIED randomized trial. Europace 2019;21(9):1360–1368. doi: 10.1093/europace/euz140. [DOI] [PubMed] [Google Scholar]
- 69. Neuzil P, Taborsky M, Holy F, Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace 2008;10(5):556–557. doi: 10.1093/europace/eun009. [DOI] [PubMed] [Google Scholar]
- 70. Spencker S, Coban N, Koch L, Schirdewan A, Müller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace 2009;11(4):483–488. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 71. Varma N. Remote monitoring for advisories: Automatic early detection of silent lead failure. Pacing Clin Electrophysiol 2009;32(4):525–527. doi: 10.1111/j.1540-8159.2009.02314.x. [DOI] [PubMed] [Google Scholar]
- 72. Ricci RP, Morichelli L, Quarta L, et al. Effect of daily remote monitoring on pacemaker longevity: A retrospective analysis. Heart Rhythm 2015;12(2):330–337. doi: 10.1016/j.hrthm.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 73. Varma N, Johnson MA. Prevalence of cancelled shock therapy and relationship to shock delivery in recipients of implantable cardioverter-defibrillators assessed by remote monitoring. Pacing Clin Electrophysiol 2009;32(Suppl. 1):S42–46. doi: 10.1111/j.1540-8159.2008.02288.x. [DOI] [PubMed] [Google Scholar]
- 74. Varma N, Love CJ, Schweikert R, Moll P, Michalski J, Epstein AE. Automatic remote monitoring utilizing daily transmissions: Transmission reliability and implantable cardioverter defibrillator battery longevity in the TRUST trial. Europace 2018;20(4):622–628. doi: 10.1093/europace/eux059. [DOI] [PubMed] [Google Scholar]
- 75. Manyam H, Burri H, Casado-Arroyo R, et al. Smartphone-based CIED Remote Monitoring: Improved compliance and connectivity. Eur Heart J Digit Health 2022;4:43–52. doi: 10.1093/ehjdh/ztac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Boston Scientific . MyLATITUDE™ Patient App. Accessed October 25, 2022. Available from https://www.bostonscientific.com/en-US/products/remote-patient-monitoring/mylatitude-patient-app.html.
- 77. Tarakji KG, Zaidi AM, Zweibel SL, et al. Performance of first pacemaker to use smart device app for remote monitoring. Heart Rhythm O2 2021;2(5):463–471. doi: 10.1016/j.hroo.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tilz RR, Shaik N, Piorkowski C, et al. Real-world adoption of smartphone-based remote monitoring using the Confirm Rx insertable cardiac monitor. J Innov Card Rhythm Manag 2021;12(8):4613–4620. doi: 10.19102/icrm.2021.120806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bautista-Mesa RJ, Lopez-Villegas A, Peiro S, et al. Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: The PONIENTE study. BMC Geriatr 2020;20(1):474. doi: 10.1186/s12877-020-01883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buchta P, Tajstra M, Kurek A, et al. The impact of remote monitoring of implanted cardioverter-defibrillator (ICD) and cardiac resynchronisation therapy device (CRT-D) patients on healthcare costs in the Silesian population: Three-year follow-up. Kardiol Pol 2017;75(6):573–580. doi: 10.5603/KP.a2017.0019. [DOI] [PubMed] [Google Scholar]
- 81. Calo L, Gargaro A, De Ruvo E, et al. Economic impact of remote monitoring on ordinary follow-up of implantable cardioverter defibrillators as compared with conventional in-hospital visits. A single-center prospective and randomized study. J Interv Card Electrophysiol 2013;37(1):69–78. doi: 10.1007/s10840-013-9783-9. [DOI] [PubMed] [Google Scholar]
- 82. Capucci A, De Simone A, Luzi M, et al. Economic impact of remote monitoring after implantable defibrillators implantation in heart failure patients: An analysis from the EFFECT study. Europace 2017;19(9):1493–1499. doi: 10.1093/europace/eux017. [DOI] [PubMed] [Google Scholar]
- 83. Chew DS, Zarrabi M, You I, et al. Clinical and economic outcomes associated with remote monitoring for cardiac implantable electronic devices: A population-based analysis. Can J Cardiol 2022;38:736–744. doi: 10.1016/j.cjca.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 84. Hummel JP, Leipold RJ, Amorosi SL, et al. Outcomes and costs of remote patient monitoring among patients with implanted cardiac defibrillators: An economic model based on the PREDICT RM database. J Cardiovasc Electrophysiol 2019;30(7):1066–1077. doi: 10.1111/jce.13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ladapo JA, Turakhia MP, Ryan MP, Mollenkopf SA, Reynolds MR. Health care utilization and expenditures associated with remote monitoring in patients with implantable cardiac devices. Am J Cardiol 2016;117(9):1455–1462. doi: 10.1016/j.amjcard.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 86. Mairesse GH, Braunschweig F, Klersy K, Cowie MR, Leyva F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: A survey from the health economics committee of the European Heart Rhythm Association. Europace 2015;17(5):814–818. doi: 10.1093/europace/euu390. [DOI] [PubMed] [Google Scholar]
- 87. Piccini JP, Mittal S, Snell J, Prillinger JB, Dalal N, Varma N. Impact of remote monitoring on clinical events and associated health care utilization: A nationwide assessment. Heart Rhythm 2016;13(12):2279–2286. doi: 10.1016/j.hrthm.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 88. Boriani G, Da Costa A, Quesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: Results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail 2017;19(3):416–425. doi: 10.1002/ejhf.626. [DOI] [PubMed] [Google Scholar]
- 89. Chew DS, Piccini JP, Au F, Frazier-Mills CG, Michalski J, Varma N. Alert-driven vs scheduled remote monitoring of implantable cardiac defibrillators: A cost-consequence analysis from the TRUST trial. Heart Rhythm 2023;20(3):440–447. doi: 10.1016/j.hrthm.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dario C, Delise P, Gubian L, Saccavini C, Brandolino G, Mancin S. Large controlled observational study on remote monitoring of pacemakers and implantable cardiac defibrillators: A clinical, economic, and organizational evaluation. Interact J Med Res 2016;5(1):e4. doi: 10.2196/ijmr.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Perl S, Stiegler P, Rotman B, et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int J Cardiol 2013;169(6):402–407. doi: 10.1016/j.ijcard.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 92. Varma N, Love CJ, Michalski J, Epstein AE. Alert-based ICD follow-up: A model of digitally driven remote patient monitoring. JACC Clin Electrophysiol 2021;7(8):976–987. doi: 10.1016/j.jacep.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 93. Amara W, Montagnier C, Cheggour S, et al. Early detection and treatment of atrial arrhythmias alleviates the arrhythmic burden in paced patients: The SETAM study. Pacing Clin Electrophysiol 2017;40(5):527–536. doi: 10.1111/pace.13062. [DOI] [PubMed] [Google Scholar]
- 94. Lappegard KT, Moe F. Remote monitoring of CIEDs-for both safety, economy and convenience? Int J Environ Res Public Health 2021;19(1). doi: 10.3390/ijerph19010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kelly SE, Campbell D, Duhn LJ, et al. Remote monitoring of cardiovascular implantable electronic devices in Canada: Survey of patients and device health care professionals. CJC Open 2020;3(4):391–399. doi: 10.1016/j.cjco.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Muniyappa AN, Raitt MH, Judson GL, et al. Factors associated with remote monitoring adherence for cardiovascular implantable electronic devices. Heart Rhythm 2022;19(9):1499–1507. doi: 10.1016/j.hrthm.2022.04.025. [DOI] [PubMed] [Google Scholar]
- 97. Mittal S, Piccini JP, Snell J, Prillinger JB, Dalal N, Varma N. Improved survival in patients enrolled promptly into remote monitoring following cardiac implantable electronic device implantation. J Interv Card Electrophysiol 2016;46(2):129–136. doi: 10.1007/s10840-016-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Varma N, Epstein AE, Schweikert R, Michalski J, Love CJ. Role of automatic wireless remote monitoring immediately following ICD implant: The Lumos-T Reduces Routine Office Device Follow-Up Study (TRUST) trial. J Cardiovasc Electrophysiol 2016;27(3):321–326. doi: 10.1111/jce.12895. [DOI] [PubMed] [Google Scholar]
- 99. Akar JG, Bao H, Jones P, et al. Use of remote monitoring of newly implanted cardioverter-defibrillators: Insights from the patient related determinants of ICD remote monitoring (PREDICT RM) study. Circulation 2013;128(22):2372–2383. doi: 10.1161/CIRCULATIONAHA.113.002481. [DOI] [PubMed] [Google Scholar]
- 100. Ren X, Apostolakos C, Vo TH, et al. Remote monitoring of implantable pacemakers: In-office setup significantly improves successful data transmission. Clin Cardiol 2013;36(10):634–637. doi: 10.1002/clc.22207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Drak-Hernández Y, Toquero-Ramos J, Fernández JM, Pérez-Pereira E, Castro-Urda V, Fernández-Lozano I. Effectiveness and safety of remote monitoring of patients with an implantable loop recorder. Rev Esp Cardiol (Engl Ed) 2013;66(12):943–948. doi: 10.1016/j.rec.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 102. Zanotto G, Melissano D, Baccillieri S, et al. Intrahospital organizational model of remote monitoring data sharing, for a global management of patients with cardiac implantable electronic devices: A document of the Italian Association of Arrhythmology and Cardiac Pacing. J Cardiovasc Med (Hagerstown) 2020;21(3):171–181. doi: 10.2459/jcm.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 103. Maines M, Tomasi G, Moggio P, et al. Implementation of remote follow-up of cardiac implantable electronic devices in clinical practice: Organizational implications and resource consumption. J Cardiovasc Med (Hagerstown) 2020;21(9):648–653. doi: 10.2459/jcm.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 104. Ryan P, McGrath C, Lawrie I, Fitzsimons C, O'Shea J, De BrÚn J. Enhancing efficiency in a cardiac investigations department by increasing remote patient monitoring. Int J Qual Health Care 2019;31(Suppl. 1):29–34. doi: 10.1093/intqhc/mzz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Andersen TO, Nielsen KD, Moll J, Svendsen JH. Unpacking telemonitoring work: Workload and telephone calls to patients in implanted cardiac device care. Int J Med Inform 2019;129:381–387. doi: 10.1016/j.ijmedinf.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 106. Liljeroos M, Thylén I, Strömberg A. Patients' and nurses' experiences and perceptions of remote monitoring of implantable cardiac defibrillators in heart failure: Cross-sectional, descriptive, mixed methods study. J Med Internet Res 2020;22(9):e19550. doi: 10.2196/19550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ricci RP, Morichelli L, D'Onofrio A, et al. Manpower and outpatient clinic workload for remote monitoring of patients with cardiac implantable electronic devices: Data from the HomeGuide Registry. J Cardiovasc Electrophysiol 2014;25(11):1216–1223. doi: 10.1111/jce.12482. [DOI] [PubMed] [Google Scholar]
- 108. Guédon-Moreau L, Finat L, Boulé S, et al. Validation of an organizational management model of remote implantable cardioverter-defibrillator monitoring alerts. Circ Cardiovasc Qual Outcomes 2015;8(4):403–412. doi: 10.1161/circoutcomes.114.001433. [DOI] [PubMed] [Google Scholar]
- 109. Ricci RP, Morichelli L, Porfili A, Quarta L, Sassi A. Diagnostic power and healthcare resource consumption of a dedicated workflow algorithm designed to manage thoracic impedance alerts in heart failure patients by remote monitoring. J Cardiovasc Med (Hagerstown) 2018;19(3):105–112. doi: 10.2459/jcm.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 110. Papavasileiou LP, Forleo GB, Panattoni G, et al. Work burden with remote monitoring of implantable cardioverter defibrillator: Is it time for reimbursement policies? J Cardiovasc Med (Hagerstown) 2013;14(2):114–119. doi: 10.2459/JCM.0b013e328354e3e1. [DOI] [PubMed] [Google Scholar]
- 111. Maines M, Tomasi G, Moggio P, et al. Scheduled vs alert transmissions for remote follow-up of cardiac implantable electronic devices: Clinical relevance and resource consumption. Int J Cardiol 2021;334:49–54. doi: 10.1016/j.ijcard.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 112. Cronin EM, Ching EA, Varma N, Martin DO, Wilkoff BL, Lindsay BD. Remote monitoring of cardiovascular devices: A time and activity analysis. Heart Rhythm 2012;9(12):1947–1951. doi: 10.1016/j.hrthm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 113. Han JK, Al-Khatib SM, Albert CM. Changes in the digital health landscape in cardiac electrophysiology: A pre-and peri-pandemic COVID-19 era survey. Cardiovasc Digit Health J 2021;2(1):55–62. doi: 10.1016/j.cvdhj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Boriani G, Burri H, Mantovani LG, et al. Device therapy and hospital reimbursement practices across European countries: A heterogeneous scenario. Europace 2011;13(Suppl. 2):ii59-ii65. doi:10.1093/europace/eur080. [DOI] [PubMed] [Google Scholar]
- 115. Grand View Research . Implantable Loop Recorders Market Size, Share & Trend Analysis Report By Application (Atrial Fibrillation, Cardiac Arrhythmia), By End-use (Hospitals, Cardiac Centers), By Region, And Segment Forecasts, 2020–2027. Accessed March 3, 2023. Available from https://www.grandviewresearch.com/industry-analysis/implantable-loop-recorders-market.
- 116. Frankel DS, Dechert-Crooks BE, Campbell K, et al. 2021. HRS educational framework for clinical cardiac electrophysiology. Heart Rhythm O2 2022;3(2):120–132. doi: 10.1016/j.hroo.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Martin GR, Beekman RH, Ing FF, et al. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2010;13(1):20–25. doi: 10.1053/j.pcsu.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 118. Gaies M, Pasquali SK, Banerjee M, et al. Improvement in pediatric cardiac surgical outcomes through interhospital collaboration. J Am Coll Cardiol 2019;74(22):2786–2795. doi: 10.1016/j.jacc.2019.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Intersocietal Accreditation Commission . Cardiac Electrophysiology Accreditation. Accessed March 3, 2023. Available from https://intersocietal.org/programs/cardiac-electrophysiology
- 120. Ahmed I, Patel AS, Balgaard TJ, Rosenfeld LE. Technician-supported remote interrogation of CIEDs: Initial use in US emergency departments and perioperative areas. Pacing Clin Electrophysiol 2016;39(3):275–281. doi: 10.1111/pace.12798. [DOI] [PubMed] [Google Scholar]
- 121. Mittal S, Younge K, King-Ellison K, Hammill E, Stein K. Performance of a remote interrogation system for the in-hospital evaluation of cardiac implantable electronic devices. J Interv Card Electrophysiol 2016;46(2):121–128. doi: 10.1007/s10840-015-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Burri H. Remote follow-up and continuous remote monitoring, distinguished. Europace 2013;15(Suppl. 1):i14–i16. doi: 10.1093/europace/eut071. [DOI] [PubMed] [Google Scholar]
- 123. Varma N, Stambler B, Chun S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol 2005;28(Suppl. 1):S133–136. doi: 10.1111/j.1540-8159.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 124. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 125. Watanabe E, Yamazaki F, Goto T, et al. Remote management of pacemaker patients with biennial in-clinic evaluation: Continuous home monitoring in the Japanese At-Home Study: A randomized clinical trial. Circ Arrhythm Electrophysiol 2020;13(5):e007734. doi: 10.1161/circep.119.007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. García-Fernández FJ, Osca Asensi J, Romero R, et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: A long-term randomized trial (RM-ALONE). Eur Heart J 2019;40(23):1837–1846. doi: 10.1093/eurheartj/ehz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zeitler EP, Patel D, Hasselblad V, Sanders GD, Al-Khatib SM. Complications from prophylactic replacement of cardiac implantable electronic device generators in response to United States Food and Drug Administration recall: A systematic review and meta-analysis. Heart Rhythm 2015;12(7):1558–1564. doi: 10.1016/j.hrthm.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Varma N. Automatic remote home monitoring of implantable cardioverter defibrillator lead and generator function: A system that tests itself everyday. Europace 2013;15(Suppl. 1):i26–i31. doi: 10.1093/europace/eut116. [DOI] [PubMed] [Google Scholar]
- 129. Ploux S, Varma N, Strik M, Lazarus A, Bordachar P. Optimizing implantable cardioverter-defibrillator remote monitoring: A practical guide. JACC Clin Electrophysiol 2017;3(4):315–328. doi: 10.1016/j.jacep.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 130. Ploux S, Swerdlow CD, Strik M, et al. Towards eradication of inappropriate therapies for ICD lead failure by combining comprehensive remote monitoring and lead noise alerts. J Cardiovasc Electrophysiol 2018;29(8):1125–1134. doi: 10.1111/jce.13653. [DOI] [PubMed] [Google Scholar]
- 131. Ahmed FZ, Sammut-Powell C, Kwok CS, et al. Remote monitoring data from cardiac implantable electronic devices predicts all-cause mortality. Europace 2022;24(2):245–255. doi: 10.1093/europace/euab160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wilkoff BL, Richards M, Sharma A, et al. A device histogram-based simple predictor of mortality risk in ICD and CRT-D patients: The heart rate score. Pacing Clin Electrophysiol 2017;40(4):333–343. doi: 10.1111/pace.13036. [DOI] [PubMed] [Google Scholar]
- 133. Böhm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: A randomized controlled trial. Eur Heart J 2016;37(41):3154–3163. doi: 10.1093/eurheartj/ehw099. [DOI] [PubMed] [Google Scholar]
- 134. Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017;38(30):2352–2360. doi: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm 2011;8(9):1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 136. Koplan BA, Kaplan AJ, Weiner S, Jones PW, Seth M, Christman SA. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: Is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol 2009;53(4):355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 137. Guédon-Moreau L, Kouakam C, Klug D, et al. Decreased delivery of inappropriate shocks achieved by remote monitoring of ICD: A substudy of the ECOST trial. J Cardiovasc Electrophysiol 2014;25(7):763–770. doi: 10.1111/jce.12405. [DOI] [PubMed] [Google Scholar]
- 138. Boulé S, Ninni S, Finat L, et al. Potential role of antitachycardia pacing alerts for the reduction of emergency presentations following shocks in patients with implantable cardioverter-defibrillators: Implications for the implementation of remote monitoring. Europace 2016;18(12):1809–1817. doi: 10.1093/europace/euv392. [DOI] [PubMed] [Google Scholar]
- 139. Otsuki S, Izumi D, Sakaguchi Y, et al. Efficacy of antitachycardia pacing alert by remote monitoring of implantable cardioverter-defibrillators for out-of-hospital electrical storm. Pacing Clin Electrophysiol 2021;44(10):1675–1682. doi: 10.1111/pace.14334. [DOI] [PubMed] [Google Scholar]
- 140. Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol 1997;29(4):744–749. doi: 10.1016/s0735-1097(96)00586-4. [DOI] [PubMed] [Google Scholar]
- 141. Karpawich PP, Justice CD, Cavitt DL, Chang CH. Developmental sequelae of fixed-rate ventricular pacing in the immature canine heart: An electrophysiologic, hemodynamic, and histopathologic evaluation. Am Heart J 1990;119(5):1077–1083. doi: 10.1016/s0002-8703(05)80237-6. [DOI] [PubMed] [Google Scholar]
- 142. Dreger H, Maethner K, Bondke H, Baumann G, Melzer C. Pacing-induced cardiomyopathy in patients with right ventricular stimulation for > 15 years. Europace 2012;14(2):238–242. doi: 10.1093/europace/eur258. [DOI] [PubMed] [Google Scholar]
- 143. Khurshid S, Epstein AE, Verdino RJ, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm 2014;11(9):1619–1625. doi: 10.1016/j.hrthm.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 144. Kiehl EL, Makki T, Kumar R, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016;13(12):2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 145. Kim JH, Kang KW, Chin JY, Kim TS, Park JH, Choi YJ. Major determinant of the occurrence of pacing-induced cardiomyopathy in complete atrioventricular block: A multicentre, retrospective analysis over a 15-year period in South Korea. BMJ Open 2018;8(2):e019048. doi: 10.1136/bmjopen-2017-019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sharma AD, Rizo-Patron C, Hallstrom AP, et al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005;2(8):830–834. doi: 10.1016/j.hrthm.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 147. Perla HT, Chandra Srinath Patloori S, Manickavasagam A, Chase D, Roshan J. Do the predictors of right ventricular pacing-induced cardiomyopathy add up? Indian Heart J 2021;73(5):582–587. doi: 10.1016/j.ihj.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Vijayaraman P, Zalavadia D, Haseeb A, et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm 2022;19(8):1263–1271. doi: 10.1016/j.hrthm.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 149. Afzal MR, Mease J, Koppert T, et al. Incidence of false-positive transmissions during remote rhythm monitoring with implantable loop recorders. Heart Rhythm 2020;17(1):75–80. doi: 10.1016/j.hrthm.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 150. Sanders P, Pürerfellner H, Pokushalov E, et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: Results from the Reveal LINQ Usability Study. Heart Rhythm 2016;13(7):1425–1430. doi: 10.1016/j.hrthm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 151. Grigoriadis CJ, Dunn A, Parikh P, Wadhwa MK. Distribution of false arrhythmic alerts from implantable loop recorders in the Philips Geneva Health Solutions implantable device remote monitoring database (Abstract). Cardiovasc Digit Health J 2022;3(4 Suppl):S16. doi: 10.1016/j.cvdhj.2022.07.038. [DOI] [Google Scholar]
- 152. de Ruvo E, Sciarra L, Martino AM, et al. A prospective comparison of remote monitoring systems in implantable cardiac defibrillators: Potential effects of frequency of transmissions. J Interv Card Electrophysiol 2016;45(1):81–90. [DOI] [PubMed] [Google Scholar]
- 153. Söth-Hansen M, Witt CT, Rasmussen M, Kristensen J, Gerdes C, Nielsen JC. Time until diagnosis of clinical events with different remote monitoring systems in implantable cardioverter-defibrillator patients. Heart rhythm 2018;15(11):1648–1654. doi: 10.1016/j.hrthm.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 154. Nishii N, Miyoshi A, Kubo M, et al. Analysis of arrhythmic events is useful to detect lead failure earlier in patients followed by remote monitoring. J Cardiovasc Electrophysiol 2018;29(3):463–470. doi: 10.1111/jce.13399. [DOI] [PubMed] [Google Scholar]
- 155. Varma N, Michalski J, TRUST Investigators . Alert notifications during automatic wireless remote monitoring of implantable cardioverter-defibrillators: Load, characteristics, and clinical utility. Heart Rhythm 2023;20:473–474. doi: 10.1016/j.hrthm.2022.11.019. [DOI] [PubMed] [Google Scholar]
- 156. Morimoto Y, Nishii N, Tsukuda S, et al. A low critical event rate despite a high abnormal event rate in patients with cardiac implantable electric devices followed up by remote monitoring. Intern Med 2019;58(16):2333–2340. doi: 10.2169/internalmedicine.1905-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Maines M, Zorzi A, Tomasi G, et al. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQTM. Europace 2018;20(6):1050–1057. doi: 10.1093/europace/eux187. [DOI] [PubMed] [Google Scholar]
- 158. Zanotto G, D'Onofrio A, Della Bella P, et al. Organizational model and reactions to alerts in remote monitoring of cardiac implantable electronic devices: A survey from the Home Monitoring Expert Alliance project. Clin Cardiol 2019;42(1):76–83. doi: 10.1002/clc.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Varma N, Ricci RP. Impact of remote monitoring on clinical outcomes. J Cardiovasc Electrophysiol 2015;26(12):1388–1395. doi: 10.1111/jce.12829. [DOI] [PubMed] [Google Scholar]
- 160. Daley C, Toscos T, Allmandinger T, Ahmed R, Wagner S, Mirro M. Organizational models for cardiac implantable electronic device remote monitoring: Current and future directions. Card Electrophysiol Clin 2021;13(3):483–497. doi: 10.1016/j.ccep.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 161. de Cock CC, Elders J, van Hemel NM, et al. Remote monitoring and follow-up of cardiovascular implantable electronic devices in the Netherlands: An expert consensus report of the Netherlands Society of Cardiology. Neth Heart J 2012;20(2):53–65. doi: 10.1007/s12471-011-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Fraiche AM, Matlock DD, Gabriel W, Rapley FA, Kramer DB. Patient and provider perspectives on remote monitoring of pacemakers and implantable cardioverter-defibrillators. Am J Cardiol 2021;149:42–46. doi: 10.1016/j.amjcard.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 163. Laurent G, Amara W, Mansourati J, et al. Role of patient education in the perception and acceptance of home monitoring after recent implantation of cardioverter defibrillators: The EDUCAT study. Arch Cardiovasc Dis 2014;107(10):508–518. doi: 10.1016/j.acvd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 164. Ricci RP, Morichelli L. Workflow, time and patient satisfaction from the perspectives of home monitoring. Europace 2013;15(Suppl. 1):i49–i53. doi: 10.1093/europace/eut113. [DOI] [PubMed] [Google Scholar]
- 165. Pedersen SS, Knudsen C, Dilling K, Sandgaard NCF, Johansen JB. Living with an implantable cardioverter defibrillator: Patients' preferences and needs for information provision and care options. Europace 2017;19(6):983–990. doi: 10.1093/europace/euw109. [DOI] [PubMed] [Google Scholar]
- 166. Strachan PH, de Laat S, Carroll SL, et al. Readability and content of patient education material related to implantable cardioverter defibrillators. J Cardiovasc Nurs 2012;27(6):495–504. doi: 10.1097/JCN.0b013e31822ad3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Timmermans I, Meine M, Zitron E, et al. The patient perspective on remote monitoring of patients with an implantable cardioverter defibrillator: Narrative review and future directions. Pacing Clin Electrophysiol 2017;40(7):826–833. doi: 10.1111/pace.13123. [DOI] [PubMed] [Google Scholar]
- 168. Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med 2012;366(9):780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 169. Catalan-Matamoros D, Lopez-Villegas A, Tore-Lappegard K, Lopez-Liria R. Patients' experiences of remote communication after pacemaker implant: The NORDLAND study. PLoS One 2019;14(6):e0218521. doi: 10.1371/journal.pone.0218521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Timmermans I, Meine M, Szendey I, et al. Remote monitoring of implantable cardioverter defibrillators: Patient experiences and preferences for follow-up. Pacing Clin Electrophysiol 2019;42(2):120–129. doi: 10.1111/pace.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Dechert BE, Bradley DJ, Serwer GA, LaPage MJ. The impact of CIEDs with automatic “wireless” remote monitoring on efficiency. Pacing Clin Electrophysiol 2021;44(10):1671–1674. [DOI] [PubMed] [Google Scholar]
- 172. Maisel WH, Sweeney MO, Stevenson WG, Ellison KE, Epstein LM. Recalls and safety alerts involving pacemakers and implantable cardioverter-defibrillator generators. JAMA 2001;286(7):793–799. doi: 10.1001/jama.286.7.793. [DOI] [PubMed] [Google Scholar]
- 173. Schwartz J, Blangy H, Zinzius PY, Freysz L, Aliot E, Sadoul N. Recall alerts in implantable cardioverter-defibrillator recipients: Implications for patients and physicians. Pacing Clin Electrophysiol 2011;34(1):96–103. doi: 10.1111/j.1540-8159.2010.02918.x. [DOI] [PubMed] [Google Scholar]
- 174. Sengupta J, Kendig AC, Goormastic M, et al. Implantable cardioverter-defibrillator FDA safety advisories: Impact on patient mortality and morbidity. Heart Rhythm 2012;9(10):1619–1626. doi: 10.1016/j.hrthm.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Blake K. Postmarket surveillance of medical devices: Current capabilities and future opportunities. J Interv Card Electrophysiol 2013;36(2):119–127. doi: 10.1007/s10840-013-9778-6. [DOI] [PubMed] [Google Scholar]
- 176. Maisel WH, Hauser RG, Hammill SC, et al. Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: Developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm 2009;6(6):869–885. doi: 10.1016/j.hrthm.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 177. Ottenberg AL, Mueller LA, Mueller PS. Perspectives of patients with cardiovascular implantable electronic devices who received advisory warnings. Heart Lung 2013;42(1):59–64. doi: 10.1016/j.hrtlng.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zeitler EP, Piccini JP. Remote monitoring of cardiac implantable electronic devices (CIED). Trends Cardiovasc Med 2016;26(6):568–577. doi: 10.1016/j.tcm.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Giannola G, Torcivia R, Airò Farulla R, Cipolla T. Outsourcing the remote management of cardiac implantable electronic devices: Medical Care Quality Improvement Project. JMIR Cardio 2019;3(2):e9815. doi: 10.2196/cardio.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Giannola G, Torcivia R, Gardini A, Glisenti F. Outsourcing of remote management of cardiac implantable electronic devices through an external remote monitoring center: An efficacy and safety evaluation (Abstract). Eur Heart J 2017;38(Suppl. 1):P4256. doi: 10.1093/eurheartj/ehx504.P4256. [DOI] [Google Scholar]
- 181. Nicolle E, Lanctin D, Rosemas S, De Melis M. Clinic time required to manage remote monitoring of cardiac implantable electronic devices: Impact of outsourcing initial data review and triage. Europace 2021;23(Suppl. 3). doi: 10.1093/europace/euab116.519. [DOI] [Google Scholar]
- 182. Nielsen JC, Kautzner J, Casado-Arroyo R, et al. Remote monitoring of cardiac implanted electronic devices: Legal requirements and ethical principles - ESC Regulatory Affairs Committee/EHRA joint task force report. Europace 2020;22(11):1742–1758. doi: 10.1093/europace/euaa168. [DOI] [PubMed] [Google Scholar]
- 183. Dechert BE, Bradley DJ, Serwer GA, Dick M, 2nd, LaPage MJ. Frequency of CIED remote monitoring: A quality improvement follow-up study. Pacing Clin Electrophysiol 2019;42(7):959–962. doi: 10.1111/pace.13707. [DOI] [PubMed] [Google Scholar]
- 184. Dechert BE, Serwer GA, Bradley DJ, Dick M, 2nd, LaPage MJ. Cardiac implantable electronic device remote monitoring surveillance in pediatric and congenital heart disease: Utility relative to frequency. Heart Rhythm 2015;12(1):117–122. doi: 10.1016/j.hrthm.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 185. Malloy LE, Gingerich J, Olson MD, Atkins DL. Remote monitoring of cardiovascular implantable devices in the pediatric population improves detection of adverse events. Pediatr Cardiol 2014;35(2):301–306. doi: 10.1007/s00246-013-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Varma N, Kondo Y, Park SJ, et al. Utilization of remote monitoring among patients receiving cardiac resynchronization therapy and comparison between Asia and the Americas. Heart Rhythm O2 2022;3(6Part B):868–870. doi: 10.1016/j.hroo.2022.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Luzi M, De Simone A, Leoni L, et al. Remote monitoring for implantable defibrillators: A nationwide survey in Italy. Interact J Med Res 2013;2(2):e27. doi: 10.2196/ijmr.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Palmisano P, Melissano D, Zanotto G, et al. Change in the use of remote monitoring of cardiac implantable electronic devices in Italian clinical practice over a 5-year period: Results of two surveys promoted by the AIAC (Italian Association of Arrhythmology and Cardiac Pacing). J Cardiovasc Med (Hagerstown) 2020;21(4):305–314. doi: 10.2459/jcm.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 189. Lau CP, Zhang S. Remote monitoring of cardiac implantable devices in the Asia-Pacific. Europace 2013;15(Suppl. 1):i65–i68. doi: 10.1093/europace/eut081. [DOI] [PubMed] [Google Scholar]
- 190. Rosenfeld LE, Patel AS, Ajmani VB, Holbrook RW, Brand TA. Compliance with remote monitoring of ICDS/CRTDS in a real-world population. Pacing Clin Electrophysiol 2014;37(7):820–827. doi: 10.1111/pace.12358. [DOI] [PubMed] [Google Scholar]
- 191. Alexander B, Baranchuk A. Remote device reprogramming: Has its time come? Circ Arrhythm Electrophysiol 2020;13(10):e008949. doi: 10.1161/circep.120.008949. [DOI] [PubMed] [Google Scholar]
- 192. Tong L, Xiong S, Hou J, et al. Cloud follow-up in patients with cardiovascular implantable electronic devices: A single-region study in China. Front Cardiovasc Med 2022;9:864398. doi: 10.3389/fcvm.2022.864398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Siddamsetti S, Shinn A, Gautam S. Remote programming of cardiac implantable electronic devices: A novel approach to program cardiac devices for magnetic resonance imaging. J Cardiovasc Electrophysiol 2022;33(5):1005–1009. doi: 10.1111/jce.15434. [DOI] [PubMed] [Google Scholar]
- 194. Ploux S, Strik M, Demonière F, et al. Remote interrogation and reprogramming of cardiac implantable electronic devices using a custom multi-vendor solution [published ahead of print, December 13, 2022]. Heart Rhythm 2022. doi: 10.1016/j.hrthm.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 195. Boriani G, Burri H, Svennberg E, Imberti JF, Merino JL, Leclercq C. Current status of reimbursement practices for remote monitoring of cardiac implantable electrical devices across Europe. Europace 2022;24:1875–1880. doi: 10.1093/europace/euac118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Mittal S, Oliveros S, Li J, Barroyer T, Henry C, Gardella C. AI filter improves positive predictive value of atrial fibrillation detection by an implantable loop recorder. JACC Clin Electrophysiol 2021;7(8):965–975. doi: 10.1016/j.jacep.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 197. Brown G, Conway S, Ahmad M, et al. Role of artificial intelligence in defibrillators: A narrative review. Open Heart 2022;9(2):e001976. doi: 10.1136/openhrt-2022-001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) (2016). Accessed March 3, 2023. Available from https://eur-lex.europa.eu/eli/reg/2016/679/oj
- 199. 21st Century Cures Act, Pub L No. 114-255, (2016). March 23, 2023. https://www.govinfo.gov/app/details/PLAW-114publ255/summary.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.