Abstract
Coronary arterial disease (CAD) is the leading cause of mortality in the world. Hyperuricemia has recently emerged as a novel independent risk factor of CAD, in addition to the traditional risk factors such as hyperlipidemia, smoking, and obesity. Several clinical studies have shown that hyperuricemia is strongly associated with the risk, progression and poor prognosis of CAD, as well as verifying an association with traditional CAD risk factors. Uric acid or enzymes in the uric acid production pathway are associated with inflammation, oxidative stress, regulation of multiple signaling pathways and the renin-angiotensin-aldosterone system (RAAS), and these pathophysiological alterations are currently the main mechanisms of coronary atherosclerosis formation. The risk of death from CAD can be effectively reduced by the uric acid-lowering therapy, but the interventional treatment of uric acid levels in patients with CAD remains controversial due to the diversity of co-morbidities and the complexity of causative factors. In this review, we analyze the association between hyperuricemia and CAD, elucidate the possible mechanisms by which uric acid induces or exacerbates CAD, and discuss the benefits and drawbacks of uric acid-lowering therapy. This review could provide theoretical references for the prevention and management of hyperuricemia-associated CAD.
Keywords: Hyperuricemia, Coronary arterial disease, Uric acid, Atherosclerosis, Pathophysiology
1. Introduction
Coronary arterial disease (CAD) is one of the common cardiovascular diseases in the world. CAD is mainly caused by coronary artery narrowing and obstruction due to atherosclerosis, which eventually causes ischemic necrosis of the myocardium [1]. Numerous studies have revealed the pathogenesis of coronary atherosclerosis and the prevention and treatment methods, but the number of deaths from CAD is still increasing. As shown in the Global Burden of Disease (GBD) study, ischemic heart disease, a consequence of coronary atherosclerosis, is the leading cause of cardiovascular death worldwide [2]. The Annual Report on Cardiovascular Health and Diseases in China (2021) projected that the number of people with cardiovascular disease in China is currently 330 million, more than 1/3 of the global number, and the highest mortality rate [3]. In order to reduce the risk level of CAD and improve the quality of life of patients, more attention should be focused on the primary and secondary prevention of CAD. Comparing to the known causative factors such as lipids and glucose in CAD prevention, little attention and investigation have been put on hyperuricemia in cardiovascular diseases [4]. Although some studies question the independent risk role of uric acid and more studies suggest that hyperuricemia has a negative impact on the onset, progression and prognosis of CAD [[5], [6], [7], [8]]. There is also evidence that hyperuricemia is inextricably linked to traditional risk factors for CAD, which may have jointly contributed to the onset and development of CAD [[9], [10], [11]].
Uric acid is derived from endogenous and exogenous purine nucleotides depending on a variety of enzymes. 30% of the uric acid synthesized in the body is excreted from the intestine and the other 70% from the kidney [12]. Uric acid is the end product of an exogenous pool of purines and endogenous purine metabolism. When uric acid production in the body increases or uric acid excretion in the kidneys and intestines decreases, resulting in an increase in uric acid concentration in the blood of more than 420 μmol/L, it will cause hyperuricemia [12] (Fig. 1). Uric acid has been considered as an important antioxidant in the human body. Some studies found that uric acid enhanced the antioxidant effect of red blood cells, which contributed more than 50% to the antioxidant effect of blood, and also had an oxidative protective effect on the intestinal lumen. Uric acid in the intestinal lumen protects the intestine mainly by regulating the composition of the intestinal flora and by binding to intestinal epithelial reactive oxygen targets, eliminating reactive oxygen species (ROS) [[13], [14], [15]]. Appropriate concentrations of uric acid can reduce oxidized low-density lipoprotein (OX-LDL)-induced vascular endothelial damage and similarly improve the oxidative stress response of endothelial cells, protecting them and thus reducing atherosclerosis [16]. In addition, uric acid has the potential to have a protective effect on the DNA of lymphocytes, lysosomal membranes, and mitigate the harmful effects of heavy metals on humans [13]. Therefore, uric acid at appropriate concentrations has antioxidant effects and high concentrations appear to cause an imbalance in oxidative stress and thus has a pro-inflammatory effect on clinical disease. In recent years, the prevalence of hyperuricemia has increased dramatically with the current occurrence rate of hyperuricemia in China at 13.3%, and hyperuricemia has become the second most common metabolic disease after diabetes [17]. Hyperuricemia is associated with several diseases such as CAD, metabolic syndrome, diabetes mellitus, and nephropathy since hyperuricemia can cause mitochondrial dysfunction, lipid accumulation, and insulin resistance in pancreatic ꞵ-cells [[18], [19], [20], [21]]. Hyperuricemia has also been proposed to be a causative factor of CAD. Oxidative stress, inflammation, endothelial dysfunction, and activation of the RAAS may also be pathological mechanisms of hyperuricemia-induced CAD [[22], [23], [24]]. At present, it is necessary to clarify the degree of association and pathophysiological link between hyperuricemia and CAD. This review will discuss the potential effects and mechanisms of hyperuricemia in CAD, in order to provide a reference for the prevention and treatment of CAD.
Fig. 1.
Synthesis and metabolism of uric acid. Abbreviations: AMP = Adenosine monophosphate, IMP = Inosine monophosphate, GMP = Guanosine monophosphate, ADA = Adenosine deaminase, PNPase = Purine nucleoside phosphorylase.
2. The correlation between hyperuricemia and CAD
2.1. Hyperuricemia is a potential pathogenic factor of CAD
The degree of coronary artery stenosis determines the severity of CAD which correlates to hyperuricemia [6,7,25]. Coronary stenosis is caused by coronary atherosclerosis and coronary plaque formation, and is positively associated with the severity of cardiac load and ischemia [26,27]. The relationship between subclinical coronary atherosclerosis and hyperuricemia has been assessed by detecting the incidence of non-calcified coronary plaque, which confirms that the incidence of coronary plaque increases with higher uric acid concentration [28]. Thus, it is controversial and confusing about the relationship between hyperuricemia and the progression of coronary stenosis.
It has been found that the severity of coronary plaques assessed by optical coherence tomography correlates with the concentration of uric acid, which in turn is positively associated with the calcification length, maximum lipid arc, and mean lipid arc of coronary plaques [29]. A prospective study showed that the gensini score for assessing coronary stenosis increased significantly with increasing uric acid levels [7], suggesting a possible concentration-dependent relationship between coronary stenosis severity and uric acid. This notion is supported by reports from different researchers. For example, Ekici et al. reported that in a cohort of 705 patients who had perfect coronary angiography and measured their pre-procedure patient’s serum uric acid levels, the severity of coronary stenosis indexed by the SYNTAX score was increased as circulating uric acid concentrations increased, suggesting a positive correlation between SYNTAX scores and uric acid concentrations [30]. Another study also showed that SYNTAX scores and the number of coronary stenoses were significantly higher in the hyperuricemia group than in the low uric acid group [31]. Elevated uric acid also increases the risk of coronary calcium deposition in middle-aged and elderly people [32]. These data indicate that elevated uric acid concentrations are associated with increases in coronary plaque formation, coronary calcium deposition, the number of coronary stenosis, and the degree of coronary stenosis lesions, suggesting that hyperuricemia is a risk factor for the occurrence and progression of CAD, in addition to many other risk factors of CAD including hypertension, diabetes mellitus, dyslipidemia, and obesity.
2.2. Hyperuricemia is closely related to the poor prognosis of CAD
Many studies have demonstrated that hyperuricemia is associated with the development of adverse cardiovascular events in patients with CAD although the degree of this association may vary between different genders and geographies [8,33,34]. Clinically, CAD can be divided into two major categories, including acute coronary syndrome (ACS) and stable CAD [35]. ACS is further divided into ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina [35]. For example, a retrospective study in women showed a statistical difference in hyperuricemia in patients with ACS compared to non-ACS patients, but excluding the effect of confounding factors (e.g., hyperlipidemia, hyperhomocysteinemia, overweight, high C-reactive protein, hypertension), hyperuricemia was not an independent risk factor in women with ACS [33]. However, it has also shown that hyperuricemia is not associated with the degree of CAD in men [36]. Moreover, a prospective study conducted in Austria showed that hyperuricemia was not associated with mortality in acute, subacute or chronic CAD [34]. In contrast, in a Polish study that evaluated the relationship between uric acid levels and its long-term prognosis in patients with NSTEMI, the authors retrospectively analyzed the association of uric acid in 549 patients hospitalized with NSTEMI and showed that uric acid was an independent risk factor for NSTEMI [8].
In addition, by comparing the mortality rates of those followed up to 6 years, they found that the percentage of patients with hyperuricemia was nearly doubled compared with patients with normal uric acid, leading to a conclusion that hyperuricemia is associated with long-term mortality in NSTEMI [8]. A Meta analysis also showed that patients with STEMI in the presence of hyperuricemia had a significantly higher incidence of in-hospital cardiovascular events, in-hospital mortality, and 1-year post-discharge mortality than the STEMI population with normal uric acid concentrations [37]. Analysis on a large number of STEMI patients and their 1-month and 1-year post-infarction adverse cardiovascular events showed that hyperuricemia was an independent predictor of 30-day and 1-year mortality in STEMI patients [38]. Elevated uric acid also contributes to the development of complications in STEMI patients. He et al. observed a strong correlation between hyperuricemia and the risk of acute kidney injury in STEMI patients [39]. Also hyperuricemia was associated with CAD serious complications such as post-infarction ventricular arrhythmias, no-/slow-reflow phenomenon, and heart failure [[40], [41], [42]]. It is of interest that the correlation between hyperuricemia and CAD varies across genders, ethnicities, and geographies, which may be attributed to different hormone levels, physiological organization, dietary habits, environment, and genetics. Although there are conflicting findings regarding the relationship between uric acid and CAD, emerging evidence suggests that hyperuricemia is an independent predictor and an independent risk factor for the incidence of mortality and complications of CAD.
2.3. Hyperuricemia is associated with traditional CAD risk factors
CAD has a variety of risk factors, and often reflected in clinical manifestations such as frequent episodes of angina pectoris, intractable heart failure, and malignant arrhythmias [35]. Elevated uric acid levels are associated with traditional CAD risk factors, including hypertension, diabetes mellitus, dyslipidemia, and obesity [[9], [10], [11]]. Previous prospective studies have shown a concomitant increase in the risk of developing hypertension with increased circulating uric acid concentrations [43]. Hyperuricemia as both a risk factor and a pathological state for the development of hypertension, since hyperuricemia causes vascular endothelial dysfunction, along with activation of the RAAS and exacerbation of arterial stiffness [44]. Also hypertension can impair renal function and decrease glomerular filtration rate, which leads to hyperuricemia [45]. It has been suggested that uric acid levels in patients with essential hypertension are J-shaped in relation to the risk of ACS [46]. This highlights the link between uric acid, hypertension, and CAD with the three perhaps mutually predisposing and influencing each other. Similarly, hyperuricemia can also affect type 2 diabetes and its comorbidities, such as diabetic kidney damage, diabetic retinopathy, and diabetic peripheral neuropathy [[47], [48], [49]]. A clinical cross-sectional study proposed a positive correlation between serum uric acid levels in type 2 diabetic patients and their insulin resistance substitutes [50]. Insulin resistance, in turn, is involved in the progression of CAD [51]. Another study on cardiac prevention confirmed that serum uric acid concentrations could independently predict death in patients at high risk of cardiovascular disease, as each 1 mg/dL increase in their serum uric acid concentrations increases the risk of cardiovascular death by approximately 40% [52]. Hyperuricemia is not only associated with hypertension and diabetes, but also has a strong correlation with visceral adiposity, metabolic syndrome, and uric acid nephropathy [53]. More studies have concluded that hyperuricemia is not only an independent risk factor for CAD, but also influences or exacerbates the development of CAD along with traditional CAD risk factors.
2.4. The potential pathophysiological association between hyperuricemia and CAD
Although hyperuricemia is widely recognized to be associated with CAD, the specific regulatory mechanisms between the two have not been elucidated. However, as coronary atherosclerosis is the leading cause of CAD [54], uric acid-induced coronary atherosclerosis is currently recognized as a major inducer of CAD. As such, several mechanisms governing coronary atherosclerosis have been are proposed to regulate uric acid-associated CAD, including oxidative stress, inflammatory regulation, RAAS activation, endothelial dysfunction, and uric acid metabolism.
3. Oxidative stress
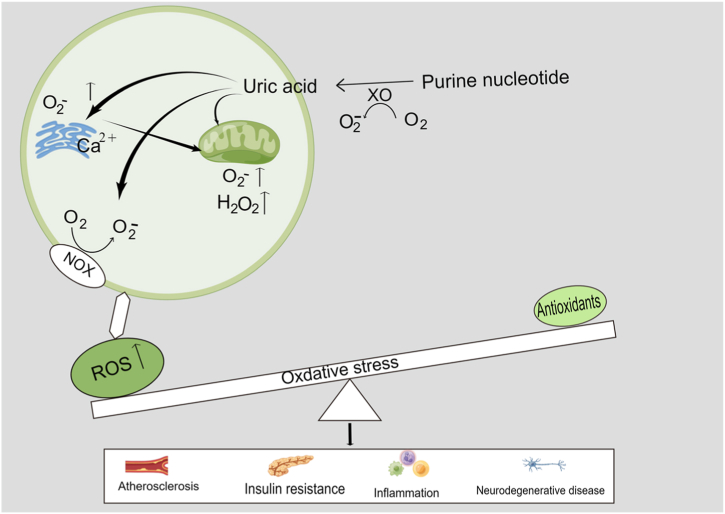
Oxidative stress is a state of oxidative and antioxidant imbalance in the body, generally accompanied by a variety of injuries (atherosclerosis, insulin resistance, inflammation, and neurodegeneration) [[55], [56], [57]]. Oxidative stress primarily induced by excessive ROS is one of the main mechanisms of atherosclerosis [57]. Uric acid is produced by the breakdown of nucleotides in the body through a variety of uricase enzymes and the consumption of adenosine triphosphate. During uric acid production, the activity of xanthine oxidase (XO) increases, prompting the elevation of ROS [58]. Elevated ROS can induce the migration and proliferation of small arterial smooth muscle cells and the production of monocyte chemotactic protein (MCP)-1, participating in the development of atherosclerosis [58]. ROS causes oxidation of low-density lipoprotein to OX-LDL, inducing an inflammatory response in vascular endothelial cells. Subendothelial macrophages can absorb OX-LDL to form foam cells, release chemokines and inflammatory factors, attract inflammatory cells, produce tumor necrosis factor-α (TNF)-α, etc., and aggregate fibroblasts to promote fibrous cap formation, thus contributing to atherosclerotic plaque formation [22]. High concentrations of uric acid are involved in the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase as well as oxidative stress, promoting the production of ROS, which stimulate the proliferation of smooth muscle cells and the expression of endothelin-1 through the activation of ERK/AP1 signaling pathway, thus inducing vasoconstriction and fibroblast proliferation, causing vascular sclerosis and impaired endothelial function [59,60]. High levels of uric acid also increases the expression level and activation level of NADPH oxidase, leading to mitochondrial dysfunction and endoplasmic reticulum stress in liver cells and renal tubular epithelial cells, thereby increasing the production of ROS in mitochondria and endoplasmic reticulum, increasing the formation of inflammatory cytokines, and inducing vascular endothelial cell senescence, regulation and lipid accumulation through inhibition of AMP-dependent protein kinase (AMPK) or activation of Rho kinase, which in turn affects the development of atherosclerosis [[61], [62], [63], [64]] (Fig. 2).
Fig. 2.
Uric acid and oxidative stress injury. Abbreviations: ROS = Reactive oxygen species, O2− = Superoxide anion, H2O2 = Hydrogen peroxide.
4. Inflammation regulation
Atherosclerosis is an inflammatory disease [65]. Uric acid itself, and ROS induced by uric acid can activate the inflammasome and multiple relevant signaling regulatory pathways, causing inflammatory factor production, vascular smooth muscle cell (VSMC) proliferation and migration, and vascular endothelial dysfunction. For example, uric acid activates the EPK/p38 MAPK cascade reaction, promoting the expression of cell adhesion molecules E-selectin, vascular cell adhesion protein-1 (VCAM)-1 and MCP-1, causing VSMC proliferation and hypertrophy as well as promoting cardiomyocyte ROS production, leading to aortic valve calcification and cardiomyocyte injury [66,67]. Activation of EPK/p38 MAPK cascade reaction induces VSMC migration and is involved in neovascularization induction and atherosclerotic plaque formation; it also promotes immune cell adhesion through chemokine receptor CXCR2 expression and inflammatory cytokine expression [68,69]. Uric acid also attenuates the activity of AMPK, a molecule that links metabolism and inflammation, and inhibition of AMPK activity induces macrophage production of inflammatory cytokines and activation of the NOD-like receptor protein (NLRP) 3 inflammasome, promoting the development of atherosclerosis [21]. Uric acid can also cause disturbances in protein metabolism, gluconeogenesis and liver fat accumulation by inhibiting AMPK activity [[19], [20], [21]]. Uric acid can also involve in the atherosclerotic process by regulating the Phosphatidylinositol-3 kinase (PI3K)-AKT pathway [[70], [71], [72]]. In human monocytes, uric acid induces AKT phosphorylation and activates the PI3K-AKT pathway, which in turn induces migration of monocytes and macrophages, lipid accumulation and endothelial dysfunction [71]. In addition, uric acid can also be involved in the progression of atherosclerosis by inhibiting AKT and causing insulin resistance [70]. Some experiments have expressed other views, and researchers have suggested that high concentrations of uric acid inhibit the PI3K/AKT signaling pathway, but the PI3K/AKT signaling pathway may not be involved in the mechanism by which hyperuricemia inhibits cardiomyocyte viability in mice, and that different cells have different regulatory pathways [66]. Relevant therapeutic targets have been proposed based on the relevant signaling pathways, and histone deacetylase inhibitors attenuate uric acid accumulation-induced vascular endothelial disorders by activating the PI3K/AKT pathway [73] (Fig. 3).
Fig. 3.
Uric acid induces arteriosclerosis as a possible signal transduction pathway. Abbreviations: NF-κB = Nuclear factor-κB, ER Stress = endoplasmic reticulum stress, UA = Uric Acid.
5. RAAS activation
Previous studies have confirmed that imbalance of the RAAS contributes the development of atherosclerosis, hypertension, and diabetes [74,75]. Angiotensin II (AngII) is a key effector of the RAAS system, acting mainly on its receptors, causing vasoconstriction, regulating acid-base homeostasis, modulating immune and inflammatory pathways (endothelial cells, renal tubular epithelial cells, smooth muscle cells), and stimulating the release of chemokines from macrophages [76]. Uric acid may activate the RAAS, up-regulate angiotensinogen (AGT), angiotensin-converting enzyme (ACE) and AngII receptor expression levels, stimulate XO, NADPH-oxidase and smooth muscle cell proliferation, and activate oxidative stress and inflammatory cascade responses [[77], [78], [79]]. An in vitro study demonstrated that either uric acid or AngII increased Toll-Like Receptor-4 (TLR4) expression and contributed to the production of MCP-1 and NADPH oxidase 4 (NOX4) [23]. In this study, the addition of TLR4 inhibitors in the uric acid, AngII and uric acid + AngII groups resulted in a decrease in the mRNA and protein levels of MCP-1 and NOX4 produced in the uric acid and AngII groups, while no significant inhibition was observed in the uric acid + AngII group [23]. Uric acid not only activates the RAAS involved in oxidative stress and inflammatory response, but also can cause a more complex inflammatory and oxidative response in combination with AngII.
6. Endothelial dysfunction
Nitric oxide (NO) is considered an anti-atherosclerotic factor in the human body, possibly by regulating vascular tone, platelet aggregation, and endothelial function [80,81]. Uric acid can reduce NO synthase (NOS) activity, reducing or depleting NO production, but generates excess ROS, aggravating endothelial dysfunction [82]. Endothelial dysfunction is the initiating factor of atherosclerosis, and high concentrations of uric acid lead to endothelial dysfunction through activation of inflammatory factors such as interleukin (IL)-1, (IL-6) and TNF-β as well as several chemokines and adhesion molecules [24]. The roles of uric acid in inducing endothelial dysfunction are supported by an in vitro observation that high concentrations of uric acid inhibited NOS expression and decreased NO production in human umbilical vein endothelial cells [83]. High concentrations of uric acid also elevated expression of high mobility group protein B1 (HMGB1), nuclear factor NF-κB, receptor for advanced glycosylation end products (RAGE), inflammatory cytokines, and adhesion molecules, the interaction of HMGB1 with RAGE and inflammatory cytokines then triggers oxidative stress and inflammation, which in turn leads to endothelial dysfunction [83]. High concentration of uric acid in the culture environment significantly inhibits endothelial progenitor cell proliferation, reduce NO production, and decreases phosphorylation levels of AKT and eNOS. Furthermore chronic hyperuricemia decreases tissue reperfusion, endothelial progenitor cell mobilization and neovascularization in the ischemic hindlimbs of mice [84]. Besides, uric acid stimulates the upregulation of renal tubular macrophage migration inhibitory factor (MIF) in rats, and MIF upregulation causes vascular inflammatory responses and inhibits vascular smooth muscle cell dedifferentiation, thus causing vascular injury and dysfunction [85].
7. Uric acid metabolism
Uric acid, as an acidic substance, is prone to precipitate urate crystals mainly consisting of monosodium urate (MSU) during acid-base imbalance in the body [86]. These crystals can directly deposit on the vascular wall damaging the endothelium, causing platelet aggregation and contributing to coronary atheroma plaque formation [86]. Similarly, MSU crystals stimulate macrophage activation, leading to the activation of NLRP-3 inflammatory vesicles and ultimately the production and release of IL-1β and IL-18, which can mediate inflammation, apoptosis and necrosis and cause an inflammatory cascade response [87]. A clinical cross-sectional study demonstrated a significantly higher incidence of severe coronary calcification in asymptomatic hyperuricemia patients with combined MSU crystals than in patients with asymptomatic hyperuricemia alone and patients with normal uric acid, suggesting that more severe CAD is associated with urate crystal deposition [88].
In summary, high concentrations of uric acid precipitate urate crystals that directly damage the vessel wall, or uric acid alters various signaling pathways, causing oxidative stress, producing a variety of inflammatory factors, and even affecting NO production and activating the RAAS, all of which processes may ultimately exacerbate coronary atherosclerosis production and progression, and lead to more serious cardiovascular events.
7.1. Clinical evidence for treatment of anti-hyperuricemia on CAD
Uric acid levels have an overall U-shaped correlation with the prognostic risk of CAD. In addition to hyperuricemia, which increases the risk of poor prognosis in patients with CAD, low uric acid level also increases this risk [[89], [90], [91]]. There are no specific criteria for a precise hypouricemia, and a uric acid concentration of less than 2 mg/dL is generally considered as hypouricemia [92]. A cohort study concluded that blood uric acid level reached a minimum risk of all-cause mortality in patients with CAD at 6.52 mg/dL in men and 5.83 mg/dL in women, with a subsequent increase in the risk of mortality regardless of whether uric acid concentrations increased or decreased. The results of the study showed that CAD with serum uric acid level in the interval (5.59 mg/dL ≤ SUA < 6.8 mg/dL) had a better prognosis [93]. However, the result of the EPOCH-JAPAN study differed, with low uric acid levels (<4.6 mg/dL in men and <3.9 mg/dL in women) increasing mortality from CAD, heart failure, and stroke [90]. The different low uric acid concentrations obtained in these studies that cause an increased risk of poor prognosis in CAD may be related to the different subgroups, ethnicity, geography, comorbidities, and exclusion criteria of the enrolled individuals. However, they share the common conclusion that when uric acid levels are reduced to a certain extent, the risk of poor prognosis of CAD increases.
Current uric acid-lowering therapy is divided into two main categories: inhibition of uric acid synthesis (e.g., XO inhibitors: allopurinol, febuxostat, etc.) and promotion of uric acid excretion (e.g., benzbromarone, etc.) [94]. However, uric acid-lowering drugs have certain side effects and may increase the risk of death in CAD [95,96]. Meanwhile, uric acid-lowering therapy with small doses of medication did not significantly correlate with the risk of death from CAD after treatment, or had a tendency to reduce the risk of death in patients with CAD [[97], [98], [99]]. Most studies have shown that uric acid-lowering therapy is beneficial for the prognosis of CAD. Uric acid-lowering therapy has a protective effect in patients with hyperuricemia with left ventricular hypertrophy and left ventricular diastolic dysfunction, and also reduces the risk of CAD, stroke, and new-onset heart failure [[100], [101], [102], [103]]. For example, the use of allopurinol for the treatment of hyperuricemia showed a trend toward lower incidence and recurrence of nonfatal myocardial infarction as the dose of allopurinol was slowly increased and the duration of treatment was prolonged [104]. In addition allopurinol may have concurrent effects in lowering blood pressure, increasing left heart ejection fraction, and improving left heart function in heart failure [105,106]. Interestingly, benzbromarone seems to have similar results to allopurinol [107]. Recent studies suggest that mice with pulmonary hypertension using benzbromarone have increased right ventricular systolic pressure and attenuated development of occlusive lesions [108]. Febuxostat has a protective effect on cardiac and renal function in a murine CKD model with hyperuricemia [109].
Is it the drug or the uric acid concentration that makes uric acid-lowering therapy effective in reducing the risk of poor prognosis in CAD, given its effect on improving the risk of poor prognosis in CAD? Clinical studies have shown a U-shaped relationship between serum uric acid and all-cause mortality in CAD in the group using uric acid-lowering drugs, with low and high levels of serum uric acid still associated with increased long-term all-cause mortality in patients with CAD, and the use of uric acid-lowering drugs did not reduce all-cause mortality [93]. Based on the available studies, it is believed that there are harmful effects of uric acid-lowering drugs that increase the risk of death from CAD and that a sharp decrease in uric acid concentration may be responsible for the harmful effects of uric acid-lowering drugs [110,111]. There are also side effects of the drugs themselves, such as febuxostat, which may increase the risk of sudden cardiac death, benzbromarone, which has severe liver and kidney toxicity, and potent inhibition of cytochrome P450 (CYP) metabolic enzymes, and even the novel uric acid transporter protein inhibitors have significant toxicity on liver and kidney [112]. This reveals that uric acid-lowering drugs may have a dual effect, and perhaps the increased risk of adverse prognosis for CAD with uric acid-lowering drugs is influenced by the concentration of the drug used and the excessive reduction in uric acid levels. Cardiovascular mortality after uric acid-lowering therapy is not linear but remains correlated with uric acid concentration, suggesting the need to monitor uric acid concentration levels during the use of uric acid-lowering drugs and to adjust treatment regimens according to uric acid levels. However, the results of the study are still deficient because uric acid-lowering drug therapy cannot be administered to the normal population. In conclusion, rational uric acid-lowering therapy seems to reduce the risk of poor prognosis in the CAD population with combined hyperuricemia to some extent.
According to the recommendations of multinational consensus, after the diagnosis of hyperuricemia or gout is confirmed, complications or co-morbidities should be screened immediately and multidisciplinary combination therapy should be given. In China, the consensus recommend that the uric acid concentration should be reduced to less than 360 μmol/L if there is one combined gout attack or any combined cardiovascular high-risk disease; for those with frequent gouty arthritis, it is recommended to reduce the uric acid concentration to less than 300 μmol/L; for patients with asymptomatic hyperuricemia, it is recommended to reduce the uric acid to 420 μmol/L [18]. The treatment opinion in the European national consensus favors an optimal uric acid level below 360 μmol/L for patients with simple hyperuricemia, but for patients with at least two of the following cardiovascular risks (hypertension, diabetes, dyslipidemia, target organ damage or previous cardiovascular events), the uric acid concentration should be less than 300 μmol/L [94]. Neither Chinese nor European national consensus recommends lowering uric acid to less than 180 μmol/L. However, it is important to note with caution that most of the clinical studies cited on the effects of uric acid-lowering therapy are of the same ethnicity and in highly defined populations, and that large randomized controlled studies are lacking. Whether uric acid-lowering therapy is effective in patients with more severe cardiovascular disease in different regions and ethnic populations, or in patients with more complex, long-term hypertensive disease, heart failure, or CAD is unknown and needs to be demonstrated by higher quality evidence-based medicine. In addition, most patients have a short duration of uric acid-lowering therapy and may not have achieved appropriate uric acid levels, which may be a possible difficulty in uric acid-lowering therapy studies [113]. The analysis of the potential mechanism of action of drugs is mostly uncontrolled, non-randomized, and lacks actual clinical estimates for populations not using uric acid-lowering drugs, which leads to some questioning of the pharmacological effects of drugs, and the next step might be to focus on the targets of drugs from different populations to clarify the dual mechanism of action of drugs.
8. Conclusions
To date, the majority of researchers accept that hyperuricemia is an independent risk factor of CAD (Fig. 4). Because of the wide range of risk factors for CAD, it is difficult to obtain a large amount of data on the presence of only a single comorbidity. The clinical studies described above combine multiple factors of CAD and if we want to obtain a clearer association for a single factor, perhaps animal studies are more likely to yield evidence. Higher uric acid concentrations (>420 μmol/L) can enhance coronary atherosclerosis formation directly or indirectly through oxidative stress, inflammatory responses, triggering of multiple signaling pathways, and activation of the RAAS. However, there is a lack of large clinical studies and disease awareness in China in this regard and the uric acid management in patients with CAD in the absence of uric acid-related complications remains poor. We believe that early intervention of abnormal uric acid levels can effectively reduce the incidence of CAD, improving the poor prognosis of the CAD population, and likely reducing the economic burden on patients. This has led to the need to strengthen education on the prevention and treatment of hyperuricemia in CAD patients, to clarify the pathophysiological link between uric acid and CAD, and to conduct more comprehensive drug studies to find the best therapeutic targets and prevention measures.
Fig. 4.
A graphical summary of this review. HUA: Hyperuricemia; CAD: coronary artery disease; ULT: uric acid-lowering therapy; VSMC: vascular smooth muscle cell; OX-LDL: Oxidized low-density lipoprotein; ROS: Reactive oxygen species; IL-1: Interleukin −1; IL-6: Interleukin −6; TNF-α:Tumor necrosis factor-α.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Discipline Construction Project of Guangdong Medical University (4SG21233G), National Natural Science Foundation of China, China (81700269), Key platform of Department of Education of Guangdong Province, China (2021LSYS007), and Science and Technology Program of Zhanjiang, China (2022E05011, 2022A01196, 2021A05158, 2021A05058, 2021A05056, 2020A01020, 2020A06003, 2020A06004).
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Contributor Information
Kaiyue Li, Email: 31178078397@qq.com.
Wei Lei, Email: leiwei@gdmu.edu.cn.
References
- 1.Lawton J., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Roth G., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Report on cardiovascular health and diseases in China 2021: an updated summary. Biomed. Environ. Sci.: BES. 2022;35(7):573–603. doi: 10.3967/bes2022.079. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Society of Cardiology of Chinese Medical Association Cardiovascular Disease Prevention and Rehabilitation Committee of Chinese Association of Rehabilitation Medicine; Cardiovascular Disease Committee of Chinese Association of Gerontology and Geriatrics; Thrombosis Prevention and Treatment Committee of Chinese Medical Doctor Association. Chinese guideline on the primary prevention of cardiovascular diseases. Chin. J. Cardiol. 2020;48(12):1000–1038. doi: 10.3760/cma.j.cn112148-20201009-00796. [DOI] [PubMed] [Google Scholar]
- 5.Whayne T. Non-traditional cardiovascular risk markers in the era of established major risk factors and multiple guidelines. Curr. Vasc. Pharmacol. 2019;17(3):270–277. doi: 10.2174/1570161116666180123112956. [DOI] [PubMed] [Google Scholar]
- 6.Lan M., et al. Evaluation of the association between hyperuricemia and coronary artery disease: a STROBE-compliant article. Medicine (Baltim.) 2018;97(44) doi: 10.1097/MD.0000000000012926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S., et al. Impact of serum uric acid levels on the clinical prognosis and severity of coronary artery disease in patients with acute coronary syndrome and hypertension after percutaneous coronary intervention: a prospective cohort study. BMJ Open. 2022;12(1) doi: 10.1136/bmjopen-2021-052031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuźma Ł., et al. The effect of serum uric acid levels on the long-term prognosis of patients with non-ST-elevation myocardial infarction. Adv. Clin. Exp. Med. 2020;29(11):1255–1263. doi: 10.17219/acem/127145. [DOI] [PubMed] [Google Scholar]
- 9.Galvão A., et al. The positive association between serum uric acid, impaired fasting glucose, impaired glucose tolerance, and diabetes mellitus in the ELSA-Brasil study. Cad. Saúde Pública. 2021;37(9) doi: 10.1590/0102-311X00255920. [DOI] [PubMed] [Google Scholar]
- 10.Piao W., et al. The prevalence of hyperuricemia and its correlates among adults in China: results from CNHS 2015-2017. Nutrients. 2022;14(19) doi: 10.3390/nu14194095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P., et al. Association between serum uric acid levels and traditional cardiovascular risk factors in Xiamen residents of China: a real-world study. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.913437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiuolo J., et al. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad S., et al. Attenuation of Hg(II)-induced cellular and DNA damage in human blood cells by uric acid. Biochem. Cell Biol.= Biochimie et biologie cellulaire. 2022;100(1):45–58. doi: 10.1139/bcb-2021-0229. [DOI] [PubMed] [Google Scholar]
- 14.Song Y., et al. Uric acid provides protective role in red blood cells by antioxidant defense: a hypothetical analysis. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3435174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wada A., et al. Protective effect of luminal uric acid against indomethacin-induced enteropathy: role of antioxidant effect and gut microbiota. Dig. Dis. Sci. 2022;67(1):121–133. doi: 10.1007/s10620-021-06848-z. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y., et al. Protective effect of uric acid on ox-LDL-induced HUVECs injury via Keap1-Nrf2-ARE pathway. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/5151168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endocrinology C. Guideline for the diagnosis and management of hyperuricemia and gout in China (2019) Chin. J. Endocrinol. Metabol. 2020;36:1–13. [Google Scholar]
- 18.Multidisciplinary Expert Task Force on Hyperuricemia and Related Diseases Chinese multidisciplinary expert consensus on the diagnosis and treatment of hyperuricemia and related diseases. Chin. Med. J. (Engl.) 2017;130(20):2473–2488. doi: 10.4103/0366-6999.216416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicerchi C., et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. Faseb. J. 2014;28(8):3339–3350. doi: 10.1096/fj.13-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma A., et al. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE mice. J. Lipid Res. 2017;58(8):1536–1547. doi: 10.1194/jlr.M073270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo C., et al. High uric acid activates the ROS-AMPK pathway, impairs CD68 expression and inhibits OxLDL-induced foam-cell formation in a human monocytic cell line, THP-1. Cell. Physiol. Biochem. 2016;40:538–548. doi: 10.1159/000452567. [DOI] [PubMed] [Google Scholar]
- 22.Kushiyama A., et al. Xanthine oxidoreductase is involved in macrophage foam cell formation and atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2012;32(2):291–298. doi: 10.1161/ATVBAHA.111.234559. [DOI] [PubMed] [Google Scholar]
- 23.Milanesi S., et al. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J. Cell. Physiol. 2019;234(7):10868–10876. doi: 10.1002/jcp.27929. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z., et al. Gout-induced endothelial impairment: the role of SREBP2 transactivation of YAP. Faseb. J. 2021;35(6) doi: 10.1096/fj.202100337R. [DOI] [PubMed] [Google Scholar]
- 25.He Q., et al. The application of Gensini score and IL-1ra in assessing the condition and prognosis of patients with coronary artery disease. Am. J. Tourism Res. 2021;13(9):10421–10427. [PMC free article] [PubMed] [Google Scholar]
- 26.Li N., et al. The quantitative relationship between coronary microcirculatory resistance and myocardial ischemia in patients with coronary artery disease. J. Biomech. 2022;140 doi: 10.1016/j.jbiomech.2022.111166. [DOI] [PubMed] [Google Scholar]
- 27.Yongguang G., et al. Diagnostic efficacy of CCTA and CT-FFR based on risk factors for myocardial ischemia. J. Cardiothorac. Surg. 2022;17(1):39. doi: 10.1186/s13019-022-01787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim D.H., et al. Serum uric acid level and subclinical coronary atherosclerosis in asymptomatic individuals: an observational cohort study. Atherosclerosis. 2019;288:112–117. doi: 10.1016/j.atherosclerosis.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Chu X., et al. Correlation between serum uric acid levels and coronary plaque characteristics on optical coherence tomography. Int. Heart J. 2022;63(5):806–813. doi: 10.1536/ihj.21-826. [DOI] [PubMed] [Google Scholar]
- 30.Ekici B., et al. The relationship between serum uric acid levels and angiographic severity of coronary heart disease. Kardiol. Pol. 2015;73(7):533–538. doi: 10.5603/KP.a2015.0024. [DOI] [PubMed] [Google Scholar]
- 31.Yu J., et al. Association between serum uric acid level and the severity of coronary artery disease in patients with obstructive coronary artery disease. Chin. Med. J. 2014;127(6):1039–1045. [PubMed] [Google Scholar]
- 32.Wang X., et al. High level of serum uric acid induced monocyte inflammation is related to coronary calcium deposition in the middle-aged and elder population of China: a five-year prospective cohort study. J. Inflamm. Res. 2022;15:1859–1872. doi: 10.2147/JIR.S353883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R., et al. The characteristics of risk factors in Chinese young women with acute coronary syndrome. BMC Cardiovasc. Disord. 2020;20(1):290. doi: 10.1186/s12872-020-01577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasak A., et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83,683 Austrian men. Clin. Chem. 2008;54(2):273–284. doi: 10.1373/clinchem.2007.094425. [DOI] [PubMed] [Google Scholar]
- 35.Gulati M., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 36.Li C., et al. [Association of serum uric acid level with coronary heart disease in men and women] Zhongguo yi xue ke xue yuan xue bao. Acta Acad. Med. Sin. 2018;40(3):338–343. doi: 10.3881/j.issn.1000-503X.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Gazi E., et al. The association between serum uric acid level and heart failure and mortality in the early period of ST-elevation acute myocardial infarction. Turk Kardiyol. Dernegi Arsivi. 2014;42(6):501–508. doi: 10.5543/tkda.2014.65507. [DOI] [PubMed] [Google Scholar]
- 38.Mandurino-Mirizzi A., et al. Elevated serum uric acid is associated with a greater inflammatory response and with short- and long-term mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Nutr. Metabol. Cardiovasc. Dis. 2021;31(2):608–614. doi: 10.1016/j.numecd.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 39.He Y., et al. Interaction between hyperuricemia and admission lactate increases the risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. Cardiorenal Med. 2022;12(5–6):189–195. doi: 10.1159/000526104. [DOI] [PubMed] [Google Scholar]
- 40.Dyrbuś M., et al. Serum uric acid is an independent risk factor of worse mid- and long-term outcomes in patients with non-ST-segment elevation acute coronary syndromes. Cardiol. J. 2021:1–11. doi: 10.5603/CJ.a2021.0156. (Ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu X., et al. Elevated uric acid is related to the no-/slow-reflow phenomenon in STEMI undergoing primary PCI. Eur. J. Clin. Invest. 2022;52(4) doi: 10.1111/eci.13719. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe S., et al. Serum uric acid level is associated with reperfusion ventricular arrhythmias in acute myocardial infarction. Diabetes Metabol. Syndr. 2021;15(4) doi: 10.1016/j.dsx.2021.102198. [DOI] [PubMed] [Google Scholar]
- 43.Han Y., et al. Serum uric acid might Be positively associated with hypertension in Chinese adults: an analysis of the China health and nutrition survey. Front. Med. 2021;8 doi: 10.3389/fmed.2021.755509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallée A. Association between serum uric acid and arterial stiffness in a large-aged 40-70 years old population. J. Clin. Hypertens. (Greenwich, Conn.) 2022;24(7):885–897. doi: 10.1111/jch.14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ejaz A., et al. The role of uric acid in acute kidney injury. Nephron. 2019;142(4):275–283. doi: 10.1159/000499939. [DOI] [PubMed] [Google Scholar]
- 46.Shen G., et al. J-shaped association between serum uric acid and acute coronary syndrome in patients with essential hypertension. Postgrad. Med. 2020;96(1132):73–78. doi: 10.1136/postgradmedj-2019-136650. [DOI] [PubMed] [Google Scholar]
- 47.Zhuang Y., et al. Serum uric acid and diabetic peripheral neuropathy: a double-edged sword. Acta Neurol. Belg. 2022 doi: 10.1007/s13760-022-01978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y., et al. Using machine learning techniques to develop risk prediction models for the risk of incident diabetic retinopathy among patients with type 2 diabetes mellitus: a cohort study. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.876559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji P., et al. Serum uric acid levels and diabetic kidney disease in patients with type 2 diabetes mellitus: a dose-response meta-analysis. Primary Care Diabetes. 2022;16(3):457–465. doi: 10.1016/j.pcd.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Han R., et al. Relationship between four non-insulin-based indexes of insulin resistance and serum uric acid in patients with type 2 diabetes: a cross-sectional study. Diabetes, Metab. Syndrome Obes. Targets Ther. 2022;15:1461–1471. doi: 10.2147/DMSO.S362248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song J., et al. Relationship among insulin therapy, insulin resistance, and severe coronary artery disease in type 2 diabetes mellitus. J. Intervent. Cardiol. 2022;2022 doi: 10.1155/2022/2450024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ioachimescu A., et al. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008;58(2):623–630. doi: 10.1002/art.23121. [DOI] [PubMed] [Google Scholar]
- 53.Sharaf El Din U.A.A., et al. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: a review. J. Adv. Res. 2017;8(5):537–548. doi: 10.1016/j.jare.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boudoulas K., et al. Coronary atherosclerosis: pathophysiologic basis for diagnosis and management. Prog. Cardiovasc. Dis. 2016;58(6):676–692. doi: 10.1016/j.pcad.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Barone E., et al. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radic. Biol. Med. 2021;176:16–33. doi: 10.1016/j.freeradbiomed.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanzillotta C., et al. Insulin resistance, oxidative stress and mitochondrial defects in Ts65dn mice brain: a harmful synergistic path in down syndrome. Free Radic. Biol. Med. 2021;165:152–170. doi: 10.1016/j.freeradbiomed.2021.01.042. [DOI] [PubMed] [Google Scholar]
- 57.Kattoor A.J., et al. Oxidative stress in atherosclerosis. Curr. Atherosclerosis Rep. 2017;19(11):42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 58.Elsayed S., et al. Protein phosphatase 2A regulates xanthine oxidase-derived ROS production in macrophages and influx of inflammatory monocytes in a murine gout model. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1033520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng T., et al. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. Int. J. Cardiol. 2010;139(1):42–49. doi: 10.1016/j.ijcard.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Chao H., et al. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol. Sin. 2008;29(11):1301–1312. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 61.Poznyak A., et al. NADPH oxidases and their role in atherosclerosis. Biomedicines. 2020;8(7) doi: 10.3390/biomedicines8070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sautin Y., et al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007;293(2):C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 63.Verzola D., et al. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi Y., et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014;94(10):1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 65.Krautter F., et al. Galectin-9: a novel promoter of atherosclerosis progression. Atherosclerosis. 2022;363:57–68. doi: 10.1016/j.atherosclerosis.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Li Z., et al. High uric acid inhibits cardiomyocyte viability through the ERK/P38 pathway via oxidative stress. Cell. Physiol. Biochem. 2018;45(3):1156–1164. doi: 10.1159/000487356. [DOI] [PubMed] [Google Scholar]
- 67.Reustle A., et al. Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. Int. J. Mol. Sci. 2018;19(12) doi: 10.3390/ijms19123761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muslin A. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin. Sci. (London, Engl.: 1979) 2008;115(7):203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kırça M., et al. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRβ. J. Recept. Signal Transduct. Res. 2017;37(2):167–173. doi: 10.1080/10799893.2016.1203941. [DOI] [PubMed] [Google Scholar]
- 70.Hu Y., et al. High uric acid promotes dysfunction in pancreatic beta cells by blocking IRS2/AKT signalling. Mol. Cell. Endocrinol. 2021;520 doi: 10.1016/j.mce.2020.111070. [DOI] [PubMed] [Google Scholar]
- 71.Crisan T.O., et al. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc. Natl. Acad. Sci. U. S. A. 2017;114(21):5485–5490. doi: 10.1073/pnas.1620910114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimura Y., et al. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 2021;22(22) doi: 10.3390/ijms222212394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K., et al. HDAC inhibitors alleviate uric acid-induced vascular endothelial cell injury by way of the HDAC6/FGF21/PI3K/AKT pathway. J. Cardiovasc. Pharmacol. 2023;81(2):150–164. doi: 10.1097/FJC.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srivastava P., et al. Imbalance between Angiotensin II - angiotensin (1-7) system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes Metabol. Syndr. 2019;13(3):2061–2068. doi: 10.1016/j.dsx.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 75.Jukema R., et al. The relation of RAAS activity and endothelin-1 levels to coronary atherosclerotic burden and microvascular dysfunction in chest pain patients. Atherosclerosis. 2022;347:47–54. doi: 10.1016/j.atherosclerosis.2022.03.017. [DOI] [PubMed] [Google Scholar]
- 76.Ames M.K., et al. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019;33(2):363–382. doi: 10.1111/jvim.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corry D., et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Y., et al. Lipoxin A4 attenuates uric acid-activated, NADPH oxidase-dependent oxidative stress by interfering with translocation of p47phox in human umbilical vein endothelial cells. Exp. Ther. Med. 2020;20(2):1682–1692. doi: 10.3892/etm.2020.8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gherghina M.E., et al. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int. J. Mol. Sci. 2022;23(6) doi: 10.3390/ijms23063188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rochette L., et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol. Ther. 2013;140(3):239–257. doi: 10.1016/j.pharmthera.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 81.Khera A., et al. Gene sequencing identifies perturbation in nitric oxide signaling as a nonlipid molecular subtype of coronary artery disease. Circul. Genom. Precis. Med. 2022;15(6) doi: 10.1161/CIRCGEN.121.003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P., et al. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: a mechanism for uric acid-induced endothelial dysfunction. Int. J. Mol. Med. 2016;37(4):989–997. doi: 10.3892/ijmm.2016.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai W., et al. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/4391920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen I., et al. Chronic hyperuricemia impairs blood flow recovery in the ischemic hindlimb through suppression of endothelial progenitor cells. Oncotarget. 2018;9(10):9285–9298. doi: 10.18632/oncotarget.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu X., et al. Blockage of macrophage migration inhibitory factor (MIF) suppressed uric acid-induced vascular inflammation, smooth muscle cell de-differentiation, and remodeling. Biochem. Biophys. Res. Commun. 2019;508(2):440–444. doi: 10.1016/j.bbrc.2018.10.093. [DOI] [PubMed] [Google Scholar]
- 86.Ciccarelli G., et al. Correlation between serum uric acid levels and residual platelet reactivity in patients undergoing PCI. Nutr. Metabol. Cardiovasc. Dis. : Nutr. Metabol. Cardiovasc. Dis. 2017;27(5):470–471. doi: 10.1016/j.numecd.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 87.Mei Y., et al. Excess uric acid induces gouty nephropathy through crystal formation: a review of recent insights. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.911968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andrés M., et al. Silent monosodium urate crystal deposits are associated with severe coronary calcification in asymptomatic hyperuricemia: an exploratory study. Arthritis Rheumatol. (Hoboken, N.J.) 2016;68(6):1531–1539. doi: 10.1002/art.39581. [DOI] [PubMed] [Google Scholar]
- 89.Chang D., et al. Association between serum uric acid level and mortality in China. Chin. Med. J. 2021;134(17):2073–2080. doi: 10.1097/CM9.0000000000001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W., et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-Japan Study. J. Atherosclerosis Thromb. 2016;23(6):692–703. doi: 10.5551/jat.31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Essex M., et al. Evaluation of the relationship between serum uric acid levels and cardiovascular events in patients with gout: a retrospective analysis using electronic medical record data. J. Clin. Rheumatol. 2017;23(3):160–166. doi: 10.1097/RHU.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 92.Otani N., et al. Hypouricemia and urate transporters. Biomedicines. 2022;10(3) doi: 10.3390/biomedicines10030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Y., et al. The U-shaped relationship between serum uric acid and long-term all-cause mortality in coronary artery disease patients: a cohort study of 33,034 patients. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.858889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borghi C., et al. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol. J. 2021;28(1):1–14. doi: 10.5603/CJ.a2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang E., et al. Cardiovascular risk associated with allopurinol vs. benzbromarone in patients with gout. Eur. Heart J. 2021;42(44):4578–4588. doi: 10.1093/eurheartj/ehab619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White W., et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N. Engl. J. Med. 2018;378(13):1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- 97.Søltoft Larsen K., et al. Impact of urate level on cardiovascular risk in allopurinol treated patients. A nested case-control study. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akkineni R., et al. Treatment of asymptomatic hyperuricemia and prevention of vascular disease: a decision analytic approach. J. Rheumatol. 2014;41(4):739–748. doi: 10.3899/jrheum.121231. [DOI] [PubMed] [Google Scholar]
- 99.Sawada S., et al. Cardiovascular risk of urate-lowering drugs: a study using the national database of health insurance claims and specific health checkups of Japan. Clin. Transl. Sci. 2022;16(2):206–215. doi: 10.1111/cts.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohta Y., et al. Effective uric acid-lowering treatment for hypertensive patients with hyperuricemia. Hypertens. Res. 2017;40(3):259–263. doi: 10.1038/hr.2016.139. [DOI] [PubMed] [Google Scholar]
- 101.Pan J., et al. Association between long-term prescription of febuxostat and the progression of heart failure with preserved ejection fraction in patients with hypertension and asymptomatic hyperuricemia. Heart Ves. 2020;35(10):1446–1453. doi: 10.1007/s00380-020-01619-8. [DOI] [PubMed] [Google Scholar]
- 102.Konishi M., et al. Effect of febuxostat on clinical outcomes in patients with hyperuricemia and cardiovascular disease. Int. J. Cardiol. 2022;349:127–133. doi: 10.1016/j.ijcard.2021.11.076. [DOI] [PubMed] [Google Scholar]
- 103.Huang Y., et al. Clinical Study on efficacy of allopurinol in patients with acute coronary syndrome and its functional mechanism. Hellenic J. Cardiol. HJC: HJC = Hellenike kardiologike epitheorese. 2017;58(5):360–365. doi: 10.1016/j.hjc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 104.de Abajo F., et al. Allopurinol use and risk of non-fatal acute myocardial infarction. Heart (British Cardiac Society) 2015;101(9):679–685. doi: 10.1136/heartjnl-2014-306670. [DOI] [PubMed] [Google Scholar]
- 105.Bredemeier M., et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018;18(1):24. doi: 10.1186/s12872-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng H., et al. Therapeutic effects of allopurinol on the function of left ventricular and activity of matrix metalloproteinase enzymes (MMPs) in patients with chronic heart failure. Cell. Mol. Biol. (Noisy-le-Grand, France) 2022;68(5):96–102. doi: 10.14715/cmb/2022.68.5.13. [DOI] [PubMed] [Google Scholar]
- 107.Nakata T., et al. Randomized, open-label, cross-over comparison of the effects of benzbromarone and febuxostat on endothelial function in patients with hyperuricemia. Int. Heart J. 2020;61(5):984–992. doi: 10.1536/ihj.20-114. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe T., et al. Increased lung uric acid deteriorates pulmonary arterial hypertension. J. Am. Heart Assoc. 2021;10(23) doi: 10.1161/JAHA.121.022712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Omizo H., et al. Cardio-renal protective effect of the xanthine oxidase inhibitor febuxostat in the 5/6 nephrectomy model with hyperuricemia. Sci. Rep. 2020;10(1):9326. doi: 10.1038/s41598-020-65706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Becker B., et al. Acute effects of hypouricemia on endothelium, oxidative stress, and arterial stiffness: a randomized, double-blind, crossover study. Physiol. Rep. 2021;9(17) doi: 10.14814/phy2.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez-Gomez M., et al. Potential dangers of serum urate-lowering therapy. Am. J. Med. 2019;132(4):457–467. doi: 10.1016/j.amjmed.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 112.Zhao T., et al. Novel human urate transporter 1 inhibitors as hypouricemic drug candidates with favorable druggability. J. Med. Chem. 2020;63(19):10829–10854. doi: 10.1021/acs.jmedchem.0c00223. [DOI] [PubMed] [Google Scholar]
- 113.Coleshill M., et al. Persistence with urate-lowering therapy in Australia: a longitudinal analysis of allopurinol prescriptions. Br. J. Clin. Pharmacol. 2022;88(11):4894–4901. doi: 10.1111/bcp.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.