Abstract
Microbial electrochemical technologies (METs) are a group of innovative technologies that produce valuables like bioelectricity and biofuels with the simultaneous treatment of wastewater from microorganisms known as electroactive microorganisms. The electroactive microorganisms are capable of transferring electrons to the anode of a MET through various metabolic pathways such as direct (via cytochrome or pili) or indirect (through transporters) transfer. Though this technology is promising, the inferior yield of valuables and the high cost of reactor fabrication are presently impeding the large-scale application of this technology. Therefore, to overcome these major bottlenecks, a lot of research has been dedicated to the application of bacterial signalling, for instance, quorum sensing (QS) and quorum quenching (QQ) mechanisms in METs to improve its efficacy in order to achieve a higher power density and to make it more cost-effective. The QS circuit in bacteria produces auto-inducer signal molecules, which enhances the biofilm-forming ability and regulates the bacterial attachment on the electrode of METs. On the other hand, the QQ circuit can effectively function as an antifouling agent for the membranes used in METs and microbial membrane bioreactors, which is imperative for their stable long-term operation. This state-of-the-art review thus distinctly describes in detail the interaction between the QQ and QS systems in bacteria employed in METs to generate value-added by-products, antifouling strategies, and the recent applications of the signalling mechanisms in METs to improve their yield. Further, the article also throws some light on the recent advancements and the challenges faced while incorporating QS and QQ mechanisms in various types of METs. Thus, this review article will help budding researchers in upscaling METs with the integration of the QS signalling mechanism in METs.
Keywords: Bioelectrochemistry, Microbial electrochemical technology, Quorum quenching, Quorum sensing, Wastewater treatment
Graphical abstract
Highlights
-
•
Bacterial quorum sensing (QS) and quorum quenching (QQ) mechanisms are elucidated.
-
•
QS enhances the transmission of electrons required for bioelectricity production.
-
•
QQ mechanism has proven efficient in preventing membranes from biofouling.
-
•
QS and QQ ameliorate the performance of microbial electrochemical technologies.
1. Introduction
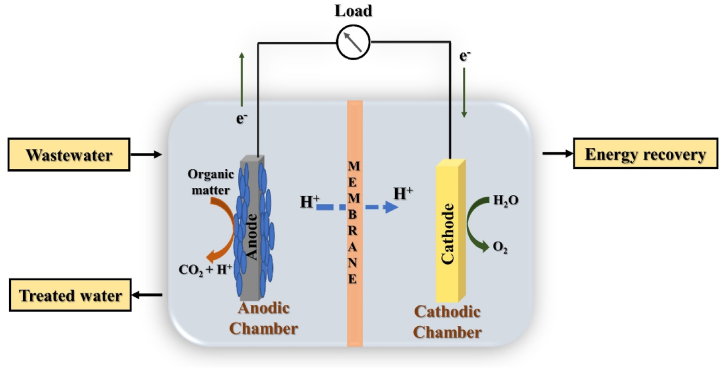
Microbial electrochemical technologies (METs) are promising techniques for wastewater treatment with concomitant value-added product recovery. The METs utilize microorganisms for catalysing different electrochemical reactions and thus can be characterized as microbial fuel cell (MFC) for bioelectricity production, microbial electrolysis cell (MEC) for hydrogen production, microbial electrosynthesis (MES) used for chemical recovery, microbial desalination cell for desalinization of saline water and microbial carbon capture for carbon sequestration with bioenergy recovery [1]. The METs are unique and clean technologies which are a combination of electrochemical and biological methods for wastewater treatment and concomitant energy recovery in terms of bioelectricity and valuable products such as; hydrogen, acetate, hydrogen peroxide and methane etc [2]. In METs, the organic compounds present in the wastewater act as the substrate for the anaerobic microbes present in the anodic chamber; on the other hand, in the cathodic chamber, oxygen acts as the terminal electron acceptor in the oxygen reduction reaction. The working mechanism of METs is purely dependent on the electroactive microorganisms formally termed as biocatalysts, which oxidizes the organic fraction of wastewater and generates electrons and protons. These produced electrons and protons are then further utilized based on the type of MET reactor used, such as in MFC; these protons and electrons are transferred through a proton exchange membrane (PEM) and an external circuit, respectively, to the cathode, where the oxygen reduction reaction takes place leading to the production of energy in the form bioelectricity. Similarly, for microbial carbon capture, oxygen required for the cathodic reaction is provided by the algal cells cultured in this chamber, thus reducing the dependence on external aeration. Whereas in the case of MEC, exoelectrogens oxidize the organic matter present in wastewater in the anodic chamber and produce CO2, protons, and electrons [3]. Further, the electrons move to the cathodic chamber via an external circuit and the proton passes through the PEM to the cathodic chamber and simultaneously gets reduced to form H2 (Fig. 1).
Fig. 1.
Schematic of a microbial fuel cell.
In recent times, METs have gained significant attention due to their promising applications for valuables recoveries such as bioelectricity, biohydrogen, biomethane, commodity chemicals and biofuels. The METs are greener technologies for resource recovery with concomitant wastewater treatment without the emission of any greenhouse gases and less sludge generation. However, the inferior yield of resources and higher fabrication cost are the major setbacks for the METs, which limits these technologies to lab-scale only. The word “quorum sensing” refers to sensing the environment by bacteria for an appropriate physiological response and gene expression during transcription under a certain threshold cell density [4]. This is operated by signal molecules known as auto-inducers (AI) produced by the bacteria and exchanged with the surrounding environment, when the cell density of autoinducers is high [5]. The production of AIs depends upon the bacterial cell density and is produced in direct proportion to the number of bacteria present in the colony [6]. Numerous signal molecules, known as QS molecules (QSM), can be found within a bacterial population and these are primarily categorized into four groups depending on the nature of AIs, namely, i) Acyl-homoserine lactones (AHLs); ii) oligopeptides or autoinducing peptides (AIP) iii) autoinducer-2 (AI-2) iv) Pseudomonas quinolone signal (PQS) and autoinducer-3 (AI-3). The mechanism of quorum sensing (QS), which is dependent upon the expression of genes, is controlled by transcription and is common in homogeneous or heterogenous bacterial communities [7,8]. The QSMs contribute to a variety of physiological changes, like pathogenicity, complex formation, symbiosis, competence, antibiotic synthesis, as well as the formation of biofilm.
Moreover, the bacterial QS mechanism is cellular communication between bacteria in response to extracellularly secreted AIs. Fundamental processes necessary for the survival of bacterial colonies viz; biofilm formation, antibiotic quorum quenching (QQ) production, sporulating capacity, bioluminescence, activation of virulence genes, and competence regulation are controlled by the QS mechanism [9,10]. The AIs are signalling molecules which are produced intracellularly and secreted by the cells either by passive or active transport. Usually, AIs are present outside the cells at a concentration low enough to be detected by other bacterial cells [10]. At low cell densities of microbial cells, the AIs are low in number; however, at high cell densities, there are enough AIs for the detection and regulation of gene expression programs [11]. In this regard, the application of QS and QQ for improving the performance of METs has gained considerable attention because of their ability to enhance the yield of valuables obtained through METs. Basically, the biofilm formation because of the attached growth on the electrode improves the performance of METs by reducing the losses associated with electron transfer. However, excessive profuse biofilm formation on the surface of the electrode and PEM deteriorates the performance of METs [12]. Therefore, the performance of MFC is dependent on the thickness of the biofilm; moreover, biofouling of membranes also gets triggered due to the random bacterial growth on the membrane surface, which diminishes the performance of membranes and results in the poor performance of the METs as well [13]. The optimal biofilm thickness on the surface of the anode and prevention of membrane biofouling can be achieved by interfering with the QS communication, thus improving the overall efficacy of the METs. Therefore, the inhibition of the autoinducer-mediated QS mechanism known as QQ can control the anodic biofilm as well as membrane biofouling [14]. Hence, due to the controlled biofilm formation, the electron transfer becomes efficient, which ultimately enhances the performance of METs.
The mechanism of QQ on the other hand, is a mechanism of inhibiting the QSMs through the production of various QQ molecules (QQM). The QQMs are found in nature (i.e., bacteria, fungi, plants), like chemical AI analogues and enzymes isolated from microorganisms. Hence, bacteria can themselves monitor their population growth by bringing variations in the concentration of signal molecules for upregulation or downregulation of a particular gene and the generated signals regulate the process of QS & QQ [15]. The QQMs also inhibit biofilm-formation by regulating the expression of gene clusters (operons) in bacteria. The bacteria form biofilms as a part of social behaviour to interact with each other via the QS-QQ signalling mechanisms and this aids in the exchange of nutrients and gases, electrons, and protection from pathogenic attacks [12].
In wastewater treatment through METs, QQ and QS plays an important role as antifouling agent to prevent the pathogenicity of biofilms, improving the longevity of the membrane [16]. The QSMs control cell-cell communication and aid in electron exchange in METs via biofilm formation, whereas the QQMs regulate the thickness of biofilms to optimise effluent treatment and value-added product recovery [17]. Bacterial biofilm formation in the anodic chamber of the MFC increases the rate of degradation of organics and electron exchange, which is advantageous for METs [18]. However, thick anodic biofilms reduce electron transportation as the bacteria in the inner layers do not have as much access to the substrate compared to the ones on the outer surface, accompanied by the loss of substrate from the outer to inner layers of the biofilm [19]. It has also been observed that electron transportation is less efficient in the inner biofilm layers [14]. Thick biofilm formation on the membrane, formally termed as membrane biofouling, also affects the proton exchange via the ion exchange membrane used in METs, thus, interfering with bioelectricity production [14]. Therefore, anodic biofilms with optimal thickness are prerequisites for the efficacious performance of METs.
Several biological or pharmacological approaches, including QQ and enzymatic processes, have proved to be effective for membrane cleaning and preventing membrane biofouling [20]. Besides, silver nanoparticles and poly-sulphones have been able to mitigate biofouling in METs [21]. It is widely known that the AHL-intervened QS plays a significant role in the formation of biofilm. As a result, numerous techniques for AHL degradation have been developed to block this QS and biofouling is mitigated [22]. Biocides and other antifouling agents have also been employed in the recent past to tackle biofouling in METs. Das et al. (2021) have shown that CuMnFe composite in a 25 L pilot-scale MFC can be successfully used as an antifouling agent [23].
2. Importance of QS/QQ assistance in the application of METs
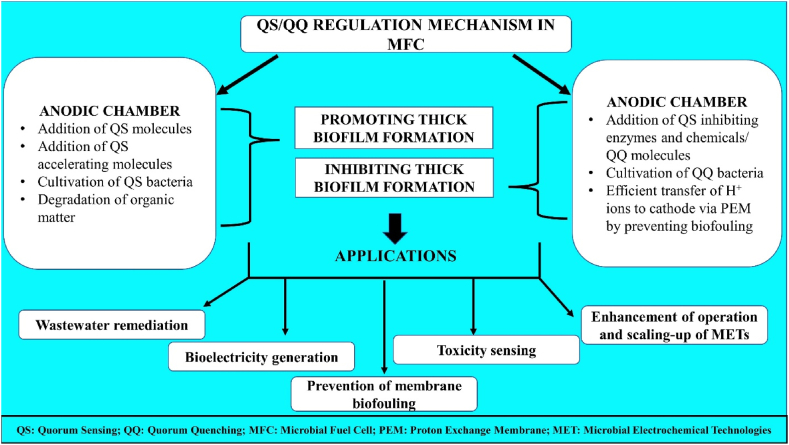
The QS is important in the formation of exo-polysaccharides (E-PS) and also in transforming the effluent into aerobic granules [24]. In contrast, a larger accumulation of AHLs promotes granule formation, whereas lower amounts can cause granule breakdown and its concomitant conversion into flocs [20]. Thus, by keeping a high concentration of QS signals and minimising the impact of QQ, the efficacy of the METs can be improved (Fig. 2), for example, by preserving the stability of aerobic granules for stable wastewater treatment [25].
Fig. 2.
Concept of bacterial QQ-QS mechanisms in microbial fuel cells and its applications in microbial electrochemical technology.
Numerous activated sludges have been studied and found to contain both QS bacteria that produce AHLs, and QQ bacteria that degrade AHLs [26,27]. This can be of keen interest for their plausible application in various types of METs. In biological wastewater treatment, AHL signals may be very helpful in detecting biofilm growth and maturation, microbial aggregation and stabilisation, exoenzyme activity, granule formation, and stability of sludge structure. All bio-membrane procedures, often referred to as fixed-film processes, rely on the adherence of microbial cells to form a biofilm on the inert support medium possessing a high specific surface area for maximal biofilm growth thus enhancing the removal of dissolved and colloidal organic contaminants [28].
In this regard, inventive QS-assisted biotechnological interventions can alleviate this ever-increasing environmental issue before it worsens further. One such advancement is the emergence of METs for the environmentally friendly generation of valuables like bioelectricity and biofuels. The most widespread type of MET is MFC which produces electricity by decomposing organic wastes, domestic wastewater, and industrial wastewater [23]. The technology of MFC is clean, eco-friendly, renewable, robust and flexible, as different types of waste material can be utilized as the substrate to generate bioelectricity [29]. This process is also termed as the “waste-to-energy” concept [29,30]. By employing the QS-QQ method, which involves live bacteria as a catalyst to transfer electrons from the wastes to the anode, the process of bio-generation of electricity in a MFC can be enhanced. The use of METs, especially MFCs integrated with QS-QQ has been proven as a cutting-edge tool for bio-electricity and bio-hydrogen production [31]. Also, utilising solid or liquid waste as feedstock integrated with microalgae [32] has the potential to revolutionise the industry [[32], [33], [34]] by treating wastewater [35], creating biosensors [36], and bioremediation of contaminants [37].
Additional research and development in multi-purpose QS technology may enhance the transmission of electrons for the generation of bioelectricity in METs in novel ways with the aid of these recent findings. The QS system may aid in the effective transmission of electrons from the exoelectrogens to the anode of MFCs by producing the QS signal molecules [38,39]. These molecules are in charge of bacterial-to-bacterial communication and carry chemical signals between cells. In the end, the anode area experiences excellent and robust biofilm development, which is imperative for the efficacious performance of METs [40]. In membrane-based technologies, biofouling is referred to as the undesired aggregation of microorganisms on the membrane surface, leading to inferior performance of the membranes [[41], [42], [43]]. Even though the strategies to prevent biofouling have been extensively researched over the last two decades, it remains a significant impediment towards the commercial scaled-up applications of membrane-based technologies [44]. Huang et al. (2016) employed AHL-based QS and QQ to improve the efficiency of biological wastewater treatment and also improvement in the organic matter removal efficiency was observed [15]. A very recent study by Güneş & Taşkan et al. (2022) to control biofouling in membrane photobioreactor (MPBR) employing the QQ mechanism of immobilized Rhodococcus sp. BH4 has shown extremely promising results [45]. In this research, biofouling was monitored according to changes in conditions such as extracellular polymeric substances (EPS), transmembrane pressure cake-layer morphology, and microbial community on the membrane surface of MPBR [45]. It was observed that transmembrane pressure in control MPBR showed 818 mbar while QQ-MPBR showed 448 mbar [45]. Therefore, it can be concluded that QQ could productively reduce EPS from MPBRs and could also be employed in various METs.
3. Bacterial signalling mechanisms
3.1. Bacterial quorum sensing mechanism
The bacterial QS and QQ are intercellular mechanisms, which control the bacterial networks in their vicinity with the help of synthesized diffusible signal molecules, that is AIs. The QS systems in Gram positive bacteria are slightly different than the ones in Gram negative bacteria (Fig. 3). The AIs are sensed by cognate receptors; however, their location usually varies depending on the Gram character of the bacterial strain. It is seen in Gram positive bacteria that a two-component QS signalling cascade takes place. The AIPs are produced by acyl synthase as pro-AIPs and transported inside the cells as mature AIPs, followed by auto-phosphorylation of histidine kinase receptor and relaying the signal (transfer of phosphate ion) to the response regulator, which aids in the expression of the target gene.
Fig. 3.
Bacterial QS mechanism in Gram positive and Gram negative bacteria.
It has been reported that the cognate receptors are present in the cytoplasm for Gram negative strains, whereas, in the case of Gram positive strains, they are membrane-bound [10,46]. The AIs, post-receptor binding, are responsible for activating cascades of downstream signalling mechanisms as well as altering the gene expression in cells. The Gram positive bacteria use AIP and a two-component signal transduction system for the QS mechanism [47].
These signalling components develop a feed-forward autoinduction loop, which has various roles, including the transition from low cell densities to high cell densities state, and an increase in overall synchronicity amongst the bacterial population. The QS-system is responsible for the competence behaviour and formation of biofilms in Streptococcus aureus [9,48]. The S. aureus accessory gene regulator's QS system has been characterised by various researchers, which gives us a clear idea about the receptor-ligand linkage in its QS system. Another Gram positive strain, Bacillus cereus, takes a slightly modified approach to the QS mechanism, when compared to S. aureus [48]. The Gram negative bacteria, in contrast, use the QS system similar to the marine bioluminescent bacterium Vibrio fischeri. The bacteria use QS to activate the luciferase operon. The autoinducer signalling molecules used by Gram negative bacteria are known as AHLs, which consist of homoserine lactones and acyl chains that contain 4 to 18 carbon atoms [49,50]. Pseudomonas aeruginosa is one such Gram negative bacterium which produces an array of virulence factors contributing to its pathogenicity. P. aeruginosa uses two LuxI/LuxR QS circuits [51], where LasI is a homolog of LuxI synthesises 3-oxo-C12-homoserine (3OC12HSL). These circuits also help in biofilm formation for P. aeruginosa. The RhlI/RhlR circuit is involved in the treatment of municipal wastewater by degrading phenolic compounds and also aids in the denitrification process [17,52]. RhlI/RhlR mutants in P. aeruginosa were seen to lack the above-mentioned processes. Also, the LuxS-A1, A3 and LuxR homolog SdiA in Escherichia coli and Salmonella sp. Induce intercellular signalling mechanisms [53,54]. Bacterial strains such as Nitrosomonas europea, and Nitrobacter winogradskyi, isolated from wastewater may have the ability to exhibit cross-interaction among QS signals, E-PS production and biomineralization to reduce wastewater sludge [55,56]. The redox reactions of E-PS, which are responsible for bioremediation by bacteria, can also be optimized through the heme-activation of E-PS [17,57]. The benefits of the QS mechanism and QQMs; therefore, make it an important prerequisite in MFCs and have huge applications in METs.
3.2. Bacterial QQ mechanism
The discovery of AI analogues has led to several studies that have come up with the concept of a QQ system, which interferes with the QS circuit [[58], [59], [60]]. These studies testify to the major breakthrough in microbial-host interaction to control microbial pathogenesis and diseases caused by them. The QQ mechanism interferes with the QS circuit by inhibiting any of the four main QS stages as follows: i) inhibiting the production of autoinducers, ii) inhibiting the transport of autoinducers, iii) degradation of autoinducers by physical and/or chemical means, and iv) preventing the recognition of autoinducers by the receptors via competitive inhibition. The QQ mechanism does not put the bacteria under selective stress like pH, temperature, moisture, or nutrient, nor does it kill the cells; therefore, it is an effective biological control to prevent bacterial growth [59,61]. The components of QQ activities are divided into two main categories, small molecule QS inhibitors (QSIs) and macromolecular QQ substances [9,11,62]. The first group structurally mimics the QS signals, such as furanone (mostly halogenated), synthetic molecules of AIPs and AHL, and flavonoids [63]. These QSIs block the binding of signals to the receptors and reduce the receptor concentration. Naringenin, a plant flavonoid, replaces odDHL by binding to LasR, reduces biofilm formation, and decreases pyocyanin, elastase and virulence factor expression in P. aeruginosa [64,65]. Chatterjee et al. (2017) have reported that Lactobacillus sp. produces 3-benzene lactic acid, a QSI which binds to the receptors PhlR and PqsR more efficiently than the P. aeruginosa's receptors [66]. The second group belongs to the enzyme inhibitors like triclosan and closantel [11]. Enoyl-ACP catalyses AHL biosynthesis and aids in the formation of an essential intermediate, and triclosan blocks the action of enoyl-ACP, whereas the histidine kinase is blocked by closantel [11,63,67]. Natural compounds like trans-cinnamaldehyde and salicylic acid inhibit QS virulence genes and biofilm formation in P. aeruginosa [68]. MacQ, an AHL-acylase isolated from Acidovorax sp. MR-S7 interfere with many AHLs, and disrupts acyl chains of C6–C14 carbon [69]. While the small molecule QSIs mainly involve in inhibiting enzyme complex formation or reducing the receptor concentration to quench the QS circuit via competitive inhibition, the macromolecular substances degrade the enzymes in the QS pathways, especially AHL signal pathways, including enzymatic hydrolysis [61,63,70]. The AHL degrading enzymes are differentiated into the following types: AHL lactonase, AHL acylase, and AHL oxidoreductase [59] and a few others (Table 1, Table 2).
Table 1.
Commonly found quorum quenching enzymes in bacteria.
| Enzyme | Mechanism | Quenching enzyme target | Examples | References |
|---|---|---|---|---|
| AHL Lactonase | Hydrolysis of AHL-lactone bonds | AHL | PON1 (mammalian liver) | [71] |
| QsdR1 (Rhizobium sp. NGR234) | [72] | |||
| QsdA (Rhodococcus erythropolis) | [73] | |||
| AhlS (Solibacillus silvestris) | [74] | |||
| MomL (Pectobacterium carotovorum) | [75] | |||
| AHL-acylase | Deacylation, hydrolysis in amide-bond of AHLs | AHL | QuiP (Pseudomonas aeruginosa PAO1) | [76] |
| AiiD (Ralstonia sp. HJ12B) | [77] | |||
| AiiO (Ochrobactrum sp. A44) | [78] | |||
| AhlM (Streptomyces sp. M664) | [79] | |||
| AHL-oxidase | Catalysis of acyl-chain oxidation | AHL | CYP102A1 (Bacillus megaterium) | [80] |
| AHL-reductase | Conversion of 3-oxo-substituted-AHLto 3-hydroxyl substituted AHLs | AHL | BpiB09 | [81] |
| Quinolone dioxygenase | Catalysis of PQS conversion to N-octanoyl-anthranilic acid and CO | Quinolones | 2-alkyl-3-hydroxy-4(1H)-quinolone 2,4-dioxygenase (Arthrobacter sp.) | [82] |
Table 2.
Development in different types of QS/QQ molecule in the field of METs.
| Organism | Type of MET | Function of the molecule | Major QS/QQ molecules produced | QS/QQ regulated activities | Energy production | Reference |
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | MFC | Electron shuttle | Overexpression of phzM-pyocyanins production | Not available | Enhanced power output up to 166.68 μW/cm2 | [83] |
| Rhodococcus sp. | Dual chamber MFC | QQ molecule | AHL-degrading bacteria | Inhibits biofilm formation | Maximum power density of 1924 mW/m2 | [84] |
| Pseudomonas aeruginosa | P. aeruginosa-inoculated MFC | QS signal | Pyocyanin | Formation of mature biofilm and enhancement of electron transportation | Maximum current density of 99.80 μA/cm2 | [85] |
| Pseudomonas aeruginosa | Dual chamber MFC | QS signal | 2-heptyl-3,4- dihydroxyquinoline inhibitor PqsE | Production of redox mediator phenazines | Maximum current density of 0.5 μA/cm2 | [86] |
| Escherichia coli | Mediator less MFC | Electron shuttle | Not available | Direct transportation of electrons | Maximum power density of 600 mW/m2 | [87] |
| Mixed consortium of electrogens | H-type two-chamber MES | Synthetic redox | QSM homologous molecule | Enhancement in electrons transportation | Maximum power density of 14.5 A/m2 | [88] |
In wastewater sludge, there is the presence of both QS and QQ bacterial colonies and there is the possibility of an interaction. For example, Song et al. (2014) investigated the induction of β-galactosidase by Agrobacterium tumefaciens [89]. When the activated sludge was incubated with AHL for a period of 6 h, they noted a minimal induction of the enzyme when compared to the absence of AHL. Also, they were able to isolate Bacillus thuringiensis from the sludge. Bacillus thuringiensis is known to possess the aiiA gene, which encodes a lactone-degrading enzyme [89,90]. Therefore, it can be inferred that QS inhibitors were present in the activated sludge, which could breakdown the AHLs and inactivate the gene expressing β-galactosidase in A. tumefaciens. However, more detailed research on the direct role of QQ circuits pertaining to the treatment of wastewater is required to comment on the interaction between the QS-QQ mechanisms.
4. Application of bacterial signalling mechanisms in METs
The METs are promising techniques that combine the principles of electrochemistry with biotechnology to recover value-added products along with concomitant wastewater remediation [91]. However, high fabrication costs and inferior yield of resources are the major setbacks towards the commercialization of METs [12,92]. To overcome these bottlenecks, researchers investigated the application of QS to accelerate intercellular interaction to enhance the efficiency of METs [12]. In this regard, numerous investigations were conducted on the application of QS in METs, which are presented in the subsequent sections.
4.1. Application of QS in MFCs to improve power generation
The MFCs are bio-electrochemical devices, which exemplify the environment-friendly production of bioelectricity along with wastewater treatment by converting the chemical energy present in the chemical bonds of organic matter present in wastewater with the help of catalytic activities of microorganisms [93]. A typical MFC consists of two chambers, specifically anodic and cathodic chambers, which are separated by a PEM [94]. Both anodic and cathodic chambers house two electrodes named anode and cathode, respectively, which are connected via an external circuit that completes the transfer of electrons from the anode to the cathode [95]. A MFC contains microorganisms that can produce electrons from organic matter by intercellular metabolism and then transfer these electrons to the anode [96]. However, the transferring of intercellular electrons from the cell membrane is difficult due to the insulation of the cell membrane [97]. For the efficient transfer of electrons from the planktonic electrogenic cells to the electrode, electron shuttles are required, which can be produced by the microbes or can be added exogenously [12]. To avoid the exogenous addition of electron shuttles, mature biofilm development is imperative, as attached microbes generally don't require shuttles to transfer electrons to the electrode [98].
In this regard, QS is advantageous for developing mature exo-electrogenic biofilm, as it is imperative for the efficacious performance of MFCs. However, too thick biofilm can reduce the conductivity of biofilm, leading to inferior power generation, so it's important to control the thickness of biofilm. In this regard, an investigation conducted by Taşkan and Taşkan, (2021) revealed that QS can be employed to control biofilm thickness. The MFC with the anodic biofilm thickness of 26 μm cultured with 40 mg Rhodococcus sp. BH4 immobilized in sodium alginate beads exhibited the maximum power density of 1924 mW/m2, which was 2.8 times higher than the control MFC (683 mW/m2) comprising of beads devoid of microbes [84]. The thickness of the biofilm was reduced with the addition of Rhodococcus sp. BH4 in the anodic chamber, which resulted in the controlled and active biofilm formation that produced more redox compounds and subsequently led to the increase in power generation [84]. Furthermore, another investigation by Cheng, Xu [99] revealed that QS signals positively affect the power output of a MFC, chlortetracycline degradation, and the structure of electroactive biofilms. The MFCs with QS molecules butanoyl homoserine lactone and PQS showed an increment in the power output by 21.57% and 13.73%, respectively, compared to MFC without QS molecules. Moreover, MFCs with butanoyl homoserine lactone and PQS experienced an augmentation in the chlortetracycline degradation efficiency by 56.53% and 50.04%, respectively, which can be attributed to the thicker biofilm formation, enhanced bacterial activities and higher biomass concentration [99].
Similarly, in another investigation conducted by Monzon, Yang [100], it was observed that the voltage generation reached 0.3 V with the addition of quinolone, in comparison to the control operated without the addition of quinolone, which had a mean value of 0.1 V corresponding to the external resistance of 510 Ω. The MFC with quinolone, the QS molecule, demonstrated a higher voltage due to the increase in electron donor concentration in the medium [100]. Furthermore, a research conducted by Yong et al. (2014) revealed that with the overexpression of phzm (methyltransferase encoding gene) for a MFC inoculated with the Pseudomonas aeruginosa-phzm produced a four-fold higher power density of 166.68 μW/cm2 corresponding to the current density of 487.97 μA/cm2; whereas, MFC inoculated with P. aeruginosa PAO1 exhibited a power density of 42.53 μW/cm2 corresponding to the current density of 142.31 μA/cm2, which can be ascribed to the overexpression of phzm resulting in the higher power production [101]. For the efficient power production, improvement in extra cellular electron transfer for the exoelectrogens can be proven as a forerunner in the way towards the development of MFC technology. In this context, a superficial resolution-contrast mixed bacterial MFC was developed to validate the redox-mediated output current. Besides, in this investigation, phenazine-1-carboxylic acid, 2,4-diacetyl phloroglucinol, 10-done, trimipramine and 1-hydroxyanthracene-9 were found to be promising redox mediators that aided in the transfer of electrons [102]. Therefore, from these investigations, it is apparent that QS mechanisms can be employed to improve the overall performance of MFC and specifically can be instrumental in enhancing its power generation. Further, the QS mechanism can also be applied for the development of stable biofilm, lowering the internal resistance and for manufacturing efficient biosensors and thus, could aid in taking this pioneering technology to the real field for pragmatic applications.
4.2. Employment of QS mechanism to ameliorate the yield of valuables in microbial electrolysis cells
The MECs are derivatives of MFCs, where the degradation of organic matter along with the concurrent recovery of valuables like hydrogen and methane can be accomplished with the application of minimal imposed potential [103,104]. For H2 evolution, mixed microbial communities are mostly used to inoculate cathodic biofilms because they exhibit better results in comparison to pure cultures [105]. However, in these mixed cultures, hydrogen scavengers like methanogens and homo-acetogens are omnipresent and in turn, it affects the electrons and protons recovery, which leads to the reduction in hydrogen yield [106]. Thus to overcome this bottleneck of inferior hydrogen yield, membrane-bound hydrogenases are employed and the efficiency of this functioning depends on the electrode potential [105]. Also, the electron transfer process in MECs is reliant on electroactive bacterial metabolism, where direct or indirect extracellular electron transfer takes place between the cells to electrodes [7]. However, poor electron transmission efficacy amid anode and biofilm is one of the major setbacks of MEC technology [93].
In this regard, to improve the efficiency of MECs, researchers employed QS and it showed an enhancement in the overall performance of a MEC along with the improvement in the extracellular electron transfer efficiency from electrogenic cells to the electrode [38]. In this veneration, a research conducted by Cai et al. (2016) revealed that with the addition of AHLs, hydrogen yield was increased by 5.57%, 38.68%, and 81.82%, corresponding to the external cell voltage of 0.8 V, 0.6 V and 0.4 V, respectively, which designated the positive effect of AHLs addition [106]. In addition to the hydrogen yield, coulombic efficiency and electron recovery efficiency were also significantly improved by the addition of AHLs. This enhancement in the hydrogen yield and efficiency can be credited to the change in microbial structure with the increase in the percentage of Gamma-proteobacter and scarcer hydrogen foragers with the addition of AHLs [106]. Similarly, in another examination conducted by Liu et al. (2015), it was demonstrated that the hydrogen yield (3.8 ± 0.2 mol H2 per mol of acetate) was amplified by 29% in MEC with the dosage of 10 μM 3O-HSL due to the accumulation of AHLs over the control MEC operated without 3OC6-HSL. Thus, the investigation indicated that the application of the short-length AHLs might be beneficial in developing mature anodic biofilms, which in turn improves the performance of MECs [38].
Furthermore, another experiment conducted by Liu et al. (2021) exposed that hydrogen yield for a MEC fed with waste activated sludge (WMEC) was 3.5 mg/g volatile suspended solid (VSS), while the same for the MEC fed with raw waste activated sludge (RMEC) was only 1.9 mg/g VSS. However, with the addition of AHL, hydrogen yield was greatly enhanced, which was noted to be 4.3 and 3.0 mg/g VSS for WMEC and RMEC, respectively [107]. The improvement in the hydrogen yield and electricity recovery can be attributed to the efficacious electron transfer between electrodes and biofilm due to effective AHL regulation (Liu et al., 2021). In one more recent investigation, Nie and co-researchers revealed that QS molecule 3-oxo-C12-HSL ameliorated the N2O recovery from incineration leachate in MEC and exhibited 80.35% enhancement in the removal efficiency of nitrate, when compared to the control MEC (67.07%), which was operated without QS molecules. The improvement in the removal efficiency of nitrite can be accredited to the increase in biomass through the early activated QS mechanism due to the dosage of 3-oxo-C12-homoserine lactone [108]. In a demonstration by Yang, Zhou [109], it was also observed that QS autoinducers contributed to microbial extracellular electron transfer by P. aeruginosa PAO1. In this investigation, three MECs were inoculated with three different mutant cultures of P. aeruginosa, individually, which showed an increase in the current generation in all the MECs due to the overproduction of PQS. Therefore, from the presented venerations, it is evident that the QS mechanism can be employed to improve hydrogen yield and electricity recovery. Furthermore, it can be influential in enhancing the extracellular electron transfer from electrogens to the electrode, which ultimately results in the improvement in the overall efficiency of MECs.
4.3. Other applications
Many other investigations were successfully conducted by researchers on the application of QS in other different derivatives of METs, suggesting that QS can be targeted for the manipulation of genes for the enhancement of wastewater treatment and resource recovery in METs [98]. In this regard, an illustration by Pan et al. (2020) revealed that when AHLs (N-hexanoyl-l-homoserine lactone, C6HSL and 3-OXO-C12- HSL) were applied in a MFC employed for toxicity sensing and higher sensing linearity was observed for a broader range of Pb2+ concentration. In addition, these MFC sensors were able to recover full voltage (405 ± 10 mV) even after shock loading of 10 mg/L of Cu2+. Whereas the control MFC operated without AHLs failed to recover its initial maximum voltage of 250 ± 10 mV, which indicated the role of QS in maintaining the stable performance of MFCs [110].
Similarly, another experiment conducted by Zhou et al. (2022) highlighted the use of C6HSL as a QS regulator in MES, a type of MET and a maximum acetate production of 1.13 ± 0.17 mM/day from CO2 reduction was observed with the increment of 94.8% compared to the control operated without C6HSL (0.58 ± 0.24 mM/day). In addition, higher electrochemical activity and lower charge transfer resistance were also noted for the biocathode and the Faradic efficiency of the MES was enhanced by 71.7%, which can be credited to the presence of more mature cathodic biofilm [111]. Similarly, in a research, Venkataraman and co-researchers demonstrated that in a MEC, anode-respiring P. aeruginosa act as a toxicity sensor directly by the production of electron shuttling QSM phenazine for sensing impurities [112]. Furthermore, genetically engineered bacterial strain A. tumefaciens A136 cultured with Luria broth in the presence of antibiotics, namely spectinomycin and tetracycline, also worked as a biosensor using QQ activity to detect a wide range of AHL compounds [113]. Moreover, for the rapid detection of acid toxicity, a single chamber MFC-based toxicity sensor using QS regulated microbial consortium was used, which sensed 80% inhibition to HCl acid after 4 h of acidic exposure [114]. Therefore, these investigations emphasise on the fact that QS-facilitated biofilm has substantial applications in the field of toxicity sensing through METs.
Majorly, attached growth takes place on the electrode surface of METs; however, planktonic cells are also present in the biotic chamber of a MET. Further, attached growth on the electrode surface can reduce the losses of electrons in parasitic reactions, which leads to the amelioration in the efficacy of METs and diminishes the losses allied with the transfer of electrons [93]. The performance of a MFC is dependent on the thickness of the biofilm because electrons are generated by electrogenic bacteria via the biodegradation of organic fraction present in the anodic chamber and concurrently, these electrons are transported to the anode directly or indirectly to produce bioelectricity in the MFCs [115]. However, excessive development of biofilm on the electrode surface and PEM, termed as biofouling, deteriorates the performance of METs [116]. In this regard, bacterial signalling has also been proven as an effective tool in regulating and preventing membrane biofouling. Thus, to prevent excessive biofilm development, researchers practised inhibition strategies against bacterial communication via blocking the QS network with the help of (i) preventing the production of QSM through their degradation, (ii) reducing the QS activity of the receptor protein, (iii) copying the QSM mainly by exercising artificial compounds like the sensing molecules [12].
In this context, a demonstration explained the degradation of organic fraction present in the anodic chamber of MFC and MEC, which enhanced bacterial growth and led to the formation of thicker biofilm over the electrode and membrane over time [116]. The thickness of anodic biofilm and the density of biofilm formed on the membrane greatly inhibits the transportation of electrons and protons, respectively. Moreover, poor electron transfer in the interior part of the biofilm compared to the outermost layer because of lesser diffusion of electron mediators leads to the inferior performance of METs [117]. In this regard, researchers found that maximum power recovery can only be achieved with the optimal biofilm thickness [12]. The interfering of QS communication can be used to achieve the optimal biofilm thickness on the surface of the electrode and to prevent biofilm formation on the membrane surface. Therefore, to control the excessive growth of anodic biofilm and to prevent membrane biofouling, autoinducer-mediated QS strategy, i.e., the QQ mechanism can be employed for the efficient operation of this technology [118]. Bacterial signalling overlays the path to enhance sluggish extracellular electron transfer rate and restricted metabolic capacity of innate electroactive microbes; or to facilitate communication between the electroactive and non-electroactive microbiome [105,119]. Therefore, it is evident that with the application of QS, the performance of different types of METs can be enhanced and could prove to be instrumental in the steady transition of these inventive technologies from the lab-scale to the commercial scale (Fig. 4).
Fig. 4.
Application of QS and QQ mechanism to improve the efficacy of METs.
5. Recent advancements
The QS-QQ mechanisms work in tandem with METs to treat wastewater and generate valuables. As QS signals are known to be generated by over 70 distinct species, it is clear that QSMs and QQMs are pivotal in the molecular mechanism systems present in multiple microorganisms [120]. These molecules control pathogenesis and regulate contaminant clearance in addition to bacterial aggregation [121]. In addition to these functions, they also assist in the formation of an optimal biofilm on the anodic surface and enhance bioelectricity production in METs. However, one of the major disadvantages of METs is high fabrication costs and low power yield, which can be circumnavigated through the application of synthetic mediators. Commonly used synthetic mediators are quinone, thionine, and methylene blue, which help to induce faster electron transportation between the biofilm and the anode of MET. Since there is a threat of synthetic redox mediators to inhibit the growth of the bacteria, scientists have proposed the use of QSMs-QQMs to solve the problem. As mentioned earlier, AHL structure, composition, and degradation are necessary for the QQ function and subsequently for wastewater treatment. Therefore, in this context, the following questions yet remain unanswered: a) What threshold concentration of AHLs is needed for a single or multiple species biofilm to form? b) What roles do bacteria that produce and quench AHL play in multi-species biofilms? c) Does the establishment of multi-species biofilms include interactions between AHLs and other factors, such as E-PS? Therefore, studies specifically addressing the ecological role of QQ and its impact on QS signalling are essential. In other words, E-PS, AHLs, or both might be responsible for the formation of the biofilm. For example, Taşkan and Taşkan [84] worked with 20 mg/L of Rhodococcus sp., an AHL-degrading bacteria, to reduce the biofilm thickness in the anodic chamber of MFCs. The bacterial strain was immobilized on sodium alginate and finally the set-up exhibited a power output of 1924 mW/m2. The output was almost twice of what was achieved with the free strain.
The main factors causing membrane biofouling in microbial bioreactors include sewage settleability and size of its organic or inorganic matter, mixed liquor flowability and zeta potential, microbial species present in the inoculum, and the characteristics and function of its EPS [122]. For the creation and use of biological-based antifouling approaches, it is envisaged that understanding and defining the interplay between three properties of enzymatic QQ, biofouling components, and biofouling control effectiveness is a prerequisite [15]. Rahimnejad et al. (2015) demonstrated that METs have the potential to remove chemical oxygen demand (COD) by about 90% with more than 80% coulombic efficiency. At the same time, the digestion of 1 kg COD anaerobically can lead to the generation of 4.16 kWh of useable electrical power [123]. However, the METs need to have similar rates of waste to energy conversion to be considered a superior alternative to other membrane bioreactors to treat wastewater with concomitant generation of power [124]. Another study employed an anode with QS modification for better interaction of electrons with the anode. Two compounds, namely, tryptophol and phenyl ethanol, were used as QSM, which yielded power densities of 156.57 mW/m2 and 159.46 mW/m2, respectively [125]. Also, some bacteria were able to produce biofilms on anodes and generate about 82% bio-hydrogen in MEC [106]. So, it has been proposed that other bio-hydrogen-producing bacterial strains like Klebsiella sp., Bacillus sp., Streptococcus sp., and Clostridium sp., will be able to form thick anodic biofilms in METs [106,126].
Moreover, a number of large-scale piloting of MFCs have also been done by some research groups, which a few years ago was a difficult feat to achieve. Firstly, Das et al. (2020) operated a cylindrical MFC for about 140 days using sewage sludge slurry as the substrate [127]. CuZn and platinum carbon Pt/C were used as cathode catalysts and it yielded power densities of 75.1 mW/m2 and 110.6 mW/m2 [127]. The MFC could also successfully remove COD by 87% and 90% simultaneously [127]. Secondly, another catalyst, Cu0.5Mn0.5Fe2O4 was used in a 25 L MFC, which could generate a power density of 7.74 mW/m2 [23]. Finally, household sewage was treated by a 720 L MFC employing Co0.5Zn0.5Fe2O4 and Sn5Cu84 as cathode and goethite as anode [128]. The MFC generated a power of 61 mW with COD removal of about 78.4% [128].
Now that the scalability of MFCs has been improved significantly, the other major drawbacks in the operation of METs are the low yield of value-added products and bio-electricity and the high cost of MET fabrication [127,128]. In this regard, an exploration revealed that the present maximum power density an MFC can produce is 20 W/m3, which is not sufficient for practical applications and it would only become beneficial if the power density rises to at least 500 W/m3 [129]. Moreover, the cost of catalysts and membranes required to fabricate scaled-up MFCs is considerably higher in comparison to conventional technologies [130]. Hence, efforts are needed to reduce the capital cost of MFCs so that this novel technology can fulfil its enormous potential at the field-scale as well. Also, the QS-QQ signalling pathways are complicated as the isolation of QSMs and QQMs from bacteria and their extraction is a long process [7]. Moreover, it has been observed that overexpression of certain QSMs can disrupt the function of other QSMs; therefore, the choice of QSM to be used is of utmost importance in METs [131]. Studies have reported that QQ-induced enzymes like homoserine acylase, PvdQ, are able to inhibit thick biofilm formation on the electrodes [132], which again is a significant finding to realize the maximum efficiency of METs. The advent of QSM and QQM homologues has proven to be more stable and feasible in terms of wastewater remediation and higher power output. Techniques such as genetic engineering, immobilization and microbial symbiosis can jointly improve the current problems [12,133]. The final perspective towards the improvement of METs by QSMs and QQMs, therefore, needs more in-depth inquiry to enable future development in the systematic operation and commercialization of METs.
6. Conclusion
The METs are an innovative set of technologies for concomitant wastewater treatment with valuable resource recovery. However, high fabrication cost and lower yield is the major obstacle to the way of commercialization of the METs. In this regard, QSM and QQM secreted by different bacterial strains can be employed to improve the yield of valuables through METs. The QSMs enhance the interaction of electron-carriers within the anodic chamber in METs and the QQMs, in turn, salvage the detrimental effects of the thick bacterial biofilms on the electrodes. Moreover, because of their networking nature, QSM is proven as an efficient tool for the development of firm biofilm, which results in the improvement in valuables recovered through METs. Furthermore, QQM has also gained considerable attention among the scientific community for its property of preventing the biofouling of membranes in METs without causing any disturbance to the beneficial microbes. Hence, the QS-QQ signalling mechanism is a viable approach for enhancing the efficacy of METs in terms of valuable resource recovery with concomitant wastewater treatment.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
VS and AA thanks CEFIPRA Indo French Project No PPMB 7133/2020 for the fellowships. VV thanks CEFIPRA Indo French Project No PPMB 7133/2020 sanctioned to her for the financial support. SD would like to thank IIT Delhi for providing the infrastructure required for this research.
Abbreviations
AHLs: Acyl-homoserine lactones; AI: Auto-inducers; AIP: Autoinducing peptides; C6HSL: N-hexanoyl-l-homoserine lactone; COD: Chemical oxygen demand; E-PS: Exo-polysaccharides; EPS: Extracellular polymeric substances; MECs: Microbial electrolysis cells; MES: Microbial electrosynthesis; METs: Microbial electrochemical technologies; MFCs: Microbial fuel cells; MPBR: Membrane photobioreactor; PEM: Proton exchange membrane; PQS: Pseudomonas quinolone signal; QQ: Quorum quenching; QQM: Quorum quenching molecules; QS: Quorum sensing; QSI: Quorum sensing inhibitors; QSM: Quorum sensing molecules; RMEC: MEC fed with raw waste activated sludge; VSS: Volatile suspended solid; WMEC: MEC fed with waste activated sludge.
References
- 1.Ganta A., Bashir Y., Das S. Dairy wastewater as a potential feedstock for valuable production with concurrent wastewater treatment through microbial electrochemical technologies. Energies. 2022;15(23):9084. [Google Scholar]
- 2.Ahirwar A., Das S., Das S., Yang Y.-H., Bhatia S.K., Vinayak V., Ghangrekar M.M. Algal Research; 2023. Photosynthetic Microbial Fuel Cell for Bioenergy and Valuable Production: A Review of Circular Bio-Economy Approach. [Google Scholar]
- 3.Das S., Mishra A., Ghangrekar M. Production of hydrogen peroxide using various metal-based catalysts in electrochemical and bioelectrochemical systems: mini review. Journal of Hazardous, Toxic, and Radioactive Waste. 2020;24(3) [Google Scholar]
- 4.Paluch E., Rewak-Soroczyńska J., Jędrusik I., Mazurkiewicz E., Jermakow K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020;104(5):1871–1881. doi: 10.1007/s00253-020-10349-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X., Yu Z., Ding T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms. 2020;8(3):425. doi: 10.3390/microorganisms8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K., Yu H., Zhang X., Choo K.-H. Quorum sensing and quenching in membrane bioreactors: opportunities and challenges for biofouling control. Bioresour. Technol. 2018;270:656–668. doi: 10.1016/j.biortech.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z., Zhu X., Liang P., Zhang X., Kimura K., Huang X. Distinction between polymeric and ceramic membrane in AnMBR treating municipal wastewater: in terms of irremovable fouling. J. Membr. Sci. 2019;588 [Google Scholar]
- 8.Liu J., Eng C.Y., Ho J.S., Chong T.H., Wang L., Zhang P., Zhou Y.J.W.r. Vol. 156. 2019. pp. 159–167. (Quorum Quenching in Anaerobic Membrane Bioreactor for Fouling Control). [DOI] [PubMed] [Google Scholar]
- 9.Rutherford S.T., Bassler B.L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine. 2012;2(11) doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng W.L., Bassler B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong Y.-H., Zhang L.-H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005;43(S):101–109. [PubMed] [Google Scholar]
- 12.Das S., Das S., Ghangrekar M.M. Bacterial signalling mechanism: an innovative microbial intervention with multifaceted applications in microbial electrochemical technologies: a review. Bioresour. Technol. 2022;344 doi: 10.1016/j.biortech.2021.126218. [DOI] [PubMed] [Google Scholar]
- 13.Czajkowski R., Jafra S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 2009;56(1) [PubMed] [Google Scholar]
- 14.Siddiqui M.F., Rzechowicz M., Harvey W., Zularisam A.W., Anthony G.F. Quorum sensing based membrane biofouling control for water treatment: a review. J. Water Proc. Eng. 2015;7:112–122. [Google Scholar]
- 15.Huang J., Shi Y., Zeng G., Gu Y., Chen G., Shi L., Hu Y., Tang B., Zhou J. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: an overview. Chemosphere. 2016;157:137–151. doi: 10.1016/j.chemosphere.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y., Guan Y., Wang D., Liang K., Wu G. Potential roles of acyl homoserine lactone based quorum sensing in sequencing batch nitrifying biofilm reactors with or without the addition of organic carbon. Bioresour. Technol. 2018;259:136–145. doi: 10.1016/j.biortech.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Maddela N.R., Sheng B., Yuan S., Zhou Z., Villamar-Torres R., Meng F. Roles of quorum sensing in biological wastewater treatment: a critical review. Chemosphere. 2019;221:616–629. doi: 10.1016/j.chemosphere.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 18.Das S., Raj R., Das S., Ghangrekar M.M. A sustainable approach for the production of green energy with the holistic treatment of wastewater through microbial electrochemical technologies: a review. Frontiers in Sustainability. 2021;2 [Google Scholar]
- 19.Kato Marcus A., Torres Ci Fau - Rittmann B.E., Rittmann B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007;98(6) doi: 10.1002/bit.21533. [DOI] [PubMed] [Google Scholar]
- 20.Kose-Mutlu B., Ergon-Can T., Koyuncu I., Lee C.-H. Quorum quenching for effective control of biofouling in membrane bioreactor: a comprehensive review of approaches, applications, and challenges. Environmental Engineering Research. 2019;24(4):543–558. [Google Scholar]
- 21.Jadhav D.A., Pandit S., Sonawane J.M., Gupta P.K., Prasad R., Chendake A.D. Effect of membrane biofouling on the performance of microbial electrochemical cells and mitigation strategies. Bioresource Technology Reports. 2021;15 [Google Scholar]
- 22.Shah S.S.A., Choo K.-H. Isolation and characterization of novel indigenous facultative quorum quenching bacterial strains for ambidextrous biofouling control. Bioresour. Technol. 2020;308 doi: 10.1016/j.biortech.2020.123269. [DOI] [PubMed] [Google Scholar]
- 23.Das I., Das S., Das S., Ghangrekar M.M. Proficient sanitary wastewater Treatment in Laboratory and field-scale microbial fuel Cell with anti-biofouling Cu0.5Mn0.5Fe2O4 as cathode catalyst. J. Electrochem. Soc. 2021;168(5) [Google Scholar]
- 24.Tan C.H., Koh K.S., Xie C., Zhang J., Tan X.H., Lee G.P., Zhou Y., Ng W.J., Rice S.A., Kjelleberg S. Community quorum sensing signalling and quenching: microbial granular biofilm assembly. npj Biofilms and Microbiomes. 2015;1(1):1–9. doi: 10.1038/npjbiofilms.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anburajan P., Kim Y., Rice S.A., Oh H.-S. Bacterial signaling and signal responses as key factors in water and wastewater treatment. J. Water Proc. Eng. 2021;44 [Google Scholar]
- 26.Kusada H., Zhang Y., Tamaki H., Kimura N., Kamagata Y. Novel N-acyl homoserine lactone-degrading bacteria isolated from penicillin-contaminated environments and their quorum-quenching activities. Front. Microbiol. 2019;10:455. doi: 10.3389/fmicb.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh H.-S., Kim S.-R., Cheong W.-S., Lee C.-H., Lee J.-K. Biofouling inhibition in MBR by Rhodococcus sp. BH4 isolated from real MBR plant. Appl. Microbiol. Biotechnol. 2013;97(23):10223–10231. doi: 10.1007/s00253-013-4933-7. [DOI] [PubMed] [Google Scholar]
- 28.Tan C.H., Koh K.S., Xie C., Tay M., Zhou Y., Williams R., Ng W.J., Rice S.A., Kjelleberg S. The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules. ISME J. 2014;8(6):1186–1197. doi: 10.1038/ismej.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deka R., Shreya S., Mourya M., Sirotiya V., Rai A., Khan M.J., Ahirwar A., Schoefs B., Bilal M., Saratale G.D. Environmental Research; 2022. A Techno-Economic Approach for Eliminating Dye Pollutants from Industrial Effluent Employing Microalgae through Microbial Fuel Cells: Barriers and Perspectives. [DOI] [PubMed] [Google Scholar]
- 30.Gude V.G. Wastewater treatment in microbial fuel cells–an overview. J. Clean. Prod. 2016;122:287–307. [Google Scholar]
- 31.Rai A., Khan M.J., Ahirwar A., Deka R., Singh N., Schoefs B., Marchand J., Varjani S., Vinayak V. Hydrogen economy and storage by nanoporous microalgae diatom: special emphasis on designing photobioreactors. Int. J. Hydrogen Energy. 2022 [Google Scholar]
- 32.Vinayak V., Khan M.J., Varjani S., Saratale G.D., Saratale R.G., Bhatia S.K. Microbial fuel cells for remediation of environmental pollutants and value addition: special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J. Biotechnol. 2021;338:5–19. doi: 10.1016/j.jbiotec.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Butti S.K., Velvizhi G., Sulonen M.L., Haavisto J.M., Koroglu E.O., Cetinkaya A.Y., Singh S., Arya D., Modestra J.A., Krishna K.V. Microbial electrochemical technologies with the perspective of harnessing bioenergy: maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016;53:462–476. [Google Scholar]
- 34.Khan M.J., Singh N., Mishra S., Ahirwar A., Bast F., Varjani S., Schoefs B., Marchand J., Rajendran K., Banu J.R. Impact of light on microalgal photosynthetic microbial fuel cells and removal of pollutants by nanoadsorbent biopolymers: updates, challenges and innovations. Chemosphere. 2022;288 doi: 10.1016/j.chemosphere.2021.132589. [DOI] [PubMed] [Google Scholar]
- 35.Khan M.J., Ahirwar A., Schoefs B., Pugazhendhi A., Varjani S., Rajendran K., Bhatia S.K., Saratale G.D., Saratale R.G., Vinayak V. Insights into diatom microalgal farming for treatment of wastewater and pretreatment of algal cells by ultrasonication for value creation. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111550. [DOI] [PubMed] [Google Scholar]
- 36.Khan M.J., Rai A., Ahirwar A., Sirotiya V., Mourya M., Mishra S., Schoefs B., Marchand J., Bhatia S.K., Varjani S. Diatom microalgae as smart nanocontainers for biosensing wastewater pollutants: recent trends and innovations. Bioengineered. 2021;12(2):9531–9549. doi: 10.1080/21655979.2021.1996748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan M.J., Suryavanshi V.J., Joshi K.B., Gangadharan P., Vinayak V. Handbook of Algal Biofuels. Elsevier; 2022. Photosynthetic microalgal microbial fuel cells and its future upscaling aspects; pp. 363–384. [Google Scholar]
- 38.Liu W., Cai W., Ma A., Ren G., Li Z., Zhuang G., Wang A. Improvement of bioelectrochemical property and energy recovery by acylhomoserine lactones (AHLs) in microbial electrolysis cells (MECs) J. Power Sources. 2015;284:56–59. [Google Scholar]
- 39.Sun M., Zhai L.-F., Li W.-W., Yu H.-Q. Harvest and utilization of chemical energy in wastes by microbial fuel cells. Chem. Soc. Rev. 2016;45(10):2847–2870. doi: 10.1039/c5cs00903k. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R., Singh L., Zularisam A.W. Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016;56:1322–1336. [Google Scholar]
- 41.Oh H.-S., Lee C.-H. Origin and evolution of quorum quenching technology for biofouling control in MBRs for wastewater treatment. J. Membr. Sci. 2018;554:331–345. [Google Scholar]
- 42.Deng L., Guo W., Ngo H.H., Zhang H., Wang J., Li J., Xia S., Wu Y. Biofouling and control approaches in membrane bioreactors. Bioresour. Technol. 2016;221:656–665. doi: 10.1016/j.biortech.2016.09.105. [DOI] [PubMed] [Google Scholar]
- 43.Abuabdou S.M., Ahmad W., Aun N.C., Bashir M.J. A review of anaerobic membrane bioreactors (AnMBR) for the treatment of highly contaminated landfill leachate and biogas production: effectiveness, limitations and future perspectives. J. Clean. Prod. 2020;255 [Google Scholar]
- 44.Nunes S.P. Block copolymer membranes for aqueous solution applications. Macromolecules. 2016;49(8):2905–2916. [Google Scholar]
- 45.Güneş G., Taşkan E. Quorum quenching strategy for biofouling control in membrane photobioreactor. Chemosphere. 2022;288 doi: 10.1016/j.chemosphere.2021.132667. [DOI] [PubMed] [Google Scholar]
- 46.Haque S., Yadav D.K., Bisht S.C., Yadav N., Singh V., Dubey K.K., Jawed A., Wahid M., Dar S.A. Quorum sensing pathways in Gram-positive and -negative bacteria: potential of their interruption in abating drug resistance. J. Chemother. 2019;31(4):161–187. doi: 10.1080/1120009X.2019.1599175. [DOI] [PubMed] [Google Scholar]
- 47.Monnet V., Gardan R. Quorum-sensing regulators in Gram-positive bacteria: 'cherchez le peptide'. Mol. Microbiol. 2015;97(2):181–184. doi: 10.1111/mmi.13060. [DOI] [PubMed] [Google Scholar]
- 48.Stapleton P.D., Taylor P.W. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 2002;85(1):57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W., Li C. Exploiting quorum sensing interfering strategies in gram-negative bacteria for the enhancement of environmental applications. Front. Microbiol. 2015;6:1535. doi: 10.3389/fmicb.2015.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papenfort K., Bassler B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14(9):576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chadha J., Harjai K., Chhibber S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ. Microbiol. 2022;24(6):2630–2656. doi: 10.1111/1462-2920.15784. [DOI] [PubMed] [Google Scholar]
- 52.Yong Y.-C., Zhong J.-J. In: Future Trends in Biotechnology. Zhong J.-J., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2013. Impacts of quorum sensing on microbial metabolism and human health; pp. 25–61. [Google Scholar]
- 53.Wetzel M.E., Olsen G.J., Chakravartty V., Farrand S.K. The repABC plasmids with quorum-regulated transfer systems in members of the rhizobiales divide into two structurally and separately evolving groups. Genome Biol Evol. 2015;7(12):3337–3357. doi: 10.1093/gbe/evv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escobar-Muciño E., Arenas-Hernández M.M.P., Luna-Guevara M.L. Mechanisms of inhibition of quorum sensing as an alternative for the control of E. coli and Salmonella. Microorganisms. 2022;10(5) doi: 10.3390/microorganisms10050884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W.-W., Zhang H.-L., Sheng G.-P., Yu H.-Q. Roles of extracellular polymeric substances in enhanced biological phosphorus removal process. Water Res. 2015;86:85–95. doi: 10.1016/j.watres.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 56.Tourney J., Ngwenya B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014;386:115–132. [Google Scholar]
- 57.Li S.W., Sheng G.P., Cheng Y.Y., Yu H.Q. Redox properties of extracellular polymeric substances (EPS) from electroactive bacteria. Sci. Rep. 2016;6 doi: 10.1038/srep39098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J., Lin J., Zhang Y., Zhang J., Feng T., Li H., Wang X., Sun Q., Zhang X., Wang Y. Activity improvement and vital amino acid identification on the marine-derived quorum quenching enzyme MomL by protein engineering. Mar. Drugs. 2019;17(5) doi: 10.3390/md17050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bzdrenga J., Daudé D., Rémy B., Jacquet P., Plener L., Elias M., Chabrière E. Biotechnological applications of quorum quenching enzymes. Chem. Biol. Interact. 2017;267:104–115. doi: 10.1016/j.cbi.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Tang K., Zhang Y., Yu M., Shi X., Coenye T., Bossier P., Zhang X.H. Evaluation of a new high-throughput method for identifying quorum quenching bacteria. Sci. Rep. 2013;3:2935. doi: 10.1038/srep02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Syafiuddin A., Boopathy R., Mehmood M.A. Recent advances on bacterial quorum quenching as an effective strategy to control biofouling in membrane bioreactors. Bioresource Technology Reports. 2021;15 [Google Scholar]
- 62.Mayer C., Muras A., Romero M., Lopez M., Tomas M., Otero A. Multiple quorum quenching enzymes are active in the nosocomial pathogen acinetobacter baumannii ATCC17978. Front. Cell. Infect. Microbiol. 2018;8:310. doi: 10.3389/fcimb.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou L., Zhang Y., Ge Y., Zhu X., Pan J. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.589640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh M.H., Choi C.H. Role of LuxIR homologue AnoIR in acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. J. Microbiol. Biotechnol. 2015;25(8):1390–1400. doi: 10.4014/jmb.1504.04069. [DOI] [PubMed] [Google Scholar]
- 65.El-Shaer S., Shaaban M., Barwa R., Hassan R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl beta-naphthylamide. J. Med. Microbiol. 2016;65(10):1194–1204. doi: 10.1099/jmm.0.000327. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee M., D'Morris S., Paul V., Warrier S., Vasudevan A.K., Vanuopadath M., Nair S.S., Paul-Prasanth B., Mohan C.G., Biswas R. Mechanistic understanding of Phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017;101(22):8223–8236. doi: 10.1007/s00253-017-8546-4. [DOI] [PubMed] [Google Scholar]
- 67.Zhong S., He S. Quorum sensing inhibition or quenching in acinetobacter baumannii: the novel therapeutic strategies for new drug development. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.558003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmed S., Rudden M., Smyth T.J., Dooley J.S.G., Marchant R., Banat I.M. Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 2019;103(8):3521–3535. doi: 10.1007/s00253-019-09618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kusada H., Tamaki H., Kamagata Y., Hanada S., Kimura N. A novel quorum-quenching N-acylhomoserine lactone acylase from Acidovorax sp. strain MR-S7 mediates. Antibiotic Resistance. 2017;83(13) doi: 10.1128/AEM.00080-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao X., Yu Z., Ding T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms. 2020;8(3) doi: 10.3390/microorganisms8030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bar-Rogovsky H., Hugenmatter A., Tawfik D.S. The evolutionary origins of detoxifying enzymes: the mammalian serum paraoxonases (PONs) relate to bacterial homoserine lactonases. J. Biol. Chem. 2013;288(33):23914–23927. doi: 10.1074/jbc.M112.427922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uroz S., Heinonsalo J. Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 2008;65(2):271–278. doi: 10.1111/j.1574-6941.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 73.Uroz S., Chhabra S.R., Camara M., Williams P., Oger P., Dessaux Y. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology. 2005;151(Pt 10):3313–3322. doi: 10.1099/mic.0.27961-0. [DOI] [PubMed] [Google Scholar]
- 74.Morohoshi T., Tominaga Y., Someya N., Ikeda T. Complete genome sequence and characterization of the N-acylhomoserine lactone-degrading gene of the potato leaf-associated Solibacillus silvestris. J. Biosci. Bioeng. 2012;113(1):20–25. doi: 10.1016/j.jbiosc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 75.Wang J., Lin J., Zhang Y., Zhang J., Feng T., Li H., Wang X., Sun Q., Zhang X., Wang Y. Activity improvement and vital amino acid identification on the marine-derived quorum quenching enzyme MomL by protein engineering. Mar. Drugs. 2019;17(5) doi: 10.3390/md17050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang J.J., Han J.I., Zhang L.H., Leadbetter J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003;69(10):5941–5949. doi: 10.1128/AEM.69.10.5941-5949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Y.H., Xu J.L., Hu J., Wang L.H., Ong S.L., Leadbetter J.R., Zhang L.H. Acylhomoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003;47:849–860. doi: 10.1046/j.1365-2958.2003.03351.x. [DOI] [PubMed] [Google Scholar]
- 78.Czajkowski R., Krzyżanowska D., Karczewska J., Atkinson S., Przysowa J., Lojkowska E., Williams P., Jafra S. Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environmental Microbiology Reports. 2011;3(1):59–68. doi: 10.1111/j.1758-2229.2010.00188.x. [DOI] [PubMed] [Google Scholar]
- 79.Park S.Y., Kang H.O., Jang H.S., Lee J.K., Koo B.T., Yum D.Y. Identification of extracellular N-acylhomoserine lactone acylase from a Streptomyces sp. and its application to quorum quenching. Appl. Environ. Microbiol. 2005;71(5):2632–2641. doi: 10.1128/AEM.71.5.2632-2641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chowdhary P.K., Keshavan N., Nguyen H.Q., Peterson J.A., Gonzalez J.E., Haines D.C. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry. 2007;46 doi: 10.1021/bi701945j. [DOI] [PubMed] [Google Scholar]
- 81.Bijtenhoorn P., Mayerhofer H., Muller-Dieckmann J., Utpatel C., Schipper C., Hornung C., Szesny M., Grond S., Thurmer A., Brzuszkiewicz E., Daniel R., Dierking K., Schulenburg H., Streit W.R. A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pustelny C., Albers A., Buldt-Karentzopoulos K., Parschat K., Chhabra S.R., Camara M., Williams P., Fetzner S. Dioxygenase-mediated quenching of quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem. Biol. 2009;16(12):1259–1267. doi: 10.1016/j.chembiol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 83.Yong X.-Y., Shi D.-Y., Chen Y.-L., Jiao F., Lin X., Zhou J., Wang S.-Y., Yong Y.-C., Sun Y.-M., OuYang P.-K. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014;152:220–224. doi: 10.1016/j.biortech.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 84.Taskan B., Taskan E. Inhibition of AHL-mediated quorum sensing to control biofilm thickness in microbial fuel cell by using Rhodococcus sp. BH4. Chemosphere. 2021;285 doi: 10.1016/j.chemosphere.2021.131538. [DOI] [PubMed] [Google Scholar]
- 85.Yong X.-Y., Yan Z.-Y., Shen H.-B., Zhou J., Wu X.-Y., Zhang L.-J., Zheng T., Jiang M., Wei P., Jia H.-H. An integrated aerobic-anaerobic strategy for performance enhancement of Pseudomonas aeruginosa-inoculated microbial fuel cell. Bioresour. Technol. 2017;241:1191–1196. doi: 10.1016/j.biortech.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 86.Wang V.B., Chua S.-L., Cao B., Seviour T., Nesatyy V.J., Marsili E., Kjelleberg S., Givskov M., Tolker-Nielsen T., Song H. Engineering PQS biosynthesis pathway for enhancement of bioelectricity production in Pseudomonas aeruginosa microbial fuel cells. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang T., Cui C., Chen S., Ai X., Yang H., Shen P., Peng Z. A novel mediatorless microbial fuel cell based on direct biocatalysis of Escherichia coli. Chem. Commun. 2006;(21):2257–2259. doi: 10.1039/b600876c. [DOI] [PubMed] [Google Scholar]
- 88.Aguirre-Sierra A., Bacchetti-De Gregoris T., Salas J.J., de Deus A., Esteve-Núñez A. A new concept in constructed wetlands: assessment of aerobic electroconductive biofilters. Environ. Sci. J. Integr. Environ. Res.: Water Research & Technology. 2020;6(5):1312–1323. [Google Scholar]
- 89.Song X.-N., Cheng Y.-Y., Li W.-W., Li B.-B., Sheng G.-P., Fang C.-Y., Wang Y.-K., Li X.-Y., Yu H.-Q. Quorum quenching is responsible for the underestimated quorum sensing effects in biological wastewater treatment reactors. Bioresour. Technol. 2014;171:472–476. doi: 10.1016/j.biortech.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 90.Pan J., Huang T., Yao F., Huang Z., Powell C.A., Qiu S., Guan X. Expression and characterization of aiiA gene from Bacillus subtilis BS-1. Microbiol. Res. 2008;163(6):711–716. doi: 10.1016/j.micres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Logan B.E., Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science. 2012;337(6095):686–690. doi: 10.1126/science.1217412. [DOI] [PubMed] [Google Scholar]
- 92.Priyadarshini M., Ahmad A., Das S., Ghangrekar M.M. Application of microbial electrochemical technologies for the treatment of petrochemical wastewater with concomitant valuable recovery: a review. Environ. Sci. Pollut. Control Ser. 2021;29:61783–61802. doi: 10.1007/s11356-021-14944-w. [DOI] [PubMed] [Google Scholar]
- 93.Logan B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009;7(5):375–381. doi: 10.1038/nrmicro2113. [DOI] [PubMed] [Google Scholar]
- 94.Ahmad A., Priyadarshani M., Das S., Ghangrekar M.M. Role of bioelectrochemical systems for the remediation of emerging contaminants from wastewater: a review. J. Basic Microbiol. 2022;62(3–4):201–222. doi: 10.1002/jobm.202100368. [DOI] [PubMed] [Google Scholar]
- 95.Ghorai A., Roy S., Das S., Komber H., Ghangrekar M.M., Voit B., Banerjee S. Preparation of sulfonated polytriazoles with a phosphaphenanthrene unit via click polymerization: fabrication of membranes and properties thereof. ACS Applied Polymer Materials. 2021;3(8):4127–4138. [Google Scholar]
- 96.Khan M.J., Das S., Vinayak V., Pant D., Ghangrekar M. Live diatoms as potential biocatalyst in a microbial fuel cell for harvesting continuous diafuel, carotenoids and bioelectricity. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.132841. [DOI] [PubMed] [Google Scholar]
- 97.Zheng S., Yang F., Chen S., Liu L., Xiong Q., Yu T., Zhao F., Schröder U., Hou H. Binder-free carbon black/stainless steel mesh composite electrode for high-performance anode in microbial fuel cells. J. Power Sources. 2015;284:252–257. [Google Scholar]
- 98.Yong Y.-C., Wu X.-Y., Sun J.-Z., Cao Y.-X., Song H. Engineering quorum sensing signaling of Pseudomonas for enhanced wastewater treatment and electricity harvest: a review. Chemosphere. 2015;140:18–25. doi: 10.1016/j.chemosphere.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 99.Cheng X.-L., Xu Q., Sun J.-D., Li C.-R., Yang Q.-W., Li B., Zhang X.-Y., Zhou J., Yong X.-Y. Quorum sensing signals improve the power performance and chlortetracycline degradation efficiency of mixed-culture electroactive biofilms. iScience. 2022;25(5) doi: 10.1016/j.isci.2022.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monzon O., Yang Y., Li Q., Alvarez P.J.J. Quorum sensing autoinducers enhance biofilm formation and power production in a hypersaline microbial fuel cell. Biochem. Eng. J. 2016;109:222–227. [Google Scholar]
- 101.Yong X.-Y., Shi D.-Y., Chen Y.-L., Jiao F., Lin X., Zhou J., Wang S.-Y., Yong Y.-C., Sun Y.-M., OuYang P.-K., Zheng T. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014;152:220–224. doi: 10.1016/j.biortech.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 102.Wang G., Wei L., Cao C., Su M., Shen J. Novel resolution-contrast method employed for investigating electron transfer mechanism of the mixed bacteria microbial fuel cell. Int. J. Hydrogen Energy. 2017;42(16):11614–11621. [Google Scholar]
- 103.Kiely P.D., Regan Jm Fau - Logan B.E., Logan B.E. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr. Opin. Biotechnol. 2011;22(2):378–385. doi: 10.1016/j.copbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Logan B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010;85(6):1665–1671. doi: 10.1007/s00253-009-2378-9. [DOI] [PubMed] [Google Scholar]
- 105.Surti P., Kailasa S.K., Mungray A.K. Genetic engineering strategies for performance enhancement of bioelectrochemical systems: a review. Sustain. Energy Technol. Assessments. 2021;47 [Google Scholar]
- 106.Cai W., Zhang Z., Ren G., Shen Q., Hou Y., Deng Y., Wang A., Liub a.W. Quorum sensing alters the microbial community of electrode-respiring bacteria and hydrogen scavengers toward improving hydrogen yield in microbial electrolysis cells. Appl. Energy. 2016;183:1133–1141. [Google Scholar]
- 107.Liu Z., Zhou A., Wang S., Cheng S., Yin X., Yue X. Quorum sensing shaped microbial consortia and enhanced hydrogen recovery from waste activated sludge electro-fermentation on basis of free nitrous acid treatment. Sci. Total Environ. 2021;766 doi: 10.1016/j.scitotenv.2020.144348. [DOI] [PubMed] [Google Scholar]
- 108.Nie H., Liu X., Dang Y., Sun D. Bioresource Technology; 2022. Early Activated Quorum Sensing Enhanced a nosZ-Deficient Strain of Pseudomonas aeruginosa for Stably Recovering Nitrous Oxide from Incineration Leachate in Microbial Electrolysis Cell. [DOI] [PubMed] [Google Scholar]
- 109.Yang Y., Zhou H., Mei X., Liu B., Xing D. Dual-edged character of quorum sensing signaling molecules in microbial extracellular electron transfer. Front. Microbiol. 2018;9:1924. doi: 10.3389/fmicb.2018.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan J., Hu J., Liu B., Li J., Wang D., Bu C., Wang X., Xiao K., Liang S., Yang J., Hou H. Environmental Research; 2020. Enhanced Quorum Sensing of Anode Biofilm for Better Sensing Linearity and Recovery Capability of Microbial Fuel Cell Toxicity Sensor; p. 181. [DOI] [PubMed] [Google Scholar]
- 111.Zhou M., Zeng C., Liu G., Luo H., Zhang R. Enhanced CO2 reduction and acetate synthesis in autotrophic biocathode by N-Hexanoyl-L-homoserine lactone (C6HSL)-based quorum-sensing regulation. Sci. Total Environ. 2022;836 doi: 10.1016/j.scitotenv.2022.155724. [DOI] [PubMed] [Google Scholar]
- 112.Venkataraman A., Rosenbaum M., Arends J.B., Halitschke R., Angenent L.T. Quorum sensing regulates electric current generation of Pseudomonas aeruginosa PA14 in bioelectrochemical systems. Electrochem. Commun. 2010;12(3):459–462. [Google Scholar]
- 113.McLean R.J., Pierson L.S., III, Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods. 2004;58(3):351–360. doi: 10.1016/j.mimet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 114.Shen Y.J., Lefebvre O., Tan Z., Ng H.Y. Microbial fuel-cell-based toxicity sensor for fast monitoring of acidic toxicity. Water Sci. Technol. 2012;65(7):1223–1228. doi: 10.2166/wst.2012.957. [DOI] [PubMed] [Google Scholar]
- 115.Das S., Mishra A., Ghangrekar M. Concomitant production of bioelectricity and hydrogen peroxide leading to the holistic treatment of wastewater in microbial fuel cell. Chem. Phys. Lett. 2020;759 [Google Scholar]
- 116.Sun D., Chen J., Huang H., Liu W., Ye Y., Cheng S. The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int. J. Hydrogen Energy. 2016;41(37):16523–16528. [Google Scholar]
- 117.Ramasamy R.P., Ren Z., Mench M.M., Regan J.M. Impact of initial biofilm growth on the anode impedance of microbial fuel cells. Biotechnol. Bioeng. 2008;101(1):101–108. doi: 10.1002/bit.21878. [DOI] [PubMed] [Google Scholar]
- 118.Sikdar R., Elias M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: a review of recent advances. Expert Rev. Anti-infective Ther. 2020;18(12):1221–1233. doi: 10.1080/14787210.2020.1794815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nath D., Das S., Ghangrekar M. High throughput techniques for the rapid identification of electroactive microorganisms. Chemosphere. 2021;285 doi: 10.1016/j.chemosphere.2021.131489. [DOI] [PubMed] [Google Scholar]
- 120.Kalia V.C. Quorum sensing inhibitors: an overview. Biotechnol. Adv. 2013;31(2):224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 121.LaSarre B., Federle M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013;77(1):73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamedi H., Ehteshami M., Mirbagheri S.A., Rasouli S.A., Zendehboudi S. Current status and future prospects of membrane bioreactors (MBRs) and fouling phenomena: a systematic review. Can. J. Chem. Eng. 2019;97(1):32–58. [Google Scholar]
- 123.Rahimnejad M., Adhami A., Darvari S., Zirepour A., Oh S.-E. Microbial fuel cell as new technology for bioelectricity generation: a review. Alex. Eng. J. 2015;54(3):745–756. [Google Scholar]
- 124.Arends J.B., Verstraete W. 100 years of microbial electricity production: three concepts for the future. Microb. Biotechnol. 2012;5(3):333–346. doi: 10.1111/j.1751-7915.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Christwardana M., Frattini D., Duarte K.D.Z., Accardo G., Kwon Y. Carbon felt molecular modification and biofilm augmentation via quorum sensing approach in yeast-based microbial fuel cells. Appl. Energy. 2019;238:239–248. [Google Scholar]
- 126.Guo Y., Wang G., Zhang H., Wen H.A.-O., Li W. Effects of biofilm transfer and electron mediators transfer on Klebsiella quasipneumoniae sp. 203 electricity generation performance in MFCs. Biotechnol. Biofuels. 2020;13(1):1–11. doi: 10.1186/s13068-020-01800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Das I., Das S., Ghangrekar M.M. Application of bimetallic low-cost CuZn as oxygen reduction cathode catalyst in lab-scale and field-scale microbial fuel cell. Chem. Phys. Lett. 2020;751 [Google Scholar]
- 128.Das I., Ghangrekar M., Satyakam R., Srivastava P., Khan S., Pandey H. On-site sanitary wastewater treatment system using 720-L stacked microbial fuel cell: case study. Journal of Hazardous, Toxic, and Radioactive Waste. 2020;24(3) [Google Scholar]
- 129.Chin M.Y., Phuang Z.X., Woon K.S., Hanafiah M.M., Zhang Z., Liu M. Vol. 320. 2022. (Life Cycle Assessment of Bioelectrochemical and Integrated Microbial Fuel Cell Systems for Sustainable Wastewater Treatment and Resource Recovery). [DOI] [PubMed] [Google Scholar]
- 130.Yadav R.K., Das S., Patil S.A. Are integrated bioelectrochemical technologies feasible for wastewater management? Trends Biotechnol. 2022 doi: 10.1016/j.tibtech.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 131.Defoirdt T. Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol. 2018;26(4):313–328. doi: 10.1016/j.tim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Fleitas Martínez O., Rigueiras P.O., Pires A.d.S., Porto W.F., Silva O.N., De la Fuente-Nunez C., Franco O.L. Interference with quorum-sensing signal biosynthesis as a promising therapeutic strategy against multidrug-resistant pathogens. Front. Cell. Infect. Microbiol. 2019;8:444. doi: 10.3389/fcimb.2018.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khandelwal A., Vijay A., Dixit A., Chhabra M. Microbial fuel cell powered by lipid extracted algae: a promising system for algal lipids and power generation. Bioresour. Technol. 2018;247:520–527. doi: 10.1016/j.biortech.2017.09.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.