Abstract
Nerve guide conduit is a promising treatment for long gap peripheral nerve injuries, yet its efficacy is limited. Drug-releasable scaffolds may provide reliable platforms to build a regenerative microenvironment for nerve recovery. In this study, an elastic hydrogel conduit encapsulating with prodrug nanoassemblies is fabricated by a continuous 3D printing technique for promoting nerve regeneration. The bioactive hydrogel is comprised of gelatin methacryloyl (GelMA) and silk fibroin glycidyl methacrylate (SF-MA), exhibiting positive effects on adhesion, proliferation, and migration of Schwann cells. Meanwhile, 7,8-dihydroxyflavone (7,8-DHF) prodrug nanoassemblies with high drug-loading capacities are developed through self-assembly of the lipophilic prodrug and loaded into the GelMA/SF-MA hydrogel. The drug loading conduit could sustainedly release 7,8-DHF to facilitate neurite elongation. A 12 mm nerve defect model is established for therapeutic efficiency evaluation by implanting the conduit through surgical suturing with rat sciatic nerve. The electrophysiological, morphological, and histological assessments indicate that this conduit can promote axon regeneration, remyelination, and function recovery by providing a favorable microenvironment. These findings implicate that the GelMA/SF-MA conduit with 7,8-DHF release has potentials in the treatment of long-gap peripheral nerve injury.
Keywords: 3D printing, Peripheral nerve repair, Nerve conduit, Biomaterials, Drug delivery
Graphical abstract
3D printed elastic GelMA/SF-MA hydrogel conduits with 7,8-DHF prodrug nanoassemblies for peripheral nerve repair.

1. Introduction
Peripheral nerve injury (PNI) is one of common diseases that often leads to loss of motor and/or sensory function with a high disability rate [1]. Although the peripheral nerves exhibit certain spontaneous regeneration potential after injury, successful reinnervation for long-gap nerve defects still cannot be achieved and remains a significant challenge. Autograft has been considered as the gold standard for long gap nerve injuries with a 50% complete recovery rate, but is still limited by mismatch in size and fascicle, inadequate donor nerves, and secondary trauma [2]. Consequently, nerve guidance conduits (NGCs) derived from biomaterials have been extensively explored as promising alternatives to bridge the injured sites [3,4]. NGCs can bridge the nerve defects to provide physical support and direction guidance for nerve regeneration. However, conventional NGCs have limitations on sensory and motor function restoration of long gap injuries due to the suboptimal growth of regenerated nerves[5]. Previous researches have suggested that a favorable microenvironment with biochemical and biophysical cues may improve the complex nerve regeneration process [6]. Therefore, new functional NGCs need to be developed to build a regenerative microenvironment for promoting peripheral nerve regeneration.
In recent years, three-dimensional (3D) printing has been applied in establishing regenerative microenvironment for nerve repair [[7], [8], [9], [10], [11]]. Through patterned-laser induced polymerization of the prepolymer, digital light processing (DLP) based 3D printing can fabricate customized scaffolds with excellent resolution and speed [12,13]. Personalized branched human facial NGC with life-size has been created by this method, which can adequately match the natural nerve anatomies [14]. Multichannel NGCs and NGCs with aligned microstructures have also been printed with this method to provide effective directional guidance for nerve regeneration [14,15]. Moreover, drug-loaded nanoparticles and platelets have also been integrated with prepolymers through DLP based 3D printing to fabricate functional conduits for promoting nerve repair [[16], [17], [18]]. In addition, a drug-loaded bandage with grating structure constructed by DLP based 3D printing has been proved to directionally release drug to the injured site [19], which significantly improve the microenvironment for nerve regeneration. However, there is still lack of photopolymers with appropriate mechanical property and biocompatibility, which are ideal for creating an optimal environment for nerve recovery.
Gelatin methacryloyl (GelMA), composed of degraded natural extracellular matrix, has been widely used in DLP based 3D printing for tissue engineering due to its excellent biocompatibility and printing performances [20,21]. Remarkably, GelMA is rich in arginine glycine aspartic acid (RGD) motifs and can promote cell adhesion and proliferation. However, the mechanical properties of GelMA cannot match nerve tissue and are insufficient for suturing with nerves. In a recent study, silk fibroin glycidyl methacrylate (SF-MA), a derivate of natural polymer produced by Bombyx mori, has been developed [22]. This photocurable material presents outstanding mechanical property and printability. Artificial trachea has been printed with SF-MA for partial defected trachea model [23], indicating that SF-MA has potential in becoming a kind of printing material for NGCs construction. Nevertheless, the lack of cell attachment sequence in SF-MA may result in poor cell adhesion [24]. Integrating GelMA with SF-MA may develop hydrogels with proper mechanical and biological properties to provide a supportive microenvironment for nerve regeneration.
Bioactive molecules such as growth factors and drugs are also important for enhancing nerve recovery [[25], [26], [27]]. Neurite sprouting and outgrowth in NGCs is essential for the re-connection of the injured stumps during the regeneration process. Tropomyosin kinase receptor B (TrkB) is associated with the activation of MAPK, PI3K, and PLCγ signaling pathways, which play a key role in stimulating neurite elongation after injury [28]. 7,8-dihydroxyflavone (7, 8-DHF), a small flavonoid molecule, has been identified as a selective TrkB agonist [29]. The treatment with 7,8-DHF results in enhanced axon regeneration and muscle reinnervation for injured peripheral nerves [30], indicating 7,8-DHF as a potential biochemical cue to improve the microenvironment in NGCs for nerve regeneration. However, the hydrophobic 7,8-DHF cannot be effectively integrated into NGCs owing to the insolubility of 7,8-DHF in the matrix material. Currently, various carrier-assisted nanoparticles have been developed to improve the water solubility of hydrophobic drugs [8,9], whereas the drug-loading capacity is low and inadequate for the long-term nerve regeneration process. Carrier-free nanoassemblies with ultrahigh drug-loading capacity may offer a better choice. Recently, prodrug nanoassemblies with impressive high loading capacities have been developed for antitumor therapy by the self-assembly of lipophilic prodrug [31,32], indicating a novel strategy for introducing 7,8-DHF into NGCs for long-gap peripheral nerve injury.
In this study, we fabricated an elastic and bioactive GelMA/SF-MA conduit with 7,8-DHF release to build a supportive microenvironment for peripheral nerve repair by DLP based 3D printing (Fig. 1). GelMA/SF-MA hydrogels with different ratios were developed by integrating GelMA with SF-MA via chemical and physical double-crosslinking. The optimal GelMA/SF-MA hydrogel for nerve repair was identified through investigation of the effect of mechanical properties and biological functions on Schwan cell behavior. Then, 7,8-DHF prodrug nanoassemblies were introduced to fabricate functional GelMA/SF-MA conduits by the flexible continuous 3D printing technology. Finally, the therapeutic efficiency of the conduits was evaluated in a 12 mm sciatic nerve defect model through electrophysiological and histopathological examinations. The conduits were demonstrated to facilitate the re-connection and re-innervation of long-gap nerve defects with function recovery.
Fig. 1.
Schematic illustration of 3D printed functional conduits for peripheral nerve repair. (a) The bioink is composed of GelMA, SF-MA, and 7,8-DHF prodrug nanoassemblies. The prodrug was prepared by conjugating 7,8-DHF with oleic acid (OA), and the nanoassemblies were formed by self-assembly of the lipophilic prodrugs. (b) The GelMA/SF-MA hydrogel conduit with 7,8-DHF release was fabricated by continuous 3D printing and further ammonium sulfate (AS) treatment. (c) The 3D printed conduit was used to bridge a 12 mm gap for peripheral nerve repair.
2. Materials and methods
2.1. Materials
Type A gelatin from porcine skin, glycidyl methacrylate (GMA), and methacrylic anhydride (MAA) were purchased from Sigma-Aldrich. B. mori cocoons were obtained from the Jiaxing Silkworm Base (Zhejiang, China). 7,8-DHF was purchased from Adamas. Lithium phenyl-2,4,6-trimethyl-benzoylphosphinate (LAP) was synthesized as previously reported [33]. All the chemical reagents were of analytical grade and used as received.
Schwann cells (S16) and rat pheoehromoeytoma cells (PC12) were purchased from BeNa Culture Collection (China). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin-streptomycin at 37 °C in a humidified and 5% CO2 incubator.
Sprague-Dawley (SD) rats were bought from Chengdu Dossy Experimental Animals Co., Ltd. and maintained under specific pathogen-free conditions. All the animal experimental studies were performed in compliance with the guidelines and regulations of Sichuan University Committee on Animal Research and Ethics.
2.2. Preparation of GelMA/SF-MA hydrogels
GelMA was synthesized as the previous report [34]. 10 g Gelatin was dissolved in 100 mL aqueous solution of 0.80 g Na2CO3 and 1.47 g NaHCO3. Then, 3 mL MAA was added dropwise to the solution under stirring at 50 °C. After 3 h, the reaction was stopped by adjusting the pH to 7 with HCl. After filtration, dialysis, and lyophilization, GelMA was obtained and stored at −20 °C.
SF-MA was prepared as previously described [22]. B. mori cocoons were degummed with Na2CO3 (0.05 M) at 100 °C for 30 min, rinsed thoroughly with distilled water, then dried at 40 °C. The degummed silk (10 g) was dissolved in 50 mL LiBr (9.3 M) at 60 °C for 1 h. GMA (3 mL) was then added dropwise under stirring at 60 °C. After 3 h, the reacted solution was filtered, dialyzed, lyophilized, and stored at −20 °C for further use.
For the preparation of GelMA/SF-MA hydrogels, GelMA and SF-MA were dissolved in deionized water with 0.75% (w/v) LAP to prepare 20% (w/v) GelMA and 20% (w/v) SF-MA, respectively. Then, 20% (w/v) GelMA and 20% (w/v) SF-MA were mixed with volume ratios of 2:0, 2:1, 1:1, 1:2, 0:2 separately to produce GelMA/SF-MA prepolymer solutions. The original hydrogels were formed by polymerization of the prepolymer solutions under the exposure (45 s) of visible light (405 nm). Subsequently, the hydrogels were soaked in ammonium sulfate solutions (AS, 30 wt%) overnight at room temperature and washed by deionized water for several times to obtain the GelMA/SF-MA hydrogels, which are GelMA, G/F 2:1, G/F 1:1, G/F 1:2, and SF-MA, respectively.
2.3. Characterization of GelMA/SF-MA hydrogels
Chemical structure of SF-MA was identified through 1H NMR (Bruker 400-MHz, German) and FITR (Nicolet 6700, America). To determine the content of protein secondary structure, deconvolution with a Gaussian curve fitting over the amide I band (1700-1600 cm−1) was performed on Peakfit software. The resulting peaks were assigned to different secondary structures respectively: 1637-1615 cm−1 (β-sheet), 1648-1637 cm−1 (random coil), 1661-1646 cm−1 (α-helix), and 1700-1660 cm−1 (β-turn) [35]. X-ray diffraction (XRD) measurements were performed to investigate the crystalline of hydrogels via an Empyrean diffractometer. The XRD patterns were obtained with Cu Kα radiation (λ = 1.5406 Å) at 40 mA and 40 kV in the range of 2θ = 5–60°. To observe the interior microstructures of hydrogels, all hydrogels were quickly frozen in liquid nitrogen and cut to expose the cross-section. After lyophilization and gold spraying, the samples were observed by SEM (JSM7500F, JEOL) operating at 15 kV.
The mechanical properties of GelMA/SF-MA hydrogels were measured by dynamic mechanical analyzer (DMA, Q800). For compressive property, cylinder-shaped hydrogels (8 mm in diameter and 4 mm in height) were tested with a ramp force (4.0 N/min to 16.0 N) in the controlled force mode at room temperature in air. The compressive modulus was calculated as the slope of the 5–20% strain (elastic region) of the stress-strain curve. For tensile property, rectangular sheet-like hydrogels (15 mm in length, 4 mm in width, and 0.5 mm in height) were fixed between two tension grips and stretched at 0.5 mm/min until failure. The tensile modulus was calculated in the 0–20% strain, which is considered as an elastic region.
2.4. Effects of GelMA/SF-MA hydrogels on schwann cells
Before in vitro experiments, prepolymer solutions were sterilized by filtration. The hydrogels were formed in a 48-well plate, then treated with AS and washed with stroke-physiological saline solution under aseptic condition. To evaluate cellular viability and adhesion, Schwann cells (2.5 × 104) were seeded on hydrogels and cultured for 24 h. After trypsinization, adherent cells were collected and incubated with cell counting kit-8 (CCK8, Meilune, China) for 1.5 h, then the OD value at 450 nm was measured by Cytation3 (BioTek, USA). For cell viability and morphology, Schwann cells were stained with Calcein AM (2 μM, KeyGen Biotech, China) and PI (8 μM, KeyGen Biotech, China) for 30 min after culture for 48 h, and observed through a fluorescence microscope (IX73, Olympus). After fixing with 4% paraformaldehyde overnight, gradient dehydration in ethanol, critical point drying and gold spraying, the morphology of Schwann cells on GelMA/SF-MA hydrogels was visualized by SEM (JSM7500F, JEOL). To further investigate the adhesion of Schwann cells on hydrogels, samples were stained with rabbit anti-vinculin antibody (1:100, Abcam, USA), anti-rabbit Alexa Fluor 488 (1:50, Zsbio, China), rhodamine phalloidin (100 nM, Cytoskeleton, USA), and DAPI (1 μg/mL, Invitrogen, USA) after culture for 48 h. Images were taken by confocal laser scanning microscope (CLSM, LSM900, Zeiss). The fluorescence intensity of vinculin, cell area, and length-width ratio were analyzed by ImageJ software.
To evaluate cell migration behavior, Schwann cells (2 × 104) were labeled with CM-Dil (5 μM, Molecular Probes) and seeded on GelMA/SF-MA hydrogels in a 24-well plate. After 12 h, samples were transferred to a live cell imaging system (DeltaVision Ultra, GE Healthcare). Images were acquired every 1 h for 24 h. Video analyses were performed with Imaris software, and the migration trajectory, migration rate, and net displacement were calculated.
To further investigate the interaction between cell and substrate, the mRNA expression levels of genes associated with cell behavior were assessed by quantitative real-time PCR (RT-qPCR). Briefly, Schwann cells (2 × 105) were seeded on GelMA/SF-MA hydrogels in a 6-well plate and harvested after culture for 72 h. The total RNA was extracted using an Animal Total RNA Isolation Kit (Foregene, China), and reverse-transcribed with RT Easy™ II kit (Foregene, China) for first strand cDNA synthesis. RT-qPCR was performed using Easy™ SYBR Green I (Foregene, China) with ABI StepOnePlus (Thermo Scientific, USA). The RT-qPCR process comprised a 3 min step at 95 °C, followed by 95 °C for 10 s, 55 °C for 10 s, and 72 °C for 20 s with 39 cycles. The relative gene expression values were calculated and normalized to GAPDH. The primers were listed in Table 1.
Table 1.
Real-time PCR primers.
| Gene | Primers Froward (3’→5′) | Primers Reverse (5’→3′) |
|---|---|---|
| NCAD | CACCCGGCTTAAGGGTGATT | CGATCCTGTCTACGTCGGTG |
| Integrin β1 | ACTCAGTGAACAGCAACGGTGAAG | TCCAAATCAGCAGCAAGGCAAGG |
| Cdc42 | GCTGTCAAGTATGTGGAGTGT | GGCTCTGGAGATGCGTTCA |
| Rac1 | CAATGCGTTCCCTGGAGAGT | AACACGTCTGTTTGCGGGTA |
| MPZ | CTGCCCTGCTCTTCTCTTCTTTGG | GAGCCCACAGCACCATAGACTTC |
| PMP22 | GGTGCTAGTGTTGCTCTTCGTCTC | CAAGGCGGATGTGGTACAGTTCTG |
| GAPDH | AACTTTGGCATCGTGGAAGG | TGGATGCAGGGATGATGTTCTG |
2.5. Preparation and characterization of 7,8-DHF-OA nanoparticles
7,8-DHF-OA was synthesized via the synthesis route shown in Fig. 1a. Briefly, anhydrous oleyl alcohol (OA, 268 mg, 1 mM) and 4-dimethylaminopyridine (DMAP, 244 mg, 2 mM) were dissolved in 20 mL anhydrous dichloromethane (DCM) at 0 °C under nitrogen. Then triphosgene (104 mg, 0.35 mM) in anhydrous DCM was added dropwise. After stirring at room temperature for 4 h, 7,8-DHF (254 mg, 1 mM) was added into the solution, followed by stirring at room temperature overnight. Finally, 7,8-DHF-OA was obtained by purifying the solution through silica gel plate chromatography (DCM: MeOH = 20:1) with a yield of 65%.
7,8-DHF-OA nanoassemblies were prepared by the nanoprecipitation method. Briefly, DSPE-mPEG2000 (1 mg/mL) and 7,8-DHF-OA (10 mg/mL) were dissolved in ethanol, then 0.2 mL of the solution was added dropwise into 2 mL water with vigorous agitation. The ethanol was removed by evaporation in vacuum to obtain the nanoassemblies. The hydrodynamic size and zeta potential of nanoassemblies were analyzed by a dynamic light scattering (DLS) instrument (Malvern Nano-ZS90). To examine the morphology of nanoassemblies, nanoassemblies were stained with uranyl acetate (0.2%, w/v) and observed by transmission electron microscopy (TEM, H-600, HITACHI).
CCK8 assay was performed to evaluate the cytotoxicity of 7,8-DHF-OA nanoassemblies. For nanoassemblies, Schwann cells (5 × 103) and PC12 cells (5 × 103) were seeded and cultured in a 96-well plate for 24 h, and then incubated with 7,8-DHF-OA nanoassemblies (0, 2.5, 5, 10, 20, 40 μg/mL) for 24 h. For nanoassemblies in hydrogels, GelMA and SF-MA were dissolved in nanoparticle solutions (0, 12.5, 25, 50, 100, 200, 300 μg/mL) and then photocured to form hydrogels in a 48-well plate. After treatment with 30% (w/v) AS, Schwann cells (1 × 104) and PC12 cells (1 × 104) were seeded and cultured on the hydrogels for 24 h. Finally, cells were incubated with CCK8 for 1.5 h and the OD values were measured as previously described.
2.6. Fabrication and characterization of functional nerve conduit
The nerve conduits were fabricated by continuous DLP printing as previous reports [16,17]. Briefly, GelMA (10%, w/v), SF-MA (10%, w/v), LAP (0.75%, w/v), 7,8-DHF-OA nanoassemblies (200 μg/mL), and vitamin B12 (0.1%, w/v) were dissolved in deionized water to prepare the prepolymer solution. 3D structure of NGCs was designed by Solidworks software and sliced into a series of images. The images were uploaded to the digital micromirror device chip to expose the designed patterns (405 nm) to the prepolymer solution. After a 3 min continuous printing process, the obtained nerve conduits (2.5 mm of outer diameter, 1.5 mm of inner diameter, 15 mm of length) were immersed into 30% (w/v) AS for physical crosslinking and then washed with deionized water. The morphology of the nerve conduits was examined by SEM (JSM7500F, JEOL) after a process of gradient dehydration in ethanol, critical point drying, cutting off, and gold spraying.
To assess the suture retention strength of NGCs, rectangular sheet-like GelMA/SF-MA hydrogels (15 mm in length, 10.5 mm in width, and 0.5 mm in thickness) were prepared. One end of the hydrogel was pierced through at 2 mm from the edge, while the other end was fixed with a clamp. The samples were stretched at the speed of 5 mm/min until failure by DMA (Q800, TA Instruments).
To investigate the enzymatic degradation properties in vitro, GelMA/SF-MA samples were incubated in 0.5 mL protease XIV solution (1.75 U/mL, pH 7.4) at 37 °C. At the predefined timepoints, the hydrogels were weighed. The degradation rate was calculated by ratio of the final hydrated weight to the original hydrated weight.
The release kinetics of 7,8-DHF from nanoassemblies and nerve conduits were investigated. 7,8-DHF-OA nanoassemblies (10 μg/mL) and nerve conduits were incubated in PBS at 37 °C, and PBS was refreshed at the designed time point. The released 7,8-DHF was measured by high performance liquid chromatography (HPLC, 1260, Angilent).
To investigate the efficacy of 7,8-DHF released from nerve conduits in axon growth in vitro, PC12 cells were cultured on the 7,8-DHF-OA nanoassemblies (0, 50, 100, 200 μg/mL) loaded GelMA/SF-MA hydrogels for 48 h. The samples were stained with rhodamine phalloidin (100 nM, Cytoskeleton, USA) and DAPI (1 μg/mL, Invitrogen, USA) for F-actin and nucleus, respectively. Images were taken by CLSM (LSM900, Zeiss). With at least 100 cells randomly chosen from the images per group, the neurite length was quantified by ImageJ software. Neurite length was defined as the distance from the end of a neurite to the cell body.
2.7. Recovery evaluation of functional nerve conduit in vivo
SD rats (male, 200–220 g) were divided into 5 groups randomly (10 in each group), including sham-operation (Sham) group, autograft (Auto) group, SF-MA conduit (SFC) group, GelMA/SF-MA conduit (GFC) group and nanoassemblies-loaded GelMA/SF-MA conduits (GFNC) group. After anesthesia, the sciatic nerve in right leg was surgically exposed. For Sham group, there was no treatment with the sciatic nerve. For other groups, a 12 mm nerve defect was constructed by removing a mid-thigh nerve segment. The conduits were implanted to bridge the gap by suturing with the two stumps of injured nerves via 8–0 absorbable vicryl sutures. While in the Auto group, 12-mm resected nerve was re-sutured with the two stumps in reversed direction.
To evaluate the function recovery of the injured nerves, the sciatic nerves in right leg of all rats were exposed 16 weeks after surgery. Electrophysiological examination was performed with an electromyograph machine (Nuocheng, China). The bipolar stimulating electrodes were contacted with the two ends of the injured nerves, and the monopolar recording electrode was placed in the gastrocnemius muscle. With an electrical stimulation of 10 mA, nerve conduction velocity (NCV) and compound muscle action potential (CMAP) were recorded.
Gastrocnemius muscles on the normal side and injured side of all rats were harvested 16 weeks after implantation. The wet weight of the gastrocnemius muscle was immediately determined to calculate the wet weight ratio, in terms of mass ratio of the injured side to the normal side. Subsequently, the tissues were fixed in 10% formalin, then dehydrated in ethanol, and embedded in paraffin to prepare tissue sections (5 μm). The sections were stained with hematoxylin and eosin (H&E), and the diameter of muscle fibers was measured by ImageJ software.
For histological evaluation, the distal segments of regenerated sciatic nerves of each group were harvested 16 weeks post-operation to prepare paraffin sections as described above. The sections were stained with H&E and Luxol fast blue (LFB), and then observed by an automatic digital slide scanner (Pannoramic MIDI, 3DHISTECH). To investigate the morphology of the regenerated nerves, the regenerated nerves were fixed in glutaraldehyde (2.5%), then stained with osmium tetroxide (1%). After embedded in resin (Epon 812 epoxy), the ultrathin sections with thickness of 80 nm were made. Followed by staining with lead citrate and uranyl acetate, the sections were observed by TEM (HT7700, Hitachi). The diameter of myelinated nerve fibers, thickness of myelin sheath, and number of myelin sheath layer were measured by ImageJ software. For the diameter of myelinated nerve fibers, the long and short diameter of each myelinated nerve fiber were measured by ImageJ software. Images from three random fields per animal (n = 3 animal per group) were analyzed. To evaluate the regeneration of the injured nerves, the paraffin sections were incubated with rabbit GAP-43 antibody (1:100, GeneTex, USA), anti-rabbit TRITC (1:50, Zsbio, China), and DAPI (1 μg/mL, Invitrogen, USA), followed by observation with CLSM (LSM900, Zeiss).
2.8. Statistical analysis
All statistical analysis was performed with Graphpad Prism 7 (Graphpad Software Inc, USA). The data was analyzed with one-way ANOVA, followed by Tukey's post hoc multiple comparison test. All data were expressed as mean values ± standard deviation (SD). P < 0.05 was considered as statistically significant (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001).
3. Results
3.1. Characterization of GelMA/SF-MA hydrogels
GelMA and SF-MA were synthesized as the previous reports [22,34], respectively (Supporting information, Figure S1). To develop hydrogels with proper physical and biological properties for nerve repair, SF-MA was chemically crosslinked with GelMA, and AS treatment was further applied to induce physical crosslinking (Fig. 2a). Thus, chemically and physically double-crosslinked GelMA/SF-MA hydrogels (GelMA, G/F 2:1, G/F 1:1, G/F 1:2, and SF-MA) were developed. The chemical structure of GelMA/SF-MA hydrogels was characterized by FTIR (Figure S2a, 3a-k). The original GelMA/SF-MA hydrogels exhibited more β-sheet structure than GelMA due to the introduction of SF-MA. After AS treatment, amide-I bands of GelMA/SF-MA hydrogels displayed blue-shifted narrow peaks with higher β-sheet conformation compared to the corresponding original hydrogels, and the β-sheet conformation content also increased with higher SF-MA concentration. It could be attributed to the transformation of SF-MA from a random-coil into the β-sheet structure. XRD spectra (Figure S2b) showed that, all the original hydrogels were amorphous with broad and obtuse peaks. After treatment with AS, G/F 1:1, G/F 1:2, and SF-MA hydrogel presented narrowed peaks at 20.6°, which could be assigned to the β-sheet crystalline structure of SF-MA induced by AS [36]. In addition, after treating with AS, the porous structure of G/F 2:1, G/F 1:1, G/F 1:2, and SF-MA was more compact than that of GelMA (Figure S4), which may be associated with the irreversible dehydration of SF-MA induced by AS.
Fig. 2.
Mechanical properties of GelMA/SF-MA hydrogels. (a) Double-crosslinked GelMA/SF-MA hydrogels were prepared by crosslinking GelMA with SF-MA and further treatment with AS. (b) The process of compression test for GelMA/SF-MA hydrogels with different ratios. (c) Representative compressive stress-strain curves for GelMA/SF-MA hydrogels. (d) Compressive elastic modulus at 20% strain. (e) Representative tensile stress-strain curves of GelMA/SF-MA hydrogels. (f) Tensile elastic modulus at 20% strain.
The mechanical properties of GelMA/SF-MA hydrogels were further characterized through compressive and tensile tests. GelMA was brittle and crushed with increasing compress stress. Compared with GelMA, GelMA/SF-MA hydrogels and SF-MA hydrogels were strong enough to support the compress stress of 16 N, which could return to their original shape after the stress was removed (Fig. 2b). A higher concentration of SF-MA could result in increased compressive strain and compressive modulus (Fig. 2c and d). At the same time, the tensile strength and breaking elongation of GelMA were significantly enhanced by chemical and physical double-crosslinking with SF-MA (Fig. 2e). The tensile modulus of GelMA was 0.027 ± 0.001 kPa, while that of G/F 2:1, G/F 1:1, G/F 1:2 and SF-MA was 0.61 ± 0.03 MPa, 1.03 ± 0.04 MPa, 1.26 ± 0.09 MPa, 3.51 ± 0.22 MPa, respectively (Fig. 2f). The results demonstrated that the introduction of SF-MA could significantly improve the compressive and tensile properties of GelMA, and form tough and elastic GelMA/SF-MA hydrogels.
3.2. Effects of GelMA/SF-MA hydrogels on schwann cells
As the principle glial cell, Schwann cell plays an essential role in peripheral nerve recovery. After PNS injury, Schwann cells proliferate and migrate rapidly to form Büngner bands for axons regrowth, and further wrap around the regenerated axons to form compact myelin sheath [37]. To select the optimal GelMA/SF-MA hydrogel for nerve repair, we investigated the effects of GelMA/SF-MA hydrogels with different ratios on proliferation, morphology, adhesion, and migration of Schwann cells.
The cell number of Schwann cells on G/F 1:1 hydrogel was higher than that of GelMA, G/F 1:2, and SF-MA hydrogels 24 h after seeding (Figure S5). The cell morphology was investigated by Calcein/PI assay and SEM (Figure S6). Schwann cells exhibited a flat oval shape and gathered to form clusters on GelMA hydrogel, while presenting a stereoscopic oval shape with poor substrate attachment on SF-MA hydrogel. Compared to GelMA and SF-MA hydrogels, more flat spindle shaped Schwann cells on G/F 2:1, G/F 1:1, and G/F 1:2 hydrogels were observed. To further investigate cell behavior of extension and adhesion, vinculin immunofluorescence was performed. Statistical analysis showed that the average cell area and cell length-width ratio on G/F 1:1 hydrogel were largest among all the hydrogels (Fig. 3b and c). Vinculin is an important actin-binding protein in cell-matrix adhesion [38]. The average fluorescence intensity of vinculin on G/F 1:1 hydrogel had no statistical difference with GelMA hydrogel but was significantly higher than that of G/F 2:1, G/F 1:2 and SF-MA hydrogels (Fig. 3a, d). GelMA was beneficial for cell adhesion but not for cell expanding, due to its high GRD content and suboptimal mechanical property. With a lower RGD concentration but proper stiffness, G/F 1:1 hydrogel was also favorable for cell attachment and extension. Thus, the vinculin expression of Schwann cells on G/F 1:1 hydrogel was comparable to that of GelMA. The results indicated that G/F 1:1 is optimal for cell adhesion and cytoskeleton due to the effect of both stiffness and adhesive property.
Fig. 3.
Morphology and adhesion of Schwann cells on GelMA/SF-MA hydrogels. (a) Immunofluorescent staining images of Schwann cells with nuclei (blue), vinculin (green), and actin (red) after culture for 48 h. Scale bar, 20 μm. (b–d) Cell area (b), cell length-width ratio (c), and average fluorescence intensity of vinculin (d) of Schwann cells on various substrates after culture for 48 h. These data are shown as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The motility of Schwann cells is crucial for guiding the elongation of axons and reconnection of the two injured stumps. A time-lapse microscopy was used to investigate the migration of Schwann cells. The results showed that Schwann cells on all hydrogels migrated randomly without direction, whereas the migration trajectories of Schwann cells on G/F 1:1 were longer than those of other groups (Fig. 4a). The mean migration rate (Fig. 4b) of G/F 1:1 (11.58 ± 4.76 μm/h) was significantly higher than that of GelMA (7.47 ± 2.56 μm/h, P < 0.001), G/F 2:1 (9.95 ± 3.27 μm/h, P < 0.01), G/F 1:2 (9.99 ± 4.11 μm/h, P < 0.01), and SF-MA (7.92 ± 5.05 μm/h, P < 0.001). The largest net displacement of Schwann cells was found on G/F 1:1 hydrogel (Fig. 4c).
Fig. 4.
Migration and gene expression of Schwann cells on GelMA/SF-MA hydrogels. (a) Migration trajectories of Schwann cells on various substrates within 24 h by time-lapse microscopy. Each color represents an individual cell. Scale bar, 30 μm. (b–c) Statistical results of cell migration rate and net displacement for Schwann cells on various substrates within 24 h. (d) RT-qPCR analysis for gene expression levels of NCAD, Integrin β1, Cdc42, Rac1, MPZ, and PMP22 for Schwann cells on various substrates after culture for 72 h. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To further understand the cell-matirx interaction of Schwann cells, we analyzed the gene expressions of some related proteins through RT-qPCR. Genes associated with adhesion (NCAD, Integrin β1) and migration (Cdc42, Rac1) were significantly upregulated on G/F 1:1 hydrogel compared to other hydrogels (Fig. 4d), which was consistent with the adhesion and migration behavior of Schwann cells. Importantly, the expression level of myelination-related genes (MPZ, PMP22) on G/F 1:1 hydrogel was the highest among all the groups (Fig. 4d), indicating that G/F 1:1 hydrogel has potentials to promote the myelin sheath formation for nerve regeneration.
These results revealed that due to the effect of adhesion performance and mechanical property, G/F 1:1 hydrogel could effectively regulate the behaviors of Schwann cells, and provide a more favorable environment for cell adhesion, spreading, elongation, and migration by upregulating the related gene expressions. Therefore, G/F 1:1 was further applied for the construction of nerve conduits.
3.3. Fabrication and characterization of functional nerve conduits
7,8-DHF is a small molecular TrkB agonist with potential to promote nerve regeneration after injury. In this study, functional NGCs were constructed by encapsulating 7,8-DHF within G/F 1:1 hydrogel. Firstly, the lipophilic prodrug (7,8-DHF-OA) was synthesized by conjugating 7,8-DHF with oleic acid (OA) (Figure S7a). 7,8-DHF-OA could self-assemble into nanoparticles when it was dispersing in water (Fig. 1a), and the drug loading capacity was about 42%. The average diameter and zeta potential of 7,8-DHF-OA nanoassemblies were 92.36 ± 0.78 nm and −26.4 ± 3.11 mV, respectively (Figure S7b). The TEM image confirmed that the 7,8-DHF-OA nanoassemblies were monodisperse with uniform particle size (Fig. 5a). Then, the cytotoxicity of 7,8-DHF-OA nanoassemblies encapsulated in G/F 1:1 hydrogel was investigated. When the concentration was less than 200 μg/mL, 7,8-DHF-OA nanoassemblies were noncytotoxic to Schwann cells and PC12 cells (Figure S7c-d). Finally, the bioink was developed by dispersing 7,8-DHF-OA nanoassemblies (200 μg/mL) in G/F 1:1, and GFNCs were fabricated rapidly through the continuous DLP-based 3D bioprinting system (Fig. 5b and c).
Fig. 5.
Fabrication and characterization of GelMA/SF-MA conduits loading with 7,8-DHF. (a) TEM image of 7,8-DHF-OA nanoassemblies. Scale bar, 50 μm. (b) Schematic illustration of continuous DLP 3D printing technology for nerve guidance conduit. (c) The photograph of the conduit. Scale bar, 5 mm. (d) Representative SEM images of the 3D printed nerve conduits. Scale bar, 200 μm. (e) Degradation of GelMA/SF-MA hydrogel conduit. (f) The cumulative release of 7,8-DHF from GelMA/SF-MA conduits. (g) Representative images of PC12 cells on GelMA/SF-MA hydrogels loaded with 0 μg/mL (ⅰ), 50 μg/mL (ⅱ), 100 μg/mL (ⅲ), and 200 μg/mL (ⅳ) 7,8-DHF-OA nanoassemblies, respectively. Red is rhodamine phalloidin, blue is DAPI, and scale bar represents 100 μm. (h) Average neurite length of PC12 cells on GelMA/SF-MA hydrogels loaded with 7,8-DHF-OA nanoassemblies after culture for 48 h. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
SEM images showed that the conduits were hollow with uniform wall thickness (Fig. 5d). The outer diameter and inner diameter of the observed conduit were 1.7 mm and 1.1 mm, respectively. Due to the shrinking during the dehydration and drying process, the conduit observed in SEM was smaller than its original shape. After incorporating the prodrug nanoassemblies into the hydrogel, the tensile modulus of the conduit was similar to that of the G:F 1:1 hydrogel, which was not significantly lower than that of rat sciatic nerve (4.03 ± 1.36 MPa). The suture retention test demonstrated that the conduit was strong enough for surgical suture process (Figure S8). At the displacement of 1 mm and stress of 0.18 MPa, the crack appeared but did not propagate. While the displacement was 1.2 mm, the crack propagated rapidly, and the samples fractured. Besides, the enzymatic degradation study indicated that the GFNCs were degradable in vitro (Fig. 5e). Although the conduit degraded after 2 weeks in the protease XIV solution, the degradation would be slower under the physiological condition in vivo. The biodegradable GFNCs with proper mechanical properties would support a physical channel for nerve regeneration.
The release profile of 7,8-DHF from GFNCs in PBS buffer was studied by HPLC. The drug was rapidly released from 7,8-DHF-OA nanoassemblies, which might be due to the increased solubility of 7,8-DHF in alkaline solution (Figure S7e). However, after integrating the nanoassemblies with GelMA/SF-MA, the drug was slowly released from the conduits (Fig. 5f) through the interaction with the molecular network of hydrogel. Even though the drug release rate in GFNC was about 40% in PBS, more drug would be gradually released from the conduit with its degradation in vivo. The efficacy of 7,8-DHF released from the GFNCs for nerve regeneration in vitro was further investigated. PC12 cells was used as a model to evaluate the drug effect on neurite outgrowth. The morphology of PC12 cells was shown in Fig. 5g. When the concentration of 7,8-DHF-OA nanoassemblies were 0, 50, 100, and 200 μg/mL, the axon lengths of PC12 cells were 48.73 ± 14.64, 58.35 ± 20.84, 68.53 ± 27.70, and 86.00 ± 32.56 μm, respectively (Fig. 5h). The results indicated that 7,8-DHF released from the GFNCs could promote neurite elongation concentration-dependently. The prepared conduits could not only provide a physical channel for axonal direction but also sustainedly release 7,8-DHF to enhance nerve regeneration.
3.4. Functional evaluation in vivo
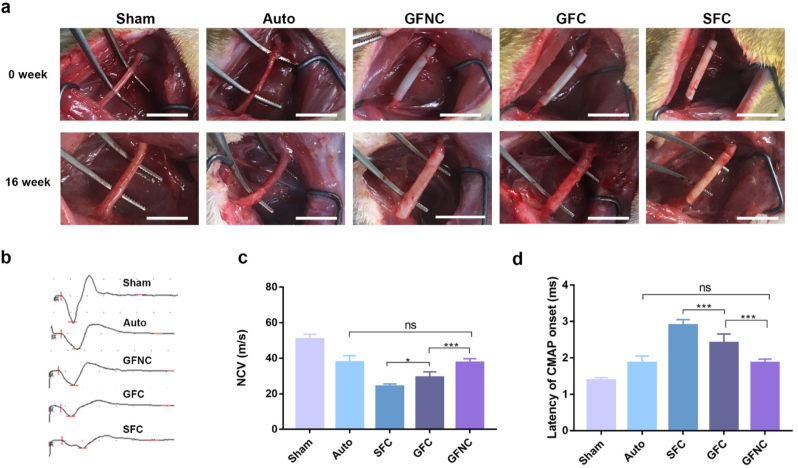
To evaluate the therapeutic efficacy of the functional NGCs in vivo, GelMA/SF-MA conduits were sutured with sciatic nerves to bridge a 12 mm defect. Due to the different surgical methods for implantation, GelMA conduit was not shown in this study. SD rats were divided into five groups randomly. After 16 weeks, the implanted sites were surgically exposed (Fig. 6a). The conduits still maintained the channel structure without collapse. The distal and proximal stumps of the injured nerves were reconnected in all experimental groups. Electrophysiological analysis was performed to assess the function recovery (Fig. 6b–d). The NCV value in GFNC group (37.6 ± 2.1 m/s) was significantly higher than that in GFC group (29.2 ± 3.1 m/s, P < 0.001), while the NCV value in GFC group was also higher than that of SFC group (24.2 ± 1.4 m/s, P < 0.05). There was no significant difference between GFNC group and Auto group (37.8 ± 3.6 m/s, P > 0.05). The latency CMAP onset of GFNC group was clearly shorter than that of GFC group and SFC group, while comparable to Auto group. In addition, gastrocnemius muscles were harvested and analyzed to assess the amyotrophy (Figure S9). The wet muscle weight ratio increased in GFNC group when compared to GFC group and SFC group. The reinnervation of gastrocnemius muscles was similar between GFNC group and Auto group. Our results revealed that GelMA/SF-MA and 7,8-DHF could effectively improve the functional recovery of injured nerves.
Fig. 6.
Function recovery of the regenerated sciatic nerves 16 weeks after surgery. (a) Representative photographs of the surgery sites at 0 week and 16 week. Scale bar, 10 mm. (b) Representative CMAP recordings of the regenerated nerves. (c–d) The value of NCV (c) and latency of CMAP onset (d) at the injured sites. These data are shown the mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
The distal segments of regenerated nerves were dissected for morphological and histological analysis. The H&E staining confirmed that the regenerated nerves were well-organized in all groups (Fig. 7a). The LFB staining identified the formation of well-aligned myelinated axons (Fig. 7b). The myelinated axons were further observed by TEM at higher magnification (Fig. 7c). The average diameter of myelinated nerve fibers increased in the order of SFC group (2.5 ± 1.2 μm), GFC group (3.9 ± 1.2 μm), and GFNC group (5.2 ± 1.4 μm) (Fig. 7d). Similarly, the thickness of myelin sheath was found to be the best in GFNC group, followed by GFC group and SFC group (Fig. 7e). No significant difference was found between GFNC group and Auto group in the average diameter of myelinated fiber and thickness of myelin sheath. Meanwhile, the number of myelin sheath layer in GFNC group was notably higher than both GFC group and SFC group, yet closed to that of Auto group (Fig. 7f).
Fig. 7.
Histological analysis for the distal segments of the regenerated nerves 16 weeks after surgery. (a–b) H&E staining (a) and LFB staining (b) of the regenerated nerves. (c) Representative TEM images for observation of the regenerated myelinated axons. (d–f) The quantitative analysis for diameter of myelinated nerve fibers (d), thickness of myelin sheath (e), and number of myelin sheath layer (f). Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Furthermore, growth associated protein 43 (GAP-43) expressed by regenerative axons and Schwann cells, was monitored by immunofluorescence staining (Figure S10). The expression of GAP-43 in GFNC group was impressively higher than all the other groups, indicating a better axon elongation and Schwann cell proliferation. The GAP-43 expression also increased in the order of Auto group, SFC group, and GFC group. Lastly, no pathological characteristics, i.e., edema, inflammation, degeneration and necrosis were detected in the main organs of any experimental groups (Figure S11). These results indicated that GelMA/SF-MA conduits with 7,8-DHF release could safely facilitate axon elongation and remyelination for functional recovery of injured nerves.
4. Discussions
Functional recovery of injured peripheral nerves still remains a great challenge in regeneration medicine. The efficacy of NGCs for functional recovery highly depends on the repairing microenvironment, which is related to conduit structure, scaffold material and bioactive molecules such as neurotrophic factors and drugs [39]. To improve the efficacy of NCGs, researchers have developed for promoting nerve regeneration. Biomimetic multichannel conduits have been designed to control axonal dispersion for increasing appropriate target reconnection [[40], [41], [42]]. Growth factors, drugs, and cells have also been introduced into NCGs to enhance axon regeneration and remyelination [10,[43], [44], [45], [46]]. However, these strategies have not provided optimal environments for nerve regeneration and require further improvements. In this work, we firstly developed an elastic hydrogel conduit with prodrug nanoassemblies by DLP 3D printing to provide a regenerative microenvironment for nerve regeneration. The bioactive hydrogel can not only provide physical support for peripheral nerve regeneration but also regulate the behavior of Schwann cells for remyelination. Meanwhile, the sustained drug release from the conduit can promote neurite outgrowth for axon regeneration. This strategy of bioactive biomaterials with drug release is advantageous in building beneficial microenvironment for nerve regeneration.
With the capacity of manufacturing complex structures, DLP based 3D printing is promising in fabrication of customized NGCs. In recent years, various photocurable derivatives of natural polymers have been developed, which are promising for fabrication of hydrogel scaffolds by DLP 3D printing, including gelatin [20], silk fibroin [22], collagen [47], alginate [48], hyaluronic acid [49], and elastin [50]. However, most of the photocurable biomaterials are unsatisfactory for mimicking the properties of natural extracellular matrix to promote nerve repair, especially for the mechanical strength and biocompatibility. In this study, we developed GelMA/SF-MA hydrogels with good mechanical property and biocompatibility by chemical and physical double-crosslinking of the two monomers. In previous studies, GelMA has been used to fabricate conduits by DLP 3D printing to bridge nerve gaps. However, the mechanical properties of these conduits are not strong enough to bear in vivo physiological stress to support nerve regeneration [14,17,51]. The in vivo implantation of these conduits was performed with surgical glue or other assisting methods. In this study, by crosslinking with SF-MA and further treatment of AS, the mechanical property of GelMA/SF-MA hydrogel has been strengthened to match with the tensile strength of sciatic nerves, and GelMA/SF-MA hydrogel can be sutured with nerves for implantation. Although silk fibroin has attracted much attention over the recent decades in the field of nerve tissue engineering, its poor cell affinity is still a limitation for clinical application [[52], [53], [54]]. In our work, the RGD sequence of GelMA can endow the GelMA/SF-MA hydrogels with excellent cell-matrix interaction to improve cell adhesion. Therefore, we report a suturable and cell-adhesive hydrogel by DLP based 3D printing for the first time, which is promising for application in personalized nerve regenerative medicine.
The physical and biochemical properties of scaffold material play an important role in building a supportive microenvironment for nerve regeneration [51]. However, the impact of the properties of GelMA/SF-MA hydrogels on nerve regeneration is still unclear. Schwann cell is the principal glial cell supporting neurons in the peripheral nervous system [55]. In this study, we have investigated the effect of hydrogel stiffness and biocompatibility on Schwann cell behavior. We found that G/F 1:1 hydrogel with moderate stiffness exhibited positive effect on the behaviors of Schwann cells by upregulating the related gene expression. Although the optimal stiffness for Schwann cells may vary with biomaterials, the effect of mechanical properties is consistent with the previous report [56]. Besides, all GelMA/SF-MA hydrogels containing RGD sequence displayed better cell adhesion compared with SF-MA. Previous studies have reported that cell adhesion and extension is dependent on RGD concentration in a non-linear relationship [57,58]. Both the stiffness and adhesive property are important for constructing an optimal microenvironment for Schwann cells, in which the stiffness may be dominant. Thus, we develop a bioactive GelMA/SF-MA hydrogel for nerve regeneration, which may provide a new parameter for designing scaffolds in nerve tissue engineering.
Although the GelMA/SF-MA hydrogels may help build a favorable microenvironment for nerve regeneration, pharmacological enhancement is also important for improving function restoration of injured nerves. 7,8-DHF is a small-molecule TrkB agonist with neurotrophic activities, which can mimic the function of BDNF [29]. English et al. found systemic treatment of 7,8-DHF could promote axon regeneration in cut peripheral nerves [30]. However, the non-specific delivery of 7,8-DHF and the associated side-effects may prevent its further application to bridge large nerve defects. Incorporating drug into a conduit can realize a local delivery to the injured sites and reduce the systemic toxicity [59]. Nevertheless, directly encapsulating hydrophobic 7,8-DHF into conduits might result in its aggregation and burst release. Previously, various attempts have been made to use micro/nano-carriers to overcome hydrophobicity of some drugs and sustain release drug from conduit, such as PLGA microspheres [60], F127–CHO micelles [61], and MPEG-PCL nanoparticles [17,18]. However, the drug-loading capacity (2–10%) of these drug carriers still needs to be improved. During the regeneration process for peripheral nerve injury, sustained and prolonged release of drug with appropriate concentration is essential for facilitating nerve recovery [51,62]. In this study, we developed 7,8-DHF prodrug nanoassemblies with good water solubility. Conjugating with a hydrophobic unsaturated fatty acid, the lipophilic prodrug can self-assemble into nanoassemblies despite the lack of a typical amphiphilic structure, leading to an impressive higher drug loading capacity (42%). Besides, the concentration of prodrug nanoassemblies in hydrogels has been optimized in vitro to maximize its effectiveness with safety. After encapsulating the nanoassemblies into GelMA/SF-MA hydrogel, 7,8-DHF could be sustainedly released from the conduit to promote neurite outgrowth. Therefore, we developed a novel prodrug nanoassemblies encapsulated hydrogel conduit, which is potential for local drug delivery in long-term recovery of injured nerves. The in vitro and in vivo results demonstrated that the GelMA/SF-MA scaffolds and the sustained drug release can provide a supportive environment for promoting nerve regeneration and remyelination. This elastic hydrogel conduit with 7,8-DHF release provides a new strategy for the treatment of long-gap peripheral nerve injury.
5. Conclusions
In this study, we designed and prepared a new elastic and bioactive GelMA/SF-MA hydrogel conduit encapsulating with 7,8-DHF prodrug nanoassemblies by DLP based 3D printing. The GelMA/SF-MA scaffold could significantly promote Schwann cell adhesion, proliferation, and migration through upregulating the related gene expressions. The drug loaded conduit could sustainedly release 7,8-DHF to improve the axonal elongation of PC12 cells. By providing a favorable microenvironment for regenerated nerves, the conduit exhibited favorable functional recovery of injured nerves via improvement of axon regeneration and remyelination. This novel drug-loaded nerve conduit would provide a new idea for clinical therapy of peripheral nerve injury.
CRediT author statement
Wenbi Wu: Methodology, Investigation, Formal analysis, Conceptualization, Writing - original draft, Writing - review & editing. Yinchu Dong: Investigation, Formal analysis, Writing - original draft. Haofan Liu: Methodology, Investigation, Writing - review & editing. Xuebing Jiang: Investigation, Validation. Ling Yang: Methodology. Jing Luo: Investigation. Yu Hu: Methodology. Maling Gou: Conceptualization, Funding acquisition, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation (32271468), Sichuan Science and Technology Program (2021JDTD0001) and Natural Science Foundation of Sichuan Province (2022NSFSC1280). The authors would like to thank Dr. Shuping Zheng from the Analytical and Testing Center, Sichuan University, P. R. China for the SEM characterization. The authors also appreciated Dr. Ruiqi Dong from National Engineering Research Center for Biomaterials, P. R. China for the technical assistance with DMA test.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2023.100652.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Liu B., Xin W., Tan J.R., et al. Myelin sheath structure and regeneration in peripheral nerve injury repair. Proc. Natl. Acad. Sci. U. S. A. 2019;116(44):22347–22352. doi: 10.1073/pnas.1910292116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H., Zhang H., Wang H., et al. Natural proteins-derived asymmetric porous conduit for peripheral nerve regeneration. Appl. Mater. Today. 2022;27 doi: 10.1016/j.apmt.2022.101431. [DOI] [Google Scholar]
- 3.Scheib J., Höke A. Advances in peripheral nerve regeneration. Nat. Rev. Neurol. 2013;9(12):668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 4.Parker B.J., Rhodes D.I., O'Brien C.M., et al. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: a commercial perspective. Acta Biomater. 2021;135:77–99. doi: 10.1016/j.actbio.2021.08.052. [DOI] [PubMed] [Google Scholar]
- 5.Lu S., Chen W., Wang J., et al. Polydopamine-decorated PLCL conduit to induce synergetic effect of electrical stimulation and topological morphology for peripheral nerve regeneration. Small Methods. 2023 doi: 10.1002/smtd.202200883. [DOI] [PubMed] [Google Scholar]
- 6.Yang S., Zhu J., Lu C., et al. Aligned fibrin/functionalized self-assembling peptide interpenetrating nanofiber hydrogel presenting multi-cues promotes peripheral nerve functional recovery. Bioact. Mater. 2022;8:529–544. doi: 10.1016/j.bioactmat.2021.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon A.R., Jariwala S.H., Bilis Z., et al. Bridging the gap in peripheral nerve repair with 3D printed and bioprinted conduits. Biomaterials. 2018;186:44–63. doi: 10.1016/j.biomaterials.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Petcu E.B., Midha R., McColl E., et al. 3D printing strategies for peripheral nerve regeneration. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aaaf50. [DOI] [PubMed] [Google Scholar]
- 9.Liu K., Yan L., Li R., et al. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv. Sci. 2022;9(12) doi: 10.1002/advs.202103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y., Wu W., Liu H., et al. 3D printing of functional nerve guide conduits. Burns Trauma. 2021;9 doi: 10.1093/burnst/tkab011. tkab011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu K., Qian Y., Gong J., et al. Biofabrication of aligned structures that guide cell orientation and applications in tissue engineering. Bio-Design and Manufacturing. 2021;4(2):258–277. doi: 10.1007/s42242-020-00104-5. [DOI] [Google Scholar]
- 12.Gou M., Qu X., Zhu W., et al. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat. Commun. 2014;5:3774. doi: 10.1038/ncomms4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koffler J., Zhu W., Qu X., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25(2):263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W., Tringale K.R., Woller S.A., et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today. 2018;21(9):951–959. doi: 10.1016/j.mattod.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pateman C.J., Harding A.J., Glen A., et al. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials. 2015;49:77–89. doi: 10.1016/j.biomaterials.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Tao J., Liu H., Wu W., et al. 3D-printed nerve conduits with live platelets for effective peripheral nerve repair. Adv. Funct. Mater. 2020;30(42) doi: 10.1002/adfm.202004272. [DOI] [Google Scholar]
- 17.Tao J., Zhang J., Du T., et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019;90:49–59. doi: 10.1016/j.actbio.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Xu X., Tao J., Wang S., et al. 3D printing of nerve conduits with nanoparticle-encapsulated RGFP966. Appl. Mater. Today. 2019;16:247–256. doi: 10.1016/j.apmt.2019.05.014. [DOI] [Google Scholar]
- 19.Zhang J., Chen Y., Huang Y., et al. A 3D-printed self-adhesive bandage with drug release for peripheral nerve repair. Adv. Sci. 2020;7(23) doi: 10.1002/advs.202002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue K., Trujillo-de Santiago G., Alvarez M.M., et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichol J.W., Koshy S.T., Bae H., et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.H., Yeon Y.K., Lee J.M., et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018;9(1):1620–1633. doi: 10.1038/s41467-018-03759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong H., Seo Y.B., Kim D.Y., et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- 24.Hasturk O., Jordan K.E., Choi J., et al. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manoukian O.S., Rudraiah S., Arul M.R., et al. Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: in vivo structural and functional assessment. Bioact. Mater. 2021;6(9):2881–2893. doi: 10.1016/j.bioactmat.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y., Chen Z., Wang H., et al. Conductive nerve guidance conduits based on morpho butterfly wings for peripheral nerve repair. ACS Nano. 2022;16(2):1868–1879. doi: 10.1021/acsnano.1c11627. [DOI] [PubMed] [Google Scholar]
- 27.Pi W., Zhang Y., Li L., et al. Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration. Biofabrication. 2022;14(3) doi: 10.1088/1758-5090/ac57a6. [DOI] [PubMed] [Google Scholar]
- 28.Jang S.W., Liu X., Yepes M., et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. U.S.A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang S.W., Liu X., Yepes M., et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. U. S. A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.English A.W., Liu K., Nicolini J.M., et al. Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves. Proc. Natl. Acad. Sci. U. S. A. 2013;110(40):16217–16222. doi: 10.1073/pnas.1303646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Wang S., Huang Y., et al. Light-activated drug release from prodrug nanoassemblies by structure destruction. Chem. Commun. 2019;55(87):13128–13131. doi: 10.1039/c9cc06673j. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Kang T., Wu Y., et al. Carbonate esters turn camptothecin-unsaturated fatty acid prodrugs into nanomedicines for cancer therapy. Chem. Commun. 2018;54(16):1996–1999. doi: 10.1039/c8cc00639c. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Zhang J., Liu X., et al. Noninvasive in vivo 3D bioprinting. Sci. Adv. 2020;6(23) doi: 10.1126/sciadv.aba7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirahama H., Lee B.H., Tan L.P., et al. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci. Rep. 2016;6 doi: 10.1038/srep31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C., Guo H., Vu V.C., et al. Thermoplastic moulding of regenerated silk. Nat. Mater. 2020;19(1):102–108. doi: 10.1038/s41563-019-0560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao W.Q., Li J.L., Qu X.H., et al. Cell-laden interpenetrating network hydrogels formed from methacrylated gelatin and silk fibroin via a combination of sonication and photocrosslinking approaches. Materials Science & Engineering C-Materials for Biological Applications. 2019;99:57–67. doi: 10.1016/j.msec.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D., Yao Y., Duan Y., et al. Surface-anchored graphene oxide nanosheets on cell-scale micropatterned poly(d,l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl. Mater. Interfaces. 2020;12(7):7915–7930. doi: 10.1021/acsami.9b20321. [DOI] [PubMed] [Google Scholar]
- 38.Motta C.M.M., Endres K.J., Wesdemiotis C., et al. Enhancing Schwann cell migration using concentration gradients of laminin-derived peptides. Biomaterials. 2019;218:119335. doi: 10.1016/j.biomaterials.2019.119335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods. Acta Biomater. 2020;106:54–69. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Yao L., de Ruiter G.C., Wang H., et al. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials. 2010;31(22):5789–5797. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Cheng Y., Wang H., et al. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater. 2020;117:180–191. doi: 10.1016/j.actbio.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Ye W.S., Li H.B., Yu K., et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020;192 doi: 10.1016/j.matdes.2020.108757. [DOI] [Google Scholar]
- 43.Yu M., Gu G., Cong M., et al. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021;134:190–203. doi: 10.1016/j.actbio.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Wu P., Tong Z., Luo L., et al. Comprehensive strategy of conduit guidance combined with VEGF producing Schwann cells accelerates peripheral nerve repair. Bioact. Mater. 2021;6(10):3515–3527. doi: 10.1016/j.bioactmat.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao Z., Yuan W., Xu J., et al. Magnesium-encapsulated injectable hydrogel and 3D-engineered polycaprolactone conduit facilitate peripheral nerve regeneration. Adv. Sci. 2022 doi: 10.1002/advs.202202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu R.S., Chen P.Y., Fang J.H., et al. Adaptable microporous hydrogels of propagating NGF-gradient by injectable building blocks for accelerated axonal outgrowth. Adv. Sci. 2019;6(16) doi: 10.1002/advs.201900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drzewiecki K.E., Parmar A.S., Gaudet I.D., et al. Methacrylation induces rapid, temperature-dependent, reversible self-assembly of type-I collagen. Langmuir. 2014;30(37):11204–11211. doi: 10.1021/la502418s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freeman F.E., Kelly D.J. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-17286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levett P.A., Melchels F.P., Schrobback K., et al. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014;10(1):214–223. doi: 10.1016/j.actbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Wang P., Berry D.B., Song Z., et al. 3D printing of a biocompatible double network elastomer with digital control of mechanical properties. Adv. Funct. Mater. 2020;30(14) doi: 10.1002/adfm.201910391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z.Z., Sakiyama-Elbert S.E. Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp. Neurol. 2019;319 doi: 10.1016/j.expneurol.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magaz A., Faroni A., Gough J.E., et al. Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Advanced Healthcare Materials. 2018;7(23) doi: 10.1002/adhm.201800308. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y., Ding F., Wu J., et al. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials. 2007;28(36):5526–5535. doi: 10.1016/j.biomaterials.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Park S.Y., Ki C.S., Park Y.H., et al. Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. Journal of Tissue Engineering and Regenerative Medicine. 2015;9(1):66–76. doi: 10.1002/term.1615. [DOI] [PubMed] [Google Scholar]
- 55.Susuki K., Raphael A.R., Ogawa Y., et al. Schwann cell spectrins modulate peripheral nerve myelination. Proc. Natl. Acad. Sci. U. S. A. 2011;108(19):8009–8014. doi: 10.1073/pnas.1019600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu Y., Ji Y.W., Zhao Y.H., et al. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials. 2012;33(28):6672–6681. doi: 10.1016/j.biomaterials.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Hersel U., Dahmen C., Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 58.Jeschke B., Meyer J., Jonczyk A., et al. RGD-peptides for tissue engineering of articular cartilage. Biomaterials. 2002;23(16):3455–3463. doi: 10.1016/s0142-9612(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 59.Manoukian O.S., Baker J.T., Rudraiah S., et al. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J. Contr. Release. 2020;317:78–95. doi: 10.1016/j.jconrel.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 60.Tajdaran K., Chan K., Shoichet M.S., et al. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater. 2019;96:211–221. doi: 10.1016/j.actbio.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 61.Deng P., Chen F., Zhang H., et al. Multifunctional double-layer composite hydrogel conduit based on chitosan for peripheral nerve repairing. Advanced Healthcare Materials. 2022 doi: 10.1002/adhm.202200115. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki K., Tanaka H., Ebara M., et al. Electrospun nanofiber sheets incorporating methylcobalamin promote nerve regeneration and functional recovery in a rat sciatic nerve crush injury model. Acta Biomater. 2017;53:250–259. doi: 10.1016/j.actbio.2017.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.