Abstract
Introduction
Muscle synergies have been proposed as a strategy employed by the central nervous system to control movements. Muscle synergy analysis is a well-established framework to examine the pathophysiological basis of neurological diseases and has been applied for analysis and assessment in clinical applications in the last decades, even if it has not yet been widely used in clinical diagnosis, rehabilitative treatment and interventions. Even if inconsistencies in the outputs among studies and lack of a normative pipeline including signal processing and synergy analysis limit the progress, common findings and results are identifiable as a basis for future research. Therefore, a literature review that summarizes methods and main findings of previous works on upper limb muscle synergies in clinical environment is needed to i) summarize the main findings so far, ii) highlight the barriers limiting their use in clinical applications, and iii) suggest future research directions needed for facilitating translation of experimental research to clinical scenarios.
Methods
Articles in which muscle synergies were used to analyze and assess upper limb function in neurological impairments were reviewed. The literature research was conducted in Scopus, PubMed, and Web of Science. Experimental protocols (e.g., the aim of the study, number and type of participants, number and type of muscles, and tasks), methods (e.g., muscle synergy models and synergy extraction methods, signal processing methods), and the main findings of eligible studies were reported and discussed.
Results
383 articles were screened and 51 were selected, which involved a total of 13 diseases and 748 patients and 1155 participants. Each study investigated on average 15 ± 10 patients. Four to forty-one muscles were included in the muscle synergy analysis. Point-to-point reaching was the most used task. The preprocessing of EMG signals and algorithms for synergy extraction varied among studies, and non-negative matrix factorization was the most used method. Five EMG normalization methods and five methods for identifying the optimal number of synergies were used in the selected papers. Most of the studies report that analyses on synergy number, structure, and activations provide novel insights on the physiopathology of motor control that cannot be gained with standard clinical assessments, and suggest that muscle synergies may be useful to personalize therapies and to develop new therapeutic strategies. However, in the selected studies synergies were used only for assessment; different testing procedures were used and, in general, study-specific modifications of muscle synergies were observed; single session or longitudinal studies mainly aimed at assessing stroke (71% of the studies), even though other pathologies were also investigated. Synergy modifications were either study-specific or were not observed, with few analyses available for temporal coefficients. Thus, several barriers prevent wider adoption of muscle synergy analysis including a lack of standardized experimental protocols, signal processing procedures, and synergy extraction methods. A compromise in the design of the studies must be found to combine the systematicity of motor control studies and the feasibility of clinical studies. There are however several potential developments that might promote the use of muscle synergy analysis in clinical practice, including refined assessments based on synergistic approaches not allowed by other methods and the availability of novel models. Finally, neural substrates of muscle synergies are discussed, and possible future research directions are proposed.
Conclusions
This review provides new perspectives about the challenges and open issues that need to be addressed in future work to achieve a better understanding of motor impairments and rehabilitative therapy using muscle synergies. These include the application of the methods on wider scales, standardization of procedures, inclusion of synergies in the clinical decisional process, assessment of temporal coefficients and temporal-based models, extensive work on the algorithms and understanding of the physio-pathological mechanisms of pathology, as well as the application and adaptation of synergy-based approaches to various rehabilitative scenarios for increasing the available evidence.
Keywords: Muscle synergy, Upper limb, Rehabilitation, Assessment, Electromyography, Patients, Clinics
Abbreviations
- ADM
Abductor digiti minimi
- AEO
Abdominal external oblique
- AIO
Abdominal internal oblique
- AMP
Amputated subjects
- ANC
Anconeus
- APB
Abductor pollicis brevis
- BIC
Biceps brachii
- BIL
Biceps brachii long head
- BIS
Biceps brachii short head
- BRA
Brachioradialis
- BRC
Brachialis
- BW
Butterworth filter
- CA
Cerebellar ataxias
- CP
Cerebral palsy
- DEA
Deltoid anterior
- DEM
Deltoid medial
- DEP
Deltoid posterior
- DYS
Dystonia children
- ECR
Extensor carpi radialis
- ECU
Extensor carpi ulnaris
- EDC
Extensor digitorum communis
- EDM
Extensor digiti minimi
- EDS
Extensor digitorum superficialis
- EIP
Extensor indicis proprius
- ERE
Erector spinae
- FCR
Flexor carpi radialis
- FCU
Flexor carpi ulnaris
- FDI
First dorsal interosseous
- FDP
Flexor digitorum profundus
- FDS
Flexor digitorum superficialis
- FIR
Finite impulse response filter
- FPB
Flexor pollicis brevis
- FSHD
Facioscapulohumeral dystrophy
- HP
Healthy participants
- INF
Infraspinatus
- INT
Interossei dorsales
- LAT
Latissimus dorsi
- LD
Limb deficiency
- LE
Lateral epicondylalgia
- MP
Muscle pain
- MS
Multiple sclerosis
- MUL
Multifidus
- PD
Parkinson's disease
- PEC
Pectoralis major
- PTE
Pronator teres
- REA
Rectus abdominis
- RHO
Rhomboid major
- SCI
Spinal cord injury
- SEA
Serratus anterior
- SIBR
Subpectoral implant breast reconstruction
- SP
Stroke patients
- STE
Sternocleidomastoid
- SUP
Supinator
- SUS
Supraspinatus
- TEA
Tears major
- TEM
Thenar eminence muscle group
- TLA
Triceps brachii lateral head
- TLO
Triceps brachii long head
- TME
Triceps brachii medial head
- TRI
Triceps brachii
- TRL
Trapezius lower
- TRM
Trapezius medialis
- TRU
Trapezius upper
1. Introduction
Neurological diseases such as stroke, Parkinson's disease (PD), or traumatic brain injury usually cause disability or disorder of limb functions, which significantly affect the quality of life of patients. Over 13 million people have a stroke every year globally and around 5.5 million people have dramatic results following the event; more than 10 million people live with PD [[1], [2], [3]]. Besides, there are other neural system diseases, like cerebral palsy (CP), Alzheimer's disease (AD), epilepsy, and multiple sclerosis (MS), which impose a great burden on families and society. How to reduce the damage and improve independence in individuals with neurological diseases is a crucial issue for society currently and in the future.
Clinical studies indicate that early rehabilitation treatment can largely improve and maintain motor function exploiting neural plasticity in critical periods [4,5]. Subjects usually show abnormal motor patterns after diseases, such as movements poorly coordinated between limbs [[6], [7], [8], [9]] and compensation movements of the body to complete motor tasks [[10], [11], [12]]. By observing the performance or capturing kinematic information when patients perform some tasks, previous studies have quantified motor function based on scales [[13], [14], [15], [16], [17]] and sensor-based assessments [[18], [19], [20], [21]]. Scale-based methods are commonly used in clinical settings, and the effectiveness and reliability of scales have been validated. However, some distinct drawbacks are also documented, such as subjective outcomes, floor and ceiling effects, poor sensitivity to changes, and time-consuming administration [22]. Sensor-based methods compensate for some of the drawbacks of scale-based methods. However, both types of methods often do not provide the information necessary to understand the pathophysiological mechanisms underlying the disease and the recovery process [23].
Surface electromyography (EMG) is a technique based on the recording of electrical physiological activity originating with muscle contraction [24,25] and has been largely used in clinical assessment and neurorehabilitation [26]. Multi-channel EMG allows for performing muscle synergy analysis [27], a technique promoting the understanding of the underlying physiological mechanisms related to muscle coordination and pathophysiological patterns of motor impairment [28]. The muscle synergy hypothesis states that the central nervous system (CNS) recruits a set of coordinated modules (muscle synergies) to control purposeful movements [[29], [30], [31]]. The neural implementation of muscle synergies has been previously demonstrated in animal experiments, including cat [30,32], frog [29,[33], [34], [35], [36], [37]], monkey [31,38], and human experiments [[39], [40], [41], [42]]. They have also been used in a variety of fields, including motor function assessment [43,44] and motor control analysis [41,45,46] in healthy subjects and patients, prosthesis control [47,48], and neurorehabilitation [[49], [50], [51]].
There is a consensus that muscle synergies can provide relevant insights that cannot be gained with standard clinical assessments and motion analysis metrics. However, muscle synergy analysis has not been widely and systematically applied in clinical scenarios and its potential is far from being fully exploited. More knowledge about the neural origin of muscle synergies and the mechanism of the CNS generating movements is required, and without a normative muscle synergy analysis pipeline including standardized experimental protocols and data processing procedures, it is difficult to compare the results among studies and translate the experimental results into general rules for clinical applications. Some review articles summarized and discussed the potential clinical applications of muscle synergies [52,53] and/or muscle synergy analysis in specific diseases such as stroke [54,55] and Parkinson's disease [56]. They focused on clinical-related output analysis and suggested lines for future research including the adoption of synergy-based rehabilitation paradigms to induce true recovery. However, how to best implement synergy-based approaches still remains an open question [52]. Moreover, no article systematically reviewed the studies in which muscle synergies were used for evaluating upper limbs in clinical applications, including a summary of experimental protocols, signal processing methods, clinical findings, and discussed how the results of a comprehensive screening may impact clinical practice.
To deepen our understanding of the muscle coordination strategies underlying human motor control and promote an effective application of muscle synergies in upper limb neurorehabilitation, we conducted a systematic review of studies employing muscle synergies extracted from the upper limb in clinical applications. Accordingly, we first introduce muscle synergy extraction and analysis methods. Then, we summarize the selected studies grouping them according to the experimental protocols (patients and participants, anatomical regions, experimental design, and tasks), signal processing and synergy extraction methods, and clinical findings. The impact of muscle synergies on upper limb function assessments and rehabilitative interventions is discussed. Finally, we highlight the opportunities and challenges of muscle synergy analysis in clinical applications.
2. Muscle synergies
Due to the complexity and redundancy of the neuromusculoskeletal system, understanding how the CNS coordinates muscle activations to produce movements is a major challenge in the field [33]. Previous studies in several animal species revealed that the CNS may adopt a modular organization to control a wide repertoire of motor behaviors. Several studies explored the control architecture of the CNS by the means of computational models that postulate a modular organization [27,57,58]. A prominent model for such modularity is the muscle synergy model [33], which hypothesizes that the CNS generates the time course of neural motor commands to activate a set of predefined modules, organized in the cerebral cortex, in the brain stem, and in the spinal cord, to produce muscle activities required for task execution. A limited set of available muscle synergies may handle effectively musculoskeletal redundancy and simplify motor control.
Over the last two decades, several synergy models and computational methods have been proposed to describe how muscle activations are coordinated. These models may capture a spatial, temporal, or spatiotemporal synergy organization [59,60]. Spatial synergies (also known as time-invariant or synchronous synergies) [33,36] represent relative levels of muscle activations that are invariant over time and conditions. Different muscle activations can be generated by scaling each synergy by task-dependent time-varying activation coefficients. The temporal synergy model [39,61] hypothesizes that muscle activation waveforms are generated by invariant muscle activation patterns, modulated by task-dependent muscle weights. The time-varying synergies (also known as spatiotemporal synergies) [62] describe specific spatiotemporal patterns that are invariant among motor tasks. Each synergy is a collection of muscle-specific activation waveforms which can be scaled in amplitude and delayed in time as a unit and combined with other synergies to generate task-dependent muscle activations. Recently, the space-by-time synergy model introduced invariant spatiotemporal patterns as a combination of spatial and temporal synergies [63].
The essence of extracting muscle synergies is dimensionality reduction. Several matrix factorization methods have been applied, such as principal component analysis (PCA) [40,[64], [65], [66]], factor analysis (FA) [39,65], non-negative matrix factorization (NMF) [33,67,68], independent component analysis (ICA) [35], autoencoder (AE) [69,70], and second-order blind identification (SOBI) [71]. These methods assume different constraints on the input signals, and factorization results are affected by the noise level, signal characteristics, and the number of channels [[72], [73], [74]]. When identifying the optimal number of synergies, a predefined threshold based on the variance accounted for (VAF) [75] or the coefficient of determination (R2) [76] is commonly used, but other criteria have also been used [29,36,59,65,[76], [77], [78], [79], [80]].
Based on the abovementioned synergy models and computational techniques, previous studies have demonstrated that many motor tasks can be executed by activating a small set of muscle synergies. For example, three to five synergies were extracted when performing reaching movements under multiple task conditions, including forearm postures, motor patterns, and force conditions [41,81,82]. A specific number of synergies could also characterize gait cycles [83,84] (typically 4 or 5, depending on the experimental condition [85]).
However, neurological lesions interfere with the neural processing of control signals occurring at all levels of the motor system, from motor cortical areas to the spinal cord [43], which causes the alteration of the recruitment and/or structure of the muscle synergies. These alterations could be valuable biomarkers for motor function assessment and effective descriptors of patients’ motor capability [43,60,75,86]. Moreover, given that muscle synergies describe how the CNS controls movement, they may also be used in various prostheses and assistive device control.
In sum, while the applications of muscle synergy analysis in neurorehabilitation are promising and muscle synergies are being employed more and more to characterize the effects of neurological diseases on motor function, few systematic literature reviews are available in the field.
3. Literature search strategies and criteria
We reviewed studies in which muscle synergies were used to analyze and assess upper limb function after various neurological diseases. The experimental protocols were limited to upper limb movements, involving the shoulder, elbow, wrist, or hand tasks. Participants included only individuals with motor impairments, e.g., stroke survivors; or the study had to aim at exploring disease-related issues based on muscle synergies, e.g., coordination in functional upper limb tasks. We identified keywords related to three different aspects: research topic (muscle synergies), body segment (upper limb or upper extremity or reaching), and application (patient or rehabilitation or clinical). Then, a literature search was conducted using the following logical combination of keywords: (“muscle synergies”) and (“upper limb” or “upper extremit*” or “reaching”) and (“patient” or “rehabilitation” or “clinical”) in Scopus, PubMed, and Web of Science based on the Title, Abstract, and Keywords. The search included studies published from January 2000 to February 2023. A preliminary screening was conducted in the papers matching the research criteria, and the exclusion criteria included: (1) duplicated papers, (2) conference, book chapters, reports, letters, and review papers, (3) papers without Journal Citation Reports (Impact Factor, IF), and (4) non-English papers. Then, a refined screening based on the full text was performed to exclude studies that did not involve muscle synergies, where the aim of the study was not clinical assessment or rehabilitation therapy, or involving only healthy participants. Afterward, we extracted the experimental protocols (e.g., the aim of the study, number and type of participants, number and name of muscles, and tasks), the methods (e.g., muscle synergy models and synergy extraction methods, signal processing methods), and the main conclusion from the selected studies to provide a comprehensive meta-analysis.
4. Results
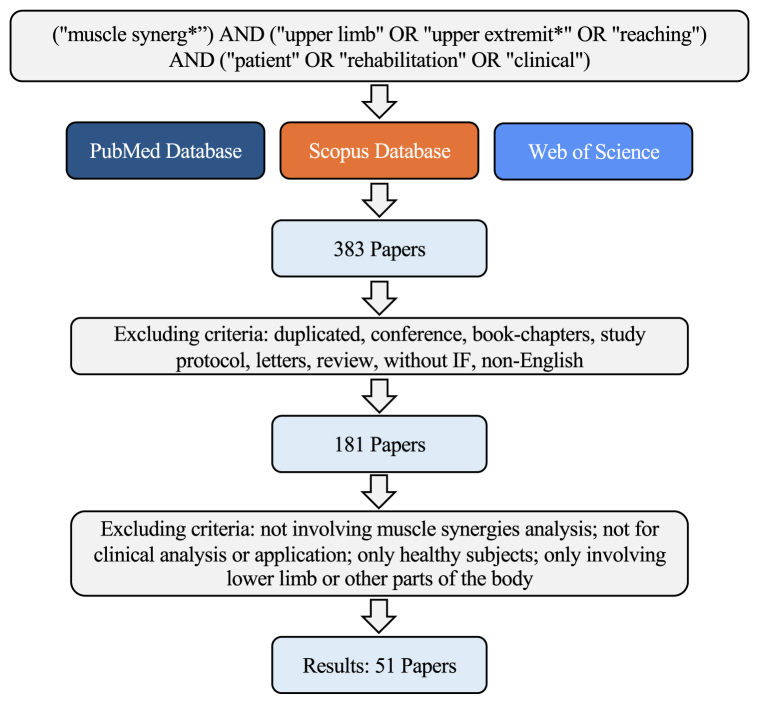
The PRISMA graph for our review is reported in Fig. 1. According to our literature search criteria, a total of 383 papers were found in our screening in Scopus, PubMed, and Web of Science. Then, the studies that did not satisfy the additional selection criteria were excluded. Finally, 51 papers were considered in this review.
Fig. 1.
PRISMA diagram for literature review.
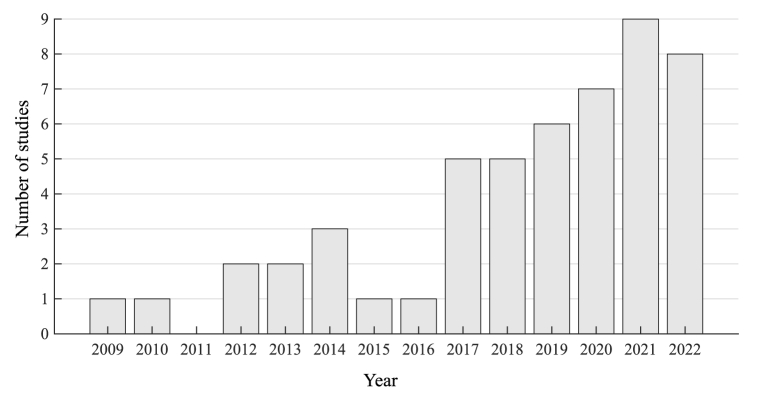
Generally, the number of studies showed an increasing trend year by year, especially since 2017 (Fig. 2). These studies covered synergy-based assessment and rehabilitative intervention of upper limb muscle synergies in various scenarios and diseases. The following subsections of the Results summarize the selected articles focusing on Patients and Participants, Recorded Muscles, Experimental Design and Tasks, Signal Processing, Number of Synergies and Types of Extraction Algorithms, and Findings Achieved with Muscle Synergies.
Fig. 2.
The number of selected studies each year in this review.
4.1. Patients and participants
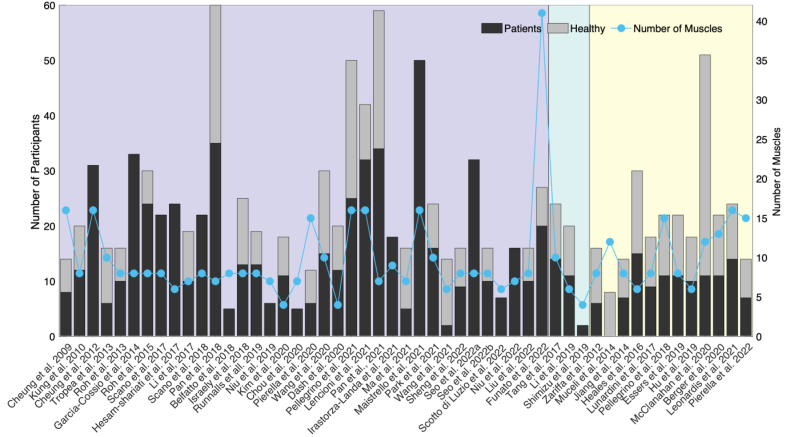
Muscle synergy analysis has been applied to various diseases affecting upper limb motor function. These studies covered a total of 1155 participants, and 748 of them were patients. Each study investigated a mean of 15 ± 10 (mean ± SD) patients. Stroke-related studies had the largest number of participants, including in total of 850 individuals, and 609 of them were patients. Seven studies recruited over 30 patients [43,[86], [87], [88], [89], [90], [91]]. The number of patients and healthy subjects in each study is shown in Fig. 3. Descriptive statistics of the participant groups can be found in Table 1. It can be observed that the number of patients enrolled is in general quite low to provide reliable statistical conclusions.
Fig. 3.
The number of recruited participants and recorded muscles in each study. The purple area shows the studies involving stroke (n = 36). The green area shows the studies including CP (n = 3). Other studies (n = 12) usually involving one type of the disease are grouped together in the yellow area, named OD (Other Diseases), which includes spinal cord injury (SCI), muscle pain (MP), limb deficiency (LD), lateral epicondylalgia (LE), dystonia children (DYS), multiple sclerosis (MS), facioscapulohumeral dystrophy (FSHD), Parkinson's disease (PD), amputated subjects (AMP), cerebellar ataxias (CA), subpectoral implant breast reconstruction (SIBR). For each study, the black bar indicates the number of patients, the gray bar the number of healthy participants, and the blue line the number of recorded muscles1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Statistics of the number of participants included in the review (CP: Cerebral Palsy, OD: Other Diseases).
| Stroke |
CP |
OD |
||||
|---|---|---|---|---|---|---|
| Patients | Healthy | Patients | Healthy | Patients | Healthy | |
| Max | 50 | 25 | 14 | 10 | 15 | 40 |
| Min | 2 | 0 | 2 | 0 | 0 | 7 |
| Med | 13 | 6 | 11 | 9 | 10.5 | 10 |
| Ave | 16.8 | 6.7 | 9.0 | 6.3 | 9.3 | 12.3 |
| SD | 11.2 | 7.1 | 6.2 | 5.5 | 4.0 | 9.0 |
This review individuated a total of 13 diseases or limb impairments, in which stroke (number of studies n = 36) was the most common, followed by CP (n = 3) [[92], [93], [94]]. Other diseases (OD) involved spinal cord injury (SCI) [95], muscle pain (MP) [96], amputated subjects (AMP) [97], dystonia children (DYS) [98], subpectoral implant breast reconstruction (SIBR) [99], PD [100], lateral epicondylalgia (LE) [101], facioscapulohumeral dystrophy (FSHD) [102], MS [103,104], cerebellar ataxias (CA) [60], and limb deficiency (LD) [105], as shown in Fig. 3. The main features of the studies are summarized in Table 2, Table 3, Table 4.
Table 2.
Main information of the studies selected in this review. Single session studies, Stroke Patients (SP).
| Study | DIS | IL | CS | N Task | N Cond | PAT | HS | MUS | ALGO | ΔN | ΔW | ΔC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheung et al., 2009 | SP | FMA 18-64 | / | 7 | 1 (free) | 8 (3 + 5); 60.1 | 6; 66.7 | (12–16) + (12–16) | NMF | Both groups showed a similar number of synergies. | Similar muscle synergies weights across arms and groups were observed. | Cortical impairment altered the activation pattern for downstream muscle synergies. |
| Kung et al., 2010 | SP | FMA 12-56 | / | 4 | 1 (robot) | 12 (11 + 1); 46.4 | 8 (6 + 2); 50.5 | 8 + 8 | PCA | / | / | There were abnormal synergies in the affected side of the stroke patients during the tracking movements. |
| Cheung et al., 2012 | SP | FMA 0-66 | Yes | 12 × 2 | 1 (free) | 31 (17 + 14); 61.6 | 0 | (10–16) + (10–16) | NMF | A reduced or increased number of synergies was observed in the affected arm compared to the unaffected arm. | Preservation, merging, or fractionation of muscle synergies was found in the affected arm. | / |
| Roh et al., 2013 | SP | FMA 12-23 | / | 54 | 1 (robot) | 10 (5 + 5); 62.3 | 6 (4 + 2); 63.2 | 8 | NMF | Four synergies were extracted from both groups. | The elbow synergies were typically retained, while shoulder-related synergies were altered in stroke individuals and were correlated with impairment level. | / |
| García-Cossio et al., 2014 | SP | FMA 0–11/24 | Yes | 6 | 1 (free) | 33 (21 + 12); 55 | 0 | 8 + 8 | NMF | The number of synergies in the paralyzed limb was slightly reduced compared to the healthy one. | The merging of healthy muscle synergies was a predominant pattern in patients. The merging or fractionation was not related to the cortex integrity. | / |
| Roh et al., 2015 | SP | FMA 12-66 | / | 54 | 1 (robot) | 24 (14 + 10); 57.5 | 6 (4 + 2); 63.2 | 8 | NMF | Four synergies were extracted from both groups. | The elbow synergies were typically retained, while shoulder-related synergies were altered in stroke individuals and were correlated with impairment level. | / |
| Scano et al., 2017 | SP | FMA 11-61 | Yes | 1 | 1 (free) | 22 (15 + 7); 56.6 | 0 | 8 | NMF | / | Five basic clusters were identified to characterize a group of stroke patients, and synergies clustering did not correlate with the clinical assessments. | / |
| Li et al., 2017 | SP | FMA 18-32 | Yes | 2 | 1 (free) | 10 (9 + 1); 60.9 | 9 (5 + 4); 57.8 | 7 | NMF | / | Pathological synergies of patients were altered from the characteristics of baseline synergy with missing or altered weights and time profiles. | The similarity indices correlated to the kinematic performance and FMA score. |
| Scano et al., 2018 | SP | FMA 12-64 | / | 1 | 2 (with and without robot) | 22 (15 + 7); - | 0 | 8 | NMF | / | Six out of seven paired synergies were very strongly similar between free and robot-assisted hand-to-mouth movement. | There was a trend of a reduction in the magnitude of the activation coefficients during interaction with the robot, though it lacked statistical significance. |
| Pan et al., 2018 | SP | FMA 9-51 | Yes | 1 | 1 (free) | 35 (27 + 8); 60.3 | 25 (13 + 12); 59.2 | 7 | NMF | Three synergies were extracted from both groups. | Stroke altered the structure of muscle synergies, which could be characterized as the merging of control group synergies. | / |
| Israely et al., 2018 | SP | FMA 50.8 | / | 9 | 1 (free) | 13 (7 + 6); - | 12; - | 8 | NMF | Four synergies were extracted from both groups. | The synergies of the study group and the control group were similar to the synergies of the representative. Two synergies were not matched between groups, but the difference was not significant. | The control group exhibited a gradual change in the synergy activation in the amplitude, whereas the study group exhibited consistently significant differences between all movement directions and the representative set of synergies. |
| Runnalls et all., 2019 | SP | FMA 9-66 | / | 14 | 3 (weight support at three levels) | 13 (9 + 4); 70.8 | 6 (4 + 2); 65.2 | 8 | NMF | Controls and patients with mild impairment showed more synergies with high-weight support. The control group expressed more synergies compared to patients in the moderate-severe group while there was no difference between the mild and moderate-severe groups. | Co-contraction of three deltoid muscles was found in the stroke group. | / |
| Kim et al., 2020 | SP | BS Ⅱ-Ⅳ | / | 8 × 2 | 1 (free) | 11 (5 + 6); 56.82 | 7; 24.86 | 4 | HALS | The study did not find a significant difference in the number of synergies between groups. | Clustering analysis showed that corresponding clusters of mild and control groups showed the highest similarity. In contrast, only three clusters showed a similarity over 0.9 between severe and control groups. | / |
| Chou et al., 2020 | SP | BS Ⅲ-Ⅳ | / | 2 | 2 (free) | 5 (5 + 0); 60 | 8 (6 + 2); 28.1 | 7 | NMF | / | / | / |
| Wang et al., 2020 | SP | FMA 27-61 | Yes | 36 × 2 | 1 (free) | 15 (9 + 6); 52.1 | 15 (10 + 5); 48.5 | 10 | PCA | / | / | / |
| Pellegrino et al., 2021 | SP | FMA 5-63 | / | 8 | 3 (three force fields) | 25 (16 + 9); 60.4 | 25; - | 16 + 16 | NMF | There was no difference in the number of synergies between groups. | In absence of force fields, the right-brain damage group had weight coefficients of muscle synergies less altered than the left-brain damage group in both arms and more similar between the two sides of the body. | The difference between the two arms of each subject in terms of activation coefficients was greater in stroke subjects than in controls for all tasks. The similarity of the activation coefficients with those of the controls was task-dependent, which was slightly higher when interacting with the resistive force field than in the other tasks. |
| Pan et al., 2021 | SP | FMA 11–51/52 | Yes | 1 | 1 (free) | 34 (26 + 8); 59.8 | 25 (13 + 12); 59.2 | 7 | NMF | Three synergies were extracted from both groups. | Compared with the control group, muscle synergies were altered in stroke subjects, and synergy patterns in the mild-to-moderate group were more similar to the control group. | / |
| Irastorza-Landa et al., 2021 | SP | FMA 2–33.5/54 | Yes | 5 | 1 (free) | 18 (12 + 6); 54.7 | 0 | 8 + 8 | NMF | The paretic limb showed a slightly lower number of optimal clusters. No significant differences were observed in the number of synergies in the paretic limb between pre- and post-therapy. | No significant differences were observed in the three muscle synergy features between pre- and post-therapy. The number of synergies showed a weak but significant correlation with motor performance. | The synergy index significantly increased after therapy and correlated with motor function. |
| Ma et al., 2021 | SP | FMA 22-60 | / | 1 | 1 (robot) | 5; 67.8 | 11; 26.7 | 7 | MCR-ALS | Three synergies were extracted from each healthy subject, while three and four synergies were identified from three and two patients, respectively. | / | / |
| Park et al., 2021 | SP | FMA 12-64 | Yes | 4 × 2 | 1 (free) | 16 (14 + 2); 51.6 | 8 (3 + 5); 54.3 | 13 | NMF | Four and five muscle synergies in the stroke and control groups were observed, respectively. | The composition of muscle synergies was comparable between the groups, except that the three heads of the deltoid muscle were co-activated and formed one synergy in the stroke group, whereas those muscles formed two synergies in the control group. | The modulation of synergy activation coefficients was altered after a stroke. |
| Wang et al., 2021 | SP | BS Ⅲ-Ⅳ | / | 1 × 2 | 1 (robot) | 2 (0 + 2); 53 | 12 (10 + 2); 25 | 6 | NMF | The number of synergies of patients decreased compared with the healthy group. | / | The activation coefficients of patients were different from the healthy group. |
| Sheng et al., 2022 | SP | FMA 13-51 | Yes | 1 × 2 | 1 (robot) | 9 (6 + 3); 67.2 | 7 (4 + 3); 47 | 8 | NMF | Two synergies were extracted from both groups. | Low synergy similarity between groups indicates that the muscle synergies of patients were different from the control group. | Patients had a lower amplitude in activation coefficients than the healthy subjects. |
| Seo et al., 2022b | SP | FMA 12-23 | / | 54 | 1 (robot) | 10 (5 + 5); 61.8 | 6 (4 + 2); 63.2 | 8 | NMF | Four synergies were extracted from both groups. | Stroke groups showed abnormal deltoid modules compared to the control group. | The PCA and the multivariate multiple linear regression analyses showed that the alterations in motor modules were associated with abnormal between-force coupling. |
| Liu et al., 2022 | SP | FMA 12-23 | / | 54 | 1 (robot) | 10 (5 + 5); 62.3 | 6 (4 + 2); 63.2 | 8 | NMF | / | / | / |
| Funato et al., 2022 | SP | FMA 6-64 | Yes | 37 | 1 (free) | 20 (20 + 0); 54.5 | 7; - | 41 | NMF | Severe stroke subjects had a significantly smaller number of synergies, while it did not differ significantly between healthy participants and those with mild stroke. The average number of synergies was close between groups. | Some synergies in stroke survivors corresponded to merged standard synergies and the merging rate increased with the impairment of stroke survivors. | / |
Table 3.
Main information of the studies selected in this review. Single session and Longitudinal studies (in gray), Other pathologies.
| Study | DIS | IL | CS | N Task | N Cond | PAT | HS | MUS | ALGO | ΔN | ΔW | ΔC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tang et al., 2017 | CP | FMA 28-61 | Yes | 3 | 1 (free) | 14 (9 + 5); 8.2 | 10 (3 + 7); 8.9 | 10 | NMF | A fewer number of synergies were recruited in CP group. | Many abnormal synergy structures specific to the CP appeared. A lower intra-group similarity was observed in the CP group. | A lower synergy activation similarity was observed in the CP group, while both were very low compared to the synergy similarity. |
| Li et al., 2019 | CP | GMFCS 1-4 | / | 1 | 1 (free) | 11 (7 + 4); 4.68 | 9 (8 + 1); 5.83 | 12 | NMF | / | Synergy structure had greater repeatability than activation curves in both the TD and CP groups. | For both muscle synergies and synergy activations, the mean similarity values of the CP group were significantly lower than those of the TD group. |
| Shimizu et al., 2019 | CP | GMFCS 3-4 | / | 1 | 1 (free) | 2 (2 + 0); 18 | 0 | 4 | NMF | / | / | / |
| Zariffa et al., 2012 | SCI | UEMS 21–25/25 | Yes | 8 | 1 (free) | 6 (6 + 0); 49.2 | 10 (10 + 0); 34.9 | 8 | NMF | / | Healthy subjects showed more co-activation of EDC and EIP, and of FDS and FCU. In contrast, the co-activation of ECR and FCR was more common among SCI subjects. | / |
| Muceli et al., 2014 | MP | / | / | 12 × 2 | 1 (free) | 0; - | 8 (8 + 0); 29.3 | 12 | NMF | The number of synergies was consistent in all conditions. | The injection of hypertonic saline had a major influence on the first synergy, mainly activating the DEA. The synergies related to the coupling of the elbow and shoulder joints were usually preserved across subjects. | The injection of hypertonic saline changed the activation signals, especially in the first synergy, a lower peak value was observed compared to the baseline level. |
| Jiang et al., 2014 | AMP | / | / | 2 | 1 (free) | 7 (7 + 0); 14-72 | 7 (4 + 3); 25-56 | 7–8 | NMF | / | / | / |
| Heales et al., 2016 | LE | PRTEE 37.7/100 (mean) | / | 4 | 1 (free) | 12 (4 + 8); 51.6 | 14 (5 + 9); 51.4 | 6 + 6 | NMF | Two synergies were extracted from both groups. | There was no difference in muscle synergies between arms in the LE group. When the synergies extracted from pooled data of the control group were used to reconstruct the muscle activation of both groups, a significantly higher VAF was observed in the control group. | / |
| Lunardini et al., 2017 | DYS | TOTAL 2-10 | / | 2 | 1 (free) | 9 (6 + 3); 13.4 | 9 (2 + 7); 15.7 | 8 | NMF | A similar number of synergies was extracted from both groups. | Muscle synergies were greatly similar between groups. | The activation profile did not significantly differ between groups, while the amplitude of the peaks presented a slight reduction in the dystonia group. |
| Pellegrino et al., 2018 | MS | EDSS 2.5–6.5/10 | / | 8 | 4 (four force levels) | 11 (2 + 9); 50 | 11 (2 + 9); 50 | 15 + 15 | NMF | The number of synergies was not significantly different between populations. | MS subjects presented a higher change in the organization of muscle synergies in the presence of external forces environment. | The correlation between activation profiles of two different tasks was significantly different between groups, and this difference depended also on the pair of tasks compared. |
| Essers et al., 2019 | FSHD | BSS 3-4 | / | 2 | 1 (free) | 11 (6 + 5); 54 | 11 (5 + 6); 55 | 8 | NMF | Two synergies were extracted from both groups. | Muscle synergies altered in FSHD individuals and showed greater diversity while controls mostly used one synergy for both tasks. | / |
| Hu et al., 2019 | PD | UPDRS 11–25/56 | / | 1 | 2 (with or without CAS) | 10 (8 + 2); 63.7 | 8 (6 + 2); 63.13 | 6 | NMF | Three synergies were extracted from both groups. | Muscle synergies were more similar in both groups with and without CAS. | Synergy activations displayed some oscillatory components in PD patients and the similarity with and without simulation was smaller than in the control group. |
| McClanahan et al., 2020 | AMP | DASH 1.67–86.67 | / | 40 | 1 (free) | 40 (28 + 12); 29.9 | 11 (11 + 0); 42.36 | 8–12 | NMF | Male subjects exhibited a larger number of synergies than female subjects in able-bodied subjects. Amputees exhibited less than four synergies, whereas able-bodied subjects commonly demonstrate five or more synergies. | / | / |
| Berger et al., 2020 | CA | ICARS 12-55 | Yes | 8 | 1 (free) | 11 (5 + 6); 44.4 | 11 (6 + 5); 49.7 | 13 | NMF | / | Cerebellar damage affects the temporal and spatiotemporal organization, but not the spatial organization, of the muscle patterns. | Cerebellar damage affected the temporal and spatiotemporal organization of synergy activations. |
| Leonardis et al., 2021 | SIBR | / | / | 40 | 1 (free) | 14 (0 + 14); - | 10 (0 + 10); - | 16 + 16 | NMF | / | Two out of three synergies were more similar than chance between the groups on the non-dominant arm, whereas only one synergy is more similar than chance on the dominant arm. | / |
| Pierella et al., 2022 | MS | EDSS 0-1 | / | 8 | 4 (four force levels) | 7 (3 + 4); 42 | 7 (3 + 4); 42 | 15 | NMF | The two populations had the same number of muscle synergies. | The two populations had similar structures of muscle synergies. | The two populations had different activation profiles. |
Table 4.
Main information of the studies selected in this review. Longitudinal studies (in gray), Stroke Patients (SP).
| Study | DIS | IL | CS | N Task | N Cond | PAT | HS | MUS | ALGO | ΔN | ΔW | ΔC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tropea et al., 2013 | SP | FMA 8-36 | / | 8 × 2 | 1 (robot) | 6 (4 + 2); 71.8 | 10 (5 + 5); 71.2 | 10 | FA | Four synergies were extracted from both groups. | Compared to the healthy group, the treatment did not significantly change patients' muscle synergies, while the consistency of muscle synergies across patients changed after treatment. | The patients' synergy activations were not consistent with the healthy subjects whatever before or after the treatment while being more anisotropic. |
| Hesam-Shariati et al., 2017 | SP | FMA 25.3–61.6 (mean) | / | 1 | 1 (VR) | 24 (16 + 8); 57.9 | 0 | 6 | NMF | The number of synergies for patients with low motor function was significantly less than for patients with high motor function at early therapy. After therapy, an increase in the number of synergies was evident for patients with low and moderate motor function, albeit not statistically significant. | Most synergy clusters changed from early to later therapy. | The synergy activation profiles were similar for patients in each level of motor function. |
| Belfatto et al., 2018 | SP | FMA 11-56 | / | 1 | 1 (free) | 5 (5 + 0); 61 | 0 | 8 | NMF | Two synergies were extracted before and after rehabilitation therapy. | Muscle synergies were very similar with a high similarity value between stages. | The first synergy activation showed a high correlation coefficient between stages, while the correlation value of the second activation was between 0.56 and 0.87. |
| Niu et al., 2019 | SP | BS Ⅲ-Ⅳ | / | 2 | 1 (free) | 6 (5 + 1); 58.7 | 1; - | 7 | NMF | / | After intervention, the synergy patterns of patients became more similar to that of normal control. | After intervention, similarity in synergy activation improved in all patients and almost all tasks. |
| Pierella et al., 2020 | SP | FMA 5-54 | / | 18 × 2 | 1 (robot) | 6 (2 + 4); 68 | 6 (2 + 4); 58 | 15 | NMF | The number of synergies of subacute stroke subjects increased after four-week training. | After training, the muscle synergies of patients became more similar to healthy subjects. | / |
| Dash and Lahiri, 2020 | SP | / | / | 1 | 1 (free) | 12 (11 + 1); 39.9 | 8 (4 + 4); 37 | 4 + 4 | NMF | / | / | / |
| Lencioni et al., 2021 | SP | FMA 14.4–49.5 (median) | / | 2 | 1 (VR + robot) | 32 (15 + 17); 63.5 (median) | 10; - | 16 + 16 | NMF | The number of synergies was not significantly different between groups. | Post-stroke subjects who followed robotic rehabilitation showed larger improvements in axial-to-proximal muscle synergies with respect to those who underwent usual care. Both treatments had negative effects on muscle synergies controlling the distal district. | At baseline, the activation profiles of stroke patients were altered compared to the healthy control. Meanwhile, the activation profile showed no significantly different values for both arms and tasks. |
| Maistrello et al., 2021 | SP | FMA 125.5 (median) | Yes | 7 | 1 (VR) | 50 (33 + 17); 63.6 | 0 | 16 + 16 | NMF | The number of synergies was not significantly changed before and after treatment. | Muscle synergies were not significantly changed before and after treatment. | / |
| Seo et al., 2022a | SP | FMA 8-40 | / | 12 | 1 (free) | 32 (17 + 15); 27-75 | 0 | 8 | NMF | After myoelectric computer interface training, there was no consistent pattern of change in the number of synergies across the patients. | The composition of muscle synergies, calculated using a traditional synergy similarity metric, did not change after the training. However, the disparity of muscle weights within synergies increased after the training in participants who responded to the training. | / |
| Scotto di Luzio et al., 2022 | SP | FMA 26-45 | / | 4 | 1 (robot) | 7 (5 + 2); 59.6 | 0 | 6 + 6 | NMF | / | A very high degree of similarity of the involved synergies between the healthy and the injured limb both before and after the treatment was observed. | / |
| Niu et al., 2022 | SP | FMA 13-56 | / | 2 | 1 (free) | 16 (15 + 1); 60.6 | 1; - | 7 | NMF | / | After FES intervention, the muscle vector exhibited more concentrated activation from individual muscles. | After the FES intervention, the time profiles showed higher magnitudes and clearer bursts. |
FMA: Fugl-Meyer Assessment Upper Extremity; GMFCS: Gross Motor Function Classification System; UEMS: Upper Extremity Motor Score; TOTAL: Total Score.
EDSS: Expanded Disability Status Scale; BSS: Brooke Scale Score; BS: Brunnstrom Stage; UPDRS: Unified Parkinson's Disease Rating Scale; DASH: Disabilities of Arm, Shoulder, and Hand; ICARS: International Cooperative Ataxia Rating Scale; CAS: Cutaneous Afferent Stimulation.
DIS: the type of disease.
IL: impairment level assessed using clinical scales, e.g., FMA 0–11/24, the impairment level was 0–11 assessed by FMA with a total score of 24 (default 66).
CS: correlation with scales, describes whether synergy-related outcomes were compared with clinical scales.
N Task: describes how many different tasks were considered (e.g., Reaching and Hand-to-mouth = 2). Reaching in multiple directions is considered as multiple different tasks (e.g., Roh 2013 reaching in 54 directions, indicate 54). “x2” means that some directions were done in going and coming back (e.g., N- > O and O- > N).
N Cond: indicates in how many conditions the task was repeated for the muscle synergy assessments. “free” means the task was performed freely except for necessary constraints. “robot” indicates that the task was performed with the assistance of a robot.
PAT: the number of patients (male + female; age: range or mean).
HS: the number of healthy subjects (male + female; age: range or mean).
MUS: the number of muscles. (12 + 12) indicates that bilateral limbs were recorded.
ALGO: the algorithm used to extract muscle synergies.
ΔN: whether the number of synergies changes?.
ΔW: whether synergy weights change?.
ΔC: whether synergy coefficients change?.
4.2. Recorded muscles
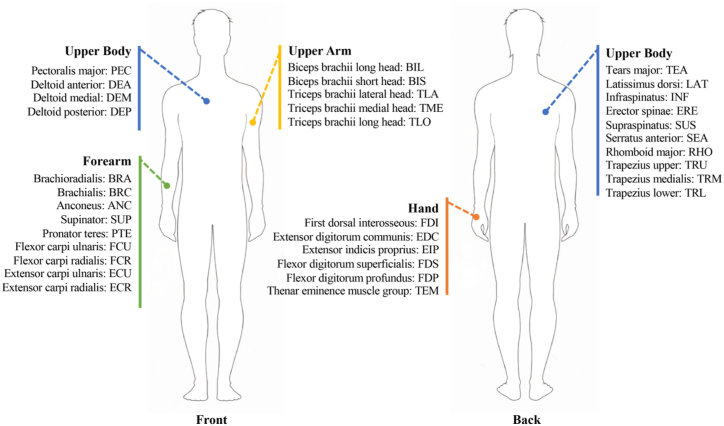
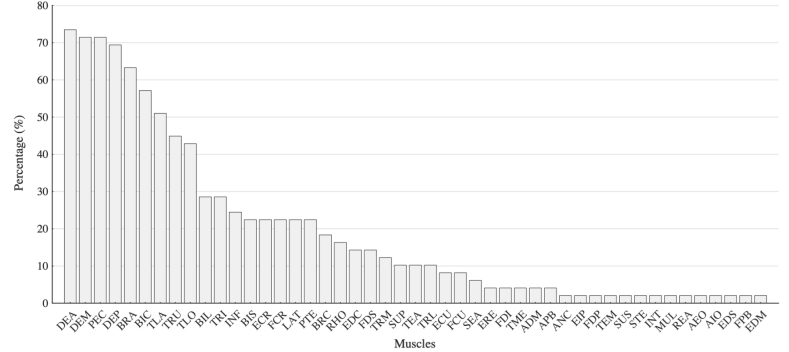
In terms of EMG signals recorded for muscle synergy analysis, four to forty-one EMG signals from the upper body, upper arm, forearm, and hand were recorded using surface electrodes. On average 10 ± 6 (mean ± SD) muscles were recorded. The highest number of recorded muscles was forty-one on both the affected and non-affected sides [106]. In all the other studies, four to sixteen muscles were recorded. The number of muscles recorded in each study is shown in Fig. 3, while Fig. 4 summarizes the most frequently recorded muscles in the selected studies. Fig. 5 shows the percentage of the studies in which each muscle was included for muscle synergy analysis. Two studies recorded EMG activity from the electrodes that were evenly spaced around the forearm [97,105] and were not related to specific muscles, thus they were excluded from the results presented in Fig. 5. We noticed that there is a set of muscles that were frequently used in muscle synergy analysis, including Deltoid (anterior, medial, posterior), Pectoralis major, Triceps brachii (lateral and long head), Biceps brachii (long and short head), Trapezius, Infraspinatus, Brachioradialis, and Latissimus dorsi for upper limb movements; Extensor carpi radialis, and Flexor carpi radialis for forearm and hand movements. In contrast, some muscles controlling hand movements were recorded only in a few studies.
Fig. 4.
Muscles recorded for upper limb muscle synergy analysis.
Fig. 5.
The percentage of studies in which corresponding muscles are recorded. Since two studies did not specify exactly the anatomical landmarks, they were excluded from the results reported in this figure (data refer to 49 studies).
4.3. Experimental Design and Tasks
The first finding of our screening regards the aims of the presented works. In fact, a vast majority of the papers are based on measurements performed in a single session aiming at characterizing patients (describing their motor capability, as in Ref. [76]); in some cases, also aiming at comparing particular experimental conditions (e.g., how muscle synergies change with and without robot assistance). However, in a limited but not negligible number of longitudinal studies, synergies are used as a metric to evaluate the course of therapy, as in Ref. [89]. Thus, it is possible to compare the design of the selected studies by dividing them according to their design and aim. Interestingly, all the studies used synergies for assessment, rather than a tool for customizing therapies or decision-making. We also noticed that most of the studies focused on stroke, and thus we investigated common features separately in the studies related to this disease in order to provide more reliable comparisons of the study outcomes.
With the aim of assessing the variability and similarity of synergy patterns, various experimental tasks were designed, involving the movement of the shoulder, elbow, wrist, and hand movements. Reaching movements were largely investigated as they are involved in most upper limb gross motor functions, and are commonly used to identify and quantify the degree of motor impairment and assess compensatory strategies [16]. In some studies, reaching motion was along a single direction, in others, more variability was explored, up to 54 directions [75]. Twenty-two studies investigated point-to-point reaching movements. In twelve of them, reaching in the horizontal plane along one or multiple directions [51,92,96,103,[107], [108], [109], [110], [111], [112], [113], [114]] was considered. Two studies were performed in the 3D upper limb workspace [115,116], and seven studies investigated reaching in the frontal plane [60,86,88,100,[117], [118], [119]]. Other studies investigated hand-to-mouth movements [120,121], upper extremity tasks used in the Fugl-Meyer Assessment [106], counterclockwise and clockwise circle-drawing movements [122], arm weight support [123], isometric force generation [44,75,124,125], handgrip and writing tasks [95,98,101,126], and crawling tasks [93]. In addition, multi-joint and multi-task upper limb movements were explored in other studies, based on multiple functional movements that resemble clinical scales [127]. Summarizing, the considered tasks are not uniform; usually, partially functional or non-functional gestures are analyzed, intended as motion primitives that represent or approximate functional gestures.
4.4. Signal processing
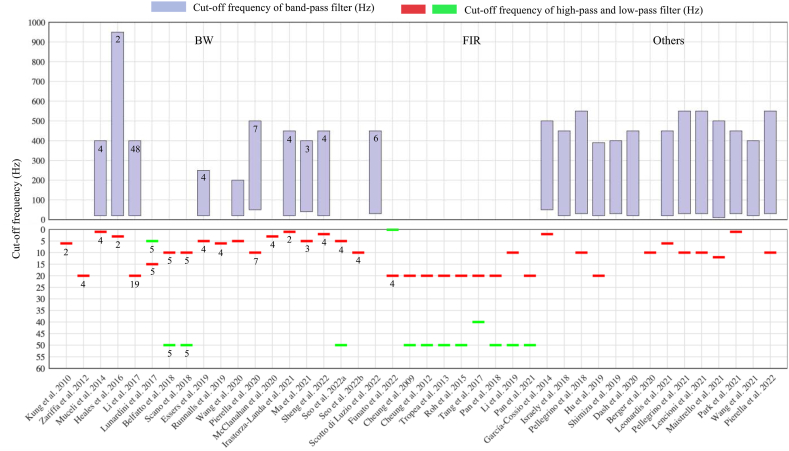
Preprocessing of the raw EMG signals is needed before synergy extraction. Preprocessing includes filtering (to remove motion artifacts and DC offset and to attenuate noise), rectifying, low-pass filtering (to obtain EMG envelopes), and normalization (to allow inter-muscle, intra-subject, and inter-subject comparisons) [74,128]. In terms of filtering, while high-pass filters and band-pass filters are commonly used to compute EMG envelopes, some studies also used band-stop filters [98,102,116] and notch filters [87,109,127,129] to eliminate interferences from the power line and electromagnetic tracking systems. The Butterworth filter was the most frequently used. Some studies also used a finite impulse response (FIR) filter [43,44,76,86,88,92,93,108] and Hilbert transform-based filter [117] to minimize the loss of the EMG signals. In this review, 42 out of 51 studies reported the filter type and/or cut-off frequency, as summarized in detail in Fig. 6.
Fig. 6.
The type of filter and cut-off frequency used in each study (42 studies reported the filter type and/or cut-off frequency). The upper panel shows the cut-off frequency of the band-pass filter (purple bars). The lower panel indicates the cut-off frequency of the low-pass filter (red line) and high-pass filter (green line). Shadow areas indicate the type of filters: BW, Butterworth filter; FIR, Finite Impulse Response filter. The studies which did not report the filter type are also shown (named as Others). The number in the bars or below the red (green) line indicates the order of the filter. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
EMG data normalization is performed to allow comparisons between subjects or sessions and for a meaningful interpretation of EMG activation levels and corresponding synergy weights. EMG signals can be normalized to maximum voluntary contraction (MVC), which is measured by performing a set of movements to elicit the maximum contraction of each muscle [130]. However, considering the limitation of practical applications, especially for patients, MVC is often difficult to measure. In this review, twenty-one studies reported the employment of alternative normalization methods, which were classified into five types: MVC (9.52%, percentage of the studies) [99,102], maximum value (MAX, 38.1%) [60,89,90,92,93,98,119,131], average value (AVE, 9.52%) [101,127], or median value (MED, 14.29%) [104,110,115] of the amplitude of the recorded EMG signals, and unit variance (UVA, 28.57%) [75,91,95,108,124,125].
4.5. Number of Synergies and Types of Extraction Algorithms
When extracting muscle synergies, NMF was the most frequently used algorithm. NMF was employed in over 90% of all studies included in this review. In addition, other factorization methods were also used, including factor analysis [108], PCA [107,119], hierarchical alternating least square algorithms (HALS) [112], and multivariate curve resolution–alternating least squares (MCR-ALS) [131]. With respect to synergy models, all studies extracted spatial synergies in upper limb muscle synergy analysis, and few studies also consider temporal and spatiotemporal synergies.
When determining the optimal number of synergies, in general, five criteria were applied based on the reconstruction VAF (or R2) curve, which represents the fraction of total variation accounted for by the synergy reconstruction. VAF is defined as 1-SSE/SSTvaf where SSE is the sum of the squared residuals, SSTvaf is the sum of the squared residuals taken with respect to zero (uncentered) and R2 is defined as 1-SSE/SSTR2, where SSTR2 is the sum of the squared residual from the mean activation vector (centered). From here on, we will use VAF as a generic term for both VAR and R2.
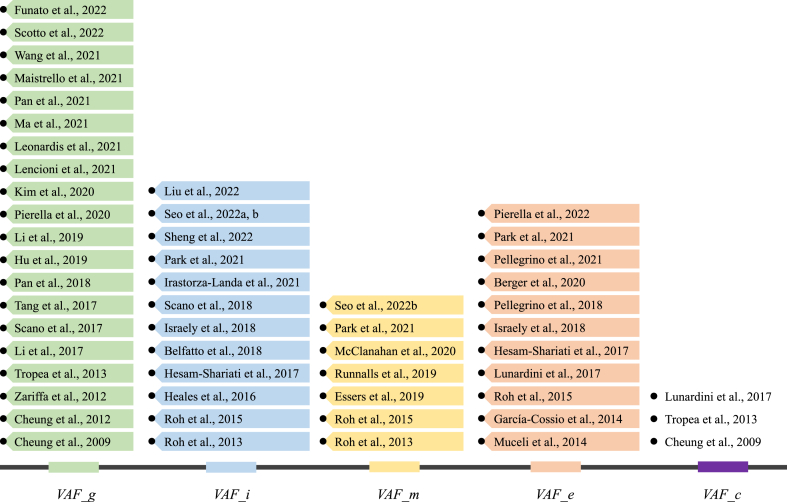
The first criterion is global VAF (VAF_g). The VAF is repeatedly computed when increasing the number of synergies from one to the maximum number of recorded muscles. Once the VAF is above a predefined threshold, the extracted synergy number is chosen. This is the simplest and most widely used criterion, and almost all studies used it. The second criterion is incremental VAF (VAF_i). In addition to satisfying the global VAF, according to this criterion when adding an additional synergy to the chosen number, the increase of the VAF should be below a predefined threshold. The third criterion is muscle VAF (VAF_m). As incremental VAF, in addition to satisfying the global VAF, to select the optimal number of synergies, this criterion requires the VAF of each individual muscle to be above a threshold. The fourth criterion is based on the linear fitting error of the VAF curve [36]. We refer to this criterion as error VAF (VAF_e). First, portions of the VAF curve are fit using the least-square technique from the first point on the VAF curve to the last point. Then, the algorithm proceeds by moving the first point to the second, and so on until the last two points are included. The number of synergies is estimated as the first point on the VAF curve for which the MSE of the linear fit is below a predefined threshold. This criterion is usually combined with one of the first three criteria. The fifth criterion is chance VAF (VAF_c) [76,98,108]. Two VAF curves are generated in this method, one for the original EMG and another for the randomly shuffled EMG samples representing the baseline VAF curve expected from chance. The slope of the baseline VAF curve is almost constant. The optimal number of synergies is defined as the point at which the slope of the VAF curve drops below 75% of the slope of the baseline VAF curve [76]. Fig. 7 summarizes the studies in which the abovementioned criteria were used.
Fig. 7.
Criteria to determine the optimal number of synergies and related studies. Some studies used multiple methods.
4.6. Findings achieved with muscle synergies
The muscle synergy framework proposes that the control of the musculoskeletal system is generated by combining a set of synergies. In the spatial model, the sole used in all the screened papers (except one [60], which also employed temporal and spatiotemporal synergies), each synergy activates a set of muscles with specific weights, and synergies are recruited with time-varying activation coefficients. Synergy weights and activations may be determined at the neural level by the activities of populations of neurons in different structures of the motor system, ranging from the motor cortex to the spinal cord [38,60,76,132]. However, neurological diseases interfere with the neural processing and the generation of appropriate motor commands, e.g., affecting the descending signals from the motor cortical areas to the spinal cord after cortical stroke [43], which may lead to abnormal muscle activations and stereotyped motor patterns. In this section, we briefly report the main characteristics and findings of the selected studies that are summarized in Table 2, Table 3, Table 4 To compare studies with a similar design, we divided them into single-session studies and longitudinal studies.
4.6.1. Single-session studies: evidence of muscle synergy modifications in stroke
In single-session studies with stroke patients (referring to Table 2), muscle synergies have been employed as biomarkers to assess motor capability and impairments, or to compare specific conditions. Papers in this section investigate a variety of topics that range from the characterization of patients with respect to healthy subjects, to testing of various assisted training conditions. Despite the studies are not homogeneous, it is still possible to identify some common findings that are summarized in the next paragraphs.
4.6.1.1. Number of synergies
Some studies aimed at characterizing synergistic patterns in pathologies comparing people with motor disability and healthy people, and showed an increase or decrease in the number of synergies when considering patients with respect to healthy controls. However, this is not always true as many studies extracted the same number of synergies from both the control and experimental groups, or did not find significant differences when the number of synergies was compared between groups. Interestingly, even the most cited studies, which have been used as a reference for many following studies and are based on similar designs, have not always reached the same conclusions. For example, no difference in the number of synergies was found in post-stroke patients in one seminal study [76], while it was found in a follow-up by the same authors [43], in which a cohort of patients with different levels of impairment was enrolled. Despite the heterogeneity which characterizes this group of studies, the conclusion shared by most of them is that the number of synergies is equal or lower in patients and tends to correlate with the increase of the severity of the pathology [43], usually being reduced in number. Thus, the number of synergies may be equal or decrease, and this proves the relevance of the number of synergies as a biomarker for motor disability.
Some studies in the stroke group aimed at comparing mainly the differences between healthy controls and patients considering synergy weights, so the number of extracted synergies was fixed a-priori in both groups. This assumption simplified the analysis, allowing us to compare synergy composition straightforwardly. However, this approach neglects modifications in the dimensionality of the motor commands, as the number of synergies is chosen a-priori. In general, for the upper limb, the number of synergies is highly dependent on the number of selected trials and the optimal criteria for the selection of the number of synergies cannot be easily determined a-priori.
4.6.1.2. Synergy structure
There is a general agreement among all studies in this group on the fact that synergies are modified when motion condition change in studies with stroke patients, based on single session design.
Cheung et al. [43] proposed three changing patterns in the structure of synergies after diseases: preservation, merging, and fractionation. They reported preservation of the muscle synergies in stroke patients with mild-to-moderate impairment (synergies are “intact” in patients); instead, merging (two or more synergies observed in the unimpaired limb appear as “fused together” into a single synergy of the impaired limb) and fractionation (one unimpaired limb synergy is split into more impaired limb synergies) were observed in severely impaired patients, and they depended on the level of the impairment [86,133]. While merging and fractionation are features that may also reflect the criteria used for extraction, it appears as a robust biomarker for motor disability. In fact, the alteration of synergy activations was also observed in subjects with upper limb impairment in further studies [75,104,108,116].
Abnormal synergy structures were characterized in various experimental studies, defining more in detail which joints are mostly affected. Roh et al. [44,75] showed the alteration of synergy weights in stroke patients during isometric force generation, especially regarding two synergies related to shoulder motion. In detail, shoulder adductor/flexor synergy was dominated by the activation of the pectoralis major, while the anterior deltoid was coactivated with medial and posterior deltoids in healthy controls. The alteration was related to the impairment level, further suggesting that synergies are valid biomarkers for motor capability [44]. In other studies, Scano and Kim compared muscle synergy structures between stroke and healthy subjects in reaching movements with clustering analysis [112,117] and found that corresponding clusters of mild and control groups showed the highest similarity compared to the severe group, thus suggesting that synergistic patterns available in functional gestures may characterize the disability of patients also in monodirectional tasks.
Many studies have shown that alterations of synergy weights and activation profiles may be biomarkers related not only with standard clinical assessments (such as clinical scales), but also to peculiar conditions of patients or the experimental constraints, and that these factors are not negligible when performing synergy analysis. Pellegrino and colleagues [110] explored the influence of hemispheric localization on muscle synergies in stroke patients when performing planar reaching movements with and without assistive or resistive forces. They reported that in the absence of force (free motion), the right brain damage group had weights of muscle synergies that were less altered than the left brain damage group in both arms and were more similar between sides [110]. They suggested that brain reorganization and compensatory strategies can induce a relevant bilateral reorganization of muscle coordination that is not mirrored with respect to the side of the lesion but is influenced by hemispheric specialization [110]. Pellegrino and colleagues [103] also indicated that although upper limb movements without external forces were a suitable and simple task to differentiate between stages of diseases, these differences were more evident in isometric tasks or when the external forces change.
García-Cossio and colleagues [87] revealed that patients with damage to the sensorimotor cortex showed high preservation of muscle synergies. On the contrary, patients with intact sensorimotor cortex showed poor muscle synergies preservation and an increase in newly generated synergies. For external constraints, such as arm weight support, a larger number of synergies were usually observed when higher support was provided in mildly impaired patients during reaching movement [123] or elbow isometric tasks [134]. However, moderate-severe patients expressed a constant single muscle synergy at all levels of support during reaching movement [123].
4.6.1.3. Temporal coefficients
A common finding of our selection of single-session studies is that the analysis mostly focused on spatial synergies rather than on temporal coefficients, which were assessed in detail only in a few studies. Available evidence indicates clearly that temporal coefficients can also be used as biomarkers to detect motor impairment [60,118,121]. It was also reported that the difference between the two arms of each subject in terms of activation coefficients was greater in stroke subjects than in controls for all tasks [110]. Moreover, the similarity of the activation coefficients with those of the controls was task-dependent, which was slightly higher when interacting with the resistive force field than in the other tasks [110].
The infrequent evaluation of temporal coefficients is a limiting factor as synergies may be invariant while their recruitment may be altered. In fact, we noted that in most of the selected studies in which temporal components are assessed, they could change in amplitude or similarity in patients compared to the control group even when no differences were found in spatial synergy structure [76]. Especially in such cases, analyzing temporal coefficients is crucial as they may convey information about motor impairments.
4.6.1.4. Synergy correlation with clinical scores and instrumental assessments
Alterations of the number of synergies, spatial synergies, and temporal coefficients can directly be used as quantitative biomarkers to classify and assess diseases. Considering the partial information achievable from single signal sources in describing the integrated neuro-biomechanical status and the dependence of assessment outcomes on assessment tasks, some studies combined kinematic and physiological signals or were based on fused and multidomain methods. Combining kinematics, kinetics, and synergy features, some studies quantified relationships between muscle synergies and clinical scales [76], by extracting synergy-related parameters from muscle synergies and/or synergy activations to assess motor function such as in Ref. [127]. They reported that synergy-based metrics were correlated with upper limb motor function and clinical scores and were able to assess motor function effectively. Wang and collaborators proposed a multi-modality fusion method, in which features extracted from muscle synergies and kinematic synergies fused to objectively quantify upper limb motor impairments of stroke patients [119], and the assessment results exhibited a significant correlation with the clinical score. In contrast, Maistrello and colleagues showed that only one synergy-based parameter could capture the same information conveyed in other domains, suggesting that synergies provide specific data that cannot be accessed or derived from traditional approaches [91]. As the available evidence is still limited and sometimes contradictory, it is indeed an open question whether and to what extent synergy-based metrics provide additional information on pathologies that cannot be deduced with standard clinical assessments.
4.6.2. Single-session studies: evidence of muscle synergy modifications in other pathologies
In single-session studies with patients with other pathologies (Table 3), muscle synergies have been employed as biomarkers to assess motor capability and impairments, or to compare specific conditions. Studies in this section investigate a variety of topics that range from the characterization of patients with respect to healthy controls, to testing of various assisted training conditions. As they analyzed different diseases, it is difficult to provide a systematic comparison. However, we summarize the common findings, when possible.
4.6.2.1. Number of synergies
For this group of studies, the number of synergies was similar between the considered groups, with the exception of studies with cerebral palsy patients [92] and amputees [97], in which synergies were lower in number for patients. It is indeed clear that not all pathologies reduce the number of synergies available to patients. However, there is a general lack of scientific evidence to draw reliable conclusions and most of the studies have no confirmation from the literature due to the lack of follow-up studies.
4.6.2.2. Synergy structure
Papers on other diseases conclude in most of the cases that synergies are altered with respect to healthy people or across experimental conditions. Although stroke and SCI constitute most of the applications, abnormal patterns were captured with synergistic approaches in other neurological diseases, as reported in Table 3. Tang et al. reported fewer abnormal synergies in children with CP than in typically developing children during three upper limb motion tasks [92]. Pellegrino et al. observed reorganization of muscle synergies and alteration of synergy activation in MS subjects but no difference in the number of synergies between the control and MS subjects [103,104]. Abnormal muscle synergies were further observed in the upper limb in other neurological diseases, such as PD [100], SCI [95], LE [101], FSHD [102], MS [104], and muscle pain [96]. As reported for stroke patients, modifications were found in shoulder synergies used for proximal control in patients with muscle pain [96], indicating that the shoulder district is critical for motion control of impaired people.
4.6.2.3. Temporal coefficients
Very few studies evaluated temporal coefficients in pathologies other than stroke. Whereas intact structures and the number of muscle synergies were found, abnormal muscle activation patterns affected the timing and amplitude in most of the cases. All the studies agree in finding altered temporal coefficients with the exception of studies on patients with dystonia, in which only the amplitude of peaks produces a slight reduction [98]. In writer's cramp, patients could generate normal and complex hand postures in non-writing tasks, which showed that these subjects were available to a full repertory of muscle synergies with a normal structure, while abnormal contractions occurred during writing, resulting from decreased surrounding inhibition at the level of the motor cortex [135,136].
A promising approach that employed multiple synergistic models (i.e., spatial, temporal, and spatiotemporal synergies) showed that cerebellar damage affects the temporal and spatiotemporal organization, but not the spatial organization, of the muscle patterns [60], suggesting that such analysis based also on temporal information should be considered in future works.
4.6.3. Muscle synergies for assessing longitudinal clinical interventions: few synergy changes were found
Some studies used muscle synergies as an outcome variable for assessing longitudinal treatments (Table 4). However, this sample of studies is limited (12 in total), and almost all of them (11/12) describe assessments on stroke patients. The design of the studies includes three main approaches, based on free movements, robot-assisted training, or virtual reality training, or a combination of those.
A group of studies used robot-assisted interventions, which allow to deliver of high-intensity and standardized training with the aim of improving motor function of patients. They examined the efficiency of robot-assisted interventions in improving motor performance and increasing the complexity of muscle synergies. However, the underlying relationship between improvements of motor outcome and the reorganization of muscle activity due to the treatment for longitudinal studies is still unclear [108]. In fact, while the results showed that the synergy-based intervention improved clinical scores and movement kinematics, the underlying muscle synergies did not show a clear trend in modification in number and/or composition after treatment. Four studies reported a difference in the number of synergies after treatment, and only two show a change in the spatial synergy composition, that was more healthy-like [115] or modified in a subject-specific way [108]. Moreover, post-stroke subjects who followed robotic rehabilitation showed larger improvements in axial-to-proximal muscle synergies with respect to those who underwent usual care, but treatments had negative effects on muscle synergies controlling the distal part of the limb [89]. No clear evidence of altered temporal coefficients is available as the available evidence is limited.
Patients from these studies cover a wide range of disability levels (severe to mild disease, even within each study) and thus no clear relationship can be deduced to correlate synergy-related measures and clinical achievements with the motor functionality of the enrolled subjects. A common conclusion is that training may affect synergy-related metrics, which is a relevant clinical result that justifies future investigations in the field. However, few studies are available for conclusive inference and most studies suffer from some limitations, which include few investigated gestures, limited assessments, few enrolled subjects, and heterogeneous training conditions, making this field of research a potentially relevant, but still largely unexplored field.
5. Discussion
5.1. Muscle synergies in upper limb clinical rehabilitation: why they are needed
Motor deficits and dysfunction caused by neurological diseases can be assessed using clinical tests such as the Brunnstrom stage, the Fugl-Meyer scale, and the Reaching Performance scale. However, these scales suffer from ceiling/floor effects and lack sensitivity in quantifying detailed characteristics of patients. These features limit the level of detail of subject-specific assessments and treatments. Scale-based assessments also require a professional therapist, and the outcomes are subjective and therapist-dependent. It was reported that in neurological rehabilitation, observing longitudinal changes in motor performance is difficult in terms of patient burden and cost but necessary for the recovery of motor function. In addition, clinical scales focus on behavioral and kinematic outcomes and only offer some descriptive information about movement execution. Limited results have been presented regarding how abnormal performance arises in the context of motor control physiology, and how the CNS regulates motor relearning and neuroplasticity induced by the rehabilitation process [22,106].
Time- and frequency-domain analyses of muscle activity can help clinicians and researchers to describe muscle contraction characteristics. Interpretation of the functional implications of multiple muscle activities during motor tasks and the high dimensionality of control space and variability are complex and multifactorial processes [53]. The combination of muscle synergies provides a framework to describe motor control and coordinated muscle activations, accounting for the hierarchical and modular organization of the CNS. Many studies have described spatial, temporal, or spatiotemporal structures reflected in muscle synergies and reported the relevance of alteration of muscle synergies as indicators of pathological impairments in the number of synergies, synergy composition, and synergy recruitment. Muscle synergy analysis extends clinical assessment and diagnosis from clinical scales, kinematics, and dynamics to the neurophysiological level, providing insights into hierarchical modular control and the interpretation of pathological spinal and cortical patterns.
In fact, with respect to kinematic assessments, synergies reveal the complexity of motor control and relate directly to the neural implementation of the control structure, assessing the dimensionality of the control space, and the spatiotemporal organization of coordinated modules. Muscle synergies also allow to separate neural and musculoskeletal deficits. Moreover, it is known that similar motor outputs can be achieved with different control patterns (as the muscle system is redundant and abundant [137]). These complex coordination features cannot be assessed with kinematics alone. On the same line, single-channel EMG is successfully used for diagnosis of the diseases, but cannot describe the coordination of control modules, which is a typical source of impairment found in neurological patients (e.g., post-stroke patients). Almost all the studies selected in this review concluded that muscle synergy analysis, despite the potential of the methods was only partially exploited, encompasses the key elements for a refined characterization of neurological diseases and, as a consequence, provides clinicians with a tool that can be tailored for effective rehabilitation.
5.2. Clinical evidence achieved with muscle synergies
Muscle synergy analysis may be a useful method for motor function assessment to discriminate a variety of pathological changes induced by lesions of the nervous system, that can be analyzed in terms of synergy number, structure, and activation, clearly adding clinical evidence on the pathophysiology of the diseases.
5.2.1. Single-session studies: stroke
In rehabilitation scenarios, starting with the work of Cheung and collaborators on the upper limbs [76] and Clark and collaborators on the lower limbs of post-stroke patients [45], muscle synergies have been viewed as biomarkers for disability and employed as an assessment method for measuring the motor capability of impaired people.
Clark and collaborators showed that a decrease in the number of synergies in the paretic lower limbs of stroke patients depended on the degree of impairment [45]. Fewer muscle synergies in the paretic limb resulted from the merging of muscle synergies in the nonparetic limbs. A reduction in the number of muscle synergies resulted in dysfunction of independent neural control of distinct biomechanical functions, resulting in a reduction of motor control complexity and poor motor performance. Changes in the number of synergies may result from abnormal descending signals from the motor cortex to the spinal cord [76], which affect the number of independent muscle synergies that can be recruited and increase co-contraction of a large group of muscles. These facts are commonly accepted and shared also in the lower limb domain.
For the upper limb, Cheung and collaborators could relate muscle synergy analysis to clinical assessment [43]. They found preservation, merging, and fractionation of muscle synergies in a group of stroke subjects with different impairment levels during upper limb reaching movements [43]. The preservation of muscle synergies was observed in mildly impaired subjects, whereas merging and fractionation were observed in patients with severe stroke. For the first time, patients could be grouped according to their neural motor control structures, and the description of pathology could be made with more detail.
The intuition that the number and composition of synergies can be successfully used as biomarkers for assessing motor disability was confirmed in recent muscle synergy analyses, that have shown that impaired motor performance (e.g., velocity, accuracy, asymmetry, and smoothness) in neurological patients is related to changes in the number of synergies [45,138,139], in synergy composition [43,44,86,109,118], and in synergy activations [43,44,109,118]. Furthermore, alterations of muscle synergies are related to clinical assessment, that is, a higher number and greater complexity usually are associated with better assessment outputs, such as the Fugl-Meyer scale [44,109] and Brunnstrom stage [86]. This is expected as the observable outcome variables (range of motion, smoothness, kinematics) are related to the control structures.
We remark however that it is difficult to draw clear and conclusive take-home messages for this section since the selected studies did not use the same design, including aim, enrolled patients, and tested conditions; and sometimes, studies with similar design, or from the same authors, did not reach the same conclusions. It is indeed likely that synergies should be evaluated in the framework of multifactorial approaches to extrapolate their informative content in a broader view that incorporate other instrumental or clinical outcome variables. Contradictory findings might be due to different cohorts of enrolled patients, and differences in the experimental design that make investigations not directly comparable. We also think that the findings from single-session studies may be sometimes biased by experimental protocols relying on few gestures and a low number of repetitions with respect to the more structured protocols typically found in synergy analysis studies involving only healthy participants, which typically involve more motion variability but are more challenging with patients. While this is understandable due to limitations found in clinical scenarios, the cooperation of patients, complexity of gestures and available times and equipment are crucial in clinical environments for allowing standardization of methods and the presentation of common patterns that are shared between studies in a reliable way.
5.2.2. Single-session studies: other diseases
There is a consensus on the fact that patients with lower functional levels are associated with modifications of spatial synergy structures and often reduced in number. In contrast to this finding, some studies also reported that spatial synergy modification was not observed with treatments using similarity analyses. The main observation for this class of works is that in other studies, variability between controls and subjects was reported mainly in the temporal or spatiotemporal organization, rather than in spatial synergies, indicating that spatial motor modules are not altered, while their temporal organization of muscle recruitment is. Interestingly, this category falls the only study that employed multiple models for evaluating synergies, comparing spatial, temporal, and spatiotemporal [60]. The spatiotemporal analysis was the one with the highest sensitivity to motor control alterations. It would be necessary in the future to explore how various algorithms and methods capture motor control features exploiting at best their characteristics for clinical applications.
The literature reports a variety of results, sometimes in disagreement, due to several factors, including the study design, the characteristics of the enrolled patients, the aims of the intervention or the evaluation, and the data processing methods. To achieve a reliable comparison of results it would beneficial to adopt standard guidelines for experimental protocols and data processing procedures. However, due to the limited amount of available evidence, it seems that modifications depend on many factors that are typical of each specific pathology and the enrolled group. Clearly, this shows a gap between the potential of the methods and the limited impact of the results achieved so far.
5.2.3. Longitudinal studies
Muscle synergy analysis has also been used in longitudinal studies to assess the effects of rehabilitation therapies. Some studies have shown that various rehabilitation interventions (traditional rehabilitation, high-intensity exercise, VR, and robot-assisted therapy) improve motor function and in some cases induce alterations of synergy-related parameters of the upper or lower limbs in stroke subjects [7,115,120,140,141], such as an increase in the number of synergies and/or improvement in synergy structures that show more complex patterns, or only modifications of temporal coefficients.
As summarized in Table 4, many studies reported alterations of muscle synergy spatial structure after diseases or rehabilitation therapies compared to the control group or baseline levels. Treatments are usually beneficial, but only in a few studies they achieved restoring of physiological synergies in patients; not only, in one of the few longitudinal studies available, it was reported that therapeutical effects may also couple with negative ones [89]. Two out of the three studies in which synergies were modified after treatment are based on multidirectional tasks [108,115]. It is possible that adopting such paradigms, based on directional variability, helps detect synergy modifications as they are more evident when a variety of directions of motion are explored. Even more interestingly, in all studies but one, temporal coefficients were modified, indicating that synergies were recruited differently. These modifications provide a rationale for employing training as it is possible to induce modifications in modular control structures; training synergies might be a matter of improving the recruitment of existing synergies rather than aiming at their reshaping, at least for some classes of patients.
Another limitation that we noticed regards the fact that the protocols for evaluating synergies might be different from the task used in the training; as an example, in Belfatto and collaborators [121], synergies for the assessment are extracted during free motion, while the study is based on a course of robot-assisted movements. This limitation is common to most studies. Moreover, the traditional multi-directional planar set-up allows to detect modulation of synergies through directions, but limits 3D movement and coordination, reducing a lot the clinical meaningfulness of the assessments for patients as the elevation of the limb against gravity is not considered. Since improvements found were often subject-specific [108], it is still an open question to understand which classes of patients can benefit more from robotic therapies.
In recent years, novel synergy-based intervention methods based on functional electrical stimulation have been proposed to restore motor function. These studies showed promising results in improving motor performance and changing the complexity of muscle synergy toward more similar patterns with respect to healthy subjects [51,111].
Another open issue is how to associate robotic or virtual reality [142] treatments with motor rehabilitation based on synergies; we expect this will be a key topic to address, and future works should expand on the neural mechanisms induced with robot assistance and other rehabilitation methods to improve the effects of longitudinal studies.
To fill the gap existing between the improvements in performance and the reduced or absent modification of synergies, recent research has focused on investigating which mechanisms drive the modification in motor control due to robotic operators. Cancrini and collaborators [143] were able to demonstrate that standard paradigms for assistance only slightly modify spatial synergies and temporal coefficients in single-session trials. These results are in line with those from Moreno and collaborators [144] that evaluated the effects of lower limb robotic assistance and support the use of robots to enhance motor recovery, as assistance (i.e., improvement of outcome performances) is achieved without a strong modification of motor patterns measured with synergy assessment. Unfortunately, the transfer of these concepts to clinical environments to create ad-hoc interventions is seen only in a few research studies and is not integrated with clinical practice. In fact, there has not been a strong rationale for using synergies as a method for customizing therapies so far; synergies have been only used for assessment.
5.3. Barriers for interpreting data and preventing systematic adoption of muscle synergies in clinical scenarios
5.3.1. Experimental protocols: a variety of approaches
Regarding patients and participants, this review investigated 13 diseases and included a total of 748 patients. Although seven studies recruited over 30 patients, on average previous studies enrolled 15 patients. Objectively, this number is not high enough to fully generalize the results, and only evidence on stroke patients is available in reasonable number to extract preliminary generalizable results. In addition, the vast majority of studies ignored some pathological information (e.g., the type of lesion and position) when analyzing and interpreting muscle synergies [86,145], while in the literature it was shown that the severity of impairment [44] and the side of the stroke lesion [110] affects the structure of muscle synergies. These studies recruited patients with different impairment levels and at different stages of recovery, which further weakened the explanation of the results which was often addressed with subject-specific findings with limited generalization power. Second, most studies recorded 4 to 16 EMG signals from the upper limbs, upper body, and hands. There was no standard set of recommended muscles for a specific task, even though previous work showed that the number and choice of muscles affect the results of muscle synergy analysis [72]. Besides, experimental tasks, biomechanical and task constraints [123,146], and body position [146] are all features that influence synergy patterns. Thus, the lack of uniformity in the various studies represents a strong limitation when attempting to summarize and generalize the results of the research performed so far.
Moreover, in the vast majority of the cases, synergy-based assessments are not driven by clinical EMG-based analysis (with the exception of [106]), and muscle synergies are only used to measure the effect of standard treatments, or to quantify differences in single session studies. Future research should test synergistic approaches “in the loop” to guide therapies rather than being used only to measure their outcomes, possibly through the design of synergy-driven treatments that aim at restoring specific modules that are missing or recruited “incorrectly”.
5.3.2. Signal processing and synergy extraction: the opportunity for new approaches
When performing EMG preprocessing, several steps are followed to obtain EMG envelopes for synergy extraction, including high-pass or band-pass filtering, rectification, low-pass filtering, and normalization. Excluding rectification, each step requires the selection of some parameters, such as the order and cut-off frequency of the filter and normalization methods, both of which affect the results of synergy extraction [73,147]. Kieliba and collaborators [74] analyzed how variations in filter cut-off frequencies and normalization methods affect synergy weights and inter-subject similarity using experimental data. They showed that the normalization methods (MVC and MAX) and band-pass filters (20–500 Hz and 50–500 Hz) did not significantly alter synergy weights. However, normalization alters the VAF, and an increase in the cut-off frequency of the low-pass filter decreased the VAF [74]; consequently, they affected the number of extracted synergies. Santuz and collaborators [78] reported that a fourth-order filter configuration with a cut-off frequency of 50 Hz (high-pass) and 20 Hz (low-pass) was the most suitable to minimize the combination of fundamental synergies for human locomotion.
Currently, despite several methods being available for determining the number of synergies to extract, clear guidelines are not available, especially when considering multiple datasets to compare. The selection of the number of synergies impacts on composition, synergy sparseness, and temporal coefficients, as well as many features related to the pathology such as merging and fractionation. Thus, it is suggested to verify the robustness of the results achieved at multiple VAF/R2 thresholds or with multiple synergy selection criteria [80]. Setting a golden standard method would allow a clear step toward standardized clinical use and inter-study data operability.
Several synergy models have been developed, but not related to pathology with a strong neurophysiological foundation. In the vast majority of papers, the NMF-based spatial model was used. The neurophysiological basis of this model was validated in pilot studies with animals [29,32,33,38] and humans [[39], [40], [41], [42]]. However, limiting the analysis to this approach can disregard neural processes related to motor learning and recovery; in about all papers, the spatial synergy model was adopted. This “limitation” is however also an opportunity as it opens the field for a variety of assessments and comparisons that may increase substantially the impact of the findings achieved so far.
The temporal model is a dual of the spatial model as it assumes that temporal synergies are invariant and the muscle weights can vary. This model is seldom used, even though there is much evidence in the literature that states that typically EMG activations are organized in bursts reflecting a specific temporal organization [39,61,148]. The space-by-time model proposes that invariant synergies are available both spatially and temporally, but it was applied only to healthy participants [149,150]. Asynchronous synergies can also be extracted with time-varying synergies [29]. This approach emphasizes an inherent spatiotemporal structure and can reveal specific alterations that may be associated with the diseases. Almost all of the above facts are based spatial synergy model that was applied in the selected studies. In clinical practice, only a few applications are available for most of the developed algorithms, such as for cerebellar patients [60]. A recent study found that three muscle synergy models (spatial synergies, temporal synergies, and spatiotemporal synergies) captured different features in the abnormal muscle patterns of cerebellar ataxia patients [60]. They reported that, while spatial muscle synergies did not differ between patients and age-matched controls, only spatiotemporal synergies could accurately capture the altered muscle patterns of the patients, and the reconstruction quality of spatiotemporal synergies correlated with the severity of impairments. At a neurophysiological level, none of these approaches was validated in a definitive way, even though their applicability in the clinical scenario may unveil mechanisms underlying motor recovery. Novel approaches include Mixed Matrix Factorization (MMF) [151], which creates a link between EMG activations and output variables (such as kinematics, output forces, or joint torques). It is possible the development of novel algorithms will foster the use of synergies in the clinical environment. It was shown that the choices and initialization of methods also influenced the synergy analysis [73,152].
5.3.3. Neural substrates of muscle synergies: limited understanding of the underlying pathophysiology
A huge gap exists in the identification of the neural substrates that implement synergies. While spatial and temporal synergies are probably implemented at the spinal level [29,33,36,37], they might be different elements of a hierarchical structure dedicated to the coordination of motor control. While spatial synergies are implemented as pools of motoneurons and interneurons connected at the spinal level, temporal synergies might be part of a controller at the cortical level dedicated to implementing motor timing. Ad-hoc experiments should be designed to provide a more comprehensive and global correlation between models and their neural implementation in order to better understand their potential applications. It would be useful to define which models better capture the specific features of a pathology depending on the lesioned structures and exploit it accordingly. Unfortunately, it should be also noted that the vast majority of muscle synergy studies are conducted by researchers with computational or bio-engineering backgrounds and the connection between the method and the pathophysiology may be partially lost when interpreting the results. We thus suggest that basic research should be fostered to increase the comprehension of the methods and connected them to the underlying physiology. Lastly, a poor or null connection between muscle synergy findings is available with motor control theories such as Latash's notion of synergies [153], the uncontrolled manifold theory [154], the internal model theory [155], the equilibrium control hypothesis [156] for global integration in a coherent framework. Lastly, gaps exist in the use of the methods and in the misinterpretation of the signals that are analyzed. EMGs are usually input to the algorithms for synergy extraction without considering that EMG is the summation of various components. In fact, EMG signals include phasic (motion-related) and tonic (static) components, that might be not generated by the same neural pathways [157]; EMG signals derive from the firing of motoneurons driven by many neural sources, such as pyramidal and reflex-based pathways, reflecting different layers of neural organization. We conclude that muscle synergy applications in clinics should be built on strong neurophysiological foundations and accurate modeling to produce shared and effective results.
5.3.4. Applicability and logistic challenges for applications of muscle synergies
5.3.4.1. Clinical vs motor control approach
Systematically performing muscle synergy analysis usually requires a large effort, including an appropriate experimental design, subject recruitment, experiment preparation, clinical time-compliant procedures, and expensive equipment to record multi-channel EMG. Moreover, the complex setup usually employed in research studies [158], as well as subjects’ availability to perform a variety of movements to elicit the repertoire of muscle synergies, cannot always be applied in clinical scenarios.
In fact, a major challenge for the field is to coherently harmonize the peculiar features of the muscle synergy method and clinical requirements when designing the experiments. Motor control studies on healthy participants aim at investigating a high variety of motions, as it is inherent in the application of the muscle synergy method that multiple directions of motion and variability should be analyzed to extract meaningful synergies; however, this is hardly achieved with patients, especially when with severe impairment. Clinicians tend to concentrate on functional movements, or “decomposed” single-joint tasks to rehabilitate, that well represent practical motion capabilities but are usually subsets of those used with healthy participants and lack repetitions and variability. Thus, such sets of movements produce sub-optimal estimations of the repertoire of available synergies, or the extraction of task-specific synergies that reflect features of EMG activations rather than invariant synergies recruited in a variety of tasks. These different philosophies are found in our selection of studies, in which clinical-driven, single, high intensity tasks [86,117], that focus on clinical needs, do not fully exploit the potential of the method as not enough motion variability is explored. On the contrary, when a rich variety of motions are analyzed, such as by Roh et al. [75], motor control studies may achieve a complexity that goes beyond the clinical needs and may be impractical with patients. Therefore, clinical tests should strive to find a compromise that captures the essence of research protocols but still remains feasible to effectively transfer the synergy method to the clinic. In such a sense, protocols that do not include enough variability, as well as standard multidirectional planar motion that misses motion variability in the 3D space, or relevant clinical requirements of functional gestures, might not be suitable for applying muscle synergies to clinical scenarios. A good compromise could be those from Irastorza-Landa et al. [127], in which synergies are extracted from a reasonable variety of movements and gestures (motor control-based design), however reflecting functional gestures useful for the clinics (clinical-based design). Thus, future directions may foresee to extract synergies from “complete” gestures, encompassing multi-joint and multi-limb complex coordination.
Following these varieties of approaches, it is not surprising that the results of muscle synergy analysis were not always consistent among studies, and the outputs were affected by this basic design choice, worsened by a further element of variability found in the methods used in signal processing and synergy extraction.
5.3.4.2. Multi-session and environmental limitations
Longitudinal assessments based on muscle synergies show the same critical issues related to signal processing and interpretation common to all muscle synergy-based studies, which worsen when inter-session assessments are considered. It was recently shown that variability between experimental sessions (i.e., before and after treatment) may affect the interpretation of the extracted synergies: given a set of tasks, even in healthy people, synergy inter-session similarity can be lower than intra-session similarity, capturing changes that are not related to neural modifications [159].
Synergy patterns (including the number of synergies, synergy weights, and synergy activations) can be used as physiological markers to assess motor function and the efficiency of rehabilitation approaches. Combining synergies with kinematic parameters, it is possible to fully characterize motor impairment at both behavioral and muscular levels in individuals with neurological diseases. However, studies have shown that muscle synergies are affected by numerous intrinsic factors and external conditions. Consequently, rehabilitation procedures aimed at correcting abnormal synergy patterns into normal patterns should consider environmental and external conditions. Even though it is still an open issue if complete recovery of motor abilities after neurological diseases may occur by recovering muscle synergies matching those from healthy subjects (or healthy side) or other new solutions [103], muscle synergies may be used for the development of personalized intervention protocols to promote the functional recovery. It is however agreed that muscle synergies can capture neural modifications at many levels and should be included more in the clinical decisional process rather than being only a metric for motor evaluation.
The partial inconsistency in the results on the one hand can be attributed to variability among the subjects. One recent study showed higher variability between subjects than intra-subjects during multidirectional reaching movements in healthy subjects in a poorly constrained scenario, indicating that large variability is commonly found in daily life movements [160]. For patients, variability may be derived from subject-specific characteristics, disease type, lesion type and position, severity, time of onset, and other factors, making it difficult to compare the outputs between studies. However, for a specific type of disease, such as cerebral stroke, the inconsistencies can be reduced by standardizing experimental protocols and analysis methods such as similar intervention approaches, movement protocols, and data processing pipelines. This process is complicated because patients may exhibit various levels of disability, resistance to fatigue, cooperativeness, and compliance to the protocol. Methodological standardization of experimental protocols has been the main obstacle impeding direct comparison of the results of the studies included in the current analysis.
5.4. Standardization of upper-limb muscle synergy analysis
As discussed above, it is hard to build a benchmark for upper limb muscle synergy analysis due to the lack of clear and uncontroversial evidence. However, standardization of these methods could promote comparisons among studies, consistent conclusions, and speed up the translation of laboratory research to clinical applications. Recently, attempts at standardizing approaches for the upper limb have been proposed for clinical practice and they include muscle synergy assessments [161].
When recruiting patients, detailed recordings including basic and pathological information, such as sex, age, onset time, degree of impairment, lesion location, and rehabilitation program, should be elaborated, which contribute to the explanation of the outputs and comparisons among studies. In terms of the experimental setups, one recent study [162] revealed that in order to capture sufficient variability of the task and reduce the influence of random factors, at least five repetitions and four reaching directions are required when performing the reaching tasks; it is in any case needed to find a synthesis between the clinical needs and the requirements of motion variability inherent to the muscle synergy method. As for the number and selection of muscles, there is also no standard recommendation, while some upper limb muscles have been largely used to record muscle activities (Fig. 5) for gross motor function, such as deltoid anterior/medial/posterior, pectoralis major, brachioradialis, biceps brachii, triceps brachii, trapezius upper, they can be regarded as a reference when characterizing muscle synergies of upper limb movements. Recommendations for fine movements are harder to provide as muscles suffer from cross-talk and should be chosen carefully.
The influence of EMG processing methods on muscle synergy analysis has not been fully assessed. Several studies reported that high-pass or band-pass filter cut-off frequency and order had few influences on the muscle synergy outputs [74,78], while low-pass filter cut-off frequency significantly affected the amount of variance accounted for, the number of identified synergies, and synergy weights and activations [74,78,[163], [164], [165]]. In general, the higher the cut-off frequency of the low-pass filter, the lower the VAF, and consequently the higher the number of extracted muscle synergies [163]. Thus, caution must be taken when choosing these parameters. Referring to previous works [44,75,86,92,109], 5–20 Hz is an option range.
For the normalization methods, previous studies only evaluated a subset of the methods considered in this review. Kieliba and colleagues [74] assessed the influence of MVC and MAX, and Shuman and colleagues [165] compared the MAX and UVA. A comprehensive evaluation is lacking. Generally, MVC is the most appropriate method, even though it is hard to measure. Thus, it is suggested to use MAX to normalize muscle activation, which is also the most used method according to our screening, at least as long as a sufficient variety of tasks and conditions is considered.
For the criteria to identify the optimal number of synergies, Hug and collaborators [163] reported a significant dependency of the number of identified synergies to the low-pass filter cut-off frequency when fixed threshold (global VAF) or linear fitting methods (error VAF) were applied, indicating the interaction relationship between cut-off frequency and criteria. Considering some unexplored factors, when determining the number of synergies, we recommend using at least two of the five criteria summarized in this review to identify the number of synergies (Fig. 7) and verify their agreement.
Even though several synergy extraction methods and synergy models were used in muscle synergy analysis, NMF and spatial synergies were used in most of the studies. A recent study reported that NMF was the most appropriate method for synergies extraction in walking and running [166]. It is easy to understand the physiological basis and pathological alterations of muscle synergies by analyzing spatial synergies. However, novel algorithms and approaches must be tested to gain new insights and develop the field as it was demonstrated that some approaches may show effects not visible with standard spatial synergies.
Muscle synergies analysis in the theoretical foundation and methodological is still an open issue, thus the above suggestions are for reference only and a large effort is needed in future studies.
We also noted that the few studies that analyzed temporal components always found their modification with respect to reference conditions. In some cases, when comparing conditions, invariant spatial synergies are matched with modified temporal coefficients; these would be evidence of structured spatial control but handled with different recruitment. The analysis of temporal coefficients when assessing synergies is then encouraged, as it is almost always done in gait studies. However, it should be mentioned that temporal coefficients might be hard to compare since they refer to paired synergies (across subjects or conditions) that share a similar, but not identical structure. Interestingly, in a study, the spatiotemporal organization was studied on cerebellar patients, and the authors noted that this analysis could reveal differences not evident with standard spatial synergies and temporal coefficients [60].
Standardization of analyses is harder to achieve with upper limbs, due to the high versatility and variability of the tasks that can be performed. However, the upper limb domain probably emphasizes the role of synergies, that allow to assess coordinated spatiotemporal parameters used for motion control.
5.5. Future work
Although muscle synergy analysis is emerging as a promising and useful tool in the clinical field, several opportunities and challenges need to be addressed in future work.
First, the main gap to fill is the lack of clear guidelines for the use of muscle synergies in clinical applications. Given that studies found alterations of muscle synergies after diseases, and muscle synergies are relevant biomarkers for clinical scenarios, there is no general consensus on how these changes are produced and how they change when patients with different lesion locations and levels or different diseases are considered [110]. The generation and recovery of disease is a long-term process, especially for neurological diseases. However, to date, studies have focused on some specific stages, such as the early or chronic stages of stroke. Tracking the evolution of a disease is more important for clinical prediction, assessment, and therapy. These require large trials, data recordings, and detailed patient information including measurements and clinical data. Moreover, so far, muscle synergies have mainly been used as biomarkers or descriptors, but therapies and treatments have rarely been designed to exploit muscle synergies. A promising new direction to exploit muscle synergies for therapy comes from myoelectric control and virtual reality [167,168]. In a virtual environment, assistance to an impaired limb may be provided by re-mapping dysfunctional muscle patterns into functional ones according to their similarity to personalized reference synergies, thus promoting adaptive plasticity [142,169]. Synergy-based myoelectric control may be also used to allow impaired individuals to control assistive devices [170]. Finally, synergies are rarely -or never-included in the clinical decision-making process, limiting the impact of muscle synergies on clinical practice. A clear trade-off between clinical needs and methodological specificity of muscle synergy analysis should be found and resolved in order to produce a meaningful study design and comparability between results.
Second, most studies usually only measured EMG signals. Although some studies captured kinematic data, they were only used for motion segmentation. Diseases usually induce multidimensional changes in physiological and kinematic measurements. The simultaneous measurement of these signals [121,171], such as EMG, EEG, ECG, kinematics, and clinical scales, is required to learn more about disease generation and recovery. Furthermore, this will contribute to the applications of muscle synergy analysis as clinical biomarkers. Further developments may also include novel algorithms that factorize EMG together with signals from other domains, such as kinematics as in the mixed-matrix factorization (MMF) algorithm [151].
Multidomain approaches are also connected to the prediction of the outcomes. Many studies have attempted to identify the relationships between biomarkers of recovery and the course of therapy. For example, Trujillo and collaborators established a relationship between EEG-based indices and the effects of therapy [172], which provided valuable information for clinical decision-making in rehabilitation fields. Similar concepts could be exploited when using muscle synergies. Muscle synergy patterns could be related to some expectation of recovery under various therapeutic methods and help clinicians to enroll patients in specific treatments that are more likely to enhance their recovery.
6. Conclusion
This review focused on muscle synergy analysis of human upper limbs in clinical assessment, diagnosis, and rehabilitative therapy. In general, the reviewed studies have demonstrated alterations of muscle synergies after disease compared to healthy subjects and revealed the feasibility and the potential of muscle synergy analysis in clinical applications for assessment and decision-making. However, the disease type, severity, lesion location, and other pathological factors might influence the expression of muscle synergies. Current studies mostly ignored these issues, which should be addressed to progress toward synergy-based rehabilitation. Moreover, the experimental protocols and methods used to extract muscle synergies varied among studies, and these differences have constituted a major obstacle to comparing the results among studies. Clear results from longitudinal studies are missing for transferring experimental research to clinical scenarios. Thus, there is a clear need for the development of standard experimental protocols and optimal signal processing methods, which would greatly increase the impact of muscle synergy analysis in clinical practice, as well as relating more directly synergy models to the specific pathologies and the underlying pathophysiological mechanisms. Even though current synergy models can explain some motor control mechanisms and capture abnormal synergies, the neural implementation of motor control processes by specific CNS structures remains largely unknown. Multimodal or multiparameter methods should be developed to deepen the understanding of the neural organization of muscle synergies and to promote the application in rehabilitation in future work.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Funding statement
This research was funded by the National Key Research and Development Program of China (Grant No., 2022YFC2405600), the Leading-edge Technology and Basic Research Program of Jiangsu (Grant No., BK20192004D), the Key Research and Development Program of Jiangsu (Grant No., BE2022160).
Declaration of competing interest
The authors declare no conflict of interest.
Contributor Information
Kunkun Zhao, Email: kkzhao@njmu.edu.cn.
Jianqing Li, Email: jqli@njmu.edu.cn.
References
- 1.Johnson C.O., Nguyen M., Roth G.A., Nichols E., Abate D., Abdelalim A. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beghi E., Giussani G., Nichols E., Abd-Allah F., Abdela J., Abdelalim A. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:357–375. doi: 10.1016/S1474-4422(18)30454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z., Yuan W. Central plasticity and dysfunction elicited by aural deprivation in the critical period. Front. Neural Circ. 2015;9 doi: 10.3389/fncir.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turon M., Fernández-Gonzalo S., de Haro C., Magrans R., López-Aguilar J., Blanch L. Mechanisms involved in brain dysfunction in mechanically ventilated critically ill patients: implications and therapeutics. Ann. Transl. Med. 2018;6:30. doi: 10.21037/atm.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson J.L., Lamontagne A., De Serres S.J. The coordination of upper and lower limb movements during gait in healthy and stroke individuals. Gait Posture. 2009;29:11–16. doi: 10.1016/j.gaitpost.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Hesam-Shariati N., Trinh T., Thompson-Butel A.G., Shiner C.T., McNulty P.A. A longitudinal electromyography study of complex movements in poststroke therapy. 2: changes in coordinated muscle activation. Front. Neurol. 2017;8:277. doi: 10.3389/fneur.2017.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues M.R.M., Slimovitch M., Chilingaryan G., Levin M.F. Does the Finger-to-Nose Test measure upper limb coordination in chronic stroke? J. NeuroEng. Rehabil. 2017;14:6. doi: 10.1186/s12984-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomita Y., Rodrigues M.R.M., Levin M.F. Upper limb coordination in individuals with stroke: poorly defined and poorly quantified. Neurorehabilitation Neural Repair. 2017;31:885–897. doi: 10.1177/1545968317739998. [DOI] [PubMed] [Google Scholar]
- 10.Mandon L., Boudarham J., Robertson J., Bensmail D., Roche N., Roby-Brami A. Faster reaching in chronic spastic stroke patients comes at the expense of arm-trunk coordination. Neurorehabilitation Neural Repair. 2016;30:209–220. doi: 10.1177/1545968315591704. [DOI] [PubMed] [Google Scholar]
- 11.Foreman M.H., Engsberg J.R. A virtual reality tool for measuring and shaping trunk compensation for persons with stroke: design and initial feasibility testing. J. Rehabil. Assist. Technol. Eng. 2019;6 doi: 10.1177/2055668318823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai S., Li G., Su E., Wei X., Huang S., Ma K., Zheng H., Xie L. Real-time detection of compensatory patterns in patients with stroke to reduce compensation during robotic rehabilitation therapy. IEEE J. Biomed. Health Inform. 2020;24:2630–2638. doi: 10.1109/JBHI.2019.2963365. [DOI] [PubMed] [Google Scholar]
- 13.Fugl-Meyer A.R., Jääskö L., Leyman I., Olsson S., Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 14.Mathiowetz V., Weber K., Kashman N., Volland G. Adult norms for the nine hole peg test of finger dexterity. Occup. Ther. J. Res. 1985;5:24–38. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 15.Wolf S.L., Catlin P.A., Ellis M., Archer A.L., Morgan B., Piacentino A. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 16.Levin M.F., Desrosiers J., Beauchemin D., Bergeron N., Rochette A. Development and validation of a scale for rating motor compensations used for reaching in patients with hemiparesis: the reaching performance scale. Phys. Ther. 2004;84:8–22. [PubMed] [Google Scholar]
- 17.de Luna Cabrai J.R., Crepaldi B.E., de Sambuy M.T.C., da Costa A.C., Abdouni Y.A., Chakkour I. Evaluation of upper-limb function in patients with obstetric palsy after modified sever-l’episcopo procedure. Revista Brasileira de Ortopedia. 2012;47:451–454. doi: 10.1016/S2255-4971(15)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S.I., Adans-Dester C., O'Brien A., Diaz G.V., Black-Schaffer R., Patel S., Zafonte R., Bonato P. Using wearable motion sensors to estimate longitudinal changes in movement quality in stroke and traumatic brain injury survivors undergoing rehabilitation. Arch. Phys. Med. Rehabil. 2016;97:e117. [Google Scholar]
- 19.Lee S., Lee Y.-S., Kim J. Automated evaluation of upper-limb motor function impairment using fugl-meyer assessment. IEEE Trans. Neural Syst. Rehabil. Eng. 2018;26:125–134. doi: 10.1109/TNSRE.2017.2755667. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.I., Adans-Dester C., Obrien A., Vergara G., Black-Schaffer R.M., Zafonte R., Dy J., Bonato P. Predicting and monitoring upper-limb rehabilitation outcomes using clinical and wearable sensor data in brain injury survivors. IEEE Trans. Biomed. Eng. 2020:1. doi: 10.1109/TBME.2020.3027853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rast F.M., Labruyère R. Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. NeuroEng. Rehabil. 2020;17:148. doi: 10.1186/s12984-020-00779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ona Simbana E.D., Sanchez-Herrera Baeza P., Jardon Huete A., Balaguer C. Review of automated systems for upper limbs functional assessment in neurorehabilitation. IEEE Access. 2019;7:32352–32367. [Google Scholar]
- 23.Schwarz A., Kanzler C.M., Lambercy O., Luft A.R., Veerbeek J.M. Systematic review on kinematic assessments of upper limb movements after stroke. Stroke. 2019;50:718–727. doi: 10.1161/STROKEAHA.118.023531. [DOI] [PubMed] [Google Scholar]
- 24.Farina D., Merletti R., Enoka R.M. The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 25.De Luca C.J., Donald Gilmore L., Kuznetsov M., Roy S.H. Filtering the surface EMG signal: movement artifact and baseline noise contamination. J. Biomech. 2010;43:1573–1579. doi: 10.1016/j.jbiomech.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Campanini I., Disselhorst-Klug C., Rymer W.Z., Merletti R. Surface EMG in clinical assessment and neurorehabilitation: barriers limiting its use. Front. Neurol. 2020;11:934. doi: 10.3389/fneur.2020.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bizzi E., Cheung V.C.K., d'Avella A., Saltiel P., Tresch M. Combining modules for movement. Brain Res. Rev. 2008;57:125–133. doi: 10.1016/j.brainresrev.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting L.H., Chiel H.J., Trumbower R.D., Allen J.L., McKay J.L., Hackney M.E., Kesar T.M. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron. 2015;86:38–54. doi: 10.1016/j.neuron.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.d'Avella A., Saltiel P., Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat. Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- 30.Torres-Oviedo G., Macpherson J.M., Ting L.H. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 2006;96:1530–1546. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- 31.Overduin S.A., d'Avella A., Roh J., Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J. Neurosci. 2008;28:880–892. doi: 10.1523/JNEUROSCI.2869-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ting L.H., Macpherson J.M. A limited set of muscle synergies for force control during a postural task. J. Neurophysiol. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- 33.Tresch M.C., Saltiel P., Bizzi E. The construction of movement by the spinal cord. Nat. Neurosci. 1999;2:162–167. doi: 10.1038/5721. [DOI] [PubMed] [Google Scholar]
- 34.Saltiel P., Wyler-Duda K., D'Avella A., Tresch M.C., Bizzi E. Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog. J. Neurophysiol. 2001;85:605–619. doi: 10.1152/jn.2001.85.2.605. [DOI] [PubMed] [Google Scholar]
- 35.Hart C.B. Modular premotor drives and unit bursts as primitives for frog motor behaviors. J. Neurosci. 2004;24:5269–5282. doi: 10.1523/JNEUROSCI.5626-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung V.C.K. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J. Neurosci. 2005;25:6419–6434. doi: 10.1523/JNEUROSCI.4904-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.d'Avella A., Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc. Natl. Acad. Sci. USA. 2005;102:3076–3081. doi: 10.1073/pnas.0500199102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overduin S.A., d'Avella A., Carmena J.M., Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron. 2012;76:1071–1077. doi: 10.1016/j.neuron.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanenko Y.P., Poppele R.E., Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion: basic muscle activation patterns. J. Physiol. 2004;556:267–282. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss E.J., Flanders M. Muscular and postural synergies of the human hand. J. Neurophysiol. 2004;92:523–535. doi: 10.1152/jn.01265.2003. [DOI] [PubMed] [Google Scholar]
- 41.d'Avella A., Portone A., Fernandez L., Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J. Neurosci. 2006;26:7791–7810. doi: 10.1523/JNEUROSCI.0830-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres-Oviedo G., Ting L.H. Muscle synergies characterizing human postural responses. J. Neurophysiol. 2007;98:2144–2156. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- 43.Cheung V.C.K., Turolla A., Agostini M., Silvoni S., Bennis C., Kasi P., Paganoni S., Bonato P., Bizzi E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. U. S. A. 2012;109:14652–14656. doi: 10.1073/pnas.1212056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh J., Rymer W.Z., Beer R.F. Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front. Hum. Neurosci. 2015;9:6. doi: 10.3389/fnhum.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark D.J., Ting L.H., Zajac F.E., Neptune R.R., Kautz S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010;103:844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung V.C.K., Cheung B.M.F., Zhang J.H., Chan Z.Y.S., Ha S.C.W., Chen C.-Y., Cheung R.T.H. Plasticity of muscle synergies through fractionation and merging during development and training of human runners. Nat. Commun. 2020;11:4356. doi: 10.1038/s41467-020-18210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artemiadis P.K., Kyriakopoulos K.J. EMG-based control of a robot arm using low-dimensional embeddings. IEEE Trans. Robot. 2010;26:393–398. [Google Scholar]
- 48.Furui A., Eto S., Nakagaki K., Shimada K., Nakamura G., Masuda A., Chin T., Tsuji T. A myoelectric prosthetic hand with muscle synergy-based motion determination and impedance model-based biomimetic control. Sci. Robot. 2019;4 doi: 10.1126/scirobotics.aaw6339. [DOI] [PubMed] [Google Scholar]
- 49.Ferrante S., Chia Bejarano N., Ambrosini E., Nardone A., Turcato A.M., Monticone M., Ferrigno G., Pedrocchi A. A personalized multi-channelchannel FES controller based on muscle synergies to support gait rehabilitation after stroke. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung V.C.K., Niu C.M., Li S., Xie Q., Lan N. A novel FES strategy for poststroke rehabilitation based on the natural organization of neuromuscular control. IEEE Rev. Biomed. Eng. 2019;12:154–167. doi: 10.1109/RBME.2018.2874132. [DOI] [PubMed] [Google Scholar]
- 51.Niu C.M., Bao Y., Zhuang C., Li S., Wang T., Cui L., Xie Q., Lan N. Synergy-based FES for post-stroke rehabilitation of upper-limb motor functions. IEEE Trans. Neural Syst. Rehabil. Eng. 2019;27:256–264. doi: 10.1109/TNSRE.2019.2891004. [DOI] [PubMed] [Google Scholar]
- 52.Torricelli D., Barroso F., Coscia M., Alessandro C., Lunardini F., Bravo Esteban E., d'Avella A. In: Emerging Therapies in Neurorehabilitation II. Pons J.L., Raya R., González J., editors. Springer International Publishing; Cham: 2016. Muscle synergies in clinical practice: theoretical and practical implications; pp. 251–272. [Google Scholar]
- 53.Safavynia S., Torres-Oviedo G., Ting L. Muscle synergies: implications for clinical evaluation and rehabilitation of movement. Top. Spinal Cord Inj. Rehabil. 2011;17:16–24. doi: 10.1310/sci1701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Israely S., Leisman G., Carmeli E. Neuromuscular synergies in motor control in normal and poststroke individuals. Rev. Neurosci. 2018;29:593–612. doi: 10.1515/revneuro-2017-0058. [DOI] [PubMed] [Google Scholar]
- 55.Hong Y.N.G., Ballekere A.N., Fregly B.J., Roh J. Are muscle synergies useful for stroke rehabilitation? Curr. Opin. Biomed. Eng. 2021;19 [Google Scholar]
- 56.Mileti I., Zampogna A., Santuz A., Asci F., Del Prete Z., Arampatzis A., Palermo E., Suppa A. Muscle synergies in Parkinson's disease. Sensors. 2020;20:3209. doi: 10.3390/s20113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee W.A. Neuromotor synergies as a basis for coordinated intentional action. J. Mot. Behav. 1984;16:135–170. doi: 10.1080/00222895.1984.10735316. [DOI] [PubMed] [Google Scholar]
- 58.Bizzi E., Mussa-Ivaldi F., Giszter S. Computations underlying the execution of movement: a biological perspective. Science. 1991;253:287–291. doi: 10.1126/science.1857964. [DOI] [PubMed] [Google Scholar]
- 59.Russo M., D'Andola M., Portone A., Lacquaniti F., d'Avella A. Dimensionality of joint torques and muscle patterns for reaching. Front. Comput. Neurosci. 2014;8 doi: 10.3389/fncom.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berger D.J., Masciullo M., Molinari M., Lacquaniti F., d'Avella A. Does the cerebellum shape the spatiotemporal organization of muscle patterns? Insights from subjects with cerebellar ataxias. J. Neurophysiol. 2020;123:1691–1710. doi: 10.1152/jn.00657.2018. [DOI] [PubMed] [Google Scholar]
- 61.Ivanenko Y.P., Cappellini G., Dominici N., Poppele R.E., Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J. Neurosci. 2005;25:7238–7253. doi: 10.1523/JNEUROSCI.1327-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.d'Avella A., Tresch M.C. Advances in Neural Information Processing Systems 14 - Proceedings of the 2001 Conference. 2002. Modularity in the motor system: decomposition of muscle patterns as combinations of time-varying synergies; pp. 141–148. [Google Scholar]
- 63.Delis I., Panzeri S., Pozzo T., Berret B. A unifying model of concurrent spatial and temporal modularity in muscle activity. J. Neurophysiol. 2014;111:675–693. doi: 10.1152/jn.00245.2013. [DOI] [PubMed] [Google Scholar]
- 64.Krishnamoorthy V., Latash M.L., Scholz J.P., Zatsiorsky V.M. Muscle synergies during shifts of the center of pressure by standing persons. Exp. Brain Res. 2003;152:281–292. doi: 10.1007/s00221-003-1574-6. [DOI] [PubMed] [Google Scholar]
- 65.Tresch M.C., Cheung V.C.K., d'Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J. Neurophysiol. 2006;95:2199–2212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- 66.Wakeling J.M., Horn T. Neuromechanics of muscle synergies during cycling. J. Neurophysiol. 2009;101:843–854. doi: 10.1152/jn.90679.2008. [DOI] [PubMed] [Google Scholar]
- 67.Lee D.D., Seung H.S. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401:788–791. doi: 10.1038/44565. [DOI] [PubMed] [Google Scholar]
- 68.Lee D.D., Seung H.S. Algorithms for non-negative matrix factorization. Adv. Neural Inf. Process. Syst. 2001;13:556–562. [Google Scholar]
- 69.Spüler M., Irastorza-Landa N., Sarasola-Sanz A., Ramos-Murguialday A. In: Artificial Neural Networks and Machine Learning – ICANN 2016. Villa A.E.P., Masulli P., Pons Rivero A.J., editors. Springer International Publishing; Cham: 2016. Extracting muscle synergy patterns from EMG data using autoencoders; pp. 47–54. [Google Scholar]
- 70.Zhao K., Wen H., Zhang Z., Atzori M., Müller H., Xie Z., Scano A. Evaluation of methods for the extraction of spatial muscle synergies. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.732156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebied A., Kinney-Lang E., Spyrou L., Escudero J. Evaluation of matrix factorisation approaches for muscle synergy extraction. Med. Eng. Phys. 2018;57:51–60. doi: 10.1016/j.medengphy.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Steele K.M., Tresch M.C., Perreault E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013;7:105. doi: 10.3389/fncom.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banks C.L., Pai M.M., McGuirk T.E., Fregly B.J., Patten C. Methodological choices in muscle synergy analysis impact differentiation of physiological characteristics following stroke. Front. Comput. Neurosci. 2017;11:78. doi: 10.3389/fncom.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kieliba P., Tropea P., Pirondini E., Coscia M., Micera S., Artoni F. How are muscle synergies affected by electromyography pre-processing? IEEE Trans. Neural Syst. Rehabil. Eng. 2018;26:882–893. doi: 10.1109/TNSRE.2018.2810859. [DOI] [PubMed] [Google Scholar]
- 75.Roh J., Rymer W.Z., Perreault E.J., Yoo S.B., Beer R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013;109:768–781. doi: 10.1152/jn.00670.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheung V.C.K., Piron L., Agostini M., Silvoni S., Turolla A., Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA. 2009;106:19563–19568. doi: 10.1073/pnas.0910114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devarajan K., Cheung V.C.K. On nonnegative matrix factorization algorithms for signal-dependent noise with application to electromyography data. Neural Comput. 2014;26:1128–1168. doi: 10.1162/NECO_a_00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santuz A., Ekizos A., Janshen L., Baltzopoulos V., Arampatzis A. On the methodological implications of extracting muscle synergies from human locomotion. Int. J. Neural Syst. 2017;27 doi: 10.1142/S0129065717500071. [DOI] [PubMed] [Google Scholar]
- 79.Severini G., De Marchis C. 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA) IEEE; Rome: 2018. Effect of SNR normalization on the estimation of muscle synergies from EMG datasets; pp. 1–5. [Google Scholar]
- 80.Ranaldi S., De Marchis C., Severini G., Conforto S. An objective, information-based approach for selecting the number of muscle synergies to be extracted via non-negative matrix factorization. IEEE Trans. Neural Syst. Rehabil. Eng. 2021;29:2676–2683. doi: 10.1109/TNSRE.2021.3134763. [DOI] [PubMed] [Google Scholar]
- 81.Roh J., Rymer W.Z., Beer R.F. Robustness of muscle synergies underlying three-dimensional force generation at the hand in healthy humans. J. Neurophysiol. 2012;107:2123–2142. doi: 10.1152/jn.00173.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao K., Zhang Z., Wen H., Wang Z., Wu J. Modular organization of muscle synergies to achieve movement behaviors. Journal of Healthcare Engineering. 2019;2019:1–9. doi: 10.1155/2019/8130297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cappellini G., Ivanenko Y.P., Poppele R.E., Lacquaniti F. Motor patterns in human walking and running. J. Neurophysiol. 2006;95:3426–3437. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- 84.Santuz A., Ekizos A., Eckardt N., Kibele A., Arampatzis A. Challenging human locomotion: stability and modular organisation in unsteady conditions. Sci. Rep. 2018;8:2740. doi: 10.1038/s41598-018-21018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lacquaniti F., Ivanenko Y.P., Zago M. Patterned control of human locomotion: control of human locomotion. J. Physiol. 2012;590:2189–2199. doi: 10.1113/jphysiol.2011.215137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan B., Sun Y., Xie B., Huang Z., Wu J., Hou J., Liu Y., Huang Z., Zhang Z. Alterations of muscle synergies during voluntary arm reaching movement in subacute stroke survivors at different levels of impairment. Front. Comput. Neurosci. 2018;12:69. doi: 10.3389/fncom.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.García-Cossio E., Broetz D., Birbaumer N., Ramos-Murguialday A. Cortex integrity relevance in muscle synergies in severe chronic stroke. Front. Hum. Neurosci. 2014;8:744. doi: 10.3389/fnhum.2014.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan B., Huang Z., Jin T., Wu J., Zhang Z., Shen Y. Motor function assessment of upper limb in stroke patients. J Healthc Eng. 2021;2021 doi: 10.1155/2021/6621950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lencioni T., Fornia L., Bowman T., Marzegan A., Caronni A., Turolla A., Jonsdottir J., Carpinella I., Ferrarin M. A randomized controlled trial on the effects induced by robot-assisted and usual-care rehabilitation on upper limb muscle synergies in post-stroke subjects. Sci. Rep. 2021;11:5323. doi: 10.1038/s41598-021-84536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seo G., Kishta A., Mugler E., Slutzky M.W., Roh J. Myoelectric interface training enables targeted reduction in abnormal muscle co-activation. J. NeuroEng. Rehabil. 2022;19:67. doi: 10.1186/s12984-022-01045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maistrello L., Rimini D., Cheung V.C.K., Pregnolato G., Turolla A. Muscle synergies and clinical outcome measures describe different factors of upper limb motor function in stroke survivors undergoing rehabilitation in a virtual reality environment. Sensors. 2021;21:8002. doi: 10.3390/s21238002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang L., Chen X., Cao S., Wu D., Zhao G., Zhang X. Assessment of upper limb motor dysfunction for children with cerebral palsy based on muscle synergy analysis. Front. Hum. Neurosci. 2017;11:130. doi: 10.3389/fnhum.2017.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li T., Chen X., Cao S., Zhang X., Chen X. Human hands-and-knees crawling movement analysis based on time-varying synergy and synchronous synergy theories. Math. Biosci. Eng. 2019;16:2492–2513. doi: 10.3934/mbe.2019125. [DOI] [PubMed] [Google Scholar]
- 94.Shimizu Y., Kadone H., Kubota S., Ueno T., Sankai Y., Hada Y., Yamazaki M. Voluntary elbow extension-flexion using single joint hybrid assistive limb (HAL) for patients of spastic cerebral palsy: two cases report. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zariffa J., Steeves J., Pai D.K. Changes in hand muscle synergies in subjects with spinal cord injury: characterization and functional implications. J. Spinal Cord Med. 2012;35:310–318. doi: 10.1179/2045772312Y.0000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muceli S., Falla D., Farina D. Reorganization of muscle synergies during multidirectional reaching in the horizontal plane with experimental muscle pain. J. Neurophysiol. 2014;111:1615–1630. doi: 10.1152/jn.00147.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McClanahan A., Moench M., Fu Q. Dimensionality analysis of forearm muscle activation for myoelectric control in transradial amputees. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lunardini F., Casellato C., Bertucco M., Sanger T.D., Pedrocchi A. Children with and without dystonia share common muscle synergies while performing writing tasks. Ann. Biomed. Eng. 2017;45:1949–1962. doi: 10.1007/s10439-017-1838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leonardis J.M., Wolff W.L., Momoh A.O., Lipps D.B. Neuromuscular compensation strategies adopted at the shoulder following bilateral subpectoral implant breast reconstruction. J. Biomech. 2021;120 doi: 10.1016/j.jbiomech.2021.110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu Z., Xu S., Hao M., Xiao Q., Lan N. The impact of evoked cutaneous afferents on voluntary reaching movement in patients with Parkinson's disease. J. Neural. Eng. 2019;16 doi: 10.1088/1741-2552/ab186f. [DOI] [PubMed] [Google Scholar]
- 101.Heales L.J., Hug F., MacDonald D.A., Vicenzino B., Hodges P.W. Is synergistic organisation of muscle coordination altered in people with lateral epicondylalgia? A case–control study. Clin. BioMech. 2016;35:124–131. doi: 10.1016/j.clinbiomech.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 102.Essers J.M.N., Peters A., Meijer K., Peters K., Murgia A. Superficial shoulder muscle synergy analysis in facioscapulohumeral dystrophy during humeral elevation tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 2019;27:1556–1565. doi: 10.1109/TNSRE.2019.2927765. [DOI] [PubMed] [Google Scholar]
- 103.Pellegrino L., Coscia M., Muller M., Solaro C., Casadio M. Evaluating upper limb impairments in multiple sclerosis by exposure to different mechanical environments. Sci. Rep. 2018;8:2110. doi: 10.1038/s41598-018-20343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pierella C., Pellegrino L., Muller M., Inglese M., Solaro C., Coscia M., Casadio M. Upper limb sensory-motor control during exposure to different mechanical environments in multiple sclerosis subjects with No clinical disability. Front. Neurorob. 2022;16 doi: 10.3389/fnbot.2022.920118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang N., Rehbaum H., Vujaklija I., Graimann B., Farina D. Intuitive, online, simultaneous, and proportional myoelectric control over two degrees-of-freedom in upper limb amputees. IEEE Trans. Neural Syst. Rehabil. Eng. 2014;22:501–510. doi: 10.1109/TNSRE.2013.2278411. [DOI] [PubMed] [Google Scholar]
- 106.Funato T., Hattori N., Yozu A., An Q., Oya T., Shirafuji S., Jino A., Miura K., Martino G., Berger D., Miyai I., Ota J., Ivanenko Y., d'Avella A., Seki K. Muscle synergy analysis yields an efficient and physiologically relevant method of assessing stroke. Brain Commun. 2022;4:fcac200. doi: 10.1093/braincomms/fcac200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kung P.-C., Lin C.-C.K., Ju M.-S. Neuro-rehabilitation robot-assisted assessments of synergy patterns of forearm, elbow and shoulder joints in chronic stroke patients. Clin. BioMech. 2010;25:647–654. doi: 10.1016/j.clinbiomech.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 108.Tropea P., Monaco V., Coscia M., Posteraro F., Micera S. Effects of early and intensive neuro-rehabilitative treatment on muscle synergies in acute post-stroke patients: a pilot study. J. NeuroEng. Rehabil. 2013;10:103. doi: 10.1186/1743-0003-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S., Zhuang C., Niu C.M., Bao Y., Xie Q., Lan N. Evaluation of functional correlation of task-specific muscle synergies with motor performance in patients poststroke. Front. Neurol. 2017;8:337. doi: 10.3389/fneur.2017.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pellegrino L., Coscia M., Pierella C., Giannoni P., Cherif A., Mugnosso M., Marinelli L., Casadio M. Effects of hemispheric stroke localization on the reorganization of arm movements within different mechanical environments. Life. 2021;11 doi: 10.3390/life11050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chou C.-H., Wang T., Sun X., Niu C.M., Hao M., Xie Q., Lan N. Automated functional electrical stimulation training system for upper-limb function recovery in poststroke patients. Med. Eng. Phys. 2020;84:174–183. doi: 10.1016/j.medengphy.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Kim H., Lee J., Kim J. Muscle synergy analysis for stroke during two degrees of freedom reaching task on horizontal plane. Int. J. Precis. Eng. Manuf. 2020;21:319–328. [Google Scholar]
- 113.Sheng Y., Tan G., Liu J., Chang H., Wang J., Xie Q., Liu H. Upper limb motor function quantification in post-stroke rehabilitation using muscle synergy space model. IEEE Trans. Biomed. Eng. 2022;69:3119–3130. doi: 10.1109/TBME.2022.3161726. [DOI] [PubMed] [Google Scholar]
- 114.Niu C.M., Chou C.-H., Bao Y., Wang T., Gu L., Zhang X., Cui L., Xuan Z., Zhuang C., Li S., Chen Z., Lan N., Xie Q. A pilot study of synergy-based FES for upper-extremity poststroke rehabilitation. Neurosci. Lett. 2022;780 doi: 10.1016/j.neulet.2022.136621. [DOI] [PubMed] [Google Scholar]
- 115.Pierella C., Pirondini E., Kinany N., Coscia M., Giang C., Miehlbradt J., Magnin C., Nicolo P., Dalise S., Sgherri G., Chisari C., Van De Ville D., Guggisberg A., Micera S. A multimodal approach to capture post-stroke temporal dynamics of recovery. J. Neural. Eng. 2020;17 doi: 10.1088/1741-2552/ab9ada. [DOI] [PubMed] [Google Scholar]
- 116.Park J.-H., Shin J.-H., Lee H., Roh J., Park H.-S. Alterations in intermuscular coordination underlying isokinetic exercise after a stroke and their implications on neurorehabilitation. J. NeuroEng. Rehabil. 2021;18:110. doi: 10.1186/s12984-021-00900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scano A., Chiavenna A., Malosio M., Molinari Tosatti L., Molteni F. Muscle synergies-based characterization and clustering of poststroke patients in reaching movements. Front. Bioeng. Biotechnol. 2017;5:62. doi: 10.3389/fbioe.2017.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Israely S., Leisman G., Machluf C.C., Carmeli E. Muscle synergies control during hand-reaching tasks in multiple directions post-stroke. Front. Comput. Neurosci. 2018;12:10. doi: 10.3389/fncom.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang C., Peng L., Hou Z.-G., Li J., Zhang T., Zhao J. Quantitative assessment of upper-limb motor function for post-stroke rehabilitation based on motor synergy analysis and multi-modality fusion. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28:943–952. doi: 10.1109/TNSRE.2020.2978273. [DOI] [PubMed] [Google Scholar]
- 120.Scano A., Chiavenna A., Malosio M., Molinari Tosatti L., Molteni F. Robotic assistance for upper limbs may induce slight changes in motor modules compared with free movements in stroke survivors: a cluster-based muscle synergy analysis. Front. Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belfatto A., Scano A., Chiavenna A., Mastropietro A., Mrakic-Sposta S., Pittaccio S., Molinari Tosatti L., Molteni F., Rizzo G. A multiparameter approach to evaluate post-stroke patients: an application on robotic rehabilitation. Appl. Sci. 2018;8:2248. [Google Scholar]
- 122.Wang C., Zhang S., Hu J., Huang Z., Shi C. Upper-limb muscle synergy features in human-robot interaction with circle-drawing movements. Appl. Bionics Biomechanics. 2021;2021:1–11. doi: 10.1155/2021/8850785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Runnalls K.D., Ortega-Auriol P., McMorland A.J.C., Anson G., Byblow W.D. Effects of arm weight support on neuromuscular activation during reaching in chronic stroke patients. Exp. Brain Res. 2019;237:3391–3408. doi: 10.1007/s00221-019-05687-9. [DOI] [PubMed] [Google Scholar]
- 124.Liu H., Gao Y., Huang W., Li R., Houston M., Benoit J.S., Roh J., Zhang Y. United States, Inter-muscular coherence and functional coordination in the human upper extremity after stroke. Math. Biosci. Eng. 2022;19:4506–4525. doi: 10.3934/mbe.2022208. Zhuoyue Honors College, Hangzhou Dianzi University, Hangzhou 310018, China, College of Automation, Hangzhou Dianzi University, Hangzhou 310018, China, Key labortory of Brain Machine Collaborative Intelligence of Zhejiang Province, Hangzhou 311247, China, Department of Biomedical Engineering, University of Houston, Houston 75835, United States, Texas Institute for Measurement Evaluation and Statistics, University of Houston, Houston 75835. [DOI] [PubMed] [Google Scholar]
- 125.Seo G., Lee S.W., Beer R.F., Alamri A., Wu Y.-N., Raghavan P., Rymer W.Z., Roh J. Alterations in motor modules and their contribution to limitations in force control in the upper extremity after stroke. Front. Hum. Neurosci. 2022;16 doi: 10.3389/fnhum.2022.937391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dash A., Lahiri U. Design of virtual reality-enabled surface electromyogram-triggered grip exercise platform. IEEE Trans. Neural Syst. Rehabil. Eng. 2020;28:444–452. doi: 10.1109/TNSRE.2019.2959449. [DOI] [PubMed] [Google Scholar]
- 127.Irastorza-Landa N., García-Cossio E., Sarasola-Sanz A., Brötz D., Birbaumer N., Ramos-Murguialday A. Functional synergy recruitment index as a reliable biomarker of motor function and recovery in chronic stroke patients. J. Neural. Eng. 2021;18 doi: 10.1088/1741-2552/abe244. [DOI] [PubMed] [Google Scholar]
- 128.Rimini D., Agostini V., Rosati S., Castagneri C., Balestra G., Knaflitz M. 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) IEEE; Seogwipo: 2017. Influence of pre-processing in the extraction of muscle synergies during human locomotion; pp. 2502–2505. [DOI] [PubMed] [Google Scholar]
- 129.Scotto di Luzio F., Cordella F., Bravi M., Santacaterina F., Bressi F., Sterzi S., Zollo L. Modification of hand muscular synergies in stroke patients after robot-aided rehabilitation. Appl. Sci. 2022;12:3146. [Google Scholar]
- 130.Boettcher C.E., Ginn K.A., Cathers I. Standard maximum isometric voluntary contraction tests for normalizing shoulder muscle EMG: MVIC tests for normalizing shoulder EMG. J. Orthop. Res. 2008;26:1591–1597. doi: 10.1002/jor.20675. [DOI] [PubMed] [Google Scholar]
- 131.Ma Y., Shi C., Xu J., Ye S., Zhou H., Zuo G. A novel muscle synergy extraction method used for motor function evaluation of stroke patients: a pilot study. Sensors. 2021;21 doi: 10.3390/s21113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bizzi E., Cheung V.C.K. The neural origin of muscle synergies. Front. Comput. Neurosci. 2013;7 doi: 10.3389/fncom.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hashiguchi Y., Ohata K., Kitatani R., Yamakami N., Sakuma K., Osako S., Aga Y., Watanabe A., Yamada S. Merging and fractionation of muscle synergy indicate the recovery process in patients with hemiplegia: the first study of patients after subacute stroke, Neural Plast. 2016;2016:1–7. doi: 10.1155/2016/5282957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Runnalls K.D., Anson G., Byblow W.D. Partial weight support of the arm affects corticomotor selectivity of biceps brachii. J. NeuroEng. Rehabil. 2015;12:94. doi: 10.1186/s12984-015-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sohn Y.H., Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann. Neurol. 2004;56:595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- 136.Sitburana O., Jankovic J. Focal hand dystonia, mirror dystonia and motor overflow. J. Neurol. Sci. 2008;266:31–33. doi: 10.1016/j.jns.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 137.Latash M. There is No motor redundancy in human movements. There is motor abundance. Mot. Control. 2000;4:259–261. doi: 10.1123/mcj.4.3.259. [DOI] [PubMed] [Google Scholar]
- 138.Allen J.L., Kesar T.M., Ting L.H. Motor module generalization across balance and walking is impaired after stroke. J. Neurophysiol. 2019;122:277–289. doi: 10.1152/jn.00561.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brough L.G., Kautz S.A., Bowden M.G., Gregory C.M., Neptune R.R. Merged plantarflexor muscle activity is predictive of poor walking performance in post-stroke hemiparetic subjects. J. Biomech. 2019;82:361–367. doi: 10.1016/j.jbiomech.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ardestani M.M., Kinnaird C.R., Henderson C.E., Hornby T.G. Compensation or recovery? Altered kinetics and neuromuscular synergies following high-intensity stepping training poststroke. Neurorehabilitation Neural Repair. 2019;33:47–58. doi: 10.1177/1545968318817825. [DOI] [PubMed] [Google Scholar]
- 141.Ambrosini E., Parati M., Peri E., De Marchis C., Nava C., Pedrocchi A., Ferriero G., Ferrante S. Changes in leg cycling muscle synergies after training augmented by functional electrical stimulation in subacute stroke survivors: a pilot study. J. NeuroEng. Rehabil. 2020;17:35. doi: 10.1186/s12984-020-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Berger D.J., d'Avella A. In: Converging Clinical and Engineering Research on Neurorehabilitation II. Ibáñez J., González-Vargas J., Azorín J.M., Akay M., Pons J.L., editors. Springer International Publishing; Cham: 2017. Towards a myoelectrically controlled virtual reality interface for synergy-based stroke rehabilitation; pp. 965–969. [Google Scholar]
- 143.Cancrini A., Baitelli P., Lavit Nicora M., Malosio M., Pedrocchi A., Scano A. The effects of robotic assistance on upper limb spatial muscle synergies in healthy people during planar upper-limb training. PLoS One. 2022;17 doi: 10.1371/journal.pone.0272813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moreno J.C., Barroso F., Farina D., Gizzi L., Santos C., Molinari M., Pons J.L. Effects of robotic guidance on the coordination of locomotion. J. NeuroEng. Rehabil. 2013;10:79. doi: 10.1186/1743-0003-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao K., Wen H., Zhang Z., He C., Wu J. Fractal characteristics-based motor dyskinesia assessment. Biomed. Signal Process Control. 2021;68 [Google Scholar]
- 146.Botzheim L., Laczko J., Torricelli D., Mravcsik M., Pons J.L., Oliveira Barroso F. Effects of gravity and kinematic constraints on muscle synergies in arm cycling. J. Neurophysiol. 2021;125:1367–1381. doi: 10.1152/jn.00415.2020. [DOI] [PubMed] [Google Scholar]
- 147.Turpin N.A., Uriac S., Dalleau G. How to improve the muscle synergy analysis methodology? Eur. J. Appl. Physiol. 2021;121:1009–1025. doi: 10.1007/s00421-021-04604-9. [DOI] [PubMed] [Google Scholar]
- 148.Chiovetto E., Berret B., Pozzo T. Tri-dimensional and triphasic muscle organization of whole-body pointing movements. Neuroscience. 2010;170:1223–1238. doi: 10.1016/j.neuroscience.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 149.Hilt P.M., Delis I., Pozzo T., Berret B. Space-by-Time modular decomposition effectively describes whole-body muscle activity during upright reaching in various directions. Front. Comput. Neurosci. 2018;12:20. doi: 10.3389/fncom.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Delis I., Hilt P.M., Pozzo T., Panzeri S., Berret B. Deciphering the functional role of spatial and temporal muscle synergies in whole-body movements. Sci. Rep. 2018;8:8391. doi: 10.1038/s41598-018-26780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scano A., Mira R.M., d'Avella A. Mixed matrix factorization: a novel algorithm for the extraction of kinematic-muscular synergies. J. Neurophysiol. 2022;127:529–547. doi: 10.1152/jn.00379.2021. [DOI] [PubMed] [Google Scholar]
- 152.Soomro M.H., Conforto S., Giunta G., Ranaldi S., De Marchis C. Comparison of initialization techniques for the accurate extraction of muscle synergies from myoelectric signals via nonnegative matrix factorization. Appl. Bionics Biomechanics. 2018;2018:1–10. doi: 10.1155/2018/3629347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Latash M.L., Scholz J.P., Schöner G. Toward a new theory of motor synergies. Mot. Control. 2007;11:276–308. doi: 10.1123/mcj.11.3.276. [DOI] [PubMed] [Google Scholar]
- 154.Scholz J.P., Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp. Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- 155.Wolpert D.M., Ghahramani Z., Jordan M.I. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- 156.Feldman A.G. Once more on the equilibrium-point hypothesis (λ model) for motor control. J. Mot. Behav. 1986;18:17–54. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- 157.d'Avella A., Fernandez L., Portone A., Lacquaniti F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J. Neurophysiol. 2008;100:1433–1454. doi: 10.1152/jn.01377.2007. [DOI] [PubMed] [Google Scholar]
- 158.Scano A., Dardari L., Molteni F., Giberti H., Tosatti L.M., D'Avella A. A comprehensive spatial mapping of muscle synergies in highly variable upper-limb movements of healthy subjects. Front. Physiol. 2019;10 doi: 10.3389/fphys.2019.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pale U., Atzori M., Müller H., Scano A. Variability of muscle synergies in hand grasps: analysis of intra- and inter-session data. Sensors. 2020;20:4297. doi: 10.3390/s20154297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao K., Zhang Z., Wen H., Scano A. Intra-subject and inter-subject movement variability quantified with muscle synergies in upper-limb reaching movements. Biomimetics. 2021;6:63. doi: 10.3390/biomimetics6040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Longatelli V., Torricelli D., Tornero J., Pedrocchi A., Molteni F., Pons J.L., Gandolla M. A unified scheme for the benchmarking of upper limb functions in neurological disorders. J. NeuroEng. Rehabil. 2022;19:102. doi: 10.1186/s12984-022-01082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao K., Zhang Z., Wen H., Scano A. Number of trials and data structure affect the number and components of muscle synergies in upper-limb reaching movements. Physiol. Meas. 2022;43:105008. doi: 10.1088/1361-6579/ac9773. [DOI] [PubMed] [Google Scholar]
- 163.Hug F., Turpin N.A., Dorel S., Guével A. Smoothing of electromyographic signals can influence the number of extracted muscle synergies. Clin. Neurophysiol. 2012;123:1895–1896. doi: 10.1016/j.clinph.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 164.van der Krogt M., Oudenhoven L., Buizer A., Dallmeijer A. The effect of EMG processing choices on muscle synergies before and after BoNT-A treatment in cerebral palsy. Gait Posture. 2016;49:31. [Google Scholar]
- 165.Shuman B.R., Schwartz M.H., Steele K.M. Electromyography data processing impacts muscle synergies during gait for unimpaired children and children with cerebral palsy. Front. Comput. Neurosci. 2017;11:50. doi: 10.3389/fncom.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rabbi M.F., Pizzolato C., Lloyd D.G., Carty C.P., Devaprakash D., Diamond L.E. Non-negative matrix factorisation is the most appropriate method for extraction of muscle synergies in walking and running. Sci. Rep. 2020;10:8266. doi: 10.1038/s41598-020-65257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Berger D.J., d'Avella A. Effective force control by muscle synergies. Front. Comput. Neurosci. 2014;8 doi: 10.3389/fncom.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Berger D.J., Gentner R., Edmunds T., Pai D.K., d'Avella A. Differences in adaptation rates after virtual surgeries provide direct evidence for modularity. J. Neurosci. 2013;33:12384–12394. doi: 10.1523/JNEUROSCI.0122-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.d'Avella A., Cesqui B., Lacquaniti F. In: Converging Clinical and Engineering Research on Neurorehabilitation. Pons J.L., Torricelli D., Pajaro M., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2013. Identifying muscle synergies from EMG decomposition: approaches, evidence, and potential application to neurorehabilitation; pp. 1243–1247. [Google Scholar]
- 170.Gurgone S., Borzelli D., de Pasquale P., Berger D.J., Lisini Baldi T., D'Aurizio N., Prattichizzo D., d'Avella A. Simultaneous control of natural and extra degrees of freedom by isometric force and electromyographic activity in the muscle-to-force null space. J. Neural. Eng. 2022;19 doi: 10.1088/1741-2552/ac47db. [DOI] [PubMed] [Google Scholar]
- 171.Brambilla C., Pirovano I., Mira R.M., Rizzo G., Scano A., Mastropietro A. Combined use of EMG and EEG techniques for neuromotor assessment in rehabilitative applications: a systematic review. Sensors. 2021;21:7014. doi: 10.3390/s21217014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trujillo P., Mastropietro A., Scano A., Chiavenna A., Mrakic-Sposta S., Caimmi M., Molteni F., Rizzo G. Quantitative EEG for predicting upper limb motor recovery in chronic stroke robot-assisted rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017;25:1058–1067. doi: 10.1109/TNSRE.2017.2678161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.