Abstract
Background.
Pulmonary nodule growth is often measured by volume doubling time (VDT) which may guide management. Most malignant nodules have a VDT of 20–400 days, with longer VDTs typically observed in indolent nodules. We assessed the utility of VDT in differentiating pulmonary carcinoids and hamartomas.
Methods.
A review was performed from January 2012-October 2021 to identify patients with pathologic diagnoses and at least two chest CTs obtained ≥ six months apart. Visualization software was used to segment nodules and calculate diameter and volume. VDT was calculated for scans with 1 mm slices. For the remainder, estimated nodule volume doubling time (eVDT) was calculated using nodule diameter. VDTs/eVDTs were placed into growth categories: <400 days; 400–600 days; and >600 days.
Results.
60 nodules were identified, 35 carcinoids and 25 hamartomas. Carcinoids were larger than hamartomas (median diameter 13.5 mm vs. 11.5 mm, p=0.05). For carcinoid tumors, median VDT (n=15) was 1485 days, and median eVDT (n=32) was 1309 days; for hamartomas, median VDT (n=8) was 2040 days, and median eVDT (n=25) was 2253 days. Carcinoid tumor eVDT was significantly shorter than hamartomas (p=0.03). By growth category, 1/25 hamartomas and 5/35 carcinoids had eVDT <400 days; and 24/25 hamartomas and 27/35 carcinoids had eVDT >600 days. Of four carcinoid tumors with metastases, two had eVDT <400 days and two had eVDT >600 days.
Conclusion.
Growth rate was not a reliable differentiator of pulmonary hamartomas and carcinoids. Slow growing carcinoids can metastasize. Radiologists should be cautious when discontinuing CT follow up based on growth rates alone.
Introduction
Pulmonary nodules are common and can represent a spectrum of benign lesions, benign neoplasms, and malignant neoplasms 1,2. The increased utilization of CT has dramatically increased the rate of nodule detection in the USA from 3.9 to 6.6/1000 person-years between 2006 and 2012 3. Pulmonary carcinoid tumors and hamartomas can present as slow-growing, round solid nodules 4. It is uncommon for primary lung cancer to present in this fashion 5.
Primary carcinoid tumors of the lung are low-to-intermediate grade malignant neuroendocrine neoplasms that constitute 0.5–5% of all lung cancers 6. Approximately 80% of pulmonary carcinoids occur in the central bronchi, and 20% occur in the lung parenchyma 6. All pulmonary carcinoids have the potential to metastasize 7. Histopathologically, typical carcinoid tumors (low-grade neuroendocrine tumors) have <2 mitoses per 2 mm2 whereas atypical carcinoid tumors (intermediate grade tumors) have 2–10 mitosis per 2 mm2 8. Typical carcinoids are more common (90% of cases), have a lower risk of metastases, and have higher survival rates: 10-year survival rates 82–87% 9–11. In contrast, atypical carcinoid tumors are less common, more likely to metastasize, and have a poorer prognosis: 10-year survival rates of 35%–56% 12,13. Surgical resection is the standard of care for both typical and atypical carcinoid tumors 14. While atypical carcinoid tumors are more common in the lung parenchyma than in the central bronchi 15, no other specific imaging findings distinguish these entities. Definitive pathological diagnosis of typical vs atypical carcinoid tumor requires surgical resection since biopsy specimens can suffer from sampling error 16.
Pulmonary hamartomas are stable to slowly growing nodules that make up 10% of benign nodules and tend to present in middle age 17–19. They can contain cartilaginous, muscle, adipose, myxomatous, and fibroblastic tissue 20. CT may show focal areas of fat and/or calcification, which if present are pathognomonic of a hamartoma 21. The classic “popcorn” calcification appearance is seen infrequently in hamartomas 22. Many hamartomas lack characteristic features on CT and present as solitary oval nodules, indistinguishable from other entities 23.
Biopsy and surgical resection are invasive but definitive methods of providing a diagnosis. There are several non-invasive ways of evaluating slowly growing pulmonary nodules. A recent study showed that peri-nodular bronchial wall thickening on CT was more common in carcinoid tumors than in hamartomas 24. However, in the absence of pathognomonic imaging features, carcinoid tumors and hamartomas can be very difficult to differentiate. Studies have shown that 18F-FDG PET-CT may help differentiate between carcinoid tumors and hamartomas 25,26. Somatostatin receptor imaging, using radiotracers such as Ga-68 DOTATATE, is mainly used for pulmonary carcinoid staging, treatment planning, and assessing for disease recurrence 27–30.
Nodule growth is often measured by the volume doubling time (VDT), with the potential to guide management by helping to differentiate between benign and malignant nodules 31,32. Most malignant nodules have a VDT between 20 and 400 days 33,34, with longer VDTs >400 days typically observed in more indolent/benign nodules 35. To our knowledge, VDT assessment to differentiate between malignant pulmonary carcinoid tumors and benign hamartomas has not been investigated. Our aim was to assess the utility of VDT to differentiate between typical carcinoids, atypical carcinoids, and hamartomas.
Materials and Methods
Patient selection.
This retrospective study was approved by the institutional review board and carried out according to HIPAA guidelines. A review of the electronic medical record of a healthcare system encompassing two large quaternary academic centers was conducted between January 2012 and October 2021 to identify patients with pulmonary carcinoid tumors and hamartomas. Inclusion criteria: pathologic diagnosis of typical carcinoid tumor, atypical carcinoid tumor, or pulmonary hamartoma, and at least two chest CTs obtained at a minimum of six months apart. Exclusion criteria: Adjacent atelectasis prohibiting accurate measurement of the nodule, nodules < 3mm in size, or failure of the pathology report to specify typical vs atypical carcinoid histology.
Nodule measurement.
A thoracic radiology fellow with 6.5 years of experience reviewed up to 3 diagnostic chest CTs for each case: the oldest CT, the most recent CT prior to resection, and up to 1 CT in between. The advanced visualization software Syngo.via (Siemens Healthcare, Erlangen, Germany) was used to semiautomatically segment the nodule and calculate average diameter and volume at each time point. Volume doubling time (VDT) was calculated only for scans with 1 mm slices, assuming a standard exponential growth rate. For scans in which 1 mm slices were not available, estimated nodule volume was calculated as the volume of a sphere using the nodule’s average diameter. An estimated VDT (eVDT) was then calculated using these estimated volumes. VDT and eVDT were capped at 10 years (3650 days), since a longer doubling time than that is likely not accurate (or simply represents a stable nodule). For each nodule, the VDT and eVDT are reported for the longest time interval between CTs that was available. We also categorized the VDTs and eVDTs into the categories used by the NELSON trial: <400 days; 400 – 600 days; and >600 days 36.
Statistics.
Study data were collected and managed using REDCap electronic data capture tools hosted at our institution 37. Data were subsequently downloaded into JMP Pro (v16, SAS Institute, Cary, NC) for further analysis. Continuous variables were compared with the Wilcoxon test, and categorical variables were compared with Fisher’s exact test. A p-value of < 0.05 was considered statistically significant.
Results
Patient and nodule characteristics.
The initial search yielded 181 patients; after exclusion criteria were applied, 57 patients remained with a total of 60 nodules. Summaries of patient and nodule characteristics are listed in Tables 1 and 2, respectively. Of the 57 patients, 32 had carcinoid tumors, and 25 had hamartomas. Three patients had two carcinoid tumors each; two of these three patients had evidence of Diffuse Idiopathic Neuroendocrine Cell Hyperplasia (DIPNECH) on pathology. Of the 57 patients, 32 were female (56%) and 25 were male (44%) with a median age of 68 (range 21 – 90) at the time of their most recent CT. In total, 6 patients had DIPNECH on pathology. Of the 60 nodules, 35 (58%) were carcinoid tumors, including 28 typical and 7 atypical carcinoids, and 25 (42%) were hamartomas. Of the carcinoid tumors, 1/7 atypical carcinoids and 2/28 typical carcinoids had evidence of positive lymph nodes at time of diagnosis. One typical carcinoid developed a recurrence in the form of metastasis to the humerus. Only two carcinoid tumors were central endobronchial lesions. Female sex was significantly more common in carcinoids than in hamartomas (74% versus 36%, p<0.01). Overall, carcinoid tumors were larger than hamartomas (median diameter of 13.5 mm versus 11.5 mm, p=0.05). Patient age was not significantly different between the carcinoid and hamartoma subgroups (p=0.38).
Table 1:
Patient Characteristics
| Patients | Carcinoids n = 32 | Hamartomas n = 25 |
|---|---|---|
|
| ||
| Male | 9 (26%) | 16 (64%) |
| Female | 26 (74%) | 9 (36%) |
| Median Age, years (range) | 68 (40 – 90) | 65 (21 – 77) |
| Positive Lymph Nodes at Diagnosis | 3 (9%) | - |
| Recurrence after Surgery | 1 (3%) | - |
| DIPNECH | 6 (19%) | - |
Table 2:
Nodule Characteristics
| Nodules | Carcinoids n = 35 | Hamartomas n=25 |
|---|---|---|
|
| ||
| Median average diameter, mm (range) | 13.5 (6 – 30.5) | 11.5 (5 – 26.5) |
| Typical Carcinoid | 28 (80%) | - |
| Atypical Carcinoid | 7 (20%) | - |
| Parenchymal Location | 33 (94%) | 25 (100%) |
| Endobronchial Location | 2 (6%) | 0 |
Volume doubling time.
For VDT based on 1 mm slices, the median interval between CTs was 604 days (range 79–2876 days). VDT was calculable for 15 carcinoid tumors, with a median VDT of 1485 days (range 50 days to >10 years) and 8 hamartomas, with a median VDT of 2040 days (range 132 days to >10 years) (Fig. 1). In this subset of patients with thin-section images, there was no significant difference between VDT for carcinoids and hamartomas (p=0.46).
Figure 1.

VDT by histology
For eVDT, the median interval between CTs was 777 days (range 79–2876 days). eVDT was calculated for all 35 carcinoid tumors (typical and atypical), with a median eVDT of 1309 days (range 56 days to >10 years) (Fig. 2). The median eVDT for all 25 hamartomas was 2253 days (range 132 days to >10 years). eVDT for all carcinoid tumors was significantly shorter than for hamartomas, indicating a faster growth rate (p=0.03). The median eVDT for 7 atypical carcinoids was 1309 days (range 378 days to >10 years) and for 28 typical carcinoids was 1380 days (range 56 days to >10 years). This difference was not significant (p=0.8). There were examples of both fast (eVDT<400 days) and slow (eVDT >600 days) growing carcinoid tumors with metastatic disease (Figs 3 and 4, respectively).
Figure 2.
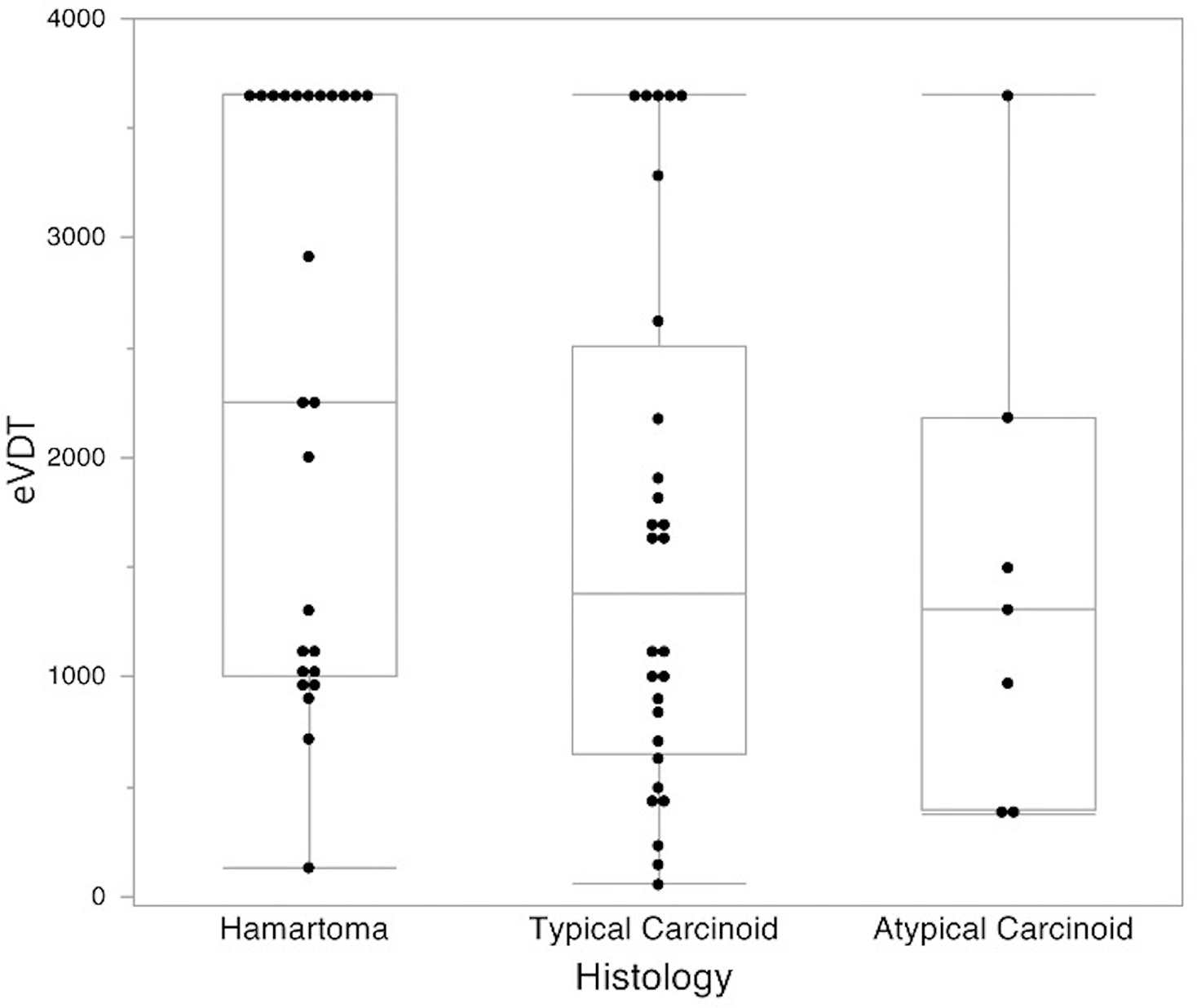
eVDT by histology
Figure 3.


Fast-growing typical carcinoid tumor with lymph node metastasis
85-year-old woman with a fast-growing typical carcinoid tumor with lymph node metastasis. (a) Axial chest CT in February 2013 and (b) axial chest CT in April 2015 show a growing nodule in the right lower lobe (white arrow). At the time of the second scan, right hilar lymphadenopathy was noted (black arrow) and was later confirmed as carcinoid metastasis on biopsy. Calculated eVDT was 233 days.
Figure 4.



Slow-growing atypical carcinoid tumor and lymph node metastasis
67-year-old man with a slow-growing atypical carcinoid tumor and lymph node metastasis. (a) Axial chest CT in April 2011 and (b) axial chest CT in September 2020 show a growing nodule in the right lower lobe (black arrow) with (c) new subcarinal lymph node metastasis (white arrow). Calculated eVDT was 1309 days.
Growth categories.
eVDT was categorized into: < 400 days; 400–600 days; or >600 days. To validate eVDT, we compared these categories for nodules in which VDT was available as well. For the 23 nodules in which it was possible to calculate VDT, 21/23 (91%) were in the same category for VDT and eVDT (two with VDT <400 days and 19 with VDT >600 days). There were two discrepancies between VDT and eVDT: one patient had a VDT of 400–600 days and an eVDT of <400 days, and the other had a VDT >600 days and an eVDT of <400 days. This latter case was an atypical carcinoid that had an eVDT >600 days when measured in the same interval used for the thin slice VDT calculation (CTs performed in 2011 and 2013); but it had an eVDT <400 days when using an even more remote prior CT from 2010 for which no thin sections were available.
For hamartomas, 1/25 had an eVDT <400 days (Fig 5) and 24/25 had eVDTs >600 days. For typical carcinoids, 3/28 had eVDTs <400; 3/28 had eVDTs 400–600; and 22/28 had eVDTs >600 days. For atypical carcinoids, 2/7 had eVDTs <400 days, and 5/7 had eVDTs >600 days. Of those carcinoid tumors with either positive nodes or recurrence, 2/4 had eVDTs <400 days, and 2/4 had eVDTs >600 days. Of the eight carcinoid tumors in patients with DIPNECH, all had eVDTs >600 days.
Figure 5.


Fast-growing hamartoma
21-year-old man with a fast-growing hamartoma, in the setting of a Succinate Dehydrogenase B Subunit (SDHB) mutation and a previously resected Gastrointestinal Stromal Tumor (GIST) of the stomach but no paraganglionomas. (a) Axial chest CT in May 2019 and (b) axial chest in September 2020 show a growing nodule in the left lower lobe (white arrow). Calculated eVDT was 132 days.
Discussion
In summary, we measured growth rates of pulmonary carcinoid tumors and hamartomas. We found a significant difference in growth rates between the two (estimated volume doubling time 1309 days for carcinoid tumors versus 2253 days for hamartomas, p=0.03). However, despite this difference, there was substantial overlap between the two groups; indeed, most carcinoids and hamartomas had volume doubling times much longer than 600 days. Additionally, one hamartoma had a VDT of <400 days, a growth rate usually associated with malignant neoplasms.
One recent study assessed the VDT of carcinoid tumors and observed a median VDT of 980 days for 12 typical carcinoids 38. While that study recorded an overall faster growth rate than ours, the medians for both that study and ours are far greater than 600 days. Carcinoids in patients with DIPNECH in our study had more indolent behaviour, with slow growth rates and no metastatic disease observed in 6 DIPNECH patients. The DIPNECH cohort was small, but the observed indolent behaviour is consistent with that previously noted in a larger case series 39. At least one previous study has looked at VDTs in hamartomas. Notably, one group calculated the VDT for nine hamartomas which, similar to our data set, showed a wide range of doubling times with one particularly fast-growing outlier. In that study, seven of the nine hamartomas had VDTs ranging from 550–6000 days and one had a VDT of <450 days 40. Despite individual VDT assessments of hamartomas and carcinoids, to our knowledge, our study is the first to compare nodule VDT between pulmonary hamartomas and carcinoid tumors.
Several guidelines suggest the use of volume doubling time in the triage of pulmonary nodules. For example, the NELSON lung cancer screening trial used an algorithm where nodules with a VDT of >600 days were placed in the benign category and were subject to annual CT follow-up 36. The British Thoracic Society recommends discontinuing follow up in solid nodules that display a <25% increase in volume at one year follow up CT 41. However, our data shows that slow-growing nodules with VDTs >600 days can be carcinoid tumors and in some cases can metastasize. Thus, radiologists must use caution in dismissing nodules with slow growth rates.
Within the carcinoid cohort, the doubling time was not useful in differentiating between typical and atypical carcinoid subtypes. Carcinoids had a wide spectrum of growth rates, with both fast and slow-growing tumors. Interestingly, metastatic disease was observed in both fast and slow-growing carcinoids, indicating that growth rate may be a poor predictor of clinical outcome. Similarly, a study comparing growth rates of 86 benign and 219 malignant nodules showed no significant difference in VDT (p=0.18) between the two groups and concluded that the diagnostic value of VDT was limited in differentiating benign from malignant nodules 42. As another example from the literature, a recent paper demonstrated that nodule growth rate assessment was inferior to the use of linear measurement-based Lung-RADS in the diagnosis of lung cancer at follow-up lung cancer screening CT 43. On the other hand, at least one study has shown that growth rate may be predictive of outcomes in pulmonary adenocarcinomas 44.
Being a retrospective study, we are limited by heterogeneity in CT acquisition technique, slice thickness, and reconstruction algorithm. This introduces errors in nodule growth rate estimation 31. Indeed, the lack of thin section images for some timepoints necessitated the use of estimated spherical nodule volumes to generate the VDT. However, we did find that, for nodules that had thin section images to compare, VDT and eVDT led to values in the same aggressiveness category in 91% of cases. The study was limited by a small sample size and selection bias as only patients who went for biopsy or surgical resection were included.
In conclusion, nodule growth rate is not a reliable differentiator of pulmonary hamartomas and carcinoid tumors. Even slow-growing nodules can be carcinoids, and in some cases, slow-growing carcinoids can metastasize. Radiologists should be cautious when considering discontinuing CT follow up based on slow growth rates alone. Future studies are needed to confirm these findings in a larger cohort.
Acknowledgements
This study was supported by NIH grant 1R01CA260889-01.
Dr Mark M Hammer is supported by NIH grant 1R01CA260889-01.
Footnotes
Declaration of Interest: None
Disclosures: This study was supported by NIH grant 1R01CA260889-01. Dr Mark M Hammer is supported by NIH grant 1R01CA260889-01.
Contributor Information
JW Ryan, Brigham and Women’s Hospital, Boston MA USA.
MM Hammer, Brigham and Women’s Hospital, Boston MA USA.
References
- 1.Winningham PJ, Martínez-Jiménez S, Rosado-de-Christenson ML, et al. Bronchiolitis: A practical approach for the general radiologist. Radiographics. 2017;37:777–794. [DOI] [PubMed] [Google Scholar]
- 2.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology. 2017;284:228–243. [DOI] [PubMed] [Google Scholar]
- 3.Gould MK, Tang T, Liu ILA, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. [DOI] [PubMed] [Google Scholar]
- 4.Meisinger QC, Klein JS, Butnor KJ, et al. CT Features of Peripheral Pulmonary Carcinoid Tumors. http://dx.doi.org/102214/AJR105954. 2012;197:1073–1080. [DOI] [PubMed] [Google Scholar]
- 5.Albert RH, Russell JJ. Evaluation of the Solitary Pulmonary Nodule. Am Fam Physician. 2009;80:827–831. [PubMed] [Google Scholar]
- 6.Herde RF, Kokeny KE, Reddy CB, et al. Primary Pulmonary Carcinoid Tumor: A Long-term Single Institution Experience. Am J Clin Oncol. 2018;41:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limaiem F, Tariq MA, Wallen JM. Lung Carcinoid Tumors. StatPearls. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537080/. 2021. Accessed January 1, 2022. [PubMed] [Google Scholar]
- 8.Melosky B Advanced typical and atypical carcinoid tumours of the lung: management recommendations. Curr Oncol. 2018;25:S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bini A, Brandolini J, Cassanelli N, et al. Typical and atypical pulmonary carcinoids: our institutional experience. Interact Cardiovasc Thorac Surg. 2008;7:415–418. [DOI] [PubMed] [Google Scholar]
- 10.Thomas CF, Tazelaar HD, Jett JR. Typical and atypical pulmonary carcinoids : outcome in patients presenting with regional lymph node involvement. Chest. 2001;119:1143–1150. [DOI] [PubMed] [Google Scholar]
- 11.Beshay M, Roth T, Stein R, et al. Synchronous bilateral typical pulmonary carcinoid tumors. Eur J Cardiothorac Surg. 2003;23:251–253. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22:934–944. [DOI] [PubMed] [Google Scholar]
- 13.Rea F, Rizzardi G, Zuin A, et al. Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients. Eur J Cardiothorac Surg. 2007;31:186–191. [DOI] [PubMed] [Google Scholar]
- 14.Filosso PL, Guerrera F, Falco NR, et al. Anatomical resections are superior to wedge resections for overall survival in patients with Stage 1 typical carcinoids. Eur J Cardiothorac Surg. 2019;55:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naalsund A, Rostad H, Strøm EH, et al. Carcinoid lung tumors--incidence, treatment and outcomes: a population-based study. Eur J Cardiothorac Surg. 2011;39:565–569. [DOI] [PubMed] [Google Scholar]
- 16.Righi L, Gatti G, Volante M, et al. Lung neuroendocrine tumors: pathological characteristics. J Thorac Dis. 2017;9:S1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348:2535–42. [DOI] [PubMed] [Google Scholar]
- 18.Higgins GA, Shields TW, Keehn RJ. The solitary pulmonary nodule. Ten-year follow-up of veterans administration-armed forces cooperative study. Arch Surg. 1975;110:570–575. [DOI] [PubMed] [Google Scholar]
- 19.Ray JF, Lawton BR, Magnin GE, et al. The coin lesion story: update 1976. Twenty years’ experience with thoracotomy for 179 suspected malignant coin lesions. Chest. 1976;70:332–336. [DOI] [PubMed] [Google Scholar]
- 20.Gjevre JA, Myers JL, Prakash UBS. Pulmonary hamartomas. Mayo Clin Proc. 1996;71:14–20. [DOI] [PubMed] [Google Scholar]
- 21.Siegelman SS, Khouri NF, Scott WW, et al. Pulmonary hamartoma: CT findings. Radiology. 1986;160:313–317. [DOI] [PubMed] [Google Scholar]
- 22.Chai JL, Patz EF. CT of the lung: patterns of calcification and other high-attenuation abnormalities. AJR Am J Roentgenol. 1994;162:1063–1066. [DOI] [PubMed] [Google Scholar]
- 23.Furuya K, Yasumori K, Takeo S, et al. Lung CT: Part 1, Mimickers of lung cancer--spectrum of CT findings with pathologic correlation. AJR Am J Roentgenol.;199. Epub ahead of print October 2012. DOI: 10.2214/AJR.10.7262. [DOI] [PubMed] [Google Scholar]
- 24.Coruh AG, Kul M, Kuru Öz D, et al. Is it possible to discriminate pulmonary carcinoids from hamartomas based on CT features? Clin Imaging. 2020;62:49–56. [DOI] [PubMed] [Google Scholar]
- 25.Uhlén N, Grundberg O, Jacobsson H, et al. 18F-FDG PET/CT Diagnosis of Bronchopulmonary Carcinoids Versus Pulmonary Hamartomas. Clin Nucl Med. 2016;41:263–267. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Wang H. Differential diagnostic value of 18F-FDG PET/CT in pulmonary carcinoids versus hamartomas. Acad Radiol. . Epub ahead of print November 9, 2020. DOI: 10.1016/J.ACRA.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Venkitaraman B, Karunanithi S, Kumar A, et al. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging. 2014;41:856–864. [DOI] [PubMed] [Google Scholar]
- 28.Sanli Y, Garg I, Kandathil A, et al. Neuroendocrine Tumor Diagnosis and Management: 68Ga-DOTATATE PET/CT. https://doi.org/102214/AJR1819881. 2018;211:267–277. [DOI] [PubMed] [Google Scholar]
- 29.Kennecke HF, Raghu P, Lin B, et al. Impact of initial imaging with gallium-68 dotatate PET/CT on diagnosis and management of patients with neuroendocrine tumors (NETs): A sequential case series. https://doi.org/101200/JCO2019374_suppl379. 2019;37:379–379. [DOI] [PubMed] [Google Scholar]
- 30.Haug AR, Cindea-Drimus R, Auernhammer CJ, et al. Neuroendocrine tumor recurrence: Diagnosis with 68Ga-DOTATATE PET/CT. Radiology. 2014;270:517–525. [DOI] [PubMed] [Google Scholar]
- 31.Devaraj A, Van Ginneken B, Nair A, et al. Use of volumetry for lung nodule management: Theory and practice1. Radiology. 2017;284:630–644. [DOI] [PubMed] [Google Scholar]
- 32.Mackintosh JA, Marshall HM, Yang IA, et al. A retrospective study of volume doubling time in surgically resected non-small cell lung cancer. Respirology. 2014;19:755–762. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Xia T, Yang X, et al. Malignant solitary pulmonary nodules: assessment of mass growth rate and doubling time at follow-up CT. J Thorac Dis. 2018;10:S797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soubani AO. The evaluation and management of the solitary pulmonary nodule. Postgrad Med J. 2008;84:459–466. [DOI] [PubMed] [Google Scholar]
- 35.Thalanayar PM, Altintas N, Weissfeld JL, et al. Indolent, potentially inconsequential lung cancers in the Pittsburgh Lung Screening Study. Ann Am Thorac Soc. 2015;12:1193–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao YR, Xie X, De Koning HJ, et al. NELSON lung cancer screening study. Cancer Imaging. 2011;11:S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russ D, Julie Barta M, Nathaniel Evans M, et al. Tumor Doubling Time of Pulmonary Carcinoid Tumors Measured by CT. Phase 1. Available from: https://jdc.jefferson.edu/si_ctr_2023_phase1/74. 2021. Accessed February 1, 2022. [DOI] [PubMed] [Google Scholar]
- 39.van den Broek MFM, Levy S, Buikhuisen WA, et al. Well-Differentiated Bronchopulmonary Neuroendocrine Tumors: More Than One Entity. J Thorac Oncol. 2021;16:1810–1820. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, ming Xu D, Jirapatnakul A, et al. CT- and computer-based features of small hamartomas. Clin Imaging. 2011;35:116–122. [DOI] [PubMed] [Google Scholar]
- 41.Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: accredited by NICE. Thorax. 2015;70:ii1–ii54. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Tian P, Qiu Z, et al. The growth feature and its diagnostic value for benign and malignant pulmonary nodules met in routine clinical practice. J Thorac Dis. 2020;12:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammer MM, Byrne SC. Cancer Risk in Nodules Detected at Follow-up Lung Cancer Screening CT. https://doi.org/102214/AJR2126927. . Epub ahead of print November 10, 2021. DOI: 10.2214/AJR.21.26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S, Lee SM, Kim S, et al. Volume doubling times of lung adenocarcinomas: Correlation with predominant histologic subtypes and prognosis. Radiology. 2020;295:703–712. [DOI] [PubMed] [Google Scholar]


