Abstract
To evaluate the release and activity of Indian jujube phenolics in vivo, its peel and pulp were subjected to simulated digestions. The phenolics content and antioxidant activity of the digested samples were determined. The results showed that the total phenolics/flavonoids in the peel were respectively 4.63 and 4.48 times higher than that in the pulp. The release of phenolics and flavonoids respectively increased by 79.75% and 39.98% in the peel and 86.34% and 23.54% in the pulp after the intestinal digestion. The correlation between the total phenolics/flavonoids and antioxidant activity was higher in the peel (r > 0.858, p < 0.01) than that in the pulp. The phenolics profiles of the peel were almost the same after the digestion, and four phenolics including naringenin tri-glycoside, quercetin-3-O-[(2-hexosyl)-6-rhamnosyl] -hexoside, quercetin-3-O-pentosylhexoside and quercetin-3-O-(2-pentosyl -rhamnoside)-4′-O-rhamnoside were found to be the main flavonoids of Indian jujube peel, and they showed high recovery (>89.88%) during the digestion, implying that these phenolics may play a vital role in the function of Indian jujubes.
Keywords: Indian jujube, Phenolics, Peel, Pulp, Simulated digestion
1. Introduction
Indian jujube (Ziziphus mauritiana Lam.) is one of the most commercialized jujube species in the world [1], belonging to the Rhamnaceae family and is widely cultivated in Asia countries, including India, Bangladesh [2]. The fruit is abundant in numerous phytochemicals including phenolics, triterpenoids, alkaloids and sterols [3]. Among them, jujube phenolics are attracting more and more attentions for their antioxidant [4], anticancer [5], antidiabetic [6], antimicrobial [7] and anti-inflammatory [8] activities. More than twenty flavonoids and phenolic acids were reported in Indian jujube fruit [9]. Most of the flavonoids in the fruit are glycoside derivatives of quercetin, luteolin and myricetin, while phenolic acids are mainly chlorogenic acid, caffeic acid and vanillic acid [7].
Due to the above beneficial functions, Indian jujube is used for both food and traditional medicine in Asian [10]. However, the stability of most phenolics in food and medicine is not high [11], and they could easily be affected by the human digestive environment with high pH and complex enzymes [12]. Therefore, digestive system is a key factor that affects the content and function of Indian jujube phenolics. To elucidate the effect of in vivo digestion on the stability and function of dietary phenolics, several simulated digestion models have been developed and widely applied to various foods [13]. These models generally contain three steps including simulated oral, gastric and intestinal digestions. By using typical digestive enzyme such as amylase, pepsin and pancreatin, these models imitate the digestive system in vitro. Several food materials such as apple [14], Lycium barbarum fruit [15] and cassava leaves [16] are subjected to simulated digestion models and most of the phenolics show low stability during the digestions. As to the effect of the digestive system on the Indian jujube phenolics, especially on the phenolics of its peel and pulp, limited information is available.
In this study, to elucidate the effect of digestive system on the phenolics of Indian jujube peel and pulp, they were separately subjected to simulated digestions. The phenolic profile and content, as well as the antioxidant activity of the digestion samples were explored.
2. Materials and methods
2.1. Chemicals and regents
Chemicals and enzymes were purchased from Aladdin Chemicals (Shanghai, China), mainly including Folin-phenol reagent, 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-Tri (2-pyridyl)-striazine (TPTZ), bile salt, and α-amylase (35,000 U/g), pepsin (479,000 U/g), and trypsin (125,000 U/g).
2.2. Pretreatment of jujube
The fresh jujube (5 kg) was bought from the local market (Xuancheng, Anhui) in May of 2022. All the jujubes were peeled and stoned after washing, and the peel and pulp were collected and homogenized (1 g sample per 5 mL water) separately by a juicer (Jiuyang Co., Ltd., China). The freshly prepared homogenates were used for next step directly, and the rest homogenates were kept in −80 °C for further use.
2.3. Simulated digestions
The simulated digestion was referred to Minekus’s procedure [17] with some modifications. The digestions consisted of three consecutive phases. In oral digestion step, nine homogenates of jujube peel or pulp (30 mL) was mixed with α-amylase solution (150 U/mL in the mixture) for 5 min after adjusting the pH to 6.5, three homogenates kept from this step were called oral digestion samples. In gastric digestion step, the six digested samples from oral step was mixed with pepsin solution (2000 U/mL in the mixture) for 2 h after adjusting pH to 2.0, three samples extracted from this step were called gastric digestion samples. In intestinal digestion step, the rest three digested samples from gastric step were mixed with pancreatin solution (200 U/mL in the mixture) for 2 h after adjusting pH to 7.4, and samples from this step were called intestine digestion samples. To stop the intestinal digestion, the samples were freeze at −80 °C, and then defrosted and centrifugated, and the supernatant were used for further analysis [18]. As controls, homogenate of jujube peel or pulp treated with the same procedure without enzymes. All the treatments were carried out on a water bath shaker at 37 °C. The volume of all the samples was made to 35 mL after adjusting the pH to 4.0. The samples for analysis in one week were stored at 4 °C, and rest samples were stored at −80 °C. All the digestions or controls were carried out in triplicate.
2.4. Total phenolics content (TPC)
The TPC was estimated according to the Singleton’s method [19] with some modifications. The diluted sample (450 μL) was mixed with Folin-Ciocalteu phenol reagent (50 μL), and kept in the dark for 5 min. After the addition of Na2CO3 (10%, 500 μL), the mixture was kept in the dark for another 10 min. The absorbance of mixture at 730 nm was read by the spectrophotometer (UV-5100, Metash, China). The TPC of the sample was expressed as gallic acid equivalents (GAE) per gram of fresh weight (FW).
2.5. Total flavonoids content (TFC)
The TFC of the samples was determined referring to Arvouet-Grand’s procedure [20] with some change. The diluted sample (500 μL) was mixed with NaNO2 (5%, w/v, 30 μL) and kept in the dark for 6 min, and followed by the addition of AlCl3 (10%, w/v, 30 μL) and kept in the dark for another 8 min. The mixture then mixed with NaOH (1 M, 200 μL) and kept in the dark for another 10 min. The absorbance of the mixture at 510 nm was read by the above spectrophotometer. The TFC of the sample was expressed as the rutin equivalent (RE) per gram of fresh weight (FW).
2.6. Antioxidant activity
2.6.1. DPPH radical scavenging activity
The evaluation of DPPH radical scavenging activity referred to Blois' protocol [21] with some modifications. Briefly, the diluted sample (25 μL) was mixed with DPPH radical solution (0.05 mM, 500 μL) and absolute ethanol (475 μL), and kept in the dark for 30 min. The absorbance of the sample at 517 nm was read by the above spectrophotometer. The DPPH radical scavenging activity of the sample was expressed as trolox equivalents (TE) per gram of fresh weight (FW).
2.6.2. Ferric reducing antioxidant power (FRAP)
The determination of FRAP of the samples referred to Benzie’s procedure [22] with some modifications. The diluted sample (10 μL) was mixed with the FRAP solution (990 μL) and kept in the dark for 10 min. The absorbance of the sample at 593 nm was read by using the above spectrophotometer. The FRAP of the samples was expressed as trolox equivalents (TE) per gram of fresh weight (FW).
2.7. HPLC and HPLC-MS analysis
The stored digestion samples were freeze-dried, extracted with methanol and filtered with 0.45 μm membrane [18]. The obtained extracts were analyzed by an iChorm5100 HPLC system (Dalian Elite, China) equipped with a reverse-phase C18 column (250 mm × 4.6, 5 μm, Dalian Elite, China) at 360 nm. The samples were eluted with a mixed solvent of 0.1% formic acid acetonitrile (solvent A) and 0.1% formic acid water (solvent B) at 1 mL/min, and the gradient was: 0–30 min, solvent A increased from 5% to 20%; 30–35 min, solvent A kept at 20%; 35–45 min, solvent A increased from 20% to 25%; 45–50 min, solvent A increased from 25% to 100%; 50–55 min, solvent A decreased from 100% to 5%; 55–60 min, solvent A maintained at 5%. ACQUITY UPLC LCT Premier XE system (Waters, USA) equipped with C18 column (250 mm × 4.6, 5 μm, Dalian Elite, China) was used for mass spectrometry, the elution condition was identical to HPLC analysis. Mass conditions: negative ionization mode with a m/z ratio of 100–1000. The ionization voltage was 3.5 kV, the drying temperature was 11 L/min, the capillary temperature and voltage were 350 °C and 4000 V (+)/3500 V (−), and the atomizer pressure was 50 psi. The phenolics in digestion samples were identified by the comparison with reference compounds or their reported mass fragments.
2.8. Quantification of phenolics
The identified phenolics were derivatives of naringenin and quercetin, therefore, these phenolics were quantified by HPLC analysis using naringenin and quercetin as reference compounds.
2.9. Statistical analysis
The samples were prepared in triplicates. The data was analyzed by one-way variance (ANOVA) and Duncan’s test at a significance level of p ≤ 0.05. Pearson correlation analysis was conducted to explore the correlations between antioxidant activity and phenolics/flavonoids contents, the significant levels were defined at p < 0.05(*) and p < 0.01(**).
3. Results and discussion
3.1. TPC and TFC
To explore the effect of simulated digestion on the phenolics and flavonoids in Indian jujube, TPC and TFC were determined. As shown in Fig. 1, the TPC/TFC in peel was obviously higher than that in pulp, and they increased significantly after simulated digestion (p ≤ 0.05). In oral digestion step, compared with control groups, the TPC increased by 84.31% in pulp and 74.00% in peel after digestion (Fig. 1A and B), while the corresponding increases of TFC were respectively 19.63% and 37.80% for pulp and peel (Fig. 1C and D), indicating that the oral digestion may degrade some polysaccharides and enhance the release of phenolics and flavonoids, and similar result also reported for sesame seeds [23]. In gastric digestion step, compared with oral step, the TPC increased by 4.13% and 7.22% in pulp and peel (Fig. 1A and B), while the corresponding increases of TFC were 3.86% and 2.50% (Fig. 1C and D), and the low pH related acid environment may be the reason for the increase: the weak interaction between the some phenolics/flavonoids and fruit matrix (e.g. protein, oligosaccharides, etc.) may be broken at the low pH, leading to the increased release of phenolics and flavonoids [24]. In small intestinal digestion step, the TPC decreased by 9.89% in pulp and 21.83% in peel as compared with gastric digestion (Fig. 1A and B), while the corresponding decreases of TFC were 15.80% and 16.76% (Fig. 1C and D), and the degradation of some phenolics and flavonoids in weak alkaline environment may be the reason [25]. The change in the TPC/TFC of Indian jujube was similar to that of persimmon fruit [26].
Fig. 1.
TPC and TFC of digested Indian jujube fruit peel and pulp. (A) TPC of the fruit pulp; (B) TPC of the fruit peel; (C) TFC of the fruit pulp; (D) TFC of the fruit peel.
3.2. Antioxidant activity
To detect the effect of simulated digestion on the antioxidant activity of digested Indian jujube pulp and peel, DPPH scavenging activity and FRAP assay were conducted. According to Fig. 2, the antioxidant activity of Indian jujube generally increased after simulated digestions, but the increases in pulp and peel were different. In oral step, the DPPH scavenging activity increased by 33.41% in pulp and 10.43% in peel as compared with control group (Fig. 2A and B), while the FRAP respectively increased by 20.34% and 8.33% in pulp and peel (Fig. 2C and D). In gastric digestion step, as compared with oral digestion, the DPPH scavenging activity increased by 12.28% and 4.29% in pulp and peel (Fig. 2A and B), while FRAP decreased 9.38% in pulp and increased 13.93% in peel (Fig. 2C and D). In small intestine digestion, the DPPH scavenging activity respectively decreased 16.95% and 23.47% in the pulp and peel (Fig. 2A and B), and the corresponding decreases of FRAP were 8.17% and 36.39% (Fig. 2C and D). The change of antioxidant activity was similar to that of TPC/TFC (Fig. 1), indicating that the phenolics and flavonoids may play an important role in the antioxidant activity of Indian jujube. Similar results were reported for the antioxidant activity of digested Lycium barbarum fruit [15].
Figure-2.
Antioxidant activity of digested Indian jujube pulp and peel. (A) DPPH scavenging activity of the fruit pulp; (B) DPPH scavenging activity of the fruit peel; (C) FRAP of the fruit pulp; (D) FRAP of the fruit peel.
3.3. Correlation analysis
As mentioned in the previous section, the change of antioxidant activity of Indian jujube were related to its TPC/TFC, therefore, the correlations between them were analyzed. As shown in Table 1, strong correlations were mainly detected in peel group, but were hardly found in pulp group. In pulp series, significant correlations were only found in control group for DPPH scavenging activity and TPC/TFC, and correlations was not high (0.686 < r < 0.732, p < 0.05). In peel series, the antioxidant activity was strongly correlated with the TPC/TFC (r > 0.858, p < 0.01). The results implied that phenolics and flavonoids may be the main antioxidants in Indian jujube peel. As to the pulp series, besides phenolics and flavonoids, polysaccharides and proteins in the pulp may contribute to the antioxidant activity to some degree, leading to the low correlations between its antioxidant activity and TPC/TFC.
Table 1.
Correlation analysis between antioxidant activity and TPC/TFC.
| TPC/TFC | DPPH | FRAP | |
|---|---|---|---|
| Pulp (control) | TPC | 0.732* | 0.197ns |
| TFC | 0.686* | 0.329ns | |
| Digested Pulp | TPC | 0.451ns | 0.453ns |
| TFC | 0.432ns | 0.422ns | |
| Peel (Control) | TPC | 0.915** | 0.958** |
| TFC | 0.947** | 0.858** | |
| Digested peel | TPC | 0.910** | 0.914** |
| TFC | 0.978** | 0.950** |
Significance: ∗∗ p ⩽ 0.01; ∗ p ⩽ 0.05; ns, non-significant.
3.4. HPLC profile
To explore the change of individual phenolics and flavonoids during the digestion, HPLC analysis was carried out. Regarding that the TPC/TFC was more abundant in Indian jujube peel (Fig. 1), therefore, HPLC analysis was mainly conducted to the peel series. According to Fig. 3, the number of the main compounds almost the same before (Fig. 3A–C) and after the simulated digestion (Fig. 3D–F), and the similarity also occurred during the digestion (Fig. 3D–F), implying that most compounds were retained during the digestion. However, the height and area of these main HPLC peaks varied greatly during the simulated digestion, and they were generally increased after simulated gastric digestion but decreased after the small intestine digestion, implying that the content of these main compounds changed with the digestion steps. Similar changes were also found in TPC/TFC (Fig. 1) and antioxidant activities (Fig. 2).
Fig. 3.
HPLC profile of Indian jujube peel. (A–C): the profiles of the control samples; (D–F): the profiles of oral, gastric and small intestine digestion samples.
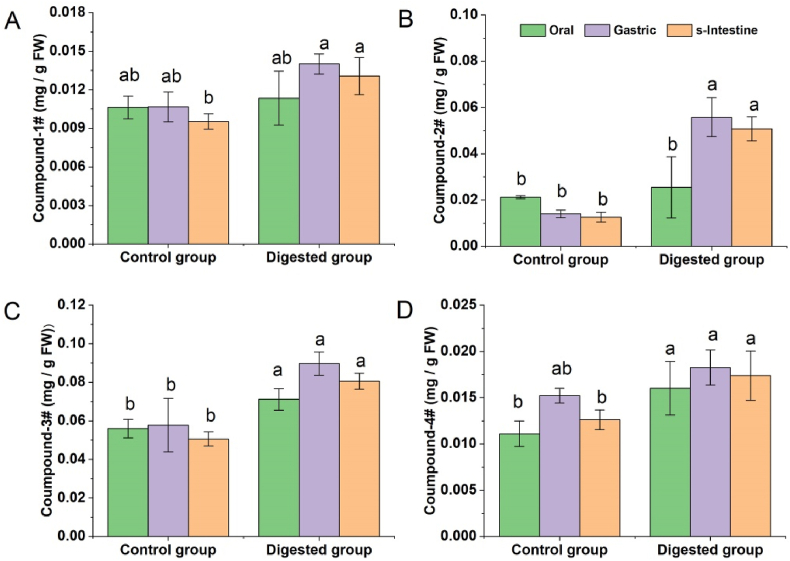
To further identify the main compounds in Fig. 3, HPLC-MS analysis was carried out, and four compounds were characterized (Table 2). Compound 1 showed the m/z of 741 ([M−H]−) and 743 ([M+H]+), and the MS fragments of 271 (naringenin) and 579 (lost a hexose group) at negative ion mode, according to the previous study on Indian jujube, it was tentatively identified as naringenin tri-glycoside [9]. Similarly, compounds 2–4 were identified as quercetin-3-O-[(2-hexosyl)-6-rhamnosyl]-hexoside (compound 2), Quercetin 3-O-pentosylhexoside (compound 3), and quercetin-3-O-(2-pentosyl-rhamnoside)-4′-O-rhamnoside (compound 4), and these compounds were previously reported in Indian jujube [9,27].
Table 2.
HPLC-MS characterization of four main compounds in Indian jujube peel.
| # | TR(min) | [M−H]−/[M+H]+ | MS fragments | Tentative identification | Reference |
|---|---|---|---|---|---|
| 1 | 30.82 | 741[M−H]− | 579/271 | Naringenin tri-glycoside | [9] |
| 743[M+H]+ | / | ||||
| 2 | 32.32 | 771[M−H]− | / | Quercetin-3-O-[(2-hexosyl)-6-rhamnosyl]-hexoside | [27] |
| 773[M+H]+ | 611/303 | ||||
| 3 | 33.02 | 595[M−H]− | / | Quercetin 3-O-pentosylhexoside | [9] |
| 597[M+H]+ | 465/303 | ||||
| 4 | 43.63 | 725[M−H]− | / | Quercetin-3-O-(2-pentosyl-rhamnoside)-4′-O-rhamnoside | [27] |
| 727[M+H]+ | 595/449/303 |
3.5. Quantification of individual phenolics
TPC and TFC analysis could only give an overview of phenolics and flavonoids in Indian jujube, but the detailed change of individual phenolics/flavonoids was unknown. To clarify the variation of the main phenolics during the simulated digestion, four identified (Table 2) phenolics were quantified. As shown in Fig. 4, the content of compounds 1–4 generally increased after simulated digestion. As to the change at different digestion steps, these compounds were mainly increased after gastric digestion but decreased after small intestine digestion. In oral step, compared with control groups, compound 1-4 separately increased 6.87%, 19.86%, 27.08% and 44.41% after digestion (Fig. 4A–D), indicating that simulated digestion could enhance the release of phytochemicals. In gastric step, compound 1–4 respectively increased 23.35%, 119.04%, 25.99% and 13.91% as compared with oral digestion (Fig. 4A–D), implying that gastric digestion further improved the release of phytochemical compounds. However, in small intestine step, compound 1-4 decreased 6.71%, 9.05%, 10.12% and 4.82% as compared with gastric digestion (Fig. 4A–D), and the decrease may be produced by alkalescence environment of this digestion step. The change of these compounds during the simulated digestion was similar to the findings reported for the digestion of Huangshan Gongju [24] and Lycium barbarum fruit [15].
Fig. 4.
The content of main individual compounds in Indian jujube peel before and after simulated digestion. (A) Naringenin tri-glycoside; (B) Quercetin-3-O-[(2-hexosyl)-6-rhamnosyl]-hexoside; (C) Quercetin 3-O-pentosylhexoside; (D) Quercetin-3-O-(2-pentosyl-rhamnoside)-4′-O-rhamnoside.
4. Conclusions
The digestive property and antioxidant activity of Indian jujube peel and pulp phenolics were explored in this study. Our data demonstrated the release of phenolics and flavonoids, as well as the antioxidant activities were enhanced after simulated digestions in both peel and pulp, and proved that Indian jujube phenolics/flavonoids were more abundant in the peel, and the main phenolics/flavonoids in the peel showed promising stability during the simulated digestion, implying that Indian jujube peel extract could be developed as potential antioxidant for food industry.
Author contribution statement
Zi-Tong Wang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yu-Ping Liu: Performed the experiments; Analyzed and interpreted the data.
Yi-Long Ma: Conceived and designed the experiments; Wrote the paper.
Shuang-Yi Pan, Jian-Kang Li, Shao-Jun Shi: Performed the experiments.
Zheng-Fang Wu, Zhi Li, Ya-Fang Shang: Contributed reagents, materials, analysis tools or data.
Zhao-Jun Wei: Conceived and designed the experiments.
Funding statement
This work was supported National Natural Science Foundation of China (32272312), National Innovation and Entrepreneurship Training Program for College Students (202210359114), National Key R&D Program of China (2022YFF1100300), and Natural Science Foundations of Ningxia (2022AAC03262).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
Contributor Information
Zi-Tong Wang, Email: 945731842@qq.com.
Yu-ping Liu, Email: 2837453519@qq.com.
Yi-Long Ma, Email: yilong.ma@hfut.edu.cn.
Shuang-Yi Pan, Email: 2392151960@qq.com.
Jian-Kang Li, Email: 3115730586@qq.com.
Shao-Jun Shi, Email: 1527297241@qq.com.
Zheng-Fang Wu, Email: 3306796325@qq.com.
Zhi Li, Email: 402996480@qq.com.
Ya-Fang Shang, Email: yafangshang19@hfut.edu.cn.
Zhao-Jun Wei, Email: zjwei@hfut.edu.cn.
Reference
- 1.Chiou C.-Y., Shih H.-C., Tsai C.-C., Jin X.-L., Ko Y.-Z., Mantiquilla J.A., Weng I.S., Chiang Y.-C. The genetic relationships of Indian jujube (Ziziphus mauritiana Lam.) cultivars using SSR markers. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afroz R., Tanvir E.M., Islam M.A., Alam F., Gan S.H., Khalil M.I. Potential antioxidant and antibacterial properties of a popular Jujube fruit: Apple Kul (Zizyphus mauritiana) J. Food Biochem. 2014;38(6):592–601. doi: 10.1111/jfbc.12100. [DOI] [Google Scholar]
- 3.Prakash O., Usmani S., Singh R., Singh N., Gupta A., Ved A. A panoramic view on phytochemical, nutritional, and therapeutic attributes of Ziziphus mauritiana Lam.: a comprehensive review. Phytother Res. 2021;35(1):63–77. doi: 10.1002/ptr.6769. [DOI] [PubMed] [Google Scholar]
- 4.Zozio S., Servent A., Cazal G., Mbéguié-A-Mbéguié D., Ravion S., Pallet D., Abel H. Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana Lamk) Food Chem. 2014;150:448–456. doi: 10.1016/j.foodchem.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Mishra T., Khullar M., Bhatia A. Anticancer potential of aqueous ethanol seed extract of Ziziphus mauritiana against cancer cell lines and ehrlich ascites carcinoma. Evid. base Compl. Alternative Med. 2011;2011 doi: 10.1155/2011/765029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia A., Mishra T. Hypoglycemic activity of Ziziphus mauritiana aqueous ethanol seed extract in alloxan-induced diabetic mice. Pharmaceut. Biol. 2010;48(6):604–610. doi: 10.3109/13880200903218935. [DOI] [PubMed] [Google Scholar]
- 7.Aldhanhani A.R.H., Ahmed Z.F.R., Tzortzakis N., Singh Z. Maturity stage at harvest influences antioxidant phytochemicals and antibacterial activity of jujube fruit (Ziziphus mauritiana Lamk. and Ziziphus spina-christi L.) Ann. Agric. Sci. (Cairo) 2022;67(2):196–203. doi: 10.1016/j.aoas.2022.12.003. [DOI] [Google Scholar]
- 8.Ramar M.K., Henry L.J.K., Ramachandran S., Chidambaram K., Kandasamy R. Ziziphus mauritiana Lam attenuates inflammation via downregulating NFκB pathway in LPS-stimulated RAW 264.7 macrophages & OVA-induced airway inflammation in mice models. J. Ethnopharmacol. 2022;295 doi: 10.1016/j.jep.2022.115445. [DOI] [PubMed] [Google Scholar]
- 9.Memon A.A., Memon N., Bhanger M.I., Luthria D.L. Assay of phenolic compounds from four species of ber (Ziziphus mauritiana L.) fruits: comparison of three base hydrolysis procedure for quantification of total phenolic acids. Food Chem. 2013;139(1):496–502. doi: 10.1016/j.foodchem.2013.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Rashwan A.K., Karim N., Shishir M.R.I., Bao T., Lu Y., Chen W. Jujube fruit: a potential nutritious fruit for the development of functional food products. J. Funct.Foods. 2020;75 doi: 10.1016/j.jff.2020.104205. [DOI] [Google Scholar]
- 11.Nayak B., Liu R.H., Tang J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit. Rev. Food Sci. Nutr. 2015;55(7):887–918. doi: 10.1080/10408398.2011.654142. [DOI] [PubMed] [Google Scholar]
- 12.Ketnawa S., Reginio F.C., Jr., Thuengtung S., Ogawa Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: a review. Crit. Rev. Food Sci. Nutr. 2022;62(17):4684–4705. doi: 10.1080/10408398.2021.1878100. [DOI] [PubMed] [Google Scholar]
- 13.Lucas-González R., Viuda-Martos M., Pérez-Alvarez J.A., Fernández-López J. In vitro digestion models suitable for foods: opportunities for new fields of application and challenges. Food Res. Int. 2018;107:423–436. doi: 10.1016/j.foodres.2018.02.055. [DOI] [PubMed] [Google Scholar]
- 14.Corona-Leo L.S., Meza-Márquez O.G., Hernández-Martínez D.M. Effect of in vitro digestion on phenolic compounds and antioxidant capacity of different apple (Malus domestica) varieties harvested in Mexico. Food Biosci. 2021;43 doi: 10.1016/j.fbio.2021.101311. [DOI] [Google Scholar]
- 15.Ma Y.-L., Wang Y., Wu Z.-F., Mei J., Zhang W.-Q., Shang Y.-F., Thakur K., Wei Z.-J. Exploring the effect of in vitro digestion on the phenolics and antioxidant activity of Lycium barbarum fruit extract. Food Biosci. 2023;51 doi: 10.1016/j.fbio.2022.102255. [DOI] [Google Scholar]
- 16.Laya A., Koubala B.B. Polyphenols in cassava leaves (Manihot esculenta Crantz) and their stability in antioxidant potential after in vitro gastrointestinal digestion. Heliyon. 2020;6(3) doi: 10.1016/j.heliyon.2020.e03567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carrière F., Boutrou R., Corredig M., Dupont D., Dufour C., Egger L., Golding M., Karakaya S., Kirkhus B., Le Feunteun S., Lesmes U., Macierzanka A., Mackie A., Marze S., McClements D.J., Ménard O., Recio I., Santos C.N., Singh R.P., Vegarud G.E., Wickham M.S.J., Weitschies W., Brodkorb A. A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 18.Ortega-Vidal J., Ruiz-Riaguas A., Fernández-de Córdova M.L., Ortega-Barrales P., Llorent-Martínez E.J. Phenolic profile and antioxidant activity of Jasonia glutinosa herbal tea. Influence of simulated gastrointestinal in vitro digestion. Food Chem. 2019;287:258–264. doi: 10.1016/j.foodchem.2019.02.101. [DOI] [PubMed] [Google Scholar]
- 19.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Methods in Enzymology. Academic Press; 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent; pp. 152–178. [DOI] [Google Scholar]
- 20.Arvouet-Grand A., Vennat B., Pourrat A., Legret P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994;49(6):462–468. [PubMed] [Google Scholar]
- 21.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 22.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 23.Luo J., Li M., Wu H., Liu Z., Barrow C., Dunshea F., Suleria H.A.R. Bioaccessibility of phenolic compounds from sesame seeds (Sesamum indicum L.) during in vitro gastrointestinal digestion and colonic fermentation. J. Food Process. Preserv. 2022;46(7) doi: 10.1111/jfpp.16669. [DOI] [Google Scholar]
- 24.Ma Y.-L., Wang Y., Wu Z.-F., Mei J., Zhang W.-Q., Shang Y.-F., Liu F.-R., Yang S.-H., Thakur K., Wei Z.-J. Profile and activity of phenolic antioxidants in chrysanthemum (Huangshan Gongju) as affected by simulated digestions. J. Food Biochem. 2022;46(12) doi: 10.1111/jfbc.14458. [DOI] [PubMed] [Google Scholar]
- 25.Lucas-Gonzalez R., Navarro-Coves S., Pérez-Álvarez J.A., Fernández-López J., Muñoz L.A., Viuda-Martos M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crop. Prod. 2016;94:774–782. doi: 10.1016/j.indcrop.2016.09.057. [DOI] [Google Scholar]
- 26.Lucas-González R., Viuda-Martos M., Pérez Álvarez J.A., Fernández-López J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018;256:252–258. doi: 10.1016/j.foodchem.2018.02.128. [DOI] [PubMed] [Google Scholar]
- 27.Sakna S.T., Mocan A., Sultani H.N., El-fiky N.M., Wessjohann L.A., Farag M.A. Metabolites profiling of Ziziphus leaf taxa via UHPLC/PDA/ESI-MS in relation to their biological activities. Food Chem. 2019;293:233–246. doi: 10.1016/j.foodchem.2019.04.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.