Abstract
In the context of the COVID-19 pandemic, antiviral drugs (AVDs) were heavily excreted into wastewater and subsequently enriched in sewage sludge due to their widespread use. The potential ecological risks of AVDs have attracted increasing attention, but information on the effects of AVDs on sludge anaerobic digestion (AD) is limited. In this study, two typical AVDs (lamivudine and ritonavir) were selected to investigate the responses of AD to AVDs by biochemical methane potential tests. The results indicated that the effects of AVDs on methane production from sludge AD were dose- and type-dependent. The increased ritonavir concentration (0.05–50 mg/kg TS) contributed to an 11.27–49.43% increase in methane production compared with the control. However, methane production was significantly decreased at high lamivudine doses (50 mg/kg TS). Correspondingly, bacteria related to acidification were affected when exposed to lamivudine and ritonavir. Acetoclastic and hydrotropic methanogens were inhibited at a high lamivudine dose, while ritonavir enriched methylotrophic and hydrotropic methanogens. Based on the analysis of intermediate metabolites, the inhibition of lamivudine and the promotion of ritonavir on acidification and methanation were confirmed. In addition, the existence of AVDs could affect sludge properties. Sludge solubilization was inhibited when exposed to lamivudine and enhanced by ritonavir, perhaps caused by their different structures and physicochemical properties. Moreover, lamivudine and ritonavir could be partially degraded by AD, but 50.2–68.8% of AVDs remained in digested sludge, implying environmental risks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-023-27045-7.
Keywords: Methane production, Microbial community, Degradation, Lamivudine, Ritonavir, Solubilization, Acidification, Methanation
Introduction
Due to the rapid development of urbanization, the scale of wastewater treatment plants (WWTPs) has dramatically increased, and the production of waste sewage sludge (WAS) has also increased sharply. The composition of WAS is complex, including organic substances (e.g., proteins, polysaccharides, and humus), as well as some pollutants (e.g., antibiotics, microplastics, and antiviral drugs (AVDs)) (Chen et al. 2021; Wu et al. 2022; Yao et al. 2021b; Zou et al. 2022). Anaerobic digestion (AD) has been widely used as a mainstream process for sludge treatment, which can simultaneously achieve reduction, resource utilization, and stabilization (Kumar Khanal et al. 2021). As a biochemical degradation process of complex organic matter (Zhu and Chen 2022), the efficiency and stability of AD mainly depend on the complex microbial communities and their activities, which are susceptible to exogenous pollutants (Luo et al. 2020; Xu et al. 2022). It was reported that the antimicrobial agent triclocarban promoted sludge solubilization but inhibited methanogens activity, which resulting in the reduction of methane production (Wang et al. 2020). Meanwhile, some aminoglycoside antibiotics may cause the reduction of acetic acid producing activity by interfering with cell wall synthesis, and RNA polymerase activity, which inhibited WAS digestion (Luo et al. 2020).
As one of the pharmaceuticals, AVDs have been used to treat viral infections, and various existing pharmaceuticals (e.g., remdesivir and lopinavir) have been tested as therapeutic agents to treat COVID-19 patients (Guan et al. 2020), which increased their use and concentration in the environment. After human consumption, parts of AVDs could be extracted into wastewater as unchanged drugs or metabolites, and WWTPs have been identified as major sources of AVDs in the environment (Kumar et al. 2021). However, WWTPs have a limited ability to degrade AVDs, and some lipophilic compounds have attracted particular attention due to their substantial accumulation in sludge. Compared to the concentration of < LOQ–1463.5 μg/L in aquatic environments, AVDs concentration levels ranging from < LOQ to 82.2 mg/kg were observed in sludge and suspended particulate matter samples (Muriuki et al. 2020; Nannou et al. 2020; Schoeman et al. 2017). Nevertheless, high prevalence and accumulation of lamivudine (3TC), a hydrophilic compound, have also been reported in sludge with concentrations of 1–31.5 mg/kg, which may cause potential impacts on subsequent sludge biological treatment (Ariyanta et al. 2022).
Some AVDs are bioactive and highly persistent in aquatic environments, which may negatively affect non-target organisms and biological activity (Kumar et al. 2021; Nannou et al. 2020; Yao et al. 2021a). Several toxicology studies have also demonstrated the toxicity of AVDs to aquatic organisms (Robson et al. 2017), and AVD ritonavir was confirmed to have high chronic toxicity, which was believed as priority AVD that need to be monitored (Czech et al. 2022). There was evidence of no adaptation of activated sludge bacteria, disruption of nitrification, and biological phosphorus removal with high AVD Tamiflu exposure in an aerobic granular sludge sequencing batch reactor (Slater et al. 2011). The active metabolite of Tamiflu, oseltamivir carboxylate, was also reported to affect bacterial community structure mainly through stimulating α, β, and γ-Proteobacteria in aquatic ecosystems (Caracciolo et al. 2010). However, the physicochemical characteristics of AVDs (e.g., water solubility, chemical structure, and polarity) vary widely, and differences between the ecotoxicities of different AVDs were observed (Azuma et al. 2015; Ngumba et al. 2016; Straub 2017). Almeida et al. found that zidovudine was the least toxic to Ceriodaphnia dubia with half maximal effective concentration (EC50) of 5.67 mg/L, while the species was sensitive to efavirenz with an EC50 of 0.03 mg/L (Almeida et al. 2021). Therefore, the effects of AVDs on sludge AD performance may be type-dependent, which requires further study.
This study aims to analyze the potential impacts of AVDs on the AD process of WAS. Firstly, the effects of different AVD types and concentrations on methane production during WAS anaerobic digestion were investigated by using biochemical methane potential (BMP) tests. Two well-known AVDs with relatively high concentrations in WWTPs were selected in this study, including lamivudine (3TC) and ritonavir (RIT). Among them, RIT is one of the candidate drugs for the therapy of COVID-19 (Barber et al. 2022). Meanwhile, the variations of microbial communities with different AVD exposures were also investigated. Thirdly, impacts of AVDs on solubilization, hydrolysis, acidification, and methanation were analyzed to reveal how AVDs affect WAS anaerobic digestion. Moreover, the degradation of AVDs during WAS anaerobic digestion was also determined. The findings of this work provide a reference guide for understanding the effects of AVDs during sludge treatment and disposal processes.
Materials and methods
Sludge properties and AVDs
The WAS used in this study was collected and concentrated at a WWTP in Shanghai, China. The inoculated sludge for AD experiments was obtained from a laboratory semi-continuous mesophilic anaerobic digester in the same WWTP mentioned above. The substrates were stored at 4 °C before use and inoculated sludge samples were incubated at 37 °C for 5 days in anaerobic conditions to improve microbial activity. The characteristics of WAS and inoculum sludge are presented in Table 1. The AVDs used in the experiments included 3TC (98% purity) and RIT (98% purity), which were purchased from Adamas Reagents Ltd., Shanghai, China. The standard lamivudine and ritonavir were stored at − 20 °C before use.
Table 1.
WAS and inoculum properties
| Parameters | WAS | Inoculated sludge |
|---|---|---|
| pH | 6.9 ± 0.1 | 7.1 ± 0.1 |
| Total solids (g/L) | 28.4 ± 1.4 | 42.5 ± 2.1 |
| Volatile solids (g/L) | 14.2 ± 0.7 | 14.8 ± 0.7 |
| TCOD (g/L) | 27.6 ± 1.4 | 37.0 ± 1.9 |
| SCOD (mg/L) | 297.0 ± 14.9 | 309.0 ± 15.5 |
| Soluble protein (mg/L) | 43.0 ± 2.2 | 27.1 ± 1.4 |
| Total polysaccharides (mg/g VS) | 32.2 ± 1.6 | 26.1 ± 1.3 |
| Total VFAs (mg COD/L) | 26.1 ± 1.3 | 41.2 ± 2.1 |
Note: “WAS” indicates “waste activated sludge”; “TCOD” indicates “total chemical oxygen demand”; “SCOD” indicates “the soluble chemical oxygen demand”; “VFAs” indicates “volatile fatty acids”
Biochemical methane potential tests
The AD experiments were carried out in 500 mL anaerobic bottles filled with 400 mL mixtures. The WAS and inoculated sludge were mixed at a volatile solids (VS) ratio of 2:1. On the control group, only WAS and inoculated sludge without supernumerary AVDs were added. It was reported that the concentration of 3TC in suspended particles and sludge was ranged from 0.1 μg/kg to 69.68 mg/kg, and that of RIT was ranged from 0.1 μg/kg to 0.01 mg/kg (Muriuki et al. 2020; Yao et al. 2021a). Thus, a wide concentration range of 3TC and RIT (0.05, 5, and 50 mg/kg TS) was chosen to disclose the potential effects on WAS anaerobic digestion. In this study, bottles with 3TC concentrations of 0.05, 5, and 50 mg/kg TS, respectively, were designated as 3TC_1, 3TC_2, and 3TC_3, whereas bottles with RIT concentrations of 0.05, 5, and 50 mg/kg TS, respectively, were designated as RIT_1, RIT_2, and RIT_3, respectively. Each experiment was carried out in triplicate. The initial pH value was maintained at 7.0, and each bottle was flushed with nitrogen gas. The bottles were then incubated in a shaker at 37 ± 1 °C and 120 r/min for 28 days. The biogas components and volume were measured, and the methane production was recorded as the volume of methane produced per kilogram of VS added (mL CH4/g VS).
Effects of AVDs on four individual steps in WAS anaerobic digestion
WAS anaerobic digestion generally includes four steps: solubilization, hydrolysis, acidification, and methanation (Wang et al. 2021). In order to investigate the effects of AVDs on each step of WAS anaerobic digestion, four batch experiments were carried out using synthetic wastewater. The dosages of 3TC and RIT were the same as for BMP tests, and each test group was carried out in triplicate.
To investigate the impacts of 3TC and RIT on solubilization process, the release of soluble protein, polysaccharide, and humus from WAS were evaluated at different 3TC and RIT concentrations. 200 mL of WAS were added, and the producers used to this experiment were the same as for the BMP tests. These batch tests were last for 1 day, and the concentrations of soluble protein, polysaccharide, and humus were quantified. Secondly, to investigate the impacts of AVDs on the hydrolysis process, 65 mL of inoculum sludge and 135 mL of synthetic wastewater were added to bottles. The synthetic wastewater contained 3.6 g/L bovine serum albumin (BSA, MW 67,000) and 0.9 g/L dextran (MW 40,000). BSA and dextran were chosen as model protein and polysaccharide compounds, given that their mass ratio was almost the same as that of protein and carbohydrate in raw WAS (Luo et al. 2015). 50 mmol/L 2-bromoethanesulfonic acid (BESA) was also dosed into the system to inhibit methanogens (Balch and Wolfe 1979; Liu et al. 2017; Wu et al. 2020). The producers were the same as the BMP tests, and the concentrations of BSA and dextran were measured after 3 days. In acidification experiments, synthetic wastewater contained 3.6 g/L L-glutamic acid (a model amino acid), 0.9 g/L glucose (a model monosaccharide), and BESA. Then, the operation was conducted with the same as described in hydrolysis test, and the production of volatile fatty acids (VFAs) was measured after 3 days of anaerobic fermentation mixed with the inoculum. In methanation experiments, the operation was same as the hydrolysis test except that BSA, and dextran was replaced with 2.16 g/L sodium acetate, and BESA was not added. Effects of AVDs on methanation were evaluated by measuring methane production after fermentation for 15 days.
Microbial community analysis
At the end of AD, sludge samples with and without AVD dosing were harvested and stored at − 20 °C. The microbial community structure was analyzed by high-throughput sequencing conducted by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The total DNA was extracted using the FastDNA Spin Kit for Soil DNA (MP Biomedicals, USA), and the extraction was amplified by PCR. The primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′)/806R (5′-GGACTACHVGGGTWTCTAAT-3′) and 524F10extF (5′-TGYCAGCCGCCGCGGTAA-3′)/Arch958RmodR (5′-YCCGGCGTTGAVTCCAATT-3′) were chosen for the bacterial and archaeal 16S rRNA gene PCR amplification, respectively.
Analytical methods
Soluble proteins and polysaccharides were determined using the modified Lowry-Folin method (Lowry et al. 1951) and the anthrone-sulfuric acid method (Jimenez et al. 2013), respectively. Soluble humic acid was measured by the modified Lowry method (Frlund et al. 1995). Total solids (TS), VS, total chemical oxygen demand (TCOD), and soluble chemical oxygen demand (SCOD) were all performed according to standard methods (Eaton et al. 1966). The VFAs were determined by gas chromatography equipped with a flame ionization detector (FID) (Shimadzu GC-1020 plus, Japan). The gas component was analyzed using gas chromatograph (Lunan GC 6890, China) equipped with a thermal conductivity detector (TCD). The methane production was calculated by multiplying integral biogas volume by the methane percentage mentioned above.
Extraction and determination of AVDs
The extraction of AVDs (3TC and RIT) from sludge samples was based on the extraction methods reported by Yao et al. (2021b) and Abafe et al. (2018) with slight modifications. Briefly, 1 mL of each sludge sample was mixed with 4 mL methanol, and the mixture was sonicated in an ultrasonic cleaner for 30 min. Afterward, the samples were centrifuged at 8000 rpm for 5 min, and the above operation was repeated twice. The supernatant was then concentrated by a hydrophilic-lipophilic balance (HLB) cartridge (200 mg, 6 mL, Milford, MA, USA), and the effluent evaporated to near dryness under a gentle nitrogen stream. Finally, the final extracts were redissolved in 2 mL of the corresponding mobile phase solution, filtered through a 0.22 μm membrane filter, and stored at − 20 °C until instrumental analysis. This concentrated method allows for a fivefold concentration.
3TC was detected by high-performance liquid chromatography (HPLC) with reference to Mallikarjuna and Gowri (Mallikarjuna Rao and Gowri Sankar 2015). The parameters are as follows: The mobile phases consisted of methanol (solvent B) and distilled water (solvent A) at a ratio of 2:8. The injection volume was 10 μL, and the chromatography column was maintained at 60 °C. The Variable Wavelength Detector (VWD) detector wavelength is 271 nm. The HYPERSIL PREP HS C18 column (Agilent, Germany) was used in this study, which had a particle size of 5 μm, internal dimensions of 4.6 mm, and a length of 250 mm. The method linearity of 3TC was examined over seven concentration levels ranging from 0.8 to 10 μg/mL. RIT was detected regarding Hiremath (Hiremath and Bhirud 2015) and conducted on a C18 column (5 μm, 250 mm × 4.6 mm). Briefly, the mobile phases consisted of acetonitrile (solvent C) and 0.05 M phosphate solution (solvent D) at a ratio of 6:4. The injection volume was 10 μL, and the chromatography column was maintained at 35 °C. The Variable Wavelength Detector (VWD) detector wavelength is 205 nm. This study’s final calibration curve for RIT ranged from 1 to 20 μg/mL. The specific HPLC detection parameters are shown in Table S1, and the standard curve is shown in Fig. S1. The detection limit (DL) and quantification limit (QL) of 3TC were 0.5 μg/mL and 1.51 μg/mL, respectively. In comparison, those of RIT were 0.79 μg/mL and 2.64 μg/mL, respectively, with a relative standard deviation of < 10%.
Statistical analysis
All experiments were conducted in triplicate, and the results were expressed as “mean ± standard deviation.” A one-way ANOVA test was used to evaluate the significance of the results, and the significant difference test (p < 0.05) was done using the least significant difference (LSD) method of multiple comparisons.
Results and discussion
Effects of AVDs on methane production from WAS digestion system
Figure 1 shows the cumulative methane production from AD of WAS with and without different AVD types and concentrations. It can be observed that the effects of AVDs on methane production were dose- and type-dependent. The cumulative methane production in the control group was 180 ± 19 mL/g VSS when stopping the experiment. With 0.05 mg/kg TS 3TC in WAS, the cumulative methane production increased by 5.56% compared with the control group. However, the cumulative methane production decreased with the further increase in 3TC. When the dose of 3TC was increased from 5 to 50 mg/kg TS, the reduction in cumulative methane production compared to the control decreased from 6.67 to 41.66%, indicating that 3TC at high dose (50 mg/kg TS) significantly inhibited the methane production during AD. Meanwhile, the impacts of RIT on methane production were different from that of 3TC. With increasing concentrations (0.05−50 mg/kg TS), RIT increased methane production by 11.28%, 34.67%, and 49.43%, respectively, compared to the control group. This indicated that RIT significantly promoted methane production at medium (5 mg/kg TS) and high doses. Similar dose- and type-dependent effects have been reported when exposed to other exogenous pollutants (Luo et al. 2020). It was reported that acetoclastic methanogens are more sensitive to the toxicity of pollutants (e.g., triclosan) than hydrotropic methanogens (Symsaris et al. 2015), and the effects of 3TC and RIT on methane production may be related to the variation of function microorganism.
Fig. 1.
Cumulative methane production from WAS with and without different AVDs types and concentrations. Different small letters (e.g., a, b, and c) indicated significant differences. The bottles with 3TC of 0.05, 5 and 50 mg/kg TS, respectively, were marked as 3TC_1, 3TC_2, and 3TC_3, while that with RIT of 0.05, 5, and 50 mg/kg TS, respectively, were marked as RIT_1, RIT_2, and RIT_3, respectively
In addition, the VS and VS removal rates during AD with different AVD exposures are shown in Fig. 2, which were used to assess the changes of volatile organic compounds in sludge. The VS removal rate continued to decrease with increasing 3TC concentrations compared to the control group (17.98 ± 0.39%), and VS removal efficiency decreased to 16.67 ± 0.34% at 3TC of 50 mg/kg TS. The change in VS showed that a low dose of 3TC did not affect the VS indicator, and high 3TC dose reduced it to 55.04%. On the contrary, the presence of RIT at medium and high concentrations increased VS removal efficiency to 23.68 ± 1.49% and 24.12 ± 1.73%, respectively, indicating that RIT significantly increased VS removal efficiency. The VS/TS values in the digester with RIT were 57.01−57.86%, indicating that RIT increased the VS of sludge. The generation of methane derived from the conversion of organic matter in sludge (Yang and Wang 2017); it is not difficult to understand that variations of VS destruction were consistent with methane production in the presence of AVDs.
Fig. 2.
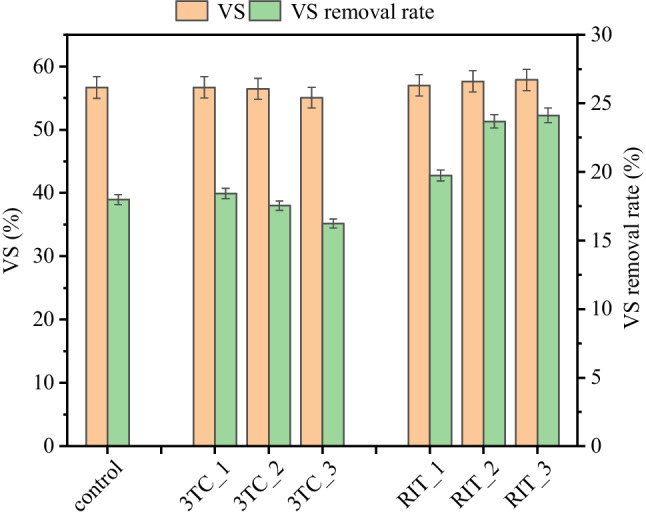
Effects of antiviral drugs on sludge VS and VS removal rate
Degradation of AVDs during anaerobic digestion
The degradation of AVDs during WAS anaerobic digestion was also investigated (Fig. 3). Given the limitations of the extraction methods and the instrumentation used in this study, 3TC and RIT at 0.05 and 5 mg/kg TS were not detected in the experimental group before and after digestion. Therefore, only the degradation of 3TC and RIT at 50 mg/kg TS during AD was analyzed. The results showed that 3TC and RIT were partially degraded after 28 days of AD, with degradation efficiencies of 31.2 ± 0.3% and 49.8 ± 0.5%, respectively. Therefore, AD can only partially degrade AVDs, and 50.2–68.8% of AVDs remained in digested sludge, which may pose ecotoxicological risks to the environment. Furthermore, it may also cause the development of antiviral drug-resistant viral strains inside the bodies of select wild animals and people. It was found that AVDs (e.g., lopinavir and RIT) have high removal efficiencies of above 90% during modified anaerobic/anoxic/oxic and oxidation ditch processes in WWTPs. Among them, AVDs were mainly removed in the anaerobic and anoxic sections with a removal efficiency of about 50% (Yao et al. 2021a), and biodegradation was considered the primary degradation pathway of AVDs (Funke et al. 2016). In addition, the degradation efficiencies were observed to vary with AVDs types. It was also reported that abacavir could be removed by biodegradation with removal efficiency of ≥ 99%, while zidovudine was biodegraded with removal efficiency of < 50%, which may be related to differences in drug structure and properties (Nannou et al. 2020).
Fig. 3.
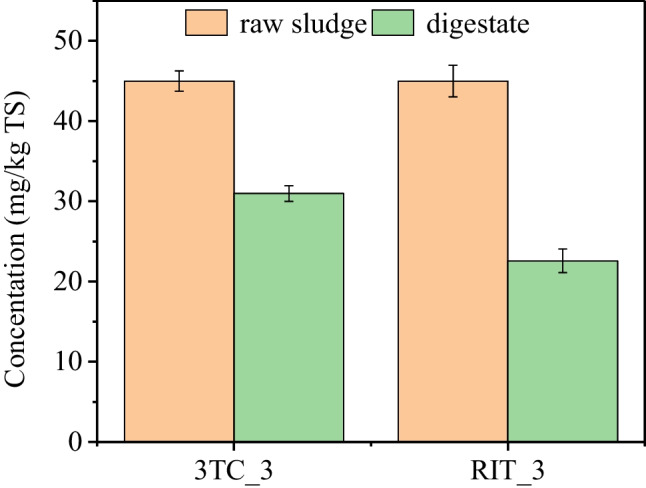
Degradation of AVDs (3TC and RIT) at 50 mg/kg (3TC_3 and RIT_3) during WAS anaerobic digestion
Effects of AVDs on microbial community
Alpha diversity
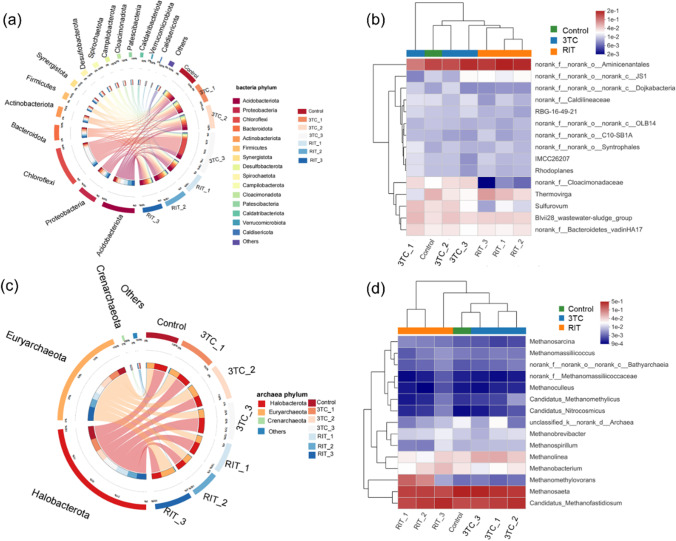
The alpha diversity of the bacterial and archaeal communities was investigated and is shown in Fig. S2, Tables S2, and S3. The Chao1 index characterizes community richness, and the presence of 3TC decreased the richness of the bacterial community and increased the richness of the archaeal community. When exposed to 3TC, the variations of the Shannon index were observed to be dose-dependent. Bacterial and archaeal diversity increased at low 3TC dose but decreased at high 3TC dose. Differently, RIT increased bacterial community richness and decreased archaeal richness at medium and high concentrations. Likewise, bacterial diversity was enhanced, but archaeal diversity was inhibited. Those results suggested that 3TC and RIT can affect bacterial and archaeal communities (Geng et al. 2022).
Bacterial community characterization
At the phylum level (Fig. 4a), Acidobacteriota (21.40%), Proteobacteria (13.68%), Chloroflexi (12.76%), Bacteroidota (9.77%), and Actinobacteriota (7.75%) were the dominant bacteria in the control group, which attribute to the degradation of organic matter in the AD system (Geng et al. 2022). Bacteroidota (11.87−13.94%), Proteobacteria (14.43−17.16%), and Firmicutes (5.25−6.00%) were enriched with low and medium concentrations of 3TC, while the relative abundance of Proteobacteria (11.58%), Actinobacteriota (4.95%), and Bacteroidota (9.20%) decreased with a high 3TC dose. It was reported that Proteobacteria, Actinobacteriota, Firmicutes, and Bacteroidota are all involved in the metabolism of organic matter and the production of VFAs (Qin et al. 2022; Wu et al. 2016; Yin and Chen 2022), which are essential in the hydrolysis, acidogenesis, and acetogenesis stages. The results suggested that 3TC may inhibit methane production by inhibiting WAS hydrolysis, acidogenesis, and acetogenesis. In the presence of RIT, the relative abundances of Proteobacteria (11.13%) and Actinobacteriota (6.67%) with low doses decreased, while the relative abundances of Acidobacteriota (22.95−30.03%), Bacteroidota (9.78−10.65%), Firmicutes (6.55%), and Synergistota (8.16%) increased at high concentrations. It was reported that Synergistota is acid-producing bacteria, mainly involving the production of VFAs (Zhu et al. 2022). The result indicated that the increase of methane production in the presence of RIT was related to the enrichment of bacteria that affected sludge acidogenesis.
Fig. 4.
Microbial community compositions of bacteria ((a) Circos plot of phylum composition; (b) major genus composition through heatmap) and archaea ((c) Circos plot of phylum composition; (d) major genus composition through heatmap)
The dominant bacteria genera included norank_f__norank_o__Aminicenantales, Thermovirga, Sulfurovum, Blvii28_wastewater-sludge_group, and norank_f__Bacteroidetes_vadinHA17, and the relative abundances of these predominant bacteria in the control group were 18.22%, 5.66%, 3.55%, 3.50%, and 2.91%, respectively (Fig. 4b). The norank_f__norank_o__Aminicenantales were reported to be related to the degradation of toxic substances, such as alkanes and N-heterocyclic compounds. It was found that high 3TC (23.85%) and RIT (20.92%) doses would increase the relative abundance of norank_f__norank_o__Aminicenantales, which may affect the degradation of AVDs. The relative abundance of Thermovirga (2.67−1.89%) was reduced in the presence of 3TC compared to the control, and norank_f__Bacteroidetes_vadinHA17 (2.55%) was inhibited with 3TC at high concentrations. Nevertheless, RIT increased the relative abundance of Blvii28_wastewater-sludge_group (3.88−4.19%) and norank_f__Bacteroidetes_vadinHA17 (3.0−3.31%). It was reported that norank_f__Bacteroidetes_vadinHA17 is a genus related to acidification (Wang et al. 2022b), while all the above-mentioned dominant genera were associated with the consumption of organic matter and the generation of VFAs (Geng et al. 2022; Granatto et al. 2021). The results of the bacterial community analysis indicated that AVDs affected bacteria associated with the metabolism of organic matter and the production of VFAs.
Archaeal community characterization
At the phylum level, Halobacterota and Euryarchaeota were the predominant archaeal phyla detected in both the control and experiment groups (Fig. 4c), which can function as hydrogenotrophic, acetoclastic, or methylotrophic methanogens (Fan et al. 2021). However, the relative abundance of the dominant archaeal phylum changed when exposed to AVDs. The relative abundance of Halobacterota (54.32−63.43%) was decreased with 3TC exposure, while that of Euryarchaeota increased to 34.58−50.32%. In presence of RIT, Euryarchaeota were enriched with a relative abundance of 35.96−57.28% with increasing RIT concentration, and Halobacterota were also slightly enriched. This difference may be related to the types of archaea and antiviral drugs. It was reported that the Halobacterota phylum is more sensitive to environmental conditions than Euryarchaeota (Funke et al. 2016), and both phyla are more susceptible to compounds with imidazole structures (Czatzkowska et al. 2021). This may cause the decrease of Halobacterota phylum in the presence of 3TC due to its imidazole structure.
At the genus level, Methanosaeta and Candidatus_Methanofastidiosum were the dominant genera in both the control and experiment groups. It was reported that Methanosaeta is the typical acetotrophic methanogen (Patil et al. 2021), while Candidatus_Methanofastidiosum is the hydrogenotrophic methanogen (Wei et al. 2019; Xu et al. 2021). Compared to the control group, the relative abundance of Methanosaeta was decreased to 44.43−40.32%, but that of Candidatus_Methanofastidiosum was increased to 38.03−45.57% at low and medium 3TC concentrations. The above results suggested that the methanogenic pathway was transferred from acetoclastic methanogens to hydrogenotrophic methanogens when exposed to 3TC at low and medium concentrations. With high 3TC concentrations exposure, the relative abundance of Methanosaeta increased, and that of Candidatus_Methanofastidiosum decreased to the abundance in control. The relative abundance of hydrogenotrophic methanogens also decreased, including Methanobrevibacter, Methanomassiliicoccus, and Methanobacterium, indicating high 3TC doses inhibited both hydrotropic methanogens and acetoclastic methanogens. In the presence of RIT, the relative abundance of Methanosaeta was decreased to 31.36−36.36%, while that of Candidatus_Methanofastidiosum (32.38−49.19%) and Methanomethylovorans (0.74−21.85%) were increased. Methanomethylovorans has been identified as a methylotrophic methanogenic bacterium (Ye et al. 2019). In addition, high RIT doses also slightly promoted the enrichment of hydrogenotrophic methanogens, including Methanolinea, Methanobacterium, Methanobrevibacter, Methanospirillum, and Methanomassiliicoccus (Geng et al. 2022; Li et al. 2019; Wang et al. 2022b; Zheng et al. 2022). Those results suggested that the presence of RIT in the anaerobic digestion system of WAS promoted the enrichment of methylotrophic and hydrotropic methanogens.
Details of how AVDs affect anaerobic digestion process
Effects of AVDs on solubilization
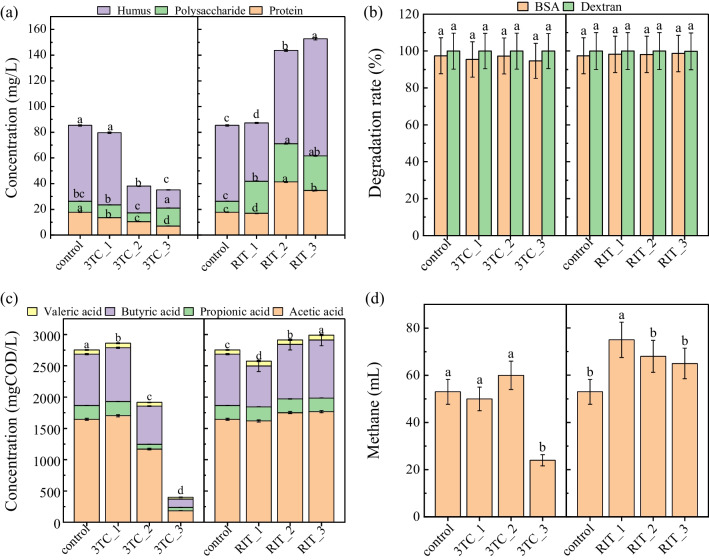
In this study, the effects of different types and concentrations of AVDs on each step of WAS anaerobic digestion were explored (Fig. 5). During the AD of WAS, the solid substrate is firstly converted into soluble substances (e.g., soluble protein, humus, and polysaccharides) (Yadav et al. 2022), but exogenous pollutants may affect the dissolution of organic matter in sludge through electrostatic interactions, affinity, hydrophobicity, and flocculation (Luo et al. 2020; Yin and Chen 2022). The soluble protein concentration was reduced by 40.9% and 60.67% in the presence of 3TC at medium and high concentrations compared to the control group, indicating that the presence of 3TC significantly inhibited the solubilization of WAS. Similar results have been reported for chitosan (Yin and Chen 2022), and it is speculated that the presence of 3TC may affect sludge flocculation in the same way as chitosan. In addition, 3TC has two resonance structures, which are positively charged at either the imine or the amine nitrogen (da Silva and Martins 2019). Therefore, 3TC may enhance the agglomeration of sludge flocs by binding to the negative charge on the surface of the sludge particles, resulting in the decrease of soluble organic matter release. In the presence of RIT, the concentrations of soluble proteins, polysaccharides, and humus were increased, suggesting that RIT promoted sludge solubilization. The high hydrophobicity of RIT may be the main reason, which was similar to previous studies for lignin (He et al. 2022). It has been reported that RIT has free electron pairs and hydrogen-bonded acceptors and donors, which can be adsorbed onto the surface of sludge floc through electrostatic interactions, hydrophobicity, and hydrogen bonding (Krasucka et al. 2022). This lowers the surface tension between the sludge floc and the WAS liquid.
Fig. 5.
Effects of different AVDs types and concentrations on metabolic steps of WAS anaerobic digestion. a Changes in the levels of soluble protein, polysaccharide, and humus after 1 day fermentation; b degradation efficiencies of BSA and dextran after 3 days fermentation; c the production of VFAs after 3 days fermentation; d methane production after 15 days fermentation. Different small letters indicate significant differences (p < 0.05)
Effects of AVDs on hydrolysis
Figure 5b exhibits the effects of AVDs on the conversion of BSA and dextran (model proteins and polysaccharides). In the control group, the degradation efficiencies of dextran and BSA were 100 ± 5% and 97.44 ± 4.87%, respectively. In the presence of 3TC and RIT, the degradation efficiencies of both BSA and dextran were not different from that in the control group, indicating that AVDs had no effects on WAS hydrolysis. It has been reported that the hydrolysis process is mainly the action of related enzymes, which primarily act on large organic substances, and contaminants (i.e., roxithromycin and AVDs) are not active sites for the enzymes (Ni et al. 2020).
Effects of AVDs on acidification
Based on the analysis of VFAs generated during acidification, the impacts of AVDs on VFAs generation were then investigated (Fig. 5c). Compared to the control, the VFAs production was inhibited by 30.43% and 85.49%, respectively, at medium and high 3TC doses. On the contrary, the presence of RIT increased VFAs generation at medium and high doses. The bacteria related to the production of VFAs are mainly Acidobacteriota and Synergistota (Wang et al. 2022a). According to the bacterial community analysis, the relative abundance of the two bacterial phyla decreased in the presence of high 3TC concentrations, while their relative abundances were increased by RIT, which may be related to their material structures. The physicochemical characteristics of AVDs (e.g., water solubility, chemical structure, and polarity) vary widely, and differences between the toxicities of different AVDs on organics have been observed. For example, the aquatic organism alga was inhibited by 4.86 mg/L nevirapine but not affected by 1000 mg/L ganciclovir (Ngumba et al. 2016; Straub 2017). Nevertheless, the mechanisms underlying their toxicities’ differences remain unknown, which need to be further studied.
Effects of AVDs on methanation
During the methanogenesis stage, methanogens play an important role, which can utilize acetate, H2, and methyl groups to produce methane (He et al. 2019). As seen in Fig. 5d, methane production with high 3TC dose exposure was lower than that in control. Consistently, the relative abundance of acetoclastic methanogens (i.e., Methanosaeta) decreased with high 3TC dose exposure. It has been reported that methanogens are more susceptible to exogenous pollutants than bacteria (Luo et al. 2020). Therefore, the presence of high 3TC concentrations inhibited the methanation step during WAS anaerobic digestion.
However, methane production was promoted with low RIT concentration and then decreased to insignificant effects with high RIT exposure. This indicated that RIT had lower biological toxicity to methanogens than 3TC. It was reported that Methanomethylovorans could consume methyl compounds, i.e., methanol, methylated amines, dimethyl sulfide, and methanethiol (Ma et al. 2021). In reactors with RIT, the degradation products of RIT may contain methyl compounds such as (thiazol-5-yl) methanol that can be utilized by Methanomethylovorans methanogens, increasing the substrate’s biogas generation (Rao et al. 2010). Low concentration of RIT may stimulate methanogens, and the stimulation effects would be diminished with the increase of concentration, as evidenced by the fact that methane production at high RIT concentration was lower than at low RIT concentration. Notably, the cumulative methane production in WAS anaerobic digestion increased with increasing RIT concentrations (Fig. 1), which was not consistent with that in the single methanation step (Fig. 5d). This implied that although high concentrations of RIT could not significantly stimulate the activity of methanogens, the accumulation of soluble organic substrates and enrichment of acidogenic bacteria with high RIT exposure provided more substrates for methanogenesis, leading to an increase in the methane production of WAS anaerobic digestion.
Potential mechanisms of AVDs affecting anaerobic digestion
Based on the results and discussion presented above, the effects of AVDs on AD were type-dependent and mainly through affecting sludge solubilization, acidification, and methanation performances. In detail, the presence of 3TC decreased the solubility of organic substrates by binding to sludge particles and enhancing the agglomeration of sludge flocs. Likewise, 3TC adversely affected the performances of the acidification and methanation steps, which was consistent with the decrease in bacteria associated with the metabolism of organic matter and the production of VFAs.
Differently, the presence of RIT promoted the release of soluble organic substrates, which can be attributed to its hydrophobic structure. Subsequently, RIT showed low toxic to acidogenic bacteria and methanogens, and increased VFAs and methane productions were observed in AD systems with RIT exposure. Correspondingly, microbial community analysis indicated the presence of RIT enriched function bacteria involved in VFAs formation and methylotrophic and hydrotropic methanogens.
Conclusion
In this study, the responses of AD to two typical AVDs (3TC and RIT) were investigated by BMP tests. The results indicated that high concentrations of 3TC adversely affected methane production by inhibiting sludge solubilization, acidification, and methanation. Meanwhile, the abundance of acetoclastic and hydrotropic methanogens decreased with the rise of 3TC levels. On the contrary, the existence of RIT increased the production of methane by promoting solubilization and acidification processes as well as enriching methylotrophic and hydrotropic methanogens. Notably, AD partly degraded AVDs, and the degradation products and pathways during AD still need further study.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors contributed to the study conception and design. The writing—review and editing were performed by Rui Wang, Wan Yang, Chen Cai, and Xiaohu Dai. And the methodology, validation, and visualization were performed by Rui Wang, Wan Yang, and Menghuan Zhong. The first draft of the manuscript was written by Rui Wang and all authors commented on previous versions of the manuscript. The supervision, project administration, and funding acquisition were performed by Xiaohu Dai. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant numbers 52131002 and 51978496).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Rui Wang and Wan Yang contribute equally to this work and share first authorship.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abafe OA, Späth J, Fick J, Jansson S, Buckley C, Stark A, Pietruschka B, Martincigh BS (2018) LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 200:660–670. http://doi.org.shiep.vpn358.com/10.1016/j.chemosphere.2018.02.105 [DOI] [PubMed]
- Almeida LC, Mattos AC, Dinamarco CPG, Figueiredo NG, Bila DM. Chronic toxicity and environmental risk assessment of antivirals in Ceriodaphnia dubia and Raphidocelis subcapitata. Water Sci Technol. 2021;84:1623–1634. doi: 10.2166/wst.2021.347. [DOI] [PubMed] [Google Scholar]
- Ariyanta HA, Chodijah S, Roji F, Kurnia A, Apriandanu DOB (2022) The role of Andrographis paniculata L. modified nanochitosan for lamivudine encapsulation efficiency enhancement and in vitro drug release study. J Drug Delivery Sci Technol 67. 10.1016/j.jddst.2021.103016
- Azuma T, Nakada N, Yamashita N, Tanaka H. Prediction, risk and control of anti-influenza drugs in the Yodo River Basin, Japan during seasonal and pandemic influenza using the transmission model for infectious disease. Sci Total Environ. 2015;521–522:68–74. doi: 10.1016/j.scitotenv.2015.03.069. [DOI] [PubMed] [Google Scholar]
- Balch WE, Wolfe RS. Specificity and biological distribution of coenzyme M (2-mercaptoethanesulfonic acid) J Bacteriol. 1979;137:256–263. doi: 10.1128/jb.137.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M, Sarpatwari A, Cepuch C. COVID-19 antivirals must not affect HIV drug supply. The Lancet HIV. 2022;9:e7–e9. doi: 10.1016/s2352-3018(21)00321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo AB, Grenni P, Saccà M. Effect of the antiviral drug oseltamivir (Tamiflu) on the bacterial community structure of a surface water ecosystem analyzed using fluorescence in situ hybridization. B Environ Contam Tox. 2010;85:443–446. doi: 10.1007/s00128-010-0114-x. [DOI] [PubMed] [Google Scholar]
- Chen H, Tang M, Yang X, Tsang YF, Wu Y, Wang D, Zhou Y (2021) Polyamide 6 microplastics facilitate methane production during anaerobic digestion of waste activated sludge. Chem Eng J 408. 10.1016/j.cej.2020.127251
- Czatzkowska M, Harnisz M, Korzeniewska E, Rusanowska P, Bajkacz S, Felis E, Jastrzebski JP, Paukszto L, Koniuszewska I (2021) The impact of antimicrobials on the efficiency of methane fermentation of sewage sludge, changes in microbial biodiversity and the spread of antibiotic resistance. J Hazard Mater 416:125773. 10.1016/j.jhazmat.2021.125773 [DOI] [PubMed]
- Czech B, Krzyszczak A, Boguszewska-Czubara A, Opielak G, Josko I, Hojamberdiev M (2022) Revealing the toxicity of lopinavir- and ritonavir-containing water and wastewater treated by photo-induced processes to Danio rerio and Allivibrio fischeri. Sci Total Environ 824:153967. 10.1016/j.scitotenv.2022.153967 [DOI] [PMC free article] [PubMed]
- da Silva CC, Martins FT. Multiple conformations and supramolecular synthons in almost fifty crystal structures of the anti-HIV/HBV drug lamivudine. J Mol Struct. 2019;1181:157–170. doi: 10.1016/j.molstruc.2018.12.099. [DOI] [Google Scholar]
- Eaton AD, Clesceri LS, Greenberg AE, Franson MAH. Standard methods for the examination of water and wastewater. Am J Public Health Nations Health. 1966;56:387–388. doi: 10.2105/AJPH.56.4.684-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Fan X, Fu P, Li Y, Zhao Y, Hua D (2021) Anaerobic digestion of wood vinegar wastewater using domesticated sludge: focusing on the relationship between organic degradation and microbial communities (archaea, bacteria, and fungi). Bioresour Technol 126384. 10.1016/j.biortech.2021.126384 [DOI] [PubMed]
- Frlund B, Griebe T, Nielsen PH. Enzymatic-activity in the activated-sludge floc matrix. Appl Microbiol Biot. 1995;43(4):755–761. doi: 10.1007/BF00164784. [DOI] [PubMed] [Google Scholar]
- Funke J, Prasse C, Ternes TA. Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Water Res. 2016;98:75–83. doi: 10.1016/j.watres.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Geng H, Xu Y, Zheng L, Liu H, Dai X (2022) Cation exchange resin pretreatment enhancing methane production from anaerobic digestion of waste activated sludge. Water Res 212:118130. 10.1016/j.watres.2022.118130 [DOI] [PubMed]
- Granatto CF, Grosseli GM, Sakamoto IK, Fadini PS, Varesche MBA (2021) Influence of cosubstrate and hydraulic retention time on the removal of drugs and hygiene products in sanitary sewage in an anaerobic Expanded Granular Sludge Bed reactor. J Environ Manage 299:113532. 10.1016/j.jenvman.2021.113532 [DOI] [PubMed]
- Guan W, Lan W, Zhang J, Zhao S, Ou J, Wu X, Yan Y, Wu J, Zhang Q. COVID-19: antiviral agents, antibody development and traditional Chinese medicine. Virol Sin. 2020;35:685–698. doi: 10.1007/s12250-020-00297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z-W, Liu W-Z, Tang C-C, Liang B, Guo Z-C, Wang L, Ren Y-X, Wang A-J (2019) Performance and microbial community responses of anaerobic digestion of waste activated sludge to residual benzalkonium chlorides. Energ Convers Manage 202. 10.1016/j.enconman.2019.112211
- He D, Zheng S, Xiao J, Ye Y, Liu X, Yin Z, Wang D (2022) Effect of lignin on short-chain fatty acids production from anaerobic fermentation of waste activated sludge. Water Res 212:118082. 10.1016/j.watres.2022.118082 [DOI] [PubMed]
- Jimenez J, Vedrenne F, Denis C, Mottet A, Déléris S, Steyer J-P, Cacho Rivero JA (2013) A statistical comparison of protein and carbohydrate characterisation methodology applied on sewage sludge samples. Water Res 47:1751–1762. 10.1016/j.watres.2012.11.052 [DOI] [PubMed]
- Hiremath SN, Bhirud CH. Development and validation of a stability indicating HPLC method for the simultaneous analysis of lopinavir and ritonavir in fixed-dose combination tablets. J Taibah Univ Med Sc. 2015;10:271–277. doi: 10.1016/j.jtumed.2014.11.006. [DOI] [Google Scholar]
- Krasucka P, Rombel A, Yang XJ, Rakowska M, Xing B, Oleszczuk P (2022) Adsorption and desorption of antiviral drugs (ritonavir and lopinavir) on sewage sludges as a potential environmental risk. J Hazard Mater 425:127901. 10.1016/j.jhazmat.2021.127901 [DOI] [PubMed]
- Kumar M, Mazumder P, Mohapatra S, Kumar Thakur A, Dhangar K, Taki K, Mukherjee S, Kumar Patel A, Bhattacharya P, Mohapatra P et al (2021) A chronicle of SARS-CoV-2: seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J Hazard Mater 405:124043. 10.1016/j.jhazmat.2020.124043 [DOI] [PMC free article] [PubMed]
- Kumar Khanal S, Lü F, Wong JWC, Wu D, Oechsner H (2021) Anaerobic digestion beyond biogas. Bioresour Technol 337:125378. 10.1016/j.biortech.2021.125378 [DOI] [PubMed]
- Li Z, Hu Y, Liu C, Shen J, Wu J, Li H, Wang K, Zuo J. Performance and microbial community of an expanded granular sludge bed reactor in the treatment of cephalosporin wastewater. Bioresour Technol. 2019;275:94–100. doi: 10.1016/j.biortech.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, He P, Shao L, Zhang H, Lu F. Significant enhancement by biochar of caproate production via chain elongation. Water Res. 2017;119:150–159. doi: 10.1016/j.watres.2017.04.050. [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/s0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Luo J, Feng L, Chen Y, Sun H, Shen Q, Li X, Chen H. Alkyl polyglucose enhancing propionic acid enriched short-chain fatty acids production during anaerobic treatment of waste activated sludge and mechanisms. Water Res. 2015;73:332–341. doi: 10.1016/j.watres.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhang Q, Zhao J, Wu Y, Wu L, Li H, Tang M, Sun Y, Guo W, Feng Q et al (2020) Potential influences of exogenous pollutants occurred in waste activated sludge on anaerobic digestion: a review. J Hazard Mater 383:121176. 10.1016/j.jhazmat.2019.121176 [DOI] [PubMed]
- Ma J, Shu L, Mitchell SM, Yu L, Zhao Q, Frear C (2021) Effects of different antibiotic operation modes on anaerobic digestion of dairy manure: Focus on microbial population dynamics. J Environ Chem Eng 9:105521. 10.1016/j.jece.2021.105521
- Mallikarjuna Rao N, Gowri Sankar D. Development and validation of stability-indicating HPLC method for simeltaneous determination of Lamivudine, Tenofovir, and Dolutegravir in bulk and their tablet dosage form. Future J Pharm Sci. 2015;1:73–77. doi: 10.1016/j.fjps.2015.11.002. [DOI] [Google Scholar]
- Muriuki C, Kairigo P, Home P, Ngumba E, Raude J, Gachanja A, Tuhkanen T (2020) Mass loading, distribution, and removal of antibiotics and antiretroviral drugs in selected wastewater treatment plants in Kenya. Sci Total Environ 743:140655. 10.1016/j.scitotenv.2020.140655 [DOI] [PubMed]
- Nannou C, Ofrydopoulou A, Evgenidou E, Heath D, Heath E, Lambropoulou D (2020) Antiviral drugs in aquatic environment and wastewater treatment plants: a review on occurrence, fate, removal and ecotoxicity. Sci Total Environ 699:134322. 10.1016/j.scitotenv.2019.134322 [DOI] [PubMed]
- Ngumba E, Gachanja A, Tuhkanen T. Occurrence of selected antibiotics and antiretroviral drugs in Nairobi River Basin, Kenya. Sci Total Environ. 2016;539:206–213. doi: 10.1016/j.scitotenv.2015.08.139. [DOI] [PubMed] [Google Scholar]
- Ni BJ, Zeng S, Wei W, Dai X, Sun J (2020) Impact of roxithromycin on waste activated sludge anaerobic digestion: methane production, carbon transformation and antibiotic resistance genes. Sci Total Environ 703:134899. 10.1016/j.scitotenv.2019.134899 [DOI] [PubMed]
- Patil SM, Kurade MB, Basak B, Saha S, Jang M, Kim SH, Jeon BH (2021) Anaerobic co-digester microbiome during food waste valorization reveals Methanosaeta mediated methanogenesis with improved carbohydrate and lipid metabolism. Bioresour Technol 332:125123. 10.1016/j.biortech.2021.125123 [DOI] [PubMed]
- Qin Y, Yang J, Wu Y, Wang D, Liu X, Du M, He D, Yi N (2022) The degradation of allyl isothiocyanate and its impact on methane production from anaerobic co-digestion of kitchen waste and waste activated sludge. Bioresour Technol 347:126366. 10.1016/j.biortech.2021.126366 [DOI] [PubMed]
- Rao RN, Ramachandra B, Vali RM, Raju SS. LC-MS/MS studies of ritonavir and its forced degradation products. J Pharm Biomed Anal. 2010;53:833–842. doi: 10.1016/j.jpba.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Robson L, Barnhoorn IEJ, Wagenaar GM. The potential effects of efavirenz on Oreochromis mossambicus after acute exposure. Environ Toxicol Pharmacol. 2017;56:225–232. doi: 10.1016/j.etap.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Schoeman C, Dlamini M, Okonkwo OJ. The impact of a Wastewater Treatment Works in Southern Gauteng, South Africa on efavirenz and nevirapine discharges into the aquatic environment. Emerging Contaminants. 2017;3:95–106. doi: 10.1016/j.emcon.2017.09.001. [DOI] [Google Scholar]
- Slater FR, Singer AC, Turner S, Barr JJ, Bond PL. Pandemic pharmaceutical dosing effects on wastewater treatment: no adaptation of activated sludge bacteria to degrade the antiviral drug Oseltamivir (Tamiflu®) and loss of nutrient removal performance. FEMS Microbiol Lett. 2011;315:17–22. doi: 10.1111/j.1574-6968.2010.02163.x. [DOI] [PubMed] [Google Scholar]
- Straub JO. Combined environmental risk assessment for the antiviral pharmaceuticals ganciclovir and valganciclovir in Europe. Environ Toxicol Chem. 2017;36:2205–2216. doi: 10.1002/etc.3758. [DOI] [PubMed] [Google Scholar]
- Symsaris EC, Fotidis IA, Stasinakis AS, Angelidaki I. Effects of triclosan, diclofenac, and nonylphenol on mesophilic and thermophilic methanogenic activity and on the methanogenic communities. J Hazard Mater. 2015;291:45–51. doi: 10.1016/j.jhazmat.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang D, Yi N, Li Y, Ni BJ, Wang Q, Wang H, Li X (2020) Insights into the toxicity of troclocarban to anaerobic digestion: sludge characteristics and methane production. J Hazard Mater 385:121615. 10.1016/j.jhazmat.2019.121615 [DOI] [PubMed]
- Wang C, Wu L, Zhang YT, Wei W, Ni BJ (2021) Unravelling the impacts of perfluorooctanoic acid on anaerobic sludge digestion process. Sci Total Environ 796:149057. 10.1016/j.scitotenv.2021.149057 [DOI] [PubMed]
- Wang F, Wu Y, Du W, Shao Q, Huang W, Fang S, Cheng X, Cao J, Luo J (2022a) How does the polyhexamethylene guanidine interact with waste activated sludge and affect the metabolic functions in anaerobic fermentation for volatile fatty acids production. Sci Total Environ 839:156329. 10.1016/j.scitotenv.2022.156329 [DOI] [PubMed]
- Wang Y, Zheng K, Guo H, Tong Y, Zhu T, Liu Y (2022b) Unveiling the mechanisms of how vivianite affects anaerobic digestion of waste activated sludge. Bioresour Technol 343:126045. 10.1016/j.biortech.2021.126045 [DOI] [PubMed]
- Wei W, Huang Q-S, Sun J, Dai X, Ni B-J. Revealing the mechanisms of polyethylene microplastics affecting anaerobic digestion of waste activated sludge. Environ Sci Technol. 2019;53:9604–9613. doi: 10.1021/acs.est.9b02971. [DOI] [PubMed] [Google Scholar]
- Wu QL, Guo WQ, Zheng HS, Luo HC, Feng XC, Yin RL, Ren NQ. Enhancement of volatile fatty acid production by co-fermentation of food waste and excess sludge without pH control: the mechanism and microbial community analyses. Bioresour Technol. 2016;216:653–660. doi: 10.1016/j.biortech.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Wu SL, Wei W, Sun J, Xu Q, Dai X, Ni BJ (2020) Medium-chain fatty acids and long-chain alcohols production from waste activated sludge via two-stage anaerobic fermentation. Water Res 186:116381. 10.1016/j.watres.2020.116381. [DOI] [PubMed]
- Wu Y, Lu M, Liu X, Chen H, Deng Z, Fu Q, Wang D, Chen Y, Zhong Y (2022) Insights into how poly aluminum chloride and poly ferric sulfate affect methane production from anaerobic digestion of waste activated sludge. Sci Total Environ 811:151413. 10.1016/j.scitotenv.2021.151413 [DOI] [PubMed]
- Xu Q, Luo TY, Wu RL, Wei W, Sun J, Dai X, Ni BJ (2021) Rhamnolipid pretreatment enhances methane production from two-phase anaerobic digestion of waste activated sludge. Water Res 194:116909. 10.1016/j.watres.2021.116909 [DOI] [PubMed]
- Xu R, Fang S, Zhang L, Cheng X, Huang W, Wang F, Fang F, Cao J, Wang D, Luo J (2022) Revealing the intrinsic drawbacks of waste activated sludge for efficient anaerobic digestion and the potential mitigation strategies. Bioresour Technol 345:126482. 10.1016/j.biortech.2021.126482 [DOI] [PubMed]
- Yadav M, Joshi C, Paritosh K, Thakur J, Pareek N, Masakapalli SK, Vivekanand V. Organic waste conversion through anaerobic digestion: a critical insight into the metabolic pathways and microbial interactions. Metab Eng. 2022;69:323–337. doi: 10.1016/j.ymben.2021.11.014. [DOI] [PubMed] [Google Scholar]
- Yang G, Wang J. Co-fermentation of sewage sludge with ryegrass for enhancing hydrogen production: performance evaluation and kinetic analysis. Bioresour Technol. 2017;243:1027–1036. doi: 10.1016/j.biortech.2017.07.087. [DOI] [PubMed] [Google Scholar]
- Yao L, Chen Z-Y, Dou W-Y, Yao Z-K, Duan X-C, Chen Z-F, Zhang L-J, Nong Y-J, Zhao J-L, Ying G-G (2021a) Occurrence, removal and mass loads of antiviral drugs in seven wastewater treatment plants with various treatment processes. Water Res 207:117803. 10.1016/j.watres.2021.117803 [DOI] [PubMed]
- Yao L, Dou W-Y, Ma Y-F, Liu Y-S (2021b) Development and validation of sensitive methods for simultaneous determination of 9 antiviral drugs in different various environmental matrices by UPLC-MS/MS. Chemosphere 282:131047. 10.1016/j.chemosphere.2021.131047 [DOI] [PubMed]
- Ye Q, Liang C, Chen X, Fang T, Wang Y, Wang H. Molecular characterization of methanogenic microbial communities for degrading various types of polycyclic aromatic hydrocarbon. J Environ Sci (china) 2019;86:97–106. doi: 10.1016/j.jes.2019.04.027. [DOI] [PubMed] [Google Scholar]
- Yin M, Chen H (2022) Unveiling the dual faces of chitosan in anaerobic digestion of waste activated sludge. Bioresour Technol 344:126182. 10.1016/j.biortech.2021.126182 [DOI] [PubMed]
- Zheng S, Yang F, Huang W, Lei Z, Zhang Z, Huang W (2022) Combined effect of zero valent iron and magnetite on semi-dry anaerobic digestion of swine manure. Bioresour Technol 346:126438. 10.1016/j.biortech.2021.126438 [DOI] [PubMed]
- Zhu S, Chen H (2022) Unraveling the role of polyferric chloride in anaerobic digestion of waste activated sludge. Bioresour Technol 346:126620. 10.1016/j.biortech.2021.126620 [DOI] [PubMed]
- Zhu R, Wang D-H, Zheng Y, Zou H, Fu S-F (2022) Understanding the mechanisms behind micro-aeration to enhance anaerobic digestion of corn straw. Fuel 318. 10.1016/j.fuel.2022.123604
- Zou M, Tian W, Zhao J, Chu M, Song T. Quinolone antibiotics in sewage treatment plants with activated sludge treatment processes: a review on source, concentration and removal. Process Saf Environ Prot. 2022;160:116–129. doi: 10.1016/j.psep.2022.02.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.