Abstract
Background:
Simple open prostatectomy is still the treatment of choice for removing large prostates; however, peri-surgical bleeding accompanied by this technique has always been a challenge for urologist surgeons. Therefore, the present study aimed to investigate the effect of surgicel on reducing bleeding in trans-vesical prostatectomy.
Materials and Methods:
The present double-blinded clinical trial included 54 patients with Benign Prostatic Hyperplasia (BPH), divided into two groups of 27, and underwent trans-vesical prostatectomy. After removing the prostate, the prostate adenoma was weighed in the first group. Then, two surgicel were inserted into the prostate loge for prostate adenomas weighing 75 g or less. For larger prostates, another surgicel was inserted for each 25 g weight higher than the limit of 75 g. However, no Surgicel was inserted in the control group. Other steps of the procedure were the same in both groups. Moreover, hemoglobin and hematocrit levels were assessed in both groups; preoperation, intra-operative, 24 h, and 48 h postoperative. In addition, all the fluid used for bladder irrigation was collected, and its hemoglobin level was assessed.
Results:
According to our results, no intergroup difference in hemoglobin level changes, hematocrit changes, International Prostate Symptom Score (IPSS), postoperative hospital stay, and number of packed cells received. However, the postoperative blood loss in bladder lavage fluid was significantly higher in the control group (120.83 ± 46.66 g) as compared to the surgicel group (72.56 ± 32.53 g) (P < 0.001).
Conclusion:
The present study concluded that using surgicel in trans-vesical prostatectomy could reduce postoperative bleeding without increasing the chance of postoperative complications.
Keywords: Absorbable cellulose, benign prostatic hyperplasia, suprapubic prostatectomy, surgicel
INTRODUCTION
Simple open prostatectomy has a history of more than 100 years. This procedure was first performed by Freyer. Despite the standardization of surgical techniques, this procedure is currently the gold standard for removing large prostates.[1] The simple open prostatectomy is usually performed in patients with benign prostate hyperplasia (BPH) and a prostate heavier than 80-100 g. However, other indications include concomitant large cystolithiasis and symptomatic bladder diverticulum, while its contraindications include prostate malignancy and small prostates.[2] Open prostatectomy is performed using two approaches: the trans-vesical (Freyer's technique) or retropubic (Millin's technique) techniques. The retropubic procedure is more suitable for hemostasis maintenance because it provides a direct view of the prostate,[3] while hemostasis maintenance is difficult in the trans-vesical procedure because it lacks a direct view of the prostate cavity. However, the trans-vesical procedure is useful in obese patients and those with concomitant large cystolithiasis and symptomatic bladder diverticulum.[4] In this procedure, skin and the underlying layers are incised under general or spinal anesthesia. Then, the bladder is opened extra-peritoneally[5] and the prostate adenoma is removed. The neck of the bladder is sutured at 5 and 7 o’ clock using the chromic catgut thread size 0 and figure-of-eight sutures to obstruct the main supplying vessels of the prostate.[6] If the hemostasis is not maintained using the basic standard techniques, the Malament technique is used by passing a nylon thread size 2 around the neck of the bladder for its temporal obstruction.[7] If the hemostasis maintenance fails again, the next step is the O’Connor technique, in which the posterior wall of the prostate cavity is plicated transversely using an absorbable thread.[8]
Insertion of surgicel sheets inside the prostate loge is another method for maintaining hemostasis.[9] According to Thumann et al., the use of cellulose oxidase in trans-vesical prostatectomy allows the surgeon to repair the bladder primarily without a cystostomy catheter. They reported that this technique was more effective than other hemostasis-maintaining techniques.[10] Moreover, Goodyear and Beard showed that using cellulose oxidase in trans-vesical prostatectomy was more effective than other techniques for hemostasis maintenance. Furthermore, they used a urethral catheter into the prostate loge in addition to cellulose oxidase.[11]
In addition to cellulose sheets, a urethral catheter balloon was inserted into the prostate loge in the previous clinical trials. However, the presence of the balloon in the prostate loge can affect postoperative blood loss. Therefore, we inserted the urethral catheter balloon into the bladder while the cellulose oxidase was inserted into the prostate loge to eliminate the mentioned effect. Moreover, we collected all fluid from bladder irrigation (from the intra-operative insertion of the urethral catheter to bladder irrigation cessation in the ward) and assessed its hemoglobin levels for precise assessment of postoperative blood loss.
MATERIALS AND METHODS
Study design and participants
The present study was a double-blinded randomized controlled clinical trial including 54 patients in two groups of 27. The patients and researchers, but not the surgeon, were blinded by the intervention assignment. The study was performed in the Al-Zahra and Khorshid Hospitals of the Isfahan University of Medical Sciences from June 2021 to June 2022, and the study protocol was approved by the Ethics Committee of the university. Furthermore, all patients gave written informed consent for participation, and the study was registered at the IRCT with the register code 20200825048515N47.
The inclusion criteria of the present study were all patients presented to the Al-Zahra and Khorshid hospitals who were indicated for simple open prostatectomy due to BPH with negative urine culture, while the exclusion criteria included a history of any surgical intervention on the prostate, current use of antiplatelet or anticoagulant drugs and any herbal medicine, a history of inherited bleeding disorders, a history of any underlying disease such as diabetes mellitus and hypertension, any intra-operative complications (prostate capsule rupture, ureter injury, etc.), use of another anesthesia technique rather than spinal anesthesia, and refusal to participate. The group allocation was performed using the simple random allocation rule by the generated random digit number by the statistical software. We generated the two comparison groups using simple randomization, with an equal allocation ratio, by referring to a generated list of random numbers. Intervention allocation concealment was done by using sealed, opaque envelopes. During the surgery and after the enucleation of the prostate adenoma, sealed, opaque envelopes were picked randomly by the circulating surgical technician without replacement, and the surgeon was informed of the patient's group assignment. The patients receiving card 1 were included in the Surgicel group, while those receiving card 2 were considered the control group.
Surgical procedure and main study outcome evaluations
All patients were operated on by a single surgeon, and the IPSS of the patients were evaluated before the surgery using seven standard IPSS questions. The patients underwent spinal anesthesia using 2.5-4 cc of bupivacaine and were then positioned in the lithotomy position. First, cystoscopy was performed using a 17 F cystoscope and 30° and 70° lenses (Karl Storz SE and Co., Germany) to evaluate the urethra, bladder, and prostate. Then, the patients were positioned in the supine position to perform the trans-vesical prostatectomy using Freyer's technique. In the first group, after enucleating the prostate adenoma and making figure-of-eight sutures at 5 and 7 o’clock in the neck of the bladder, a 2-way 20 F urethral catheter was inserted. Then, two Surgicel sheets (Emosist 10 × 20 cm, Mascia Brunelli Spa, Italy) were inserted into the prostate loge for prostate adenomas weighing 75 g or less. For larger prostates, another Surgicel sheet was inserted for each 25 g weight higher than the limit of 75 g. However, no Surgicel sheet was inserted in the control group. Other steps of the procedure were the same in both groups. Finally, an 18 F Malecot catheter was inserted in the cystostomy for bladder irrigation.
Hemoglobin and hematocrit levels of the patients were assessed in both groups before the operation, during the insertion of the urethral catheter, 24 h, and 48 h after the surgery by assessing the venous blood samples (2 cc). Moreover, the patients with hemoglobin levels lower than 9 g/dL or symptoms indicating insufficient oxygenation were indicated for blood transfusion, and the number of packed cells received by each patient was recorded. Furthermore, all fluids from bladder irrigation (from the intra-operative insertion of urethral catheter to bladder irrigation cessation in the ward) were collected, and its hemoglobin levels were assessed directly by centrifuging the collected fluid using a hematology cell counter (Cell-Dyn 3200). Any urethral catheter obstruction was recorded, and the urethral catheters were removed at the first outpatient visit 7 days after discharge. Then, any potential urinary retention after the urethral catheter removal was evaluated. Finally, the IPSS of the patients was assessed in the third outpatient visit (4 weeks postoperation) using 7 standard IPSS questions.
Statistical analysis
The quantitative variables were described using the mean and standard deviation, whereas the qualitative variables were described using frequency and percentage. Data normality was investigated using the Kolmogorov − Smirnov test and Q-Q chart. Moreover, comparisons of the basic quantitative variables were performed using the independent samples t-test, while the Chi-square test was used for basic qualitative variables. The main study variables (hemoglobin and hematocrit) underwent intergroup and intragroup comparisons using the repeated measures analysis of variance (ANOVA). The assumption of sphericity was evaluated using Mauchly's test, and if not confirmed, multivariate ANOVA was used. The mean values of the main study variables were compared between the groups in each assessment using the independent samples t-test, and the obtained P value value was adjusted using the Bonferroni method. Mean change of the value of IPSS was evaluated in each group compared to before intervention using paired sample t-test and between group difference was evaluated using independent samples t-test. In addition, postoperative hemoglobin lost in bladder lavage fluid was compared using the independent samples t-test at the end of the study. Furthermore, intragroup and intergroup comparisons of IPSS were performed using the paired t-test and independent samples t-test, respectively. The number of packed cells received by the patients and complications, including urethral catheter obstruction and urinary retention urethral catheter removal, was compared using the Chi-square or Fisher's exact tests.
RESULTS
The present study included 54 patients with a mean age of 71.98 ± 6.2 years [Figure 1]. The patients were divided into two groups of 27, and there was no significant intergroup difference in age, body mass index, prostate size, prostate-specific antigen, and preoperative IPSS, hemoglobin level, and hematocrit [Table 1].
Figure 1.
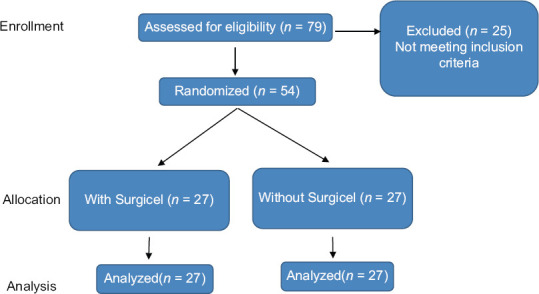
Patients’ enrollment algorithm.
Table 1.
Demographic and basic clinical characteristics of study participants
| Variables | Total (n=54) | G1 (n=27) | G2 (n=27) | P** |
|---|---|---|---|---|
| BMI | 24.82±1.75 | 24.46±2 | 25.18±1.3 | 0.13 |
| Age | 71.98±6.28 | 72.44±5.6 | 71.51±6.9 | 0.59 |
| Hb1 | 13.61±1.5 | 13.24±1.2 | 13.98±1.64 | 0.07 |
| Hct1 | 40.3±4.3 | 39.36±4.1 | 41.24±4.36 | 0.11 |
| Prostate weight | 111.61±35.7 | 104.1±33.4 | 119.11±36.92 | 0.12 |
| PSA | 5.61±2.9 | 5.2±2.6 | 5.9±3.1 | 0.37 |
| IPSS | 26.72±3.18 | 27.19±3.1 | 26.26±3.25 | 0.28 |
**Resulted from independent samples t-test. Hb1=Preoperative Hb; Hct1=Preoperative Hct. BMI=Body mass index; PSA=Prostate-specific antigen; IPSS=International prostate symptom score; Hb=Hemoglobin; Hct=Hematocrit
According to our findings, hematocrit and hemoglobin levels decreased significantly in both groups compared to preoperative assessment (Ptime < 0.001), while there was no between-group difference in hematocrit and hemoglobin levels (Pgroup > 0.05) even after adjusting the potential confounding effects of preoperative hemoglobin and hematocrit. For both hematocrit and hemoglobin levels significant interaction between time and intervention was seen (Ptime × group) [Tables 2 and 3].
Table 2.
Course of hemoglobin during the study
| Group | Hb1 | Hb2 | Hb3 | Hb4 | P-time* | P-group* | P-time × group* |
|---|---|---|---|---|---|---|---|
| 1 | 13.24±1.2 | 12.45±1.24 | 11.88±1.38 | 11.24±1.16 | 0.001 | 0.36 | <0.001 |
| 2 | 13.98±1.64 | 13.11±1.52 | 12.1±1.79 | 10.98±1.38 | <0.001 | ||
| P** | 0.28 | 0.36 | >0.99 | >0.99 |
*Resulted from repeated measures ANOVA; **Resulted from independent samples t-test with Bonferroni adjustment. Hb1=Preoperative Hb; Hb2=Hb after urethral catheter insertion; Hb3=Hb at 24 h after surgery; Hb4=Hb at 48 h after surgery. ANOVA=Analysis of variance; Hb=Hemoglobin
Table 3.
Course of hematocrit during the study
| Group | Hct1 | Hct2 | Hct3 | Hct4 | P-time* | P-group* | P-time × group* |
|---|---|---|---|---|---|---|---|
| 1 | 39.36±4.1 | 35.93±3.15 | 34.88±3.84 | 33.33±3.2 | <0.001 | 0.44 | 0.009 |
| 2 | 41.24±4.36 | 36.91±3.90 | 35.45±3.92 | 32.88±3.9 | <0.001 | ||
| P** | 0.33 | >0.99 | >0.99 | >0.99 |
*Resulted from repeated measures ANOVA. **Resulted from independent samples t-test with Bonferroni adjustment. Hct1=Preoperative Hct; Hct2=Hct after urethral catheter insertion; Hct3=Hct at 24 h after surgery; Hct4=Hct at 48 h after surgery. ANOVA=Analysis of variance; Hct=Hematocrit
According to our findings, postoperative blood loss was significantly higher in the control group (120.83 ± 46.66 g) compared to the intervention group (72.56 ± 32.53 g) (P < 0.001) [Figure 2]. Moreover, the mean postoperative hospital stay was 3.26 ± 0.52 days in the intervention group and 3.15 ± 0.45 days in the control group, showing no significant difference (P = 0.258). Also, the intervention and control groups had a comparable mean operation duration of 78.70 ± 7.17 min and 82.14 ± 8.93 min, respectively, which were not significantly different (P = 0.124).
Figure 2.
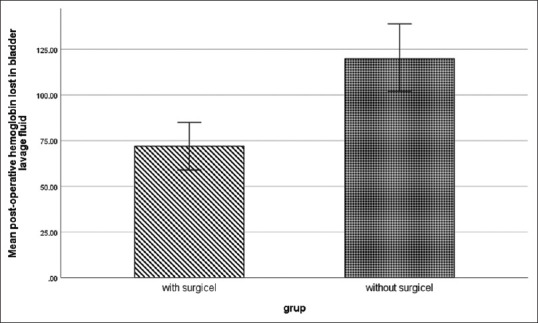
Postoperative hemoglobin lost in bladder lavage fluid
In both groups, IPSS decreased significantly after the operation compared to preoperative values (P < 0.001). However, there was no between-groups difference in the IPSS decrease (P = 0.58) [Table 4]. Moreover, the groups were not significantly different in the number of packed cells received after the surgery (intervention group: 6 units and 22.2%, control group: 7 units and 25.9%) and the number of postoperative Foley catheter inactivation (intervention group: 7 patients, control group: 6 patients). In addition, no acute urinary retention was observed after pulling out the urethral catheter in the patients of the two groups.
Table 4.
International prostate symptom score changes
| Group | IPSS1* | IPSS2* | P a | P b |
|---|---|---|---|---|
| 1 | 27.19±3.1 | 3.7±1.1 | 0.001 | 0.58 |
| 2 | 26.26±3.25 | 3.48±1.28 | 0.001 |
*IPSS1: Preoperative IPSS; IPSS2: IPSS 4 week after surgery, aResulted from paired samples t-test; bResulted from independent samples t-test. IPSS=International prostate symptom score
DISCUSSION
Trans-vesical open prostatectomy is a gold standard for the surgical removal of large prostates. However, intra- and postoperative blood loss accompanied by this technique have always been a challenge for urologist surgeons. Various techniques have been suggested to manage this problem, one of them is the application of surgicel. The present study investigated the effectiveness of this method in reducing intra- and postoperative blood loss due to trans-vesical prostatectomy.
Oxidized cellulose (Surgicel) is a chemical hemostasis-maintaining material with applications in several surgical procedures.[12,13,14] It was first introduced in 1949 as a hemostatic agent.[15] Moreover, upon absorbing the blood, it turns brown and can grow up to 10 times in weight and volume. In fact, this material is a false clot and stops the bleeding by exerting pressure resulting from its increased volume.[16,17] Surgicel is mainly used to prevent capillary and venous bleeding.[18] In recent years, absorbable hemostatic materials, such as collagen, gelatin, and surgicel, have been increasingly used due to their high biocompatibility and not damaging the surrounding structures.[19,20,21,22]
According to a study by Lin et al., surgicel is superior to other absorbable hemostatic materials due to its higher biocompatibility.[19] Moreover, it can be used in spinal,[23] gynecologic,[18] thoracic,[24] adenoid,[25] and colorectal[26] surgeries. Furthermore, this material is used in partial open nephrectomy, laparoscopy,[27,28,29] and radical laparoscopic prostatectomy.[30] The use of these materials is a standard method in neurosurgery.[19,20,21,22,23,24,31] The blood absorption of surgicel starts following 24 h postoperation and lasts for weeks.[18] In addition, it has acidic properties and exerts an antibiotic effect by reducing the pH of the surrounding tissues.[12] It also acts as an antibacterial against aerobic and anaerobic bacteria.[32] However, this method has disadvantages as well. There have been reports of remaining surgicel parts after the surgery, which can be misdiagnosed with a mass or abscess, in addition, in some studies a slight increase in the rate of infection has been reported when using Surgicel, also in rare cases, the increase in the volume and pressure of the tubercles on the adjacent organs has caused problems, so it is recommended to use this substance in sufficient and necessary amounts.[18,19,20,21,22,23,24,31]
According to a study by Goodyear and Beard, using cellulose oxidase in trans-vesical prostatectomy was more effective than other techniques for hemostasis maintenance,[11] which was compatible with our results. However, the techniques used in the study by Goodyear et al. and the present study were different. Moreover, Goodyear et al. inserted the urethral catheter balloon into the prostate loge in addition to cellulose oxidase, while we inserted the urethral catheter balloon into the bladder. Both studies assessed the hemoglobin and hematocrit changes, which depended on the patient's hydration status at sampling time. However, we collected all fluid from bladder irrigation (from the intra-operative insertion of the urethral catheter to bladder irrigation cessation in the ward) and assessed its hemoglobin levels for precise assessment of postoperative blood loss, showing a significantly higher blood loss in the control group (120.83 ± 46.66 g) compared to the intervention group (72.56 ± 32.53 g, P < 0.001)
Riba et al. reported the case of obstruction in the posterior urethra after using surgicel in open prostatectomy.[33] This was also a concern of ours in the present study. According to a study by Uribe et al., surgicel turns into a mucoid material after exposure to urine for 5 days or longer,[34] which resolved our concern because the researchers of the present study kept the urethral catheter in its place for 7 days after the trans-vesical prostatectomy. Moreover, we did not report any case of urinary retention after urethral catheter removal due to the urethral placement of surgicel.
The present study's strengths included using a precise method for postoperative blood loss assessment. Moreover, the present study was a randomized controlled trial. However, the study had a limitation as well. In the present study, the urethral catheter was routinely removed 7 days after the surgery. Therefore, regarding potential complications, such as urethral catheter obstruction, our results cannot be generalized to cases in which the urethral catheter is removed in the first days after the operation.
CONCLUSION
According to our results, cellulose sheets can reduce intra- and postoperative blood loss in trans-vesical prostatectomy without increasing the chance of postoperative complications, such as urethral catheter obstruction and urinary retention after urethral catheter removal. However, further studies are needed to confirm the validity of Surgicel application in reducing postoperative blood loss due to trans-vesical prostatectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The present study was supported by Isfahan University of Medical Sciences.
REFERENCES
- 1.Fitzpatrick JM. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Nocick AC, editors. Campbell-Walsh Urology. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 2.Han M, Partin AW. Retropubic and suprapubic open prostatectomy. In: Wein AJ, editor. Campbell-Walsh Urology. Philadelphia: Elsevier; 2012. [Google Scholar]
- 3.Millin T. Retropubic prostatectomy; a new extravesical technique; report of 20 cases. Lancet. 1945;2:693–6. doi: 10.1016/s0140-6736(45)91030-0. [DOI] [PubMed] [Google Scholar]
- 4.Bernie JE, Schmidt JD. Simple perineal prostatectomy: Lessons learned from a modern series. J Urol. 2003;170:115–8. doi: 10.1097/01.ju.0000071681.03755.b3. [DOI] [PubMed] [Google Scholar]
- 5.Yu GW, Miller HC. Critical Operative Maneuvers in Urologic Surgery. St. Louis: Mosby; 1996. Suprapubic prostatectomy; pp. 199–206. [Google Scholar]
- 6.Moddler JK, McVary KT. Suprapubic prostatectomy. In: Smith J, Howards S, Preminger G, editors. Hinman's Atlas of Urologic Surgery. Philadelphia: Elsevier; 2012. [Google Scholar]
- 7.Malament M. Maximal hemostasis in suprapubic prostatectomy. Surg Gynecol Obstet. 1965;120:1307–12. [PubMed] [Google Scholar]
- 8.O’Connor VJ. Urologic Surgery. Philadelphia: JB Lippincott; 1983. Suprapubic prostatectomy; pp. 853–60. [Google Scholar]
- 9.Ellis WJ, Wright JL. Complications of simple prostatectomy. In: Taneja SS, editor. Complications of Urologic Surgery. Philadelphia, USA: WB Saunders; 2010. pp. 497–501. [Google Scholar]
- 10.Thumann RC, Jr, Stump GD. Suprapubic transvesical prostatectomy with primary closure of the bladder using oxidized cellulose: An analysis of 100 cases. J Urol. 1952;67:95–100. doi: 10.1016/S0022-5347(17)68320-4. [DOI] [PubMed] [Google Scholar]
- 11.Goodyear WE, Beard DE. Blood loss in prostatectomy. J Urol. 1949;62:849–57. doi: 10.1016/S0022-5347(17)69012-8. [DOI] [PubMed] [Google Scholar]
- 12.Rustagi T, Patel K, Kadrekar S, Jain A. Oxidized cellulose (Surgicel) causing postoperative cauda equine syndrome. Cureus. 2017;9:e1500. doi: 10.7759/cureus.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Hong W, Wu W, Ni H, Zhou M. Delayed absorption of oxidized cellulose (Surgicel) in post-thyroidectomy patients. J Ultrasound Med. 2016;35:1349–51. doi: 10.7863/ultra.15.08014. [DOI] [PubMed] [Google Scholar]
- 14.Hu YY, Mazer LM, Yule SJ, Arriaga AF, Greenberg CC, Lipsitz SR, et al. Complementing operating room teaching with video-based coaching. JAMA Surg. 2017;152:318–25. doi: 10.1001/jamasurg.2016.4619. [DOI] [PubMed] [Google Scholar]
- 15.Allouni A, Dujon D. A novel use of surgicel® as a spacer for intraoperative contour defect. JPRAS Open. 2017;13:46–8. [Google Scholar]
- 16.Valsangkar NP, Eppstein AC, Lawson RA, Taylor AN. Effect of lean processes on surgical wait times and efficiency in a tertiary care veterans affairs medical center. JAMA Surg. 2017;152:42–7. doi: 10.1001/jamasurg.2016.2808. [DOI] [PubMed] [Google Scholar]
- 17.Hoschander AS, Salgado CJ, Kassira W, Thaller SR, editors. Operative Procedures in Plastic, Aesthetic and Reconstructive Surgery. Florida, USA: CRC Press; 2015. pp. 265–268. [Google Scholar]
- 18.Cormio L, Cormio G, Di Fino G, Scavone C, Sanguedolce F, Loizzi V, et al. Surgicel® granuloma mimicking ovarian cancer: A case report. Oncol Lett. 2016;12:1083–4. doi: 10.3892/ol.2016.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin B, Yang H, Cui M, Li Y, Yu J. Surgicel™ application in intracranial hemorrhage surgery contributed to giant-cell granuloma in a patient with hypertension: Case report and review of the literature. World J Surg Oncol. 2014;12:1–5. doi: 10.1186/1477-7819-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Chen P. Surgicel® (oxidized regenerated cellulose) granuloma mimicking local recurrent gastrointestinal stromal tumor: A case report. Oncol Lett. 2013;5:1497–500. doi: 10.3892/ol.2013.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haki BK, Eftekhari J, Alizadeh V, Tizro P. Comparison of hemodynamic stability, bleeding, and vomiting in propofol-remifentanil and isoflurane-remifentanil techniques in septorhinoplasty surgery. Jentashapir Journal of Health Research. 2014;5:3. [Google Scholar]
- 22.Anvari HM, Haki BK, Ghorbanian N. Evaluation of patients’ awareness for elective surgery referred to Al-Zahra and Imam Reza hospitals in Tabriz on spinal and general anesthesia and their selection factors in the preoperative anesthetic in 2011-2012. Jentashapir J Health Res. 2013;4:1. [Google Scholar]
- 23.Choi DH, Lee JW, Kim CH, Choi YS. Indirect repair with surgicel® and fibrin glue for postoperative cerebrospinal fluid leakage after cervical anterior foraminotomy: A case report. J Korean Soc Spine Surg. 2016;23:171–6. [Google Scholar]
- 24.Badenes D, Pijuan L, Curull V, Sánchez-Font A. A foreign body reaction to Surgicel® in a lymph node diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Med. 2017;12:55–6. doi: 10.4103/1817-1737.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma J, Zangmo R, Kumar S, Roy K. Use of oxidized regenerated cellulose (Surgicel Nu-Knit) as a hemostat in laparoscopic endometriotic cystectomy: A case report. Int J Reprod Contracept Obstet Gynecol. 2015;4:283–5. [Google Scholar]
- 26.Myung YS, Ko BM, Han JP, Hong SJ, Jeon SR, Kim JO, et al. Effectiveness of Surgicel® (Fibrillar) in patients with colorectal endoscopic submucosal dissection. Surg Endosc. 2016;30:1534–41. doi: 10.1007/s00464-015-4369-5. [DOI] [PubMed] [Google Scholar]
- 27.Breda A, Stepanian SV, Lam JS, Liao JC, Gill IS, Colombo JR, et al. Use of haemostatic agents and glues during laparoscopic partial nephrectomy: A multi-institutional survey from the United States and Europe of 1347 cases. Eur Urol. 2007;52:798–803. doi: 10.1016/j.eururo.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Klingler CH, Remzi M, Marberger M, Janetschek G. Haemostasis in laparoscopy. Eur Urol. 2006;50:948–56. doi: 10.1016/j.eururo.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 29.Lewis KM, McKee J, Schiviz A, Bauer A, Wolfsegger M, Goppelt A. Randomized, controlled comparison of advanced hemostatic pads in hepatic surgical models. ISRN Surg 2014. 2014:930803. doi: 10.1155/2014/930803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahlering TE, Eichel L, Chou D, Skarecky DW. Feasibility study for robotic radical prostatectomy cautery-free neurovascular bundle preservation. Urology. 2005;65:994–7. doi: 10.1016/j.urology.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Brotfain E, Korn A, Gidon M, Zlotnik A, Klein M, Melamed I. Surgicel induced intraoperative cardiovascular collapse in a child with midbrain glioma. Case Rep Clin Med. 2015;4:36. [Google Scholar]
- 32.Oto A, Remer EM, O’Malley CM, Tkach JA, Gill IS. MR characteristics of oxidized cellulose (Surgicel) AJR Am J Roentgenol. 1999;172:1481–4. doi: 10.2214/ajr.172.6.10350276. [DOI] [PubMed] [Google Scholar]
- 33.Riba LW. Urethral obstruction with oxidized cellulose following retropubic prostatectomy. J Am Med Assoc. 1949;141:532. doi: 10.1001/jama.1949.62910080006009a. [DOI] [PubMed] [Google Scholar]
- 34.Uribe CA, Eichel L, Khonsari S, Finley DS, Basillote J, Park HK, et al. What happens to hemostatic agents in contact with urine?. An in vitro study. J Endourol. 2005;19:312–7. doi: 10.1089/end.2005.19.312. [DOI] [PubMed] [Google Scholar]


