Abstract
Background:
The inflammation accelerates the progression of bipolar disorder. Supplementation of anti-inflammatory supplements in adjuvant with medications may alleviate disorder signs. This study aimed to investigate the effects of omega-3 fatty acid supplementation on the serum concentrations of pro-inflammatory cytokines and depression status in patients with bipolar disorder.
Materials and Methods:
This randomized clinical trial study was conducted in Zahedan city in 2021. Patients with bipolar disorder (n = 60) were grouped into two groups: omega-3 fatty acid supplement group (n = 30, 15 men and 15 women) and placebo one using a permuted block stratified randomization. The patients in the omega-3 group received 2 g of omega-3 fatty acids daily for 2 months while patients in the placebo group received 2 g soft gels daily in the same form. Depression score and the serum concentrations of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP) were assessed before and after the study.
Results:
Depression score and the serum concentrations of TNF-α, IL-6, and hs-CRP were decreased after intervention in the omega-3 fatty acid group also compared with the placebo group (P < 0.001). The results also show a positive correlation between the serum concentrations of TNF-α, IL-6, and hs-CRP with depression scores (P < 0.001).
Conclusion:
Prescription of omega-3 fatty acids can decrease inflammatory parameters and help to decrease depression in patients with bipolar disorder. This supplement can be used along with medications for decreasing the inflammatory markers in these patients.
Keywords: Bipolar disorder, depression, inflammation, interleukin-6, omega-3
INTRODUCTION
Bipolar disorder is a psychiatric disorder accompanied by other psychiatric disorders that is seen in 0.5%–1% of the general population.[1] It has been reported a prevalence of 0.96% of mood disorders in Iran that comprised 22.37% of mood disorders.[2] It is characterized by episodes of mania or hypomania and depression that cause cognitive faults and decrease quality of life.[1] The pathogenesis of the disorder is not still elucidated, but inflammatory signaling is involved in this disorder. The inflammation accelerates the progression of the disorder, and the presence of inflammation is the main proof of bipolar disorder.[3] Patients with bipolar disorder show a significant increase in central and peripheral pro-inflammatory elements such as cytokines. The increases in tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP) have been observed in patients with bipolar disorder compared with healthy people.[4] The decreasing inflammation can help the treatment of bipolar disorder.[3] Omega-3 polyunsaturated fatty acids are administrated for the treatment of bipolar disorder.[5,6]
Omega-3 fatty acids decrease inflammation via various mechanisms such as prevention of leukocyte chemotaxis and production of eicosanoids and inflammatory cytokines.[7] A meta-analysis has shown that omega-3 fatty acid supplementation significantly decreases the levels of TNF-α, IL-6, and hs-CRP in patients with heart failure.[8] It was also reported that omega-3 fatty acid supplementation significantly reduces the levels of hs-CRP in patients with gestational diabetes mellitus.[9] Other studies have not reported the positive effects of omega-3 fatty acid supplementation on inflammatory factors in patients with metabolic syndrome[10] and with type 2 diabetes and nonalcoholic fatty liver.[11]
With regard to the effects of omega-3 fatty acids in decreasing the inflammation and its anti-inflammatory properties in the alleviation of symptoms and signs of bipolar disorder and also conflicts for the effects of omega-3 fatty acids on inflammatory factors, we aimed to investigate the effects of omega-3 fatty acids on the serum concentrations of pro-inflammatory cytokines and depression status in patients with bipolar disorder. Based on our searches, there are few studies on the effects of omega-3 fatty acids on the serum concentrations of pro-inflammatory cytokines and depression status in patients with bipolar disorder.
MATERIALS AND METHODS
Participants
This randomized clinical trial study was conducted on 60 patients with bipolar disorder in the depressive phase in 2021. The patients referred to Baharan Psychiatric Clinic in Zahedan under the supervision of an academic member psychiatrist of Zahedan University of Medical Sciences were selected for this study. The participants were selected based on inclusion and exclusion criteria. Inclusion criteria were as follows: Age limit of 16-60 years, at least 6 months history of bipolar disorder, willingness to participate in this study, lack of receiving of omega-3 fatty acid supplement for several months before the start of the study, lack of programmed surgery for three next months, receiving drugs interfering with omega-3 fatty acids such as anticoagulant medications, lack of high hypertension, and patients who did not consume alcohol. Exclusion criteria included changed dosage and medication type, showing sensitivity to omega-3 fatty acid supplement, consuming lesser than 80.00% supplement, having infectious and inflammatory disorders, and lack of willingness for continuing the study. Delusional patients with bipolar were also excluded. The patients were randomly grouped into two 30-people groups with an equal ratio of both sexes. The patients in the omega-3 fatty acid group (OG) daily consumed 2 capsules of 1000 mg of omega-3 fatty acids (180 mg EPA, 120 mg DHA), for 2 months 12 after meals with a sufficient amount of water, while patients in the placebo group (PG) daily consumed 2 capsules of placebo (pure edible paraffin oil) in the same of form for OG. Paraffin and omega-3 fatty acids were prepared from Barij Essence Company.
Figure 1 depicts a flow diagram of participants in this study. Sample sizes were calculated based on previous studies[13] and based on the below equation:
Figure 1.
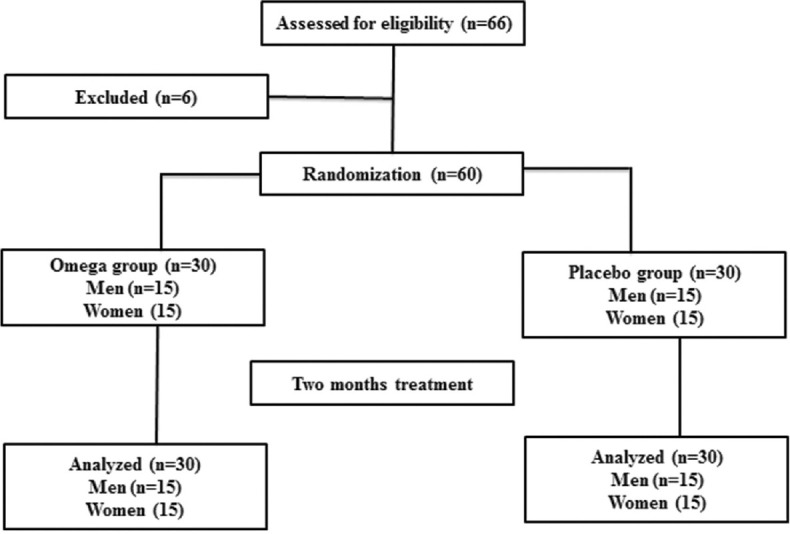
Participants’ flow diagram of the study
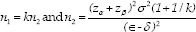
Where n1 and n2 show the number of samples in the omega-3 and placebo groups, respectively. Furthermore, κ, α, 1−β, σ2, є, and δ show the number of samples in omega-3 to placebo, probability of type 1 error, statistical power, the mixed variance of groups, real difference, and clinical effective limit, respectively. Based on the same study, the values for κ, α, 1−β, σ2, є, and δ were 1, 0.05, 0.80, 88.5, 2.2, and 5, respectively.[13]
To avoid gastrointestinal side effects, we suggested consuming supplements with the main meals (lunch and dinner). The patients were monitored for clinical abnormalities and weekly controlled for consumption of supplements. In addition, to avoid error effect, it was recommended families and patients who do not feed foods containing omega-3 and not follow an anti-inflammatory diet. A complete list of familis was given to them and followed weekly.
Randomization
The patients were allocated into groups by using permuted block stratified randomization. The patients were classified based on age and gender. Quadruple blocks (both groups and two replications for each group) were randomly selected from permutes and assigned to groups by help R software (version 4.0.2) (R Foundation for statistical computing, Vienna, Austria).
Matching and blinding
Both groups were matched for sex and age based on frequency matching and with the help of permuted block stratified randomization. Double blinding was conducted so that Interventionists, and technicians did not have any information for the study.
Measurements
To measure the depression status, the Hamilton test was used.[14] It has a Persian version with a reliability of 81%. This questionnaire is a 24-item scale with items for feelings of sadness, suicide, sleep, work and activity, psychological anxiety, body anxiety, weight loss, frustration, etc. Items were scored from 0 as lack and 1–4 for other conditions. Total scores were gathered and scored as 0–6 (the absence or remission of depression), 7–17 (mild depression), 18–24 (moderate depression), and 25 and above (severe depression). The interviewer completed the questionnaire and also requested the data for age, married status, and job. All the patients were diagnosed by a psychiatrist, and they had a history of the disorder.
To assess the serum concentrations of TNF-α, IL-6, and hs-CRP, blood samples (5 mL) were collected from each patient before and after the study and centrifuged at 3500 rpm for 10 min. Serum samples were analyzed for the serum concentrations of TNF-α, IL-6, and hs-CRP by commercial kits (LDN Company, Germany), as suggested by producer companies.
Data analysis
We analyzed the data for normality by Kolmogorov–Smirnov test and Q_Q plot, and the data for depression score and the serum concentrations of TNF-α, IL-6, and hs-CRP were analyzed by paired t-test for intragroup comparison and T-independent for comparisons between groups, because the data were normal. Demographic characteristics were reported as mean ± standard deviation and distribution frequency. Furthermore, to compare basic characteristics between the intervention and the control groups, independent sample t-test and Chi-square test were used for continuous and categorical variables, respectively. In addition, the correlation between serum concentrations and depression score was investigated by Pearson correlation. P < 0.05 was considered statistically significant.
Ethical considerations
This study was approved by the Ethical Committee of Zahedan University of Medical Sciences (IR.ZAMUS.REC.1400.319). The study was registered at the Iranian Registry of Clinical Trials IRCT (IRCT20211220053469N1; www.irct.ir). All the participants signed informed consent, and subjects who did not have willing to participate in this study were allowed to leave the study. Informed consent was taken from all the participants.
RESULTS
Demographic data
Table 1 depicts the results for demographic data. The results show that most participants in the OG were housekeepers (n = 12, 40.00%) and unemployed (n = 11, 36.70%) while most participants in the control group were housekeepers (n = 13, 43.30%). The results showed that most participants had an education lower than diploma in both groups. The participants were middle aged and did not have significant differences in age. The results did not show significant differences between omega-3 fatty acids and placebo for height (P = 0.72), weight before the study (P = 0.99), disease duration (P = 0.576), and weight after the study (P = 0.94).
Table 1.
Demographic data for participants in both groups
| Parameters | Omega-3 fatty acids | Placebo | P* |
|---|---|---|---|
| Job | Housekeeper (n=12; 40.00%) Worker (n=1; 3.30%) Employee (n=1; 3.30%) Freelance (n=5; 6.70%) Unemployment (n=11; 36.70%) | Housekeeper (n=13; 43.30%) Freelance (n=7; 23.30) Unemployment (n=8; 26.70) Retired (n=2; 6.70%) | 0.345 |
| Education | <Diploma (n=21; 70.00%) Diploma (n=8; 25.00%) BSc. and higher (n=1; 3.30%) | <Diploma (n=25; 83.30%) Diploma (n=5; 16.70%) | 0.658 |
| Disease’s duration (months) | 23.2±7.8 | 26.7±9.3 | 0.576 |
| Age (years) | 36.93±10.03 | 37.83±10.72 | 0.74 |
| Height (cm) | 169.37±8.04 | 165.10±22.00 | 0.32 |
| Weight (kg), preintervention | 67.35±14.68 | 67.28±11.26 | 0.99 |
| Weight (kg), postintervention | 67.27±14.94 | 67.50±10.28 | 0.94 |
*P values were obtained from independent samples t-test for continuous variables and Chi-square test for categorical ones. The results are reported as mean±SD. SD=Standard deviation
The serum concentrations of interleukin-6, tumor necrosis factor-α and high-sensitivity C-reactive protein
Table 2 presents the effects of omega-3 fatty acid supplementation on the serum concentrations of IL-6, TNF-α, and hs-CRP between groups, before and after the study. For the serum concentrations of IL-6 (P = 0.652), TNF-α (P = 0.196), and hs-CRP (P = 0.083), significant differences were not seen between the OG and the placebo group before intervention. The serum concentrations of IL-6 (P < 0.001), TNF-α (P < 0.001), and hs-CRP (P < 0.001) were significantly reduced in the OG after the study compared with before the study. The serum concentrations of IL-6, TNF-α, and hs-CRP were decreased by 50.00%, 32.00%, and 43.00%, respectively, after the intervention compared with before intervention in the case group. Significant differences were not seen in the serum concentrations of IL-6 (P = 0.124), TNF-α (P = 0.865), and hs-CRP (P = 0.641) between the placebo group before and after the intervention. There were significant differences between the two groups after intervention for all inflammatory factors (P < 0.001).
Table 2.
The effects of omega-3 fatty acid supplementation on the serum concentrations of interleukin-6, tumor necrosis factor-α, and high-sensitivity C-reactive protein of patients with bipolar disorder
| Parameters | Groups | n | Before | After | Changes | P* |
|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | Omega-3 | 30 | 3.46±0.54 | 2.30±0.40 | −1.16±0.68 | <0.001 |
| Placebo | 30 | 3.39±0.61 | 3.51±0.64 | 0.11±0.39 | 0.124 | |
| P** | 0.652 | <0.001 | ||||
| TNF-α (pg/mL) | Omega-3 | 30 | 7.23±1.02 | 5.46±1.05 | −1.76±0.75 | <0.001 |
| Placebo | 30 | 6.94±0.66 | 6.95±0.91 | 0.01±0.60 | 0.865 | |
| P** | 0.196 | <0.001 | ||||
| hs-CRP (pg/mL) | Omega-3 | 30 | 2.82±1.02 | 1.97±0.57 | −0.85±0.68 | <0.001 |
| Placebo | 30 | 3.02±0.40 | 3.04±0.29 | 0.02±0.27 | 0.641 | |
| P** | 0.083 | <0.001 |
*P value for independent t-test, **P value for dependent t-test. The results are reported as mean±SD. SD=Standard deviation; IL-6=Interleukin-6; TNF-α=Tumor necrosis factor-α; hs-CRP=High-sensitivity C-reactive protein
Depression score
The effects of omega-3 fatty acid supplementation on depression scores are also reported in Table 3. There were no significant differences between the OG and the placebo group at the start of the study for the depression score on the Hamilton test (P = 0.128). The results showed that the depression score (P < 0.001) was significantly decreased before the study compared with after intervention in the OG. Regarding depression scores, there were no significant differences between the placebo group before and after intervention (P = 0.590). There were significant differences between the two groups after intervention (P < 0.001).
Table 3.
The effects of omega-3 fatty acid supplementation on depression score of patients with bipolar disorder
| Parameters | Groups | n | Before | After | Changes | P* |
|---|---|---|---|---|---|---|
| Depression score | Omega-3 | 30 | 18.93±9.04 | 13.70±5.32 | −5.23±3.22 | <0.001 |
| Placebo | 30 | 19.36±9.72 | 20.43±8.26 | 1.06±5.22 | 0.590 | |
| P** | 0.128 | <0.001 |
*P value for independent t-test, **P value for dependent t-test. The results are reported as mean±SD. SD=Standard deviation
The relation between depression score and the serum concentrations of interleukin-6, tumor necrosis factor-α, and high-sensitivity C-reactive protein
We observed a positive correlation between depression scores with the serum concentrations of IL-6, TNF-α, and hs-CRP before and after intervention in both groups [Table 4].
Table 4.
Correlation between pro-inflammatory cytokines with depression score in patients with bipolar disorder
| Groups | n | IL-6 | TNF-α | hs-CRP | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| R | P | R | P | R | P | |||
| Before | Omega-3 | 30 | 0.385 | 0.032 | 0.421 | 0.019 | 0.682 | <0.001 |
| Before | Placebo | 30 | 0.521 | <0.001 | 0.512 | <0.001 | 0.711 | <0.001 |
| After | Omega-3 | 30 | 0.414 | 0.012 | 0.365 | 0.039 | 0.811 | <0.001 |
| After | Placebo | 30 | 0.445 | <0.001 | 0.551 | <0.001 | 0.351 | 0.039 |
The results are reported as correlation coefficient. IL-6=Interleukin-6; TNF-α=Tumor necrosis factor-α; hs-CRP=High-sensitivity C-reactive protein
DISCUSSION
This study was conducted to investigate the effects of omega-3 fatty acid supplementation on depression score and the serum concentrations of TNF-α, IL-6, and hs-CRP and depression scores in patients with bipolar disorder. The results for serum concentration of pro-inflammatory factors in patients with bipolar disorder are similar to results reported by other studies that have shown a positive correlation between depression status and serum concentrations of pro-inflammatory cytokines.[15] The results elucidate the role of cytokines in the pathogenesis and progression of the disorder. Based on our findings, the increased serum concentration of TNF-α, IL-6, and hs-CRP increases depression score while the decreased serum levels decrease depression score. The results are also in line with findings reported by Bai et al. who compared inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder and showed a significant relationship between inflammatory dysregulation and progression of bipolar disorder.[16] In the current study, patients in both groups had scores higher than 25 before intervention which shows severe depression based on the Hamilton score. Previous studies have attributed the increased depression to a fault in immunological responses and activation of the inflammatory response system.[17] Other studies have also confirmed the relation between pro-inflammatory cytokines with depression in patients with bipolar disorder and showed to be high depression in patients and attributed it to an increase in the serum concentrations of cytokines.[18] The increase in serum concentrations of pro-inflammatory cytokines might be a response to patient status during the involvement with the disorder. The relation between concentrations of pro-inflammatory cytokines and depression has been previously reported. Cytokines might be involved in the hypothalamus–pituitary axis (HPA) and cause neurogenesis in major depression.[17] Thus, a fault in the HPA axis has a significant role in the relationship between mood disorders and inflammation. Cytokines penetrate in central nervous system via the blood–brain barrier, binding to receptors in the peripheral afferent nerve and promoting cortisol and other hormones and transmitters distributing the HPA axis.[17] TNF-α has negative effects on systems involved in depression, such as dopaminergic and serotonergic systems.[19] The increased levels of hs-CRP and IL-6 elevate vulnerability to inflammatory responses and are associated with mood disorders.[20] In sum, cytokine levels are higher in response to disorder and these are involved in mood disorders such as depression in patients with bipolar disorders.
Our findings showed that administration of omega-3 fatty acid supplement significantly reduced the serum concentrations of TNF-α, IL-6, and hs-CRP. The results are in agreement with findings reported by Dezfouli et al. who conducted a meta-analysis review on the effects of omega-3 supplementation on serum levels of inflammatory biomarkers in 371 patients with hemodialysis and showed that omega-3 fatty acid supplementation significantly decreased the serum concentrations of TNF-α, IL-6, and hs-CRP.[21] The results are also similar to results reported by Singh who reviewed the effects of omega-3 fatty acid supplementation on cognition and inflammation and showed that omega-3 supplementation decreased the serum concentrations of IL-6 and TNF-α in parallel with improving cognitive performance.[22] In contrast to our findings, Salehi et al. investigated the effects of omega-3 fatty acid supplementation for 6 months in patients with hemodialysis and did not report significant effects of omega-3 fatty acid supplementation on the serum concentrations of IL-6 and TNF-α.[23] The mechanism of omega-3 fatty acids in decreasing inflammation could be attributed to its effects in downregulating inflammatory cytokines and enzymes[24] and attenuating the production of pro-inflammatory cytokines.[25]
Our findings also showed decreased depression scores in patients receiving omega-3 fatty acid supplements. Similar to our findings, Osher et al.[26] assessed the effects of daily supplementation of omega-3 fatty acids (1.5–2 g/day) for 6 months on depression scores with the help of the Hamilton questionnaire. They reported a decrease of 50.00% depression score compared to preintervention. Frangou et al.[27] supplemented ethyl eicosapentaenoic acid in bipolar depression for 12 weeks and assessed depression score by the Hamilton questionnaire. They observed a reduction in depression scores in patients receiving omega-3 fatty acid supplements and also a decrease in depression scores in the control group. The antidepressant mechanism of omega-3 fatty acids is significantly associated with the anti-inflammatory properties of omega-3 fatty acids. Pro-inflammatory and inflammatory cytokines decrease neurotransmitter precursor availability, activate the HPA axis, and change neurotransmitter metabolism which all have significant roles in the progression of depression.[28] Our results showed a positive correlation between the serum concentrations of cytokines and depression scores. The supplementation of omega-3 fatty acids decreased the serum concentrations of cytokines parallel with decreased depression scores. It highlights the role of anti-inflammatory omega-3 fatty acid supplement and also its role in decreasing the depression via the anti-inflammatory system. Omega-3 fatty acids also maintain membrane integrity and fluidity which are essential for neurotransmitter binding and signaling within the cell.[29]
This study has several limitations such as assessing cytokines in serum may not reflect their concentrations in brain. Although studies have reported that changes in peripheral levels may partly reflect the changes in brain cytokine secretion, we did not find any definitive conclusion on this matter.[30] This study investigated three inflammatory factors which is a limitation in the current study, but assessing other factors can be an idea for future studies. The lack of a follow-up period is another limitation of the current study. The strength point of the current study is to be novel and can open new doors for future researchers and help to manage the treatment of bipolar disorder.
CONCLUSION
This study suggests a positive relationship between the serum concentrations of TNF-α, IL-6, and hs-CRP and score depression in patients with bipolar disorders. Our findings also showed that supplementation of omega-3 fatty acids decreased depression scores via a possible decrease in inflammatory responses. Although future studies are required for showing the relation between peripheral cytokines, depression, and omega-3 in patients with bipolar disorder, omega-3 fatty acids can be suggested as a supplement for the treatment of inflammation and depression in patients with bipolar disorder.
Financial support and sponsorship
This study was financially supported by the Vice Chancellor for Research and Technology of Zahedan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kheradmand A, AbediYekta AH, Safarzadeh H, Ganjalikhani M. Physical fitness in patients with bipolar disorder compared with a population-based sample. Health Sci Rep. 2022;5:e507. doi: 10.1002/hsr2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi MR, Davidian H, Noorbala AA, Malekafzali H, Naghavi HR, Pouretemad HR, et al. An epidemiological survey of psychiatric disorders in Iran. Clin Pract Epidemiol Ment Health. 2005;1:1–8. doi: 10.1186/1745-0179-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saccaro LF, Schilliger Z, Dayer A, Perroud N, Piguet C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: A narrative review. Neurosci Biobehav Rev. 2021;127:184–92. doi: 10.1016/j.neubiorev.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Panaccione I, Spalletta G, Sani G. Neuroinflammation and excitatory symptoms in bipolar disorder. Neuroimmunol Neuroinflammation. 2015;2:215–27. [Google Scholar]
- 5.Gabriel FC, Oliveira M, Martella BM, Berk M, Brietzke E, Jacka FN, et al. Nutrition and bipolar disorder: A systematic review. Nutr Neurosci. 2022;24:1–15. doi: 10.1080/1028415X.2022.2077031. [DOI] [PubMed] [Google Scholar]
- 6.Kishi T, Sakuma K, Okuya M, Ikeda M, Iwata N. Omega-3 fatty acids for treating residual depressive symptoms in adult patients with bipolar disorder: A systematic review and meta-analysis of double-blind randomized, placebo-controlled trials. Bipolar Disord. 2021;23:730–1. doi: 10.1111/bdi.13115. [DOI] [PubMed] [Google Scholar]
- 7.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Meng Q, Zheng L, Yu P, Hu H, Zhuang R, et al. Effect of omega-3 polyunsaturated fatty acids on left ventricular remodeling in chronic heart failure: A systematic review and meta-analysis. Br J Nutr. 2022;4:1–35. doi: 10.1017/S0007114521004979. [DOI] [PubMed] [Google Scholar]
- 9.Jamilian M, Tabassi Z, Reiner Ž, Panahandeh I, Naderi F, Aghadavod E, et al. The effects of n-3 fatty acids from flaxseed oil on genetic and metabolic profiles in patients with gestational diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Br J Nutr. 2020;123:792–9. doi: 10.1017/S0007114519003416. [DOI] [PubMed] [Google Scholar]
- 10.Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141:2166–71. doi: 10.3945/jn.111.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orang Z, Mohsenpour MA, Mozaffari-Khosravi H. Effect of Omega-3 fatty acid supplementation on inflammatory markers and insulin resistance indices in patient with type 2 diabetes and nonalcoholic fatty liver: A randomized double-blind clinical trial. Obes Med. 2020;19:100278. [Google Scholar]
- 12.Perunicic-Pekovic GB, Rasic ZR, Pljesa SI, Sobajic SS, Djuricic I, Maletic R, et al. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrology (Carlton) 2007;12:331–6. doi: 10.1111/j.1440-1797.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 13.Safa M, Tafti SF, Boroujerdi FG, Talischi F. Clinical trial in the treatment of 80 Iranian patients with major depression disorder by the combination of omega 3 fatty acid and a selective serotonin reuptake inhibitor. Ther Adv Psychopharmacol. 2013;3:186–90. doi: 10.1177/2045125312471667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton M. The hamilton depression scale-accelerator or break on antidepressant drug discovery. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp-2013-306984. [DOI] [PubMed] [Google Scholar]
- 15.Brietzke E, Stertz L, Fernandes BS, Kauer-Sant’anna M, Mascarenhas M, Escosteguy Vargas A, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–7. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Bai YM, Su TP, Tsai SJ, Wen-Fei C, Li CT, Pei-Chi T, et al. Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J Affect Disord. 2014;166:187–92. doi: 10.1016/j.jad.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, et al. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disord. 2015;17:269–77. doi: 10.1111/bdi.12259. [DOI] [PubMed] [Google Scholar]
- 19.Müller N, Ackenheil M. Psychoneuroimmunology and the cytokine action in the CNS: Implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1–33. doi: 10.1016/s0278-5846(97)00179-6. [DOI] [PubMed] [Google Scholar]
- 20.Queissner R, Pilz R, Dalkner N, Birner A, Bengesser SA, Platzer M, et al. The relationship between inflammatory state and quantity of affective episodes in bipolar disorder. Psychoneuroendocrinology. 2018;90:61–7. doi: 10.1016/j.psyneuen.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Dezfouli M, Moeinzadeh F, Taheri S, Feizi A. The effect of omega-3 supplementation on serum levels of inflammatory biomarkers and albumin in hemodialysis patients: A systematic review and meta-analysis. J Ren Nutr. 2020;30:182–8. doi: 10.1053/j.jrn.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Singh JE. Dietary sources of omega-3 fatty acids versus omega-3 fatty acid supplementation effects on cognition and inflammation. Curr Nutr Rep. 2020;9:264–77. doi: 10.1007/s13668-020-00329-x. [DOI] [PubMed] [Google Scholar]
- 23.Salehi Y, Moinzadeh F, Mortazavi M, Hoseini M, Dolatkhah S, Taheri S, et al. The effect of omega-3 supplementation on serum inflammatory factors in hemodialysis patients. BJMMR. 2017;20:1–0614. [Google Scholar]
- 24.Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2014;7:a016311. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, et al. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28:845–55. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Osher Y, Bersudsky Y, Belmaker RH. Omega-3 eicosapentaenoic acid in bipolar depression: Report of a small open-label study. J Clin Psychiatry. 2005;66:726–9. doi: 10.4088/jcp.v66n0608. [DOI] [PubMed] [Google Scholar]
- 27.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: Randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 28.Logan AC. Omega-3 fatty acids and major depression: A primer for the mental health professional. Lipids Health Dis. 2004;3:25. doi: 10.1186/1476-511X-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su KP. Biological mechanism of antidepressant effect of omega-3 fatty acids: How does fish oil act as a ‘mind-body interface’? Neurosignals. 2009;17:144–52. doi: 10.1159/000198167. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Kastin AJ. Penetration of neurotrophins and cytokines across the blood-brain/blood-spinal cord barrier. Adv Drug Deliv Rev. 1999;36:291–8. doi: 10.1016/s0169-409x(98)00086-6. [DOI] [PubMed] [Google Scholar]


