Highlights
-
•
This review provides an overview of all the MLI studies performed to date in adults aged 45 years and older.
-
•
The main outcomes of MLI studies are currently related to cognition rather than AD prevalence.
-
•
MLIs may reduce the risk of cognitive decline.
-
•
Longer MLI studies should be conducted to establish the effect on the prevention of cognitive decline and AD.
Keywords: Multidomain lifestyle intervention, Cognition, Cognitive decline, Alzheimer's disease, Modifiable risk factor
Abstract
Alzheimer's disease (AD) is a neurodegenerative disorder with an increasing incidence and currently without a cure. It is speculated that targeting multiple modifiable risk factors (MRFs) could be a beneficial strategy for the prevention of cognitive decline and AD. This study provides an overview and discusses the existing literature on multidomain lifestyle interventions in relation to cognitive decline and the prevention of AD. A literature search was performed in PubMed and Scopus, for studies published in English up to 31 May 2021. We identified nine relevant studies on the effect of multidomain lifestyle interventions on cognition (n = 8) and/or AD incidence or risk scores (n = 4). The studies included a combination of the separate intervention components diet (n = 8), physical activity (n = 9), cognitive activity (n = 6), metabolic or cardiovascular risk factor reduction strategies (n = 8), social activity (n = 2), medication (n = 2), and/or supplementation (n = 1). Global cognition was improved significantly in four of the eight studies that had global cognition as the outcome. Moreover, significant improvements were shown for cognitive domains in two of the three studies with specific cognitive domains as an outcome. No effect on AD incidence was observed, although positive results were shown for AD risk scores. The results suggest that multidomain lifestyle intervention studies may be partially effective in preventing cognitive decline. However, studies were heterogeneous and limited in follow-up. Future research on the effect of multidomain lifestyle interventions on cognitive decline and AD incidence must be conducted with a longer follow-up period.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder and is the most common form of dementia. Currently, more than 50 million people worldwide have been diagnosed with dementia, with AD contributing to 60–70% of those cases [1]. Furthermore, this number is expected to increase to 152 million by 2050, mainly due to the increasing aging population. Dementia has an enormous economic impact, with annual global costs of 263 billion US dollars attributable to dementia [26]. For this reason, dementia has been recognized as a public health priority by the [40].
Although no cure currently exists for AD, more than twenty well-known risk factors have been identified, including modifiable risk factors (MRFs) such as lack of exercise, lack of cognitive activity, smoking, and a poor diet [3]. According to one meta-analysis, around one-third of AD cases worldwide might be attributed to the MRFs diabetes, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, and a low level of education [25]. In addition, a more recent meta-analysis proposed nineteen modifiable factors for AD prevention [44]. These findings support the idea that preventive strategies are important for limiting the rise in AD prevalence. The importance of targeting single MRFs for the risk reduction of cognitive decline and AD has already been shown in observational studies and randomized controlled trials (RCTs) [[42], [44]]. For example, positive associations between cognitive function and single MRFs have been established for physical activity [45] and cognitive training [[13], [17]]. Nevertheless, other single prevention trials on potential risk factors for cognitive decline and AD, like hypertension and obesity, have found mostly discouraging results [37]. Thus, the targeting of multiple risk factors simultaneously in the prevention of AD has been proposed as a more effective strategy for AD prevention [16].
Since the aforementioned meta-analysis of Norton et al., AD prevention studies have focused on multidomain lifestyle interventions, wherein risk factors are targeted by a combination of separate intervention components, such as diet, physical activity, cognitive activity, or cardiovascular risk management. To date, three literature reviews have examined the evidence of RCTs, cohort and/or experimental/cross-sectional multidomain lifestyle intervention studies in relation to cognitive decline and AD prevention [[4], [14], [34]]. The most recent review in 2019 discussed all completed and ongoing prospective multidomain lifestyle intervention studies and concluded that multidomain lifestyle interventions have significant potential to enhance cognitive reserve and reduce the risk of AD [4]. However, only studies up until August 2019 were included, since which multiple prospective multidomain lifestyle interventions have been completed.
Thus, the aim of the current review is to provide an overview, evaluate, and discuss the evidence of completed RCT multidomain lifestyle intervention studies published up to 31 May 2021 in relation to cognitive decline and the prevention of AD in adults aged 45 years and older.
2. Methods
2.1. Literature search
The literature search was conducted in the databases Scopus and PubMed for multidomain lifestyle intervention studies published in English up to 31 May 2021. The search terms used included keywords related to multidomain, AD or cognitive decline, and prevention (Appendix 1). The literature search resulted in a total of 379 articles after removing duplicates. Furthermore, bibliographies of prior literature reviews [[4], [15], [34]] were examined, resulting in five additional studies. The literature search was performed by SN under the supervision of BW.
2.2. Study selection
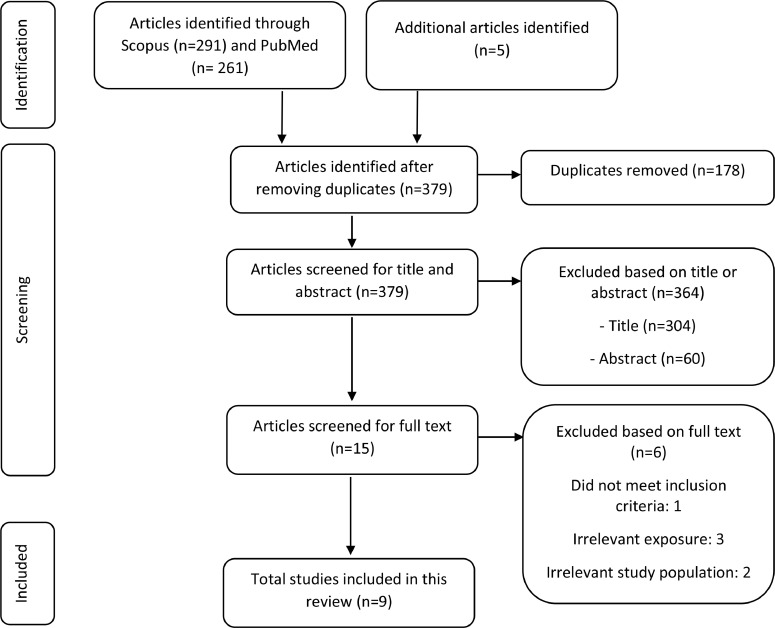
Fig. 1 exhibits the flow diagram of the study selection process. Studies were included if: 1) they had a control group, 2) the participants were randomized, 3) there were at least three combined interventions, 4) the study interval was six months or longer, and 5) studies had clear cognitive outcome measures (i.e., neuropsychological tests, AD incidence, or risk scores) as the outcomes. Only studies with participants aged 45 years and older were included because cognitive decline has been shown to be already present from this age [31]. Studies were first screened based on the title followed by abstract, resulting in fifteen potentially relevant studies. After full-text screening, nine studies were included. Studies were excluded if: 1) they were study protocols, non-human studies, reviews, or meta-analyses, 2) they had irrelevant exposures and outcomes, or 3) they had an uncommon study population (e.g., cognitive decline because of underlying disease), or 4) the study population comprised individuals with severe cognitive impairment or already diagnosed AD. One researcher (SN) assessed study eligibility for inclusion twice.
Fig. 1.
Flow diagram of the identified, screened and included multidomain lifestyle intervention studies on cognitive decline and AD prevention outcome.
2.3. Quality assessment
The quality of the involved studies was independently reviewed by two reviewers (BW and SN) using the revised Cochrane risk-of-bias tool for randomized trails [33]. The quality assessment evaluated the risk of bias in the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and reported result selection. The risk of bias judgement comprised the categories: “high”, “low”, and “some concerns”. There was a 100% agreement in the interpretation by the two reviewers. Six studies had a low risk of bias, and three studies had some concerns. The results are shown in Table 1.
Table 1.
Overview and characteristics of the included multidomain lifestyle intervention studies relating to cognitive decline and the prevention of AD.1
| Author, year, country, study title | Study design | Quality assessment2 | Study sample (n,%women, mean age, characteristics) | Study length | Intervention components and frequency | Outcome measures | Main results |
|---|---|---|---|---|---|---|---|
| Ngandu et al. 2015 [24] Finland FINGER | Double blind RCT | LR | n = 1260 (46% women) Mean age = 69.4 y At risk of dementia (age 60–77 y, CAIDE Dementia Risk Score ≥ 6 points, mean or slightly lower cognition) | 2 years | Diet (daily) Physical activity (strength 1–3 x/week, aerobic 2–5 x/week) Cognitive training (3 x/week individual + 10 group sessions) Social activities Metabolic and vascular risk management (3 x check-up) |
Global cognition (NTB Z score) Executive functioning, processing speed, memory (NTB domain Z scores) |
The intervention significantly improved global cognition (p = 0.03, mean change in NTB total Z of 0.20 in the intervention group vs 0.16 in the control group) (mean between group difference of 0.222 NTB total Z score per year), executive functioning (p = 0.039), and processing speed (p = 0.029) compared to control. The intervention significantly reduced the risk of cognitive decline compared to control for NTB total score (OR 1.31, 95% CI 1.01–1.71), executive functioning and processing speed. The intervention had no significant effect on memory. |
| van Charante et al. 2016 [36] The Netherlands preDIVA | Open label cluster RCT | SC |
n = 3526 (55% women) Mean age = 74.5 y General population registered with a general practice (age 70–78 y) |
6 years | Physical activity Management of cardiovascular risk factors (e.g. targeting healthy BMI, healthy diet, no smoking, low cholesterol, normal blood pressure) Medical interventions when needed (1 visit every month for individual tailored lifestyle advice) |
Cumulative incidence of dementia, disability score (ALDS) Incidence CVD, cognitive decline (MMSE and VAT) |
The intervention had no effect on incident all-cause dementia, (HR 0.92, 95% CI 0.71–1.19), incidence AD (HR 1.05, 95% CI 0.78–1.41), disability (p = 0.93), incidence CVD (p = 0.57) or cognitive decline (MMSE score p-value = 0.73, VAT A p = 0.48) compared to control. |
| Andrieu et al. 2017 [2] France, Monaco MAPT | Multicentre, randomized, placebo controlled superiority trial | LR |
n = 1525 (64% women) Mean age = 75.3 y Community-dwelling (age ≥ 70 y, at least one of the following criteria: spontaneous memory complaint expressed to their physician, limitation in one IADL, slow gait speed) |
3 years | Diet (daily) Physical activity (walking 5 times/week) Cognitive training Cardiovascular risk factor management Omega 3 PUFAs supplementation (daily) (12 two-hour sessions (twice per week in the first month, once per week in the second)) |
Composite Z score of different neuropsychological tests (free and total recall of the FCSRT, 10 MMSE orientation items, DSST score from the WAIS-R, CNT)) MMSE, TMT A and B, COWAT, VAS-scales for memory function and daily life functioning, ADCS-ADL, CDR sum of boxes |
The intervention groups had no significant difference in CD compared to control (combined intervention: mean difference 0.093, 95% CI 0.001- 0.184; adjusted p = 0.142, only multidomain intervention: 0.079, 95% CI –0.012–0.170; p = 0.179) The multidomain intervention + omega 3 PUFAs, compared to control, improved cognition, but not significantly (mean difference 0.093, 95% CI 0.001–0.184; p = 0.142). Post-hoc analysis of pooled multidomain intervention showed significant less cognitive decline with the composite Z score for groups contain the multidomain intervention compared to groups who did not receive the multidomain intervention (p = 0.015). There was less decline in the 10 MMSE orientation items with the multidomain interventions groups compared to the groups who did not. receive the multidomain intervention, but only significant for the multidomain intervention + omega 3 PUFAs group compared to control (mean difference 0.131, 95% CI 0.029–0.233; p = 0.036) |
| Chen et al. 2020 [6] Taiwan Taiwan Multidomain Intervention Efficacy Study |
Complementary cluster randomized trial | LR |
n = 1082 (68.7% women) Mean age = 75.1 y Prefrail/frail community-dwelling (age ≥ 65 y, subjective memory impairment and/or loss of ≥ 1 IADL, and or slow gait speed |
12 months | Diet (daily) Physical activity Cognitive training (16 two-hour sessions, 4 in the first month, 2 in the second, and one in the 10 months thereafter) |
General cognitive performance (MoCA) Concentration, delayed recall |
The intervention significantly improved concentration at six months (interaction 0.23, 95% CI 0.04, 0.42; p = 0.019) and twelve months (interaction 0.46, 95% CI 0.22, 0.70; p = <0.001) compared to control. Intervention resulted in higher, not significantly, general cognitive performance compared to control (MoCAadj 1.03, 95% CI −0.19, 2.24; p = 0.094). However, significant improvement on cognition was seen among participants ≥ 75 years (1.96, 95% CI 0.25 – 3.68; p = 0.027). |
| Richard et al. 2019 [27] The Netherlands, Finland, France HATICE |
Multinational RCT | SC |
n = 2724 (47.6% women) Mean age = 69 y Community-dwelling (age ≥ 65 y, at least 2 CVD risk factors or a history of CVD and/or diabetes) |
18 months | Diet Physical activity Cardiovascular risk factor management (non-stop access to online coach-supported interactive platform) |
Dementia risk score (CAIDE) Cognitive functioning (global (MMSE), composite Z score (MMSE, Stroop 1–3, RAVLT delayed recall/recognition, verbal fluency) |
The intervention decreased the risk of dementia with 0.19 compared to control (mean difference −0.15, 95% CI −0.28, −0.03; p = 0.02) The intervention had no effect on cognitive functioning, global (mean difference −0.05; p = 0.49) and composite Z score (mean difference 0.01; p = 0.44) compared to control. |
| Williamson et al. 2019 [38] United States, Puerto Rico SPRINT MIND |
Multicentre RCT | LR |
n = 9361 (35.6% women) Mean age = 67.9 y Adults (age ≥ 50 y, hypertension) |
4 years | Diet Physical activity Other components of a healthy lifestyle (e.g., reduction of alcohol; cessation of smoking; healthy BMI; low cholesterol) Antihypertensive medication(s) |
Occurrence of probable dementia and MCI Composite outcome of occurrence of probable dementia or MCI |
The intervention resulted in less occurrence, not significant, probable dementia compared to control (7.2 vs 8.6 cases per 1000 person-years; HR 0.83, 95% CI 0.67–1.04; p = 0.10). The intervention reduced significant the risk of MCI compared to control (14.6 vs 18.3 cases per 1000 person-years; HR 0.81, 95% CI 0.69–0.95; p = 0.007)) The intervention reduced significant the composite outcome of probable dementia or MCI compared to control (20.2 vs 24.1 cases per 1000 person-years, HR 0.85, 95% CI, 0.74–0.97; p = 0.01)) |
| De Souto Barreto et al. 2021 [8] France eMIND |
Pilot RCT | LR |
n = 120 (57.5% women) Mean age = 74.2 y Community-dwelling (age ≥ 65 y, subjective memory complaints (MMSE ≥ 24)) |
6 months | Diet Physical activity Cognitive training (non-stop access to online intervention platform) |
Cognitive composite Z score (MMSE, DSST of WAIS-R, total recall of the FCSRT, CNT) MMSE, DSST of WAIS-R, total recall of FCSRT, CNT, COWAT |
The intervention did not have any significant effects on cognition compared to control (cognitive composite score: −0.20, 95% CI −0.41, 0.002; p = 0.053). |
| Clare et al. 2015 [7] Wales Age Well |
Pilot RCT | LR |
n = 75 (86.7% women) Mean age = 68.2 y Community-dwelling (age > 50 y) |
12 months | Diet Physical activity Cognitive activity Vascular risk factor management Social engagement (bi-monthly telephone calls, 2 structured interviews (12 and 24 month)) |
Cognition function (MoCA), immediate and delayered recall ability (CVLT), executive functioning (D-KEFS, TMT Verbal Fluency) | Cognitive function, immediate recall and delayed recall were improved in the intervention groups and the control group, but only significant results were seen for cognitive function in the goal setting with mentoring condition (p = 0.03) and delayed recall in the control group. |
| Z. Xu et al. 2020 [43] China |
Pilot feasibility RCT | SC |
n = 19 (73.7% women) Mean age = 74.0 y Older adults with MCI (age 60–80 y, HK–MoCA score of 19–21 |
6 months | Diet Physical activity (mind-body) (3 x per week) Cognitive training (3 x per week) Metabolic and vascular risk factors management |
Cognitive function (ADAS-Cog, HK-MoCA, CDR sum of box, DAD) |
RFM intervention resulted in significant higher HK-MoCA scores compared to control (Group X time interaction, p = <0.001) |
AD, Alzheimer's disease; FINGER, Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; CAIDE, Cardiovascular Risk Factors, Aging and Incidence of Dementia; NTB, neuropsychological test battery; OR, Odds Ratio; preDIVA, Prevention of Dementia by Intensive Vascular care; ALDS, Academic Medical Center Linear Disability Score; CVD, cardiovascular disease; MMSE, Mini-Mental State Examination; VAT, Visual Association Test; HR, Hazard Ratio; MAPT, Multidomain Alzheimer Preventive Trial; IADL, instrumental activity of daily living; PUFAs, polyunsaturated fatty acids; FCSRT, Free and Cued Selective Reminding Test; DSST, Digit Symbol Substitution test; WAIS-R, Wechsler Adult Intelligence Scale-Revised; CNT, Category Naming Test; TMT, Trail Making Test; COWAT, Controlled Oral Word Association Test; VAS, Visual Analogue Scale; ADCS-ADL, Alzheimer's Disease Cooperative Study Activities of Daily Living; CDR, Clinical Dementia Rating; MoCA, Montreal Cognitive Assessment; HATICE, healthy aging through internet counselling in the elderly; LDL, low-density lipoprotein; BMI, Body Mass Index; RAVL, Rey Auditory Verbal Learning Test; SPRINT, Systolic Blood Pressure Intervention Trial; CVLT, California Verbal Learning Test; D-KEFS, Delis-Kaplan Executive Function System; MCI, mild cognitive impairment; HK-MoCA, Montreal Cognitive Assessment Hong Kong version; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; CDR, Clinical Dementia Rating; DAD, Disability Assessment for Dementia; CPR, cognitive training, physical exercise and risk factor modification; RFM, Risk Factor Modification.
Cochrane Risk of Bias tool 2 was used with the risk-of-bias judgements low risk (LR), high risk (HR) and some concerns (SC).
2.4. Cognition and Alzheimer's disease outcomes
Outcomes on global cognition (Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), or composite Z score), cognitive function (single neuropsychological tests or neuropsychological domains), AD incidence, and AD risk scores were obtained. The cognitive domains reported are part of one of the neurocognitive key domains of cognitive function as defined by the DSM-5 [28]. Only two studies reported results on the incidence of AD. Therefore, cognitive decline was used as an indicator of future risk of AD. The data were extracted from eligible papers by researcher SN, exported to Microsoft Word, and summarized per study. Only relevant results for cognitive decline or AD outcomes are shown in Table 1.
3. Results
3.1. Included studies
A total of nine multidomain lifestyle intervention studies, three of which were pilot studies were included [[7], [8], [43]]. An overview of the key characteristics of these studies is provided in Table 1. All studies were RCTs [[2], [6], [7], [8], [24], [27], [36], [38], [43]], two of which were cluster RCTs [[6], [36]]. Most of the studies were conducted in Europe, namely Finland, Germany, the Netherlands, Wales, and France. One study was conducted in both the USA and Puerto Rico, and the remaining were in Taiwan and China. The studies differed in the type of intervention component (Appendix 2); all studies included the component physical activity, eight studies diet, and six studies cognitive training. Besides physical activity and healthy diet strategies, eight targeted metabolic or cardiovascular risk factors, such as reduction of alcohol use, cessation of smoking, and cholesterol lowering methods. Two studies included social activities/engagement, two other studies medication, and one study provided an omega-3 polyunsaturated fatty acids (PUFAs) supplement.
3.2. Cognition and cognitive decline
The effect of multidomain lifestyle interventions on cognition was investigated in eight studies [[2], [6], [7], [8], [24], [27], [36], [43]], although the assessment methods were heterogeneous. Cognition was assessed with different single neuropsychological tests in all eight studies, and cognitive domains were investigated in three studies [[6], [7], [24]]. The aforementioned studies also looked at global cognition, measured with MMSE or MoCA [[2], [6], [27], [36], [43]], or expressed as a composite Z score which was calculated from the combination of different neuropsychological tests [[2], [8], [24], [27]].
In two of the studies that measured global cognition based on a composite Z score, the intervention group had a significant improvement in global cognition compared to the control group [[2], [24]]. In the FINGER study, 1260 Finnish older adults (mean age 69.4 y, 46% women) at risk of dementia were investigated. The intervention group received the components diet, physical activity, cognitive training, social activities, and metabolic and vascular risk factor management, whereas the control group received only general health advice. After two years, cognition improved significantly (mean difference 0.022 neuropsychological test battery (NTB) total Z score per year; p = 0.03) whilst risk of cognitive decline (Odds Ratio (OR) 1.31, 95% confidence interval (CI) 1.01–1.71) was significantly reduced in the intervention group compared to the control group [24].
Similar effects on global cognition were found in a three-year multidomain lifestyle intervention study (MAPT) among 1525 community-dwelling older adults (mean age 75.3 y, 64% women) with subjective memory complaints. The same intervention components as in the FINGER study were included, with the exception of social activities and the addition of the dietary supplement omega-3 PUFAs in one of the two multidomain intervention groups. The multidomain intervention groups were compared with a group using only omega-3 PUFAs and one using only a placebo, the control group. Initially, the multidomain intervention groups had less cognitive decline after three years, but this did not reach a statistical significance (mean difference 0.093, 95% CI 0.001–0.184; p = 0.142 and mean difference 0.079, 95% CI −0.012–1.70; p = 0.179). However, significantly less cognitive decline was observed in these intervention groups compared to the omega-3 PUFAs and placebo group when doing an analysis of pooled multidomain interventions (p = 0.015) [2].
Global cognition, as measured with MMSE or MoCA, improved significantly in three studies [[2], [6], [43]]. In the Taiwan Multidomain Intervention Efficacy Study, an intervention involving diet, physical activity, and cognitive training lasting 12 months resulted in an improvement in cognition (mean difference 1.96, 95% CI 0.25 – 3.68; p = 0.027, as measured with MoCA) among the 1082 (pre)frail older adults (mean age 75.1 y, 68.7% women) participants aged 75 years and older with subjective memory complaints [6]. Xu et al. [43] divided nineteen older adults (mean age 74.0 y, 73.7% women) with mild cognitive impairment (MCI) into two intervention groups (cognitive training, mind-body physical exercise, and metabolic risk factor modification (CPR) vs risk factor modification (RFM) and one control group (health advice)). An improvement in cognition (measured with Montreal Cognitive Assessment Hong Kong version (HK-MoCA)) was shown after six months, but this was only significant in the RFM intervention group (containing the components diet and metabolic risk factor management) compared with the control group (p < 0.001) [43].
Besides global cognition, cognitive domains were investigated in three studies [[6], [7], [24]]. Data on neuropsychological domains were extracted from individual neuropsychological tests [[6], [7]] as well as domain composite Z scores [24]. Significant improvements of different cognitive domains were found in two studies [[6], [24]]. Processing speed (estimated mean difference 0.030, 95% CI 0.003–0.057, p = 0.029) and executive functioning (estimated mean difference 0.027, 95% CI 0.001–0.052, p = 0.039) improved significantly in the intervention group compared to the control group in the FINGER study [24], and concentration was significantly improved in the Taiwan Multidomain Intervention Efficacy Study (mean difference 0.46, 95% CI 0.22- 0.70; p < 0.001) [6].
In contrast to the positive results mentioned earlier, however, some studies failed to find significant effects for interventions on cognition or cognitive decline. In a study among 3526 Dutch older adults (mean age 74.5 y, 55% women) from the general population (preDIVA), the multidomain lifestyle intervention components physical activity, metabolic or cardiovascular risk factor reduction strategies showed no difference after six years in cognitive functioning, as measured with MMSE and Visual Association Test (VAT), compared to usual care [36]. Moreover, the Age Well study also failed to show an improvement of cognitive function (MoCA) after 12 months in the intervention groups (containing a combination of diet, physical activity, cognitive training, vascular risk factor management, and social engagement) compared to the control group in 75 community-dwelling older adults (mean age 68.2 y, 86.7% women) from the general population [7]. Similar results were seen in two web-based multidomain lifestyle intervention studies [[8], [27]]. In the eMIND study, the effect of a web-based multidomain intervention (including the components diet, physical activity, and cognitive training) was studied in 120 French community-dwelling older adults (mean age 74.2 y, 5.7% women) with subjective memory complaints. After 6 months, effects were found on neither global cognition (composite Z score) nor on several neuropsychological tests in the intervention group [8]. Lasty, in the HATICE study, an interactive supportive web-based intervention (containing diet, physical activity, and cardiovascular risk factor management) was compared with a static online information platform in 2724 community-dwelling older adults (mean age 69 y, 47.6% women) from the general population with a higher risk of cardiovascular disease (CVD). The intervention did not have any effect on the measured cognitive outcomes, cognitive functioning (based on global MMSE), or composite Z score after 18 months [27].
3.3. Alzheimer's disease
Almost all studies included assessment of cognitive function, but only three studies looked also at outcomes specific to AD [[27], [36], [38]]. Two of them included the incidence of (probable) AD based on internationals established criteria (Diagnostic and Statical Manual of Mental Disorders (DMS IV) and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria), and one included neuropsychological tests with AD risk scores [27].
No significant effects for a lower occurrence of AD (based on DSM IV) were found in the preDIVA study (Hazard Ratio (HR) 0.92, 95% CI 0.71–1.19) [36], and a lower occurrence of probable dementia (based on NINCS-ADRDA) could not be concluded in the SPRINT MIND study among 9361 older adults with hypertension (mean age 67.9y, 35.6% women) after four years (HR 0.83, 95% CI 0.67–1.04) [38]. The SPRINT MIND study included a combination of diet physical activity, additional strategies targeting metabolic or cardiovascular risk factors, and antihypertensive medication(s) as intervention components.
Unlike the studies which reported outcomes on AD incidence, significant results were reported in the HATICE study, which investigated the effect of a multidomain lifestyle intervention on AD risk scores. In this study, a decreased 20-year risk of dementia (based on the Cardiovascular Risk Factors, Aging and Incidence of Dementia (CAIDE) Risk Score) of 0.19 was seen in the intervention group compared to 0.04 in the control group (mean difference −0.15, 95% CI −0.28, −0.03; p = 0.02) [27]. The CAIDE Dementia Risk Score is a validated tool to predict late-life dementia risk [30].
4. Discussion
This literature review provided an overview and evaluated the evidence of multidomain lifestyle intervention studies published up to the 31st of May 2021 in relation to cognitive function and the prevention of AD in adults aged 45 years and older. Significant effects of multidomain lifestyle intervention studies on global cognition were observed in half of the studies [[2], [6], [24], [43]]. Furthermore, significant improvements in cognition were shown for specific cognitive domains [[6], [24]]. Cognitive decline precedes AD and may start 7.5 years before the onset of dementia [39], meaning cognitive decline could be an important indicator of the future risk of AD, highlighting the importance of these encouraging findings. While significant results were shown for AD risk scores [27], clinical effects were small, and significant differences in the incidence of AD outcomes were not observed [[36], [38]]. Thus, these results suggest that multidomain lifestyle interventions may improve cognition and thereby reduce the risk of cognitive decline, although evidence for their reduction of the incidence of AD outcomes is lacking. Our study findings are consistent with previous reviews reporting that multidomain lifestyle interventions are effective in the delay and/or prevention of cognitive decline [[4], [14], [34]] Moreover, our study builds on the existing evidence in terms of the efficacy and impact of the interventions on the outcomes of AD.
The observed differences in results between the studies may be attributed to methodological differences. Although the study designs were in large part comparable (i.e. most were RCTs), there were essential differences in study populations (e.g., cognitive status and age), follow-up duration, intervention components, and outcome measures, making the results difficult to compare and interpret.
Methodological differences may also explain the lack of significant differences in cognition between some of the studies [[7], [8], [27], [36]]. For instance, the eMIND study did not find an effect on cognition, possibly due to the short study duration, different intervention components and frequencies, and lack of statistical power, as the study had a small sample size and duration [8]. The lack of statistical power was also observed in the studies investigating the effect on global cognition measured with MMSE or MoCA. In one, improvement of cognition was observed only in the RFM intervention group that included the components diet and metabolic risk factor management [43]. However, this study had a very small sample size of nineteen participants and lasted for only six months, potentially resulting in a low statistical power. Moreover, in the Taiwan Multidomain Intervention efficacy study, global cognition was only improved in participants aged 75 years and older. This could be because individuals aged 75 years and older have a higher prevalence of frailty, which could lead to larger effect sizes of the intervention in this age group [6]. The findings suggest that methodological differences should be considered when interpreting the results of multidomain lifestyle intervention studies.
With respect to the outcome measures, no significant results were observed in studies including individual cognitive tests. Since study outcomes depend on the types of tests used, a better alternative measurement for cognition might be the use of a composite score, which is mostly used to assess global cognition and consists of a combination of different cognitive tests (e.g., MMSE, VAT, Digit Symbol Substitution test (DSST), Rey Auditory Verbal Learning Test RAVL, etc.). Despite the fact that such composite scores still differ in composition between each other, they are more sensitive to detect changes compared to single neuropsychological tests [5].
Only two studies reported results on the incidence of AD due to the lack of follow-up data that is needed to obtain such results. The fact that no significant results were found in these two studies might be attributable to several study limitations. Firstly, the SPRINT MIND was stopped early because of the cardiovascular health benefits. Moreover, participants were lost to follow up during the extended follow-up visits, which could both have led to underestimating of the AD occurrence [38]. In the preDIVA study, the most plausible reason that no significant results were found might be the fact that the usual cardiovascular care was already of high quality. As a result, the differences between the intervention group and the control group were quite small in terms of management of cardiovascular risk factors, meaning the impact of the intervention could have been too low to affect lifestyle change [36].
Based on studies with AD risk scores, multidomain lifestyle interventions reduce the risk of AD significantly [27]. Although encouraging, it should be noted that this outcome was only studied in one study in patients with a higher risk of CVD. CVD is associated with AD, and thus targeting CVD risk factors (e.g., high blood pressure) might be an effective strategy for the prevention of AD [32]. However, the effect of lowering blood pressure for the reduction in cognitive decline and the prevention of AD is still uncertain, and further work is required in order to draw more definitive conclusions [10].
Finally, it is worth mentioning that none of the studies on AD outcomes included cognitive training in their intervention. It is of interest for future clinical research to study the effect of long-term cognitive training in these patient groups. The studies may also benefit from the addition of the component sleep to the intervention group, since it has been shown that sleep management might serve as a promising target for dementia prevention [41].
The results are important for policymakers and healthcare professionals because they contribute to evidence of the association between healthy lifestyles and the lower risk of AD [9]. However, as discussed above, stronger evidence is required in order to make concrete clinical guidelines based on multidomain lifestyle intervention studies.
Although the lack of evidence could be interpreted as discouraging, in our opinion this may be the results of the variations in methodology of the included studies rather than evidence of the lack of effect of lifestyle interventions. Previous research has demonstrated the positive effect of diets such as the Mediterranean diet on cognition [35] and physical activity on cognitive health outcomes [18]. Besides, it is also true that statistically significant study outcomes do not necessarily translate into meaningful clinical improvements. Thus, clinicians should be aware that multidomain lifestyle interventions may be beneficial in the treatment of patients predisposed for cognitive decline and AD, despite the need for further research to definitively confirm this.
Whilst some multidomain lifestyle interventions are free and easy to implement, other are less so, particularly for older adults with cognitive impairment or mobility issues, and longer term implementation feasibility research is lacking [23]. Besides this, cognitive decline or AD may be a long-term condition due to slow mental decline, and the long-term sustainability of multidomain lifestyle interventions has not been well studied. Therefore, multidomain lifestyle intervention studies must focus more on patient selection, clearly documented intervention strategies and well documented outcome measures, and long term (> 10 years) exposure.
4.1. Limitations and strengths
This study has some limitations. Firstly, it is not a systematic review but a literature review. Therefore, potentially relevant studies could have been missed because the search strategy was not performed in a systematic way and did not adhere to prescribed guidelines of the COCHRANE manual for systematic reviews for interventions [12]. Secondly, in four studies, cognition or AD incidence/risk score was not the primary outcome [[7], [8], [27], [43]]. This could have influenced the power of the study because sample size calculations are based on the primary outcome(s) [11]. This was the case in one study [27], and sample size calculations were not mentioned in two studies [[7], [43]]. Thirdly, the studies included in this review were quite heterogeneous with respect to sample size, adherence, study outcomes, and intervention components. For instance, not all the intervention components included were well-defined. This is particularly the case with the metabolic or cardiovascular risk factor reduction strategies. Although we do realize that there should be an individualized strategy to reduce personal risk factors, such strategies (e.g., targeting a healthy BMI and cholesterol lowering interventions) will interfere with both psychical activity and diet intervention components This made comparing results between the studies difficult. Fourthly, the cognitive statuses of the participants in the included studies varied. Since the results of multidomain lifestyle intervention studies may differ depending on the target population, similar results between studies should be interpreted with caution. Lastly, the studies were performed mostly in European (6/9 studies) or in other high-income countries. Therefore, the results of this review may not directly be generalizable to low- and middle-income countries. Additionally, since only articles written in English were included, valuable studies performed in low- and middle-income countries published in other languages may have been missed. Nevertheless, since more than 60% of the people with dementia live in low-and middle-income countries [29], such results could be of important value.
This review also has several strengths. To date, it is the most comprehensive review of multidomain lifestyle intervention studies in relation to cognitive decline and the prevention of AD. Moreover, the quality of the review is higher than previous literature reviews of multidomain lifestyle interventions due to the inclusion of data with statistical outcomes in the results, in contrast to two previous reviews for which this was lacking [[4], [34]].
5. Conclusion and future directions
In conclusion, the results of this study indicate that multidomain lifestyle interventions may reduce the risk of cognitive decline, although it cannot currently be concluded that multidomain lifestyle interventions can prevent AD. While the interventions did not result in a significant decrease in occurrence of AD, they were associated with a significantly reduced risk of AD risk scores. Therefore, multidomain lifestyle interventions might still be important in the prevention of AD in the future.
Currently, several multidomain lifestyle intervention studies investigating the effect of multiple MRFs on cognition and/or dementia are running or planned to take place [[19], [20], [21], [22]]. This is important because future research on longitudinal multidomain lifestyle intervention studies is required to establish the effect of multidomain lifestyle interventions, specifically on AD incidence. Especially, studies with long-term follow-up (> 10 years), clear study outcomes, well-defined intervention components, and the addition of the intervention component sleep would benefit the literature. Finally, studies performed in LMICs are also recommended.
Declaration of Competing Interest
None.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2023.100166.
Appendix. Supplementary materials
References
- 1.Alzheimer's Disease International, 2018. World Alzheimer Report 2018 - The state of the art of dementia research: new frontiers.
- 2.Andrieu S., Guyonnet S., Coley N., Cantet C., Bonnefoy M., Bordes S., Bories L., Cufi M.N., Dantoine T., Dartigues J.F., Desclaux F., Gabelle A., Gasnier Y., Pesce A., Sudres K., Touchon J., Robert P., Rouaud O., Legrand P., Payoux P., Caubere J.P., Weiner M., Carrié I., Ousset P.J., Vellas B., Vellas B., Guyonnet S., Carrié I., Brigitte L., Faisant C., Lala F., Delrieu J., Villars H., Combrouze E., Badufle C., Zueras A., Andrieu S., Cantet C., Morin C., Van Kan G.A., Dupuy C., Rolland Y., Caillaud C., Ousset P.J., Fougère B., Willis S., Belleville S., Gilbert B., Fontaine F., Dartigues J.F., Marcet I., Delva F., Foubert A., Cerda S., Noëlle-Cuffi M., Costes C., Rouaud O., Manckoundia P., Quipourt V., Marilier S., Franon E., Bories L., Pader M.L., Basset M.F., Lapoujade B., Faure V., Li M., Tong Y., Malick-Loiseau C., Cazaban-Campistron E., Desclaux F., Blatge C., Dantoine T., Laubarie-Mouret C., Saulnier I., Clément J.P., Picat M.A., Bernard-Bourzeix L., Willebois S., Désormais I., Cardinaud N., Bonnefoy M., Livet P., Rebaudet P., Gédéon C., Burdet C., Terracol F., Pesce A., Roth S., Chaillou S., Louchart S., Sudres K., Lebrun N., Barro-Belaygues N., Touchon J., Bennys K., Gabelle A., Romano A., Touati L., Marelli C., Pays C., Robert P., Le Duff F., Gervais C., Gonfrier S., Gasnier Y., Bordes S., Begorre D., Carpuat C., Khales K., Lefebvre J.F., El Idrissi S.M., Skolil P., Salles J.P., Dufouil C., Lehéricy S., Chupin M., Mangin J.F., Bouhayia A., Allard M., Ricolfi F., Dubois D., Paule M., Martel B., Cotton F., Bonafé A., Chanalet S., Hugon F., Bonneville F., Cognard C., Chollet F., Payoux P., Voisin T., Peiffer S., Hitzel A., Allard M., Zanca M., Monteil J., Darcourt J., Molinier L., Derumeaux H., Costa N., Vincent C., Perret B., Vinel C., Olivier-Abbal P. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong R.A. Risk factors for Alzheimer's disease. Folia Neuropathol. 2019;57:87–105. doi: 10.5114/FN.2019.85929. [DOI] [PubMed] [Google Scholar]
- 4.Bott N.T., Hall A., Madero E.N., Glenn J.M., Fuseya N., Gills J.L., Gray M. Face-to-face and digital multidomain lifestyle interventions to enhance cognitive reserve and reduce risk of alzheimer's disease and related dementias: a review of completed and prospective studies. Nutrients. 2019 doi: 10.3390/nu11092258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnham S.C., Raghavan N., Wilson W., Baker D., Ropacki M.T., Novak G., Ames D., Ellis K., Martins R.N., Maruff P., Masters C.L., Romano G., Rowe C.C., Savage G., Macaulay S.L., Narayan V.A. Novel statistically-derived composite measures for assessing the efficacy of disease-modifying therapies in prodromal Alzheimer's disease trials: an AIBL study. J. Alzheimer's Dis. 2015;46:1079–1089. doi: 10.3233/JAD-143015. [DOI] [PubMed] [Google Scholar]
- 6.Chen L.K., Hwang A.C., Lee W.J., Peng L.N., Lin M.H., Neil D.L., Shih S.F., Loh C.H., Chiou S.T. Efficacy of multidomain interventions to improve physical frailty, depression and cognition: data from cluster-randomized controlled trials. J. Cachexia. Sarcopenia Muscle. 2020;11:650–662. doi: 10.1002/jcsm.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clare L., Nelis S.M., Jones I.R., Hindle J.V., Thom J.M., Nixon J.A., Cooney J., Jones C.L., Tudor Edwards R., Whitaker C.J. The Agewell trial: a pilot randomised controlled trial of a behaviour change intervention to promote healthy ageing and reduce risk of dementia in later life. BMC Psychiatry. 2015;15 doi: 10.1186/s12888-015-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souto Barreto P., Pothier K., Soriano G., Lussier M., Bherer L., Guyonnet S., Piau A., Ousset P.J., Vellas B. A web-based multidomain lifestyle intervention for older adults: the eMIND randomized controlled trial. J. Prev. Alzheimer's Dis. 2021;8:142–150. doi: 10.14283/jpad.2020.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhana K., Evans D.A., Rajan K.B., Bennett D.A., Morris M.C. Healthy lifestyle and the risk of Alzheimer dementia: findings from 2 longitudinal studies. Neurology. 2020;95:E374–E383. doi: 10.1212/WNL.0000000000009816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias M.F., Torres R.V., Davey A. Clinical trials of blood pressure lowering and antihypertensive medication: is cognitive measurement state-of-the-art? Am. J. Hypertens. 2018 doi: 10.1093/ajh/hpy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickey G.L., Grant S.W., Dunning J., Siepe M. Statistical primer: sample size and power calculations-why, when and how? Eur. J. Cardio-thoracic Surg. 2018 doi: 10.1093/ejcts/ezy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., Welch V. Cochrane; 2022. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 13.Kelly M.E., Loughrey D., Lawlor B.A., Robertson I.H., Walsh C., Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res. Rev. 2014;15:28–43. doi: 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018 doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 15.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018 doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 16.Kivipelto M., Mangialasche F., Snyder H.M., Allegri R., Andrieu S., Arai H., Baker L., Belleville S., Brodaty H., Brucki S.M., Calandri I., Caramelli P., Chen C., Chertkow H., Chew E., Choi S.H., Chowdhary N., Crivelli L., Torre R.D.La, Du Y., Dua T., Espeland M., Feldman H.H., Hartmanis M., Hartmann T., Heffernan M., Henry C.J., Hong C.H., Håkansson K., Iwatsubo T., Jeong J.H., Jimenez-Maggiora G., Koo E.H., Launer L.J., Lehtisalo J., Lopera F., Martínez-Lage P., Martins R., Middleton L., Molinuevo J.L., Montero-Odasso M., Moon S.Y., Morales-Pérez K., Nitrini R., Nygaard H.B., Park Y.K., Peltonen M., Qiu C., Quiroz Y.T., Raman R., Rao N., Ravindranath V., Rosenberg A., Sakurai T., Salinas R.M., Scheltens P., Sevlever G., Soininen H., Sosa A.L., Suemoto C.K., Tainta-Cuezva M., Velilla L., Wang Y., Whitmer R., Xu X., Bain L.J., Solomon A., Ngandu T., Carrillo M.C. World-Wide FINGERS Network: A global Approach to Risk Reduction and Prevention of Dementia. 2020;16:1078–1094. doi: 10.1002/alz.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lampit A., Hallock H., Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin M., Ma C., Zhu J., Gao J., Huang L., Huang J., Liu Z., Tao J., Chen L. Effects of exercise interventions on executive function in old adults with mild cognitive impairment: a systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 2022;82 doi: 10.1016/J.ARR.2022.101776. [DOI] [PubMed] [Google Scholar]
- 19.National Library of Medicine (US), 2021a. South Korean Study to Prevent Cognitive Impairment and Protect Brain Health Through Lifestyle Intervention [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT03980392?term=multidomain&cond=Cognitive+Decline&draw=2&rank=1 (accessed 6.22.21).
- 20.National Library of Medicine (US), 2021b. Preventing Cognitive Decline: the Go-On Multi-domain Intervention Study [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT04840030?term=multidomain&cond=Cognitive+Decline&draw=2&rank=5 (accessed 6.22.21).
- 21.National Library of Medicine (US), 2021c. Multimodal Preventive Trial for Alzheimer's Disease (MIND)-ADmini [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT03249688 (accessed 6.22.21).
- 22.National Library of Medicine (US), 2020. Evaluation of Pilot Community-based Multi-domain Program Older Adults at Risk of Cognitive Impairment [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT04440969?term=multidomain&cond=Cognitive+Decline&draw=2&rank=3 (accessed 6.22.21).
- 23.Ng P.E.M., Nicholas S.O., Wee S.L., Yau T.Y., Chan A., Chng I., Yap L.K.P., Ng T.P. Implementation and effectiveness of a multi-domain program for older adults at risk of cognitive impairment at neighborhood senior centres. Sci. Rep. 2021:1–11. doi: 10.1038/S41598-021-83408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., Lindström J., Mangialasche F., Paajanen T., Pajala S., Peltonen M., Rauramaa R., Stigsdotter-Neely A., Strandberg T., Tuomilehto J., Soininen H., Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 25.Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 26.Pedroza P., Miller-Petrie M.K., Chen C., Chakrabarti S., Chapin A., Hay S., Tsakalos G., Wimo A., Dieleman J.L. Global and regional spending on dementia care from 2000−2019 and expected future health spending scenarios from 2020−2050: an economic modelling exercise. eClinicalMedicine. 2022;45 doi: 10.1016/j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richard E., Moll van Charante E.P., Hoevenaar-Blom M.P., Coley N., Barbera M., van der Groep A., Meiller Y., Mangialasche F., Beishuizen C.B., Jongstra S., van Middelaar T., Van Wanrooij L.L., Ngandu T., Guillemont J., Andrieu S., Brayne C., Kivipelto M., Soininen H., Van Gool W.A. Healthy ageing through internet counselling in the elderly (HATICE): a multinational, randomised controlled trial. Lancet Digit. Heal. 2019;1:e424–e434. doi: 10.1016/S2589-7500(19)30153-0. [DOI] [PubMed] [Google Scholar]
- 28.Sachdev P.S., Blacker D., Blazer D.G., Ganguli M., Jeste D.V., Paulsen J.S., Petersen R.C. Classifying neurocognitive disorders: the DSM-5 approach. Nat. Rev. Neurol. 2014:634–642. doi: 10.1038/NRNEUROL.2014.181. [DOI] [PubMed] [Google Scholar]
- 29.Salthouse T.A. What and when of cognitive aging. Curr Dir Psychol Sci. 2004 doi: 10.1111/j.0963-7214.2004.00293.x. [DOI] [Google Scholar]
- 30.Sindi S., Calov E., Fokkens J., Ngandu T., Soininen H., Tuomilehto J., Kivipelto M. The CAIDE Dementia Risk Score App: the development of an evidence-based mobile application to predict the risk of dementia. Alzheimer's Dement. Diagn. Assess. Dis. Monit. 2015;1:328. doi: 10.1016/J.DADM.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh-Manoux A., Kivimaki M., Glymour M.M., Elbaz A., Berr C., Ebmeier K.P., Ferrie J.E., Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344 doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stampfer M.J. Cardiovascular disease and Alzheimer's disease: common links. J. Intern. Med. 2006 doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 33.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Toman J., Klímová B., Vališ M. Multidomain lifestyle intervention strategies for the delay of cognitive impairment in healthy aging. Nutrients. 2018 doi: 10.3390/nu10101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valls-Pedret C., Sala-Vila A., Serra-Mir M., Corella D., De La Torre R., Martínez-González M.Á., Martínez-Lapiscina E.H., Fitó M., Pérez-Heras A., Salas-Salvadó J., Estruch R., Ros E. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern. Med. 2015;175:1094–1103. doi: 10.1001/JAMAINTERNMED.2015.1668. [DOI] [PubMed] [Google Scholar]
- 36.van Charante E.P.M., Richard E., Eurelings L.S., van Dalen J.W., Ligthart S.A., van Bussel E.F., Hoevenaar-Blom M.P., Vermeulen M., van Gool W.A. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 37.Williams, J.W., Brenda Plassman, M.L., Burke, J., Holsinger, T., Benjamin, S., 2010. Evidence Report/Technology Assessment Preventing Alzheimer's Disease and Cognitive Decline. [PMC free article] [PubMed]
- 38.Williamson J.D., Pajewski N.M., Auchus A.P., Bryan R.N., Chelune G., Cheung A.K., Cleveland M.L., Coker L.H., Crowe M.G., Cushman W.C., Cutler J.A., Davatzikos C., Desiderio L., Erus G., Fine L.J., Gaussoin S.A., Harris D., Hsieh M.K., Johnson K.C., Kimmel P.L., Tamura M.K., Launer L.J., Lerner A.J., Lewis C.E., Martindale-Adams J., Moy C.S., Nasrallah I.M., Nichols L.O., Oparil S., Ogrocki P.K., Rahman M., Rapp S.R., Reboussin D.M., Rocco M.V., Sachs B.C., Sink K.M., Still C.H., Supiano M.A., Snyder J.K., Wadley V.G., Walker J., Weiner D.E., Whelton P.K., Wilson V.M., Woolard N., Wright J.T., Wright C.B. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: a Randomized Clinical Trial. JAMA - J. Am. Med. Assoc. Am. Med. Assoc. 2019:553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson R.S., Segawa E., Boyle P.A., Anagnos S.E., Hizel L.P., Bennett D.A. The natural history of cognitive decline in Alzheimer's disease. Psychol. Aging. 2012;27:1008–1017. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization and Alzheimer's disease International . World Heal. Organ.; 2012. DEMENTIA A public Helath Priority; pp. 1–4. [Google Scholar]
- 41.Xu W., Tan C.C., Zou J.J., Cao X.P., Tan L. Original research: sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2020;91:236. doi: 10.1136/JNNP-2019-321896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu W., Tan Lan, Wang H.F., Jiang T., Tan M.S., Tan Lin, Zhao Q.F., Li J.Q., Wang J., Yu J.T. Meta-analysis of modifiable risk factors for Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2015;86:1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 43.Xu Z., Zhang D., Lee A.T.C., Sit R.W.S., Wong C., Lee E.K.P., Yip B.H.K., Tiu J.Y.S., Lam L.C.W., Wong S.Y.S. A pilot feasibility randomized controlled trial on combining mind-body physical exercise, cognitive training, and nurse-led risk factor modification to reduce cognitive decline among older adults with mild cognitive impairment in primary care. PeerJ. 2020;8 doi: 10.7717/peerj.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu J.T., Xu W., Tan C.C., Andrieu S., Suckling J., Evangelou E., Pan A., Zhang C., Jia J., Feng L., Kua E.H., Wang Y.J., Wang H.F., Tan M.S., Li J.Q., Hou X.H., Wan Y., Tan Lin, Mok V., Tan Lan, Dong Q., Touchon J., Gauthier S., Aisen P.S., Vellas B. Evidence-based prevention of Alzheimer's disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry. 2020;91:1201–1209. doi: 10.1136/jnnp-2019-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng G., Xia R., Zhou W., Tao J., Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016;50:1443–1450. doi: 10.1136/bjsports-2015-095699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.