Abstract
Blood–brain barrier (BBB) interface with multicellular structure controls strictly the entry of varied circulating macromolecules from the blood-facing surface into the brain parenchyma. Under several pathological conditions within the central nervous system, the integrity of the BBB interface is disrupted due to the abnormal crosstalk between the cellular constituents and the recruitment of inflammatory cells. Exosomes (Exos) are nano-sized extracellular vesicles with diverse therapeutic outcomes. These particles transfer a plethora of signaling molecules with the potential to modulate target cell behavior in a paracrine manner. Here, in the current review article, the therapeutic properties of Exos and their potential in the alleviation of compromised BBB structure were discussed.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01142-z.
Keywords: Blood–brain-barrier, Exosomes, Therapeutic outcomes, Integrity, Neuroangiogenesis outcomes
Background
The central nervous system (CNS) is a vascularized system with a close relationship between vascular structure and neurons, indicating the importance of neuro-angiogenesis in the functionality of the CNS [1]. In this regard, a selective border, namely, the blood–brain barrier (BBB), consists of numerous multicellular structures that block the flow of bulky chemicals and immune cells from the circulatory system to the brain [2]. The BBB integrates spatially several cell types to generate functional neurovascular units [1]. Microvascular endothelial cells (ECs) with basal membrane cover the luminal surface while pericytes and astrocytes wrap the abluminal surface [3]. It is suggested that neuronal terminals, as well as microglial cells and localized macrophages, can be juxtaposed with the BBB structure [4]. In the BBB structure, ECs, pericytes, and end-foot astrocytes collaborate to create a natural barrier that limits blood cells traveling to the brain parenchyma [5]. The physiological properties of BBB also regulate the transit of nutrients, chemicals, and medicines from blood to the brain [6]. The phenomenon of transcellular mechanisms or transcytosis mainly relies on the selective interaction of ligands with specific receptors on the luminal surface of endothelial layers [7]. Besides, intracellular metabolic activity can help the inward transport of varied compounds for delivery into the brain parenchyma. BBB not only protects neural tissue from harmful compounds and pollutants in the blood but also constricts the crossing route of therapeutics [8]. Compared to the other tissues, the transfer of varied compounds, especially hydrophilic molecules, is limited through the BBB wall because of the existence of tight junctions between the ECs. The junctional adhesion molecules (JAMs) tightly link the ECs [9]. Productive proteins at tight junctions (TJs) that actively participate in biological interaction include claudins, occludin, and JAMs. Several cytoplasmic factors connect the transmembrane adhesion compounds to the cytoskeleton [10]. TJs are connected to the actin/vinculin-based cytoskeleton and engage with basal endothelium (Fig. 1) [11]. It was thought that BBB is disturbed by conditions like ischemia and other neurological illnesses such as myelin deficiency [12]. These characteristics have the potential to trigger an inflammatory process and oligodendrocyte disruption by causing the continual movement of immune cells from the circulation into the CNS area [13]. Under pathological conditions, microglia can secrete reactive oxygen species (ROS) in response to brain damage or immunological activation, resulting in the disruption of BBB and leakage of plasma into the CNS parenchyma (Fig. 1) [14]. At the BBB level, neurons can interact with astrocytes to control the circulation rate [8]. CNS equilibrium and performance are crucially regulated by the astrocyte's function. Reactive astrocytes are generated in response to CNS damage. Astrocytes can polarize into the neurotoxic or pro-inflammatory phenotype (A1) and the neuroprotective or anti-inflammatory phenotype (A2) [12]. Even though, the straightforward distinction of the A1/A2 phenotypes does not accurately represent the full spectrum of astrocytic phenotypes, it helps us comprehend how astrocytes behave in different CNS illnesses [15]. To this end, specific transport systems have been developed in the BBB interface to regulate the inward and outward movement of molecules between the blood and brain parenchyma. It has been indicated that both paracellular and especially transcellular mechanisms actively participate in the transfer of molecules via BBB interface [1]. The occurrence of neurological pathologies such as multiple sclerosis, encephalitis, Alzheimer’s disease, and ischemic stroke can affect the continuity of the endothelial barrier, leading to several neurological abnormalities [16]. Some parts of the brain harbor the neurovascular units because of the necessity for the perception of fluctuations within the blood and subsequent feedback [17]. Commensurate with these findings, maintenance of cross-talk between the cellular components of BBB under pathological conditions is mandatory to preserve its selective barrier capacity and prevent several neurological deficiencies.
Fig. 1.
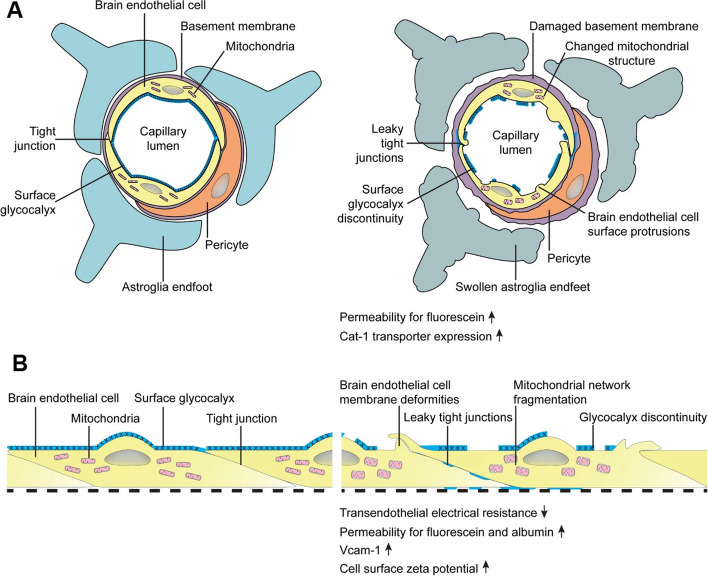
The structure of BBB in in vivo A and in vitro B conditions. Brain ECs covers the luminal surface of the BBB interface and are in close contact with blood components. ECs are juxtaposed tightly via the activity of tight junctions, resulting in the formation of a selective barrier interface. ECs are laid on the basal membrane layer which is wrapped by pericytes. Pericytes are sandwiched between ECs at the luminal surface and astrocyte endfeet at the abluminal face. Under pathological conditions, the continuity of the BBB interface is disrupted, leading to the loosening of EC‒to‒EC and EC‒to‒pericyte connections. The production of glycocalyx is not normal on the luminal surface and ECs exhibit several protrusions. Astrocyte endfeet are swollen and lost integrity with pericytes. Along with these changes, the function of several transporters such as cationic amino acid transporter-1 (Cat-1), etc. is disrupted. The increase of adhesion molecules such as VCAM-1 increases the probability of immune cell recruitment and entry via the BBB. The up-regulation of inflammatory cytokines contributes to the abnormal function of tight junction molecules and BBB leakage. Reprinted with permission [18], Copyright 2022. Fluids and Barriers of the CNS
Exo biogenesis
Among different subclasses of extracellular vesicles (EVs), Exos with an average size of 40–150 nm are secreted into the extracellular matrix (ECM) and actively participate in paracrine interaction between the cells [19, 20]. These nano-sized particles originated from an endosomal system and are involved in varied cellular functions [21]. From a morphological aspect, Exos exhibit a relatively spherical appearance with a lipid bilayer membrane [21, 22]. Molecular investigations have revealed that fundamental components are attached to the lipid membrane in the process of Exo biogenesis inside the cells. For instance, flotillin, several tetraspanin types (CD63, CD9, CD81, and CD82), Alix, tumor susceptibility gene 101 (TSG101), membrane fusion proteins such as RABs, and ARF along with different types of HSPs (HSP60, 70, and 90) can be detected in the abluminal surface of Exo membrane (Fig. 2) [23, 24] Besides, lipid compounds such as ganglioside GM3, cholesterol, sphingomyelin, and ceramides decorate the Exo surface [25]. The existence of these compounds makes the Exo natural shuttles carry the biomolecules between the cells in a paracrine manner [26–29]. Within the cytosol, the procedure of Exo biogenesis is initiated with the early endosome formation. In the latter steps, early endosomes can mature into late endosomes and multivesicular bodies (MVBs) where the numerous intraluminal vesicles (ILVs) are generated via the invagination of the membrane into the lumen. Following the secretion into the ECM, ILVs are known hereafter as Exos [30, 31]. It has been indicated that several types of molecular machinery can expedite the phenomenon of Exo biogenesis by using the endosomal system. Among these effectors, ESCRT machinery is composed of four complexes (ESCRT-0, I, II, and III) and the assistant factor namely AAA ATPase Vps4 has a crucial role in Exo biogenesis. Along with these effectors, ALIX, TSG101, and HRS are touted as ESCRT subunits [32]. ALIX collaborates with the ESCRT-III complex and interacts with the ATG12–ATG3 compound to regulate Exo synthesis. MVB morphology and trafficking can be changed by the inhibition of the ATG12–ATG3 complex [33]. In the ESCRT-dependent pathway, ESCRT-0, I, and II with several ubiquitin-binding domains partake in cargo sorting into the ILVs while the activity of ESCRT-III regulates membrane flexibility, the fission process, and ILVs formation in the lumen of MVBs [34]. It was suggested that the activity of ESCRT-III can be regulated by AAA ATPase Vps4. Unlike ESCRT-dependent mechanisms, tetraspanins such as CD63 and lipids (ceramides) are ESCRT-independent effectors can accelerate the invagination of MVBs membrane and ILV formation [35, 36]. Upon the formation of MVBs, these vesicles can be designated for degradation and fusion with the plasma membrane to release Exos [29]. For lysosomal degradation, the activity of Rab7 is critical while this factor can orchestrate the formation of autophagolysosomes after the activation of autophagic response [31, 36]. To be specific, the Rab-GTPases family including several Rab types are critical components of intracellular trafficking machinery [37]. For instance, Rabs such as Rab35, 11, and 27 in collaboration with SNARE proteins can accelerate the fusion of MVBs with the plasma membrane and Exo release (Fig. 2) [38]. Exo can penetrate target cells by engaging several mechanisms. In direct fusion, the exosomal membrane is merged with the host cell plasma membrane. Receptor-ligand interaction is another strategy that Exo use for internalization. Along with these mechanisms, unspecific phenomena such as clathrin-dependent endocytosis macropinocytosis, micropinocytosis, and lipid raft-dependent have been also detected in terms of Exo internalization [31, 39].
Fig. 2.
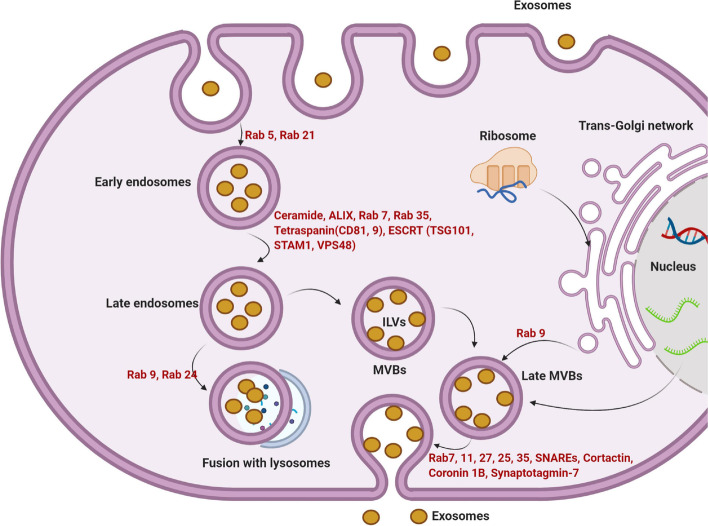
The molecular machinery of Exo biogenesis. Newly generated early endosomes are produced via cell membrane invagination to support the intracellular transmission of uptaken Exos. In the next step, early endosomes mature into later Exos and eventually MVBs. Along with these changes, the invagination of the endosomal membrane into the lumen leads to the formation of several ILVs, known as Exos after secretion into the ECM. The participation of ESCRT independent mechanisms and/or ESCRT complex (ESCRT-0, -I, -II, and -III) with several tetraspanin types is mandatory for the sequestration of new cargo into the ILVs. By engaging the SNARE system and several GTPases, MVBs are directly fused with the cell membrane to release their contents. Exos have nano-sized bi-lipid layer structures with specific molecular identities. ESCRT: endosomal sorting complex transport; MHC: major histocompatibility complex; MVB: multi-vesicular body; Rab: Ras-associated binding proteins. Reprinted with permission [29], Copyright 2021. Frontiers in Cell and Developmental Biology
Treatment of neurological diseases has a significant hurdle in the effective transport of macromolecular therapeutic medicines across the BBB to the CNS [40]. Exos have been shown to penetrate the BBB with the help of ECs (Fig. 3). These particles are natural nanomaterials with properties to carry specific genetic and proteomic cargoes to the brain. Besides, the inherent macromolecular transport and targeting characteristics of Exos have been clearly described. Because a load of several compounds is applicable in the Exo surface and lumen, this approach paves the way to cure a variety of neurological diseases by systemic medication transport all over the BBB [41]. It has been shown that bEnd.3 brain endothelial progenitor cells can release Lipocalin-2-loaded Exos from principal brain tumors [42]. Exos also improve bEnd.3 cells membrane permeability in an LCN2-dependent manner via the modulation of the JAK-STAT3 signaling axis. The suppression of LCN2 led to the inhibition of Exo therapeutic effects on BBB ECs. Of note, Exos can act as natural bioshuttles to carry different signaling molecules or regulate the transit of several components through the BBB structure. For instance, it was suggested that the delivery of nanocapsules composed of 2-methacryloyloxyethyl phosphorylcholine, monoclonal antibody, and cross-linker from BBB is closely associated with the activity of released Exos from brain glioma tumor cells. To be specific, the inhibition of Exo using an Akt inhibitor release from tumor mass can efficiently decrease the BBB entry of nanocapsules [43]. Whether cancer Exos can regulate the entry of blood components through BBB should be addressed.
Fig. 3.
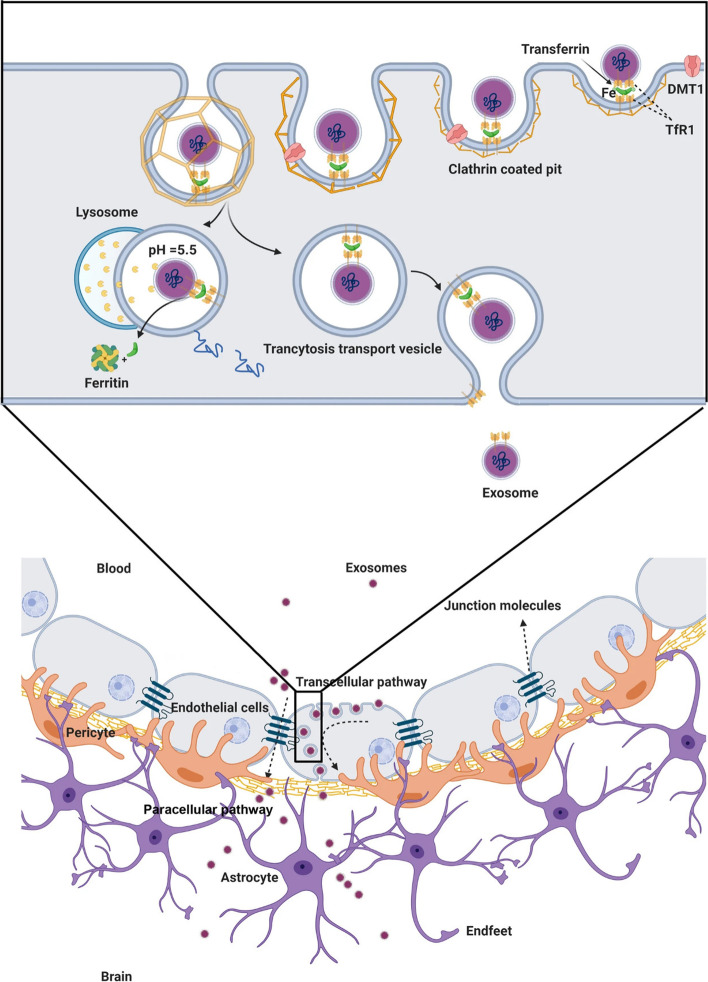
Exos can cross the BBB interface using paracellular and transcellular pathways. Due to the existence of transferrin on the Exo surface, these nanoparticles can attach to the transferrin receptor (TFR) at the luminal surface of brain ECs, leading to the formation of clathrin-coated pits and Exo internalization. The internalized Exos are direct to lysosomal degradation where the released cargo can regulate specific signaling pathways inside the ECs or cross the endothelial barrier and exist through the abluminal surface. After the production of inflammatory cytokines and certain physiological conditions, Exos can cross the BBB via a paracellular route where the function of tight junctions is not abnormal. Reprinted with permission [1], Copyright 2021. Cell & Bioscience
Exo isolation and enrichment methods
To date, several isolation technologies have been developed for the isolation and enrichment of Exo for biofluids [44]. Because Exo are small-sized particles (40–150 nm) with low density (1.13–1.19 g/ml), the existence of cell fragments and varied protein types may affect the purification of Exo [45]. At present, ultracentrifugation, ultrafiltration, immunoaffinity-based isolation, size exclusion chromatography (SEC), and polymer precipitation are the most common Exo isolation methods in in vitro and in vivo experiments [44]. Near half of the experiments associated with Exo are done via ultracentrifugation methods. This procedure consists of preliminary centrifugation steps at lower speeds, 300, 2000, and 10,000 g to eliminate cell debris and fragments which follows by centrifugations at higher speeds (100,000–120,000 g) for 1–2 h. It seems that the existence of subcellular organelles, infectious viruses, and protein aggregates can reduce the purity of isolated Exo. Besides, Exo isolated by high-speed centrifugation steps exhibited some morphological abnormalities [46].
In ultrafiltration methods, Exo are enriched after passing through filtering membranes with certain pore sizes. The ultrafiltration process is done via engaging forces such as pressure, centrifugation, and electric stimulation [47]. It is thought that Exo and particles smaller than Exo can pass the membranes with permeate. Morphological studies have shown Exo deformation isolated by the ultrafiltration method. Because of Exo absorbance within the membranes, the total number of isolated Exo is less which difficult the application of this method in small volume samples [48].
In the SEC method, biofluids are passed through columns with porous beads and small-sized particles can enter the pores with lower elution speed. Based on the previous data, SEC can yield Exo with high-rate purity like the ultrafiltration technique [49]. The isolation of Exo via immunoaffinity is conducted based on the physical interaction of exosomal antigens (tetraspanins, etc.) with antibodies. For this purpose, antibodies are conjugated with magnetic beads, and chromatography substrates [50]. This technique helps the researchers to fraction the specific types of Exo, i.e. cancer Exo from normal counterparts, based on specific exosomal surface antigens [51]. It was suggested that price, genetic instability, and lack of homogeneity after antibody functionalization limit the bulk application of immunoaffinity-based technologies for Exo isolation [52]. The application of a single antibody for Exo isolation can increase the enrichment of the target Exo population but reduce the minimum number of Exo required for biological analyses [51].
Using several polymers such as polyethylene, acetate, protamine, etc. Exo can be precipitated in low centrifugation rates for large sample sizes [53]. In recent years, several isolation kits have been developed for Exo enrichment from biofluids. For instance, “Exosome Isolation kit”, “Eloquence”, and “Exo-spin” are the most common available kits. Despite the ease of use, the Exo values such as purity, number, and size distribution are so variable [54]. Along with approaches, other modalities such as membrane-based separation, microfluidics, and other modalities can be used for the isolation of Exo [55].
Exo and BBB crossing
The existence of an impenetrable BBB interface limits the exchanges of compounds (400–600 Da <) between the blood and cerebrospinal fluid, resulting in the maintenance of neuronal homeostasis in CNS [56]. In this regard, several molecular mechanisms are engaged to orchestrate the BBB crossing of varied molecules [57]. Several mechanisms such as direct fusion, clathrin- and caveolin-mediated endocytosis, ligand-receptor interaction, macropinocytosis, and phagocytosis are involved in the entry of Exo into the brain parenchyma [58, 59]. Which mechanisms are dominant Exo entry routes under physiological and pathological conditions remained unknown. In an experiment, it was shown that bovine milk eGFP+ EVs can be easily uptaken by mouse bEnd.3 cells in an in vitro chamber model and reached BV2 microglia on the bottom surface [57]. Brain microvascular ECs are located at the blood side of the BBB structure and are in close contact with circulating Exos [60]. It has been indicated that Exo can release their content into EC cytosol after direct fusion [61]. It is possible that entering Exo are directed to lysosomal degradation and/or early and late endosomes [62]. Besides, Exo can be transferred to the luminal surface of brain ECs [63]. Of note, the Exo subpopulation less than 50 nm in diameter, known also as exomers, can reach the EC abluminal side compared to large-size Exo (50–150 nm) [64]. Compared to paracytosis, it seems that transcytosis is the main route of Exo entry to brain ECs. In an in vitro Transwell Insert assay, treatment of ECs with an endocytosis inhibitor namely Dynasore prohibited the number of labeled Exo at the abluminal surface, indicating the importance of endocytosis in BBB crossing of circulating Exo [59].
Several data have indicated the existence of clathrin pits on the luminal surface of brain EC which are involved in clathrin-based transcytosis [65]. Upon the attachment of Exo ligands to cognate receptors, clathrin vesicles containing both receptors and ligands are generated and can deliver their contents at the abluminal surface [66]. For example, exosomal tetraspanins can interact directly with surface integrins such as integrin α4 [67]. It was indicated that the interaction of macrophage Exo LFA-1 with endothelial ICAM-1 promotes Exo diapedesis across the BBB [68]. Other receptors such as the transferrin receptor (TfR) are also involved in the delivery of Exo into brain microvascular ECs and BBB crossing via the dynamic endocytosis cycle [69]. These data indicate that multiple molecular mechanisms may participate in the BBB crossing of Exo. Further investigations are highly recommended to address the exact role of each entry route under physiological and pathological conditions.
Anti/inflammatory properties of Exos
Inflammation is a natural biological process that occurs in response to several pathological conditions to reestablish tissue homeostasis (Table 1) [70]. Neuroinflammation is a common hallmark reported under neurodegenerative conditions such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), etc. [71]. Under inflammatory conditions, the stimulation of glial cells can lead to the local or extensive elevation of pro-inflammatory cytokines within the brain parenchyma. If the host tissue is not able to remove the stimuli or the inflammatory response continues over long periods, uncontrolled inflammatory reactions cause injuries [72].
Table 1.
The role of inflammatory factors in neurodegenerative diseases
| Neurodegenerative diseases | Neuronal cargo | Activity | Inflammatory factors | Ref |
|---|---|---|---|---|
| Alzheimer's disease (AD) | neurofibrillary tangles (NFT) and amyloid-beta peptide (Aβ) | Aggregates of hyperphosphorylated tau lead to ROS production and inflammatory responses | IL-1, IL-6, TNF-α, and PGE2 | [73] |
| Parkinson’s disease (PD) | alpha-synuclein (α-syn) leads to the loss of dopaminergic neurons | Control of neurotransmitter release, through effects on the SNARE complex | C-reactive protein (CRP), IL-6, and TNF-α | [74] |
| Amyotrophic lateral sclerosis | p75ECD phosphorylated neurofilament heavy (pNfH) neurofilament light (NfL) | Involved in neuroprotective actions against Aβ toxicity | CRP, IL-6, IL-8, TNF-α, IL-1β, IL-17, IL-33, IL-10, Monocyte chemoattractant protein 1 (MCP-1), and IFNγ | [75] |
| Huntington's disease | rab11 activity | Affects the recycling of transferrin receptor and neuronal glutamate/cysteine transporter EAAC1 | IL-1, IL-6, TNF-α, TGF-β | [76] |
| Lewy body disease | alpha-synuclein | localizes specifically to the nerve terminal and inhibits neurotransmitter release when over-expressed | IL-1β, TNF-α, IL-6, and IL-10 | [77] |
| Multiple sclerosis (MS) | neutrophil-to-lymphocyte ratio | oligodendrocyte damage and demyelination | IL-6, IFN-γ, TNF-α, and granulocyte–macrophage colony-stimulating factor (GM-CSF) | [78] |
Exos are practically able to transport biomolecules and signaling payloads to different targeting cells [28]. Unlike synthetic nanoparticles, Exos are relatively stable vesicles without toxicity and can cross several biological barriers such as BBB [1, 26]. Due to their potential to carry several biomarkers associated with diseases such as AD [β-amyloid (Aβ), tau] and Parkinson's disease, (α-synuclein), etc., Exos are considered important biological elements for early-stage detection and monitoring of pathological conditions [79]. Notably, microglia-related Exos harboring tau and Aβ can transmit pro-inflammatory signals to remote sites [80]. Several aspects uncovered that Exos can transfer specific biomolecules which are identical to certain pathological conditions [22]. Molecular identification of exosomal cargo has revealed the existence of inflammatory modulators such as inflammatory miRNAs and varied relevant proteins with the potential to act in close and remote sites [81]. In an experiment conducted by Fitzpatrick et al., it was indicated that Exos isolated from Staphylococcus aureus exposed human aortic ECs increased the expression of CD11b and MHCII in THP-1 monocytes [82]. Based on the data, exosomal transfer of miR-99a and miR-99b inhibits the mTOR activity in monocytes, resulting in increased production of IL-6, and reduction of IL-10 (Fig. 4) [82]. These findings are consistent with the fact that direct exposure of BBB ECs to sepsis and inflammatory conditions can contribute to the release of Exos with certain signaling molecules that recruit immune cells and affect the integrity of the BBB interface. To be specific, the exposure of brain ECs to infectious agents and noxious compounds supports the production and release of certain Exo types that lead to the worsening of pathological conditions. Of note, the occurrence of adaptive immune system response with regulated inflammatory cascades can alleviate brain injuries [83]. Within the CNS, the transfer of α-synuclein, Aβ, and prions account for the distribution of these biomarkers between the cells, resulting in the dissemination of inflammatory cascades [84]. Ngolab and co-workers indicated that the injection of Exos from Dementia with Lewy Bodies patients into the murine brain parenchyma promotes α-synuclein aggregation in MAP2+, Rab5+ neurons (Fig. 5) [85]. Thus, one could hypothesize that Exo functions as disease-promoting or restraining modulators, and these features are closely associated with the entity of exosomal cargo and cell state [84]. Besides, examining the content of Exos in several CNS injuries with different severity of cell damages can help us to predict the side effects or protective properties of these particles. It has been shown that exosomal miR-124-3p can lead to the suppression of inflammatory response within the nervous system and induction of neurite formation. From the molecular aspect, this miRNA regulates M1 inflammatory cytokines via engaging the PI3K/Akt/NF-κB axis [86]. Besides, miR-124-3p protects brain microvascular cells via the promotion of autophagy [87]. Therefore, the induction of autophagy by Exos is touted as an alternative supporting mechanism for cell survival during pathological conditions [29]. Due to the existence of heterogeneous Exos in the circulation system, it is postulated the direct contact of these particles in the bloody face of BBB can regulate the EC activity via the modulation of distinct signaling pathways [88]. In support of this notion, the injection of healthy rat serum Exos diminished the infarct volume via the reduction of apoptotic changes in BBB ECs in rats with ischemic stroke [88]. Protein levels of ZO-1 and Claudin-5 were induced and coincided with the reduction of MMP-9 and Evans blue leakage to the affected sites, highlighting the restoration of BBB continuity. Consistent with these data, the excessive autophagic response was indicated by reduced LC3-II/LC3-I ratio and autophagosome number in bEnd.3 cells after being exposed to oxygen–glucose deprivation [88]. The inhibition of autophagic response using chemical compounds like 3-methyladenine blunted uncontrolled autophagic activity and degradation of occludins in a rat model of stroke [89]. On contrary, Yang and co-workers indicated that the stimulation of autophagy in zebrafish can restore the function of BBB ECs via recycling aggregated claudin 5 distributed by caveolin 1 related mechanisms [90]. Whether the activation or inhibition of autophagy can restore the function of BBB and ECs needs further investigations. Any interpretations should be in accordance with the stage of pathological conditions and the intensity of autophagy response [91]. It confirmed that the source of Exos from different cellular source after the promotion of brain injury is integral to BBB function and continuity [92]. In addition to the effect of glia Exos on brain ECs, emerging data have pointed to the fact that EC Exos can exert neuroprotective effects on injured neurons and other cell types [93]. In an in vitro co-culture system using human umbilical cord ECs and primary mouse hippocampal neurons, the apoptotic changes were diminished in neurons exposed to hypoxic and hypoglycemic conditions. Data indicated that hypoxic neurons can uptake EC Exos via caveolin-1 based endocytosis, leading to the alteration of miR-1290. The inhibition of Exo secretion via chemical inhibitor namely GW4869 from ECs led to the promotion of apoptosis rate in injured neurons [93]. These data indicate the crucial role of brain microvascular ECs in the neuroprotection in a paracrine manner. Whether the release of Exos by ECs precedes the brain injury or is done in response to the signaling molecules should be addressed. The activation of Exo production is possibly a compensatory mechanism to prevent the extension of pathological conditions to other brain sites like BBB interface and to normalize the excessive behavior of glial cells after being exposed to injured neurons. Interestingly, EC can shed Exos with a pro-neural factor namely Ascl1 under the ischemic stroke. The entry of these Exos in astrocytes can intensify astrocyte trans-differentiation into neural progenitors (Fig. 6) [94]. These features show the vascular cell-based regulation of neuroplasticity via the secretion of Exos under pathological conditions. This phenomenon occurred at the levels of certain factors transferred by Exos inside the astrocytes in a paracrine manner.
Fig. 4.
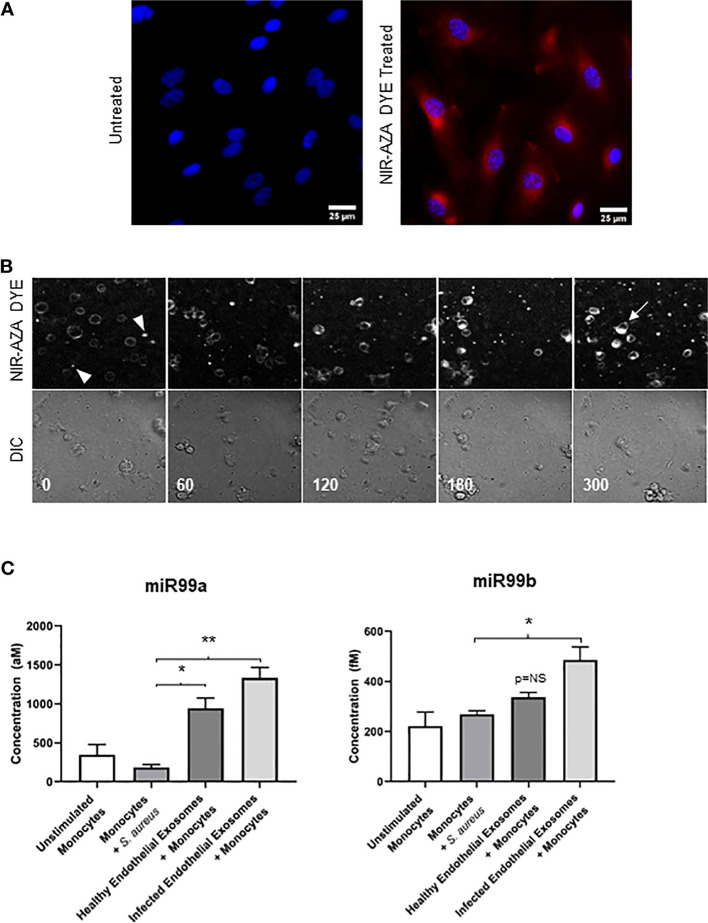
Effect of inflamed EC Exos on THP-1 monocytes A-C. Human aortic ECs were incubated with Near-Infrared BF2-azadipyrromethene (NIR-AZA) dye (red). Nuclei were stained with DAPI A. THP-1 monocytes were incubated with Exos isolated from normal and inflamed ECs inside the ibidi chambers and imaged every 5 min over 16 h [time-lapse across 300 min] B [top panel: Cy5 channel, bottom panel: DIC channel]. At time 0, no detectable fluorescence signals can be achieved indicating the lack of interaction between Exos and monocytes (arrowheads). The intensity of fluorescence signals reached the maximum levels after 300 min. The existing materials inside the cells indicate the successful uptake of Exos (arrow). Real-time PCR analysis C. The direct exposure of monocytes to Staphylococcus aureus did not yield statistically significant differences in terms of miR-99a/b. The incubation of monocytes with normal Exos up-regulated the expression of miR-99a as compared to the non-treated monocytes. Inflamed Exos isolated from ECs contributed to the higher levels of miR-99a and miR-99b in monocytes. These data indicate the uptake of introduced Exos by monocytes (n = 3); One-Way ANOVA; *p < 0.05, **p < 0.01; NS: not significant. Reprinted with permission [82], Copyright 2022. Frontiers in Cellular and Infection Microbiology
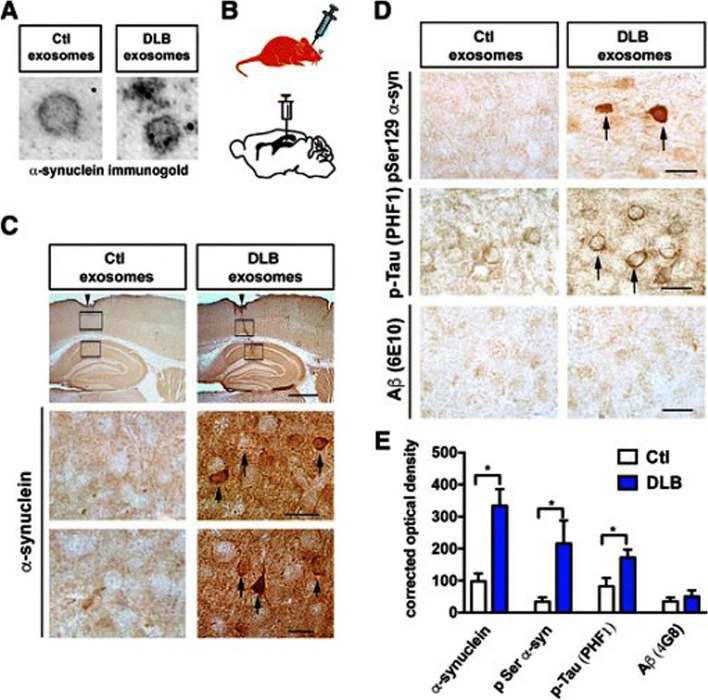
Fig. 5.
Monitoring the effect of dementia with Lewy bodies patient Exos on intracellular accumulation of phosphorylated proteins. Detection of exosomal α-synuclein using immuno-gold labeling and TEM images (Ctl: Control; DLB: dementia with Lewy bodies patient) A. Stereotaxic injection of prepared Exos into C57BL/6 N × DBA/2 F1 mouse hippocampus B. Bright-field imaging of brain slides after injection of Exos C [row 1: Sagittal view of the hippocampus. The site of needle injection is highlighted by arrowheads (Scale bar: 150 μm). Arrows indicate immuno-labeled cell bodies (Scale bar: 25 μm). Brain samples were stained using anti- phosphorylated α-synuclein (pSer129), PHF1 and Aβ (6E10) [(Scale bar: 25 μm]. Semi-quantification of immunohistochemical stains using an optical density. *p < 0.05. Reprinted with permission [85], Copyright 2017. Acta Neuropathologica Communications
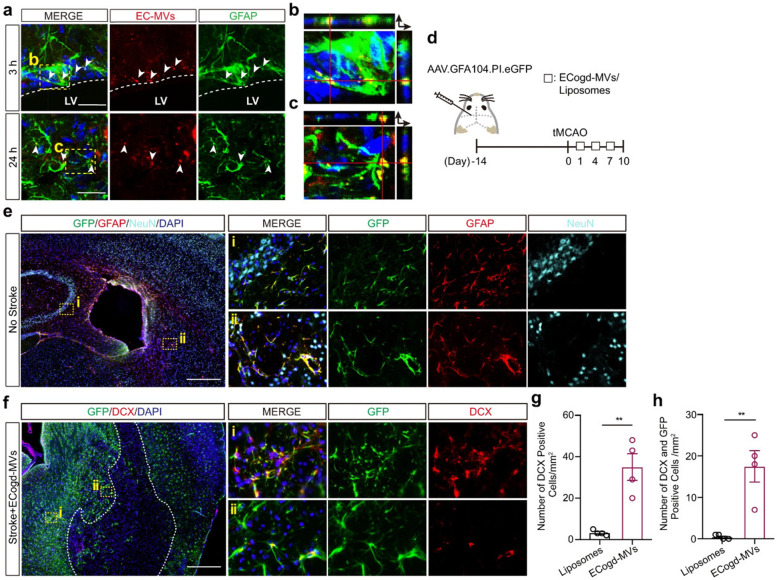
Fig. 6.
Hypoxia and hypoglycemia-treated ECs produce microvesicles (EC-MVs) with the potential to induce astrocyte differentiation toward neural progenitor cells in a mouse model of ischemia (a-h). Confocal images revealed the successful entry of red color Cy3-labeled EC microvesicles (EC-MVs) by astrocytes after 3 (a, b; Scale bar: 25 μm) and 24 h (a, c; Scale bar: 25 μm) after injection into the lateral ventricle (White color dash lines: lateral ventricle border). Schematic of experimental procedure (d). The expression of GFP, GFAP, and NeuN 14 days after administration of AAV.GFA104.PI.eGFP vector (e; Scale bar: 200 μm). In panel f, GFP and DCX positive cells were monitored on day 10 with transient MCAO (Scale bar: 200 μm). The number of DCX+ neurons/mm2 was measured in the peri-infarct zone (g) ROIs were calculated per each Sect. (3 sections per animal; each in 4 mice; p = 0.0026). DCX+/GFP+ cells per mm2 of in peri-infarct zone (3 sections per animal; each in 4 mice; p = 0.0043) (h). Three (a) and four (e, f) sets of experiments were performed in this study. Abbreviation: Doublecortin: DCX; Glial fibrillary acidic protein: GFAP; Green fluorescent protein: GFP; Neuronal nuclei: NeuN; Regions of Interests; ROIs. Reprinted with permission, [94] Copyright 2022. Nature Communications
The source and metabolic status of parent glial cells are important in the production of Exos affecting the BBB interface [95, 96]. For instance, recent works have indicated that inflamed astrocyte-derived Exos harbor large amounts of IL-1β and HMGB1 with the potential to induce an inflammatory response in the target cells [49, 97]. In support of this statement, the incubation of human brain ECs with AD mouse astrocyte EVs led to the reduction of electrical resistance, and down-regulation of tight junction proteins such as claudin-5, occludin, and ZO-1 when compared to the healthy astrocytes [98, 99]. Commensurate with these comments, the differential contents of Exos from varied cell types may contribute to the suppression and/or induction of inflammation in vascular cells within the BBB structure during pathological conditions [22].
Angiogenic and arteriogenic properties of Exos
The formation of de novo blood vessels is defined as angiogenesis or neo-angiogenesis [100]. The lack of blood nourishment can contribute to ischemic changes. Therefore, the promotion of angiogenesis seems critical issue following ischemic stroke [100]. Several studies have proved the eligibility of Exos in the promotion and inhibition of angiogenesis in either in vitro or in vivo conditions (Table 2) [101, 102]. It has been indicated that stem cells and mature cell types can release angiocrine via the secretion of Exos [103–106]. Exos may transport a wide range of macromolecules from origin cells to acceptor cells, including microRNAs, nucleotide sequences, proteins, lipids, and even psychoactive agents [107]. Exos can control the intracellular levels of angiogenesis-related RNAs and proteins after the induction of ischemic response. For example, it was suggested that exosomal cargo increases the expression of VEGF, leading to diminished infarct size [108]. Upon the attachment of VEGF to tyrosine kinase VEGFR-2, the formation of new blood and lymphatic vessels is initiated [109]. Along with these changes, the endothelial basal membrane is disrupted by the activity of MMP-2 and -9 to accelerate ECs migration in the direction of cytokines and proangiogenic factors [110]. The promotion of angiogenesis coincides with the induction of permeability in the endothelial layer [111]. In an experiment conducted by Liu and co-workers, they proved that brain EC monolayer integrity was restored in 5 × FAD mouse (animal model applicable to AD) after exposure to neural stem cell Exo in a Transwell insert system [112]. Yet, the influence of adaptive and excessive angiogenesis during pathological conditions in terms of BBB continuity remains unknown. Irrespective of being adaptive or pathological angiogenesis response, the local or systemic elevation of angiogenic factors can disrupt the EC‒to-EC integrity because of the production of pro-angiogenesis factors like VEGF, etc. [113]. To be specific, the tightly regulation of pro-angiogenic factors at early stages after angiogenesis initiation is mandatory to control BBB leakage and unwanted outcome in response to varied pathological conditions [113]. Higher VEGF levels in ischemic conditions led to the destruction of basal lamina (IV type collagen), activation of MMP-2, and -9, and abnormal BBB leakage [113]. As a correlate, the angiogenic properties of Exos in the alleviation of compromised BBB structure should be interpreted logically. Following ischemic stroke, the elevation of miRNA212 and miRNA132 in brain ECs and exosomal levels of these miRNAs blunts the angiogenic levels and reduces the barrier continuity via the suppression of claudin-1, junctional adhesion molecule 3, and tight junction-associated protein 1 [114]. Noteworthy, it would be possible that the changes in Exo cargoes during pathological conditions within the brain parenchyma and other tissues. Therefore, it seems that the intensity and type of angiogenic response can be different in brain ECs after exposure to Exos from normal and injured or inflamed cells.
Table 2.
The role of Exo in the diagnosis, therapy, and function in angiogenesis
| Exosomal cargo | Mechanisms | Target cell | Function | References |
|---|---|---|---|---|
| Annexin A2 | Making of plasmin | Macrophages and ECs | Pro-angiogenesis | [115] |
| Angiopoietin-2 | Tie2-unrelated route | HUVECs | Pro-angiogenesis | [116] |
| Alcohol acetyltransferase II, Metastasis Associated 1, Seryl-TRNA Synthetase 1, Rho-associated coiled-coil containing protein kinase 1/2 | Increased VEGF and HIF-1 expression | HUVECs | Pro-angiogenesis | [117] |
| Carbonic anhydrase 9 | Enhancing the transcription of MMP-2 | HUVECs | Pro-angiogenesis | [118] |
| SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase, insulin-like growth factor 1 receptor, Focal adhesion kinase | VEGF overexpression | Endothelial cells | Pro-angiogenesis | [119] |
| The cluster of differentiation 147 | increase in VEGF and MMP | HUVECs | Pro-angiogenesis | [120] |
| Delta Like Canonical Notch Ligand 4 | Stopping notch indication | U373 and HUVECs | Pro-angiogenesis | [121] |
| EGF Like Repeats And Discoidin Domains 3 | encourage cell migration and expansion | Pro-angiogenesis | [122] | |
| Epidermal growth factor receptor III | By initiating the MAPK and Akt paths, the interpretation of the VEGF gene is increased | U373 and HUVECs | Pro-angiogenesis | [123] |
| Family With Sequence Similarity 225 Member A lncRNA | By disappointing its receptors NETO2 and FOXP1 as a result of miR-206 mooching, the PI3K/Akt/NF-B/Snail axis is activated | HUVECs | Pro-angiogenesis | [124] |
| High Mobility Group Box 3 | In vivo and in vitro, nEXOs that included HMGB3 sped up vasculature | HUVECs | Pro-angiogenesis | [125] |
| Influence of interleukin-8 | Regulation via MAPK | HUVECs | Pro-angiogenesis | [126] |
| Intercellular Adhesion Molecule 1, CD44v5 | p38 MAPK, RhoA/ROCK, ERK1/2 kinase, Src kinase, and eNOS | HUVECs | Pro-angiogenesis | [127] |
| lncRNA UCA1 | The mechanism for AMOTL2/ERK1/2 Trafficking | HUVECs | Pro-angiogenesis | [128] |
| IL-6, VEGF, and MMP-2 | Route for WNT5A signal | Endothelial cells | Pro-angiogenesis | [129] |
| IL-8, PDGF | PI3K/AKT Trafficking | Endothelial cells | Pro-angiogenesis | [130] |
| GM-CSF, HIF1α, HIF-2α | Increased VEGF transcription | ECs and M1/M2 macrophages | Pro-angiogenesis | [131] |
| miR-148a-3p | Constraining ERRFI1 to activate the EGFR/MAPK transcription factors | HUVECs | Pro-angiogenesis | [132] |
| miR-373 | β-catenin/ Wnt Trafficking | Endothelial cells | Pro-angiogenesis | [133] |
| miR-155 | VEGF-A expression is increased through the VHL/HIF-1 route | ARPE-19 | Pro-angiogenesis | [134] |
| miR-182-5p | concentrating on Elements 2 and 4 as Kruppel | HUVECs | Pro-angiogenesis | [135] |
| miR-9 | JAK-STAT mechanism | Endothelial cells | Pro-angiogenesis | [136] |
| miR-497 | VEGF and HIF-1 production are both downregulated | HUVECs | Anti-angiogenesis | [137] |
| miR-135b | Elimination of FIH-1 | Endothelial cells | Pro-angiogenesis | [138] |
| miR-141 | miR-141/KLF12 passageway in Exos | HUVECs | Pro-angiogenesis | [139] |
| miR-10b | reduction in the amount of HOXD10 and KLF4 proteins | HUVECs | Promotes cell invasion | [140] |
| miR-145 | STIM1 stimulates vasculature by inhibiting the IRS1-targeting exosomal miR-145 | HUVECs | Pro-angiogenesis | [141] |
| miR-21 | VEGF overexpression | HUVECs | Pro-angiogenesis | [142] |
| miR-27a | MiR-27a-loaded Exos from RCCC promote tumorigenesis and suppress SFRP1 transcription | HUVECs | Pro-angiogenesis | [143] |
| miR-92a-3p | KLF2 is the target of exosomal-released miR-92a-3p, which controls morphogenesis | HUVECs | Pro-angiogenesis | [144] |
| miR-122 | a reduction in PKM | Normal cells in the pre-metastatic niche | Promotes metastasis, before angiogenesis | [145] |
| miR-210 | VEGF overexpression | Endothelial cells | Pro-angiogenesis | [146] |
| miR-1290 | SMEK1 is targeted by miR-1290 to produce an angiogenesis phenotype | HUVECs | Pro-angiogenesis | [147] |
| miR-549a | Exosomal miR-549a inhibits HIF1 in HUVECs, which has an impact on the vasculature and endothelial mobility | HUVECs | Pro-angiogenesis | [148] |
| miR-21 | enhancing M2 polarity signal transcription in TAMs | CD14 + human monocytes | Pro-angiogenesis | [31] |
| M-phase-related transcripts | Stimulation of cellular proliferation and modification of the M-phase of the cell growth | Endothelial cells | Initiate angiogenesis | [36] |
| miR-23a | By attacking prolyl, exosomal miR-23a promoted vasculature and dilation of blood vessels. adhesion molecules protein ZO-1 and hydroxylase | HUVECs | Pro-angiogenesis | [149] |
| miR-181a | Exos released by the hypoxic PTC carried miR-181a, which inhibits DACT2 by decreasing the expression MLL3 and causes YAP-VEGF-mediated vasculature | HUVECs | Pro-angiogenesis | [150] |
| miR-221-3p | Exos produced from CC cells that contained miR-221-3p increased MVEC vasculature in CC via lowering MAPK10 | MVECs | Pro-angiogenesis | [31] |
| miR-210 | Diminishment of EFNA3 | Endothelial cells | Pro-angiogenesis | [151] |
| miR-130a | reduction of c-MYB | HUVECs | Pro-angiogenesis | [36] |
| miR-21 | Rho decreased the expression | HUVECs | Anti-angiogenesis | [152] |
| miR-141-3p | NF-B and JAK/STAT3 communication mechanisms activation | HUVECs | Pro-angiogenesis | [153] |
| Neuraminidase | Overexpression of MMP-9 and CXCR4 | 786–0 | Enhance migration and invasion | [154] |
| Neuraminidase | Src route | HUVECs | Pro-angiogenesis | [155] |
| Neuraminidase | VEGF production is upregulated, whereas hepaCAM transcription is downregulated | HUVECs | Pro-angiogenesis | [31] |
| PTCH 1, SMO, SHH, Ihh | Exos from CC cells stimulate pro-angiogenic responses in endothelium by upregulating Hh-GLI signaling and modifying target genes for angiogenesis upstream side | HUVECs | Pro-angiogenesis | [156] |
| Profilin 2 | In H446 and ECs, t PFN2 stimulated Smad2/3 and pERK | HUVECs | Pro-angiogenesis | [31] |
| PFKFB-3 | The development of lactose and Fru-2,6-P2 is rising | HUVECs | Pro-angiogenesis | [157] |
| RAMP2-AS1 lncRNA | By depressing miR-2355-target 5p's VEGFR2 by miR-2355-5p syphoning, angiogenesis cell membrane receptors are increased | HUVECs | Pro-angiogenesis | [31] |
| TIE2 | Exo-mediated induction of TIE2-expressing macrophages by TIE2-high cancer cells occurs | HUVECs | Pro-angiogenesis | [158] |
| TGF-β | Transmission reliant on SMAD | Fibroblasts | Pro-angiogenesis and pro-tumorigenesis | [159] |
| Tetraspanin Tspan8 (D6.1A) | MMP, VEGF, and VEGFR transcription is increased | Endothelial cells | Pro-angiogenesis | [160] |
| VEGF | uses its tyrosine kinase domains to effect action | HUVECs | Pro-angiogenesis | [31] |
| VEGF-A | The increased angiogenic capacity of brain ECs | Brain microvascular Endothelial cells | Pro-angiogenesis | [22] |
| Vasorin | encourage cell migration and expansion | HUVECs | Pro-angiogenesis | [161] |
| Wnt4 | β-catenin/ Wnt circuit | Endothelial cells | Pro-angiogenesis | [22] |
Among several cell types, the paracrine activity of stem cells has been documented in terms of angiogenesis and tissue healing [162]. Gao and co-workers indicated that injection of endothelial progenitor cell (EPC) Exos via the tail vein can restore the function of injured ECs in mouse traumatic brain injury [163]. Histological analysis revealed the successful uptake of PKH67-labeled Exos by brain ECs coincided with the reduction of brain edema. In the acceptor cells, the levels of PTEN and phosphorylated Akt were reduced with the increase of occludin and ZO-1 [163]. Because EPCs can be recruited into the brain after cerebrovascular damage, it is logical to mention that Exos from EPC sources are valid therapeutic tools to restore BBB integrity. Of note, progressive loss of homeostasis in EPCs can affect the therapeutic effects of ECs [164]. In a study, it was confirmed that pre-treatment with TNF-α can trigger apoptotic changes in EPCs. Incubation of brain ECs with Exos from apoptotic EPCs led to significant oxidative stress and expression of miR126 and eNOS compared to Exos isolated from starved EPCs [164]. Besides the direct effect of EPC Exos on brain ECs, astrocytes are other target cells with the ability to uptake regenerative Exos [165]. In in vivo conditions, ECs are frontline cells to uptake the EPC Exos while Exos can be also uptaken in a dose- and time-dependent manner by astrocytes at the abluminal surface after exposure to ischemic conditions, leading to the suppression of lipid peroxidation and oxidative stress [165]. Similar conditions were shown in ischemic mice after injection of MSC Exos [166]. It confirmed that intracellular levels of ROS were reduced in brain ECs and these effects were more evident when miR-132-3p-loaded Exos from the same cellular source was used [166]. It should not be forgotten that juxtaposed ECs can be valid cell sources to donate Exos to adjacent ECs to modulate cell behavior and activity [167]. The works have revealed that a large number of filamentous structures namely tunneling nanotubes constitute a scaffolding network at the apicollateral sides of brain ECs to transfer Exos (Fig. 7) [167]. These data showed that both endosomal systems along with tunneling nanotube networks are involved in the Exo donation between the brain ECs. Taken together, these data indicate the potential of Exos in the modulation of EC function through the BBB under physiological and pathological conditions. Yet, the mechanism of action and various effects of Exos from different sources should be addressed.
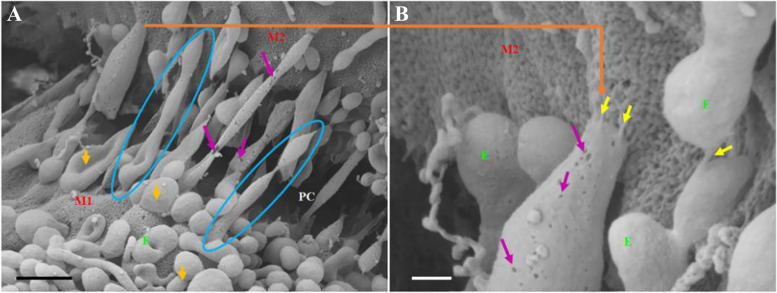
Fig. 7.
Formation of long tunneling nanotubes between brain ECs to transfer Exis. Exos can be transferred to adjacent ECs via the formation of long, hollow tubes (A; Scale bar: 1000 nm). Magnification of target area B. Tunneling nanotubes with sticky ends attach to juxtaposed cell surfaces (Scale bar: 200 nm). M1: propose membrane of cells with originating tunneling nanotubes; M2: target cell membrane interacted with tunneling nanotubes from other cells. PC: paracellular space between juxtaposed brain ECs. Blue circles: tunneling nanotube formation; Purple arrow indicates several pores on the membrane of tunneling nanotubes. Yellow arrows indicate the interaction between tunneling nanotubes and target BCs at the extremities. Reprinted with permission [167], Copyright 2021. Frontiers in Neuroanatomy
Exos and myelination
Myelin is a highly technical sheath created by oligodendrocytes with distinct architecture and structure. Proteolipid proteins (PLP) and myelin basic protein (MBP) are prevalent myelin proteins [168]. Oligodendrocytes can respond to suitable physiological stimuli with an inherent ability to generate specific myelin sheath membranes and create a 3D myelin structure [169]. Even though a large number of myelin proteins were discovered more than 50 years ago, nothing is known about their 3D structure or the molecular processes behind their role in myelination. In a multilayer proteolipid membrane, molecular structure and dynamics are crucial to operating physiologically. Proteins on adjacent membranes interact to form multilayers, and proteins and lipids combine to form nanoclusters, which are the building blocks for membrane organization [170]. The membrane lipid content affects the behavior of adhesion proteins. The terrestrial nervous system's myelin, a multilayered proteolipid membrane that covers certain axons, allows nerve signals to travel salutatory. Myelin is a repeating structure composed of lipid bilayers that are closely bound and are maintained together via certain proteins. Myelin has a special lipid makeup that is high in cholesterol and poor in water [168]. Animal studies suggest that myelin proteins have similar functions even if they are genetically unrelated. Proteins with comparable activities have been localized in myelin throughout evolution to ensure stability in a range of conditions. Although having a comparable structure and function, the myelin of the CNS and peripheral nervous systems (PNS) carries distinct proteins [169]. Degenerative brain illnesses are associated with defects in myelin durability and integrity. For instance, Charcot-Marie-Tooth disease (CMT) is the most prevalent hereditary neurotoxicity, whereas multiple sclerosis (MS) is an immunological illness of the CNS in which the immune response assaults the myelin layer [171]. It has been shown that close cell–cell contact and certain molecular interactions control dynamic contact between oligodendrocytes and axons in the internodal, paranodal, and juxtaparanodal areas [172]. Schwann cells (SCs) play a significant role in regional nervous circuit axonal regeneration. SCs dedifferentiate to a progenitor-like state following nerve damage and successfully direct axons to their originally focal areas. Throughout axonal regeneration, interface and soluble mediators are involved in the crosstalk between SCs and axons. Unexpectedly, Exos from SCs significantly boost axonal regeneration in vitro and improve regeneration following sciatic nerve damage in vivo [173]. There is proof that following an axotomy, SC dedifferentiates and releases Exos that particularly internalize by DRG axons and support axonal regeneration. Typical Exos markers like CD63, Tsg101, Hsp70, Hsp90, and flotillin-1 were present in any of these Exos. Additionally, they had the p75-neurotrophin receptor (p75NTR), which is a dedifferentiated SC phenotypic feature and is often generated in response to nerve injury. Data have proved the critical role of Exos in paracrine interaction between the neuron–neuron and neuron-glia interactions within the CNS, having significant implications for myelin development and restoration [174]. MBP, PLP, 2′3'-cyclic-nucleotide phosphodiesterase (CNPase), and myelin oligodendrocyte glycoprotein are dateable in large levels in oligodendroglia Exos. Besides, myelin-specific lipids, peroxiredoxin, and heat shock proteins can be transferred via Exos [175]. Data suggest oligodendroglia Exos had favorable impacts on the neuronal pulse, mortality, and antioxidant capacity under respiratory failure. So, Exos play an important role in myelin sheath driven by neuron-oligodendrocyte contact [176]. Although the same mechanisms participate in the control of Exo secretion and myelination in both oligodendrocytes in CNS and Schwann cells in PNS, the number of Exos can differ in both cells. Like other Exo types, markers such as Hsp70, 90, Tsg101, flotillin-1, and CD63, can be detected in SC Exos. The adjacent axons' internalization of SC-derived Exos encoding CD63-GFP was initially demonstrated. Following sciatic nerve compression, these SC-derived Exos boosted the rate of axonal regeneration in dorsal root ganglion (DRG) neurons both in vitro and in vivo, demonstrating the function of Exos in axonal restoration [169]. Restoration SC-derived Exos facilitated the result of increasing neurite growth from DRG plantlets by transferring exosomal miRNA-21, concurrently with PTEN beneath and PI3K stimulation in the neurons [31]. By the downregulation of c-Jun and Sox2 transcriptional activity using retroviral transduction, it was further proven that the impact of healing SC-derived Exos is reliant on their production [124]. In line with these data, Exos play a role in axon-glia interaction and the workable differences among both Exos derived from distinctive and repair SCs suggest that the exosomal cargo content varies. This distinction clarifies the distinctive ability of restorative SCs to aid in axonal rebuilding and improved function following nerve damage [173]. The use of the caspase-3 inhibitor z-DEVD-FMK revealed that conditioned response apoptosis might be the principal process for this Exo-mediated increased cell survival. The promotion of SC injury coincides with the alteration of the exosomal profile and the release of specific markers such as the p75-neurotrophin receptor (p75NTR) [173]. This factor is associated with the dedifferentiation of SCs during post-nerve injury [177]. Molecular and cellular investigations have revealed that SCs use a calmodulin-dependent pathway in response to DRG-derived glutamate stimulus for Exo biogenesis and abscission [173]. Previously, it was demonstrated that SCs produced Exos in a calcium-dependent way in response to a glutamate stimulation generated from the DRG, increasing the function of nerve cells. Protein disulfide-isomerase A3, fibronectin, flotillin-2, the major vault protein (MVP), monocarboxylate transporter 1, neuropilin-2 (NRP2), septin-7, and syntenin-1 were all found in Exos from the treated cells of SCs [178]. These compounds are strongly connected to axon renewal. B-crystallin and galectin-1, which have anti-inflammatory properties, were also present. Bioinformatics analyses have suggested the existence of molecules involved in axon renewal, including fatty acid-binding protein, fibronectin, MVP, carboxypeptidase E, NRP2, galectin-1, and protein disulfide-isomerase B-crystallin, both of which have anti-inflammatory properties [179]. Likewise, CNS epithelial cells can alter the composition of their Exo after myelin injury [178]. The function of the GTPase RhoA, which is responsible for development cone collapse and axon retraction, is reduced by Exos, and the development cone shape is changed to a pro-regenerating pattern [171].
Conclusion
Exos are valid cell byproduct nanoparticles with pleiotropic effects on several cell lineages. Emerging data have indicated Exos are valid de novo reservoirs with certain biomarkers for monitoring the type, intensity, and progression of pathological conditions, especially in CNS with specific molecular identities. The existence of discontinuities in the BBB barrier interface is one of the most important issues found in several CNS injuries and inflammatory responses. Exos with unique cargos can be used for the alleviation of inflammatory response and restoration of BBB cell normal activities. Of course, the type, sources, and dose of Exos for in vivo application remain to be elucidated using several future studies.
Despite the several advantages associated with the application of Exos, it seems that delivery of circulating Exos to the target sites is one of the challenging issues [180, 181]. That said, Exos lack an appropriate targeting capacity and systemic administration leads to off-target effects and accumulation in non-specific organs such as pulmonary, hepatic, and splenic tissues. Of note, allogeneic Exos are sequestrated by alloreactive T lymphocytes in lymphoid tissues [182]. These features indicate the superiority of autologous Exos over allogeneic and xenogeneic Exos for therapeutic purposes. Commensurate with these comments, the application of certain loading techniques and surface modification approaches is mandatory to increase the on-target therapeutic effects of Exos [181]. Besides these challenges, isolation, and purification of Exos using conventional and available approaches are problematic. For instance, high-rate pelleting can affect the morphology and increase the possibility of damage to the Exo membrane and protein contamination [47, 183]. It is suggested inappropriate Exo concentrations and the existence of specific factors such as tissue factor and other procoagulant factors can contribute to thrombosis and hemostatic perturbations [184]. Exos isolated from biofluids exhibits more heterogeneity with higher pro-thrombotic capacity compared to Exos purified from in vitro conditions [184]. Taken together, attempts should be concentrated to develop GMP-grade isolation and preparation technologies in terms of Exo to circumvent current limitations associated with BBB and CNS therapy.
Acknowledgements
Authors wish to thank the personnel of the Faculty of Advanced Medical Sciences for guidance and help.
Abbreviation
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- BBB
Blood-brain barrier
- BBB
Blood-brain barrier
- CNS
Central nervous system
- CMT
Charcot-Marie-Tooth disease
- DRG
Dorsal root ganglion
- ECs
Endothelial cells
- EPC
Endothelial progenitor cell
- Exos
Exosomes
- ECM
Extracellular matrix
- EVs
Extracellular vesicles
- ILVs
Intraluminal vesicles
- JAMs
Junctional adhesion molecules
- MVP
Major vault protein
- MMPs
Matrix metalloproteinases
- MS
Multiple sclerosis
- MVBs
Multivesicular bodies
- MBP
Myelin basic protein
- NRP2
Neuropilin-2
- PNS
Peripheral nervous systems
- PLP
Proteolipid proteins
- ROS
Reactive oxygen species
- SCs
Schwann cells
- SEC
Size exclusion chromatography
- TJs
Tight junctions
- TSG101
Tumor susceptibility gene 101
- VEGF
Vascular endothelial growth factor
- Aβ
β-Amyloid
Authors’ contributions
"L.S., F.S., M.K., H.M., N.M., and M.T. collected data and wrote the manuscript. R.R. supervised the study." The author(s) read and approved the final manuscript.
Funding
This study was supported by a grant (67049) from Tabriz University of Medical Sciences with an ethical code of IR.TBZMED.VCR.REC.1400.043.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leila Salimi and Mohammad Karimipour contributed as co-first authors to this study.
References
- 1.Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M, Eslami Abriz A, Zarebkohan A, Rahbarghazi R, Sokullu E. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):1–28. doi: 10.1186/s13578-021-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linville RM, DeStefano JG, Sklar MB, Xu Z, Farrell AM, Bogorad MI, Chu C, Walczak P, Cheng L, Mahairaki V. Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials. 2019;190:24–37. doi: 10.1016/j.biomaterials.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10(1):1–17. doi: 10.1038/s41467-019-13812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oztop-Cakmak O, Solaroglu I, Gursoy-Ozdemir Y. The role of pericytes in neurovascular unit: emphasis on stroke. Curr Drug Targets. 2017;18(12):1386–1391. doi: 10.2174/1389450117666160613104523. [DOI] [PubMed] [Google Scholar]
- 6.Li YN, Pan R, Qin XJ, Yang WL, Qi Z, Liu W, Liu KJ. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J Neurochem. 2014;129(1):120–129. doi: 10.1111/jnc.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mäger I, Meyer AH, Li J, Lenter M, Hildebrandt T, Leparc G, Wood MJ. Targeting blood-brain-barrier transcytosis–perspectives for drug delivery. Neuropharmacology. 2017;120:4–7. doi: 10.1016/j.neuropharm.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Hajal C, Le Roi B, Kamm RD, Maoz BM. Biology and Models of the Blood–brain Barrier. Annu Rev Biomed Eng. 2021;23:359–384. doi: 10.1146/annurev-bioeng-082120-042814. [DOI] [PubMed] [Google Scholar]
- 9.Garrido-Urbani S, Bradfield P, Imhof B. Tight junction dynamics: the role of junctional adhesion molecules (JAMs) Cell Tissue Res. 2014;355(3):701–715. doi: 10.1007/s00441-014-1820-1. [DOI] [PubMed] [Google Scholar]
- 10.Berndt P, Winkler L, Cording J, Breitkreuz-Korff O, Rex A, Dithmer S, Rausch V, Blasig R, Richter M, Sporbert A. Tight junction proteins at the blood–brain barrier: far more than claudin-5. Cell Mol Life Sci. 2019;76(10):1987–2002. doi: 10.1007/s00018-019-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter FR, Harazin A, Tóth AE, Veszelka S, Santa-Maria AR, Barna L, Kincses A, Biczó G, Balla Z, Kui B. Blood–brain barrier dysfunction in L-ornithine induced acute pancreatitis in rats and the direct effect of L-ornithine on cultured brain endothelial cells. Fluids Barriers CNS. 2022;19(1):1–20. doi: 10.1186/s12987-022-00308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geranmayeh MH, Rahbarghazi R, Farhoudi M. Targeting pericytes for neurovascular regeneration. Cell Commun Signal . 2019;17(1):1–13. doi: 10.1186/s12964-019-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winek K, Soreq H, Meisel A. Regulators of cholinergic signaling in disorders of the central nervous system. J Neurochem. 2021;158(6):1425–1438. doi: 10.1111/jnc.15332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho MS. Microglia in Parkinson’s Disease. In: Verkhratsky A, Ho MS, Zorec R, Parpura V (eds) Neuroglia in Neurodegenerative Diseases. Singapore: Springer Singapore; 2019. pp 335–53. 10.1007/978-981-13-9913-8_13.
- 15.Fan Y-Y, Huo J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem Int. 2021;148:105080. doi: 10.1016/j.neuint.2021.105080. [DOI] [PubMed] [Google Scholar]
- 16.Vinters HV, Kleinschmidt-DeMasters B. General pathology of the central nervous system. In: Greenfield's Neuropathology-Two Volume Set. CRC Press. 2018; pp 25–82.
- 17.Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter FR, Harazin A, Tóth AE, Veszelka S, Santa-Maria AR, Barna L, Kincses A, Biczó G, Balla Z, Kui B, Maléth J, Cervenak L, Tubak V, Kittel Á, Rakonczay Z, Deli MA. Blood–brain barrier dysfunction in l-ornithine induced acute pancreatitis in rats and the direct effect of l-ornithine on cultured brain endothelial cells. Fluids Barriers of the CNS. 2022;19(1):16. doi: 10.1186/s12987-022-00308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Niel G, d'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 20.Seyedaghamiri F, Salimi L, Ghaznavi D, Sokullu E, Rahbarghazi R. Exosomes-based therapy of stroke, an emerging approach toward recovery. Cell Commun Signal . 2022;20(1):110. doi: 10.1186/s12964-022-00919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajabi H, Konyalilar N, Erkan S, Mortazavi D, Korkunc SK, Kayalar O, Bayram H, Rahbarghazi R. Emerging role of exosomes in the pathology of chronic obstructive pulmonary diseases; destructive and therapeutic properties. Stem Cell Res Ther. 2022;13(1):144. doi: 10.1186/s13287-022-02820-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyedaghamiri F, Salimi L, Ghaznavi D, Sokullu E, Rahbarghazi R. Exosomes-based therapy of stroke, an emerging approach toward recovery. Cell Commun Signal . 2022;20(1):1–18. doi: 10.1186/s12964-022-00919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezabakhsh A, Cheraghi O, Nourazarian A, Hassanpour M, Kazemi M, Ghaderi S, Faraji E, Rahbarghazi R, Avci ÇB, Bagca BG. Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over-activity of the autophagic pathway. J Cell Biochem. 2017;118(6):1518–1530. doi: 10.1002/jcb.25814. [DOI] [PubMed] [Google Scholar]
- 24.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donoso-Quezada J, Ayala-Mar S, González-Valdez J. The role of lipids in exosome biology and intercellular communication: Function, analytics and applications. Traffic. 2021;22(7):204–20. 10.1111/tra.12803. [DOI] [PMC free article] [PubMed]
- 26.Heidarzadeh M, Sokullu E, Saghati S, Karimipour M, Rahbarghazi R. Insights into the critical role of exosomes in the brain; from neuronal activity to therapeutic effects. Mol Neurobiol. 2022;1–13. [DOI] [PubMed]
- 27.Bagi HM, Ahmadi S, Tarighat F, Rahbarghazi R, Soleimanpour H. Interplay between exosomes and autophagy machinery in pain management: state of the art. Neurobiol Pain . 2022;12:100095. doi: 10.1016/j.ynpai.2022.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostafazadeh M, Kahroba H, Haiaty S, TazeKand AP, Samadi N, Rahbarghazi R, Nouri M. In vitro exosomal transfer of Nrf2 led to the oxaliplatin resistance in human colorectal cancer LS174T cells. Cell Biochem Funct. 2022;40(4):391–402. doi: 10.1002/cbf.3703. [DOI] [PubMed] [Google Scholar]
- 29.Amini H, Rezabakhsh A, Heidarzadeh M, Hassanpour M, Hashemzadeh S, Ghaderi S, Sokullu E, Rahbarghazi R, Reiter RJ. An examination of the putative role of melatonin in exosome biogenesis. Front Cell Dev Biol. 2021;9:686551. doi: 10.3389/fcell.2021.686551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafari R, Rahbarghazi R, Ahmadi M, Hassanpour M, Rezaie J. Hypoxic exosomes orchestrate tumorigenesis: molecular mechanisms and therapeutic implications. J Transl Med. 2020;18(1):1–14. doi: 10.1186/s12967-020-02662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salimi L, Akbari A, Jabbari N, Mojarad B, Vahhabi A, Szafert S, Kalashani SA, Soraya H, Nawaz M, Rezaie J. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell Biosci. 2020;10:1–18. doi: 10.1186/s13578-020-00426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100. doi: 10.1111/cas.14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buratta S, Tancini B, Sagini K, Delo F, Chiaradia E, Urbanelli L, Emiliani C. Lysosomal exocytosis, exosome release and secretory autophagy: The autophagic-and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21(7):2576. doi: 10.3390/ijms21072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III–dependent sorting of tetraspanins to exosomes. J Cell Biol . 2020;219(3):e201904113. doi: 10.1083/jcb.201904113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal S, Agarwal V, Agarwal M, Singh M. Exosomes: structure, biogenesis, types and application in diagnosis and gene and drug delivery. Curr Gene Ther. 2020;20(3):195–206. 10.2174/1566523220999200731011702. [DOI] [PubMed]
- 36.Rahbarghazi R, Jabbari N, Sani NA, Asghari R, Salimi L, Kalashani SA, Feghhi M, Etemadi T, Akbariazar E, Mahmoudi M. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun Signal . 2019;17(1):1–17. doi: 10.1186/s12964-019-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng C, Huang Y, Zheng J. Renal clearable nanocarriers: Overcoming the physiological barriers for precise drug delivery and clearance. J Control Release. 2020;322:64–80. doi: 10.1016/j.jconrel.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stalder D, Gershlick DC Direct trafficking pathways from the Golgi apparatus to the plasma membrane. In: Seminars in cell & developmental biology, 2020. Elsevier, [DOI] [PMC free article] [PubMed]
- 39.Jia G. Sowers JR (2020) Targeting endothelial exosomes for the prevention of cardiovascular disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866(8):165833. doi: 10.1016/j.bbadis.2020.165833. [DOI] [PubMed] [Google Scholar]
- 40.Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood–brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):1801362. doi: 10.1002/adma.201801362. [DOI] [PubMed] [Google Scholar]
- 41.Schwarzenbach H, Gahan PB. MicroRNA shuttle from cell-to-cell by exosomes and its impact in cancer. Non-coding RNA. 2019;5(1):28. doi: 10.3390/ncrna5010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asil SM, Ahlawat J, Barroso GG, Narayan M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater Sci. 2020;8(15):4109–4128. doi: 10.1039/D0BM00809E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C, Wu Y, Wang L, Li S, Zhou J, Tan Y, Song J, Xing H, Yi K, Zhan Q, Zhao J, Wang Q, Yuan X, Kang C. Glioma-derived exosomes hijack the blood–brain barrier to facilitate nanocapsule delivery via LCN2. J Control Release. 2022;345:537–548. doi: 10.1016/j.jconrel.2022.03.038. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, Du L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. 2022;9:811971. doi: 10.3389/fbioe.2021.811971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, Zhang D, Song J, Cui D. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small. 2020;16(9):1903916. doi: 10.1002/smll.201903916. [DOI] [PubMed] [Google Scholar]
- 46.Yakubovich E, Polischouk A, Evtushenko V. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem (Mosc) Suppl Ser A Membr Cell Biol . 2022;16(2):115–126. doi: 10.1134/S1990747822030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 49.You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles. 2020;9(1):1706801. doi: 10.1080/20013078.2019.1706801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruivo CF, Adem B, Silva M, Melo SA. The biology of cancer exosomes: insights and new perspectivesbiology of cancer exosomes. Can Res. 2017;77(23):6480–6488. doi: 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- 51.Filipović L, Spasojević M, Prodanović R, Korać A, Matijaševic S, Brajušković G, de Marco A, Popović M. Affinity-based isolation of extracellular vesicles by means of single-domain antibodies bound to macroporous methacrylate-based copolymer. New Biotechnol. 2022;69:36–48. doi: 10.1016/j.nbt.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y, Hanayama R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6(1):33935. doi: 10.1038/srep33935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dash M, Palaniyandi K, Ramalingam S, Sahabudeen S. Raja N (2021) Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim Biophys Acta Biomembr . 1863;1863(2):183490. doi: 10.1016/j.bbamem.2020.183490. [DOI] [PubMed] [Google Scholar]
- 54.Soares T, Martins J, Catita I, Martins Rosa AB, Cruz da e Silva O, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE. 2018;13(6):e0198820. doi: 10.1371/journal.pone.0198820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meggiolaro A, Moccia V, Brun P, Pierno M, Mistura G, Zappulli V, Ferraro D. Microfluidic strategies for extracellular vesicle isolation: towards clinical applications. Biosensors. 2022;13(1):50. doi: 10.3390/bios13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Ital J Pediatr. 2018;44(2):131. doi: 10.1186/s13052-018-0563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou F, Ebea P, Mutai E, Wang H, Sukreet S, Navazesh S, Dogan H, Li W, Cui J, Ji P, Ramirez DMO, Zempleni J. Small Extracellular Vesicles in Milk Cross the Blood-Brain Barrier in Murine Cerebral Cortex Endothelial Cells and Promote Dendritic Complexity in the Hippocampus and Brain Function in C57BL/6J Mice. Front Nutr. 2022;9:838543. doi: 10.3389/fnut.2022.838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):1–19. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto J, Stewart T, Banks WA, Zhang J. The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des. 2017;23(40):6206–6214. doi: 10.2174/1381612823666170913164738. [DOI] [PubMed] [Google Scholar]
- 60.Heidarzadeh M, Gürsoy-Özdemir Y, Kaya M, Eslami Abriz A, Zarebkohan A, Rahbarghazi R, Sokullu E. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):142. doi: 10.1186/s13578-021-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toth AE, Holst MR, Nielsen MS. Vesicular transport machinery in brain endothelial cells: what we know and what we do not. Curr Pharm Des. 2020;26(13):1405–1416. doi: 10.2174/1381612826666200212113421. [DOI] [PubMed] [Google Scholar]
- 62.Haqqani AS, Delaney CE, Brunette E, Baumann E, Farrington GK, Sisk W, Eldredge J, Ding W, Tremblay T-L, Stanimirovic DB. Endosomal trafficking regulates receptor-mediated transcytosis of antibodies across the blood brain barrier. J Cereb Blood Flow Metab. 2018;38(4):727–740. doi: 10.1177/0271678X17740031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–1495. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 64.Wang YI, Abaci HE, Shuler ML. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017;114(1):184–194. doi: 10.1002/bit.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HR, Gil S, Andrieux K, Nicolas V, Appel M, Chacun H, Desmaele D, Taran F, Georgin D, Couvreur P. Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells. Cell Mol Life Sci. 2007;64:356–364. doi: 10.1007/s00018-007-6390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. 2020;21(12):4407. doi: 10.3390/ijms21124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y, Ho W. (−)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J Neuroinflammation. 2012;9(1):1–13. doi: 10.1186/1742-2094-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J Control Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 70.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bahlakeh G, Rahbarghazi R, Mohammadnejad D, Abedelahi A, Karimipour M. Current knowledge and challenges associated with targeted delivery of neurotrophic factors into the central nervous system: focus on available approaches. Cell Biosci. 2021;11(1):181. doi: 10.1186/s13578-021-00694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salarinasab S, Salimi L, Alidadiani N, Shokrollahi E, Arzhanga P, Karbasforush S, Marofi F, Nasirzadeh M, Rahbarghazi R, Nourazarian A. Interaction of opioid with insulin/IGFs signaling in Alzheimer's disease. J Mol Neurosci. 2020;70(6):819–834. doi: 10.1007/s12031-020-01478-y. [DOI] [PubMed] [Google Scholar]
- 74.Ludtmann MH, Abramov AY. Mitochondrial calcium imbalance in Parkinson’s disease. Neurosci Lett. 2018;663:86–90. doi: 10.1016/j.neulet.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 75.Staats KA, Borchelt DR, Tansey MG, Wymer J. Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Mol Neurodegener. 2022;17(1):1–19. doi: 10.1186/s13024-022-00515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oosterloo M, Bijlsma EK, Van Kuijk SM, Minkels F, Christine E. Clinical and genetic characteristics of late-onset Huntington's disease. Parkinsonism Relat Disord. 2019;61:101–105. doi: 10.1016/j.parkreldis.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Matar E, Ehgoetz Martens KA, Halliday GM, Lewis SJ. Clinical features of Lewy body dementia: insights into diagnosis and pathophysiology. J Neurol. 2020;267(2):380–389. doi: 10.1007/s00415-019-09583-8. [DOI] [PubMed] [Google Scholar]
- 78.Sen MK, Almuslehi MS, Shortland PJ, Coorssen JR, Mahns DA. Revisiting the pathoetiology of multiple sclerosis: has the tail been wagging the mouse? Front Immunol. 2020;11:572186. doi: 10.3389/fimmu.2020.572186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 80.Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, Riganti L, Corradini I, Francolini M, Garzetti L. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol. 2012;72(4):610–624. doi: 10.1002/ana.23627. [DOI] [PubMed] [Google Scholar]
- 81.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11(1):1–10. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fitzpatrick G, Nader D, Watkin R, McCoy CE, Curley GF, Kerrigan SW. Human endothelial cell-derived exosomal microRNA-99a/b drives a sustained inflammatory response during sepsis by inhibiting mTOR expression. Front Cell Infect Microbiol. 2022;12:854126. doi: 10.3389/fcimb.2022.854126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jian Z, Liu R, Zhu X, Smerin D, Zhong Y, Gu L, Fang W, Xiong X. The involvement and therapy target of immune cells after ischemic stroke. Front Immunol. 2019;10:2167. doi: 10.3389/fimmu.2019.02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan Y, Chen Z, Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med. 2022;20(1):1–15. doi: 10.1186/s12967-022-03493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ngolab J, Trinh I, Rockenstein E, Mante M, Florio J, Trejo M, Masliah D, Adame A, Masliah E, Rissman RA. Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology. Acta Neuropathol Commun. 2017;5(1):46. doi: 10.1186/s40478-017-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S, Zhou Z, Rong Y, Wang J, Ji C. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology . 2020;18(1):1–20. doi: 10.1186/s12951-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao J, Wang Y, Wang D, Yan W, Zhang S, Li D, Han Z, Chen F, Lei P. MiR-124-3p attenuates brain microvascular endothelial cell injury in vitro by promoting autophagy. Histol Histopathol. 2022;37(2):159–168. doi: 10.14670/hh-18-406. [DOI] [PubMed] [Google Scholar]
- 88.Huang LY, Song JX, Cai H, Wang PP, Yin QL, Zhang YD, Chen J, Li M, Song JJ, Wang YL, Luo L, Wang W, Qi SH. Healthy Serum-Derived Exosomes Improve Neurological Outcomes and Protect Blood-Brain Barrier by Inhibiting Endothelial Cell Apoptosis and Reversing Autophagy-Mediated Tight Junction Protein Reduction in Rat Stroke Model. Front Cell Neurosci. 2022;16:841544. doi: 10.3389/fncel.2022.841544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim K-A, Kim D, Kim J-H, Shin Y-J, Kim E-S, Akram M, Kim E-H, Majid A, Baek S-H, Bae O-N. Autophagy-mediated occludin degradation contributes to blood–brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models. Fluids Barriers of the CNS. 2020;17(1):21. doi: 10.1186/s12987-020-00182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang Z, Lin P, Chen B, Zhang X, Xiao W, Wu S, Huang C, Feng D, Zhang W, Zhang J. Autophagy alleviates hypoxia-induced blood-brain barrier injury via regulation of CLDN5 (claudin 5) Autophagy. 2021;17(10):3048–3067. doi: 10.1080/15548627.2020.1851897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jung S, Jeong H, Yu S-W. Autophagy as a decisive process for cell death. Exp Mol Med. 2020;52(6):921–930. doi: 10.1038/s12276-020-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie R, Zeng X, Yan H, Huang X, Deng C. Effects and Mechanisms of Exosomes from Different Sources in Cerebral Ischemia. Cells. 2022;11(22):3623. doi: 10.3390/cells11223623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yue K-Y, Zhang P-R, Zheng M-H, Cao X-L, Cao Y, Zhang Y-Z, Zhang Y-F, Wu H-N, Lu Z-H, Liang L, Jiang X-F, Han H. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019;10(12):869. doi: 10.1038/s41419-019-2100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li W, Mandeville ET, Durán-Laforet V, Fukuda N, Yu Z, Zheng Y, Held A, Park J-H, Nakano T, Tanaka M, Shi J, Esposito E, Niu W, Xing C, Hayakawa K, Lizasoain I, van Leyen K, Ji X, Wainger BJ, Moro MA, Lo EH. Endothelial cells regulate astrocyte to neural progenitor cell trans-differentiation in a mouse model of stroke. Nat Commun. 2022;13(1):7812. doi: 10.1038/s41467-022-35498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pivoriūnas A, Verkhratsky A. Astrocyte-derived extracellular vesicles mediate intercellular communications of the neurogliovascular unit. Neural Regen Res. 2021;16(7):1421. doi: 10.4103/1673-5374.300994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang W, Hong J, Zhang H, Zheng W, Yang Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY) 2021;13(17):21642–21658. doi: 10.18632/aging.203508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, Shetty AK. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav Immun. 2019;81:430–443. doi: 10.1016/j.bbi.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 98.Kriaučiūnaitė K, Kaušylė A, Pajarskienė J, Tunaitis V, Lim D, Verkhratsky A, Pivoriūnas A. Immortalised hippocampal astrocytes from 3xTG-AD mice fail to support BBB integrity in vitro: role of extracellular vesicles in glial-endothelial communication. Cell Mol Neurobiol. 2021;41(3):551–562. doi: 10.1007/s10571-020-00871-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rocchio F, Tapella L, Manfredi M, Chisari M, Ronco F, Ruffinatti FA, Conte E, Canonico PL, Sortino MA, Grilli M. Gene expression, proteome and calcium signaling alterations in immortalized hippocampal astrocytes from an Alzheimer’s disease mouse model. Cell Death Dis. 2019;10(1):1–18. doi: 10.1038/s41419-018-1264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geranmayeh MH, Nourazarian A, Avci ÇB, Rahbarghazi R, Farhoudi M. Stem cells as a promising tool for the restoration of brain neurovascular unit and angiogenic orientation. Mol Neurobiol. 2017;54(10):7689–7705. doi: 10.1007/s12035-016-0286-4. [DOI] [PubMed] [Google Scholar]
- 101.Rezabakhsh A, Rahbarghazi R. Putative role of stem cells in the alleviation of ischemic heart disease; a review article. Studies Med Sci. 2021;32(9):691–706. doi: 10.52547/umj.32.9.691. [DOI] [Google Scholar]
- 102.Baruah J, Wary KK. Exosomes in the regulation of vascular endothelial cell regeneration. Front Cell Dev Biol. 2020;7:353. doi: 10.3389/fcell.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38(1):1–14. doi: 10.1186/s13046-019-1384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie F, Wen G, Sun W, Jiang K, Chen T, Chen S, Wen J. Mechanical stress promotes angiogenesis through fibroblast exosomes. Biochem Biophys Res Commun. 2020;533(3):346–353. doi: 10.1016/j.bbrc.2020.04.159. [DOI] [PubMed] [Google Scholar]
- 105.Rezaie J, Mehranjani MS, Rahbarghazi R, Shariatzadeh MA. Angiogenic and restorative abilities of human mesenchymal stem cells were reduced following treatment with serum from diabetes mellitus type 2 patients. J Cell Biochem. 2018;119(1):524–535. doi: 10.1002/jcb.26211. [DOI] [PubMed] [Google Scholar]
- 106.Zhang L, Dong Z-f, Zhang J-y. Immunomodulatory role of mesenchymal stem cells in Alzheimer's disease. Life Sci. 2020;246:117405. doi: 10.1016/j.lfs.2020.117405. [DOI] [PubMed] [Google Scholar]
- 107.Gutierrez-Millan C, Calvo Díaz C, Lanao JM, Colino CI. Advances in exosomes-based drug delivery systems. Macromol Biosci. 2021;21(1):2000269. doi: 10.1002/mabi.202000269. [DOI] [PubMed] [Google Scholar]
- 108.Johnson T, Zhao L, Manuel G, Taylor H, Liu D. Approaches to therapeutic angiogenesis for ischemic heart disease. J Mol Med. 2019;97(2):141–151. doi: 10.1007/s00109-018-1729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beaulieu E VEGF increases the fibrinolytic activity of endothelial cells within fibrin matrices: Involvement of VEGFR-2, ti. [DOI] [PubMed]
- 111.Zhou Y, Zhu X, Cui H, Shi J, Yuan G, Shi S, Hu Y. The role of the VEGF family in coronary heart disease. Front Cardiovasc Med. 2021;8:738325. doi: 10.3389/fcvm.2021.738325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu Y, Huber CC, Wang H. Disrupted blood-brain barrier in 5× FAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem Biophys Res Commun. 2020;525(1):192–196. doi: 10.1016/j.bbrc.2020.02.074. [DOI] [PubMed] [Google Scholar]
- 113.Zhang HT, Zhang P, Gao Y, Li CL, Wang HJ, Chen LC, Feng Y, Li RY, Li YL, Jiang CL. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol Med Rep. 2017;15(1):57–64. doi: 10.3892/mmr.2016.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burek M, König A, Lang M, Fiedler J, Oerter S, Roewer N, Bohnert M, Thal SC, Blecharz-Lang KG, Woitzik J. Hypoxia-induced microRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res. 2019;10(6):672–683. doi: 10.1007/s12975-018-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, Vishwanatha JK. Exosomal annexin II promotes angiogenesis and breast cancer metastasisexosomal anx II in angiogenesis and metastasis. Mol Cancer Res. 2017;15(1):93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thiriet M. Cardiovascular risk factors and markers. In: Vasculopathies. Springer, 2018; pp 91–198
- 118.Horie K, Kawakami K, Fujita Y, Sugaya M, Kameyama K, Mizutani K, Deguchi T, Ito M. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun. 2017;492(3):356–361. doi: 10.1016/j.bbrc.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 119.Belli S, Esposito D, Servetto A, Pesapane A, Formisano L, Bianco R. c-Src and EGFR inhibition in molecular cancer therapy: what else can we improve? Cancers. 2020;12(6):1489. doi: 10.3390/cancers12061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int J Mol Sci. 2020;21(16):5840. doi: 10.3390/ijms21165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Traustadóttir GÁ, Lagoni LV, Ankerstjerne LBS, Bisgaard HC, Jensen CH, Andersen DC. The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is conserved in mammals, and serves a growth modulatory role during tissue development and regeneration through Notch dependent and independent mechanisms. Cytokine Growth Factor Rev. 2019;46:17–27. doi: 10.1016/j.cytogfr.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 122.Sagkan RI, Akin DFB. Epidermal growth factor-like repeats and discoidin I-like domains 3 is a novel regulator of epithelial-mesenchymal transition in clear cell renal cell carcinoma. in silico analysis. Erciyes Medical J. 2021;43(2):122–130. [Google Scholar]
- 123.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im S-A, Petrakova K, Bianchi GV, Esteva FJ, Martín M. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 124.Dai W, Shi Y, Hu W, Xu C. Long noncoding RNA FAM225B facilitates proliferation and metastasis of nasopharyngeal carcinoma cells by regulating miR-613/CCND2 axis. Bosn J Basic Med Sci. 2022;22(1):77. doi: 10.17305/bjbms.2021.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andersson U, Yang H, Harris H. Seminars in immunology. Elsevier; 2018. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells; pp. 40–48. [DOI] [PubMed] [Google Scholar]
- 126.Gonzalez-Aparicio M, Alfaro C. Influence of interleukin-8 and neutrophil extracellular trap (NET) formation in the tumor microenvironment: is there a pathogenic role? J Immunol Res. 2019;2019 [DOI] [PMC free article] [PubMed]
- 127.Chan YK, Zhang H, Liu P, Tsao SW, Lung ML, Mak NK, Ngok-Shun Wong R, Ying-Kit Yue P. Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int J Cancer. 2015;137(8):1830–1841. doi: 10.1002/ijc.29562. [DOI] [PubMed] [Google Scholar]
- 128.Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B, Huang C, Zhao Q, Qiu Z. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Molecular Therapy-Nucleic Acids. 2020;22:179–195. doi: 10.1016/j.omtn.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with α5β1 integrin in glioma. Oncogene. 2013;32(3):327–340. doi: 10.1038/onc.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hood JL. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med Hypotheses. 2016;94:118–122. doi: 10.1016/j.mehy.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang M, Zhao Y, Yu Z-Y, Zhang R-D, Li S-A, Zhang P, Shan T-K, Liu X-Y, Wang Z-M, Zhao P-C. Glioma exosomal microRNA-148a-3p promotes tumor angiogenesis through activating the EGFR/MAPK signaling pathway via inhibiting ERRFI1. Cancer Cell Int. 2020;20(1):1–16. doi: 10.1186/s12935-020-01566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eichelser C, Stückrath I, Müller V, Milde-Langosch K, Wikman H, Pantel K, Schwarzenbach H. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget. 2014;5(20):9650. doi: 10.18632/oncotarget.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoon C, Kim J, Park G, Kim S, Kim D, Hur DY, Kim B, Kim YS. Delivery of miR-155 to retinal pigment epithelial cells mediated by Burkitt’s lymphoma exosomes. Tumor Biology. 2016;37(1):313–321. doi: 10.1007/s13277-015-3769-4. [DOI] [PubMed] [Google Scholar]
- 135.Li J, Yuan H, Xu H, Zhao H, Xiong N. Hypoxic cancer-secreted exosomal miR-182-5p promotes glioblastoma angiogenesis by targeting kruppel-like factor 2 and 4miR-182-5p promotes glioblastoma angiogenesis. Mol Cancer Res. 2020;18(8):1218–1231. doi: 10.1158/1541-7786.MCR-19-0725. [DOI] [PubMed] [Google Scholar]
- 136.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 137.Wu Z, Cai X, Huang C, Xu J, Liu A. miR-497 suppresses angiogenesis in breast carcinoma by targeting HIF-1α. Oncol Rep. 2016;35(3):1696–1702. doi: 10.3892/or.2015.4529. [DOI] [PubMed] [Google Scholar]
- 138.Liang M, Zhu B, Wang M, Jin J. Knockdown of long non-coding RNA DDX11-AS1 inhibits the proliferation, migration and paclitaxel resistance of breast cancer cells by upregulating microRNA-497 expression. Mol Med Rep. 2022;25(4):1–14. doi: 10.3892/mmr.2022.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mao S, Lu Z, Zheng S, Zhang H, Zhang G, Wang F, Huang J, Lei Y, Wang X, Liu C. Exosomal miR-141 promotes tumor angiogenesis via KLF12 in small cell lung cancer. J Exp Clin Cancer Res. 2020;39(1):1–16. doi: 10.1186/s13046-020-01680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crudele F, Bianchi N, Reali E, Galasso M, Agnoletto C, Volinia S. The network of non-coding RNAs and their molecular targets in breast cancer. Mol Cancer. 2020;19(1):1–18. doi: 10.1186/s12943-020-01181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan S, Zhao X, Shao C, Fu B, Huang Y, Zhang N, Dou X, Zhang Z, Qiu Y, Wang R. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis. 2021;12(1):1–15. doi: 10.1038/s41419-020-03304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taverna S, Fontana S, Monteleone F, Pucci M, Saieva L, De Caro V, Cardinale VG, Giallombardo M, Vicario E, Rolfo C. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget. 2016;7(21):30420. doi: 10.18632/oncotarget.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mao W, Wang K, Wu Z, Xu B, Chen M. Current status of research on exosomes in general, and for the diagnosis and treatment of kidney cancer in particular. J Exp Clin Cancer Res. 2021;40(1):1–13. doi: 10.1186/s13046-021-02114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen S, Chen X, Luo Q, Liu X, Wang X, Cui Z, He A, He S, Jiang Z, Wu N. Retinoblastoma cell-derived exosomes promote angiogenesis of human vesicle endothelial cells through microRNA-92a-3p. Cell Death Dis. 2021;12(7):1–11. doi: 10.1038/s41419-021-03986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuan X, Qian N, Ling S, Li Y, Sun W, Li J, Du R, Zhong G, Liu C, Yu G. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11(3):1429. doi: 10.7150/thno.45351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jung KO, Youn H, Lee C-H, Kang KW, Chung J-K. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8(6):9899. doi: 10.18632/oncotarget.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang Q, Wang G, Niu L, Zhao S, Li J, Zhang Z, Jiang H, Zhang Q, Wang H, Sun P. Exosomal MiR-1290 promotes angiogenesis of hepatocellular carcinoma via targeting SMEK1. J Oncol. 2021;2021:6617700. doi: 10.1155/2021/6617700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xuan Z, Chen C, Tang W, Ye S, Zheng J, Zhao Y, Shi Z, Zhang L, Sun H, Shao C. TKI-resistant renal Cancer secretes low-level Exosomal miR-549a to induce vascular permeability and angiogenesis to promote tumor metastasis. Front Cell Dev biology. 2021;1460. 10.3389/fcell.2021.689947. [DOI] [PMC free article] [PubMed]
- 149.Hsu Y, Hung J, Chang W, Lin Y, Pan Y, Tsai P, Wu C, Kuo P. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Cen A, Yang Y, Ye H, Li J, Liu S, Zhao L. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis. Molecular Therapy-Nucleic Acids. 2021;24:610–621. doi: 10.1016/j.omtn.2021.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roma-Rodrigues C, Fernandes AR, Baptista PV. Counteracting the effect of leukemia exosomes by antiangiogenic gold nanoparticles. Int J Nanomed. 2019;14:6843. doi: 10.2147/IJN.S215711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hsieh C-H, Tai S-K, Yang M-H. Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia. 2018;20(8):775–788. doi: 10.1016/j.neo.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Masoumi-Dehghi S, Babashah S, Sadeghizadeh M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J Cell Commun Signal. 2020;14(2):233–244. doi: 10.1007/s12079-020-00548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen G, Zhang Y, Wu X. 786–0 Renal cancer cell line-derived exosomes promote 786–0 cell migration and invasion in vitro. Oncol Lett. 2014;7(5):1576–1580. doi: 10.3892/ol.2014.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shen Y, Xue C, Li X, Ba L, Gu J, Sun Z, Han Q, Zhao RC. Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signaling pathway. Stem Cells Dev. 2019;28(7):464–476. doi: 10.1089/scd.2018.0125. [DOI] [PubMed] [Google Scholar]
- 156.Bhat A, Yadav J, Thakur K, Aggarwal N, Tripathi T, Chhokar A, Singh T, Jadli M, Bharti AC. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog–GLI signaling components. Cancer Cell Int. 2021;21(1):1–15. doi: 10.1186/s12935-021-02026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, Liu L. Exosome-mediated cell-cell communication in tumor progression. Am J Cancer Res. 2018;8(9):1661. [PMC free article] [PubMed] [Google Scholar]
- 158.Yadav J, Aggarwal N, Chaudhary A, Tripathi T, Baruah D, Chhakara S, Janjua D, Chhokar A, Thakur K, Senrung A. Role of exosomes in tumor induced neo-angiogenesis. 2022
- 159.Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-β/Smad signaling pathway. Onco Targets Ther. 2019;12:6897. doi: 10.2147/OTT.S209413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bonnet M, Maisonial-Besset A, Zhu Y, Witkowski T, Roche G, Boucheix C, Greco C, Degoul F. Targeting the tetraspanins with monoclonal antibodies in oncology: focus on Tspan8/Co-029. Cancers. 2019;11(2):179. doi: 10.3390/cancers11020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang A, Dong J, Li S, Wang C, Ding H, Li H, Su X, Ge X, Sun L, Bai C. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci. 2015;11(8):961. doi: 10.7150/ijbs.11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rahbarghazi R, Nassiri SM, Khazraiinia P, Kajbafzadeh A-M, Ahmadi SH, Mohammadi E, Molazem M, Zamani-Ahmadmahmudi M. Juxtacrine and paracrine interactions of rat marrow-derived mesenchymal stem cells, muscle-derived satellite cells, and neonatal cardiomyocytes with endothelial cells in angiogenesis dynamics. Stem Cells Dev. 2013;22(6):855–865. doi: 10.1089/scd.2012.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gao W, Li F, Liu L, Xu X, Zhang B, Wu Y, Yin D, Zhou S, Sun D, Huang Y. Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp Neurol. 2018;307:99–108. doi: 10.1016/j.expneurol.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 164.Wang J, Chen S, Ma X, Cheng C, Xiao X, Chen J, Liu S, Zhao B, Chen Y. Effects of Endothelial Progenitor Cell-Derived Microvesicles on Hypoxia/Reoxygenation-Induced Endothelial Dysfunction and Apoptosis. Oxid Med Cell Longev. 2013;2013:572729. doi: 10.1155/2013/572729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Halurkar MS, Wang J, Chen S, Bihl JC. EPC-EXs improve astrocyte survival and oxidative stress through different uptaking pathways in diabetic hypoxia condition. Stem Cell Res Ther. 2022;13(1):91. doi: 10.1186/s13287-022-02766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J, Wang Y, Chen Y, Bihl J, Chen Y, Zhao B, Ma X. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11(1):260. doi: 10.1186/s13287-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mentor S, Fisher D. Exosomes form tunneling nanotubes (TUNTs) in the blood-brain barrier: a nano-anatomical perspective of barrier genesis. Front Mol Neurosci. 2022;15:938315. doi: 10.3389/fnmol.2022.938315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hawkins RD, Byrne JH. Associative learning in invertebrates. Cold Spring Harb Perspect Biol. 2015;7(5):a021709. doi: 10.1101/cshperspect.a021709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Domingues HS, Cruz A, Chan JR, Relvas JB, Rubinstein B, Pinto IM. Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia. 2018;66(1):5–14. doi: 10.1002/glia.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Di Gioacchino M, Bianconi A, Burghammer M, Ciasca G, Bruni F. Campi G Myelin basic protein dynamics from out-of-equilibrium functional state to degraded state in myelin. Biochim Biophys Acta Biomembr. 2020;1862(6):183256. doi: 10.1016/j.bbamem.2020.183256. [DOI] [PubMed] [Google Scholar]
- 171.Liu X-A, Rizzo V, Puthanveettil S. Pathologies of axonal transport in neurodegenerative diseases. Transl Neurosci. 2012;3(4):355–372. doi: 10.2478/s13380-012-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elazar N, Vainshtein A, Rechav K, Tsoory M, Eshed-Eisenbach Y, Peles E. Coordinated internodal and paranodal adhesion controls accurate myelination by oligodendrocytes. J Cell Biol. 2019;218(9):2887–2895. doi: 10.1083/jcb.201906099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hu M, Hong L, Liu C, Hong S, He S, Zhou M, Huang G, Chen Q. Electrical stimulation enhances neuronal cell activity mediated by Schwann cell derived exosomes. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-41007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krämer-Albers E-M, Hill AF. Extracellular vesicles: interneural shuttles of complex messages. Curr Opin Neurobiol. 2016;39:101–107. doi: 10.1016/j.conb.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 175.Wang X, Shang H, Ma C, Chen L. A fluorescence assay for exosome detection based on bivalent cholesterol anchor triggered target conversion and enzyme-free signal amplification. Anal Chem. 2021;93(24):8493–8500. doi: 10.1021/acs.analchem.1c00796. [DOI] [PubMed] [Google Scholar]
- 176.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18(5):628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Imbschweiler I, Seehusen F, Peck CT, Omar M, Baumgärtner W, Wewetzer K. Increased p75 neurotrophin receptor expression in the canine distemper virus model of multiple sclerosis identifies aldynoglial Schwann cells that emerge in response to axonal damage. Glia. 2012;60(3):358–371. doi: 10.1002/glia.22270. [DOI] [PubMed] [Google Scholar]
- 178.Domingues HS, Falcão AM, Mendes-Pinto I, Salgado AJ, Teixeira FG. Exosome Circuitry During (De)(Re) Myelination of the Central Nervous System. Front Cell Dev Biol. 2020;8:483. doi: 10.3389/fcell.2020.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wei Z, Fan B, Ding H, Liu Y, Tang H, Pan D, Shi J, Zheng P, Shi H, Wu H. Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem. 2019;457(1):51–59. doi: 10.1007/s11010-019-03511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bagheri E, Abnous K, Farzad SA, Taghdisi SM, Ramezani M, Alibolandi M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261:118369. doi: 10.1016/j.lfs.2020.118369. [DOI] [PubMed] [Google Scholar]
- 181.Jafari D, Shajari S, Jafari R, Mardi N, Gomari H, Ganji F, Forouzandeh Moghadam M, Samadikuchaksaraei A. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34(5):567–586. doi: 10.1007/s40259-020-00434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Prunevieille A, Babiker-Mohamed MH, Aslami C, Gonzalez-Nolasco B, Mooney N, Benichou G. T cell antigenicity and immunogenicity of allogeneic exosomes. Am J Transplant. 2021;21(7):2583–2589. doi: 10.1111/ajt.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rezabakhsh A, Sokullu E, Rahbarghazi R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther. 2021;12(1):521. doi: 10.1186/s13287-021-02596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Silachev DN, Goryunov KV, Shpilyuk MA, Beznoschenko OS, Morozova NY, Kraevaya EE, Popkov VA, Pevzner IB, Zorova LD, Evtushenko EA. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells. 2019;8(3):258. doi: 10.3390/cells8030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.