Abstract
Background
Reducing the risk of recurrent Plasmodium vivax malaria is critical for malaria control and elimination. Primaquine (PQ) is the only widely available drug against P. vivax dormant liver stages, but is recommended as a 14-day regimen, which can undermine adherence to a complete course of treatment.
Methods
This is a mixed-methods study to assess socio-cultural factors influencing adherence to a 14-day PQ regimen in a 3-arm, treatment effectiveness trial in Papua, Indonesia. The qualitative strand, consisting of interviews and participant observation was triangulated with a quantitative strand in which trial participants were surveyed using a questionnaire.
Results
Trial participants differentiated between two types of malaria: tersiana and tropika, equivalent to P. vivax and Plasmodium falciparum infection, respectively. The perceived severity of both types was similar with 44.0% (267/607) perceiving tersiana vs. 45.1% (274/607) perceiving tropika as more severe. There was no perceived differentiation whether malaria episodes were due to a new infection or relapse; and 71.3% (433/607) acknowledged the possibility of recurrence. Participants were familiar with malaria symptoms and delaying health facility visit by 1–2 days was perceived to increase the likelihood of a positive test. Prior to health facility visits, symptoms were treated with leftover drugs kept at home (40.4%; 245/607) or bought over the counter (17.0%; 103/607). Malaria was considered to be cured with ‘blue drugs’ (referring to dihydroartemisinin-piperaquine). Conversely, ‘brown drugs,’ referring to PQ, were not considered malaria medication and instead were perceived as supplements. Adherence to malaria treatment was 71.2% (131/184), in the supervised arm, 56.9% (91/160) in the unsupervised arm and 62.4% (164/263) in the control arm; p = 0.019. Adherence was 47.5% (47/99) among highland Papuans, 51.7% (76/147) among lowland Papuans, and 72.9% (263/361) among non-Papuans; p < 0.001.
Conclusion
Adherence to malaria treatment was a socio-culturally embedded process during which patients (re-)evaluated the characteristics of the medicines in relation to the course of the illness, their past experiences with illness, and the perceived benefits of the treatment. Structural barriers that hinder the process of patient adherence are crucial to consider in the development and rollout of effective malaria treatment policies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-023-04578-3.
Keywords: Primaquine adherence, Malaria radical cure, Vivax treatment, Malaria in Papua, Malaria Indonesia
Background
Outside of sub-Saharan Africa, the burden of malaria is falling, although the relative proportion of malaria due to Plasmodium vivax is rising [1]. Malaria control efforts focused on reducing P. vivax infections and their recurrences will consequently be critical in achieving malaria elimination targets [2, 3]. Plasmodium vivax is particularly difficult to eliminate since it forms dormant liver stages (hypnozoites) that can reactivate weeks to months after the initial infection causing relapses and maintaining ongoing transmission. For more than half a century, primaquine (PQ) has been the only widely available drug that kills P. vivax hypnozoites [4, 5]. Currently PQ treatment is provided over the course of 14 days in combination with a shorter 3-day course of schizontocidal treatment to eliminate the blood stages of P. vivax (called radical cure) [6]. While well tolerated in most recipients, PQ can cause severe haemolysis in individuals with glucose-6-dehydrogenase (G6PD) deficiency [7]. The World Health Organization (WHO) recommends screening patients for G6PD deficiency prior to PQ based radical cure [8]. In practice, G6PD testing is rarely available resulting in under-prescription of PQ for fear of haemolysis [9, 10]. When radical cure is prescribed, the long course of PQ can undermine patient adherence impacting on drug effectiveness [11].
Studies about patient adherence to antimalarials yielded varied results related to patient characteristics, the nature of their consultation with the provider, and methodological variations of the study [12]. Assessment of adherence relied on subjective measures such as self-reporting or a combination of self-reporting and more objective measures such as pill count, presenting empty pill bags, or directly measuring the level of PQ in the blood [13–16]. Treatment supervision to improve adherence has been studied, including in the trial where this study is embedded [17, 18]. As such, trial procedures can have an impact on adherence outcomes [12].
Decision on whether to adhere to treatment is often influenced not only by individual but also societal factors [14, 19]. Community perceptions of malaria, its recurrence and treatment influence the uptake of and adherence to appropriate malaria treatment [11, 20]. In the high malaria endemic area of Mimika, Papua, Indonesia health provider factors (such as prescriber adherence to treatment protocol) were found to influence uptake of malaria testing and treatment service and treatment-seeking behaviour [21, 22]. However, little is known about the socio-cultural factors driving treatment-seeking behaviour and adherence in the complex context of Papua.
A better understanding of adherence to treatment at the community level inform context-specific approaches to health care delivery to ensure that they will have the greatest possible impact on patient outcomes and the burden of disease. This study assessed malaria perceptions, treatment-seeking behaviour, and adherence to 14-day radical cure for P. vivax malaria in Mimika, Papua, Indonesia.
Methods
Study context
This mixed-methods study was embedded in a cluster-randomized, controlled, open label trial in Mimika District in Papua, Indonesia (Fig. 1) [18]. Patients with microscopy confirmed malaria were enrolled into the trial at 10 local public healthcare centres. All patients, irrespective of malaria species, were treated with dihydroartemisinin-piperaquine (DP) followed by 14 days of PQ after screening for haemoglobin (Hb) and G6PD levels two days after diagnosis and initial treatment [18]. The PQ used in this study was administered at a total dose of 7 mg/kg, which, although recommended by the WHO for areas with high relapse risk, is not current policy in Indonesia, which recommends a total dose of 3.5 mg/kg (equivalent to a dose of 0.25 mg/kg per day for 14 days) for patients with P. vivax and Plasmodium ovale malaria (single or mixed infection). Patients with Plasmodium falciparum malaria were treated with DP and a single dose of 15 mg PQ on the day of diagnosis. In the current clinical practice outside of this trial, malaria treatment is given without testing Hb levels and G6PD activity.
Fig. 1.
Map indicating the location of Timika (marked with a star). Map source: gpspointsplotter.com
The study area was divided into 21 clusters, which were randomized to a supervised and unsupervised PQ arm. In the supervised arm, participants were provided with two daily doses of PQ on alternate days, one dose to be taken in front of the study team and the other the next day and this was repeated for seven visits (14 PQ doses in total). In the unsupervised arm, participants were provided with 14 PQ doses while receiving a more thorough explanation on the importance of finishing the PQ course than is common in clinical practice. At each monthly visit, a microscopic blood film examination was undertaken to detect recurrent parasitaemia. All patients were provided with a phone number to contact the study team if they had symptoms suggestive of a malaria episode and/or a haemolytic event between the scheduled visits.
Seven months after the first enrollment in the supervised and unsupervised trial arms, additional patients from all 21 clusters were enrolled into a third, non-randomized, observational control arm. Participants received standard care (species-specific treatment and low dose PQ), as per usual practice without the extensive trial-specific follow up. Participants of the control arm were only followed up once six months after enrolment. Arm specific procedures are outlined in Table 1.
Table 1.
Treatment and supervision procedures of study arms
| Supervised | Unsupervised | Control | ||
|---|---|---|---|---|
| Total PQ dose (radical cure) | 7 mg/kg (max. 2 tablets/day) over 14 days | 3.5 mg/kg (max. 1 tablet/day) over 14 days | ||
| PQ prescription | All patients irrespective of malaria species |
Only patients with P. vivax and P. ovale (single or mixed) species received radical cure P. falciparum patients received single dose 15 mg PQ |
||
| Treatment steps and supervision | Supervised DP for 3 days followed by 14 days of daily PQ supervised on alternate days | Supervised DP for 3 days followed by unsupervised PQ | Supervised first dose of DP followed by unsupervised DP and PQ | |
| Follow-up visits | D1, D2, D4, D6, D8, D10, D12, D14, D16, M1, M2, M3, M4, M5, M6 | D1, D2, D16, M1, M2, M3, M4, M5, M6 | M6 | |
PQ, primaquine; mg, milligram; kg, kilogram; max., maximum; DP, dihydroartemisinin piperaquine; D, day; M, month
Study design
A mixed-methods study was conducted using qualitative and quantitative research methods for data triangulation and complementarity purposes. The study had a sequential design, comprising of three consecutive strands, which in standard annotation can be presented as [qualQUANqual] [23]. Figure 2 explained the data collection timeline within the trial.
Fig. 2.
Timeline of mixed-methods study within trial period
Quantitative strand
Data collection
A structured questionnaire was developed for the quantitative patient survey based on insights from the exploratory qualitative strand. The questionnaire was pre-tested in the same setting. The survey aimed to assess adherence to PQ and other malaria treatments, perceptions of malaria and its recurrences, treatment-seeking behaviour, and perceptions of the trial. Data collection was conducted in collaboration with AKVO (https://akvo.org, last accessed on 12.12.2022) using software installed on electronic tablets for automatic data entry and continuous monitoring to ensure data quality. Six research assistants with knowledge of the local culture and some of the local languages who had been involved in the clinical trial were recruited and trained as enumerators.
Sampling
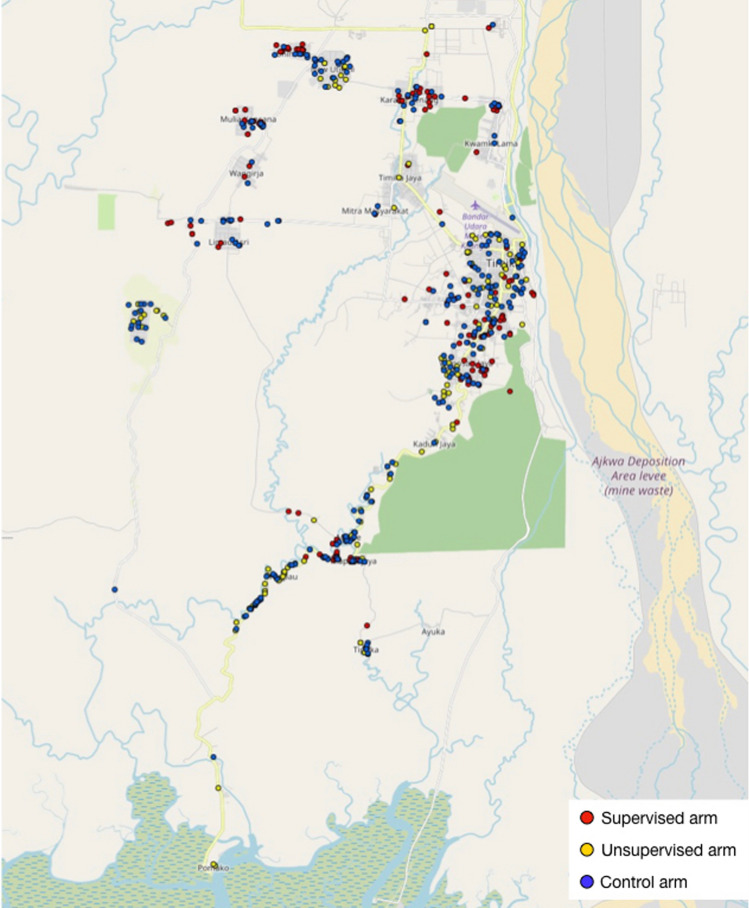
This study was embedded in a trial conducted in 21 clusters across different districts in and around the town of Timika (Fig. 3). All patients registered in the trial database were included in the quantitative strand of this study. Enumerators first attempted to contact patients by telephone to arrange an appointment for an interview in their homes; however, interviews were also conducted at participants’ school or workplace. Patients who could not be contacted by phone or who did not have a mobile phone were visited without an appointment.
Fig. 3.
The spread of survey participants in and around the town of Timika. Map generated on gpspointplotter.com
Data analysis
Preliminary data cleaning was conducted concurrently to the survey to resolve data entry errors and double entries using automated data entry software (Akvo™, Amsterdam, The Netherlands). Subsequent cleaning and analyses were conducted in R (R version 3.3.0, The R Foundation for Statistical Computing). Descriptive statistics were used to indicate proportions which were compared by the chi-square test. Post-hoc analyses using Marascuilo procedure were performed to test which specific proportions are different from each other in multiple comparisons of proportions.
Qualitative strand
Data collection
The qualitative strand explored people’s perceptions of malaria, malaria treatment-seeking behaviour, adherence to malaria treatment, and perceptions of the ongoing trial. Trained Indonesian social scientists (AR, CL) collected data through interviewing and participant observation with community members of all ethnicities.
Sampling
A theoretical sampling strategy gradually included informants purposively selected in relation to emerging results based on malaria status, ethnicity, age, occupation, including trial participants and household members, other members of target communities, trial field supervision staff, healthcare staff from the various healthcare facilities in this region (not limited to participating facilities), and alternative healthcare providers (e.g., traditional health providers).
Data analysis
The iterative nature of qualitative data collection required data collection tools to be adapted continuously by intermittent analysis of raw data. Abductive analysis—a continuous interplay between emerging findings and theory—resulted in a theoretical model of factors influencing adherence to radical cure in this context [24]. NVivo 12 qualitative data analysis software (QSR International Pty Ltd. Cardigan, UK) was used to construct a final coding tree, which was used for thematic content analysis of all raw data and analytical memos.
Concept operationalization
Ethnicity
The Island of Papua has diverse ethnicities, both internal migrant (from the western islands of Indonesia) and indigenous groups. In Indonesia, the concept of “indigenous” is highly contested because of the government view that everyone is indigenous, however, in Mimika regency, the sense of ethnic identity differentiating Papuans and non-Papuans are widely held [25, 26]. Among the Papuans, the many ethnic groups can be divided into highland and lowland Papuans based on linguistic and genetic characteristics [26–28]. In Mimika, the traditional owners of the lands that make up the regency from coastline to mountains are the Kamoro (a lowland ethnic group) and Amungme (a highland ethnic group) peoples. The non-Papuans are commonly called ‘pendatang’ or the ‘incomers,’ regardless of how many generations they have lived in the area. They are comprised of different ethnic groups from various parts of Indonesia and arrived in Mimika regency through different modes of migration for assorted reasons for decades [26, 29]. In this study, the study population was classified into three categories: (1) highland Papuans, (2) lowland Papuans, and (3) non-Papuans as has been done in other malaria studies [18, 21, 30].
Adherence
In this study, adherence was defined as the aggregate of several factors quantified in the questionnaire. A patient was considered adherent if they: (1) stated to have finished all the drugs given during the trial/in the last malaria episode AND (2) mentioned ‘blue drugs’ PLUS any of the following: ‘brown drugs,’ ‘small drugs,’ ‘spleen drugs,’ or ‘primaquine’ when asked which drugs they have finished. Several variables potentially influencing self-reported adherence were considered, including trial arm, ethnicity, initial diagnosis during trial, and time between end of trial and interview.
Public health facility
Public health facilities were defined as primary health care clinics and the hospital, both of which were owned and run by the government. These included Puskesmas (public health center), Pustu (satellite health center), and the general hospital. For the Puskesmas and Pustu, priority access is given by area of residence. Registered Indonesian citizens (presenting a citizen card) can apply for national health insurance to get free basic healthcare at government health facilities and certain private health facilities that collaborate in the national health insurance system. In this system, clients are assigned one clinic as their primary health care provider—for most people this will be the Puskesmas nearest to their registered address.
Private health facility
Private health facilities were defined as non-governmental primary health care clinics and hospitals and these included malaria control clinics and the Caritas hospital (both owned by the biggest mining company in the area) as well as the private practices of local doctors and midwives. The mining company provides free access to its health facilities for Kamoro and Amungme peoples as well as five other highland ethnic groups regarded to be directly affected by mining operations (Damal, Dani, Mee, Moni, and Nduga peoples).
Ethical considerations
The trial and the mixed-methods study were conducted according to the principles stated in the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects) as amended in 2008, all applicable regulations and according to established international scientific standards. The study protocol was reviewed and approved by the ethical committees of the Faculty of Medicine Gadjah Mada University in Indonesia (KE/FK/522/EC/2016), the Human Research Ethics Committee (HREC) of the Northern Territory (NT) Department of Health and Menzies School of Health Research in Australia (15-2517), and the Institute of Tropical Medicine in Belgium (1163/17).
For the qualitative strand, oral consent was collected from all participants and, for minors, from a legal guardian. For the quantitative survey, written informed consent was obtained from all participants or legal guardians in case they were underage. Illiterate participants willing to participate provided consent by a fingerprint in the presence of a witness.
Results
Study participants
Qualitative strand
In total 47 interviews (both in-depth and informal) and 12 observations were conducted and transcribed. Audio-recorded interviews were transcribed ad verbatim and informal conversations were summarized.
Quantitative strand
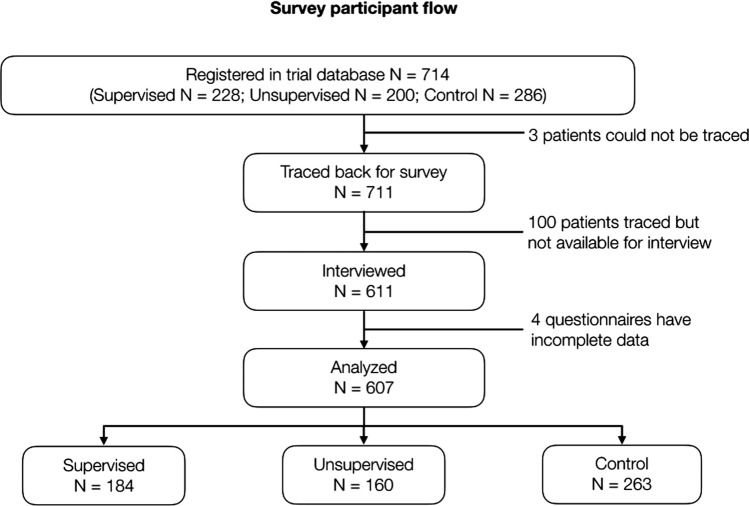
85.6% (611/714) of trial participants from all three arms participated in the survey. After data cleaning four participant records were excluded due to missing answers. In total 607 questionnaires were analysed (Fig. 4) and 50.4% (306/607) of all respondents were women. The median age was 17 years old (interquartile range (IQR): 8–33); Additional file 1: Table S1. Of all participants, 16.3% (99/607) were highland Papuans including 11.0% (67/607) who self-identified as Dani, 24.2% (147/607) were lowland Papuans with 15% (93/607 Kamoro, and 59.5% (361/607) were non-Papuans including 13% (77/607) Kei, from the neighbouring archipelago of Maluku, and 15% (88/607) Java/Sunda, the largest ethnic groups in Indonesia.
Fig. 4.
Survey participant flow for the quantitative strand
Malaria types, etiologies, and recurrences
Malaria types
Participants had a clear understanding of the presence of various kinds of malaria in their region. The types of malaria that people referred to were derived from medical terms that healthcare workers at public health centres used amongst themselves and in communication with patients, such as malaria tropika and malaria tersiana. Healthcare workers used the term tropika to refer to P. falciparum and tersiana for P. vivax malaria. In terms of symptoms, patients perceived malaria tersiana to cause body pains, prolonged fevers, and the inability to do any work or other activities. Malaria tropika was perceived to cause headaches, a shorter febrile illness, and a lack of appetite. Some participants regarded malaria tersiana as being more serious due to its repeated episodes and it being perceived to make one weaker, despite having milder or no symptoms. A community member explained: “for malaria tersiana, we don’t really get a fever, but it is very serious because it takes a long time.” In the survey, the proportion of respondents perceiving one malaria type as more severe than the other was similar with 44.0% (267/607) perceiving tropika as more severe vs. 45.1% (274/607) perceiving tersiana as more severe. 47.9% (291/607) of respondents acknowledged the possibility of asymptomatic malaria while 28.8% (175/607) perceived it to be impossible to have malaria in the blood without symptoms (Table 2).
Table 2.
Malaria types, etiologies, and treatment perceptions mentioned in survey (N = 607)
| Frequency (n) | Proportion (%) | |
|---|---|---|
| Knowledge of several types of malaria | ||
| Awareness of different malaria species | 587 | 96.7 |
| Types of malaria mentioned | ||
| Malaria tersiana | 573 | 94.4 |
| Malaria tropika | 571 | 94.1 |
| Mixed infection | 25 | 4.1 |
| Malariae malaria | 18 | 3.0 |
| Bone malaria | 17 | 2.8 |
| Others (quartana, haemorrhagic fever, batana, gamete) | 28 | 4.6 |
| Perceived malaria severity | ||
| Tersiana is more severe | 267 | 44.0 |
| Tropika is more severe | 274 | 45.1 |
| Other types of malaria are more severe | 14 | 2.3 |
| Do not know | 18 | 3.0 |
| No answer | 34 | 5.6 |
| Perception of the cause of a malaria episode | ||
| Fatigue | 284 | 46.8 |
| Mosquito bite | 224 | 36.9 |
| Weather-related (rain/cold/heat/etc.) | 138 | 22.7 |
| Eating too late in the evening | 107 | 17.6 |
| Worked too hard | 57 | 9.4 |
| Food-related (ate cold food/street food/cane and coconut) | 6 | 1.0 |
| Not using bed net | 31 | 5.1 |
| Perception of malaria illness | ||
| Possible to have malaria in blood and feeling healthy | 291 | 47.9 |
| Not possible to have malaria in blood and feeling healthy | 175 | 28.8 |
| Do not know | 141 | 23.2 |
| Perception of malaria treatment effectiveness | ||
| Make body feel better | 581 | 95.7 |
| Does not make body feel better | 21 | 3.5 |
Malaria aetiologies
Only 36.9% (N = 224) of respondents mentioned mosquito bites as the cause of their last malaria episode, as participants perceived malaria episodes to have multiple etiologies. A malaria episode could be caused by being near bodies of water (e.g., rivers, ditches) or triggered by working too hard and not eating well. Among survey respondents (Table 2), 46.8% (N = 284) reported that the fatigue caused by working too hard made people vulnerable to malaria, especially in combination with adverse weather conditions (22.7%, N = 138). The physical conditions that were perceived to favor a malaria episode included lower general body fitness and a weakened immune system.
Perception of recurrences
Participants frequently experienced repeated episodes of malaria, but there was no perceived difference between a primary malaria episode and a relapse. The length of time between one malaria episode and another was not considered an indicator of whether a malaria episode was a reinfection or a relapse. Papuan populations who had lived in the area for generations reported that repeated malaria episodes cause belly swelling due to spleen enlargement. They told their children not to hit someone in the belly to avoid breaking the spleen, and the term “poro limpa” (literally “spleen belly”) is used to mock people with rotund midsections.
Participants who were interviewed during the trial mentioned repeated malaria episodes were due to not finishing the drugs. As expressed by a male patient from the supervised trial arm: “Probably the malaria (parasite) just fainted. So, if we do not finish the medicines, it would be stronger in our body.” In the survey, most respondents agreed that recurrences are possible after a fully treated malaria infection (71.3%, 433/607) (Table 3).
Table 3.
Self-reported recurrence of trial participants (N = 607)
| Frequency (n) |
Proportion (%) | |
|---|---|---|
| Perception of recurrence | ||
| Malaria can come back after finishing medication | 433 | 71.3 |
| Malaria cannot come back after finishing medication | 162 | 26.7 |
| Do not know | 12 | 2.0 |
| Self-reported recurrence during trial period (participant recall) | ||
| Had another malaria episode during the trial | 139 | 22.9 |
| Did not have another malaria episode during the trial | 439 | 72.3 |
| Do not remember malaria episode | 2 | 0.3 |
| Recurrences recorded during the trial* (N = 409) | ||
| 1 or more recurrences | 174 | 42.5 |
| No recurrences | 235 | 57.5 |
*Recurrences during the trial follow-up period were only recorded in the supervised and unsupervised arms; in the control arm these data were not recorded as they were only visited once at the end of the 6-month period since enrolment
Therapeutic itineraries
Biomedical and alternative treatment
The qualitative analyses showed that malaria was frequently self-diagnosed based on symptom recognition, with fever being the most widely reported characteristic of malaria. Associated with self-diagnosis was the common use of various home treatments (including paracetamol, traditional treatments, and leftover antimalarials) to counter the first signs of malaria. In the survey (Table 4), most participants reported that prior to visiting a health service, they relieved symptoms with leftover drugs kept at home (40.4%, 245/607) or with non-prescription drugs purchased over the counter (17.0%, 103/607). Visiting a health facility was the second stage of the therapeutic itinerary, usually occurring later than 24 h after symptom onset. In the qualitative interviews, people reported this delay to be intentional to increase the likelihood that their malaria test would be positive. The test was thought to be negative if done in the initial stages of illness onset, causing disappointment because this meant they would get no treatment to take home. Negative test results, therefore, drove people to visit different health centres to disprove the initial test results. Health care workers often have no explanation as to the cause of fever in the event of negative malaria test results.
Table 4.
Treatment-seeking behaviour and drug of choice for malaria symptoms (N = 607)
| Frequency (n) |
Proportion (%) |
|
|---|---|---|
| Steps of treatment before visiting health provider (can give multiple answers) | ||
| Use remaining drugs at home | 245 | 40.4 |
| Do nothing | 214 | 35.3 |
| Buy over-the-counter drugs (pharmacy/kiosk/etc.) | 103 | 17.0 |
| Herbal treatments | 59 | 9.7 |
| Blood letting | 26 | 4.3 |
| Others (pray, drink, get warm, apply wet cloth, etc.) | 32 | 5.3 |
| Time of visit to health provider | ||
| Same day as onset of symptoms | 44 | 7.2 |
| Day after onset of symptoms | 131 | 21.6 |
| Two days or more after onset of symptoms | 425 | 70.0 |
| Do not remember | 6 | 1.0 |
| First choice of health provider | ||
| Public health centre | 415 | 68.4 |
| Malaria control clinic* | 138 | 22.7 |
| Private clinics/private practice | 17 | 2.8 |
| General hospital | 6 | 1.0 |
| Caritas hospital* | 21 | 3.5 |
| Others (over-the-counter drugs, community health worker, etc.) | 8 | 1.3 |
| Do not know | 2 | 0.3 |
| First choice of drug ( can give multiple answers) | ||
| Blue drugs | 537 | 88.5 |
| Brown drugs [without blue drugs] | 334 [21] | 55.0 [3.5] |
| Green drugs | 64 | 10.5 |
| Yellow drugs | 136 | 22.4 |
| Primaquine | 46 | 7.6 |
| Spleen drugs | 7 | 1.2 |
| Small drugs | 24 | 4.0 |
| Paracetamol | 111 | 18.3 |
| White drugs | 149 | 24.5 |
| Chloroquine/quinine | 11 | 1.8 |
| Antibiotics | 4 | 0.7 |
| Supplements/vitamins | 7 | 1.2 |
| Herbal e.g., papaya leaves, cat’s whiskers, … | 56 | 9.2 |
| Use of herbal medication | ||
| Oral/topical, complementary to medical drug | 46 | 7.6 |
*Funded by the mining company; free healthcare for seven ethnic groups directly affected by mining operations
Public and private health facilities
Significant differences between the experiences of care at the government public health centre and at the malaria control clinics were reported. According to community members and healthcare workers, tests were only conducted if the clinical symptoms warranted such a test at public health centres; while at malaria control clinics, people were tested for malaria immediately and regardless of symptoms, which increased the perceived quality of care. Participants were less certain that medication would be prescribed at the public health centre and did not trust its quality of service and drugs. Malaria control clinics were also perceived to be less crowded and have shorter waiting times and longer opening hours into the afternoon, which suited people who farm in the morning.
Despite the perception of less favourable service at government public health centres, 68.4% (415/607) stated that government public health centres were their first choice of health provider, whereas 22.7% (138/607) reported that a malaria control clinic was their first choice (Table 4).
Perceptions of anti-malaria medicines
DHA-piperaquine (DP)
The standard schizontocidal treatment, DP, was locally known as the ‘blue drug’ due to its colour. The blue drug invoked confidence, which was related to expectations of the type of malaria it targeted, its perceived side effects, its perceived effectiveness, and the duration of the treatment. Some people remembered the first time in 2006 when DP became widely used and considered it to be highly efficacious in relieving symptoms compared to the previous malaria drugs. Over time, this treatment has become socially acceptable, and adhering is considered socially responsible—the mother who gives DP to her child is seen as providing ‘good’ care for her children. The treatment was reported to produce side effects such as nausea, and the large size and the number of tablets taken per day (based on bodyweight, an adult typically needs to take 3–4 tablets/day) was considered unpleasant, but these aspects had become part of the common knowledge on how DP worked and were accepted as part of the package of care that comes with the ‘blue drug’ regimen.
Primaquine (PQ)
Unlike the ‘blue drug,’ PQ was not identified as being a malaria drug. Among some indigenous populations, PQ is called the ‘spleen drug,’ while DP is called ‘malaria drug.’ PQ was also commonly referred to as the ‘brown drug’ and its earthy colour did not stand out as much as the bright blue DP tablets. PQ tablets are smaller than DP, a characteristic which was associated with the drug being less ‘strong’ and less ‘important.’ The prescription of PQ is inconsistent, since not all malaria patients would get it, or different durations would be prescribed. While patients were advised to finish all PQ tablets, the role of the drug for malaria was often not stated. Inconspicuous drug appearance, combined with inconsistent prescription of PQ and its long duration of treatment resulted in the meaning of PQ being re-interpreted at community level and harmonized with perceptions of vitamins or supplements. As recalled by one trial participant: “…yes, just vitamin, the small one, for malaria parasite, the colour was brown. I just took one tablet per night.”
These qualitative findings were confirmed in the survey in which 88.5% (537/607) of respondents provided the ‘blue drugs’ as the first answer when asked about their first choice of malaria drugs, followed by 55.0% (334/607) mentioning the ‘brown drugs’ (Table 4). Only 3.5% (21/607) referred to the ‘brown drugs’ without the ‘blue drugs.’ Some patients mentioned ‘green drugs’ (10.5%; N = 64). The first DP tablets distributed in the area in 2006 were green, so it could be assumed that green drugs refer to DP as well. Other names/colours that could indicate PQ were ‘yellow drugs,’ ‘small drugs,’ and ‘spleen drugs.’ A small proportion of participants (7.6%; N = 46) mentioned ‘primaquine’ by name.
Alternative therapies
Almost all participants believed in the effectiveness of biomedical malaria treatment and the use of traditional or herbal therapeutics was seen as complementary and used to relieve symptoms instead of as a cure. A variety of nettles were applied on the skin, generating a sensation of itching or heat to alleviate the feeling of joint pain and general body discomfort. Some people grew them in their garden. One type of nettle (Laportea decumana) was sold at the markets, typically by vendors from the highland Papuan populations. Blood letting was another customary practice among highland populations to reduce the feeling of pain in certain areas of the body (for example, blood letting from the forehead in the case of headaches).
Malaria treatment adherence
Perceptions and experiences with treatment completion
In line with the perceptions of malaria medicines, completion of malaria treatment was only associated with DP, and not with PQ. Furthermore, the long duration of PQ treatment, that exceeded resolution of malaria symptoms, reinforced the belief of the potency of DP and the lack of importance of PQ. When asked which drugs participants received in the last malaria episode during the trial, 86.8% (527/607) mentioned the ‘blue drugs’ and 58.8% (357/607) the ‘brown drugs (Table 5).’ A follow-up question to the participants who claimed that they had only completed some of the drugs (N = 88) found that 86.4% (N = 76) of participants specified finishing the ‘blue drugs’ but only 22.7% (N = 20) the ‘brown drugs.’
Table 5.
Perception and experience of completing course of drugs (N = 607)
| Frequency (n) |
Proportion (%) |
|
|---|---|---|
| Perception on completing course of drugs | ||
| Should finish all drugs prescribed/given | 522 | 86.0 |
| Should stop once you feel better | 79 | 13.0 |
| Depending on the type of malaria | 2 | 0.3 |
| Do not know | 4 | 0.6 |
| Drugs received during the trial/in the last malaria episode (can have multiple answers) | ||
| Blue drugs | 527 | 86.8 |
| Brown drugs | 357 | 58.8 |
| Yellow drugs | 136 | 22.4 |
| Small drugs | 32 | 5.3 |
| Spleen drugs | 8 | 13.2 |
| Primaquine | 41 | 6.8 |
| Green drugs | 39 | 6.4 |
| Paracetamol (incl. Dumin*/drugs for fever) | 212 | 34.9 |
| White drugs | 136 | 22.4 |
| Drugs finished during the trial/in the last malaria episode | ||
| All drugs b | 511 | 84.2 |
| Some of the drugs a c | 88 | 14.9 |
| Did not finish any drugs c | 6 | 1.0 |
| Do not remember | 2 | 0.3 |
| Which drugs were finished (asked to those who finished some of the drugs, N = 88) a | ||
| Blue drugs | 76 | 86.4 |
| Brown drugs | 20 | 22.7 |
| Small drugs | 2 | 2.3 |
| Primaquine | 4 | 4.6 |
| Yellow drugs | 5 | 5.7 |
| Green drugs | 3 | 3.4 |
| Paracetamol (incl. Dumin*) | 6 | 6.8 |
| White drugs | 6 | 6.8 |
| Others (antibiotics/drugs for nausea) | 2 | 2.3 |
| Reasons for finishing the drugs (asked to those who finished all drugs, N = 511) b | ||
| Wanting to get better | 298 | 58.3 |
| Not wanting to get malaria again | 126 | 24.7 |
| The doctor/trial staff told me to | 87 | 17.0 |
| To stay healthy after feeling better | 12 | 13.6 |
| To kill the germs/the illness | 5 | 1.0 |
| Liking taking drugs | 2 | 0.4 |
| Considering it poor practice not to finish the drugs | 1 | 0.2 |
| I do not know | 3 | 0.6 |
| Reasons for not finishing (asked to those who finished some of the drugs and who did not finish any drugs, N = 94) c | ||
| Feeling better/no more fever or nausea | 69 | 73.4 |
| Forgetting to take the drug | 14 | 14.9 |
| The taste of the drug/Not liking drugs | 12 | 12.8 |
| Nausea/dizziness | 5 | 5.3 |
| Others (course was too long/took other drugs/someone told me not to/prefer the blue drugs) | 5 | 5.3 |
*A local brand of paracetamol
a Which drugs were finished (asked to those who finished some of the drugs, N = 88)
b Reasons for finishing the drugs (asked to those who finished all drugs, N = 511)
c Reasons for not finishing (asked to those who finished some of the drugs and who did not finish any drugs, N = 94)
Completing the full course of treatment was perceived as important by 86.0% (522/607) of participants and 84.2% (N = 511) stated they finished all drugs in their last malaria episode (Table 5). ‘Wanting to get better’ was the main motivation for completion (58.3%; 298/511), followed by ‘not wanting to get malaria again’ (24.7%; 126/511). The most cited reason for not completing treatment was feeling better or no longer having fever (73.4%; 69/94). In addition, perceptions of what malaria treatments were needed and were received during the trial varied, indicating that adherence to the package of treatments included in radical cure was not straightforward.
Aggregated adherence predictors
Trial participants in all three arms reported different adherence to the treatment. Measure of adherence to calculate predictors was derived from the survey questions on completion of both PQ and DP. Adherence was low in all arms: 71.2% (131/184) in the supervised arm, 56.9% (91/160) in the unsupervised arm, and 62.4% (164/263) in the control arm; overall difference p = 0.019, Table 6. Adherence did not differ between patients who were interviewed in the survey within the 6 months trial (before the last follow-up visit, or for control arm, the only follow-up visit) or after (p = 0.417, Table 6).
Table 6.
Aggregated measure of adherence* in different study arms and ethnic groups (N = 607)
| Adherence N (%) |
Non-adherence N (%) |
p-value** | |
|---|---|---|---|
| Study intervention arm | 0.019 | ||
| Supervised (N = 184) | 131 (71.2) | 53 (28.8) | |
| Unsupervised (N = 160) | 91 (56.9) | 69 (43.1) | |
| Control (N = 263) | 164 (62.4) | 99 (37.6) | |
| Malaria species at trial enrolment | 0.084 | ||
| P. vivax single/mix (N = 260) | 179 (68.8) | 81 (31.2) | |
| P. falciparum (N = 307) | 182 (59.3) | 125 (40.7) | |
| Other species (N = 2) | 1 (50) | 1 (50) | |
| Species details not available (N = 38) | 24 (63.2) | 14 (36.8) | |
| Group of ethnicities | < 0.001 | ||
| Highland Papuans (N = 99) | 47 (47.5) | 52 (52.5) | |
| Lowland Papuans (N = 147) | 76 (51.7) | 71 (48.3) | |
| Non-Papuans (N = 361) | 263 (72.9) | 98 (27.1) | |
| Time between trial enrolment and survey | 0.417 | ||
| Before/at 6-month follow-up (N = 195) | 129 (66.2) | 66 (33.9) | |
| After 6-month follow-up (N = 412) | 257 (62.4) | 155 (37.6) |
*Measure of adherence was calculated from survey participants who said they finished all or some of the drugs given during trial/in the last malaria episode AND mentioned both ‘blue drugs’ PLUS any of the following: ‘brown drugs,’ ‘small drugs,’ ‘spleen drugs,’ or ‘primaquine’
**p-values were calculated with Fisher’s exact test for malaria species and chi-square tests for the other variables
In both the supervised and unsupervised arms all patients were given 14-day PQ regardless of malaria species whereas in the control arm only those with single or mixed P. vivax or P. ovale infections received 14-day PQ. For this reason, whether adherence differed with the species of malaria at enrolment was explored. There was no significant difference between malaria diagnosis and self-reported adherence (p = 0.084, Table 6). There was a significant difference between the different ethnicities in terms of reported adherence (p < 0.001), with both highland (Amungme, Damal, Dani, Mee, Moni, Nduga, Wamena) and lowland (Kamoro, Asmat, Biak, Fakfak, Jayapura, Merauke, Moor, Serui, Sorong) Papuans reporting lower adherence: 47.5% (47/99) and 51.7% (76/147) for highland and lowland Papuans, respectively than non-Papuans (72.9%; 263/361, Table 6).
Malaria species at enrolment (Fisher’s exact test p = 0.011) and ethnicity (chi-square test p = 0.005) differed significantly between the study arms. In the post hoc analysis using the Marascuilo procedure, the difference in adherence between unsupervised and control groups and between highland-Papuan and non-Papuan ethnic groups, remained significant.
Reported influence of supervision strategy on adherence
In the supervised arm, qualitative inquiries indicated that both trial participants and field supervision staff considered that supervised treatment on alternate days was suboptimal because participants were dependent on meeting field supervision staff to receive their tablets. If they were not reached at home for supervision visits, they could not take the PQ dose for that day. The qualitative strand also revealed that language barriers complicated the social relations between participants and field researchers of different ethnicities, since some Papuans spoke little Indonesian and few field researchers spoke any of the local languages. Moreover, high population mobility among certain ethnicities, such as travelling for mangrove forest fishing (Kamoro) and travelling between mountain villages and the mine (Dani, Damal, Moni), complicated both the supervision of the adherence by field researchers and the adherence by participants.
Discussion
This mixed-methods study investigated adherence to the radical cure treatment of P. vivax, including both a 3-day regimen of DP plus a 14-day regimen of PQ in Papua’s Mimika District, Indonesia. The findings highlight that in this location, (i) ethnicity is a strong predictor of adherence and influenced the effectiveness of treatment supervision; (ii) perceptions of and experiences with different healthcare providers, together with (iii) perceptions of medicines, influenced the process of treatment adherence.
-
(i)
Ethnicity In Papua, the perceptions of medicines and of malaria must be interpreted in the context of a remarkably diverse ethnic setting. Furthermore, the presence of a local mine dominates the socioeconomic landscape. As is frequently found in mining communities, the mine attracts people from other parts of the country to form the main mine workforce [31], connecting local and non-local workforces with the same healthcare services. There were interethnic differences in adherence to malaria medication, with lower adherence among Papuan populations as compared to non-Papuan ethnicities. The qualitative strand of this study shows that ethnicity-related structural factors could explain the interethnic difference in treatment adherence. First, language barriers complicated an efficient dialogue between healthcare workers and patients enabling a shared understanding of the importance of adherence to P. vivax malaria treatment [24]. Many healthcare workers are not Papuan and do not speak any of the local languages. Second, Papuan populations are socially and economically marginalized, with lower educational and employment opportunities compared to non-Papuans [26]. Issues of trust and hierarchical healthcare worker-patient relationship—that are well documented in other health interventions—can contribute to explaining lower adherence in highland and lowland Papuans [9]. Third, the mobility associated with mining work and the subsistence strategies practiced by both highland and lowland Papuans are influencing the feasibility of adhering to long duration treatments. Treatment supervision and encouragement provided by someone living in the same village or neighborhood (for example a community health worker)—adapting both the visiting hours and the explanation about the medicines to the patient’s socio-ethnic context—has potential to minimize this problem.
-
(ii)
Perceptions of and experiences with different healthcare providers In this complex social context, people often self-diagnose malaria based on symptoms and usually only visit a health provider more than 24 h after onset of symptoms, with the purpose of confirming their self-diagnosis and to receive treatment. The high prevalence of malaria often makes people consider malaria when they have a fever, and this prompts the expectation of a positive diagnosis and associated DP treatment when they visit a health facility. The aversion to having a negative diagnosis and hence not receiving treatment is compounded by the fact that examination for other probable causes of fever is rarely done at any clinic. Gaps in diagnosis for acute febrile illness at primary healthcare settings are common in the global south [32–34]. In Indonesia, like in many other countries, few studies have investigated the epidemiology of febrile illness etiologies [35]. Availability of point-of-care diagnostic tools for other probable causes of fever would lead to more efficient malaria control [36].
In this setting, the mining company-funded health providers represent an important alternative to public health providers, as they have existed for longer and were perceived as more likely to provide a positive diagnosis than public health centers. The choice of health provider is also driven by factors of familiarity, cost of treatment and transport, waiting time, and perceived quality of service [37, 38]. Prior to a visit to a health provider, the severity of the symptoms was usually assessed by a self-treatment with drugs leftover from previous illnesses or with over-the counter drugs [39, 40]. Herbal topical and oral remedies were complementary medicines used to alleviate symptoms rather than replace biomedical treatment [41].
-
(iii)
Perception of medicines Local healthcare workers and community members differentiated between two types of malaria (tropika and tersiana), against which ‘blue drugs’ (referring to DP) were perceived to be efficacious while the ‘brown drugs’ (referring to PQ) were considered less potent, associated to vitamins or supplements. PQ had been around long before DP was introduced as a malaria treatment and has accompanied different drugs that were used as treatment for acute stage malaria. However, DP was invariably perceived as malaria drug and the self-reported adherence to DP was far better than to PQ. This suggested a high level of acceptance of DP as a new drug introduced in 2006 to replace other schizontocidal drugs that were inefficacious due to resistance [42, 43].
Inconsistent prescribing of PQ by healthcare providers and the long duration of PQ treatment helped shape conceptualizations of PQ as supplements rather than as malaria treatment. Since 2006, clinical guidelines have recommended that patients diagnosed with P. falciparum get a single dose of PQ, whilst patients diagnosed with P. vivax malaria get a 14-day low dose PQ regimen. Inconsistent and inadequate dosage of PQ prescriptions also happened due to the unfamiliarity of health workers with treatment guidelines or due to supply shortages [30]. In the context of this trial, the recommended low dose was used in the control arm, while the supervised and unsupervised arms were treated with a higher dose PQ regimen. Without supervision, the challenges of the long duration and higher number of daily tablets received by participants in the unsupervised arm could consequently have reduced adherence.
This study also found that the physical appearance of the PQ (smaller size and earthy colour) in contrast to DP evoked a perception of it being less important. The relationship between drug colour and size and perceived efficacy has been studied in other settings, and the colour and size that is perceived to be “strong” might vary across cultures [44, 45]. An appearance that evoked a perception of a drug being “strong” could prove essential in increasing drug adherence.
Limitations
This study has several limitations. The sample only included patients recruited from public health centres into a clinical trial and this may have introduced selection and participation bias. Although very few patients screened for enrolment were subsequently excluded from the study, caution is needed in over-interpreting the study results particularly regarding treatment seeking behaviour. The proportion of community members that prefer mining company-funded health providers might be higher and there may be a part of the community that was not sampled since they did not access healthcare despite having malaria. Most of the study population were young (as expected as malaria is more prevalent in children), however the treatment-seeking behaviour for adult or child patients (through their guardian) were not differentiated. This study did not conduct multivariable analysis to quantify potential interactions between the factors influencing adherence. Other potential confounders such as gender and economic status were also not analysed in this paper.
Conclusion
Although oral supervision of a 14-day PQ course for P. vivax malaria may improve adherence to a prolonged treatment regimen, the supervision strategy was challenged by both socio-cultural factors (language and other interethnic encounter barriers, high mobility of patients of certain ethnicities/occupations, perceptions of medicines) and practical bottlenecks (dosage change, difficult implementation of home visits by fieldworkers to remote locations) resulting in lower adherence among indigenous populations compared to non-indigenous populations. Both socio-cultural logics and practical challenges that underlie patient decisions to adhere to or to abandon PQ treatment are important to address. The lack of representation of indigenous populations among healthcare workers is a likely contributor. Structural barriers that hinder the process of patient adherence are crucial to consider in the development and roll-out of effective malaria treatment policies such as oral supervision strategies.
Supplementary Information
Additional file 1. Table S1. Demographic characteristics of survey participants
Acknowledgements
We thank all the patients, health staff, and the study team who took part in this study. We thank Mark T. Westra and Satya Nugraha (www.akvo.org, Amsterdam, The Netherlands) for the technical support in survey data management, and Niamh Meagher (Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, VIC, Australia) for providing the trial data. We are grateful for Pak Reynold Rizal Ubra (District Health Office, Papua, Indonesia) and Prof Yati Soenarto (Universitas Gadjah Mada, Yogyakarta, Indonesia) for their continuing support to our works in Timika, Indonesia.
Author contributions
JRP, KT, AD, RNP, KPG, BL, and CG designed and supervised the study. AR, CURL, and MR collected the qualitative data and carried out the preliminary analysis. AR, JRP, EK and FHB facilitated and supervised the quantitative survey. AR, MR, KPG, and CG analysed the data and wrote the first draft of the manuscript. All authors contributed to writing the final manuscript. All authors read and approved the final manuscript.
Funding
The trial that this study was embedded in was funded by the Gates Foundation (INV-007122) and the Department of Foreign Affairs of the Australian Government (72904). RNP is a Wellcome Trust Senior Fellow in Clinical Science (200909), and KT is a CSL Centenary Fellow. The trial was supported by the Australian Centre for Research Excellence on Malaria Elimination (ACREME), funded by the NHMRC of Australia 91134989. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed in the quantitative strand of this study are included in this published article [and its supplementary information files]. The data that support the findings and analyzed in the qualitative strand of this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The trial and the mixed-methods study were conducted according to the principles stated in the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects) as amended in 2008, all applicable regulations and according to established international scientific standards. The study protocol was reviewed and approved by the ethical committees of the Faculty of Medicine Gadjah Mada University in Indonesia (KE/FK/522/EC/2016), the Human Research Ethics Committee (HREC) of the Northern Territory (NT) Department of Health and Menzies School of Health Research in Australia (15-2517), and the Institute of Tropical Medicine in Belgium (1163/17). For the qualitative strand, oral consent was collected from all participants and, for minors, from a legal guardian. For the quantitative survey, written informed consent was obtained from all participants or legal guardians in case they were underage. Illiterate participants willing to participate provided consent by a fingerprint in the presence of a witness.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Price RN, Commons RJ, Battle KE, Thriemer K, Mendis K. Plasmodium vivax in the era of the shrinking P. falciparum map. Trends Parasitol. 2020;36:560–70. doi: 10.1016/j.pt.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. World Malaria Report 2019. Geneva. World Health Organization, 2019.
- 3.Thriemer K, Ley B, Bobogare A, Dysoley L, Alam MS, Pasaribu AP, et al. Challenges for achieving safe and effective radical cure of Plasmodium vivax: a round table discussion of the APMEN Vivax Working Group. Malar J. 2017;16:141. doi: 10.1186/s12936-017-1784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernando D, Rodrigo C, Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malar J. 2011;10:351. doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John GK, Douglas NM, Von Seidlein L, Nosten F, Baird JK, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012;11:280. doi: 10.1186/1475-2875-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thriemer K, Ley B, von Seidlein L. Towards the elimination of Plasmodium vivax malaria: implementing the radical cure. PLoS Med. 2021;18:e1003494. doi: 10.1371/journal.pmed.1003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Seidlein L, Auburn S, Espino FE, Shanks D, Cheng Q, McCarthy J, et al. Review of key knowledge gaps in glucose-6-phosphate dehydrogenase deficiency detection with regard to the safe clinical deployment of 8-aminoquinoline treatment regimens: a workshop report. Malar J. 2013;12:112. doi: 10.1186/1475-2875-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale malaria. Geneva. World Health Organization, 2016.
- 9.Chu CS, White NJ. Management of relapsing Plasmodium vivax malaria. Expert Rev Anti Infect Ther. 2016;14:885–900. doi: 10.1080/14787210.2016.1220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.John CC. Primaquine plus artemisinin combination therapy for reduction of malaria transmission: promise and risk. BMC Med. 2016;14:65. doi: 10.1186/s12916-016-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeters Grietens K, Soto V, Erhart A, Muela Ribera J, Toomer E, Tenorio A, et al. Adherence to 7-day primaquine treatment for the radical cure of P. vivax in the peruvian Amazon. Am J Trop Med Hyg. 2010;82:1017–23. doi: 10.4269/ajtmh.2010.09-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruxvoort K, Goodman C, Kachur SP, Schellenberg D. How patients take malaria treatment: asystematic review of the literature on adherence to antimalarial drugs. PLoS One. 2014;9:e84555. doi: 10.1371/journal.pone.0084555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saint-Gerons DM, Rodovalho S, Barros Dias ÁL, de Carvalho A, Beratarrechea ALU, Monteiro WM, et al. Strengthening therapeutic adherence and pharmacovigilance to antimalarial treatment in Manaus, Brazil: a multicomponent strategy using mHealth. Malar J. 2022;21:28. doi: 10.1186/s12936-022-04047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiteh F, Okebe J, Masunaga Y, D’Alessandro U, Achan J, Gryseels C, et al. Understanding adherence to reactive treatment of asymptomatic malaria infections in the Gambia. Sci Rep. 2021;11:1746. doi: 10.1038/s41598-021-81468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheoymang A, Ruenweerayut R, Muhamad P, Rungsihirunrat K, Na-Bangchang K. Patients’ adherence and clinical effectiveness of a 14-day course of primaquine when given with a 3-day chloroquine in patients with Plasmodium vivax at the Thai-Myanmar border. Acta Trop. 2015;152:151–6. doi: 10.1016/j.actatropica.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Pereira EA, Ishikawa EA, Fontes CJ. Adherence to Plasmodium vivax malaria treatment in the brazilian Amazon Region. Malar J. 2011;10:355. doi: 10.1186/1475-2875-10-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi R, Lawpoolsri S, Imwong M, Kobayashi J, Kaewkungwal J, Pukrittayakamee S, et al. Directly-observed therapy (DOT) for the radical 14-day primaquine treatment of Plasmodium vivax malaria on the Thai-Myanmar border. Malar J. 2010;9:308. doi: 10.1186/1475-2875-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poespoprodjo JR, Burdam FH, Candrawati F, Ley B, Meagher N, Kenangalem E, et al. Supervised versus unsupervised primaquine radical cure for the treatment of falciparum and vivax malaria in Papua, Indonesia: a cluster randomised, controlled, open-label superiority trial. Lancet Infect Dis. 2022;22:367–76. doi: 10.1016/S1473-3099(21)00358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehr A, Nieto-Sanchez C, Muela J, Jaiteh F, Ceesay O, Maneh E, et al. From informed consent to adherence: factors influencing involvement in mass drug administration with ivermectin for malaria elimination in the Gambia. Malar J. 2021;20:198. doi: 10.1186/s12936-021-03732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muela Ribera J, Hausmann-Muela S, Gryseels C, Peeters Grietens K. Re-imagining adherence to treatment from the “other side”: local interpretations of adverse anti-malarial drug reactions in the peruvian Amazon. Malar J. 2016;15:136. doi: 10.1186/s12936-016-1193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karyana M, Devine A, Kenangalem E, Burdarm L, Poespoprodjo JR, Vemuri R, et al. Treatment-seeking behaviour and associated costs for malaria in Papua, Indonesia. Malar J. 2016;15:536. doi: 10.1186/s12936-016-1588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devine A, Kenangalem E, Burdam FH, Anstey NM, Poespoprodjo JR, Price RN, et al. Treatment-seeking behavior after the implementation of a unified policy of dihydroartemisinin- piperaquine for the treatment of uncomplicated malaria in Papua, Indonesia. Am J Trop Med Hyg. 2018;98:543–50. doi: 10.4269/ajtmh.17-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse JM. Approaches to qualitative-quantitative methodological triangulation. Nurs Res. 1991;40:120–3. doi: 10.1097/00006199-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Jaccard J, Jacoby J. Theory construction and model-building skills: a practical guide for social scientists. New York: Guilford Press; 2010. [Google Scholar]
- 25.Hadiprayitno II. The limit of narratives: ethnicity and indigenous rights in Papua, Indonesia. Int J Minor Gr Rights. 2017;24:1–23. doi: 10.1163/15718115-02401004. [DOI] [Google Scholar]
- 26.Upton S. The impact of migration on the people of Papua, Indonesia. A historical demographic analysis. University of New South Wales. 2009.
- 27.Ploeg A. Pathways of change. Marseille: Pacific-Credo Publications; 2020. p. 163. [Google Scholar]
- 28.Diamond J. Guns, germs and steel: a short history of everybody for the last 13,000 years. Surrey: Vintage; 1998. p. 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auwalin I. Ethnic identity and internal migration decision in Indonesia. J Ethn Migr Stud. 2020;46:2841–61. doi: 10.1080/1369183X.2018.1561252. [DOI] [Google Scholar]
- 30.Douglas NM, Poespoprodjo JR, Patriani D, Malloy MJ, Kenangalem E, Sugiarto P, et al. Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: a hospital-based cohort study. PLoS Med. 2017;14:e1002379. doi: 10.1371/journal.pmed.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petkova-Timmer V, Ivanova G, Rolfe J. Mining developments and social impacts on communities: Bowen basin case studies. Rural Soc. 2009;19:211–28. doi: 10.5172/rsj.19.3.211. [DOI] [Google Scholar]
- 32.Shrestha P, Roberts T, Homsana A, Myat TO, Crump JA, Lubell Y, et al. Febrile illness in Asia: gaps in epidemiology, diagnosis and management for informing health policy. Clin Microbiol Infect. 2018;24:815–26. doi: 10.1016/j.cmi.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Maze MJ, Bassat Q, Feasey NA, Mandomando I, Musicha P, Crump JA. The epidemiology of febrile illness in sub-saharan Africa: implications for diagnosis and management. Clin Microbiol Infect. 2018;24:808–14. doi: 10.1016/j.cmi.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira J, Bressan CS, Brasil P, Siqueira AM. Epidemiology of acute febrile illness in Latin America. Clin Microbiol Infect. 2018;24:827–35. doi: 10.1016/j.cmi.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasem MH, Kosasih H, Tjitra E, Alisjahbana B, Karyana M, Lokida D, et al. An observational prospective cohort study of the epidemiology of hospitalized patients with acute febrile illness in Indonesia. PLoS Negl Trop Dis. 2020;14:e0007927. doi: 10.1371/journal.pntd.0007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoler J, Awandare GA. Febrile illness diagnostics and the malaria-industrial complex: a socio-environmental perspective. BMC Infect Dis. 2016;16:683. doi: 10.1186/s12879-016-2025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thandar MM, Kyaw MP, Jimba M, Yasuoka J. Caregivers’ treatment-seeking behaviour for children under age five in malaria-endemic areas of rural Myanmar: a cross-sectional study. Malar J. 2015;14:1. doi: 10.1186/1475-2875-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mujica Mota RE, Lara AM, Kunkwenzu ED, Lalloo DG. Health seeking behavior after fever onset in a malaria-endemic area of Malawi. Am J Trop Med Hyg. 2009;81:935–43. doi: 10.4269/ajtmh.2009.08-0361. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed SM, Haque R, Haque U, Hossain A. Knowledge on the transmission, prevention and treatment of malaria among two endemic populations of Bangladesh and their health-seeking behaviour. Malar J. 2009;8:173. doi: 10.1186/1475-2875-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naing PA, Maung TM, Tripathy JP, Oo T, Wai KT, Thi A. Awareness of malaria and treatment-seeking behaviour among persons with acute undifferentiated fever in the endemic regions of Myanmar. Trop Med Health. 2017;45:31. doi: 10.1186/s41182-017-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gryseels C, Uk S, Erhart A, Gerrets R, Sluydts V, Durnez L, et al. Injections, cocktails and diviners: therapeutic flexibility in the context of malaria elimination and drug resistance in Northeast Cambodia. PLoS One. 2013;8:e80343. doi: 10.1371/journal.pone.0080343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karyana M, Burdarm L, Yeung S, Kenangalem E, Wariker N, Maristela R, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poespoprodjo JR, Kenangalem E, Wafom J, Chandrawati F, Puspitasari AM, Ley B, et al. Therapeutic response to dihydroartemisinin-piperaquine for P. falciparum and P. vivax nine years after its introduction in southern Papua, Indonesia. Am J Trop Med Hyg. 2018;98:677–82. doi: 10.4269/ajtmh.17-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brieger WR, Salami KK, Oshiname FO. Perceptions of drug color among drug sellers and consumers in rural southwestern Nigeria. Res Soc Adm Pharm. 2007;3:303–19. doi: 10.1016/j.sapharm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Tao D, Wang T, Wang T, Qu X. Influence of drug colour on perceived drug effects and efficacy. Ergonomics. 2018;61:284–94. doi: 10.1080/00140139.2017.1349935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Demographic characteristics of survey participants
Data Availability Statement
All data generated or analyzed in the quantitative strand of this study are included in this published article [and its supplementary information files]. The data that support the findings and analyzed in the qualitative strand of this study are available from the corresponding author on reasonable request.