Abstract
We have isolated cold-sensitive fermentation mutants (Csf mutants) of a commercial baker's yeast that have practically no fermentation capacity at 5°C and return to their normal capacity at 25 to 40°C. CSF1 was cloned by functional complementation of the Csf phenotype. CSF1 contain an open reading frame of 8,874 nucleotides, encoding a protein of 2,958 amino acids. The nucleotide sequence was identical to that of the YLR087C gene in the Saccharomyces genome database, but there was no information about the function of the predicted CSF1 (YLR087C) protein. Gene disruption shows that CSF1 is required for growth and fermentation only at low temperatures. Permeabilized cells of the disruptant showed nearly the same ethanol production rate as those of the parent strain, even at 10°C. The disruptant cells had the same glucose uptake rates as the parental cells at 30°C, but three- to fivefold-lower rates than the parental cells at 10°C. These findings suggest that CSF1 associates with a new nutrient transport system which exists on the plasma membrane and is required only at low temperature.
Recently, we have established the refrigerated-dough process for bread making by using cold-sensitive fermentation (Csf) mutants of a baker's yeast. Some of them are now practically employed by bakeries in Japan. These Csf mutants produce much less CO2 gas in dough than the parental strain at refrigeration temperatures but return to normal fermentation activity at room temperature or higher (7).
Cold-sensitive mutants of the yeast Saccharomyces cerevisiae have been reported, but they are mostly cold sensitive for growth, cell division cycling, or RNA splicing. One class of cdc mutants are well known as having a cold-sensitive cell division cycle. The cdc50 mutant encoded a cold-sensitive mutation of the Cdc protein which was essential for growth at low temperature for an unknown mechanism (9; http://genome-www.stanford.edu/Saccharomyces/). The LTE1 gene (16) was essential for growth at low temperature for an unknown mechanism. NSR1 (6), a cold-shock-inducible gene, was reported as being related to growth at low temperature when cells were exposed to an abrupt temperature drop. NSR1 protein was required for normal pre-rRNA processing. The LTG3 gene (3) was essential for growth at low temperature only in a tryptophan auxotroph. The uptake of tryptophan seems to be a rate-limiting step in growth at low temperature. There are many other genes related to growth at low temperature. However, there was little information about the function of genes related to growth and fermentation only at low temperature.
In this study, we cloned CSF1, which complemented the csf1 mutation in a baker's yeast strain. Our observations indicate that CSF1 is related to a new, unknown mechanism for nutrient uptake at low temperature.
MATERIALS AND METHODS
Strains and genomic libraries.
RZT3 (7) is a csf1 mutant strain derived from commercial baker's yeast strain KY5649 (Dia-Yeast YST; Kyowa Hakko Kogyo Co., Tokyo, Japan), and RZT3u is a ura3 mutant of RZT3. YHK142 has the genotype MATα ura3 gal2 mal (5). YHK1144 is a CSF1 disruptant derived from YHK142 in this study. The library of genomic DNA used in this work contained DNA inserts of Saccharomyces cerevisiae X2180-1A and X2180-1B in the low-copy-number vector YCp50.
Media and genetic methods.
YPD medium for cultivation contained 1% yeast extract (Difco Laboratories, Detroit, Mich.), 2% Bactopeptone (Difco), and 2% glucose. SGlu was a synthetic medium containing 2% glucose and 0.67% yeast nitrogen base without amino acids (Difco); when necessary, it was supplemented with appropriate nutrients. Buffered medium (BM) was SGlu medium with 100 mM potassium phosphate buffer (pH 5.0). Genetic manipulation was performed basically by the method of Matsuzaki et al. (10). Crude plasmid DNAs were prepared from yeast transformants by rapid glass bead disruption and phenol extraction (15). Contour-clamped homogenous electric field gel electrophoresis (CHEF) was performed by the method of Chu et al. (2) with CHEF DRII (Bio-Rad Laboratories, Richmond, Calif.). The protocols for labeling, hybridizing, and detecting DNA were described previously (4).
Plasmid construction.
pHK162 is an originally cloned plasmid carrying CSF1. Plasmid pHK188, used for the production of URA+ cells carrying a disruption of the chromosomal CSF1, was constructed from pHK162 by inserting an 8-kb BamHI-Sau3AI/BamHI (nucleotides 1292 to 9589) fragment of CSF1 from pHK162 into the BamHI site of pUC19 and replacing a 0.6-kb MluI-SpeI (nucleotides 4379 to 5018) internal fragment of CSF1 with the 1.2-kb HindIII-HindIII URA3 fragment. Where necessary, DNA fragments were blunt-ended with the Klenow enzyme before the filled-in ligation.
Fermentation assay.
The fermentative activity of yeast cells was measured as described previously (7).
DNA sequence analysis.
DNA fragments were subcloned in the pUC19 vector, and a deletion set for DNA sequence determination was constructed using the Kilo-Sequence deletion kit (Takara Shuzo, Kyoto, Japan) according to the manufacturer's protocol. The sequencing reaction was done by the dideoxy chain termination method (12) with the T7 Sequencing kit (Pharmacia Biotech, Uppsala, Sweden). Both strands were sequenced by using the ALF DNA sequencer (Pharmacia Biotech) according to the protocol of the manufacturer. Computer analysis of the nucleotide and deduced amino acid sequences was performed with the DNASIS (Hitachi Software Engineering Co. Ltd., Yokohama, Japan). A homology search analysis of the deduced amino acid sequence was performed with the SWISS-PROT protein data bank.
Ethanol fermentation with permeabilized cells.
Permeabilized cells were prepared as described by Takeshige and Ouchi (13). These cells were suspended in a buffer containing 5 mM MgCl2, 2 mM ATP, 1 mM NAD+, 30 mM Pi, 1 mM arsenate, and 150 mM MES (morpholinoethanesulfonic acid, pH 6.9). These suspensions were preincubated at assay temperatures for 10 min. Ethanol fermentation was initiated by addition of glucose (final concentration, 2%). A portion (50 μl) of the reaction mixture was periodically withdrawn, and the ethanol content of the reaction mixture was measured with a gas chromatograph (Shimadzu GC-14A; Shimadzu Co., Kyoto, Japan). Ethanol fermentation in intact cells was assayed in the same conditions as for permeabilized cells.
Glucose uptake assay.
The method for assay of glucose uptake was slightly modified from reference 14. Cells were grown in BM medium to an optical density at 600 nm of 3.0. Cells were collected by centrifugation, washed three times with 100 mM potassium phosphate buffer (pH 6.5), and suspended in the same buffer at a final concentration of 50 mg of cells (wet weight) per ml. Portions (50 μl) of the suspension and fivefold concentrates of 14C-labeled d-glucose (12.5 μl; purchased from Japan Radioisotope Association) were preincubated at the assay temperature and then mixed and incubated for 5 s, the period during which uptake was in the linear range. Uptake was terminated by addition of 10 ml of a quenching solution (100 mM potassium phosphate buffer [pH 6.5] containing 500 mM unlabeled glucose) at 0°C. Cells were then collected rapidly and washed on glass fiber filters (Whatman GF/F; purchased from Whatman Japan Ltd., Tokyo, Japan) with 20 ml of the quenching solution. Filters were placed in 3.5 ml of scintillant, and radioactivity was measured with a Beckman LS335 liquid scintillation counter. The control blank in each experiment consisted of labeled glucose added to the quenching solution before the yeast cells were added. The glucose concentration for the experiment ranged from 0.1 to 100 mM (specific radioactivity, 6 to 740 kBq/μmol).
Leucine uptake assay.
Leucine uptake was measured by the method of Ramos et al. (11). 14C-labeled l-leucine was purchased from the Japan Radioisotope Association. The conditions for the leucine uptake assay were the same as those for the glucose uptake assay except for the incubation time (1 min) and the quenching solution (distilled water).
RESULTS AND DISCUSSION
Cloning and nucleotide sequence of CSF1.
The genomic library was screened for a gene which complemented the csf1 mutation using RZT3u as the host strain by a color plate assay (7). Out of about 40,000 colonies, one positive clone was obtained that carried a plasmid named pHK162. pHK162 contained a 12-kb inserted fragment. Reintroduction of the plasmid into RZT3u restored wild-type fermentation at 10°C. The complete nucleotide sequence of the 12-kb inserted fragment was determined on both strands by using overlapping deletion mutants. The fragment contained a sole open reading frame (ORF) of 8,874 bp that encoded a polypeptide of 2,958 amino acids with a calculated molecular mass of 338 kDa. The complementation analysis with various deletion plasmids indicated that this ORF encoded CSF1 (data not shown). The nucleotide sequence was identical to that of the YLR087C gene in the Saccharomyces genome database (http://genomewww.stanford.edu/Saccharomyces/). YLR087C is located on chromosome XII. This information was coincident with chromosome mapping data for the csf1 mutation which we had obtained by a specific chromosome loss technique (4, 5) (data not shown). The predicted CSF1 (YLR087C) protein contained four transmembrane motifs, but there was no information about the function of the protein. When the amino acid sequence of CSF1 was compared with available protein sequences in the GenBank, SwissProt, PIR, PRF, and TIGR databases using TBLASTN (1), only Candida albicans Con4-3103 (positions 41995 to 33164) in the TIGR database was found to be similar to CSF1 (29% identity, 48% similarity). However, the TIGR database is constructed with unfinished microbial genome sequences and still contains errors. There was no information about the function of this gene in C. albicans.
We failed to detect CSF1 mRNA in wild-type cells cultured at both 10 and 30°C by Northern blotting hybridization analysis (data not shown), presumably because the transcription level of CSF1 was below detection.
Phenotypes of a CSF1 disruptant strain.
Plasmid pHK188 was digested with BamHI, and the linear fragment containing CSF1::URA3 obtained was integrated into the CSF1 allele in strain YHK142. Several yeast transformants prototrophic for uracil were selected on SGlu plates. One of these strains, YHK1144, was used for the following experiments. The CSF1 disruption in strain YHK1144 was confirmed by Southern blot analysis of genomic DNA from strains YHK1144 and YHK142 with a BamHI-SphI 6.4-kb DNA fragment (nucleotides 1292 to 7670) of pHK162 as a probe (data not shown). These findings indicate that CSF1 is not essential for cell growth under normal conditions.
The shape and size of cells, mating ability (MATα), and growth rate at 30°C were indistinguishable between YHK1144 and YHK142. When cultured at 10°C, however, YHK1144 cells did not apparently grow even after 170 h, while the parental cells grew and reached maximum growth in 120 h (data not shown). These results indicate that CSF1 is required for cell growth at low temperature.
Growth of the CSF1 disruptant YHK1144 and the csf1 mutant RZT3 at 30°C was more sensitive to hygromycin B than that of either parent strain, but no difference was observed in the sensitivity to vanadate (data not shown).
Ethanol production in permeabilized cells.
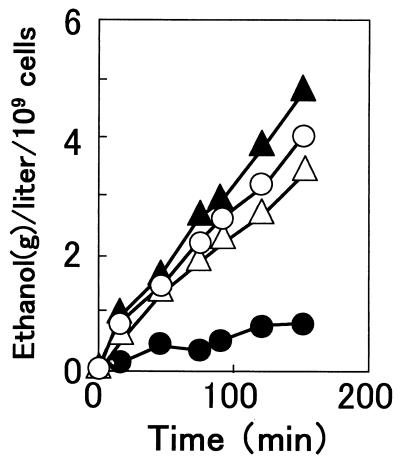
Permeabilized cells are those which have lost their plasma membrane semipermeability but whose enzymes remain virtually intact within the cells or cellular boundary. Therefore, by comparing the fermentation activities of intact and permeabilized cells at low temperature, it may be possible to determine the sites in cells which are cold sensitive for fermentation. As shown in Fig. 1, permeabilized cells of YHK142 showed nearly the same ethanol production rate as those of YHK1144 even at 10°C. This finding was quite different from that in the control experiment, in which intact cells of YHK1144 showed a fivefold-lower rate than those of YHK142. These results suggested that the primary site of cold-sensitive fermentation was not inside the cells but on the plasma membrane.
FIG. 1.
Ethanol production by permeabilized (open symbols) and intact (solid symbols) cells of the parent strain YHK142 (triangles) and the disruptant strain YHK1144 (circles) at 10°C. Cells were suspended in a buffer containing 5 mM MgCl2, 2 mM ATP, 1 mM NAD+, 30 mM Pi, 1 mM arsenate, and 150 mM MES (pH 6.9). Ethanol fermentation was initiated by addition of glucose (final concentration, 2%).
Kinetics of glucose and leucine uptake.
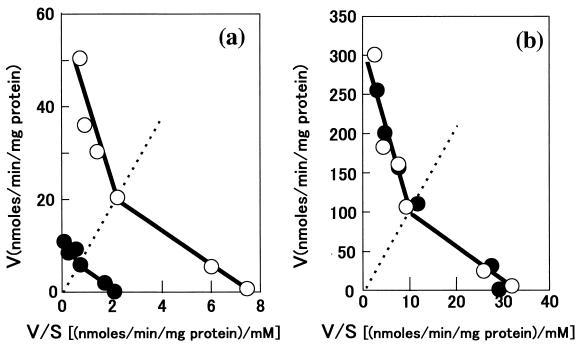
In S. cerevisiae, glucose uptake is mediated by low- and high-affinity transport systems (Kms of approximately 20 and 1 mM, respectively). Therefore, we examined the kinetics of glucose uptake by YHK142 and YHK1144 in the range from 1 to 100 mM d-glucose at 10 and 30°C. YHK1144 showed the same uptake rates as YHK142 at 30°C but three- to fivefold-lower rates than YHK142 at 10°C at any glucose concentration tested (Fig. 2). These results indicated that both low- and high-affinity transport systems for glucose were cold sensitive in YHK1144.
FIG. 2.
Eadie-Hofstee plots of glucose transport in wild-type strain YHK142 (○) and CSF1 disruptant strain YHK1144 (●) at 10°C (a) and 30°C (b). The glucose concentration ranged from 0.1 to 100 mM (specific radioactivity, 6 to 740 kBq/μmol). The assay mixture was incubated for 5 s, the period during which uptake was in the linear range. Dashed lines represent a substrate concentration of 10 mM. Data were obtained from duplicate experiments.
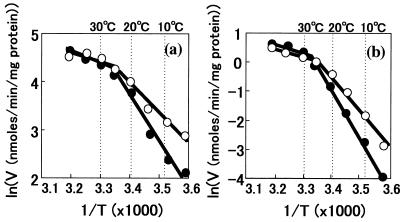
We estimated activation energies for the transport of glucose based on the kinetic data from uptake assays with 20 mM glucose at temperatures from 5 to 40°C. As shown in Fig. 3, there was a break point on each slope of the Arrenius plots at about 25°C. The activation energies calculated from the slopes were 5 and 8 kcal/mol for YHK142 and YHK1144, respectively, below 25°C, but there was no difference above 25°C.
FIG. 3.
Arrenius plots of glucose (a) and l-leucine (b) transport at various temperatures in wild-type strain YHK142 (○) and CSF1 deletion mutant YHK1144 (●). Glucose transport was measured for 5 s with 20 mM glucose. l-Leucine transport was measured for 1 min with 1 mM l-leucine. The experiment was repeated twice.
Activation energies were also different for l-leucine transport above 25°C between YHK142 and YHK1144, 12 and 17 kcal/mol, respectively. Break points were again found at about 25°C, and above 25°C there was no difference in l-leucine uptake rates (Fig. 3).
In addition, our preliminary experiment showed that RZT3, a csf1 mutant, exhibited cold-sensitive maltose fermentation (data not shown). These findings suggest that CSF1 is required for the transport not only of glucose but also of other nutrients at low temperature in S. cerevisiae.
It is clear that the function of CSF1 is different from that of any previous reported genes, but the mechanism by which CSF1 associates with the nutrient transport systems at low temperature is still unknown. The lipid composition of the plasma membrane and H+-ATPase activity are well known as important factors affecting membrane potential and most transport systems. Recently, many hygromycin B-sensitive mutants were reported, and some mutations affected membrane potential (8). The CSF1 disruptant strain was more sensitive to hygromycin B than the parent strain. Therefore, it was deduced that the glucose uptake activity of CSF1 disruptants decreased as plasma membrane H+-ATPase activity decreased. However, our preliminary data showed no difference in lipid composition and plasma membrane ATPase activity at 10°C between YHK142 and YHK1144 (data not shown).
To understand the actual functions of CSF1 or the Csf1 protein, further studies are required: (i) cloning and sequence analyses of the mutant csf1 gene of RZT3, (ii) analyses of transcription level and transcriptional control of CSF1 by using a reporter gene connected to the CSF1 promoter or a multicopy plasmid carrying CSF1, and (iii) determination of the location of Csf1 protein with a specific antibody.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu G, Vollrath D, Davis W R. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986;234:1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura D, Yamashita I, Nimi O, Toh-e A. Cloning and nucleotide sequence of a gene conferring ability to grow at low temperature on Saccharomyces cerevisiae tryptophan auxotrophs. J Ferment Bioeng. 1994;77:1–9. [Google Scholar]
- 4.Kawasaki H, Ouchi K. A DNA construct useful for specific chromosome loss in Saccharomyces cerevisiae. J Ferment Bioeng. 1994;77:125–130. [Google Scholar]
- 5.Kawasaki H, Ouchi K. Effect of chromosome loss in diploid strains of Saccharomyces cerevisiae on the growth of the resultant aneuploid cells. Seibutsu-Kogaku Kaishi. 1996;74:11–16. [Google Scholar]
- 6.Kondo K, Kowalski L R Z, Inouye M. Cold shock induction of yeast NSR1 protein and its role in pre-rRNA processing. J Biol Chem. 1992;267:16259–16265. [PubMed] [Google Scholar]
- 7.Kyogoku Y, Ouchi K. Isolation of a cold-sensitive fermentation mutant of a baker's yeast strain and its use in a refrigerated dough process. Appl Environ Microbiol. 1995;61:639–642. doi: 10.1128/aem.61.2.639-642.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madrid R, Gomez M J, Ramos J, Rodriguez-Navarro A. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J Biol Chem. 1998;273:14838–14844. doi: 10.1074/jbc.273.24.14838. [DOI] [PubMed] [Google Scholar]
- 9.Maftahi M, Gaillardin C, Nicaud J-M. Generation of Saccharomyces cerevisiae deletants and basic phenotype analysis of eight novel genes from the left arm of chromosome XIV. Yeast. 1998;14:271–280. doi: 10.1002/(SICI)1097-0061(199802)14:3<271::AID-YEA218>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki H, Nakajima R, Nishiyama J, Araki H, Oshima Y. Chromosome engineering in Saccharomyces cerevisiae by using a site-specific recombination system of a yeast plasmid. J Bacteriol. 1990;172:610–618. doi: 10.1128/jb.172.2.610-618.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos E H, De Bongioanni L C, Wainer S R, Stoppani A O M. Amino acid uptake by yeast. IV. Effect of thiol reagents on l-leucine transport in Saccharomyces cerevisiae. Biochim Biophys Acta. 1983;731:361–372. doi: 10.1016/0005-2736(83)90029-9. [DOI] [PubMed] [Google Scholar]
- 12.Sanger F, Nicklen S, Coulsen A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1987;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeshige K, Ouchi K. Reconstruction of ethanol fermentation in permeabilized cells of the yeast Saccharomyces cerevisiae. J Ferment Bioeng. 1995;79:11–16. [Google Scholar]
- 14.Walsh M C, Smits H P, Scholte M, Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176:953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward C A. Single-step purification of shuttle vectors from yeast for high frequency back-transformation into E. coli. Nucleic Acids Res. 1990;18:5319. doi: 10.1093/nar/18.17.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickner R B, Koh T J, Crowley J C, O'Neil J, Kaback D B. Moleculer cloning of chromosome I DNA from Saccharomyces cerevisiae: isolation of the MAK16 gene and analysis of an adjacent gene essential for growth at low temperature. Yeast. 1987;3:51–57. doi: 10.1002/yea.320030108. [DOI] [PubMed] [Google Scholar]