Abstract
Calcific aortic valve disease (CAVD) and stenosis have a complex pathogenesis, and no therapies are available that can halt or slow the progression of this disease. Several studies have shown the presence of apolipoprotein-related amyloid deposits in close proximity to calcified areas in diseased aortic valves. In this Perspective article, we explore a possible relationship between amyloid deposits, calcification and the development of aortic valve stenosis. These amyloid deposits might contribute to amplification of the inflammatory cycle in the aortic valve, including extracellular matrix remodeling and myofibroblast and osteoblast-like cell proliferation. Further investigation in this area is needed to characterize the amyloid deposits associated with CAVD, which could allow the use of antisense oligonucleotides and/or isotype gene therapies for possible prevention and/or treatment of CAVD.
Introduction
Numerous mechanisms, including mechanical stress, lipoprotein oxidation, inflammation, altered calcium–phosphorus homeostasis, metabolic changes, extracellular vesicle release and immune–inflammatory responses, have been proposed to explain the pathogenesis of calcific aortic valve disease (CAVD)1. Disrupted communication between valve interstitial cells and valve endothelial cells promotes endothelial-to-mesenchymal transition, in which valve endothelial cells expressing octamer-binding transcription factor 4 (OCT4) adopt an osteogenic phenotype. Perpetuating this process, OCT4 expression leads to the generation and release of pro-inflammatory cytokines that promote further endothelial-to-mesenchymal transition, especially in an experimental hypercholesterolaemic milieu2. Overall, 25–50% of patients with CAVD have concomitant coronary artery disease, and some of the risk factors for these two conditions are shared;3-5 however, clinical trials using lipid-lowering therapies to treat CAVD have been inconclusive6-8.
Given that amyloid deposits, which are known to be pro-inflammatory, have been observed in close proximity to areas of calcification in heart valves9, we hypothesized that amyloid deposition might have a role in the pathogenesis of CAVD. In this Perspective article, we explore the possible relationship between valvular amyloid deposition, calcification of the aortic valve and the development of aortic valve stenosis. Our analysis is specifically focused on CAVD and does not cover other aetiologies of aortic valve stenosis, such as rheumatic heart disease.
Amyloidoses
Amyloidoses are protein-aggregation diseases characterized by the extracellular or intracellular deposition of normally soluble proteins or peptides as insoluble amyloid fibrils. Amyloid deposits are often detected using Congo red, a diagnostic dye whose binding to amyloid leads to a characteristic yellow–green birefringence observed by polarized light microscopy10. Imaging the atomic structures of hundreds of different amyloids with the use of solid-state nuclear magnetic resonance and cryogenic electron microscopy has revealed that the amyloid core contains stacks of flattened protein molecules in a cross-β-sheet amyloid fold11, and the dye molecules can intercalate between the protein molecules. Fibril visualization using transmission electron microscopy is a sensitive and specific tool in the diagnosis of amyloidosis; amyloid fibrils formed in vivo are typically short and non-branching, with an approximate width of 10 nm. 10 Mass spectrometry allows accurate identification of the amino acid sequences of amyloid protein and, together with immunohistochemistry of tissue deposits, is used for amyloid typing. Of the nearly 40 human amyloid proteins that have been identified to date, some, such as monoclonal immunoglobulin light chains or transthyretin (TTR), affect various organs except the brain, whereas others, such as amyloid β (Aβ) peptide or tau protein, are localized only to the central nervous system10.
Irrespective of the type, all amyloid deposits contain a major protein as well as additional components termed amyloid signature proteins; the latter include apolipoproteins, serum amyloid P (SAP) component and heparin sulfate proteoglycans (HSPGs)10. How exactly these additional components are integrated in the amyloid deposits is unclear, but compelling evidence suggests that they can stabilize amyloid (apolipoprotein E (apoE) and SAP) and/or augment its formation (HSPGs) via direct binding to amyloid12-14.
Amyloidosis of the heart is often part of a generalized systemic disorder, in which the typical major proteins that are deposited are immunoglobulin light chains or TTR and their fragments15. The deposition of other misfolded proteins, such as apolipoprotein A-I (apoA-I), has also been reported as a causative agent in several types of cardiac amyloidosis15-17. First, variants in the region of the gene encoding apoA-I amino acid residues 170–178 can lead to a rare form of hereditary cardiac apoA-I amyloidosis, a systemic disease that is caused by the deposition of apoA-I N-terminal fragments as amyloid and can involve severe atherosclerosis16. Second, in non-hereditary apoA-I amyloidosis, which is associated with ageing and is much more prevalent than its hereditary counterpart, wild-type full-length apoA-I deposits as amyloid under inflammatory conditions of oxidative stress and acidic pH; these deposits are found in atherosclerotic arteries and contribute to atherosclerosis17. Of note, in vitro studies show that acidification induces amyloid formation by most proteins, whereas oxidation greatly accelerates amyloid formation by both wild-type apoA-I and wild-type TTR18, 19. These findings suggest that local acidification and oxidation at the sites of inflammation contribute to amyloid deposition by wild-type apoA-I and wild-type TTR in vivo. Separate from these systemic amyloidoses, an amyloid protein derived from the precursor atrial natriuretic peptide can be deposited specifically in the atria of the heart.10
Amyloid in calcified aortic valves
Multiple pathology studies over the past four decades have demonstrated the presence of isolated amyloid deposits in CAVD, located in close proximity to calcified areas9, 20-23 Amyloid deposits have also been noted near calcification at other sites such as nodular calcification in coronary artery, mitral annular calcification, and nodular pulmonary amyloidosis (Fig. 1, Table 1). Amyloid deposits were first described in aortic and mitral valves surgically removed from patients with chronic valvular disease9. Histochemical and immunofluorescence studies subsequently showed the presence of SAP in association with CAVD, but the absence of amyloid immunoglobulin light chains or serum amyloid A in the deposits20, 21. One study reported that amyloid deposits coexist with calcification and hyalinization in 88% of aortic valves and 45% of mitral valves24. Whereas all patients above 50 years of age had both calcium phosphate and amyloid deposits, only minimal deposits were present in the aortic valve of one of three patients under 30 years of age. Of the fourteen patients between the ages of 31 and 50, amyloid deposits and calcium phosphate was present in 10 and 11 patients, respectively. 24 Overall, 75% of aortic valves surgically excised from patients with calcific aortic valve stenosis had moderate-to-severe amyloid deposition, defined as 3–10% or >10% of the sectional area involved in the amyloidosis. 22 Valvular amyloid deposition correlated with hyperlipidaemia, coronary artery disease, and obesity suggesting the involvement of lipids. Indeed, lipids are not only common constituents of amyloid deposits but also influence amyloid formation by various proteins, particularly apolipoproteins and other lipophilic proteins such as Aβ peptide25. Moreover, the role of lipids, particularly phospholipids and lipid oxidation products, in the pathology of CAVD has been firmly established26.
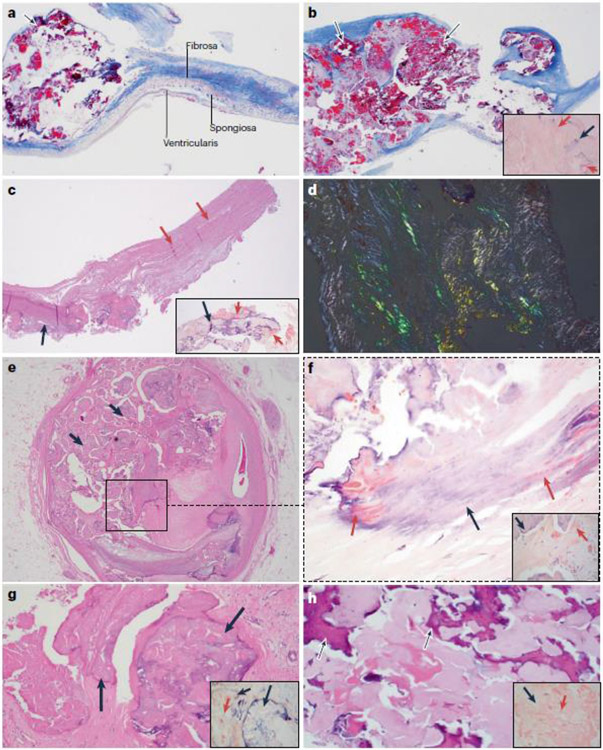
Fig. 1. Association between amyloid deposits and calcification.
a–d ∣ Calcification in native and bioprosthetic aortic valves is associated with amyloid deposits. Parts a and b are from a man aged 58 years with a bicuspid aortic valve who underwent aortic valve replacement for symptomatic aortic stenosis. The patient never smoked and he had no history of coronary artery disease, obesity, chronic kidney disease, diabetes mellitus or hypertension; a plasma lipid profile was not available. Part a shows trichrome staining of the excised aortic valve, revealing small deposits of nodular calcification (arrow) in the cusp (2× magnification). Adjacent is the layered architecture of the valve. On the aortic side is the fibrosa, which is rich in collagen fibres and provides tensile strength to the valve, and on the ventricular side is the ventricularis, which is rich in elastic fibres. Between the two layers is the spongiosa, which is primarily composed of proteoglycans. The valvular endothelial cells line both sides of the cusp. As seen in this image, the calcification starts in the fibrosa. Part b shows an area of calcification in the same valve, which now involves the full thickness of the cusp (arrows). In the inset image, Congo red staining for amyloid shows salmon-pink deposits of amyloid (red arrows) in the vicinity of calcification (black arrow). Part c is from a man aged 56 years who underwent aortic valve replacement with a bovine bioprosthetic valve 15 years ago and who recently presented to the hospital for evaluation of gradually worsening exertional dyspnoea over the past 2 years. The patient had no coronary risk factors and no history of major adverse cardiac events. A transthoracic echocardiogram showed severe aortic stenosis of the bioprosthetic valve, and he underwent aortic valve replacement again. Haematoxylin and eosin (H&E) staining of the excised aortic valve shows areas of nodular calcification (black arrow) that involves the full thickness of the acellular bioprosthetic valve (pink arrows) (4× magnification). In the inset image, Congo red staining shows salmon-pink deposits of amyloid (red arrows) adjacent to the calcified area (black arrow). Part d shows the apple-green birefringence that is characteristic of amyloid when examined by polarization microscopy using a polarizer and an analyser in the bioprosthetic valve (Congo red staining; 10× magnification). e–h ∣ Calcification at sites other than the aortic valve is also associated with amyloid deposits. Parts e and f are from a patient who underwent heart transplantation for severe coronary artery disease. Part e shows H&E staining of a section of the left main coronary artery that has fibrocalcific plaque with nodular calcification (black arrows) (2× magnification). Part f shows Congo red staining of the region in the square in part e, revealing salmon-pink amyloid deposits (red arrows) within the calcified areas (black arrow) and in the vicinity of calcification (inset image) (10× magnification). As the calcification increases, the salmon-pink deposits of amyloid seem to decrease. Part g shows H&E staining of a mitral valve from a man aged 79 years with mitral regurgitation due to mitral annular calcification and mitral valve prolapse. Nodular areas of calcification (black arrows) are present (10× magnification). In the inset image, Congo red staining shows salmon-pink deposits of amyloid (red arrow) within and adjacent to the calcified area (black arrows). Part h is from a woman aged 70 years with multiple calcified nodules in the lung, as seen on a CT scan. H&E staining of a biopsy sample from one of the nodules is positive for amyloid with areas of calcification (black arrows) (10× magnification). In the inset image, Congo red staining highlights salmon-pink deposits of amyloid (red arrow) and adjacent calcification (black arrow). In all the patients, the classic apple-green birefringence was seen with polarizing microscopy. Liquid chromatography and mass spectrometry (LC-MS) to subtype the amyloid deposits showed that the amyloid associated with the calcified valves (a-d and g) contained the signature proteins apolipoprotein A-IV, apolipoprotein E and serum amyloid P. Whereas the patients with valvular amyloid did not have one specific amyloid protein, LC-MS revealed that the amyloid found in the lung nodule (part h) was amyloid immunoglobulin λ-light chain. LC-MS was not performed on the amyloidosis present in the coronary artery calcification (parts e,f).
Table 1 ∣.
Previous reports of amyloid deposits in valvular tissue
| Study | Number of surgical valves |
Valve type (n) | Specimen type | Number of valves with amyloid |
Type of amyloid |
Ref. |
|---|---|---|---|---|---|---|
| Goffin et al. (1980) | 226 | Aortic (106), mitral (107), tricuspid (13) | Any valvulopathy: post-inflammatory, congenital, sclerotic, mucoid, unknown | 33 | Not defined | 9 |
| Iwata et al. (1982) | 131 | Aortic (59), mitral (67), tricuspid (5) | All surgically resected valves | 58 | Negative for AA, AL | 20 |
| Cooper et al. (1983) | 152 | Aortic (90), mitral (60), pulmonary (2) | All surgically resected valves | 81 | Not defined | 21 |
| Ladefoged et al. (1984) | 100 | Aortic (51), mitral (49) | All surgically resected valves | 67 | Not defined | 24 |
| Kristen et al. (2010) | 150 | Aortic (119), mitral (31) | All surgically resected valves | 83 | Negative for AL, AA, β2M, TTR; weak apoA-I staining | 22 |
| Audet et al. (2012) | 70 | Aortic (70) | All surgically resected valves for calcific aortic valve disease with stenosis | 70 | Positive for apoA-I; other types not tested | 23 |
AA, amyloid A; AL, amyloid immunoglobulin light chain; ApoA-I, apolipoprotein A-I; β2M (beta-2 microglobulin); TTR, transthyretin.
Importantly, infrared spectroscopy and scanning electron microscopy performed on human calcified aortic valves showed that the severity of CAVD correlated with oxidative stress, which was partially attributable to lipid peroxidation, and with secondary structural changes in proteins, including an α-helix to β-sheet conversion, which suggests amyloid formation27. Taken together, these studies suggest a causative link between oxidation, calcification and amyloid deposition.
Apolipoproteins and amyloid in CAVD
Immunohistochemical staining has allowed protein identification in calcified aortic valves28 (Fig. 2). Immunoreactivity with an antibody to apoA-I was observed to co-localize with the amyloid deposits in the valves and apoptotic cells near to mineralized areas; however, major amyloid proteins commonly associated with systemic or myocardial amyloidosis, such as TTR or amyloid immunoglobulin light chains, were not observed22. The severity of valvular amyloidosis correlated positively with the plasma levels of HDL and apoA-I, and correlated inversely with the aortic valve area obtained by comprehensive Doppler echocardiography-verified aortic valve area. Importantly, the amyloid extracts from the calcified aortic valves induced apoptosis and mineralization of aortic valve interstitial cells in vitro, suggesting a possible link between apolipoprotein deposition and calcification23.
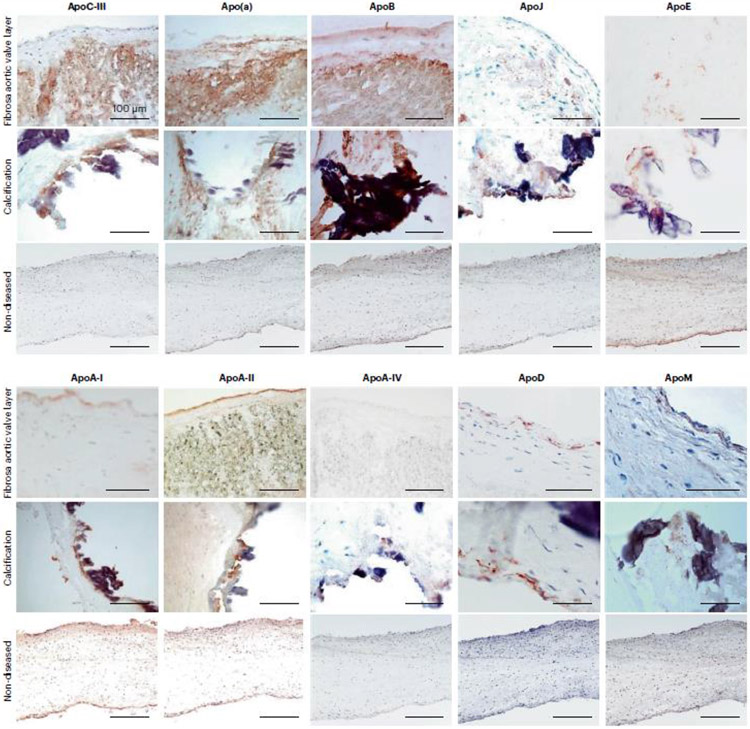
Fig. 2. Apolipoproteins in human aortic valve leaflets.
Aortic valve leaflets obtained from valve replacement surgeries were used for mass spectrometric analysis. Three areas were dissected from eachs leaflet: the calcific and fibrotic areas, and the area with no discernible pathology (referred to as non-diseased). The tissues were lysed and the protein constituents proteolyzed for first unbiased label-free mass spectrometry (modified from reference 32), and then for labeled-based targeted mass spectrometry (modified from reference 28), both performed on a benchtop quadrupole Orbitrap mass spectrometer (Q Exactive). The initial unbiased proteome profiling demonstrated that several apolipoproteins were present in aortic valve leaflets, most of which were enriched in the calcific portions: ApoA-I, ApoA-II, ApoA-IV, ApoB, ApoC-III, ApoD, ApoL-I and ApoM, all of which were verified using targeted mass spectrometry (Schlotter F, JBC 2021). While the other apolipoproteins, Apo(a), ApoE, ApoH and ApoJ were not differentially enriched in any leaflet area, they were nonetheless detected in all aortic valve leaflets.
This figure demonstrates representative immunohistochemistry images of aortic valve leaflets’ cryosections stained with antibodies against ApoCIII, Apo(a), ApoB, ApoJ, ApoE, ApoA-I, ApoA-II, ApoA-IV, ApoD and ApoM. The images show positive diffuse staining in the fibrosa layer (top panel, fibrosa facing up) for ApoCIII, Apo(a) and ApoB antibodies (red-brown reaction product) and cellular expression for ApoE, ApoA-I, ApoA-II, ApoA-IV, ApoD and ApoM. Most apolipoproteins are highly expressed around calcific nodules (middle panel). Non-diseased leaflets (bottom panel) show negative antibody reaction, except a weak staining for ApoE, ApoA-I and ApoA-II. Our mass spectrometric and immunohistochemistry analyses indicate that valves from patient with calcific aortic stenosis are highly enriched in apolipoproteins, particularly localized to the calcification-prone fibrosa and calcific nodules, thus suggesting their contribution to calcification process. (Modified from reference 28).
Other apolipoproteins, including apolipoprotein B (apoB), apolipoprotein E (apoE) and apolipoprotein(a) (apo(a)), have also been found in amyloid deposits associated with calcification in the aortic valve . 28 ApoB and apo(a) colocalized in most regions of the aortic valve, regardless of the severity of calcification; apoE colocalized with apoB and apo(a), but was also present independently. 28 Whereas apoB was almost always present extracellularly, apoE was observed both intracellularly in macrophages as well as extracellularly, 28 suggesting that apoE either originated from circulating VLDL and HDL or was synthesized locally by macrophages infiltrating into the valves. Indeed, macrophage production of apoE has been shown to increase in response to increased intracellular cholesterol levels30. Of note, the presence of particular APOE alleles (specifically apoE4) is an independent predictor of an increased risk of aortic valve stenosis29. The presence of apoE4 is also a major risk factor for neurodegenerative amyloidoses, such as late-onset Alzheimer disease12.
Furthermore, apoA-I, apoB and apoE have been shown to colocalize with osteoprotegerin and biglycan in the stenotic valves. The concentration of apoA-I as determined by enzymatic immunoassays was higher in control valves than in stenotic valves, suggesting a protective role for apoA-I in retarding calcification by inducing secretion of osteoprotegerin, which inhibits tumour necrosis factor (TNF)-induced aortic valve calcification31.
Precision-targeted proteomic studies and immunohistochemical staining have revealed differential expression of apolipoproteins at different stages of CAVD28, 32. Whereas apo(a), apoB, apoC-III, apoE and apoJ were present in the fibrosa of the valve and were enriched around the calcified regions, apoA-I, apoA-II, apoA-IV, apoD and apoM colocalized with the calcific deposits. Moreover, apoC-III promoted calcification in valve interstitial cells in culture by inducing mitochondrial dysfunction and inflammation28. These effects could stem, in part, from the inhibitory action of apoC-III on triglyceride hydrolysis and the ensuing compositional, structural and functional alterations to the pro-atherogenic apoB-containing lipoproteins. Furthermore, levels of apoC-III–lipoprotein(a) (Lp(a)) complexes in combination with oxidized phospholipids can be used to predict aortic valve stenosis and the need for aortic valve replacement33, highlighting the active role of apoC-III and the pro-atherogenic lipoproteins in aortic valve stenosis and CAVD.
In a longitudinal population-based study, the plasma level of Lp(a) was shown to be associated with new onset of aortic valve calcification and calcific aortic valve stenosis34. Moreover, genome-wide association studies suggest a possible causal relationship4. Plasma Lp(a) levels were associated with both the presence and the progression of pre-existing mild–moderate aortic valve stenosis35, 36. Approximately 30–35% of individuals with aortic valve stenosis have elevated plasma levels of Lp(a)37. A major contributor to the pathophysiology of aortic valve stenosis is the number of oxidized phospholipids present in Lp(a)38, 39.
An apolipoprotein proteomics study has revealed increased tissue levels of apoA-I in patients with CAVD; however, apoA-I did not induce calcification in valve interstitial cells in culture. Despite the uncertain mechanisms, a potential link between calcification and amyloid disease is emerging. Specifically, a multiomic integrative study in CAVD identified several genes such as APOA1, APP and TTR that also have important roles in non-valvular amyloid diseases40. A computational proteomic network analysis has shown a link between Alzheimer disease and CAVD. 40 Together, these studies suggest a possible role for apolipoprotein-related amyloid deposits in CAVD.
[H2] Factors influencing amyloid formation by apolipoproteins
Of the nearly 40 human proteins that are currently known to form pathological amyloid, five (apoA-I, apoA-II, apoA-IV, apoC-II and apoC-III) are exchangeable apolipoproteins, and two related amyloid-forming proteins (serum amyloid A and α-synuclein) contain apolipoprotein-like amphipathic α-helices that bind to lipids10, 25. Although no atomic structures of apolipoprotein fibrils are currently available, extensive studies of apolipoproteins in their native functional states have established a link between their high propensity to form amyloid and their normal function of binding to lipid surfaces25.
Lipoproteins contain a core of apolar lipids surrounded by a surface of polar lipids and apolipoproteins. The major apolipoproteins associated with VLDL are apoB, apoC and apoE; apoB is the major LDL-associated protein, whereas apoA-I and apoA-II are the major HDL-associated proteins. Unlike apoB, which is water insoluble and therefore non-exchangeable, the other apolipoproteins are water soluble and can reversibly dissociate from lipoprotein surfaces. These dissociated lipid-poor or free apolipoproteins are structurally unstable and metabolically labile; they can either be recruited back to a lipid surface or be degraded (Fig. 3). Alternatively, free apolipoproteins can misfold and deposit as amyloid25. Most free apolipoproteins are molten globular and/or contain large intrinsically disordered regions. Low structural stability and high conformational flexibility of these proteins combined with their high hydrophobicity, which is a prerequisite for lipid binding, augment apolipoprotein misfolding and aggregation into amyloid25. These properties help to explain why exchangeable apolipoproteins are overrepresented in amyloidoses.
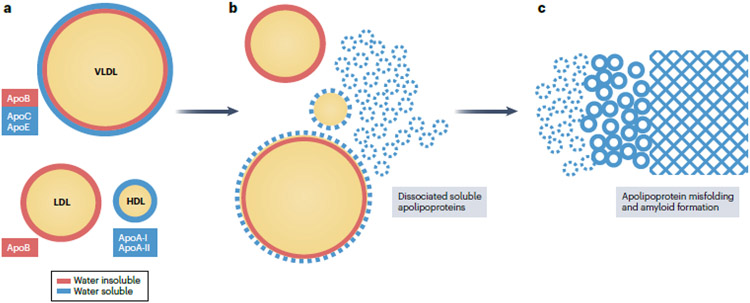
Fig. 3. The possible involvement of lipoproteins and apolipoproteins in amyloidogenesis.
Lipoproteins contain a core of apolar lipids (cholesterol esters and triglycerides surrounded by an amphipathic surface comprised of polar lipids (phospholipids and cholesterol) as well as exchangeable (water-soluble, green) and non-exchangeable (water-insoluble, purple) apolipoproteins. Exchangeable apolipoproteins can reversibly dissociate from the lipoprotein surface. These free apolipoproteins can re-bind to another lipoprotein, or misfold to form amyloid. Apo, apolipoprotein. Modified with permission from ref.25
In addition to their role as major proteins in amyloid fibrils, apolipoproteins also act as ‘amyloid signature proteins’ that comprise minor, non-fibrillary components in various types of amyloid deposit. Perhaps best known is the role of apoE in Alzheimer disease, in which apoE interactions with Aβ amyloid stabilize amyloid deposits and are a proposed therapeutic target12. Therapeutic intervention aimed at depleting apoE with the use of immunotherapy or antisense oligonucleotides was reported to decrease the amyloid plaque burden and improve cerebrovascular function in a mouse model with both amyloid plaques and cerebral amyloid angiopathy41. Interestingly, despite the prominence of apoE in Alzheimer disease and other amyloid diseases, no amyloidoses involving apoE as the major fibrillary protein have been reported10, which is probably due to the relatively low propensity of the amino acid sequence of apoE to form amyloid25.
Lipids influence amyloid formation in general and apolipoprotein amyloid formation in particular25. Normally, binding to lipoproteins such as HDL or VLDL stabilizes the native apolipoprotein structure against unfolding, proteolysis, or misfolding to form amyloid. Conversely, the transient release of apolipoproteins from the surface of lipoproteins in a labile, lipid-poor or free state seems to be a required early step in misfolding and aggregation in amyloid. Therefore, factors that promote the release of apolipoproteins are expected to contribute to amyloidosis. These factors can include modifications to apolipoproteins and/or lipids. Pro-amyloidogenic protein modifications include genetic variants, which can occur in hereditary amyloidoses involving apoA-I or apoA-II, or mild oxidation, which destabilizes HDL and promotes the release of wild-type apoA-I42. Mildly oxidized apoA-I is prone to misfolding; in particular, methionine oxidation induces amyloid formation by wild-type apoA-I in vitro and probably in vivo18. Indeed, apoA-I is rendered dysfunctional by oxidation in atherosclerotic plaques, which can result from the action of oxidative enzymes such as myeloperoxidase that are abundantly expressed in the macrophages contained in human atheromas18.
In addition to apolipoprotein modifications, altered lipid composition, such as increased free fatty acid content in the lipoprotein surface or increased triglyceride content in the core, can also destabilize lipoprotein assembly and promote apolipoprotein release in a labile, free form that is prone to misfolding42. Therefore, factors that decrease oxidation, lipolysis and triglyceride content in plasma lipoproteins might provide potential therapeutic avenues to treat apolipoprotein-associated amyloidoses. Conversely, conditions such as diabetes mellitus, obesity and inflammation, which are associated with lipolysis, oxidative stress and elevated triglyceride levels, are expected to contribute to apolipoprotein amyloid formation42. These factors, particularly local inflammation, oxidation and lipolysis, are relevant to CAVD.
Amyloid fibril formation, inflammation and mineralization
A direct, causative link between inflammation and amyloid deposition in vivo was established circa 1980 for serum amyloid A, an HDL-associated apolipoprotein that is elevated in inflammation and forms a protein precursor in serum amyloid A amyloidosis, a major complication of chronic inflammation10. Subsequent studies of other amyloidoses have shown that a pro-inflammatory environment, in particular local acidification and oxidative stress, is pro-amyloidogenic. In vitro studies have demonstrated that oxidation induces amyloid formation by wild-type apoA-I and wild-type TTR18, 19, and an acidic pH greatly accelerates amyloid formation 10, 13. Conversely, amyloid fibrils formed by apolipoproteins can initiate a range of pro-inflammatory responses43. For example, apoC-III activates a macrophage signalling cascade and calcification in valve interstitial cells28, involving CD36 scavenger receptors and the production of reactive oxygen species and TNF43. Similarly, in Alzheimer disease, amyloid aggregates and fibrils of Aβ peptide and tau protein can induce a microglial inflammatory response by activating the NLRP3 inflammasome44.
A comprehensive proteomic and transcriptomic study demonstrated that CAVD progresses from the activation of valve interstitial cells and involves inflammation-related and calcification-related pathways32. Extracellular matrix (ECM) remodelling with differential expression of biglycan, decorin, lumican, periostin and prolargin has been reported during the development of CAVD 45. ECM proteoglycans such as HSPGs promote amyloid formation and are found in all extracellular amyloid deposits 13. The changes in the valve ECM promote interaction with Toll-like receptors (2 and 4), detected in valve interstitial cells in stenotic valves to induce expression of pro-inflammatory mediators and the upregulation of osteogenesis-associated factors46. Therefore, amyloid-related changes in the inflammatory cascade together with subsequent ECM changes can promote the inflammation–mineralization process in valves (Fig. 5 moved to the end).
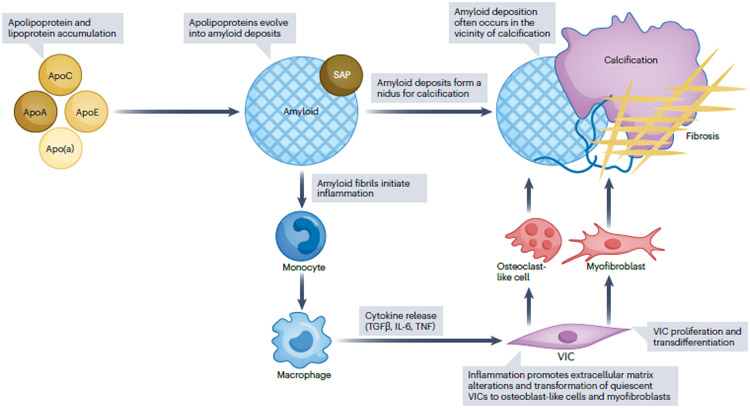
Fig. 5. Aortic valve amyloid deposits and aortic stenosis.
Amyloid deposits are commonly seen in calcific aortic valve disease and are located in the vicinity of calcification, suggesting a possible relationship between amyloid deposition and calcification. Apolipoprotein and lipoprotein accumulation has frequently been described in calcific aortic valve disease. A potential mechanism of amyloid formation from dissociated soluble apolipoproteins has been described. Amyloid deposits could function as a nidus for calcification. Amyloid fibrils perpetuate inflammation and extracellular matrix remodelling, resulting in valve fibrosis, calcification and, consequently, aortic valve stenosis. Apo, apolipoprotein; SAP, serum amyloid P; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor; VIC, valve interstitial cell.
Biomineralogical analysis of the morphological and chemical features of calcified aortic and mitral valves has shown that the deposits are composed of calcium phosphate with an apatite mineral crystal structure 47. Polished thin sections of heavily calcified valves showed circular cavities or holes of various sizes filled with disorganized and mineralized collagen and birefringent organic fibres, suggestive of amyloid. 47 A multiomics study of CAVD has demonstrated a network linking CAVD to Alzheimer disease; the presence of TTR and Aβ precursor protein in the deposits in calcified aortic valves was confirmed by immunohistochemistry40. Importantly, the TTR and Aβ precursor protein expression was not seen in either the non-diseased portion of valves from patients with CAVD or in healthy valves. A high prevalence of aortic valve stenosis has been shown in patients with TTR cardiac amyloidosis48. The presence of TTR-enriched amyloid-like deposits in the aortic valves of patients with CAVD40 suggests that the current transthyretin stabilizing therapies for TTR amyloidosis, such as diflunisal and tafamidis might benefit patients with CAVD. Importantly, even in the absence of calcification, amyloid deposition alone in the aortic valve could lead to valve leaflet thickening and restriction. This suggestion might be clinically relevant and help to explain the discordance between aortic valve calcium score and the severity of aortic valve stenosis.
Protein amyloids might be part of the interface between the organic and mineral phases in calcified aortic valves. Chemical modifications to proteins in the local pro-inflammatory environment surrounding amyloid deposits might have a role in calcification. Infrared spectroscopy, scanning electron microscopy and X-ray diffraction studies of calcified aortic valves suggest that oxidative protein–protein crosslinking occurs at the initiation sites for calcification and that oxidative stress might be linked to mineralization of the amyloid proteins49. The infrared spectral changes of calcified aortic valves were related to progression of the disease; these changes included a shift to lower frequencies of amide-I/amide-II bands, suggesting an α-helix to β-sheet conversion. 49 A similar shift of the amide-I band has also been seen in regions of mineralization in explanted bioprosthetic valves that corresponded to the presence of β-sheet conformation50. These spectral changes are consistent with protein misfolding in amyloid.
Endomyocardial biopsy samples from patients with amyloid deposition show microcalcifications in both the TTR and immunoglobulin light chain types of amyloidosis, particularly the former, which may be related to the time course of amyloid deposition being much more rapid with the amyloid light chain. .51 It seems that amyloid deposits that are localized and form slowly might develop more calcification than those that are distributed systemically, consistent with a hypothetical spatiotemporal link between amyloid deposition and calcification. Furthermore, in individuals with a similar severity of aortic valve stenosis, women have a predominance of fibrosis, whereas men have a predominance of calcification52. It would be interesting to explore how sex-related differences in TTR amyloid deposition in the heart, which is more prevalent in men than in women, 53 (added this reference) relates to the extent of calcification.
Localized amyloidosis associated with calcification has been reported in the skin, mediastinum, breast, neck and lungs54. Amyloid deposits derived from several apolipoproteins, including apoA-I, apoA-II, apoA-IV, apoE and SAP, have been shown to be localized in atherosclerotic plaques55-57. Imaging with 18F–flutemetamol, which specifically binds to amyloid, has identified amyloid-positive areas in human atherosclerotic plaques58. In a longitudinal 18F-fludeoxyglucose PET–CT study, arterial inflammation preceded calcification in the arterial wall59. Data suggest that calcification can be present in all types of amyloid.
Given that HSPGs, apolipoproteins and SAP are found in all types of amyloid, they might contribute to calcification10. Furthermore, common structural features observed in amyloid fibrils confer distinct stereochemical properties, such as periodic arrays of closely spaced, charged residues running along the fibril surface, which can act as templates for the binding of calcium phosphate ions during biomineralization. In the current models of biomineralization of tooth enamel, which contains bundles of aligned apatite crystals, amyloid-like nanoribbons of amelogenin protein have been shown to act as potent nucleators of calcium phosphate deposits60, 61. This finding strongly supports our hypothesis that amyloid fibrils can act as templates for calcification in various organs and tissues, perhaps including the aortic valve. How the stereochemical properties of amyloid fibrils might contribute to calcium binding and biomineralization is described below.
Potential mechanisms of calcification
Throughout evolution, acidic arrays formed from proteins and proteoglycans have been crucial for biomineralization by calcium carbonate or calcium phosphate; these arrays can control the local concentrations of salt ions and provide scaffolds for mineral deposition by acting as templates for the nucleation and growth of the mineral phase62.
Amyloid fibrils contain periodic arrays of charged residues on their surface11, 13, 63 and are associated with HSPGs, which are periodic polyanions. It is possible that anionic arrays in amyloid fibrils, HSPGs and other amyloid signature proteins might act as templates for calcium biomineralization.
Indeed, calcium is well known to bind to anionic moieties of proteoglycans, such as HSPGs. Furthermore, calcium can probably bind directly to amyloid fibrils. Polyvalent metal ions are often found in amyloid deposits and can bolster their formation, most notably for Aβ peptide. The underlying mechanism is suggested by the atomic structure of ex vivo Aβ(1–42) amyloid, which showed extra densities on cryogenic electron microscopy, probably corresponding to bound divalent metal ions such as calcium64. In this structure, periodic arrays formed by pairs of adjacent acidic residues coordinate arrays of metal ions running along the fibril surface. Given that periodic arrays of closely spaced, uncompensated charged residues are often seen on the surfaces of amyloid fibrils13, it is possible that calcium binding to acidic arrays occurs not only in Aβ(1–42) peptide, but also in other amyloids.
Amyloid signature proteins can also contribute to calcium binding even if these proteins are well folded. In their native state, these proteins contain class A amphipathic α-helices with a characteristic charged residue distribution in which acidic residues are located along the middle of the polar helical face, whereas basic residues cluster at its edges25. These surface arrays of acidic residues are expected to bind to calcium and other divalent cations; indeed, HDL particles are greatly destabilized by micromolar calcium concentrations (O.G., unpublished observations), suggesting specific binding of calcium to acidic residues in the α-helices of apoA-I.
SAP, another calcium-binding amyloid signature protein, might also provide a potential link between amyloid and calcium deposition. SAP is a member of the C-reactive protein family and is a cyclic pentamer that has a calcium-binding site. Unlike C-reactive protein, the concentration of SAP in the plasma is relatively constant. Under physiological conditions with normal levels of calcium, SAP can bind in a calcium-dependent manner to both protein and non-protein ligands, including apolipoproteins65. Like apoE, SAP was proposed to bind to amyloid and stabilize it against proteolysis; this idea is consistent with the clinical trial in which a small-molecule SAP ligand followed by IgG anti-SAP antibodies was used to deplete plasma SAP levels in patients with systemic amyloidosis and led to a reduction in amyloid deposits in the liver and other tissues66. Although the exact mechanism of calcium-dependent binding of SAP to amyloid is uncertain, detailed knowledge of the atomic structures of SAP and amyloid fibrils might provide a basis for future studies of the SAP–calcium–amyloid complex and its potential role in calcification.
Potential therapeutic targets
The involvement of lipoproteins in the pathogenesis of CAVD suggests a potential benefit of lipid-lowering therapies. However, the results of clinical trials using statins have been inconclusive. The ASTRONOMER3, SALTIRE4 and SEAS5 studies showed that intense lipid-lowering therapy did not halt the progression of calcific aortic stenosis. In these trials, the statins might have been introduced too late to reverse or slow the calcification process. Conversely, rosuvastatin in the RAAVE trial67 was shown to slow the progression of aortic stenosis, as assessed by echocardiographic haemodynamic measurements. Therefore, more studies of cholesterol-lowering drugs in patients with CAVD are warranted. Furthermore, triglyceride-lowering approaches such as fibrates, low-fat diet and triglyceride-lowering dietary supplements hold potential promise, especially if the causal link between apolipoprotein amyloid deposition and calcification is established. Indeed, as explained above, decreased plasma levels of triglycerides prevent apolipoprotein release from lipoproteins in a labile lipid-poor or free state that is the protein precursor of amyloid42.
In view of the emerging evidence of an association between Lp(a) and CAVD, there are two potential implications for prognosis and future therapies. First, measuring and identifying elevated plasma Lp(a) levels in patients with pre-existing aortic stenosis would identify them as having a 30–50% higher rate of disease progression, as measured by peak aortic valve velocity, and in need of early replacement of the aortic valve35, 36. Therefore, these patients can be considered candidates for closer follow-up, including with imaging tests. Second, the association implies that a substantial proportion of patients with aortic valve stenosis might benefit from Lp(a)-lowering therapies. The potent reduction of plasma Lp(a) levels by up to 80% with mRNA-targeted therapies. 68 can be harnessed to design studies to assess the effect of Lp(a)-lowering therapies on the progression of aortic valve stenosis and the need for aortic valve replacement. Whether there is a role for interference in amyloidogenesis and subsequent calcification remains to be investigated.
Conclusions
The development of CAVD is a complex process, with no currently available strategy to slow its progression. The presence of amyloid deposits adjacent to calcified areas and their potential relationship with an inflammatory milieu suggest a role of these amyloid deposits in the initiation and/or propagation of calcification.(Figure 5) Further research in this area is warranted to delineate the role of amyloid deposits in the mineralization of the aortic valve. The role of amyloid deposits in precipitating calcification in the tissues of other organs also needs to be explored. These investigations might help to guide therapeutic targeting of (apo)lipoprotein-related amyloids and prevent or slow the progression of calcific aortic valve stenosis.
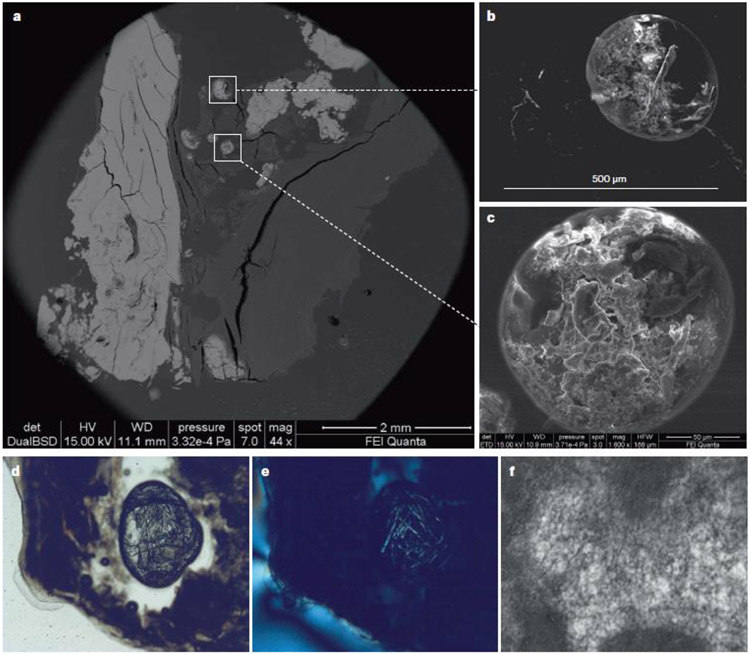
Fig. 4. Biomineralogical features of calcification in heart valves.
Low magnification of a polished thin section of a tricuspid aortic valve shows fully mineralized portions of the valve, seen as bright areas (part a). In the centre of the image, circular cavities are seen. Higher magnification of these circular cavities by backscatter electron microscopy (part b) and scanning electron microscopy (part c) shows the presence of disorganized and mineralized collagen in these cavities. Images of the pockets seen under polarized light microscopy (part d) and cross polarized light (part e) show the presence of birefringent organic fibres that are morphologically similar to amyloid fibrils. An ultrastructural examination of another valve confirms the presence of amyloid fibrils (part f). Parts a–e modified with permission from ref. 47
Acknowledgements
E.Aikawa has received support from the NIH (grant numbers R01 HL136431, R01 HL141917 and R01 HL147095). S.G. has received support from the National Institute on Aging – Designated Alzheimer’s Disease Research Center (P50 AG005138 and P30 AG066514). O.G. has received support from the NIH (grants R01 GM067260 and R01 GM135158).
Footnotes
Competing interests
P.P. has received grant funding from Cardiac Phoenix, Edwards Lifesciences, Medtronic and Pi-Cardia for echocardiography core laboratory analyses and research studies in the field of transcatheter valve therapies, for which he received no personal compensation; he has also received lecture fees from Edwards Lifesciences and Medtronic. R.R. has received research grants to his institution from Amgen, Arrowhead, NIH, Novartis and Regeneron; he is a member of the advisory board of Amgen, Novartis, Regeneron and 89 Bio; he has received honoraria for non-promotional speaking from Kowa; he has stock holdings with MediMergent; and he receives royalties from Wolters Kluwer (UpToDate). S.T. is a co-inventor who receives royalties from patents owned by University of California, San Diego on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins; he is a co-founder who has equity interests in Kleanthi Diagnostics and in Oxitope and affiliates. Although these relationships have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Kleanthi and Oxitope, the research findings included in this particular publication do not necessarily relate to the interests of Kleanthi and Oxitope. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with conflict of interest policies. The other authors declare no competing interests.
References
- 1.Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aikawa E, Hutcheson JD. The developmental origin of calcific aortic stenosis. N Engl J Med. 2022;386:1372–1374 [DOI] [PubMed] [Google Scholar]
- 3.Rapp AH, Hillis LD, Lange RA, Cigarroa JE. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol. 2001;87:1216–1217; A1217 [DOI] [PubMed] [Google Scholar]
- 4.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, et al. Association between cardiovascular risk factors and aortic stenosis: The canheart aortic stenosis study. J Am Coll Cardiol. 2017;69:1523–1532 [DOI] [PubMed] [Google Scholar]
- 6.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (astronomer) trial. Circulation. 2010;121:306–U247 [DOI] [PubMed] [Google Scholar]
- 7.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. New Engl J Med. 2005;352:2389–2397 [DOI] [PubMed] [Google Scholar]
- 8.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. New Engl J Med. 2008;359:1343–1356 [DOI] [PubMed] [Google Scholar]
- 9.Goffin Y Microscopic amyloid deposits in the heart valves: A common local complication of chronic damage and scarring. J Clin Pathol. 1980;33:262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, et al. Amyloid nomenclature 2018: Recommendations by the international society of amyloidosis (isa) nomenclature committee. Amyloid. 2018;25:215–219 [DOI] [PubMed] [Google Scholar]
- 11.Sawaya MR, Hughes MP, Rodriguez JA, Riek R, Eisenberg DS. The expanding amyloid family: Structure, stability, function, and pathogenesis. Cell. 2021;184:4857–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisniewski T, Drummond E. Apoe-amyloid interaction: Therapeutic targets. Neurobiol Dis. 2020;138:104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewkowicz E, Jayaraman S, Gursky O. Protein amyloid cofactors: Charged side-chain arrays meet their match? Trends Biochem Sci. 2021;46:626–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao Y, Sun Y, Lv S, Xia W, Zhao K, Xu Q, et al. Heparin induces alpha-synuclein to form new fibril polymorphs with attenuated neuropathology. Nat Commun. 2022;13:4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittleson MM, Maurer MS, Ambardekar AV, Bullock-Palmer RP, Chang PTP, Eisen HJ, et al. Cardiac amyloidosis: Evolving diagnosis and management: A scientific statement from the american heart association. Circulation. 2020;142:E7–E22 [DOI] [PubMed] [Google Scholar]
- 16.Obici L, Franceschini G, Calabresi L, Giorgetti S, Stoppini M, Merlini G, et al. Structure, function and amyloidogenic propensity of apolipoprotein a-1. Amyloid. 2006;13:191–205 [DOI] [PubMed] [Google Scholar]
- 17.Rocken C, Tautenhahn J, Buhling F, Sachwitz D, Vockler S, Goette A, et al. Prevalence and pathology of amyloid in atherosclerotic arteries. Arterioscler Thromb Vasc Biol. 2006;26:676–677 [DOI] [PubMed] [Google Scholar]
- 18.Wong YQ, Binger KJ, Howlett GJ, Griffin MD. Methionine oxidation induces amyloid fibril formation by full-length apolipoprotein a-i. Proc Natl Acad Sci U S A. 2010;107:1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, Buxbaum JN, Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry-Us. 2013;52:1913–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata T, Nakamura H, Nagasawa T, Kamei T, Fujihara S, Yokota T, et al. Amyloid deposits in heart-valves. Acta Pathol Japon. 1982;32:23–29 [DOI] [PubMed] [Google Scholar]
- 21.Cooper JH. Localized dystrophic amyloidosis of heart-valves. Hum Pathol. 1983;14:649–653 [DOI] [PubMed] [Google Scholar]
- 22.Kristen AV, Schnabel PA, Winter B, Helmke BM, Longerich T, Hardt S, et al. High prevalence of amyloid in 150 surgically removed heart valves-a comparison of histological and clinical data reveals a correlation to atheroinflammatory conditions. Cardiovasc Pathol. 2010;19:228–235 [DOI] [PubMed] [Google Scholar]
- 23.Audet A, Cote N, Couture C, Bosse Y, Despres JP, Pibarot P, et al. Amyloid substance within stenotic aortic valves promotes mineralization. Histopathology. 2012;61:610–619 [DOI] [PubMed] [Google Scholar]
- 24.Ladefoged C, Rohr N. Amyloid deposits in aortic and mitral-valves - a clinicopathological investigation of material from 100 consecutive heart-valve operations. Virchows Arch A. 1984;404:301–312 [DOI] [PubMed] [Google Scholar]
- 25.Das M, Gursky O. Amyloid-forming properties of human apolipoproteins: Sequence analyses and structural insights. Adv Exp Med Biol. 2015;855:175–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tintut Y, Hsu JJ, Demer LL. Lipoproteins in cardiovascular calcification: Potential targets and challenges. Front Cardiovasc Med. 2018;5:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamarelis I, Koutoulakis E, Kotoulas C, Dritsa V, Mamareli V, Anastassopoulou J. Ft-ir spectroscopic study of amyloid protein formation and aortic valve calcification. Hell J Cardiol. 2017;58:148–150 [DOI] [PubMed] [Google Scholar]
- 28.Schlotter F, Freitas RCCD, Rogers MA, Blaser MC, Wu PJ, Higashi H, et al. Apoc-iii is a novel inducer of calcification in human aortic valves. Journal of Biological Chemistry. 2021;296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novaro GM, Sachar R, Pearce GL, Sprecher DL, Griffin BP. Association between apolipoprotein e alleles and calcific valvular heart disease. Circulation. 2003;108:1804–1808 [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman SH, Evans GF, Oneal L. Cytokine regulation of macrophage apo-e secretion - opposing effects of gm-csf and tgf-beta. Atherosclerosis. 1992;96:203–214 [DOI] [PubMed] [Google Scholar]
- 31.Lommi JI, Kovanen PT, Jauhiainen M, Lee-Rueckert M, Kupari M, Helske S. High-density lipoproteins (hdl) are present in stenotic aortic valves and may interfere with the mechanisms of valvular calcification. Atherosclerosis. 2011;219:538–544 [DOI] [PubMed] [Google Scholar]
- 32.Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation. 2018;138:377–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capoulade R, Torzewski M, Mayr M, Chan KL, Mathieu P, Bosse Y, et al. Apociii-lp(a) complexes in conjunction with lp(a)-oxpl predict rapid progression of aortic stenosis. Heart. 2020;106:738–745 [DOI] [PubMed] [Google Scholar]
- 34.Kaiser Y, van der Toorn JE, Singh SS, Zheng KH, Kavousi M, Sijbrands EJG, et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur Heart J. 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capoulade R, Chan KL, Yeang C, Mathieu P, Bosse Y, Dumesnil JG, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246 [DOI] [PubMed] [Google Scholar]
- 36.Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73:2150–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia HS, Ma GS, Taleb A, Wilkinson M, Kahn AM, Cotter B, et al. Trends in testing and prevalence of elevated lp(a) among patients with aortic valve stenosis. Atherosclerosis. 2022;349:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamstrup PR, Hung MY, Witztum JL, Tsimikas S, Nordestgaard BG. Oxidized phospholipids and risk of calcific aortic valve disease: The copenhagen general population study. Arterioscler Thromb Vasc Biol. 2017;37:1570–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torzewski M, Ravandi A, Yeang C, Edel A, Bhindi R, Kath S, et al. Lipoprotein(a) associated molecules are prominent components in plasma and valve leaflets in calcific aortic valve stenosis. JACC Basic Transl Sci. 2017;2:229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heuschkel MA, Skenteris NT, Hutcheson JD, van der Valk DD, Bremer J, Goody P, et al. Integrative multi-omics analysis in calcific aortic valve disease reveals a link to the formation of amyloid-like deposits. Cells-Basel. 2020;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong M, Jiang H, Serrano JR, Gonzales ER, Wang C, Gratuze M, et al. Apoe immunotherapy reduces cerebral amyloid angiopathy and amyloid plaques while improving cerebrovascular function. Science Translational Medicine. 2021;13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayaraman S, Sanchez-Quesada JL, Gursky O. Triglyceride increase in the core of high-density lipoproteins augments apolipoprotein dissociation from the surface: Potential implications for treatment of apolipoprotein deposition diseases. Biochim Biophys Acta Mol Basis Dis. 2017;1863:200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medeiros LA, Khan T, El Khoury JB, Pham CLL, Hatters DM, Howlett GJ, et al. Fibrillar amyloid protein present in atheroma activates cd36 signal transduction. Journal of Biological Chemistry. 2004;279:10643–10648 [DOI] [PubMed] [Google Scholar]
- 44.Luciunaite A, McManus RM, Jankunec M, Racz I, Dansokho C, Dalgediene I, et al. Soluble abeta oligomers and protofibrils induce nlrp3 inflammasome activation in microglia. J Neurochem. 2020;155:650–661 [DOI] [PubMed] [Google Scholar]
- 45.Martin-Rojas T, Mourino-Alvarez L, Alonso-Orgaz S, Rosello-Lleti E, Calvo E, Lopez-Almodovar LF, et al. Itraq proteomic analysis of extracellular matrix remodeling in aortic valve disease. Sci Rep-Uk. 2015;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng XZ, Ao LH, Song Y, Babu A, Yang XP, Wang MR, et al. Expression of functional toll-like receptors 2 and 4 in human aortic valve interstitial cells: Potential roles in aortic valve inflammation and stenosis. Am J Physiol-Cell Ph. 2008;294:C29–C35 [DOI] [PubMed] [Google Scholar]
- 47.Cottignoli V, Cavarretta E, Salvador L, Valfre C, Maras A. Morphological and chemical study of pathological deposits in human aortic and mitral valve stenosis: A biomineralogical contribution. Patholog Res Int. 2015;2015:342984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ternacle J, Krapf L, Mohty D, Magne J, Nguyen A, Galat A, et al. Aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2019;74:2638–2651 [DOI] [PubMed] [Google Scholar]
- 49.Mamarelis I, Koutoulakis E, Kotoulas C, Mamareli V, Dritsa V, Anastassopoulou J. The role of oxidative stress on amyloid-like protein formation and aortic valve calcification. Eur Heart J. 2016;37:313–313 [Google Scholar]
- 50.Dittfeld C, Mieting A, Welzel C, Jannasch A, Matschke K, Tugtekin SM, et al. Molecular spectroscopic imaging offers a systematic assessment of pathological aortic valve and prosthesis tissue in biomineralization. Crystals. 2020;10 [Google Scholar]
- 51.Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in attr and al cardiac amyloidosis: Implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol. 2016;25:413–417 [DOI] [PubMed] [Google Scholar]
- 52.Simard L, Cote N, Dagenais F, Mathieu P, Couture C, Trahan S, et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: Is valvular fibrosis the explanation? Circ Res. 2017;120:681–691 [DOI] [PubMed] [Google Scholar]
- 53.Caponetti AG, Rapezzi C, Gagliardi C, Milandri A, Dispenzieri A, Kristen AV, et al. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: Insights from thaos. JACC Heart Fail. 2021;9:736–746 [DOI] [PubMed] [Google Scholar]
- 54.Jenkins MC, Potter M. Calcified pseudotumoural mediastinal amyloidosis. Thorax. 1991;46:686–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westermark P, Mucchiano G, Marthin T, Johnson KH, Sletten K. Apolipoprotein a1-derived amyloid in human aortic atherosclerotic plaques. Am J Pathol. 1995;147:1186–1192 [PMC free article] [PubMed] [Google Scholar]
- 56.Li XA, Hatanaka K, Ishibashi-Ueda H, Yutani C, Yamamoto A. Characterization of serum amyloid p component from human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1995;15:252–257 [DOI] [PubMed] [Google Scholar]
- 57.Howlett GJ, Moore KJ. Untangling the role of amyloid in atherosclerosis. Curr Opin Lipidol. 2006;17:541–547 [DOI] [PubMed] [Google Scholar]
- 58.Hellberg S, Silvola JMU, Liljenback H, Kiugel M, Eskola O, Hakovirta H, et al. Amyloid-targeting pet tracer [(18)f]flutemetamol accumulates in atherosclerotic plaques. Molecules. 2019;24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: A longitudinal fdg-pet/ct study. Circ Cardiovasc Imaging. 2013;6:747–754 [DOI] [PubMed] [Google Scholar]
- 60.Akkineni S, Zhu C, Chen J, Song M, Hoff SE, Bonde J, et al. Amyloid-like amelogenin nanoribbons template mineralization via a low-energy interface of ion binding sites. Proc Natl Acad Sci U S A. 2022;119:e2106965119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bai Y, Yu Z, Ackerman L, Zhang Y, Bonde J, Li W, et al. Protein nanoribbons template enamel mineralization. Proc Natl Acad Sci U S A. 2020;117:19201–19208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ajili W, Tovani CB, Fouassier J, de Frutos M, Laurent GP, Bertani P, et al. Inorganic phosphate in growing calcium carbonate abalone shell suggests a shared mineral ancestral precursor. Nat Commun. 2022;13:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE. A new era for understanding amyloid structures and disease. Nat Rev Mol Cell Biol. 2018;19:755–773 [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Arseni D, Zhang W, Huang M, Lovestam S, Schweighauser M, et al. Cryo-em structures of amyloid-beta 42 filaments from human brains. Science. 2022;375:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poulsen ET, Pedersen KW, Marzeda AM, Enghild JJ. Serum amyloid p component (sap) interactome in human plasma containing physiological calcium levels. Biochemistry-Us. 2017;56:896–902 [DOI] [PubMed] [Google Scholar]
- 66.Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, et al. Therapeutic clearance of amyloid by antibodies to serum amyloid p component. N Engl J Med. 2015;373:1106–1114 [DOI] [PubMed] [Google Scholar]
- 67.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Goncalves F, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255 [DOI] [PubMed] [Google Scholar]