Abstract
The objective was to provide an updated profile of the bovine acute-phase response to include recent advancements in technologies and expanded hematological, cytokine, and serum chemistry variables. Beef steers (n = 32; body weight [BW] = 251 ± 19.5 kg) were fitted with indwelling jugular catheters 1 d before lipopolysaccharide (LPS; 0.25 µg LPS/kg BW from Escherichia coli O111:B4) administration to facilitate serial blood collection. Rectal temperature was measured using indwelling probes, and ocular temperature was measured using infrared thermal imaging. Blood samples were collected for subsequent analysis of serum chemistry, hematology, and cytokine concentrations. Pearson correlation of rectal temperature and ocular infrared temperature was 0.61 (P < 0.01) and the Spearman correlation coefficient was 0.56 (P < 0.01). Interactions of hour × method were observed for ocular and rectal measurements of body temperature in response to endotoxin exposure. Maximum observed temperature was 39.6 °C at 2.5 h for both rectal and ocular measurements. Body temperature differed by method at hours 0.5, 2.5, 4.5, 7.5, 12.5, 36.5, and 47.5 (P < 0.01), but were not different otherwise. All variables of serum chemistry and complete blood count were influenced by LPS administration, except creatinine, serum glucose, and percent basophils (P ≤ 0.02). Alanine aminotransferase and alkaline phosphatase peaked at hour 2 relative to LPS administration, returned to baseline at hour 12 and continued to decrease below the baseline value at hour 48 (P < 0.01). Total protein concentration decreased 3% in response to LPS (P = 0.01). Total white blood cell count decreased 75% after LPS administration at hour 1 (P < 0.01). Lymphocyte count recovered to baseline at hour 6; sooner than neutrophil count at hour 36. Serum cortisol concentration increased 294% relative to baseline at hour 1 followed by a sustained decrease and return to normal concentration at hour 4 (P < 0.01). Additionally, circulating cytokine concentrations changed with time in response to the LPS challenge, excluding aFGF, bFGF, IGF-1, IL-2, IL-4, MCP-1, and ANG-1 (P ≤ 0.08). Maximum observed concentration of TNF-α at hour 1 was 117% greater than the pre-challenge value (P < 0.01). Data presented herein add to existing works to understand the endocrine and immune responses of beef steers administered exogenous LPS, and incorporate recent technologies, additional biomarkers, and an expanded cytokine profile that can be used as referential data in future research.
Keywords: acute phase response, bovine, complete blood count, cytokines, lipopolysaccharide, serum chemistry
The work herein contributes to the greater understanding of the bovine acute phase response, including multiple physiological markers and differing methods of body temperature measurement.
Introduction
The acute phase response (APR) is a component of innate immunity wherein the body attempts to prevent further tissue damage, destroy the assaultive pathogen, and return to homeostasis (Baumann and Gauldie, 1994). This dynamic response is early and nonspecific, and can include systemic, metabolic changes with variation by breed, sex, and temperament (Petersen et al., 2004; Hughes et al., 2014). Characteristics of inflammation and the APR can be mimicked in a controlled setting by administration of exogenous bacterial lipopolysaccharide (LPS), and thus effects on immune, growth, and stress systems can be closely studied (Waldron et al., 2003). Application of these methods contribute to the greater understanding of the bovine response to inflammatory diseases. The greatest challenge to animal health management in beef production systems is bovine respiratory disease (BRD), a complicated inflammatory disease characterized by stress-induced immunosuppression, polymicrobial infection leading to bronchopneumonia, and added environmental effects (Duff and Galyean, 2007; Richeson and Falkner, 2020; Galyean et al., 2022). These compounding effects yield greater than $2 billion USD in economic losses annually from mortality, growth performance losses, decreased carcass value, and labor inputs (Wilson et al., 2017).
Carroll et al. (2009b) authored the foremost expansive profile of the bovine APR after an LPS challenge. All variables measured were influenced by the LPS challenge (2.5 μg LPS/kg BW intravenously), except IL-2, IL-4, acid soluble protein, α-acid glycoprotein, and rump sweat rate. Thus, this research indicated exposure to exogenous LPS produces a pronounced APR in bovine. Serum tumor necrosis factor-α (TNF-α) concentration peaked 1.4 h following LPS administration, followed by peak IL-1β, IL-6, IFN-γ, and cortisol from 3.0 to 4.3 h. Increases in respiration rate, ear temperature, and serum cortisol concentration preceded the spike in serum TNF-α, which indicated the early local response to endotoxin was not mediated by circulating TNF-α, and rather elevated cortisol was likely the effector molecule in upregulating subsequent immune activity.
Following the work of Carroll et al. (2009b), several advancements in assay sensitivity, additional biomarkers, and new technologies have been established. Thus, the objective of the present study was to update the encompassing profile of the bovine APR to reflect advancements from the last 14 yr, including quantification of 30 cytokines, serum chemistry, hematology, and body temperature measured using infrared thermography to better understand the physiology of diseases affecting cattle.
Materials and Methods
Use of animal subjects
Experimental procedures followed the “Guide for the Care and Use of Agricultural Animals in Research and Teaching” and were approved by the Livestock Issues Research Unit Institutional Animal Care and Use Committee (Protocol #2121F). The study was conducted in June 2021 at the USDA-ARS Livestock Issues Research Unit Bovine Immunology Research and Development facility, located approximately 24 km northeast of Lubbock, TX.
Animal preparation and challenge procedures
On day −1 relative to the LPS challenge, 32 crossbred beef steers were weighed (body weight [BW] = 251 ± 19.5 kg), fitted with an indwelling rectal temperature probe (TidbiT v2 temperature logger, Part UTBI-001, Onset Corp., Pocasset, MA) to record body temperature in 5-min intervals (Reuter et al., 2010), and an indwelling jugular catheter was inserted to facilitate serial collection of blood samples. Steers were housed in individual bleeding stalls (2.28 m × 0.76 m) with rubber flooring inside an environmentally controlled barn allowing for normal postural movements and maintenance behaviors (standing, lying, eating, individual grooming, etc.). The ambient temperature inside the barn was 27.4 ± 3.42 °C throughout the data collection period. One steer was removed for extreme temperament; thus 31 steers were used in the experiment. Ad libitum water and feed was individually accessible. Steers were fed a 65% concentrate diet composed of 63.26% wet corn gluten feed (Cargill Inc., Blair, NE), 17.1% steam-flaked corn, 15.23% chopped alfalfa hay, 1.44% limestone, 1.47% supplement, and 1.5% yellow grease. The next day at 0900 h (0 h), steers were challenged intravenously with 0.25 µg LPS/kg BW from Escherichia coli O111:B4 (Sigma Aldrich, St. Louis, MO).
Determination of body temperature
Ocular infrared temperature was measured at hours −1.5, −0.5, 0.5, 1.5, 2.5, 3.5, 4.5, 5.0, 5.5, 6.5, 7.5, 8.5, 12.5, 24.5, 36.5, and 47.5 using a thermal imaging camera with a 24° lens (Model E95, FLIR Systems, Wilson, OR) measured at a distance of approximately 1 m from the lacrimal region of the eye at an angle of 45°–90°. Images were recaptured if the image was blurry or an improbable temperature was recorded (<36.7 °C or >42.8 °C). The acceptable range in body temperature of beef cattle is within 36.7–39.1 °C, excluding times of febrile response (Fielder, 2015). Data from the indwelling rectal thermometers was summarized as the average of 1-h intervals and reported at equivalent time points.
The FLIR E95 thermal imaging camera contains settings for emissivity, reflected temperature, atmospheric temperature, relative humidity, and distance which were calibrated before use for accurate measurement of body temperature. Before the experiment began, the emissivity settings were calibrated such that infrared temperature was within ±0.3 °C of rectal temperature. Reflective temperature was recorded from 180° behind where temperature was being measured. As described previously, this study was conducted inside an environmentally controlled barn, and the camera settings were calibrated using the ambient temperature and relative humidity before each collection time point. Thermal imaging systems are sensitive to major atmospheric changes; thus, the camera settings should be adjusted when atmospheric temperature changes ±5 °C or relative humidity changes ±10%.
Blood sampling
Blood samples for complete blood count analysis were collected via jugular catheter in 1-h intervals from −2 to 4 h, and again at hours 6, 8, 12, 24, 36, and 48 into a 4 mL evacuated tube containing 7.2 mg ethylenediaminetetraacetic acid (BD Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ). Red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelets, white blood cells, and leukocyte differentials were quantified using an automated hemocytometer (ProCyte Dx Hematology Analyzer; IDEXX Laboratories, Westbrook, ME) with bovine specific analogs. Two additional samples were collected into 9 mL evacuated tubes with no additive (BD Vacutainer), allowed to clot for 30 min, then centrifuged at 1,500 × g for 20 min at 4 °C. Triplicate aliquots of serum were decanted and stored at −80 °C for subsequent analysis of cortisol and cytokine concentrations at hours −2, 0, 1, 2, 3, 4, 6, and 8 relative to LPS administration. Cortisol concentration was determined using a commercially available enzyme-linked immunoassay kit (Arbor Assays, Ann Arbor, MI) relative to a standard curve established using known cortisol concentrations. The minimum detectable concentration was 27.6 pg/mL, and the intra-assay and inter-assay CV were 14.7% and 15.1%, respectively. Serum cytokine concentrations were quantified by a full-service commercial laboratory using a bovine-specific antibody array (Quantibody Bovine Cytokine Antibody Array, Ray Biotech, Peachtree Corners, GA). A final blood sample was collected into a 4-mL evacuated tube coated with lithium heparin. Plasma was processed in the same manner as serum, except for the 30-min clotting time, and used for chemistry analysis of plasma alanine aminotransferase, alkaline phosphatase, creatinine, glucose, total protein, and blood urea nitrogen (Prep Profile II, VetScan VS2, Abaxis, Union City, CA) at hours 0, 2, 12, and 48 relative to LPS administration.
Statistical analyses
Animal was the experimental unit for all analyses. Pearson and Spearman correlation coefficients between ocular and rectal temperature measurements were determined using PROC CORR (SAS v9.4, SAS Institute Inc., Cary, NC). The effects of temperature measurement method, hour, and their interaction were analyzed as fixed effects using PROC MIXED, where animal was included as a random effect and animal within method was the subject of the repeated measures. The autoregressive-1 multiple covariance structure was used because it resulted in the smallest Akaike and Schwartz Bayesian criteria. Serum chemistry, hematology, and cytokine data were analyzed as repeated measures in PROC MIXED, where hour was repeated, and the subject was individual animal. Results were considered statistically significant when P ≤ 0.05. Data are presented as LSM ± SEM. Percentage change was calculated between the peak value observed and the pre-challenge value.
Results
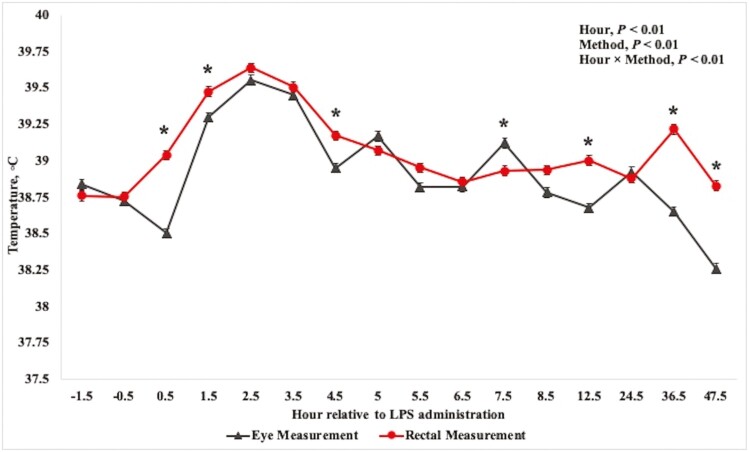
The Pearson correlation coefficient between rectal and ocular infrared temperatures was 0.61 (P < 0.01) and the Spearman correlation coefficient was 0.56 (P < 0.01). At −0.5 h relative to LPS administration, average body temperature was 38.7 °C for both ocular and rectal measurements (Table 1). Rectal temperature increased above the pre-challenge values at 0.5 h and returned to baseline values by 6.5 h (P < 0.01). The ocular temperature did not increase above the pre-challenge temperature until 1-h post-challenge but returned to baseline values 1 h sooner than rectal temperature at 5.5 h (P < 0.01). Peak observed temperature was recorded at 2.5 h and was 39.6 °C for both ocular and rectal measurements. Numerous interactions of hour × method were noted for ocular and rectal measurements of body temperature (Fig. 1). Additionally, ocular and rectal measurements differed from one another at hours 0.5, 1.5, 4.5, 7.5, 12.5, 36.5, and 47.5 (P < 0.01), wherein rectal temperature was greater than ocular temperature at all-time points except hour 7.5. Otherwise, body temperature did not differ by method.
Table 1.
Body temperature, serum chemistry, and complete blood count response to an intravenous dose of 0.25 µg lipopolysaccharide/kg body weight in beef steers
| Item | Pre-challenge1 | Maximum | Minimum | SEM2 | Max. hour | Min. hour | Δ%3 | P-value4 |
|---|---|---|---|---|---|---|---|---|
| Body temperature, °C | ||||||||
| Rectal | 38.8 | 39.6 | 38.7 | 0.271 | 2.5 | −0.5 | 2 | <0.01 |
| Ocular | 38.8 | 39.6 | 38.3 | 0.342 | 2.5 | 47.5 | 2 | <0.01 |
| Serum chemistry | ||||||||
| Alanine aminotransferase, U/L | 19.0 | 20.3 | 18.5 | 0.65 | 2 | 48 | 7 | <0.01 |
| Alkaline phosphatase, U/L | 127.7 | 178.5 | 103.8 | 6.94 | 2 | 48 | 40 | <0.01 |
| Creatinine, mg/dL | 0.77 | 0.82 | 0.76 | 0.027 | 12 | 48 | 6 | 0.22 |
| Glucose, mg/dL | 95.6 | 102.4 | 87.9 | 4.44 | 2 | 12 | 7 | 0.13 |
| Total protein, g/dL | 7.6 | 7.6 | 7.4 | 0.07 | 0 | 2 | −3 | 0.01 |
| Blood urea nitrogen, mg/dL | 9.5 | 10.7 | 9.5 | 0.34 | 12 | 0 | 13 | <0.01 |
| Complete blood count | ||||||||
| Red blood cells, M/µL | 7.3 | 7.6 | 7.0 | 0.14 | 3 | 36 | 4 | 0.02 |
| Hemoglobin, g/dL | 9.8 | 10.2 | 9.4 | 0.16 | 3 | 24 | 4 | <0.01 |
| Hematocrit, % | 28.1 | 29.3 | 27.2 | 0.57 | 3 | 36 | 4 | 0.02 |
| Platelets, K/µL | 506 | 516 | 381 | 24.8 | −2 | 2 | −25 | <0.01 |
| White blood cells, K/µL | 11.4 | 12.6 | 2.9 | 0.55 | 36 | 1 | −75 | <0.01 |
| Neutrophils, K/µL | 4.2 | 4.3 | 0.4 | 0.41 | −2 | 1 | −90 | <0.01 |
| Lymphocytes, K/µL | 5.1 | 7.0 | 2.0 | 0.43 | 12 | 1 | −62 | <0.01 |
| Monocytes, K/µL | 1.9 | 2.6 | 0.3 | 0.11 | 48 | 1 | −84 | <0.01 |
| Eosinophils, K/µL | 0.23 | 0.32 | 0.17 | 0.059 | 2 | 1 | −29 | <0.01 |
| Basophils, K/µL | 0.0029 | 0.0037 | 0.0000 | 0.00075 | 36 | 4 | −100 | <0.01 |
| Neutrophils, % | 35.3 | 36.0 | 14.4 | 3.21 | −2 | 1 | −59 | <0.01 |
| Lymphocytes, % | 45.8 | 69.1 | 45.4 | 2.81 | 1 | −2 | 51 | <0.01 |
| Monocytes, % | 16.8 | 21.1 | 9.3 | 0.76 | 48 | 3 | −45 | <0.01 |
| Eosinophils, % | 2.0 | 7.8 | 1.5 | 0.63 | 2 | 48 | 292 | <0.01 |
| Basophils, % | 0.025 | 0.028 | 0.000 | 0.0095 | 36 | 4 | −100 | 0.34 |
| Neutrophil:Lymphocyte | 0.96 | 0.99 | 0.22 | 0.099 | −2 | 1 | −77 | <0.01 |
1Average of hours −2 and 0.
2Pooled standard error of the least squares mean.
3Percentage change between the peak value observed and the pre-challenge value.
4Observed significance level for the hour comparison.
Figure 1.
Mean (±SEM) body temperature measured by ocular infrared thermography or rectal probe in 31 beef steers administered intravenous exogenous lipopolysaccharide to induce a febrile response. Ocular temperature was measured using an infrared camera (FLIR E95; FLIR Systems, Wilson, OR) at a distance of ≤1 m and angle between 45° and 90°. Indwelling rectal thermometers were affixed according to Reuter et al. (2010). Significance (P ≤ 0.05) by method is denoted by *.
All serum chemistry and hematology variables were influenced by the LPS challenge, with the exception of creatinine, serum glucose, and percentage basophils (P ≤ 0.02; Table 2). After the peak observed concentration at hour 2, both alanine aminotransferase (ALT) and alkaline phosphatase (ALP) returned to baseline concentration at hour 12 (P ≥ 0.10), however, ALP concentration continued to decrease such that concentration at hour 48 was less than baseline (P < 0.01). Maximum concentration of ALT was 7% greater than the pre-challenge value, and ALP concentration increased 40%. Concentration of total protein decreased 3% in response to the LPS challenge at hour 2 and did not return to baseline until hour 48. Blood urea nitrogen (BUN) concentration did not increase above baseline until hour 12 (P < 0.01), and decreased slightly at hour 48 (P = 0.03), but did not return to baseline within the data collection period (hours 0, 2, 12, and 48; P = 0.03).
Table 2.
Cortisol and cytokine responses to an intravenous dose of 0.25 µg lipopolysaccharide/kg body weight in beef steers
| Item | Pre-challenge1 | Maximum | Minimum | SEM2 | Max. hour | Min. hour | Δ%3 | P-value4 |
|---|---|---|---|---|---|---|---|---|
| Cortisol, ng/mL | 12.4 | 48.9 | 11.1 | 3.01 | 1 | 0 | 294 | <0.01 |
| Cytokines, pg/mL | ||||||||
| IFN-αA | 82 | 176 | 40 | 35.8 | 3 | 6 | 113 | <0.01 |
| IFN-γ | 233 | 372 | 117 | 40.4 | 3 | 6 | 60 | <0.01 |
| IL-13 | 1150 | 1807 | 599 | 218.9 | 3 | 6 | 57 | <0.01 |
| IL-1α | 47 | 97 | 24 | 10.8 | 3 | 6 | 107 | <0.01 |
| IL-1F5 | 77 | 170 | 40 | 17.2 | 3 | 6 | 119 | <0.01 |
| IL-21 | 314 | 824 | 155 | 85.8 | 3 | 6 | 162 | <0.01 |
| IP-10 | 415 | 558 | 269 | 56.4 | 3 | 6 | 35 | <0.01 |
| MIG | 247 | 818 | 217 | 65.2 | 3 | 6 | 232 | <0.01 |
| MIP-1β | 32 | 291 | 20 | 15.5 | 3 | −2 | 803 | <0.01 |
| TNF-α | 661 | 1433 | 393 | 133.3 | 1 | 6 | 117 | <0.01 |
| aFGF | 732 | 1013 | 621 | 259.5 | 3 | 6 | 38 | 0.32 |
| bFGF | 8 | 14 | 2 | 4.0 | −2 | 4 | −76 | 0.43 |
| GASP-1 | 56 | 116 | 41 | 18.2 | 2 | 0 | 107 | <0.01 |
| IGF-1 | 167 | 325 | 108 | 129.5 | 8 | 4 | 95 | 0.68 |
| IL-2 | 2611 | 3331 | 1892 | 927.1 | 0 | −2 | 28 | 0.30 |
| IL-4 | 235 | 307 | 221 | 97.6 | 8 | 0 | 30 | 0.76 |
| IL-15 | 2,738 | 3,510 | 1,941 | 947.7 | 3 | 6 | 28 | 0.01 |
| MCP-1 | 31,947 | 36,741 | 20,822 | 8,709.1 | 0 | 6 | −35 | 0.21 |
| NCAM1 | 8,679 | 8,908 | 6,880 | 570.6 | −2 | 6 | −21 | <0.01 |
| VEGF | 563 | 1,035 | 413 | 482.0 | 8 | 0 | 84 | 0.08 |
| ANG1 | 30 | 47 | 26 | 10.7 | 4 | 0 | 54 | 0.12 |
| CD40L | 7,702 | 10,054 | 5,611 | 3,245.8 | 2 | 6 | 31 | 0.07 |
| Decorin | 564 | 695 | 474 | 28.1 | 1 | 8 | 23 | <0.01 |
| IFN-β | 219 | 369 | 204 | 46.3 | 2 | 0 | 68 | 0.02 |
| IL-1β | 290 | 383 | 275 | 48.4 | 2 | 0 | 32 | <0.01 |
| IL-10 | 473 | 975 | 433 | 179.2 | 4 | 8 | 106 | <0.01 |
| IL-17A | 180 | 250 | 158 | 38.3 | 2 | 0 | 39 | <0.01 |
| IL-18 | 1,152 | 3,710 | 888 | 345.9 | 2 | 8 | 222 | <0.01 |
| LIF | 565 | 821 | 506 | 372.7 | 8 | 6 | −10 | 0.06 |
| RANTES | 144 | 1,772 | 128 | 79.3 | 3 | −2 | 1,127 | <0.01 |
1Average of hours −2 and 0.
2Pooled standard error of the least squares mean.
3Percentage change between the peak value observed and the pre-challenge value.
4Observed significance level for the hour comparison.
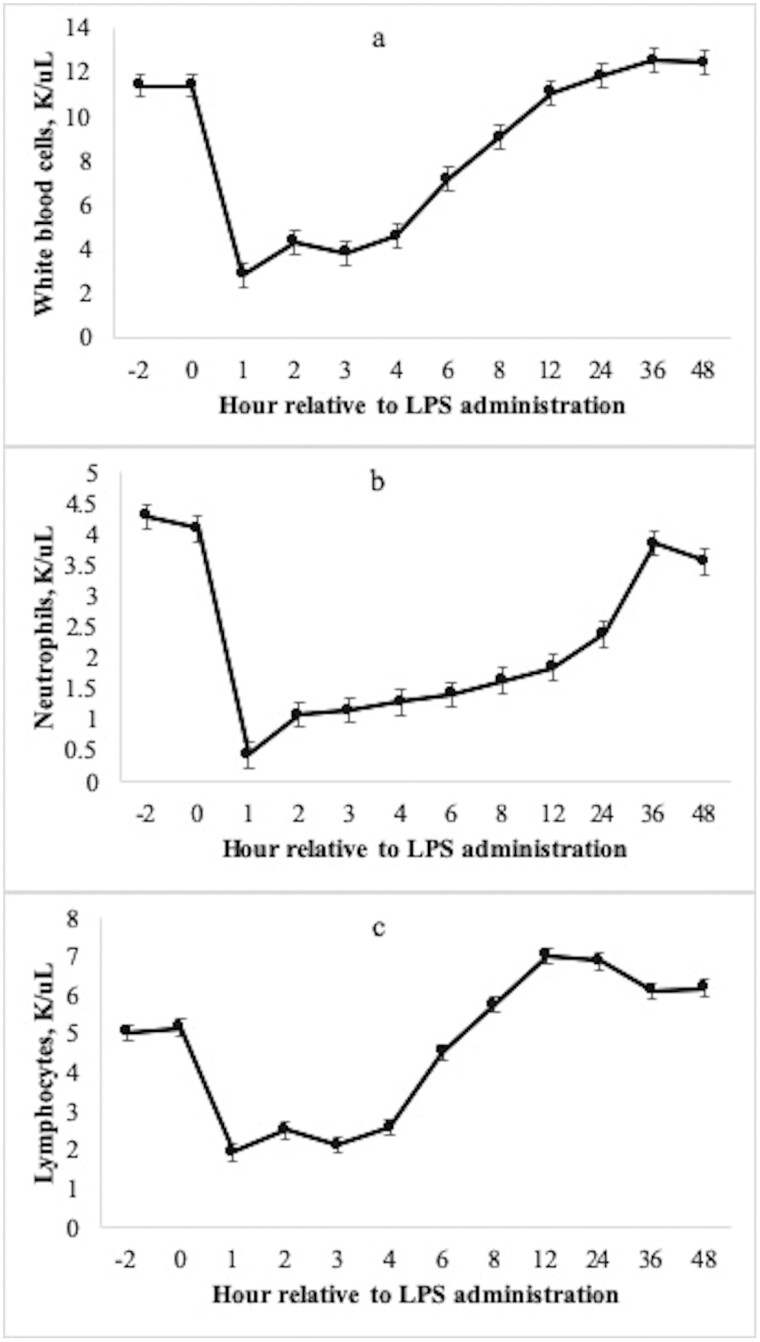
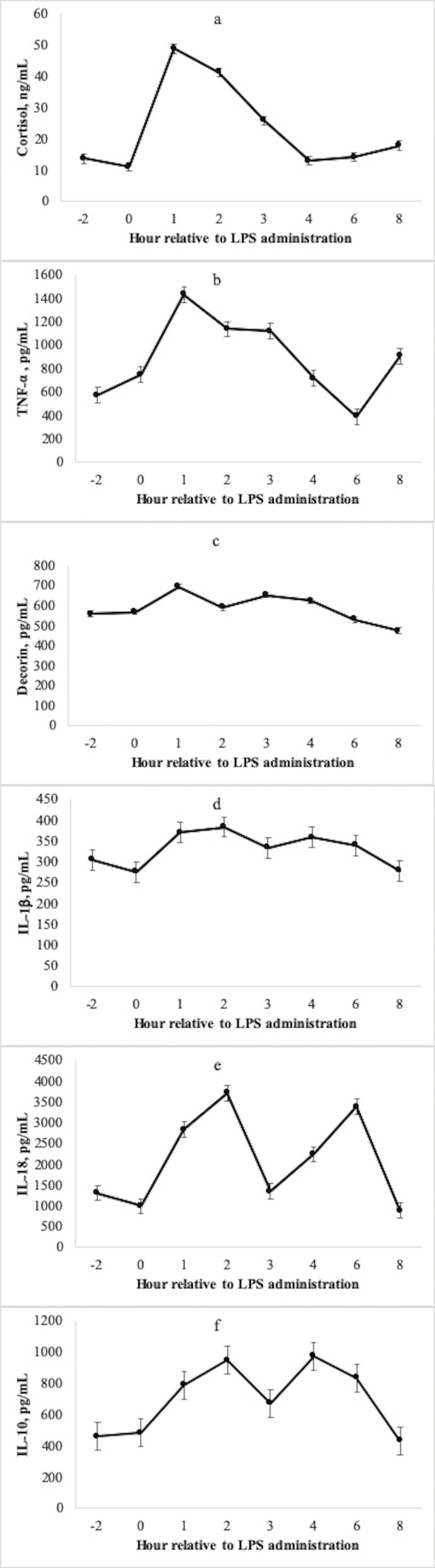
Total white blood cell count decreased 75% at hour 1 after LPS administration (Fig. 2a, P < 0.01). Accordingly, neutrophil count decreased 88% and lymphocyte count decreased 62% at hour 1 relative to the pre-challenge concentration (Fig. 2b and Fig. 2c; P < 0.01). Following the acute decrease at hour 1, neutrophil count increased gradually and returned to baseline at hour 36, whereas lymphocyte count recovered to baseline sooner at hour 6. Serum cortisol concentration increased 294% above the pre-challenge concentration at hour 1, followed by a gradual decrease and return to baseline at hour 4 (Fig. 3a; P < 0.01).
Figure 2.
Mean (±SEM) total circulating white blood cell count (a), neutrophil count (b), and lymphocyte count (c) of 31 beef steers following an intravenous challenge with lipopolysaccharide (LPS; 0.25 μg/kg body weight). All measurements changed with time following administration of LPS at hour 0 (P < 0.01).
Figure 3.
Mean (±SEM) serum cortisol (a), TNF-α (b), decorin (c), IL-1β (d), IL-18 (e), and IL-10 (f) concentration of 31 beef steers following an intravenous challenge with lipopolysaccharide (LPS; 0.25 μg/kg body weight). All measurements changed with time following administration of LPS at hour 0 (P < 0.01).
The reader is directed to Table 3 for descriptions of individual cytokine, chemokine, and growth factor functions discussed herein. Measures of circulating cytokine concentrations changed with time throughout the LPS challenge, excluding aFGF, bFGF, IGF-1, IL-2, IL-4, MCP-1, and ANG-1 (P ≤ 0.08; Table 2). Concentration of TNF-α peaked at hour 1 and was 117% greater than the pre-challenge value (Fig. 3b; P < 0.01) and returned to baseline at hour 4. Another pro-inflammatory cytokine, decorin (Fig. 3c), also peaked in concentration at hour 1. Interestingly, the concentration returned to baseline at hour 2, followed by a secondary peak at hour 3. The concentration then declined to less than the pre-challenge value throughout the remainder of the data collection period (8 h).
Table 3.
General functions of cytokines measured in the present study
| Item | Class | Function | Citation |
|---|---|---|---|
| IFN-αA | Anti-inflammatory Pro-inflammatory |
- Inhibits multiplication of viruses - Stimulation of innate and adaptive immune cells and antibody class switching - Immunosuppressive with prolonged activity |
Cha et al. (2014)
Tilg and Peschel (1996) |
| IFN-γ | Pro-inflammatory | - Upregulates activity of CD4 helper T cells, CD8 cytotoxic T cells, NK cells, dendrites, and macrophages - Potentiates pro-inflammatory signaling and antigen presentation - Antagonizes IL-10 and TGF-β |
Castro et al. (2018)
Kopitar-Jerala (2017) |
| IL-13 | Anti-inflammatory | - Inhibits pro-inflammatory cytokine production from macrophages - Central mediator of IgE |
Dembic (2015)
de Vries (1998) |
| IL-1α | Pro-inflammatory | - Simulates activity of genes related to inflammation and immune processes - Cell recruitment by promoting expression of adhesion molecules and chemokine production |
Gabay et al. (2010)
Medline (2022) |
| IL-1F5 | Pro-inflammatory | - Also known as IL-36RN - Proposed to play a role in skin inflammation - Might be involved in innate immune response to fungal pathogens |
Blumberg et al. (2007) |
| IL-21 | Anti-inflammatory Pro-inflammatory |
- Increases production of IL-17 by promoting differentiation of naïve Th cells to Th17 cells, upregulates IL-6 - Produced by activated CD4+ T cells; initiates and sustains antibody production and mediates antibody class switching - Promotes migration of neutrophils and NK cells to cite of inflammation, terminal conversion of B cells to plasma cells - In the presence of bacterial LPS: inhibits B cell proliferation and dendrite activation - Balances inflammatory response by increasing IL-10 production and decreasing TNF-α production |
Davis et al. (2007)
Mootha et al. (2021) |
| IP-10 | Chemokine | - Chemotactic agent for T cells, NK cells, monocytes, macrophages, and dendrites - Produced by many cell types in response to IFN-γ and LPS |
Chen et al. (2020)
Dufour et al. (2002) |
| MIG | Chemokine Pro-inflammatory |
- “Monokine induced by gamma”, or CXCL9 - Recruitment of activated T cells to sites of infection - Induced by IFN-γ, might amplify the IFN-γ signal - May be a functional measure of bioactive IFN-γ activity that is more sensitive than direct measurement of IFN-γ |
Berthoud et al. (2009) |
| MIP-1β | Pro-inflammatory | - Attracts several types of immune cells to the site of microbial infections - Stimulates production of pro-inflammatory cytokines, mast cell degranulation, and NK cell activation |
Menten et al. (2002) |
| TNF-α | Pro-inflammatory | - Among the most important pro-inflammatory cytokines in mounting a proper, robust immune response - Participates in vasodilatation, expression of adhesion molecules and leukocyte recruitment, regulates blood coagulation, contributes to oxidative stress, indirectly induces fever - Activation of neutrophils and platelets and enhances killing abilities of NK cells and macrophages. Upregulates subsequent cytokine production - Bacterial LPS is one of the main stimulants of TNF-α production |
Idriss and Naismith (2000)
Zelová and Hošek (2013) |
| aFGF bFGF |
Growth factor | - Fibroblast growth factors. Prototypic members have different isoelectric points: - aFGF = acidic. Greatest expression in brain, retina, bone matrix, osteosarcomas - bFGF = basic. Greatest expression in pituitary gland, neural tissue, adrenal cortex, corpus luteum, placenta - Regulation of cell proliferation and differentiation, critical for normal fetal development, tissue maintenance, and somatic stem cell development - During inflammation: members of the FGF family facilitate the repair process |
DePhillips and Lenhoff (2004)
Marega et al. (2021) Yun et al. (2010) |
| GASP-1 | Myokine | - Negative regulation of muscle mass - Regulates activation of myostatin via proteolytic cleavage |
Elkina et al. (2011)
Périè et al. (2017) |
| IGF-1 | Growth factor Anti-inflammatory Pro-inflammatory |
- Hormone that is a major mediator of somatic growth and anabolic responses in several cells and tissues - During inflammation: can modulate immunity in various immune cells, primarily lymphocytes and monocytes, with pro- and anti-inflammatory effects depending on the magnitude of the stimulus and the type of immune cell - Mechanisms of growth hormone and IGF-1 effects on the inflammatory response are not completely understood |
Johnson et al. (1996)
Wolters et al. (2017) |
| IL-2 | Pro-inflammatory | - Produced by activated T lymphocytes and upregulates the growth and activity of T lymphocytes, B lymphocytes, NK cells, and development of the immune system - Strong activator of TNF-α, IFN-γ, and IL-1 production - Depending on the quantity of interaction with the IL-2 receptor, can be immunosuppressive via production of effector immune cells and regulatory T cells |
Hodi and Soiffer (2002)
Mitoma et al. (2021) |
| IL-4 | Immunoregulator | - “Prototypic immunoregulatory cytokine”: regulation of antibody production, hematopoiesis, inflammation, effector T-cell responses. Mainly produced by activated T cells - Augments expression of major histability complexes, promotes secretion of IgE and IgG - Inhibits production of inflammatory cytokines, primarily TNF-α, IL-6, and IL-1 - Pathways to determine the fate of Th lymphocytes, and promoting Th2 differentiation while inhibiting Th1 differentiation |
Brown and Hural (1997)
Curtis (2006) Luzina et al. (2012) Smiley and Grusby (1998) |
| IL-15 | Pro-inflammatory | - Important during the innate inflammatory response to microbial and parasitic pathogens, and mounting a protective immune response - Critical to the development, differentiation, and survival of NK cells - In mice: expressed by monocytes and macrophages primed with microbial PAMPs or IFN-γ - Is not downregulated by inhibitory cytokines, such as IL-4 or IL-13 - Closely related to IL-2 |
Marks-Konczalik et al. (2000)
Perera et al. (2012) |
| MCP-1 | Chemokine | - “Monocyte chemoattractant protein” - Regulates the migration and translocation of monocytes and macrophages |
Deshmane et al. (2009) |
| NCAM-1 | Adhesion molecule | - “Neural cell adhesion molecule” - Part of the immunoglobulin superfamily that is expressed in neurons and glial cells - Important for the development of the central nervous system and synaptic plasticity - Mode of action includes regulation of cell adhesion, migration, and neurite growth |
Hübschmann et al. (2005)
Jesudas et al. (2020) |
| VEGF | Pro-inflammatory | - “Vascular endothelial growth factor” - Enhances endothelial permeability by upregulating the expression of adhesion molecules on endothelial cells, acts as chemoattractant for monocytes - During inflammation: produced by T cells, synovial cells, smooth muscle cells, and epithelial cells at the site of inflammation that promotes angiogenesis and exacerbates the severity of the reaction |
Angelo and Kurzrock (2007)
Reinders et al. (2003) |
| ANG-1 | Anti-inflammatory Pro-inflammatory |
- “Angiogenic growth factor” - Upregulates the growth, maturation, and structural integrity of blood vessels - Involved in cellular signaling that activates production of pro-inflammatory cytokines and macrophage differentiation - Assists in endothelial integrity, which can temper the inflammatory response by preventing vascular leakage - Involved in neutrophil recruitment via production of IL-8, a neutrophil chemotactic factor |
Pizurki et al. (2003)
Seok et al. (2013) |
| CD40 | Ligand | - Expressed almost exclusively by CD4+ cells - Belongs to the tumor necrosis factor superfamily - Mediates a variety of activities, including the activation of B cells and subsequent isotype switching, immunoglobulin production, and memory, and monocyte activation - Without CD40, system cannot execute immunoglobulin class switching, and only IgM class antibodies can be produced |
Alegre et al. (1998)
Chan and Rainer (2013) Manzoor (2015) |
| Decorin | Pro-inflammatory | - Involved in extensive signaling network to regulate cytokine and growth factor production - Upregulates synthesis of TNF-α and IL-12 following binding to TLR2 and TLR4 on the surface of macrophages |
Dong et al. (2022) |
| IFN-β | Anti-inflammatory Pro-inflammatory |
- Regulates cytokine and chemokine production, which in turn regulate inflammation - Regulates the development of nearly all effector cells of both innate and adaptive immunity - Modulates expression of TNF-α and IL-10 in peripheral mononuclear cells, decreases TNF-α expression in monocytes, and increases expression of IL-10 in dendrites |
Bolívar et al. (2018) |
| IL-1β | Pro-inflammatory | - “Master regulator” of inflammation via regulation of innate immunity processes - Expression of IL-1β considered a “priming step”: activated cell must encounter further PAMP or DAMP - Leukocytic pyrogen, mediates fever, and induces several components of the APR - Critical in mounting a robust inflammatory response, but chronic exposure exacerbates tissue damage - Stimulus is not well-defined; purportedly continuously secreted, with serum concentration reflective of the strength of the stimulus and the extracellular requirement |
Lopez-Castejon and Brough (2011)
Kaneko et al. (2019) |
| IL-10 | Anti-inflammatory | - Potent anti-inflammatory agent - Inhibits synthesis of pro-inflammatory cytokines, and downregulates antigen presentation to monocytes, macrophages, and dendrites - Limits T cell activation and proliferation - Monocytes and macrophages are the primary targets |
Islam et al. (2021)
Iyer and Cheng (2012) |
| IL-17A | Pro-inflammatory | - Potentiates the inflammatory response by inducing granulopoiesis factors and neutrophil-specific chemokines - Production of several mediators of the APR, such as IL-6, and pro-inflammatory cytokines TNF-α and IL-1β - Insufficient to produce a robust immune response on its own, synergistic with other pro-inflammatory mediators to yield sustained neutrophil recruitment to the site of inflammation |
Zenobia and Hajishengallis (2015) |
| IL-18 | Pro-inflammatory | - Important in local and systemic inflammation - Promotes the expression of TNF-α and IFN-γ - Regulates activity of Th1 cells, cytotoxic T cells, B cells, NK cells, and macrophages - Stimulates IL-8 production as a neutrophil chemoattractant |
Biet et al. (2002) |
| LIF | Anti-inflammatory Pro-inflammatory |
- “Leukemia inhibitory factor” - In humans: A protective cytokine produced early in the inflammatory response that downregulates expression of pro-inflammatory cytokines and growth factors - Promotes the production of APP and is a monocyte chemoattractant |
Banner et al. (1998)
Yue et al. (2015) |
| RANTES | Chemokine | - “Regulated on activation normal T cell expressed and secreted” - Chemoattractant for monocytes, NK cells, and eosinophils - Homing and migration factor of effector and memory T cells during the inflammatory response |
Crawford et al. (2011) |
Peak concentration of IL-1β was observed at hour 2 and increased 32% above the pre-challenge concentration (Fig. 3d; P < 0.01), followed by a return to baseline at hour 3 and for the remainder of the data collection period. Pro-inflammatory cytokine IL-17A followed a similar temporal pattern as IL-1β, wherein peak concentration was observed at hour 2 (P < 0.01) and concentration returned to baseline at hour 3. With both pro- and anti-inflammatory properties, IFN-β followed a similar temporal pattern by peaking in concentration at hour 2 and returning to baseline at hour 3 (P < 0.01). Another pro-inflammatory cytokine, IL-18, displayed a distinct biphasic response, wherein the first peak at hour 2 was 222% greater than the pre-challenge value (Fig. 3e; P < 0.01) and returned to baseline at hour 3. A secondary peak of similar magnitude was observed again at hour 6 (P < 0.01), followed by a return to baseline at hour 8. Myostatin inhibitor growth and differentiation factor-associated serum protein (GASP)-1 likewise increased 107% from the pre-challenge value at hour 2 (P < 0.01). The circulating GASP-1 concentration returned to baseline at hours 3, 4, and 6, but increased in concentration again at hour 8 (P = 0.03). Circulating concentration of ligand CD40 tended to increase above the pre-challenge value at hour 2 (P = 0.06), followed by a return to baseline at hour 3.
At hour 3, peak concentration in pro-inflammatory cytokines (IFN-γ, IL-1a, IL-1F5, MIP-1β), cytokines with both pro- and anti-inflammatory functions (IFN-αA, IL-21), anti-inflammatory cytokine (IL-13), and chemokines (IP-10, MIG, RANTES) were observed. There was some variation in the duration of response. Pro-inflammatory cytokine IL-1F5 and chemokine MIG both returned to baseline at hour 4 and remained at this concentration through hour 8. Conversely, IL-21 returned to baseline at hour 4 and MIP-1β at hour 6 but were both slightly elevated relative to the pre-challenge value at hour 8. Additionally, after the peak at hour 3, both IL-13 and IP-10 decreased below the pre-challenge value at hour 6 but following an increase at hour 8 were not different from the pre-challenge value. Chemokine RANTES did not return to baseline value within the 8 h data collection period, following a 1,127% increase in concentration at hour 3 and a sustained decrease thereafter. Finally, IL-10 first peaked in concentration at hour 2 (P = 0.01), returned to baseline at hour 3, then peaked again at hour 4 (P = 0.02) before returning to baseline again at hour 8 (Fig. 3f).
Discussion
Generally, the initial APR is activated when TNF-α, IL-1β, and IL-6 are produced from monocytes and neutrophils (Elsasser et al., 2008; Carroll et al., 2009b). Subsequently, acute phase proteins (APP) are produced from hepatocytes in attempt to return the animal to a pre-challenge state via activation of the compliment system and binding to pathogens for eradication (Carroll et al., 2009b). For example, haptoglobin is among the most responsive APP in bovine, and functions by binding free hemoglobin into a stable complex, preventing iron loss and thus limiting bacterial growth (Huntoon et al., 2008). Additionally, stress, including intravenous LPS administration, activates the sympathetic nervous system, which serves as the body’s “alarm system”, therefore depressing feed intake, activating the hypothalamic-pituitary-adrenal (HPA) axis, and the fight or flight response. Thus, the acute increase in production of catecholamines and cortisol can yield increased respiration rate, body temperature, sweat rate, and increased catabolic effects. This sympathetic response is initially immunostimulatory, but chronic exposure (>24 h) can interfere with immunity by inhibiting L-selectin expression thereby preventing immune cells ability to translocate from circulation to tissue (Dhabhar, 2009). Immune challenge models, such as the LPS challenge used herein, can be used in research to produce a known response that mimics inflammatory diseases. Gram-negative endotoxemia is a major component to many livestock diseases, and although an LPS challenge cannot replicate complicated polymicrobial infections that are often exacerbated by additional environmental effects, some local effects of acute inflammation can be closely studied using this model.
Hallmark clinical signs of the APR include lethargy, depression, anorexia, increased rate of respiration, and decreased sexual and aggressive behavior. The APR is nonspecific and critical for animal survival, with variations among- and within-species as a result of sex, breed, and temperament (Hughes et al., 2014). Stimulus of the APR can include tissue damage and platelet aggregation, inflammation, infection, or presence of bacterial products such as LPS. Within minutes, macrophages (blood monocytes and tissue macrophages) and neutrophils produce IL-1 and IL-6 type cytokines. Likewise, fibroblasts and endothelial cells produce IL-1, IL-6, and IL-8 type cytokines, stimulating neutrophil recruitment and further production of local TNF-α. Within hours after the stimuli, these local responses yield a systemic response. Hepatic APP are synthesized, with multiple targets and expansive functions. Likewise, the HPA axis is activated, yielding production of glucocorticoids from the adrenal cortex. Initial exposure to elevated concentration of glucocorticoids is immunostimulatory via the production of APP, activation of immune cells, and stimulation of cytokine production (Carroll et al., 2009b). As glucocorticoid concentration is elevated for several hours (5–8 h), the effects become anti-inflammatory, resulting in downregulation of the initial local inflammatory response (Petersen et al., 2004; Burdick et al., 2012). Additionally, because pro-inflammatory cytokine receptors extend to cell types beyond the immune system, direct mechanisms exist for altered nutrient metabolism, interrupted anabolic processes, and upregulated catabolism during an immune challenge (Spurlock, 1997).
Body temperature
Fever is widely recognized as a response to acute inflammation to accelerate pathogen eradication (Carroll and Forsberg, 2007). Carroll et al. (2009b) reported a mean maximum rectal temperature of 40.2 °C at hour 4.9 relative to LPS administration, a 4% difference between maximum observed and pre-challenge rectal temperature. In the present experiment, peak temperature and percentage change was slightly less than reported by Carroll et al. (2009b), but were not unanticipated values as the febrile response is highly adaptive, and because a lesser dose of LPS was used in the present study (0.25 µg/kg BW vs. 2.5 µg/kg BW).
Other works have reported an increase in rectal temperature within 1 h after LPS administration, whereas temperatures may not return to pre-challenge values until approximately 8 h post-challenge (Waldron et al., 2003; Carroll et al., 2009b). Interestingly, the ocular temperature measurement increased above the pre-challenge temperature 1-h later than rectal temperature in the present study at 1.5 h. In contrast, George et al. (2014) reported similar timing of an LPS-induced temperature increase among rectal, vaginal, ocular, and muzzle measurements, wherein the maximum temperature at all locations was reported at hour 6. Furthermore, ocular temperature of the present study tended to be less than rectal temperature throughout the study, even when temperatures were not statistically different. This is in agreement with George et al. (2014), who noted ocular and muzzle temperatures were generally less than both rectal and vaginal temperature measurements.
Infrared thermography is a measurement of surface temperature quantified by changes in heat transfer and blood flow, rather than a direct measurement of core body temperature like rectal or vaginal probe methods (McManus et al., 2016). An acute stressor redirects blood circulation from extremities to the brain, vital organs, and skeletal muscle. This occurs as an important survival mechanism, as these tissues take precedent in the hierarchy of nutrient partitioning during times of stress, and likewise is a response to potential blood loss during predation events (Johnson, 2002; Carroll and Burdick Sanchez, 2014). These concepts have been previously demonstrated using infrared thermography on the ear of rabbits (Blessing, 2003) and the tail and paw of rats (Vianna and Carrive, 2005). In bovine, surface temperature has been determined using radio-frequency implants located at the base of the ear, posterior to the poll, and beneath the umbilical fold (Reid et al., 2012), and using subcutaneous temperature loggers in various locations in the neck (Lee et al., 2016). Thus, vasoconstriction of ocular capillary beds after administration of intravenous LPS in the present study likely explains the delayed increase in ocular temperature relative rectal temperature, the generally lower ocular temperatures relative to rectal temperature, and moderate correlation of measurement methods (Burdick Sanchez et al., 2023). Limitations of infrared technology include inaccurate results caused by environmental interference such as direct sunlight, humidity, and wind, or overall image quality (McManus et al., 2016). Nonetheless, environmental impacts in the present experiment should be minimal as the facility was enclosed and temperature controlled, and any temperature or humidity changes were programmed into the camera.
The ability to detect small differences in body temperature is of paramount importance to animal health management. Post-weaning, BRD is the predominant disease challenge in beef cattle (Smith, 1998; Griffin, 2014), for which our current real-time diagnostic sensitivity is poor (Timsit et al., 2016). No method of real-time BRD diagnosis is considered gold standard; however, the most widely used approach begins with visual identification of clinical signs of illness (depression, inappetence, labored respiration, fever) followed by confirmation and thus qualification for antimicrobial treatment with rectal temperature (Thomson and White, 2006; Griffin et al., 2010). White and Renter (2009) evaluated performance of these and other methods using Bayesian estimation in a population of cattle experiencing high rates of clinical disease. Based on this analysis, 38% of truly diseased animals in any given population could go undiagnosed, and conversely, 37% of therapeutically treated animals could have been misdiagnosed. In the present study, both rectal and ocular measurements were sensitive to changes in temperature induced by LPS, evidenced by the low hour × method SEM (±0.067), similar to previous reports in small ruminants using vaginal temperature (SEM ± 0.06; Godfrey et al., 2013). However, beef production would benefit from advanced technologies to precisely diagnose BRD in real time.
Infrared thermography has been previously investigated in bovine. Using a challenge model with bovine viral diarrhea virus in beef heifers, Schaefer et al. (2004) reported the eye to be most consistent and sensitive surface to measure body temperature using infrared thermography compared to lateral, dorsal, hoof, ear, and nasal measurements. Schaefer et al. (2007) used ocular temperature to investigate infrared thermography in early detection of BRD in high-risk beef calves. Using a prediction model, infrared thermography, clinical illness score, core temperature, and leukocyte differential analysis were equivalently accurate in identifying illness when clinical signs were already evident. However, infrared thermography was a better predictor of disease when measured 4–6 d before appearance of clinical signs according to the developed prediction model (Schaefer et al., 2007). Data reported in the present study appear to contradict these findings, as the ocular temperature measured using infrared thermography yielded a delayed increase in temperature after LPS administration relative to rectal temperature. Potentially, this could be a difference in models used between studies and the resulting observed temperatures, because maximum ocular temperature in calves that were true positives in Schaefer et al. (2007) were never truly febrile 4–6 d before observed illness (37.49 ± 0.94 °C), at the onset of observed illness (38.34 ± 1.11 °C), or in the absolute temperatures used to create the prediction model (38.1 °C). However, at each of the above time points, the ocular temperature was lesser than the rectal temperature, which is similar to observations presented in this study.
George et al. (2014) evaluated the relationship of temperatures measured via rectal probe or thermal imaging of the eye or muzzle in multiparous, non-lactating, pregnant cows in tropical conditions and reported a correlation coefficient of 0.58 among rectal and ocular measurements. Similarly, in a previous study (data unpublished) we evaluated the correlation of rectal temperature and ocular temperature using infrared thermography in steers intravenously injected with exogenous LPS using the same facilities and procedures as the present study. Body temperature differed by measurement method at 11 of the 16 time points measured. The Pearson correlation coefficient was 0.71 and the Spearman correlation was 0.66 among measurement methods. These works are consistent with the results of the present study, which reported moderate correlation coefficients and temperature differences by method at 7 of the 16 time points.
Serum chemistry
Alanine aminotransferase is an enzyme predominantly found in the liver, with lesser quantities also found in the kidneys, heart, and skeletal muscle. In humans, increased serum ALT is a sensitive indicator of hepatocellular diseases including, but not limited to hepatitis and cirrhosis. Several pharmaceuticals may also cause increased concentrations of ALT, none of which were used during cannulation surgery of the present study. Similarly, ALP is in greatest concentration in Kupffer cells of the liver and in bone, and concentration is especially increased during obstructive biliary disease and cirrhosis. In addition to detection of liver disease, ALP has potent anti-inflammatory effects and an ability to attenuate toxicity of bacterial LPS via dephosphorylation of the lipid A moiety (Presbitero et al., 2018). In rats challenged with LPS orally, Koyama et al. (2002) reported a 2-fold increase in serum LPS of rats administered an ALP inhibitor simultaneously with the LPS challenge. Likewise, in vitro viability of human aortic endothelial cells improved when supplemented with intestinal ALP during exposure to LPS (Koyama et al., 2002). In bovine, Smock et al. (2020) reported increased serum ALT concentration, but decreased ALP concentration in high-risk calves at feedlot arrival. It is expected that high-risk calves harbor some chronic stress and inflammation at feedlot arrival associated with marketing and transportation (Richeson et al., 2019). Therefore, one might expect serum ALP to increase in response to an inflammatory immune challenge as was observed in the present study, but not corroborated by Smock et al. (2020). In the present study, serum ALP concentration increased 40% at hour 2 relative to LPS administration. Some differences in ALP mobilization may perhaps exist during acute vs. chronic inflammation that could explain this difference, as that was a major difference in stress models between those studies. The role of ALP during inflammatory livestock diseases, such as BRD, could be an area that would benefit from further research.
The response of serum glucose did not change over time in the present study. Given the profound effect of glucocorticoids on glucose homeostasis, this was not anticipated. During a stress event, glucocorticoid production promotes gluconeogenesis and decreases glucose uptake by tissues in order to prioritize glucose for the brain and immune systems. Therefore, plasma glucose increases to ensure an adequate supply of glucose to the brain, as it is the brain’s primary energy source, while glucose is used for energy by immune cells to mount the immune response (Kuo et al., 2015). In fact, is has been noted that approximately 0.5–1 kg of glucose is used by beef steers and lactating dairy cattle in response to an endotoxin challenge (Kvidera et al., 2016), thus noting the importance of glucose during immune activation. Carroll and Burdick Sanchez (2014) reported peak change in glucose was less and occurred later during an LPS challenge relative to a challenge with corticotropin releasing hormone and vasopressin. In the present study, maximum glucose concentration at hour 2 (102.4 mg/dL) was not different from the pre-challenge value (95.6 mg/dL); however, all glucose concentrations reported were near or greater than the upper reference range for bovine (40–100 mg/dL; Merck Veterinary Manual). This could be attributed to a combination of the high plane of nutrition and glycogen stores, overall pre-challenge health, weight of the cattle, and the relatively low dose of LPS used in the present study, all of which can buffer a negative serum glucose response.
Total serum protein decreased 3% at hour 2 relative to LPS administration in the present study, likely indicative of protein catabolism for amino acid utilization in the production of APP, cytokines, and other cellular immune components. Additionally, BUN increased 13% with a peak at hour 12. The concomitant increase in APP production is to be expected after an acute immune challenge, evidenced by the 241% increase in serum amyloid-A at hour 7.5 reported by Carroll et al. (2009b). However, some APP such as haptoglobin may not peak for several days after the original insult (Holland et al., 2011). Nutrition and stress are inextricably linked, and thus the relationship of nutrient requirements and BRD has long been intensively studied. While a stress response can exacerbate nutritional deficiencies, likewise nutritional deficiencies can yield a stress response (Reuter et al., 2008; Krehbiel, 2020). Protein requirements for hematopoiesis and APP production is supplied in part by skeletal muscle catabolism and subsequent uptake of free amino acids, which could explain increased BUN in the present study (Christ et al., 1994). In bovine, these concepts have been demonstrated in high-risk calves at feedlot arrival (Smock et al., 2020), and high-risk calves newly received to a stocker facility administered estradiol implants (Richeson et al., 2015).
Taken together, inflammatory disease, the APR, and production of APP alter indicators of metabolic function in bovine. Measurements of serum chemistry included herein are unable to account for efflux to target tissues and are limited to the 4 time points analyzed. Nonetheless, the long-term effects of naturally acquired BRD and resulting growth performance and carcass characteristics is a prodigious area of study, and data presented herein can provide context and reference ranges to some serum chemistry responses of bovine following an acute immune challenge with LPS.
Complete blood count
During a challenge with exogenous LPS, it is well-documented that cattle experience significant leukopenia, lymphopenia, and neutropenia within 1 h following LPS administration (Burdick Sanchez et al., 2014). Circulating endotoxin is recognized by the innate immune system as a foreign antigen, and thus the acute decrease in white blood cell count indicates cellular translocation from circulation to tissue, decreased hematopoiesis, and increased apoptosis of neutrophils (Carroll and Burdick Sanchez, 2014). In the present study, total white blood cell count and all differentials decreased 1 h following the LPS dose, with the most pronounced decrease being neutrophils (88% decrease) and blood monocytes (84% decrease). The total white blood cell count remained depleted for several hours, and following a gradual increase returned to pre-challenge concentration at hour 12. We also observed differing recovery patterns between neutrophil and lymphocyte populations. As described previously, neutrophil population decreases dramatically in response to the initial challenge, but the population recovery was more sustained over time and did not return to pre-challenge values until hour 36. Conversely, while circulating lymphocyte population decreased 62% following the LPS dose, the population recovered sooner at hour 6, and remained greater than the pre-challenge value for the remainder of the data collection period (48 h). These results are consistent with those reported previously by our lab in beef cattle administered exogenous LPS (Burdick Sanchez et al., 2014; Burdick Sanchez et al., 2020).
An ongoing challenge in animal health and immunology research is that of complete blood count reference data that is specific to growing and finishing beef cattle. Reference ranges published by Merck (2022) are often used by researchers and veterinarians for context; however, these reference ranges do not differentiate between critically different production scenarios, age, genotype, or sex. Moreover, these data were populated using means within the nonspecific population and the corresponding ±95% confidence interval and thus, do not necessarily indicate a normal or healthy range, but rather a commonly reported value (Herrick et al., 2020). Therefore, because the data reported herein are consistent with prior works, and because the cattle type and production phase are known, these data can reasonably be used as reference ranges for complete blood count of beef cattle at risk of developing inflammatory diseases, such as BRD.
Cortisol response
In agreement with Carroll et al. (2009b), the pre-challenge cortisol concentration was normal (12.4 ng/mL), indicating steers did not experience a stress response to the housing conditions or investigator handling. The serum cortisol response in the present study was notably less than reported by Carroll et al. (2009b), wherein the peak was 99 ng/mL, a 3,127% increase relative to the pre-challenge value. Comparatively, in the present study we observed a 294% increase in serum cortisol with a peak concentration of 49 ng/mL at hour 1. Indeed, the LPS dose administered in the present study was 10-fold less than Carroll et al. (2009b), however, internal observations within our lab have noted increased sensitivity to LPS in both cattle and pigs in the past 20 yr (Carroll et al., 2005, 2009a; Burdick Sanchez et al., 2020). It is well-documented that genetic selection for growth and productivity unfavorably selects against health and fitness traits in food producing animals (Rauw et al., 1998; Reverter et al., 2021). Thus, this heightened sensitivity to exogenous bacterial LPS could reflect the aggressive selection for growth in beef cattle herds. Therefore, LPS challenge doses have decreased in recent years so as to avoid mortality by attenuating the inflammatory response, and to not mask important biological differences.
Pro-inflammatory cytokine responses to LPS
A major characteristic of pro-inflammatory cytokines is recruitment and activation of immune cells (Table 3). Additionally, several cytokines have both pro- and anti-inflammatory functions. In the present study, circulating TNF-α and decorin concentrations peaked quickest at hour 1 relative to other cytokines after the LPS challenge. Among the most important pro-inflammatory cytokines, TNF-α has several complicated, and sometimes contradictory biological functions, which include vasodilatation, activation of neutrophils and platelets, adhesion molecule expression, improved killing ability of macrophages and NK cells, oxidative stress, and indirect induction of fever. Although high concentrations can be toxic to the host, an insufficient TNF-α response can allow progressive disease. Bacterial LPS is a primary stimulant of TNF-α production, which is attenuated by IL-4 via decreasing levels of cAMP (Idriss and Naismith, 2000; Zelová and Hošek, 2013). In TNFR-knockout mice challenged with bacterial endotoxin, Acton et al. (1996) reported increased susceptibility to infection and a suppressed inflammatory response, demonstrating the important role of TNF-α in mounting an adequate immune response. The temporal response of TNF-α in the present study was similar to that of Carroll et al. (2009b) and Carroll and Burdick Sanchez (2014) in bovine after exogenous LPS administration, wherein peak concentration was observed at hours 1.4 and 1, respectively. However, there is a notable difference in magnitude of the TNF-α response among these studies. Carroll et al. (2009b) reported a 10,289% increase relative to the pre-challenge concentration and a peak of 8,847 pg/mL. Comparatively, peak TNF-α concentration in the present study was 1,433 pg/mL, a 117% increase above the pre-challenge concentration. This is likely a result of the differing doses of LPS administered, whereas Carroll et al. (2009b) administered a 2.5 µg/kg BW dose, and a 0.25 µg/kg BW dose was used in the present study.
Decorin is produced by fibroblasts, stressed vascular endothelium, and smooth muscle cells. With peak concentration at hour 1, decorin is involved in an extensive signaling network that mediates downstream signal transduction of other cytokines and growth factors. When decorin binds to toll-like receptors 2 and 4 on the surface of macrophages, the synthesis of TNF-α and IL-12 are upregulated. Thus, decorin participates in the pro-inflammatory response (Dong et al., 2022). In septic human patients and LPS-challenged mice, Merline et al. (2011) reported that within 0.5 h after LPS administration, decorin concentration increased and peritoneal macrophages increased decorin mRNA expression. Likewise, Chatterjee et al. (2021) reported upregulated decorin expression of CD4+ T and CD8+ T cells in mouse models and asthmatic human patients. Although the contribution of decorin during inflammation is not yet comprehensively understood, data in the present study is in agreement with murine and human models indicating it is an early responder within the acute pro-inflammatory response in bovine.
The initial pro-inflammatory response was proceeded by peaks at hour 2 (IL-1β, IL-17A, IL-18) and hour 3 (IFN-αA, IFN-γ, IL-1α, IL-1F5, IL-21, MIP-1β). Among these, IL-1β is considered the master regulator of inflammation as it controls several processes of innate immunity, including fever, upregulation of several APR components, and activation of lymphocytes (Kaneko et al., 2019). In humans, a primed cell must encounter further pathogen-associated molecular pattern or damage-associated molecular pattern stimulation leading to IL-1β expression. As such, the stimulus remains unclear and, purportedly, secretion of IL-1β may occur on a continuum depending on the strength of the stimulus (Lopez-Castejon and Brough, 2011). In bovine, IL-1β has been linked to regulation of ciliary motility during pneumonia (Jain et al., 1995), and to increase circulating leukocytes, increase serum haptoglobin and fibrinogen, and decrease plasma zinc concentration (Godson et al., 1995). Carroll et al. (2009b) reported a greater percentage change in IL-1β concentration (279%) than the present study (32%), however this is likely related to the lesser LPS dosage used presently.
Anti-inflammatory cytokine responses to LPS
While inflammation is a critical component of the innate immune response, a prolonged or exaggerated inflammatory response ultimately limits the host’s ability to remove the pathogen, causes tissue damage, and prevents return to normal tissue homeostasis. Therefore, anti-inflammatory cytokines work to ensure the inflammatory response is proportional to the threat and prevent collateral damage from unmitigated inflammation.
In 7-mo-old beef calves challenged with low- or high-virulence type 2 bovine viral diarrheal virus (BVDV-2), Palomares et al. (2014) reported gene expression of anti-inflammatory cytokines IL-4, IL-10, and TGF-β were upregulated in calves administered high-virulence BVDV-2, but not the low-virulence strain. Similarly, Risalde et al. (2011) challenged 8 to 9-mo-old dairy calves with bovine herpesvirus-1 after a subclinical challenge with BVDV to emulate complicated secondary infections. In calves challenged with both viruses, IL-10 and TNF-α concentration peaked at hour 4, while the IL-10 response was delayed to hour 7 in calves challenged with subclinical BVDV only. These results demonstrate the proportional nature of the anti-inflammatory response to the magnitude of the pro-inflammatory response, which is similar to the present study although differing challenge methods were used. Interestingly, in both treatment groups by Risalde et al. (2011), there was no temporal response of IL-4, an immunoregulatory cytokine. Before enrollment, calves were confirmed negative of BVDV and BHV-1 antigen and antibodies by enzyme-linked immunosorbent assay. Thus, because IL-4 is predominantly produced by activated T cells, this might be a result of the calves lack of existing immunity to these pathogens (Luzina et al., 2012). Although the challenge methods differed between studies, there were likewise no differences in the IL-4 response of the present study.
As discussed previously, peak TNF-α concentration was observed 1 h after LPS administration, with remaining pro-inflammatory cytokine peaks proceeding at hours 2 or 3. In response, peak concentrations of prototypic anti-inflammatory cytokines, IL-13 and IL-10, were observed at hours 3 and 4, respectively. Because it is within the purview of both IL-13 and IL-10 to limit production of pro-inflammatory cytokines, TNF-α concentration decreased markedly after hour 3, and minimum concentration was observed at hour 6. Additionally, several cytokines with both pro- and anti-inflammatory roles were responsive to LPS at hours 2 (IFN-β) and hour 3 (IFN-αA, IL-21). These observations suggest the anti-inflammatory cytokines evaluated herein were efficacious in attenuating the bovine inflammatory response to LPS.
Chemokine and other protein responses to LPS
Chemokines and other proteins that responded earliest to LPS included GASP-1 and CD40L at hour 2. Because GASP-1 is a myostatin inhibitor, its role during an immune response is somewhat novel, but is proposed as a mediator of skeletal muscle repair via recruitment of regenerative immune cells such as M1 and M2 macrophages (Estrellas et al., 2018). Additionally, because a challenge with exogenous LPS produces an acute response, CD40 ligand is critical to initiation of subsequent adaptive immunity and is almost exclusively produced by CD4+ T cells. In particular, CD40 is required for immunoglobulin class switching and, without which only IgM class antibodies can be produced. Moreover, CD40 mediates several critical activities including surface antigen presentation, immunoglobulin secretion, and generation of memory in B cells (Alegre et al., 1998; Manzoor, 2015).
At hour 3, peak concentrations of chemoattractants IP-10, MIG, and RANTES were observed. Particularly notable is the 1,127% increase in RANTES relative to the pre-challenge value. This potent chemoattractant is important for homing and migration of effector and memory T cells, monocytes, and natural killer cells during an acute immune response, and thus is especially relevant in the regulation of protective immunity (Crawford et al., 2011). Additionally, in mice during respiratory infections, RANTES was involved in dendritic cell migration, survival of resident alveolar macrophages, and recruitment of effector and memory T cells (Tyner et al., 2005; Grayson et al., 2007; Kohlmeier et al., 2008).
Conclusions
These data collectively demonstrate a pronounced bovine APR to exogenous LPS exposure. Although ocular infrared temperature was generally less than rectal temperature, both methods of body temperature measurement were sensitive to changes in body temperature and the methods were moderately correlated. Liver enzyme, total protein, and BUN responses to acute inflammation demonstrate the link of metabolism to inflammatory responses of bovine and offer an interesting area of further research. The pro-inflammatory response was initiated by cortisol, TNF-α, and decorin at hour 1, which was tempered by the anti-inflammatory effects of IL-13 and IL-10 at hours 3 and 4. Additionally, complete blood count data reported herein is consistent with additional works in beef calves during an inflammatory challenge, and thus could be useful reference ranges in subsequent works. Therefore, these data provide updates to the physiological, endocrine, and immune responses of beef steers administered exogenous LPS to incorporate recent technologies and an expanded cytokine profile, which can be used as foundational data in future research.
Acknowledgments
This project was funded in part by the Foundation for Food and Agriculture Research (Grand ID: ICASATWG-0000000038). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the Foundation for Food and Agriculture Research. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Glossary
Abbreviations:
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANG
angiopoietin
- APP
acute phase protein
- APR
acute phase response
- BHV
bovine herpesvirus
- BRD
bovine respiratory disease
- BUN
blood urea nitrogen
- BVDV
bovine viral diarrhea virus
- BW
body weight
- ELISA
enzyme-linked immunosorbent assay
- FGF
fibroblast growth factor
- GASP
growth and differentiation factor-associated serum protein
- HPA
hypothalamus-pituitary-adrenal
- IFN
interferon
- IGF
insulin-like growth factor
- IL
interleukin
- IP
interferon-gamma induced protein
- LIF
leukemia inhibitory factor
- LPS
lipopolysaccharide
- MCP
monocyte chemoattractant protein
- MIG
monokine induced by gamma
- MIP
macrophage inflammatory protein
- NCAM
neural cell adhesion molecule
- RANTES
regulated on activation normal T cell expressed and secreted
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Contributor Information
Taylor M Smock, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX 79409, USA.
P Rand Broadway, United States Department of Agriculture, Agriculture Research Service, Livestock Issues Research Unit, Lubbock, TX 79403, USA.
Nicole C Burdick Sanchez, United States Department of Agriculture, Agriculture Research Service, Livestock Issues Research Unit, Lubbock, TX 79403, USA.
Jeffery A Carroll, United States Department of Agriculture, Agriculture Research Service, Livestock Issues Research Unit, Lubbock, TX 79403, USA.
Miles E Theurer, Veterinary Research and Consulting Services, Hays, KS 67601, USA.
Kristin E Hales, Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX 79409, USA.
Conflict of Interest Statement
The authors have no real or perceived conflicts of interest.
Literature Cited
- Acton, R. D., Dahlberg P. S., Uknis M. E., Klaerner H. G., Fink G. S., Norman J. G., and Dunn D. L.. 1996. Differential sensitivity to Escherichia coli infection in mice lacking tumor necrosis factor p55 or interleukin-1 p80 receptors. Arch. Surg. 131:1216–1221. doi: 10.1001/archsurg.1996.01430230098017. [DOI] [PubMed] [Google Scholar]
- Alegre, M. L., Thompson C. B., and Gajewski T. F.. 1998. Second signals for lymphocyte activation. Encyclopedia of immunology. 2nd ed. Academic Press, Cambrige, MA. [Google Scholar]
- Angelo, L. S., and Kurzrock R.. 2007. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin. Cancer Res. 13:2825–2830. doi: 10.1158/1078-0432.ccr-06-2416. [DOI] [PubMed] [Google Scholar]
- Banner, L. R., Patterson P. H., Allchorne A., Poole S., and Woolf C. J.. 1998. Leukemia inhibitory factor is an anti-inflammatory and analgesic cytokine. J. Neurosci. 18:5456–5462. doi: 10.1523/JNEUROSCI.18-14-05456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, H., and Gauldie J.. 1994. The acute phase response. Immunol. Today. 15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Berthoud, T. K., Dunachie S. J., Todryk S., Hill A. V., and Fletcher H. A.. 2009. MIG (CXCL9) is a more sensitive measure than IFN-γ of vaccine induced T-cell responses in volunteers receiving investigated malaria vaccines. J. Immunol. Methods. 340:33–41. doi: 10.1016/j.jim.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biet, F., Locht C., and Kremer L.. 2002. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 80:147–162. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- Blessing, W. W. 2003. Lower brainstem pathways regulating sympathetically mediated changes in cutaneous blood flow. Cell. Mol. Neurobiol. 23:527–538. doi: 10.1023/a:1025020029037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg, H., Dinh H., Trueblood E. S., Pretorius J., Kugler D., Weng N., Kanaly S. T., Towne J. E., Willis C. R., Kuechle M. K., et al. 2007. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolívar, S., Anfossi R., Humeres C., Vivar R., Boza P., Muñoz C., Pardo-Jimenez V., Olivares-Silva F., and Díaz-Araya G.. 2018. IFN-β plays both pro-and anti-inflammatory roles in the rat cardiac fibroblast through differential STAT protein activation. Front. Pharmacol. 9:1368. doi: 10.3389/fphar.2018.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M. A., and Hural J.. 1997. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- Burdick, N. C., Bernhard B. C., Carroll J. A., Rathmann R. J., and Johnson B. J.. 2012. Enhancement of the acute phase response to a lipopolysaccharide challenge in steers supplemented with chromium. Innate Immun. 18:592–601. doi: 10.1177/1753425911428964. [DOI] [PubMed] [Google Scholar]
- Burdick Sanchez, N. C., Buntyn J. O., Carroll J. A., Wistuba T., DeHann K., Sieren S. E., Jones S. J., and Schmidt T. B.. 2014. Enhancement of the acute phase response to lipopolysaccharide in feedlot steers supplemented with OmniGen-AF. J. Anim. Sci. 92:E37–E38. [Google Scholar]
- Burdick Sanchez, N. C., Carroll J. A., Broadway P. R., Edrington T. S., Yoon I., and Belknap C. R.. 2020. Some aspects of the acute phase immune response to a lipopolysaccharide (LPS) challenge are mitigated by supplementation with a Saccharomyces cerevisiae fermentation product in weaned beef calves. Transl. Anim. Sci. 4:txaa156. doi: 10.1093/tas/txaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick Sanchez, N. C., Dailey J. W., Broadway P. R., Davis E. M., Bowen B. M., Petry A. L., Ballou M. A., Hales K. E., and Carroll J. A.. 2023. A viable less-invasive alternative for continuous temperature measurement in weaned pigs. Livest. Sci. 267:105126. doi: 10.1016/j.livsci.2022.105126. [DOI] [Google Scholar]
- Carroll, J. A., Arthington J. D., and C. C.Chase, Jr. 2009a. Early weaning alters the acute-phase reaction to an endotoxin challenge in beef calves. J. Anim. Sci. 87:4167–4172. doi: 10.2527/jas.2009-2016. [DOI] [PubMed] [Google Scholar]
- Carroll, J. A., and Burdick Sanchez N. C.. 2014. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: overlapping physiological responses and endocrine biomarkers that are indicative of stress responsiveness and immune function in beef cattle. J. Anim. Sci. 92:5311–5318. doi: 10.2527/jas.2014-8123. [DOI] [PubMed] [Google Scholar]
- Carroll, J. A., Carter D. B., Korte S. W., and Prather R. S.. 2005. Evaluation of the acute phase response in cloned pigs following a lipopolysaccharide challenge. Domest Anim. Endocrinol. 29:564–572. doi: 10.1016/j.domaniend.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Carroll, J. A., and Forsberg N. E.. 2007. Influence of stress and nutrition on cattle immunity. Vet. Clin. North Am. Food Anim. Pract. 23:105–149. doi: 10.1016/j.cvfa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Carroll, J. A., Reuter R. R., C. C.Chase, Jr, Coleman S. W., Riley D. G., Spiers D. E., Arthington J. D., and Galyean M. L.. 2009b. Profile of the bovine acute-phase response following an intravenous bolus-dose lipopolysaccharide challenge. Innate Immun. 15:81–89. doi: 10.1177/1753425908099170. [DOI] [PubMed] [Google Scholar]
- Castro, F., Cardoso A. P., Gonçalves R. M., Serre K., and Oliveira M. J.. 2018. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, L., Berry C. M., Nolan D., Castley A., Fernandez S., and French M. A.. 2014. Interferon-alpha, immune activation and immune dysfunction in treated HIV infection. Clin. Transl. Immunol. 3:e10. doi: 10.1038/cti.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, C. P., and Rainer T. H.. 2013. Advances in clinical chemistry. Chapter 2: pathophysiological roles and clinical importance of biomarkers in acute coronary syndrome. Vol. 59; p. 23–63. Elsevier, Amsterdam, Netherlands. doi: 10.1016/B978-0-12-405211-6.00002-4 [DOI] [PubMed] [Google Scholar]
- Chatterjee, J., Sanapala S., Cobb O., Bewley A., Goldstein A. K., Cordell E., Ge X., Garbow J. R., Holtzman M. J., and Gutmann D. H.. 2021. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat. Commun. 12:1–12. doi: 10.1038/s41467-021-27455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Wang J., Liu C., Su L., Zhang D., Fan J., Yang Y., Xiao M., Xie J., and Xu Y.. 2020. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 26:1–12. doi: 10.1186/s10020-020-00230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ, B., Nath A., Heinrich P. C., and Jungermann K.. 1994. Inhibition by recombinant human interleukin-6 of the glucagon-dependent induction of phosphoenolpyruvate carboxykinase and of the insulin-dependent induction of glucokinase gene expression in cultured rat hepatocytes: regulation of gene transcription and messenger RNA degradation. Hepatology. 20:1577–1583. doi: 10.1002/hep.1840200629 [DOI] [PubMed] [Google Scholar]
- Crawford, A., Angelosanto J. M., Nadwodny K. L., Blackburn S. D., and Wherry E. J.. 2011. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog. 7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, J. L. 2006. Interleukins: IL-4. Encyclopedia of respiratory medicine. p. 354–359. Academic Press, Cambrige, MA. doi: 10.1016/B0-12-370879-6/00475-0 [DOI] [Google Scholar]
- Davis, I. D., Skak K., Smyth M. J., Kristjansen P. E., Miller D. M., and Sivakumar P. V.. 2007. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin. Cancer Res. 13:6926–6932. doi: 10.1158/1078-0432.CCR-07-1238. [DOI] [PubMed] [Google Scholar]
- de Vries, J. E. 1998. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Clin. Immunol. 102:165–169. doi: 10.1016/S0091-6749(98)70080-6. [DOI] [PubMed] [Google Scholar]
- Dembic, Z. 2015. Cytokines of the immune system. Chapter 6: interleukins. p. 143–239. doi: 10.1016/B978-0-12-419998-9.00006-7. [DOI] [Google Scholar]
- DePhillips, P., and Lenhoff A. M.. 2004. Relative retention of the fibroblast growth factors FGF-1 and FGF-2 on strong cation-exchange sorbents. J. Chromat. 1036:51–60. doi: 10.1016/j.chroma.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Deshmane, S. L., Kremlev S., Amini S., and Sawaya B. E.. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar, F. S. 2009. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 16:300– 317. doi: 10.1159/000216188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y., Zhong J., and Dong L.. 2022. The role of decorin in autoimmune and inflammatory diseases. J. Immunol. Res. 22:11. doi: 10.1155/2022/1283383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff, G. C., and Galyean M. L.. 2007. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour, J. H., Dziejman M., Liu M. T., Leung J. H., Lane T. E., and Luster A. D.. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Elkina, Y., von Haehling S., Anker S. D., and Springer J.. 2011. The role of myostatin in muscle wasting: an overview. J. Cachexia Sarcopenia Muscle. 2:143–151. doi: 10.1007/s13539-011-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser, T. H., Caperna T. J., Li C. J., Kahl S., and Sartin J. L.. 2008. Critical control points in the impact of the proinflammatory immune response on growth and metabolism. J. Anim. Sci. 86:E105–E125. doi: 10.2527/jas.2007-0634. [DOI] [PubMed] [Google Scholar]
- Estrellas, K. M., Chung L., Cheu L. A., Sadtler K., Majumdar S., Mula J., Wolf M. T., Elisseeff J. H., and Wagner K. R.. 2018. Biological scaffold–mediated delivery of myostatin inhibitor promotes a regenerative immune response in an animal model of Duchenne muscular dystrophy. J. Biol. Chem. 293:15594–15605. doi: 10.1074/jbc.RA118.004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielder, S. E. 2015. Normal rectal temperature ranges. Merck veterinary manual. https://www.merckvetmanual.com/special-subjects/reference-guides/normal-rectal-temperature-ranges?query=normal%20temperature. Accessed June 27, 2022.
- Gabay, C., Lamacchia C., and Palmer G.. 2010. IL-1 pathways in inflammation and human diseases. Nat. Rev. 6:232–241. doi: 10.1038/nrrheum.2010.4 [DOI] [PubMed] [Google Scholar]
- Galyean, M. L., Duff G. C., and Rivera J. D.. 2022. Galyean Appreciation Club Review: revisiting nutrition and health of newly received cattle—what have we learned in the last 15 years? J. Anim. Sci. 100:skac067. doi: 10.1093/jas/skac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, W. D., Godfrey R. W., Ketring R. C., Vinson M. C., and Willard S. T.. 2014. Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. J. Anim. Sci. 92:4949–4955. doi: 10.2527/jas.2014-8087. [DOI] [PubMed] [Google Scholar]
- Godfrey, R. W., Vinson M. C., and Ketring R. C.. 2013. The effect of a split feeding regimen and breed on body temperature of hair sheep ewes in the tropics. J. Anim. Sci. 91:5202–5207. doi: 10.2527/jas.2013-6559. [DOI] [PubMed] [Google Scholar]
- Godson, D. L., Baca-Estrada M. E., Van Kessel A. G., Hughes H. P. A., Morsy M. A., Van Donkersgoed J., Harland R. J., Shuster D. E., Daley M. J., and Babiuk L. A.. 1995. Regulation of bovine acute phase responses by recombinant interleukin-1β. Can. J. Vet. Res. 59:249–255. [PMC free article] [PubMed] [Google Scholar]
- Grayson, M. H., Ramos M. S., Rohlfing M. M., Kitchens R., Wang H. D., Gould A., Agapov E., and Holtzman M. J.. 2007. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J. Immunol. 179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- Griffin, D. 2014. The monster we don’t see: subclinical BRD in beef cattle. Anim. Health Res. Rev. 15:138–141. doi: 10.1017/S1466252314000255. [DOI] [PubMed] [Google Scholar]
- Griffin, D., Chengappa M. M., Kuszak J., and McVey D. S.. 2010. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. Food Anim. Pract. 26:381–394. doi: 10.1016/j.cvfa.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Herrick, R. T., Jones T. P., Sperber J. L., Richeson J. T., Brown T. R., and Lawrence T. E.. 2020. Assessment of changes in complete blood count and serum chemistry in fed Holstein steers with or without liver abscesses. Appl. Anim. Sci. 36:256–264. doi: 10.15232/aas.2019-01954. [DOI] [Google Scholar]
- Hodi, S. F., and Soiffer R. J.. 2002. Encyclopedia of cancer (second edition). Chapter 11: interleukins. p. 523–535. doi: 10.1016/B0-12-227555-1/00110-6. [DOI] [Google Scholar]
- Holland, B. P., Step D. L., Burciaga-Robles L. O., Fulton R. W., Confer A. W., Rose T. K., Laidig L. E., Richards C. J., and Krehbiel C. R.. 2011. Effectiveness of sorting calves with high risk of developing bovine respiratory disease on the basis of serum haptoglobin concentration at the time of arrival at a feedlot. Am. J. Vet. Res. 72:1349–1360. doi: 10.2460/ajvr.72.10.1349. [DOI] [PubMed] [Google Scholar]
- Hübschmann, M. V., Skladchikova G., Bock E., and Berezin V.. 2005. Neural cell adhesion molecule function is regulated by metalloproteinase-mediated ectodomain release. J. Neurosci. Res. 80:826–837. doi: 10.1002/jnr.20530. [DOI] [PubMed] [Google Scholar]
- Hughes, H. D., Carroll J. A., Sanchez N. C. B., and Richeson J. T.. 2014. Natural variations in the stress and acute phase responses of cattle. Innate Immun. 20:888–896. doi: 10.1177/1753425913508993. [DOI] [PubMed] [Google Scholar]
- Huntoon, K. M., Wang Y., Eppolito C. A., Barbour K. W., Berger F. G., Shrikant P. A., and Baumann H.. 2008. The acute phase protein haptoglobin regulates host immunity. J. Leukoc. Biol. 84:170–181. doi: 10.1189/jlb.0208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss, H. T., and Naismith J. H.. 2000. TNFα and the TNF receptor superfamily: structure-function relationships. Microsc. Res. Tech. 50:184–195. doi:. [DOI] [PubMed] [Google Scholar]
- Islam, H., Chamberlain T. C., Mui A. L., and Little J. P.. 2021. Elevated interleukin-10 levels in COVID-19: potentiation of pro-inflammatory responses or impaired anti-inflammatory action? Front. Immunol. 12:2485. doi: 10.3389/fimmu.2021.677008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, S. S., and Cheng G.. 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, B., Rubinstein I., Robbins R. A., and Sisson J. H.. 1995. TNF-alpha and IL-1 beta upregulate nitric oxide-dependent ciliary motility in bovine airway epithelium. Am. J. Physiol. Lung Cell Mol. 268:L911–L917. doi: 10.1152/ajplung.1995.268.6.L911 [DOI] [PubMed] [Google Scholar]
- Jesudas, B. R., Nandeesha H., Menon V., and Allimuthu P.. 2020. Relationship of elevated neural cell adhesion molecule 1 with interleukin-10 and disease severity in bipolar disorder. Asian J. Psychiatr. 47:101849. doi: 10.1016/j.ajp.2019.101849. [DOI] [PubMed] [Google Scholar]
- Johnson, R. W. 2002. The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopath. 87:443–450. doi: 10.1016/S0165-2427(02)00069-7 [DOI] [PubMed] [Google Scholar]
- Johnson, B. J., Hathaway M. R., Anderson P. T., Meiske J. C., and Dayton W. R.. 1996. Stimulation of circulating insulin-like growth factor I (IGF-I) and insulin-like growth factor binding proteins (IGFBP) due to administration of a combined trenbolone acetate and estradiol implant in feedlot cattle. J. Anim. Sci. 74:372–379. doi: 10.2527/1996.742372x. [DOI] [PubMed] [Google Scholar]
- Kaneko, N., Kurata M., Yamamoto T., Morikawa S., and Masumoto J.. 2019. The role of interleukin-1 in general pathology. Inflamm. Res. 39:1–16. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier, J. E., Miller S. C., Smith J., Lu B., Gerard C., Cookenham T., Roberts A. D., and Woodland D. L.. 2008. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 29:101–113. doi: 10.1016/j.immuni.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala, N. 2017. The role of interferons in inflammation and inflammasome activation. Front. Immunol. 8:873. doi: 10.3389/fimmu.2017.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, I., Matsunaga T., Harada T., Hokari S., and Komoda T.. 2002. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin. Biochem. 35:455–461. doi: 10.1016/s0009-9120(02)00330-2. [DOI] [PubMed] [Google Scholar]
- Krehbiel, C. R. 2020. Bovine respiratory disease influences on nutrition and nutrient metabolism. Vet. Clin. N. Am. Food Anim. Pract. 36:361–373. doi: 10.1016/j.cvfa.2020.03.010 [DOI] [PubMed] [Google Scholar]
- Kuo, T., McQueen A., Chen T.-C., and Wang J.-C.. 2015. Regulation of glucose homeostasis by glucocorticoids, glucocorticoid signaling. Springer Publishing, New York City, NY; p. 99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvidera, S. K., Horst E. A., Abuajamieh M., Mayorga E. J., Sanz Fernandez M. V., and Baumgard L. H.. 2016. Technical note: a procedure to estimate glucose requirements of an activated immune system in steers. J. Anim. Sci. 94:4591–4599. doi: 10.2527/jas.2016-0765. [DOI] [PubMed] [Google Scholar]
- Lee, Y., Bok J. D., Lee H. J., Lee H. G., Kim D., Lee I., Kang S. K., and Choi Y. J.. 2016. Body temperature monitoring using subcutaneously implanted thermo-loggers from Holstein steers. Asian Australas. J. Anim. Sci. 29:299. doi:10.5713%2Fajas.15.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon, G., and Brough D.. 2011. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 22:189–195. doi: 10.1016/j.cytogfr.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzina, I. G., Keegan A. D., Heller N. M., Rook G. A., Shea-Donohue T., and Atamas S. P.. 2012. Regulation of inflammation by interleukin-4: a review of “alternatives”. J. Leukoc. Biol. 92:753–764. doi: 10.1189/jlb.0412214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor, A. M. 2015. Developing costimulatory molecules for immunotherapy of diseases. Chapter 1: introduction to costimulation and costimulatory molecules. p. 1–43. doi: 10.1016/B978-0-12-802585-7.00001-7. [DOI] [Google Scholar]
- Marega, M., Chen C., and Bellusci S.. 2021. Cross-talk between inflammation and fibroblast growth factor 10 during organogenesis and pathogenesis: lessons learnt from the lung and other organs. Front. Cell Dev. Biol. 9:656–883. doi: 10.3389/fcell.2021.656883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Konczalik, J., Dubois S., Losi J. M., Sabzevari H., Yamada N., Feigenbaum L., Waldmann T. A., and Tagaya Y.. 2000. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. USA. 97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, C., Tanure C. B., Peripolli V., Seixas L., Fischer V., Gabbi A. M., Menegassi S. R., Stumpf M. T., Kolling G. J., Dias E., et al. 2016. Infrared thermography in animal production: an overview. Comput. Electron. Agric. 123:10–16. doi: 10.1016/j.compag.2016.01.027. [DOI] [Google Scholar]
- Medline. 2022. National Library of Medicine. IL1A gene. https://medlineplus.gov/genetics/gene/il1a/. Accessed January 10, 2023.
- Menten, P., Wuyts A., and Van Damme J.. 2002. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 13:455–481. doi: 10.1016/S1359-6101(02)00045-X [DOI] [PubMed] [Google Scholar]
- Merck. 2022. Veterinary manual. Hematalogical reference ranges. https://www.merckvetmanual.com/special-subjects/reference-guides/hematologic-reference-ranges. AccessedSeptember 18, 2022.
- Merline, R., Moreth K., Beckmann J., Nastase M. V., Zeng-Brouwers J., Tralhão J. G., Lemarchand P., Pfeilschifter J., Schaefer R. M., Iozzo R. V., et al. 2011. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci. Signal. 4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma, S., El-Khaiat H. M., Uto T., Sato K., Sekiguchi S., and Norimine J.. 2021. Characterization of bovine interleukin-2 stably expressed in HEK-293 cells. J. Vet. Med. Sci. 83:134–141. doi: 10.1292/jvms.20-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha, A., Malaiappan S., Milstein D. M., Karthikeyan G., Varghese S. S., and Jayakumar N. D.. 2021. Comparison of interleukin-21 levels and its correlation with clinical parameters among healthy individuals, chronic periodontitis, and aggressive periodontitis patients. J. Clin. Transl. Res. 7:84. doi: 10.18053/jctres.07.202101.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomares, R. A., Brock K. V., and Walz P. H.. 2014. Differential expression of pro-inflammatory and anti-inflammatory cytokines during experimental infection with low or high virulence bovine viral diarrhea virus in beef calves. Vet. Immunol. Immunopath. 157:149–154. doi: 10.1016/j.vetimm.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Perera, P. Y., Lichy J. H., Waldmann T. A., and Perera L. P.. 2012. The role of interleukin-15 in inflammation and immune responses to infection: implications for its therapeutic use. Microbes Infect. 14:247–261. doi: 10.1016/j.micinf.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périè, L., Parenté A., Baraige F., Magnol L., and Blanquet V.. 2017. Alterations in adiposity and glucose homeostasis in adult GASP-1 overexpressing mice. Cell. Physiol. Biochem. 44:1896–1911. doi: 10.1159/000485878. [DOI] [PubMed] [Google Scholar]
- Petersen, H. H., Nielsen J. P., and Heegaard P. M. H.. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Pizurki, L., Zhou Z., Glynos K., Roussos C., and Papapetropoulos A.. 2003. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br. J. Pharmacol. 139:329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presbitero, A., Mancini E., Brands R., Krzhizhanovskaya V. V., and Sloot P. M.. 2018. Supplemented alkaline phosphatase supports the immune response in patients undergoing cardiac surgery: clinical and computational evidence. Front. Immunol. 9:2342. doi: 10.3389/fimmu.2018.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauw, W. M., Kanis E., Noordhuizen-Stassen E. N., and Grommers F. J.. 1998. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livest. Prod. Sci. 56:15–33. doi: 10.1016/S0301-6226(98)00147-X. [DOI] [Google Scholar]
- Reid, E. D., Fried K., Velasco J. M., and Dahl G. E.. 2012. Correlation of rectal temperature and peripheral temperature from implantable radio-frequency microchips in Holstein steers challenged with lipopolysaccharide under thermoneutral and high ambient temperatures. J. Anim. Sci. 90:4788–4794. doi: 10.2527/jas.2011-4705. [DOI] [PubMed] [Google Scholar]
- Reinders, M. E., Sho M., Izawa A., Wang P., Mukhopadhyay D., Koss K. E., Geehan C. S., Luster A. D., Sayegh M. H., and Briscoe D. M.. 2003. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Invest. 112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, R. R., Carroll J. A., Dailey J. W., Cook B. J., and M. L. Galyean. 2008. Effects of dietary energy source and level and injection of tilmicosin phosphate on immune function in lipopolysaccharide-challenged beef steers. J. Anim. Sci. 86:1963–1976. doi: 10.2527/jas.2007-0838. [DOI] [PubMed] [Google Scholar]
- Reuter, R. R., Carroll J. A., Hulbert L. E., Dailey J. W., and Galyean M. L.. 2010. Development of a self-contained, indwelling rectal temperature probe for cattle research. J. Anim. Sci. 88:3291–3295. doi: 10.2527/jas.2010-3093. [DOI] [PubMed] [Google Scholar]
- Reverter, A., Hine B. C., Porto-Neto L., Li Y., Duff C. J., Dominik S., and Ingham A. B.. 2021. ImmuneDEX: a strategy for the genetic improvement of immune competence in Australian Angus cattle. J. Anim. Sci. 99:skaa384. doi: 10.1093/jas/skaa384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson, J. T., Beck P. A., Hughes H. D., Hubbell D. S., Gadberry M. S., Kegley E. B., Powell J. G., and Prouty F. L.. 2015. Effect of growth implant regimen on health, performance, and immunity of high-risk, newly received stocker cattle. J. Anim. Sci. 93:4089–4097. doi: 10.2527/jas.2014-8835. [DOI] [PubMed] [Google Scholar]
- Richeson, J. T., and Falkner T. R.. 2020. Bovine respiratory disease vaccination: what is the effect of timing? Vet. Clin. Food Anim. Pract. 36:473–485. doi: 10.1016/j.cvfa.2020.03.013 [DOI] [PubMed] [Google Scholar]
- Richeson, J. T., Samuelson K. L., and Tomczak D. J.. 2019. BEEF SPECIES–RUMINANT NUTRITION CACTUS BEEF SYMPOSIUM: energy and roughage levels in cattle receiving diets and impacts on health, performance, and immune responses. J. Anim. Sci. 97:3596–3604. doi: 10.1093/jas/skz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risalde, M. A., Molina V., Sánchez-Cordón P. J., Pedrera M., Panadero R., Romero-Palomo F., and Gómez-Villamandos J. C.. 2011. Response of proinflammatory and anti-inflammatory cytokines in calves with subclinical bovine viral diarrhea challenged with bovine herpesvirus-1. Vet. Immunol. Immunopath. 144:135–143. doi: 10.1016/j.vetimm.2011.07.022 [DOI] [PubMed] [Google Scholar]
- Schaefer, A. L., Cook N. J., Church J. S., Basarab J., Perry B., Miller C., and Tong A. K. W.. 2007. The use of infrared thermography as an early indicator of bovine respiratory disease complex in calves. Res. Vet. Sci. 83:376–384. doi: 10.1016/j.rvsc.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, A. L., Cook N., Tessaro S. V., Deregt D., Desroches G., Dubeski P. L., Tong A. K. W., and Godson D. L.. 2004. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 84:73–80. doi: 10.4141/A02-104. [DOI] [Google Scholar]
- Seok, S. H., Heo J. I., Hwang J. H., Na Y., Yun J. H., Lee E. H., Park J. W., and Cho C. H.. 2013. Angiopoietin-1 elicits pro-inflammatory responses in monocytes and differentiating macrophages. Mol. Cell. 35:550–556. doi: 10.1007/s10059-013-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley, S. T., and Grusby M. J.. 1998. Interleukin 4. Encyclopedia of Immunology. 2nd ed. p. 1451–1453. Elsevier, Amsterdam, Netherlands. doi: 10.1006/rwei.1999.0368. [DOI] [Google Scholar]
- Smith, R. A. 1998. Impact of disease on feedlot performance: a review. J. Anim. Sci. 76:272–274. doi: 10.2527/1998.761272x. [DOI] [PubMed] [Google Scholar]
- Smock, T. M., Samuelson K. L., Wells J. E., Hales K. E., Hergenreder J. E., Rounds P. W., and Richeson J. T.. 2020. Effects of Bacillus subtilis PB6 and/or chromium propionate supplementation on serum chemistry, complete blood count, and fecal Salmonella spp. count in high-risk cattle during the feedlot receiving and finishing periods. Transl. Anim. Sci. 4:txaa164. doi: 10.1093/tas/txaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlock, M. E. 1997. Regulation of metabolism and growth during immune challenge: an overview of cytokine function. J. Anim. Sci. 75:1773–1783. doi: 10.2527/1997.7571773x. [DOI] [PubMed] [Google Scholar]
- Thomson, D. U., and White B. J.. 2006. Backgrounding beef cattle. Vet. Clin. Food Anim. Pract. 22:373–398. doi: 10.1016/j.cvfa.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Tilg, H., and Peschel C.. 1996. Interferon-alpha and its effects on the cytokine cascade: a pro-and anti-inflammatory cytokine. Leuk. Lymph. 23:55–60. doi: 10.3109/10428199609054802 [DOI] [PubMed] [Google Scholar]
- Timsit, E., Dendukuri N., Schiller I., and Buczinski S.. 2016. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: a systematic literature review and hierarchical Bayesian latent-class meta-analysis. Prev. Vet. Med. 135:67–73. doi: 10.1016/j.prevetmed.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Tyner, J. W., Uchida O., Kajiwara N., Kim E. Y., Patel A. C., O’Sullivan M. P., Walter M. J., Schwendener R. A., Cook D. N., Danoff T. M., et al. 2005. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med. 11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna, D. M. L., and Carrive P.. 2005. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 21:2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]
- Waldron, M. R., Nishida T., Nonnecke B. J., and Overton T. R.. 2003. Effect of lipopolysaccharide on indices of peripheral and hepatic metabolism in lactating cows. J. Dairy Sci. 86:3447–3459. doi: 10.3168/jds.S0022-0302(03)73949-6. [DOI] [PubMed] [Google Scholar]
- White, B. J., and Renter D. G.. 2009. Bayesian estimation of the performance of using clinical observations and harvest lung lesions for diagnosing bovine respiratory disease in post-weaned beef calves. J. Vet. Diagn. 21:446–453. doi: 10.1177%2F104063870902100405 [DOI] [PubMed] [Google Scholar]
- Wilson, B. K., Richards C. J., Step D. L., and Krehbiel C. R.. 2017. Beef species symposium: best management practices for newly weaned calves for improved health and well-being. J. Anim. Sci. 95:2170–2182. doi: 10.2527/jas.2016.1006. [DOI] [PubMed] [Google Scholar]
- Wolters, T. L. C., Netea M. G., Hermus A. R. M. M., Smit J. W. A., and Netea-Maier R. T.. 2017. IGF1 potentiates the pro-inflammatory response in human peripheral blood mononuclear cells via MAPK. J. Mol. Endocrin. 59:129–139. doi: 10.1530/JME-17-0062 [DOI] [PubMed] [Google Scholar]
- Yue, X., Wu L., and Hu W.. 2015. The regulation of leukemia inhibitory factor. Cancer Microenviron. 2:877. doi: 10.14800/ccm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, Y. -R., Won J. E., Jeon E., Lee S., Kang W., Jo H., Jang J. -H., Shin U. S., and Kim H. -W.. 2010. Fibroblast growth factors: biology, function, and application for tissue regeneration. J. Tissue Eng. 1:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelová, H., and Hošek J.. 2013. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res. 62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- Zenobia, C., and Hajishengallis G.. 2015. Basic biology and role of interleukin-17 in immunity and inflammation. Peridon. 69:142–159. doi: 10.1111/prd.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]