Abstract
Background:
Qualifying comorbidity sets (QCS) are tools used to identify multimorbid patients at increased surgical risk. It is unknown how the QCS framework for multimorbidity affects surgical risk in different racial groups.
Methods:
This retrospective cohort study included Medicare patients age ≥ 65.5 who underwent an emergency general surgery operation from 2015–2018. Our exposure was race and multimorbidity, included in our model as an interaction term. The primary outcome of the study was 30-day mortality. Secondary outcomes included routine discharge, 30-day readmission, length of stay, and complications.
Results:
In total, 163,148 patients who underwent and operation were included in this study. Of these, 13,852 (8.5%, p<0.001) were Black, and 149,296 (91.5%, p<0.001) were White. Black multimorbid patients had no significant differences in 30-day mortality, routine discharge or 30-day readmission when compared to White multimorbid patients after risk-adjustment. Black multimorbid patients had significantly lower odds of complications (OR 0.89, p=0.014) compared to White multimorbid patients.
Conclusions:
Our study of universally insured patients highlights the critical role of pre-operative health status and its association with surgical outcomes.
Keywords: Multimorbidity, older age, race, Black, White, emergency, general surgery
Introduction
Over the past several decades, evidence has emerged demonstrating that Black patients tend to fare worse than White patients after surgery.1, 2 Studies have found that Black patients in the US have a 20–50% higher rate of mortality when compared to White patients undergoing major operations.3 One of the potential causes for these disparities is the omission of key patient level factors, leading to insufficient risk adjustment. Minority patients often present for surgery with a greater number of comorbidities and more advanced disease than non-minority patients, a distinction that is most pronounced among the older adult population.4, 5 Therefore, it is challenging to elucidate whether observed racial disparities are the result of residual confounding from insufficient risk adjustment, possibly due to unmeasured structural or systemic factors.
Multimorbidity is commonly defined as the presence of multiple diseases or conditions, often with a cut-off of two or more.6 The presence of two or more comorbidities by absolute count may be insufficient to risk stratify patients when making treatment recommendations.7 In 2018, Silber et al. identified a multimorbid patient population with substantially increased risk of surgical mortality compared to typical, older patients.8 This definition of multimorbidity differentiates those with multiple, common comorbidities from those with specific combinations of comorbidities, known as Qualifying Comorbidity Sets (QCS), that confer a substantially increased surgical risk. The QCS framework provides greater discrimination of patient’s surgical risk when compared to the Elixhauser comorbidity index.9
Given the recent acknowledgement of structural racism10, we sought to examine the impact of multimorbidity on surgical outcomes in a population that is universally insured and thus should have greater access to surgical care than an uninsured population. Insurance coverage does not fully address all the structural barriers Black patients may face when receiving care; however, it is an important factor in receiving care. To date, the impact of multimorbidity on surgical risk in different racial groups remains unknown. In this study, we aimed to examine the postoperative outcomes of older multimorbid Black patients compared to older multimorbid White patients. This was done in a group of universally insured Medicare beneficiaries, who presented to the hospital with an emergency general surgery diagnosis. We hypothesized that 1) older Black multimorbid patients will have higher mortality and worse post-operative outcomes than older White multimorbid patients and 2) that controlling for multimorbidity will reduce apparent racial disparities seen in post-operative outcomes but will not eliminate them completely.
Methods
Data source and population:
The CMS Master Beneficiary Summary File (MBSF), Inpatient, Carrier (Part B) and Durable Medical Equipment (DME) files were utilized for this study. We included patients with a principal diagnosis of an emergency general surgery (EGS) condition who were ≥ 65.5 years of age, enrolled in Medicare fee-for-service (FFS), and underwent a general surgery operation between July 1, 2015, and June 30, 2018. Our cohort was drawn from a 100% sample of Medicare FFS claims. The coding strategy is detailed in Supplemental Section 1. Patients who did not have continuous Part A & B coverage or who were enrolled in a health maintenance organization (HMO) at any time between 6 months before and 6 months after their index EGS admission were excluded to ensure that their available claims represented all billable encounters (see Figure 1). The study was conducted according to the STROBE guidelines.
Fig. 1.
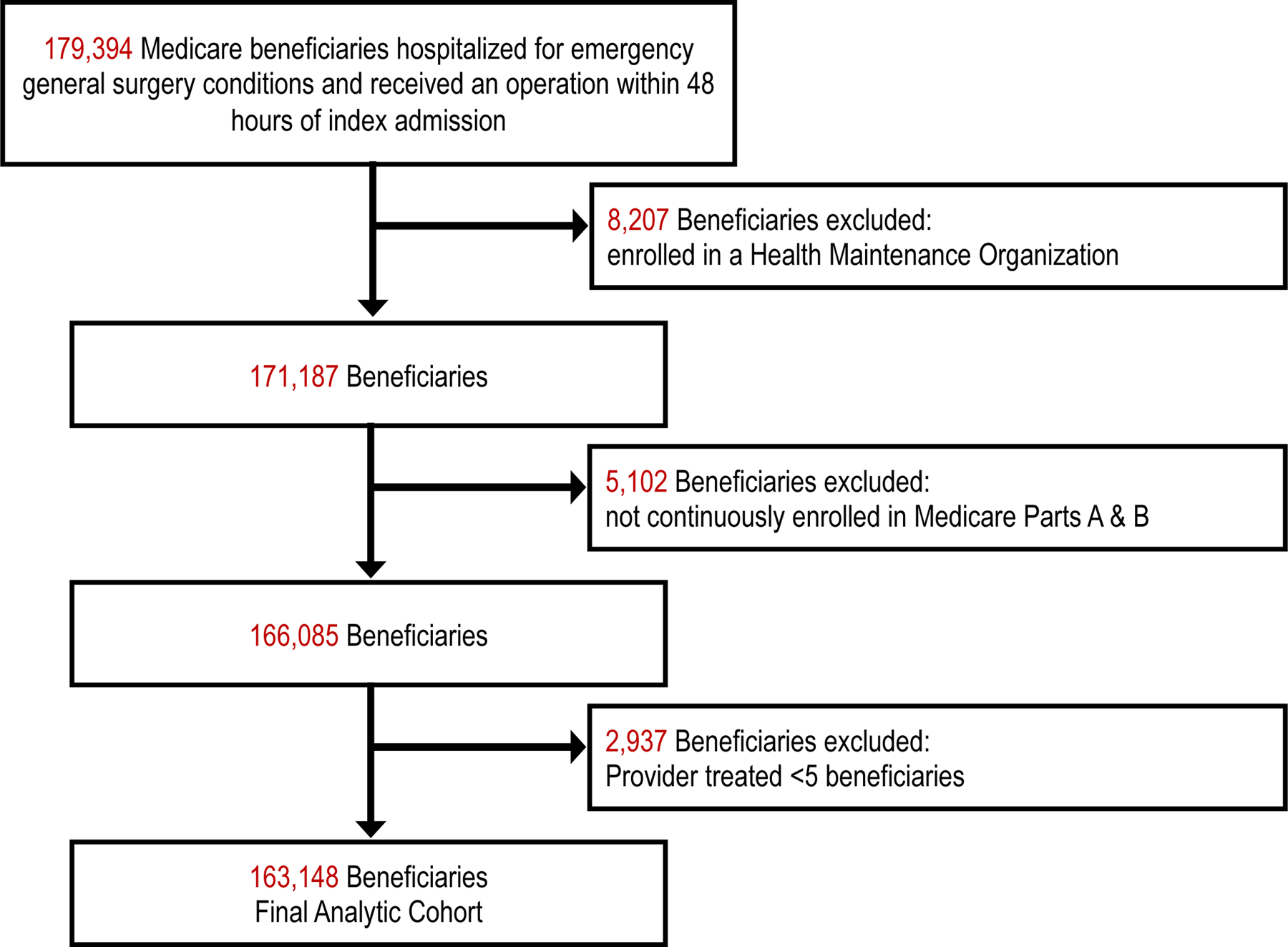
CONSORT flow diagram of patients screened and included in analysis.
Multimorbidity:
We defined multimorbidity using the qualifying comorbidity sets (specific combinations of comorbid conditions) identified by Silber et al.8 to be associated with an increased risk of surgical mortality.8 The QCSs used in this analysis included those defined using ICD 9 codes and those subsequently established using ICD 10 codes. See Supplemental Section 2 and 3 for the individual qualifying comorbidity sets used in our analysis along with associated abbreviations and acronyms. Patients were defined as multimorbid if their specific combinations of comorbid conditions fit any of the QCSs. Patients were defined as non-multimorbid if their combination of comorbidities did not fit into one of the QCSs (including those with no comorbidities).
It is important to note that QCSs are combinations of comorbidities that are associated with an increased risk of mortality. QCSs have been shown to be more specific in identifying high risk patients than simply counting the number of comorbid conditions.11 Further, each QCS each impart a different level of increased risk. For example, using ICD-9 era QCSs, the QCS of COPD, Renal Dysfunction and Thrombocytopenia has been shown to have a 7.64 (95%CI: 6.51,8.95) times higher mortality rate when compared to a patient without that specific QCS. In comparison, a patient with the QCS of Coagulopathy, Complicated Hypertension and Stroke/TIA has a 4.60 (95%CI: 3.68, 5.76) increased risk of mortality. Both of these QCS impart increased mortality risk for a patient; however, the first QCS in the example imparts a greater risk.
Covariates:
Race was ascertained from Medicare claims data, which obtains enrollee information from the social security association master beneficiary report. Medicare claims have been shown to be very accurate in identifying White (k=0.90) and Black (k=0.96) individuals when compared to self-reported surveys.12 . Emergency general surgery conditions were identified using the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification codes (ICD-9/10-CM). Dates and ages allowed a 6-month “look-back” period to confirm the index admission and gather information regarding comorbidities. Patients were classified into one of five emergency general surgery condition categories based on the principal diagnosis codes: colorectal, general abdominal, hepatopancreaticobiliary (HPB), intestinal obstruction, and upper gastrointestinal. Diagnoses included in each category were drawn from the work of Shafi et al.13 and are shown in Supplemental Section 4. General surgery operations were identified using standard Current Procedural Terminology (CPT) codes.14 Age, gender, EGS condition type, procedure type, the number of individual co-morbid conditions, dual-eligibility, a claims-based frailty index score, preoperative sepsis, and the presence of Alzheimer’s/dementia conditions were abstracted or calculated from the dataset. The claims-based frailty index (CFI) is a composite score based on patient mobility, use of skilled nursing care, and ADL disability. CFI has been validated in Medicare data and has been shown to predict mortality and the number of hospital days.15 Dual-eligibility was used to identify patients who utilized both Medicare and Medicaid coverage to obtain medical services. Dual-eligible beneficiaries have been shown to have a lower socioeconomic status, experience greater barriers to accessing healthcare, and have a higher prevalence of chronic medical conditions.16
Outcomes:
The primary outcome of the study was 30-day mortality, defined as death that occurred within 30 days of the index EGS hospitalization. Secondary outcomes included routine discharge, 30-day readmission, length of stay at the index hospital admission, and complication rates. Routine discharge was defined as discharge to home (excluding discharge to home with services). Patients were determined to have experienced a complication if the diagnostic or procedural code indicating the complication was not present on admission and did not appear in their medical history within the past 6 months.
Descriptive statistics were reported as the number of patients from each racial group and by multimorbidity status. Continuous variables were reported as the mean ± standard deviation. Unadjusted analysis of continuous variables was performed using one-way ANOVA and categorical variables were analyzed using the chi-square test. Risk-adjusted outcomes were estimated using mixed effects, multivariable logistic regression for binary outcomes and mixed effects (30d mortality, routine discharge, complications, 30d readmission) , and multivariable linear regression for continuous outcomes (LOS). The relationship between multimorbidity (binary: yes/no) and race (binary: White/Black) was included in the model as an interaction term. The models were run both with and without the inclusion of the individual QCSs. We clustered the data by hospital as a random effect in the models to account for within-hospital correlations that might understate the standard errors. Statistical significance was set at P-values < 0.05. Data were analyzed using Stata Version 17.0 (StataCorp, College Station, TX, USA). This study was approved by the institutional review board of the University of Pennsylvania (protocol number 832059).
Results
In total, 163,148 patients who underwent and operation were included in this study. Of these, 13,852 (8.5%, p<0.001) were Black, and 149,296 (91.5%, p<0.001) were White. Among Black patients, 7,204 (52.0%, p<0.001) were multimorbid. Among White patients, 72,115 (48.3%, p<0.001) were multimorbid. The mean age of Black multimorbid patients was 70.2 years. The mean age of White multimorbid patients was 75.8 years.
Black patients had higher rates of intestinal obstruction in both the non-multimorbid (B:26.9% W:22.9%; p<0.001) and multimorbid groups (B:31.0%, W:26.9%; p<0.001). Black non-multimorbid (B:45.5% W:47.2%; p<0.001) and multimorbid (B:30.1%, W:32.2%; p<0.001) patients had lower rates of HPB conditions than their White counterparts. Black non-multimorbid patients had a higher Angus sepsis score than White non-multimorbid patients (B:2.9%, W:2.0%; p<0.001). In the multimorbid population there was no difference in the Angus sepsis score between Black and White patients (B:20.3% W:20.6%, p=0.470). Black multimorbid patients were also significantly more likely to be dual-eligible than White multimorbid patients (B:48.4%, W:19.2%, p<0.001). (Table 1)
Table 1:
Characteristics of White and Black Patients by Multimorbid status
| White | Black | |||||
|---|---|---|---|---|---|---|
| Non-MM | MM | P-value | Non-MM | MM | P-value | |
| Total Number n (%) | 77,192 (51.7) | 72,186 (48.3) | <0.001 | 6,649 (48.0) | 7,212 (52.0) | <0.001 |
| Age, mean +/− SD | 72.4 +/− 11.4 | 75.8 +/− 11.0 | <0.001 | 66.4 +/− 14.3 | 70.2 +/− 13.2 | <0.001 |
| Gender, n (%) | <0.001 | <0.001 | ||||
| Male, n (%) | 33,162 (43.0) | 35,420 (49.1) | 2,510 (37.8) | 3,266 (45.3) | ||
| Female, n (%) | 44,030 (57.0) | 36,766 (50.9) | 4,139 (62.2) | 3,946 (54.7) | ||
| Number of Comorbidities*, n (%) | <0.001 | <0.001 | ||||
| 0 | 4,846 (6.3) | 0 (0.0) | 283 (4.3) | 0 (0.0) | ||
| 1 | 10,015 (13.0) | 417 (0.6) | 772 (11.6) | 30 (0.4) | ||
| 2 | 14,578 (18.9) | 1,674 (2.3) | 1,130 (17.0) | 128 (1.8) | ||
| ≥3 | 47,753 (61.9) | 70,095 (97.1) | 4,464 (67.1) | 7,054 (97.8) | ||
| EGS Condition, n (%) | <0.001 | <0.001 | ||||
| Colorectal | 4,017 (5.2) | 5,077 (7.0) | 285 (4.3) | 445 (6.2) | ||
| General Abdominal | 2,518 (3.3) | 9,783 (13.6) | 312 (4.7) | 982 (13.6) | ||
| HPB | 36,472 (47.2) | 23,256 (32.2) | 3,023 (45.5) | 2,167 (30.0) | ||
| Intestinal Obstruction | 17,669 (22.9) | 19,437 (26.9) | 1,785 (26.8) | 2,237 (31.0) | ||
| Upper GI | 16,516 (21.4) | 14,633 (20.3) | 1,244 (18.7) | 1,381 (19.1) | ||
| Claims-Based Frailty Index, mean (sd) | 0.128 +/− 0.032 | 0.169 +/− 0.060 | <0.001 | 0.133 (0.033) | 0.175 (0.064) | <0.001 |
| Angus Sepsis (Preoperative) (Yes), n (%) | 1,569 (2.0) | 14,904 (20.6) | <0.001 | 193 (2.9) | 1,463 (20.3) | <0.001 |
| Alzheimer’s/Dementia (Yes), n (%) | 4,273 (5.5) | 8,933 (12.4) | <0.001 | 378 (5.7) | 928 (12.9) | <0.001 |
| Medicaid Dual-Eligible (Yes), n (%) | 11,907 (14.6) | 12,961 (19.2) | 2,909 (41.7) | 3,326 (48.4) | ||
| Procedure Type, n (%) | <0.001 | <0.001 | ||||
| Adrenalectomy, n (%) | < 10 (.) | < 10 (.) | 0 (.) | 0 (.) | ||
| Appendectomy, n (%) | 1,787 (2.3) | 1,268 (1.8) | 155 (2.3) | 155 (2.1) | ||
| Appendectomy - Lap, n (%) | 10,030 (13.0) | 5,325 (7.4) | 684 (10.3) | 490 (6.8) | ||
| Bariatric, n (%) | < 10 (.) | < 10 (.) | 0 (.) | 0 (.) | ||
| Biliary Common Duct, n (%) | 532 (0.7) | 457 (0.6) | 36 (0.5) | 32 (0.4) | ||
| Biliary Other, n (%) | 26 (0.0) | 42 (0.1) | < 10 (.) | < 10 (.) | ||
| Cholecystectomy, n (%) | 35,615 (46.1) | 24,275 (33.6) | 2,945 (44.3) | 2,242 (31.1) | ||
| Closure of Enterostomy, n (%) | 19 (0.0) | 41 (0.1) | < 10 (.) | < 10 (.) | ||
| Closure of Enterostomy with Resection, n (%) | 34 (0.0) | 86 (0.1) | < 10 (.) | 12 (0.2) | ||
| Colectomy Partial - Lap, n (%) | 1,140 (1.5) | 1,138 (1.6) | 94 (1.4) | 86 (1.2) | ||
| Colectomy Partial - Open, n (%) | 6,754 (8.7) | 11,354 (15.7) | 532 (8.0) | 1,025 (14.2) | ||
| Colectomy Total - Lap, n (%) | 13 (0.0) | 29 (0.0) | < 10 (.) | < 10 (.) | ||
| Colectomy Total - Open, n (%) | 184 (0.2) | 813 (1.1) | 19 (0.3) | 110 (1.5) | ||
| Esophagectomy, n (%) | < 10 (.) | 20 (0.0) | 0 (0.0) | < 10 (.) | ||
| Esophagomyotomy, n (%) | < 10 (.) | < 10 (.) | 0 (.) | 0 (.) | ||
| Hernia Abdominal - Lap, n (%) | 1,069 (1.4) | 909 (1.3) | 130 (2.0) | 110 (1.5) | ||
| Hernia Abdominal - Open, n (%) | 4,208 (5.5) | 4,292 (5.9) | 455 (6.8) | 598 (8.3) | ||
| Hernia Groin - Lap, n (%) | 490 (0.6) | 367 (0.5) | 42 (0.6) | 23 (0.3) | ||
| Hernia Groin - Open, n (%) | 3,677 (4.8) | 3,531 (4.9) | 374 (5.6) | 364 (5.0) | ||
| Large Bowel Other, n (%) | 456 (0.6) | 700 (1.0) | 36 (0.5) | 77 (1.1) | ||
| Liver Other, n (%) | 34 (0.0) | 33 (0.0) | < 10 (.) | < 10 (.) | ||
| Liver Partial Hepatectomy, n (%) | 84 (0.1) | 132 (0.2) | 11 (0.2) | 21 (0.3) | ||
| Lysis of Adhesions, n (%) | 2,334 (3.0) | 2,643 (3.7) | 258 (3.9) | 348 (4.8) | ||
| Mastectomy, n (%) | 0 (0.0) | < 10 (.) | 0 (0.0) | < 10 (.) | ||
| PD Access Procedure, n (%) | < 10 (.) | 15 (0.0) | < 10 (.) | < 10 (.) | ||
| Pancreatectomy, n (%) | 21 (0.0) | 52 (0.1) | 0 (0.0) | < 10 (.) | ||
| Parathyroidectomy, n (%) | 0 (0.0) | < 10 (.) | 37 (0.6) | 50 (0.7) | ||
| Proctectomy, n (%) | 577 (0.7) | 704 (1.0) | < 10 (.) | 10 (0.1) | ||
| Proctectomy - Lap, n (%) | 178 (0.2) | 92 (0.1) | < 10 (.) | < 10 (.) | ||
| Proctopexy, n (%) | 60 (0.1) | 36 (0.0) | < 10 (.) | < 10 (.) | ||
| Pyloroplasty, n (%) | 20 (0.0) | 58 (0.1) | 90 (1.4) | 126 (1.7) | ||
| Small Bowel Other, n (%) | 769 (1.0) | 1,238 (1.7) | 490 (7.4) | 806 (11.2) | ||
| Small Bowel Resection, n (%) | 4,825 (6.3) | 7,604 (10.5) | < 10 (.) | 12 (0.2) | ||
| Splenectomy, n (%) | 40 (0.1) | 131 (0.2) | 10 (0.2) | 14 (0.2) | ||
| Stomach Anti-Reflux, n (%) | 32 (0.0) | 40 (0.1) | 12 (0.2) | 39 (0.5) | ||
| Stomach Gastric Bypass (non-bariatric), n (%) | 81 (0.1) | 162 (0.2) | 29 (0.4) | 78 (1.1) | ||
| Stomach Other, n (%) | 126 (0.2) | 256 (0.4) | < 10 (.) | < 10 (.) | ||
| Stomach Partial Gastrectomy, n (%) | 189 (0.2) | 495 (0.7) | 0 (0.0) | < 10 (.) | ||
| Stomach Total Gastrectomy, n (%) | < 10 (.) | 38 (0.1) | 0 (0.0) | < 10 (.) | ||
| Ulcer, n (%) | 1,762 (2.3) | 3,793 (5.3) | 177 (2.7) | 333 (4.6) | ||
QCS Comorbid Conditions:
A total of 57 individual comorbid conditions were included in the QCS. Thirty-two conditions were more common among Black patients. Twenty-one conditions were less common among Black patients. There was no difference in the proportion of Black and White patients with opportunistic infections, other neurological conditions, or acute myocardial infarction. The patient counts and frequencies are described in Supplemental Section 5.
Qualifying Comorbidity Sets by Race
Regarding QCS distribution, Black and White multimorbid patients differed in several ways. Black multimorbid patients were more likely to have “chronic kidney disease requiring dialysis” (B:23.6% W:8.3%, p<0.001) and “diabetes with complications with congestive heart failure” (B:17.2% W:9.9%, p<0.001). Black multimorbid patients were less likely to have “congestive heart failure with cardiac arrythmia” (B:16.5% W:20.7%, p<0.001) or “cardiac arrythmia with chronic lung disease” (B:8.7% W:15.3%, p<0.001) than White multimorbid patients. See Table 2 for a representative list of the QCS distributions. See Supplemental Section 6 for a full list of the QCS distributions.
Table 2.
Representative Sample of Individual QCS Frequencies Among Multimorbid Black and White Patients
| White | Black | P-value | |
|---|---|---|---|
| Amputation & Complications (Yes), n (%) | 941 (1.4) | 204 (3.0) | <0.001 |
| CKD Stage 4–5 & Dialysis (Yes), n (%) | 5,629 (8.3) | 1,626 (23.6) | <0.001 |
| Liver Diseases (Yes), n (%) | 4,143 (6.1) | 529 (7.7) | <0.001 |
| Oxygen (Yes), n (%) | 7,147 (10.6) | 495 (7.2) | <0.001 |
| Pressure Ulcer, Skin (Yes), n (%) | 2,255 (3.3) | 314 (4.6) | <0.001 |
| Sepsis/Shock (Yes), n (%) | 19,346 (28.6) | 1,822 (26.5) | <0.001 |
| Acute Renal Failure | Wheelchair/Beds (Yes), n (%) | 959 (1.4) | 173 (2.5) | <0.001 |
| CHF | Acute Renal Failure (Yes), n (%) | 7,895 (11.7) | 1,054 (15.3) | <0.001 |
| CHF | Vascular Diseases (Yes), n (%) | 10,731 (15.9) | 1,313 (19.1) | <0.001 |
| CHF | Wheelchair/Beds (Yes), n (%) | 1,319 (2.0) | 228 (3.3) | <0.001 |
| Diabetes w/Complications | Chronic Ulcer, Skin, Not Pressure (Yes), n (%) | 2,013 (3.0) | 303 (4.4) | <0.001 |
| Diabetes w/Complications | Complications Implants Graft (Yes), n (%) | 897 (1.3) | 274 (4.0) | <0.001 |
| Diabetes w/Complications | CHF (Yes), n (%) | 6,713 (9.9) | 1,183 (17.2) | <0.001 |
| Diabetes w/Complications | Other Hematological (Yes), n (%) | 2,593 (3.8) | 361 (5.2) | <0.001 |
| Endocrine & Metabolic Disorders | Other Hematological (Yes), n (%) | 1,273 (1.9) | 184 (2.7) | <0.001 |
| Heart Arrhythmias | Acute Renal Failure (Yes), n (%) | 8,161 (12.1) | 698 (10.1) | <0.001 |
| Heart Arrhythmias | Chronic Lung Diseases (Yes), n (%) | 10,371 (15.3) | 600 (8.7) | <0.001 |
| Other Hematological | Complications Implants Graft (Yes), n (%) | 520 (0.8) | 127 (1.8) | <0.001 |
| Other Hematological | Heart Arrhythmias (Yes), n (%) | 5,016 (7.4) | 312 (4.5) | <0.001 |
| Vascular Diseases | Acute Renal Failure (Yes), n (%) | 8,742 (12.9) | 1,042 (15.2) | <0.001 |
| Vascular Diseases | Chronic Ulcer, Skin, Not Pressure (Yes), n (%) | 2,787 (4.1) | 350 (5.1) | <0.001 |
| CHF | Heart Arrhythmias (Yes), n (%) | 13,987 (20.7) | 1,132 (16.5) | <0.001 |
| CHF | Heart Arrhythmias | Coronary Artery Disease (Yes), n (%) | 8,804 (13.0) | 659 (9.6) | <0.001 |
| Diabetes w/Complications | Vascular Diseases | Chronic Lung Diseases (Yes), n (%) | 2,764 (4.1) | 359 (5.2) | <0.001 |
| Endocrine & Metabolic Disorders | Complications Implants Graft | Cor (Yes), n (%) | 293 (0.4) | 111 (1.6) | <0.001 |
| Endocrine & Metabolic Disorders | CHF | Heart Arrhythmias (Yes), n (%) | 1,296 (1.9) | 201 (2.9) | <0.001 |
| Vascular Diseases | Chronic Lung Diseases | Complications Implants G (Yes), n (%) | 619 (0.9) | 107 (1.6) | <0.001 |
| Vascular Diseases | Chronic Lung Diseases | Coronary Artery Disease (Yes), n (%) | 6,467 (9.6) | 506 (7.4) | <0.001 |
| Vascular Diseases | Complications Implants Graft | Coronary Artery D (Yes), n (%) | 796 (1.2) | 147 (2.1) | <0.001 |
Unadjusted outcomes
Black multimorbid patients had lower rates of 30-day mortality when compared to White multimorbid patients (B:8.3% W:9.7%, p<0.001), but there was no difference in mortality between Black and White 30-day mortality among non-multimorbid patients (B:1.4% W:1.4%, p=0.901). There was no significant difference in routine discharge between Black and White multimorbid patients (B:41.9% W:41.8%, p=0.956); however, in the non-multimorbid population Black patients had a lower rate of routine discharge compared to White patients (B:71.8% W:73.9%, p<0.001). Black multimorbid patients had higher 30-day readmission compared to White multimorbid patients (B:25.1%, W:22.2%, p<0.001). Black patients had longer lengths of stay (excluding patients who died in-hospital) than White patients, regardless of multimorbidity status. Further details are provided in Table 3.
Table 3.
Unadjusted Outcomes of Multimorbid and Non-Multimorbid patients compared by Race
| Multimorbid | Non-Multimorbid | |||||
|---|---|---|---|---|---|---|
| Black | White | P value | Black | White | P value | |
| Died within 30 days of index EGS discharge (Yes), n (%) | 593 (8.2) | 6,908 (9.6) | <0.001 | 81 (1.2) | 903 (1.2) | 0.725 |
| Routine Discharge (Yes), n (%) | 3,023 (41.9) | 30,537 (42.3) | 0.526 | 4,866 (73.2) | 58,098 (75.3) | <0.001 |
| 30d Readmission (Yes), n (%) | 1,802 (25.0) | 15,808 (21.9) | <0.001 | 827 (12.4) | 8,651 (11.2) | 0.002 |
| Length of Stay (w/o pts who died in-hospital), mean (sd) | 8.4 +/− 7.5 | 7.4 +/− 6.3 | <0.001 | 5.1 +/− 4.5 | 4.3 +/− 3.6 | <0.001 |
Adjusted outcomes
Black multimorbid patients had no significant differences in 30-day mortality, routine discharge or 30-day readmission when compared to White multimorbid patients after adjustment for potential confounders. Black multimorbid patients had significantly lower odds of complications (OR 0.89, p=0.014) compared to White multimorbid patients. In addition, it was estimated that Black multimorbid patients stayed 0.30-days longer in the hospital than White multimorbid patients (p<0.001). (Table 4) Inclusion of individual QCSs into the model did not affect any of the outcomes; these results are reported in Supplemental Section 7.
Table 4:
Adjusted Outcomes of Black Multimorbid patients when Compared to White Multimorbid patients
| Died within 30 days of index EGS discharge | Routine Discharge | Complication | 30d Readmission | Length of Stay (w/o pts who died in-hospital) | |
|---|---|---|---|---|---|
| Black, OR, (p value) | 0.92 (0.455) | 1.02 (0.632) | 0.89** (0.014) | 1.07 (0.180) | 0.30*** (<0.001) |
Discussion
This national retrospective Medicare cohort study examined the impact of race and multimorbidity on EGS surgical outcomes. The study demonstrates insured, Black multimorbid patients have similar outcomes to White multimorbid patients after adjustment for potential confounders, and Black multimorbid patients had comparatively lower rates of complications. These findings were unexpected given the wealth of literature indicating negative health disparities among minority patients.17, 18
Many factors have been shown to impact surgical health disparities. Some of these include socioeconomic status (SES), insurance status, patient’s preoperative health status, hospital quality, biases in referral patterns, and implicit bias by providers.17 With adjustment for these potential confounders, we expected to see a reduction or elimination of racial disparities in our model, similar to the results seen in other studies.19, 20 For example, Schoenfeld et al.21 studied an equally insured population of Black and White patients and found no difference in post-operative outcomes between the Black and White cohorts after adjustment for age, sex, comorbidity, and SES. In our models, we controlled for many of these factors, including dual-eligibility, which likely played a role in the lack of differences observed between racial groups. Our results are consistent with existing literature that shows a reduction in surgical disparities among older universally insured EGS patients.22, 23
In our adjusted models of multimorbid patients, the lower rate of complications observed in the Black patient population was unexpected. One possible explanation for these findings is the differential distribution of QCSs between Black and White multimorbid patient populations. To date, these risks have not been quantified and published for the ICD 10 era codes. Black multimorbid patients in our study may have had a distribution of QCSs that imparts a lower post-operative risk for complications than their White multimorbid counterparts.
One critical factor in a patient’s post-operative course is their pre-operative health status. In our study, both Black and White multimorbid patients had significantly higher rates of 30-day mortality when compared to their non-multimorbid counterparts. Mortality in this population may have influenced the conditional effects of race on multimorbidity. In general, patients with better functional status and lower rates of co-morbidities have lower rates of morbidity and mortality following surgery.24, 25 Surgical health disparities are often attributed to the fact that Black patients present for surgery with a greater number and higher severity of co-morbid conditions.26 In our study, we see that in a population of multimorbid patients (with significant comorbidities) Black patients have lower complication rates.
For many non-emergent/elective surgical conditions patients present to outpatient clinics, where it has been shown that Black patients are referred for surgery at lower rates than White patients.27, 28 The referral patterns among other factors lead to disparities in the receipt of surgery.29 For example, bariatric surgery is utilized at a lower rate among minority patients, even though a greater percentage of patients who are eligible are Black and come from lower socioeconomic backgrounds30 Findings demonstrating referral bias have been replicated in several surgical domains such as total knee arthroplasty31, kidney transplantation32 and many cancer operations.33–35 Even among Black patients that do undergo surgery, their operations are often delayed when compared to White patients.36 In cancer populations, wait times can have a strong effect on survival.37
In our study, the emergent nature of the EGS conditions eliminates the need for an elective referral for surgery which should eliminate referral bias in this setting. However, in the emergency department, a surgical consultation is still required to access surgical care. In this setting, it has been demonstrated that Black Medicare patients receive lower rates of surgical consultations than similar White patients, even after controlling for differences in patient comorbidities, diagnostic imaging, socioeconomic factors, and individual hospital factors.38 Disparate rates of consultation may impart selection bias into the operative cohort of Black and White patients with EGS conditions.
A strength of our study is the use of the QCS framework which has been demonstrated to predict which patients are at elevated surgical risk more efficiently than other comorbidity indicies.9 The QCS framework allows us to identify combinations of comorbidities that place surgical patients at significantly greater surgical risk, thus allowing for more effective risk adjustment during analysis. Our results support existing literature that shows universally insured older EGS patients, after risk adjustment, do not experience significant health disparities.23
Implications
Racial disparities continue to exist across countless aspects of healthcare; however, they do not appear to be as prevalent among the older, multimorbid patient population undergoing emergency general surgery. This suggests that multimorbidity is one of the few factors that affects patients similarly across racial groups. Our study results highlight the critical role of pre-operative health status and its effects on surgical outcomes. Interventions aimed at improving prehospital health status may offer a fruitful opportunity to reduce observed disparities.
Limitations
This study is subject to the usual limitations of a retrospective study that utilizes claims data: granularity regarding the severity of individual co-morbidities cannot be extracted. Additionally, as with all observational studies there is the potential for unmeasured residual confounding. Our use of the QCS framework combats this challenge by offering a greater predictive capacity regarding surgical risk than that previously available in claims data. Our study also examined only those patients who underwent surgery. It has been documented that Black and White patients are offered operative treatment at different rates39, 40 which may introduce selection bias into results. Our real-world data reflects the current state and attempts to overcome this form of selection bias in care delivery.
Conclusions
In our adjusted model, Black multimorbid patients have equivalent risk of mortality when compared to White multimorbid patients, and lower rates of complications than their White multimorbid counterparts. The QCS framework offers a more targeted approach to determine a patient’s postoperative risk profile; however, more research is needed to determine the impact of specific QCSs on surgical outcomes.
Supplementary Material
No differences in 30-day mortality between Black and White multimorbid patients
Black multimorbid patients had significantly lower odds of complications
Risk-adjusted Medicare EGS patients do not experience significant health disparities
Use of the QCS framework allows for more effective risk adjustment
Funding/Disclosures:
The research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG060612. Dr. Claire Rosen was supported by the National Institute on Aging of the National Institutes of Health under award number F32AG074614. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Access Statement: Research data supporting this is available upon request
Meeting Presentation: American College of Surgeons Clinical Congress, Virtual, October 2021
Conflicts of interest: none
This manuscript is not under consideration for publication in any other journal and the authors have no financial conflicts in relation to the content of the manuscript.
References
- 1.Metcalfe D, Castillo-Angeles M, Olufajo OA, et al. Failure to rescue and disparities in emergency general surgery. Journal of Surgical Research 2018/11/01/ 2018;231:62–68. doi: 10.1016/j.jss.2018.04.047 [DOI] [PubMed] [Google Scholar]
- 2.Udyavar NR, Salim A, Cornwell EE, Cooper Z, Haider AH. Do outcomes in emergency general surgery vary for minority patients based on surgeons’ racial/ethnic case mix? The American Journal of Surgery 2019/07/01/ 2019;218(1):42–46. doi: 10.1016/j.amjsurg.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 3.Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg Feb 2006;243(2):281–6. doi: 10.1097/01.sla.0000197560.92456.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho VP, Schiltz NK, Reimer AP, Madigan EA, Koroukian SM. High-Risk Comorbidity Combinations in Older Patients Undergoing Emergency General Surgery. J Am Geriatr Soc Mar 2019;67(3):503–510. doi: 10.1111/jgs.15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry RG, Mitchell JA, Hawkins J, Johnson-Lawrence V. The Role of Age and Multimorbidity in Shaping Older African American Men’s Experiences with Patient(−)Provider Communication. Geriatrics (Basel) Oct 24 2018;3(4)doi: 10.3390/geriatrics3040074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston MC, Crilly M, Black C, Prescott GJ, Mercer SW. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health Feb 1 2019;29(1):182–189. doi: 10.1093/eurpub/cky098 [DOI] [PubMed] [Google Scholar]
- 7.Rosen CBWC, Keele LJ, et al. Multimorbidity confers greater risk for older patients in emergency general surgery than the presence of multiple comorbidities: A retrospective observational study Medical Care 2022;In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silber JH, Reiter JG, Rosenbaum PR, et al. Defining Multimorbidity in Older Surgical Patients. Med Care Aug 2018;56(8):701–710. doi: 10.1097/MLR.0000000000000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen CB, Wirtalla C, Keele LJ, et al. Multimorbidity Confers Greater Risk for Older Patients in Emergency General Surgery Than the Presence of Multiple Comorbidities: A Retrospective Observational Study. Medical Care 2022;60(8):616–622. doi: 10.1097/mlr.0000000000001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts SE. I Can’t Breathe - Race, Violence, and COVID-19. Ann Surg Sep 1 2020;272(3):e191. doi: 10.1097/SLA.0000000000004256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen CB, Wirtalla C, Keele LJ, et al. Multimorbidity Confers Greater Risk for Older Patients in Emergency General Surgery Than the Presence of Multiple Comorbidities: A Retrospective Observational Study. Med Care Aug 1 2022;60(8):616–622. doi: 10.1097/MLR.0000000000001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarrin OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of Race and Ethnicity Codes in Medicare Administrative Data Compared With Gold-standard Self-reported Race Collected During Routine Home Health Care Visits. Med Care Jan 2020;58(1):e1–e8. doi: 10.1097/MLR.0000000000001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafi S, Aboutanos MB, Agarwal S Jr., et al. Emergency general surgery: definition and estimated burden of disease. J Trauma Acute Care Surg Apr 2013;74(4):1092–7. doi: 10.1097/TA.0b013e31827e1bc7 [DOI] [PubMed] [Google Scholar]
- 14.Kelz RR, Niknam BA, Sellers MM, et al. Duty Hour Reform and the Outcomes of Patients Treated by New Surgeons. Ann Surg Apr 2020;271(4):599–605. doi: 10.1097/SLA.0000000000003304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci Jun 14 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niefeld MR, Kasper JD. Access to ambulatory medical and long-term care services among elderly Medicare and Medicaid beneficiaries: organizational, financial, and geographic barriers. Med Care Res Rev. Jun 2005;62(3):300–19. doi: 10.1177/1077558705275418 [DOI] [PubMed] [Google Scholar]
- 17.Haider AH, Scott VK, Rehman KA, et al. Racial Disparities in Surgical Care and Outcomes in the United States: A Comprehensive Review of Patient, Provider, and Systemic Factors. Journal of the American College of Surgeons 2013;216(3):482–492.e12. doi: 10.1016/j.jamcollsurg.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health May 2012;102(5):953–66. doi: 10.2105/AJPH.2012.300773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silber JH, Rosenbaum PR, Kelz RR, et al. Examining Causes of Racial Disparities in General Surgical Mortality. Medical Care 2015;53(7):619–629. doi: 10.1097/mlr.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zogg CK, Jiang W, Chaudhary MA, et al. Racial disparities in emergency general surgery: Do differences in outcomes persist among universally insured military patients? J Trauma Acute Care Surg May 2016;80(5):764–75; discussion 775–7. doi: 10.1097/TA.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld AJ, Jiang W, Harris MB, et al. Association Between Race and Postoperative Outcomes in a Universally Insured Population Versus Patients in the State of California. Ann Surg Aug 2017;266(2):267–273. doi: 10.1097/SLA.0000000000001958 [DOI] [PubMed] [Google Scholar]
- 22.Armenia SJ, Pentakota SR, Merchant AM. Socioeconomic factors and mortality in emergency general surgery: trends over a 20-year period. Journal of Surgical Research 2017/05/15/ 2017;212:178–186. doi: 10.1016/j.jss.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 23.Zogg CK, Jiang W, Chaudhary MA, et al. Racial disparities in emergency general surgery: Do differences in outcomes persist among universally insured military patients? Journal of Trauma and Acute Care Surgery 2016;80(5):764–777. doi: 10.1097/ta.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 24.Van Cleave JH, Egleston BL, McCorkle R. Factors Affecting Recovery of Functional Status in Older Adults After Cancer Surgery. Journal of the American Geriatrics Society 2011;59(1):34–43. doi: 10.1111/j.1532-5415.2010.03210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laor A, Tal S, Guller V, Zbar AP, Mavor E. The Charlson Comorbidity Index (CCI) as a Mortality Predictor after Surgery in Elderly Patients. The American Surgeon 2016;82(1):22–27. doi: 10.1177/000313481608200113 [DOI] [PubMed] [Google Scholar]
- 26.Tammemagi CM. Comorbidity and Survival Disparities Among Black and White Patients With Breast Cancer. JAMA 2005;294(14):1765. doi: 10.1001/jama.294.14.1765 [DOI] [PubMed] [Google Scholar]
- 27.Oddone EZ, Horner RD, Monger ME, Matchar DB. Racial variations in the rates of carotid angiography and endarterectomy in patients with stroke and transient ischemic attack. Arch Intern Med Dec 27 1993;153(24):2781–6. [PubMed] [Google Scholar]
- 28.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors Associated With Decisions to Undergo Surgery Among Patients With Newly Diagnosed Early-Stage Lung Cancer. JAMA 2010;303(23):2368–2376. doi: 10.1001/jama.2010.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenfeld AJ, Sturgeon DJ, Dimick JB, et al. Disparities in Rates of Surgical Intervention Among Racial and Ethnic Minorities in Medicare Accountable Care Organizations. Ann Surg Mar 2019;269(3):459–464. doi: 10.1097/SLA.0000000000002695 [DOI] [PubMed] [Google Scholar]
- 30.Livingston EH, Ko CY. Socioeconomic characteristics of the population eligible for obesity surgery. Surgery Mar 2004;135(3):288–96. doi: 10.1016/j.surg.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 31.Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national medicare data. Annals of the Rheumatic Diseases 2014;73(12):2107–2115. doi: 10.1136/annrheumdis-2013-203494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy KA, Jackson JW, Purnell TS, et al. Association of Socioeconomic Status and Comorbidities with Racial Disparities during Kidney Transplant Evaluation. Clin J Am Soc Nephrol Jun 8 2020;15(6):843–851. doi: 10.2215/CJN.12541019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bliton JN, Parides M, Muscarella P, Papalezova KT, In H. Understanding Racial Disparities in Gastrointestinal Cancer Outcomes: Lack of Surgery Contributes to Lower Survival in African American Patients. Cancer Epidemiol Biomarkers Prev Mar 2021;30(3):529–538. doi: 10.1158/1055-9965.EPI-20-0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osuoha CA, Callahan KE, Ponce CP, Pinheiro PS. Disparities in lung cancer survival and receipt of surgical treatment. Lung Cancer Aug 2018;122:54–59. doi: 10.1016/j.lungcan.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 35.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst Mar 6 2002;94(5):334–57. doi: 10.1093/jnci/94.5.334 [DOI] [PubMed] [Google Scholar]
- 36.de Angelis P, Kaufman EJ, Barie PS, Narayan M, Smith K, Winchell RJ. Disparities in Timing of Trauma Consultation: A Trauma Registry Analysis of Patient and Injury Factors. J Surg Res Oct 2019;242:357–362. doi: 10.1016/j.jss.2019.04.073 [DOI] [PubMed] [Google Scholar]
- 37.Neroda P, Hsieh MC, Wu XC, et al. Racial Disparity and Social Determinants in Receiving Timely Surgery Among Stage I-IIIA Non-small Cell Lung Cancer Patients in a U.S. Southern State. Front Public Health 2021;9:662876. doi: 10.3389/fpubh.2021.662876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts SE, Rosen CB, Keele LJ, et al. Rates of Surgical Consultations After Emergency Department Admission in Black and White Medicare Patients. JAMA Surg Oct 12 2022;doi: 10.1001/jamasurg.2022.4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranjit A, Chaudhary MA, Jiang W, et al. Disparities in receipt of a laparoscopic operation for ectopic pregnancy among TRICARE beneficiaries. Surgery May 2017;161(5):1341–1347. doi: 10.1016/j.surg.2016.09.029 [DOI] [PubMed] [Google Scholar]
- 40.Ransome E, Tong L, Espinosa J, Chou J, Somnay V, Munene G. Trends in surgery and disparities in receipt of surgery for intrahepatic cholangiocarcinoma in the US: 2005–2014. J Gastrointest Oncol Apr 2019;10(2):339–347. doi: 10.21037/jgo.2018.12.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


