Abstract
Background:
Gastric cancer incidence rates overall in the U.S. have declined over recent decades and are predicted to continue declining. However, there have been mixed recent findings regarding potential stabilization of rates, and potential divergent trends by age group. We used the most recent cancer data for the U.S. and examined trends in gastric cancer (GC) between 1992 and 2019, overall and in important sub-groups of the population.
Methods:
Age-adjusted GC incidence rates and trends in adults ≥20 years were calculated using data from the SEER 12 program. Secular trends were examined overall and by age group, sex, race/ethnicity, SEER registry, and tumor location. We used joinpoint regression to compute annual percent changes, average annual percent changes, and associated 95% confidence intervals.
Results:
GC rates decreased by 1.23% annually from 1992 to 2019. Despite overall decreases, GC incidence rates increased for age groups below 50 years, predominately driven by non-cardia GC (74.3% of all GCs). Cardia GC (26.7% of GC) rates decreased in all age groups except for 80-84 years. Overall GC rates decreased for both sexes, all races, and for all SEER registry regions, with the largest decreases occurring in males, Asians and Pacific Islanders, and in Hawaii. Age-period-cohort analysis revealed that birth cohorts prior to 1940 and after 1980 both had increased rates of GC compared to the reference birth cohort of 1955.
Conclusion:
GC rates overall have continued to decline through 2019, despite increases in the rate of non-cardia GC for younger age groups.
Keywords: gastric cancer, incidence, birth cohort, minorities
INTRODUCTION
In line with an overall decline of cancer incidence and mortality rates in the United States during recent decades, rates of gastric cancer (GC) have also decreased significantly during this time. Furthermore, GC rates in the U.S. have been previously forecast to decrease through 2030 due to large population-level reductions in the prevalence of Helicobacter pylori infections, the primary risk factor for GC globally, and in smoking, and an improvement in diet quality in high-risk areas.1 We have previously demonstrated that overall, GC rates in the U.S. decreased at a rate of 1.55% annually from 1999 to 2007 and then have remained stable thereafter through 2013. However, the burden and secular trends of GC have differed by age-group, sex, race/ethnicity, and region. For example, in contrast to decreasing rates among persons aged ≥50 years in the U.S., we and others have noted that the rates of GC are increasing among persons <50 years. Previous studies have also shown that rates of GC have decreased more in males compared to females and decreased more in non-Hispanic whites (NHW) compared to all other races and ethnicities in the U.S. GC can be divided based on anatomic location into non-cardia GC (NCGC) and cardia GC (CGC). The epidemiology of the two main subtypes of GC is different in several aspects. The increase in GC rates among young adults in the U.S. was predominantly due to an increase in the rate of NCGC, in particular advanced NCGC2, while the rate of CGC remained relatively stable in all age groups.3 Previous work by our group has identified NHWs <50 years of age as having the largest increase in GC incidence rates when compared to other races (0.50% annual increase from 1999 to 2013).3 Risk factors for NCGC include H. pylori infection and high intake of salty and smoked foods. Risk factors for CGC are predominantly obesity and gastro-esophageal reflux disease. Male sex and tobacco use are risk factors for both anatomic subtypes of GC.4
In this paper, we examined recent trends of GC incidence (overall, and separately for NCGC and CGC) using the most recent high-quality cancer registry data for the U.S. Utilizing this population-based resource, with cases through 2019, we assessed trends in GC incidence rates overall and among subgroups of the population by age group, sex, race/ethnicity, and geographic region.
METHODS
We used cancer incidence data from the Surveillance, Epidemiology, and End Results (SEER) program. SEER, maintained by the National Institutes of Health, is a comprehensive, national database containing de-identified data for the incidence and outcomes for cancers in the U.S., including GC. We utilized the most recent SEER 12 release (04/15/2022).5 SEER 12 includes the registries previously included in SEER 13 (data releases prior to 2022) but with Detroit no longer included.6 Data were obtained through SEER*Stat version 8.4.0 software.7 We included incident primary GC cases aged ≥20 years at diagnosis and diagnosed between 1992 and 2019. We identified GC cases within the SEER 12 registries (Alaska Native Tumor Registry, Connecticut, Atlanta, Rural Georgia, San Francisco-Oakland, San Jose-Monterey, Hawaii, Iowa, Los Angeles, New Mexico, Seattle-Puget Sound, and Utah) using a combination of International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site code C16.0-C16.9 and ICD-O-3 histology codes 9050–9055, 9140 and 9590–9992. CGC cases were those cases with site code C16.0 and NCGC cases those with site codes C16.1-C16.9.
Statistical analysis
We calculated annual age-standardized incidence rates of GC both overall and stratified by sex, race/ethnicity, and SEER registry (geography). Rates were standardized to those of the U.S. population in 2000 using the direct method and reported per 100,000 person-years. Corresponding 95% confidence intervals (CIs) were calculated using the modification described in Tiwari et al.8 We also examined age-specific GC rates based on defined age groups at the time of cancer diagnosis. Starting with the November 2021 data submission (data released 04/15/2022), race and ethnicity in SEER 12 are reported in five mutually exclusive categories: NHW, non-Hispanic Black (NHB), Asian and Pacific Islander (API), non-Hispanic American Indian/Alaska Native (AI/AN), and Hispanic.9 Where possible, we examined secular trends within each race/ethnicity group.
We evaluated secular trends in GC (as well as NCGC, CGC separately) incidence rates via the National Cancer Institute’s (NCI) Joinpoint program (version 4.9.1.0; available at: https://surveillance.cancer.gov/joinpoint/), which tests whether an apparent change in trend is statistically significant using a Monte Carlo Permutation method. We tested a single line model (i.e., no joinpoints), and then assessed if more joinpoints should be added to the model based on their statistical significance. We allowed a maximum of two joinpoints with a minimum of two observations per segment.10 The best joinpoint model was identified using log-transformed data. We obtained the annual percentage change (APC) in incidence rates over a single linear segment and the average annual percentage change (AAPC) over the entire study period for each joinpoint model. The 95% CIs were calculated using a normal approximation.11 The joinpoint model determines whether age-standardized incidence is best explained by single segment or multiple linear segments when a significant difference in the linear slope of the temporal trend developed. When the slope of the trend (APC or AAPC) was statistically significant, the trend was considered increasing (slope >0) or decreasing (slope <0). A parallelism test was used to examine whether the slopes of the change in trend between groups were similar (or not) in direction. The parallelism test provides an analysis for testing if the fitted models between groups (e.g., between females and males) have the same shape, but are shifted along the X-axis (i.e., year of diagnosis). A statistically significant p-value on this test indicates that the two trends in terms of AAPCs compared were statistically significantly different from each other.12 All tests were two-sided with a statistical significance level of α=0.05.
Finally, we used age-period-cohort models to search for patterns in secular incidence trends accounting for age at GC diagnosis (age), year of GC diagnosis (period), and year of birth (cohort). Age-period-cohort models help to describe the mathematical associations among the rate of cancer and age, period (calendar year of diagnosis), and birth cohort. In addition, plots generated from these models allow visualization of trends by these factors after accounting for the competing factors (e.g., birth cohort effects controlled for age and period effects). These models were fit using the NCI’s Age-Period-Cohort web tool (https://analysistools.cancer.gov/apc/), which provided estimates of net drifts (APC in expected age-adjusted rates over time), local drifts (APC in expected age-specific rates over time), and cohort rate ratios (ratio of age-specific rates in each birth cohort relative to the reference cohort), plus enables testing of equality of observed trends.13 We used 13 5-year age groups (20-24 years through 80-84 years), and five 5-year calendar periods (1995-1999 through 2015-2019). Default reference groups were used for comparisons (i.e., calendar period, 2005-2009; and birth cohort, 1955).
RESULT
Overall Trends
Between 1992 and 2019, there were 80,143 newly diagnosed GC cases in the SEER 12 registries (25.7% CGC, 74.3% NCGC). The annual number of new GC cases increased from 2,612 in 1992 to 2,967 in 2019, an increase of 13.6% (Table 1). However, despite the increase in the frequency of cases, the incidence rate decreased over the study period. The age-adjusted rate for the entire study period was 11.35 per 100,000 (95% CI 11.27, 11.43), decreasing from 13.61 per 100,000 (95% CI 13.09, 14.15) in 1992 to 9.43 per 100,000 (95% CI 9.10, 9.78) in 2019. The AAPC in age-adjusted incidence rates of GC from 1992 to 2019 was −1.23% (95% CI −1.33, −1.14). Joinpoint regression did not identify any statistically significant inflection points (Table 2), such that there was a linear decline in GC rates between 1992 and 2019. Likewise, overall CGC (AAPC −0.52%; 95% CI −0.72, −0.31; Table 3) and NCGC (AAPC −1.48%; 95% CI −1.57, −1.38; Table 4) rates both decreased linearly from 1992 to 2019 as well, without any statistically significant inflection points.
Table 1.
Annual frequencies and age-adjusted incidence rates of gastric cancer in the SEER 12 registries between 1992 and 2019.
| Year | Incident gastric cancer |
Age-adjusted rate per 100,000 (95% CI) |
|---|---|---|
| Total | 80,143 | 11.35 (11.27-11.43) |
| 1992 | 2,612 | 13.61 (13.09-14.15) |
| 1993 | 2,646 | 13.52 (13.03-14.07 |
| 1994 | 2,655 | 13.33 (12.82-13.84) |
| 1995 | 2,607 | 12.90 (12.41-13.40) |
| 1996 | 2,662 | 12.92 (12.43-13.42) |
| 1997 | 2,664 | 12.69 (12.21-13.18) |
| 1998 | 2,691 | 12.60 (12.13-13.09) |
| 1999 | 2,788 | 12.80 (12.33-13.29) |
| 2000 | 2,751 | 12.42 (11.96-12.90) |
| 2001 | 2,644 | 11.74 (11.29-12.19) |
| 2002 | 2,759 | 12.01 (11.57-12.47) |
| 2003 | 2,750 | 11.75 (11.31-12.20) |
| 2004 | 2,843 | 11.97 (11.53-12.42) |
| 2005 | 2,758 | 11.39 (10.96-11.82) |
| 2006 | 2,844 | 11.56 (11.13-11.99) |
| 2007 | 2,838 | 11.26 (10.84-11.68) |
| 2008 | 2,797 | 10.87 (10.47-11.29) |
| 2009 | 3,012 | 11.48 (11.06-11.90) |
| 2010 | 2,926 | 10.85 (10.46-11.28) |
| 2011 | 3,013 | 11.87 (10.84-11.27) |
| 2012 | 3,095 | 10.97 (10.58-11.37) |
| 2013 | 3,080 | 10.68 (10.30-11.08) |
| 2014 | 3,083 | 10.42 (10.05-10.80) |
| 2015 | 3,043 | 10.02 (9.66-10.39) |
| 2016 | 3,243 | 10.26 (9.90-10.63) |
| 2017 | 3,164 | 10.09 (9.73-10.45) |
| 2018 | 2,987 | 9.63 (9.29-9.98) |
| 2019 | 2,967 | 9.43 (9.10-9.78) |
CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
Table 2.
Annual percent change (APC) and average annual percent change (AAPC) in gastric cancer incidence rates over time, overall, and by age group, sex, and race/ethnicity.
| Characteristics | Joinpoint segment year start |
Joinpoint segment year end |
APC (95% CI) | P-value | |
|---|---|---|---|---|---|
| Overall | 1992 | 2019 | −1.23 (−1.33, −1.14) | <0.001 | |
| Age-group | |||||
| <40 | 1992 | 2019 | 1.30 (0.87, 1.73) | <0.001 | |
| 40-44 | 1992 | 2019 | 0.80 (0.22, 1.38) | 0.009 | |
| 45-49 | 1992 | 2019 | 0.63 (0.20, 1.06) | 0.005 | |
| 50-54 | 1992 | 2019 | −0.09 (−0.51, 0.32) | 0.642 | |
| 55-59a | 1992 | 1997 | −5.84 (−9.35, −2.19) | 0.003 | |
| 1997 | 2019 | −0.35 (−0.70, 0.01) | 0.057 | ||
| 60-64 | 1992 | 2019 | −1.17 (−1.52, −0.81) | <0.001 | |
| 65-69 | 1992 | 2019 | −1.65 (−1.91, −1.38) | <0.001 | |
| 70-74 | 1992 | 2019 | −1.73 (−1.96, −1.51) | <0.001 | |
| 75-79 | 1992 | 2019 | −1.45 (−1.68, −1.22) | <0.001 | |
| 80-84 | 1992 | 2019 | −1.59 (−1.84, −1.34) | <0.001 | |
| 85+ | 1992 | 2019 | −2.37 (−2.59, −2.15) | <0.001 | |
| Sex | |||||
| Female | 1992 | 2019 | −0.79 (−0.95, −0.62) | <0.001 | |
| Male | 1992 | 2019 | −1.67 (−1.77, −1.57) | <0.001 | |
| Race/Ethnicity | |||||
| NHW | 1992 | 2019 | −1.51 (−1.64, −1.37) | <0.001 | |
| NHBb | 1992 | 2012 | −1.44 (−1.90, −0.98) | <0.001 | |
| 2012 | 2019 | −3.64 (−5.68, −1.56) | 0.002 | ||
| Hispanic | 1992 | 2019 | −1.44 (−1.62, −1.25) | <0.001 | |
| AI/AN | 1992 | 2019 | −0.43 (−1.44, 0.58) | 0.386 | |
| API | 1992 | 2019 | −3.11 (−3.32, −2.89) | <0.001 | |
Abbreviations: AI/AN, American Indian/Alaska Native; API, Asian and Pacific Islanders; CI, confidence interval; NHB, non-Hispanic Black; NHW, non-Hispanic White.
AAPC for 55-59 year age-group: −1.39 (95% CI, −2.10, −0.67) p<0.001.
AAPC for NHBs: −2.02 (95% CI, −2.62, −1.41) p<0.001.
Table 3.
Annual percent change (APC) and average annual percent change (AAPC) in cardia gastric cancer incidence rates over time, overall, and by age group, sex, and race/ethnicity.
| Characteristics | Joinpoint segment year start |
Joinpoint segment year end |
APC (95% CI) | P-value | |
|---|---|---|---|---|---|
| Overall | 1992 | 2019 | −0.52 (−0.72, −0.31) | <0.001 | |
| Age-group | |||||
| 45-49 | 1992 | 2019 | −0.63 (−1.43, 0.17) | 0.117 | |
| 50-54 | 1992 | 2019 | −0.59 (−1.26, 0.09) | 0.084 | |
| 55-59 | 1992 | 2019 | −1.31 (−1.88, −0.75) | <0.001 | |
| 60-64 | 1992 | 2019 | −0.63 (−1.24, −0.02) | 0.044 | |
| 65-69 | 1992 | 2019 | −0.99 (−1.47, −0.51) | <0.001 | |
| 70-74 | 1992 | 2019 | −0.50 (−0.97, −0.03) | 0.037 | |
| 75-79 | 1992 | 2019 | −0.40 (−0.91, 0.10) | 0.111 | |
| 80-84 | 1992 | 2019 | 0.02 (−0.60, 0.63) | 0.958 | |
| 85+ | 1992 | 2019 | −0.44 (−1.09, 0.20) | 0.169 | |
| Sex | |||||
| Female | 1992 | 2019 | 0.03 (−0.35, 0.40) | 0.883 | |
| Male | 1992 | 2019 | −0.83 (−1.06, −0.59) | <0.001 | |
| Race/Ethnicity | |||||
| NHW | 1992 | 2019 | −0.17 (−0.40,0.05) | 0.127 | |
| Hispanic | 1992 | 2019 | −0.72 (−1.38, −0.06) | 0.035 | |
| API | 1992 | 2019 | −1.44 (−1.90, −0.98) | <0.001 | |
Abbreviations: API, Asian and Pacific Islanders; CI, confidence interval; NHW, non-Hispanic White.
Note: <40 year age-group, NHBs, and AI/AN were excluded due to small numbers of cases.
Table 4.
Annual percent change (APC) and average annual percent change (AAPC) in non-cardia gastric cancer incidence rates over time, overall, and by age group, sex, and race/ethnicity.
| Characteristics | Joinpoint segment year start |
Joinpoint segment year end |
APC (95% CI) | P-value | |
|---|---|---|---|---|---|
| Overall | 1992 | 2019 | −1.48 (−1.57, −1.38) | <0.001 | |
| Age-group | |||||
| <40 | 1992 | 2019 | 1.25 (0.78,1.72) | <0.001 | |
| 40-44 | 1992 | 2019 | 0.88 (0.30, 1.47) | 0.004 | |
| 45-49a | 1992 | 2007 | 2.35 (1.20, 3.52) | <0.001 | |
| 2007 | 2019 | −0.56 (1.92, 0.83) | 0.411 | ||
| 50-54 | 1992 | 2019 | 0.09 (−0.35, 0.53) | 0.688 | |
| 55-59b | 1992 | 1997 | −6.73 (−11.3, −1.9) | 0.009 | |
| 1997 | 2019 | −0.04 (−0.51, 0.43) | 0.869 | ||
| 60-64c | 1992 | 2008 | −2.32 (−3.29, −1.35) | <0.001 | |
| 2008 | 2019 | −0.00 (−1.48, 1.49) | 0.995 | ||
| 65-69d | 1992 | 2004 | −0.70 (−1.91, 0.53) | 0.252 | |
| 2004 | 2019 | −2.80 (−3.61, −1.97) | <0.001 | ||
| 70-74 | 1992 | 2019 | −2.20 (−2.46, −1.93) | <0.001 | |
| 75-79 | 1992 | 2019 | −1.80 (−2.09, −1.52) | <0.001 | |
| 80-84 | 1992 | 2019 | −2.03 (−2.26, −1.80) | <0.001 | |
| 85+ | 1992 | 2019 | −2.76 (−3.01, −2.51) | <0.001 | |
| Sex | |||||
| Female | 1992 | 2019 | −0.93 (−1.09, −0.77) | <0.001 | |
| Male | 1992 | 2019 | −2.06 (−2.15, −1.97) | <0.001 | |
| Race/Ethnicity | |||||
| NHW | 1992 | 2019 | −2.34 (−2.50, −2.18) | <0.001 | |
| NHB | 1992 | 2019 | −2.01 (−2.29, −1.72) | <0.001 | |
| Hispanic | 1992 | 2019 | −1.56 (−1.75, −1.37) | <0.001 | |
| API | 1992 | 2019 | −3.32 (−3.54, −3.11) | <0.001 | |
Abbreviations: AI/AN, American Indian/Alaska Native; API, Asian and Pacific Islanders; CI, confidence interval; NHB, non-Hispanic Black; NHW, non-Hispanic White.
AAPC for 45-49 year age-group: 1.05 (95% CI, 0.21, 1.89) p=0.014.
AAPC for 55-59 year age-group: −1.31 (95% CI, −2.25, −0.36) p=0.007.
AAPC for 60-64 year age-group: −1.39 (95% CI, −2.17, 0.59) p=0.001.
AAPC for 65-69 year age-group: −1.87 (95% CI, −2.54, −1.20) p=<0.001.
Age Group
The highest age-specific incidence rates for GC were observed among persons aged ≥85 (60.65 per 100,000), 80-84 (57.55 per 100,000), and 75-79 (47.83 per 100,000) years. While age-specific rates decreased among all age groups aged 55 years and older, rates were stable among 50-54-year-olds, and increased among persons aged <50 years. Specifically, between 1992 and 2019, age-specific incidence rates for GC increased at a rate of 1.30% (95% CI 0.87,1.73), 0.80% (95% CI 0.22,1.38) and 0.63% (95% CI 0.20, 1.06) annually for persons aged <40, 40-44, and 45-49 years, respectively. Rates of GC decreased with increasing age above 55 years, with individuals aged ≥85 years seeing the largest decrease over the study period (AAPC −2.37%; 95% CI −2.59, −2.15). We observed a statistically significant inflection point for the 55-59 age group in 1997; rates decreased rapidly from 1992-1997 (APC −5.84%; 95% CI −9.35, −2.19) and then continued to decrease but at a lesser rate from 1997-2019 (APC −0.35%; 95% CI −0.70, 0.01). CGC incidence rates decreased between 1992 and 2019 in all age groups, except for the 80-84 age group (APC 0.02; 95% CI −0.60, 0.63) (Table 3). This contrasts with NCGC incidence rates, which reflect overall GC trends, with increasing rates for younger age groups (<50 years) and decreasing rates for older age groups (Table 4).
Sex
There were overall decreases in age-standardized incidence rates for GC in both females and males over the study period. Among females, incidence rates decreased from 9.21 per 100,000 in 1992 to 7.13 per 100,000 in 2019. Among males, incidence rates decreased from 15.6 per 100,000 in 1992 to 12.26 per 100,000 in 2019. Joinpoint regression analysis showed different negative AAPCs in females (−0.79%; 95% CI −0.95, −0.62) and males (−1.67% 95% CI −1.77, −1.57) for GC overall. A parallelism test indicated that secular trends in GC rates were statistically significantly different between males and females (p<0.001). Females compared to males also showed a smaller rate of decline in incidence rates for CGC (AAPCs: 0.03%; 95% CI −0.35, 0.40 vs. −0.83; 95% CI-1.06, −0.59%) and NCGC (AAPCs: −0.93%; 95% CI −1.09, −0.77 vs −2.06%; 95% CI −2.15, −1.97). Similar to the overall findings, we did not observe any inflection points with Joinpoint analysis for females or males in relation to GC, CGC, or NCGC.
Race/Ethnicity
Over the entire study period, AAPCs were negative (indicating decreasing rates) for NHWs, Hispanics, and APIs for GC, CGC, and NCGC. AAPCs were negative for NHBs for GC and NCGC, but the sample size was insufficient for analysis of CGC for NHBs. Rates of GC among AI/AN were stable between 1992 and 2019 (AAPC −0.43; 95% CI, −1.44, 0.58), but the sample size was insufficient for analysis of NCGC and CGC groups individually. For GC overall, Joinpoint only identified NHBs as having a statistically significant inflection point (2012); GC incidence decreased by 1.44% (95% CI −1.90, −0.98) annually from 1992-2012 but decreased by 3.64% (95% CI −5.68, −1.56) annually from 2012-2019. No other statistically significant inflection points were identified for any race/ethnicity subgroup for GC, NCGC, and CGC, decreasing at a linear rate (Table 2).
Geography
We examined incidence trends separately for SEER 12 registries with sufficient available data. Over the entire study period, except for Atlanta, GC incidence rates decreased (statistically significant and negative AAPCs) over time. Joinpoint identified one inflection point for only Connecticut (2016) and Los Angeles (2013). Connecticut had a decrease in the incidence of GC (APC −0.97; 95% CI, −1.26, −0.68) from 1992 to 2016 and an even larger decrease from 2016-2019 (APC −6.71; 95% CI, −13.33, 0.41). Los Angeles followed a similar pattern, where GC rates decreased by 0.90% annually from 1992-2013 (95% CI, −1.14, −0.65) but with a larger decrease occurring from 2013 to 2019 (APC −2.79; CI 95%, −4.32, −1.24). All other registries experienced a linear decrease in GC incidence between 1992 and 2019 (Table 5).
Table 5.
Annual percent change (APC) and average annual percent change (AAPC) in gastric cancer incidence rates over time by SEER 12 registry.
| SEER 12 registry |
Joinpoint segment year start |
Joinpoint segment year end |
APC (95% CI) | P-value |
|---|---|---|---|---|
| Connecticuta | 1992 | 2016 | −0.97 (−1.26, −0.68) | <0.001 |
| 2016 | 2019 | −6.71 (−13.3, 0.41) | 0.063 | |
| Atlanta | 1992 | 2019 | −0.02 (−0.47, 0.43) | 0.926 |
| San Francisco – Oakland | 1992 | 2019 | −1.38 (−1.61, −1.16) | <0.001 |
| San Jose – Monterey | 1992 | 2019 | −1.00 (−1.34, −0.66) | <0.001 |
| Hawaii | 1992 | 2019 | −3.49 (−3.76, −3.22) | <0.001 |
| Iowa | 1992 | 2019 | −1.23 (−1.55, −0.91) | <0.001 |
| Los Angelesb | 1992 | 2013 | −0.90 (−1.14, −0.65) | <0.001 |
| 2013 | 2019 | −2.79 (−4.32, −1.24) | 0.001 | |
| New Mexico | 1992 | 2019 | −0.96 (−1.58, −0.35) | 0.004 |
| Seattle – Puget Sound | 1992 | 2019 | −0.96 (−1.24, −0.68) | <0.001 |
| Utah | 1992 | 2019 | −1.11 (−1.59, −0.63) | <0.001 |
Note: Alaska and Rural Georgia were excluded from Registry stratified analyses due to too suppressed cells for years except 2017 and 2019 for Alaska and 2018 for Rural Georgia.
AAPC for Connecticut registry: −1.63 (95% CI, −2.42, −0.82) p<0.001.
AAPC for Los Angeles registry: −1.32 (95% CI, −1.70, −0.95) p<0.001.
Age-Period-Cohort Models
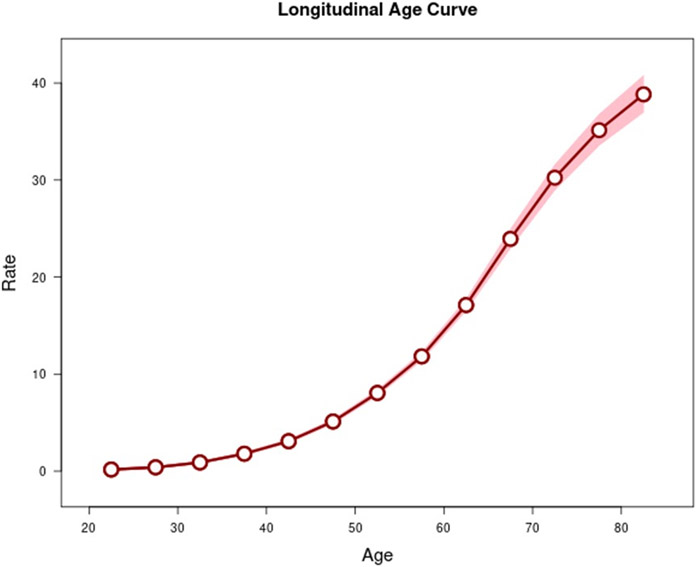
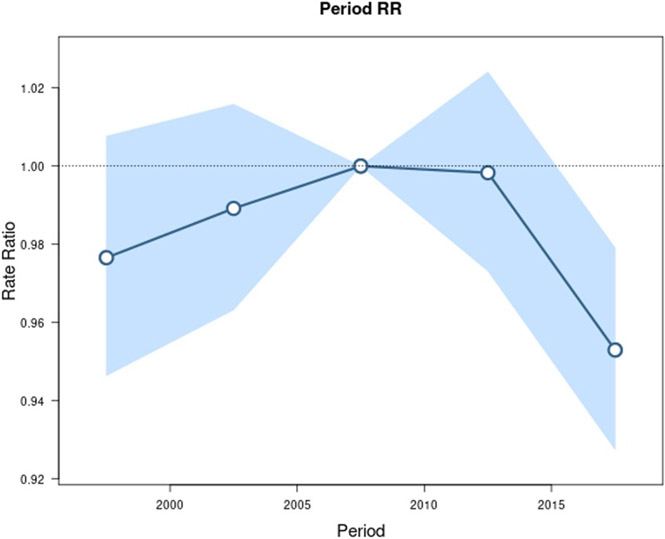
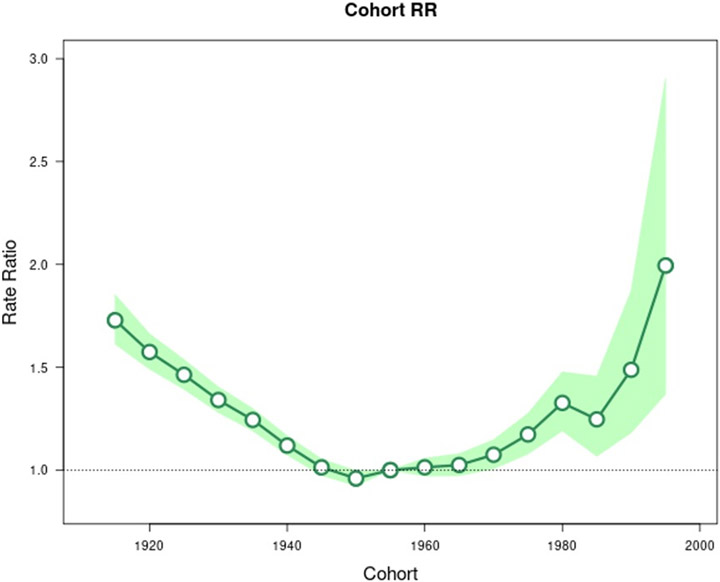
Age-period-cohort analysis was conducted for GC, CGC, and NCGC. The longitudinal age curve, seen in Figure 1, highlights that the highest rates of GC occur in older age groups. Similar age effects were seen for CGC and NCGC (Supplemental Figures 1-2). Figures 2 and 3 show the estimated period rate ratio (RR) and cohort RR, respectively. Compared to the reference period (2005 to 2010), the period RRs were similar, never exceeding a 7% increase or decrease for GC. When examined separately, we found no period effect for CGC (Supplemental Figure 3) but a strong period effect for NCGC (Supplemental Figure 4). There was a strong birth cohort effect for GC. Compared to persons born circa 1955 for GC, persons born from 1945 to 1970 displayed similar cohort RRs. Overall, the graph for GC cohort RRs is U shaped, with higher RRs for cohorts further distanced from the reference cohort. The 1915 cohort had a RR of 1.73 (95% CI 1.61, 1.85). Similarly, the 1995 birth cohort had a RR of 1.99 (95% CI 1.37, 2.90). Like the period effect, we observed differences in the birth cohort effects for CGC and NCGC. The cohort RRs for NCGC were similar to the cohort RRs for GC, whereby NCGC rates decreased in successive birth cohorts through 1955, were stable for multiple birth cohorts, and then increased (Supplemental Figures 5-6).
Figure 1.
Longitudinal age curves of gastric cancer in SEER 12 from 1992 to 2019 and corresponding 95% confidence intervals.
Figure 2.
Incidence rate ratios by period (reference cohort 2005-2009) for gastric cancer incidence in SEER 12. Shaded bands indicate the 95% confidence interval.
Figure 3.
Incidence rate ratios by birth cohort (reference = cohort 1955) for gastric cancer incidence in SEER 12 database. Shaded bands indicate the 95% confidence interval.
DISCUSSION
In our population-based study, we found that overall GC incidence rates in the U.S. have been steadily decreasing from 1992 through 2019 at a rate of 1.23% annually. This decrease was predominantly driven by decreases in NCGC which decreased by 1.48% annually, versus CGC which decreased 0.52% annually across this time frame. The decline in GC rates was observed in females and males, and across racial and ethnic groups. Males, NHBs and APIs had especially high rates of decline in GC incidence. The rate of decline was higher with increasing age. However, we also observed an increase in the rates of GC most prominent in the <40 age group, but also present for the 40-44 and 45-49 age groups to a lesser degree. Our previous paper had indicated that this increase in persons <50 is driven by increases in NHWs and Hispanics, with females increasing more than males.2 This is a concerning finding, given that GC is predominately thought of as a cancer affecting older males.
Of note, there were significant differences in the rate of decline from differing geographical regions. While GC rates declined in all other regions, GC rates remained stable in Atlanta between 1992 and 2019. This is of interest given Atlanta’s high NHB population and that the overall decline in GC for NHBs during our study period was 2.01% annually. Similar trends were noted for New Mexico (−0.96% annually) and San Jose (−1.00% annually) regions, regions with large Hispanic populations, compared to Hispanics overall (1.44% annually) and with Seattle-Puget Sound (−0.96% annually), a region with a large API population, compared to APIs overall (−3.11% annually). These stark differences highlight a geographical impact on GC rates, independent of race and ethnicity, that should continue to be investigated.
Our data build upon prior publications describing the decline in overall GC rates in the U.S. In a previous paper by our group analyzing rates of GC from 1999 to 2013, a statistically significant inflection point was noted in 2007.3 We did not observe this with newer data through 2019, instead observing a linear decline during 1992-2019. This is likely due to additional data points, providing a clearer picture of the GC trend in the U.S. Our study is in line with the findings of Lin et al. that predicted decreasing overall rates of GC through 2030.1 However, while rates are decreasing among persons aged >50 years, previous papers have characterized an increase in incidence of GC, predominately NCGC, among persons aged <50 in the U.S. We noted similar findings in our current study, with the 45-49 age group. These findings emphasize the need for further preventative measures in younger age groups.
While we were unable to examine specifically the cause of the decline in GC rates, we posit that it likely reflects the decline in use of tobacco products over the last two decades as well as an improvement in diet secondary to a decrease in the consumption of preserved foods. The increase in the incidence of NCGC individuals <50 is potentially due to the changing population demographics of the U.S. with growth of NHWs, increased healthcare barriers preventing testing and eradication of H. pylori infections, or additional non-H. pylori related causes.14 In addition, our age-period-cohort analysis highlighted a definite cohort effect for GC, which points to differences in exposure prevalence in successive birth cohorts contributing to secular trends in GC rates. However, as hypothesized, secular trends in NCGC rates are driven mostly by a birth cohort effect because it is H. pylori related. For the most recent birth cohorts (individuals born circa 1985 and onwards), the increase across successive birth cohorts was especially striking. However, it is worth noting that the risk estimates for these cohorts were less precise (i.e., had wider confidence intervals) due to smaller numbers of cancers. Conversely, CGC does not have a strong birth cohort effect, and secular trends are mostly driven by period and age effects.
The strengths of our study include that the data were population-based and that there is a very low risk of information or recall bias, as data was collected prospectively and independently of our study. A limitation of our study is that it was based on the SEER 12 cancer registry data and that no information on individual GC risk factors was available. As such, our study is unable to provide any direct evidence about the role of specific exposures or interventions effects we noted for the GC incidence trends.
In conclusion, we found that overall GC incidence rates in the U.S. are continuing to decline. The magnitude of decline has been similar across all years studied. The decreasing trend was observed almost uniformly across subgroups of sex, race/ethnicity, and geography, although the magnitude of the decline in rates varied and there were divergent trends in persons aged <50 (increasing rates) vs. ≥50 (decreasing rates). The decreasing trend was also observed when separating GC into NCGC and CGC subtypes. We continue to monitor trends of GC with a particularly close eye on the increasing rates in persons aged <50 years.
Supplementary Material
Funding:
This work was funded (in part) by a Research Training Award from the Cancer Prevention & Research Institute of Texas (CPRIT) for the Systems Epidemiology of Cancer Training (SECT) Program (RP210037; PI: A. Thrift).
Footnotes
Competing Financial Interests: The authors declare no potential conflicts of interest.
References
- 1.Lin Y, Zheng Y, Wang HL, Wu J. Global Patterns and Trends in Gastric Cancer Incidence Rates (1988-2012) and Predictions to 2030. Gastroenterology. 2021. Jul;161(1):116–127.e8. doi: 10.1053/j.gastro.2021.03.023. Epub 2021 Mar 18. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, El-Serag HB, Thrift AP. Increasing Incidence of Advanced Non-cardia Gastric Cancers Among Younger Hispanics in the USA. Dig Dis Sci. 2021. May;66(5):1669–1672. doi: 10.1007/s10620-020-06397-x. Epub 2020 Jun 16. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Graham DY, Khan A, Balakrishnan M, Abrams HR, El-Serag HB, Thrift AP. Incidence of gastric cancer in the USA during 1999 to 2013: a 50-state analysis. Int J Epidemiol. 2018. Jun 1;47(3):966–975. doi: 10.1093/ije/dyy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karimi Parisa et al. “Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention.” Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology vol. 23,5 (2014): 700–13. doi: 10.1158/1055-9965.EPI-13-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SEER. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 12 Registries, Nov 2021 Sub (1992-2019) - Linked To County Attributes - Time Dependent (1990-2019) Income/Rurality, 1969-2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission. 2021. [Google Scholar]
- 6.SEER. Registry Groupings in SEER Data and Statistics. Available from https://seer.cancer.gov/registries/terms.html 2022.
- 7.Surveillance Research Program-National cancer Institute [internet]. SEER*Stat Software Version 8.4.0. 2022. April 15. [accessed 2022 April 15]. Available from https://seer.cancer.gov/seerstat,2022.
- 8.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547–69. [DOI] [PubMed] [Google Scholar]
- 9.SEER. Race and Hispanic Ethnicity Changes. Available from https://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/.2022.
- 10.Yu B, Barrett MJ, Kim H-J, et al. Estimating joinpoints in continuous time scale for multiple change-point models. Computational Statistics & Data Analysis 2007;51:2420–2427. [Google Scholar]
- 11.Walters KA, Li Y, Tiwari RC, et al. A Weighted-Least-Squares Estimation Approach to Comparing Trends in Age-Adjusted Cancer Rates Across Overlapping Regions. J Data Sci 2011;8:631–644. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HJ, Fay MP, Yu B, et al. Comparability of segmented line regression models. Biometrics 2004;60:1005–14. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014. Nov;23(11):2296–302. doi: 10.1158/1055-9965.EPI-14-0300. Epub 2014 Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dis. 2020;38(4):280–285. doi: 10.1159/000506509. Epub 2020 Feb 17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.