Abstract
Aims
Variants of the junctional cadherin 5 associated (JCAD) locus associate with acute coronary syndromes. JCAD promotes experimental atherosclerosis through the large tumor suppressor kinase 2 (LATS2)/Hippo pathway. This study investigates the role of JCAD in arterial thrombosis.
Methods and results
JCAD knockout (Jcad−/−) mice underwent photochemically induced endothelial injury to trigger arterial thrombosis. Primary human aortic endothelial cells (HAECs) treated with JCAD small interfering RNA (siJCAD), LATS2 small interfering RNA (siLATS2) or control siRNA (siSCR) were employed for in vitro assays. Plasma JCAD was measured in patients with chronic coronary syndrome or ST-elevation myocardial infarction (STEMI). Jcad−/− mice displayed reduced thrombogenicity as reflected by delayed time to carotid occlusion. Mechanisms include reduced activation of the coagulation cascade [reduced tissue factor (TF) expression and activity] and increased fibrinolysis [higher thrombus embolization episodes and D-dimer levels, reduced vascular plasminogen activator inhibitor (PAI)-1 expression]. In vitro, JCAD silencing inhibited TF and PAI-1 expression in HAECs. JCAD-silenced HAECs (siJCAD) displayed increased levels of LATS2 kinase. Yet, double JCAD and LATS2 silencing did not restore the control phenotype. si-JCAD HAECs showed increased levels of phosphoinositide 3-kinases (PI3K)/ proteinkinase B (Akt) activation, known to downregulate procoagulant expression. The PI3K/Akt pathway inhibitor—wortmannin—prevented the effect of JCAD silencing on TF and PAI-1, indicating a causative role. Also, co-immunoprecipitation unveiled a direct interaction between JCAD and Akt. Confirming in vitro findings, PI3K/Akt and P-yes-associated protein levels were higher in Jcad−/− animals. Lastly, as compared with chronic coronary syndrome, STEMI patients showed higher plasma JCAD, which notably correlated positively with both TF and PAI-1 levels.
Conclusions
JCAD promotes arterial thrombosis by modulating coagulation and fibrinolysis. Herein, reported translational data suggest JCAD as a potential therapeutic target for atherothrombosis.
Keywords: Arterial thrombosis, JCAD, KIAA1462, Cardiovascular disease, PAI-1, Tissue factor
Structured Graphical Abstract
Structured Graphical Abstract.
JCAD promotes arterial thrombosis through Akt modulation. PAI-1, plasminogen activator inhibitor-1; TF, tissue factor; YAP, yes-associated protein 1. Created with BioRender.com.
This paper was handled by Guest Editor Prof. Tomasz Guzik (University of Glasgow, United Kingdom)See the editorial comment for this article ‘JCAD: a new GWAS target to reduce residual cardiovascular risk?’, by T. J. Guzik and K. M. Channon, https://doi.org/10.1093/eurheartj/ehac708.
Translational perspective.
This study illustrates a novel role for JCAD in arterial thrombosis by regulating the coagulation cascade and fibrinolytic activity through tissue factor and plasminogen activator inhibitor-1 modulation. Our results shed new light on the role of JCAD in arterial thrombus formation, a dreadful but common complication of atherosclerotic cardiovascular disease. Indeed, the present work suggests a potential therapeutic role of JCAD inhibition to impinge on arterial thrombogenicity. However, as therapeutic JCAD inhibition may cause untoward effects, its physiological function beyond the cardiovascular system warrants further study. The development of pharmacological JCAD inhibitors will be of utmost importance to set the stage for preclinical studies to assess the efficacy and safety of JCAD blockage in established models of atherosclerotic cardiovascular disease.
Permissions information.
The authors do hereby declare that all illustrations and figures in the manuscript are original and not require reprint permission.
Introduction
Arterial thrombosis is the key event underlying most acute cardio- and cerebrovascular events.1 Endothelial erosion or plaque rupture leads to the exposure of collagenous plaque components as well as vascular tissue factor (TF), activating the coagulation cascade and triggering platelet aggregation. Fibrin deposition then stabilizes the arterial clot, leading to vascular occlusion and ischaemic damage of downstream parenchyma.2 Spontaneous plasmin-mediated fibrinolysis may limit the formation of an occlusive thrombus and restore blood flow. Unveiling hitherto unknown molecular mechanisms of arterial thrombosis may result in the identification of novel molecular targets for the prevention and management of myocardial infarction and ischaemic stroke, which still account for the majority of deaths worldwide.3
Genome-wide association studies (GWAS) have consistently linked genetic variants at the JCAD [junctional cadherin 5 associated, also known as KIAA1462 or junctional protein associated with coronary artery disease (CAD)] locus to an increased risk of CAD and incident acute coronary syndrome (ACS).4,5 Xu et al.6 recently investigated the direction of such an association showing that the lead risk variants increase the expression of this protein. Previous studies showed that JCAD co-localizes with cell–cell junctions and is mainly expressed by endothelial cells, where it regulates angiogenesis under pathological circumstances, rather than developmental ones.7 Experimental models explored such associations and reported an important role for JCAD in atherogenesis by facilitating interactions between the endothelium and inflammatory cells at the site of shear-stress, thereby accelerating plaque growth.6,8 Mechanistically, JCAD signals within endothelial cells by activating the Hippo pathway through large tumor suppressor kinase 2 (LATS2), thereby triggering the expression of several pro-atherogenic and pro-inflammatory genes.6,7 GWAS further indicate an association of JCAD with acute thrombotic events (i.e. myocardial infarction) for which an experimental explanation is still missing.
The present study aimed to investigate the role of JCAD in arterial thrombosis. To this end, we assessed the effect of genetic JCAD deletion in an in vivo murine model of arterial thrombosis based on an endothelial-specific injury.9 Next, to increase the translational relevance of this work and gain mechanistic insight, we confirmed the animal findings in JCAD-depleted primary human aortic endothelial cells (HAECs). Finally, we assessed circulating levels of JCAD in patients with ACS and chronic coronary syndrome (CCS).
Methods
Detailed methods are provided in the Supplementary material online, Appendix. The study complies with the Declaration of Helsinki. All procedures were approved by the Cantonal Veterinary Authority of Zurich, Switzerland. Animal experiments were performed conforming to Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. The data underlying this article will be shared on reasonable request with the corresponding author.
Animals
3- to 5-month-old JCAD knockout (Jcad−/−) mice together with corresponding wild-type (WT, Jcad+/+) littermates were used for experiments. Jcad−/− mice were generated, and their genotype was confirmed by routine genomic polymerase chain reaction (PCR) as described previously.6
In vivo carotid artery thrombosis model
Jcad−/− and Jcad+/+ mice underwent photochemical injury of the common carotid artery to induce arterial thrombosis as previously described.10–12
Treatment with wortmannin
A separate set of mice received wortmannin (0.5 mg/kg body weight) 24 h and 1.5 h before undergoing photochemical-induced carotid artery thrombosis.13,14 A vehicle was used as control.
Echocardiographic measurements
A Vevo 3100 system (VisualSonics, Toronto, Canada) equipped with an MS550D 40 MHz linear array transducer was used for vascular and cardiac measurements in animals at baseline.
Platelet aggregation and reactivity in mice
Platelet aggregation in response to collagen and thrombin was assessed by light transmission aggregometry, as previously detailed.15 Platelet reactivity towards collagen and adenosine diphosphate (ADP) was also assessed via fluorescence-activated cell sorting (FACS) analysis of surface protein expression.
Determination of tissue factor activity and expression in arterial samples
As previously described, TF activity was determined in carotid artery lysate from uninjured vessels as previously described by the colorimetric ACTICHROME ® TF assay according to the manufacturer’s recommendation (American Diagnostica, Stamford, CT, USA).9,16 TF expression was instead measured in arterial lysates by ELISA (R&D system).
Cell culture experiments
Primary HAECs transfected with JCAD small interfering RNA (siJCAD), LATS2 siRNA (siLATS2), scrambled siRNA (siSCR), or a combination of them were employed for in vitro experiments.17,18 The proteinkinase B (Akt) inhibitor wortmannin was added to the culturing medium in a separate set of experiments. Culturing and transfection protocols are detailed in the Supplementary material online, Appendix.
Western blotting
Murine uninjured arteries and HAECs were lysed in lysis buffer and protein separated on sodium dodecyl sulphate -polyacrylamide gel electrophoresis as previously described.19
Quantitative real-time polymerase chain reaction
Quantification of JCAD, F3, and plasminogen activator inhibitor (PAI)-1 mRNA levels was done by real-time PCR.
Study population
The study cohort consisted of two groups of patients: (i) 39 consecutive patients admitted to the coronary care unit of Policlinico Universitario ‘A. Gemelli’ within 6 h after the onset of chest pain, presenting with ST-elevation myocardial infarction (STEMI) defined according to current guidelines and confirmed at coronary angiography; (ii) 22 CCS patients admitted to the cardiology ward with symptoms of stable effort angina lasting more than 12 months, angiographically confirmed CAD and no overt ischaemic episodes during the previous 48 h.
Plasma sampling
Murine D-dimer and human JCAD, TF, and PAI-1 were assessed in EDTA plasma samples by ELISA following the manufacturers’ instructions.20
Statistical analysis
Statistical analysis was performed as detailed in the Supplementary material online, Appendix.
Results
Loss of JCAD delays time to arterial thrombotic occlusion and blunts thrombus firmness in vivo.
To investigate the potential role of JCAD in arterial thrombosis, time to carotid artery occlusion was analysed in Jcad−/− mice and control littermates following photochemical-induced carotid endothelial injury. Time to stable thrombotic occlusion was significantly delayed in Jcad−/− male animals as compared with Jcad+/+ littermates by around 40% (P < 0.05, Figure 1A). The irregular blood flow dynamic observed during the thrombosis experiments reflects the dissolving action of the fibrinolytic system on the forming thrombus (Figure 1B). Thrombus embolization and blood flow restoration above 0.1 mL/min occurred more often in Jcad−/− male mice than in controls (P < 0.05, Figure 1C, Supplementary material online, Figure S1), suggesting blunted thrombus firmness. Importantly, no significant differences in initial blood flow, heart rate and body weight were observed between the two groups (Figure 1D–F). Similar findings were reported in female animals, specifically an increased time to thrombotic occlusion was observed in female Jcad−/− mice as compared with littermates (P < 0.05, Supplementary material online, Figure S1) as well as a tendency towards more episodes of thrombus embolization (see Supplementary material online, Figure S1). No significant differences were found between the experimental groups in terms of carotid arterial stiffness and heart function at the time of the experiment as assessed by echocardiography (Table 1).
Figure 1.
Effects of JCAD deletion on carotid arterial thrombosis in vivo in male mice. (A) Jcad−/− mice showed delayed time to formation of an occlusive thrombus in their carotid arteries after endothelial-specific damage as compared with Jcad+/+ littermates. (B) Representative trace of mean blood flow until occlusion (mean flow ≤ 0.1 mL for 1 min) in the two study groups. (C) JCAD deletion increased the number of episodes of thrombus embolization. (D–F) No difference in initial heart rate, blood flow and weight was reported among Jcad−/− and wild-type littermates. n = 7 different mice per group. A, C—F: unpaired two-tailed Student’s t-test. *P < 0.05.
Table 1.
Ultrasound characterization of vascular and cardiac phenotype
| Jcad+/+ (n = 7) | Jcad−/− (n = 7) | P | |
|---|---|---|---|
| Pulse wave velocity (m/s) | 3.8 ± 0.3 | 3.2 ± 0.5 | NS |
| Left ventricle volume; systole (µL) | 21.9 ± 4.9 | 23.2 ± 2.5 | NS |
| Left ventricle volume; diastole (µL) | 61.9 ± 5.4 | 62.0 ± 4.4 | NS |
| Left ventricle diameter; systole (mm) | 2.4 ± 0.2 | 2.5 ± 0.1 | NS |
| Left ventricle diameter; diastole (mm) | 3.7 ± 0.1 | 3.8 ± 0.1 | NS |
| Left ventricle mass (mg) | 131 ± 10 | 127 ± 8 | NS |
| Left ventricle anterior wall; systole (mm) | 1.4 ± 0.1 | 1.3 ± 0.1 | NS |
| Left ventricle anterior wall; diastole (mm) | 0.9 ± 0.1 | 0.8 ± 0.1 | NS |
| Left ventricle posterior wall; systole (mm) | 1.4 ± 0.1 | 1.4 ± 0.1 | NS |
| Left ventricle posterior wall; diastole (mm) | 0.9 ± 0.1 | 1.0 ± 0.1 | NS |
| Ejection fraction (%) | 67 ± 4 | 63 ± 2 | NS |
| Fractional shortening (%) | 37 ± 3 | 34 ± 1 | NS |
| Stroke volume (µL) | 40.0 ± 1.1 | 38.9 ± 2.2 | NS |
| Cardiac output (mL/min) | 18.9 ± 0.4 | 18.6 ± 0.9 | NS |
| Isovolumetric contraction time (ms) | 14.8 ± 1.4 | 19.9 ± 2.4 | NS |
| Isovolumetric relaxation time (ms) | 17.9 ± 0.8 | 18.7 ± 2.3 | NS |
| Mitral valve deceleration time (ms) | 16.6 ± 2.6 | 17.0 ± 3.1 | NS |
| E/A ratio | 1.3 ± 0.1 | 1.3 ± 0.1 | NS |
| E/E’ ratio | 23.2 ± 2.3 | 22.3 ± 3.4 | NS |
JCAD deficiency does not affect platelet count, volume, and function
Given the fundamental role of platelets in arterial thrombosis, we initially investigated the effect of JCAD deletion on their count, morphology and function. Complete blood count analysis revealed that platelet numbers and volumes were unchanged in Jcad−/− and Jcad+/+ mice (Figure 2A and B, respectively). Similarly, red blood cell and white blood cell counts were not affected by JCAD deficiency in animals at the experimental age (see Supplementary material online, Table S1). Collagen, thrombin and ADP trigger platelet aggregation during atherothrombosis. The reactivity of washed platelets to such mediators was then assessed by ex vivo light transmission aggregometry and FACS analysis. Loss of JCAD did not affect collagen-induced platelet reactivity as demonstrated by similar maximal aggregation, rate (slope) of aggregation and lag phase in Jcad−/− mice and control littermates (Figure 2C–F). Similar results were obtained after stimulation with thrombin, where again Jcad−/− platelets did not show any overt phenotype when compared with WT cells (Figure 2G–J). Furthermore, we assessed by FACS the expression of the platelet activation marker P-selectin (CD62P) and the activated glycoprotein IIb/IIIa (JON/A) receptor at resting state and after ex vivo ADP- and collagen-mediated activation. In accordance with our aggregometry data, Jcad+/+ and Jcad−/− showed similar P-selectin and JON/A expression levels at baseline. As expected, both groups increased the expression of activation markers after stimulation with ADP and collagen. Nevertheless, we did not detect any differences between Jcad+/+ and Jcad−/− platelets upon activation (Figure 2K–N).
Figure 2.
Impact of JCAD deletion on platelet count, volume and ex vivo activation and aggregation. (A and B) Jcad−/− animals did not show any difference in terms of platelet count or mean platelet volume as compared with Jcad+/+ animals. (C–E) Ex vivo collagen-induced platelet aggregation as assessed by light transmission aggregometry did not show any difference in terms of maximal aggregation, rate (slope) of aggregation and lag phase in Jcad−/− animals and WT littermates. (G–J) Ex vivo thrombin-induced platelet aggregation as assessed by light transmission aggregometry did not show any difference in terms of maximal aggregation, rate (slope) of aggregation and lag phase in Jcad−/− animals and WT littermates. (K—N) FACS analysis of platelet reactivity markers after ex vivo ADP (10 µM, 20 µM) and collagen (20 µg/mL) activation in Jcad+/+ and Jcad−/− mice. Mean fluorescence intensity of CD62P was similar between groups at baseline (K and L), after ADP (K) and collagen (L) stimulation. Mean fluorescence intensity of JON/A was similar between groups at baseline (M and N), after ADP (M) and collagen (N) stimulation. n = 5 different mice per group. A-B, D–F, H–J: unpaired two-tailed Student’s t-test. K–N: paired two-tailed Student’s t-test. *P < 0.05, **P < 0.01.
JCAD deletion associates with reduced activation of the coagulation cascade and increased fibrinolysis
After endothelial damage, TF triggers the activation of the coagulation cascade, eventually leading to thrombin and fibrin formation to stabilize platelet clots (Figure 3A). Consistent with a reduced thrombogenicity, carotid artery lysates from Jcad−/− mice showed reduced TF activity (i.e. reduced formation of factor X activated at the functional assay) as compared with those from control littermates (P < 0.05, Figure 3B). Accordingly, ELISA quantification of TF in the artery detected reduced levels of the procoagulant by 20% in Jcad−/− samples as compared with Jcad+/+ (P < 0.05, Figure 3C). On the contrary, levels of thrombomodulin did not change between the study groups (see Supplementary material online, Figure S2A).
Figure 3.
Effects of JCAD deletion on extrinsic coagulation pathway and fibrinolytic system. (A) Schematic of extrinsic coagulation pathway activation (B) JCAD deletion reduced carotid artery TF activity. (C) Accordingly, arterial levels of TF were lower in Jcad−/− mice as compared with control littermates. (D) Schematic of fibrinolytic cascade (E) Plasma D-dimer concentration was significantly increased in Jcad−/− animals as compared with controls (F) Jcad−/− animals exhibited reduced arterial levels of PAI-1. (G) Phosphorylation levels of YAP were higher in Jcad−/− animals than in Jcad+/+ controls. n = 7 different mice per group. B-C, E-G: unpaired two-tailed Student’s t-test. *P < 0.05 **P < 0.01. GAPDH = glyceraldehyde 3-phosphate dehydrogenase, PAI-1 = plasminogen activator inhibitor-1; TF = tissue factor; YAP = yes-associated protein 1.
Together with coagulation, fibrinolysis gets activated to digest fibrin into fibrin degradation products (such as D-dimer) and dissolve the clot. Tissue- or urokinase-type plasminogen activators (tPA and uPA) are released to activate plasmin—the major fibrinolysin—but gets rapidly inhibited by PAI-1 (Figure 3D). Alongside reduced thrombus firmness, Jcad−/− mice also displayed increased fibrinolysis as shown by higher plasma levels of D-dimer (P < 0.01, Figure 3E). Accordingly, Jcad−/− animals displayed reduced levels of the anti-fibrinolytic PAI-1 as compared with WT littermates (P < 0.05, Figure 3F), while no differences were detected in terms of tPA and uPA (see Supplementary material online, Figure S2B and C).
In keeping with its known role as an activator of the Hippo pathway, animals lacking JCAD expression showed increased yes-associated protein (YAP) phosphorylation, suggesting its cytoplasmic segregation (P < 0.05, Figure 3G).
Silencing of JCAD reduces TF and PAI-1 expression in stimulated primary human aortic endothelial cells
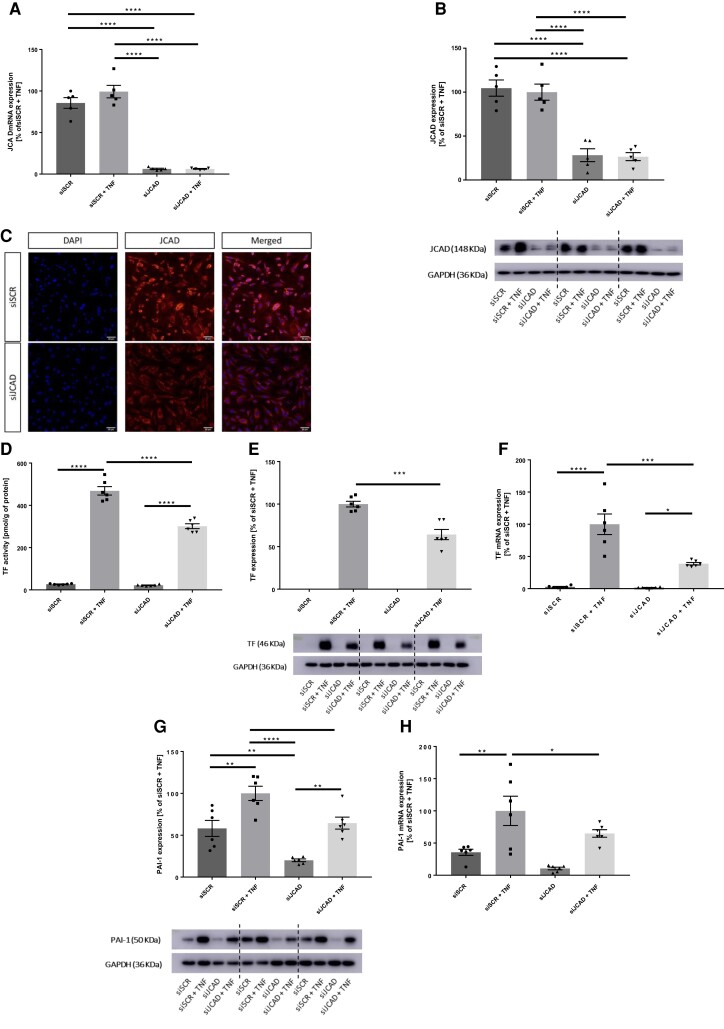
To test the translational relevance of our in vivo findings, TF and PAI-1 expression were assessed in JCAD-silenced primary HAECs under basal conditions and after stimulation with tumour necrosis factor (TNF)-α. In HAECs, lipofectamine-mediated small interfeiring RNA (siRNA) transfection induced JCAD gene silencing in cells receiving JCAD siRNA (siJCAD) as compared with those receiving the scrambled control (siSCR) (P < 0.0001, Figure 4A). Western blotting (WB) and immunocytochemistry confirmed JCAD reduction upon silencing also at the protein level (P < 0.0001 for all, Figure 4B and C, respectively).
Figure 4.
Effects of JCAD silencing in primary HACEs. (A) In HAECs, JCAD mRNA levels were significantly reduced after transfection with JCAD small interfering RNA (siJCAD) as compared with control siRNA (siSCR). (B-C) Western blotting and Immunostaining of HAECs confirmed JCAD silencing at the protein level. (D) TF activity is induced in siSCR HAECs by treatment with 10 ng/mL TNF-α, this effect is reduced upon JCAD silencing. (E) Accordingly, TF protein levels are increased in stimulated siSCR-treated cells with higher protein levels than stimulated JCAD-silenced ones. (F) TF mRNA levels follow a similar trend (G) Similarly, also TNF-α-related induction of PAI-1 is reduced in siJCAD-treated cells irrespective of TNF-α stimulation (H) PAI-1 mRNA are higher in stimulated siSCR-treated cells as compared with stimulated siJCAD ones. n = 5–6 independent experiments. A, D-H: one-way analysis of variance (ANOVA) with Tukey post hoc test (number of comparisons = 6). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HAEC = human aortic endothelial cell; PAI-1 = plasminogen activator inhibitor-1; TF = tissue factor; TNF-α = tumour necrosis factor-α.
As expected, the activity of TF increased in human aortic endothelial cell (HAEC) lysates upon TNF-α stimulation both in cells receiving siSCR and in those silenced for JCAD (P < 0.0001 for both, Figure 4D). Yet, TNF-α-induced TF activity remained significantly lower in siJCAD HAECs as compared with control ones (P < 0.0001, Figure 4D). Accordingly, WB analysis detected lower levels of TF protein in stimulated siJCAD cells as compared with siSCR (P < 0.001, Figure 4E). Indicating a transcriptional modulation, levels of TF mRNA remained lower in siJCAD cells (P < 0.0001, Figure 4F) despite their significant induction upon TNF-α stimulation (P < 0.05 for siJCAD cells and P < 0.0001 for siSCR, Figure 4F).
TNF-α increased the expression of PAI-1 both in siSCR and siJCAD cells (P < 0.01 for both, Figure 4G). Yet, when compared with siSCR HAECs, JCAD-silenced cells expressed lower levels of the procoagulant both at baseline and after stimulation (P < 0.01 and P < 0.05, respectively). Again, quantitative PCR analysis of cell lysates detected lower levels of PAI-1 mRNA in siJCAD endothelial cells as compared with those treated with siSCR (P < 0.05, Figure 4H).
LATS2 kinase does not mediate the effect of JCAD on TF and PAI-1 levels
LATS2 kinase was previously shown to mediate the effect of JCAD silencing on endothelial cells. Specifically, JCAD inhibits LATS2, thereby reducing YAP phosphorylation and allowing its nuclear translocation with a net positive effect on the Hippo pathway. Accordingly, LATS2 silencing of endothelial cells lacking JCAD resulted in the rescue of the control phenotype.7 To investigate the role of LATS2 in JCAD-induced TF and PAI-1 modulation, HAECs were double silenced for the two genes, the efficacy of the silencing protocol was confirmed by WB (Figure 5B). Yet, silencing of LATS2 did neither affect TNF-α-induced TF activity nor expression in JCAD-silenced cells and remained significantly lower as compared with siSCR HAECs (Figure 5C and D). Similar effects were reported for PAI-1, whose expression was not modified by LATS2 knockdown in JCAD-silenced cells (Figure 5E).
Figure 5.
LATS2 as a mediator of JCAD in HACEs. (A) Schematic of JCAD regulation of the Hippo pathway (B) Efficacy of double JCAD and LATS2 silencing by JCAD and LATS2 small interfering RNA (siJCAD and siLATS2) as compared with control siRNA (siSCR). (C-E) Double JCAD and LATS2 silencing did not retrieve TF activity, TF expression and PAI-1 levels of siJCAD cells to those observed in siSCR-treated ones. n = 6 independent experiments. BE: one-way analysis of variance (ANOVA) with Tukey post hoc test (number of comparisons = 15 for all, exception made for D where the number of comparisons = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HAEC = human aortic endothelial cell; LATS-2 = large tumour suppressor kinase 2; PAI-1 = plasminogen activator inhibitor-1; TF = tissue factor; TNF-α = tumour necrosis factor-α.
Increased PI3K/Akt activation mediates the effect of JCAD on procoagulants
Phosphoinositide 3-kinases (PI3K)/Akt is an ubiquitous anti-apoptotic protein kinase with a key role in different cellular processes. Known to modulate both TF and PAI-1 expression, recent work demonstrated that Akt can directly phosphorylate YAP, thereby down-regulating its function as transcriptional regulator. Here we demonstrated that TNF-α activated JCAD-silenced cells show higher levels of PI3K as compared with activated siSCR cells (P < 0.05, Figure 6A). As expected, increased levels of PI3K accompanied with higher activated Akt both at baseline and upon stimulation with TNF-α (P < 0.05 and P < 0.001, respectively, Figure 6B). Demonstrating a causal role for this pathway in JCAD-mediated TF and PAI-1 regulation, pre-treatment with the PI3K/Akt inhibitor wortmannin rescued the control phenotype of stimulated HAECs. Specifically, pre-treatment with wortmannin significantly increased TF levels of JCAD-silenced cells (P < 0.0001, Figure 6C) up to the level observed in stimulated siSCR cells (Figure 6C). Accordingly, TF expression as assessed by WB was also significantly increased in stimulated siJCAD upon Akt inhibition (P < 0.0001, Figure 6D). Similarly to what was shown for TF, the disruption of Akt signalling significantly increased PAI-1 expression of stimulated siJCAD cells (P < 0.0001, Figure 6E) to the level observed in siSCR ones (Figure 6E). To confirm the involvement of JCAD in the regulation of the PI3K/Akt pathway, we found a direct protein–protein interaction as shown by co-immunoprecipitation experiments (Figure 6F).
Figure 6.
PI3K/Akt pathway as a mediator of JCAD effects on HACEs. (A) JCAD silencing by mean of small interfering RNA (siJCAD) increased the levels of PI3K in HAECs stimulated with TNF-α (10 ng/mL). (B) siJCAD HAECs showed increased phosphorylation of Akt both when unstimulated or stimulated (TNF-α, 10 ng/mL) (C-E) Treatment with the PI3K/Akt inhibitor Wortmannin rescued the siSCR phenotype in siJCAD-treated HAECs in terms of TF activity, TF expression and PAI-1 levels. (F) Representative immunoblot of co-immunoprecipitation of Akt and JCAD from whole-cell lysates of stimulated and unstimulated siSCR-treated cells showing direct protein-to-protein interaction. n = 6 independent experiments. A-F: one-way analysis of variance (ANOVA) with Tukey post hoc test (number of comparisons = 6 for A-B, = 15 for C and E, = 3 for D). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HAEC = human aortic endothelial cell; PAI-1 = plasminogen activator inhibitor-1; PI3K = phosphatidylinositol 3-kinase; TF = tissue factor; TNF-α = tumour necrosis factor-α.
Treatment with wortmannin rescues the wild-type thrombotic phenotypes in jcad−/− mice
Mirroring the in vitro results, genetic deletion of JCAD is associated with increased levels of PI3K/Akt also in murine arteries (Figure 7A and B). In order to prove the causal role of PI3K/Akt on JCAD thrombosis modulation, Jcad−/− animals were treated with wortmannin before undergoing carotid photochemical-induced thrombosis in a separate set of experiments. Treatment with wortmannin significantly reduced the time to occlusion of Jcad−/− animals (P < 0.01, Figure 7C) to the level observed in Jcad+/+ mice. Of importance, pre-treatment with wortmannin did not affect initial heart rate and blood flow (Figure 7E and F).
Figure 7.
Effect of PI3K inhibition on carotid arterial thrombosis in vivo in Jcad-/− mice. (A) JCAD deletion increases arterial levels of PI3K in mice. (B) JCAD deletion increases arterial levels of phosphorylated Akt in mice. (C) Jcad−/− mice showed delayed time to formation of an occlusive thrombus in their carotid arteries after endothelial-specific damage as compared with Jcad+/+ littermates. Such an effect was eliminated by pre-treatment with the Akt inhibitor wortmannin. (D) Pre-treatment with wortmannin associated with a trend toward a reduced number of thrombus embolization episodes in Jcad−/− mice. (E-F) No difference in terms of initial heart rate and blood flow was recorded among the three study groups. n = 8 different mice per group. A-B: unpaired two-tailed Student’s t-test. C-F: one-way analysis of variance (ANOVA) with Tukey post hoc test (number of comparisons = 3). **P < 0.01. PI3K = phosphatidylinositol 3-kinase.
Patients with STEMI have high circulating JCAD protein levels which positively correlate with TF and PAI-1
To test the translational relevance of our data, plasma levels of JCAD were assessed in patients presenting with acute (i.e. STEMI) or chronic (i.e. CCS) manifestations of CAD. Given JCAD’s being mostly expressed in endothelial cells and in consideration of the fact that endothelial cells could not be easily obtained in ACS patients, we chose plasma levels of this protein as a surrogate for cellular expression. A total of 61 patients were included in the analysis, of which 39 and 22 presented with STEMI and CCS, respectively (Table 2). Interestingly, STEMI patients showed markedly elevated JCAD plasma levels as compared with those with CCS (Figure 8A), a phenomenon that was consistently observed after controlling for pre-interventional LDL cholesterol (LDL-C), high-sensitivity troponin I (hs-TnI) and dual antiplatelet therapy (DAPT) use (adjusted mean, 1.71 vs. 1.09 ng/mL; P = 0.014). When we stratified all patients according to JCAD tertiles, both TF and PAI-1 levels markedly increased across JCAD tertiles (P for linear trend = 0.0065 and 0.0183, respectively). Compared with the lowest tertile, patients in the highest JCAD tertile showed elevated TF (407.80 vs. 86.18 pg/mL; adj. P = 0.0129) and PAI-1 levels (16.57 vs. 9.45 ng/mL; adj. P = 0.037; Figure 8B and C). Notably, these differences in TF and PAI levels remained consistent after controlling for potential confounders, including DAPT use and individual LDL-C as well as hs-TnI levels (adj. mean, 378.38 vs. 94.47 pg/mL, adj. P = 0.039, and 17.31 vs. 8.68 ng/mL, adj. P = 0.036).
Table 2.
Demographic and clinical characteristics of the study cohort
| Whole cohort (n = 61) | CCS (n = 22) | ACS (n = 39) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 66 ± 12 | 71 ± 10 | 63 ± 13 | 0.016 |
| Male sex, n (%) | 53 (86.9) | 19 (86.4) | 34 (87.2) | NS |
| Clinical and biochemical | ||||
| Weight (kg) | 77 ± 13 | 78 ± 13 | 76 ± 14 | NS |
| Height (m) | 1.72 ± 0.08 | 1.72 ± 0.08 | 1.71 ± 0.09 | NS |
| BMI (kg/m2) | 26.8 ± 6.8 | 28.2 ± 9.6 | 26.1 ± 4.6 | NS |
| Haemoglobin (g/dL) | 13.7 ± 1.6 | 13.8 ± 1.6 | 13.7 ± 1.6 | NS |
| White blood cells (103/mL) | 9.36 ± 4.11 | 7.10 ± 2.22 | 10.64 ± 4.40 | 0.001 |
| Platelets (103/mL) | 221 ± 67 | 204 ± 57 | 231 ± 71 | NS |
| Total cholesterol (mg/dL) | 153 ± 34 | 149 ± 30 | 156 ± 36 | NS |
| HDL-C (mg/dL) | 42 ± 15 | 49 ± 19 | 38 ± 11 | 0.003 |
| LDL-C (mg/dL) | 89 ± 29 | 88 ± 22 | 89 ± 32 | NS |
| Triglycerides (mg/dL) | 134 ± 79 | 109 ± 42 | 148 ± 91 | 0.045 |
| Glycemia (mg/dL) | 107 ± 32 | 101 ± 32 | 110 ± 32 | NS |
| History, n (%) | ||||
| Previous ACS | 15 (24.6) | 5 (22.7) | 10 (25.6) | NS |
| Smoking | 42 (68.9) | 15 (68.2) | 27 (69.2) | NS |
| Diabetes | 18 (29.5) | 7 (31.8) | 11 (28.2) | NS |
| Hypertension | 41 (67.2) | 18 (81.8) | 23 (59.0) | NS |
| Obesity | 6 (9.8) | 1 (4.5) | 5 (12.8) | NS |
| Dyslipidaemia | 38 (62.3) | 17 (77.3) | 21 (53.8) | NS |
| Familiarity | 22 (36.1) | 8 (36.4) | 14 (35.9) | NS |
| Medications, n (%) | ||||
| Aspirin | 35 (57.4) | 19 (86.4) | 16 (41.0) | 0.001 |
| Thienopyridine | 1 (1.6) | 0 (0) | 1 (2.6) | NS |
| Clopidogrel | 10 (16.7) | 9 (40.9) | 1 (2.6) | <0.0001 |
| Prasugrel | 1 (1.6) | 0 (0) | 1 (2.6) | NS |
| Ticagrelor | 3 (4.9) | 1 (4.5) | 2 (5.1) | NS |
| Anticoagulant | 4 (6.7) | 2 (9.1) | 2 (5.1) | NS |
| ACE-I | 16 (26.2) | 7 (31.8) | 9 (23.1) | NS |
| ARB | 19 (31.1) | 10 (45.5) | 9 (23.1) | NS |
| β-blockers | 23 (37.7) | 14 (63.6) | 9 (23.1) | 0.003 |
| Calcium channel blocker | 12 (19.7) | 4 (18.2) | 8 (20.5) | NS |
| Antidiabetics | 11 (18.0) | 4 (18.2) | 7 (17.9) | NS |
| Statins | 34 (55.7) | 18 (81.8) | 16 (41.0) | 0.003 |
CCS, chronic coronary syndrome; ACS, acute coronary syndrome; BMI, body mass index; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker. Bold values are statistically significant.
Figure 8.
JCAD, TF and PAI-1 expression in patients with STEMI. (A) Circulating JCAD levels are increased in STEMI patients as compared with CCS controls. (B-C) TF and PAI-1 protein levels are increased across JCAD tertiles. n = 22 patients for CCS and n = 39 patients for STEMI. A-C: Analysis of covariance (ANCOVA). CCS = chronic coronary syndrome; PAI-1 = plasminogen activator inhibitor-1; STEMI = ST-elevation myocardial infarction; TF = tissue factor.
Discussion
This work for the first time demonstrates the deleterious effect of the recently characterized protein JCAD in regulating arterial thrombosis in vivo as well as its potential role as a therapeutic target in atherothrombosis. Data obtained in mice and translated to primary HAECs and blood samples obtained from patients with STEMI corroborate the following conclusions: (i) occluding arterial thrombosis triggered by endothelial-specific injury is delayed in mice lacking JCAD and occurs only after several cycles of thrombus formation and lysis; (ii) JCAD knockout reduced the activation of the coagulation cascade and increased fibrinolysis by acting on TF and PAI-1 without affecting platelet aggregation; (iii) JCAD silencing impairs TNFα-induced TF activity and TF and PAI-1 protein expression in primary HAECs; (iv) the intracellular PI3K/Akt pathway, but not LATS2, mediates the effect of JCAD in this setting as shown by treatment with the PI3K inhibitor wortmannin both in vitro in human cells and in vivo in mice; (v) in patients with STEMI, a clinical condition characterized by acute intracoronary thrombosis, circulating levels of JCAD are increased and they positively correlate with those of TF and PAI-1 (Structured Graphical Abstract).
The identification of novel therapeutic targets for the management of atherothrombosis remains of utmost importance in consideration of the high morbidity and mortality of its clinical complications. Here, we hypothesized that JCAD, a recently characterized endothelial junctional protein localizing at cell–cell junctions, may play a role in arterial thrombosis. This hypothesis is based on internal and external data demonstrating that ageing, vascular dysfunction and age-dependent cardiovascular conditions such as CAD are mediated by similar molecular mechanisms signalling through inflammation and free radicals.21–24 Indeed, JCAD is one of the genes described to be associated with such conditions in GWAS.4,5 Genetic variants at the JCAD locus were significantly associated with CAD and myocardial infarction. Yet, although previous experimental studies detailed the role of JCAD in favouring vascular dysfunction and atherosclerosis through the regulation of inflammation,6,8 to date no specific studies investigated whether the association between JCAD and unfavourable cardiovascular outcomes could be driven by a facilitating action on thrombus formation.
In line with our initial hypothesis, we hereby show that mice lacking functional JCAD display delayed formation of an occlusive thrombus in the carotid artery after endothelial-specific damage, irrespective of their sex. Mechanistically, the potential involvement of all three major players involved in arterial thrombosis (i.e. platelet aggregation, coagulation cascade and fibrinolysis) was investigated. Ex vivo aggregometry, FACS analysis of membrane protein expression and morphological characterization ruled out a possible contribution of platelets to the observed effects. Of interest, recent proteomic characterization of circulating platelets reported negligible levels of JCAD expression, suggesting a marginal role for this protein in regulating their function.25 Instead, significant differences were found in: (i) the activation of the coagulation cascade as proved by reduced formation of factor X activated by Jcad−/− carotid lysates at the functional assay; (ii) the fibrinolytic cascade as demonstrated by increased thrombus embolization episodes and circulating levels of D-dimer—a well-established read-out of fibrin degradation—in genetically modified animals. We identified increased TF and PAI-1 expression as mediators for the abovementioned effects. Both showing procoagulant activity, subendothelial TF works as a cofactor for circulating factor VII directly cleaving factor X and triggering the coagulation cascade when exposed after endothelial damage2 while PAI-1 is the major endogenous regulator of fibrinolysis blocking tPA- and uPA-mediated plasminogen activation.26 Of translational relevance and mirroring the in vivo data, JCAD silencing reduced TF and PAI-1 expression in vitro in stimulated primary HAECs. Furthermore, with these experiments, we could demonstrate that JCAD-mediated procoagulant modulation takes place at the transcriptional level. JCAD was previously shown to regulate the transcription of several genes involved in apoptosis, inflammation and oxidative stress.6,7 Of interest, the previously reported modulatory role exerted by JCAD on inflammatory mediators through NF-kB coupled with our experimental evidence in the experimental model of thrombosis suggests this protein is an important player in the context of thromboinflammation. Mechanistically, JCAD signals within endothelial cells through the Hippo pathway, which is involved in several downstream phenotypes.27 Specifically, JCAD inhibits the Hippo kinase LATS2, thereby blocking the phosphorylation of YAP/TAZ, which is free to enter the nucleus and activate the transcription factors of the TEAD family. Accordingly, the P-YAP/YAP ratio was increased in the arterial lysate of Jcad−/− animals as compared with control littermates. Previously, Jones et al. used LATS2 silencing to rescue the wild-type phenotype in JCAD-silenced endothelial cells.7 Yet in our hands, double JCAD and LATS2 silencing did not result in increased TF and PAI-1 transcription as seen in siSCR-treated cells, implying a secondary role for the Hippo pathway in the observed phenotypes. Our results confirmed the previously reported upregulation of LATS2 signalling however, rescue experiments focusing on LATS2 silencing did not rescue the effect on TF and PAI-1, indicating that in this specific case the observed effect is mediated by a different regulatory pathway, i.e. PI3K/Akt. On the other hand, arteries of Jcad−/− mice as well as JCAD-silenced HAECs show increased activation of the intracellular PI3K/Akt pathway, known to regulate the levels of both TF and PAI-1 at the transcriptional and post-transcriptional levels.28–30 To confirm the causative role of this pathway, we pre-treated HAECs with the selective PI3K inhibitor wortmannin, abolishing the protective effects of JCAD knockdown. Of much interest, pre-treatment of Jcad−/− mice with wortmannin rescued the wild-type thrombotic phenotype also in knockout mice undergoing photochemical carotid thrombosis. Lastly, we report that JCAD co-immunoprecipitates with Akt, implying a direct protein–protein interaction, which may account for the modulating effect of JCAD on its activation. Indeed, we and others previously showed how proteins at the cell–cell junction have not only structural roles, but they also signal by directly interacting with intracellular transduction pathways such as PI3K/Akt.20,31,32
As a proof-of-principle test for the translational relevance of our findings in the clinical setting, we investigated JCAD plasma levels in patients with STEMI or CCS. Circulating plasma levels of the protein were chosen as a surrogate marker for its endothelial expression given the difficulty of obtaining endothelial cells from living patients and were used to investigate the relationship between JCAD, TF and PAI-1 at the clinical level. Interestingly, JCAD levels were found to be increased in STEMI patients, in whom acute thrombosis of a major coronary artery is the underlying cause, as compared with those with CCS, where coagulation is not activated. These findings were consistently observed after controlling for individual lipid levels (i.e. LDL-C), antithrombotic treatment (i.e. DAPT) and estimated infarct size (i.e. hs-TnI levels). GWAS previously associated the JCAD locus with the occurrence of ACS and endothelial JCAD expression was previously reported to be increased in coronary plaques as compared with normal segments.6 Yet, to the best of our knowledge, this is the first report showing a significant upregulation of such a protein after STEMI onset. Considering previous reports showing an association between plasma procoagulant levels and major adverse cardiovascular events in patients with CAD,33 here we additionally report that patients in the highest tertile of JCAD also had the highest TF and PAI-1 levels suggesting common regulating mechanisms. Again, such differences persisted after adjustment for lipid levels, antithrombotic treatments and estimated myocardial infarct size. These data are in line with in vivo and in vitro findings and support the role of JCAD in the pathophysiology of arterial thrombosis.
The current report presents some limitations which should be considered. First, deletion of JCAD was not specific to endothelial cells; thus, we cannot fully exclude the contribution of other cell types to the reduced arterial thrombogenicity. Yet, JCAD previously showed preferential endothelial expression,6 and in our investigation we provide in additional to the in vivo data, a detailed mechanistic analysis carried using primary HAECs. Furthermore, here we ruled out a role for platelets, which are considered major regulators of arterial thrombosis together with endothelial cells. Also, although widely accepted, the use of constitutive knockout models does not exclude the possibility of compensatory adaptive mechanisms taking place over the course of their life and has limited translational value. Such limitations could be overcome by using inducible genetic models or, of higher translational relevance, by treating the animals with specific JCAD inhibitors which are currently unavailable. Finally, we consider the clinical data reported herein as hypothesis-generating and acknowledge the observational character of these results, which require external validation in independent cohorts. Indeed, observational data are subject to biases from selection, confounding, and measurement, which can result in over- or underestimation of the effect of interest. Specifically, acute myocardial infarction is a hugely inflammatory event with strong effects on acute-phase proteins.34 Although it appears unlikely that the associations between plasma JCAD and TF and PAI-1 levels are driven by such inflammatory responses, we consider the clinical data presented herein only as hypothesis-generating, which warrants external validation.
Conclusions
This study demonstrates that the inhibition of JCAD is protective against arterial thrombosis by selectively modulating coagulation and fibrinolysis, but not platelet aggregation through endothelial TF and PAI-1 expression. Our translational findings support the importance of JCAD as a novel potential therapeutic target for cardiovascular prevention and support further investigations to validate its clinical relevance.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Contributor Information
Luca Liberale, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; First Clinic of Internal Medicine, Department of Internal Medicine, University of Genoa, 6 viale Benedetto XV, 16132 Genoa, Italy.
Yustina M Puspitasari, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Stefano Ministrini, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Internal Medicine, Angiology and Atherosclerosis, Department of Medicine and Surgery, University of Perugia, piazzale Gambuli 1, 06124 Perugia, Italy.
Alexander Akhmedov, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Simon Kraler, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Nicole R Bonetti, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8092 Zurich, Switzerland.
Georgia Beer, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Ana Vukolic, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland.
Dario Bongiovanni, Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale (EOC), Lugano, Switzerland; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Department of Cardiovascular Medicine, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy; Department of Internal Medicine I, School of Medicine, University Hospital rechts der Isar, Technical University of Munich, Munich, Germany.
Jiaying Han, Department of Internal Medicine I, School of Medicine, University Hospital rechts der Isar, Technical University of Munich, Munich, Germany.
Kilian Kirmes, Department of Internal Medicine I, School of Medicine, University Hospital rechts der Isar, Technical University of Munich, Munich, Germany.
Isabell Bernlochner, Department of Internal Medicine I, School of Medicine, University Hospital rechts der Isar, Technical University of Munich, Munich, Germany.
Jaroslav Pelisek, Department of Vascular Surgery, University Hospital Zurich, Zurich, Switzerland.
Jürg H Beer, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Department of Internal Medicine, Cantonal Hospital of Baden, Im Ergel 1, 5404 Baden, Switzerland.
Zheng-Gen Jin, Department of Medicine, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA.
Daniela Pedicino, Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli-IRCCS, Largo A. Gemelli 8, Rome 00168, Italy; Cardiovascular and Pulmonary Sciences, Catholic University, Largo G. Vito, 1 - 00168 Rome, Italy.
Giovanna Liuzzo, Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli-IRCCS, Largo A. Gemelli 8, Rome 00168, Italy; Cardiovascular and Pulmonary Sciences, Catholic University, Largo G. Vito, 1 - 00168 Rome, Italy.
Konstantinos Stellos, Biosciences Institute, Vascular Biology and Medicine Theme, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, UK; Department of Cardiology, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, UK; Department of Cardiovascular Research, European Center for Angioscience (ECAS), Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany; German Centre for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislauf-Forschung, DZHK), Heidelberg/Mannheim Partner Site, Mannheim, Germany; Department of Cardiology, University Hospital Mannheim, Mannheim, Germany.
Fabrizio Montecucco, First Clinic of Internal Medicine, Department of Internal Medicine, University of Genoa, 6 viale Benedetto XV, 16132 Genoa, Italy; IRCCS Ospedale Policlinico San Martino Genoa—Italian Cardiovascular Network, L.go R. Benzi 10, 16132 Genoa, Italy.
Filippo Crea, Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli-IRCCS, Largo A. Gemelli 8, Rome 00168, Italy; Cardiovascular and Pulmonary Sciences, Catholic University, Largo G. Vito, 1 - 00168 Rome, Italy.
Thomas F Lüscher, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Heart Division, Royal Brompton and Harefield Hospitals and Nationl Heart and Lung Institute, Imperial College, London, United Kingdom.
Giovanni G Camici, Center for Molecular Cardiology, Schlieren Campus, University of Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland; Department of Research and Education, University Hospital Zurich, Rämistrasse 100, 8092 Zurich, Switzerland.
Funding
The present work was supported by Swiss Heart Foundation grants to L.L. and G.G.C., the Swiss National Science Foundation (G.G.C. [310030_197510]) to G.G.C., the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology to G.G.C. G.G.C. is the recipients the recipients of a H.H. Sheikh Khalifa bin Hamad Al Thani Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich. D.P. and G.L. thank the Ministry of health (Italy) ‘Ricerca corrente’ RC2021 and ‘Ricerca di Rete Cardiovascolare’ RCC2022 for the support. G.L. is also supported by PRIN (Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale) 2017, Prot. 2017WJBKKW_001. This study is partially supported by National Institutes of Health (NIH) grants (HL130167, HL141171) to Z.G.J.References
References
- 1. Wang M, Hao H, Leeper NJ, Zhu L, Early Career C. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol 2018;38:e90–e95. 10.1161/ATVBAHA.118.310367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breitenstein A, Camici GG, Tanner FC. Tissue factor: beyond coagulation in the cardiovascular system. Clin Sci (Lond) 2009;118:159–172. 10.1042/CS20080622 [DOI] [PubMed] [Google Scholar]
- 3. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, et al. European Society of cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–579. 10.1093/eurheartj/ehx628 [DOI] [PubMed] [Google Scholar]
- 4. Erdmann J, Willenborg C, Nahrstaedt J, Preuss M, Konig IR, Baumert J, et al. Genome-wide association study identifies a new locus for coronary artery disease on chromosome 10p11.23. Eur Heart J 2011;32:158–168. 10.1093/eurheartj/ehq405 [DOI] [PubMed] [Google Scholar]
- 5. Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet 2017;49:1385–1391. 10.1038/ng.3913 [DOI] [PubMed] [Google Scholar]
- 6. Xu S, Xu Y, Liu P, Zhang S, Liu H, Slavin S, et al. The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis. Eur Heart J 2019;40:2398–2408. 10.1093/eurheartj/ehz303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones PD, Kaiser MA, Ghaderi Najafabadi M, Koplev S, Zhao Y, Douglas G, et al. JCAD, a gene at the 10p11 coronary artery disease locus, regulates hippo signaling in endothelial cells. Arterioscler Thromb Vasc Biol 2018;38:1711–1722. 10.1161/ATVBAHA.118.310976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Douglas G, Mehta V, Al Haj Zen A, Akoumianakis I, Goel A, Rashbrook VS, et al. A key role for the novel coronary artery disease gene JCAD in atherosclerosis via shear stress mechanotransduction. Cardiovasc Res 2020;116:1863–1874. 10.1093/cvr/cvz263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gebhard C, Stampfli SF, Gebhard CE, Akhmedov A, Breitenstein A, Camici GG, et al. Guggulsterone, an anti-inflammatory phytosterol, inhibits tissue factor and arterial thrombosis. Basic Res Cardiol 2009;104:285–294. 10.1007/s00395-008-0757-5 [DOI] [PubMed] [Google Scholar]
- 10. Holy EW, Akhmedov A, Speer T, Camici GG, Zewinger S, Bonetti N, et al. Carbamylated low-density lipoproteins induce a prothrombotic state via LOX-1: impact on arterial thrombus formation in vivo. J Am Coll Cardiol 2016;68:1664–1676. 10.1016/j.jacc.2016.07.755 [DOI] [PubMed] [Google Scholar]
- 11. Breitenstein A, Stampfli SF, Reiner MF, Shi Y, Keller S, Akhmedov A, et al. The MAP kinase JNK2 mediates cigarette smoke-induced arterial thrombosis. Thromb Haemost 2017;117:83–89. 10.1160/TH16-05-0351 [DOI] [PubMed] [Google Scholar]
- 12. Stampfli SF, Akhmedov A, Gebhard C, Lohmann C, Holy EW, Rozenberg I, et al. Aging induces endothelial dysfunction while sparing arterial thrombosis. Arterioscler Thromb Vasc Biol 2010;30:1960–1967. 10.1161/ATVBAHA.110.206920 [DOI] [PubMed] [Google Scholar]
- 13. Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt Pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol 2004;24:1963–1969. 10.1161/01.ATV.0000143096.15099.ce [DOI] [PubMed] [Google Scholar]
- 14. Yu J, Liu R, Huang J, Wang L, Wang W. Inhibition of phosphatidylinositol 3-kinease suppresses formation and progression of experimental abdominal aortic aneurysms. Sci Rep 2017;7:15208. 10.1038/s41598-017-15207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dowling MR, Josefsson EC, Henley KJ, Hodgkin PD, Kile BT. Platelet senescence is regulated by an internal timer, not damage inflicted by hits. Blood 2010;116:1776–1778. 10.1182/blood-2009-12-259663 [DOI] [PubMed] [Google Scholar]
- 16. Camici GG, Steffel J, Amanovic I, Breitenstein A, Baldinger J, Keller S, et al. Rapamycin promotes arterial thrombosis in vivo: implications for everolimus and zotarolimus eluting stents. Eur Heart J 2010;31:236–242. 10.1093/eurheartj/ehp259 [DOI] [PubMed] [Google Scholar]
- 17. Payeli SK, Schiene-Fischer C, Steffel J, Camici GG, Rozenberg I, Luscher TF, et al. Cyclophilin A differentially activates monocytes and endothelial cells: role of purity, activity, and endotoxin contamination in commercial preparations. Atherosclerosis 2008;197:564–571. 10.1016/j.atherosclerosis.2007.08.025 [DOI] [PubMed] [Google Scholar]
- 18. Namdar M, Gebhard C, Studiger R, Shi Y, Mocharla P, Schmied C, et al. Globotriaosylsphingosine accumulation and not alpha-galactosidase-a deficiency causes endothelial dysfunction in fabry disease. PLoS One 2012;7:e36373. 10.1371/journal.pone.0036373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liberale L, Bertolotto M, Carbone F, Contini P, Wust P, Spinella G, et al. Resistin exerts a beneficial role in atherosclerotic plaque inflammation by inhibiting neutrophil migration. Int J Cardiol 2018;272:13–19. 10.1016/j.ijcard.2018.07.112 [DOI] [PubMed] [Google Scholar]
- 20. Diaz-Canestro C, Merlini M, Bonetti NR, Liberale L, Wust P, Briand-Schumacher S, et al. Sirtuin 5 as a novel target to blunt blood-brain barrier damage induced by cerebral ischemia/reperfusion injury. Int J Cardiol 2018;260:148–155. 10.1016/j.ijcard.2017.12.060 [DOI] [PubMed] [Google Scholar]
- 21. Camici GG, Shi Y, Cosentino F, Francia P, Luscher TF. Anti-aging medicine: molecular basis for endothelial cell-targeted strategies—a mini-review. Gerontology 2011;57:101–108. 10.1159/000314227 [DOI] [PubMed] [Google Scholar]
- 22. Shi Y, Camici GG, Luscher TF. Cardiovascular determinants of life span. Pflugers Arch 2010;459:315–324. 10.1007/s00424-009-0727-2 [DOI] [PubMed] [Google Scholar]
- 23. Liberale L, Kraler S, Camici GG, Luscher TF. Ageing and longevity genes in cardiovascular diseases. Basic Clin Pharmacol Toxicol 2020;127:120–131. 10.1111/bcpt.13426 [DOI] [PubMed] [Google Scholar]
- 24. Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020;41:2974–2982. 10.1093/eurheartj/ehz961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeiler M, Moser M, Mann M. Copy number analysis of the murine platelet proteome spanning the complete abundance range. Mol Cell Proteomics 2014;13:3435–3445. 10.1074/mcp.M114.038513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin H, Xu L, Yu S, Hong W, Huang M, Xu P. Therapeutics targeting the fibrinolytic system. Exp Mol Med 2020;52:367–379. 10.1038/s12276-020-0397-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ye J, Li TS, Xu G, Zhao YM, Zhang NP, Fan J, et al. JCAD Promotes progression of nonalcoholic steatohepatitis to liver cancer by inhibiting LATS2 kinase activity. Cancer Res 2017;77:5287–5300. 10.1158/0008-5472.CAN-17-0229 [DOI] [PubMed] [Google Scholar]
- 28. Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells: role of rho/rho-kinase and akt pathways. Circulation 2002;105:1756–1759. 10.1161/01.cir.0000015465.73933.3b [DOI] [PubMed] [Google Scholar]
- 29. Blum S, Issbruker K, Willuweit A, Hehlgans S, Lucerna M, Mechtcheriakova D, et al. An inhibitory role of the phosphatidylinositol 3-kinase-signaling pathway in vascular endothelial growth factor-induced tissue factor expression. J Biol Chem 2001;276:33428–33434. 10.1074/jbc.M105474200 [DOI] [PubMed] [Google Scholar]
- 30. Mukai Y, Wang CY, Rikitake Y, Liao JK. Phosphatidylinositol 3-kinase/protein kinase akt negatively regulates plasminogen activator inhibitor type 1 expression in vascular endothelial cells. Am J Physiol Heart Circ Physiol 2007;292:H1937–H1942. 10.1152/ajpheart.00868.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol 2007;94:29–65. 10.1016/j.pbiomolbio.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 32. Liberale L, Gaul DS, Akhmedov A, Bonetti NR, Nageswaran V, Costantino S, et al. Endothelial SIRT6 blunts stroke size and neurological deficit by preserving blood-brain barrier integrity: a translational study. Eur Heart J 2020;41:1575–1587. 10.1093/eurheartj/ehz712 [DOI] [PubMed] [Google Scholar]
- 33. Jung RG, Motazedian P, Ramirez FD, Simard T, Di Santo P, Visintini S, et al. Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thromb J 2018;16:12. 10.1186/s12959-018-0166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liebetrau C, Hoffmann J, Dorr O, Gaede L, Blumenstein J, Biermann H, et al. Release kinetics of inflammatory biomarkers in a clinical model of acute myocardial infarction. Circ Res 2015;116:867–875. 10.1161/CIRCRESAHA.116.304653 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.