Abstract
The World Health Organization (WHO) has established a target to eliminate mother-to-child-transmission (EMTCT) of hepatitis B virus (HBV), defined as a prevalence of hepatitis B surface antigen (HBsAg) of ≤0.1% among children, by 2030. Using nationally representative serosurveys to verify achievement of this target requires large sample sizes and significant resources. We assessed the feasibility of a potentially more efficient two-phase method to verify EMTCT of HBV in Colombia. In the first phase, we conducted a risk assessment to identify municipalities at the highest risk of ongoing HBV transmission. We ranked the 1122 municipalities of Colombia based on the reports of HBV infection in pregnant women per 1000 population. Municipalities with ≥0.3 reports per 1000 persons (equating to the top quartile) were further assessed based on health facility birth rates, coverage with three doses of hepatitis B vaccine (HepB3) and seroprevalence data. Hepatitis B risk was considered to be further increased for municipalities with HepB3 coverage or health facility birth rate <90%. In the second phase, we conducted a multistage household serosurvey of children aged 5–10 years in 36 municipalities with the highest assessed HBV risk. HBsAg was not detected in any of 3203 children tested, yielding a 90% upper confidence bound of <0.1% prevalence. Coverage with HepB3 and hepatitis B birth dose was high at 97.5% and 95.6%, respectively. These results support the conclusion that Colombia has likely achieved EMTCT of HBV.
Keywords: Colombia, hepatitis B, hepatitis B vaccines, immunization, seroprevalence
1 |. INTRODUCTION
Globally, an estimated 296 million people live with chronic hepatitis B virus (HBV) infection, with approximately 820,000 deaths attributed to these infections annually.1 The probability of developing chronic HBV infection is inversely related to the age at which the infection is acquired; up to 90% of persons infected as infants develop chronic HBV infection compared with <5% of persons infected after the age of 5 years.2 To prevent these infections, WHO recommends that all children receive 3–4 doses of the hepatitis B vaccine with the first dose given as soon as possible after birth, ideally within 24 h of birth.2
The WHO Global Health Sector Strategy on Viral Hepatitis which aims to eliminate hepatitis B as a public health problem by 2030 includes a target to reduce HBV incidence by 95% as measured by a hepatitis B surface antigen (HBsAg) prevalence of ≤0.1% among children.3 The Pan American Health Organization (PAHO) included a similar target in their framework to achieve elimination of mother-to-child transmission (EMTCT) of HIV, syphilis, hepatitis B and Chagas disease (EMTCT-PLUS).4
Nationally representative serosurveys have been used to verify the achievement of HBsAg prevalence targets of 1%–2% that were established for hepatitis B control in several regions.5 However, using this approach to estimate HBsAg prevalence levels of 0.1% with precision of ±0.5% requires large sample sizes (approximately 15,000 children) not feasible in most settings. With the issuance of interim global guidance for the verification of viral hepatitis elimination,6 more resource-efficient approaches for collecting relevant data could benefit countries seeking evidence to verify achievement of EMTCT of HBV.
A two-phased approach used to verify maternal and neonatal tetanus elimination (MNTE), which is defined as <1 case of neonatal tetanus (NT) per 1000 births, provides an example of an alternative strategy to verify occurrence of rare events.7 First, a risk assessment is conducted to identify the areas of a country that are considered to have the highest risk of NT based on programmatic indicators (e.g., tetanus immunization coverage among women of reproductive age, health facility births). Second, a community-based survey is conducted in the areas assessed to be at the highest risk to identify any NT deaths. The rationale for this two-phased approach is that if the highest risk areas have met the MNTE target, it can be assumed that areas of lower risk and therefore, the whole country will have met the goal. In Colombia, the availability of years of geographically detailed data relevant to hepatitis B, including immunization coverage, maternal and child health indicators and surveillance/seroprevalence data provided the opportunity to pilot a similar approach to assess EMTCT of HBV.
Hepatitis B vaccine was introduced in Colombia in high-risk areas in 1992 and nationwide in 1994, with hepatitis B vaccine birth dose (HepB-BD) added in 2001.8 From 2005 to 2020, average coverage with three doses of hepatitis B vaccine (HepB3) was 91% (range: 85%–94%), while coverage with HepB-BD was 84% (range: 74%–93%).9 A serosurvey done in 1999 in the Colombian Amazon, a region with high rates of chronic HBV infection, showed a reduction of 72% in the prevalence of HBV infection, chronic or resolved, in children ages 5–9 years compared with 199210 and that the prevalence of chronic HBV infection in children under 8 years of age was 0.4% compared with 3% in children aged 8–11 years. Given these results in a high-risk area and the high rates of hepatitis B vaccination maintained during the last decade in Colombia, the prevalence of HBV infection in children was thought to be near the elimination target of 0.1 percent. This study aims to provide evidence to confirm if Colombia has achieved HBV elimination among children and to assess the feasibility and usefulness of a two-phase approach to validate the EMTCT of HBV.
2 |. METHODS
2.1 |. Phase 1: Identification of municipalities in Colombia with the highest likelihood of HBV infection in children
A group of national and international experts on hepatitis B reviewed existing data relevant to assessing the current risk of HBV transmission in Colombia. The criteria considered in evaluating the potential usefulness of each data source for inclusion in the risk assessment included completeness, validity and level of geographic detail. Data were compiled from public health monitoring systems, including the National Public Health Surveillance System (SIVIGILA—its acronym in Spanish) of the National Institute of Health; the Vital Statistics Record (EEVV—its acronym in Spanish) of the National Administrative Department of Statistics, and the Expanded Immunization Program (PAI—its acronym in Spanish) of the Ministry of Health and Social Protection. The main variables included in the final risk assessment were the number of HBV infections in pregnant women per 1000 inhabitants (hereafter called HBV infections in pregnant women) reported through SIVIGILA, coverage with three doses of pentavalent vaccine (which includes hepatitis B) reported by PAI, and the percentage of births occurring in health facilities (hereafter called facility birth rate) reported in EEVV. Municipality-level data for these data sources were available for the last 10 years. Published seroprevalence data were also considered. Data sources that were reviewed but not incorporated in the risk assessment included reports of HBsAg reactivity from blood banks and surveillance data for groups other than pregnant women. These data were not used because they were not geographically linked or were considered to predominantly represent transmission among adults in high-risk groups for HBV infection (e.g., people who inject drugs, men who have sex with men). HepB-BD coverage was not included because those data were linked to the municipality of the delivery hospital, which often differed from the mother’s municipality of residence. Instead, the facility birth rate was considered more geographically valid and used as a proxy for the likelihood of receiving HepB-BD.11
For the assessment, we first ranked the municipalities of Colombia according to the average number of HBV infections in pregnant women reported during the years 2008–2013. We identified the municipalities with a rate equal to or above the top 75th percentile of infection rates. The HBV risk in those municipalities was further characterized based upon the average HepB3 coverage and facility birth rate during the same years; the assessed risk level was increased for municipalities where either indicator was less than 90 percent.
2.2 |. Phase 2: Targeted serological survey
2.2.1 |. Survey design
We conducted a population-based multistage cluster survey of households in the identified high-risk municipalities to enroll children aged 5–10 years. The minimum age of 5 years was used because infections acquired through these years reflect the cumulative burden of infections due to MTCT and early childhood transmission.2 The target age range was expanded to include children aged up to 10 years to improve the feasibility of the study, with the expectation that, since immunization coverage has been high in Colombia for more than 10 years, the children in those five age cohorts would have had similar access to immunization and reflect the overall impact of vaccination during that 5-year period.
The survey was designed with the primary purpose of classification12 to assess the null hypothesis that the prevalence of HBsAg in children aged 5–10 years living in those areas was at or below the EMTCT HBV target of 0.1%. Using the Fleiss formula13 with a continuity correction, and assuming a type I error of 0.05 and type II error of 0.10, the sample size needed to assess the null hypothesis against an alternative threshold of 0.4% was 2284 children. This sample size has a high probability (90% power) of rejecting the null hypothesis of ≤0.1% if the true prevalence is ≥0.4%, but the power is lower if the true prevalence is between 0.1% and 0.4%. Balancing the goals of the assessment and the resources required for implementing the survey, this was considered an acceptable compromise. Adjusting for an estimated design effect of 1.1 and a 20% non-response rate, the minimum sample size was 3015 children. Based on 2018 population projections indicating an average of 0.41 children 5–10 years old per household and a desired cluster size of 30 households per enumeration area (EA), an estimated 395 EAs were needed to achieve the target sample size. In the first stage, EAs, stratified by urban/rural, were randomly selected with probability proportional to size from a list of all EAs in the selected municipalities from the 2018 National Households, Homes and Persons Population Census (VIHOPE—its acronym in Spanish) from the National Administrative Department of Statistics (DANE—its acronym in Spanish).14 EAs were divided into segments of approximately 30 households, of which one segment was randomly selected. All inhabited buildings in the selected segment were visited to identify households with age-eligible children. In households with more than one 5–10-year-old child, one was randomly selected to participate in the study.
2.2.2 |. Survey planning and implementation
The survey was conducted in February–March 2019 by eight teams. Teams included a supervisor and an enumerator experienced in implementing population-based studies and four nurses/nursing assistants who interviewed participants and performed the tests. Team members received a 7-day training on study implementation.
Before implementation, the project was discussed with local government authorities and official representatives of Indigenous Peoples and Organizations to obtain their support. A plan for referral of infected persons for further care was established with the departmental/municipal health bureaus and health care providing institutions/companies.
During the survey, the teams visited each household in the selected segment where they verified the age and residence for each selected child, described the goals and methods of the study and provided information about hepatitis B. After obtaining informed consent of the parent/caretaker, the team administered a questionnaire to collect sociodemographic information (ethnicity, place of birth, country of birth, school enrollment and coverage with Social Security Healthcare System [SSHS]) and the immunization history of the selected child. Immunization history was verified with the vaccination card when available; however, in absence of a card, the oral report of the parent/caretaker was recorded. In Colombia, there are four types of affiliation to SSHS: contribution-based, subsidized, special and excepted schemes. The contribution-based scheme is for employed workers or retirees who can pay their contributions; the subsidized regime covers persons who cannot afford payment. Excepted and special programs cover certain groups (e.g., military, teachers) outside of SSHS. Data were collected by electronic tablets, using an application developed in CsPro.
With the assent of the child, a blood sample of approximately 75 μL was taken by fingerprick and tested using the VIKIA® HBsAg rapid test (bioMérieux SA). This test, with a reported sensitivity of 98.9% and specificity of 99.8%,15 was WHO-prequalified and provides results to participants in 30 min.
2.2.3 |. Data analysis
Survey data were analysed with SAS® software, Version 9.4. (SAS® Institute Inc.). In addition to basic frequencies describing the survey sample, we calculated population-level estimates of immunization coverage and HBsAg seroprevalence accounting for the stratified cluster design and sampling weights based on the probability of selection of each child.
We analysed vaccination coverage based on vaccination card, oral report by caregivers, and either source. Timely HepB-BD vaccine was defined as one received on the day of birth or the day after, as indicated by the vaccination records or oral report. Rao-Scott Chi-square statistics were calculated to assess associations between socio-demographic factors and vaccination coverage.
2.2.4 |. Ethical considerations
The survey protocol was reviewed by the U.S. Centers for Disease Control and Prevention (CDC) under human research protection procedures and was approved by the Profamilia Research Ethics Committee and the PAHO/WHO Research Ethics Review Committee.
3 |. RESULTS
3.1 |. Phase 1: Risk assessment of municipalities
The 1122 municipalities of Colombia were ranked based on the number of reported HBV infections in pregnant women; a total of 102 municipalities had ≥0.3 reports per 1000 population which equated to the 75th quartile. Subsequently, we identified 34 municipalities at the highest risk of hepatitis B based on HepB3 coverage <90% and/or facility birth rates <90%. Six of those 34 municipalities were excluded for security reasons and replaced by the next highest-risk municipality from the same department. Two municipalities (Mitú and Fundación) from the departments of Vaupés and Magdalena, respectively, were added based on input from national experts and historical evidence indicating high-risk for hepatitis B in those areas.16,17 The 36 municipalities assessed as most at risk of having ongoing HBV transmission were located across 16 of Colombia’s 32 departments. Of those departments, 11 (Amazonas, Antioquia, Cesar, Chocó, Guainía, Guaviare, La Guajira, Magdalena, Nariño, Norte de Santander and Vaupes) had been previously documented to have moderate or high burden of hepatitis B16,17,18,19,20,21 and five (Boyacá, Cundinamarca, Huila, Putumayo and Santander) had not. Figure 1 shows the municipalities included in the study. The parameters used for the classification and selection of municipalities can be found in Appendix A. Among the 36 selected municipalities, reports of HBV infection in pregnant women were ≥0.3 per 1000 population in 34, the facility birth rate was <90% in 17, and HepB3 coverage was <90% in 24 municipalities. Twenty-nine municipalities met two or more of these criteria. Based on the 2018 demographic projections, these municipalities represent 137,660 children aged 5–10 years or approximately 4% of the children in this age group in Colombia.
FIGURE 1.
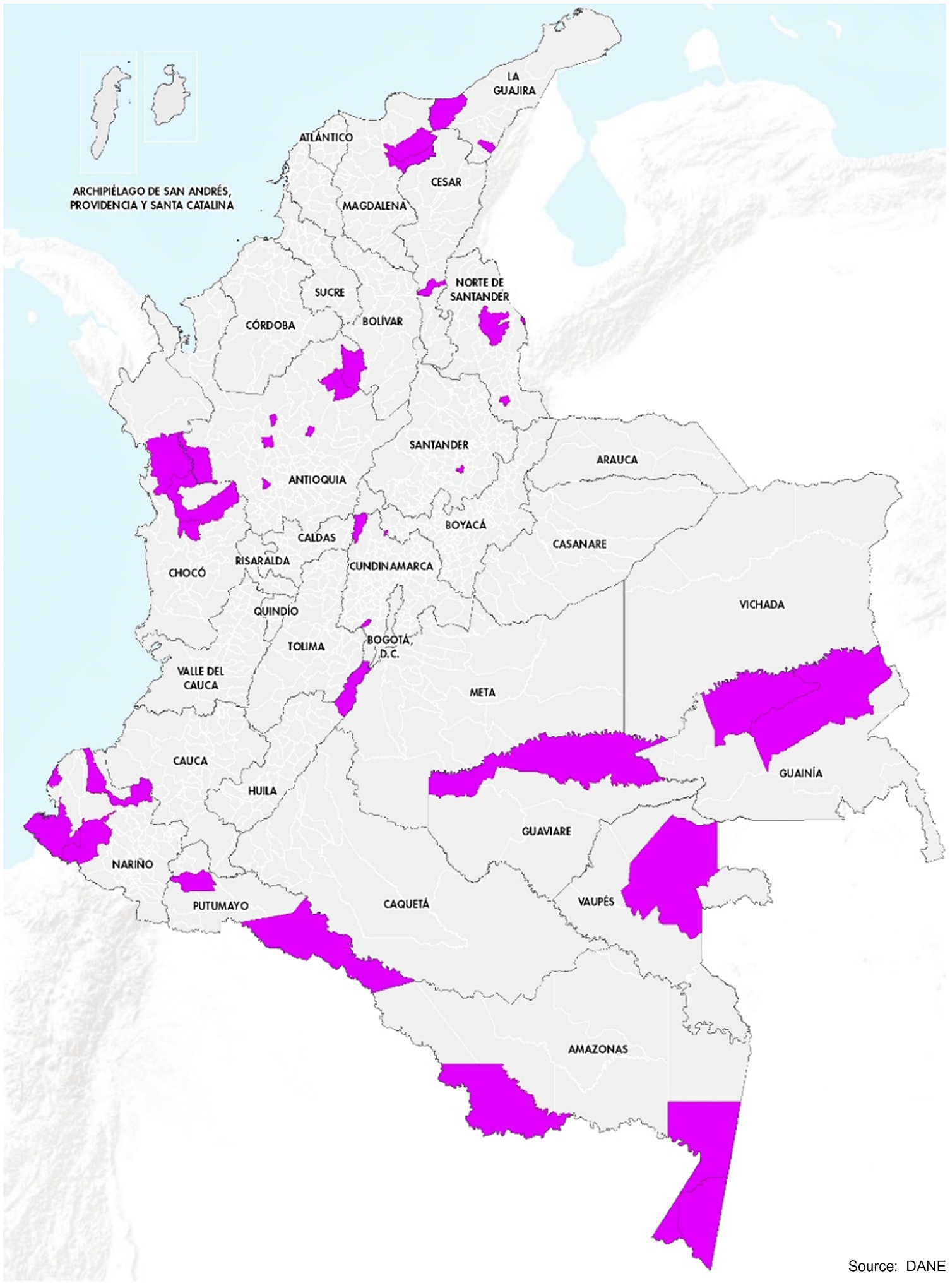
Municipalities that were considered at high-risk for HBV transmission and included in the serosurvey to assess progress towards elimination of hepatitis B virus infection in children aged 5–10 years in Colombia, 2019
3.2 |. Phase 2: Serological survey
3.2.1 |. Characteristics of enrolled children
Of the 36 targeted municipalities, one (Puerto Leguizamo) was not surveyed because the supply of HBsAg test kits was exhausted. In two selected segments of Bojayá and Inírida municipalities, no occupied households were found. In total, 3409 households with eligible children were identified and 3203 children aged 5–10 years were enrolled for a participation rate of 94%.
Of the 3203 children enrolled (Table 1), 96.3% were born in Colombia and of the 3.7% born elsewhere, 76% were born in Venezuela; 41.7% were of Afro-Colombian origin, 14.5% were indigenous and 43.7% did not identify as any particular ethnic group. According to the reports of parents/guardians, 87.1% of the children enrolled were born in a health institution and 12.5% were born at home. A skilled birth attendant was present for 95.3% of the births (84.5% were attended by doctors, 8.0% by midwives and 2.8% by nurses). The majority (79%) were covered by the SSHS subsidized scheme, while 14.6% were covered by contribution-based affiliates or through exception/special programs and 5.7% had no coverage. Approximately 96% of participating children were enrolled in school.
TABLE 1.
Sociodemographic characteristics of children aged 5–10 years enrolled in serosurvey to assess HBV infection—Colombia, 2019
| Characteristic | Children enrolled N = 3203 | Percent (%) |
|---|---|---|
| Location of residence | ||
| Rural | 1741 | 54.4 |
| Urban | 1462 | 45.6 |
| Ethnicity | ||
| Indigenous | 464 | 14.5 |
| Afrocolombian | 1337 | 41.7 |
| Other identified ethnicity | 2 | 0.1 |
| No identified ethnicity | 1400 | 43.7 |
| Type of health insurancea | ||
| Contributive | 383 | 12.0 |
| Subsidized | 2529 | 79.0 |
| Exception/Special | 85 | 2.7 |
| Unaffiliated/None | 183 | 5.7 |
| Unknown | 23 | 0.7 |
| Country of birth | ||
| Colombian born | 3084 | 96.3 |
| Foreign born | 119 | 3.7 |
| Location of delivery | ||
| Born in health facility | 2789 | 87.1 |
| Born at home | 401 | 12.5 |
| Born elsewhere or unknown | 13 | 0.4 |
| Immunization card | ||
| Yes, and available | 1741 | 54.4 |
| Yes, but not available | 1193 | 37.2 |
| No | 269 | 8.4 |
| Education level | ||
| Preschool or less | 970 | 30.3 |
| Primary | 2109 | 65.8 |
| Secondary | 6 | 0.2 |
| Not in school | 115 | 3.6 |
| Unknown | 3 | 0.1 |
In Colombia, there are four types of affiliation to Social Security Healthcare System (SSHS): contribution-based, subsidized, special and excepted schemes. The contribution-based scheme is for persons with a work contract, retirees and independent workers who can pay their contributions; the subsidized scheme is for persons who cannot afford payment and are granted a subsidy amount. Excepted/special programs provide coverage to certain groups (e.g., military, teachers) outside of SSHS.
3.2.2 |. Immunization history
Among the children enrolled, 54.4% had a vaccination card available for review by the survey team; 37.2% reportedly had a card but it was unavailable for review and 8.4% had no card. Card retention was highest among indigenous children (72.6%) and lowest among children not covered by SSHS (30.6%) and foreign-born children (45.4%).
Coverage with HepB3 was 95.4% as shown in Table 2. Overall coverage with HepB-BD was 95.6%; coverage with a timely HepB-BD was 71.1% (Table 2). Among those with card confirmed receipt of HepB-BD for whom complete information was available, 71.4% were vaccinated on their day of birth or the day after, 10.3% were vaccinated 2–7 days after birth, and 18.4% received the first dose of hepatitis B vaccine more than 7 days after birth.
TABLE 2.
Coverage with hepatitis B vaccines among children 5–10 years in selected municipalities at high-risk for HBV transmission—Colombia, 2019
| Vaccination card (n = 1741) | Verbal report (n = 1462) | Either source (n = 3203) | ||||
|---|---|---|---|---|---|---|
| Vaccine | N | % (Wilson 95% CI) | N | % (Wilson 95% CI) | N | % (Wilson 95% CI) |
| Hepatitis B Birth Dose | ||||||
| Any | 1663 | 50.3 (47.4–53.3) | 1371 | 45.3 (42.4–48.3) | 3034 | 95.6 (94.7–96.4) |
| Timelya | 1099 | 32.2 (29.9–34.6) | 1157 | 38.9 (36.0–41.9) | 2256 | 71.1 (68.5–73.6) |
| Pentavalent vaccineb | ||||||
| Any | 1713 | 51.8 (48.8–54.7) | 1389 | 45.7 (42.9–48.6) | 3102 | 97.5 (96.7–98.1) |
| 3 doses (HepB3) | 1687 | 51.0 (48.1–53.9) | 1348 | 44.4 (41.6–47.2) | 3035 | 95.4 (94.3–96.3) |
Defined as Days 0 or 1 for card confirmed, ≤24 h of birth for oral report.
Includes hepatitis B, diphtheria, pertussis, tetanus and H. influenzae type B.
Compared to the relevant reference groups indicated in Table 3, receipt of HepB-BD was significantly lower among children who were indigenous (any dose: 89.0%; timely dose: 55.4%, p ≤ 0.0001 for both) or were foreign-born (any dose: 86.3%; timely dose 55.4%, p ≤ 0.0001 and p = 0.003, respectively), those born at home (any dose: 83.8%; timely dose: 23.0%, p ≤ 0.0001 for both), and those not covered by SSHS (any dose: 90.1%; timely dose: 63.4% p ≤ 0.0001 and p = 0.003, respectively). Overall coverage with HepB-BD was similar in urban and rural areas (96.4% vs. 94.3%); however, rural children were significantly (p ≤ 0.0001) less likely to receive a timely birth dose (66.6% vs. 77.8%). Lower HepB3 coverage was noted in children not covered by SSHS (87.6%, p ≤ 0.0001), those born at home (87.8%, p ≤ 0.0001) and those who were foreign-born (76.6%), p ≤ 0.0001).
TABLE 3.
Sociodemographic characteristics associated with Hepatitis B birth dose (HepB-BD) and three dose (HepB3) coverage among children 5–10 years of age in selected high-risk municipalities—Colombia, 2019
| HepB-BD (any) | HepB-BD (timelya) | HepB3 | ||||
|---|---|---|---|---|---|---|
| n | % (Wilson 95% CI) | n | % (Wilson 95% CI) | n | % (Wilson 95% CI) | |
| Zone | ||||||
| Urban (N = 1462) | 1400 | 96.4 (95.0–97.4) | 1115 | 77.8 (74.8–80.6) | 1374 | 95.2 (93.5–96.4) |
| Rural (N = 1741) | 1634 | 94.3 (92.7–95.6) | 1141 | 66.6 (62.2–70.7)** | 1661 | 95.8 (94.4–96.9) |
| Ethnic group | ||||||
| Indigenous (N = 464) | 403 | 89.0 (84.8–92.2)** | 246 | 55.4 (47.0–63.4)** | 431 | 93.8 (90.2–96.1) |
| Afrocolombian (N = 1337) | 1271 | 95.6 (94.0–96.8) | 964 | 74.6 (70.2,78.5) | 1280 | 95.6 (93.9–96.8) |
| Neither of the above (N = 1402) [ref] | 1360 | 97.3 (96.0–98.2) | 1046 | 77.4 (74.6–79.9) | 1324 | 95.6 (93.9–96.8) |
| Type of health insuranceb | ||||||
| Contributive (N = 383) [ref] | 371 | 97.8 (95.7–98.8) | 307 | 80.5 (74.5–85.3) | 370 | 98.0 (96.1–99.0) |
| Subsidized (N = 2529) | 2396 | 95.4 (94.4–96.3) | 1750 | 72.5 (69.5–75.3) | 2409 | 95.3 (93.9–96.3) |
| Special/Excepted (N = 85) | 84 | 99.5 (94.8–100.0) | 69 | 89.1 (80.6–94.1) | 84 | 99.7 (95.1–100.0) |
| Unaffiliated (n = 183) | 162 | 90.1 (83.7–94.2)** | 114 | 63.4 (52.4–73.3)* | 152 | 87.6 (80.7–92.2)** |
| Place of birth | ||||||
| Health institution (N = 2789) | 2704 | 97.4 (96.6–98.1) | 2188 | 80.4 (78.0–82.6) | 2665 | 96.6 (95.7–97.3) |
| Home (N = 401) | 323 | 83.8 (78.6–87.9)** | 65 | 23.0 (16.7–30.7)** | 362 | 87.8 (81.3–92.3)** |
| Country of birth | ||||||
| Colombia (N = 3084) | 2935 | 96.0 (95.0–96.7) | 2196 | 74.4 (71.9–76.8) | 2946 | 96.1 (95.0–97.0) |
| Other (N = 119) | 99 | 86.3 (77.3–92.1)** | 60 | 55.4 (42.4–67.6)* | 89 | 76.6 (64.7–85.5)** |
Defined as Days 0 or 1 after birth for card confirmed vaccination, ≤24 h of birth for oral report.
In Colombia, there are four types of affiliation to Social Security Healthcare System SSHS). The contribution-based scheme is for persons with a work contract, retirees and independent workers who can pay their contributions; the subsidized scheme is for persons who cannot afford payment and are granted a subsidy amount. Excepted/special programmes provide coverage to certain groups (e.g., military, teachers) outside of SSHS.
Rao-Scott chi square, p < =0.005.
Rao-Scott chi square, p < =0.0001.
3.2.3 |. Hepatitis B prevalence
Of 3203 children tested, none were found to have HBsAg. The upper bound of the 90% confidence interval was 0.093%, estimated using the Korn and Graubard method.22 When p = 0, the adjusted n is equivalent to the sample size, and the formula is then equivalent to the Clopper–Pearson exact method. The Clopper–Pearson is known to be conservative.23,24 This result supports the conclusion that the prevalence among children in the surveyed areas was likely to be ≤0.1%.
4 |. DISCUSSION
Using a novel approach to verify the elimination of hepatitis B in children in Colombia, we found no HBV infections among a representative sample of 3203 children from 36 municipalities most at risk of having ongoing transmission of HBV, with an upper confidence limit for the prevalence of HBsAg that was <0.1%. Assuming that the prevalence of HBV in the rest of the country would be the same or lower, this result accompanied by a finding of high coverage with HepB3 and HepB-BD, despite being in some of the most remote and economically challenged parts of Colombia, provide strong evidence that Colombia has achieved the regional and global target for EMTCT of HBV. This conclusion was further supported by a modelling exercise done by the Ministry of Health with the Center for Disease Analysis Foundation which complemented and corroborated our study findings [personal communication, Center for Disease Analysis Foundation].
Although immunization coverage was generally high, some disparities were noted. Coverage with HepB3 was lower among those unaffiliated with SSHS, those born at home, or those who were foreign-born. These children as well as indigenous children were also more likely to have not received hepB-BD or to have received it late. Although coverage with HepB-BD was similar in rural and urban areas, rural children were more likely to receive delayed HepB-BD. More than 25% of the children confirmed by card as having received a HepB-BD received it two or more days after birth. The efficacy of HepB-BD in preventing MTCT of HBV is the highest when given within 24 h of birth and diminishes steadily with no added benefit when given after 7 days.2 Given the high proportion of births occurring in facilities, efforts should be made to ensure all infants receive HepB-BD before being discharged. In addition, educating pregnant women during antenatal care visits regarding the need for a timely HepB-BD and providing the HepB-BD during post-natal care visits as has been done in the Western Pacific Region could be helpful to reach children who are born at home.25 Our findings also highlight the importance of addressing disparities in vaccination coverage among indigenous children and of vaccinating all children coming from neigh-bouring countries with at least three doses of hepatitis B vaccine.
This novel approach for verifying EMTCT of HBV, based on a similar approach used to validate MNTE, proved to be feasible in Colombia. We focused the risk assessment at the municipality level because national experts thought the risk of HBV transmission was unlikely to be evenly distributed across an entire department and that, given the expected low frequency of HBV infections in children, the planned serosurvey should be focused on smaller areas of well-defined risk to be able to have a high probability of identifying these rare cases. The risk assessment identified many areas that were previously known to have a high HBV burden but also identified areas not previously highlighted as having significant HBV transmission. We excluded six municipalities due to security issues but the potential for bias was minimized by replacing them with municipalities in the same department which were similar to the excluded municipalities in terms of the assessed indicators (Appendix B).
Given the data requirements needed to conduct a useful risk assessment, the approach used in Colombia might not be applicable in all settings and is likely to be most practical in countries where surveillance and immunization coverage data are available for lower sub-national levels. Even in Colombia, although there were many types of data sources available for consideration in the risk assessment, several could not be used because of limitations in geographic detail or representativeness. For example, although geographically detailed information on the receipt of HepB-BD was available, we were not able to use this important variable in the risk assessment because the location recorded was linked to the municipality where the vaccine was received, which was often not the same as the location of residence. Similarly, although pregnant women in Colombia are screened for HBsAg during antenatal care, there is no database linking the results of those tests. For this evaluation, we used facility birth rate as a proxy for likelihood of receiving HepB-BD11 and assumed reports of hepatitis B in pregnant women received through the national notifiable disease surveillance system largely represented the results of ANC screening.
There were also challenges associated with the serosurvey. Although the geographic scope of the survey was less than that of a nationally representative survey, the resources required were still substantial because of the logistic challenges associated with accessing remote or insecure areas. Given the high levels of school attendance, implementation of the survey in a school setting might have reduced the resources required. However, at the time of survey design, available information suggested that there were geographic disparities in school attendance that might have affected the representativeness of results obtained from a school-based survey.
Because of the range of circumstances and data sources in different countries, a variety of approaches will likely be necessary to verify hepatitis B elimination. The integration of hepatitis B testing into other large representative surveys (e.g., multi-indicator cluster surveys or Demographic and Health surveys [MICS/DHS], population-based HIV surveys) would facilitate the collection of data to assess progress towards elimination if they include an adequate number of children to detect low-frequency events. Stored specimens collected for previous surveys or other purposes might also be used if there are sufficient specimens and they are representative of the population of interest. The integration of hepatitis B screening during pregnancy as part of PAHO’s EMTCT-PLUS strategy,4 and the establishment of systems to track HBV infection among pregnant women and follow-up testing infants born to HBsAg positive women would also facilitate collection of evidence needed for future assessments regarding the elimination of MTCT of HBV.
In conclusion, this study used a novel two-phased approach to provide evidence to validate the elimination of MTCT of HBV in Colombia. The inclusion of a risk assessment in the first phase allowed available survey resources to be focused on the areas at the highest risk for HBV, maximizing the likelihood of detecting these rare events. Countries with sub-national level data on hepatitis B prevalence in women of reproductive age, hepatitis B vaccination coverage, and other useful indicators for risk assessment could consider this method to validate hepatitis B elimination. This survey could inform the development of methods for country validation of viral hepatitis elimination which are currently underway.6
ACKNOWLEDGEMENTS
Brock Stewart, CDC; Viviana Calderón and Jenny Carolina Peralta, PAHO; Sidia Caicedo, MoH; Rodolfo González, DANE; and Danny Rivera, Profamilia. Additionally, many thanks go to the epidemiologic surveillance and virology laboratory departments of the National Institute of Health of Colombia, the participating representatives from PAI and SSR in the departments included in the serosurvey, the survey teams of Profamilia and the families and children that participated in the study.
Funding information
This study was funded by the U.S. Centers for Disease Control and Prevention (CDC) through a cooperative agreement with the Pan American Health Organization (PAHO).
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- EA
enumeration area
- EMTCT
elimination of mother-to-child-transmission
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HepB3
three doses of hepatitis B vaccine
- HepB-BD
hepatitis B vaccine birth dose
- MNTE
maternal and neonatal tetanus elimination
- MTCT
mother-to-child transmission
- NT
neonatal tetanus
- PAHO
Pan American Health Organization
- WHO
World Health Organization
APPENDIX A. Epidemiologic Characteristics of Municipalities at high-risk for HBV transmission included in Serosurvey to Assess the Burden of HBV infection in children—Colombia, 2019
| Average 2008–2013 | Historical Prevalence Studies | ||||||
|---|---|---|---|---|---|---|---|
| HBV in pregnant women (per 1000 inhabitants) | 3rd dose coverage with pentavalent vaccine (%) | Institutional delivery rate (%) | |||||
| ALL MUNICIPALITIES (n = 1122) |
25% quartile, median, and 75% quartile |
0.09 0.17 0.33 |
71.2%, 82.8% 92.6% |
87.6% 95.1% 98.1% |
|||
| SELECTED MUNICIPALITIES (n = 36) | Municipality | HBsAg | antiHBc | References | |||
| Department | |||||||
| AMAZONAS | Puerto Nariño | 2.82 | 70.5% | 60.2% | 8.0% | 54.6% | 16 |
| ANTIOQUIA | Armenia | 1.89 | 73.5% | 93.2% | 6.5% | 17,18 | |
| GUAINÍA | Inírida | 1.84 | 99.7% | 87.6% | 67.6% | 17 | |
| ANTIOQUIA | El Bagre | 1.83 | 92.1% | 96.5% | 6.5% | 17,18 | |
| ANTIOQUIA | Vigía Del Fuerte | 1.81 | 91.7% | 96.0% | 6.5% | 17,18 | |
| ANTIOQUIA | Guadalupe | 1.59 | 83.2% | 93.4% | 6.5% | 17,18 | |
| AMAZONAS | Leticia | 1.57 | 99.9% | 88.4% | 8.0% | 54.6% | 16 |
| AMAZONAS | El Encanto | 1.44 | 50.3% | 50.5% | 15.0% | 61.8% | 16,19 |
| GUAINÍA | Barranco Minas | 1.42 | 33.0% | 69.2% | 67.6% | 17 | |
| GUAVIARE | San José Del Guaviare | 1.31 | 81.6% | 97.7% | 13.5% | 20 | |
| ANTIOQUIA | Liborina | 1.23 | 89.9% | 93.4% | 6.5% | 17 | |
| SANTANDER | Confines | 1.23 | 83.3% | 100.0% | NA | ||
| ANTIOQUIA | Zaragoza | 1.07 | 85.0% | 98.0% | 6.5% | 17,18 | |
| CUNDINAMARCA | Tibacuy | 1.04 | 75.7% | 87.5% | 0.8% | 17 | |
| BOYACÁ | La Victoria | 1.00 | 84.1% | 85.0% | NA | ||
| NARIÑO | San Andrés De Tumaco | 0.92 | 72.0% | 99.5% | 35.0% | 17 | |
| NARIÑO | Barbacoas | 0.91 | 80.4% | 90.6% | 35.0% | 17 | |
| CHOCÓ | Quibdó | 0.89 | 92.8% | 98.6% | 4.4% | 18.4% | 16,18 |
| HUILA | Colombia | 0.84 | 82.7% | 69.5% | NA | ||
| AMAZONAS | Tarapacá | 0.82 | 55.4% | 61.6% | 8.0% | 54.6% | 16 |
| ANTIOQUIA | Toledo | 0.82 | 75.6% | 90.9% | 6.5% | 17,18 | |
| NORTE DE SANTANDER | Cácotaa | 0.79 | 86.9% | 100.0% | 53.0% | 93.0% | 17 |
| NORTE DE SANTANDER | Puerto Santandera | 0.69 | 98.8% | 82.6% | 53.0% | 93.0% | 17 |
| NORTE DE SANTANDER | Sardinataa | 0.66 | 88.2% | 74.5% | 53.0% | 93.0% | 17 |
| CUNDINAMARCA | Puerto Salgar | 0.66 | 53.6% | 93.6% | 0.8% | 17 | |
| CHOCÓ | Río Quito | 0.59 | 63.1% | 71.5% | 3.7% | 18.4% | 16 |
| LA GUAJIRA | Dibulla | 0.58 | 94.8% | 94.2% | 4.4% | 35.0% | 17 |
| CESAR | Pelaya | 0.57 | 102.6% | 96.6% | 18.6% | 56.6% | 17 |
| CHOCÓ | Bojayá | 0.50 | 88.0% | 75.1% | 3.7% | 18.4% | 16 |
| NARIÑO | El Charco | 0.47 | 71.2% | 66.3% | 5.3% | 17%−35% | 17 |
| MAGDALENA | Aracataca | 0.44 | 97.8% | 98.5% | 5.1% | 33.3% | 16,21 |
| PUTUMAYO | Puerto Leguízamo | 0.43 | 98.5% | 84.1% | NA | ||
| LA GUAJIRA | El Molino | 0.42 | 73.0% | 98.4% | 4.4% | 35.0% | 17 |
| PUTUMAYO | Villagarzón | 0.32 | 93.3% | 60.4% | na | ||
| MAGDALENA | Fundaciónb | 0.15 | 92.4% | 99.9% | 8.4% | 31.6% | 16 |
| VAUPÉS | Mitúb | 64.9% | 58.0% | 25.3% | 43.7% | 17 | |
| ≥0.3/1000 | <90% | <90% | |||||
These municipalities replaced initially selected municipalities listed in Appendix B which were excluded due to security issues.
These municipalities were included based on expert opinion.
Purple: HBV rate in pregnant women ≥ 0.3/1000 inhabitants; Yellow: 3rd dose coverage with pentavalent vaccine (<90%); Pink: Institutional delivery rate (<90%).
APPENDIX B. Epidemiologic Characteristics of Municipalities at high-risk for HBV transmission that were excluded from the Serosurvey to Assess the Burden of HBV infection in children because of security issues—Colombia, 2019
| HBV rate in pregnant women (per 1000 inhabitants) | 3rd dose coverage with pentavalent vaccine (%) | Institutional delivery rate (%) | ||
|---|---|---|---|---|
| NORTE DESANTANDER | El Tarra | 1.69 | 105.2% | 88.6% |
| NORTE DESANTANDER | Convención | 1.14 | 106.2% | 95.8% |
| NORTE DESANTANDER | Teorama | 1.1 | 75.0% | 84.9% |
| ARAUCA | Saravena | 1.06 | 83.5% | 95.9% |
| NORTE DESANTANDER | Ocaña | 0.86 | 101.5% | 99.7% |
| NORTE DESANTANDER | HacarI | 0.8 | 87.8% | 74.3% |
| ≥0.3/1000 | <90% | <90% |
Purple: HBV rate in pregnant women ≥ 0.3/1000 inhabitants; Yellow: 3rd dose coverage with pentavalent vaccine (<90%); Pink: Institutional delivery rate (<90%).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
DECLARATION OF RESPONSIBILITY
The findings and conclusions in this paper are those of the authors and do not necessarily reflect the position of CDC nor PAHO.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.World Health Organization (WHO). Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. World Health Organization; 2021. Accessed June 22, 2022. https://www.who.int/publications/i/item/9789240027077 [Google Scholar]
- 2.World Health Organization(WHO). Hepatitis B vaccines: WHO position paper - July 2017. WER. 2017;27(92):369–392. Accessed June 22, 2022. https://apps.who.int/iris/bitstream/handle/10665/255841/WER9227pdf?sequence=1 [Google Scholar]
- 3.World Health Organization (WHO). Global Health Sector Strategy on Viral Hepatitis 2016–2021: World Health Organization; 2016. Accessed June 22, 2022. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=08F696C68D4F7E62CF24A3DEE1C6D227?sequence=1 [Google Scholar]
- 4.Pan American Health Organization. EMTCT Plus. Framework for Elimination of Mother-Tochild Transmission of HIV, Syphilis, Hepatitis B, and Chagas PAHO; 2017. Accessed June 22, 2022. https://iris.paho.org/bitstream/handle/10665.2/34306/PAHOCHA17009-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- 5.World Health Organization (WHO). Documenting the Impact of Hepatitis B Immunization: Best Practices for Conducting a Serosurvey. World Health Organization; 2011. Accessed June 22, 2022. http://whqlibdoc.who.int/hq/2011/WHO_IVB_11.08_eng.pdf [Google Scholar]
- 6.World Health Organization (WHO). Interim Guidance for Country Validation of Viral Hepatitis Elimination. World Health Organization; 2021. Accessed June 22, 2022. https://www.who.int/publications/i/item/9789240028395 [Google Scholar]
- 7.World Health Organization (WHO). Validation of maternal and neonatal tetanus elimination, including a guide to the use of lot quality assurance – cluster sample surveys to assess neonatal tetanus mortality. Updated Field Version – 2014.
- 8.Ropero Álvarez AM, Pérez-Vilar S, Pacis-Tirso C, et al. Progress in vaccination towards hepatitis B control and elimination in the region of the Americas. BMC Public Health. 2017;17:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). WHO and UNICEF Estimates Time Series for Colombia. World Health Organization; 2020. Accessed June 22, 2022. https://immunizationdata.who.int/pages/coverage/HEPB.html?CODE=COL&ANTIGEN=HEPB_BD+HEPB3&YEAR= [Google Scholar]
- 10.De la Hoz F, Pérez L, de Neira M, Salón AJ. Eight years of hepatitis B vaccination in Colombia with a recombinant vaccine: factors influencing hepatitis B virus infection and effectiveness. J Int J Infect Dis. 2008;12:183–189. [DOI] [PubMed] [Google Scholar]
- 11.Allison RD, Patel MK, Tohme RA. Hepatitis B vaccine birth dose coverage correlates worldwide with rates of institutional deliveries and skilled attendance at birth. Vaccine. 2017;35:4094–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. World Health Organization Vaccination Coverage Cluster Surveys: Reference Manual. World Health Organization; 2018. Accessed June 22, 2022. https://apps.who.int/iris/handle/10665/272820. License: CC BY-NC-SA 3.0 IGO [Google Scholar]
- 13.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions, Third Edition. John Wiley & Sons; 2003. [Google Scholar]
- 14.Censo Nacional de Población y Vivienda – CNPV 2018 – DANE. Accessed June 22, 2022. https://www.dane.gov.co/index.php/estadisticas-por-ema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018
- 15.WHO. BioMérieux VIKIA® HBs Ag WHO reference number: PQDx 0284–016–00. 2018. Accessed June 22, 2022. https://cupdf.com/document/who-prequalification-of-in-vitro-diagnostics-public-report-rapid-test-for-the.html?page=1
- 16.Alvarado-Mora MV, Gutierrez Fernandez MF, Gomes-Gouveˆa MS, de Azevedo Neto RS, Carrilho FJ, et al. Hepatitis B(HBV), hepatitis C(HCV) and Hepatitis Delta (HDV) viruses in the Colombian population—how is the epidemiological situation? PLoS ONE. 2011;6(4):e18888. doi: 10.1371/journal.pone.0018888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buitrago GB. Patología Geográfica - Historia Natural de las Hepatitis B y D en Colombia. Biomédica. 1991;11:1–4. [Google Scholar]
- 18.Ríos Patiño D, di Filippo D, Insuasty M, Rendón JC, Ríos WA, et al. Infección por el virus de la hepatitis B en individuos con factores de exposición en Quibdó y Apartadó. Rev Col Gastroenterol. 2015;30(1):11. [Google Scholar]
- 19.De la Hoz F, Duran M, Gamarra A, Velandia MP, Rojas M. Factores de riesgo en la transmision de la hepatitis B en la Amazonia Colombiana. Biomedica. 1992;12(1):5–9. [Google Scholar]
- 20.Cedeño D, Serna MT, Parra O, Díaz BS, Llanos García F, et al. Estudio descriptivo del brote de hepatitis B en el resguardo indígena El Refugio, San José del Guaviare, 2008. IQEN. 2009;14(3):33–37. [Google Scholar]
- 21.Ljunggren KE, Patarroyo ME, Engle R, Purcell RH, Gerin JL. Viral hepatitis in Colombia: a study of the “hepatitis of the Sierra Nevada de Santa Marta”. Hepatology. 1985;5(2):299–304. [DOI] [PubMed] [Google Scholar]
- 22.Ward B kg_nchs: a command for Korn-Graubard confidence intervals and National Center for Health Statistics’ data presentation standards for proportions. Stata J. 2019;19(3):510–522. doi: 10.1177/1536867X19874221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LD, Cai TT, DasGupta A. Confidence intervals for a binomial proportion (with discussion). Stat Sci. 2001;16:101–133. [Google Scholar]
- 24.He Xiaomin, Wu Shwu-Jen. Confidence intervals for the binomial proportion with zero frequency; Paper SP10–2009, SESUG. 2009.
- 25.World Health Organization. Regional Office for the Western Pacific. (2006). Preventing Mother-to-Child Transmission of Hepatitis B: Operational Field Guidelines for Delivery of the Birth Dose of Hepatitis B Vaccine. WHO Regional Office for the Western Pacific. Accessed June 22, 2022. https://apps.who.int/iris/handle/10665/272905 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


